-
废水生物脱氮是水环境治理中一个重要的方向[1]。近年来,工艺流程短、能耗低、污泥产量少的新型生物自养脱氮工艺——厌氧氨氧化(anaerobic ammonium oxidation, Anammox)已成为这个方向的研究热点。
厌氧氨氧化菌(anaerobic ammonium oxidation bacteria,AnAOB)由于世代周期长、对环境条件要求较高,导致厌氧氨氧化工艺启动时间较长。选择和控制适合AnAOB 生长的条件,使反应器快速启动和稳定运行是目前Anammox应用于实际污水处理中亟待解决的难题[2]。Anammox生物膜工艺在一定程度上可以避免微生物的流失,并持留足够的生物量[3],是常用的Anammox工艺类型。Anammox生物膜反应器高效运行的前提是AnAOB能附着于填料表面,形成有活性的生物膜,因此,填料性能对Anammox生物膜反应器的快速启动与稳定运行有重要的影响。
现阶段对于生物膜填料的研究主要针对不同工艺类型的填料,如流化床中的聚乙烯填料[4],固定床中的海绵[5]、木质碳[5]、火山岩[5-6]、陶粒[6]等,膜生物反应器中的无纺布[7]以及混合填料[8]等;对于生物填料的改性多采用具有强氧化性的无机或有机试剂,通过化学氧化改变材料表面分子结构,或在惰性填料表面引入羧基、羰基、磺酸基等极性基团,改善材料亲水性;改性过程中须使用大量化学药剂,如硫酸、硝酸等强氧化性酸,高锰酸钾、重铬酸钾等易制毒强氧化剂及氯磺酸、甲基磺酸等有机溶剂,易对环境造成严重污染[9]。曾涛涛[6]分别以火山岩和陶粒为填料,研究2种填料对Anammox生物滤柱的脱氮效果的影响,发现火山岩填料的总氮去除率相比于陶粒提高20%,其表面富集的AnAOB相对丰度提高25%,菌群多样性更高;刘杰等[10]曾采用添加生物酶促进剂配方的新型高分子改性填料BMTM研究了其对上流式填料床生物膜反应器Anammox工艺启动的影响,发现添加改性填料BMTM后,反应器启动时间可以缩短57 d。而低温等离子体技术是对材料进行干式处理的表面处理技术[11],与传统的湿法处理不同,它无须添加化学药剂,对环境无二次污染,且处理高效,效果较好。目前低温等离子体技术主要应用于高分子材料、生物功能材料和无机填料性能的优化,以及废气、废水中各类污染物的净化处理和杀菌等方面[12]。陈冰等[13]采用低温等离子体处理苯二甲酸乙二醇酯(PET)高分子材料薄膜表面后,发现材料表面C=O和C=C双键数量增加,酯基减少,亲水性明显改善;田冶等[14]利用低温等离子体技术成功在壳聚糖膜表面接枝了N-乙烯基吡咯酮,使其接触角变小、粗糙度增加,进而导致材料表面细胞亲和性提高;侯建等[15]在处理H2S和CS2等废气问题时,发现使用低温等离子体技术对H2S和CS2的去除率可分别达到90%和70%。
由于低温等离子体改性技术无须添加化学药剂,对环境无二次污染,且对高分子材料表面性能有较好的改性效果,故本研究采用低温等离子体技术对聚氨酯填料进行了改性,对比了改性前后填料表面特性、Anammox工艺生物膜量、长期运行脱氮效果,并结合MiSeq高通量测序技术和实时荧光定量PCR(qPCR)技术对改性前后Anammox生物膜系统的微生物群落结构特征和功能基因相对丰度的变化进行了分析,为低温等离子体技术应用于Anammox生物膜工艺提供参考。
全文HTML
-
未改性填料为网状聚氨酯泡沫塑料(江苏艾勤环保科技有限公司)填料,其主要性能指标为:孔隙率97.3%,丝径0.98 mm,堆密度27 kg·m−3,将其剪切为1.0 cm×1.0 cm×1.0 cm正方体块,备用。
改性填料为未改性填料经低温等离子体技术表面处理后的填料,等离子体表面改性装置采用德国Plasma Technology GmbH公司研发的MiniFlecto®科研用小型真空等离子清洗机。低温等离子体表面处理样品时,选用氧气作为产生等离子体的气体分子,工作功率300 W,气体流量为标况(0 ℃,101.33 kPa)下6 cm3·min−1,真空度20 Pa,处理时间2 min[16]。
-
测试前,采用热压成型工艺对填料进行预处理,将其热压成膜,处理后的膜样使用JCL2000D静态接触角测量仪测试其表面的静态接触角(θ);测试液体为蒸馏水,用微量进样器将液滴缓慢滴于膜样表面,液滴与材料表面接触的瞬间采集图像,用五点拟合法计算接触角,并选择样品不同位置测量5次取平均值,测量误差控制在±0.5°;接触角测试原理参考韩玉香等[17]的研究;材料表面自由能在接触角测试的基础上依据Young方程[18]计算得到。
改性前后填料的比表面积和孔径分布采用全自动比表面积和孔隙分析仪(3Flex surface characterization analyzer from micromeritics,USA)[19],通过N2吸脱附测试进行测定。在液氮温度77 K下,以He为载气,N2为吸附气体,在相对压力p/p0为0.01~1.00,测定吸附等温线,结合BET方程计算比表面积;采用BJH法[20]计算孔容和孔径分布。
-
采用2套有效容积为300 mL的血清瓶作为Anammox生物膜小试实验装置。S1反应器内装填未改性聚氨酯固定填料,S2反应器内装填改性聚氨酯固定填料,填料填充比均为12%。接种污泥为已稳定运行1a以上的SBR中试反应器中的Anammox颗粒污泥,接种污泥浓度为2 000 mg·L−1。
实验进水为人工配水,配水组成参考相关方法[21],主要包括:(NH4)2SO4和NaNO2按需配制,KH2PO4 30 mg·L−1,MgSO4·7H2O 300 mg·L−1,NaHCO3 500 mg·L−1,CaCl2·2H2O 150 mg·L−1;微量元素Ⅰ(1 mL·L−1):乙二胺四乙酸二钠(EDTA·2Na) 6.39 g·L−1,FeSO4·7H2O 5 g·L−1;微量元素Ⅱ(1 mL·L−1):EDTA·2Na 19.11 g·L−1,H3BO3 0.014 g·L−1,ZnSO4·7H2O 0.43 g·L−1,CoCl2·6H2O 0.24 g·L−1,MnCl2·4H2O 0.99 g·L−1,CuSO4·5H2O 0.25 g·L−1,NiCl2·6H2O 0.19 g·L−1,NaMoO4·2H2O 0.22 g·L−1。进水前,对人工配水曝氮气(N2)30 min,控制溶解氧(DO)小于0.3 mg·L−1,pH为7.8~8.0。
小试实验运行周期24 h,进水15 min,反应时间23 h,静沉30 min,排水15 min,排水90%。将血清瓶放置在恒温振荡摇床中进行实验,控制摇床转速为150 r·min−1,温度为30 ℃。前10 d为启动期,进水氮负荷为100 mg·(L·d)−1,其中氨氮(
${\rm{NH}}_4^ + $ -N)为50 mg·L−1和亚硝酸盐氮(${\rm{NO}}_2^ - $ -N)为50 mg·L−1;10~70 d进水氮负荷为200 mg·(L·d)−1,其中${\rm{NH}}_4^ + $ -N 100 mg·L−1,${\rm{NO}}_2^ - $ -N 100 mg·L−1;70~120 d进水氮负荷为100 mg·(L·d)−1,其中${\rm{NH}}_4^ + $ -N 50 mg·L−1,${\rm{NO}}_2^ - $ -N 50 mg·L−1。 -
常规指标均参照国家环境保护局发布的标准方法[22]测定。氨氮(
${\rm{NH}}_4^ + $ -N):纳氏试剂分光光度比色法;亚硝酸盐氮(${\rm{NO}}_2^ - $ -N):N-(1-萘基)-乙二胺分光光度法;硝酸盐氮(${\rm{NO}}_3^ - $ -N):紫外分光光度法;总氮(TN):碱性过硫酸钾氧化紫外分光光度法;pH采用JENCO便携式酸度计测定,DO采用哈希便携式溶氧仪测定。反应器稳定运行至第45天,取挂膜填料用无菌水冲洗,锡纸包裹放入105 ℃烘箱中,恒温干燥,冷却后称质量,测定其干质量;再将干燥后的填料置于1 mol·L−1 NaOH溶液中,70 ℃条件下水浴1 h,40 Hz超声波(KQ-300DE型数控超声波清洗器)处理1 h,水洗数遍至脱落生物膜被洗掉,填料放回称量瓶烘干、冷却,称剩余物质干质量、填料干质量。填料表面生物量[23]按式(1)计算。
式中:q为1 g填料表面生物量,g;mTS为冷却后的干质量,g;mRS为剩余物后干质量,g;mMS为填料干质量,g。
稳定运行至第70天,取填料放于50 mL离心管,并取反应器出水上清液浸没,于小型自动离心机离心振荡10 min,至生物膜完全脱落,泥水混合物在转速3 000 r·min−1下离心3 min,弃去上清液,保留泥样提取DNA,做高通量测序和qPCR检测。
DNA的提取:采用土壤DNA提取试剂盒(Omega Bio-Tek,Inc.,Norcross,GA,USA),提取微生物基因组DNA。
MiSeq高通量测序:对所扩增的16S rRNA中的V3~V4区域,进行小片段基因文库的构建,并基于Illumina HiSeq测序平台对该文库进行双末端测序,经过读写拼接过滤,对所有样本的有效数据,以97%的一致性进行OTUs(operational taxonomic units)聚类,然后对OTUs的序列进行物种注释丰度分析;通过α多样性和β多样性分析揭示样本的物种组成和群落结构的差异。
1.1. 填料
1.2. 改性前后填料性能表征
1.3. 改性前后填料Anammox挂膜与脱氮性能实验
1.4. 分析方法
-
改性前后聚氨酯泡沫塑料填料的静态接触角和BET比表面积测试结果表明,改性前填料的接触角为99.24°,单点比表面积是8.98 m2·g−1,平均孔径集中在3.01 nm,经低温等离子体改性以后,接触角降为65.97°,填料的比表面积提高至9.66 m2·g−1,孔径分布集中于4.98 nm。依据Young方程计算改性前后样品表面自由能,分别由29.31 mN·m−1升高到41.39 mN·m−1,比表面积增加8%。根据低温等离子体技术处理的工作原理,在电磁场的作用下,且在一定的真空度中,氧气气体变稀薄,分子间距及分子或离子的自由运动距变长,氧气分子发生碰撞形成氧等离子体,氧等离子体在电磁场内空间运动,轰击被处理聚氨酯样品表面,引入含氧官能团(—OH、—OOH) [24],产生表面刻蚀,生成表面自由基,这使得材料表面粗糙度增加,填料亲水性能得以改善,因此,接触角会显著降低,固体表面自由能增加,提高了聚氨酯填料的生物相容性,从而更有利于微生物附着于其表面生长。
-
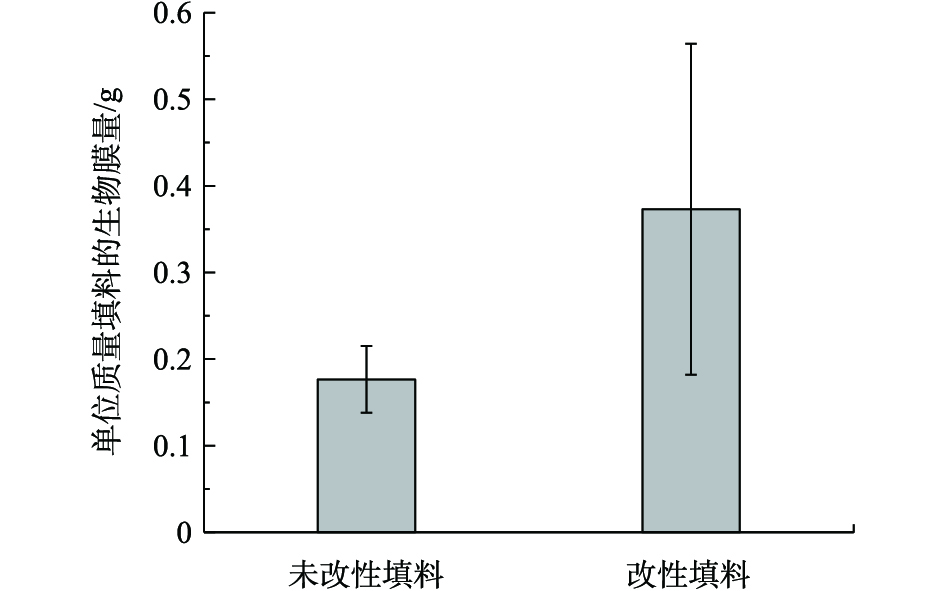
反应器运行至第45天时,取改性前后填料进行生物膜量的检测,其结果如图1所示。在稳定运行期间,未改性单位质量填料生物膜平均干质量为0.18 g,改性后填料的质量填料生物膜平均干质量为0.37 g,改性后,相同时间内单位质量填料上的生物膜量相比于改性前提高了53%。这主要是因为采用低温等离子体技术表面处理后,填料表面粗糙度增加,提高了填料的亲水性,有利于细胞黏附生长,故加速生物膜的形成过程,加快了填料挂膜启动速度。同时,改性处理增加了填料比表面积,改性后填料表面生物膜量亦显著增加。
-
未改性填料S1反应器和改性填料S2反应器同时接种Anammox种泥,进水N负荷为100 mg·(L·d)−1,
${\rm{NH}}_4^ + $ -N为50 mg·L−1,${\rm{NO}}_2^ - $ -N为50 mg·L−1(如图2所示)。第3天开始,S1、S2中的${\rm{NH}}_4^ + $ -N和${\rm{NO}}_2^ - $ -N同步去除,对TN的去除率均可以达到70%左右,消耗${\rm{NO}}_2^ - $ -N与消耗${\rm{NH}}_4^ + $ -N的比值和生成${\rm{NO}}_3^ - $ -N与消耗${\rm{NH}}_4^ + $ -N的比值分别为1.12、0.22和2.02和0.13,与理论的比值1.32和0.26接近,此时2个系统的TN去除主要由AnAOB完成,AnAOB的活性开始恢复。从第4天开始,S1和S2中TN去除率在80.07%~91.84%,对${\rm{NO}}_2^ - $ -N的去除率维持于96.84%以上,对${\rm{NH}}_4^ + $ -N的去除率维持在57.88%~83.67%。当反应器稳定运行至第10天时,提升进水N负荷至200 mg·(L·d)−1,
${\rm{NH}}_4^ + $ -N为100 mg·L−1,${\rm{NO}}_2^ - $ -N为100 mg·L−1,此后连续运行60 d。在此期间,S1反应器${\rm{NH}}_4^ + $ -N、${\rm{NO}}_2^ - $ -N、TN平均去除率分别为81.07%、97.14%、84.97%,S2反应器${\rm{NH}}_4^ + $ -N、${\rm{NO}}_2^ - $ -N、TN平均去除率分别为80.51%、96.08%、84.20%;消耗${\rm{NO}}_2^ - $ -N和消耗${\rm{NH}}_4^ + $ -N的实际比值在理论比值1.32左右,但生成${\rm{NO}}_3^ - $ -N与消耗${\rm{NH}}_4^ + $ -N的比值均低于理论比值0.26,这可能是存在部分反硝化,反硝化利用的有机碳源可能来源于微生物死亡释放的有机碳[25-26]。当运行至第71天,将进水N负荷降至100 mg·(L·d)−1,目的是为后期处理低氨氮实际废水做驯化。此间S1反应器
${\rm{NH}}_4^ + $ -N、${\rm{NO}}_2^ - $ -N、TN平均去除率分别为86.45%、98.98%、84.73%,S2反应器${\rm{NH}}_4^ + $ -N、${\rm{NO}}_2^ - $ -N、TN平均去除率分别为88.28%、99.30%、87.01%。在整个运行期间,2个体系脱氮效率基本相同,这可能是由于本研究所采用的进水基质负荷较低,水力停留时间较长,使得整个运行期间2个系统保持了基本一致的高脱氮效率。 -
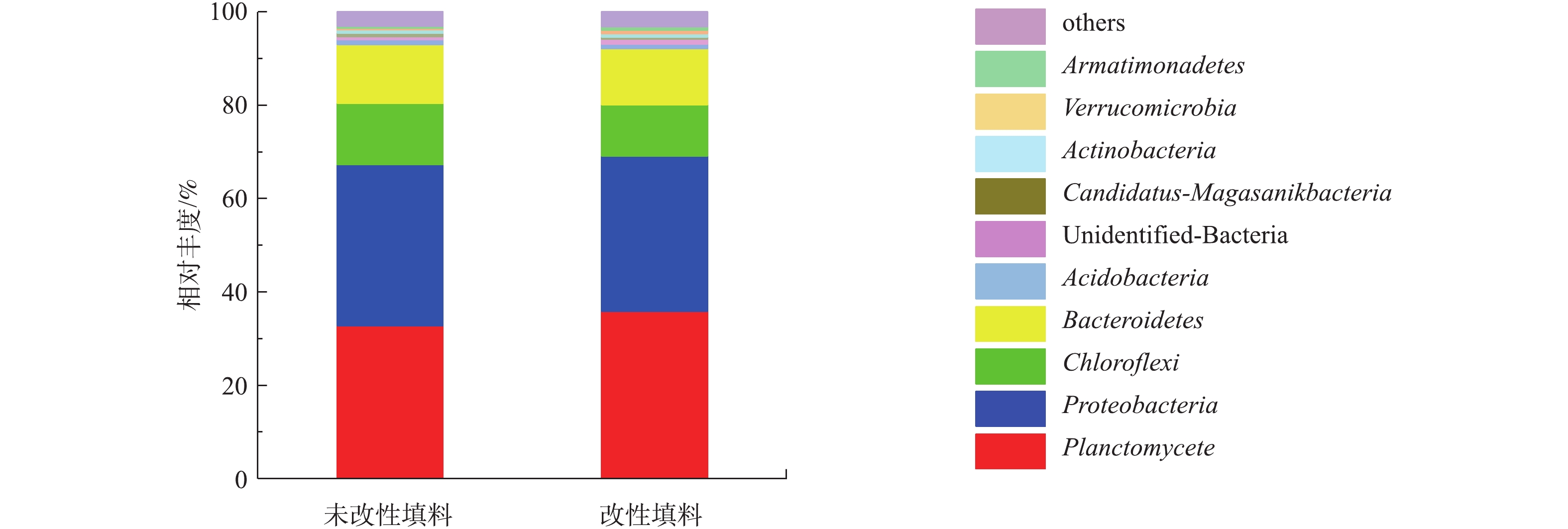
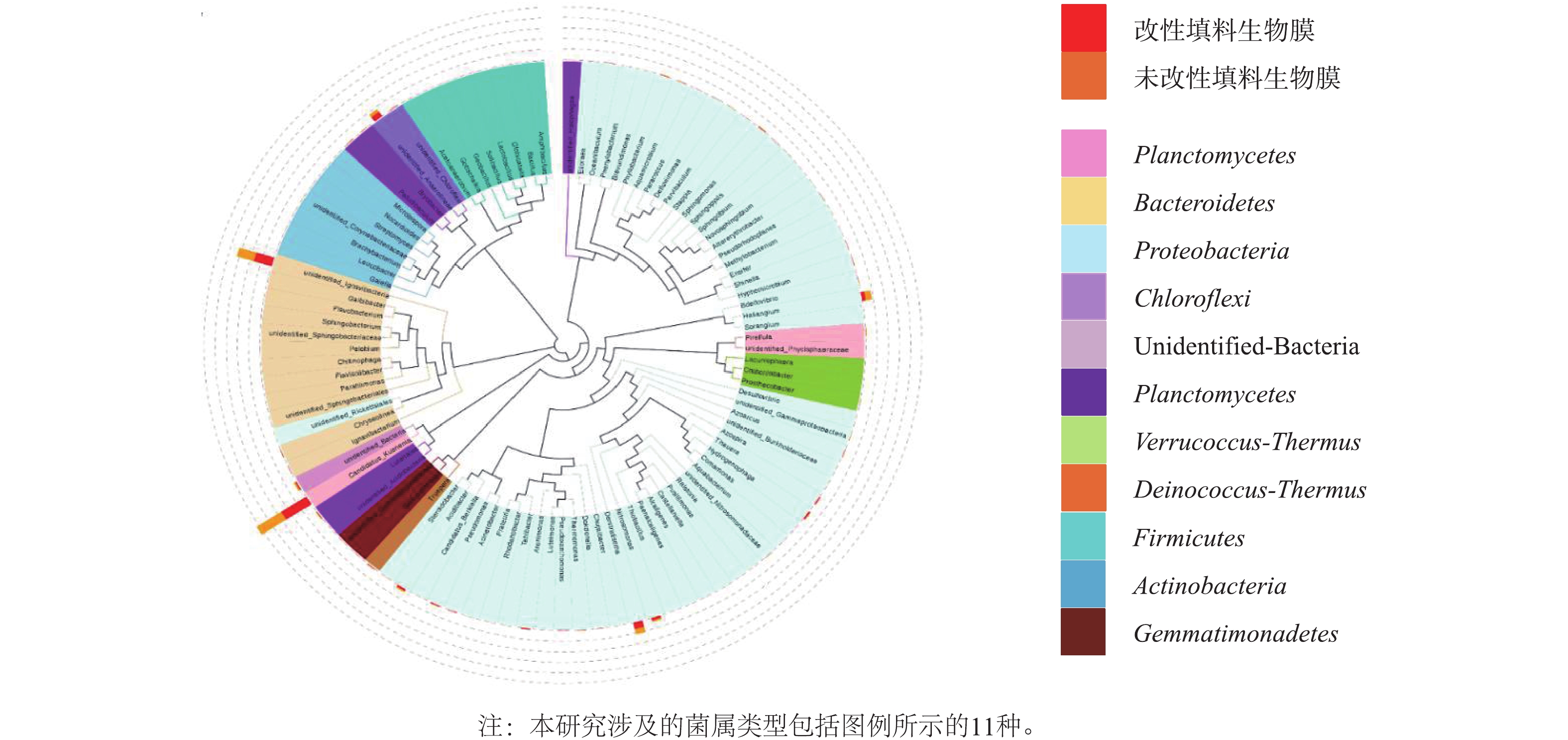
1)生物膜微生物群落结构特征分析。采用MiSeq高通量测序技术分析填料改性前后生物膜上的特异性DNA片段;对样本的有效数据,以97%的一致性进行OTUs聚类,并对OTUs的序列进行物种注释,根据注释结果对改性前后填料上的细菌在各分类水平(门、纲、目、科、属)上进行结构组成和组间对比分析,其中门水平上的物种相对丰度如图3所示;并选取样品序列比对后的top100属的代表序列,绘制属水平物种系统发育树如图4所示。
由图3可知,填料改性前后生物膜上的微生物菌群结构在门水平上相似,其所含优势微生物菌群主要包括浮霉菌门(Planctomycetes)、变形菌门(Proteobacteria)、绿弯菌门(Chloroflexi)、拟杆菌门(Bacteroidetes)、酸杆菌门(Acidobacteria)等[27]。其中,填料改性前后生物膜上浮霉菌门(Planctomycetes)相对丰度均最高,分别是17.55%和17.56%,王衫允[27]研究亦发现,低温低氨氮条件下Anammox颗粒污泥的优势菌群以浮霉菌门为主,其相对丰度为16.69%;AnAOB属于分支很深的浮霉菌目的厌氧氨氧化菌科[28],故其丰度最高。除此之外,拟杆菌门(Bacteroidetes)、变形菌门(Proteobacteria)和绿弯菌门(Chloroflexi)的相对丰度也较高;拟杆菌门(Bacteroidetes)在2种填料生物膜上分别占10.93%和10.72%,拟杆菌门是一类对有机碳源适应性较强的菌群,属于化能有机营养菌,能够代谢碳水化合物,降解复杂有机物[29];变形菌门(Proteobacteria)分别占9.58%和9.50%,有研究[30-31]表明,变形菌门是在大多数生物脱氮过程中起主要作用的微生物种类。
为进一步揭示改性前后微生物的变化,从属水平上对物种分析(图4)发现,Candidatus Kuenenia是主要的AnAOB功能菌属,相对丰度分别是17.55%和17.56%;其中填料改性前后变化较大的菌群是变形菌门(Proteobacteria),其氨氧化菌(AOB)亚硝化单胞菌Nitrosomonas由3.72%降至1.96%,反硝化菌(DNB)脱硝酸盐单胞菌属Denitratisoma[32]由3.45%升至4.04%,DNB的Haliangium属由3.72%降至1.96%。填料改性后生物膜上氨氧化菌(AOB)亚硝化单胞菌Nitrosomonas丰度降低,Denitratisoma属相对丰度增加的原因可能是改性填料相比于未改性填料,生物膜生长速度更快,生物膜内层的厌氧环境为该菌属的快速繁殖提供了有利条件;Haliangium属是一类与有机质降解相关的反硝化菌群[33-34],填料改性前生物膜上含量较多的原因可能是未经低温等离子体处理的聚氨酯填料表面残留有机质,为Haliangium属提供了有利的生长环境。可见,填料改性前后表面生物膜AnAOB丰度远高于其他菌属,保证了2个体系较高的氮去除率,且体系中均含有AOB、DNB,它们与AnAOB共同组成脱氮功能菌群,以适应进水氮浓度的微小波动,保证了稳定的脱氮效果。
2) 生物膜微生物群落多样性分析。采用MiSeq高通量测序技术测定填料改性前后生物膜上的特异性DNA片段,对2个样品所得有效序列进行归一化(64 695)处理后,按照97%的相似度计算Alpha多样性指数,如表1所示,Alpha多样性指数包括ACE指数、Chao1指数、Shannon指数和Simpson指数,指数越大,说明物种丰富度越高[35-36]。由Alpha多样性分析指数结果可知,2个样品的测序深度指数覆盖率均高于99%,这说明测序结果可以全面有效地分析微生物群落结构;根据厌氧氨氧化污泥菌群丰富度指数ACE和Chao1的分析结果可知,改性填料的ACE和Chao1指数为866.03和847.85,其分别高于未改性填料对应的816.57和803.05,这表明填料改性后填料上生物膜菌群丰富度增加;填料改性后,指示种群多样性和群落复杂程度的Shannon和Simpson指数均增加,这说明改性填料生物膜上的功能性微生物种群多样性提高。
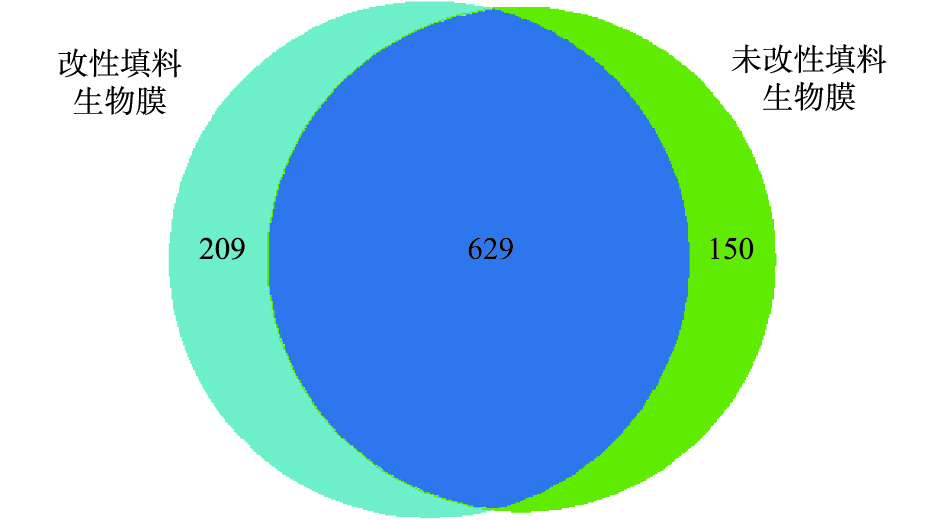
基于填料改性前后2个样品的OTUs数目及分布水平绘制OTUs的Venn图,结果如图5所示。Venn图可以直观地反映样品间的OTUs数目及不同样品间的差异性水平[37]。由图5可知,在2个典型的改性前后填料的样品中,一共含有988条OTUs,两者共有的OTUs占OTUs总数的63.66%,共计629条,并分别占各自OTUs总数的75.06%(改性填料OTUs总数838)和80.74%(未改性填料OTUs总数779);对于不同填料独有OTUs而言,改性填料含有209个OTUs,未改性填料含有150个OTUs,分别占各自OTUs的24.94%和19.26%,这进一步说明改性填料上附着的生物膜有更丰富的生物多样性。
-
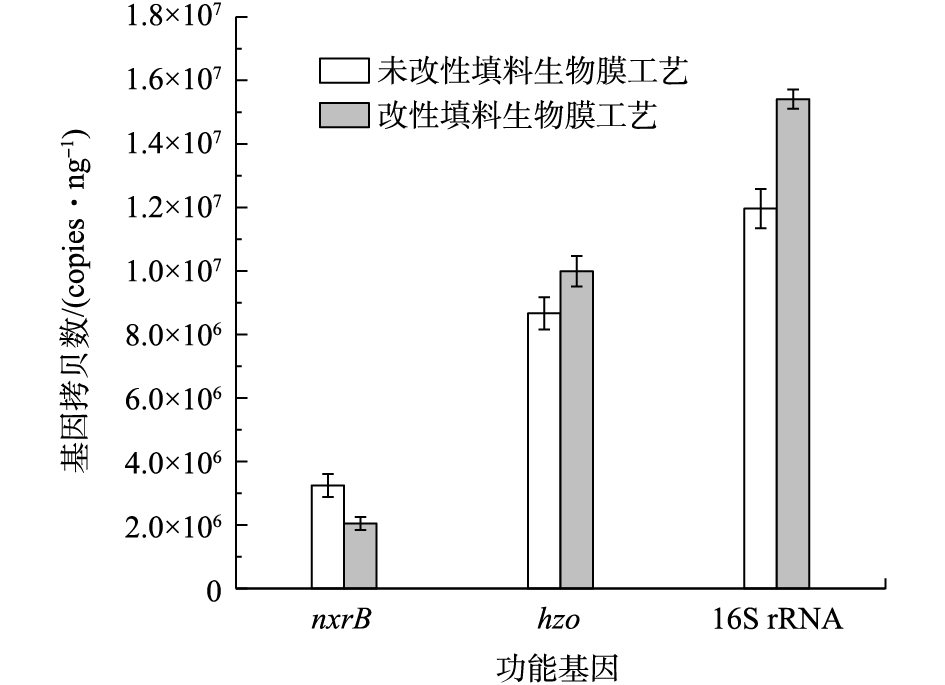
应用qPCR技术,对填料改性前后生物膜上功能微生物的基因丰度进行检测,选择的目的功能基因包括hzo基因、nxrB基因及16S rRNA的基因。16S rRNA是全细菌目的基因,以此作为hzo基因、nxrB基因相对丰度的基准值,各基因定量结果如图6所示。其中hzo基因是联氨氧化还原酶(hydrazine oxidoreductase)的功能基因,联氨氧化还原酶可以将厌氧氨氧化过程中的中间产物联氨N2H4催化氧化成N2[6, 38];NXR基因是亚硝酸氧化还原酶(nitrite oxidoreductase)的功能基因,它既可以催化亚硝酸盐氧化为硝酸盐,也可以催化硝酸盐还原为亚硝酸盐,因此,有研究[39]将NXR作为NOB的生物标记,而nxrB是编码NXR的β亚纲活性位点的功能基因之一。由图6可以看出,hzo基因和nxrB基因拷贝数都在1×106copies·ng−1的数量级,改性前后hzo基因相对丰度分别为59.50%、73.50%,改性前后nxrB基因相对丰度分别为21.10%和17.70%。由此可见,改性后hzo基因丰度提高了14%,nxrB基因丰度减少了3%,这表明改性后的系统含hzo基因的菌群数量增多,进一步提高改性后Anammox生物膜工艺系统的进水氮负荷,系统可能仍会维持较高的氮去除率。含nxrB基因菌群减少,可能是由于NOB受到一定的抑制。
2.1. 改性前后填料表面性能
2.2. 改性前后填料应用于Anammox生物膜工艺启动和稳定运行
2.3. 填料改性前后对Anammox工艺启动和稳定运行期间脱氮效果分析
2.4. 稳定运行期间,改性前后填料生物膜微生物群落结构和多样性分析
2.5. 改性前后功能微生物基因丰度的变化
-
1)经低温等离子体技术改性后,聚氨酯泡沫塑料接触角由99.24°降至65.97°,比表面积由8.98 m2·g−1提高至9.65 m2·g−1,材料表面粗糙度增加,亲水性得到明显改善。
2)以改性前后聚氨酯泡沫塑料作填料,启动Anammox生物膜工艺,工艺稳定运行120 d,TN去除率均在80%以上。经低温等离子体技术处理后的聚氨酯泡沫塑料的填料,相同时间内其单位质量填料生物膜干质量为0.37 g,较未改性前的0.18 g提高了53%,生物挂膜速度显著提升。
3)高通量测序分析结果表明,改性前后生物膜上存在的主要功能菌属是Candidatus Kuenenia,改性填料生物膜上微生物种群多样性更高;qPCR结果表明,改性后hzo基因丰度提高了14%,nxrB基因丰度减少了3%,改性后的系统含hzo基因的菌群数量增多,进一步提高改性后Anammox生物膜工艺系统的进水氮负荷,系统可能仍会维持较高的氮去除效率。含nxrB基因菌群减少,可能是由于NOB受到一定的抑制。



 下载:
下载: