-
畜禽养殖废水含有高浓度有机物、氨氮、悬浮固体和相当数量的病原体及特定结构的有毒物质[1-2],经过好氧处理后,色度亦急剧升高,是地表水及地下水的主要污染源[3]。目前,有研究者[4]认为,畜禽养殖废水处理是一个难题,并给予了极大的关注。处理畜禽养殖废水的传统技术主要有还田技术、生态修复技术和生化处理技术[5],但是对其中多环有机物去除效果不太理想,因其分子结构复杂、化学性质稳定,使用传统方法无能为力,处理后的出水COD和色度仍然居高不下。因此,因水制宜,建立不同区域养殖废水处理模式已经成为当前的研究重点。
臭氧氧化法因其具有高效、经济、操作简便和脱色效果明显等优点,在提高难生化降解有机废水的可生化性以及显色废水的脱色方面得到了广泛应用。而单独臭氧氧化法一直面临臭氧利用效率低下、污染物去除效果不佳以及成本高昂等缺点。在非均相催化条件下,已有研究[6]表明,能够促进臭氧向羟基自由基(HO·)的转变,可提高臭氧利用率及有机物矿化效率。非均相催化臭氧化技术不仅能更加高效地分解O3产生HO·,而且催化剂以固态形式存在,在弱酸至弱碱环境中,金属元素基本不溶出[7-8],具有工艺流程简单、催化效率高、易分离、可重复利用等优点。许珊珊等[9]研究了MgO/GAC在臭氧化敌草隆和乙酸中的活性,结果表明MgO/GAC能使臭氧化的效率提高约15%~35%;将Fe-Ce/GAC催化剂用于催化降解模拟高浓度腐殖酸废水,可使COD、腐殖酸去除率分别比单纯臭氧氧化提高了40.3%、31.8%[10];使用负载铈的黄铁矿烧渣催化臭氧氧化水中活性黑5,在pH为3~10时,Ce-PyC均能保持稳定高效的催化活性,TOC去除率可达到80%[11]。但有关负载型Mn-Fe-Ce/γ-Al2O3催化剂的制备以及三元催化剂的应用目前仍鲜有研究,尤其是奶牛养殖废水应用微气泡臭氧非均相催化氧化的方法进行处理,其在单独臭氧氧化、普通催化剂/臭氧体系与三元催化剂/臭氧体系的降解效果与机理都值得深入研究。
本研究采用浸渍焙烧法制备了负载型Mn-Fe-Ce/γ-Al2O3催化剂,分别对制备条件和工艺条件进行了优化,通过SEM、XRF、BET和XRD等分析手段表征了催化剂的结构和物理化学特性,并与微气泡臭氧构成了非均相催化臭氧氧化体系,以某奶牛养殖基地一级生化处理出水为处理对象,考察了该非均相臭氧催化剂的催化活性。
-
将γ-Al2O3小球用去离子水清洗,后放于烘箱中干燥,在65 ℃的条件下干燥。通过浸渍焙烧法制备三元催化剂,取一定量的硝酸锰、硝酸铁和硝酸铈,将其溶于蒸馏水中,充分搅拌,定容至100 mL后,形成前驱体浸渍液,溶液中硝酸锰、硝酸铁和硝酸铈的质量分数分别为1%、1%和1.5%;将20 g γ-Al2O3小球浸入前驱体浸渍液中,分别经振荡浸渍和静态浸渍,其中振荡浸渍为12 h,振荡条件:振荡温度为30 ℃,振荡速度为180 r·min−1;静态浸渍12 h,静态温度为30 ℃条件下放置。将金属负载于γ-Al2O3小球表面,滤去浸渍液,取出催化剂并将其放入温度为65 ℃的烘箱中干燥12 h;将干燥后的催化剂置于坩埚后,放于温度为600 ℃的马弗炉中焙烧4 h,焙烧后即制得三元负载型Mn-Fe-Ce/γ-Al2O3催化剂。
-
采用场发射扫描电子显微镜(SEM,SU8220型,日立公司,日本)观察催化剂的微观形貌;催化剂中的元素含量使用能量色散型X射线荧光光谱仪(XRF,EDX-7000,岛津公司,日本)测定;使用全自动快速比表面与孔隙度分析仪(BET,ASAP2460型,Micromeritics公司,美国)测定催化剂的比表面积;催化剂的晶态结构使用X射线衍射仪(XRD,D8 ADVANCE型,Bruker公司,德国)进行分析。
-
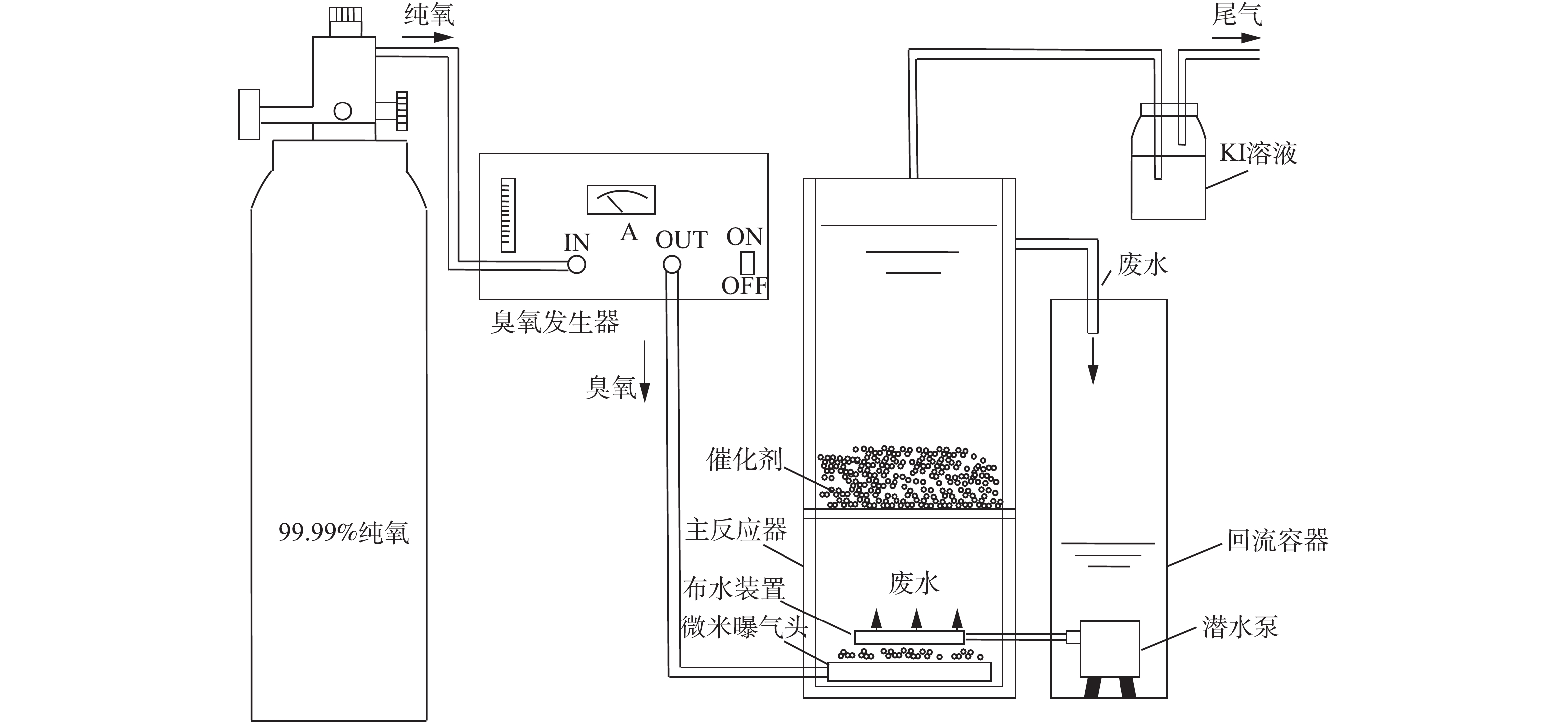
实验所用废水为实际废水,取自广州市某奶牛养殖基地的一级好氧池出水,COD平均值为460 mg·L−1,且可生化性低,二级生化池COD几乎没有降低,其中的有机物难以通过传统生物方法降解。实验所用装置如图1所示。
臭氧发生器的型号为CH-ZTW5G,臭氧反应器为2个自制的有机玻璃反应器,其中左方反应器高度为300 mm,直径为120 mm,有效容积为4 L,在高度为80 mm的地方装有孔径为2 mm不锈钢多空分散网,布气装置为反应器底部的微米级曝气头。右方反应器高度为200 mm,直径为100 mm,内部安装一个潜水泵,型号为HJ-611。
所有实验均在室温下进行,采用半连续方式进行,将约为3.6 L的废水倒入右方反应器,开启潜水泵,使右方容器的废水进入左方的反应器中,待水位到达出水口位置,废水再次流入右方容器中,废水形成循环的流动状态,有利于臭氧与废水的混合。在反应过程中,调节水样pH至9,臭氧投加量为12.5 mg·(L·min)−1,催化氧化时间为20 min,催化剂投加量为60 g,分别在5、10、15、20 min时进行取样,再分别测定COD和色度。
-
COD的测定采用紫外分光光度法;色度的测定采用稀释倍数法;pH的测定采用比色法。
-
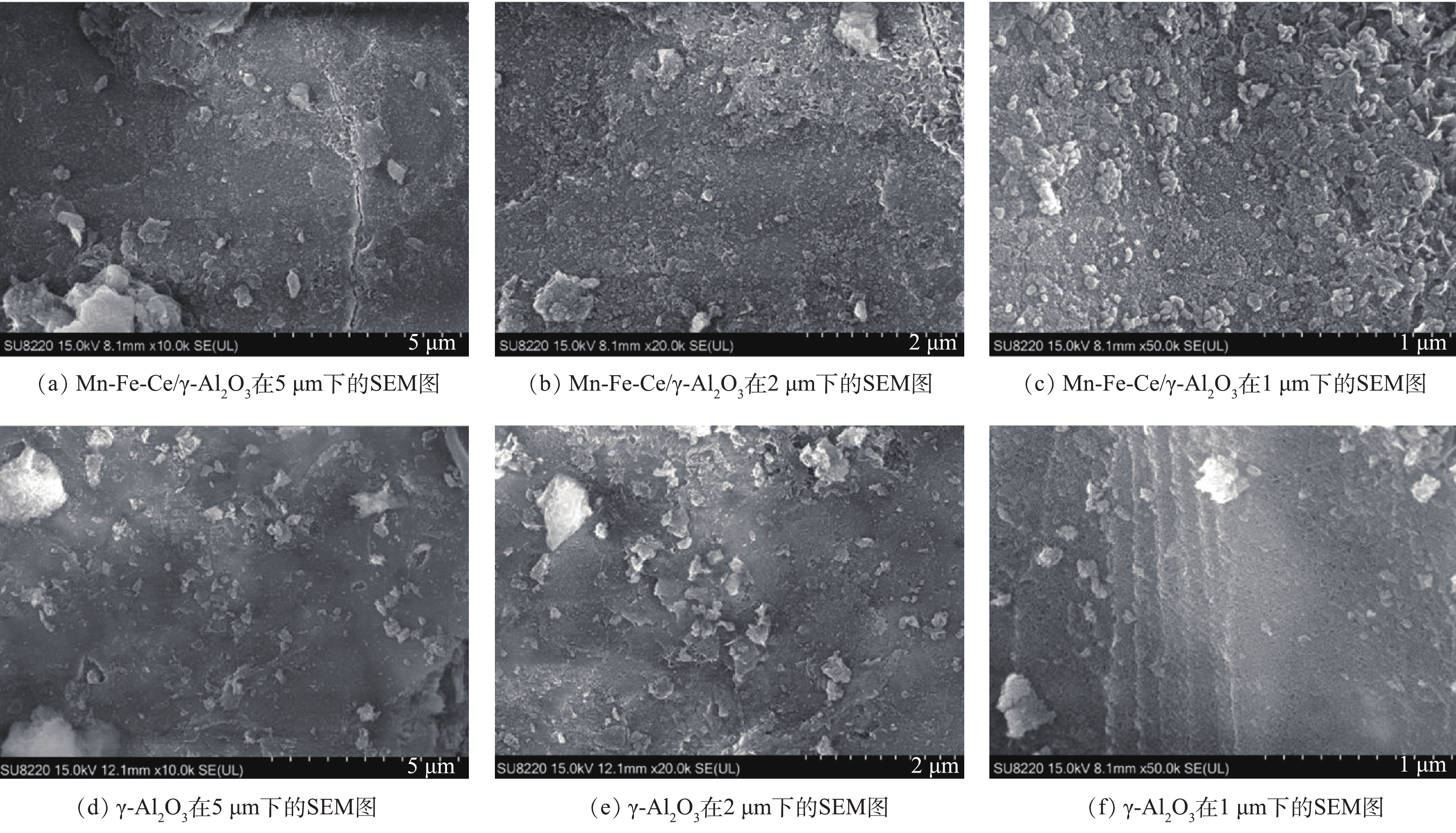
图2(a)~图2(f)分别为Mn-Fe-Ce/γ-Al2O3催化剂及γ-Al2O3在5、2和1 μm下的SEM图。由图2(a)~图2(c)可以明显看出,2种材料表面呈现出不同的形貌特征,Mn-Fe-Ce/γ-Al2O3催化剂表面多呈现出椭圆形的细小颗粒,经过浸渍焙烧后,生成的活性组分均匀附着在载体表面。催化剂在高温条件下发生物化反应,生成了金属氧化物。由图2(d)~图2(f)可知,γ-Al2O3载体表面明显较光滑,并稀疏地分布有一些不规则的小颗粒。以上结果能够证明活性组分负载成功。
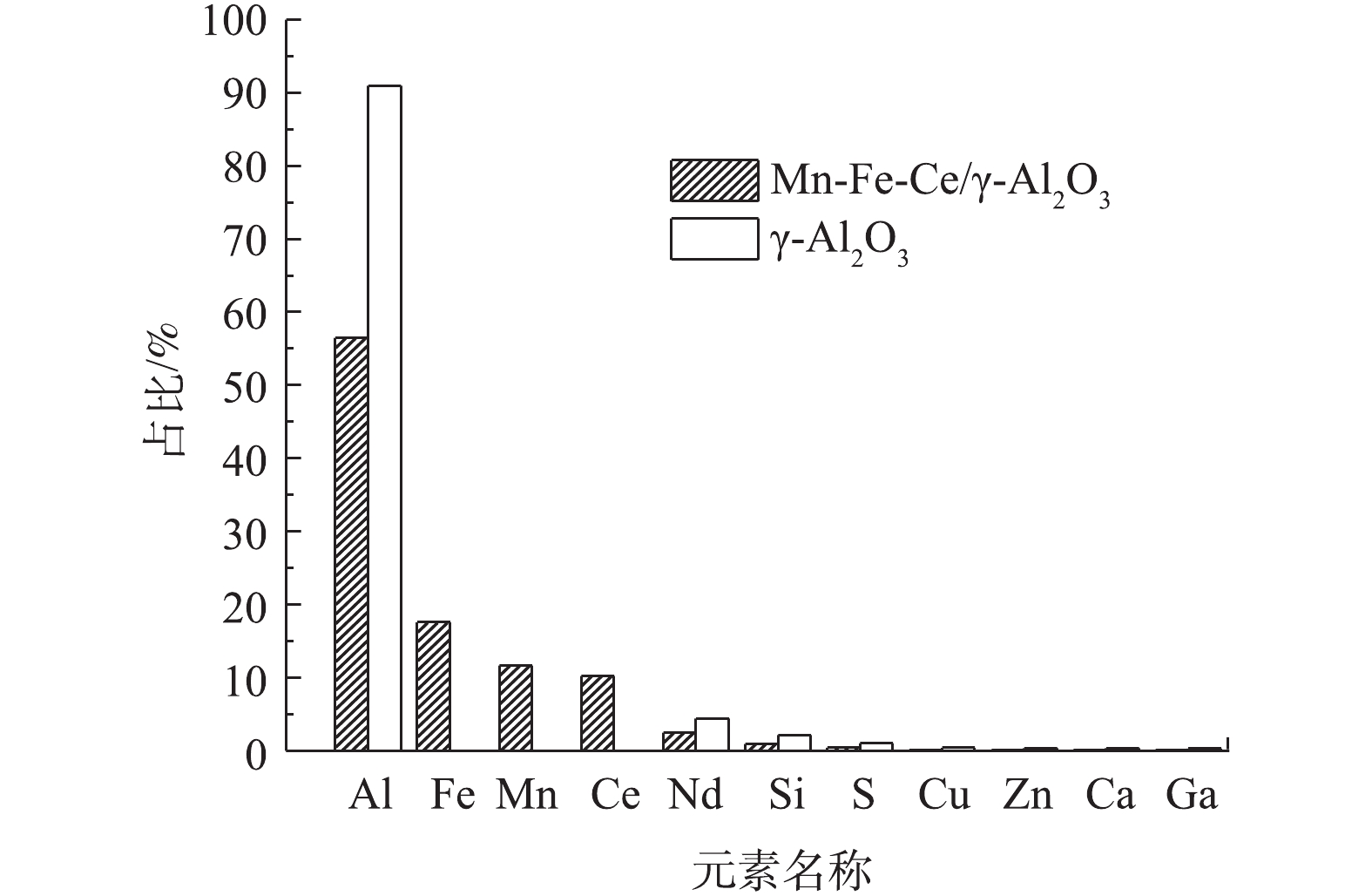
通过XRF能谱分析了Mn-Fe-Ce/γ-Al2O3催化剂及γ-Al2O3的元素组成,测定结果如图3所示。由图3可知,Mn-Fe-Ce/γ-Al2O3催化剂中除了主要元素Al以外,检测到了Fe、Mn、Ce的百分含量为17.553%、11.649%、10.241%;而γ-Al2O3中存在90%以上的Al和微量的其他元素,但并未检测到Fe、Mn、Ce,说明Fe、Mn、Ce能有效地负载在γ-Al2O3的表面,与SEM形貌观测相一致。
对Mn-Fe-Ce/γ-Al2O3催化剂及γ-Al2O3进行了BET测试,如表1所示。负载了Mn、Fe、Ce的γ-Al2O3催化剂相比于γ-Al2O3,其比表面积和总孔体积都有所下降,这是因为经过活性组分的负载后,载体上的部分孔道和缝隙被占据[12],这也可以从SEM分析中得到证实。
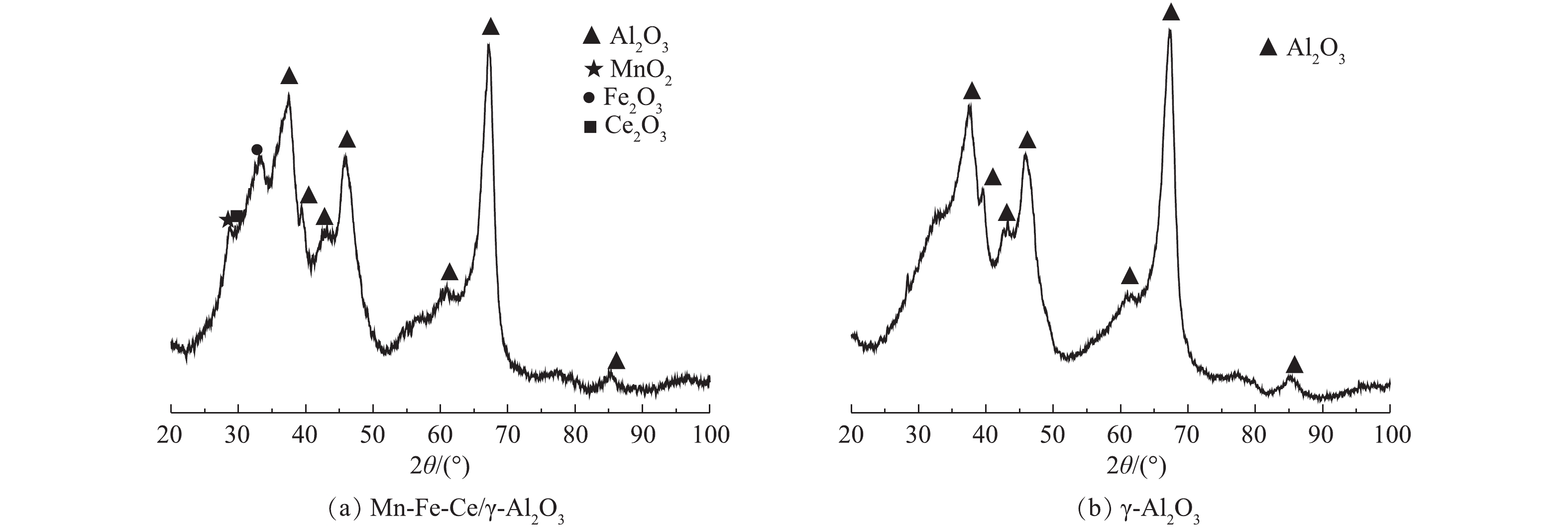
Mn-Fe-Ce/γ-Al2O3催化剂及γ-Al2O3的XRD图如图4所示。在图4(b)中,位于2θ=37.441º、39.672º、42.823º、45.788º、60.457º、67.306º、85.005º处的衍射峰与JCPDS标准卡片中的Al2O3的特征峰吻合并且强度一致;而在图4(a)中,可以明显看出,衍射角为28º~30º以及32º处出现部分衍射峰,且同样表现出了Al2O3的特征峰,从标准卡片中可以看出,衍射角为28.68º的特征峰属于无定型的MnO2,31.0º的特征峰属于Fe2O3,28.7º的特征峰属于Ce2O3。但所检测出来的衍射峰强度均较弱,这可能与负载量和结晶效果有关。
-
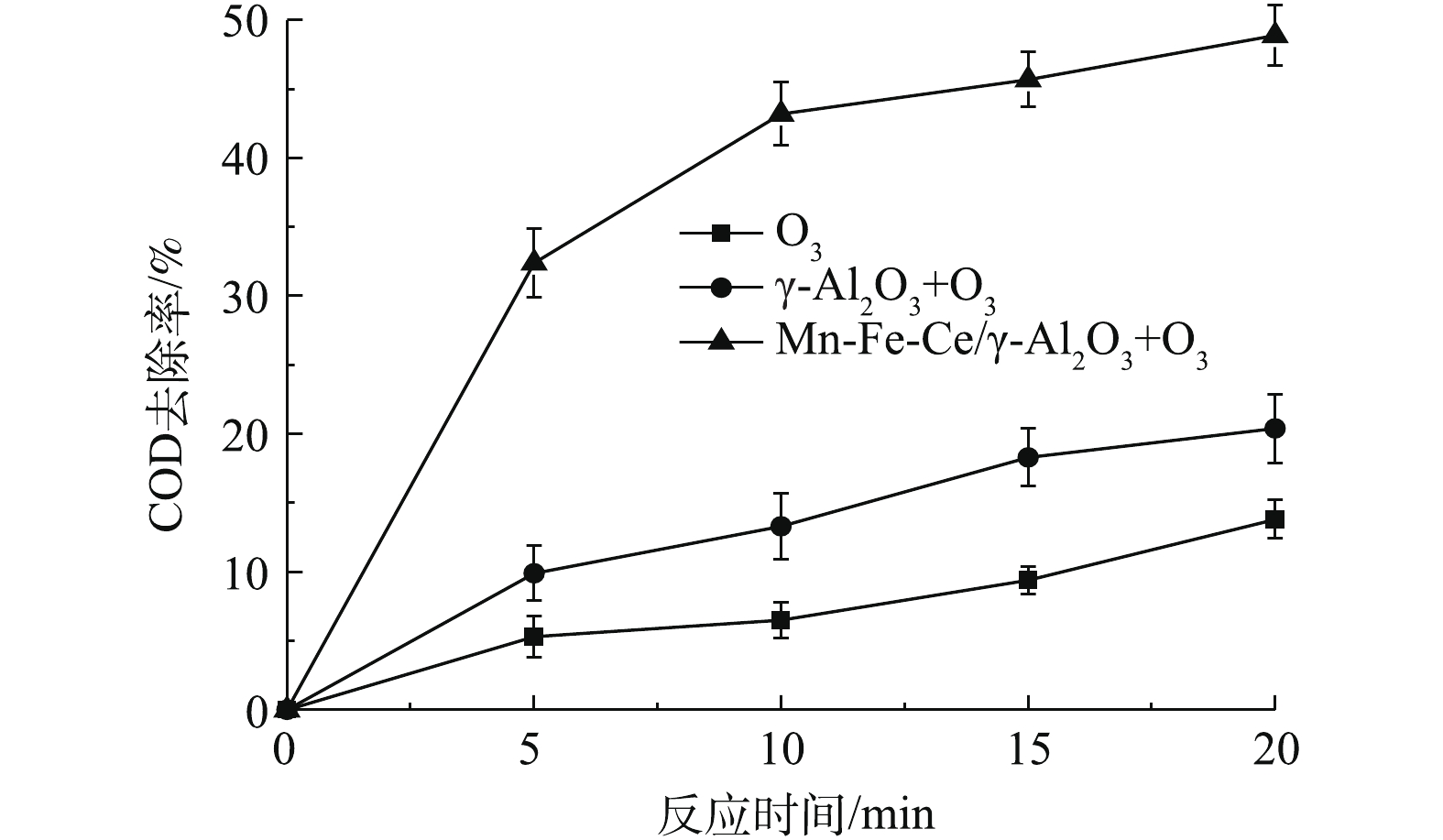
以废水COD和色度去除率为考察指标,分别通过单独臭氧氧化和微气泡臭氧非均相催化氧化奶牛养殖废水,对比其催化效果,微气泡臭氧非均相催化氧化分别以未改性的γ-Al2O3和Mn-Fe-Ce/γ-Al2O3作为非均相臭氧催化剂。通过测定Mn-Fe-Ce/γ-Al2O3+O3反应前后废水的BOD5/COD,验证微气泡臭氧非均相催化氧化对奶牛养殖废水可生化性的影响(见图5)。
在单独臭氧处理时,5 min内COD的去除率仅为5.3%,处理进行到20 min时,COD的去除率也只达到13.8%;在采用γ-Al2O3+O3处理时,COD去除率相比于单独臭氧氧化有少许提升,5 min之后的COD的去除速率达到了9.9%,反应结束后,COD的去除率达到了20.4%,略高于单独臭氧氧化时的去除率;在采用Mn-Fe-Ce/γ-Al2O3+O3处理时,5 min内,去除率已经达到32.4%,COD的去除率在反应结束时为48.9%,相比γ-Al2O3处理,COD去除效果得到显著提升。
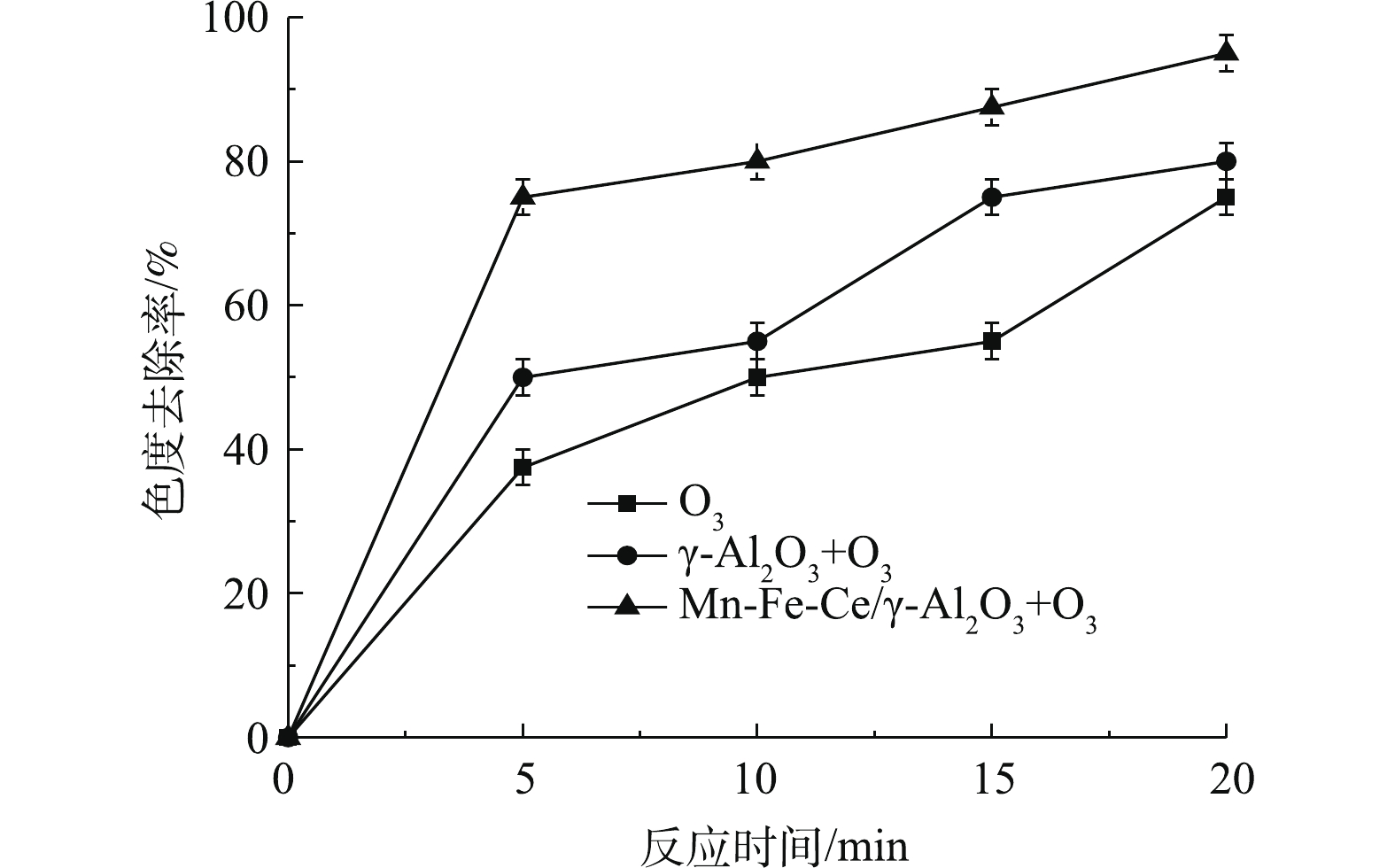
如图6所示,单独臭氧处理的色度去除率为75%,采用γ-Al2O3+O3处理时,色度去除率为80%,当采用Mn-Fe-Ce/γ-Al2O3+O3,色度去除率可达95%,总体呈现出较好的脱色效果。使用Mn-Fe-Ce/γ-Al2O3作为催化剂的去除效果相比前两者更为突出。由COD和色度去除率的结果可以说明,经过改性后的Mn-Fe-Ce/γ-Al2O3催化剂具有较高的臭氧催化活性。
为验证Mn-Fe-Ce/γ-Al2O3+O3体系对奶牛养殖废水可生化性的影响,分别测定反应前废水与反应后废水的BOD5、COD、NH3-N、TP和色度,具体水质指标如表2所示。Mn-Fe-Ce/γ-Al2O3+O3体系处理后,NH3-N与TP浓度变化很小,COD有大幅降低,而且BOD5/COD由原来的0.21提高至0.54,可生化性得到明显提高,表明Mn-Fe-Ce/γ-Al2O3+O3体系将奶牛养殖废水中部分难生化降解的有机物已经转化为可生化降解的有机物。
-
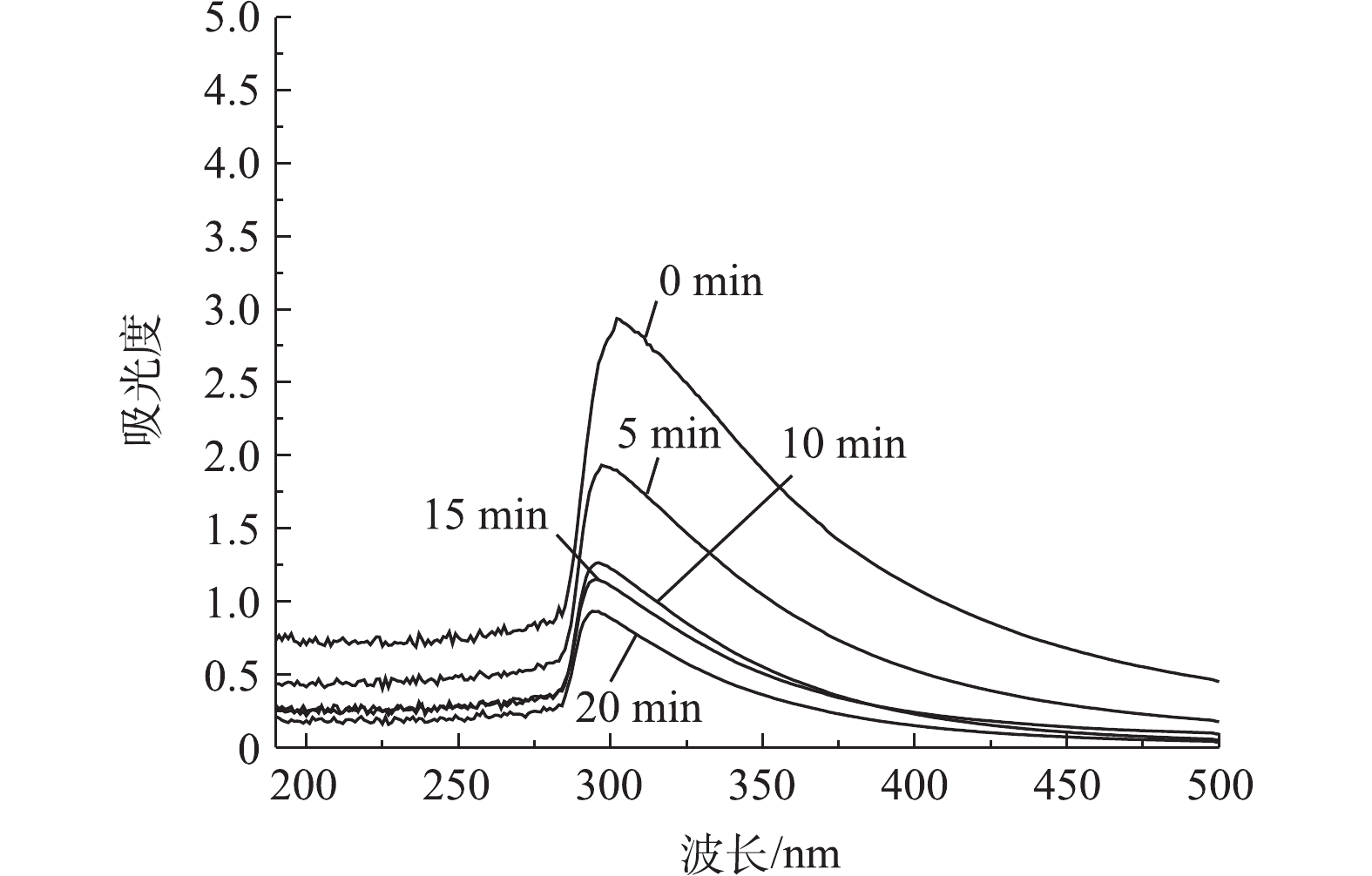
废水中的难生化降解有机物可能来源于饲料、奶牛或厌氧微生物的代谢产物,由于饲料主要来自于当地,从而养殖废水水质也具有一定的地域性和特殊性。使用质谱仪对水质进行分析,结果显示有多达6 000种有机物,且大部分为较复杂的含苯环结构的有机物。不同反应时间的水样紫外光谱扫描结果如图7所示。由图7可知,接近300 nm处出现明显的吸收峰。有研究[13]表明,250 nm处的紫外吸收说明芳环的存在;250~300 nm处的紫外吸收说明有苯环的存在。该奶牛养殖废水在接近300 nm处有紫外吸收,且吸收峰明显,吸光度质较高,也说明体系中存在的难以生化降解的有机物含有苯环结构。另外,随着反应时间从0到20 min,紫外吸收峰逐渐降低,说明含苯环有机物被破坏,难生化降解有机物越来越少[14]。
为验证反应体系中HO·的存在,分析HO·在臭氧非均相催化氧化降解奶牛养殖废水的过程中的作用,以叔丁醇(TBA)作为淬灭剂[15-16],考察该反应是否为HO·介导的氧化机理。分别向O3反应体系和Mn-Fe-Ce/γ-Al2O3+O3体系加入20 mg·L−1的TBA,结果如图8所示。加入TBA后,2个体系表现出了不同程度的抑制作用。对于O3体系,加入叔丁醇,对COD的去除率降低了不到1%,而在相同的条件下,对于Mn-Fe-Ce/γ-Al2O3+O3体系,加入叔丁醇后,在15 min内,COD的去除率只有23.0%;未加入叔丁醇,在15 min内,COD的去除率为45.7%。总体上说明了TBA抑制了体系中大部分HO·的氧化作用,表明在Mn-Fe-Ce/γ-Al2O3+O3体系中,HO·是主要的氧化剂。
在叔丁醇存在条件下,Mn-Fe-Ce/γ-Al2O3+O3体系反应进行到20 min时,COD去除率反而下降,仅有3.7%,推测可能的原因是:叔丁醇作为HO·淬灭剂,显著减少了体系中的HO·,但也不可能完全淬灭体系产生的所有HO·。在这种情况下,HO·与难生化降解有机物的作用过程将变得比较缓慢。在前15 min内,废水中的难降解有机物在HO·的作用下,分子结构尚未发生重大变化(如开环),也不能被重铬酸钾氧化;进行到20 min时,在HO·的持续作用下,这些难降解有机物分子结构发生了重大变化(如开环),中间产物也可以被重铬酸钾氧化,因而COD不降反升,其去除率下降。但上述推测尚须进一步的实验数据证实。
目前,对于臭氧非均相催化氧化的机理研究,尽管仍存在少许特例提出反应过程中不涉及HO·的产生,如KASPRZYK-HORDERN等[17]认为,Fe2O3/Al2O3催化臭氧氧化草酸,叔丁醇的投加对反应过程不造成影响,是臭氧分子直接氧化的作用,但绝大多数臭氧非均相催化氧化的反应都为HO·介导的氧化机理[18-20],并且在反应体系中发挥着主要作用[21-23]。采用Mn-Fe-Ce/γ-Al2O3作为臭氧催化剂,能提高对奶牛养殖废水中难生化降解有机物的氧化效果,关键原因就在于负载了金属氧化物的负载型催化剂在载体表面提供了大量活性位点,从而表现出更高的活性[24],通过锰、铁和铈的氧化物的负载,活性组分可以发挥协同催化作用[25],使体系中生成了更多氧化能力较强的HO·。稀土元素中的4f轨道被发现有助于臭氧的催化[26],二氧化铈作为一种立方萤石多孔型氧化物,可使反应体系中离子和臭氧快速分散。此外,BOARO等[27]提出氧空穴理论,Ce在氧化反应中能在三价与四价中进行转换,使晶格形成氧空穴[28-29],从而促进HO·的生成。在微气泡臭氧非均相催化氧化的过程中,由于催化剂表面电荷不平衡,水中的金属氧化物(如二氧化锰、氧化铁)会强烈吸附水分子[30],水分子发生电离生成H+和OH−,会与金属氧化物产生表面羟基,溶解在水中的臭氧可与表面羟基发生反应生成HO·[21, 31-32]。其机理[33]如式(1)~式(3)所示。
本研究结果支持了第2种观点,即遵循HO·介导的氧化机理。臭氧分子吸附在Mn-Fe-Ce/γ-Al2O3催化剂的表面,与表面羟基发生上述链式反应,从而进一步分解产生HO·。
-
1)经含锰化合物、含铁化合物和含铈化合物的前驱体浸渍液浸渍并焙烧后制得Mn-Fe-Ce/γ-Al2O3催化剂,在pH为9,O3投加量为12.5 mg·(L·min)−1,反应时间为20 min,催化剂投加量为60 g的条件下进行臭氧催化氧化,奶牛养殖废水COD去除率和色度去除率分别可达到48.9%和95%,较单独臭氧氧化时的COD去除率和色度去除率分别提高了35.1%和20%。
2) SEM结果显示,在Mn-Fe-Ce/γ-Al2O3经过改性后,活性组分均匀附着在载体表面;XRF分析显示Fe、Mn、Ce 3种元素能有效地负载在γ-Al2O3的表面;BET的结果经过活性组分的负载后,载体上的部分孔道和缝隙被占据,与SEM的结果相呼应;XRD分析表明,催化剂表明形成了活性组分,形成较弱的衍射峰与负载量和结晶效果有关。
3)通过添加TBA作淬灭剂,验证反应体系中HO·的存在,结果表明,单独O3体系中加入叔丁醇对COD的去除率降低了不到1%,而在相同条件下,对于Mn-Fe-Ce/γ-Al2O3+O3体系,加入叔丁醇后COD的去除率从48.9%降至3.7%,明显抑制了水中有机污染物的氧化。HO·在Mn-Fe-Ce/γ-Al2O3+O3体系中是主要的氧化剂。
Mn-Fe-Ce/γ-Al2O3催化剂的制备及其在奶牛养殖废水处理中的臭氧催化氧化性能
Preparation of Mn-Fe-Ce/γ-Al2O3 catalyst and its ozone catalytic oxidation performance in dairy farming wastewater treatment
-
摘要: 不同地区的奶牛养殖废水水质具有地域性。在南方地区,常规处理工艺出水COD和色度普遍偏高,臭氧催化氧化是一种非常有潜力的技术。采用优化的浸渍焙烧的制备方法,以γ-Al2O3为载体,制备了Mn-Fe-Ce/γ-Al2O3催化剂,并对其性状进行了表征。将该催化剂用于实际奶牛养殖废水一级好氧池出水的臭氧氧化中,结果表明:经含锰、铁和铈化合物的前驱体浸渍液浸渍并进行焙烧方法得到的Mn-Fe-Ce/γ-Al2O3催化剂对奶牛养殖废水有较好的催化性能;在臭氧投加量为12.5 mg·(L·min)−1,催化剂投加量为60 g,反应20 min的条件下,COD去除率由使用γ-Al2O3时的20.4%提高到48.9%,单独使用臭氧时仅为13.8%;色度去除率可达95%;BOD5/COD达到0.54。臭氧催化氧化不仅可以去除COD和色度,而且有效改善了可生化性,为氧化出水继续使用生化法创造了条件。添加TBA作为HO·的淬灭剂实验结果表明,HO·在体系中起主要作用。研究结果可为奶牛养殖废水处理提供新的技术方法。
-
关键词:
- Mn-Fe-Ce/γ-Al2O3 /
- 非均相 /
- 臭氧催化氧化 /
- 奶牛养殖废水 /
- HO·介导
Abstract: The quality of dairy farming wastewater has regional characteristic. In the southern region, COD and chroma in the effluents of conventional treatment processes are generally high. Catalytic ozonation is a kind of promising technology. The Mn-Fe-Ce/γ-Al2O3 catalyst was prepared with a γ-Al2O3 carrier and the optimized impregnation-calcination method, and its properties were characterized, then it was used to ozone oxidize the effluent from the first aerobic tank treating the actual dairy farming wastewater. The results showed that the catalyst prepared by impregnation with precursor impregnating solution containing manganese compound, iron compound and cerium compound, and calcination treatment, had good catalytic performance on the dairy farming wastewater degradation. At the ozone dosage of 12.5 mg·(L·min)−1 and the catalyst dosage of 60 g, the COD removal efficiency increased to 48.9% after 20 min oxidation, while the COD removal efficiency was only 20.4% when using γ-Al2O3, and was 13.8% when using ozone alone. The removal efficiency of chroma reached 95% and the BOD5/COD reached 0.54. Catalytic ozonation could not only remove COD and chroma, but also ameliorate biodegradability effectively, which created the conditions for the subsequent biochemical treatment of above oxidized effluent. Further experiment results of adding HO· quenchers of TBA showed that HO· plays a major role in the system. The above results provide a new technical method for the treatment of dairy farming wastewater.-
Key words:
- Mn-Fe-Ce/γ-Al2O3 /
- heterogeneous /
- ozone catalytic oxidation /
- dairy farming wastewater /
- HO· mediation
-

-
表 1 催化剂的比表面积和总孔体积分析
Table 1. Analysis of specific surface area and total pore volume of catalyst
催化剂 比表面积/(m2·g−1) 总孔体积/(cm3·g−1) Mn-Fe-Ce/γ-Al2O3 159.969 8 0.046 016 γ-Al2O3 200.208 2 0.059 603 表 2 微气泡臭氧非均相催化氧化对奶牛养殖废水可生化性的影响
Table 2. Effect of heterogeneous catalytic oxidation of microbubble ozone on biodegradability of dairy farming wastewater
阶段 COD/(mg·L−1) BOD5/(mg·L−1) BOD5/COD NH3-N/(mg·L−1) TP/(mg·L−1) 色度/倍 处理前 460 98.4 0.21 59.09 7.52 160 处理后 235 126.9 0.54 56.30 7.25 8 -
[1] JUTEAU P, TREMBLAY D, OULD-MOULAYE C B, et al. Swine waste treatment by self-heating aerobic thermophilic bioreactors[J]. Water Research, 2004, 38(3): 539-546. doi: 10.1016/j.watres.2003.11.001 [2] YANG P Y, CHEN H J, KIM S J. Integrating entrapped mixed microbial cell (EMMC) process for biological removal of carbon and nitrogen from dilute swine wastewater[J]. Bioresource Technology, 2003, 86(3): 245-252. doi: 10.1016/S0960-8524(02)00171-2 [3] FERREIRA F L A, LUCAS J J D, AMARAL L A D. Partial characterization of the polluting load of swine wastewater treated with an integrated biodigestion system[J]. Bioresource Technology. 2003, 90(2): 101-108. [4] LOEHR R C. Pollution Control for Agriculture[M]. Orland, USA: Academic Press, 1984. [5] MINER J R. Alternatives to minimize the environmental impact of large swine production unites[J]. Journal of Animal Science, 1999, 77(2): 440-444. doi: 10.2527/1999.772440x [6] 王利平, 沈肖龙, 倪可, 等. 非均相催化臭氧氧化深度处理炼油废水[J]. 环境工程学报, 2015, 9(5): 2297-2302. [7] TONG S P, LIU W P, LENG W H, et al. Characteristics of MnO2 catalytic ozonation of sulfosalicylic acid and propionic acid in water[J]. Chemosphere, 2003, 50(10): 1359-1364. doi: 10.1016/S0045-6535(02)00761-0 [8] WU J J, MURGANDHAM M, CHANG L T, et al. Catalytic ozonation of oxalic acid using SrTiO3 catalyst[J]. Ozone Science & Engineering, 2011, 33(1): 74-79. [9] 许珊珊, 林存旺, 丁亚磊, 等. MgO/活性炭催化臭氧化降解有机物的作用机制[J]. 环境科学, 2018, 39(2): 838-843. [10] 李民, 陈炜鸣, 蒋国斌, 等. Fe-Ce/GAC催化臭氧降解高浓度腐殖酸废水[J]. 环境科学学报, 2017, 37(9): 3409-3418. [11] 何宏平, 吴德礼, 马鲁铭, 等. 改性黄铁矿烧渣催化臭氧氧化水中活性黑5[J]. 同济大学学报(自然科学版), 2015, 43(11): 1728-1734. doi: 10.11908/j.issn.0253-374x.2015.11.018 [12] 张耀辉, 涂勇, 唐敏, 等. Fe2O3-TiO2-MnO2/Al2O3催化臭氧化催化剂的制备及表征[J]. 中国环境科学, 2016, 36(10): 3003-3009. doi: 10.3969/j.issn.1000-6923.2016.10.023 [13] 尚海英. 典型合成橡胶废水有机物分析方法及生物降解特性[D]. 兰州: 兰州交通大学, 2016. [14] 张悦, 王兵, 任宏洋. O3/Mn2O3对钻井废水多相催化臭氧化试验研究[J]. 环境科学学报, 2015, 35(10): 3185-3192. [15] DONG Y, YANG H, HE K, et al. Catalytic activity and stability of Yzeolite for phenol degradation in the presence of ozone[J]. Applied Catalysis B, 2008, 82(3): 163-168. [16] YANG D, YUAN J, XIA H. Effect of hydroxyl radical inhibitor on ozonation of phenol[J]. Environmental Protection of Chemical Industry, 2014, 34(1): 24-27. [17] KASPRZYK-HORDERN B, ZIÓLEK M, NAWROCKI J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment[J]. Appied Catalysis B, 2003, 46(4): 639-669. doi: 10.1016/S0926-3373(03)00326-6 [18] MARTINS R C, QUINTA-FERREIRA R M. Catalytic ozonation of phenilic acids over a Mn-Ce-O catalyst[J]. Applied Catalysis B, 2009, 90(1/2): 268-277. [19] EROL F, ÖZBELGE T A. Catalytic ozonation with non-polar bonded alumina phases for treatment of aqueous dye solutions in a semi-batch reactor[J]. Chemical Engineering Journal, 2008, 139(2): 272-283. doi: 10.1016/j.cej.2007.07.100 [20] LIU X Y, ZHOU Z M, JING G H, et al. Catalytic ozonation of acid red B in aqueous solution over a Fe-Cu-O catalyst[J]. Separation and Purification Technology, 2013, 115(2): 129-135. [21] 王兵, 周鋆, 任宏洋, 等. MgO催化臭氧氧化降解苯酚机理研究[J]. 环境科学学报, 2016, 36(11): 4009-4016. [22] 王莹. MnO2/ Al2O3催化臭氧微气泡氧化深度处理煤化工废水[D]. 哈尔滨: 哈尔滨工业大学, 2017. [23] 潘璐阳, 王树涛, 张兰河, 等. 掺杂型纳米MnO2/Al2O3催化剂的制备及催化臭氧化处理驱油污水二级出水[J]. 硅酸盐通报, 2015, 34(8): 2260-2266. [24] 张莉莉. 臭氧-催化协同脱除挥发性有机物的试验研究[D]. 杭州: 浙江大学, 2012. [25] PARK C, KEANE M A. Catalyst support effects: Gas-phase hydrogenation of phenol over palladium[J]. Journal of Colloid and Interface Science, 2003, 266(1): 183-194. doi: 10.1016/S0021-9797(03)00171-1 [26] TROVARELLI A, DE L C, DOLCETTI G. Design better cerium-based oxidation catalysts[J]. Chemical Technology, 1997, 27(6): 32-37. [27] BOARO M, GIORDANO F, RECCHIA S, et al. On the mechanism of fast oxygen storage and release in ceria-zirconia model catalysts[J]. Applied Catalysis B, 2014, 52(3): 225-237. [28] CARLA A O, JOSE J M, MANUEL F R, et al. Ozonation of model organic compounds catalysed by nanostructured cerium oxides[J]. Applied Catalysis B, 2011, 103(1/2): 190-199. [29] CARLA A O, JOSE J M, MANUEL F R, et al. Ceria and cerium-based mixed oxides as ozonation catalysts[J]. Chemical Engineering Journal, 2012, 200-202: 499-505. [30] JOSEPY Y, RANKE W, WEISS W. Water on FeO(Ⅲ) and Fe3O4(Ⅲ): Adsorption behavior on different surface terminations[J]. Journal of Physical Chemistry B, 2000, 104(14): 3224-3236. doi: 10.1021/jp9932012 [31] SUI M H, LI S, LU K X, et al. FeOOH catalytic ozonation of oxalic acid and the effect of phosphate binding on its catalytic activity[J]. Applied Catalysis B, 2010, 96(1/2): 94-100. [32] ERNST M, LUROT F, SCHROTTER J. Catalytic ozonation of refractory organic model compounds in aqueous solution by aluminum oxide[J]. Applied Catalysis B, 2004, 47(1): 15-25. doi: 10.1016/S0926-3373(03)00290-X [33] ZHAO L, MA J, SUN Z Z, et al. Mechanism of heterogeneous catalytic ozonation of nitrobenzene in aqueous solution with modified ceramic honeycomb[J]. Applied Catalysis B, 2009, 89(3): 326-334. -



 下载:
下载: