-
近年来,基于硫酸根自由基氧化原理的活化过硫酸盐高级氧化技术受到研究人员的广泛关注[1]。相较于其他常见的氧化剂,如过氧化氢(H2O2)[2]、高锰酸盐(
$ {\rm{MnO}}_4^-$ )[3]和臭氧(O3)[4]等,过硫酸盐($ {{\rm{S}}_{\rm{2}}}{\rm{O}}_{\rm{8}}^{{\rm{2 - }}}$ )[5]因其成本低廉、高效稳定、持续时间长等优点在降解有机污染物的研究中得到越来越多的应用[6]。通过活化过硫酸盐产生更高氧化还原电位的高级氧化技术可以大幅提高污染物的去除率,在土壤地下水有机污染修复方面具有不可忽略的优势[7],尤其是针对多氯联苯(PCBs)[8]、多环芳烃(PAHs)[9]、石油烃(TPH)[10]等有机污染场地,其优势更加明显。由于过硫酸盐自身对污染物的氧化能力有限,探索不同活化方法提高过硫酸盐的污染物去除率成为研究发展的热点。有研究[7]表明,通过热[11]、碱[12]、电力[13]、超声[14]、过渡金属[15]以及紫外光[16]等手段激活过硫酸盐,体系中可以同时产生2种强氧化剂:硫酸根自由基(
$ {\rm{SO}}_{\rm{4}}^{{\rm{ - }} }\cdot$ ,E0=2.5~3.1 V)和羟基自由基(OH·,E0=2.8 V)[1]。与羟基自由基不同的是,硫酸根自由基更加稳定,它的半衰期(40 μs)远大于羟基自由基(<1 μs),可以在复杂多变环境中获得更持久的存留时间,特别是在土壤-地下水有机污染修复中,可以实现多介质输移且保持其强氧化活性[17]。为了更好地跟踪活化过硫酸盐氧化技术在全球范围内的研究趋势,本研究通过对1986—2019年以来Web of Science核心数据库中相关刊物的数量、发展趋势、研究团队(国家、机构、期刊和作者)以及文献被引频次等进行了回顾与分析;结合关键词共现关系图谱,梳理了过硫酸盐的研究热点及未来发展趋势,总结了过硫酸盐不同活化方法的特点,并系统归纳了33年以来有关过硫酸盐高级氧化法的研究动态及面临的挑战,以期为今后的相关研究提供有益参考。
全文HTML
-
所有数据来源于Web of Science核心合集,数据检索策略为:TS=(“persulfate” OR “persulfates” OR “persulphate” OR “persulphates” OR “
$ {{\rm{S}}_{\rm{2}}}{\rm{O}}_{\rm{8}}^{{\rm{2 - }}}$ ” OR “perdisulfate” OR “peroxysulfate” OR “peroxydisulfate”),所有的搜索截至2019年10月14日,以避免数据库每日更新导致文献数量偏差。限制文献类型为Article(论文)和Review(综述),并将出版时间在2020年的3篇文章进行了剔除,对语言和数据类别没有限制,并在检索结果中筛选与过硫酸盐研究有关的文章,共得到文献7 734篇(系统时间范围为1986—2019年)。使用Microsoft Excel 2010分析出版数量的趋势,采用多项式模型[18](三项式模型)预测下一年发表量的增长趋势。同时,使用VOSviewer(1.6.13版)(引文网络分析软件)进行文献、关键词共现频次和可视化分析,揭示我国对于过硫酸盐研究的热点以及趋势。网络图中每个节点代表一个关键词,节点的大小表示关键词出现的频次,节点之间连线的粗细表示关键词共现强度的高低,彼此邻近的关键词表示他们通常出现在相似的文献中。本研究中,英格兰、北爱尔兰、苏格兰和威尔士的文章归类到英国,中国香港、澳门和台湾的文章归类入中国。VOSviewer的计数方法采用分数计数,限制每篇文章最大作者数量为25个。
-
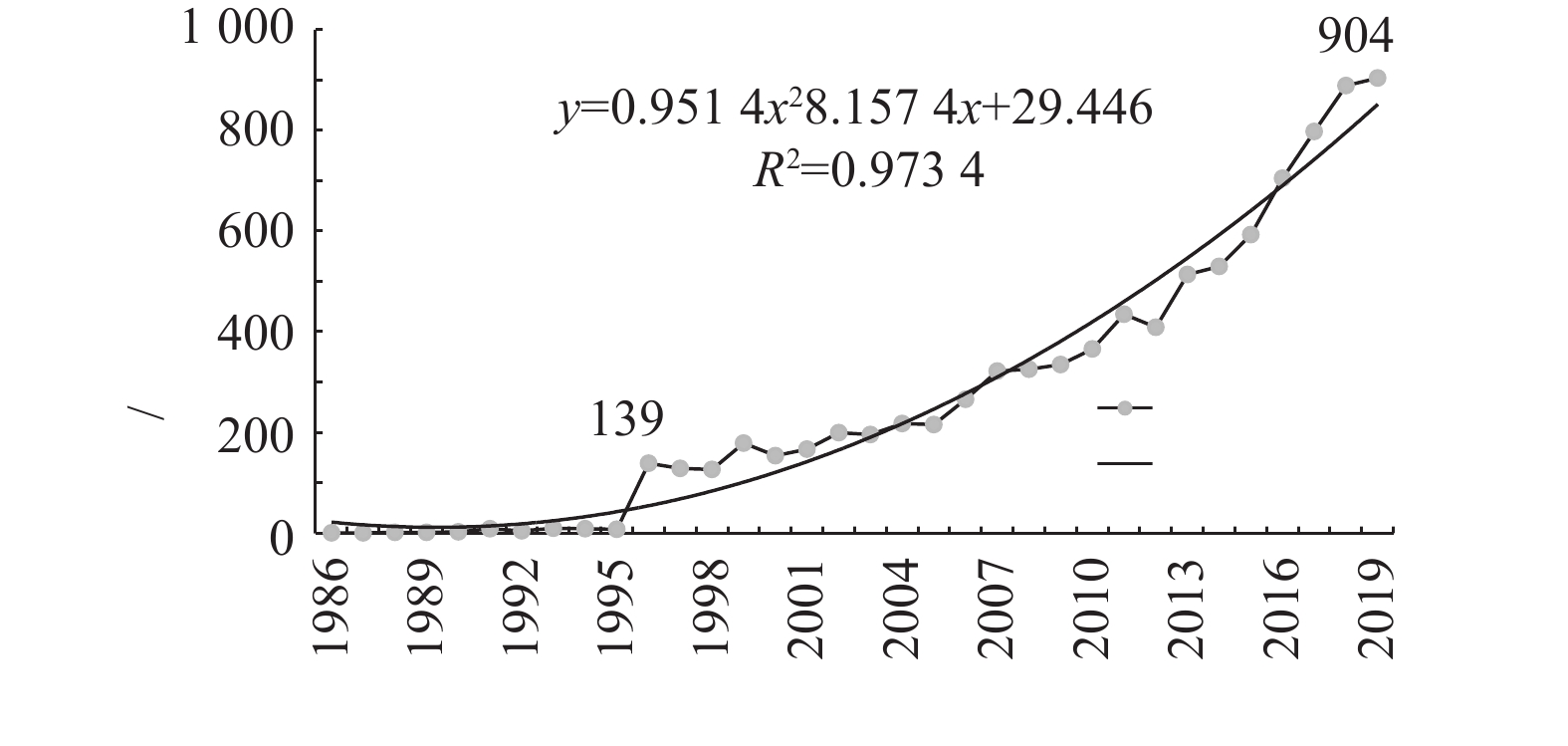
图1为全球每年发表过硫酸盐研究文献的数量以及增长趋势。结果显示,在1996年之前,文献数量增长缓慢。这一阶段是过硫酸盐研究的初级阶段,主要关注点在于过硫酸盐性质的探索及其在氧化过程中的反应机理。但从1996年开始,过硫酸盐的应用研究突然猛增,仅这一年的文献数量达到139篇,此后保持均匀上升的趋势。2007年文献发表数量超过300篇,且增长趋势仍在继续。值得关注的是,仅在2019年(截至10月)发表文献数量达到904篇。
用多项式趋势线模型模拟了33年中发表年份与发表文献数量之间的关系。发文量随年份的增加而持续增多,且增加幅度越来越大。根据目前的线性趋势,预测2020年发表文献数量将会突破1 000篇,表明对于过硫酸盐的研究仍然在持续发展中。趋势线方程见式(1)。
式中:y为发文数量,篇;x为年份;决定系数R2=0.973 4,且其趋势线模型拟合结果良好。
-
共有100个国家参与了过硫酸盐的相关研究。表1总结了过硫酸盐研究最多的10个国家。可以看出,中国发表的研究论文最多(3 508篇,45.36%),说明中国对过硫酸盐的研究方面高度关注。研究表明,我国土壤和地下水均受到不同程度的有机污染,如,土壤中六六六、DDT、多环芳烃3类有机污染物点位超标率分别为0.5%、1.9%、1.4%[19];地下水的开采量以每年2.6×109 m3的速度迅猛增加,其所带来的潜在有机污染问题也越来越严重。目前,北京的浅层地下水中监测中已检出六六六、DDT、多环芳烃、单环芳烃等多种有机污染物[20],而通过研究新型高级氧化技术达到对有机污染场地的高效修复就显得尤为重要。
全球范围内有4 272家机构在过硫酸盐的研究中做出贡献。如表2所示,在前10的研究机构中,中国科学院以发表相关文献311篇位居第一,占比为4.02%;排名第二的机构是同济大学,发文数量为134篇,占比1.73%;第三是哈尔滨工业大学,发文数量为120篇,占比为1.56%。结果表明,对本领域的研究主要集中在高等院校,这些机构不仅发文数量多,且文章所属期刊分区主要集中在Q1(《Chemical Engineering Journal》《Chemosphere》《Water Research》等)。在综合总被引次数以及篇均被引次数方面,中国科学院、同济大学、哈尔滨工业大学以及印度理工大学在过硫酸盐的应用研究领域具备较强的实力。
-
这些过硫酸盐的研究文献共涉及25 703名作者,其中发文数量在10篇及以上的作者有225位,20篇及以上有34位,排在前10位的作者发文数量均大于35篇(表3)。辛辛那提大学的DIONYSIOU Dionysio Dion的篇均被引次数居于榜首(65.25),h指数也位居前列;阿德莱德大学的WANG Shaobin的h指数高达96,篇均被引次数位列第二,由此展现出他们对于过硫酸盐研究实力较强,其文章具有影响意义。同时,排在前10的作者都对本领域的研究做出了杰出的贡献,其中,哈尔滨工业大学马军教授影响力尤为突出。
本研究检索出关于过硫酸盐的研究期刊共有1 100种。按照发表文章的数量,选取了排名前10的期刊,并依次罗列了该期刊的引用数量、出版国家和2018年的影响因子(表4)。发表论文数量超过60篇的期刊共有24种,超过100篇的有12种。其中,最为突出的是《Journal of Applied Polymer Science》,共发表了495篇有关过硫酸盐研究的文章,占所纳入刊物数量的5.3%;《Chemical Engineering Journal》上发表的有关过硫酸盐研究的文章引用次数高达13 040次,以其影响因子8.355居于榜首,是本领域研究最具影响力的期刊。另外,在排名前10的期刊中,发文较多的期刊多属于英国,总体影响因子较高,这说明英国在本领域的研究综合实力较强。
-
由表5中统计的前10篇被引文献和被引量可以看出,来自辛辛那提大学的ANIPSITAKIS G P于2004年发表的文章《Radical generation by the interaction of transition metals with common oxidants》被引次数最多,达到610次,位居第一。此文章主要是研究不同过渡金属对氧化剂的活化作用之间的比较,以此来探索最有效的活化方式,为活化技术在土壤-地下水有机污染物修复应用奠定基础。来自中国台湾中兴大学的梁晨居(LIANG C J)2007年发表的《Influence of pH on persulfate oxidation of TCE at ambient temperatures》、2008年发表的《A rapid spectrophotometric determination of persulfate anion in ISCO》和2009年发表的《Identification of sulfate and hydroxyl radicals in thermally activated persulfate》3篇文章均为前10高被引参考文献。余下的6篇文章的共同被引用量均超过了300次。这说明过硫酸盐的应用研究已经成为当前的研究热点问题。
排名前10的被引文献分布在8种期刊上,其中被引次数最高的前3篇文章分别刊登在《Environmental Science & Technology》和《Journal of Physical and Chemical Reference Data》上,这2种期刊均有2篇文章上榜。同时,《Environmental Science & Technology》还上榜了过硫酸盐研究的前10期刊。该刊位于环境科学类期刊Q1区,影响因子7.149,这也说明其刊登的文章具有一定的影响力和权威性。
-
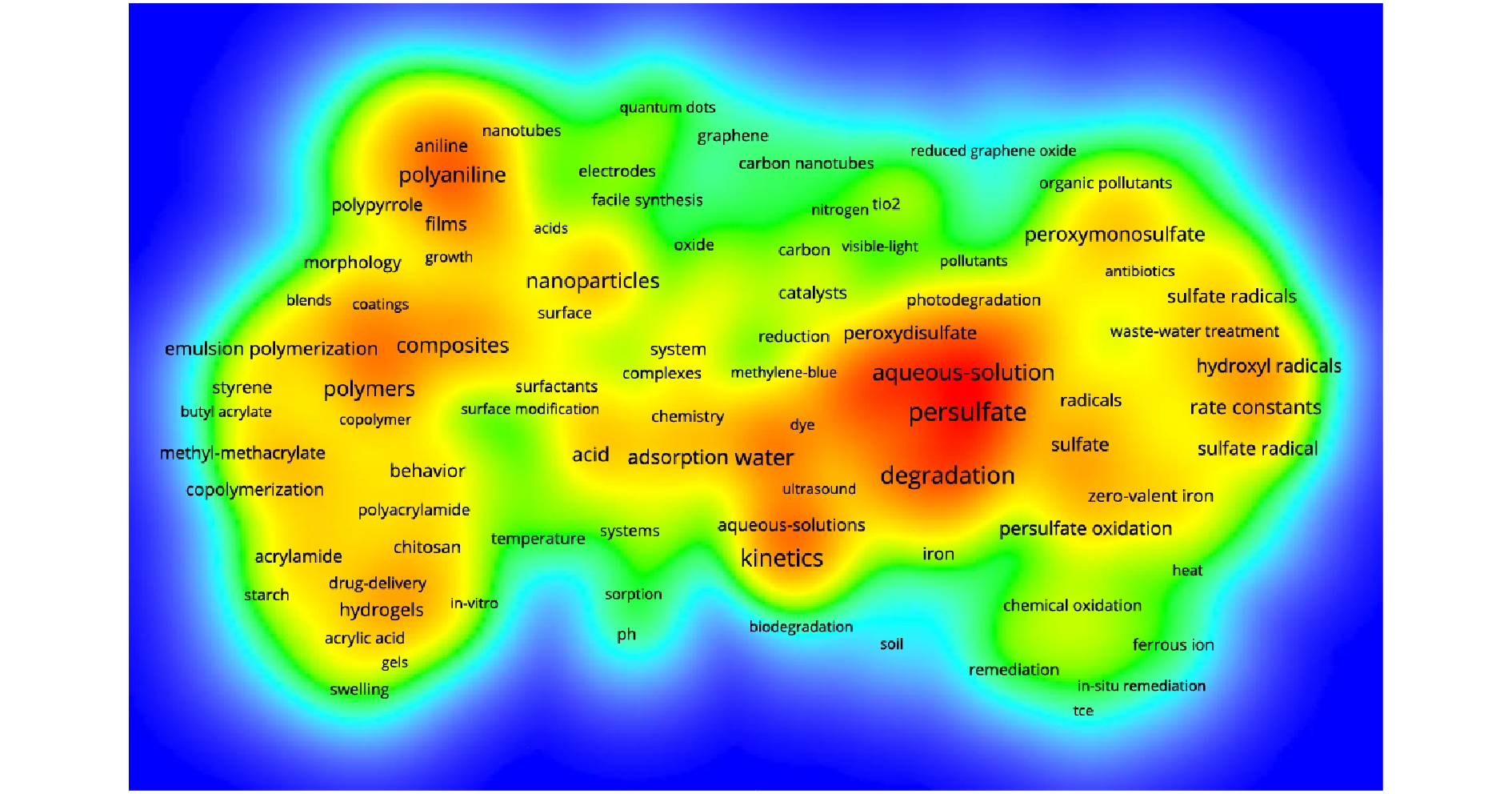
对检索出的7 734篇文章中出现的24 270个关键词进行可视化分析,关键词出现频率大于300次的共有29个。图2显示,常见关键词有过硫酸盐、氧化、降解、动力学、水、机理、硫酸根、过一硫酸盐、效率、芬顿反应、释放、原位修复、热、紫外线、pH、温度等,可为分析过硫酸盐的研究方向提供参考。
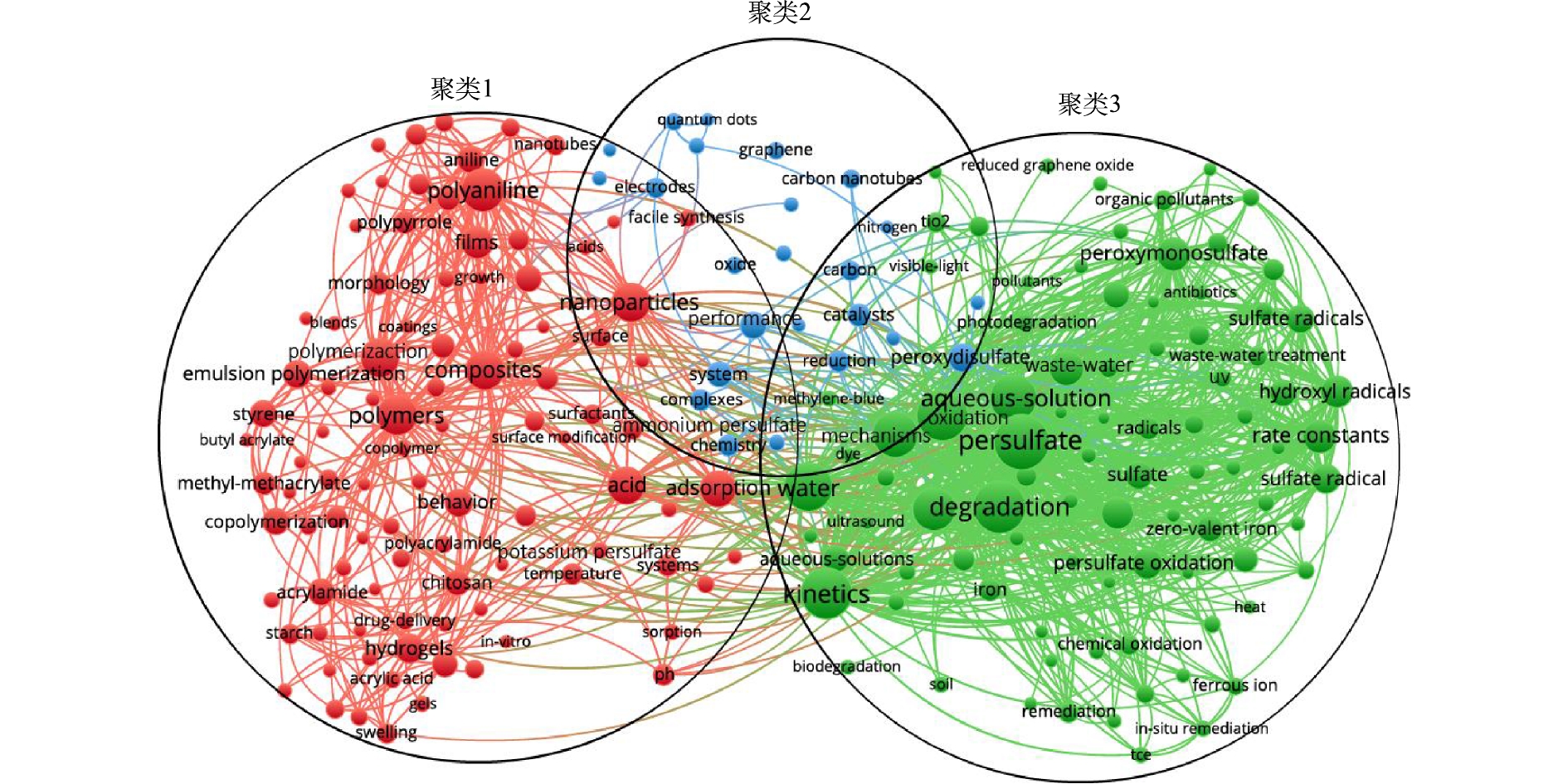
图3反映了出现频率大于60的关键词聚类分析的结果,210个关键词被分为3个聚类。聚类1的主要关注点在于聚合反应、聚苯胺、酸性、温度、纳米微粒、过硫酸铵、过硫酸钾。这一部分学者们主要研究了过硫酸盐最初在工业方面的应用和影响因素,如过硫酸盐引发甲基丙烯酸甲酯的乳液聚合、过硫酸钾引发苯乙烯的乳液聚合。聚类2中的常见关键词主要是过二硫酸盐、性能、还原反应、效率、碳、催化剂、电化学和复合物。这一部分主要描述了活化过硫酸盐的过程中涉及到的相关反应、对有机污染物的去除率以及不同反应过程中催化剂对于整个反应的影响。聚类3关注过硫酸盐、机理、降解、化学氧化、活化、废水处理、土壤、原位修复、动力学、硫酸根自由基和羟基自由基。该部分主要归纳了活化过硫酸盐的各种方法及其反应机理。
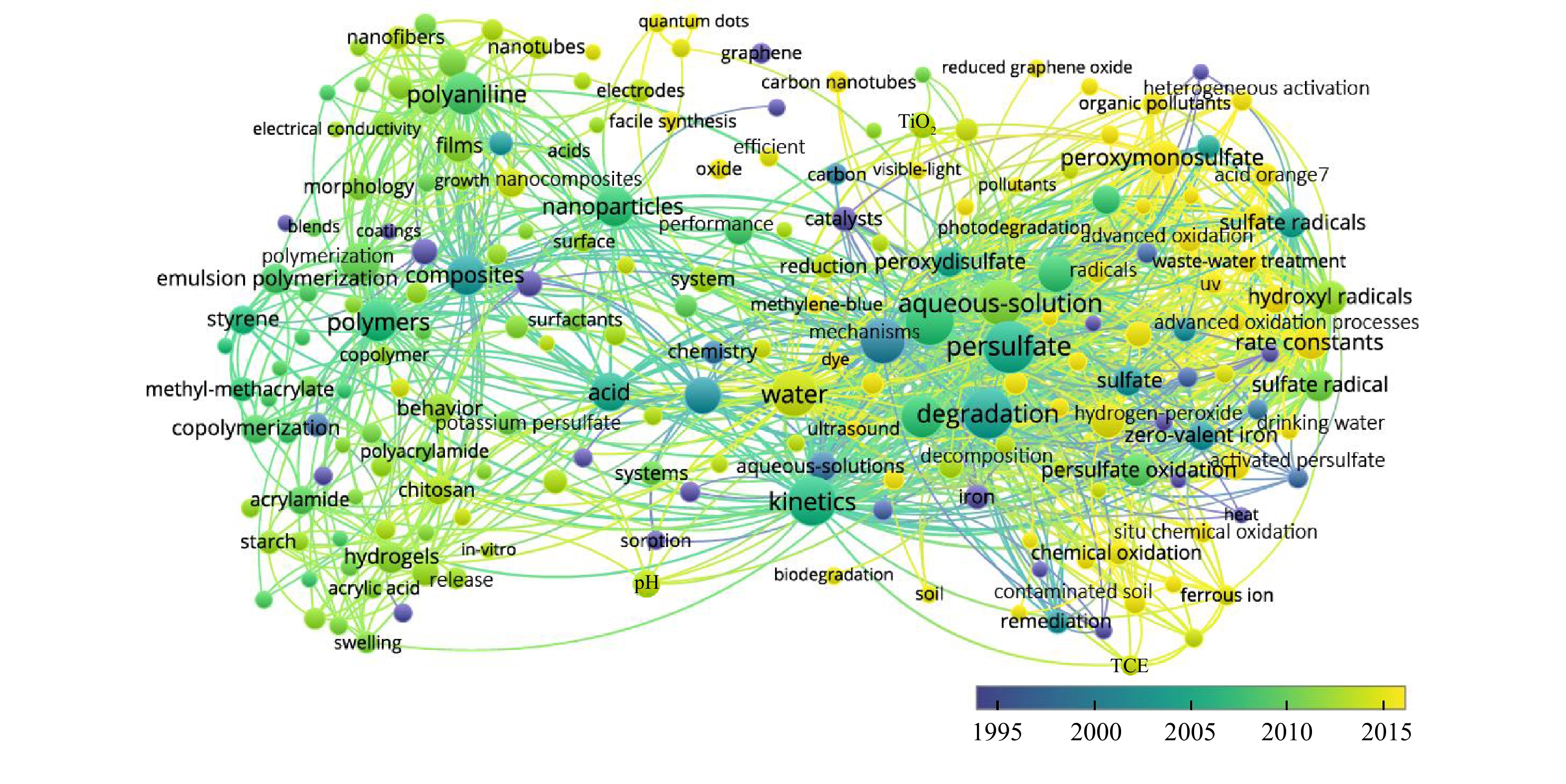
关键词热点现象的出现也将代表过硫酸盐的未来研究趋势和发展方向。通过对图4中的共现关键词出现时间进行分析发现,1995年之后,关键词聚合反应、纳米微粒、过硫酸铵、过硫酸钾等关键词显示出过硫酸盐在工业领域中的研究概况,如工业生产过硫酸铵、过硫酸钾的原理与技术。随着时间的推移,研究人员开始转向过硫酸盐在环境污染治理中的应用研究。高级氧化、活化过硫酸盐、原位化学氧化等关键词逐渐成为了过硫酸盐的研究热点。其中,过硫酸盐的活化方法、活化过硫酸盐的反应动力学机理、过硫酸盐高级氧化技术在原位场地污染修复应用以及过硫酸盐纳米复合材料对于反应过程的影响成为研究的重要关注点。
-
1)关于过硫酸盐活化方法的文献分析。活化过硫酸盐是指使用活化助剂生成活性中间体(如硫酸根自由基、羟基自由基等),以此降解环境介质中的有机污染物。未活化的过硫酸根阴离子也会和其他有机化学物质发生反应,但由于其氧化电位较低(E0=2.01 V),对污染物的去除率远不如活化后的过硫酸盐[21]。因此,研究者们致力于探索不同的活化方法,以期达到经济可行、技术可行、环境友好的目的。
过硫酸盐吸收热能后过氧键断裂,形成2个硫酸盐自由基[22]。一般来说,热活化过硫酸盐的方法对去除有机物是十分高效的,且不存在催化剂分散和失活等问题,尤其是针对土壤中高浓度难降解的有机污染物[23]。但适当的热源限制了土壤系统的实际应用,反应温度是最关键的因素。在控制温度范围内,污染物的反应速率随着温度的升高而增大,并且适当提高反应温度可以大大降低氧化剂的用量[24]。与热活化法不同的是,碱活化过硫酸盐除了可以产生硫酸根自由基以外,还可以产生羟基自由基[25]。通常,使用氢氧化钾和氢氧化钠来提高pH,当pH大于12时,羟基自由基占主导作用,从而可以更广泛地用于原位降解各类污染物[26]。此外,过硫酸盐还可被银、铜、铁、钒、钛、钴、锰等过渡金属有效活化,通过单电子转移生成硫酸根自由基,这是有关过硫酸盐活化方法中最普遍的方法之一[27]。该方法没有特殊的环境要求,其中Fe2+和Fe3+是最常见的过渡金属离子活化剂,但过量的Fe2+与
$ {\rm{SO}}_{\rm{4}}^{{\rm{ - }} }\cdot$ 和$ {{\rm{S}}_{\rm{2}}}{\rm{O}}_{\rm{8}}^{{\rm{2 - }}}$ 发生快速反应,从而使反应停止,导致污染物去除效率较低,并且在环境pH为5时还会转化为Fe (OH)3沉淀[28]。电活化过硫酸盐是在阴极产生硫酸根自由基,然后在电流作用下,通过电迁移、电渗透和电泳去除环境中的污染物,是一种可行且有效的原位修复技术[29]。其机理与过渡金属活化过硫酸盐的单电子转移氧化还原反应类似,电迁移和电渗透流动可以克服低渗透土壤中氧化剂传递差的问题。一般情况下,选择合适的氧化剂注射部位,可以减少原位修复中氧化剂消耗、提高污染物去除率[7]。紫外光可显著促进过硫酸盐分解成硫酸根自由基,与热活化过硫酸盐的机理类似。在众多影响因素中,波长和紫外辐射率在过硫酸盐的激活和有机降解中起着重要的作用[30]。此外,太阳光中含有约5%的紫外光,足已活化过硫酸盐[31],该技术具有经济成本低、环境友好的优点。但紫外光的穿透能力有限,在实际应用中会受到诸多限制。超声波活化过硫酸盐是指通过超声空化产生的局部高温高压,使O—O键断裂,并产生2个硫酸根自由基,其能量诱导机制与热活化和紫外光活化产生自由基相同[17]。有研究[32]表明,超声波活化过硫酸盐可显著提高非生物降解污染物的降解率。
表6归纳总结了6种常见的过硫酸盐活化方法。通过对纳入过硫酸盐活化方法的文章进行筛选,共得到1 107篇文章,并就其进行计量学分析,得到关键词的共现频次(表6),其中最常出现的关键词是过渡金属活化的相关方法(如Fe2+、Fe3+、Cu2+、Ag2+等),达到557次。由此可知,就活化过硫酸盐的方法而言,热活化、过渡金属活化和紫外光活化法是主要的研究焦点,而碱活化、超声波活化以及电活化方面的研究相对薄弱。
2)过硫酸盐高级氧化法的实际应用情况。结合图2和图3可知,关于过硫酸盐的研究应用常见于修复被污染的水、土壤、大气环境,相比之下,过硫酸盐降解废水中各类有机污染物(如,菲、蒽、芘等多环芳烃以及苯胺、三氯乙烯、双酚A等有机物)的研究进行得更为全面,而在土壤地下水修复中的应用显得尚未充分[38]。在对活化过硫酸盐不同方法(表6)进行比较的基础上,对不同活化方法的文献使用关键词进行分类,统计结果见表7。以此为依据梳理出关于过渡金属活化的文献466篇,紫外光活化的文献210篇、热活化的文献175篇、碱活化的文献101篇、超声波活化的文献89篇、电活化的文献35篇,同时还涉及不同活化方法间的联合修复的文献45篇以及少量关于磁性纳米颗粒活化、地下矿物活化和污泥生物炭活化等相关活化方法的文献。根据上述结果,表7归纳部分基于过硫酸盐的高级氧化法的实际应用,其中过渡金属活化的实际应用以Fe2+为例,联合修复以
$ {{\rm{S}}_{\rm{2}}}{\rm{O}}_{\rm{8}}^{{\rm{2 - }}}$ /Fe2+/CA和电化学/环形铁皮/PS 2个应用为例。除前面介绍的各类活化过硫酸盐的方法外,为了获得更大污染物的去除效率,各活化方法之间可以相互结合使用。其中过渡金属和其他活化过硫酸盐方法的结合是迄今为止过硫酸盐领域最常见的应用技术,并已被证明可以有效去除内分泌干扰物、药物和个人护理品等污染物。有研究[39]报道,以硫酸亚铁和氢氧化钠为活化剂,共同激活过硫酸盐以减少长叶莴苣表面上的O157: H7大肠杆菌和李斯特菌。这表明运用过渡金属和碱活化过硫酸盐可能成为一种可替代农产品去污消毒剂的环境友好方法。另有研究[40]证明,将过渡金属与热活化法相结合后,对酿酒厂废水COD的去除效率高于其中任意单一方法。KHANDARKAEVA等[41]指出,使用含有亚铁离子和过硫酸盐的太阳强化氧化体系去除农药阿特拉津的效率远远高于仅用亚铁离子或太阳光活化方法的效率。同时,铁化合物被证明不仅是催化剂,而且是光敏剂,能够导致溶液的吸光度增加。
当然,热、电力、碱、紫外、超声波等活化方法之间也可以相互结合使用。XUE等[42]将电化学助剂引入到热活化过硫酸盐工艺中,再与Fe3+相结合,用于超滤后的垃圾渗滤液纳滤浓缩液进行处理。对比有无电化学辅助前后的去除差别,发现引入电化学法可大大提高COD的去除率(从66%到87%)且几乎完全消除其急性毒性,使之有利于接下来的生物处理。紫外光和热活化也可以结合使用,有研究[43]报道,增加紫外辐射功率、提高活化温度将会促进吸收-氧化烟气中的NO和SO2。然而,在实际的水和燃煤烟气中,各种杂质、灰尘或污垢广泛存在,并且会附着在石英管的表面,可能会阻碍紫外线的有效传输。为了克服这些问题,LIU等[44]使用超声波、二价铁与热共活化过硫酸盐体系也能达到吸收烟气中的NO和SO2的目的,实验表明28 kHz的超声波比40 kHz更有效。这是因为低频率超声波会产生更强的空化效应,导致更高的传质效率和自由基的产量。
总而言之,随着研究的不断深入,越来越多的活化方法、新型材料以及新兴污染物将会被研究者们探索发现。基于过硫酸盐的高级氧化法正在进行并持续朝着高效、经济、环境友好的方向不断发展。但是到目前为止,大量研究主要集中在水污染修复问题中。相对而言,成分更为复杂多变的土壤地下水环境污染修复中应用的挑战不容小觑,未来应给予更多关注。
2.1. 发表文献数量及增长趋势
2.2. 主要发文国家与机构
2.3. 主要发文期刊与作者
2.4. 文献被引频次分析
2.5. 关键词分析
2.6. 基于关键词分析的研究热点与发展趋势
-
1)从33年来过硫酸盐研究的文章数量增长趋势得出,研究初期发文数量少并且增长趋势较为波动,但随着对环境污染治理的不断重视,近些年增长趋势明显。同时,根据趋势线进一步预测未来对于过硫酸盐的研究将持续高涨。
2)就发文国家和机构而言,我国对于过硫酸盐研究的重视程度在全球范围内居于榜首,中国的发文量在全球100个国家发表的7 734篇文章中约占一半(45.36%)。7个中国高等院校及机构排在了前10位,以997篇的发文量占纳入文献的12.89%。
3)过硫酸盐研究主要的发文期刊集中在环境类影响因子较高的几种刊物,《Chemical Engineering Journal》引文频次达到13 040,影响因子也是前10期刊中最高(8.355),可见其在整个领域内的影响力以及对于活化过硫酸盐实际应用的重要性。主要的发文作者分布在中国,排名前10的作者中6位中国作者发文量达到264篇。
4)关键词聚类分析聚焦为3类:第1类是关于过硫酸盐在工业领域的应用;第2类是在活化过硫酸盐过程中所涉及到不同阶段的相关反应,及其反应物对于整个体系的影响;第3类集中在活化过硫酸盐的氧化技术、活化方法和机理等,为其后的研究热点提供相关参考。
5)从关键词的共现频次和出现的时间节点锁定过硫酸盐的研究热点主要集中在活化过硫酸盐的方法、在土壤-地下水等有机污染场地进行原位修复的应用研究。通过对不同的活化方法进行对比,活化过硫酸盐的氧化技术将随着对过渡金属活化和热活化的进一步研究而得到更有利的发展。同时,就不同的活化技术在反应过程中存在各种的缺陷,考虑将不同的活化方法进行结合使用,在反应过程中以优补缺提高活化效率。



 下载:
下载: