-
燃煤汞污染是目前面临的重要污染问题之一[1]。烟气中的汞主要以3种形式存在:颗粒态汞Hgp、氧化态汞Hg2+和单质汞Hg0[2]。Hgp可随飞灰一起被电除尘器捕集[3];Hg2+性质稳定,易溶于水,可通过湿法脱硫系统脱除[4-6];而Hg0不溶于水且易挥发,现有污染控制设备无法直接将其脱除[7-9]。目前常用的脱除Hg0方法有吸附法和湿式氧化法[10]。其中,氯系氧化剂氧化性强,具有较强脱除效率,同时有良好的选择性,受到研究者的广泛关注。MINDAL等[11]发现,NaClO氧化剂能有效氧化脱除Hg0。赵毅等[12-13]研究了NaClO2氧化脱汞,最优条件下脱汞效率为65%;除单一氧化剂外,复合氧化剂能进一步提高脱汞性能。阮长超[14]利用NaClO/NaClO2、NaClO2/H2O2复合吸收液进行脱汞研究,其最优脱汞效率达到90%。
上述研究结果表明,单一或复合型氯酸盐系列氧化剂均具有较好的氧化脱汞能力。但目前开发的氯酸盐脱汞氧化剂成本昂贵,在降低成本的同时兼顾实现资源化将具有重要意义[15-16]。我国现有大型燃煤电厂90%以上采用石灰石-石膏湿法脱硫,利用现有脱硫设备实现烟气高效除汞是最为经济合理的途径[17-18]。石灰石-石膏湿法脱硫产生的脱硫废水氯离子含量高[19-20],是目前的治理难点。钱凯凯[21]采用双膜三室法对高氯离子含量脱硫废水进行电解[22-23],其电解产生的电解产物HClO和Cl2可作为经济高效的脱汞氧化剂,可在实现资源循环利用的同时降低氧化脱汞的成本。
本研究以脱硫废水电解产物活性氯(HClO和Cl2)为脱汞氧化剂,在自制鼓泡反应器中,探究其脱除Hg0能力,考察了电解产物活性氯浓度、反应温度、pH等参数对氧化Hg0的影响,以期为脱硫废水资源化利用以及WFGD同时脱硫脱汞提供参考。
-
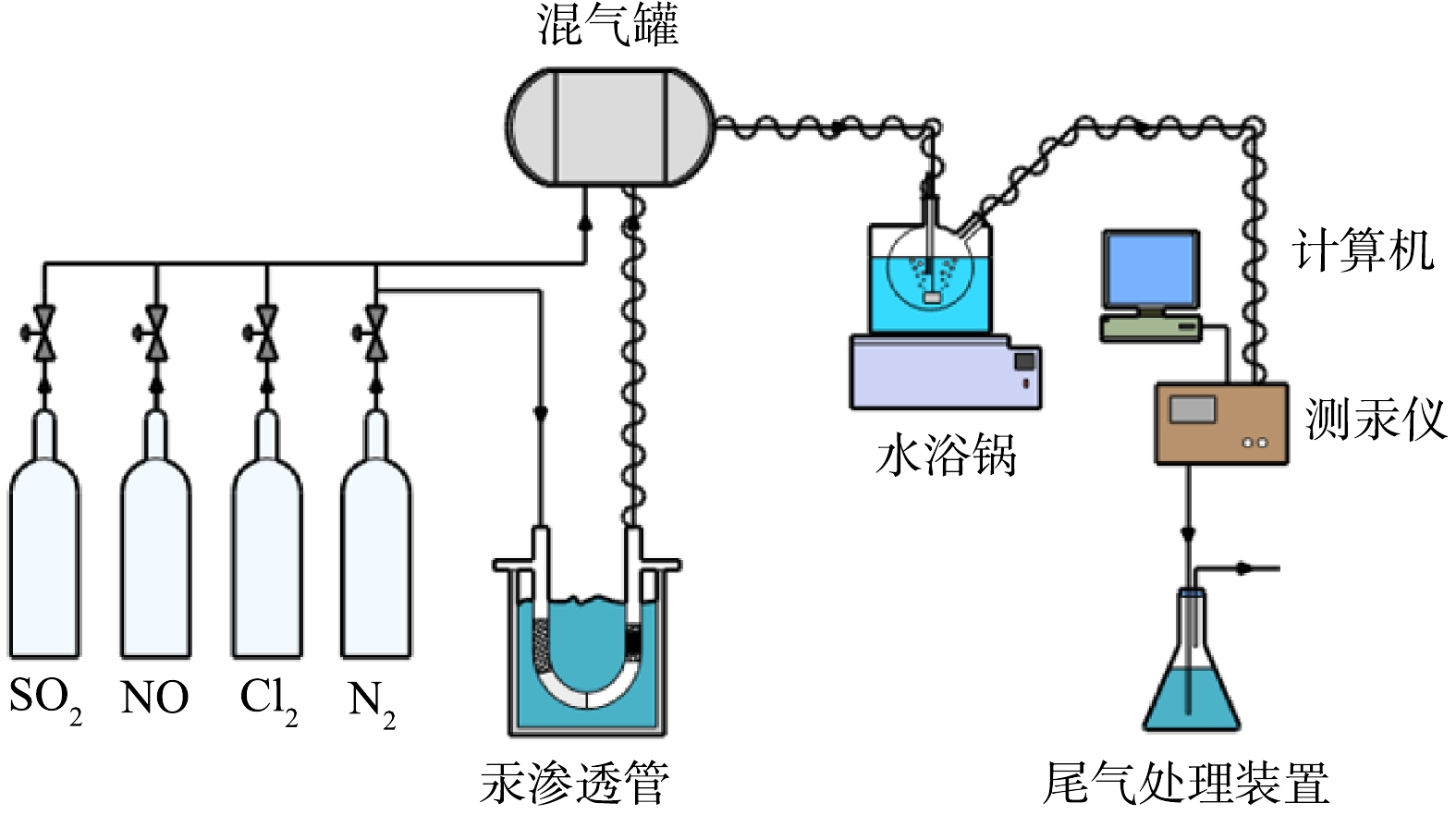
试剂包括:次氯酸钠(AR级)、氯化钠(AR级)、无水氯化钙(AR级)、六水合氯化镁(AR级)、无水硫酸钠(AR级)、高锰酸钾(AR级)、氢氧化钠(AR级)、硫酸(98%)、盐酸(37%);仪器包括:圆底烧瓶(500 mL)、汞渗透管(HE-SR,美国VICI Metronics 公司)、恒温水浴锅(HH-2,常州普天仪器制造有限公司)、原子吸收测汞仪(SG-921,北京吉天仪器有限公司)。
如图1所示,实验装置由模拟烟气部分,汞发生装置,鼓泡反应装置,保温系统以及尾气处理部分组成。模拟烟气均由标准气瓶提供,通过转子流量计控制其流量大小;汞蒸汽发生源为HE-SR型汞渗透管,采用氮气携带和恒温水浴方式加热汞渗透管,获得较为稳定的单质汞含量;氧化反应在自制鼓泡发生器进行,并且由水浴锅加热,检测装置为在线原子吸收测汞仪。
吸收液:采用双膜三室电解池[21]在电流密度25 mA∙cm−2,电极间距4.5 cm条件下电解氯离子质量浓度为15 000 mg∙L−1脱硫废水,通过控制电解时间控制其生成的有效氯质量浓度。将电解生成的Cl2经分离纯化后储存在压缩钢瓶中。本实验所需的试剂均为分析级,电解产物有效氯溶液由次氯酸钠(有效氯>10%)溶液配制,吸收液的pH通过0.1 mol·L−1的HCl调节。
-
实验中氮气一路作为载气,以100 mL·min−1吹扫U型管中挥发出的汞蒸气作为模拟烟气的汞气源,另一路作为平衡气体,2路气体在混气瓶混合后通入鼓泡反应器,气体总流量保持1 L·min−1。Hg源温度设定为60 ℃,其Hg0质量浓度为40 µg·m−3。按化学计量比配置一定浓度的HClO吸收液,置入鼓泡反应器中,吸收液体积为500 mL,反应后的尾气用高锰酸钾溶液吸收净化。所有汞经过的管路均采用聚四氟乙烯,为防止汞在管路内冷凝,同时保证进入反应器的气体温度与反应温度相同,在相应的连接管路上用温控电热带包裹住。
-
电解产物氧化Hg0为Hg2+性能评价计算公式见式(1)。
式中:C0为氧化反应前的Hg0的质量浓度,µg·m−3;C1为氧化反应后的Hg0的质量浓度,µg·m−3。
-
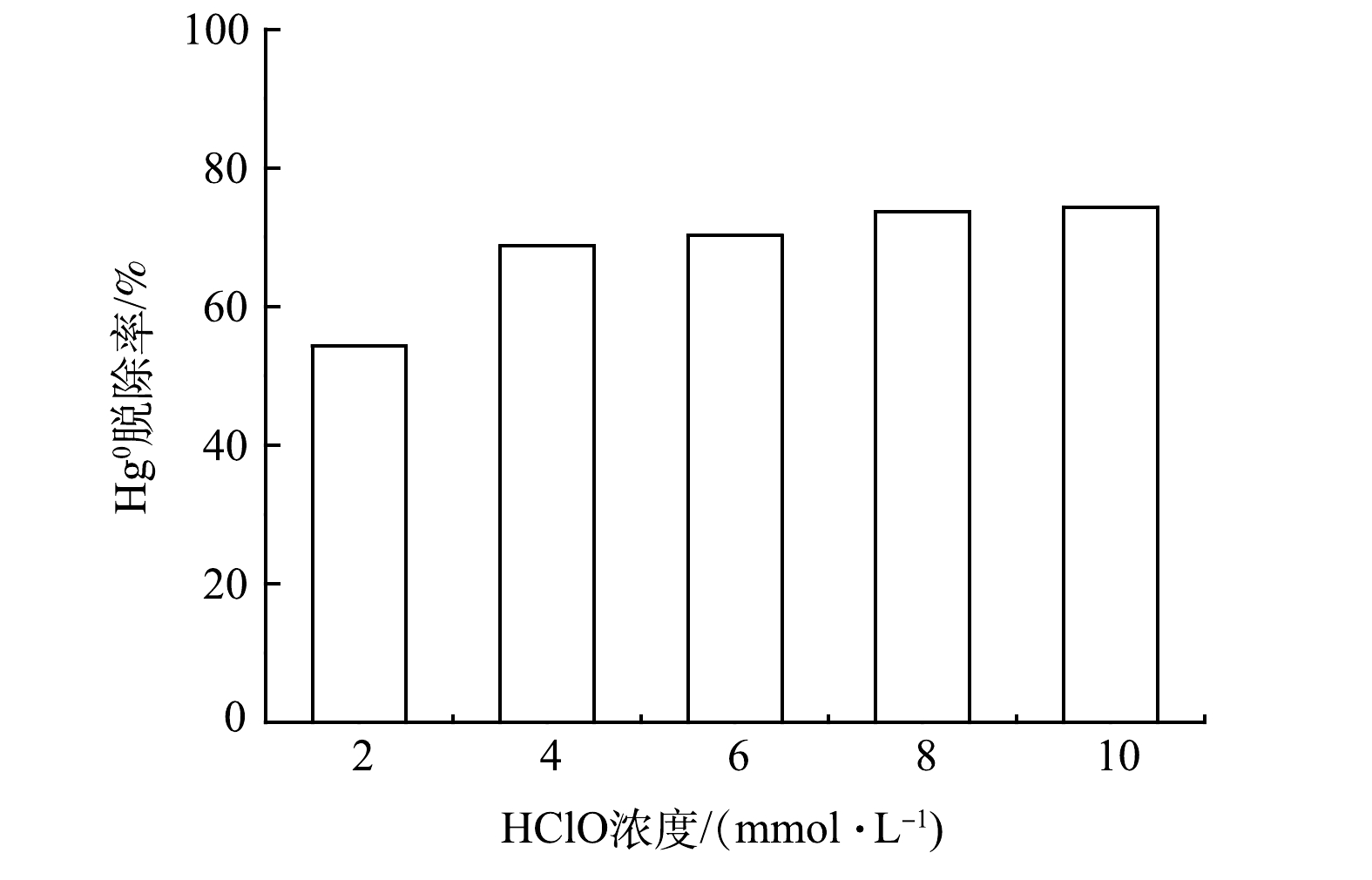
1) HClO浓度对脱汞效率的影响。固定反应温度为45 ℃,pH为4.35,以研究不同HClO浓度对脱汞效率的影响,结果如图2所示。Hg0氧化效率随着电解产物HClO浓度的增大而逐渐提高。当HClO浓度由2 mmol·L−1升至4 mmol·L−1时,Hg0氧化效率明显增强,其脱除率提升了15%。随着HClO浓度进一步提高,Hg0脱除率无明显升高,其脱除率最高可达75%。这主要归因于,HClO浓度增大,溶液中解离的有效氯浓度增大,反应液对Hg0的氧化能力增强;Hg0去除率增大的幅度并不与HClO浓度成正比,对于低浓度的HClO溶液,Hg0的去除率增大幅度较快,但CHClO> 4 mmol·L−1时,Hg°的脱除效率增加缓慢。因此,从经济因素考虑,选取HClO最优浓度为4 mmol·L−1。
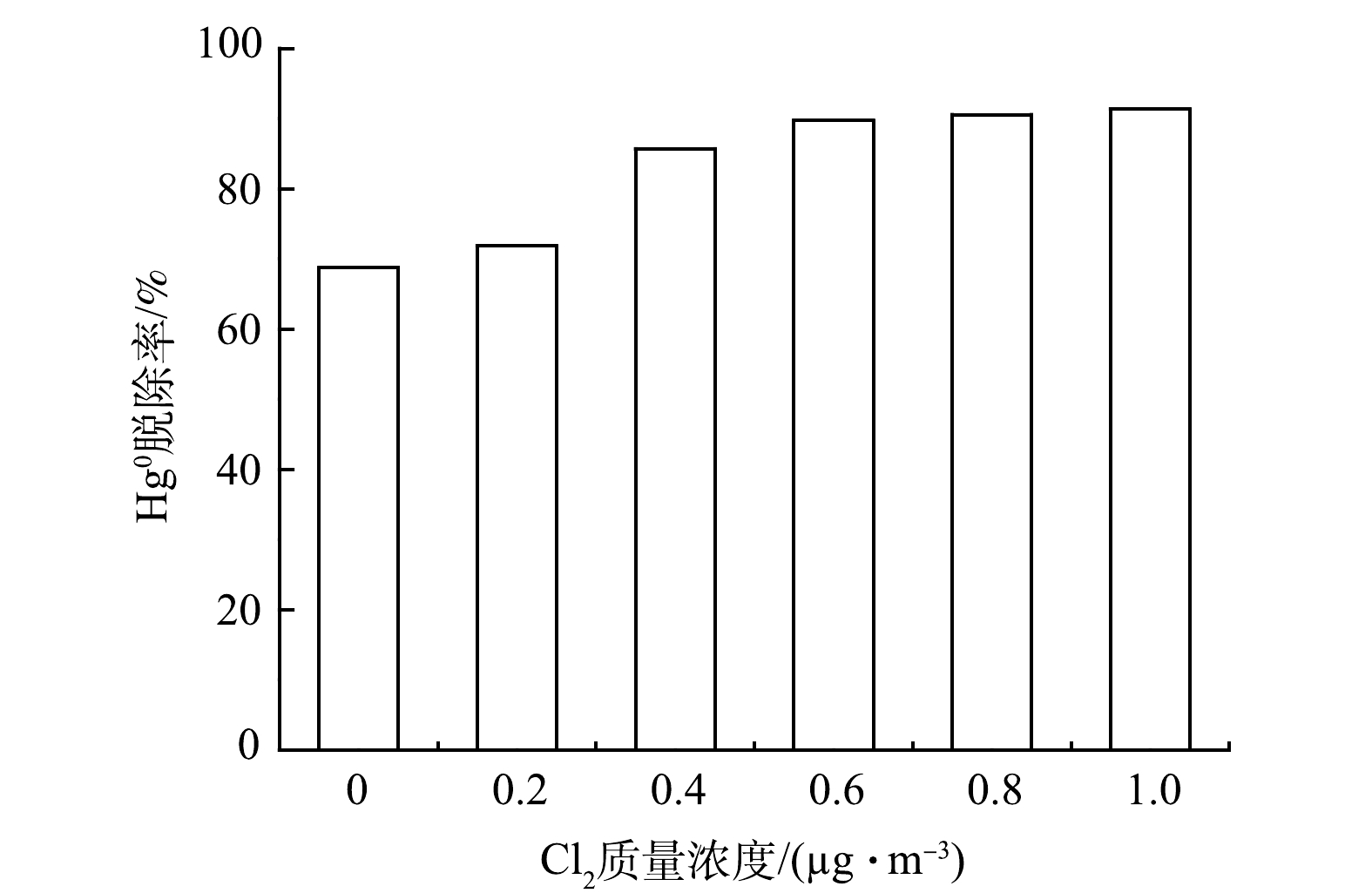
2)Cl2质量浓度对脱汞效率的影响。在最佳HClO浓度为4 mmol·L−1,研究Cl2质量浓度对Hg0氧化效率的影响。反应温度为45 ℃,pH为4.35,结果如图3所示。当Cl2质量浓度为0.2 μg·m−3时,Hg0氧化效率有小幅度提升,Cl2质量浓度升高至0.4 μg·m−3时,Hg0氧化效率显著增强。Cl2质量浓度由0.4 μg·m−3上升至1 μg·m−3时,Hg0脱除率逐渐趋于稳定。而Cl2质量浓度由0上升至0.4 μg·m−3时,脱汞效率提升约20%。这主要是由于Cl2作为强氧化剂,能快速将Hg0氧化为Hg2+,进而被溶液吸收。同时,一部分氯气溶解在吸收液中,生成HClO,在较短时间内pH降为2.07,在酸性条件下,ClO−具有更强的氧化性,促使Hg0向Hg2+转化。考虑反应吸收液在实际工程应用中对设备的腐蚀,选取最优电解产物Cl2质量浓度为0.4 μg·m−3。
-
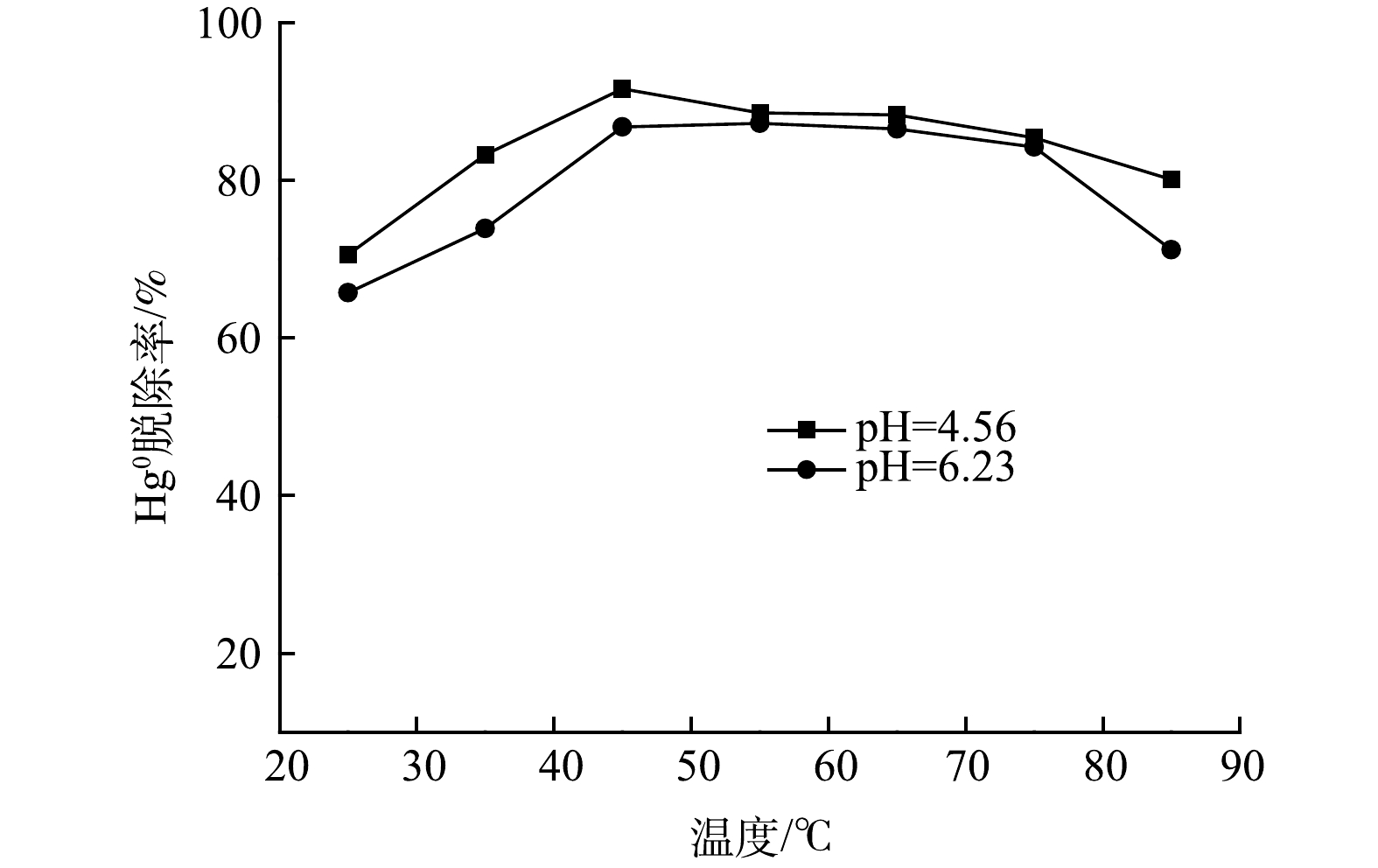
电解产物HClO浓度为4 mmol·L−1,反应温度对脱汞效率的影响如图4所示。可知,Hg0脱除率随温度升高呈现先升高后递减趋势。电解溶液pH为4.56,45 ℃时Hg0去除率达到92%。相同温度下,pH=6.23时电解溶液脱汞率为86%。不同pH下电解液脱汞率均在45 ℃左右达到峰值。随着温度继续升高,Hg0脱除率逐渐下降。考察Hg0在水中的溶解度发现,80 °C时Hg0 溶解度系数为0.999×10−7 mol·(Pa L)−1,30 °C时为8 639×10−7 mol·(Pa L)−1,二者相差约9倍,这说明反应温度升高可降低Hg0在溶液中的溶解度[24]。另一方面,电解溶液对Hg0的氧化属于放热反应[25],温度升高可抑制该反应的进行,因此,升高温度不利于Hg0的氧化吸收。最佳反应温度为45 ℃。
-
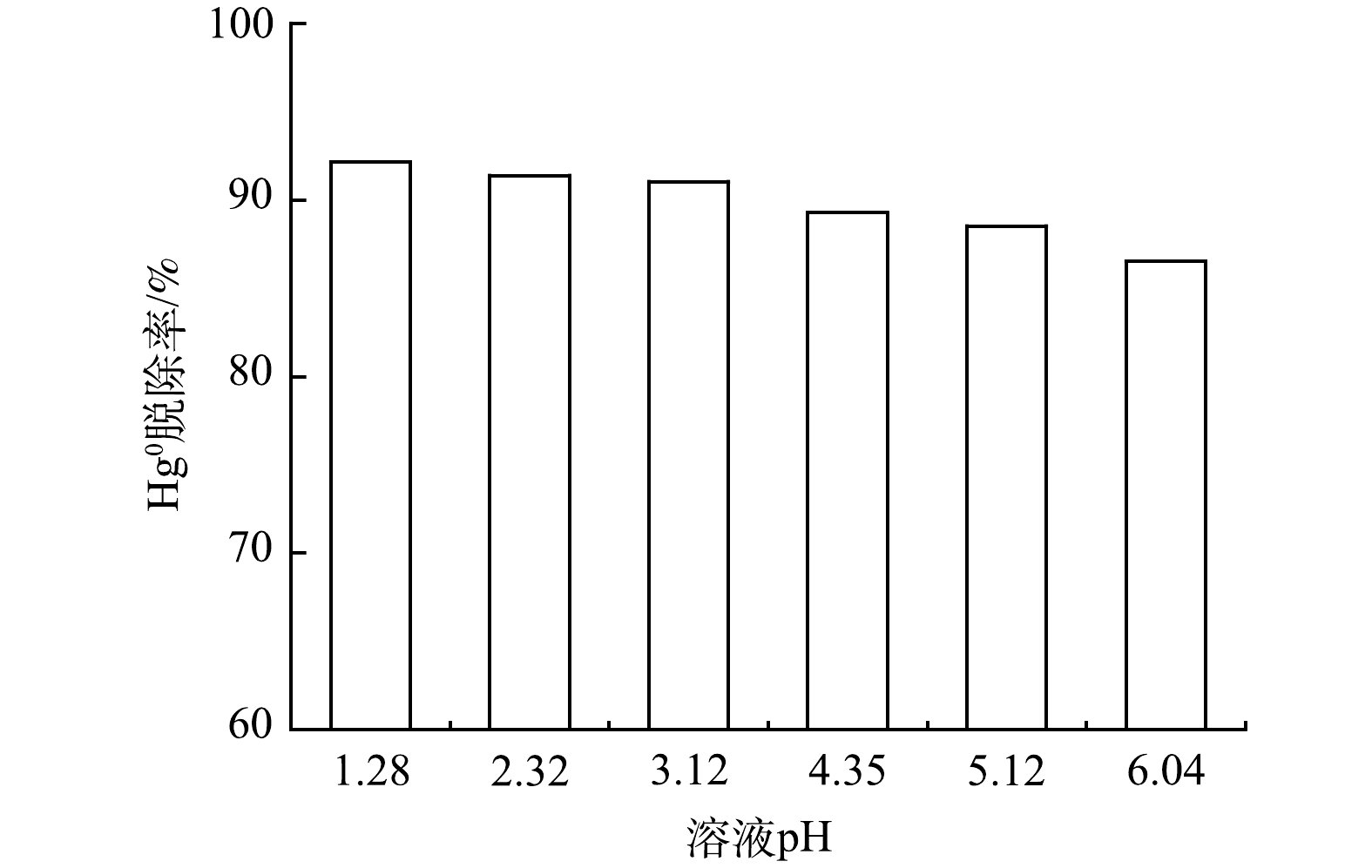
pH是影响氧化剂氧化脱除Hg0的关键因素之一。在HClO浓度为4 mmol·L−1、反应温度为45 ℃条件下,研究pH对Hg0脱除效率的影响,结果如图5所示。可知,Hg0的脱除效率随pH升高逐渐降低,其脱汞率维持在86%~92%,这表明酸性条件更有利于Hg0氧化脱除。其主要原因是,ClO−在酸性条件具有更强的氧化性[26],有利于Hg0氧化吸收。此外,酸性条件下ClO−/Cl−标准电极电势为1.48 V[27-28],Hg2+/Hg0氧化还原电位为0.85 V,其远高于Hg2+/Hg0氧化还原电位,故使脱汞效率维持在较高水平。综上所述,pH越低,Hg0脱除效果越好。但考虑实际工程应用中溶液对设备的腐蚀程度,所选取最优pH为4.35~6.04。
-
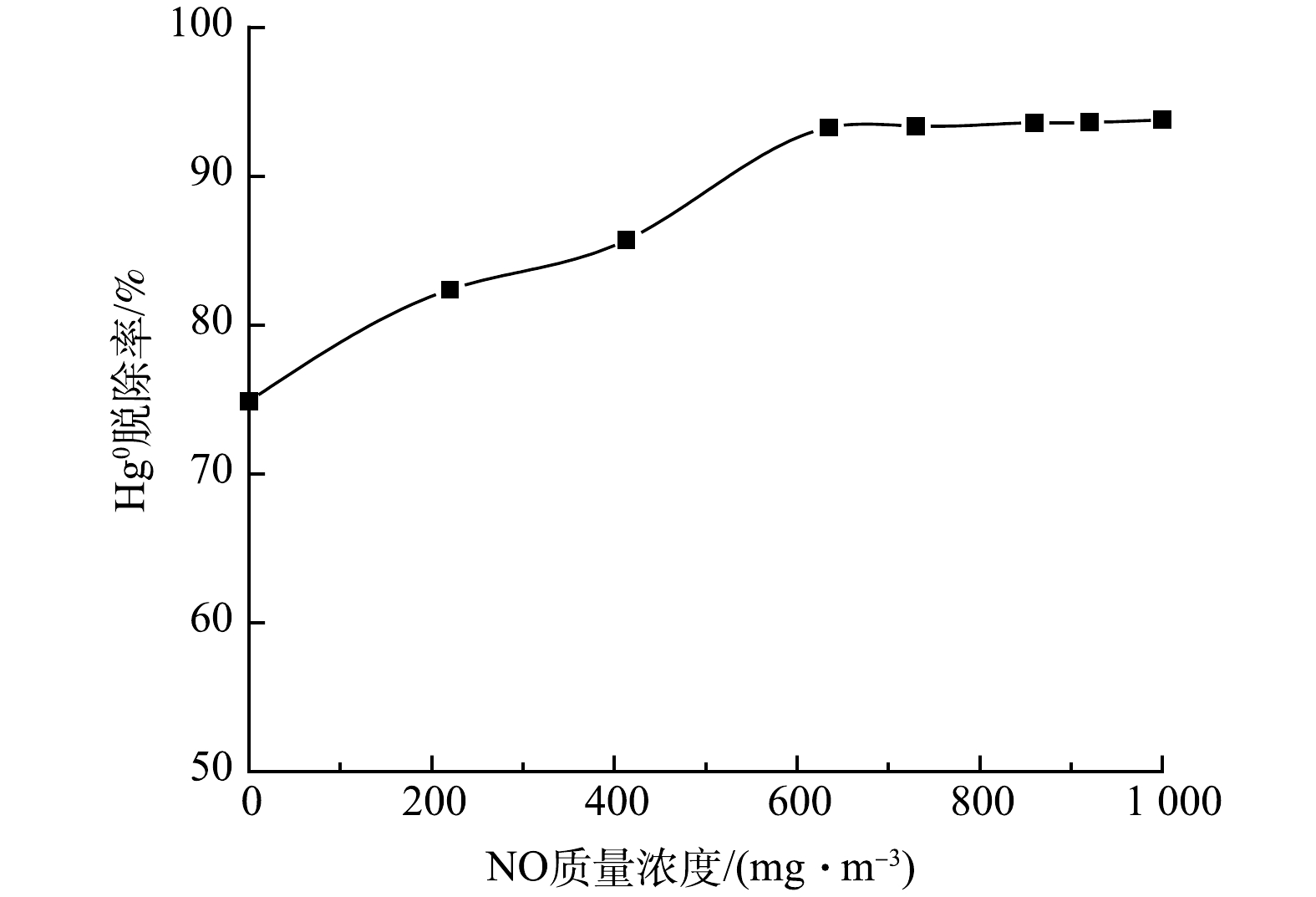
在HClO浓度为4 mmol·L−1、Cl2质量浓度为0.4 μg·m−3、反应温度为45 ℃条件下,研究烟气中不同NO质量浓度对Hg0脱除效率的影响,结果如图6所示。由图可知,NO的存在对Hg0的氧化脱除表现出促进作用,随着NO质量浓度的增加,Hg0脱除效率逐渐升高趋于平稳。NO质量浓度在0~600 mg·m−3时,Hg0脱除率显著增强。NO浓度逐渐增强,脱汞率保持稳定,其最高值可达93%。其原因一方面可能为,NO与氯系溶液形成一种中间产物NOCl[29],该物质在室温下可与Hg0发生快速反应,将Hg0转化为Hg2+[30],见式(2);另一方面,NO氧化反应发生在气-液相界面上,随着NO浓度增大,吸收液中生成HNO2和HNO3[11,13, 31-32],见式(3)~式(6),这使得吸收液的pH在短时间内急剧下降,酸性条件促进Hg0 的氧化脱除。吸收液中Hg2+含量升高,硝酸溶液中,Hg2+可以催化氧化Hg0 [30, 33-34],从而使脱汞效率维持在较高的水平。
-
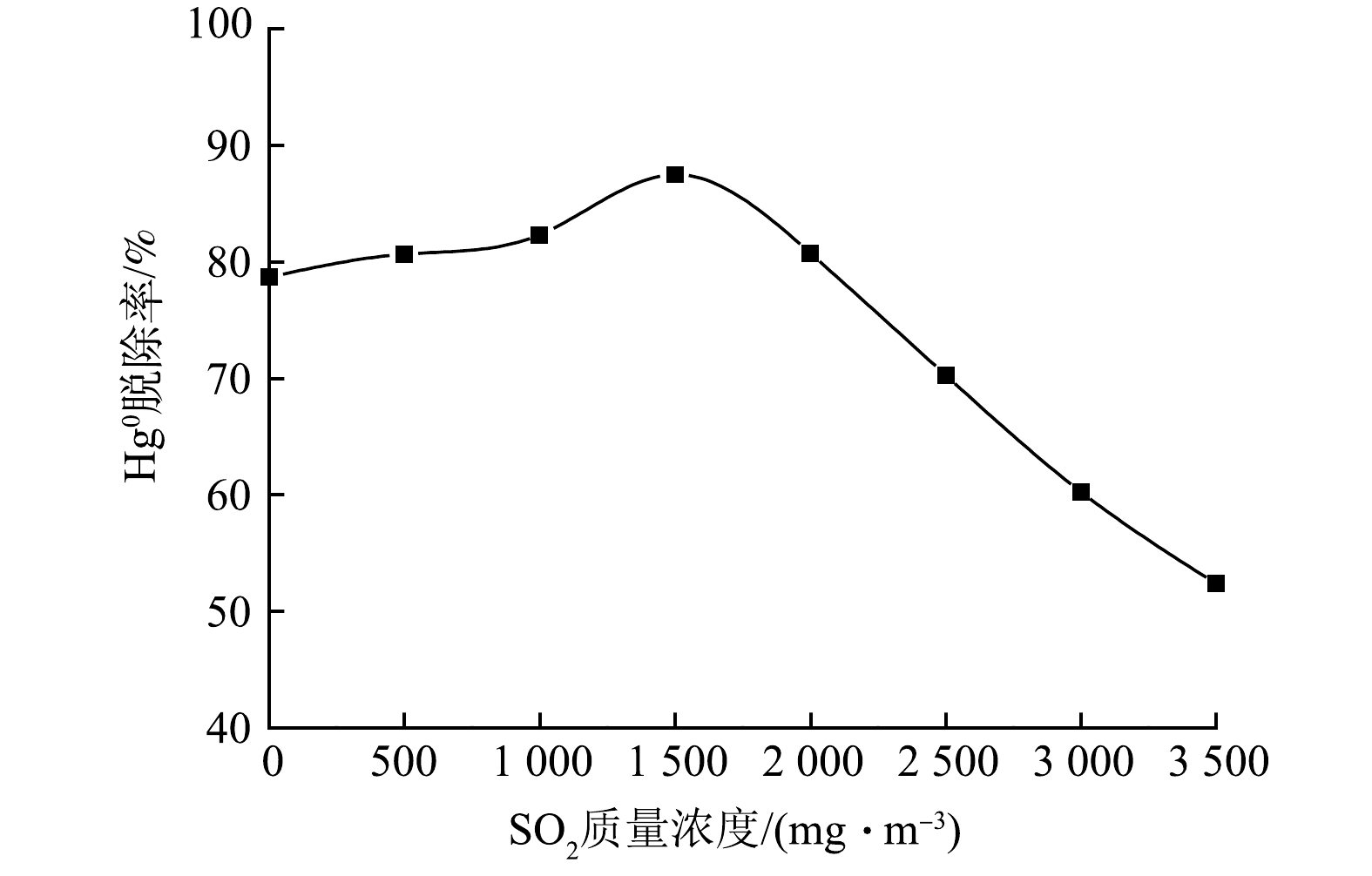
烟气含硫量是影响脱汞的重要因素,其他反应条件不变,研究SO2浓度对Hg0脱除率的影响,结果如图7所示。可知,SO2的存在对烟气脱汞呈现先促进后抑制的作用,SO2质量浓度高于1 500 mg·m−3时,Hg0脱除率呈直线下降。呈现该现象的原因是,随着SO2质量浓度的增加,使更多的SO2进入吸收液,SO2易与Cl2和ClO−发生反应,反应液呈酸性,促进Hg0向Hg2+转化(见式(7)~式(9)),Hg0氧化率显著提高,达到88%。随着SO2浓度进一步增强,溶液中SO32−、 SO42− 和HSO3−含量增加,这些离子的存在抑制Hg0氧化[35],见式(10)~式(11),导致Hg0脱除率降低。实际工程应用中可优先将SO2脱除。
-
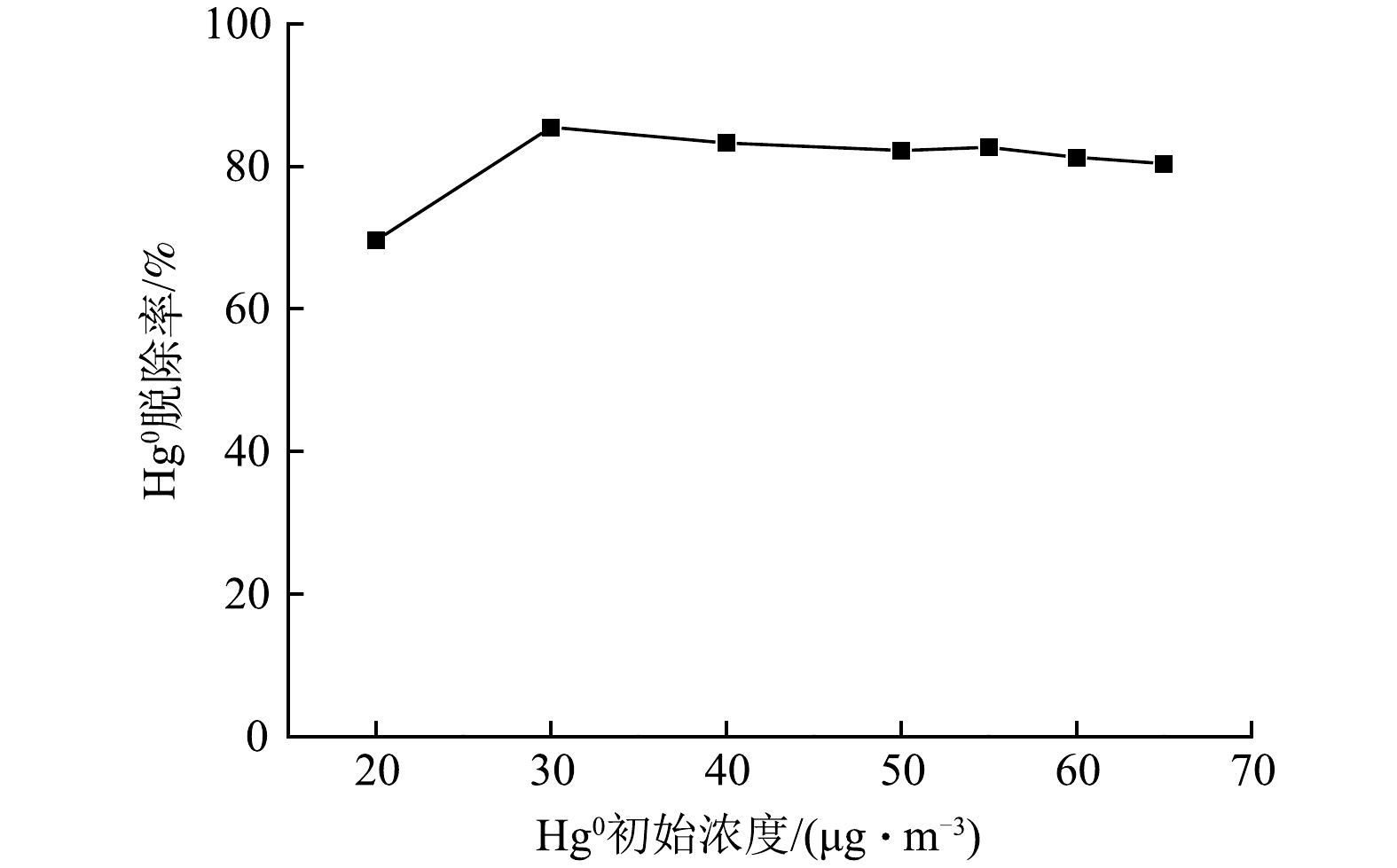
为检测该方法在实际工程应用中的适应性,研究了不同汞入口浓度对电解产物脱汞效率的影响。固定反应温度为45 ℃,HClO浓度为4 mmol·L−1,Cl2质量浓度为0.4 μg·m−3,结果如图8所示。可知,汞入口浓度在20~70 μg·m−3范围内,电解产物对其有较强的脱除效果,脱除率为70%~85%。因此,该方法对不同入口汞浓度有良好适应性。
-
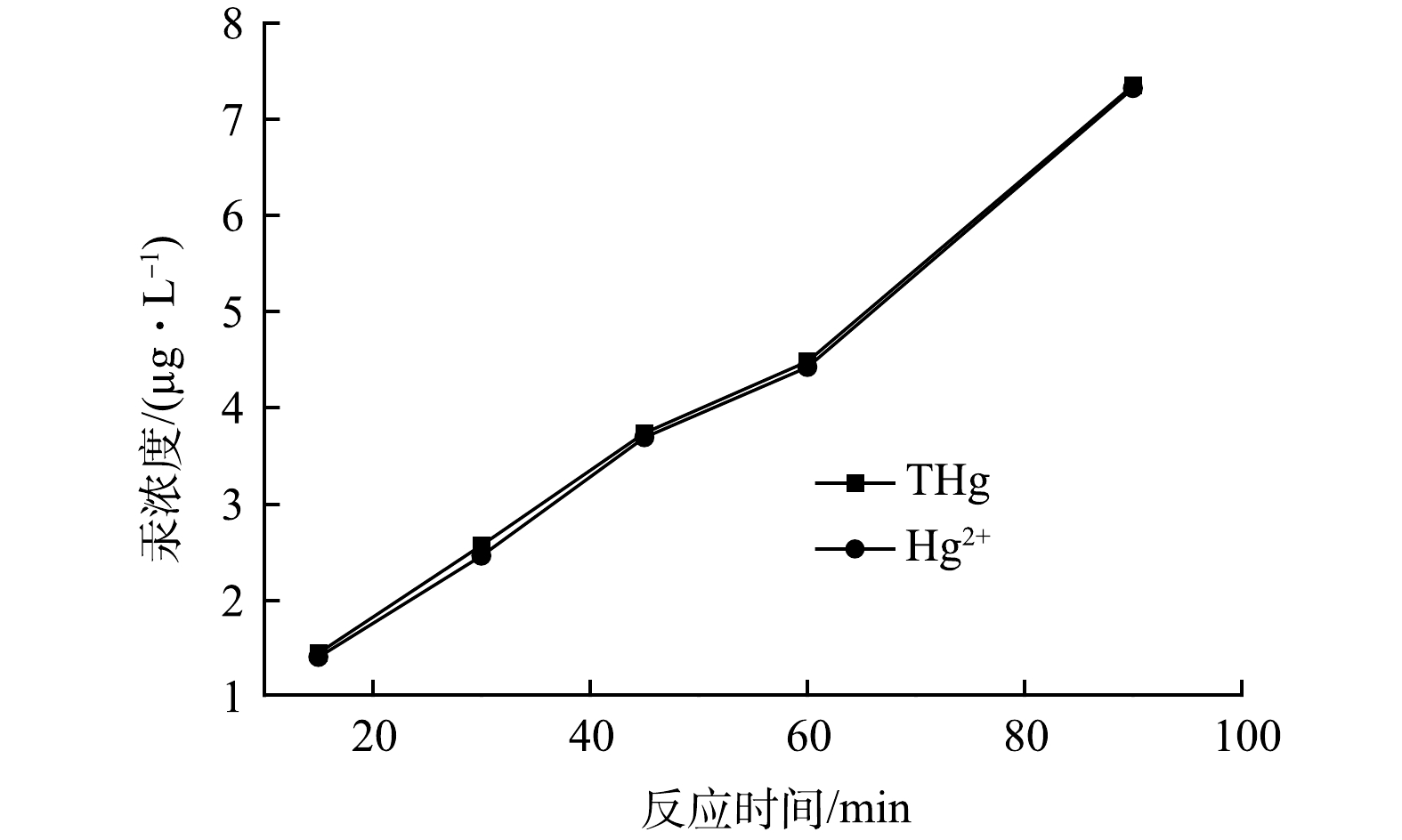
电解产物溶液主要成分是ClO−,其氧化性随着溶液的pH和反应温度变化,在酸性条件下表现出较强的氧化性。分析了相同时间点溶液中Hg2+浓度和总汞浓度(THg)。结果如图9所示,在实验允许误差范围内Hg2+浓度和总汞浓度保持一致。这表明,在电解产物氧化Hg0的过程中,氧化产物只存在Hg2+,无其他类型的产物生成。此外,在酸性条件下,Hg2+/ Hg0(0.85V)氧化还原电位低于Cl2/Cl−(1.229V)和ClO−/Cl−(1.48V)氧化还原电位,这进一步证实了电解产物对Hg0的氧化反应。
根据电解产物反应溶液离子测定结果,同时基于前人的研究结果[36],脱硫废水电解产物活性氯氧化脱除Hg0过程中可能发生的反应见式(12)~式(16)。
-
1) Hg0氧化效率随电解产物活性氯浓度的增加而增大。考虑设备腐蚀和经济效益,最佳电解产物HClO浓度为4 mmol·L−1,Cl2质量浓度为0.4 μg·m−3,此条件Hg0脱除率可达85.69%。Hg0氧化最佳温度为45 ℃。酸性条件可促使Hg0向Hg2+转化,使脱汞率显著提高,最适宜pH为4.35~6.04。
2)烟气中的硫氧化物和氮氧化物对其氧化脱汞有显著影响。Hg0脱除率随SO2浓度的增加呈先增加后逐渐下降,Hg0脱除率最高达87.46%,SO2质量浓度高于1 500 mg·m−3时将抑制汞氧化脱除;NO存在有助于Hg0氧化为Hg2+。
3)此氧化剂在不同汞浓度下均有较好的去除效果。
以脱硫废水电解产物活性氯为氧化剂的湿法脱汞工艺
Experimental study on wet demercury removal by active chlorine electrolysis products in desulfurization wastewater
-
摘要: 采用脱硫废水电解产物活性氯(HClO和Cl2)作为氧化剂,在自制鼓泡反应器中研究反应温度、pH、入口汞浓度、NO浓度、SO2浓度等主要参数对Hg0氧化效果的影响,并简要探究其反应机理。结果表明,电解产物活性氯能有效促进Hg0的氧化脱除,反应中最佳HClO浓度为4 mmol·L−1,Cl2质量浓度为0.4 μg·m−3,其最适宜反应温度为45 ℃;酸性条件有助于Hg0的氧化吸收,最适宜氧化的pH为4.35~6.04;Hg0氧化效率随SO2质量浓度增加呈现先升高后降低的趋势,其脱除率最高可达87.46%。NO的存在对Hg0氧化有一定的促进作用,该方法对不同入口汞浓度有较强适应性。本研究结果可为WFGD同步脱硫脱汞提供参考。Abstract: The effects of the main parameters such as reaction temperature, pH, inlet mercury concentration, NO concentration and SO2 concentration on the oxidation effect of Hg0 were studied in self-made bubble reactors using activated chloride (HClO and Cl2) as oxidants in desulfurized wastewater electrolytic products, and the reaction mechanism was briefly explored. The results showed that the active chlorine of the electrolytic product effectively promoted the oxidation and removal of Hg0, determined that the optimal HClO concentration was 4 mmol·L−1, the Cl2 concentration was 0.4 μg·m−3, and the optimum reaction temperature was 45 °C; acidic conditions were conducive to the oxidation absorption of Hg0, which was the most suitable. The oxidation pH range was 4.35~6.04; the oxidation efficiency of Hg0 increased first and then decreased with the increase of SO2 concentration, with a removal rate of 87.46%. The existence of NO had a certain promotional effect on the oxidation of Hg0. This method had strong adaptability to different inlet mercury concentrations and a good industrial application prospect. The results of this study can provide a reference for the simultaneous desulfurization and mercury removal of WFGD.
-

-
-
[1] LIU Y X, ZHOU J F, WANG Q, et al. A novel process for removal of Hg0 from flue gas using urea/persulfate activated by high temperature in a spray reactor[J]. Chemical Engineering Research & Design, 2015, 104: 828-834. [2] 马宵颖, 赵毅. 类过氧化物酶Fe-TAML催化H2O2脱除烟气中的单质汞[J]. 化工环保, 2018, 38(4): 425-430. doi: 10.3969/j.issn.1006-1878.2018.04.011 [3] 王运军, 段钰锋, 杨立国, 等. 燃煤电站布袋除尘器和静电除尘器脱汞性能比较[J]. 燃料化学学报, 2008, 36(1): 7. [4] ZHAO Y, QI M, HAO R L, et al. A novel catalytic oxidation process for removing elemental mercury by using diperiodatoargentate (Ⅲ) in the catalysis of trace ruthenium (Ⅲ)[J]. Journal of Hazardous Materials, 2020, 381(5): 120964. [5] WANG Y J, LIU Y, Wu Z B, et al. Experimental study on the absorption behaviors of gas phase bivalent mercury in Ca-based wet flue gas desulfurization slurry system[J]. Journal of Hazardous Materials, 2010, 183(1/2/3): 902-907. [6] 商永强, 赵永椿. 磁性吸附剂脱汞机理及技术研究进展[J]. 华电技术, 2019, 41(12): 8-15. [7] LI Y, ZHANG J Y, ZHAO Y C, et al. Volatility and speciation of mercury during pyrolysis and gasification of five Chinese coals[J]. Energy & Fuels, 2011, 25(9): 3988-3996. [8] DRANGA B A, LAZAR L, KOESER H. Oxidation Catalysts for Elemental Mercury in Flue Gases-A Review[J]. Catalysts, 2012, 2(1): 139-170. doi: 10.3390/catal2010139 [9] 吴笑男, 卢金荣, 梁英华. 硅胶复合材料吸附重金属汞的研究进展[J]. 化工环保, 2017, 37(6): 616-621. doi: 10.3969/j.issn.1006-1878.2017.06.003 [10] SJOSTROM S, EBNER T, LEY T, et al. Assessing sorbents for mercury control in coal-combustion flue gas.[J]. Journal of the Air & Waste Management Association, 2002, 52(8): 902-911. [11] MONDAL M K, CHELLUBOYANA V R. New experimental results of combined SO2 and NO removal from simulated gas stream by NaClO as low-cost absorbent[J]. Chemical Engineering Journal, 2013, 217: 48-53. doi: 10.1016/j.cej.2012.12.002 [12] ZHAO Y, JIE Y, MA X Y. Absorption behavior and removal of gaseous elemental mercury by sodium chlorite solutions[J]. Journal of Environment Engineering, 2012, 138(6): 620-624. doi: 10.1061/(ASCE)EE.1943-7870.0000519 [13] ZHAO Y, GUO T X, CHEN Z Y, et al. Simultaneous removal of SO2 and NO using M/NaClO2 complex absorbent[J]. Chemical Engineering Journal, 2010, 160(1): 42-47. doi: 10.1016/j.cej.2010.02.060 [14] 阮长超. 氧化剂协同石灰石湿法烟气脱硫脱汞实验研究[D]. 武汉: 华中科技大学, 2017. [15] 李兵, 王宏亮, 许月阳, 等. 燃煤电厂湿法脱硫设施对烟气中微量元素的减排特性[J]. 煤炭学报, 2015, 40(10): 2479-2483. [16] 郑逸武, 段钰锋, 汤红健, 等. 燃煤烟气污染物控制装置协同脱汞特性研究[J]. 中国环境科学, 2018, 38(3): 862-870. doi: 10.3969/j.issn.1000-6923.2018.03.009 [17] 武成利. 燃煤烟气中汞再析出及抑制研究[D]. 淮南: 安徽理工大学, 2010. [18] 梁大镁. 湿法脱硫系统协同脱除汞的实验研究[D]. 武汉: 华中科技大学, 2011. [19] 刘政修, 李磊, 王斌. 燃煤电厂锅炉烟气湿法脱硫废水深度处理工艺选择[J]. 全面腐蚀控制, 2016. [20] 李行, 陆海伟, 黄河清. 燃煤电厂湿法脱硫废水零排放处理工艺[J]. 广东化工, 2018, 45(18): 2. [21] 钱凯凯. 电化学处理脱硫废水协同去除燃煤烟气零价汞的研究[D]. 武汉: 武汉大学, 2020. [22] 刘诗杰. 复合催化材料制备及其在高氯电厂烟气脱硫废水处理中应用研究[D]. 武汉: 湖北大学, 2018. [23] 吴火强, 刘亚鹏, 王璟, 等. 脱硫废水膜浓缩浓水电解制氯工艺分析[J]. 热力发电, 2016, 45(9): 109-115. [24] LAUDAL D L, BROWN T D, NOTT B R. Effects of flue gas constituents on mercury speciation[J]. Fuel Processing Technology, 2000, 65(99): 157-165. [25] 能子礼超, 杨红, 海来伍加, 等. 次氯酸钾氧化去除烟气中单质汞的反应机理[J]. 化工环保, 2020, 40(5): 507-511. [26] BYOUN S K, SHIN D N, MOON I S, et al. Quick vaporization of sprayed sodium hypochlorite (NaClO(aq)) for simultaneous removal of nitrogen oxides (NOx), sulfur dioxide (SO2), and mercury (Hg0)[J]. Journal of the air & waste management association, 2019, 69(7): 857-866. [27] KACZUR J J. Oxidation chemistry of chloric acid in NOx/SOx and air toxic metal removal from gas streams[J]. Environmental Progress & Sustainable Energy, 2010, 15(4): 245-254. [28] SADA E, KUMAZAWA H, YAMANAKA Y, et al. Kinetics of absorption of sulfur dioxide and nitric oxide in aqueous mixed solutions of sodium chlorite and sodium hydroxide.[J]. Journal of chemical engineering of Japan, 1978, 11(4): 276-282. doi: 10.1252/jcej.11.276 [29] MARTIN K, GONZALEZ E, ZHOU C Q, et al. Elemental mercury removal using a wet scrubber[J]. Office of Scientific & Technical Information Technical Reports, 1999, 61(1/2): 180-185. [30] 马宵颖. 液相烟气脱汞实验研究[D]. 保定: 华北电力大学(河北), 2008. [31] WANG J, ZHONG W Q. Simultaneous desulfurization and denitrification of sintering flue gas via composite absorbent[J]. Chinese Journal of Chemical Engineering, 2016, 24(8): 1104-1111. doi: 10.1016/j.cjche.2016.04.005 [32] GUSTIN M, LADWIG K. Laboratory investigation of Hg release from flue gas desulfurization products[J]. Environmental Science & Technology, 2010, 44(10): 4012. [33] O’DRISCOLL N J, SICILIANO S D, LEAN DRS. Continuous analysis of dissolved gaseous mercury in freshwater lakes[J]. Science of the Total Environment, 2003, 304(1/2/3): 285-294. [34] WANG Q F, LIU Y, WU ZB. Laboratory Study on Mercury Release of the Gypsum from the Mercury Coremoval Wet Flue Gas Desulfurization System with Additives[J]. Energy & Fules, 2018, 32(2): 1005-1011. [35] ZHAO Y, HAO R L, QI M. Integrative process of preoxidation and absorption for simultaneous removal of SO2, NO and Hg0[J]. Chemical Engineering Journal, 2015, 269: 159-167. doi: 10.1016/j.cej.2015.01.064 [36] I FÁBIÁN, D SZÜCS, GORDON G. Unexpected Phenomena in the Mercury(II) Chlorite Ion System: Formation and Kinetic Role of the HgClO2+ Complex[J]. Journal of Physical Chemistry A, 2000, 104(34): 8045-8049. doi: 10.1021/jp0015832 -



 下载:
下载: