-
受人类活动如金属采矿冶炼、污水灌溉、农业生产等影响,农田土壤重金属污染问题日益突出。2014年国家环境保护部与国土资源部联合发布的《全国土壤污染状况调查公报》[1]显示,我国土壤中镉(Cd)污染物点位超标率为7.0%,位于无机污染物之首;砷(As)污染物点位超标率为2.7%,位于无机污染物第3;二者属于耕地土壤的主要污染物。土壤中As、Cd的累积不仅会导致粮食减产,带来经济损失[2],还会通过食物链富集并进入人体,从而对人群健康造成威胁[3]。然而,由于As、Cd的元素性质和赋存形态不同,在土壤pH-Eh的影响下,二者具有不同的迁移特性和生物有效性,致使Cd污染土壤的修复材料和方法普遍不适用于As污染土壤[4],因此,As、Cd复合污染土壤的联合修复是目前的研究难点。
一般的土壤修复手段有物理、化学和生物技术。其中,化学钝化具有简单、快速、相对成本低等优点[5],被广泛应用于重金属污染土壤的修复中。常见的化学钝化材料有金属及其氧化物、含硫物质、含磷物质、硅钙物质、黏土矿物、有机物料、生物炭等[6]。而金属及其氧化物、含硫、含磷材料及硅钙物质会造成土壤中金属、磷、钙的积累,易引发土壤二次污染的问题[7-10];有机物料、生物炭对As、Cd复合污染土壤的修复效果欠佳[11-12],对二者同时吸附的能力和亲和力不足,若要大规模应用就会导致较高的修复成本。层状双金属氢氧化物(layered double hydroxides,LDHs)是一种典型的阴离子层状黏土矿物,通常被称为类水滑石化合物,一般是由2种金属的氢氧化物层构成主体,层间填充带负电荷的阴离子和水,结构通式为[M2+1−xM3+x(OH)2]x+(An−)x/n·mH2O[13]。该材料具有良好的热稳定性、亲水性、记忆效应和较高的阴离子交换能力,受到了国内外学者的广泛关注[14-17]。铁锰类LDHs具有治理土壤As、Cd复合污染的可能性。这是因为,铁氧化物对As(V)具有亲和力和高选择性,而锰氧化物具有将As (III)氧化为As(V)的作用,进而可以在较宽的pH范围内提高对As的吸附能力[18-21];且LDHs属于碱性材料,可促使土壤中Cd形成氢氧化物或碳酸盐沉淀,降低Cd的移动性[22-23]。此外,铁、锰都是环境友好型的金属元素,以铁、锰元素制成的LDHs材料对环境污染小,而且制备成本低。
然而,锰的氢氧化物在碱性条件下极易被氧化,这会破坏晶体生长[24],难以制备出完整层状结构的铁锰类LDHs,故需引入第3种元素保持其稳定性。周宏光[24]采用Mg2+介入的共沉淀法制备出具有良好层状结构且具有极高吸附容量和酸碱双功能性的FeMnMg-LDHs材料,对Cd2+的最大吸附量为59.99 mg·g−1,远高于其他同类型的LDHs材料,但此材料在使用过程中的Mg2+溶出率较大。廖玉梅等[25]对FeMnMg-LDHs进行了改性,引入Ni2+制备出具有良好层状结构和较高稳定性的FeMnNi-LDHs材料,对As(Ⅲ)的最大吸附量为240.86 mg·g−1,明显高于其他层状双金属氢氧化物,但该研究仅停留在重金属废水处理领域,还未应用于土壤重金属修复领域。
考虑到Ca2+的离子半径(0.098 nm)与Mg2+(0.065 nm)相差不大,且与Fe2+(0.076 nm)、Mn2+(0.080 nm)接近,故预期Ca2+能和Fe、Mn良好结合,提高材料的稳定性[26-27];另外,Ca2+还会和As发生沉淀作用,形成Ca3As2O8,从而降低As的有效性[28]。故本研究尝试引入Ca2+,采用共沉淀法制备FeMnCa-LDHs材料,通过XRD、SEM、FT-IR手段对材料的微观结构进行表征和分析;并分别开展土壤培养实验和小白菜盆栽试验,以考察FeMnCa-LDHs材料对土壤-作物体系中As和Cd的钝化效果,为铁锰类LDHs材料用于修复土壤砷镉复合污染的实际应用提供参考。
-
选取Ca作为稳定剂采用共沉淀法[25]制备FeMnCa-LDHs材料。制备流程为:准确称取5.549 gCaCl2、6.758 gFeCl3·6H2O、4.947 gMnCl2·4H2O溶于50 mL无氧水中,搅拌均匀,再向500 mL的三颈烧瓶加入100 mL无氧水,之后用2 mol·L−1NaOH溶液调节无氧水pH为12,持续通入氮气10 min;将混合金属离子溶液以0.5 mL·min−1的速度滴入三颈烧瓶内,同时使用2 mol·L−1NaOH调节溶液pH为12,并用机械搅拌器搅拌;滴加完成后,持续通入氮气,机械搅拌24 h;搅拌完成后用塑料膜封闭三颈烧瓶瓶口,放入水浴锅,60 ℃恒温水浴加热12 h;反应后的固体产物用无氧水冲洗至中性,离心,冷冻干燥;干燥后的产物用玛瑙研钵磨碎过100目筛,妥善密封保存于干燥箱中,备用。
-
对材料进行FT-IR分析的预处理方法为:准确称取0.050 gFeMnCa-LDHs材料于100 mL锥形瓶中,分别加入50 mL质量浓度为100 mg·L−1的CdCl2、NaAsO2溶液,使用0.1 mol·L−1HCl调节溶液pH为5.5±0.1,将锥形瓶置于25 ℃恒温振荡器振荡24 h,转速为180 r·min−1,经0.4 μm滤膜抽滤,过滤后的材料冷冻干燥、研磨后,进行检测分析。
-
供试土壤采自重庆市江津区白沙镇河口村稻田,土壤均采自耕层0~20 cm,将土壤去除杂物后自然风干,磨碎过10目筛,保存备用。土壤基本理化性质为:pH 5.03、有机质质量分数7.93 g·kg−1、总氮质量分数4.22 g·kg−1、总磷质量分数0.63 g·kg−1、总钾质量分数9.56 g·kg−1、总Cd质量分数0.45 mg·kg−1、总As质量分数7.93 mg·kg−1。其中,总Cd质量分数超过了《土壤环境质量 农用地土壤污染风险管控标准(试行)》(GB 15618-2018)[29]的风险筛选值,超标倍数为1.5倍;总As质量分数在风险筛选值以下。供试作物选用小白菜,品种为长江五号快菜(十字花科芸薹属,Brassica chinensis)。
-
参考《土壤环境质量 农用地土壤污染风险管控标准(试行)》(GB 15618-2018)[29],分别设置了低、中和高污染3种水平,并分别以不同浓度的CdCl2和NaAsO2溶液,将溶液加入土壤中搅拌均匀来调控As和Cd的污染水平,控制土壤含水率为田间最大持水率的60%,室温下避光培养45 d,待土壤中As和Cd形态稳定后,使土壤自然风干,研磨过2 mm筛进行测定,结果如表1所示。
-
在上述不同污染水平的土壤中,按照材料∶土壤(质量百分比)=0.1%、0.5%、1.0%的比例分别添加一定质量的FeMnCa-LDHs,以不施加材料为对照(0),充分混合均匀,一共分为12个处理组。用底径10 cm、外径14 cm、高12 cm的圆柱形塑料盆种植小白菜,每组种3盆,每盆装1.0 kg土并施入1.0 g含N15%、含P2O515%、含K2O15%的复合肥,陈化7 d。每盆播种6~10粒小白菜种子,待出苗后根据大小和长势情况进行间苗,每盆留3株。在整个生长期按60%的田间持水量浇水。50 d后,收获小白菜植株并采集盆栽土壤。将植株用去离子水洗净、擦干,分为地上部分和根部于冰箱中保存备用。
-
采用XRD分析材料的晶体结构;采用SEM观察材料的形态特征;采用FT-IR分析材料表面官能团;采用《土壤 pH值的测定 电位法》(HJ 962-2018)[30]测定土壤pH;按照土壤农化分析常规方法[31]测定全氮、全磷、全钾和有机质;土壤总As和总Cd分别按《土壤质量 总汞、总砷、总铅的测定 原子荧光法》(GB/T 22105.2-2008)[32]和《土壤质量 铅、镉的测定 石墨炉原子吸收分光光度法》(GB/T 17141-1997)[33]测定;采用BCR连续提取法[34]对As、Cd形态分级进行测定;植物各部位的As和Cd质量分数分别按《食品安全国家标准 食品中镉的测定》(GB 5009.15-2014)[35]和《食品安全国家标准 食品中总砷及无机砷的测定》(GB 5009.11-2014)[36]进行测定;土壤及植物中的As质量分数均采用原子荧光光谱仪(LC-AFS-8230、SA-20,北京吉天仪器有限公司)测定,Cd质量分数均采用石墨炉原子吸收分光光度计(GFA-6880,岛津仪器有限公司)测定。所有测定过程均进行空白实验,每个处理设置3个平行,土壤样品中As和Cd的加标回收率分别为89%~104%和94%~103%,植物样品中As和Cd的加标回收率分别为88%~105%和95%~102%。
-
FeMnCa-LDHs材料的XRD表征结果如图1(a)所示。材料在2θ为11.26 °、22.58 °、32.54 °时均有特征峰,分别对应003晶面、006晶面和009晶面[37-38],这3个峰值间有较好的倍数关系,表明3个晶面相互平行,这说明合成的FeMnCa-LDHs材料具有层状双氢氧化物的典型特征结构[39]。
FeMnCa-LDHs材料的SEM分析结果如图1(b)所示。从图中可以看出,FeMnCa-LDHs材料的分子之间排列不规则,暴露出许多孔隙,且有一定的片层结构。经测定,材料的比表面积为63.05 m3·g−1、孔体积为0.29 cm3·g−1、孔径为18.46 nm。
FeMnCa-LDHs材料吸附水中As和Cd前后的FT-IR图谱如图1(c)所示。经测定,材料对As、Cd的饱和吸附容量分别为216.08和193.18 mg·g−1。对比其在水中吸附前后的图谱可以发现,在3 300~3 600 cm−1处出现很强的吸收峰,这是由2~3个羟基和层间水分子的伸缩振动叠加而形成[40]。在3 595 cm−1附近的羟基特征峰偏移非常大,这表明羟基在吸附As、Cd过程中起主导作用。其他特征峰偏移较小,这表明其他官能团起辅助作用。吸附后的图谱可以在874 cm−1处明显观察到As的伸缩振动特征峰,这说明FeMnCa-LDHs对As的吸附作用非常明显。
-
图2显示了在低、中、高3种污染水平土壤上施加不同量的FeMnCa-LDHs材料并进行盆栽培养50 d后,土壤pH的变化情况。结果表明,FeMnCa-LDHs材料能显著提高土壤pH,随着材料施用量的增加,土壤pH在不断增大。与CK相比,在低、中、高污染水平下,土壤pH分别提高了2.04、1.97和1.90。施用材料后土壤从酸性变为弱碱性。这是因为,FeMnCa-LDHs是碱性材料,其等电点为10.1,层间富含大量—OH,能降低土壤中交换性氢离子,有效提高土壤pH,改善土壤酸性环境[41]。在各材料施用量下,污染程度越高的土壤,pH越低。这可能是由于有更多的Cd与土壤胶体和材料中的—OH结合,导致进入土壤溶液中的—OH减少,使得土壤pH略低。
-
在不同污染水平下,FeMnCa-LDHs材料施用量对土壤中As和Cd形态的影响见图3。由图3(a)可知,施用FeMnCa-LDHs材料改变了土壤中As的形态分布,1.0%的材料施用量使弱酸提取态As向残渣态As转化。与CK相比,1.0%的材料施用量降低了弱酸提取态As的质量分数,在低、中、高污染水平下分别下降2.66%、6.65%和12.1%;同时,残渣态As的质量分数分别增加4.73%、5.04%和21.9%。这可能是由于FeMnCa-LDHs材料对As产生了氧化、离子交换和配位络合等吸附作用,在吸附过程中层板上的Mn可将土壤中的As(Ⅲ)氧化成As(V),同时层板上的Fe可以高效吸附氧化生成的As(V)和部分未被氧化的As(Ⅲ)[24];As还会与Fe表面的羟基形成羟基化金属离子([MOH]n+)[42],被高效吸附到材料层间;Ca2+的存在也会与As形成Ca3(AsO4)2和CaHAsO3沉淀[43],使土壤中As的生物有效性降低,起到钝化效果。
由图3(b)可知,随着材料施用量的增加,弱酸提取态Cd的质量分数不断降低,弱酸提取态Cd向更稳定的形态转化,土壤Cd的活性降低。与CK相比,在低、中、高污染水平下,1.0%的材料施用量使弱酸提取态Cd的质量分数分别下降11.6%、22.6%和28.9%;残渣态Cd的质量分数分别增加10.1%、8.92%和8.38%;可氧化态Cd在中、高污染水平下也有一定程度的增加。这是因为,Cd能与FeMnCa-LDHs材料中大量存在的—OH形成氢氧化物沉淀,吸附在材料表面而被去除[25];此外,土壤pH是影响土壤中Cd的生物有效性和迁移性的重要因素,从图2可知,FeMnCa-LDHs材料的施用能显著提高土壤pH,增加土壤表面负电荷,从而促进土壤对Cd2+的吸附,并促进Cd形成氢氧化物和碳酸盐沉淀[16,44-45],使Cd的生物有效性降低。
-
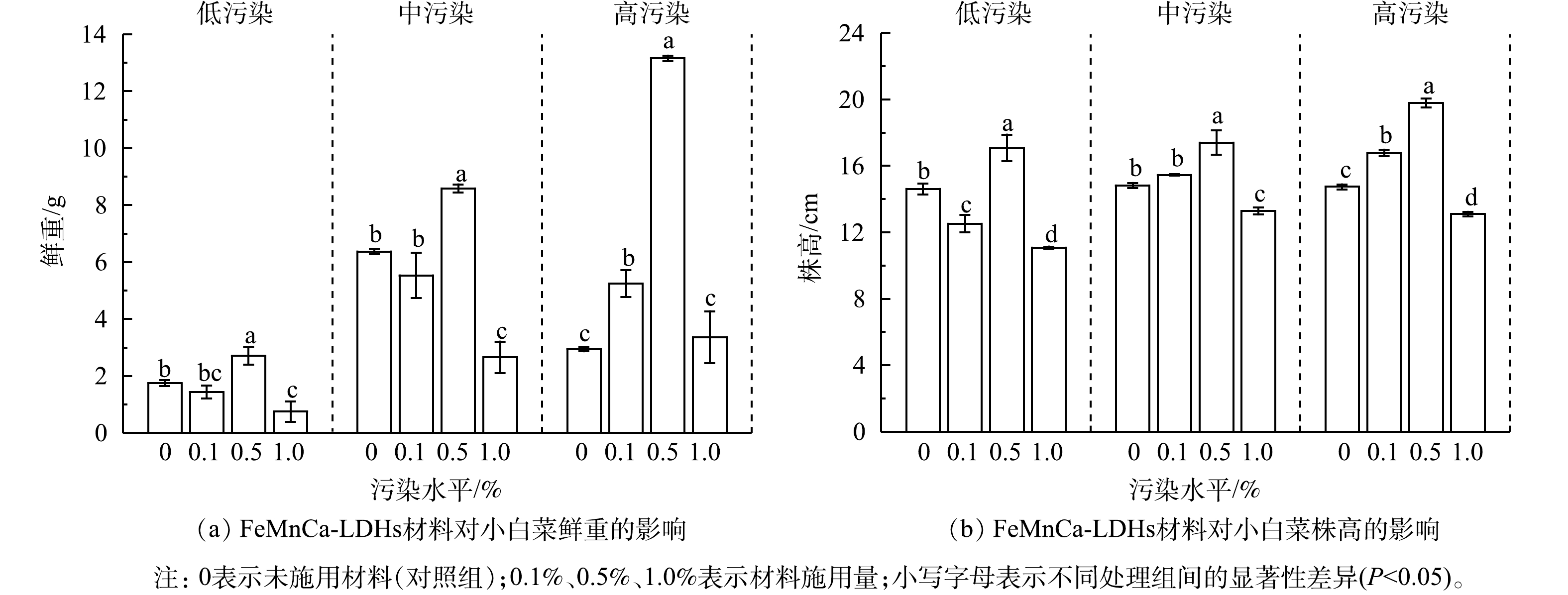
图4显示了在低、中、高3种污染水平下,FeMnCa-LDHs材料施用量对小白菜生长状况的影响。可以看出,适量施用FeMnCa-LDHs材料对小白菜的生长发育有促进作用。0.5%的材料施用量使小白菜的鲜重和株高显著增加,在高污染水平下其鲜重和株高分别是CK组的4.5和1.3倍;而以1.0%的比例施用时,小白菜鲜重和株高显著低于0.5%的施用组,因此,寻求最佳的材料施用量对于小白菜的生长有重要意义。小白菜在中、高污染水平下的生长状况普遍优于低污染水平。孙光闻等[46]发现,小白菜的产量具有随着土壤中Cd质量分数的增加而增加的趋势,土壤在低于6 mg·kg−1污染水平时,对小白菜的生长无影响或促进其生长;而在本实验中,高污染水平的Cd质量分数在未施加材料前仅为2.09 mg·kg−1,远低于6 mg·kg−1的污染水平。另有研究表明,Cd对小白菜生长的影响因品种的耐性强弱而有所不同,对耐性强的品种而言,Cd对其生长有促进作用[47-48]。此外,中高污染土壤的平均pH低于低污染土壤(图2),而As的生物有效性与土壤pH呈正相关[49],在酸性土壤中As对植物的毒害作用受到抑制。因此,本实验在中、高污染水平的土壤上种植的小白菜生长状况更好。
-
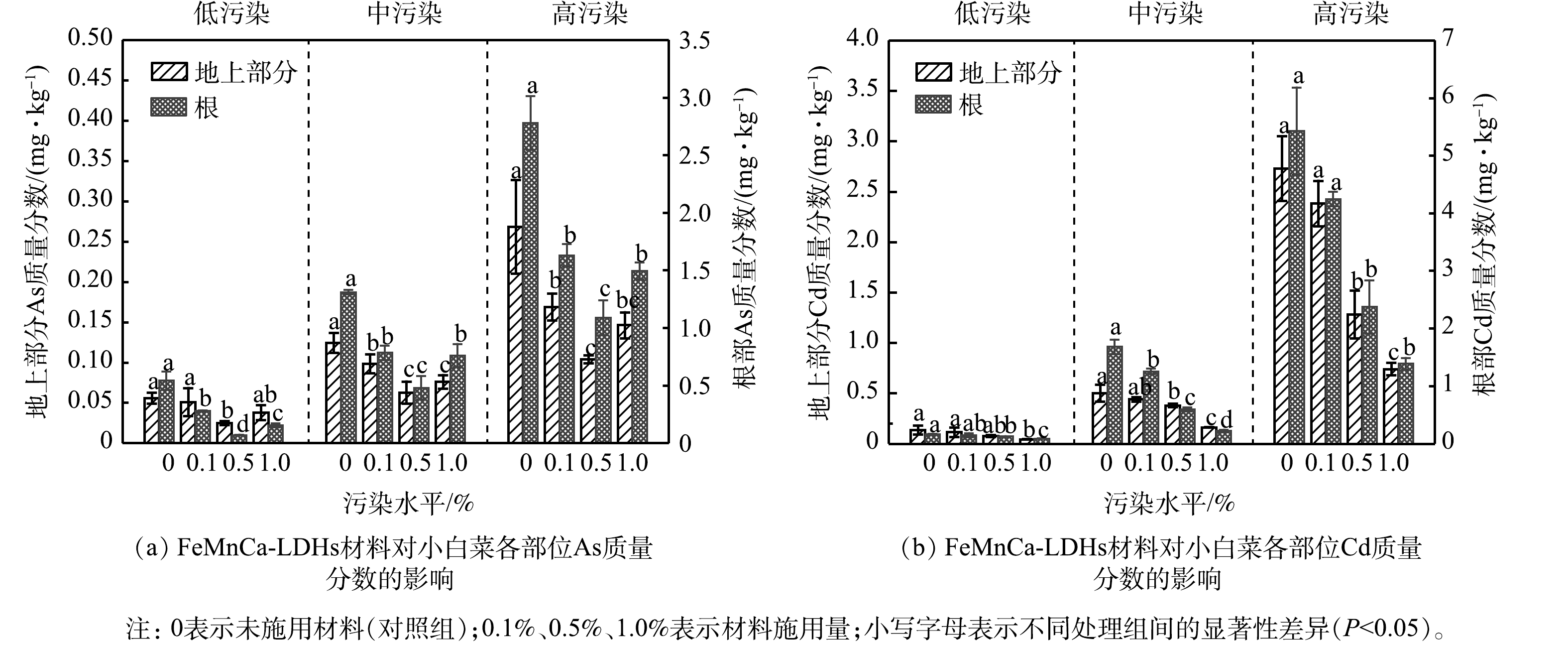
在低、中、高3种污染水平下,FeMnCa-LDHs材料施用量对小白菜地上部分和根部中As和Cd质量分数的影响见图5。如图5(a)所示,小白菜各部位中As的质量分数均随着FeMnCa-LDHs施用量的增加呈现出先降低再增加的趋势。与CK相比,0.5%的施用量使小白菜地上部分和根部中As的质量分数显著降低,在高污染水平下分别降低61.2%和60.8%。从2.3节的实验结果已知,FeMnCa-LDHs材料能降低As的生物有效性,故小白菜各部位中As的质量分数显著降低。当施用量为1.0%时,FeMnCa-LDHs材料对小白菜吸收As的抑制作用减弱,地上部分和根部中As的质量分数高于0.5%的施用组;然而,此时土壤中弱酸提取态As的质量分数却低于0.5%的施用组(图3(a))。这可能是由于在材料施用量较高时,过量的Fe、Mn元素会影响植物对重金属胁迫响应的调节机制[50],致使各部位中As的质量分数有所回升。据我国食品安全标准[51],蔬菜中的As阈值为0.5 mg·kg−1,在低污染水平下,施用材料后小白菜地上部分和根部中As的质量分数均未超标;在中污染水平下,0.5%的施用组亦未超标。
由图5(b)可知,FeMnCa-LDHs材料对小白菜各部位中Cd的质量分数的降低效果不同于As,在3种污染水平下,小白菜各部位中Cd的质量分数均随材料施用量的增加而不断降低。在高污染水平下,0.5%的材料施用量显著降低了小白菜地上部分和根部中Cd的质量分数,降幅分别为53.0%和56.2%。从2.2节和2.3节的实验结果已知,随着材料施用量的增加,土壤pH在不断上升,土壤中弱酸提取态Cd也在向更稳定的形态转化,Cd的生物有效性不断降低,故小白菜各部位中Cd的质量分数不断减少。值得注意的是,1.0%的材料施用量使中污染水平下小白菜地上部分中Cd的质量分数由0.50 mg·kg−1降到0.16 mg·kg−1,低于国家食品安全标准0.2 mg·kg−1[51]。
-
化学提取法和生物学评价法是目前最常用的两种评价重金属生物有效性的方法[52-53],为探究两种方法之间的相关性,本研究以高污染水平为例,对盆栽土壤中各形态As、Cd的质量分数及小白菜地上部分和根部中As、Cd的质量分数作Pearson相关性分析,结果如表2。由表2可知,小白菜地上部分和根部中As和Cd的质量分数与弱酸提取态As和弱酸提取态Cd的质量分数呈显著正相关,与残渣态As和残渣态Cd的质量分数呈显著负相关。FeMnCa-LDHs材料通过促使弱酸提取态As和弱酸提取态Cd向残渣态As和残渣态Cd转化,降低了弱酸提取态As和弱酸提取态Cd的质量分数,同时提高了残渣态As和残渣态Cd的质量分数,使小白菜地上部分和根部对As和Cd的吸收量降低。
-
1) FeMnCa-LDHs材料具有层状双金属氢氧化物的特征峰和典型结构,材料层间富含—OH,能使土壤由酸性变为弱碱性,且随着材料施用量的增加,土壤pH不断增大。
2) FeMnCa-LDHs材料能改变土壤中As和Cd的形态分布,降低弱酸提取态As和弱酸提取态Cd的质量分数并提高残渣态As和残渣态Cd的质量分数,起到了同时钝化As、Cd的效果。
3) 0.5%和1.0%的FeMnCa-LDHs材料施用量分别能最大程度降低小白菜各部位中As、Cd的质量分数,由于0.5%的施用量能显著促进小白菜生长,故认为该施用量对As、Cd复合污染土壤的农业生产应用更具有实际意义。
4)小白菜各部位中As和Cd的质量分数与土壤中弱酸提取态As和弱酸提取态Cd呈显著正相关,与残渣态As和残渣态Cd呈显著负相关。FeMnCa-LDHs材料通过促使土壤中As和Cd由弱酸提取态转化为残渣态的方式降低As、Cd的生物有效性,从而修复土壤As、Cd复合污染。
FeMnCa-LDHs材料对不同程度砷镉复合污染土壤的钝化修复
Passivation remediation of arsenic-cadmium contaminated soils with different pollution levels by FeMnCa-LDHs materials
-
摘要: 针对土壤砷镉复合污染同时修复较为困难的问题,制备了新型FeMnCa-LDHs材料以实现对土壤中As、Cd的同时钝化。通过土壤培养实验和小白菜盆栽实验,研究了在不同污染水平下FeMnCa-LDHs材料施用量对土壤As、Cd的形态转化及对小白菜各部位中As、Cd质量分数的影响,并分析了两者的相关性。结果表明,在高污染水平下施用1.0%的FeMnCa-LDHs材料,可使弱酸提取态As和弱酸提取态Cd的质量分数分别下降12.1%和28.9%,As和Cd由弱酸提取态向更稳定的形态转化;材料对As的吸附作用及对土壤pH的提高是其同时钝化As和Cd的主要原因。在高污染水平下,0.5%的材料施用量可使小白菜地上部分中As和Cd的质量分数分别减少61.2%和53.0%。相关性分析结果表明,小白菜各部位中As和Cd的质量分数与土壤中弱酸提取态As和弱酸提取态Cd的质量分数呈显著正相关,与残渣态As和残渣态Cd的质量分数呈显著负相关,这说明FeMnCa-LDHs材料通过改变土壤中As、Cd的形态分布降低了As、Cd的生物有效性。本研究可为土壤As、Cd复合污染提供参考。Abstract: In view of the difficulty in simultaneous remediation of arsenic and cadmium pollution in soil, a novel material FeMnCa-LDHs was prepared to achieve simultaneous immobilization of arsenic and cadmium in the soil. Soil culture experiments and a pot-planting experiment of Brassica chinensis were carried out to explore the effects of different addition rates of FeMnCa-LDHs on the inactivation dynamics of arsenic and cadmium species in soil, as well as the mass fraction of arsenic and cadmium in Brassica chinensis under different heavy metal pollution levels, and the correlation between the two was analyzed. The results showed that 1.0% addition rate reduced the mass fraction of weak acid extractable arsenic and cadmium by 12.1% and 28.9%, respectively, for the highly polluted soil and both arsenic and cadmium transformed from weak acid-extractable form to residual state. The material's adsorption of arsenic and the increase of soil pH were the main reasons for its simultaneous immobilization of arsenic and cadmium. 0.5% addition rate could reduce the mass fractions of arsenic and cadmium in the aboveground part of Brassica chinensis by 61.2% and 53.0% for the highly polluted soil. The correlation analysis showed that the mass fractions of arsenic and cadmium in various parts of Brassica chinensis significantly and positively correlated with those extracted by weak acid in the soil, while negatively correlated with the residual forms. It showed that the FeMnCa-LDHs material reduced the bioavailability of arsenic and cadmium by changing the morphological distribution of arsenic and cadmium in soil. This study could provide a new solution to this issue in simultaneous remediation of arsenic and cadmium pollution in soil.
-
Key words:
- arsenic /
- cadmium /
- layered double hydroxides /
- bioavailability
-

-
表 1 3种污染水平土壤As和Cd的质量分数
Table 1. As and Cd mass content of soil at three pollution levels
mg·kg−1 污染水平 As Cd 低污染 7.93 0.45 中污染 150 0.6 高污染 230 2.0 注:低污染为不加外源重金属的原土实测值。 表 2 土壤各形态As、Cd与小白菜各部位中As、Cd的相关性分析
Table 2. Correlation analysis of As and Cd in different soil forms and in different parts of Brassica chinensis
供试指标 地上部分As质量分数 根部As质量分数 供试指标 地上部分Cd质量分数 根部Cd质量分数 弱酸提取态As质量分数 0.709**1) 0.741** 弱酸提取态Cd质量分数 0.964** 0.962** 可还原态As质量分数 0.505 0.574 可还原态Cd质量分数 −0.536 −0.519 可氧化态As质量分数 −0.284 −0.293 可氧化态Cd质量分数 −0.491 −0.466 残渣态As质量分数 −0.853** −0.892** 残渣态Cd质量分数 −0.851** −0.782** 注:1)**表示在0.01水平下显著相关(P<0.01)。 -
[1] 环境保护部, 国土资源部. 全国土壤污染状况调查公报[EB/OL]. (2014-04-17)[2021-07-01]. http://www.mee.gov.cn/gkml/sthjbgw/qt/201404/W020140417558995804588.pdf. [2] 朱维, 周航, 吴玉俊, 等. 组配改良剂对稻田土壤中镉铅形态及糙米中镉铅累积的影响[J]. 环境科学学报, 2015, 35(11): 3688-3694. [3] 徐珺, 曾敏, 王光军, 等. 2种组配改良剂修复镉砷复合污染稻田土壤的研究[J]. 环境科学学报, 2018, 38(5): 2008-2013. [4] 贺玉龙. 镉砷在土壤中的赋存形态及生物有效性研究[J]. 绿色科技, 2019(10): 108-109. [5] WANG R, SHAFI M, MA J, et al. Effect of amendments on contaminated soil of multiple heavy metals and accumulation of heavy metals in plants[J]. Environmental Science and Pollution Research, 2018, 25(28): 28695-28704. doi: 10.1007/s11356-018-2918-x [6] 胡灿洋. 铁硫复合试剂对砷镉污染农田土壤的钝化修复[D]. 大连: 大连理工大学, 2019. [7] SONG Y, HOU D, ZHANG J, et al. Environmental and socio-economic sustainability appraisal of contaminated land remediation strategies: A case study at a mega-site in China[J]. Science of the Total Environment, 2018, 610: 391-401. [8] HOU D, GU Q, MA F, et al. Life cycle assessment comparison of thermal desorption and stabilization/solidification of mercury contaminated soil on agricultural land[J]. Journal of Cleaner Production, 2016, 139: 949-956. doi: 10.1016/j.jclepro.2016.08.108 [9] 陈远其, 张煜, 陈国梁. 石灰对土壤重金属污染修复研究进展[J]. 生态环境学报, 2016, 25(08): 1419-1424. [10] 曹胜, 欧阳梦云, 周卫军, 等. 石灰对土壤重金属污染修复的研究进展[J]. 中国农学通报, 2018, 34(26): 109-112. [11] 李园星露, 叶长城, 刘玉玲, 等. 生物炭耦合水分管理对稻田土壤As-Cd生物有效性及稻米累积的影响[J]. 农业环境科学学报, 2018, 37(4): 696-704. [12] GONZALEZ V, GARCIA I, DEL MORAL F, et al. Effectiveness of amendments on the spread and phytotoxicity of contaminants in metal-arsenic polluted soil[J]. Journal of Hazardous Materials, 2012, 205: 72-80. [13] KOVANDA F, GRYGAR T, DORNICAK V. Thermal behaviour of Ni-Mn layered double hydroxide and characterization of formed oxides[J]. Solid State Sciences, 2003, 5(7): 1019-1026. doi: 10.1016/S1293-2558(03)00129-8 [14] RAHMAN M T, KAMEDA T, MIURA T, et al. Removal of Mn and Cd contained in mine wastewater by Mg-Al-layered double hydroxides[J]. Journal of Material Cycles and Waste Management, 2019, 21(5): 1232-1241. doi: 10.1007/s10163-019-00875-9 [15] CHOONG C E, WONG K T, JANG S B, et al. Granular Mg-Fe layered double hydroxide prepared using dual polymers: Insights into synergistic removal of As(III) and As(V)[J]. Journal of Hazardous Materials, 2021, 403: 123883. [16] ZHANG X, SHAN R, LI X, et al. Effective removal of Cu(II), Pb(II) and Cd(II) by sodium alginate intercalated MgAl-layered double hydroxide: Adsorption properties and mechanistic studies[J]. Water Science and Technology, 2021, 83(4): 975-984. doi: 10.2166/wst.2021.013 [17] MIYATA S, KUMURA T. Synthesis of new hydrotalcite-like compounds and their physicochemical properties[J]. Chemistry Letters, 1973(8): 843-848. [18] LÓPEZ-GARCÍA M, MARTÍNEZ-CABANAS M, VILARIÑO T, et al. New polymeric/inorganic hybrid sorbents based on red mud and nanosized magnetite for large scale applications in As(V) removal[J]. Chemical Engineering Journal, 2017, 311: 117-125. doi: 10.1016/j.cej.2016.11.081 [19] TALEB K, MARKOVSKI J, MILOSAVLJEVIĆ M, et al. Efficient arsenic removal by cross-linked macroporous polymer impregnated with hydrous iron oxide: Material performance[J]. Chemical Engineering Journal, 2015, 279: 66-78. doi: 10.1016/j.cej.2015.04.147 [20] HONG J, ZHU Z, LU H, et al. Synthesis and arsenic adsorption performances of ferric-based layered double hydroxide with α-alanine intercalation[J]. Chemical Engineering Journal, 2014, 252: 267-274. doi: 10.1016/j.cej.2014.05.019 [21] LOU Z, CAO Z, XU J, et al. Enhanced removal of As(III)/(V) from water by simultaneously supported and stabilized Fe-Mn binary oxide nanohybrids[J]. Chemical Engineering Journal, 2017, 322: 710-721. doi: 10.1016/j.cej.2017.04.079 [22] LOMBI E, HAMON R E, MCGRATH S P, et al. Lability of Cd, Cu, and Zn in polluted soils treated with lime, beringite, and red mud and identification of a non-labile colloidal fraction of metals using isotopic techniques[J]. Environmental Science & Technology, 2003, 37(5): 979-984. [23] FRIESL W, FRIEDL J, PLATZER K, et al. Remediation of contaminated agricultural soils near a former Pb/Zn smelter in Austria: Batch, pot and field experiments[J]. Environmental Pollution, 2006, 144(1): 40-50. doi: 10.1016/j.envpol.2006.01.012 [24] 周宏光. FeMnMg-LDH的制备及其对环境铅镉污染的钝化效应研究[D]. 重庆: 西南大学, 2017. 46-47. [25] 廖玉梅, 余杰, 魏世强, 等. FeMnNi-LDHs对水中As(Ⅲ)的吸附性能与机制[J]. 环境科学, 2021, 42(1): 293-304. [26] DRAGOI B, UNGUREANU A, CHIRIEAC A, et al. Hydrogenation of unsaturated carbonyl compounds on non-calcined LDHs. I. synthesis and characterization of ZnNiCuAl hydrotalcite-like materials[J]. Acta Chimica Slovenica, 2010, 57(3): 677-685. [27] VELU S, SUZUKI K, OSAKI T. Selective production of hydrogen by partial oxidation of methanol over catalysts derived from CuZnAl-layered double hydroxides[J]. Catalysis Letters, 1999, 62(2/3/4): 159-167. [28] 袁峰, 唐先进, 吴骥子, 等. 两种铁基材料对污染农田土壤砷铅镉的钝化修复[J]. 环境科学, 2021, 42(7): 3535-3548. [29] 中华人民共和国生态环境部. 土壤环境质量 农用地土壤污染风险管控标准(试行): GB 15618-2018[S]. 北京: 中国环境科学出版社, 2018. [30] 环境保护部南京环境科学研究所, 江苏省环境监测中心. 土壤 pH 值的测定 电位法: HJ 962-2018[S]. 北京: 中国环境科学出版社, 2018. [31] 鲁如坤, 土壤农业化学分析方法[M]. 北京:中国农业科技出版社, 2000. [32] 中华人民共和国国家质量监督检验检疫总局,中国国家标准化管理委员会. 土壤质量 总汞、总砷、总铅的测定 原子荧光法 第2部分: 土壤中总砷的测定: GB/T 22105.2-2008[S]. 北京:质检出版社, 2008. [33] 中华人民共和国生态环境部. 土壤质量 铅、镉的测定 石墨炉原子吸收分光光度法: GB/T 17141-1997[EB/OL].(1998-05-01)[2021-07-01].http://www.mee.gov.cn/image20010518/1949.pdf. [34] RUBAN V, LOPEZ-SANCHEZ J F, PARDO P, et al. Selection and evaluation of sequential extraction procedures for the determination of phosphorus forms in lake sediment[J]. Journal of Environmental Monitoring, 1999, 1(1): 51-56. doi: 10.1039/a807778i [35] 中华人民共和国国家卫生和计划生育委员会. 食品安全国家标准 食品中镉的测定: GB 5009.15-2014[S]. 北京: 质检出版社, 2015. [36] 中华人民共和国国家卫生和计划生育委员会, 食品安全国家标准 食品中总砷及无机砷的测定: GB 5009.11-2014[S]. 北京: 质检出版社, 2015. [37] THOMAS G S, KAMATH P V. Line broadening in the PXRD patterns of layered hydroxides: The relative effects of crystallite size and structural disorder[J]. Journal of Chemical Sciences, 2006, 118(1): 127-133. doi: 10.1007/BF02708774 [38] TICHIT D, LORRET O, COQ B, et al. Synthesis and characterization of Zn/Al and Pt/Zn/Al layered double hydroxides obtained by the sol-gel method[J]. Microporous and Mesoporous Materials, 2005, 80(1/2/3): 213-220. doi: 10.1016/j.micromeso.2004.12.015 [39] 邓欣, 方真, 张帆, 等. 纳米Zn-Mg-Al水滑石的制备、表征及记忆功能[J]. 材料导报, 2010, 24(S1): 41-43. [40] CAVANI F, TRIFIRO F, VACCARI A. Hydrotalcite-type anionic clays: Preparation, properties and applications[J]. Catalysis Today, 1991, 11(2): 173-301. doi: 10.1016/0920-5861(91)80068-K [41] 曹青青, 宋敏, 孟凡跃, 等. FeAl-LDHs/生物炭修复镉污染土壤及作用机制[J]. 生态环境学报, 2020, 29(4): 834-41. [42] 袁林. 铁锰复合氧化物对重金属铅镉吸附解吸特征及其影响因素研究[D]. 重庆: 西南大学, 2010. [43] ZHANG D, YUAN Z, WANG S, et al. Incorporation of arsenic into gypsum: Relevant to arsenic removal and immobilization process in hydrometallurgical industry[J]. Journal of Hazardous Materials, 2015, 300: 272-280. doi: 10.1016/j.jhazmat.2015.07.015 [44] ZHAI W, DAI Y, ZHAO W, et al. Simultaneous immobilization of the cadmium, lead and arsenic in paddy soils amended with titanium gypsum[J]. Environmental Pollution, 2020, 258: 113790. [45] YUAN Y, CHAI L, YANG Z, et al. Simultaneous immobilization of lead, cadmium, and arsenic in combined contaminated soil with iron hydroxyl phosphate[J]. Journal of Soils and Sediments, 2017, 17(2): 432-439. doi: 10.1007/s11368-016-1540-0 [46] 孙光闻, 朱祝军, 方学智. 镉污染土壤对小白菜生长及镉和养分含量的影响[J]. 华北农学报, 2011, 26(S1): 60-63. doi: 10.7668/hbnxb.2011.S1.013 [47] 张菊平, 崔文朋, 焦新菊, 等. 低浓度镉对小白菜生长及营养元素吸收积累的影响[J]. 江西农业大学学报, 2011, 33(1): 22-28. doi: 10.3969/j.issn.1000-2286.2011.01.005 [48] OBATA H, UMEBAYASHI M. Effects of cadmium on mineral nutrient concentrations in plants differing in tolerance for cadmium[J]. Journal of Plant Nutrition, 1997, 20(1): 97-105. doi: 10.1080/01904169709365236 [49] 代允超. 土壤中镉、砷生物有效性影响因素及评价方法研究[D]. 咸阳: 西北农林科技大学, 2018. [50] DING Y, DING L, XIA Y, et al. Emerging roles of microRNAs in plant heavy metal tolerance and homeostasis[J]. Journal of Agricultural and Food Chemistry, 2020, 68(7): 1958-1965. doi: 10.1021/acs.jafc.9b07468 [51] 中华人民共和国国家卫生和计划生育委员会,国家食品药品监督管理总局. 食品安全国家标准 食品中污染物限量: GB 2762-2017[S]. 北京:质检出版社, 2017. [52] MA Q, ZHAO W, GUAN D X, et al. Comparing CaCl2, EDTA and DGT methods to predict Cd and Ni accumulation in rice grains from contaminated soils[J]. Environmental Pollution, 2020, 260: 114042. doi: 10.1016/j.envpol.2020.114042 [53] SCHRODER J L, BASTA N T, SI J T, et al. In vitro gastrointestinal method to estimate relative bioavailable cadmium in contaminated soil[J]. Environmental Science & Technology, 2003, 37(7): 1365-1370. -




 下载:
下载: