-
磷是生物生长的重要营养元素,也是农业肥料中不可替代的养分[1]。然而,磷作为不可再生资源,预计将在2035年达到磷产量峰值后面临供不应求的风险,影响着全球粮食供应[2]。另一方面,工业、农业和生活废水中过量排入水体的磷不仅未能有效回收,反而导致水中藻类等水生生物过度生长,引起水体富营养化,造成水体水质恶化等严重问题[3-4]。因此,如何实现磷的去除与资源回收成为水处理行业的研究重点。常用的除磷技术有化学沉淀法、生物法、吸附法等[5]。其中,化学沉淀法一般是利用铝盐、铁盐和钙盐等化学药剂形成不溶性磷酸盐沉淀,并通过固液分离除磷的传统技术[6]。该方法操作简单、占地面积小、除磷效率高,但生成的化学污泥产量大且成分复杂,导致磷资源回收困难,污泥处置成本增加,还可能造成二次污染[6-8]。生物法是利用聚磷菌在厌氧和好氧交替的条件下过量吸收磷,并通过排泥除磷的方法[9]。生物除磷无需添加化学药剂,产生的污泥可直接用作肥料,具有磷资源回收的潜力,但对环境条件要求高,除磷后的出水稳定性较差,工艺运行成本高[8-10]。相比之下,吸附法因操作简单、效率高、能耗低、无二次污染、磷资源可回收等优点而受到广泛关注[11]。目前用于水体除磷的吸附材料主要有生物炭、双层氢氧化物、水凝胶等[12],但生物炭吸附量低,双层氢氧化物、水凝胶等材料成本较高,因此,寻求高效低成本的吸附材料成为废水除磷的关键[13]。
给水厂污泥(drinking water treatment residues,DWTR)是饮用水生产过程中相对清洁安全的副产品,对PO43−-P具有选择性吸附,被认为是一种有前景的廉价除磷材料[14]。DWTR主要含有铝、铁等元素,并以无定型的非晶形态存在,具有较大的比表面积和孔隙率,能够通过表面官能团与PO43−-P发生配体交换吸附水中的磷,可以作为人工湿地的除磷填料[14-17]。另外,提高DWTR吸附性能的改性技术也受到研究者的关注[18-20]。譬如,镧的负载可以显著提高DWTR对PO43−-P的吸附量[21],但镧属于稀土金属,价格较高;铁是一种来源广泛、价格相对较低的金属,其对PO43−-P的吸附能力较弱[22]。
为了发挥镧和铁的协同作用,本研究采用共沉淀法制备出一种镧铁复合给水厂污泥吸附材料(LaFe-DWTR)。目前,DWTR的除磷研究一般采用静态吸附和固定床吸附模式进行,采用完全混合式反应器(continuous stirred tank reactor,CSTR)的研究相对较少[23-24]。由于CSTR可以提供充足的反应空间和运行时间,能够实现较高的传质速率和连续稳定运行[25],因此,本研究采用自主设计的CSTR实验装置,研究了LaFe-DWTR在CSTR中对模拟废水和城市污水处理厂二沉池出水的除磷效果,探讨了停留时间(hydraulic retention time,HRT)、LaFe-DWTR投加量和水力学条件对CSTR运行效果的影响,以期为LaFe-DWTR应用于水体富营养化控制提供参考。
-
实验所用给水厂污泥取自北京市第九水厂。将自然风干后的DWTR于105℃烘箱干燥24 h,研磨过120目筛保存。称取一定质量的水合氯化镧和氯化铁于250 mL去离子水,然后加入5 g DWTR搅拌均匀,并缓慢滴加3.0 mol·L−1 NaOH溶液,待pH稳定至11后停止。采用500 r·min−1转速连续搅拌5 h,再静置24 h后,倾去上清液,用去离子水洗涤沉淀至中性,于105 ℃干燥12 h,研磨过120目筛得到LaFe-DWTR。其理化性质采用X射线能谱仪(EDS)、粒度测定仪、比表面积分析仪(BET)等表征确定,LaFe-DWTR主要由镧和铁组成(27.79%La、15.03%Fe、4.24%Al、3.95%Si、3.55%Mg、16.12%C、19.28%O),平均粒径为22.70 μm,比表面积为98.57 m2·g−1,孔径为6.00 nm,孔体积为0.17 cm3·g−1,镧和铁的质量浓度分别为175.10 mg·g−1和107.60 mg·g−1。
-
1) CSTR系统的搭建与运行。以CSTR为主体的动态系统如图1所示,包括进水池、加药池、CSTR和再生罐4个部分。其中,进水池和加药池分别与CSTR底部的进水口和加药口相连,再生罐与CSTR的材料回收口和再生回流口相连。CSTR结构包括反应区和沉淀区2部分,总有效容积为19.27 L;中心的圆柱形反应槽是反应区,容积为1.85 L;圆柱形反应槽与反应器主体外壳之间是沉淀区,容积为17.42 L。沉淀区中间设置导流板以增强沉淀效果。
采用蠕动泵控制CSTR系统的运行。运行过程中,材料回收管和再生材料回流管的阀门处于关闭状态,含磷废水从进水池流入CSTR反应区,加药池内搅拌均匀的LaFe-DWTR悬浊液同步流入CSTR反应区,含磷废水和LaFe-DWTR在反应区内充分接触,待混合溶液充满反应区后溢流到沉淀区,混合液在导流板的作用下往下流,此时LaFe-DWTR在沉淀区底部富集,上清液继续向上流动,待整个反应器充满后从出水孔流出。运行一段时间后,部分吸附PO43−-P后的LaFe-DWTR通过材料回收口输送至再生罐进行再生,回收量控制在CSTR中LaFe-DWTR总量的10%左右,再生后的LaFe-DWTR回流至CSTR反应区进行下1次吸附。
2) CSTR系统运行条件的优化。配制PO43−-P质量浓度为100.00 mg·L−1的模拟废水于进水池中,并用去离子水配制一定质量浓度的LaFe-DWTR悬浊液于加药池中,加药池采用500 r·min−1的转速持续搅拌。将模拟废水和LaFe-DWTR悬浊液以相同的流速输送到CSTR反应器内,使CSTR的进水PO43−-P质量浓度稀释至50.00 mg·L−1。分别控制HRT、LaFe-DWTR投加量、水力学条件3个变量进行实验,优化CSTR系统的运行条件,具体运行参数见表1。每个实验连续运行220 h,每5 h测1次出水PO43−-P质量浓度。
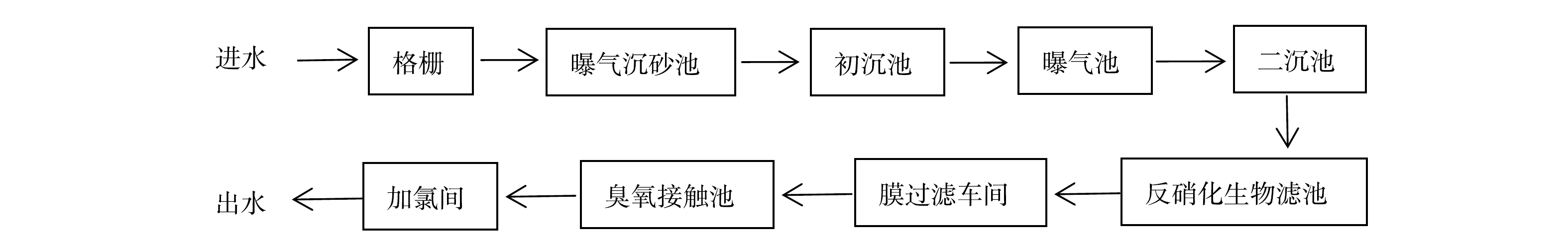
3) LaFe-DWTR处理城市污水处理厂二沉池出水的吸附除磷效果。为探究LaFe-DWTR对实际含磷污水的去除效果,选取北京市某再生水厂的二沉池出水作为CSTR的进水。该污水处理厂的工艺流程见图2。二沉池出水的水质参数如下:pH=7.90、4.00 mg·L−1 PO43−-P、19.07 mg·L−1 COD、13.44 mg·L−1 NO3−、79.55 mg·L−1 Cl−、132.36 mg·L−1 SO42−、78.00 mg·L−1 SiO32−、85.40 mg·L−1 HCO3−、10.84 mg·L−1 K+、3.44 mg·L−1 Ca+、4.67 mg·L−1 Na+、16.69 mg·L−1 Mg+。由于所取的城市污水处理厂二沉池出水中PO43−-P含量较低,为模拟大多数污水处理厂生物除磷后的二沉池出水PO43−-P浓度,水样经过滤后,通过实验室添加磷酸二氢钾使CSTR进水PO43−-P浓度达到2.00 mg·L−1[26]。CSTR系统运行时,控制HRT为3 h,LaFe-DWTR投加量为0.14 g·L−1,反应区搅拌转速为150 r·min−1,连续运行220 h,探究LaFe-DWTR对实际城市污水处理厂出水的除磷效果。
-
采用钼酸铵分光光度法测定PO43−-P质量浓度。根据水溶液中PO43−-P质量浓度差法计算吸附量。LaFe-DWTR对的PO43−-P吸附量根据式(1)进行计算。
式中:Qt为在t时刻的PO43−-P吸附量,mg·g−1;C0和Ct分别为CSTR系统的PO43−-P进水质量浓度和t时刻的出水质量浓度,mg·L−1;V为CSTR系统处理的水溶液体积,L;M为所使用的LaFe-DWTR质量,g。
在t时刻的PO43−-P去除率(R)根据式(2)进行计算。
-
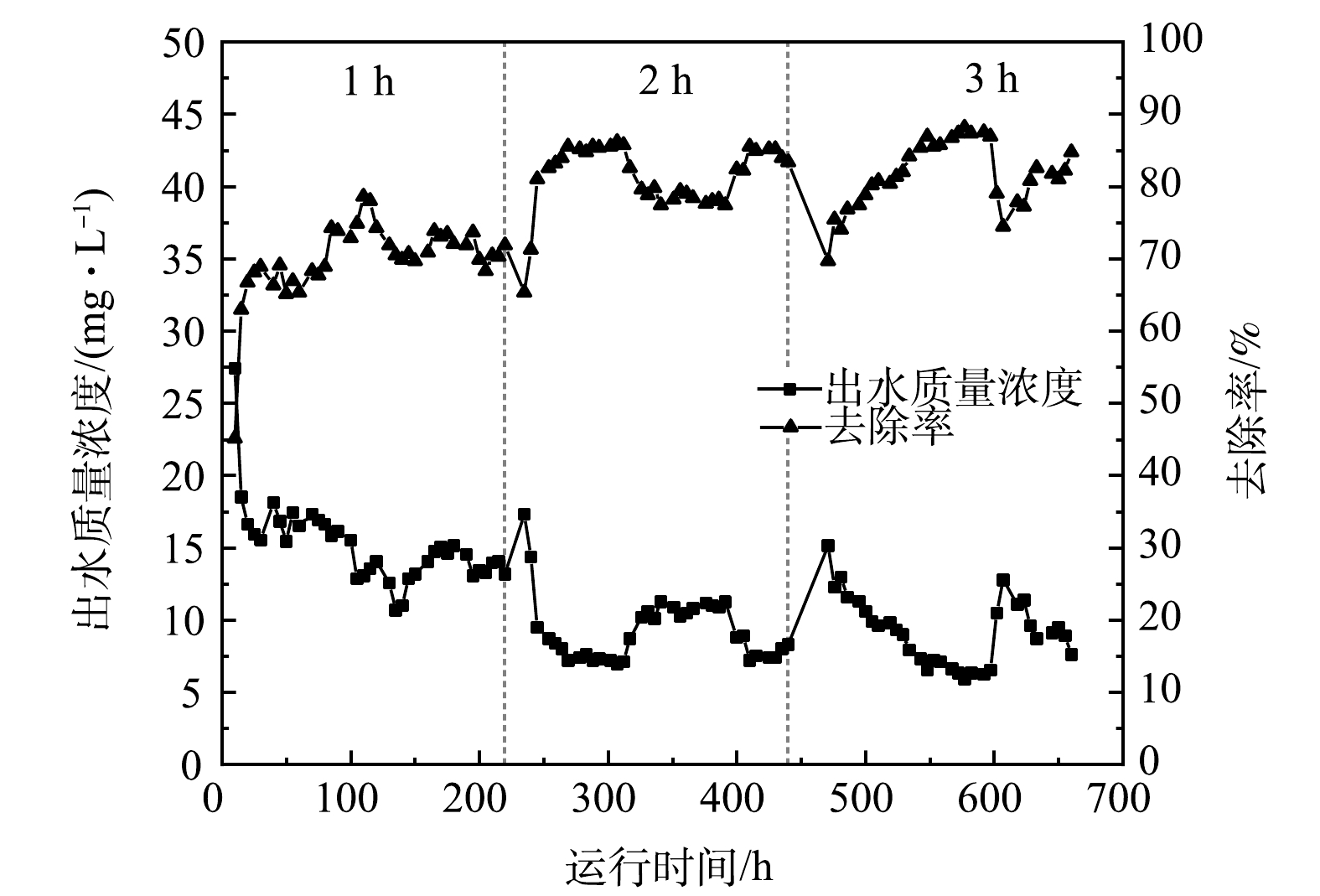
1)水力停留时间的影响。水力停留时间(HRT)对LaFe-DWTR吸附PO43−-P的影响如图3所示。当HRT由1 h增加到3 h时,平均出水PO43−-P质量浓度由15.13 mg·L−1降低到9.21 mg·L−1,平均去除率由69.97%增加到81.58%,吸附量由17.49 mg·g−1增加到20.44 mg·g−1。可见,HRT越长,出水PO43−-P质量浓度越低,去除率越高,LaFe-DWTR吸附效率越高。当HRT为1 h时,反应区的PO43−-P还未被充分吸附便溢流到沉淀区,因此,PO43−-P去除率低,LaFe-DWTR利用率低,出水效果差。而HRT在2 h和3 h时的除磷效果无明显差别,去除率均达到80%以上,吸附量仅相差0.04 mg·g−1。此现象与给水厂污泥(DWTR)和大理石粉末(PMW)在CSTR中吸附PO43−-P的趋势一致[24, 27]。结合静态吸附特征分析,LaFe-DWTR对PO43−-P的吸附动力学是快速吸附、缓慢趋于平衡的过程,快速吸附发生在LaFe-DWTR表面,慢速吸附发生在LaFe-DWTR颗粒内部,LaFe-DWTR在2 h内已经完成快速吸附,此后吸附逐渐趋于平衡。因此,CSTR的HRT大于2 h后,除磷效果提升变缓,这种现象与CSTR中海草纤维(POF)、磷矿废石(PMS)等吸附除磷的特征类似[25,28]。在本研究中,HRT为2 h和3 h的除磷效果接近,但HRT为3 h的LaFe-DWTR投加量(272 g)仅为2 h的67%,且沉淀时间(28.16 h)更长,出水水质更好。因此,在CSTR运行过程中选择HRT为3 h更合适。
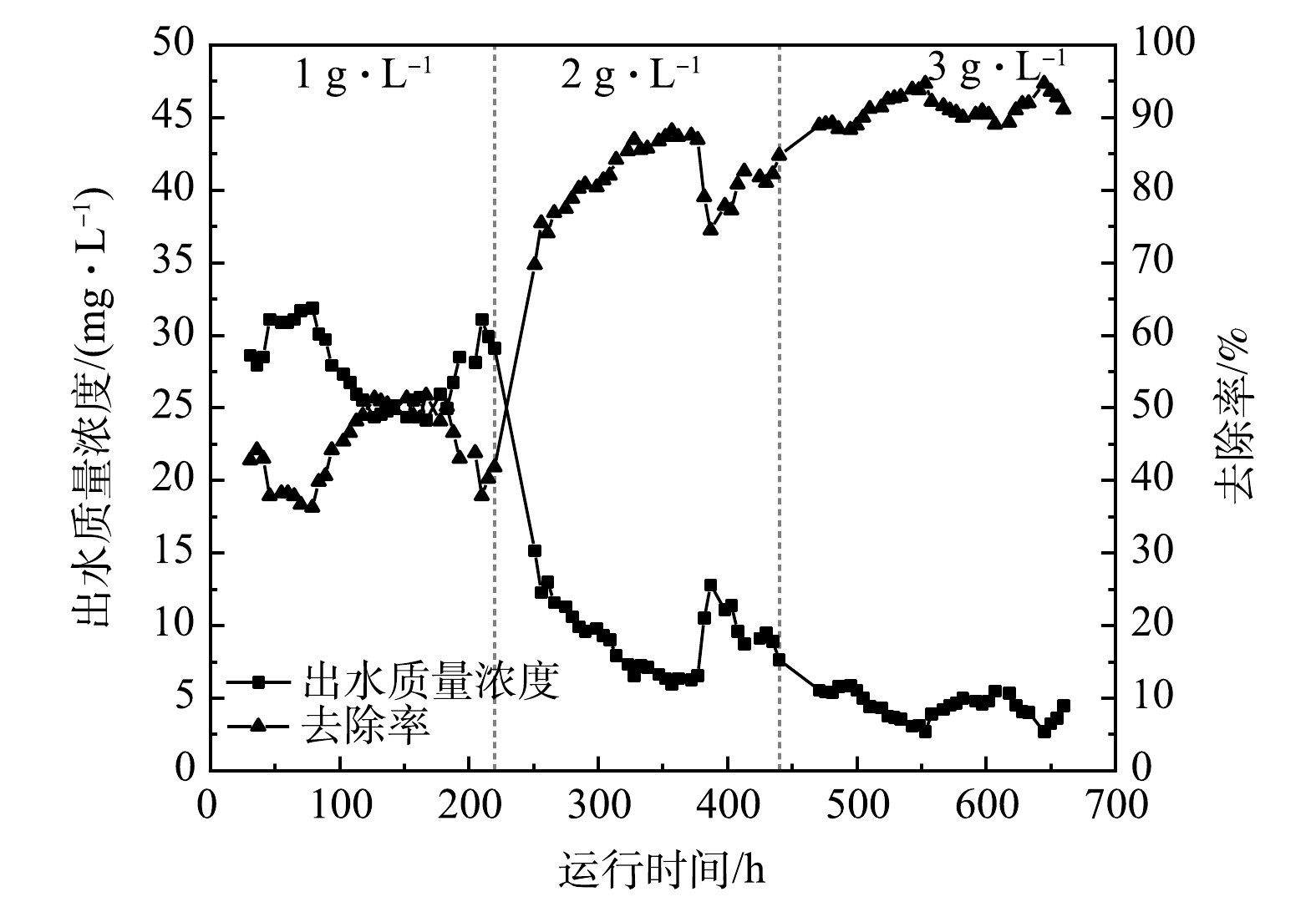
2) LaFe-DWTR投加量的影响。投加量对LaFe-DWTR吸附PO43−-P的影响如图4所示。随着投加量的增加,平均出水PO43−-P质量浓度随之降低,去除率相应增加。投加量对给水厂污泥(DWTR)与海草纤维(POF)吸附PO43−-P的影响也有相同的结论[24, 28]。当LaFe-DWTR投加量由1.0 g·L−1增加到2.0 g·L−1时, PO43−-P平均出水质量浓度由27.78 mg·L−1降至9.21 mg·L−1,平均去除率由44.45%升至81.58%,除磷效果明显增强。这是由于LaFe-DWTR投加量的增加可为PO43−-P提供更多的吸附位点,因此,2.0 g·L−1投加量的吸附效果优于1.0 g·L−1。当LaFe-DWTR投加量由2.0 g·L−1增加到3.0 g·L−1时,平均出水PO43−-P质量浓度由9.21 mg·L−1降低到4.40 mg·L−1,平均去除率由81.58%增至91.19%,除磷效果有所改善,但整个运行过程中投加的LaFe-DWTR总量由272 g增加到408 g,PO43−-P平均吸附量由20.44 mg·g−1降低到15.20 mg·g−1, LaFe-DWTR利用效率降低,出现了吸附位点未充分利用的现象。因此,综合考虑PO43−-P去除率和LaFe-DWTR利用效率,在进水PO43−-P质量浓度为50 mg·L−1时,选择2.0 g·L−1 LaFe-DWTR作为除磷的最佳投加量。
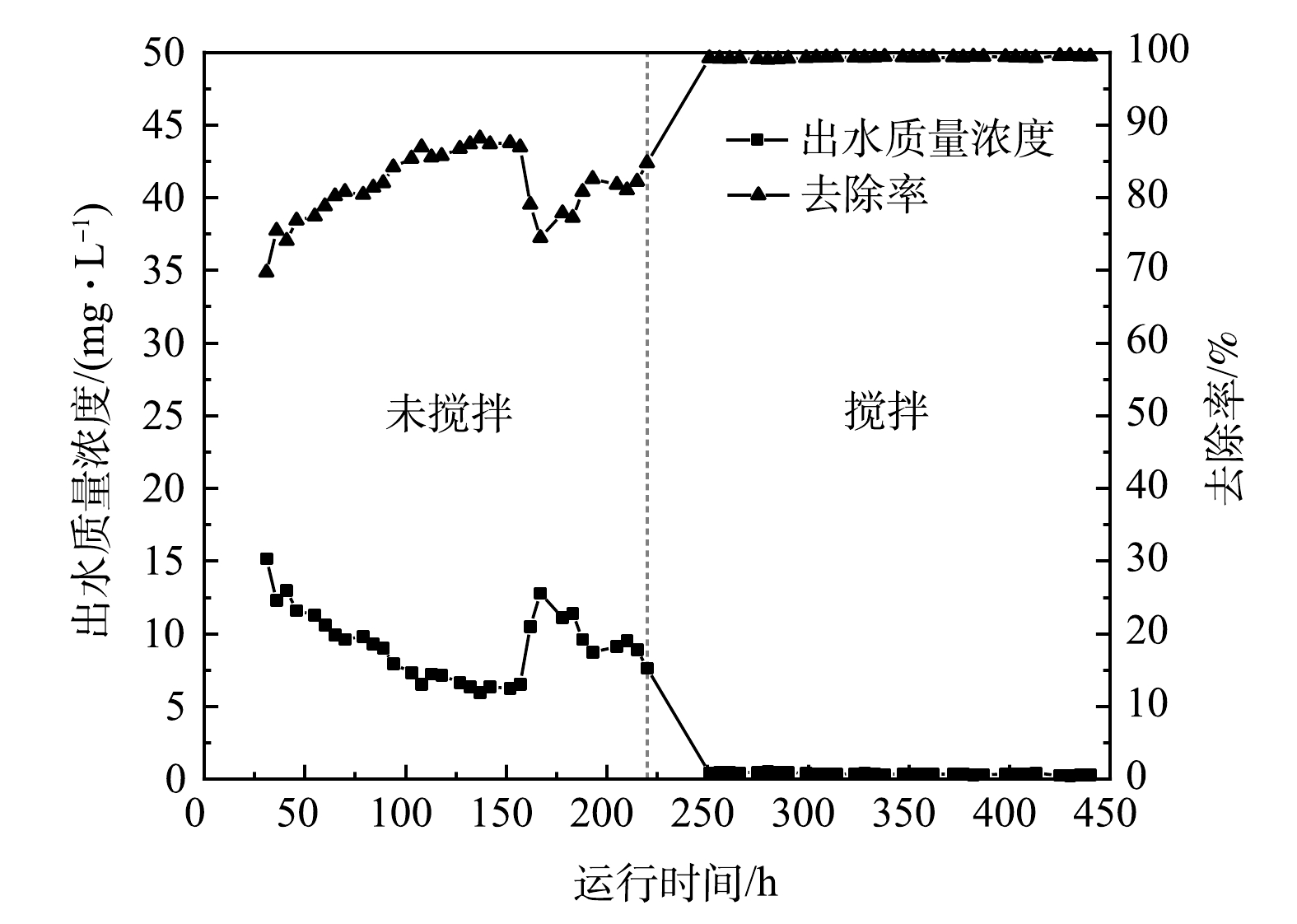
3)水力学条件的影响。由图5可见,在CSTR反应区无搅拌时,平均出水PO43−-P质量浓度是9.21 mg·L−1,平均吸附量为20.44 mg·g−1,平均去除率为81.58%。当反应区采用搅拌方式改变水力条件后,CSTR的除磷效果显著提高,平均出水PO43−-P质量浓度降至0.37 mg·L−1,平均去除率稳定在99%以上,吸附量提高到24.82 mg·g−1。由此可见,在无搅拌时,吸附材料进入CSTR反应区后自然沉降,LaFe-DWTR分布不均匀,部分沉淀到反应区下层无法与PO43−-P充分接触;而通过搅拌改变水力条件后,LaFe-DWTR在悬浮状态下做无规则运动,与PO43−-P的接触面积增加,为PO43−-P提供了更多的吸附位点,强化了LaFe-DWTR对PO43−-P的吸附效果[29]。因此,采用搅拌改变反应区水力条件可以增强CSTR的除磷效果。
-
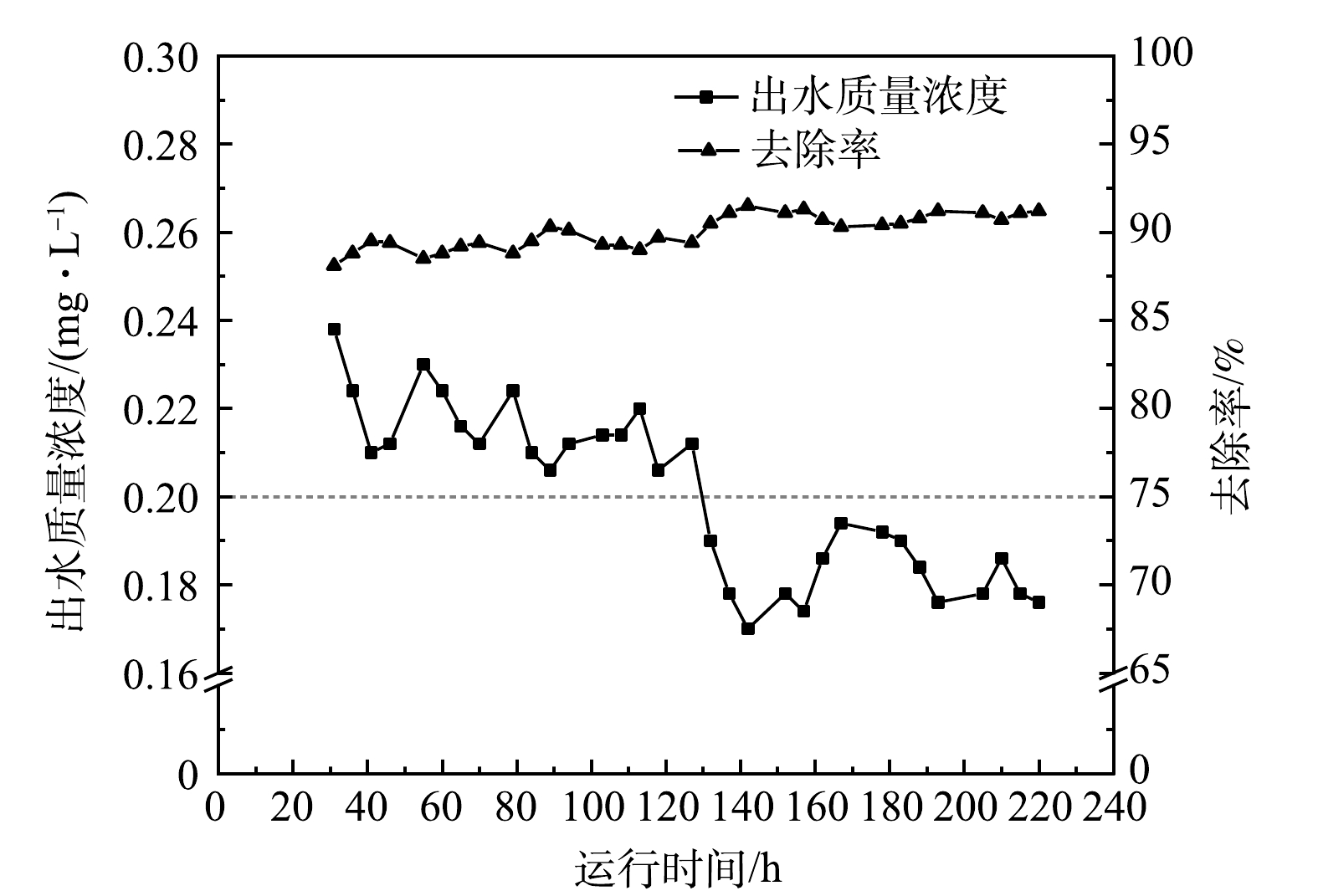
CSTR处理城市污水处理厂二沉池出水的吸附除磷效果如图6所示。运行初期,CSTR系统启动31 h后开始出水,此时出水PO43−-P质量浓度为0.24 mg·L−1,运行41 h后出水PO43−-P质量浓度降至0.21 mg·L−1;在41~127 h内,出水PO43−-P质量浓度稳定在0.20~0.22 mg·L−1;在132~220 h内,出水PO43−-P质量浓度降至0.20 mg·L−1以下,最低出水质量浓度可降至0.17 mg·L−1,最高去除率达到91.50%。这说明随着CSTR运行时间的延长,对二沉池出水的除磷效果逐渐变好。由于低质量浓度含磷污水所需的LaFe-DWTR投加量较少,反应区的LaFe-DWTR与PO43−-P接触更充分,出水稳定性增强;同时,随着CSTR运行时间的延长,在沉淀区底部积累的LaFe-DWTR对反应区溢流的混合液可以进一步净化,出水逐渐变好的趋势能够明显观察到。在整个运行周期内,LaFe-DWTR对PO43−-P的平均吸附量为12.86 mg·g−1,平均去除率为90.02%,CSTR平均出水PO43−-P质量浓度为0.20 mg·L−1,达到了《城市污水处理厂污染物排放标准》(GB 18918–2002)一级A标准的限值(0.5 mg·L−1)。
-
LaFe-DWTR和其他天然材料或工业副产品在动态条件下吸附PO43−-P的效果见表2。对比后可发现,当初始PO43−-P质量浓度不低于50 mg·L−1时,LaFe-DWTR投加量仅为2 g·L−1,远低于海草纤维(POF)、磷矿废石(PMS)、大理石粉末(PMW)等吸附材料的投加量,LaFe-DWTR对PO43−-P的吸附量和去除率均优于其他材料;当初始PO43−-P质量浓度低于10 mg·L−1时,LaFe-DWTR投加量(0.14 g·L−1)同样低于给水厂污泥(DWTR)和酸矿排水污泥(AMD)等吸附材料,表现出明显的优势。因此,LaFe-DWTR是一种较有前途的除磷材料。
-
1)CSTR可以实现对LaFe-DWTR吸附除磷的稳定运行。对于含有50 mg·L−1 PO43−-P的模拟废水,在HRT为3 h、LaFe-DWTR投加量为2 g·L−1和反应搅拌的条件下,PO43−-P的去除率稳定在99%以上,LaFe-DWTR对PO43−-P吸附量可达24.82 mg·g−1。
2)对于PO43−-P初始质量浓度为2 mg·L−1的城市污水处理厂二沉池出水,在CSTR中采用LaFe-DWTR动态吸附除磷,平均出水PO43−-P质量浓度可降至0.20 mg·L−1。
镧铁负载给水厂污泥复合材料在完全混合式反应器中的动态吸附除磷特征
Dynamic phosphorus removal from aqueous solutions by lanthanum/iron-loaded drinking water treatment residues in a continuous stirred tank reactor
-
摘要: 采用共沉淀法制备出一种镧铁负载给水厂污泥复合材料(LaFe-DWTR),研究其在完全混合式反应器(CSTR)中对模拟废水和城市污水处理厂二沉池出水的除磷效果以及水力停留时间(HRT)、LaFe-DWTR投加量和水力学条件的影响。结果表明,当CSTR进水PO43−-P质量浓度为50 mg·L−1,HRT为3 h,LaFe-DWTR投加量为2 g·L−1,反应区采用搅拌时,LaFe-DWTR对PO43--P的去除率稳定在99%以上,吸附量可达24.82 mg·g−1。对于CSTR进水初始PO43−-P质量浓度为2 mg·L−1的城市污水处理厂二沉池出水,在3 h HRT,0.14 g·L−1投加量时,CSTR出水的PO43−-P质量浓度稳定在0.2 mg·L−1左右,已达到《城市污水处理厂污染物排放标准》(GB 18918–2002)一级A标准的要求。Abstract: A lanthanum/iron-loaded drinking water treatment residues (LaFe-DWTR) composite material was prepared by the co-precipitation method, and its performance on phosphorus removal from the simulated wastewater and the secondary effluent of municipal wastewater treatment plant(WWTP) in a continuous stirred tank reactor (CSTR) was studied. The effects of hydraulic retention time (HRT), LaFe-DWTR dosage and hydraulic conditions on PO43−-P removal were also discussed. The results indicated that PO43−-P removal efficiency in the CSTR with stirring at the reaction zone was over 99%, and the corresponding adsorption capacity of LaFe-DWTR toward PO43−-P reached 24.82 mg·g−1 at the initial PO43−-P concentration of 50 mg·L−1, HRT of 3 h and LaFe-DWTR dosage of 2 g·L−1. For the secondary effluent of municipal WWTP with an initial PO43−-P concentration of 2 mg·L−1 as the influent of CSTR, when the HRT was 3 h and LaFe-DWTR dosage was 0.14 g·L−1, the concentration of PO43−-P in CSTR effluent stably maintained about 0.2 mg·L−1, which could meet the requirements of the Class A Standard of the Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant (GB 18918-2002).
-
Key words:
- lanthanum/iron loading /
- drinking water treatment residues /
- phosphorus adsorption /
- dynamic /
- CSTR
-

-
表 1 CSTR系统运行参数
Table 1. Operational parameters of CSTR system
控制变量 进水PO43−-P
浓度/
(mg·L−1)LaFe-DWTR
投加量/
(g·L−1)HRT/
h水力学
条件运行
时长/hHRT 50.00 2 1/2/3 无搅拌 220 投加量 50.00 1/2/3 3 无搅拌 220 水力学条件 50.00 2 3 无/有搅拌 220 表 2 动态条件下LaFe-DWTR与其他吸附剂对PO43--P吸附性能的比较
Table 2. Comparison of phosphate adsorption capacities onto LaFe-DWTR with other adsorbents under dynamic conditions
吸附材料 CSTR反应区
体积/L初始PO43−-P
质量浓度/(mg·L−1)HRT/h 材料投加量/
(g·L−1)磷吸附量/
(mg·g−1)磷去除率/% 参考文献 海草纤维(POF) 1.2 50 0.5 5 3.03 80 [28] 磷矿废石(PMS) 1.2 50 0.5 5 5.63 81 [25] 大理石粉末(PMW) 1.2 100 8.8 12 17.0 88.3 [27] 羟基磷灰石(HAP) — 72.9 2 — — 52.4 [30] 给水厂污泥(DWTR) 1.0 10 2 10 0.95 95 [24] 粉煤灰/钢渣复合材料(PSPRC) 375 0.5 3 10 — 84 [31] 酸矿排水污泥(AMD) 2.0 1.8 1 1 1.79 99.3 [5] LaFe-DWTR 1.85 50 3 2 24.82 99 本研究 -
[1] CORDELL D, ROSEMARIN A, SCHRODER J J, et al. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options[J]. Chemosphere, 2011, 84(6): 747-758. doi: 10.1016/j.chemosphere.2011.02.032 [2] CORDELL D, DRANDERT J O, WHITE S. The story of phosphorus: Global food security and food for thought[J]. Global Environmental Change, 2009, 19(2): 292-305. doi: 10.1016/j.gloenvcha.2008.10.009 [3] SHEN C, ZHAO Y Q, LIU R B, et al. Enhancing wastewater remediation by drinking water treatment residual-augmented floating treatment wetlands[J]. Science of the Total Environment, 2019, 673(10): 230-236. [4] AYELE H S, ATLABACHEW, Review of characterization, factors, impacts, and solutions of lake eutrophication: Lesson for lake Tana, Ethiopia[J]. Environmental Science and Pollution Research, 2021, 28(12): 14233-14252. [5] WEI X C, JRR C V, BHOJAPPA S. Phosphorus removal by acid mine drainage sludge from secondary effluents of municipal wastewater treatment plants[J]. Water Research, 2008, 42(13): 3275-3284. doi: 10.1016/j.watres.2008.04.005 [6] WANG Q P, LIAO Z Y, YAO D X, et al. Phosphorus immobilization in water and sediment using iron-based materials: A review[J]. Science of the Total Environment, 2020: 767. [7] YE Y Y, NGO H H, GUO W S, et al. Insight into chemical phosphate recovery from municipal wastewater[J]. Science of the Total Environment, 2017, 576: 159-171. doi: 10.1016/j.scitotenv.2016.10.078 [8] RECEPOGLU Y K, GOREN A Y, OROOJI Y, et al. Carbonaceous materials for removal and recovery of phosphate species: Limitations, successes and future improvement[J]. Chemosphere, 2022, 287(2): 132177. [9] PERERA M K, ENGLEHARDT J D, DVORAK A C. Technologies for Recovering Nutrients from Wastewater: A Critical Review[J]. Environmental Engineering Science, 2019, 36(5): 511-529. doi: 10.1089/ees.2018.0436 [10] 徐颖, 叶志隆, 叶欣, 等. 给水污泥对水中磷的吸附性能. 环境工程学报[J]. 2018, 12(3): 712-719. [11] BHATNAGAR A, SILLANPAA M. A review of emerging adsorbents for nitrate removal from water[J]. Chemical Engineering Journal, 2011, 168(2): 493-504. doi: 10.1016/j.cej.2011.01.103 [12] ZHOU Y Q, WANG Y L, DONG S X, et al. Phosphate removal by a La(OH)3 loaded magnetic MAPTAC-based cationic hydrogel: Enhanced surface charge density and Donnan membrane effect[J]. Journal of Environmental Sciences, 2022, 113: 26-39. doi: 10.1016/j.jes.2021.05.041 [13] 高欢, 韦安磊, 郑晓青, 等. 乙酰化小麦秸秆吸附水中六价铬[J]. 环境工程学报, 2016, 10(9): 4753-4760. doi: 10.12030/j.cjee.201601205 [14] YANG Y, ZHAO Y. Q, KEARNEY P. Influence of ageing on the structure and phosphate adsorption capacity of dewatered alum sludge[J]. Chemical Engineering Journal, 2008, 145(2): 276-284. doi: 10.1016/j.cej.2008.04.026 [15] TAHMAZI T A, BABATUNDE A O. Mechanistic study of P retention by dewatered waterworks sludges[J]. Environmental Technology and Innovation, 2016, 6: 38-48. doi: 10.1016/j.eti.2016.05.002 [16] YANG Y, ZHAO Y Q, BABATUNDE A O, et al. Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge[J]. Separation and Purification Technology, 2006, 51(2): 193-200. doi: 10.1016/j.seppur.2006.01.013 [17] ZHAO X H, ZHAO Y Q, WANG W K, et al. Key issues to consider when using alum sludge as substrate in constructed wetland[J]. Water Science and Technology, 2015, 71(12): 1775-1782. doi: 10.2166/wst.2015.138 [18] XU H, DING M M, SHEN K L, et al. Removal of aluminum from drinking water treatment sludge using vacuum electrokinetic technology[J]. Chemosphere, 2017, 173: 404-410. doi: 10.1016/j.chemosphere.2017.01.057 [19] KEELEY J, SMITH A D, JUDD S J, et al. Acidified and ultrafiltered recovered coagulants from water treatment works sludge for removal of phosphorus from wastewater[J]. Water Research, 2016, 88: 380-388. doi: 10.1016/j.watres.2015.10.039 [20] LI X Q, CUI J, PEI Y S. Granulation of drinking water treatment residuals as applicable media for phosphorus removal[J]. Journal of Environmental Management, 2018, 213: 36-46. [21] WANG C H, WU Y, WANG Y Q, et al. Lanthanum-modified drinking water treatment residue for initial rapid and long-term equilibrium phosphorus immobilization to control eutrophication[J]. Water Research, 2018: 173-183. [22] YU J, XIANG C, ZHANG G, et al. Activation of lattice oxygen in LaFe(Oxy)hydroxides for efficient phosphorus removal[J]. Environmental Science and Technology, 2019, 53(15): 9073-9080. doi: 10.1021/acs.est.9b01939 [23] BABATUNDE A O, ZHAO Y. Q, DOYLE R. J, et al. Performance evaluation and prediction for a pilot two-stage on-site constructed wetland system employing dewatered alum sludge as main substrate[J]. Bioresource Technology, 2011, 102(10): 5645-5652. doi: 10.1016/j.biortech.2011.02.065 [24] BAI L L, WANG C H, PEI Y S, et al. Reuse of drinking water treatment residuals in a continuous stirred tank reactor for phosphate removal from urban wastewater[J]. Environmental Technology, 2014, 35(21-24): 2752-2759. [25] JELLALI S, WAHAB M A, ANANE M, et al. Phosphate mine wastes reuse for phosphorus removal from aqueous solutions under dynamic conditions[J]. Journal of Hazardous Materials, 2010, 184(1/2/3): 226-233. [26] HAO H T, WANG Y L, SHI B Y. NaLa(CO3)2 hybridized with Fe3O4 for efficient phosphate removal: Synthesis and adsorption mechanistic study[J]. Water Research, 2019, 155: 1-11. doi: 10.1016/j.watres.2019.01.049 [27] JAOUADI S, WAHAB M A, ANANE M, et al. Powdered marble wastes reuse as a low-cost material for phosphorus removal from aqueous solutions under dynamic conditions[J]. Desalination and Water Treatment, 2014, 52(7/8/9): 1705-1715. [28] WAHAB M A, HASSINE R B, ELLALI S. Removal of phosphorus from aqueous solution by Posidonia oceanica fibers using continuous stirring tank reactor[J]. Journal of Hazardous Materials, 2011, 189(1/2): 577-585. [29] 王君, 王瑶, 黄星, 等. 基于流态化作用的吸附反应动力学和穿透特征[J]. 环境科学, 2014, 35(2): 678-683. [30] KIM E H, YIM S B, JUNG H C, et al. Hydroxyapatite crystallization from a highly concentrated phosphate solution using powdered converter slag as a seed material[J]. Journal of Hazardous Materials, 2006, 136(3): 690-697. doi: 10.1016/j.jhazmat.2005.12.051 [31] LIU Y, ZHANG L M, SINGH R P. Enhanced phosphorus removal from wastewater using RSPRC and a novel reactor[J]. Applied Sciences, 2020, 10(10). -




 下载:
下载: