-
锰是一种自然界中常见的元素。在化工产业当中,新型锰基正极材料发展前景广阔[1];对生物而言,锰可以参与多种酶的合成[2],但也能引起儿童注意缺陷与多动障碍[3]、影响胎儿的神经发育[4]。地下水锰含量超标已经引起了国内外学者的广泛关注[5];然而大部分水库水均存在季节性铁锰超标现象,对不同pH条件下高铁锰地表水的处理机制仍需进一步的探究[6]。
目前,使用较多的除锰方法有自然氧化除锰[7]、化学氧化除锰[8]、生物除锰[9]以及接触氧化除锰[10]。自然氧化除锰需要大量的药剂和新建构筑物;化学氧化除锰常用二氧化氯或高锰酸钾等氧化二价锰,其投量掌握困难,易产生多种消毒副产物;生物除锰借助自然生长的铁锰细菌,通过扩散、吸附与生物氧化3个阶段除锰,但目前还需要对其机理及培养过程进行进一步的研究。一项将生物与重力驱动膜结合以去除Mn2+的研究表明,其与自催化氧化锰氧化物(MnOx)共同作用可以实现对Mn2+优良的去除率(>94.6%)[11]。
接触氧化除锰利用 “锰质活性滤膜”吸附并催化氧化二价锰,但滤膜自然成熟通常需要长达数月的时间,且会出现锰穿透的现象。为缩短成熟期,许多研究对影响接触氧化法除锰效果的因素进行了系统讨论。在多种环境下使用MnOx对滤料进行涂覆,结果均证明高pH能带来更高的锰去除率[12-13]。有研究通过生化手段处理高锰地下水,成功减少了滤料成熟期[14],并说明pH是控制MnOx生成反应速率的关键。一项对高铁锰氨氮井水的现场研究在启动过程中向滤池投加高锰酸钾,证明化学氧化法可有效缩短滤池启动时间[15]。对于滤料,沸石是一种生产量大、成本低、孔隙率高,被广泛用作水处理中的过滤介质,且因催化与离子交换的潜力受到广泛关注[16]。然而,使用沸石滤料去除地表水中锰的研究仍大多停留在实验室阶段,pH条件对地表水除锰的影响也尚未形成较为全面的理论体系,使用次氯酸钠减少滤料成熟期的工程应用经验也较少。
基于此,本研究使用改性沸石作为滤料,以某水库地表水作为原水,进行静态吸附实验与pH影响实验。为进一步缩短滤柱成熟期,采用次氯酸钠预氧化辅助滤柱启动,对在不同pH条件下启动的改性沸石滤柱滤料表面的形貌和组成进行表征分析,探讨pH对改性沸石滤料的吸附过程及预氧化辅助滤柱启动过程的影响,并在不同pH条件下对次氯酸钠投加量进行优化,以期为预氧化辅助沸石滤柱启动的实际应用提供参考。
-
主要化学试剂:次氯酸钠(NaClO)、氯化锰(MnCl2)、氯化亚铁(FeCl2·4H2O)、氯化钠(NaCl)购自广东西陇科学股份有限公司;盐酸(HCl)、氢氧化钠(NaOH)购自天津大陆化学试剂厂;本研究中所有试剂均为分析纯。实验水质:本研究在季节性铁锰超标的C水库进行,选用水库表层下5~9 m处的地表水作为实验原水,水温为20~32 ℃。向原水中定量投加FeCl2、MnCl2和NH4Cl,将铁锰质量浓度稳定在0.4~0.6 mg·L−1,氨氮质量浓度稳定在0.3~0.4 mg·L−1。
主要仪器:扫描电子显微镜(SEM,Sigma300,蔡司,德国);能量色散 X 射线光谱仪(EDS,XFlash 6130,布鲁克,德国);X 射线光电子能谱分析仪(XPS,ESCALAB250Xi,赛默飞世尔科技,美国);X射线衍射仪(XRD,D8,ADVANCE,德国)。
-
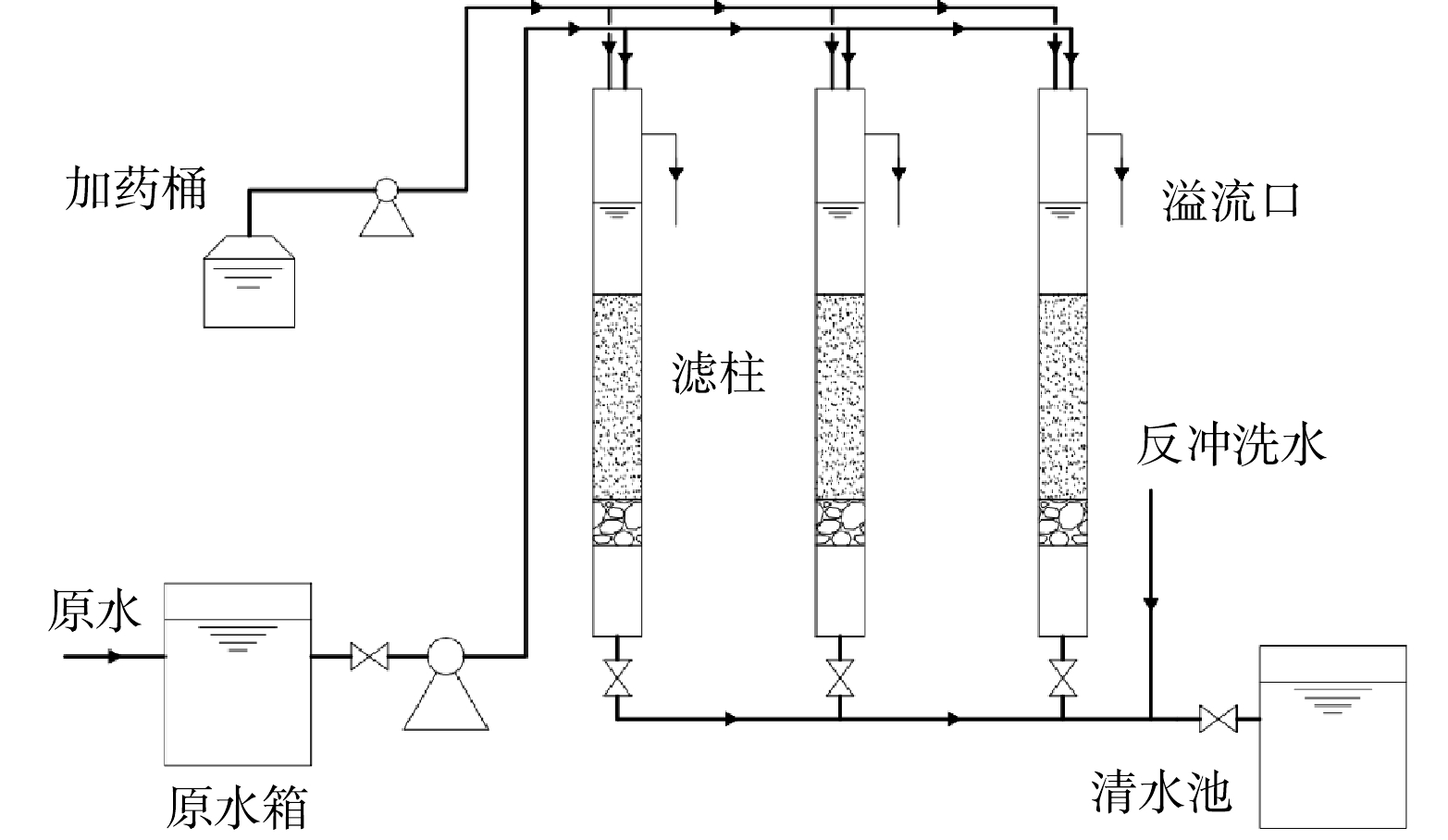
实验装置主要由潜水泵、原水箱、滤柱、清水池与加药系统组成(图1)。实验原水经潜水泵提升至原水箱,再抽送至内径65 mm、高约2.4 m的PVC制滤柱。滤柱底部承托层为各5 cm的粒径2~4 mm和4~8 mm的天然石英砂,上端滤料层为高度90 cm、粒径为0.8~1.5 mm的改性沸石。次氯酸钠由蠕动泵加至滤柱与原水混合,采用下向流流经滤柱。滤柱运行时将滤速控制在10 m·h−1,每24 h进行1次时长5 min、强度为10 L ·(s·m2)−1的单水冲反冲洗。
-
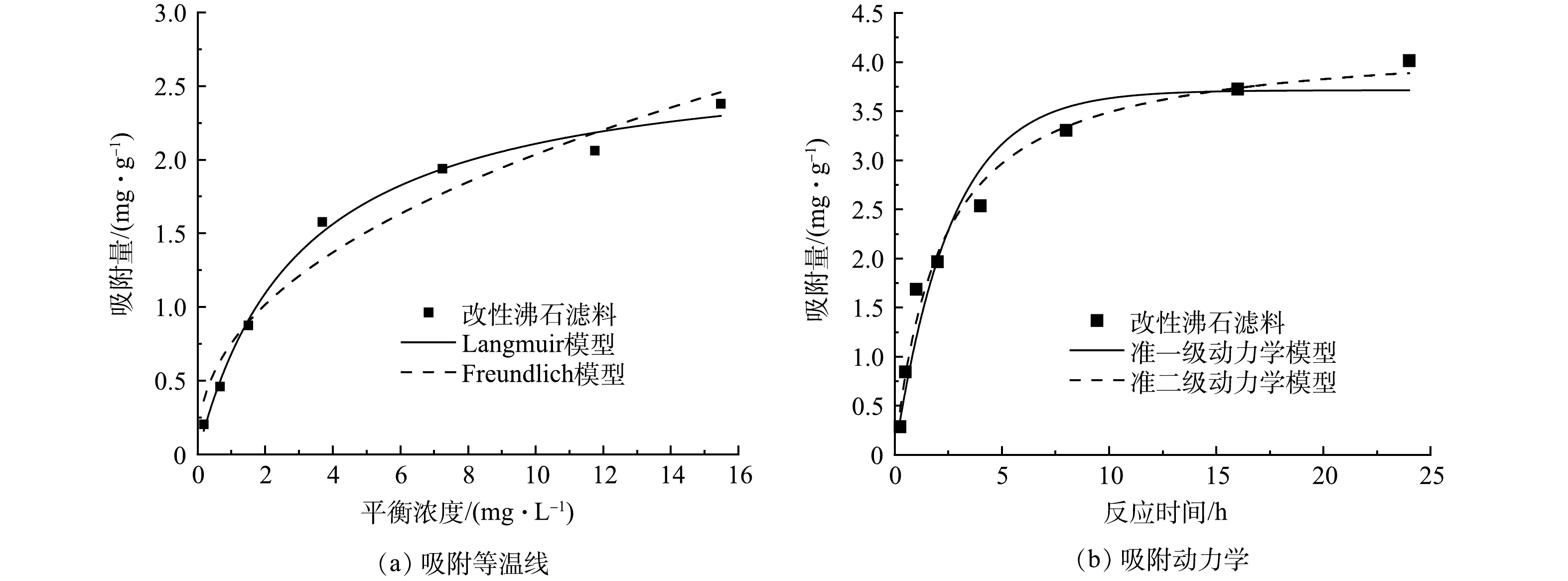
1)改性沸石滤料对Mn2+的静态吸附实验。配制质量浓度为1.0~25.0 mg·L−1的Mn2+溶液,分别取不同浓度的Mn2+溶液100 mL置于250 mL的锥形瓶中,并加入0.2 g改性沸石,过0.45 μm滤膜过滤样品,使用过硫酸铵法检测Mn2+的质量浓度,并对所得实验结果分别进行Langmuir(式(1))、Freundlich模型(式(2))拟合。
将100 mL 10.0 mg·L−1的Mn2+溶液加入250 mL的锥形瓶中,投加0.4 g改性沸石,过0.45 μm滤膜过滤,并检测残留Mn2+质量浓度,对实验结果分别进行准一级动力学(式(3))、准二级动力学(式(4))拟合[17]。
式中:qe为滤料对锰的吸附量,mg·g−1;qm为滤料对锰的饱和吸附容量,mg·g−1;Ce为吸附达到平衡时溶液中锰质量浓度,mg·L−1;KL为平衡常数,L·mg−1。
式中:KF为Freundlich吸附特征常数,mg·g−1;n为与吸附过程与吸附位点有关的常数。
式中:qe为平衡时刻滤料理论吸附量,mg·g−1;qt为t时刻滤料对锰的吸附量,mg·g−1;k1为准一级吸附动力学平衡常数,min−1。
式中:k2为准二级吸附动力学平衡常数,g·(mg·min)−1。
2)改性沸石滤料对Mn2+吸附的pH影响实验。分别配制pH为5.5~8.0,质量浓度为10 mg·L−1的Mn2+溶液,将上述溶液分别加入100~250 mL锥形瓶中,并投加0.4 g改性沸石,过0.45 μm滤膜并检测Mn2+质量浓度。
3) pH对滤柱启动的影响实验。设置3根滤柱,向进水中分别投加不同质量浓度的HCl或NaOH,以控制其pH分别为6.5、7.0、7.5。在实验进行阶段,当滤柱出水Mn2+质量浓度第1次超过0.1 mg·L−1后,向滤柱中投加0.1 mg·L−1的次氯酸钠溶液。每7 d将次氯酸钠溶液质量浓度提高0.1 mg·L−1,当出水Mn2+质量浓度低于0.1 mg·L−1后,停止提升加药质量浓度,并持续运行。
4)次氯酸钠最佳投加量优化研究实验。设置3根滤柱,调节其进水pH为6.5、7.0、7.5,投加1.0 mg·L−1的次氯酸钠。待其出水Mn2+质量浓度稳定且低于0.1 mg·L−1即成熟后进行实验,每7 d减少0.1 mg·L−1的次氯酸钠,每天检测出水Mn2+质量浓度直至其大于0.1 mg·L−1,此时的次氯酸钠加药质量浓度加0.1 mg·L−1即为当前pH下的最佳投药量。
-
滤料吸附Mn2+的能力会影响前期滤柱的除锰效果。为探究改性沸石滤料对Mn2+的吸附能力与吸附特性,进行吸附等温线与吸附动力学实验,拟合结果如图2所示,拟合计算得到的各项参数如表1和表2所示。由此可见,改性沸石滤料对Mn2+的吸附更符合Langmuir模型。这说明吸附过程中伴随着化学反应的发生,为化学吸附[18]。化学吸附吸附热较高、吸附力较强,说明Mn2+与沸石结合紧密,一旦吸附就不易解附。吸附过程更符合准二级动力学,且实际吸附能力也与二级动力学得出的结果(4.24 mg·g−1)更为接近,这说明吸附过程中Mn2+在改性沸石滤料表面形成单分子层。一项使用NaOH对天然沸石进行改性的研究表明,天然沸石对Mn2+的吸附更符合Langmuir模型,而改性后沸石对Mn2+的吸附则更符合Freundlich模型[19],这说明对沸石的改性并没有改变沸石本身对Mn2+的吸附特性。值得一提的是,还有研究表明,沸石对水中的氨氮也有一定的吸附效果[20],且可能与多种金属离子发生离子交换[21],这可能影响其在实际除锰应用中的效果。
-
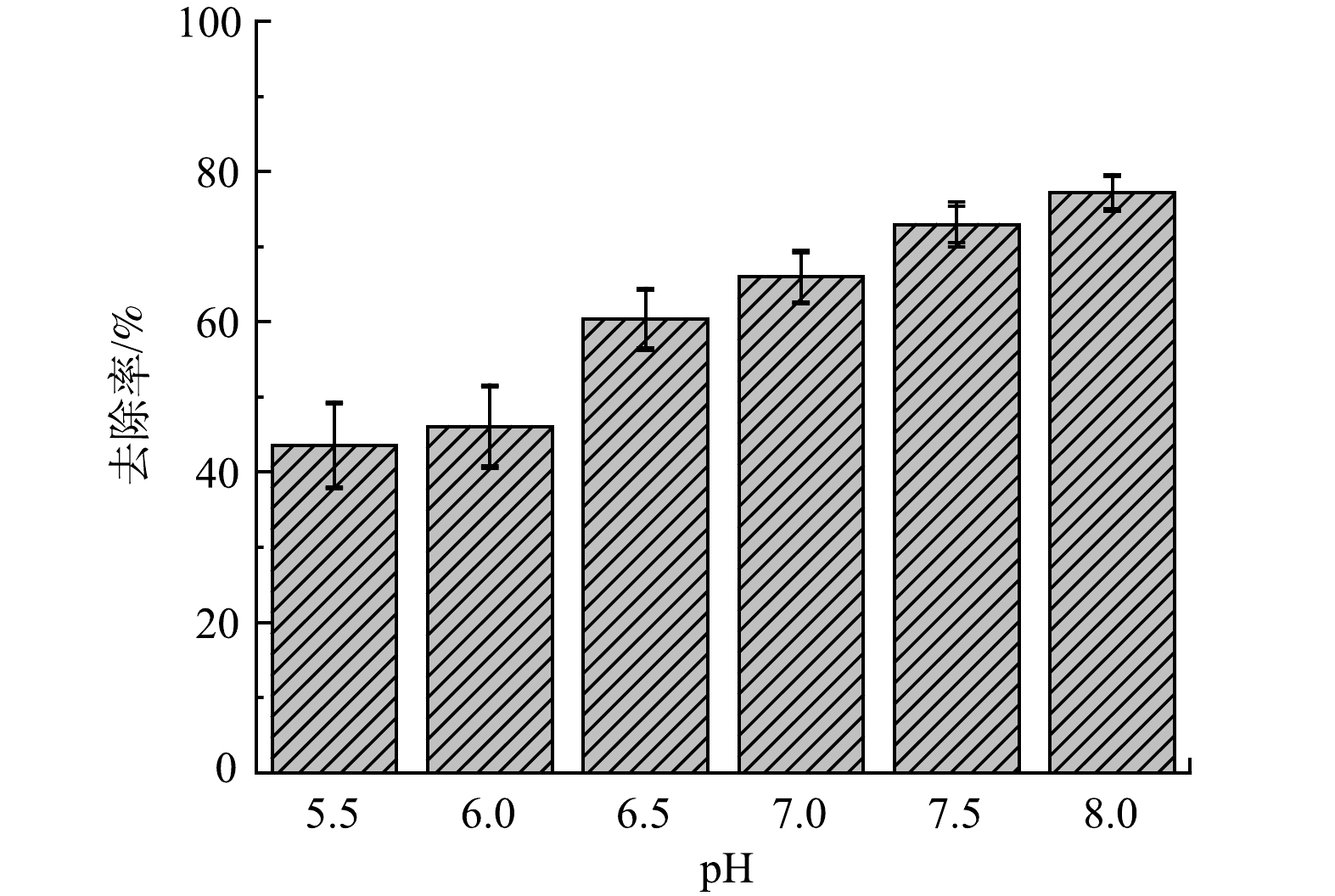
不同pH条件下改性沸石滤料对锰的吸附结果如图3所示。在pH为5.5和6.0时,去除率仅为43.53%和46.05%,但pH继续上升时去除率也随之逐渐升高,由pH为6.5时的60.34%上升至pH为8.0时的77.14%。改性沸石吸附锰的能力随pH的升高逐渐变强,这可能是因为pH会影响改性沸石滤料表面的电荷[22]。随着pH升高,溶液中的H+浓度降低,改性沸石滤料表面的正电荷密度也随之降低,从而减小滤料表面与Mn2+之间的静电排斥力,使Mn2+更容易附着在其表面且被吸附。此外,H+也会与Mn2+抢夺吸附位点[23],当pH升高时,由于与H+的竞争减弱,改性沸石滤料吸附Mn2+的能力也会有所上升。
-
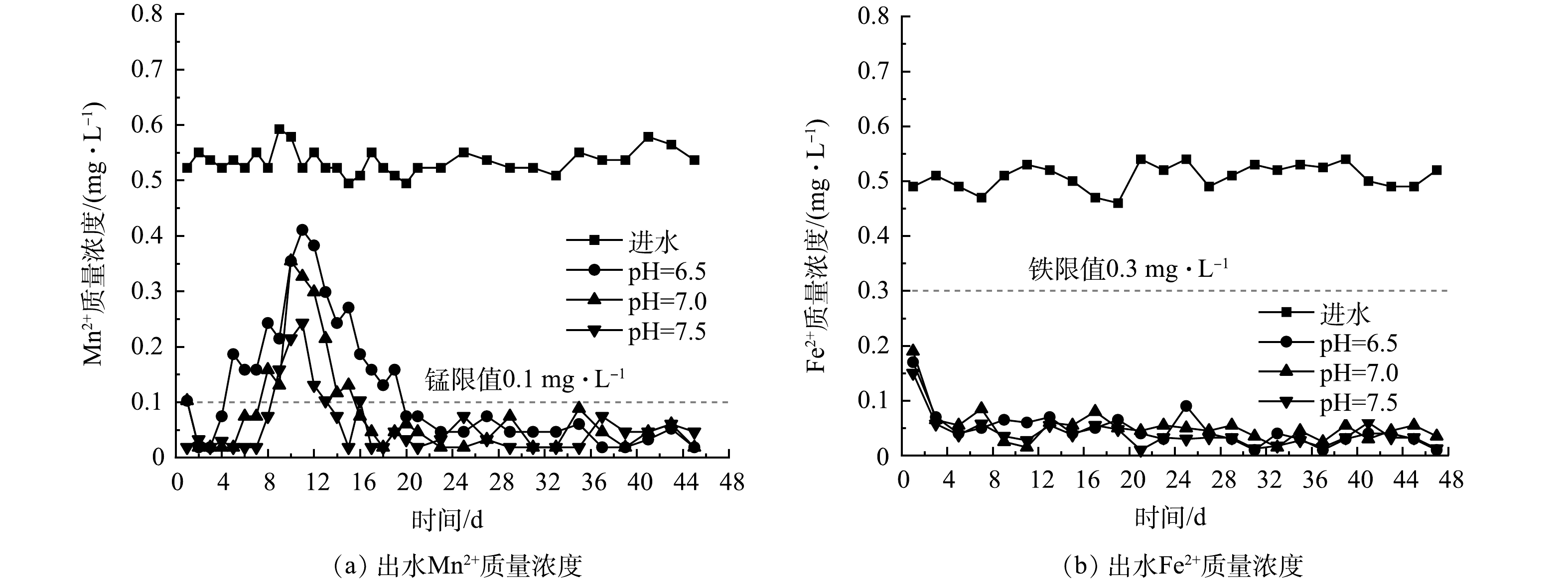
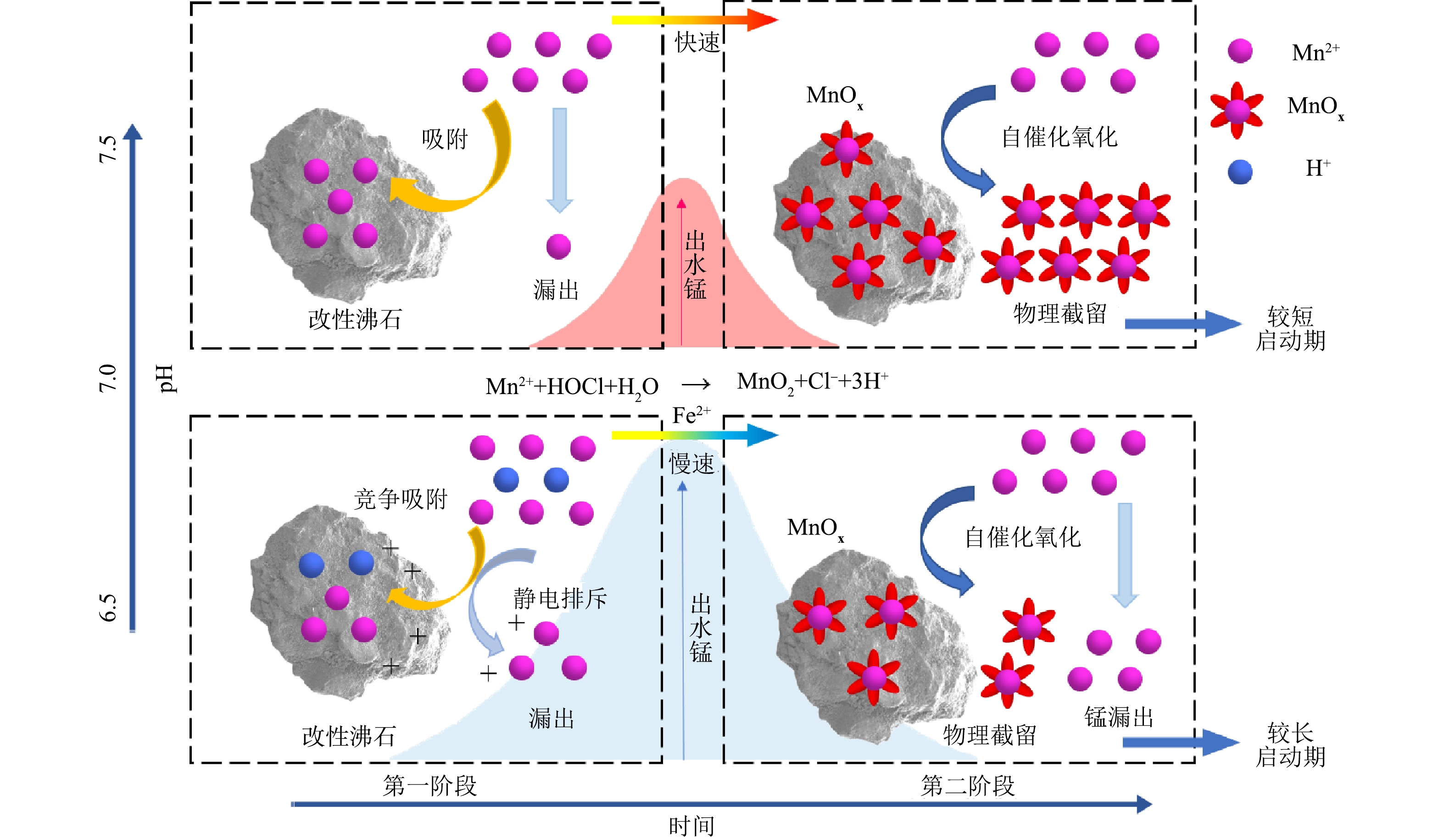
有研究表明[24],滤料表面的锰氧化物也可以催化氯氧化剂对Mn2+的氧化反应。为进一步减少滤柱的启动时间,使用次氯酸钠预氧化的方法辅助滤柱启动,在45 d内监测其出水锰与出水铁质量浓度,结果如图4所示。3种滤柱按pH从低到高顺序,分别在第5、8和9天时出现了锰穿透的情况,并均在第10天和第11天出水Mn2+达到峰值,又在第20、16、14天启动成功。此后出水锰质量浓度虽均随原水波动,但波动幅度很小,持续在锰限值(0.1 mg·L−1)以下。这表明成功启动的滤柱具有良好的稳定性。一项使用Mn(IV)催化降解水体中微量有机污染物的研究[25]表明,HClO被用作再生剂成功将Mn(II)与Mn(III)复原为Mn(IV),证明了次氯酸有恢复高价锰催化剂的能力。pH分别为6.5、7.0、7.5的3根滤柱的最终次氯酸钠投加量分别为0.3、0.2和0.1 mg·L−1,低pH下运行的滤柱其投氯较早、同一时间的加氯量也较多,但启动期仍然较长、同一时间的锰去除率仍较低,这说明在该实验中pH是影响除锰效果的关键因素。3根滤柱启动期分别为15、8和5 d,说明pH会显著影响实验中滤柱的启动期。此外,3根滤柱的出水Fe2+质量浓度除第1天外均低于0.1 mg·L−1,远低于《生活饮用水卫生标准》(GB 5749-2022)中所规定的标准0.3 mg·L−1,说明滤柱表现出优良的除铁能力。3种滤柱出水Fe2+质量浓度稳定且不随pH的变化而变化,而出水Mn2+质量浓度却随pH变化,这可能是由于滤柱的除铁过程先于除锰。一项对高铁、高锰、高氨氮地下水的研究[26]表明,在低pH下锰被催化氧化的速率更慢,滤料表面更倾向于生成铁质滤膜而不是锰质活性滤膜;而且,未能被氧化的铁将随过滤而渗透至滤层下部,并生成铁质滤膜,压缩滤柱除锰段的长度,进一步降低除锰的效果。
-
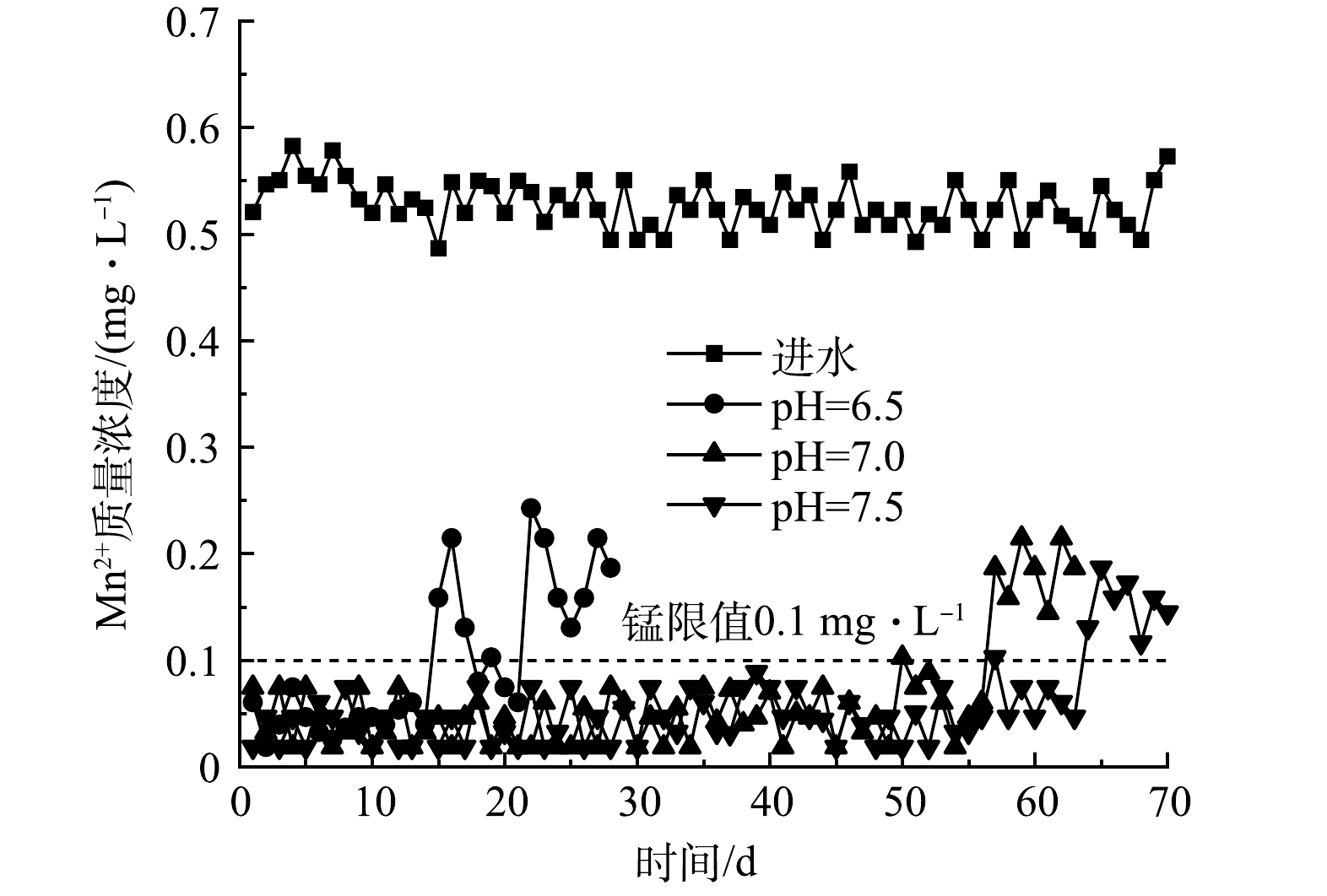
为探究持续运行中次氯酸钠对不同pH下滤柱除锰效果的影响、同时减少运行过程中加药量,进行次氯酸钠最佳投加量优化研究,结果如图5所示。对pH=6.5的滤柱,次氯酸钠投加量为0.8 mg·L−1时便出现超标,之后又在5 d内减少,并返回锰限值0.1 mg·L−1以下,推测在该过程中可能又有新的活性层成熟。进一步减少投加量,出水Mn2+质量浓度没有再一次回到锰限值以下,说明滤柱已无法负担,认定pH=6.5时次氯酸钠最低投加量为0.8 mg·L−1。对于pH为7.0和7.5的滤柱,两者的最低次氯酸钠投量分别为0.3 mg·L−1和0.2 mg·L−1,远小于pH=6.5的滤柱。在滤柱启动成功后,适当减少次氯酸钠投加量,出水Mn2+仍可保持达标,这说明投加的次氯酸钠量已大于所需。3根滤柱启动时投氯量均为1.0 mg·L−1,但滤柱成熟后,所需的次氯酸钠投加量随pH的升高而减少,这表明pH对稳定运行过程中所需的次氯酸钠投加量具有较大的影响。在加氯条件下启动的3根滤柱形成的锰质活性滤膜均无法靠其自身的催化能力将原水中的锰去除至锰限值以下,而持续投加次氯酸钠可以保证滤柱的出水Mn2+质量浓度维持在0.1 mg·L−1以下。次氯酸的氧化性虽随pH升高而降低,但在碱性环境下次氯酸氧化Mn2+的产物H+被快速消耗,使Mn2+的氧化反应平衡右移,导致高pH下除锰所需的次氯酸钠持续投加量降低[27]。有研究将沸石与自催化氧化锰氧化物(MnOx)耦合以处理高铁锰地下水,在加氯条件下其反冲洗水中的Mn2+质量浓度随着运行pH的升高而减少[28],说明氯可以将Mn2+氧化为高价锰并以固体形式被截留。由此可以推断,在本研究中次氯酸钠可氧化Mn2+,加速滤膜成熟,且结论与地下水除铁锰研究中的一致。
-
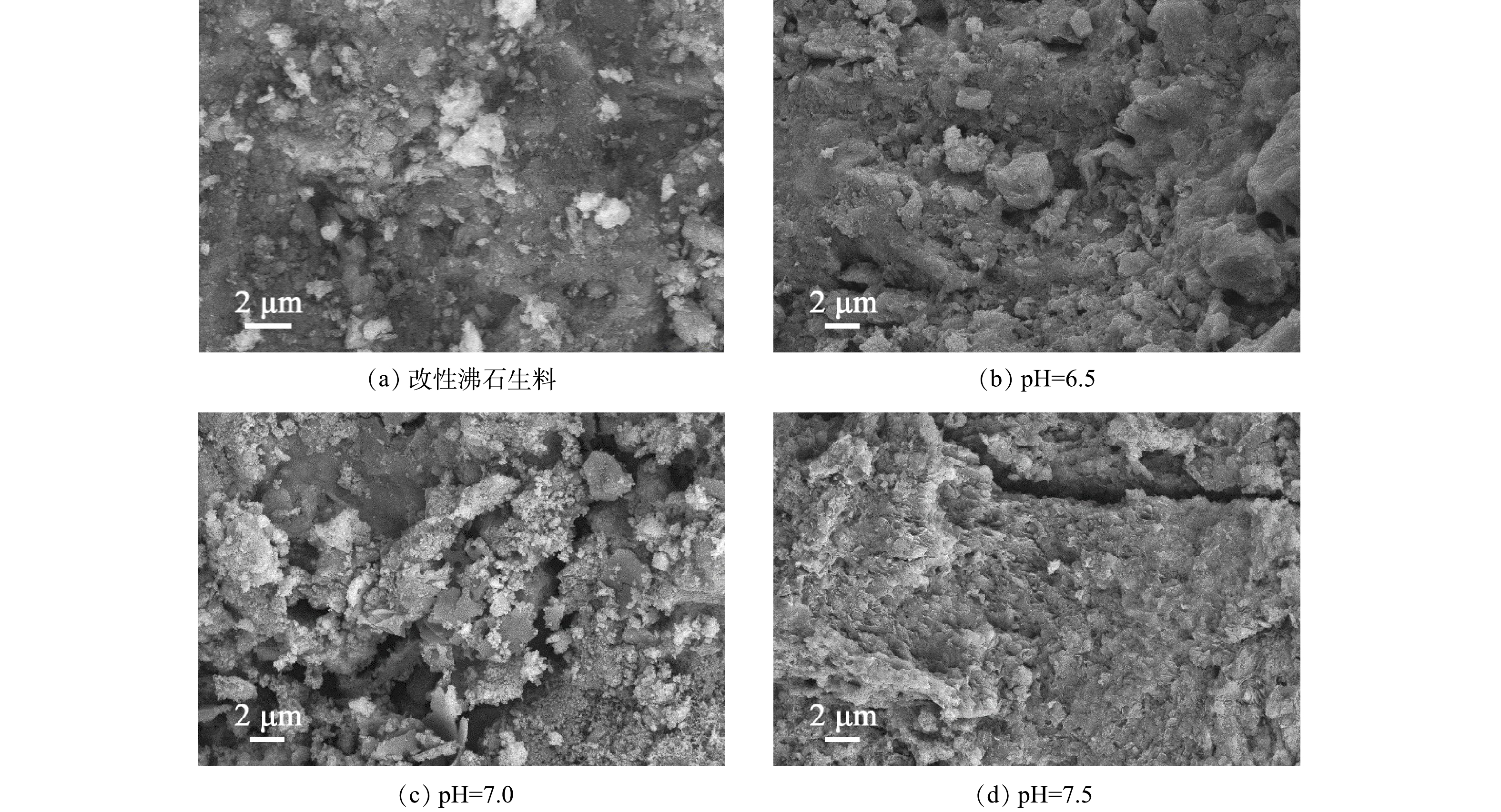
图6为改性沸石生料与pH=6.5、7.0、7.5下经过过滤运行后的改性沸石滤料的SEM图像。在改性沸石生料表面的孔隙是沸石的特征,而其上的颗粒物则是在改性过程中负载的MnO2颗粒。在经过过滤除锰过程后,附着在表面的颗粒物是催化氧化过程中形成的锰氧化物。由图6可知,随着pH的增大,滤料表面颗粒物呈现增加趋势,表面形态从平整变得更加细致而紧密,这样的结构有利于锰离子的吸附、催化氧化以及去除。这表明pH的升高有利于滤膜的生成。在滤料表面没有观察到微生物,这是由于投加的次氯酸钠作为氧化剂的同时也具有消毒作用,证明在本实验中锰的去除依靠的是物理化学作用而非生物作用。
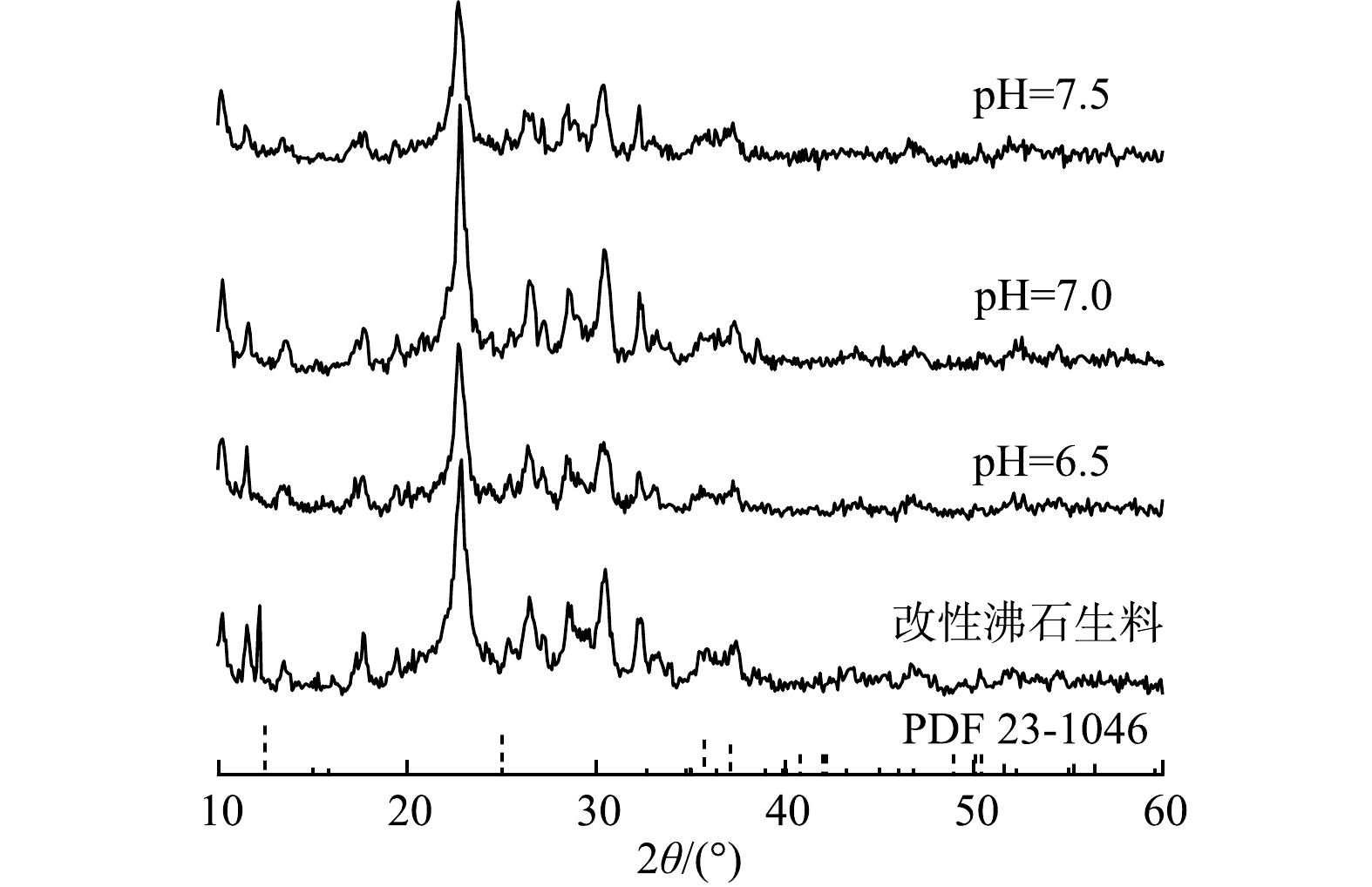
不同pH运行条件下滤料的XRD分析结果如图7所示。3种滤料的最高衍射峰(20.86°处)为SiO2的特征峰,这是沸石的主要成分。与标准PDF卡片(JCPDS PDF23-1046)进行比较,发现在12.47°与37.12°等处观测到的衍射峰为水钠锰矿的特征峰。以上特征峰在不同滤料中出现的位置相同并且没有新的特征峰出现,这说明原水pH的变化并没有影响改性沸石滤料和锰质活性滤膜的结构。
对不同pH条件下运行的滤料进行EDS分析,其表面元素组成如表3所示。经过过滤除锰过程,改性沸石滤料表面的碳、锰、铁的含量有显著升高,而铝、钙、氧、硅含量出现不同程度的降低。铁和锰含量的升高是由于滤料表面生成的大量铁质与锰质活性滤膜,而碳含量的升高则是由于沸石滤料的多孔结构吸附水体中的有机物所致[29]。锰含量的升高表明随着pH升高,滤料表面生成了更多的滤膜,这与SEM的表征结果一致。
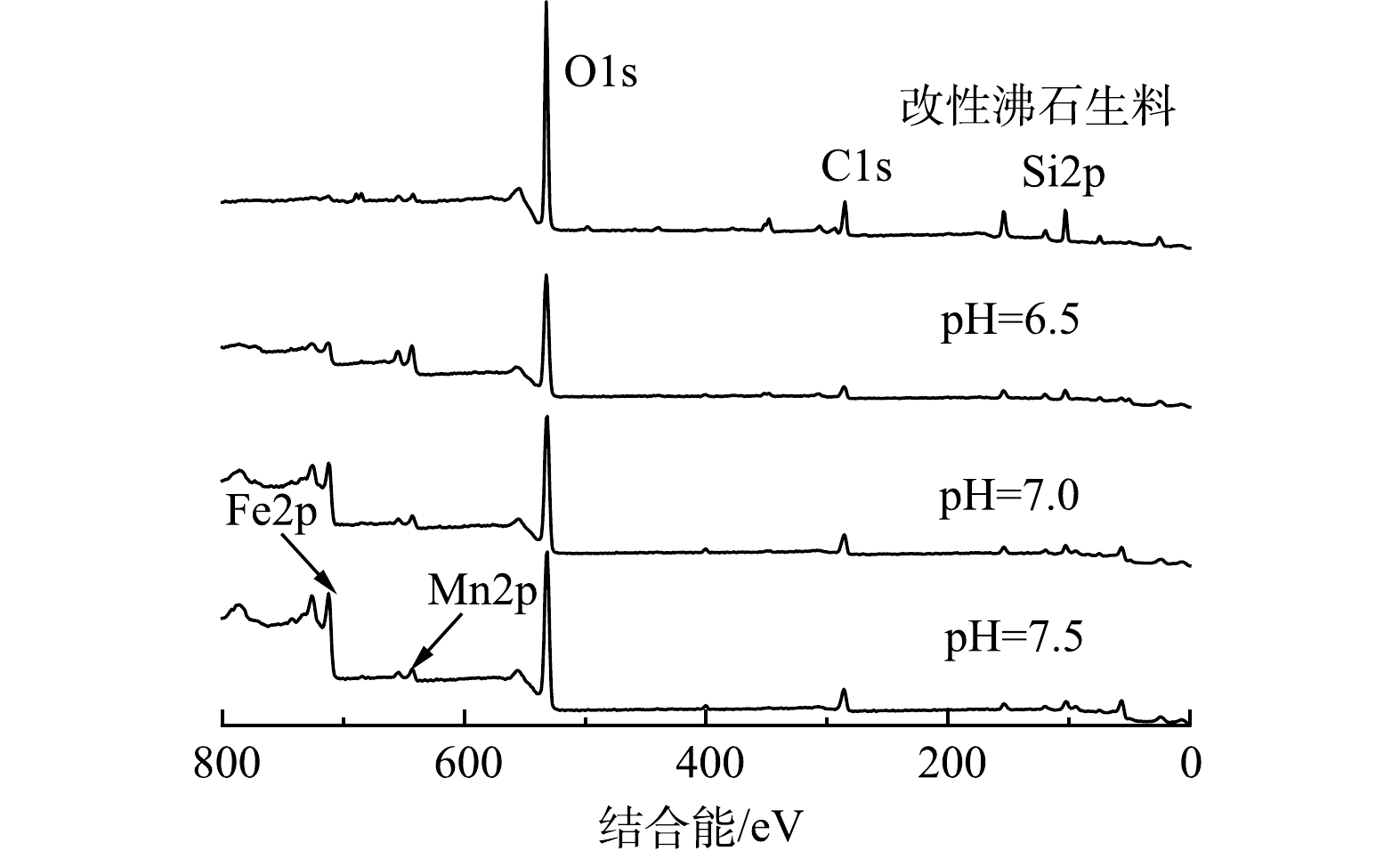
为进一步研究滤料表面各元素价态变化,对在不同pH条件下运行后的改性沸石滤料进行XPS全扫图谱分析,结果如图8所示。天然沸石滤料为铝硅酸盐矿物,故存在Si2p、C1s、O1s峰;而生料中的Mn2p峰则是由于在改性过程中MnO2颗粒附着在滤料表面。与改性沸石生料相比,经过滤后的3种沸石滤料的Si2p、O1s、C1s峰均有所下降,而Fe2p、Mn2p峰则明显升高。这是因为在过滤过程中铁和锰生成了滤膜且附着在滤料表面。随着pH的升高,Fe2p、Mn2p峰峰值也变高。这说明滤膜生成效率也在逐渐提高,这与SEM和EDS的表征分析结果一致。
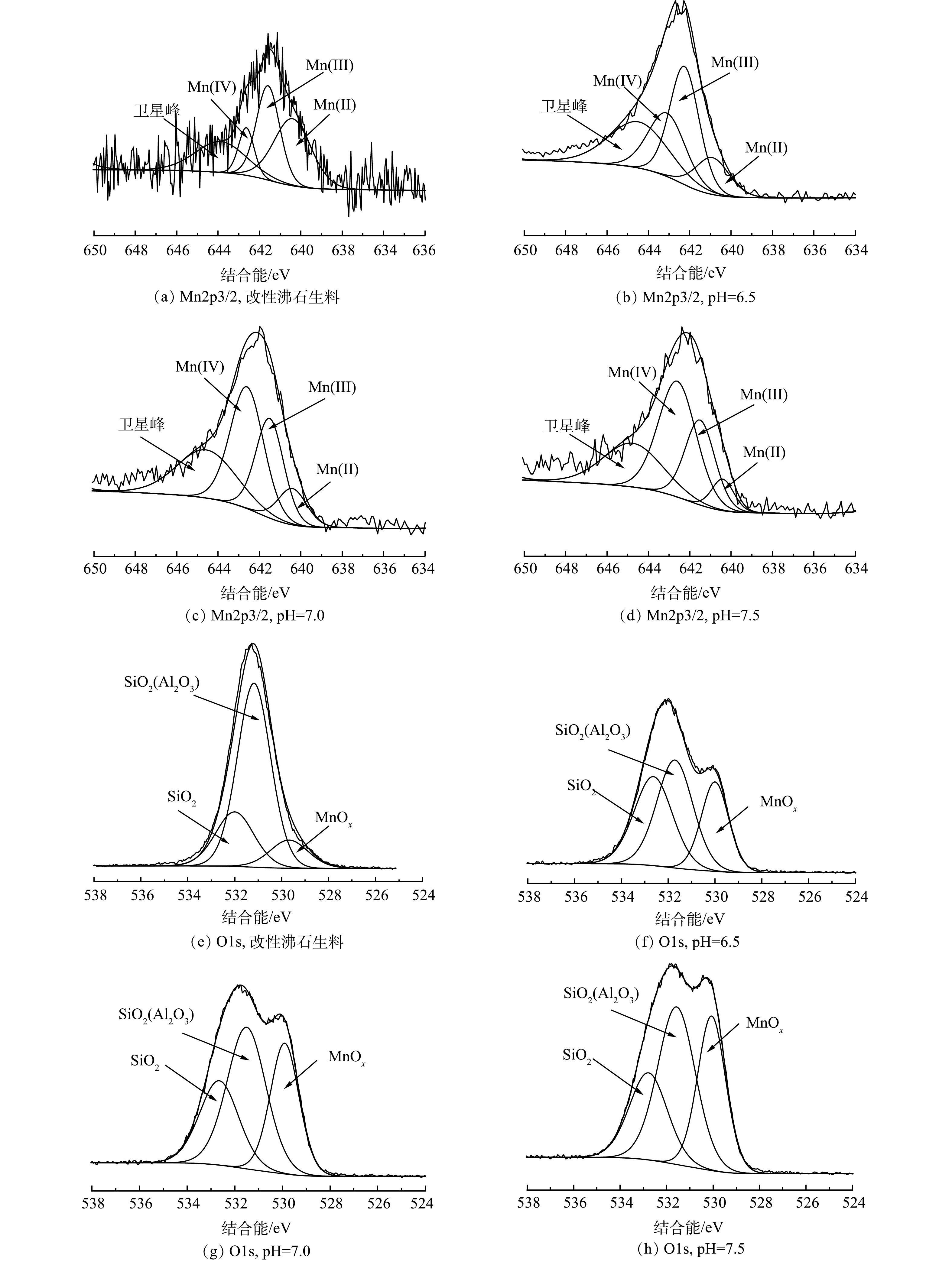
为探究在不同pH条件下生成的锰质活性滤膜中锰的价态组成,使用XPS对4种样品进行Mn2p和O1s高分辨谱图分析,结果如图9所示。Mn2p由于自旋会分裂成Mn2p1/2和Mn2p3/2 2个峰[30],对Mn2p3/2峰进行分峰拟合,结果如图9 (a)~(d)所示。4种滤料的谱图中均存在4个峰,说明滤料表面的锰不仅有Mn(IV),而是多种价态同时存在,以MnOx的形式附着在滤料表面。经计算[31]可得,pH分别为6.5、7.0和7.5的滤料表面Mn(II)含量逐渐下降,由18.67%、13.23%下降至7.48%;而Mn(IV)含量则由24%上升至51.4%与52.86%。这说明滤膜的催化氧化性能得到大幅提升。一项对滤池除锰过程中滤料表面催化活性物质进行的研究发现,除Mn(IV)外,Mn(III)含量的上升也可说明催化活性物质的增加[32]。综上所述,推测本研究中Mn(III)与Mn(IV)共同起到催化氧化Mn2+的作用。图9(e)~(h)为改性沸石滤料的O1s谱图,在531.70 eV附近与532.50 eV附近的2个峰分别为沸石主要成分SiO2、SiO2(Al2O3);而528.50~530.40 eV处的峰则是改性过程中添加在其上的锰氧化物产生的。与之相比,经过滤之后的改性沸石滤料SiO2(Al2O3)峰减弱,这与EDS分析中Al含量的降低相吻合。随pH升高,锰氧化物的含量也不断升高,而据上述分析,Mn(II)的含量并没有显著变化。由此可知,滤料表面锰质活性滤膜有效催化物质含量即Mn(III)与 Mn(IV)随着pH的升高而增加,且会带来催化氧化效率的提升。
-
在本研究中,滤柱启动阶段滤柱除锰的方式包括沸石滤料的吸附作用、次氯酸钠的氧化作用和滤料表面的锰质活性滤膜对Mn2+的催化氧化作用。3种滤柱锰穿透期的pH条件与启动期时长虽有所不同,但出水锰质量浓度均先上升后下降,并具有1~2 d的高峰期,可由此将滤柱的启动过程依时间分为2个阶段(图10)。在出水锰达到峰值之前为第1阶段,此时改性沸石滤料的吸附起主要作用。过滤初期,锰质活性滤膜尚未生成,无法将Mn2+完全催化氧化。一部分Mn2+被沸石滤料所吸附;另一部分则被次氯酸钠氧化形成MnOx并附着在滤料表面,这部分Mn2+成为Mn(III)与Mn(IV)催化氧化水中的Mn2+[33-34];剩余Mn2+则随水流流出滤柱。随着过滤的进行,滤料中可与Mn2+发生吸附与离子交换的位点逐渐被耗尽,最终达到吸附-解吸平衡。但MnOx生成量仍较低,催化氧化能力的上升无法弥补由于滤料吸附能力下降导致除锰效率的下降,此时出水锰便会不断上升。可以推断,改性沸石滤料吸附Mn2+性能的下降是第1阶段中影响滤柱除锰速率的主要因素。而在高pH下,由于沸石表面的电荷发生变化以及Mn2+与H+的竞争吸附也变弱,导致沸石对Mn2+的吸附能力变强;次氯酸与Mn2+的反应平衡向有利于Mn2+被氧化的方向进行,从而使启动期变短;同时滤料表面相较铁质滤膜更倾向于生成锰质滤膜,生成的滤膜也更加细致紧密,导致与低pH下相比较低的出水锰质量浓度。另外,在滤柱实际运行过程中,改性沸石滤料对Mn2+的吸附容量明显低于静态吸附实验当中得到的结果(约4 mg·g−1)。这是由于滤料不仅吸附Mn2+,还可吸附原水中的Fe2+、氨氮以及有机污染物[35],使滤料表面含有铁、碳等元素,这些粒子与Mn2+竞争吸附位点,使沸石在使用天然地表水的实验当中对Mn2+的吸附能力下降;此外,较高的滤速(10 m·h−1)也会导致吸附剂表面的活性位点被快速覆盖,进而导致吸附性能的下降。
但是,由于次氯酸钠的投加,改性沸石滤料上吸附的大量Mn2+同时经由MnOx的催化作用被原位氧化,使得锰质活性滤膜的生成速率得到大幅提升,当锰质活性滤膜的催化能力逐渐上升,出水锰达到峰值之后为第2阶段,此阶段锰质活性滤膜与次氯酸钠的催化氧化作用起主要作用。此时滤膜接近成熟,尽管滤料的吸附能力因已吸附水中的大量颗粒和锰质活性滤膜的生成而严重下降,但由于锰质活性滤膜的自催化氧化作用得到强化,滤柱去除Mn2+的整体性能呈现上升趋势,直至滤柱启动成功。在第2阶段, pH高时次氯酸氧化Mn2+反应较快;锰质活性滤膜的生成速率较高,导致pH=7.5时滤料表面高价锰含量几乎为pH=6.5时的2倍,最终可缩短启动期。而在持续运行过程中,较低pH下生成的滤膜由于MnOx的有效催化成分含量较低,所需的次氯酸钠投加量也较高,需借助次氯酸钠的辅助氧化作用维持较高的锰去除率;同时,低pH条件下锰质活性滤膜的生成速率也较低,减少次氯酸钠投加量时达到新平衡的速率也较慢。以上2点为低pH条件下持续运行时所需次氯酸钠投加量高、减量投氯后出水锰波动大的原因。
-
1)随pH的升高,改性沸石滤料对Mn2+的吸附能力也有所上升,在应用实验中直接导致前期除锰效率升高和更长的锰穿透期。
2) pH较低时,锰被催化氧化的速率较慢,滤料表面更倾向于生成铁质滤膜。随着pH升高,更快的锰质活性滤膜生成速率带来了高pH时滤柱的快速启动。
3)滤料表面生成的锰质滤膜有效含量随pH降低而降低,使得持续运行过程中高pH条件下成熟的滤柱所需的次氯酸钠投加量远远小于低pH条件下的投加量。
pH对预氧化沸石滤柱启动过程中除锰效率的影响
Effect of pH on manganese removal efficiency during the start-up process of pre-oxidation zeolite filter column
-
摘要: 针对当前接触氧化除锰法启动期较长的问题,采用改性沸石滤料耦合次氯酸钠预氧化辅助滤柱启动,采用扫描电子显微镜(SEM)、能量色散 X 射线光谱(EDS)、X射线衍射(XRD)、X 射线光电子能谱(XPS)对滤料进行分析,探究了原水pH对滤柱启动中锰去除效率的影响,并对持续运行过程中次氯酸钠的投加量进行了优化,对改性沸石滤料进行静态吸附实验,并在进水pH分别为6.5、7.0、7.5时进行滤柱启动实验。结果表明,改性沸石滤料对Mn2+的吸附符合Langmuir模型与准二级动力学方程,当pH为5.5~8.0时,随pH升高改性沸石滤料对Mn2+的去除率上升。在3种pH条件下,滤柱的锰穿透期分别为5、8 和9 d,并分别在第20、16和14天启动成功;次氯酸钠预氧化启动的3种滤柱次氯酸钠最低投加量分别为0.8、0.3与0.2 mg·L−1。SEM与XRD表征结果证实了水钠锰矿在滤料表面的出现;而EDS与XPS结果分别表明,更高的pH会同时带来滤料表面锰元素含量和锰价态的升高。以上研究结果可为地表水水厂中对除锰有利的pH范围及沸石在启动过程中对锰的吸附作用提供参考。Abstract: In view of the long start-up period of the current contact oxidation manganese removal method, modified zeolite filter media coupled with sodium hypochlorite preoxidation was used to assist the start-up of the filter column. Scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS) were used to analyze the filter materials, the effect of raw water pH on the start-up of the filter column was investigated, and the dosage of sodium hypochlorite for continuous operation was optimized. Static adsorption experiments were conducted on modified zeolite filter media, and column start-up experiments were conducted at feed water pH of 6.5, 7.0 and 7.5, respectively. The results showed that the adsorption of Mn2+ by the modified zeolite filter media was in accordance with the Langmuir model and quasi-secondary kinetic equation, and the removal rate of Mn2+ by the modified zeolite filter media increased with increasing pH when the pH was 5.5~8.0. The manganese penetration periods of the filter columns at the three different pHs were 5, 8 and 9 days, respectively, and were successfully activated on day 20th, 16th and 14th; and the minimum doses of sodium hypochlorite were 0.8, 0.3 and 0.2 mg·L−1 for the three filter columns with sodium hypochlorite preoxidation, respectively. SEM and XRD results confirmed the appearance of Birnessite on the surface of the filter media, while the EDS and XPS results showed that higher pH resulted in both higher manganese content and higher average valence on the surface of the filter media, respectively. The results of this study can provide a reference for the pH range favorable for Mn removal in surface water plants and the adsorption of Mn by zeolites during start-up process.
-
Key words:
- pH /
- manganese removal /
- modified zeolite filter media /
- pre-oxidation
-

-
表 1 改性沸石滤料吸附 Mn2+的等温吸附方程参数拟合值
Table 1. Fitting parameters of adsorption isotherms of Mn2+ on modified zeolite filter media
Langmuir模型 Freundlich模型 qm/(mg·g−1) KL /(L·mg−1) R2 KF/(mg·g−1) n R2 2.75 0.33 0.993 0.75 0.23 0.961 表 2 改性沸石滤料吸附 Mn2+的吸附动力学参数拟合值
Table 2. Fitting parameters of adsorption kinetics of Mn2+ on modified zeolite filter media
准一级动力学 准二级动力学 qe/(mg·g−1) k1/h−1 R2 qe/(mg·g−1) k2/(g·(mg·min)−1) R2 3.71 0.38 0.954 4.24 0.11 0.983 表 3 在不同pH运行条件下滤料表面元素组成及含量
Table 3. Elemental composition and content of filter material surface under different pH operation conditions
滤料种类 元素含量/% C O Mg Al Si Ca Mn Fe 生料 2.68 40.62 0.77 7.55 42.07 2.89 3.41 0.00 pH=6.5 12.52 30.31 0.46 4.68 32.14 1.40 10.68 7.81 pH=7.0 7.10 27.51 0.10 2.71 25.24 0.55 17.69 19.10 pH=7.5 10.11 27.22 0.32 3.25 20.77 0.64 21.86 15.82 -
[1] ORTIZ-VITORIANO N, DREWETT N E, GONZALO E, et al. High performance manganese-based layered oxide cathodes: Overcoming the challenges of sodium ion batteries[J]. Energy & Environmental Science, 2017, 10(5): 1051-1074. [2] 刘明朝. 小胶质细胞活化在锰诱导的多巴胺能神经元损伤中的作用及可能机制[D]. 西安: 第四军医大学, 2007. [3] CHUNG S E, CHEONG H K, HA E H, et al. Maternal blood manganese and early neurodevelopment: The mothers and children’s environmental health (MOCEH) study[J]. Environmental Health Perspectives, 2015, 123(7): 717-722. doi: 10.1289/ehp.1307865 [4] CLAUS HENN B, BELLINGER D C, HOPKINS M R, et al. Maternal and cord blood manganese concentrations and early childhood neurodevelopment among residents near a mining-impacted superfund site[J]. Environmental Health Perspectives, 2017, 125(6): 067020. doi: 10.1289/EHP925 [5] RAMACHANDRAN M, SCHWABE K A, YING S C. Shallow groundwater manganese merits deeper consideration[J]. Environmental Science & Technology, 2021, 55(6): 3465-3466. [6] HOYLAND V W, KNOCKE W R, FALKINHAM III J O, Effect of drinking water treatment process parameters on biological removal of manganese from surface water[J]. Water Research, 2015, 69: 154-161. [7] 杨炜, 王明辉, 原书文, 等. 郑州市石佛水厂除铁除锰滤池运行机理讨论[J]. 中州大学学报, 1999(4): 80-82. [8] GREGORY D, CARLSON K. Effect of soluble Mn cconcentration on oxidation kinetics[J]. Journal American Water Works Association, 2003, 95(1): 98. doi: 10.1002/j.1551-8833.2003.tb10273.x [9] CHENG Q, HUANG Y, NENGZI L, et al. Performance and microbial community profiles in pilot-scale biofilter for ammonia, iron and manganese removal at different dissolved oxygen concentrations[J]. World Journal of Microbiology and Biotechnology, 2019, 35(3). [10] 白朗明, 赵煊琦, 丁俊文, 等. 改性硅铝矿石除锰效能及机制研究[J]. 给水排水, 2020, 56(S2): 7. [11] CHANG H L, SUN W Y, WANG Y R, et al. Effects of organics concentration on the gravity-driven membrane (GDM) filtration in treating iron- and manganese-containing surface water[J]. Water Research, 2022, 226: 119223. doi: 10.1016/j.watres.2022.119223 [12] CERRATO J M, KNOCKE W R, HOCHELLA M F, et al. Application of XPS and solution chemistry analyses to investigate soluble manganese removal by MnOx(s)-coated media[J]. Environmental Science & Technology, 2011, 45: 10068-10074. [13] FUNES A, VICENTE J D, CRUZ-PIZARRO L, et al. The influence of pH on manganese removal by magnetic microparticles in solution[J]. Water Research, 2014, 53: 110-122. doi: 10.1016/j.watres.2014.01.029 [14] BRUINS J H, PETRUSEVSKI B, SIOKAR Y M, et al. Biological and physico-chemical formation of Birnessite during the ripening of manganese removal filters[J]. Water Research, 2015, 69: 154-161. doi: 10.1016/j.watres.2014.11.019 [15] 武俊槟, 黄廷林, 程亚, 刘杰. 催化氧化除铁锰氨氮滤池快速启动的影响因素[J]. 中国环境科学, 2017, 37(3): 1003-1008. [16] JHA V K, NAGAE M, MATSUDA M, et al. Zeolite formation from coal fly ash and heavy metal ion removal characteristics of thus-obtained Zeolite X in multi-metal systems[J]. Journal of Environmental Management, 2009, 90(8): 2507-2514. doi: 10.1016/j.jenvman.2009.01.009 [17] HALIU S L, NAIR B U, REDI-ABSHIRO M, et al. Preparation and characterization of cationic surfactant modified zeolite adsorbent material for adsorption of organic and inorganic industrial pollutants[J]. Journal of Environmental Chemical Engineering, 2017, 5(4): 3319-3329. doi: 10.1016/j.jece.2017.06.039 [18] 赵济金, 戚菁, 吉庆华, 等. 铁锰改性铜绿微囊藻对锑的吸附性能[J]. 环境工程学报, 2019, 13(7): 1573-1583. [19] ATES A, AKGUL G. Modification of natural zeolite with NaOH for removal of manganese in drinking water[J]. Powder Technology, 2016, 287: 285-291. doi: 10.1016/j.powtec.2015.10.021 [20] FU HAILIU, LI YI, YU ZIYAO, et al. Ammonium removal using a calcined natural zeolite modified with sodium nitrate[J]. Journal of Hazardous Materials, 2020, 393: 122481. doi: 10.1016/j.jhazmat.2020.122481 [21] KARADAG D, KOC Y, TURAN M, OZTURK M. A comparative study of linear and non-linear regression analysis for ammonium exchange by clinoptilolite zeolite[J]. Journal of Hazardous Materials, 2007, 144: 432-437. doi: 10.1016/j.jhazmat.2006.10.055 [22] MALAMIS S, KATSOU E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms[J]. Journal of Hazardous Materials, 2013, 252: 428-461. [23] LIN J W, ZHAN Y H, ZHU Z L, et al. Adsorption of tannic acid from aqueous solution onto surfactant-modified zeolite[J]. Journal of Hazardous Materials, 2011, 193: 202-111. [24] KNOCKE W R, OCCIANO S C, HUNGATE O R. Removal of soluble manganese by oxide-coated filter media: sorption rate and rwmoval mechanism issues[J]. Journal American Water Works Association, 1991, 83(8): 64-69. doi: 10.1002/j.1551-8833.1991.tb07201.x [25] CHARBONNET J A. Chemical regeneration of manganese oxide-coated sand for oxidation of organic stormwater contaminants[J]. Environmental Science & Technology, 2018, 52: 10728-10736. [26] 陈天意, 陈志和, 金树峰, 等. pH 值对滤池处理高浓度铁、锰及氨氮地下水的影响[J]. 中国给水排水, 2015, 31(23): 5-9. [27] 雷晓玲, 秦颖, 文永林, 等. 预氧化强化混凝工艺处理含锰水实验研究[J]. 应用化工, 2022, 51(1): 110-113. [28] WANG P P, WANG H, ZHANG Y F, et al. Accelerated catalytic oxidation of dissolved manganese(II) by chlorine in the presence of in situ-growing 3D manganese(III)/(IV) oxide nanosheet assembly in zeolite filter[J]. Water Research, 2021, 201: 117223. doi: 10.1016/j.watres.2021.117223 [29] ALVER E, METIN A U. Anionic dye removal from aqueous solutions using modified zeolite: Adsorption kinetics and isotherm studies[J]. Chemical Engineering Journal, 2012, 200(202): 59-67. [30] BIESINGER M C, PAYNE B P, GROSVENOR B P, et al. Resolving surface chemical states in XPS analysis of-first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni[J]. Applied Surface Science, 2011, 257(7): 2717-2730. doi: 10.1016/j.apsusc.2010.10.051 [31] ILTON E, POST J, HEANEY P, et al. XPS determination of Mn oxidation states in Mn (hydr)oxides[J]. Applied Surface Science, 2016, 366: 475-485. doi: 10.1016/j.apsusc.2015.12.159 [32] 张云飞. 原位锰改性沸石氯催化氧化过滤去除水中溶解锰效能[D]. 哈尔滨: 哈尔滨工业大学, 2019. [33] YANG H Y, TANG X B, LUO X S, et al. Oxidants-assisted sand filter to enhance the simultaneous removals of manganese, iron and ammonia from groundwater: Formation of active MnOx and involved mechanisms[J]. Journal of Hazardous Materials, 2021, 415: 125707. doi: 10.1016/j.jhazmat.2021.125707 [34] YANG H Y, YAN Z S, DU X, et al. Removal of manganese from groundwater in the ripened sand filtration: Biological oxidation versus chemical auto-catalytic oxidation[J]. Chemical Engineering Journal, 2020, 382: 123033. doi: 10.1016/j.cej.2019.123033 [35] LIN L, LEI Z F, WANG L, et al. Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites[J]. Separation and Purification Technology, 2013, 103: 15-20. doi: 10.1016/j.seppur.2012.10.005 -



 下载:
下载: