-
磷过量是水华爆发重要的诱因之一[1]。近年来,随着排污管控和面源污染治理,水体外源磷已得到逐步控制,内源成为水体中磷的主要来源,而底泥中磷向上覆水的释放是内源磷的重要成因之一[2-3]。
原位钝化技术具有操作简便、见效快等优势,可有效控制底泥中磷的释放,近年来得到了广泛的推广应用[4-5]。镧系钝化剂是目前使用最为广泛的底泥磷钝化剂,其效果远优于传统的铝盐和钙盐[6-8]。笔者在先前研究中,合成了具有强控磷能力的新型钝化剂——镧沸石,与市售锁磷剂Phoslock®相比具有一定的优势[9-12]。
钝化剂使用过程中,泥水界面有机质对其效果有显著影响[13-14]。从比重分组的角度,有机质可以分为轻组和重组有机质[15-18]。其中,轻组有机质由动植物残体、微生物等组成,具有周转时间短(最短为数周)和碳氮比高等特点,其含量呈现明显的季节变化[19-22]。将土壤或沉积物分散在密度大于轻组有机质的重液(如NaI、Na6(H2W12O40)溶液中,轻组有机质就会悬浮在溶液中,实现其与土壤或沉积物中其他组分的分离[23]。轻组有机质虽然占比不足有机质总量的10%,但其对底泥的磷释放行为有着重要影响[24]。有研究表明,去除轻组有机质后太湖贡湖湾底泥磷释放量增加了12.8倍,吸附磷的速率减少到原先的40%[25];底泥对磷的饱和吸附量也有了明显的降低[26]。但是,底泥轻组有机质对于钝化剂控磷效果的影响,目前仍然不清楚。
本研究以黑臭河道底泥为对象,考察了镧沸石对原底泥(raw sediment,简称R-S)和去除轻组有机质底泥(light fraction organics removal sediment,简称LFOR-S)磷吸附性能的影响,分析了镧沸石对R-S和LFOR-S磷释放的控制效果及其对温度变化的响应,并通过XPS和磷分级揭示了相关机理。研究结果将为原位钝化技术在河湖磷污染控制中的应用提供参考。
-
镧沸石的制备按照文献[9]进行。具体来说,25 g粉煤灰与150 mL NaOH溶液(浓度2 mol·L−1)充分混合,在水热反应釜中以95 ℃反应24 h。反应结束后,向得到的固液混合物中逐滴加入浓度为0.67 mol·L−1的LaCl3溶液150 mL,同时保持搅拌。之后离心分离,弃去上清液。用纯水和乙醇清洗固体2~3次,冻干研磨过80目筛备用。
-
底泥样品于2022年4月在新河西安鄠邑区黑臭段(34°13′34.95″N、108°39′57.672″E)使用重力取泥器采集。采集后尽快冻干,过80目筛获得原始底泥(R-S)。去除轻组有机质的方法参考文献[22],具体方法为:向5 g底泥中加入20 mL比重为1.7的NaI溶液,超声状态下混合10 min,之后在4 000 r·min−1条件下离心10 min,弃去上清液。将上述步骤重复2次后,使用去离子水将固体残渣清洗3次,冻干获得去除轻组有机质底泥(LFOR-S)。使用低温外热重铬酸钾氧化法测定R-S和LFOR-S的有机质含量,使用过硫酸钾-硫酸消解法测定R-S和LFOR-S的总磷含量,使用比表面与孔隙度分析仪(北京金埃谱公司F-sorb3400型)测定BET比表面积。
-
分别称取0.4 g R-S或LFOR-S于50 mL离心管中,添加质量分数为2%(0.008 0 g)或4%(0.016 0 g)的镧沸石。加入40 mL质量浓度为0~100 mg·L−1的KH2PO4溶液(包含0.01 M的NaCl离子强度)。在25 ℃下恒温振荡(180 r·min−1)24 h,取出后在3 000 r·min−1下离心15 min,使用钼锑抗分光光度法分析上清液正磷酸盐浓度,根据式(1)计算底泥对磷的吸附量;使用Langmuir(式(2))和Freundlich模型(式(3))拟合吸附等温线[27]。
式中:Qe为平衡吸附量,mg·kg−1;C0和Ce为反应前后的磷质量浓度,mg·L−1;V为液体体积,L;m为底泥或底泥/镧沸石混合物的重量,g。
式中:KL为Langmuir常数;Qmax为根据Langmuir模型计算出的最大吸附量,mg·kg−1;KF为Freundlich常数;其余符号意义同前。
为明确镧沸石对底泥吸附量增加的贡献,通过差减,得到投加镧沸石前后底泥磷吸附量的增加量,再除以投加的镧沸石质量,再根据式(4)计算镧沸石的吸附量。
式中:QL为镧沸石的吸附量,mg·g−1;QS+L为投加镧沸石后底泥的吸附量,mg·kg−1;QS为底泥自身的磷吸附量,mg·kg−1;m为底泥质量,g;mL为镧沸石投加量,g;1 000为单位转换系数。
-
分别称取0.4 g R-S或LFOR-S于50 mL离心管中,添加质量分数2%(0.008 0 g)或4%(0.016 0 g)的镧沸石。加入40 mL浓度为0.01 mmol·L−1的NaCl溶液,恒温振荡(180 r·min−1)一定时间。反应结束后,在3 000 r·min−1下离心15 min,测定上清液的正磷酸盐浓度。在磷释放动力学实验中,反应时间为0.25、0.5、1.5、3、5、8、12、24 h,反应温度为25 oC。在不同温度下的磷释放实验中,反应时间为24 h,反应温度为5、10、15和30 ℃。
通过实验后水中磷的浓度计算磷的释放量(式(5))。通过特定温度下,底泥在投加镧沸石前后磷释放量的变化,来计算镧沸石在该温度下的磷释放控制率(式(6))。
式中:R为磷释放量,mg·kg−1;C为释放实验结束后水中磷的质量浓度,mg·L−1。
式中:K为特定温度下的磷释放控制率,%;RS+L和RS分别为底泥在投加镧沸石后和投加前的磷释放量,mg·kg−1。
-
按照1.4中方法,不添加镧沸石的情况下获得R-S或LFOR-S磷释放上清液,反应时间为24 h。上述两种上清液中正磷酸盐质量浓度分别为1.79和2.89 mg·L−1。将2种上清液分别与0.008 0 g镧沸石混合,恒温振荡(180 r·min−1)24 h,离心分离得到反应后的镧沸石,45 oC下烘干。使用日本Ulvac-Phi公司PHI Quantera SXM 扫描成像X射线光电子能谱仪测定XPS图谱,按照C1s峰结合能为248.8 eV进行校正,并使用casaXPS进行处理。
-
分别称取1 g R-S或LFOR-S于50 mL离心管中,添加质量分数2%(0.020 0 g)或4%(0.040 0 g)的镧沸石。添加40 mL去离子水,在25 ℃下恒温振荡(180 r·min−1)24 h,取出后在3 000 r·min−1的条件下离心15 min,分析剩余底泥的磷形态,同时测定水相中磷的浓度。磷形态主要采用连续提取法测定[28],将底泥中的磷分为6种形态,其中NH4Cl-P和BD-P形态具有较强的释放潜力,NaOH-P和Org-P形态具有中等释放潜力,HCl-P和Res-P形态较为稳定一般不会释放。通过磷形态转化实验前后特定形态磷含量的变化计算转化率(式(7))。
式中:T为某一形态磷在转化实验前后的转化率,%;Qf和Qi分别为转化实验结束后和开始前该形态磷的含量,mg·kg−1。
-
去除轻组有机质前后底泥理化性质的变化如表1所示。可以看出,去除轻组有机质后,底泥中有机质含量下降了0.18%。此处下降的有机质属于轻组有机质。换算后发现,黑臭河道底泥中轻组有机质占总有机质的比例约为7%,远高于在西辽河[29]、西湖[15]、洱海[24]、太湖和鄱阳湖[19]等自然水体底泥中得到结果,而低于玄武湖、月湖[19]等城市内湖底泥中的水平。这也印证了前人有关重污染城市河湖底泥中轻组有机质含量较高的观点[19]。去除轻组有机质后,底泥中总磷从2 272.11下降到2 116.22 mg·kg−1,下降的部分应随轻组有机质的去除离开。底泥的比表面积在去除轻组有机质前后没有发生明显的变化。底泥的pH在去除轻组有机质后有了明显了上升。根据磷分级结果(见2.6),R-S和LFOR-S中BD-P和NaOH-P含量之和占到总磷的一半左右。研究表明,这些与底泥中铁、铝氧化物结合的磷,在碱性条件下会被水中OH−基团置换从而释放出来[26, 30-31]。因此,LFOR-S对磷的“束缚”能力相比R-S会出现明显的下降,进而产生较高的磷释放量。
-
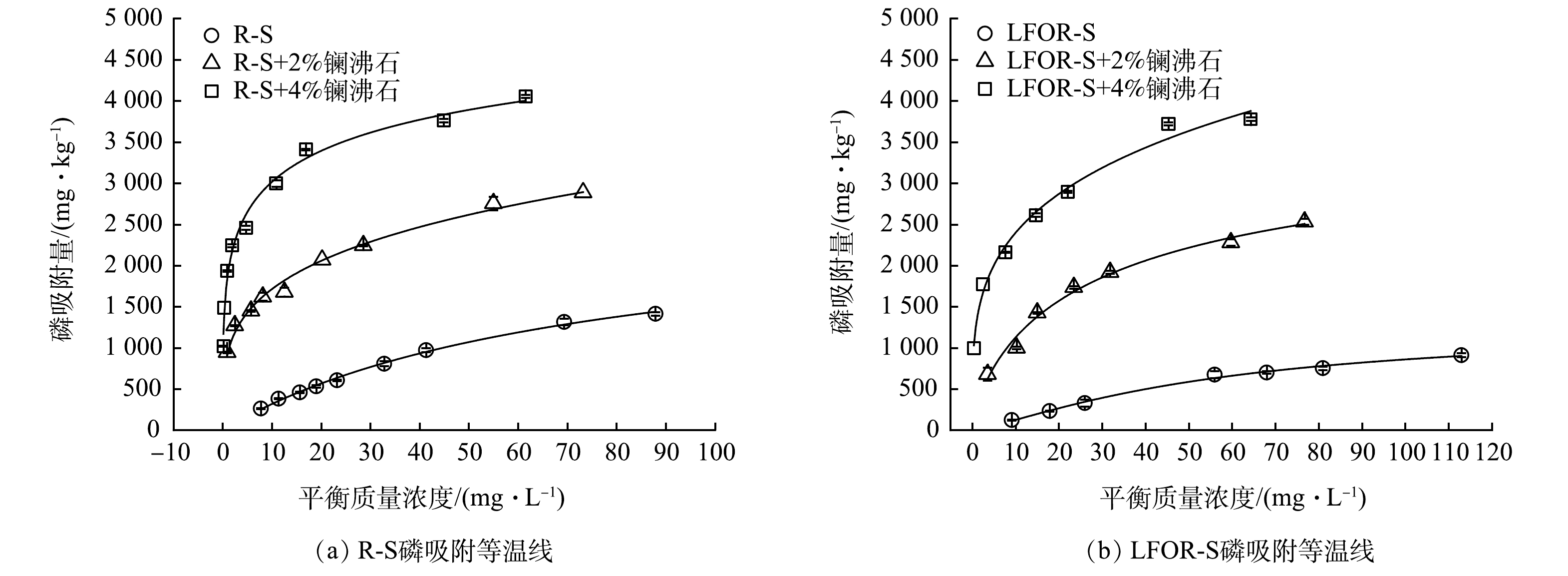
为了解投加镧沸石对R-S和LFOR-S磷吸附能力的影响,测定了R-S和LFOR-S在投加镧沸石前后的吸附等温线,并使用Langmuir和Freundlich模型对其进行了拟合,结果如图1和表2所示。结果表明,R-S和LFOR-S在投加镧沸石前后的吸附等温线均可以被上述两个模型较好地拟合。去除轻组有机质后,底泥的最大磷吸附量从2 568下降到2 071 mg·kg−1。Freundlich模型拟合的结果表明,R-S和LFOR-S的Freundlich常数分别为68.13和13.57,同样说明去除轻组有机质后底泥对磷的吸附能力有所下降[32]。这与WANG等(2011)对太湖贡湖湾、梅梁湾和武汉月湖底泥进行类似研究的结果相近[26]。底泥去除轻组有机质后磷吸附量的下降主要是由于以下2个原因:1)磷酸盐可与底泥中铁铝氧化物表面的OH基团发生配体交换被吸附,同时释放出OH−,pH升高时水中OH−浓度增加会抑制这一反应;2)有机质对底泥中铁铝矿物表面吸附的磷起到一定的“保护”作用,轻组有机质的去除使得部分磷直接暴露于固液界面,其释放趋势因而增强[26]。
投加镧沸石后,无论是R-S还是LFOR-S的磷吸附量都有了显著的提升。对于R-S,投加质量百分数为2%和4%的镧沸石后,磷最大吸附量上升到3 055 mg·kg−1和4 054 mg·kg−1;对于LFOR-S,在相同的条件下,磷的最大吸附量则上升到3 004 mg·kg−1和4 014 mg·kg−1。镧沸石中的有效组分为水合氧化镧,其中La3+可以通过与PO43-形成沉淀的方式去除水中的磷,且沉淀产物LaPO4不会受到环境中pH和氧化还原电位变化的影响[9, 11]。在包括太湖、滇池、洱海等多个湖泊底泥中,镧沸石已经显示出较好的磷钝化能力[10-12]。
为明确镧沸石对底泥磷吸附量提升的贡献,根据式(4)计算了镧沸石在R-S和LFOR-S中的实际磷吸附量。结果表明,投加2%和4%镧沸石后,镧沸石在R-S中的实际磷吸附量分别为27.41 mg·g−1和41.20 mg·g−1,在LFOR-S中的实际磷吸附量为49.65 mg·g−1和52.59 mg·g−1。LFOR-S中镧沸石的实际吸附量大于R-S,说明镧沸石的吸附能力在LFOR-S中可得到更好地发挥。
-
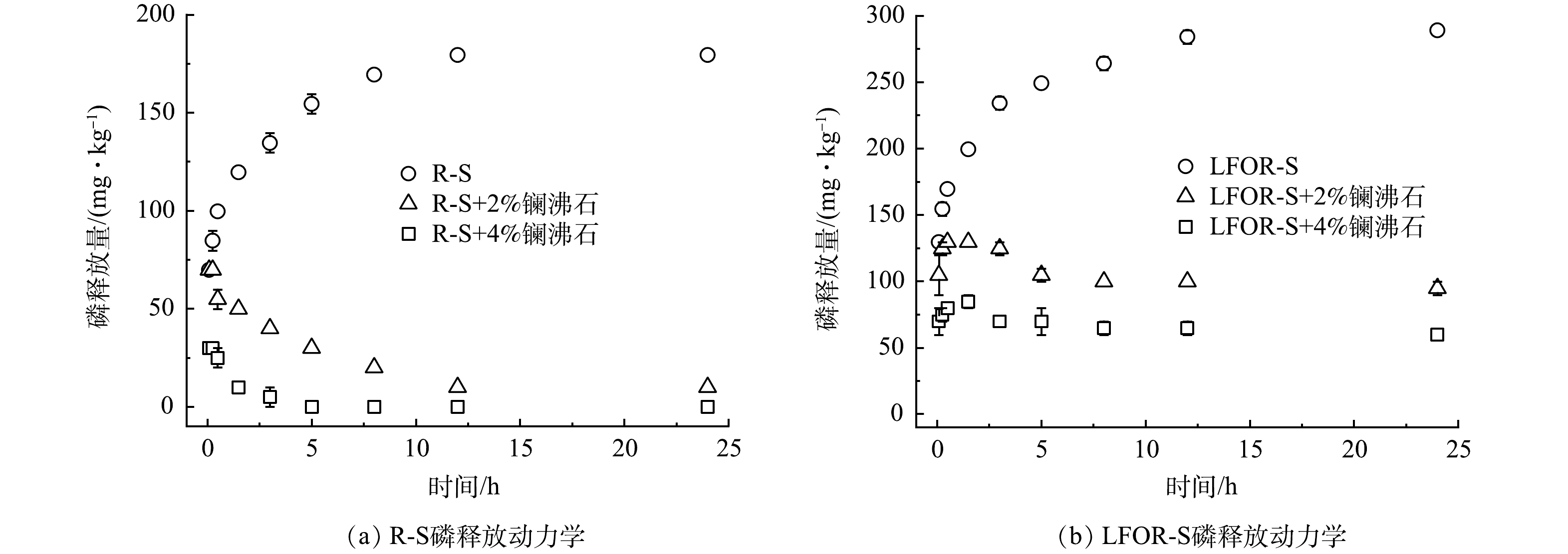
R-S和LFOR-S的磷释放动力学过程如图2所示。可以看出,两种底泥磷释放量逐随时间逐渐上升,在12 h后逐渐平衡。R-S的磷释放量179.40 mg·kg−1,相比前人在我国浅水湖泊中得到的结果[19],本研究中底泥的磷释放量明显更高,这可能是与本研究采取的底泥中磷的本底值较高有关。去除轻组有机质后,底泥的磷释放量上升到289.03 mg·kg−1,相比R-S上升了1.61倍,这与易文利等在武汉月湖、太湖五里湖和贡湖开展的研究结果相近[25]。去除轻组有机质后,底泥磷释放量的增加主要与pH的升高有关,原因详见本文2.1节。
投加镧沸石可以有效控制底泥中磷的释放。如图2(a)所示,投加2%的镧沸石可以将磷释放量降低到9.97 mg·kg−1,而镧沸石投加量在4%时则可以完全控制R-S的磷释放。去除轻组有机质后,镧沸石对磷释放的控制效果明显变差,投加2%和4%的镧沸石分别仅可将释放量降低到94.68 mg·kg−1和59.80 mg·kg−1(图2(b))。如前所述,镧沸石在LFOR-S中有着更高的吸附量,因此LFOR-S磷释放量过高应该是控磷效果较差的原因。要实现对LFOR-S磷释放的有效控制,需增大镧沸石投量[33]。
-
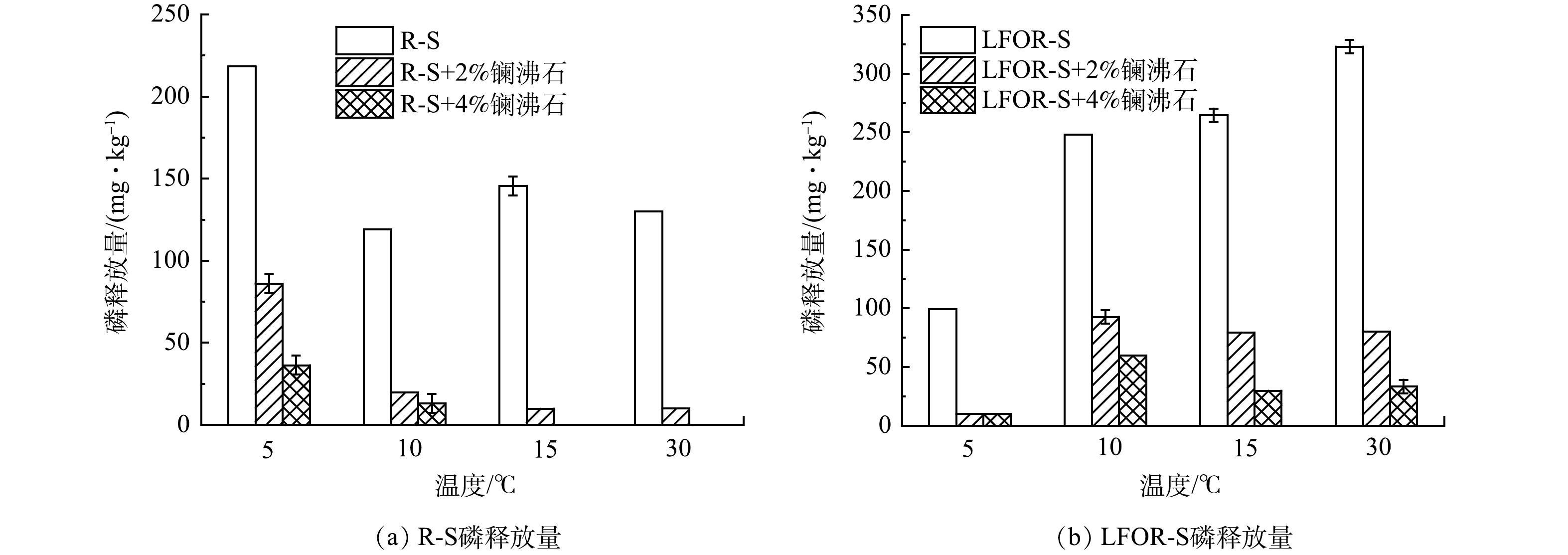
不同温度下R-S和LFOR-S的磷释放情况如图3所示。对于R-S来说,温度在5 oC时磷释放量较大(218.24 mg·kg−1)。当温度上升到10 oC以上时,释放量降至119.04~145.49 mg·kg−1,并保持相对稳定。对LFOR-S来说,当温度由5 oC上升到30 oC的过程中,磷释放量持续上升。具体来说,5 oC时磷释放量为99.20 mg·kg−1,之后磷释放量稳步上升,到30 oC时达到322.96 mg·kg−1。底泥中磷的释放是底泥对磷的吸附和释放2个过程“净”的结果,即二者的差值[5]。从吸附来说,高温条件有利于底泥对磷的吸附[34];从释放来说,温度升高微生物活性增强会形成局部厌氧环境并生成CO2,导致Fe/Mn结合磷和Ca结合磷的释放[35, 36]。推测环境温度上升到10 oC以上时,R-S由于吸附作用增强磷释放量明显降低;而对LFOR-S,温度升高引起释放量的增加占主导作用。
投加镧沸石后,R-S和LFOR-S的磷释放均得到不同程度的控制。通过式(6)计算得到磷释放控制率(表3)。对R-S来说,温度从5 oC上升到30 oC,投加质量分数2%的镧沸石时,磷释放控制率从60.61%逐步上升到92.31%,这主要是由于镧沸石的吸附属于吸热反应,其性能会随着温度的升高而增加[37]。对LFOR-S来说,随温度升高磷释放控制率呈现先降低后增高的趋势,具体来说当镧沸石投加量为2%、温度由5 oC上升到10 oC时磷释放控制率由90%下降到62.67%,而温度继续升高控制率则逐步回升到75.26%。如前所述,随着温度升高,镧沸石的吸附性能会增加,但LFOR-S的磷释放量也会随之升高。因此,磷释放控制率随温度的变化,取决于以上2个因素中影响更大者。推测在5~10 oC,底泥磷释放量的增加影响更大,而在10 oC以上镧沸石的吸附性能的提升则占主导作用。
-
由于底泥成分复杂,很难直接使用光谱学手段表征镧沸石控制底泥磷释放的机理[38]。因此,使用R-S和LFOR-S释放24 h后得到的上清液与镧沸石反应,测定了反应前后镧沸石的XPS谱图,探讨实际环境中的控磷机理。
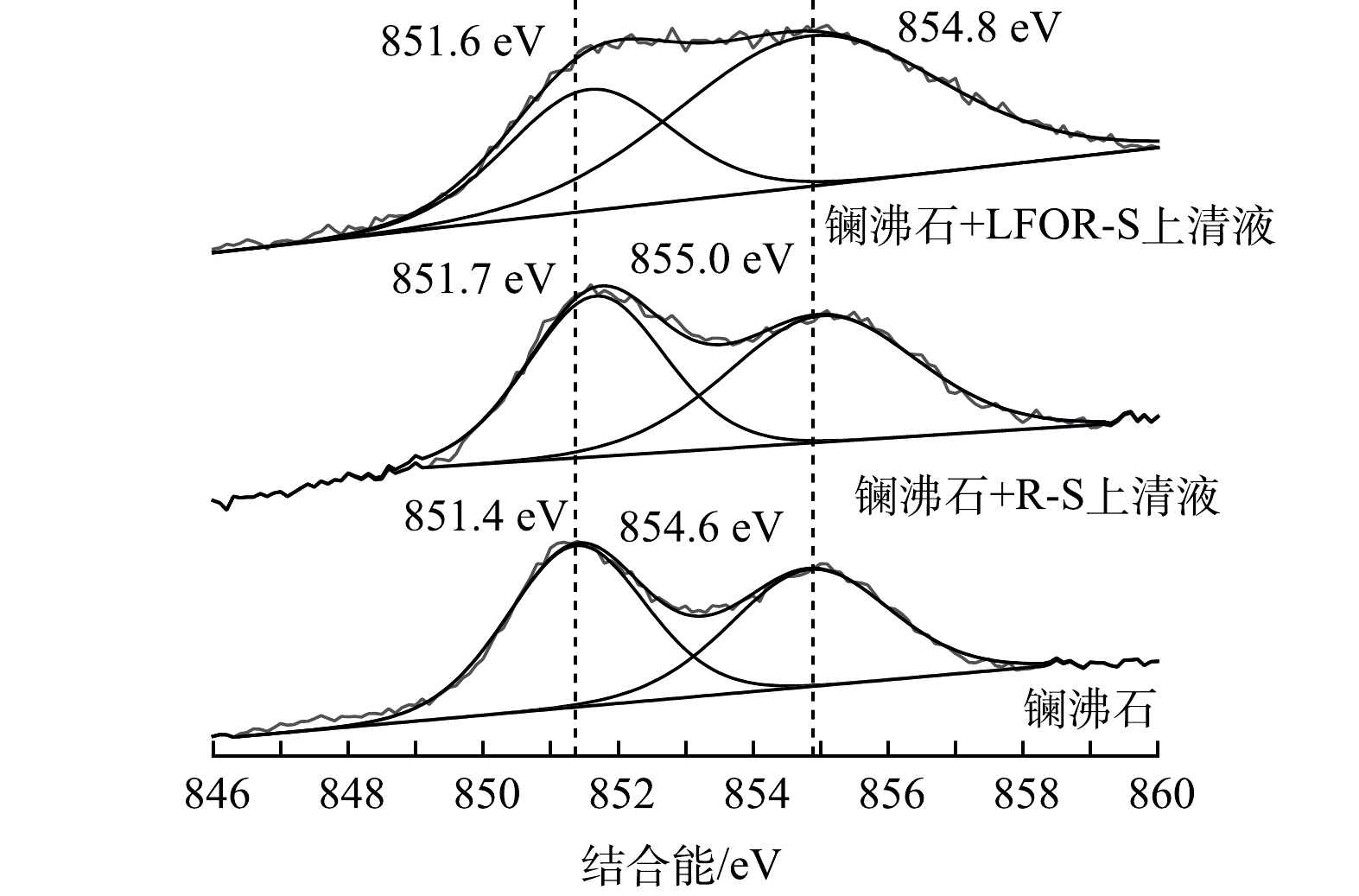
通过XPS宽谱获得的3种样品表面元素组成如表4所示。可以看出,镧沸石和R-S/LFOR-S释放上清液反应后,表面均有磷元素检出,说明镧沸石可有效吸附2种上清液中的磷。镧沸石反应后表面镧、氧原子摩尔比明显降低,侧面证实了具有较高氧原子含量的PO43-在镧沸石表面吸附[39]。镧沸石与R-S和LFOR-S释放上清液反应前后的La3d轨道XPS精细谱(图4)表明,镧沸石在反应后,La3d轨道结合能均有了明显的上升。有研究[40-42]表明,镧沸石中水合氧化镧组分表面的羟基,可以与PO43-之间发生配体交换形成内圈络合物。发生配体交换反应后,La原子的化学环境发生了明显的变化,其轨道结合能也会随之上升。因此,镧沸石与2种上清液中磷的作用机制均应为配体交换[40]。
-
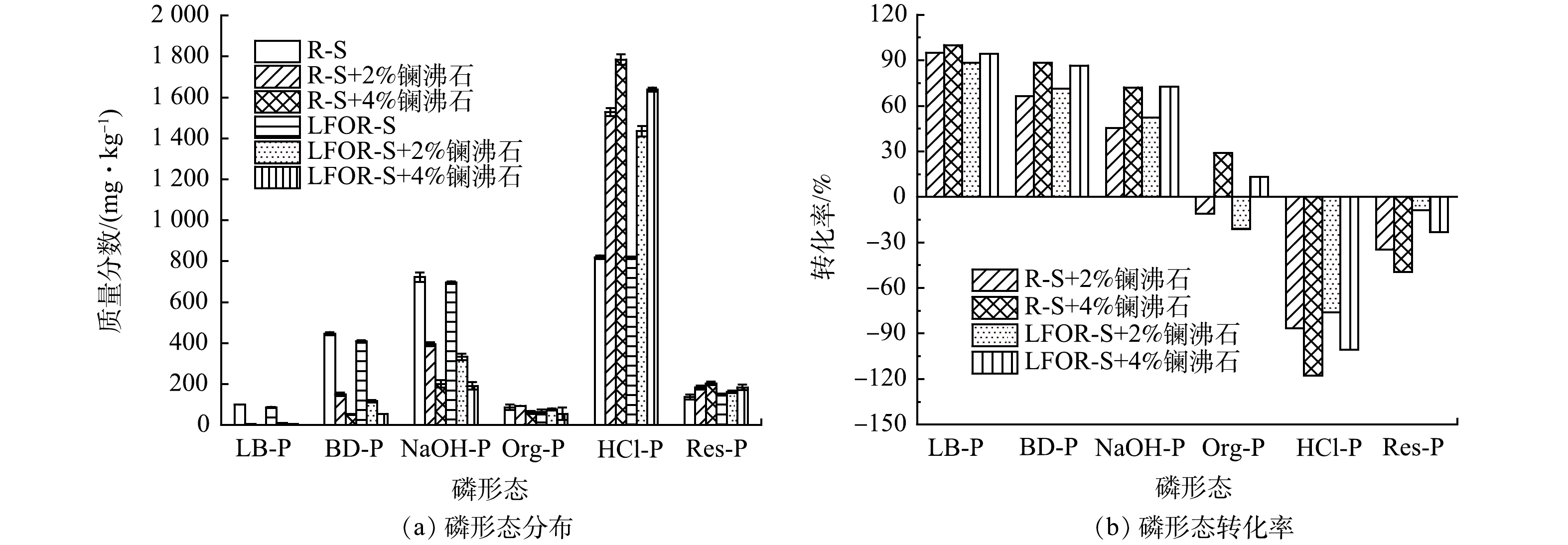
投加镧沸石前后R-S和LFOR-S中磷形态分级情况如图5(a)所示。可以看出,无论对于R-S还是LFOR-S,投加镧沸石后非稳定态磷(LB-P、BD-P、NaOH-P、Org-P)的含量均有了明显的下降,而稳定形态磷(HCl-P和Res-P)的含量则有了明显的上升,说明镧沸石将底泥中非稳定态磷转化为了稳定态。为进一步比较镧沸石在R-S和LFOR-S中磷形态转化能力,根据式(7)计算了每一种磷形态的转化率[43],结果如图5(b)所示。从非稳定态磷来看,投加镧沸石后R-S和LFOR-S中的转化率基本上为正值且大小相近,说明两种底泥中非稳定态磷含量的下降幅度接近。从稳定形态磷来看,投加镧沸石后转化率均为负值,且LFOR-S中转化率的绝对值小于R-S。具体来说,投加2%的镧沸石时,R-S中HCl-P的转化率为-86.58%,LFOR-S中HCl-P的转化率仅为-75.92%;R-S中Res-P的转化率为-33.73%。LFOR-S中Res-P的转化率仅为-8.89%。这说明投加镧沸石后,LFOR-S中稳定形态磷含量的增幅小于R-S。衡算发现,投加2%镧沸石后R-S中非稳定态磷的含量降低了710.85 mg·kg−1,而稳定态磷的含量增加了755.48 mg·kg−1;在相同处理下,LFOR-S中非稳定态磷的含量降低了717.51 mg·kg−1,而稳定态磷的含量仅增加了632.52 mg·kg−1。也就是说,LFOR-S中减少的非稳定形态磷并未完全转化为稳定形态。
为明确LFOR-S中非稳定态磷的去向,测定了磷形态转化实验结束后水相中的磷含量。结果表明,实验后R-S中进入上清液的磷为79.91 mg·kg−1,投加2%镧沸石和4%镧沸石可以将水相中的磷分别降至22.64 mg·kg−1和25.30 mg·kg−1。而LFOR-S中进入水相的磷达到312.98 mg·kg−1,投加2%镧沸石和4%镧沸石仅能将磷降至106.55 mg·kg−1和74.58 mg·kg−1。因此,可以推断在磷形态转化过程中,LFOR-S中部分非稳定态磷直接进入了水相,由于其浓度较高无法被镧沸石有效控制,最终导致磷释放控制率有所下降。
-
本文的研究结果对于实际的原位钝化控磷工程具有一定的意义。根据谢锦升等[44]的研究,轻组有机质组分具有明显的季节变化特征,其含量在冬春季含量高于夏季58%~122%[44]。LI等[21]发现,在6~23 oC内,轻组有机质含量与环境温度呈反比关系。因此,春夏季气温逐渐升高时,底泥中轻组有机质含量会逐渐下降,磷的释放量则随之上升。在此条件下,镧沸石控磷效果相比升温前将有所下降。事实上,近期已有学者观测到夏季钝化控磷效果较差的现象[45]。根据2.3中的结果,对于LFOR-S,增大镧沸石投加量可以有效提高其磷释放控制率。因此,在底泥轻组有机质含量下降导致镧沸石控磷效果变差时,可以通过增大投量来保障钝化效果。总之,在使用镧沸石控制河湖内源磷的实践中,特别是在轻组有机质含量较高的重污染城镇河湖修复工程中,温度上升对钝化效果的抑制作用需要引起重视,并及时补充投加钝化剂。
-
1)投加镧沸石可以有效提高R-S和LFOR-S的磷吸附能力,且镧沸石在LFOR-S可以发挥出较好的固磷能力。LFOR-S的磷释放量为R-S的1.6倍,在相同投加量下控磷效果差于R-S。
2)无论在R-S还是LFOR-S中,镧沸石的控磷机制均为生成内圈络合物。镧沸石可以驱动底泥中非稳定形态的磷转化为稳定形态,但在LFOR-S中不能实现完全转化,这可能是镧沸石对LFOR-S磷释放控制效果不佳的原因。
3)在实际原位钝化工程中,如存在轻组有机质含量下降的情况,应考虑适当增加镧沸石的投量以实现较好的控磷效果。
底泥轻组有机质对镧沸石控磷效果的影响
Effect of light fraction organic matter in sediment on its phosphate releasing controlling by lanthanum modified zeolite
-
摘要: 底泥中轻组有机质对其中磷的吸附-释放行为影响显著。镧沸石可有效地控制底泥磷释放,但轻组有机质对控磷效果的影响尚不清楚。因此,考察了镧沸石对原底泥(raw sediment,简称R-S)和去除轻组有机质底泥(light fraction organic removal sediment,简称LFOR-S)中磷释放的控制效果及机理。R-S和LFOR-S的最大磷吸附量分别为2 568 mg·kg−1和2 071 mg·kg−1,投加镧沸石可将其提高到4 054 mg·kg−1和4 014 mg·kg−1。LFOR-S在24 h内磷释放量为R-S的1.61倍。温度从5 oC上升到30 oC时,镧沸石对R-S的磷释放控制率逐渐升高,而对LFOR-S的磷释放控制率由90%下降到76%后又回到原水平。XPS表征发现,配体交换是镧沸石控制2种底泥磷释放的主要机制。镧沸石可将底泥中非稳定态磷转化为稳定态,但LFOR-S中减少的非稳定磷的并未完全转化为稳定态,而是进入了水相中使其磷释放量高于R-S,导致控磷效果变差。在原位钝化实际工程中,温度升高引起底泥轻组有机质含量下降时,需提高镧沸石投量以获得稳定的控磷效果。Abstract: Light fraction organic matter in sediment has a significant impact on its phosphorus releasing behavior. Lanthanum Modified Zeolite (LMZ) could effectively control phosphorus release from sediment, but the effect of light fraction organic matter in sediment on its performance was still unclear. Therefore, control of phosphate release from raw sediment (R-S) and light fraction organic removal sediment (LFOR-S) by LMZ were investigated in the present study. The results showed that the maximum phosphate adsorption capacities of R-S and LFOR-S were 2568 and 2071 mg·kg−1, respectively, and they could increase to 4054 and 4014 mg·kg−1 after dosing LMZ, respectively. Phosphate release amount of LFOR-S was 1.61 times of that of R-S within 24 hours, leading to a lower controlling efficiency of LMZ in LFOR-S. Controlling performance of LMZ on phosphate release from R-S gradually became better when the temperature rose from 5 oC to 30 oC, while that of LFOR-S decreased from 90% to 76% and then returned to the original level under the same condition. XPS characterization showed that ligand exchange was the main mechanism of phosphate releasing inhibition by LMZ. LMZ could convert unstable phosphorus in R-S to stable forms, while a completely conversion of decreased liable phosphorus did not occur in LFOR-S, some unstable phosphorus entered water phase which led to a higher release than RS and worse phosphorus release control effect of LMZ. In engineering practice, it is necessary to increase the dosage of LMZ to maintain a stable controlling performance when the amount of light fraction organic matter in the sediment decreased under elevated temperature.
-
Key words:
- lanthanum modified zeolite /
- sediment /
- light fraction organic matter /
- phosphate
-

-
表 1 去除轻组有机质前后底泥的基本理化性质
Table 1. Basic physical and chemical parameters of sediment before and after light fraction organic matter removal
底泥 有机质
含量/%总磷/
(mg·kg−1)比表面积/
(m2·g−1)pH
(固液比1:5)R-S 2.54 2 272.11 8.12 7.35 LFOR-S 2.36 2 116.22 8.15 10.21 表 2 投加镧沸石前后R-S和LFOR-S对磷的吸附等温线的拟合结果
Table 2. Fitting results of adsorption isotherms of R-S and LFOR-S before and after LMZ dosing
实验组合 Langmuir模型 Freundlich模型 Qmax/(mg·kg−1) KL/(L·mg−1) R2 KF/(mg·kg−1)·(mg·L−1)−1/n 1/n R2 R-S 2 568 0.014 0.984 68.13 0.696 0.992 R-S+2% 镧沸石 3 055 0.152 0.986 965.16 0.252 0.988 R-S+4% 镧沸石 4 054 0.532 0.996 1 805.09 0.209 0.966 LFOR-S 2 071 0.007 0.938 13.67 0.998 0.990 LFOR-S+2% 镧沸石 3 004 0.059 0.989 400.22 0.437 0.974 LFOR-S+4% 镧沸石 4 014 0.201 0.987 1 307.37 0.262 0.992 表 3 不同温度下镧沸石对R-S和LFOR-S磷释放控制率
Table 3. Phosphate releasing control efficiency of LMZ for R-S and LFOR-S at different temperatures %
温度/oC R-S LFOR-S 2%镧沸石 4%镧沸石 2%镧沸石 4%镧沸石 5 60.61 83.33 90.00 90.00 10 83.33 88.89 62.67 76.00 15 93.18 100.00 70.00 88.75 30 92.31 100.00 75.26 89.69 表 4 不同反应条件下镧沸石表面元素组成及摩尔比值
Table 4. Surface element composition and molar ratios of LMZ under different reaction conditions.
样品 元素原子百分比/% La:O La Si C O P 镧沸石 1.240 47.130 21.300 26.890 — 0.046 镧沸石+R-S释放上清液 1.120 42.920 24.130 28.460 0.870 0.039 镧沸石+LFOR-S
释放上清液0.830 36.580 29.530 28.730 0.650 0.029 -
[1] XIAO M, BURFORD M A, WOOD S A, et al. Schindler's legacy: From eutrophic lakes to the phosphorus utilization strategies of cyanobacteria[J]. FEMS Microbiology Reviews, 2022, 46(6): 1-24. [2] YIN H, ZHANG M, YIN P, et al. Characterization of internal phosphorus loading in the sediment of a large eutrophic lake (Lake Taihu, China)[J]. Water Research, 2022, 225: 119-125. [3] YAN Q, CHENG T, SONG J, et al. Internal nutrient loading is a potential source of eutrophication in Shenzhen Bay, China[J]. Ecological Indicators, 2021, 127: 107736. doi: 10.1016/j.ecolind.2021.107736 [4] 杨海全, 陈敬安, 刘文, 等. 草海底泥原位钝化工程示范及其生态环境效应[J]. 环境工程学报, 2017, 11(7): 4437-4444. [5] XUE W, LU S-Y. Effects of inactivation agents and temperature on phosphorus release from sediment in Dianchi Lake, China[J]. Environmental Earth Sciences, 2015, 74(5): 3857-3865. doi: 10.1007/s12665-014-3910-5 [6] 马鑫雨, 杨盼, 张曼, 等. 湖泊沉积物磷钝化材料的研究进展[J]. 湖泊科学, 2022, 34(1): 1-17. [7] MEIS S, SPEARS B M, MABERLY S C, et al. Sediment amendment with Phoslock® in Clatto Reservoir (Dundee, UK): Investigating changes in sediment elemental composition and phosphorus fractionation[J]. Journal of Environmental Management, 2012, 93(1): 185-193. doi: 10.1016/j.jenvman.2011.09.015 [8] FUNES A, ÁLVAREZ-MANZANEDA I, ARCO A D, et al. Evaluating the effect of CFH-12® and Phoslock® on phosphorus dynamics during anoxia and resuspension in shallow eutrophic lakes[J]. Environmental Pollution, 2021, 269: 116093. doi: 10.1016/j.envpol.2020.116093 [9] XIE J, WANG Z, FANG D, et al. Green synthesis of a novel hybrid sorbent of zeolite/lanthanum hydroxide and its application in the removal and recovery of phosphate from water[J]. Journal of Colloid and Interface Science, 2014, 423: 13-19. doi: 10.1016/j.jcis.2014.02.020 [10] WANG Z, FAN Y, LI Y, et al. Synthesis of zeolite/hydrous lanthanum oxide composite from coal fly ash for efficient phosphate removal from lake water[J]. Microporous and Mesoporous Materials, 2016, 222: 226-234. doi: 10.1016/j.micromeso.2015.10.028 [11] WANG Z, LU S, WU D, et al. Control of internal phosphorus loading in eutrophic lakes using lanthanum-modified zeolite[J]. Chemical Engineering Journal, 2017, 327: 505-513. doi: 10.1016/j.cej.2017.06.111 [12] 王哲, 朱俊, 李雯, 等. 镧沸石对磷和重金属的吸附与底泥钝化性能[J]. 环境科学, 2022, 43(11): 5106-5114. [13] LüRLING M, WAAJEN G, VAN OOSTERHOUT F. Humic substances interfere with phosphate removal by lanthanum modified clay in controlling eutrophication[J]. Water Research, 2014, 54: 78-88. doi: 10.1016/j.watres.2014.01.059 [14] DITHMER L, NIELSEN U G, LUNDBERG D, et al. Influence of dissolved organic carbon on the efficiency of P sequestration by a lanthanum modified clay[J]. Water Research, 2016, 97: 39-46. doi: 10.1016/j.watres.2015.07.003 [15] 李静, 朱广伟, 朱梦圆, 等. 杭州西湖“香灰土”沉积物轻、重有机质组成特征及其环境意义[J]. 环境科学, 2015, 36(6): 2038-2045. [16] 赵萱, 成杰民, 鲁成秀. 不同生态类型富营养化湖泊沉积物中有机质赋存形态[J]. 环境化学, 2012, 31(3): 302-307. [17] NDZELU B S, DOU S, ZHANG X, et al. Molecular composition and structure of organic matter in density fractions of soils amended with corn straw for five years[J/OL]. Pedosphere, 1-11,https://doi.org/10.1016/j.pedsph.2022.06.057, 2022-06-07. [18] 黄桥明, 吕茂奎, 聂阳意, 等. 武夷山不同海拔森林表层土壤轻组有机质特征[J]. 生态学报, 2020, 40(17): 6215-6222. [19] 易文利, 王圣瑞, 金相灿, 等. 长江中下游浅水湖沉积物中有机质及其组分的赋存特征[J]. 西北农林科技大学学报(自然科学版), 2008, 36(5): 141-148. [20] 赵海超, 王圣瑞, 焦立新, 等. 洱海沉积物有机质及其组分空间分布特征[J]. 环境科学研究, 2013, 26(3): 243-249. [21] LI X, YANG T, HICKS L C, et al. Latitudinal patterns of light and heavy organic matter fractions in arid and semi-arid soils[J]. CATENA, 2022, 215: 106293. doi: 10.1016/j.catena.2022.106293 [22] MAYER S, KöLBL A, VöLKEL J, et al. Organic matter in temperate cultivated floodplain soils: Light fractions highly contribute to subsoil organic carbon[J]. Geoderma, 2019, 337: 679-690. doi: 10.1016/j.geoderma.2018.10.014 [23] 张雨洁. 会稽山香榧林土壤有机碳特征研究[D]. 北京, 中国林业科学研究院, 2019. [24] 赵海超, 王圣瑞, 张莉, 等. 有机质含量及其组分对洱海沉积物磷吸附-释放影响[J]. 环境科学学报, 2014, 34(9): 2346-2354. [25] 易文利, 王圣瑞, 金相灿, 等. 去除轻组有机质对湖泊沉积物磷释放速率的影响研究[J]. 南开大学学报(自然科学版), 2008, 41(4): 1-7. [26] WANG S, YI W, YANG S, et al. Effects of light fraction organic matter removal on phosphate adsorption by lake sediments[J]. Applied Geochemistry, 2011, 26(3): 286-292. doi: 10.1016/j.apgeochem.2010.12.001 [27] 陈星, 陆莹, 黄威. 氧化-载钠改性黑臭河道底泥对磷削减的效果及机制[J]. 环境工程学报, 2017, 11(12): 6282-6289. [28] LIN J, ZHAO Y, ZHANG Z, et al. Immobilization of mobile and bioavailable phosphorus in sediments using lanthanum hydroxide and magnetite/lanthanum hydroxide composite as amendments[J]. Science of the Total Environment, 2019, 687: 232-243. doi: 10.1016/j.scitotenv.2019.06.042 [29] 王而力, 王嗣淇. 西辽河沉积物有机组分对磷的吸附影响[J]. 中国环境科学, 2012, 32(4): 687-694. [30] XU D, YAN P, LIU Z, et al. Spatial distribution of phosphorus forms and the release risk of sediments phosphorus in West Lake, Hangzhou, China[J]. Ecological Engineering, 2021, 173: 106421. doi: 10.1016/j.ecoleng.2021.106421 [31] 黄威, 靳郑海, 凃成琪, 等. 城市河网区河流沉积物磷形态分布特征及释放贡献[J]. 环境科学学报, 2022, 42(12): 171-185. [32] 金彦任, 黄振兴. 吸附与孔径分布[M]. 北京, 国防工业出版社, 2015. [33] VAN OOSTERHOUT F, LURLING M. The effect of phosphorus binding clay (Phoslock (R)) in mitigating cyanobacterial nuisance: A laboratory study on the effects on water quality variables and plankton[J]. Hydrobiologia, 2013, 710(1): 265-277. doi: 10.1007/s10750-012-1206-x [34] 王若凡, 田甜, 刘骅, 等. 黄河兰州段消落带表层沉积物对磷的吸附[J]. 环境工程学报, 2023, 17(1): 343-350. [35] 张义, 刘子森, 张垚磊, 等. 环境因子对杭州西湖沉积物各形态磷释放的影响[J]. 水生生物学报, 2017, 41(6): 1354-1361. [36] KONG M, HAN T, CHEN M, et al. High mobilization of phosphorus in black-odor river sediments with the increase of temperature[J]. Science of the Total Environment, 2021, 775: 145595. [37] XIE J, LAI L, LIN L, et al. Phosphate removal from water by a novel zeolite/lanthanum hydroxide hybrid material prepared from coal fly ash[J]. Journal of Environmental Science and Health, Part A:Toxic/Hazardous Substances and Environmental Engineering, 2015, 50(12): 1298-1305. [38] YIN H, ZHU J. In situ remediation of metal contaminated lake sediment using naturally occurring, calcium-rich clay mineral-based low-cost amendment[J]. Chemical Engineering Journal, 2016, 285: 112-120. doi: 10.1016/j.cej.2015.09.108 [39] MALLET M, BARTHELEMY K, RUBY C, et al. Investigation of phosphate adsorption onto ferrihydrite by X-ray Photoelectron Spectroscopy[J]. Journal of Colloid and Interface Science, 2013, 407: 95-101. doi: 10.1016/j.jcis.2013.06.049 [40] FANG L, LIU R, LI J, et al. Magnetite/Lanthanum hydroxide for phosphate sequestration and recovery from lake and the attenuation effects of sediment particles[J]. Water Research, 2018, 130: 243-254. doi: 10.1016/j.watres.2017.12.008 [41] WU Y, LI X, YANG Q, et al. Hydrated lanthanum oxide-modified diatomite as highly efficient adsorbent for low-concentration phosphate removal from secondary effluents[J]. Journal of Environmental Management, 2019, 231: 370-390. [42] WU B, FANG L, FORTNER J D, et al. Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)(3)/Fe3O4 nanocomposites[J]. Water Research, 2017, 126: 179-188. doi: 10.1016/j.watres.2017.09.034 [43] XU Y, HU H, LIU J, et al. pH dependent phosphorus release from waste activated sludge: contributions of phosphorus speciation[J]. Chemical Engineering Journal, 2015, 267: 260-265. doi: 10.1016/j.cej.2015.01.037 [44] 谢锦升, 杨玉盛, 杨智杰, 等. 退化红壤植被恢复后土壤轻组有机质的季节动态[J]. 应用生态学报, 2008, 19(3): 557-563. [45] YANG C, YANG P, YIN H. In situ control of internal nutrient loading and fluxes in the confluence area of an eutrophic lake with combined P inactivation agents and modified zeolite[J]. Science of the Total Environment, 2021, 775: 145745. doi: 10.1016/j.scitotenv.2021.145745 -



 下载:
下载: