-
染料废水种类繁多、排放量大,具有组成复杂、难生物降解的特点[1-2],在自然环境中将会导致富营养化继而影响生态平衡,同时部分染料的致癌作用会对人体健康造成严重影响[3]。染料废水常用处理方法包括吸附[4]、膜处理[5]、生物降解[6]、混凝[7]、高级氧化处理(AOPs)等。吸附法工艺简单,但材料的最大吸附量及再生能力使其限制性较强;膜处理法虽然可行,但操作过程中需较高压力和反复清洗导致其寿命较短[8];生物降解对环境要求较高,常需与其他工艺联合使用[9]。传统技术均存在运行时间长,运行成本高及二次污染等问题,因此,开发高效深度处理染料废水的方法至关重要。
电催化氧化可通过阴极还原与阳极氧化反应产生氧化活性基团,实现染料污染废水脱色和降解反应,具有操作简单、反应条件温和、适用范围广的特点 [10-12]。除阳极氧化可产生强氧化自由基外,电化学过程中阴极两电子氧还原反应(oxygen reduction reaction, ORR)可原位产生H2O2[13-14](式(1)),阳极产生的自由基和阴极产生的H2O2形成有机物污染物降解[15-16]的协同效应,使废水中有机污染物转变为低毒的中间产物或矿化。但ORR反应受制于阴极的活性位点,活性位点少会导致H2O2产生受限,为了解决这一问题,国内外学者开展大量研究工作。研究发现以炭黑[17]、多壁碳纳米管(MWCNT)[18]、石墨烯[19]等碳材料复合碳质基底制备的气体扩散电极(gas diffusion electrode, GDE),因其表面丰富的含氧官能团,对ORR反应选择性较高[20-22],有利于O2的吸附和还原[23],促进羟基自由基产生,从而提升体系氧化能力。上述碳材料中,炭黑的热化学不稳定性会限制其耐久性、碳纳米管价格昂贵,相比之下三维多层片状结构的石墨烯具有价格适中、导电性好、比表面积大和化学惰性强[24]的优势,表现出优异的H2O2生成效率,是ORR反应的理想材料,但石墨烯在GDE结构下的电化学反应性能及其机理仍需进一步评估和探索。
本研究以活性碳纤维为基底,采用高导电、层状物理剥离的石墨烯为复合材料,PTFE为粘结剂,碳酸氢钠为造孔剂,通过超声-浸渍法制备石墨烯复合活性碳纤维气体扩散电极(GO/ACF)。以甲基橙废水为目标污染物组建电催化氧化与还原耦合处理体系,研究材料制备过程中GO与PTFE质量比、GO浆液中无水乙醇添加量、煅烧温度、煅烧时间对材料降解性能的影响,通过扫描电子显微镜、X-射线光电子能谱仪、傅里叶变换红外光谱仪对材料进行测试表征。考察不同的实验条件(初始电压、电解质浓度)下甲基橙电催化降解率,并探究GO/ACF产双氧水的性能、电化学性能及循环稳定性。本研究开发了一种制备简单、高效的气体扩散电极,可在甲基橙高效降解领域实现绿色、低成本处理,有望应用于更多废水处理。
-
无水乙醇(CH3CH2OH)、碳酸氢钠(NaHCO3)、硫酸钠(Na2SO4)、均为分析纯;甲基橙(C14H14N3SO3Na,98%)、聚四氟乙烯分散液(PTFE,60%)、石墨烯(GO,湖南丰恩新材料科技有限公司)、活性碳纤维(ACF,昆山易众天新材料有限公司)
-
722光栅分光光度计、管式炉(合肥科晶)、ZC-220AD超声清洗机(上海智悦)、RXN-605D直流电源(深圳兆信)、SB-948曝气泵(松宝)、CS350M型电化学工作站(武汉科思特仪器股份有限公司)
-
1)材料制备过程及优化。采用超声-浸渍法制备石墨烯复合活性碳纤维气体扩散电极。对活性碳纤维进行加热煮沸2次并烘干的预处理,记为ACF。在无水乙醇中加入2.2 g GO和0.88 g NaHCO3,超声15 min使其均匀分散即得GO浆液,继续超声15 min并缓慢逐滴加入PTFE分散液,后将ACF浸入GO浆液超声25 min,对ACF进行翻面继续超声25 min,取出黏附GO的ACF置于管式炉中进行煅烧,控制升温速率为10 ℃·min−1,即得GO/ACF。
通过改变制备过程中GO与PTFE质量比(C:P)、GO浆液中无水乙醇添加量、煅烧温度和煅烧时间对GO/ACF制备过程进行优化。配置100 mg·L−1的甲基橙废水,以Na2SO4为电解质,分别使用下述GO/ACF作为阴极对甲基橙废水进行降解,并在阴极侧进行曝气,控制阴阳极间间距为20 mm,恒定电压为6 V,Na2SO4质量浓度为2%,处理时间为90 min,每隔15 min进行取样测定吸光度并计算浓度。首先,固定无水乙醇添加量100 mL、煅烧温度360 ℃、煅烧时间30 min,按照C:P分别为1:2、1:4、1:5、1:6添加PTFE分散液;其次采用最佳CP比,其他条件相同,改变无水乙醇添加量分别为80、90、100、110、120 mL;最后采用最佳C:P比及无水乙醇添加量,分别改变煅烧温度为320、340、360、380、400 ℃及煅烧时间为10、20、30、40、50 min,优化制备参数。
2)产双氧水实验。由于甲基橙溶液颜色较为明显,所以在测定H2O2产量过程中,以相同体积去离子水进行替代。以250 mL烧杯作为反应容器,钌铱电极为阳极,ACF和GO/ACF分别为阴极,配置质量分数为2% 硫酸钠溶液200 mL,进行电催化产H2O2实验。实验过程中控制阴阳极间间距为20 mm,恒定电压为6 V,反应时间为90 min。每隔15 min进行取样,并采用硫酸钛分光光度法在波长为400 nm处测定水样H2O2质量浓度[27]。
3)实验条件优化。以GO/ACF为阴极,其余条件与2)相同,研究不同初始电压及电解质浓度对甲基橙降解性能影响。首先改变初始电压分别为4、5、6、7、8 V,通过测定甲基橙电催化降解率,得出最优电压;然后固定初始电压为最优电压,改变Na2SO4质量浓度为0.5%、1%、1.5%、2%、2.5%,得到降解过程最佳Na2SO4质量浓度。
4)电化学表征。使用电化学工作站对ACF、优化制备的GO/ACF进行伏安循环测试(CV)和恒电位阻抗(EIS)测试。以三电极体系进行测试,ACF及GO/ACF分别为工作电极,钌铱电极为对电极,饱和甘汞电极(SCE)为参比电极,工作电极与对电极的间距为20 mm,溶液中甲基橙浓度为100 mg·L−1、Na2SO4质量百分比为2%。CV测试过程采用的窗口电压为-0.5~1.2 V,以不同扫速(40、60、80 mV·s−1)分别对ACF、GO/ACF电极连续扫描10个周期,并选取稳定的第7周期做CV曲线。EIS测试采用的电压为1.25 V,频率为100 000~0.01 Hz。
5)循环稳定性实验。以优化制备的GO/ACF为阴极,采用最佳实验条件进行循环稳定实验。配置甲基橙浓度为100 mg·L−1、Na2SO4质量浓度为2%的溶液,GO/ACF电催化降解甲基橙90 min为一次处理,处理完成后,将GO/ACF烘干并更换相同参数溶液进行再次处理,如此循环10次,测定每次处理后的甲基橙浓度和第2、4、6、8、10次处理前后体系中COD值。
-
用扫描电子显微镜(SEM)观察ACF及GO/ACF电极表面的形貌特征;通过X-射线光电子能谱仪(XPS)分析获得电极表面元素组成等微观性质;通过傅里叶变换红外光谱仪(FTIR)分析电极表面官能团;通过电化学工作站对ACF及GO/ACF的电容及导电性能进行分析。采用重铬酸钾法(HJ 828-2017)测定废水的COD,采用722光栅分光光度计测定甲基橙在最大波长处(464 nm)的吸光度,其降解率根据式(2)计算。
式中:η为降解率,%;C0和Ct分别为甲基橙的初始浓度和t时的浓度,mg·L−1;t 为降解时间,min。
-
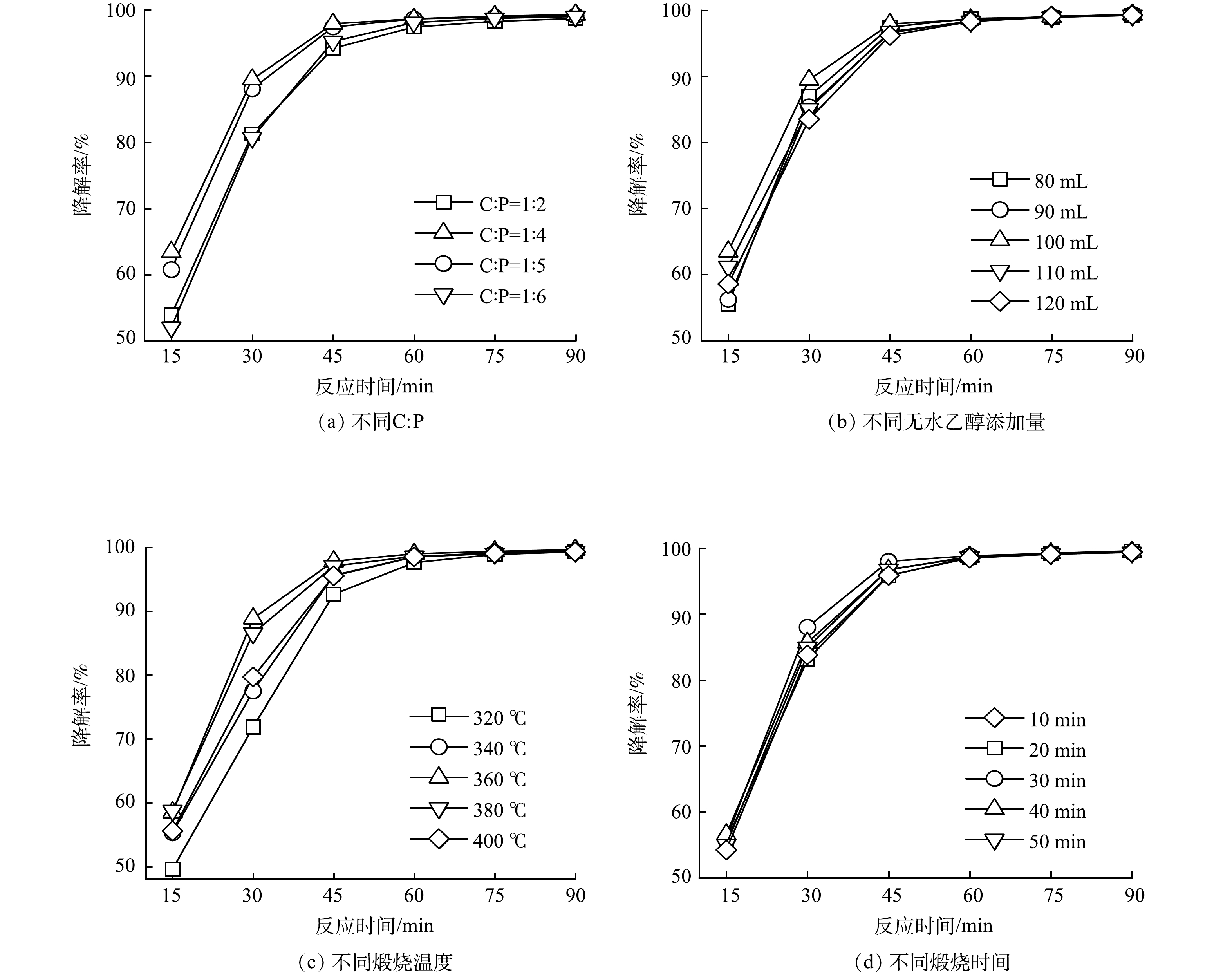
GO/ACF制备过程中C:P、GO浆液中无水乙醇添加量、煅烧温度、煅烧时间对GO/ACF甲基橙电催化降解性能影响如图1,可以看出不同条件下制备的GO/ACF甲基橙电催化降解性能有所差别,其中C:P对降解性能影响最为显著。图1(a)为不同C:P制备的GO/ACF为阴极时甲基橙电催化降解性能。由图1(a)可知,在反应初始阶段(0~45 min)随C:P的比例增大(C:P=1:2、1:4、1:5、1:6),体系的甲基橙降解率分别为94.16%、97.86%、97.37%、95.21%,呈现出先增大后减小的趋势。这是因为PTFE与GO在ACF表面形成了具有多孔、疏水性的催化层,随C:P比增加,PTFE增加,材料更具疏水性,减少阴极泛洪,进而促进氧分布[25],提升电化学反应活性位点;继续增加PTFE时GO负载过多,催化层变厚,氧气传输受到阻碍,活性位点相应减小,导致甲基橙降解率略有下降。C:P为1:4时,甲基橙最终降解率最高(100%),表明该条件下制备的GO/ACF具有较好的甲基橙降解性能,后续实验选择C:P为1:4。
图1(b)为不同无水乙醇添加量制备的GO/ACF甲基橙电催化降解性能。可见,随无水乙醇含量增多(80、90、100、110、120 mL),GO浆液浓度下降,在反应初始阶段(0~45 min)体系对甲基橙的降解率分别为96.59%、97.44%、97.93%、96.76%、96.20%,呈现为先增大后减小的趋势。这是因为无水乙醇添加量较少(<100 mL)时,GO浆液浓度大,ACF负载有效GO含量较高,GO相互覆盖导致催化剂层厚,进而堵塞多孔结构影响氧的传输,增加传质阻力,减弱氧还原反应,自由基生成效率变慢[26];而无水乙醇添加量较高时(>100 mL),GO浆液浓度小,ACF负载的有效GO含量低,GO/ACF表面含氧官能团含量降低,所提供的活性位点少故降解率下降。无水乙醇添加量为100 mL时,甲基橙最终降解率最高(100%),表明该条件下制备的GO/ACF具有较好的甲基橙降解性能,后续实验选择无水乙醇添加量为100 mL。
图1(c)为不同煅烧温度条件下制备的GO/ACF甲基橙电催化降解性能。PTFE与GO混合后需要煅烧熔化PTFE,提高GO的粘合性能并在ACF表面构成整个薄膜[27]。如图1(c)所示,在煅烧温度为320、340、360、380、400 ℃时,甲基橙在45 min内降解率分别为92.66%、95.63%、97.83%、97.10%、95.56%,呈现出先增大后减小的趋势。这是因为随着煅烧温度的增加,电极表面的粘合性能及薄膜的覆盖提高,气体传输较为畅通,从而使降解效率提升;而在煅烧温度大于360 ℃后,降解率逐步下降,可能是因为此时PTFE逐渐发生裂解,破坏了气体传输的骨架结构,导致降解性能下降。煅烧温度为360 ℃时,甲基橙最终降解率(100%)最高,表明该条件下制备的GO/ACF具有较好的甲基橙降解性能,后续实验选择煅烧温度为360 ℃。图1(d)为不同煅烧时间制备的GO/ACF甲基橙电催化降解性能。可见,煅烧时间为10、20、30、40、50 min时,甲基橙在45 min内降解率分别为95.86%、95.83%、98.01%、96.72%、96.77%,降解率最大差值小于3%,对材料的降解性能无显著影响,在90 min内降解率均保持在100%,从节能环保以及经济效益的角度考虑,本实验选用煅烧时间为10 min进行后续测试。
-
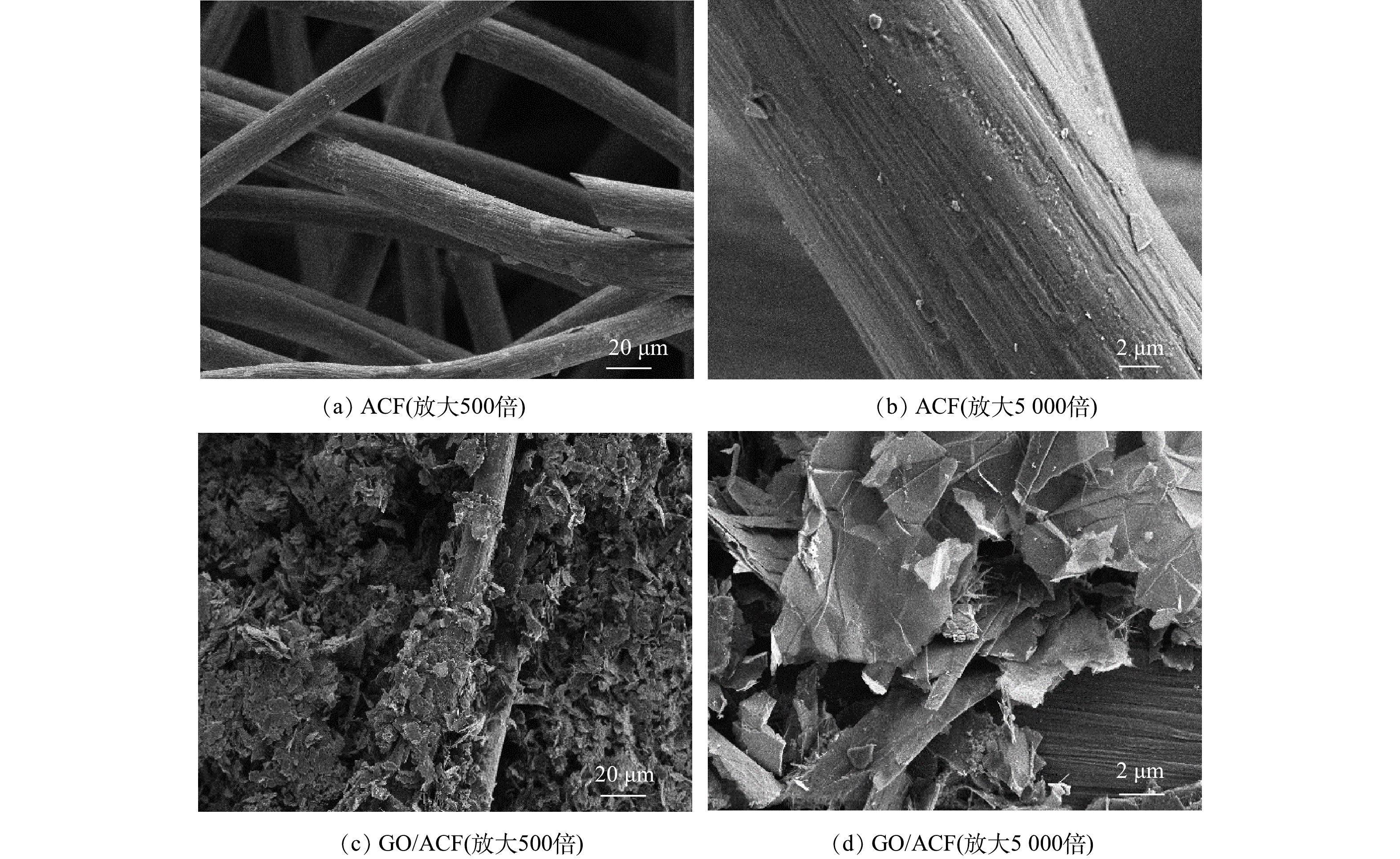
采用透射电镜观察ACF、GO/ACF的微观结构,结果如图2所示。图2(a)、图2(b)为ACF在不同尺度下的SEM图像,通过图2(a)可以看出ACF内部纤维分布疏松,呈现三维网络形态,具有丰富的空隙,可以为氧气传输提供路径[28]。图2(b)可看出纤维结构干净,尺寸均匀、表面光滑无杂质。图2(c)、图2(d)为不同尺度下GO/ACF的SEM照片,可以看出ACF表面出现大量GO的薄片层,并随机附着于纤维条表面,鳞片状结构较为显著,优于XIAO等人报道的微观形貌[1],这证明通过超声-浸渍法成功将GO复合于ACF表面,使电极表面粗糙并附着活性物质,具有更大的比表面积和反应位点有利于接触并降解污染物。
-
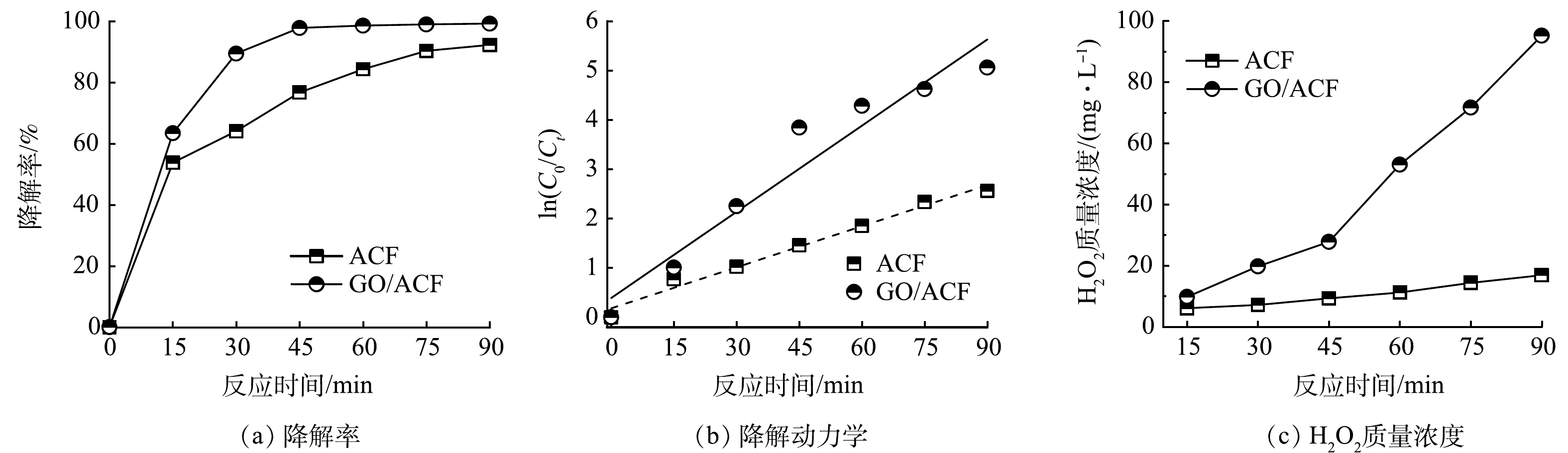
图3为ACF及GO/ACF甲基橙电催化降解率、降解动力学、双氧水产量对比。在除阴极不同,其余条件相同的情况下,ACF、GO/ACF对甲基橙电催化的降解效果如图3(a)所示。可以看出,GO/ACF的降解率明显优于ACF,随着反应时间增加,降解速率逐渐降低而后趋于平缓,是因为污染物浓度减小导致了传质性能下降。在90 min内ACF、GO/ACF甲基橙电催化降解率分别达到92.30%和99.37%,在45 min时ACF甲基橙电催化降解率为76.75%,而GO/ACF的降解率已达97.86%,不仅显著优于ACF,也优于LIU等光催化甲基橙降解性能[1]。图3(b)为ACF、GO/ACF体系降解动力学拟合的准一级动力学方程。通过对比表1中ACF及GO/ACF 的反应速率系数k可知,GO/ACF体系对模拟废水的降解速率高于ACF体系。GO/ACF体系的相关系数相对较低,其原因是该体系在45 min时甲基橙的降解率已超过97% ,此时体系中甲基橙的浓度较低对拟合产生了影响[29]。
ACF、GO/ACF体系在90 min内产生双氧水质量浓度如图3(c)所示,可以看出GO/ACF体系产生的双氧水质量浓度显著优于ACF,且随反应时间增加质量浓度差值逐渐增大。反应时间为90 min时,ACF体系中双氧水质量浓度为16.84 mg·L−1,而GO/ACF双氧水质量浓度达到95.23 mg·L−1,高出ACF约4.65倍,且仍有上升趋势,这与前文所述GO对ORR的高选择性[22]一致。
-
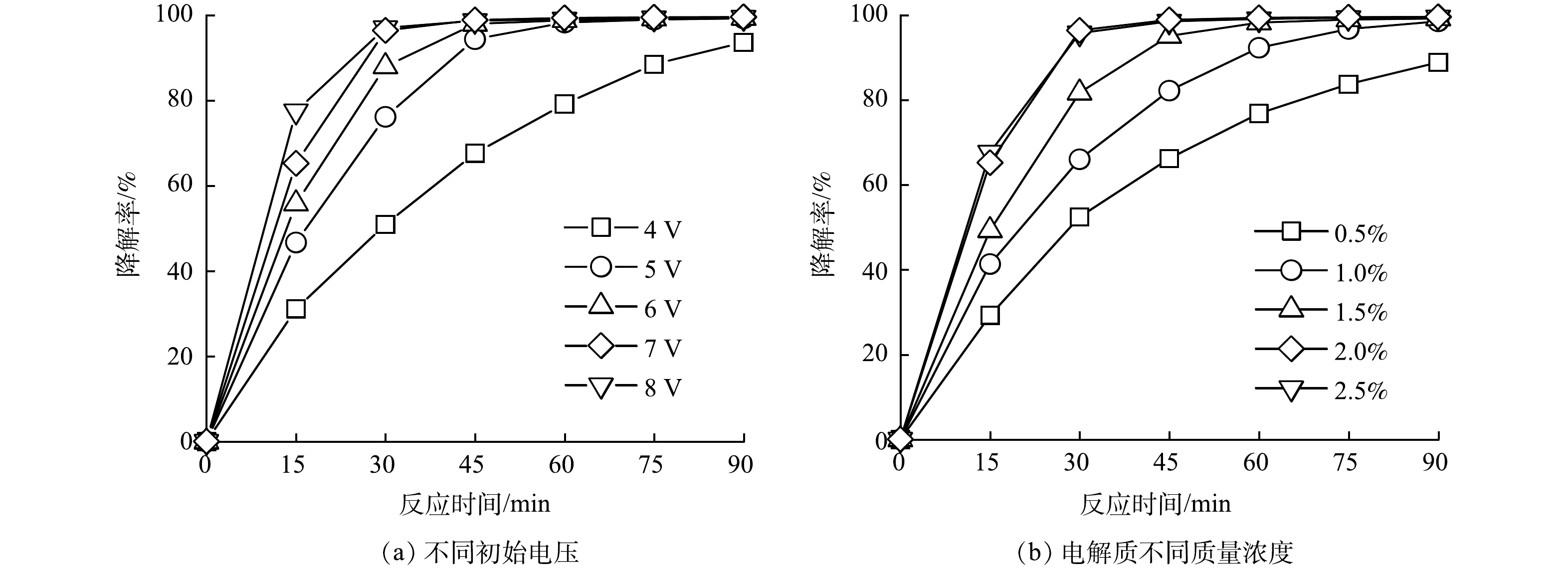
图4为不同初始电压、不同电解质浓度时GO/ACF甲基橙电催化降解性能。可以看出实验条件不同时GO/ACF甲基橙电催化降解性能差异显著,其中不同初始电压对降解性能影响最为显著。图4(a)为不同初始电压时GO/ACF甲基橙电催化降解性能。可以看出随初始电压的增加(4、5、6、7、8 V),甲基橙降解率在一定时间(0~30 min)范围内均有增加。反应时间为15 min时,甲基橙降解率在4 V和8 V电压下分别为31.10%和77.54%。可以看出提高初始电压会大幅提升甲基橙溶液的降解效率,这是由于高电压使更多电荷参与反应,增加了电子传输速率,从而提升H2O2的生成速率 [30],有利于甲基橙的氧化降解。而当反应时间达到30 min时,随初始电压(4、5、6、7、8 V)的增加,甲基橙降解率分别为50.95%、76.18%、88.01%、96.45%、97.06%,整体呈现升高的趋势。而在7 V增到8 V时无明显变化,这是因为30 min时这2个电压下的甲基橙降解率已超过95%,溶液中传质大幅减弱,增加初始电压对甲基橙降解影响较小。但电解过程中的电压越大,则能耗越大,且会增加副反应的发生[31],综合考虑,采用7 V作为最适电压。
不同的电解质质量百分比下GO/ACF对甲基橙电催化降解性能如图4(b)所示。由图4(b)可知,当电解质质量百分比为0.5%、1.0%、1.5%、2%、2.5%的条件下,在30 min时甲基橙降解率分别为52.46%、66.05%、81.73%、96.45%、95.19%。可以看出电解质质量百分比在0.5%~2.0%内,甲基橙降解率与电解质质量百分比呈正向关系,这是因为电解质浓度越大,溶液的电流越大, H2O2的生成速率快且生成量高,可促进甲基橙的氧化降解,而当电解质的质量百分比超过2.0%时,增大电解质浓度对甲基橙降解率的提高不明显,是由于溶液中SO42−的浓度过高可能会消耗生成的羟基自由基(式(3))[32]。从经济及降解过程考虑,确定电解质最适宜的质量百分比为2.0%。
-
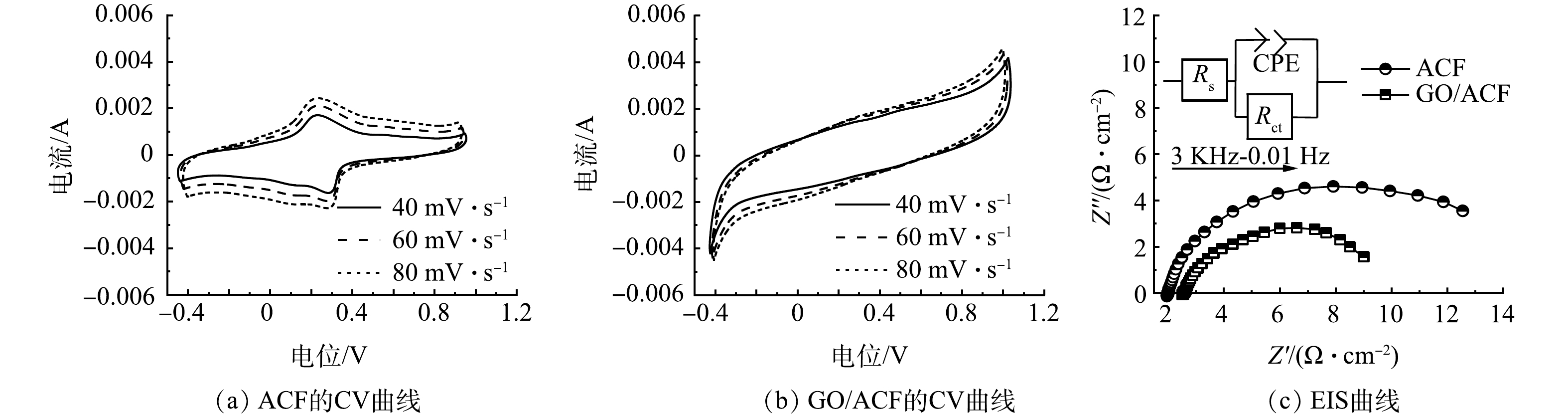
1)电化学性能测试。ACF及GO/ACF在不同扫速下的CV曲线及EIS曲线如图5中所示。ACF及GO/ACF的CV曲线如图5(a)和图5(b)所示。可以看出ACF的峰值电流明显弱于GO/ACF,以扫速40 mV·s−1的CV曲线为例,ACF的峰值电流为0.001 7 A·cm2,而GO/ACF的峰值电流为0.004 18 A·cm2,约为ACF峰值电流的2.46倍,反映了GO/ACF的电子传递速率更快,可能是GO复合后电极活性位点增加,从而提高了催化活性[33]。使用电化学工作站对扫速40 mV·s−1的CV曲线进行电容分析,可知ACF的电容值为17.213 mF·cm−2,而GO/ACF电容值为26.252 mF·cm−2高于ACF,说明GO/ACF电极有更大的伏安电荷量,其表面发生的了更多的电化学反应,双氧水产生量和双电层反应均高于ACF[34]。
图5(c)为ACF及GO/ACF的EIS曲线。对两者的电化学阻抗谱进行拟合分析,可计算出对应的电荷转移电阻Rct(ACF)为13.193 Ω、Rct(GO/ACF)为7.908 Ω,可以发现GO/ACF电极比ACF电极电阻降低了40.06%。这是由于高导电性石墨烯的复合改善了电极的电导率,提高电极导电性,减小了电荷传输阻力,有助于电子转移和氧气传输。
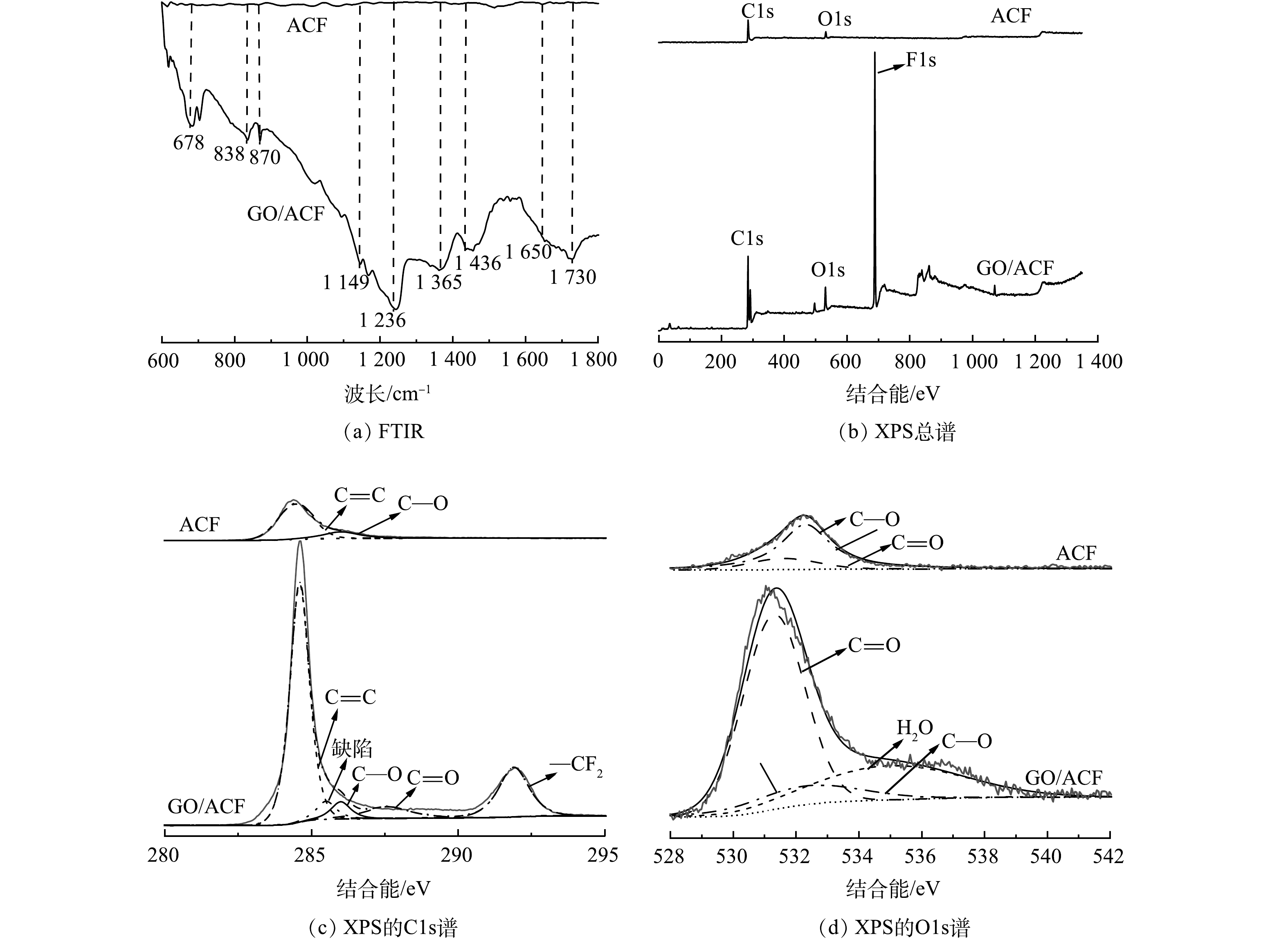
2) FTIR分析。已有研究[13, 26]表明,含氧官能团可以提高ORR反应的活性,促进O2吸附,有利于H2O2的形成,提升污染物的降解效果,因此,可以通过FTIR来研究GO复合前后电极官能团的变化,分析GO/ACF的强化机制。如图6(a)所示,聚四氟乙烯(PTFE)的3个主要吸收峰分别位于678、1 149、1 236 cm−1处,其中678 cm−1为F—C—F的弯曲振动,1 149 cm−1和1 236 cm−1为F—C—F的伸缩振动[35],这证明经过煅烧后,PTFE作为粘合剂仍存在于GO/ACF电极上。1 650 cm−1和1 730 cm−1处的峰处于双键伸缩振动区,分别对应着C=C和C=O的伸缩振动,其中C=C基团的存在有利于改善材料导电性,C=O基团存在有利于H2O2形成,对比ACF和GO/ACF的图谱可知,GO/ACF在1 650 cm−1和1 730 cm−1处的峰显著强于ACF,表明GO复合可增加含氧官能团的数量,使GO/ACF的导电性及双氧水催生产率均优于ACF[36],进而提升了甲基橙降解效率。GO复合可保证ORR过程的高效进行,为电极长期运行提供有力保障。
3) XPS分析。通过XPS测试研究ACF和GO/ACF的元素组成和化学状态。由图6(b)可以看出ACF主要由C、O元素构成,GO/ACF主要由C、O、F元素构成,而GO/ACF中C、O元素对应的峰值高于ACF峰值。这证明GO的引入增加了电极中C和O元素的含量。ACF、GO/ACF的C1s光谱如图6(c)所示。有研究[36]表明,C=C存在不成对电子和π—π*跃迁,可以提高材料导电性,而284.5 eV处存在一个强的C=C峰,计算可知该处GO/ACF的峰面积约为ACF的4倍,结合前文的EIS分析,证实GO的引入可减小电荷传输阻力,提高体系的导电性。GO/ACF光谱中另一个强峰291.9 eV对应着聚四氟乙烯的—CF2,表明了聚四氟乙烯的存在。图6(d)为ACF、GO/ACF的O1s光谱。可以看出GO/ACF含氧官能团的峰面积大于ACF,2个电极在531.6 eV和532.3 eV处表现出相同的含氧官能团(C=O和C—O), GO/ACF中C=O与C—O的峰面积比也显著高于ACF。结合文献报道[37]及前文中不同电极H2O2产率,可知C=O的引入促进了ORR反应进行。综上可知GO复合使电极有特定的含氧官能团,更有利于电催化氧化反应进行。
-
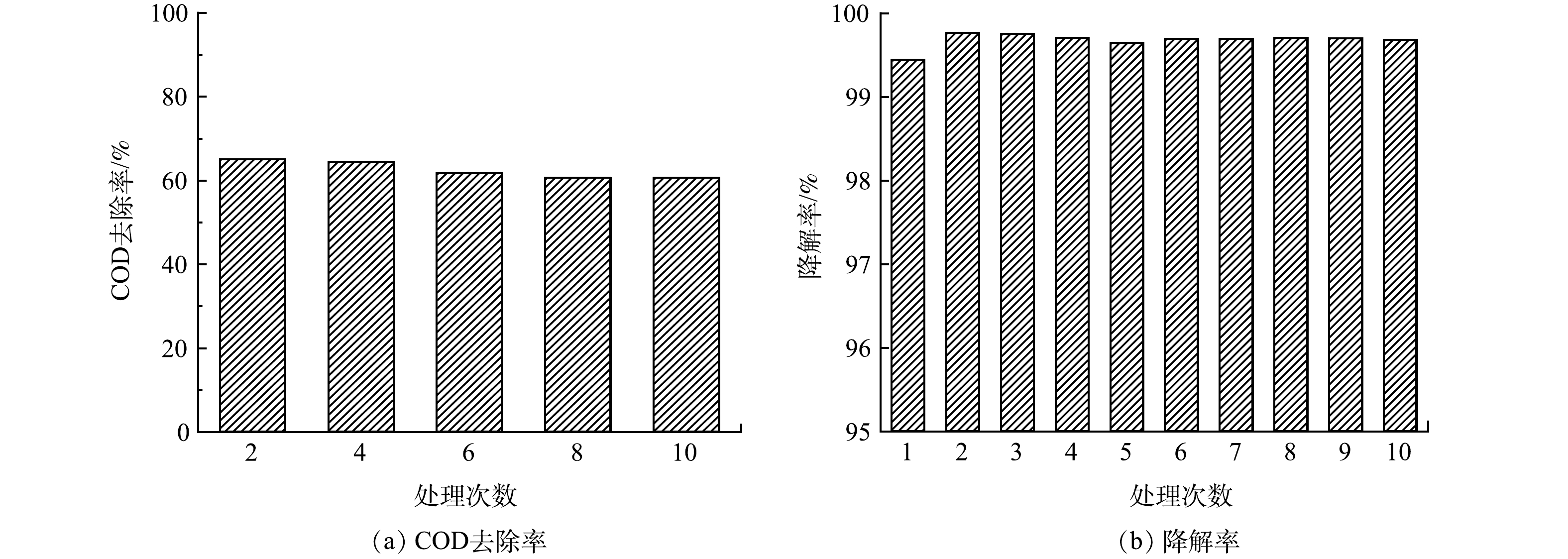
使用GO/ACF处理10批次甲基橙废水,甲基橙废水电催化CODCr去除率与降解率如图7(a)和图7(b)所示。甲基橙的降解并不代表被完全矿化。由图7(a)可以看出,甲基橙废水COD去除率整体呈现下降趋势,第2次处理甲基橙废水时COD去除率为65.00%,第10次COD去除率为60.56%,相差不超过5%,说明GO/ACF电极具有良好的稳定性[1]。由图7(b)可以看出,甲基橙降解率随循环次数增加整体呈现出微弱下降的趋势,第2次循环降解率较第1次略有提高。这可能是GO/ACF在第1次处理过程中表面略有脱落,运行一段时间使表面气体传输结构更加稳定。在10次处理中,甲基橙降解率均超过99%,降解率最高和最低分别为99.77%和99.64%,相差仅为0.13%,说明GO/ACF降解效果较好[38],具有较好的稳定性,应用前景较好。
-
1)采用超声-浸渍法制备的GO/ACF与ACF相比,GO/ACF在45 min内对甲基橙的降解率高于ACF 21.11%,动力学拟合近似符合一级动力学方程,反应速率系数约为ACF的2倍。相比ACF,GO/ACF的H2O2产量从16.84 mg·L−1提升至95.23 mg·L−1,高出ACF的H2O2产量4.65倍,说明GO复合能有效强化2电子ORR反应产H2O2,进而提升体系降解率。
2) C:P=1:4、GO浆液中无水乙醇添加量100 mL、煅烧温度360 ℃、煅烧时间10 min是制备GO/ACF气体扩散电极的最佳条件;通过条件优化实验确定了初始电压7 V、电解质质量浓度2%时, GO/ACF体系的处理效果最好。
3) ACF及GO/ACF电化学性能测试结果表明GO/ACF相比于ACF有效提高了峰值电流及电容值并减小电极电阻,说明GO复合促进了电子传递过程。GO均匀负载于ACF表面,并在电极表面引入C=O含氧官能团,可较好的促进H2O2产生。
4) GO/ACF在循环处理10次后,甲基橙废水COD去除率下降4.45%,降解率仅下降0.13%,表明该电极具有长期循环降解能力及良好的稳定性。
石墨烯复合活性碳纤维气体扩散电极对甲基橙的降解性能
Degradation of methyl orange using reduced graphene combined activated carbon fiber gas diffusion electrode
-
摘要: 采用超声-浸渍法制备石墨烯(GO)复合活性碳纤维(ACF)气体扩散电极,对其微观形貌、表面元素及表面官能团等进行表征,通过甲基橙批量降解处理实验阐述阴极双氧水产生性能与机理。结果表明,制备条件GO与聚四氟乙烯分散液(PTFE)质量比为1:4、GO浆液中无水乙醇添加量100 mL、煅烧温度360 ℃、煅烧时间10 min得到的电极性能最优,GO/ACF较ACF在45 min电催化反应甲基橙降解率提升了21.11%,双氧水最终的质量浓度提高了4.65倍,GO/ACF对甲基橙的最终降解率为100.0%,扫描电子显微镜微观形貌表征结果表明,石墨烯可均匀负载于活性碳纤维表面,X-射线光电子能谱、傅里叶变幻红外光谱仪、循环伏安及电化学阻抗测试结果表明,石墨烯复合增加了电极表面C=O官能团含量,电极电阻减小了40.06%,有利于产生H2O2。对初始电压、电解质浓度影响降解因素进行了优化,使用10次后GO/ACF对甲基橙降解率仍保持在100.0%、甲基橙废水COD去除率仅下降了4.45%,表明GO/ACF具有较高循环稳定性。以上研究结果可为印染废水电催化高效脱色处理与有机污染物氧化降解提供理论依据与技术借鉴。Abstract: The ultrasonic-impregnation method was used to prepare the graphene (GO) combined activated carbon fiber (ACF) gas diffusion electrode, and its micro-morphology, surface elements, and surface functional groups were characterized. The batch tests of methyl orange degradation were conducted to clarify the performance and mechanism of hydrogen peroxide production at cathode. The experimental results showed that the optimal electrode performance occurred when the mass ratio of GO to polytetrafluoroethylene dispersion (PTFE) was 1:4, the amount of anhydrous ethanol added to GO slurry was 100 mL, the calcination temperature was 360 ℃, and the calcination time was 10 min. After 45 min of the electrocatalytic reaction, the degradation rate of methyl orange by GO/ACF increased by 21.11% compared with ACF, the final mass concentration of hydrogen peroxide increased by 4.65 times, and the degradation rate of methyl orange by GO/ACF reached 100.0%. The microscopic morphology observation of scanning electron microscopy showed that graphene could be uniformly loaded on the surface of activated carbon fibers. According to the analysis of X-ray photoelectron spectroscopy, Fourier transform infrared spectroscopy, cyclic voltammetry, and electrochemical impedance testing results, graphene combination increased the content of C=O functional groups on the electrode surface and decreased electrode resistance by 40.06%, which was conducive to H2O2 production. The initial voltage and degradation factors of electrolyte concentration were optimized. After 10 recycles, GO/ACF still degrade methyl orange completely, while the CODCr removal rate of methyl orange wastewater only decreased by 4.45%, which indicated that GO/ACF had a good cycling stability. The results of the study can provide a theoretical basis and technical reference for highly efficient decolorization treatment of printing and dyeing wastewater by the electro-catalysis and the oxidative degradation of organic contaminants.
-
Key words:
- gas diffusion electrode /
- electrocatalytic oxidation /
- graphene /
- activated carbon fiber /
- methyl orange
-

-
表 1 ACF、GO/ACF甲基橙降解动力学方程及相关系数
Table 1. ACF, GO/ACF methyl orange degradation kinetic equations and the correlation coefficients
阴极材料 动力学方程 反应速率系数k/min−1 R2 ACF y= 0.027 7x+0.181 6 0.027 7 0.983 2 GO/ACF y = 0.058 2x+0.390 5 0.058 2 0.937 2 -
[1] LIU Y, JIN W, ZHAO Y, et al. Enhanced catalytic degradation of methylene blue by alpha-Fe2O3/graphene oxide via heterogeneous photo-Fenton reactions[J]. Applied Catalysis B: Environmental, 2017, 206: 642-652. doi: 10.1016/j.apcatb.2017.01.075 [2] 李燕, 杨建花, 李宝库, 等. 漆酶-介体系统对多种不同结构染料的脱色效果[J]. 环境工程学报, 2020, 14(12): 3308-3316. doi: 10.12030/j.cjee.201912179 [3] SHARMA M, AGARWAL S, MALIK R A, et al. Recent advances in microbial engineering approaches for wastewater treatment: A review[J]. Bioengineered, 2023, 14(1): 2184518. doi: 10.1080/21655979.2023.2184518 [4] TANG Q, ZHANG F J, CHEN W, et al. Floating-separation adsorbent for methylene blue and Pb (II) removal: Structure construction and adsorption mechanism[J]. Separation and Purification Technology, 2022, 295: 121332. doi: 10.1016/j.seppur.2022.121332 [5] LIU L, YU L, BORJIGIN B, et al. Fabrication of thin-film composite nanofiltration membranes with improved performance using β-cyclodextrin as monomer for efficient separation of dye/salt mixtures[J]. Applied Surface Science, 2021, 539: 148284. doi: 10.1016/j.apsusc.2020.148284 [6] BABU S S, MOHANDASS C, VIJAYARAJ A S, et al. Detoxification and color removal of Congo red by a novel Dietzia sp (DTS26) - A microcosm approach[J]. Ecotoxicology and Environmental Safety, 2015, 114: 52-60. doi: 10.1016/j.ecoenv.2015.01.002 [7] VERMA A K, DASH R R, BHUNIA P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters[J]. Journal of Environmental Management, 2012, 93(1): 154-168. [8] MORADIHAMEDANI P. Recent advances in dye removal from wastewater by membrane technology: A review[J]. Polymer Bulletin, 2021, 79(4): 2603-2631. [9] BHATIA D, SHARMA N R, SINGH J, et al. Biological methods for textile dye removal from wastewater: A review[J]. Critical Reviews in Environmental Science and Technology, 2017, 47(19): 1836-1876. doi: 10.1080/10643389.2017.1393263 [10] SIRES I, BRILLAS E, OTURAN M A, et al. Electrochemical advanced oxidation processes: today and tomorrow. A review[J]. Environmental Science and Pollution Research, 2014, 21: 8336-8367. doi: 10.1007/s11356-014-2783-1 [11] CHONG M N, SHARMA A K, BURN S, et al. Feasibility study on the application of advanced oxidation technologies for decentralised wastewater treatment[J] Journal of Cleaner Production, 2012, 35: 230-238. [12] VALLEJO M, ROMAN M F S, ORTIZ I, et al. Overview of the PCDD/Fs degradation potential and formation risk in the application of advanced oxidation processes (AOPs) to wastewater treatment[J] Chemosphere, 2015, 118: 44-56. [13] 雷丽丹, 周正伟, 高雅, 等. 电化学氧化改性石墨毡电芬顿体系对三氯乙烯的降解研究[J]. 安全与环境工程, 2021, 28(3): 108-116. doi: 10.13578/j.cnki.issn.1671-1556.20201197 [14] ZHAN J H, WANG H J, PAN X J, et al. Simultaneous regeneration of p-nitrophenol-saturated activated carbon fiber and mineralization of desorbed pollutants by electro-peroxone process[J]. Carbon, 2016, 101: 399-408. doi: 10.1016/j.carbon.2016.02.023 [15] CHEN J, WAN J F, GONG Y G, et al. Effective electro-Fenton-like process for phenol degradation on cerium oxide hollow spheres encapsulated in porous carbon cathode derived from skimmed cotton[J]. Chemosphere, 2021, 270: 128661. doi: 10.1016/j.chemosphere.2020.128661 [16] CUI Y P, ZHOU Z W, GAO Y, et al. Energy saving intermittent electro-Fenton system combined with commercial MoS2 for effective rhodamine B degradation[J]. Journal of the Cleaner Production, 2021, 289: 125807. doi: 10.1016/j.jclepro.2021.125807 [17] LUO H J, LI C L, WU C Q, et al. Electrochemical degradation of phenol by in situ electro-generated and electro-activated hydrogen peroxide using an improved gas diffusion cathode[J]. Electrochimica Acta, 2015, 186: 486-493. doi: 10.1016/j.electacta.2015.10.194 [18] GENDEL Y, ROTH H, ROMMERSKIRCHEN A, et al. A microtubular all CNT gas diffusion electrode[J]. Electrochemistry Communications, 2014, 46: 44-47. doi: 10.1016/j.elecom.2014.06.006 [19] ZHANG Z H, MENG H S, WANG Y J, et al. Fabrication of graphene@graphite-based gas diffusion electrode for improving H2O2 generation in Electro-Fenton process[J]. Electrochimica Acta, 2018, 260: 112-120. doi: 10.1016/j.electacta.2017.11.048 [20] DARVISHI CHESHMEH SOLTANI R, MASHAYEKHI M. Decomposition of ibuprofen in water via an electrochemical process with nano-sized carbon black-coated carbon cloth as oxygen-permeable cathode integrated with ultrasound[J]. Chemosphere, 2018, 194: 471-480. doi: 10.1016/j.chemosphere.2017.12.033 [21] KONDRATOWICZ I, NADOLSKA M, SAHIN S, et al. Tailoring properties of reduced graphene oxide by oxygen plasma treatment[J]. Applied Surface Science, 2018, 440: 651-659. doi: 10.1016/j.apsusc.2018.01.168 [22] WU G, SANTANDREU A, KELLOGG W, et al. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition[J]. Nano Energy, 2016, 29: 83-110. doi: 10.1016/j.nanoen.2015.12.032 [23] LEI Z D, CHEN H B, YANG M, et al. Boron and oxygen-codoped porous carbon as efficient oxygen reduction catalysts[J]. Applied Surface Science, 2017, 426: 294-300. doi: 10.1016/j.apsusc.2017.07.183 [24] MOOSTE M, KIBENA-POLDSEPP E, OSSONON B D, et al. Oxygen reduction on graphene sheets functionalised by anthraquinone diazonium compound during electrochemical exfoliation of graphite[J]. Electrochimica Acta, 2018, 267: 246-254. doi: 10.1016/j.electacta.2018.02.064 [25] YU F K, ZHOU M H, YU X M. Cost-effective electro-Fenton using modified graphite felt that dramatically enhanced on H2O2 electro-generation without external aeration[J]. Electrochimica Acta, 2015, 163: 182-189. doi: 10.1016/j.electacta.2015.02.166 [26] TAN L J, LIU Y M, ZHU G W, et al. Metal-free electro-Fenton degradation of perfluorooctanoic acid with efficient ordered mesoporous carbon catalyst[J]. Science of the Total Environment, 2023, 875: 162725. doi: 10.1016/j.scitotenv.2023.162725 [27] 赵晗露. 电化学原位产过氧化氢耦合紫外系统降解罗丹明B[D]. 威海: 哈尔滨工业大学 (威海校区) , 2021. [28] BERENGUER R, NISHIHARA H, ITOI H, et al. Electrochemical generation of oxygen-containing groups in an ordered microporous zeolite-templated carbon[J]. Carbon, 2013, 54: 94-104. doi: 10.1016/j.carbon.2012.11.007 [29] 朱询. 基于活性材料/rGO水凝胶粒子电极的三维电催化法处理模拟印染废水的研究[D]. 北京: 北京化工大学, 2021. [30] PANIZZA M. , CERISOLA G. Electro-Fenton degradation of synthetic dyes[J]. Water Research, 2009, 43(2): 339-344. doi: 10.1016/j.watres.2008.10.028 [31] 张妮, 任松宇, 张燕羽, 等. 缓释铁源耦合气体扩散电极强化电芬顿降解环丙沙星[J]. 环境工程学报, 2022, 16(11): 3596-3605. doi: 10.12030/j.cjee.202208056 [32] ZHOU M H, YU Q H, LEI L C, et al. Electro-Fenton method for the removal of methyl red in an efficient electrochemical system[J]. Separation and Purification Technology, 2007, 57(2): 380-387. doi: 10.1016/j.seppur.2007.04.021 [33] YANG D S, BHATTACHARJYA D, INAMDAR S, et al. Phosphorus-doped ordered mesoporous carbons with different lengths as efficient metal-free electrocatalysts for oxygen reduction reaction in alkaline media[J]. Journal of the American Chemical Society, 2012, 134(39): 16127-16130. doi: 10.1021/ja306376s [34] IMRAN M, PRAKASH O, PUSHKAR P, et al. Performance enhancement of benthic microbial fuel cell by cerium coated electrodes[J]. Electrochimica Acta, 2019, 295: 58-66. doi: 10.1016/j.electacta.2018.08.158 [35] 王志彬, 董勇修, 刘显贞, 等. 聚四氟乙烯微粉表面改性对氢化丁腈橡胶性能影响[J]. 高分子材料科学与工程, 2023, 39(1): 70-74. doi: 10.16865/j.cnki.1000-7555.2023.0014 [36] CORDEIRO P J M, JIMENEZ C S, LANZA M R D, et al. Electrochemical production of extremely high concentrations of hydrogen peroxide in discontinuous processes[J]. Separation and Purification Technology, 2022, 300: 121847. doi: 10.1016/j.seppur.2022.121847 [37] SUN F, YANG C W, QU Z B, et al. Inexpensive activated coke electrocatalyst for high-efficiency hydrogen peroxide production: Coupling effects of amorphous carbon cluster and oxygen dopant[J]. Applied Catalysis B: Environmental, 2021, 286: 119860. doi: 10.1016/j.apcatb.2020.119860 [38] LIU N, XIE H D, WEI J, et al. Catalytic activity of a composite metal electrode catalyst for the degradation of real dyeing wastewater by a heterogeneous electro-Fenton process[J]. Journal of Environmental Chemical Engineering, 2019, 7(1): 102930. doi: 10.1016/j.jece.2019.102930 -



 下载:
下载: