-
高盐废水一般指含盐量大于30 g·L−1的废水[1]。近年来,随着炼油、纺织和食品加工等行业的快速发展,高盐废水的排放也日益增多[1-2]。在有机污染物处理过程,生物法具有经济环保的优点;然而,高盐度带来的高渗透压会抑制微生物活性,高密度也会影响活性污泥沉降性能,因此高盐胁迫条件下活性污泥法的废水处理效能不佳[3-4]。相比之下,好氧颗粒污泥(AGS)结构密实,具有较强的抗盐度和负荷冲击能力[5-6],在榨菜废水[7]和页岩气采出水[8]等不同高盐废水处理过程展现出广阔应用潜力。
研究表明,运行模式对于AGS的形成和稳定性起着至关重要的作用[9-12]。以往的研究已经考察了好氧模式以及厌氧/好氧模式(厌氧推流进水、快速进水厌氧静置、快速进水厌氧搅拌)对于AGS的形成以及稳定性的影响[10-11,13]。相比而言,单一好氧模式由于丝状菌骨架生长快、剪切时间长等原因,AGS造粒时间较短[14-15];厌氧/好氧模式下,聚磷菌(PAO)和聚糖菌(GAO)等慢速生长微生物在厌氧期摄入大量碳源,限制了好氧期好氧异养菌的生长,有助于形成更为致密、稳定的AGS,但造粒时间更长[10-11]。目前世界上大部分AGS工程应用所采用的Nereda®工艺,即为代尔夫特理工大学Mark C.M. van Loosdrecht等提出的厌氧推流进水/好氧模式[16-17]。进一步,国内外学者针对厌氧推流进水时间进行细化研究,发现短厌氧推流进水/好氧模式的造粒效果居于单一好氧和长厌氧推流进水/好氧模式之间[13,18]。然而,上述研究大多在实际或模拟市政污水体系下展开,鲜有研究报道高盐废水体系运行模式对于AGS造粒过程的影响。鉴于高盐胁迫条件下,聚磷菌、聚糖菌、反硝化菌(DNB)等均会受到不同程度的抑制[19-20],厌氧推流进水期小分子碳源去除效果下降对造粒稳定性的影响犹未可知[10];此外,高盐条件下,胞外聚合物(extracellular polymeric substances, EPS)分泌较多且颗粒更为致密,一定程度上提升了单一好氧模式所培养颗粒的稳定性[21-23]。因此,高盐废水体系运行模式对活性污泥颗粒化过程的影响亟待研究。
本研究采用3个完全相同的序批式反应器(SBR),系统研究了不同运行模式(R1为好氧模式、R2为厌氧/好氧模式(厌氧推流进水30 min)、R3为厌氧/好氧模式(厌氧推流进水120 min))下活性污泥颗粒化过程及高盐废水处理效能,通过比较污泥性质(粒径、MLSS、SVI30和SVI30/SVI5)、颗粒形态、污染物去除率、EPS及微生物多样性,分析高盐废水体系下不同运行模式的优势与短板,为高盐废水体系活性污泥造粒过程提供有益参考。
-
接种污泥取自江苏中法水务城东水质净化厂好氧池(江苏常熟),接种污泥呈现黄褐色,污泥浓度(MLSS)为3.1 g·L−1,污泥体积指数(SVI30)为80.3 mL·g−1,D50为88.2 μm。
废水选用人工配水,进水化学需氧量(COD)维持在1 000 mg·L−1,对应的总有机碳(TOC)浓度为345 mg·L−1,碳源选用葡萄糖、乙酸钠混合碳源,其COD比值为3∶1;加入191 mg·L−1 NH4Cl以及88 mg·L−1 KH2PO4以保证COD∶N∶P = 100∶5∶2;同时添加40 mg·L−1 CaCl2、37 mg·L−1 MgSO4·7H2O、37 mg·L−1 FeSO4·7H2O以及0.1 mL·L−1微量元素液,其中微量元素液由500 mg·L−1 H3BO3、500 mg·L−1 ZnCl2、300 mg·L−1 CuCl2、1000 mg·L−1 MnCl2·4H2O、500 mg·L−1 (NH4)6Mo7O24·4H2O、500 mg·L−1 AlCl3、500 mg·L−1 CoCl2·6H2O以及500 mg·L−1 NiCl2组成;同时,加入30 g·L−1 NaCl提供3%盐度环境。此外,第46~66 d,配水中添加了500 mg·L−1 NaHCO3补充碱度。
-
使用3个完全相同的SBR培养AGS,反应器内径8 cm,总高度115 cm,有效容积4 L。
3个SBR (R1为好氧模式,R2为厌氧/好氧模式(厌氧推流进水30 min),R3为厌氧/好氧模式(厌氧推流进水120 min))的运行周期均为6 h,包含进水、曝气、沉降、排水和静置5步,具体运行参数如表1所示。除进水时间和曝气时间外,其他参数均保持一致,换水比(VER)为60%,有机负荷为2.4 kg COD·m−3·d−1,表观气速为1.2 cm·s−1,温度为23±2 ℃。
-
有机物浓度由TOC分析仪(Sievers InnovOx Laboratory, SUEZ, USA)通过超临界水氧化法(375 ℃)测定,以防止高盐条件下Cl−对COD测试的干扰。NH4+-N采用纳氏试剂分光光度法(HJ 535-2009)测定;NO3−-N采用紫外分光光度法(HJ/T 346-2007)测定;NO2−-N采用N-(1-萘基)-乙二胺光度法(GB 7493-87)测定。
混合液悬浮固体浓度(MLSS)、混合液挥发性悬浮固体浓度(MLVSS)及污泥容积指数(SVI5 and SVI30)均使用标准方法测定[24]。颗粒粒径使用马尔文激光粒度仪(Mastersizer 3000, Malvern, England)测定。污泥形貌特征分别使用普通相机及体视显微镜(SteREO Discovery. V20, ZEISS, Germany)观察;污泥微观形貌特征使用扫描电子显微镜(SEM) (Quanta FEG 650, ThermoFisher, USA)观察[25-26]。胞外聚合物(EPS)使用改进的热提取方法提取,并采用Bradford法测定胞外蛋白(PN)、苯酚硫酸法测定胞外多糖(PS),以两者之和计算EPS含量[25,27]。
微生物多样性分析分别选取接种污泥与3个SBR运行66 d时的污泥作为样品(分别命名为S0、S1、S2和S3),采用引物338F和806R扩增16S rRNA基因V3-V4区,纯化后的扩增产物在Illumina MiSeq PE300 (Illumina, San Diego, USA)平台测序,该步骤由上海美吉生物医药科技有限公司完成。
-
D50为污泥颗粒粒径中值,可以直观地表征活性污泥造粒过程(图1(a)),一般认为,D50 > 200 μm是颗粒形成的标志[9]。3个SBR (R1为好氧模式,R2为厌氧/好氧模式(厌氧推流进水30 min),R3为厌氧/好氧模式(厌氧推流进水120 min))接种的活性污泥D50均为88.2 μm,在较强的水力选择压下(高剪切、短沉降时间等),颗粒粒径在运行过程中均显著提高。就R1而言,D50在第10 d超过200 μm (271 μm),然后在13 d急剧增加至1 210 μm,并在第22 d增加至最高值1 560 μm,随后逐渐下降并在28 d以后在823~1 280 μm之间波动。R2的D50在第13 d超过200 μm (906 μm),在第19 d增加至最高值1 390 μm,最终稳定在1 030~1 220 μm。R3的D50变化趋势与R1和R2相似,在第10 d超过200 μm (229 μm),随后在第16 d急剧增加至1 110 μm,并在第28 d增加至最高值1 480 μm,最终在989~1 410 μm之间波动。总体而言,在3%盐度高盐废水中,R1、R2和R3分别在第10、13和10 d达到颗粒污泥形成的标准(D50 > 200 μm)。相比于前人在无盐废水体系的研究(活性污泥在厌氧/好氧模式下的颗粒化时间大多为好氧模式的2倍以上)[9,28-31],本研究在高盐废水体系下厌氧/好氧模式的造粒时间短,达到了与好氧模式类似的效果,推测原因在于高盐条件对于造粒过程的强化作用(促进EPS分泌、压缩双电层等)[7,21,25]以及配水中葡萄糖碳源加速了造粒[18]。
接着,分析污泥浓度和沉降性能以反映颗粒污泥成熟情况(图1(b)、图1(c)和图1(d))。3个SBR的初始接种污泥MLSS和SVI30相同,分别为3.1 g·L−1和80.3 mL·g−1。接种后,MLSS均有所上升,在第7 d R1、R2和R3分别提高至3.8、4.2以及3.7 g·L−1。随着沉降时间在第9 d缩短至4 min,部分沉降性能差的絮状污泥在高水力选择压下被排出反应器,导致MLSS在第13 d分别下降至1.0、0.8和2.5 g·L−1,SVI30下降至34.4、44.0和24.4 mL·g−1,SVI30/SVI5比值全部上升至1。相较于R3,R1以及R2中的生物量下降尤为显著,表明长时间厌氧推流进水有利于污泥拥有更好的沉降性能,从而有利于污泥的持留。根据颗粒污泥造粒过程完全颗粒化的定义(D50 > 200 μm,SVI30 < 50 mL·g−1,SVI30/SVI5 > 0.9)[9,26,32],3个SBR的颗粒污泥分别在第13 d (D50 = 1 210 μm,SVI30 = 34.4 mL·g−1,SVI30/SVI5 = 1)、13 d (D50 = 906 μm,SVI30 = 44.0 mL·g−1,SVI30/SVI5 = 1)和10 d (D50 = 640 μm,SVI30 = 24.4 mL·g−1,SVI30/SVI5 = 1)实现完全颗粒化。在实现完全颗粒化之后,3个SBR中的颗粒尺寸和污泥浓度均不断上升,SVI30均维持在50.0 mL·g−1以下,其中R1及R2中的AGS相对R3较蓬松(图2),SVI30也略高(33.6、35.1 mL·g−1 v.s. 19.7 mL·g−1);对于SVI30/SVI5而言,R1波动最大(0.84~1.08),也体现了纯好氧模式培养获得的颗粒性质相对不稳定,R3的SVI30/SVI5比值最为稳定(保持在1.0),说明长厌氧推流进水时间培养的颗粒最为致密且稳定性最好,这与先前文献报道是一致的[11]。52 d左右,3个SBR先后进入稳定期(R1:D50~823 μm,MLSS~12.0 g·L−1,SVI30~29.7 mL·g−1,SVI30/SVI5~1。R2:D50~1 060 μm,MLSS~11.9 g·L−1,SVI30~25.1 mL·g−1,SVI30/SVI5~1。R3:D50~1 130 μm,MLSS~12.4 g·L−1,SVI30~20.2 mL·g−1,SVI30/SVI5~1)。
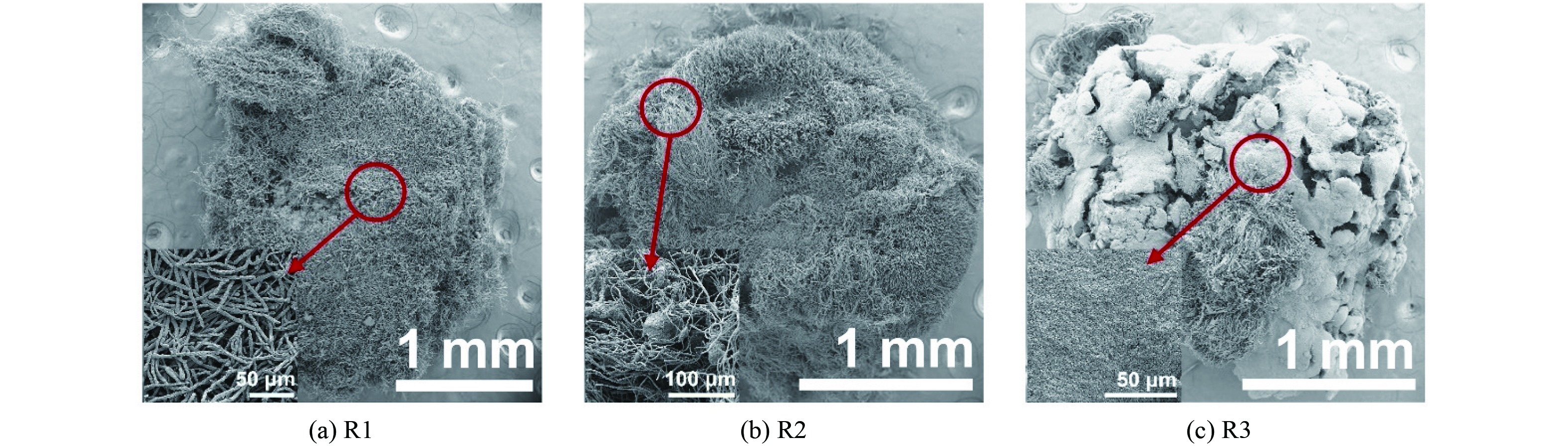
图2展示了高盐废水体系造粒过程污泥形态变化。第0 d,接种到3个SBR中的普通活性污泥呈黄褐色絮体状态。R1、R2和R3分别在培养4、6、6 d后出现了颗粒(~800 μm),此时大量絮状污泥仍然存在。第9 d,缩短沉降时间(5~4 min),大量絮状污泥被排出反应器,造粒速度加快。第13 d,R1和R2中迅速出现了较多边界清晰的颗粒(R1:~870 μm,R2:~1 210 μm),絮状污泥鲜有存在;相比之下,R3中出现的团聚体尺寸较小(~450 μm)。随着沉降时间逐步缩短为2 min,3个SBR中的颗粒数量急剧增多,体积逐渐变大;R1和R2的颗粒外表呈黄褐色,但部分颗粒外表呈毛绒状,其中R1存在许多大颗粒(~5 018 μm);R3颗粒相对较黑,表面更为致密圆滑。
图3展示了3个SBR运行66 d时的AGS形貌。从外表面看,R1和R2的颗粒微观结构相似,表面较为蓬松,以丝状菌为主导,R2丝状菌上缠绕有更多的细菌菌团(图3(a)和图3(b))。相比之下,R3颗粒表面排布了致密的细菌层,外表仅有少量丝状菌,结构相对密实(图3(c)),其主要原因在于,配水中同时存在葡萄糖和乙酸钠,大部分有机物可以被PAO、GAO等转化为聚-β-羟基丁酸酯(PHB),进而抑制丝状微生物等好氧异养菌生长,使得颗粒光滑圆润;部分残余的有机物(葡萄糖及其发酵产物等)导致颗粒外层少量丝状菌生长[10]。3个SBR内颗粒污泥形貌差异较大的主要原因在于,R1和R2中进水有机物在厌氧期没有或较少被转化为PHB等物质,大部分有机物在好氧期被颗粒外层丝状微生物为代表的好氧异养菌利用。
总体而言,在高盐环境下,单一好氧与厌氧/好氧模式造粒时间差异不大,R1、R2和R3中的颗粒分别在第4、6、6 d首次出现,在第13、13、10 d实现完全颗粒化,而厌氧/好氧模式培养的颗粒致密性和稳定性均较优。长厌氧推流进水时间(120 min)相比于短厌氧推流进水时间(30 min)会提升颗粒结构强度,强化AGS稳定性。
与先前无盐废水条件文献报道相比[11],本研究结果有相似之处,但也有所差异。本研究中,好氧模式形成的颗粒污泥较为蓬松且不稳定,而厌氧/好氧模式下的颗粒污泥结构致密且稳定,这与无盐废水体系文献报道基本一致。本研究中,好氧模式与厌氧/好氧模式下颗粒造粒速度并无显著差异,与先前无盐废水体系文献报道的厌氧/好氧模式造粒速度慢这一结论并不相同。
-
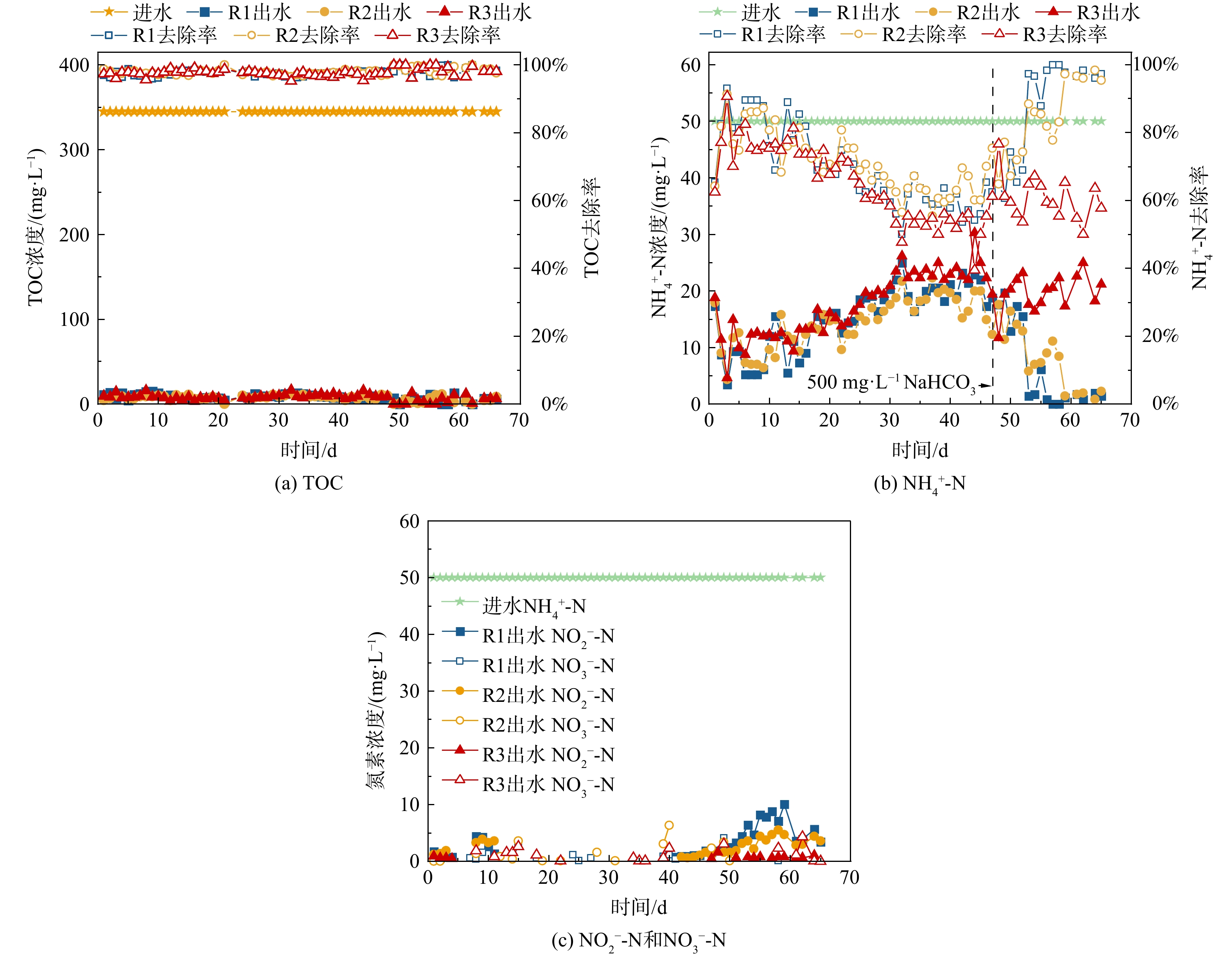
接着,对3个SBR的污染物去除效能进行评估(图4)。在66 d的运行过程中,3个SBR的进水TOC均为345 mg·L−1,NH4+-N均为50 mg·L−1。就TOC去除效能而言(图4(a)),接种空曝1 d的市政活性污泥后,第1 d,3个SBR的TOC去除率均高于97%,可能由于本批次活性污泥的盐度耐受性较好。之后,运行过程中,3个SBR的去除率基本稳定保持在97%以上,即使在第13 d左右反应器中生物量较低时,TOC去除率仍然保持在97%以上。
就NH4+-N去除效能而言(图4(b)),第1 d,3个SBR的NH4+-N去除率分别为65.4%、64.2%和62.4%;第3 d NH4+-N去除率分别上升至93.1%、91.3%和90.7%;之后,NH4+-N去除率逐渐下降,这说明前期较高的NH4+-N去除率可能是污泥吸附-排泥导致的[33]。随着反应器的运行,3个SBR的NH4+-N去除率不断下降且较为相似,直至第30 d以后达到相对稳定,R1、R2和R3分别稳定在59%、62%和53%左右。第46 d,为了提高NH4+-N去除率,向3个SBR中投加500 mg·L−1 NaHCO3 (NaHCO3∶NH4+-N = 10∶1)。第46~52 d,NH4+-N去除率略有提高。第53 d后,R1的NH4+-N去除率迅速提高至97.2%,并能基本稳定保持在97%以上;R2上升略慢,但59 d后也可以基本稳定保持在96%以上。这说明,前期反应器NH4+-N去除率受限是由于碱度的缺乏,限制了硝化细菌的碳源补给[34]。相比之下,投加NaHCO3后,R3的NH4+-N去除率仍没有显著提升,依旧在50%~70%间波动。R3反应器在好氧曝气阶段的溶氧为7~8 mg·L−1、pH为7.5~8.3,在碱度充足的情况下理论上适合AOB和NOB的生长;鉴于曝气开始时,笔者在R3反应器闻到了臭鸡蛋气味,且在反应器排气口检测到了51.2 mg·L−1的H2S气体(R1、R2未测得),且后续16S rRNA高通量测序证实了R3中硫酸盐还原菌(SRB)的存在(Desulfovibrio (1.06%)和Fusibacter (5.44%)),因此推测硫酸盐还原菌在厌氧推流进水阶段产生的S2-会抑制硝化菌的生长和硝化反应的进行。研究表明,S2-会抑制硝化细菌生长代谢活性,其原因可能在于破坏细胞膜完整性或抑制关键酶[35-36]。此外,在运行过程的前40 d,R1和R2的出水中NO2−-N较低,从第40 d开始出水中NO2−-N逐渐增高,最终分别在4.0和2.7 mg·L−1左右波动,而R3整个过程中基本没有NO2−-N存在;3个SBR整个过程中基本没有NO3−-N存在;总体而言,3个反应器的TN去除率最终分别稳定在86.6%、89.8%和54.9%左右。上述结果表明,在高盐废水进水有机负荷为2.4 kg COD·m−3·d−1、硫酸根负荷为0.06624 kg SO42-·m−3·d−1时,长厌氧推流(120 min)会产生S2-,影响生物脱氮的顺利进行。
-
胞外聚合物(EPS)在微生物聚集、造粒和AGS稳定性方面发挥着重要作用[37-38],图5从EPS的组成和浓度角度对高盐废水体系运行模式对活性污泥颗粒化过程的影响进行分析。
接种的活性污泥EPS浓度为26.9 mg·g−1 VSS,此后,3个SBR的EPS含量波动上升,并分别在第40、34、34 d达到最大值(108.3、54.1、76.8 mg·g−1 VSS),R2和R3的EPS含量显著低于R1,可能是由于R1中较长的曝气时间会刺激EPS产生[12],也可能是由于大量释放EPS的微生物属于好氧菌[39]。上述阶段,EPS含量的增加有助于微生物粘附,促进了污泥颗粒化[40]。随后在第46~66 d,3个SBR的EPS含量开始下降并最终稳定在30~50 mg·g−1 VSS,这与我们先前稳定期的现象是一致的[25-26]。总体而言,3个SBR的EPS含量普遍较低,这可能是由于提取方法造成的[25]。
PS可以形成交叉网状结构的主干,促进桥接,而PN可以降低表面负电荷,增强疏水性[23,41]。文献报道,更高的PN/PS值提升了颗粒的疏水性,有助于获得致密稳定的颗粒[42-44]。总体而言,3个SBR的PN/PS值在整个运行过程中呈先上升后波动稳定的趋势,表明污泥自身黏附性能得到了提高。其中第0~40 d,R1和R3的比值要略高于R2;第46~66 d,R3的PN/PS值(~1.6)显著高于R1 (~0.8)和R2 (~0.6)。上述结果表明,长厌氧推流进水/好氧模式有利于AGS的结构稳定。总体而言,本研究中PN/PS值低于大多数AGS研究,其原因可能在于盐度会刺激PS分泌[45],也可能是高浓度的Na+取代Ca2+进而抑制PN分泌[40,46]。
-
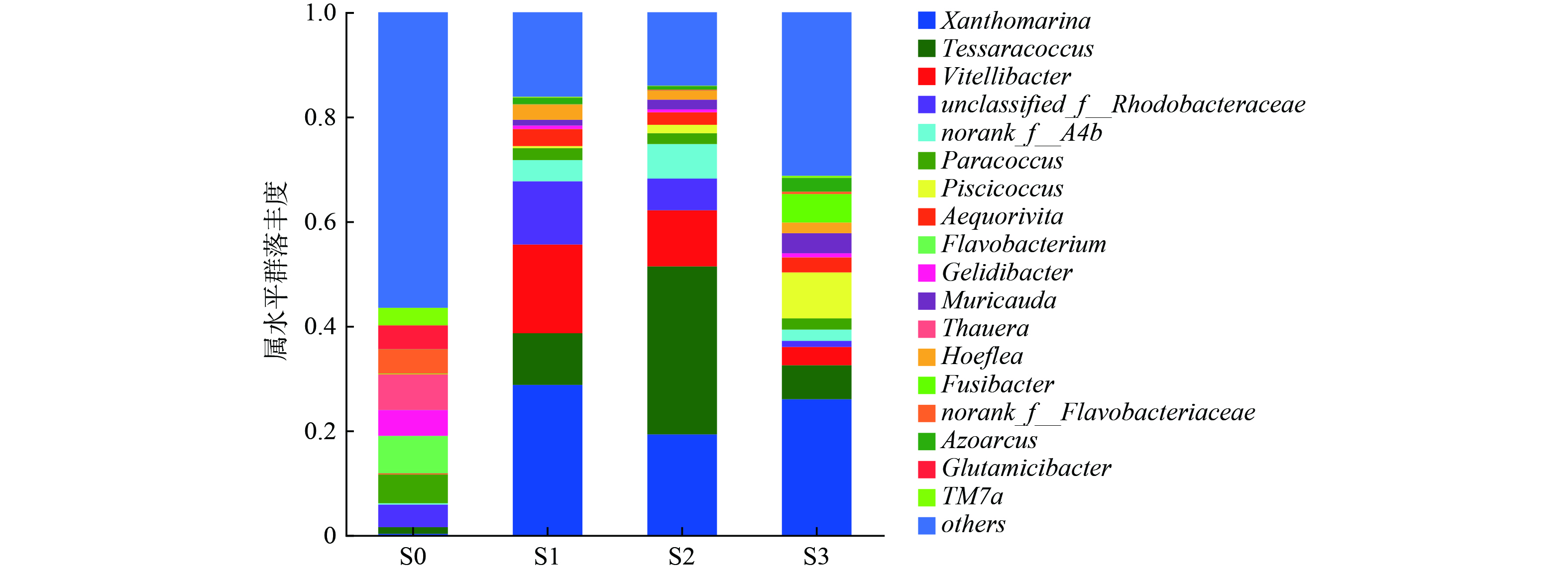
图6从属水平上对接种污泥及3个SBR运行66 d时的污泥微生物群落组成进行了分析。结果发现,第66 d 3个SBR中的微生物群落较接种污泥已经有了较大变化。接种污泥S0的微生物多样性较为丰富,其中优势属主要包括Flavobacterium (7.2%)、Gelidibacter (4.9%)、Thauera (6.8%)、Glutamicibacter (4.5%)和TM7a (3.4%)等,但是这些属在S1~S3中几乎检测不到,可能是由于这些微生物对盐度比较敏感,也有可能是颗粒化过程中生态位的变化导致的[47]。Xanthomarina (28.7%、19.3%、26.0%)、Tessaracoccus (9.9%、32.1%、6.5%)、Vitellibacter (17.0%、10.7%、3.5%)、unclassified_f__Rhodobacteraceae (12.1%、6.1%、1.2%)、norank_f__A4b (4.0%、6.6%、2.1%)、Paracoccus (2.2%、2.1%、2.1%)、Aequorivita (3.2%、2.3%、2.9%)、Muricauda (1.1%、1.9%、3.8%)以及Hoeflea (2.8%、1.6%、2.0%)为3个SBR中均能检测到的主要菌属,意味着这些微生物可以在不同运行模式处理高盐废水。
表2进一步在属水平对主要功能菌群进行分析。在3个SBR中共检测到了5种PAO (Tessaracoccus、 Corynebacterium、Halomonas、Micropruina、Pseudomonas)和1种GAO (Kineosphaera),PAO等慢速生长微生物的富集有助于提升AGS稳定性[10]。R2中Tessaracoccus丰度高达32.11%,说明了该条件造粒的稳定性;而R3 PAO含量低于R2,其原因可能在于SRB的抑制作用。对于硝化菌而言,3个SBR的AOB (Nitrosomonas)丰度分别为0.04%、0.03%、0.01%,这与R3较低的NH4+-N去除率一致。对于反硝化菌而言,unclassified_f__Rhodobacteraceae、Aequorivita、Azoarcus总体丰度相对较高,其中Aequorivita还具有降解复杂有机物的能力[48]。值得注意的是,R3中检测到了较高浓度的SRB: Fusibacter (5.44%)和Desulfovibrio (1.06%),相比之下,R1与R2中SRB含量很少,说明在较高进水有机物浓度条件下,长厌氧推流进水模式容易引发SRB富集。SRB代谢产生的S2-会抑制硝化菌等功能微生物代谢活性,其机制及调控策略有待深入研究。此外,Piscicoccus在R3中拥有较高的丰度,有利于有机物的降解[49]。Xanthomarina被报道在高盐废水体系下与TN去除有关[26];Vitellibacter与Hoeflea具有降解有机物的能力[50-52];Paracoccus与碳代谢和无机氮转化密切相关[53];Muricauda能够分泌多种可以水解复杂碳源的胞外酶[54],并且有助于EPS的合成[55]。
-
1)就污泥性质而言,在高盐废水体系下,R1 (好氧模式)、R2 (厌氧/好氧模式:厌氧推流进水30 min)和R3 (厌氧/好氧模式:厌氧推流进水120 min)中的颗粒分别在第4、6、6 d首次出现,在第13、13、10 d实现完全颗粒化,单一好氧与厌氧/好氧模式造粒时间无显著差异。相比于R1和R2,R3中的颗粒结构致密稳定,PN/PS含量较高。
2)就高盐废水处理效能而言,3个SBR均可实现97%以上的TOC去除率;R1和R2的NH4+-N去除率大于96%,而R3在50%~70%波动,表明硫酸盐还原产生的S2-对硝化细菌产生了抑制。
3)生物多样性分析表明,在3%盐度不同运行模式下Xanthomarina、Tessaracoccus、Vitellibacter、unclassified_f__Rhodobacteraceae、norank_f__A4b、Paracoccus、Aequorivita、Muricauda及Hoeflea是3个SBR中的主要菌属。Tessaracoccus是主要的PAO,在R2中丰度较高;Nitrosomonas是主要的AOB,在R3中丰度相对较低;R3中检测到了较高丰度的SRB (Desulfovibrio和Fusibacter),其产生的S2-抑制了硝化反应。
4)综合污泥性质及高盐废水处理效果来看,单一好氧模式容易引起丝状菌主导的颗粒污泥的形成,结合先前研究经验,丝状菌主导的颗粒污泥不利于反应器长期稳定运行;在较高进水有机物浓度条件下,长厌氧推流进水/好氧模式容易引发SRB富集,SRB代谢产生的S2-会抑制硝化菌等功能微生物代谢活性;相比之下,短厌氧推流进水/好氧模式更适合应用于高盐废水处理过程。
高盐废水体系运行模式对活性污泥颗粒化过程的影响
Effect of operation modes on granulation process of activated sludge in treatment of hypersaline wastewater
-
摘要: 好氧颗粒污泥(AGS)技术在高盐废水处理领域具有广阔应用潜力,高盐胁迫条件下运行模式对造粒过程影响尚不清晰。采用3个序批式反应器(SBR),探究好氧模式(R1)、厌氧/好氧模式(R2为厌氧推流进水30 min;R3为厌氧推流进水120 min)对活性污泥颗粒化过程及高盐废水处理效能的影响。结果发现,R1、R2和R3造粒时间无显著差异(分别在第13、13、10 d实现完全颗粒化),而R3培养的颗粒结构更致密、胞外蛋白/胞外多糖(PN/PS)含量较高。3个SBR均可在高盐废水体系实现高TOC去除率(>97%);R1和R2的NH4+-N去除率大于96%,而R3在50%~70%波动,可能是硫酸盐还原菌(Desulfovibrio和Fusibacter)在长厌氧推流进水过程产生的S2-抑制了硝化反应。本研究结果可为高盐废水体系活性污泥造粒过程提供参考。Abstract: Aerobic granular sludge (AGS) technology has shown great application potentials in the treatment of hypersaline organic wastewater. The effect of operation modes on granulation process under hypersaline stress was still unknown. In this study, three sequencing batch reactors (SBRs) were used to investigate the granulation process of aerobic activated sludge and the efficiency of hypersaline wastewater treatment under different operation modes, including aerobic mode (R1) and anaerobic/aerobic mode (R2: anaerobic plug-flow feeding for 30 min, R3: anaerobic plug-flow feeding for 120 min). The results showed that the granulation time of R1, R2, and R3 was similar (granulation completion on Day 13, 13, and 10), whereas the particles cultivated in R3 had dense structure and high PN/PS content. Under hypersaline conditions, all three SBRs could achieve high TOC removal efficiencies (>97%), NH4+-N removal efficiencies of R1 and R2 were both greater than 96%, while R3 was fluctuated between 50%~70%, which might be due to the inhibition of nitrification reaction by sulfate-reducing bacteria (Desulfovibrio and Fusibacter) with the generation of S2- during the long anaerobic plug-flow feeding period. This study can provide a useful reference for aerobic granulation in hypersaline wastewater.
-

-
表 1 反应器运行参数
Table 1. Operational conditions for three SBRs
反应器 进水时间/min 曝气时间/min 沉降时间/min 排水时间/min 静置时间/min R1 4 348~351 5~2* 2 1 R2 30 322~325 5~2* 2 1 R3 120 232~235 5~2* 2 1 注:*表示第0~8 d沉降时间为5 min,第9~16 d为4 min,第17~22 d为3 min,第23~66 d为2 min。 表 2 属水平功能微生物分布
Table 2. Functional groups on genus level
关键物种(属水平) 相对丰度/% 参考文献 S0 S1 S2 S3 GAO Kineosphaera 0 0 0.01 0.03 [56] PAO Tessaracoccus 1.26 9.87 32.11 6.48 [57] Corynebacterium 0.37 0.01 0.01 2.14 [58] Halomonas 1.9 0.12 0.17 0.03 [59] Micropruina 1 0.38 0.97 1.15 [60] Pseudomonas 0.1 0 0.07 0.06 [58] AOB Nitrosomonas 0.06 0.04 0.03 0.01 [61] DNB unclassified_f__Rhodobacteraceae 4.33 12.1 6.06 1.17 [51] Aequorivita 0.18 3.27 2.39 2.87 [62] Azoarcus 0.03 1.24 0.67 2.64 [63] SRB Fusibacter 0.08 0.06 0.05 5.44 [64] Desulfovibrio 0 0.01 0.02 1.06 [65] -
[1] HE H J, CHEN Y J, LI X, et al. Influence of salinity on microorganisms in activated sludge processes: A review[J]. International Biodeterioration & Biodegradation, 2017, 119: 520-527. [2] SARVAJITH M, NANCHARAIAH Y V. Biological nutrient removal by halophilic aerobic granular sludge under hypersaline seawater conditions[J]. Bioresource Technology, 2020, 318: 124065. doi: 10.1016/j.biortech.2020.124065 [3] WANG Z H, GAO M C, SHE Z L, et al. Effects of salinity on performance, extracellular polymeric substances and microbial community of an aerobic granular sequencing batch reactor[J]. Separation and Purification Technology, 2015, 144: 223-231. doi: 10.1016/j.seppur.2015.02.042 [4] 于建国, 韩昫身, 金艳. 页岩气压裂返排液生物处理技术研究进展[J]. 石油与天然气化工, 2022, 51(5): 131-138. [5] 赵佳睿. 盐度对好氧颗粒污泥形成过程及胞外聚合物特性的影响[D]. 哈尔滨: 哈尔滨工业大学, 2022. [6] 雷蕾. 同步脱氮除碳耐盐好氧颗粒污泥培育过程性能研究[D]. 上海: 华东理工大学, 2021. [7] YUE J X, HAN X S, JIN Y, et al. Performance and Bacterial Characteristics of Aerobic Granular Sludge in Treatment of Ultra-Hypersaline Mustard Tuber Wastewater[J]. Fermentation, 2023, 9(3): 224. doi: 10.3390/fermentation9030224 [8] 陈翱翔. 好氧颗粒污泥SBR处理页岩气开采水力压裂返排废水的研究[D]. 重庆: 重庆大学, 2017. [9] PISHGAR R, DOMINIC J A, SHENG Z Y, et al. Influence of operation mode and wastewater strength on aerobic granulation at pilot scale: Startup period, granular sludge characteristics, and effluent quality[J]. Water Resarch, 2019, 160: 81-96. doi: 10.1016/j.watres.2019.05.026 [10] PRONK M, ABBAS B, AL-ZUHAIRY S H, et al. Effect and behaviour of different substrates in relation to the formation of aerobic granular sludge[J]. Applied Microbiology and Biotechnology, 2015, 99(12): 5257-5268. doi: 10.1007/s00253-014-6358-3 [11] IORHEMEN O T, LIU Y. Effect of feeding strategy and organic loading rate on the formation and stability of aerobic granular sludge[J]. Journal of Water Process Engineering, 2021, 39: 101709. doi: 10.1016/j.jwpe.2020.101709 [12] P. S. G. DA SILVA V E, DE SOUSA ROLLEMBERG S L, BEZERRA DOS SANTOS A. Impact of feeding strategy on the performance and operational stability of aerobic granular sludge treating high-strength ammonium concentrations[J]. Journal of Water Process Engineering, 2021, 44: 102378. [13] 李冬, 曹思雨, 王琪, 等. 厌氧推流进水对反硝化除磷好氧颗粒污泥系统的影响[J]. 环境科学, 2021, 42(09): 4399-4405. [14] BEUN J J, HENDRIKS A, VAN LOOSDRECHT M C M, et al. Aerobic granulation in a sequencing batch reactor[J]. Water Research, 1999, 33(10): 2283-2290. doi: 10.1016/S0043-1354(98)00463-1 [15] TAY J H, LIU Q S, LIU Y. Microscopic observation of aerobic granulation in sequential aerobic sludge blanket reactor[J]. Journal of Applied Microbiology, 2001, 91(1): 168-175. doi: 10.1046/j.1365-2672.2001.01374.x [16] DE BRUIN L, DE KREUK M, VAN DER ROEST H, et al. Aerobic granular sludge technology: an alternative to activated sludge?[J]. Water Science and Technology, 2004, 49(11-12): 1-7. doi: 10.2166/wst.2004.0790 [17] 郝晓地, 孙晓明, LOOSDRECHT M V. 好氧颗粒污泥技术工程化进展一瞥[J]. 中国给水排水, 2011, 27(20): 9-12. [18] YUAN Q, GONG H, XI H, et al. Strategies to improve aerobic granular sludge stability and nitrogen removal based on feeding mode and substrate[J]. Journal of Environmental Sciences, 2019, 84: 144-154. doi: 10.1016/j.jes.2019.04.006 [19] DINçER A R, KARGI F. Salt Inhibition of Nitrification and Denitrification in Saline Wastewater[J]. Environmental Technology, 1999, 20(11): 1147-1153. doi: 10.1080/09593332008616912 [20] WELLES L, LOPEZ-VAZQUEZ C M, HOOIJMANS C M, et al. Impact of salinity on the anaerobic metabolism of phosphate-accumulating organisms (PAO) and glycogen-accumulating organisms (GAO)[J]. Applied Microbiology and Biotechnology, 2014, 98(17): 7609-7622. doi: 10.1007/s00253-014-5778-4 [21] YUE J X, HAN X S, JIN Y, et al. Potential of direct granulation and organic loading rate tolerance of aerobic granular sludge in ultra-hypersaline environment[J]. Environmental Research, 2023, 228: 115831. doi: 10.1016/j.envres.2023.115831 [22] NIU X Y, HAN X S, JIN Y, et al. Aerobic granular sludge treating hypersaline wastewater: Impact of pH on granulation and long-term operation at different organic loading rates[J]. Journal of Environmental Management, 2023, 330: 117164. doi: 10.1016/j.jenvman.2022.117164 [23] LI X L, LUO J H, GUO G, et al. Seawater-based wastewater accelerates development of aerobic granular sludge: A laboratory proof-of-concept[J]. Water Research, 2017, 115: 210-219. doi: 10.1016/j.watres.2017.03.002 [24] 国家环境保护总局. 水和废水监测分析方法[M]. 第4版. 北京: 中国环境科学出版社, 2002: 211-213. [25] TANG R, HAN X S, JIN Y, et al. Do increased organic loading rates accelerate aerobic granulation in hypersaline environment?[J]. Journal of Environmental Chemical Engineering, 2022, 10(6): 108775. doi: 10.1016/j.jece.2022.108775 [26] HAN X S, TANG R, LIU C S, et al. Rapid, stable, and highly-efficient development of salt-tolerant aerobic granular sludge by inoculating magnetite-assisted mycelial pellets[J]. Chemosphere, 2023, 339: 139645. doi: 10.1016/j.chemosphere.2023.139645 [27] GENG M Y, MA F, GUO H J, et al. Enhanced aerobic sludge granulation in a Sequencing Batch Reactor (SBR) by applying mycelial pellets[J]. Journal of Cleaner Production, 2020, 274: 123037. doi: 10.1016/j.jclepro.2020.123037 [28] 金正宇, 郗皓, 苑泉, 等. 进水模式对强化脱氮好氧颗粒污泥培养的影响[J]. 中国环境科学, 2018, 38(3): 935-942. [29] HAN X S, JIN Y, YU J. Rapid formation of aerobic granular sludge by bioaugmentation technology: A review[J]. Chemical Engineering Journal, 2022, 437: 134971. doi: 10.1016/j.cej.2022.134971 [30] 陈希, 袁乙卜, 张建民, 等. 大分子有机物对除磷颗粒污泥特性及菌群结构的影响[J]. 环境科学学报, 2021, 41(4): 1309-1322. [31] HE Q L, ZHOU J, WANG H Y, et al. Microbial population dynamics during sludge granulation in an A/O/A sequencing batch reactor[J]. Bioresource Technology, 2016, 214: 1-8. doi: 10.1016/j.biortech.2016.04.088 [32] HAMZA R A, SHENG Z Y, IORHEMEN O T, et al. Impact of food-to-microorganisms ratio on the stability of aerobic granular sludge treating high-strength organic wastewater[J]. Water Research, 2018, 147: 287-298. doi: 10.1016/j.watres.2018.09.061 [33] BASSIN J P, PRONK M, KRAAN R, et al. Ammonium adsorption in aerobic granular sludge, activated sludge and anammox granules[J]. Water Research, 2011, 45(16): 5257-5265. doi: 10.1016/j.watres.2011.07.034 [34] 彭永臻, 李璐凯, 李夕耀, 等. 不同pH值及碱性物质对短程硝化的影响[J]. 北京工业大学学报, 2017, 43(10): 1554-1562. [35] WANG D H, XU S J, JIANG C C, et al. The effects, mechanisms, and applications of sulfide as both an inhibitor and electron donor in novel biological nitrogen removal process[J]. Science of the Total Environment, 2023, 894: 164784. doi: 10.1016/j.scitotenv.2023.164784 [36] ZHANG Z, ZHANG C H, YANG Y, et al. A review of sulfate-reducing bacteria: Metabolism, influencing factors and application in wastewater treatment[J]. Journal of Cleaner Production, 2022, 376: 134109. doi: 10.1016/j.jclepro.2022.134109 [37] ZHANG B, WU L, SHI W X, et al. A novel strategy for rapid development of a self-sustaining symbiotic algal-bacterial granular sludge: Applying algal-mycelial pellets as nuclei[J]. Water Research, 2022, 214: 118210. doi: 10.1016/j.watres.2022.118210 [38] SHI Y J, LIU Y. Evolution of extracellular polymeric substances (EPS) in aerobic sludge granulation: Composition, adherence and viscoelastic properties[J]. Chemosphere, 2021, 262: 128033. doi: 10.1016/j.chemosphere.2020.128033 [39] HU B, WANG T, YE J H, et al. Effects of carbon sources and operation modes on the performances of aerobic denitrification process and its microbial community shifts[J]. Journal of Environmental Management, 2019, 239: 299-305. [40] CORSINO S F, CAPODICI M, TORREGROSSA M, et al. Physical properties and Extracellular Polymeric Substances pattern of aerobic granular sludge treating hypersaline wastewater[J]. Bioresource Technology, 2017, 229: 152-159. doi: 10.1016/j.biortech.2017.01.024 [41] HOU M, LI W, LI H, et al. Performance and bacterial characteristics of aerobic granular sludge in response to alternating salinity[J]. International Biodeterioration & Biodegradation, 2019, 142: 211-217. [42] LIU X Y, PEI Q Q, HAN H Y, et al. Functional analysis of extracellular polymeric substances (EPS) during the granulation of aerobic sludge: Relationship among EPS, granulation and nutrients removal[J]. Environmental Research, 2022, 208: 112692. doi: 10.1016/j.envres.2022.112692 [43] ZHANG Y H, DONG X C, NURAMKHAAN M, et al. Rapid granulation of aerobic granular sludge: A mini review on operation strategies and comparative analysis[J]. Bioresource Technology Reports, 2019, 7: 100206. doi: 10.1016/j.biteb.2019.100206 [44] ZHAO Z W, LIU S, YANG X J, et al. Stability and performance of algal-bacterial granular sludge in shaking photo-sequencing batch reactors with special focus on phosphorus accumulation[J]. Bioresource Technology, 2019, 280: 497-501. doi: 10.1016/j.biortech.2019.02.071 [45] LEI L, YAO J C, LIU Y D, et al. Performance, sludge characteristics and microbial community in a salt-tolerant aerobic granular SBR by seeding anaerobic granular sludge[J]. International Biodeterioration & Biodegradation, 2021, 163: 105258. [46] HE Q L, WANG H Y, CHEN L, et al. Elevated salinity deteriorated enhanced biological phosphorus removal in an aerobic granular sludge sequencing batch reactor performing simultaneous nitrification, denitrification and phosphorus removal[J]. Journal of Hazardous Materials, 2020, 390: 121782. doi: 10.1016/j.jhazmat.2019.121782 [47] HUANG J L, WANG H H, ALAM F, et al. Granulation of halophilic sludge inoculated with estuarine sediments for saline wastewater treatment[J]. Science of the Total Environment, 2019, 682: 532-540. doi: 10.1016/j.scitotenv.2019.05.197 [48] LIU J J, ZHANG X Q, PAN J, et al. Aequorivita viscosa sp. nov. , isolated from an intertidal zone, and emended descriptions of Aequorivita antarctica and Aequorivita capsosiphonis[J]. International Journal of Systematic and Evolutionary Microbiology, 2013, 63: 3192-3196. [49] CHEN D Y, WANG Z M, ZHANG M L, et al. Effect of increasing salinity and low C/N ratio on the performance and microbial community of a sequencing batch reactor[J]. Environmental Technology, 2021, 42(8): 1213-1224. doi: 10.1080/09593330.2019.1660417 [50] LIANG J H, WANG Q H, LI Q X, et al. Aerobic sludge granulation in shale gas flowback water treatment: Assessment of the bacterial community dynamics and modeling of bioreactor performance using artificial neural network[J]. Bioresource Technology, 2020, 313: 123687. doi: 10.1016/j.biortech.2020.123687 [51] WANG D B, TAO L J, YANG J N, et al. Understanding the interaction between triclocarban and denitrifiers[J]. Journal of Hazardous Materials, 2021, 401: 123343. doi: 10.1016/j.jhazmat.2020.123343 [52] LIU J, GAO F, GAO M C, et al. Effects of flow mode on the performance and microbial community in tidal flow constructed wetland treating mariculture wastewater[J]. Journal of Water Process Engineering, 2022, 47: 102850. doi: 10.1016/j.jwpe.2022.102850 [53] JIANG Y, SHI X Q, NG H Y. Aerobic granular sludge systems for treating hypersaline pharmaceutical wastewater: Start-up, long-term performances and metabolic function[J]. Journal of Hazardous Materials, 2021, 412: 125229. doi: 10.1016/j.jhazmat.2021.125229 [54] CHEN Y Y, HU T H, XIONG W, et al. Enhancing robustness of activated sludge with Aspergillus tubingensis as a protective backbone structure under high-salinity stress[J]. Journal of Environmental Management, 2021, 297: 113302. doi: 10.1016/j.jenvman.2021.113302 [55] GHOSH S, CHAKRABORTY S. Aerobic granulation of single strain oil degraders: Salt tolerance enhancing organics and nitrogen removal from high-strength refinery wastewater[J]. Journal of Water Process Engineering, 2021, 42: 102104. doi: 10.1016/j.jwpe.2021.102104 [56] LIU W T. Kineosphaera limosa gen. nov. , sp. nov. , a novel Gram-positive polyhydroxyalkanoate-accumulating coccus isolated from activated sludge[J]. International Journal of Systematic and Evolutionary Microbiology, 2002, 52(5): 1845-1849. [57] STOKHOLM-BJERREGAARD M, MCILROY S J, NIERYCHLO M, et al. A Critical Assessment of the Microorganisms Proposed to be Important to Enhanced Biological Phosphorus Removal in Full-Scale Wastewater Treatment Systems[J]. Frontiers in Microbiology, 2017, 8: 718. doi: 10.3389/fmicb.2017.00718 [58] TARAYRE C, CHARLIER R, DELEPIERRE A, et al. Looking for phosphate-accumulating bacteria in activated sludge processes: a multidisciplinary approach[J]. Environmental Science and Pollution Research, 2017, 24(9): 8017-8032. doi: 10.1007/s11356-017-8490-y [59] NGUYEN H T T, NIELSEN J L, NIELSEN P H. ‘Candidatus Halomonas phosphatis’, a novel polyphosphate‐accumulating organism in full‐scale enhanced biological phosphorus removal plants[J]. Environmental Microbiology, 2012, 14(10): 2826-2837. doi: 10.1111/j.1462-2920.2012.02826.x [60] MCILROY S J, ONETTO C A, MCILROY B, et al. Genomic and in Situ Analyses Reveal the Micropruina spp. as Abundant Fermentative Glycogen Accumulating Organisms in Enhanced Biological Phosphorus Removal Systems[J]. Frontiers in Microbiology, 2018, 9: 1004. doi: 10.3389/fmicb.2018.01004 [61] ARP D J, SAYAVEDRA-SOTO L A, HOMMES N G. Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea[J]. Archives of Microbiology, 2002, 178(4): 250-255. doi: 10.1007/s00203-002-0452-0 [62] ZENG D F, MIAO J, WU G X, et al. Nitrogen removal, microbial community and electron transport in an integrated nitrification and denitrification system for ammonium-rich wastewater treatment[J]. International Biodeterioration & Biodegradation, 2018, 133: 202-209. [63] SCHIE P M V, YOUNG L Y. Isolation and Characterization of Phenol-Degrading Denitrifying Bacteria[J]. Applied and Environmental Microbiology, 1998, 64(7): 2432-2438. doi: 10.1128/AEM.64.7.2432-2438.1998 [64] BRIOUKHANOV A L, KADNIKOV V V, BELETSKY A V, et al. Aerotolerant Thiosulfate-Reducing Bacterium Fusibacter sp. Strain WBS Isolated from Littoral Bottom Sediments of the White Sea—Biochemical and Genome Analysis[J]. Microorganisms, 2023, 11(7): 1642. doi: 10.3390/microorganisms11071642 [65] HEIDELBERG J F, SESHADRI R, HAVEMAN S A, et al. The genome sequence of the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough[J]. Nature Biotechnology, 2004, 22(5): 554-559. doi: 10.1038/nbt959 -




 下载:
下载: