-
为有效防治农业杂草,实现农作物保护和农产品产量的提高,近年来除草剂的使用量逐渐增加,成为全球用量最大的一类农药[1-3]。莠去津作为三嗪类除草剂的代表被广泛用于小麦、甘蔗、玉米、高粱等多种作物田间阔叶杂草的防治[4-6]。莠去津水溶性好且半衰期长(约30—100 d),通过地表径流和雨水冲刷等方式很容易进入水体,造成其在环境中的残留问题[7]。残留在环境中的莠去津对非靶标生物和人类均有致癌致畸风险[8-9]。此外,由于残效期长、生物可降解性较低,残留在环境中的莠去津还会对后茬作物产生药害[10-11]。因此,亟需开发经济高效、二次污染少的莠去津去除技术。
目前,莠去津的去除方法主要有物理吸附、生物降解和化学降解等。Yan等[12]利用碳纳米管实现对莠去津的吸附,结果表明,莠去津的吸附量不仅与碳纳米管的比表面积大小有关,材料的表面性质也会对吸附能力产生影响。Boruah等[13]设计合成了Fe3O4/石墨烯复合材料,并用于水溶液中莠去津的吸附,磁性材料的加入,使得分离过程更易实现。郑妍婕[14]通过制备多种生物炭材料,实现土壤中莠去津的吸附。生物降解主要通过筛选各种降解菌实现,耗时较长,且微生物易受到环境因素的影响[15-16]。化学降解法具有较高稳定性,其中光催化降解技术近年来发展迅速[17-19]。TiO2、ZnO、Ag3PO4、CdS等金属纳米材料及其复合材料[20-24]等被应用于莠去津的光催化降解。由于金属催化剂自身对环境具有污染性,紫外光响应的特点又限制了光能的充分利用。因此从环境保护和能源利用角度出发,可见光响应催化剂具有更大的应用潜力。
石墨型氮化碳(g-C3N4)材料是一种新型非金属纳米催化剂,可见光响应特性使g-C3N4纳米材料对自然光的利用率大大提升[25]。g-C3N4可被用于多种污染物的光催化降解。但基本型g-C3N4材料受到比表面积较低,光致空穴和电子对重组率高等的限制,光催化活性较低。因此在实现比表面积增大的基础上,调整材料的能带结构,提高光能利用率[26-27],有助于提高材料的光催化性能。从环境保护角度出发,非金属元素掺杂在提高g-C3N4催化活性的同时还能避免金属材料的二次污染问题。
基于此,开发一种高效、环保、经济的莠去津降解技术具有重要意义。本文制备了多孔氮化碳纳米材料,并将其应用于莠去津的可见光催化降解。比较水热处理和浸泡处理两种方式对材料性能的影响,并对材料进行了系统表征分析,明确材料属性与降解性能的相互关系。同时研究莠去津浓度、pH值变化及催化剂用量对降解过程的影响。结合活性物种捕获实验,对降解机理进行了阐述。以期为高残留除草剂的污染治理提供理论和技术支持。
-
莠去津标准品(纯度
$\geqslant $ 98%,First Standard);乙腈、甲醇(色谱纯,德国Merck公司);甲酸(色谱纯,Sigma-aldrich公司);三聚氰胺、磷酸(H3PO4)(分析纯,国药集团化学试剂有限公司);叔丁醇(TBA)、碘化钾(KI)、苯醌(BQ)(阿拉丁化学试剂公司);蒸馏水,符合GB/T6682中一级水的要求。 -
BS214S分析天平(Sartorius公司,德国);X射线衍射仪(Bruker D8,美国);扫描电子显微镜(Zeiss Sigma 500,德国);UV-Vis分光光度计(PE Lambda 950,美国);X射线光电子能谱仪(Thermo Fisher K-Alpha,美国);全自动比表面积及孔隙度分析仪(Micromeritics ASAP 2460,美国);氙灯平行光源(CEL-HXF-300,北京中教金源)。
-
将10 g三聚氰胺置于50 mL坩埚内,加盖密封,放入马弗炉中。以10 °C·min−1的速率升温至550 °C并保持4 h。反应结束后,冷却至室温,收集所得产物,研磨均匀备用。所得材料标记为MCN。将2.0 g MCN材料置于80 mL, 1.0 mol·L−1的H3PO4水溶液中,搅拌15 min。混合物转移至聚苯酚内衬的高温反应釜中,200 °C反应12 h。反应后离心并收集产物,水洗至中性,最后用乙醇洗涤,60 °C烘干,标记为PCN-H。相同混合物置于烧杯中,室温浸泡12 h,离心收集产物,水洗至中性,最后用乙醇洗涤,60 °C烘干,标记为PCN-S。
-
光照反应在体积为150 mL并配有循环冷凝水的夹层烧杯中进行,采用的光照波段为可见光(λ> 400 nm)。在100 mL莠去津水溶液(2.0 mg·L−1)中加入50 mg催化剂材料,黑暗条件下搅拌30 min,使催化剂材料与目标降解物达到吸附-解吸附平衡。给予光照后,在15、30、45、60 min取出0.7 mL反应液。样品离心(10000 r·min−1,5 min)处理,取上清液过膜,待进样分析。根据光照过程中莠去津浓度的变化判断降解率。C代表取样时目标物的浓度,C0代表初始目标物的浓度。每组实验重复3次。
-
莠去津浓度利用超高效液相色谱系统(Waters, 美国)配离子阱质谱(AB SCIEX, 美国)进行检测。液相色谱柱ACQUITY UPLC BEH C18(1.7 μm, 2.1 mm × 50 mm, Waters, 美国)。流动相为(A)乙腈,(B)0.1%甲酸水。梯度洗脱程序0 min, 10% A + 90% B; 1 min, 10% A + 90% B; 4.5 min, 90% A + 10% B; 5.5 min, 90% A + 10% B; 5.6 min, 10% A + 90% B; 6.5 min, 10% A + 90% B。莠去津的定性和定量离子对分别是216.0/104.0 (m/z) 和216.0/174.1 (m/z),去簇电压(DP)为70 eV,碰撞电压(CE)为24 eV。
-
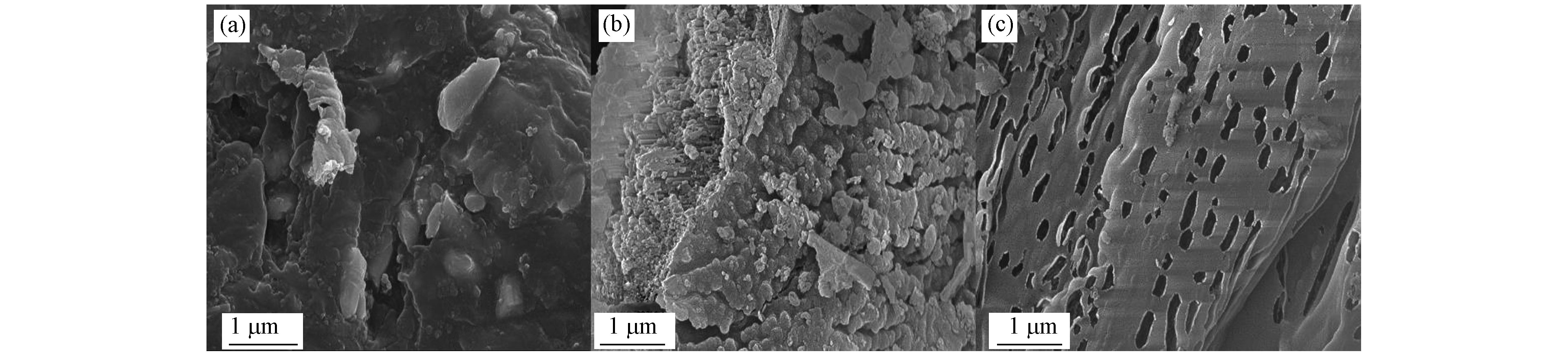
通过扫描电子显微镜(SEM)表征催化剂材料的形貌和微观结构。由图1所示,MCN(a)表面致密,呈现块状堆积结构。经过H3PO4水溶液浸泡,PCN-S(b)的表面形貌发生变化,材料表面变得粗糙。而水热反应所得材料PCN-H(c)的堆积结构被剥离为片状,且分布有众多孔状结构。
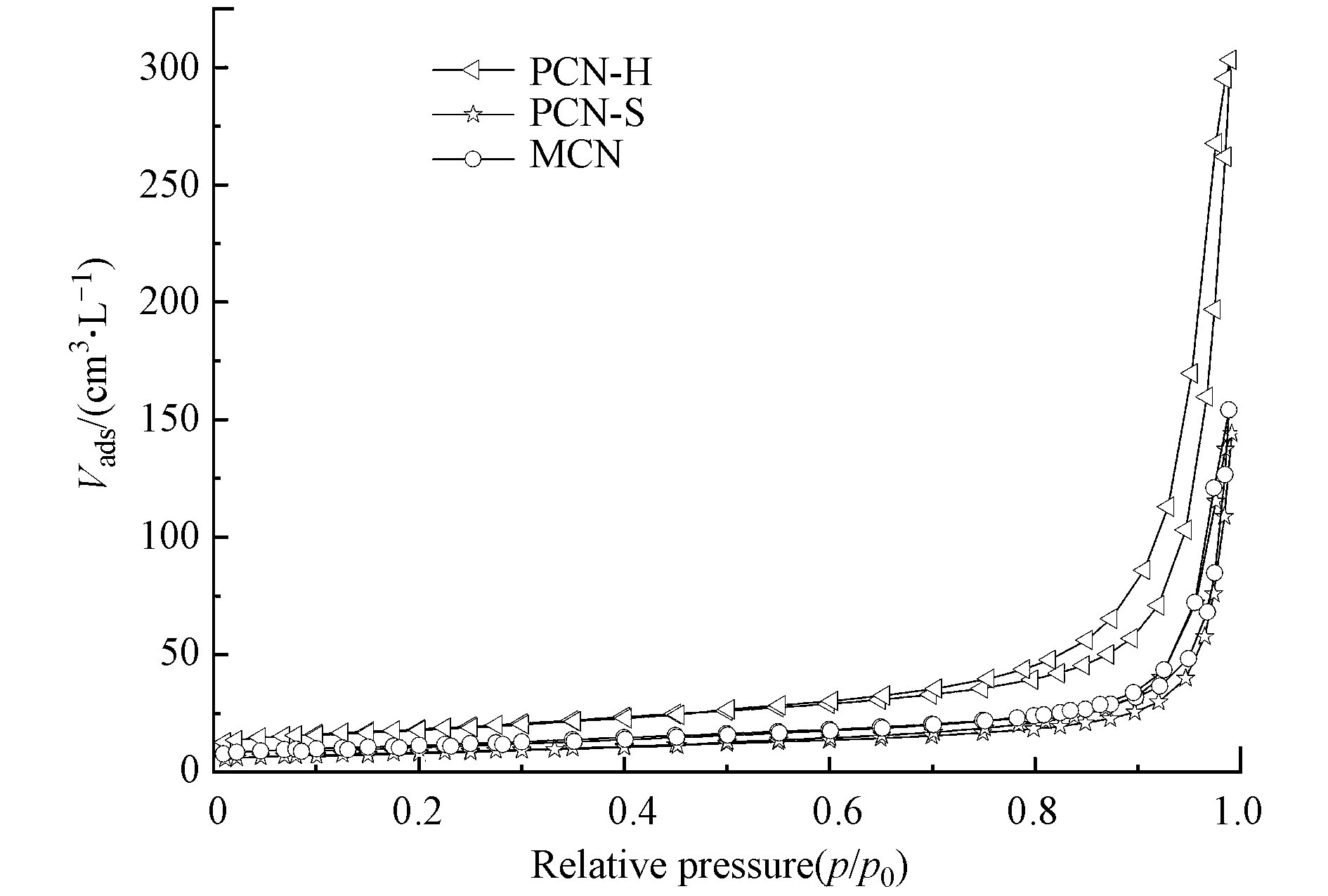
分析N2吸附-解吸附等温线(图2)可以得知,MCN,PCN-S和PCN-H均属于IV型等温线(BDDT分类),说明材料中含有介孔,与表1平均孔径数据相吻合。水热反应可以增加氮化碳材料的比表面积,PCN-H的比表面积是MCN的4.3倍,是PCN-S的3.0倍,孔体积也有显著增加。
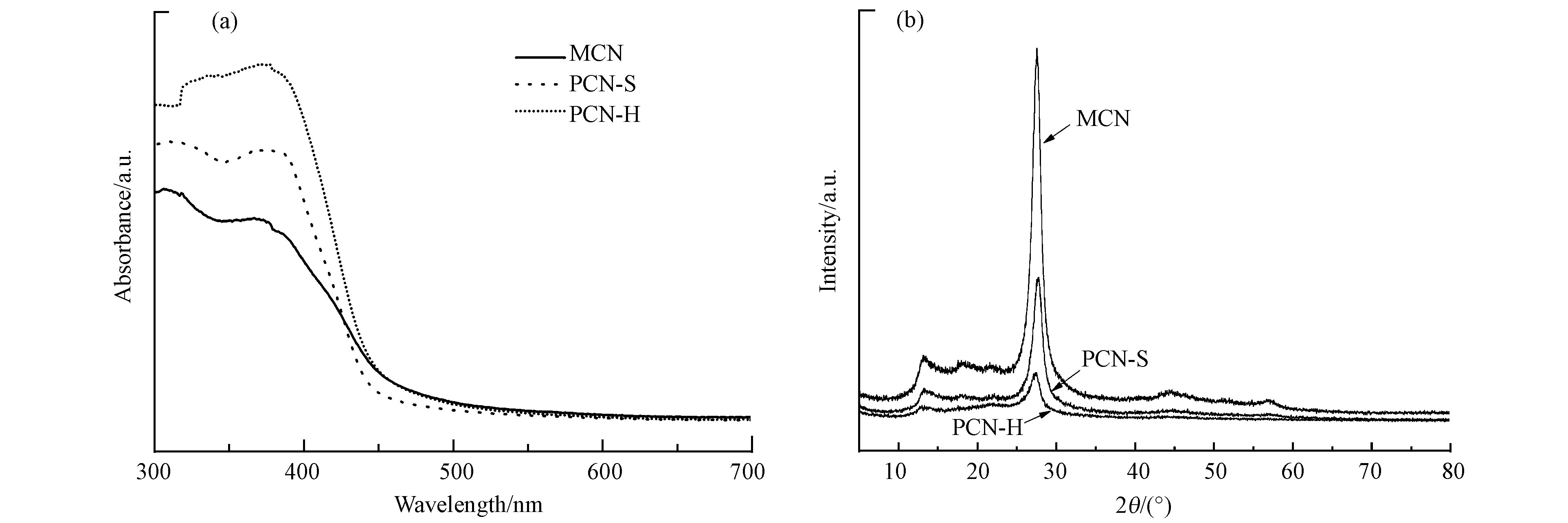
MCN,PCN-S和PCN-H的UV-Vis吸光光谱如图3(a)所示。在300—450 nm范围呈现典型的吸收峰。于MCN和PCN-S相比,PCN-H的吸光强度明显增加。X射线衍射(XRD)图谱如图3(b)所示,3种材料在12.7°和27.4°有两个特征衍射峰。位于12.7°处的衍射峰是平面内堆积的三嗪结构特征峰,位于27.4°处的衍射峰为层间堆积芳香结构的特征峰[28]。与MCN相比,PCN-S和PCN-H的衍射峰强度减弱,说明材料堆积的紧密程度下降[29],与SEM照片中观察到的形貌变化相符合。
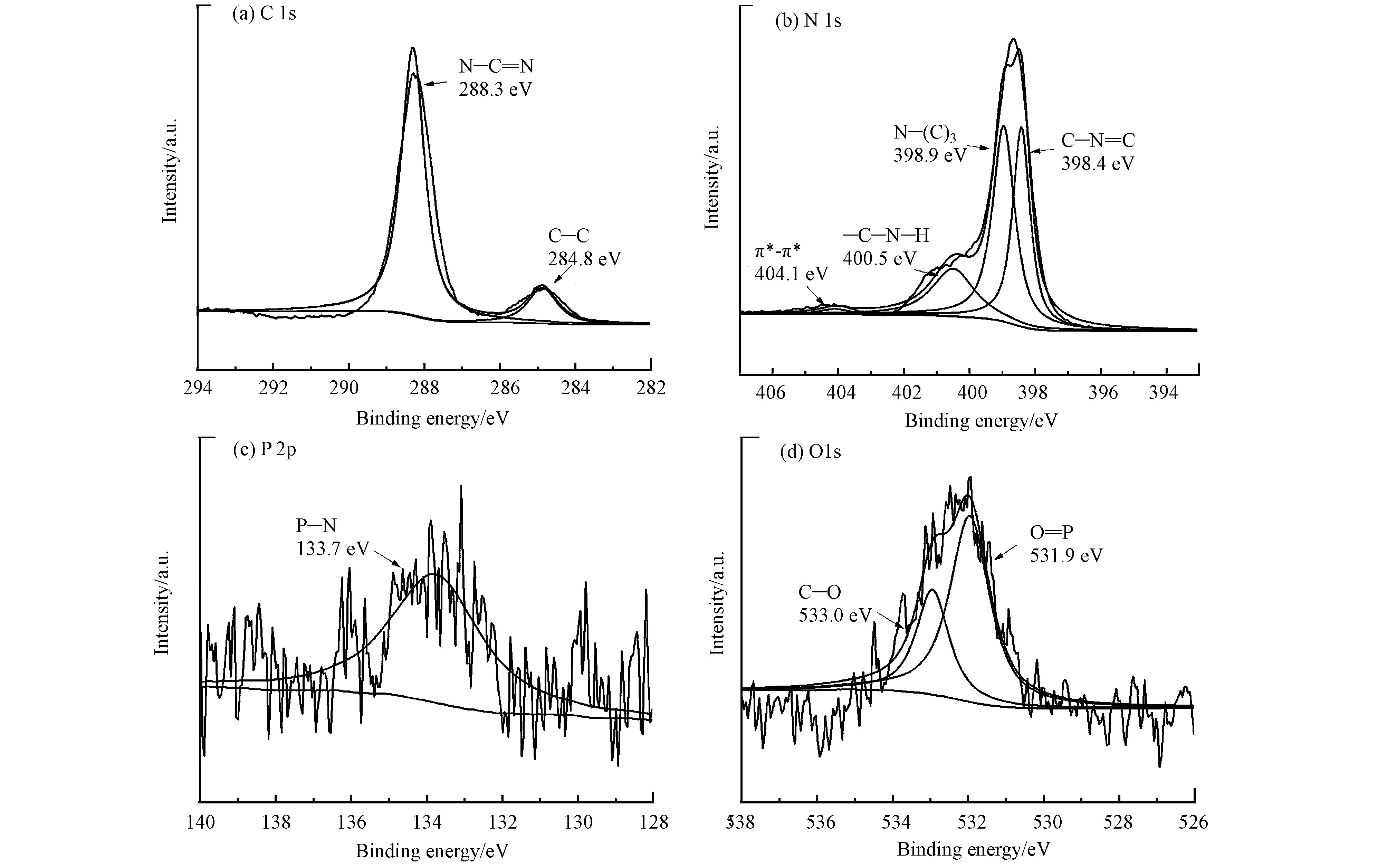
通过X射线光电子能谱(XPS)进一步明确PCN-H的元素组成及形态。从图4可知,PCN-H材料含有C、N、P、O元素。其中C 1s的分峰拟合在284.8 eV、288.3 eV处有2个峰,分别对应C—C、N—C=N。N 1s的分峰拟合在398.4 eV、398.9 eV、400.5 eV、404.1 eV处有4个峰,分别对应C—N=C、N—(C)3、C—N—H、π—π堆积[30]。P 2p的XPS分峰拟合结果表明,P元素通过化学键与氮化碳材料结合(398.9 eV),改变了材料的能带结构,进而影响可见光吸收能力,与图3(a)PCN-H材料UV-Vis吸光能力明显增强的结果相符合[31]。O 1s的分峰拟合在531.9 eV、533.0 eV处有2个峰,分别对应O=P—、C—O[32],进一步证实P的成功掺杂。
-
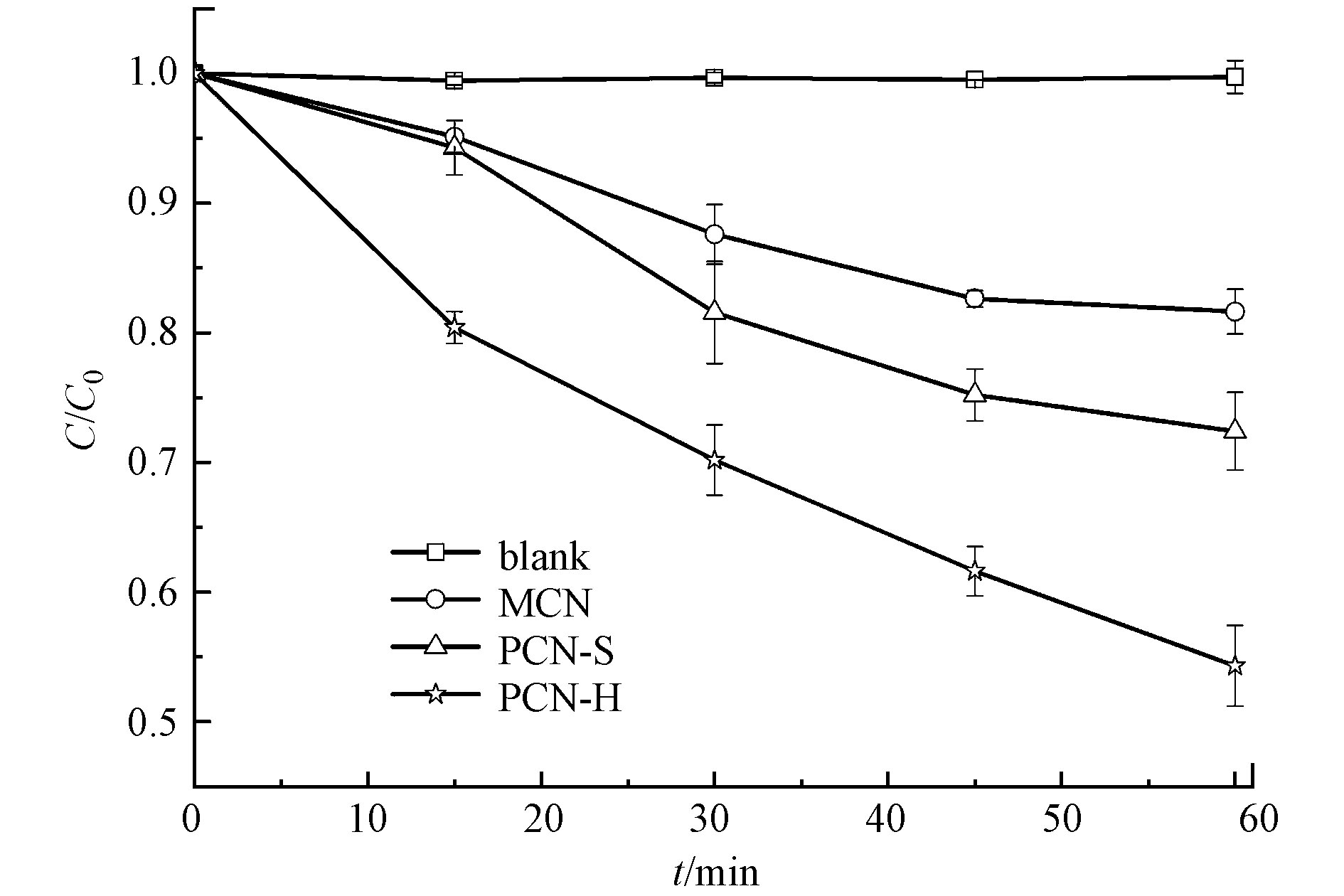
将50 mg催化剂均匀分散于起始浓度为2.0 mg·L−1的莠去津水溶液中,黑暗条件下达到吸附-解吸附平衡后,给予光照。在光照15、30、45、60 min时取样检测。从图5可以看出,莠去津在无催化剂参与时,60 min内的自身分解可以忽略。MCN对莠去津的降解率为18.4%,PCN-S对莠去津的降解率为27.6%,PCN-H对莠去津的降解率为45.7%。PCN-H对莠去津的催化效率显著提高,是MCN的2.5倍。
-
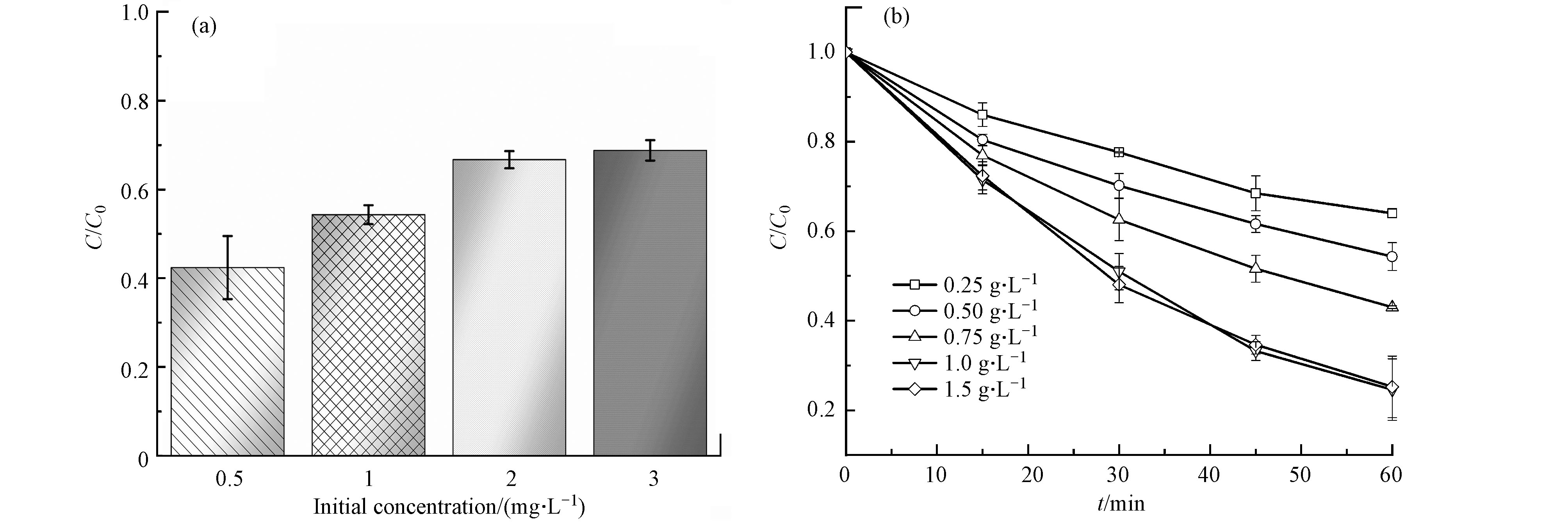
实验研究了莠去津初始浓度为0.5、1.0、2.0、3.0 mg·L−1时对降解的影响(PCN-H催化剂用量固定为50 mg)。由图6(a)可知,莠去津的降解比例在浓度为0.5 mg·L−1最高(1 h降解率为45.7%),即低浓度更有利于目标物降解。初始浓度为2.0 mg·L−1和3.0 mg·L−1时的降解效率差别较小,说明此时体系内催化剂的量与目标降解物达到饱和,在不增加光照时长的条件下,50 mg催化剂不能再降解更多的目标物。此外,实验考察的PCN-H用量范围为0.25—1.5 g L−1。由图6(b)所示,在一定范围内,莠去津的降解率随催化剂用量的增加而提高,当PCN-H用量达到1.0 g L−1时,莠去津1 h的降解率达到75.4%。继续增加PCN-H用量至1.5 g L−1时, 莠去津降解率无明显提高。
-
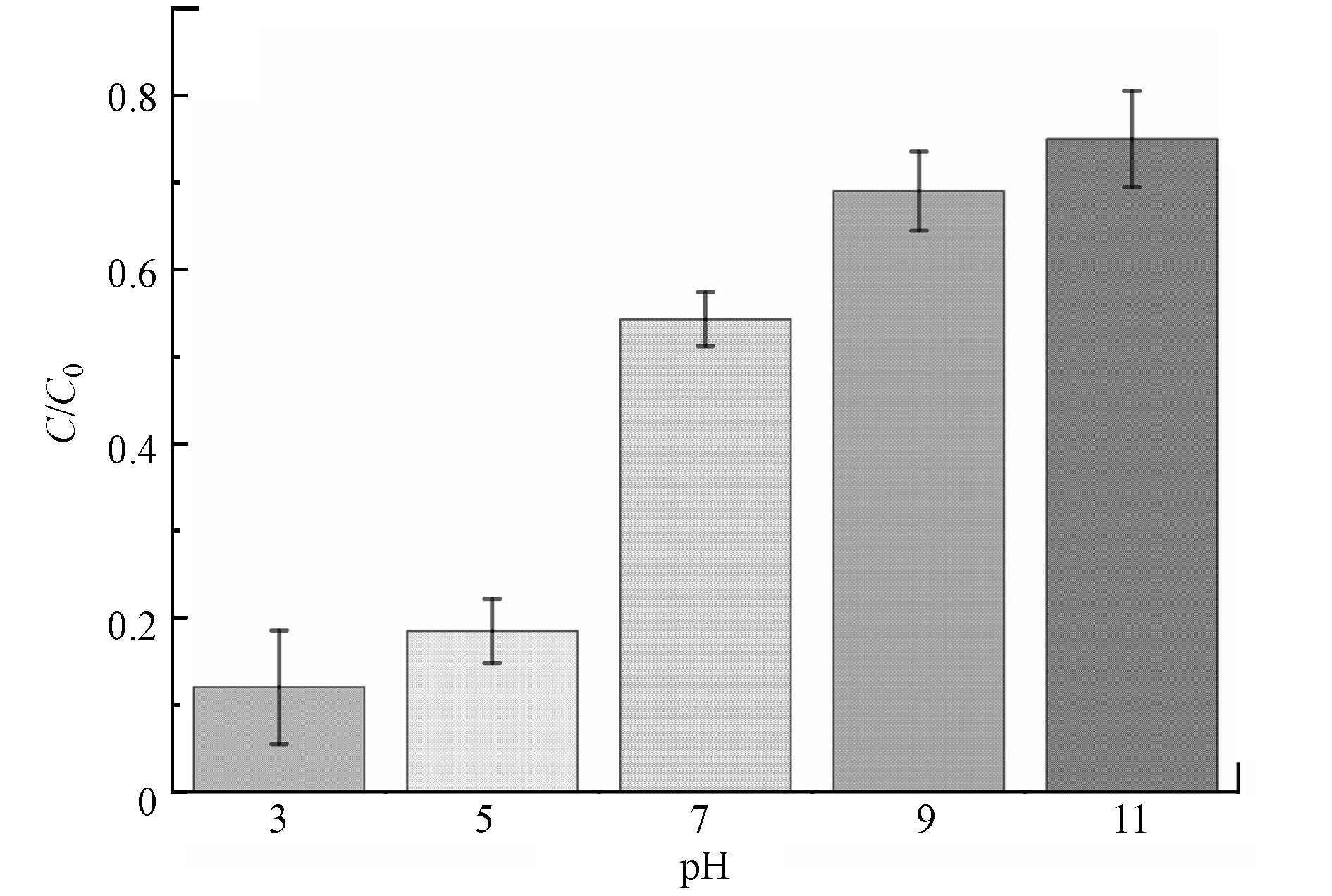
考虑到实际环境中pH值变化可能对PCN-H光催化降解莠去津性能的影响,设计实验考察了pH 3—11对莠去津降解率的影响。从图7可以看出,酸性条件下莠去津的降解率更高,而碱性条件不利于莠去津的降解。
-
催化剂PCN-H对莠去津光催化降解性能的提高主要有3方面的原因,一是材料比表面积的增加提供了更多反应活性位点。MCN的紧密堆积结构,使得材料与目标降解物的有效接触面积减小。而通过水热反应,PCN-H结构被有效剥离,呈现薄层状,且表面富孔状结构。片层状结构有利于光致空穴和电子的分离,降低重组率[33]。其次,PCN-H的孔体积(0.48 m3·g−1)是MCN(0.11 m3·g−1)的4.4倍,多孔结构不仅为光致电子的分离和传输提供了通道,还增加了可见光在材料内部的反射与吸收,使得光能利用率进一步提高[34]。第三,水热釜提供了高温高压环境,使得团聚的MCN材料实现良好的分散[35],P元素介入到氮化碳结构单元中,而非通过物理吸附结合在表面。通过与PCN-S对莠去津降解效率对比可以看出,水热反应比单纯的磷酸溶液浸泡对催化剂的性能提升更为明显。本实验选的择磷酸为磷源,成本低,且酸性条件有利于水热反应的进行。由于P元素的掺杂,催化剂的UV-Vis吸收边也发生了明显变化,PCN-H可见光吸收能力得到显著增强。与已有的金属元素掺杂及金属化合物复合等方式相比,本研究采用的非金属元素掺杂手段制备简便且更具环保性。
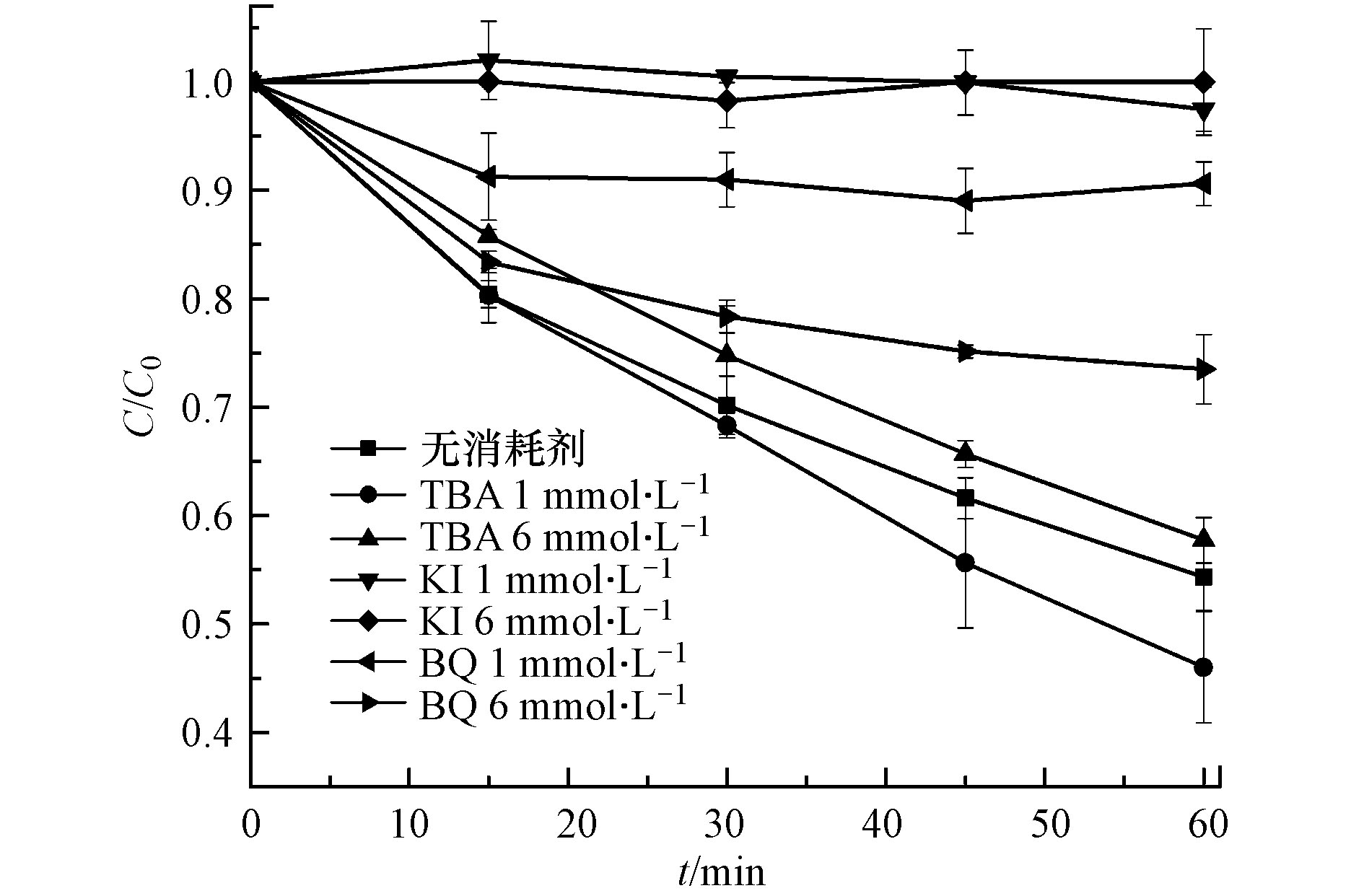
可见光催化降解过程中的主要活性物种有羟基自由基(•OH)、光致空穴(h+)和超氧自由基(•O2−)[36]。通过活性物种捕获实验对莠去津的降解机理进行了分析。由图8可知,当体系加入低浓度(1 mmol·L−1)TBA消耗•OH时,莠去津的降解效率与未加入消耗剂时相比没有发生明显变化,增大TBA的浓度至6 mmol·L−1时,莠去津的降解受到轻微抑制。由此可知,在PCN-H可见光催化莠去津降解过程中,•OH并不发挥主要作用,这与传统的TiO2等金属催化剂在紫外光下降解莠去津的机理有所不同[37]。当h+和•O2−分别被KI和BQ消耗后,莠去津的降解受到极大抑制。尤其在h+被消耗后,莠去津几乎不能被降解。值得注意的是,•O2−消耗剂浓度增加时,对莠去津的降解抑制率反而下降。由此推测,•O2−消耗量的增大伴随着更多e−的消耗,在一定程度上促进了h+和e−分离,使更多h+参与到莠去津的降解中,从而使得降解率提高。因此在PCN-H可见光催化降解莠去津的过程中,活性物种的作用排序为h+ > •O2− > •OH。
可能的降解机理归纳为式(1)—(5)。由前述实验结果可知,酸性条件更有利于莠去津的降解,结合降解机理式(2)分析,酸性条件下,反应体系内存在较多游离的H+,抑制了h+与水的反应,使更多h+可以参与到莠去津的光催化降解过程中,进而提高降解效率。而当反应体系为碱性时,产生的游离H+被不断消耗,更多的h+参与到式(2)的反应中,而不能参与莠去津的降解,因此降解率下降,碱性越大,抑制越明显,与“2.3.2”的实验结果相一致。
-
(1)磷酸水热处理可以显著提升氮化碳纳米材料的比表面积,形成表面多孔的形貌特点。比表面积增加使催化剂更好的与莠去津分子反应,进而提高降解效率。非金属磷元素掺杂起到了调节材料能带隙的作用,增强了氮化碳材料的可见光吸收能力。
(2)50 mg PCN-H催化剂在1 h内对莠去津的降解率可达45.7%(起始浓度为2.0 mg·L−1)。光致空穴起到主要的降解作用。
(3)酸性反应条件和增大催化剂用量,更有利于莠去津的降解。由于工农业废水多为酸性废水,PCN-H在酸性条件下对莠去津降解活性高的特点使其具有更好的应用前景。
多孔氮化碳纳米材料光催化降解莠去津的性能及机理研究
Fabrication porous carbon nitride for photocatalytic degradation of atrazine: Influencing parameters and mechanism
-
摘要: 本文利用磷酸水热法制备了多孔氮化碳(PCN-H)纳米材料,通过紫外-可见吸光光谱、扫描电子显微镜、X射线衍射、X射线光电子能谱和比表面积分析等多种手段表征催化剂的形貌、光学属性及结构特点等。在可见光照射下光催化降解除草剂莠去津,评价材料对莠去津的催化降解活性。分别测试最优化氮化碳材料在不同pH条件下对莠去津降解效率的变化,并分析催化剂用量和除草剂浓度对降解率的影响。结合活性物种捕获实验,阐述莠去津可见光降解的机理。通过材料表征结果分析,PCN-H表现为独特的多孔结构,比表面积分别是基础石墨型氮化碳(MCN)和磷酸浸泡氮化碳(PCN-S)的4.3倍和3.0倍。磷酸水热处理成功实现磷元素的掺杂,可见光利用率明显提高,1 h内即可将莠去津降解率从18.4%提升至45.7%。酸性条件有助于PCN-H对莠去津催化降解。在PCN-H可见光催化降解莠去津的过程中,光致空穴和超氧自由基发挥主要作用。该方法制备的材料光能利用率高,避免了金属催化剂自身对环境的潜在污染,酸性条件下降解更为高效,有助于减轻农药污染对农业生态环境及非靶标生物造成的负面影响。Abstract: To improve the photocatalytic efficiency, bulk carbon nitride (MCN) was treated by hydrothermal process with phosphoric acid in the present study. The resulting sample was denoted as PCN-H. The morphology, optical properties, and structure of prepared CN samples were characterized by UV-Vis spectrophotometer, scanning electron microscope, X-ray diffraction, X-ray photoelectron spectroscopy, and BET analysis. The effect of the degradation of atrazine by PCN-H was investigated. The reaction mechanism was identified via active species trapping experiments. PCN-H with porous structure was successfully fabricated. The Brunauer-Emmett-Teller (BET) surface area of PCN-H is about 4.3 times and 3.0 times than that of MCN and PCN-S, respectively. Compared to PCN-S and MCN, the enhanced light absorption ability was obtained via P doping. The degradation percentage increased from 18.4% to 45.7% just in an hour. Acidic condition is conducive to high degradation efficiency. Light induced hole and superoxide radical are the main active species of PCN-H during the photocatalytic degradation of atrazine. The as-prepared PCN-H exhibits considerably high photocatalytic activity under visible light irradiation, which can also overcome contamination problem caused by metal catalyst. This work proved that PCN-H has great potential to be applied in alleviating negative effects of pesticide on agricultural ecological environment and non-target organisms.
-
Key words:
- carbon nitride /
- doping /
- atrazine /
- photocatalysis /
- mechanism
-

-
表 1 催化剂CN材料的比表面积,孔体积和平均孔径
Table 1. The SBET, pore volume and average pore diameter of the prepared CN samples
材料 Sample 比表面积 SBET/(m2·g−1) 孔体积 /(m3 ·g−1) Pore volumes 平均孔径/nm Average pore diameters MCN 14.10 0.11 32.17 PCN-S 20.36 0.21 28.19 PCN-H 60.25 0.48 23.73 -
[1] ESTEVEZ E, CABERA M D, FERNÁNDEZ-VERA J R, et al. Monitoring priority substances, other organic contaminants and heavy metals in a volcanic aquifer from different sources and hydrological processes [J]. Science of The Total Environment, 2016, 551/552: 186-196. doi: 10.1016/j.scitotenv.2016.01.177 [2] SCHNOOR B, ELHENDAWY A, JOSEPH S, et al. Engineering atrazine loaded poly (lactic-co-glycolic acid) nanoparticles to ameliorate environmental challenges [J]. Journal of Agricultural and Food Chemistry, 2018, 66(30): 7889-7898. doi: 10.1021/acs.jafc.8b01911 [3] TANG H, DAI Z, XIE X, et al. Promotion of peroxydisulfate activation over Cu0.84Bi2.08O4 for visible light induced photodegradation of ciprofloxacin in water matrix [J]. Chemical Engineering Journal, 2019, 356: 472-482. doi: 10.1016/j.cej.2018.09.066 [4] BEAN B W, PICCINNI G, SALISBURY C D. Wheat cultivar tolerance to atrazine [J]. Journal of Production Agriculture, 1999, 12(4): 597-600. doi: 10.2134/jpa1999.0597 [5] SINGH S, KUMAR V, CHAUHAN A, et al. Toxicity, degradation and analysis of the herbicide atrazine [J]. Environmental Chemistry Letters, 2018, 16(1): 211-237. doi: 10.1007/s10311-017-0665-8 [6] SOLOMON K R, BAKER D B, RICHARDS R P, et al. Ecological risk assessment of atrazine in North American surface water [J]. Environmental Toxicology and Chemistry, 1996, 15(1): 31-74. doi: 10.1002/etc.5620150105 [7] COMBER S D W. Abiotic persistence of atrazine and simazine in water [J]. Pesticide Science, 1999, 55(7): 696-702. doi: 10.1002/(SICI)1096-9063(199907)55:7<696::AID-PS11>3.0.CO;2-7 [8] HAYES T, HASTON K, TSUI M, et al. Herbicides: Feminization of male frogs in the wild [J]. Nature, 2002, 419(6910): 895-896. doi: 10.1038/419895a [9] FAN W, YANASE T, MORINAGA H, et al. Atrazine-induced aromatase expression is SF-1 dependent: Implications for endocrine disruption in wildlife and reproductive cancers in humans [J]. Environmental Health Perspectives, 2007, 115(5): 720-727. doi: 10.1289/ehp.9758 [10] 范润珍, 钱传范, 卢向阳. 敌克松解除莠去津对水稻药害的使用技术研究 [J]. 农药, 1999, 38(5): 21-23. FAN R Z, QIAN C F, LU X Y. Study on the elimination of the phytotoxicity of atrazine to rice using fenaminosulf [J]. Agrochemicals, 1999, 38(5): 21-23(in Chinese).
[11] 郝文波, 李丽春, 韩云, 等. 6种长效除草剂土壤残留致烟草药害症状及其致害临界值 [J]. 广东农业科学, 2013, 9: 80-82. doi: 10.3969/j.issn.1004-874X.2013.10.024 HAO W B, LI L C, HAN Y, et al. Study of critical concentration and symptoms in tobacco phytotoxicity caused by six soil residual herbicides [J]. Guangdong Agricultural Sciences, 2013, 9: 80-82(in Chinese). doi: 10.3969/j.issn.1004-874X.2013.10.024
[12] YAN X M, SHI B Y, LU J J, et al. Adsorption and desorption of atrazine on carbon nanotubes [J]. Journal of Colloid and Interface Science, 2008, 321(1): 30-38. doi: 10.1016/j.jcis.2008.01.047 [13] BORUAH P, SHARMA B, HUSSAIN N, et al. Magnetically recoverable Fe3O4/graphene nanocomposite towards efficient removal of triazine pesticides from aqueous solution: Investigation of the adsorption phenomenon and specific ion effect [J]. Chemosphere, 2017, 168: 1058-1067. doi: 10.1016/j.chemosphere.2016.10.103 [14] 郑妍婕. 生物炭对莠去津在土壤中的吸附及后茬作物的影响研究[D]. 北京: 中国农业科学院, 2019. ZHENG Y J. Effects of biochars on atrazine adsorption in soil and succession crops[D]. Beijng: Chinese Academy of Agricultural Sciences, 2019 (in Chinese).
[15] SINGH S, KUMAR V, UPADHYAY N, et al. The effects of Fe(Ⅱ), Cu(Ⅱ) and humic acid on biodegradation of atrazine [J]. Journal of Environmental Chemical Engineering, 2020, 8(2): UNSP103539. doi: 10.1016/j.jece.2019.103539 [16] SÁNCHEZ-SÁNCHEZ R, AHUATZI-CHACÓN D, GALÍNDEZ-MAYER J, et al. Removal of triazine herbicides from aqueous systems by a biofilm reactor continuously or intermittently operated [J]. Journal of Environmental Management, 2013, 128: 421-426. doi: 10.1016/j.jenvman.2013.05.050 [17] CHONG M N, JIN B, CHOW C W K, et al. Recent developments in photocatalytic water treatment technology: A review [J]. Water Research, 2010, 44(10): 2997-3027. doi: 10.1016/j.watres.2010.02.039 [18] STYLIDI M, KONDARIDES D I, VERYKIOS X E. Visible light-induced photocatalytic degradation of Acid Orange 7 in aqueous TiO2 suspensions [J]. Applied Catalysis B:Environmental, 2007, 47(3): 189-201. [19] ZHANG H, LV X J, LI Y M, et al. P25-graphene composite as a high performance photocatalyst [J]. ACS Nano, 2010, 4(1): 380-386. doi: 10.1021/nn901221k [20] PARRA S, STANCA S E, GUASAQUILLO I, et al. Photocatalytic degradation of atrazine using suspended and supported TiO2 [J]. Applied Catalysis B:Environmental, 2004, 51(2): 107-116. doi: 10.1016/j.apcatb.2004.01.021 [21] CHATTERJEE D, MAHATA A. Evidence of superoxide radical formation in the photodegradation of pesticide on the dye modified TiO2 surface using visible light [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2004, 165(1/2/3): 19-23. [22] FENOLL J, HELLÍN P, MARTÍNEZ C M, et al. Semiconductor-sensitized photodegradation of s-triazine and chloroacetanilide herbicides in leaching water using TiO2 and ZnO as catalyst under natural sunlight [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2012, 238(15): 81-87. [23] MOHAGHEGH N, TASVIRI M, RAHIMI E, et al. Comparative studies on Ag3PO4/BiPO4-metal-organic framework-graphene-based nanocomposites for photocatalysis application [J]. Applied Surface Science, 2015, 351: 216-224. doi: 10.1016/j.apsusc.2015.05.135 [24] JO W K, LEE J Y, SELVAM N C S. Synthesis of MoS2 nanosheets loaded ZnO-g-C3N4 nanocomposites for enhanced photocatalytic applications [J]. Chemical Engineering Journal, 2016, 289: 306-318. doi: 10.1016/j.cej.2015.12.080 [25] WANG X C, MAEDA K, THOMAS A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light [J]. Nature Materials, 2009, 8(1): 76-80. doi: 10.1038/nmat2317 [26] JIANG W J, RUAN Q S, XIE J J, et al. Oxygen-doped carbon nitride aerogel: A self-supported photocatalyst for solar-to-chemical energy conversion [J]. Applied Catalysis B:Environmental, 2018, 236: 428-435. doi: 10.1016/j.apcatb.2018.05.050 [27] 常方, 黄韬博, 陈龙, 等. 不同光波长对类石墨相氮化碳催化降解莫西沙星的机理探究 [J]. 环境化学, 2020, 39(3): 593-600. doi: 10.7524/j.issn.0254-6108.2019102206 CHANG F, HUANG T B, CHEN L, et al. Photocatalytic degradation mechanism of moxifloxacin by g-C3N4 under various light wavelengths [J]. Environmental Chemistry, 2020, 39(3): 593-600(in Chinese). doi: 10.7524/j.issn.0254-6108.2019102206
[28] 谢治杰, 冯义平, 张钱新, 等. Z型MoO3/g-C3N4复合催化剂用于可见光降解萘普生的机制研究 [J]. 环境化学, 2019, 38(8): 1724-1734. doi: 10.7524/j.issn.0254-6108.2018100101 XIE Z J, FENG Y P, ZHANG Q X, et al. Photocatalytic degradation mechanism of naproxen using Z-scheme MoO3 /g-C3N4 under visiblelight irradiation [J]. Environmental Chemistry, 2019, 38(8): 1724-1734(in Chinese). doi: 10.7524/j.issn.0254-6108.2018100101
[29] NIU P, ZHANG L L, LIU G, et al. Graphene-like carbon nitride nanosheets for improved photocatalytic activities [J]. Advanced Functional Materials, 2012, 22(22): 4763-4770. doi: 10.1002/adfm.201200922 [30] HE N, CAO S, ZHANG L, et al. Enhanced photocatalytic disinfection of Escherichia coli K-12 by porous g-C3N4 nanosheets: Combined effect of photo-generated and intracellular ROSs [J]. Chemosphere, 2019, 235: 1116-1124. doi: 10.1016/j.chemosphere.2019.07.007 [31] GUO S E, DENG Z P, LI M X, et al. 2016. Phosphorus-doped carbon nitride tubes with a layered micro-nanostructure for enhanced visible-light photocatalytic hydrogen evolution [J]. Angewandte Chemie-International Edition, 2016, 55: 1830-1834. doi: 10.1002/anie.201508505 [32] Albero J, Vidal A, Migani A, et al. Phosphorous-doped graphene as a metal-free material for thermochemical water reforming at unusually mild conditions [J]. ACS Sustainable Chemistry & Engineering, 2019, 7: 838-846. [33] HOU Y, LI J, WEN Z, et al. N-doped graphene/porous g-C3N4 nanosheets supported layered-MoS2 hybrid as robust anode materials for lithium-ion batteries [J]. Nano Energy, 2014, 8: 157-164. doi: 10.1016/j.nanoen.2014.06.003 [34] TIAN J, NING R, LIU Q, et al. Three-dimensional porous supramolecular architecture from ultrathin g-C3N4 nanosheets and reduced graphene oxide: Solution self-assembly construction and application as a highly efficient metal-free electrocatalyst for oxygen reduction reaction [J]. ACS Applied Materials & Interfaces, 2014, 6(2): 1011-1017. [35] 殷婷婷, 王国宏, 韩德艳, 等. 水热法制备纳米二氧化钛的研究进展 [J]. 广州化工, 2012, 40(5): 10-12. doi: 10.3969/j.issn.1001-9677.2012.05.005 YIN T T, WANG G H, HAN D Y, et al. Research progress in hydrothermal synthesis of nano-titania [J]. Guangzhou Chemical Industry, 2012, 40(5): 10-12(in Chinese). doi: 10.3969/j.issn.1001-9677.2012.05.005
[36] LEI H, ZHANG H H, ZOU Y, et al. Synergetic photocatalysis/piezocatalysis of bismuth oxybromide for degradation of organic pollutants [J]. Journal of Alloys and Compounds, 2019, 809: UNSP151840. doi: 10.1016/j.jallcom.2019.151840 [37] PELIZZETTI E, MAURINO V, MINERO C, et al. Photocatalytic degradation of atrazine and other s-triazine herbicides [J]. Environmental Science & Technology, 1990, 24(10): 1559-1565. -



 下载:
下载: