-
随着工业发展和人类活动的增加,采矿、电镀、冶金等行业所造成的重金属排放已经成为全球性的环境污染问题之一. 如含有铅、锌、汞、铬等重金属离子的工矿业废水会进入各种水域或土壤中. 重金属会通过生物积累和食物链的富集对人体健康造成严重危害[1-3]. 而Cd2+作为最具毒性的重金属离子之一[4-5],很容易在各种器官和组织中积累,从而导致骨骼、肾脏损伤和癌症等病症[6-8]. 生物质炭具有比表面积大、孔隙结构丰富等优点[9-11],已经被证实能够吸附水体中的重金属离子[12-14]. 虽然生物质炭对重金属的吸附效果显著,但是其吸附效果受到原材料和制备温度的影响. 如炭化温度的增加会造成生物质炭表面官能团减少[15],但却会适量增加孔隙结构,从而影响生物质炭对重金属离子的吸附性能. 而原材料的差异是影响生物质炭特性的主要因素,如原材料中所含的元素种类越多,越有利于生物质炭的化学沉淀的形成. 因此原材料的种类也将是影响生物质炭固定重金属的因素之一.
园林废弃物是固体废弃物之一,在城市垃圾填埋场倾倒的垃圾中约有25%是花园垃圾[16]. 城市地区的木质生物质是一个潜在的、巨大的、未被充分利用的资源,这种资源随着城市和绿地面积的扩大而不断增加[17]. 园林废弃物常用的处理方式有堆肥、掩埋、粉碎覆盖和直接燃烧等[18]. 但是这些方法存在降解速率慢和对环境有二次污染等缺点. 而将其制备成生物质炭不仅可以有效缓解园林固体废弃物处置问题,而且有利于降低环境污染修复的成本. 如今已经有研究证实园林类生物质炭能有效吸附固定重金属,例如600 ℃制备的硬木生物质炭被证实能吸附水溶液中47.66 mg·g−1的Pb2+[13]. Ippolito 等[19]发现美国黑松生物质炭能通过提高pH值形成重金属矿物沉淀来降低Cd2+的生物可利用度. 周润娟[20]发现不同原材料的生物质炭对Cd2+的最大吸附容量有很大差异 (水葫芦茎粉:84.79 mg·g−1、根粉:103.57 mg·g−1和植株:142.59 mg·g−1). 汤嘉雯等[21]发现加拿大一枝黄花的茎或茎叶混合物制备而成的生物质炭对水中Cd2+的吸附机理包括离子交换、络合反应、沉淀作用和物理吸附等,但是没对主要机理进行剖析. 园林废弃物除了种类外,其自身的不同部位的差异也会对重金属修复产生影响,然而针对这方面的研究十分有限.
本研究旨在探讨梧桐不同部位废弃物的生物质炭吸附固定Cd2+的性能差异和机理. 本研究假设梧桐不同部位废弃物的初始结构和元素释放是影响Cd2+固定的主要因素. 采用X射线衍射 (XRD)、衰减全反射红外光谱 (ATR-IR)、扫描电镜 (SEM)、比表面积及孔隙分析 (BET) 和电感耦合等离子光谱发生仪 (ICP-OES) 等技术探究梧桐不同部位废弃物生物质炭对Cd2+的吸附性能和矿物学特征.
-
生物质取自江苏省南京市的法国梧桐树,其为落叶乔木,在植物分类学上属悬铃木科,适应性强,广泛应用于城市绿化. 将自然脱落的梧桐树皮、枝条、叶片分类,去除杂质,经过去离子水清洗后分别置于烘箱中在80 ℃条件下烘干至恒重. 分别将3种不同的样品用粉碎机磨碎,取100目筛以下,放入密封袋中待用.
-
利用元素分析仪 (Elementar Vario EL Ⅲ, Germany) 测定生物质炭中的C、H、S元素含量. 比表面积孔径分析仪 (ASAP 3020, America) 检测生物质炭的比表面积和孔隙度等指标. pH值由SG98 InLab pH计 (梅特勒-托利多国际公司) 和专业的Pro-ISM-IP67探针测量. 用电感耦合等离子体发射光谱法 (ICP-OES) 测定滤液中Cd2+的浓度. 用0、1、5、10、25、50 mg·L−1标准品校准Cd2+. 本标准由国家有色金属电子材料分析测试中心提供 (中国),内标曲线的R2>0.999. ATR-IR分析应用于Nicolet iS5傅里叶变换红外光谱仪和ThermoFisher OMNIC软件 (ThermoFisher Scientific Inc, Madison, USA). 以4 cm−1的光谱分辨率对每个样品进行16次扫描,记录400—2500 cm−1的光谱区域. 用X射线衍射仪 (Bruker D8, Germany) 检查生物质炭上结晶的矿物学特征 (Cu-Ka; 40 kV; 40 mA; scanned from 5°—80° at a speed of 0.02(°)·s−1). 样品镀金后被用于扫描电镜分析 (Hitachi, Japan, 加速电压为5—15 kV的Carl Zeiss Supra 55系统中),采用Oxford-Aztec X-Max-150型能谱仪 (EDS) 进行半定量分析 (采集时间90 s). 其他仪器:烘箱 (GFL-125)、可编程箱式电炉 (XMTA-8000)、数显恒温磁力加热搅拌器 (HJ-6A)、超声清洗机 (PS-40A) 等.
硝酸镉 (Cd(NO3)2, AR)、乙酸钠 (NaOAC, AR) 和氯化镁 (MgCl2, AR) 购自国药集团化学试剂有限公司,乙醇 (CH3CH2OH, AR)、醋酸 (HOAC, AR)和硝酸钠 (NaNO3) 购买自淄博文海工贸有限公司,氢氧化钠 (NaOH, AR) 购自西陇化工股份有限公司,硝酸 (HNO3, AR) 购买自上海凌峰化学试剂有限公司. 在整个实验过程中使用由上海和泰仪器有限公司购买的10寸双级过滤器制备的纯水.
-
称量2.5 g的梧桐树生物质样品置于50 mL的坩埚,将坩埚用锡纸包裹密封,在450 ℃下热解3 h. 将制备得到的生物质炭研磨、封存、取100—500目编号备用. 一共制备出3种生物质炭,处理编号设为:树皮炭、枝条炭、叶片炭.
-
生物质炭元素的测定:采用元素分析仪测定生物质炭中C、N、S元素的含量和C/N[22]. 生物质炭灰分的测定:称量生物质炭样品10—20 mg,置于烘箱中加热至105 ℃,持续加热40 min,除去水分.然后在N2条件下将样品加热到900 ℃,升温速率为10 ℃·min−1,持续加热20 min,最后将样品在900 ℃条件下暴露在空气中30 min.挥发分和固定炭的测定:样品在900 ℃持续加热30 min后经过N2吹扫前及吹扫后的质量损失率来计算[23]. 生物质炭的比表面积 (BET) 和孔隙的测定:利用高速自动比表面与孔隙度分析仪测定生物质炭比表面积,将样品于机器中升温至300 ℃,真空脱气4 h,然后回填氦气,并进行等温吸附-脱附测定 (−196.15 ℃),P/P0=0.05—0.35 (P为氮气的分压;P0为液氮温度下,氮气的饱和蒸气压).
-
(1) 吸附动力学实验
称取0.1 g的生物质炭投加到150 mL的Cd2+溶液中 (1 g·L−1),调节至pH 5,于26 ℃恒温条件下在磁力搅拌器中以500 r·min−1分别搅动0.5、1、2、4、6、12、24 h. 以注射器取出样品后过0.22 μm的滤膜,并利用ICP-OES测定滤液中Cd2+的浓度,每个处理设置3个重复.
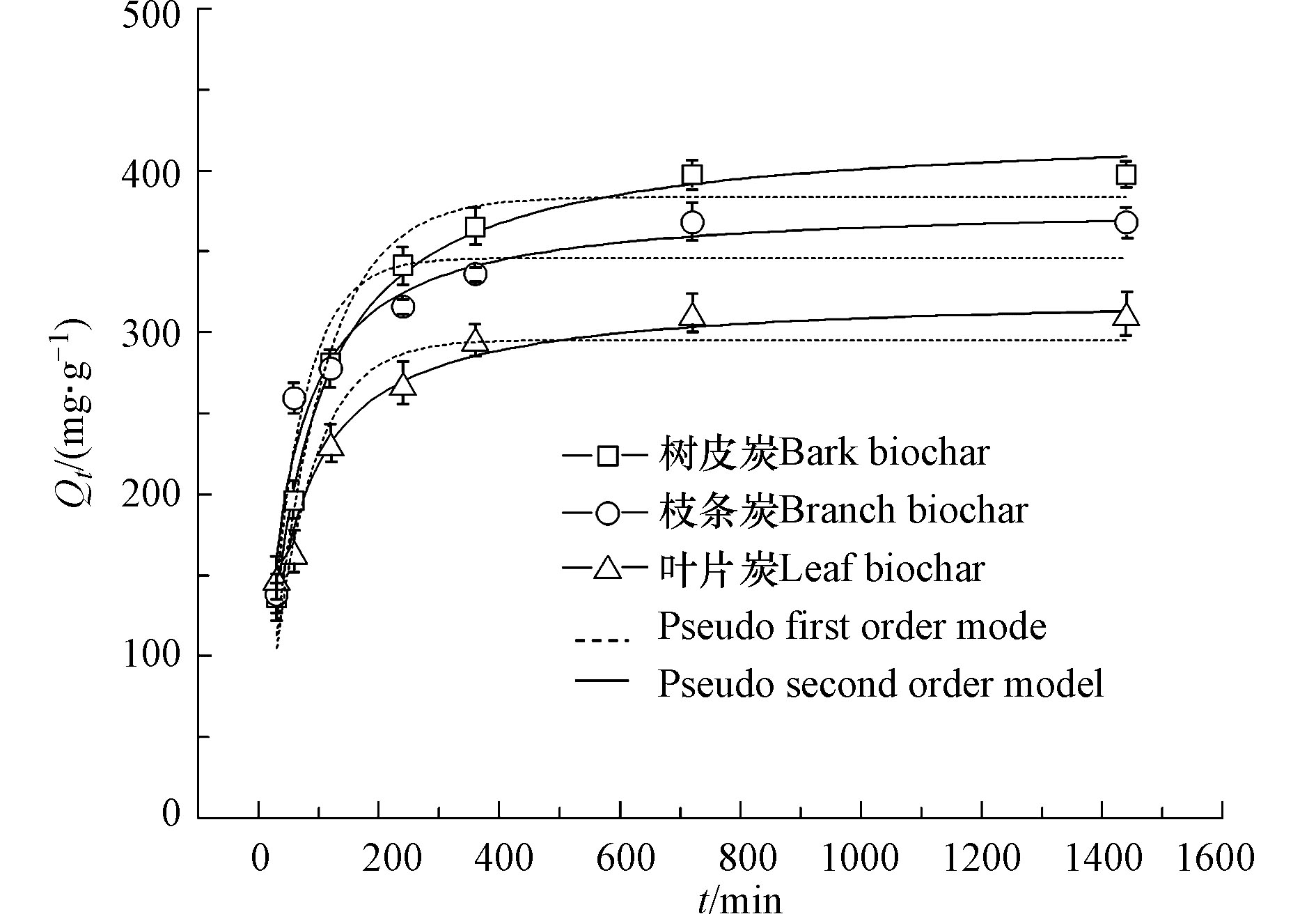
采用Lagergren准一级动力学模型、准二级动力学模型对动力学吸附结果进行拟合,其表达公式如下:
准一级动力学方程[24]:
准二级动力学方程[25]:
式中,k1、k2分别为准一级 (h−1)、准二级速率常数 (mg·g·h−1);t (h) 为反应时间;Qt为t对应的吸附量 (mg·g−1),Qe为吸附平衡时的吸附量 (mg·g−1).
(2) 等温吸附实验
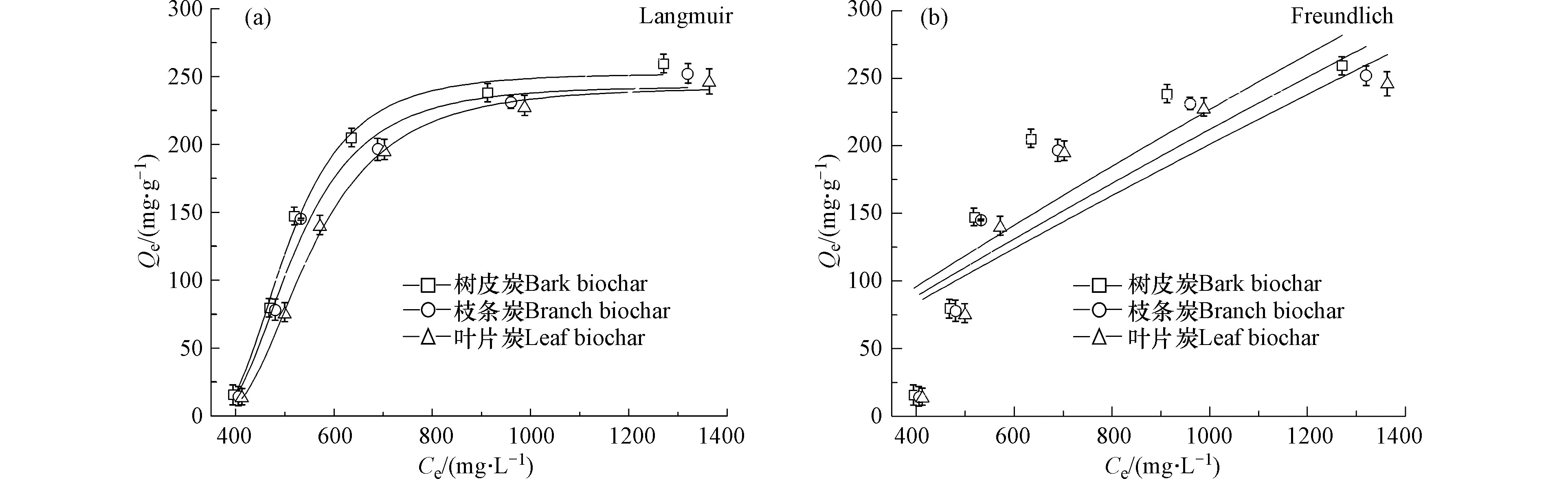
称取0.1 g的生物质炭投加到150 mL浓度分别为0.5、1.0、1.5、2.0、2.5、3.0 g·L−1的Cd2+溶液中,调节溶液至pH 5,26 ℃恒温在磁力搅拌器中以500 r·min−1搅动,24 h后取出混合液体过0.22 μm的滤膜,并测定滤液中Cd2+的浓度,每个处理设置3个重复. 利用Langmuir和Freundlich等温吸附模型进行模拟. Langmuir模型理论的假设条件为,在均一表面进行的单分子层吸附,且被吸附分子之间无任何相互作用[26],可用如下公式进行表示:
Freundlich模型描述的是多层吸附,在高浓度时吸附容量持续增加,常用于描述物理吸附,公式如下:
两个模型式中:Ce为吸附平衡后剩余溶液中重金属离子的浓度 (mg·L−1);Qe和Qmax分别为平衡吸附量和最大吸附量 (mg·g−1);b与吸附强度有关 (mg·L−1);n和K为与吸附强度和吸附量有关的参数.
(3) pH的影响实验
称取0.01 g的3种生物质炭投加到150 mL Cd2+溶液 (1 g·L−1),调节溶液初始pH值至3.0、4.0、5.0、6.0、7.0、8.0,于26 ℃恒温条件下在磁力搅拌器中以500 r·min−1搅拌,24 h后取出样品过滤膜测定Cd2+的浓度,每个处理设置3个重复.
-
生物质炭吸附的重金属形态测定:本研究采用四步萃取法以确定生物质炭吸附Cd2+后的形态 (水溶态、可交换态、酸溶态和非生物利用态),具体实验步骤参照Shen等[27]的研究. 首先将吸附反应后的生物质炭用20 mL纯水快速洗涤2次,洗涤溶液中Cd2+的浓度低于检测线,表明洗涤对吸附的Cd2+总量的影响可忽略不计. 将剩余的炭固体在60 ℃烘箱中干燥48 h,称取0.1 g烘干后的生物质炭样品于50 mL离心管中,作为萃取的初始样品,所有步骤和处理均设3个处理. 四步萃取法[28]:(1) 水溶态组分:首先将上述步骤的离心管中加入20 mL纯水,然后使用恒温振荡箱在室温 (20 ℃) 和200 r·min−1的条件下振荡24 h. 振荡完成后,将样品于8000 r·min−1离心机上离心2 min,最后取上清液过0.22 μm水系滤膜,测定其Cd2+浓度. (2) 可交换态组分:取前一步的固体残留物并在其中加入8 mL的0.5 mol·L−1 MgCl2溶液 (用NaOH或HCl调节到pH 7.0),置于200 r·min-1的恒温振荡箱中室温 (20 ℃) 振荡20 min,取出后于8000 r·min-1离心机上离心2 min,取上清液过0.22 μm水系滤膜,测定其Cd2+浓度. (3) 酸溶态组分:取步骤二的固体残留物加入8 mL的1 mol·L−1 NaOAc (用HOAc调节到pH 5.0),置于200 r·min−1的恒温振荡箱中室温振荡5 h,取出于8000 r·min−1离心机上离心2 min,取上清液过0.22 μm水系滤膜,测定其Cd2+浓度. (4) 非生物利用组分:将第3步的固体残留物在室温下用9 mL 36%的HCl和3 mL 70%的HNO3消解16 h,然后在95 ℃下加热2 h.取上清液过0.22 μm水系滤膜,测定Cd2+浓度.
-
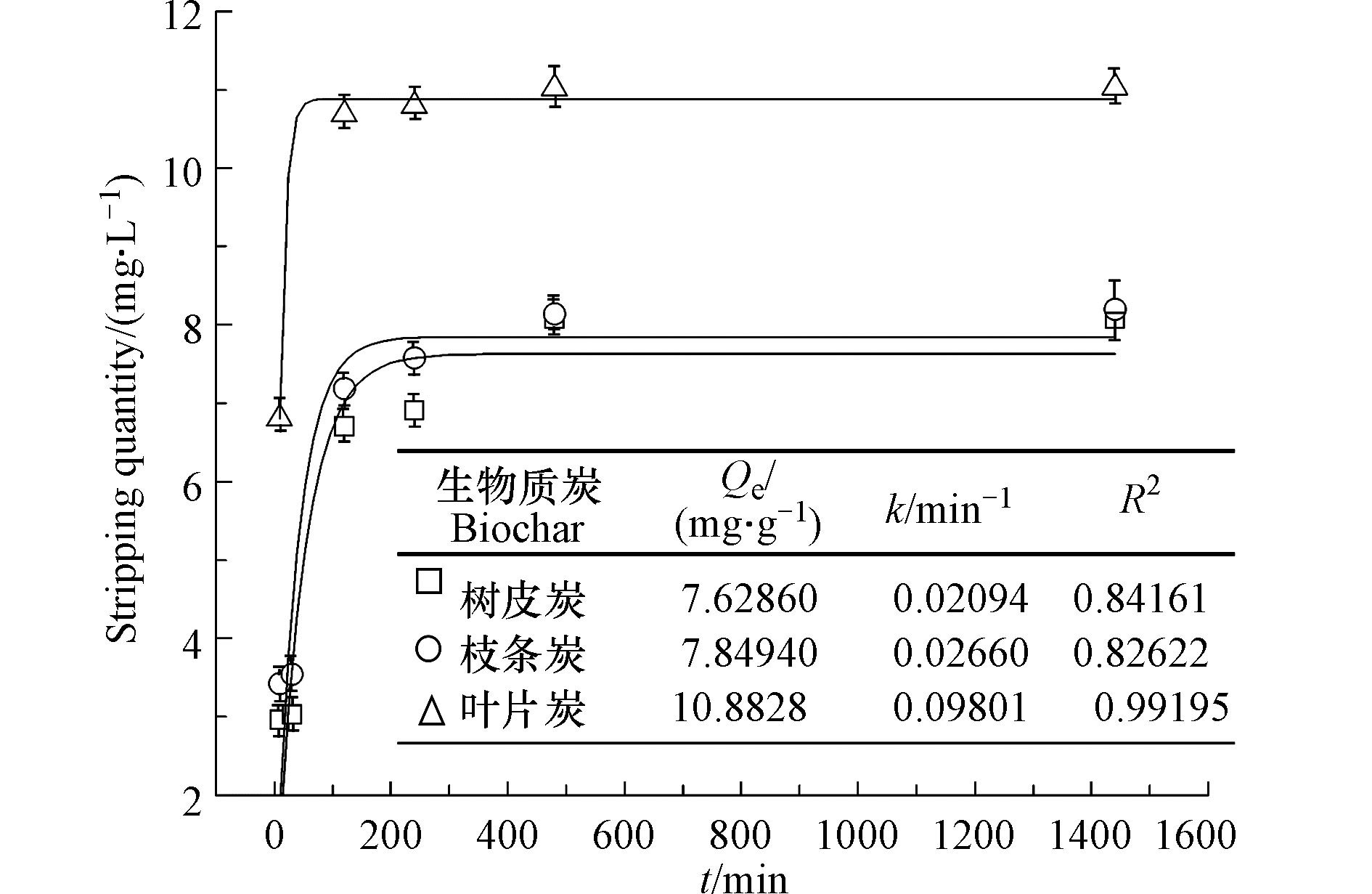
使用与吸附试验背景溶液相同浓度的NaNO3制备脱附剂,在50 mL离心管中加入0.1 g吸附饱和后的生物质炭,加入20 mL上述脱附剂,放入20 ℃恒温振荡箱中振荡10 min、30 min、2 h、4 h、8 h、24 h后,于8000 r·min−1离心机上离心2 min,取上清液过0.22 μm水系滤膜,测定其Cd2+浓度,每个处理设置3个重复.
-
(1) 工业分析和元素分析
不同生物质炭的工业分析和元素分析结果见表1. 3种梧桐不同部位的生物质炭灰分和固定碳含量呈现叶片炭>枝条炭>树皮炭的趋势,而挥发分含量、C/N和原料中木质素含量的趋势则完全相反. 树叶炭的产率最高,枝条炭的产率最低. SEM的EDS结果表明树皮炭中的元素更加丰富,K、Ca等元素以灰分的形式富集 (图1).
(2) 生物质炭比表面积和孔隙结构
不同组分生物质炭的孔隙结构特征如表2所示. 叶片炭的比表面积具备显著的优势,而枝条炭的总孔隙和中孔体积较高. SEM结果也观察到梧桐树皮炭表面粗糙,孔隙偏大. 枝条炭呈蜂窝状,表面有明显的管束结构,排列较为均匀. 叶片炭主要呈块状和粒状. 且3种生物质炭中树皮炭表面附着更多的灰分颗粒,可能含有很多的无机矿物盐组分[29].
-
吸附动力学研究结果表明随着吸附时间的增加,生物质炭的吸附速率呈现出先快后慢,最后趋于平衡的趋势,3种生物质炭的平衡时间较为相近,约为8 h (图2). 不同部位生物质炭的吸附量呈现树皮炭>枝条炭>叶片炭,枝条炭的前期吸附速率最快. 采用Lagergren准一级动力学模型、准二级动力学模型进一步分析不同材料生物质炭对Cd2+的吸附情况(表3),发现所有生物质炭处理的准二级拟合曲线中的R2明显高于准一级方程拟合曲线的R2. 因此准二级方程能更好的描述生物质炭对Cd2+的吸附过程,证明3种生物质炭对于重金属Cd的吸附以化学吸附为主[28,30].
根据Langmuir方程和Freundlich方程的拟合结果 (图3),溶液初始浓度为0.5—2 g·L−1时Cd2+的吸附量呈线性增长趋势,在2.5—3 g·L−1范围内逐渐平缓,吸附量开始趋于饱和. Langmuir和Freundlich方程对Cd2+吸附模型拟合结果见表4. Langmuir模型能更好的描述梧桐生物质炭对Cd2+的等温吸附行为. Langmuir等温模型说明梧桐生物质炭吸附Cd2+的过程中单分子层吸附和非均匀表面吸附同时存在,但单分子层吸附占主导地位. 对比不同处理生物质炭的Langmuir模型拟合参数 (表5),梧桐树废弃物类生物质炭的吸附量具有明显的优势.
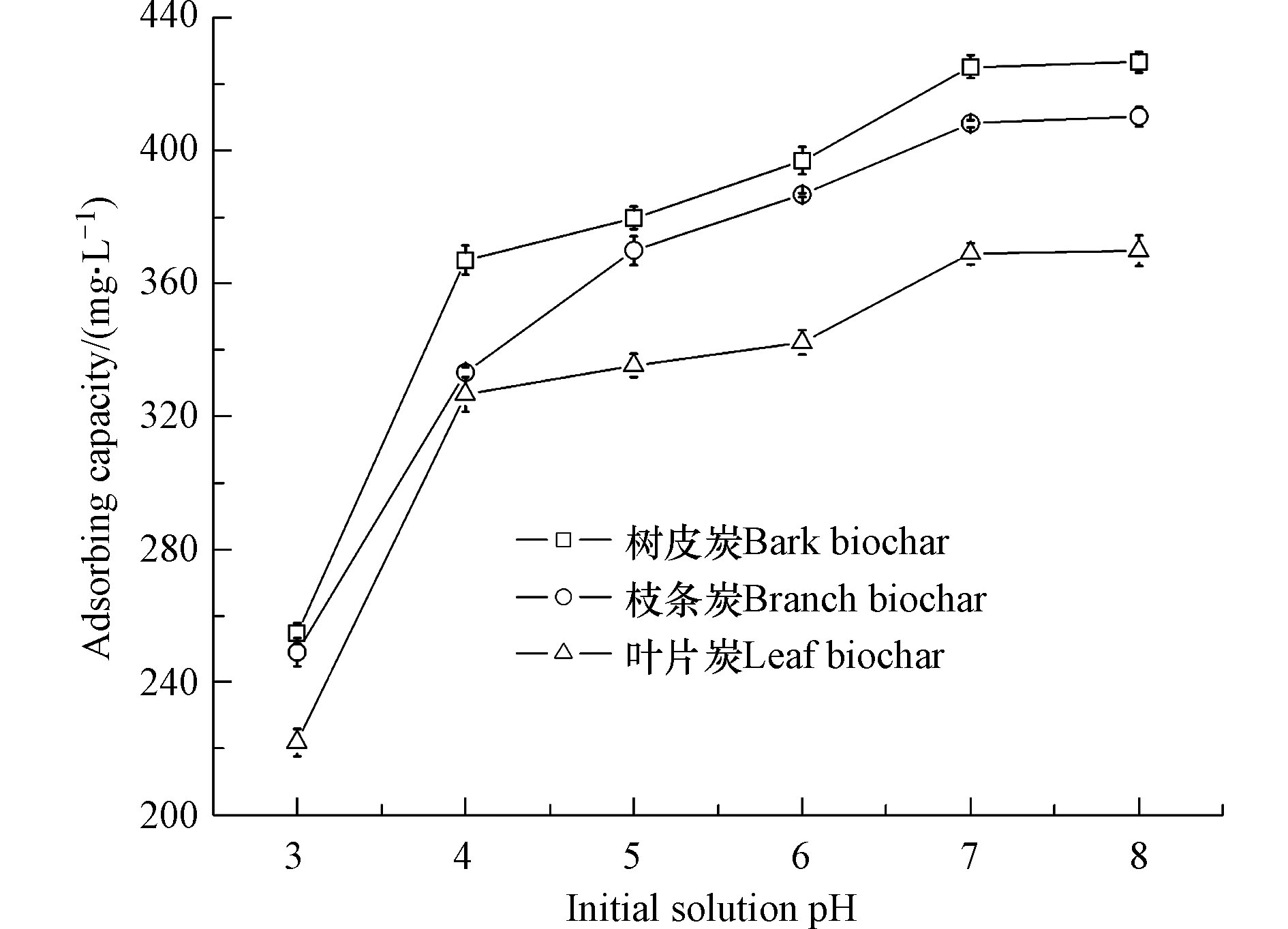
不同pH的溶液环境会影响溶液中重金属离子的形态,从而影响生物质炭对重金属吸附. 研究结果表明(图4),当pH值为3—5时,3种部位的生物质炭Cd2+吸附量均随着pH的升高而升高,且吸附率增长显著;在pH值为5—8时,吸附上升的趋势逐渐平稳,在pH>6时,生物质炭对Cd2+的吸附量又有明显升高,这可能是由于碱性条件引起的重金属络合造成的[37]. 黄菲等[38]的研究结果也表明当溶液pH值大于6时,Cd2+会以CdOH+或Cd(OH)2的形式产生沉淀,从而影响生物质炭对于Cd的吸附. 在相同pH下,3种不同材料的生物质炭吸附量大小始终为树皮炭>叶片炭>枝条炭.
-
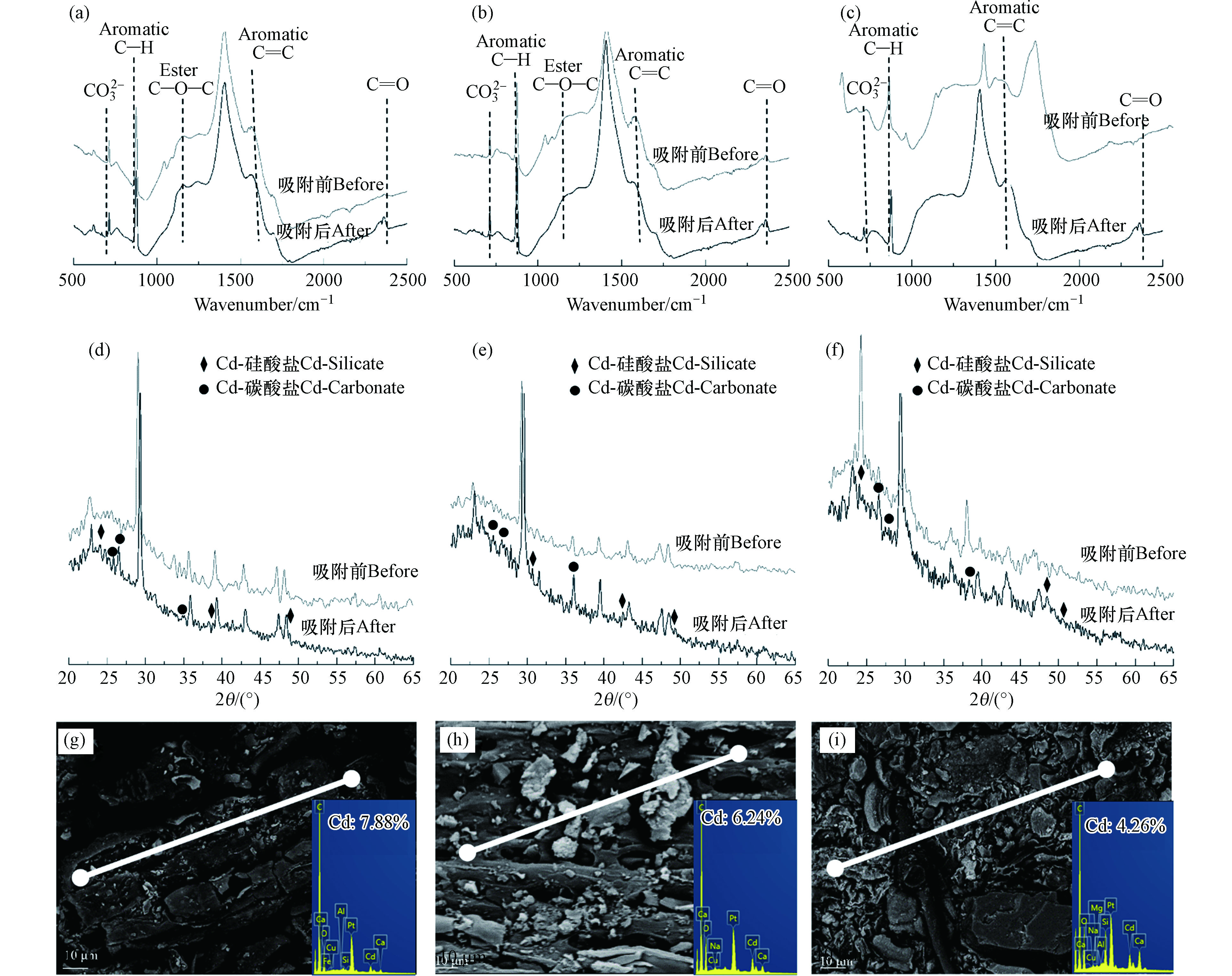
不同部位的生物质炭吸附Cd2+前后的红外分析如图5所示. 结果表明,生物质炭上存在主要官能团有芳香族C—H (875 cm−1)[39],脂类C—O—C (1185 cm−1)[40],芳香族C=C (1575−1585 cm−1)[41] 和脂肪类C=O (2380 cm−1)[42] 等官能团. 吸附后3种生物质炭表面均出现明显的
$ {\rm{CO}}_3^{2 - }$ 峰 (676 cm−1). 树皮炭的官能团相对明显和丰富,且数量要比枝条炭和叶片炭的峰多.XRD的结果表明吸附前生物质炭中无明显的Cd重金属矿物存在 (图5). 吸附Cd2+后,在23.60o,30.26°、49.67°和47.40°出现了显著的CdCO3峰[43-45],证明吸附过后生物质炭上有丰富的CdCO3生成. 除了碳酸盐的峰外,其他的峰如硅酸盐38.08°、27.02°也被检出[46-47]. 而SEM/EDS的线扫结果也证实在生物质炭表面有明显的Cd矿物被吸附 (4.8%—14.23%),并且SEM下明显观察到Cd矿物呈现均匀分布的状态,与之前吸附前初始结构中的灰分分布相吻合 (图5).
-
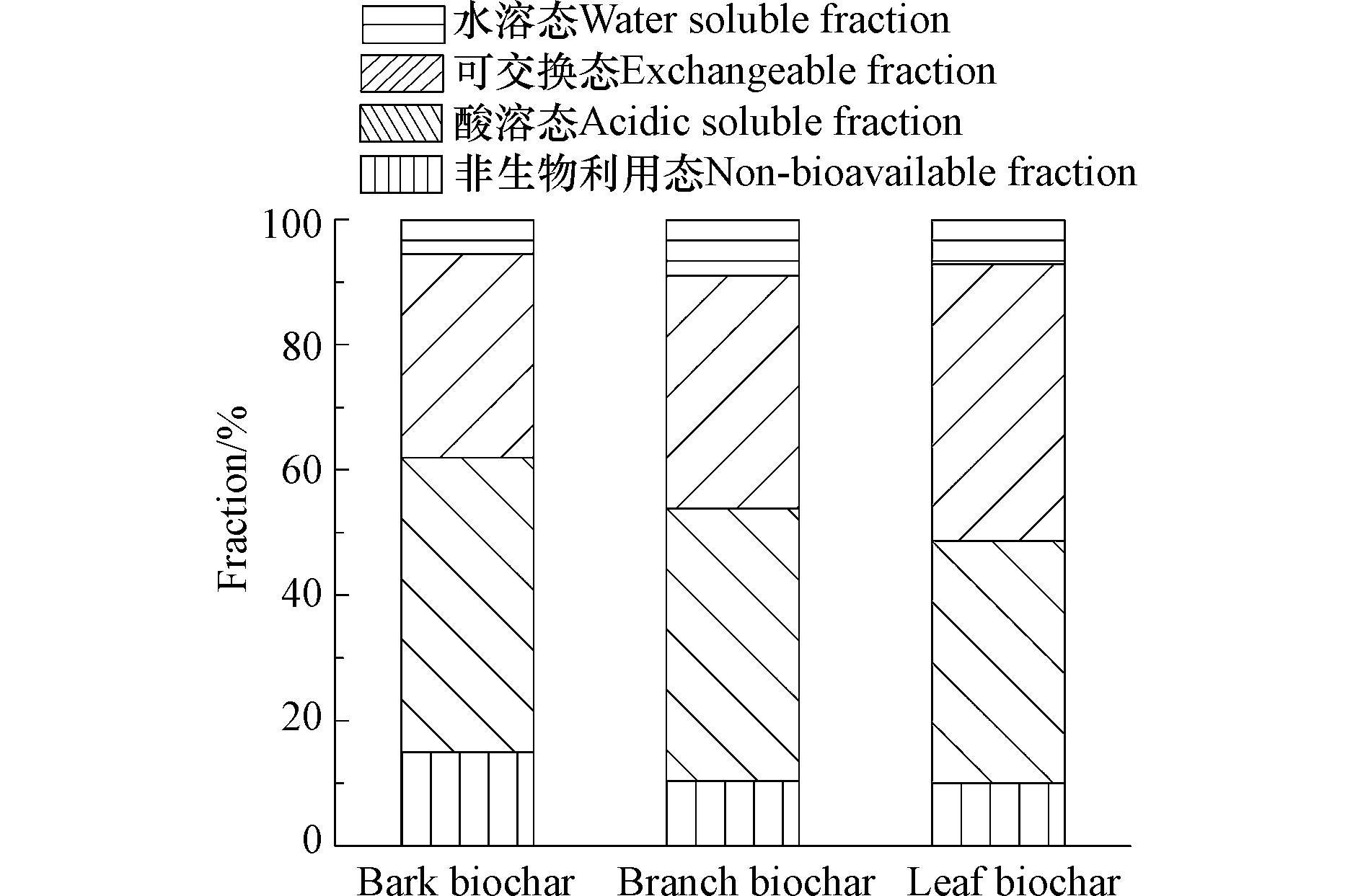
不同处理生物质炭的重金属形态萃取结果如图6所示,3种不同部位生物质炭的可交换态 (32.56%—44.13%) 和酸溶态 (47.01%—56.16%) 明显比水溶态组分 (5.48%—8.97%) 和非生物利用态 (10.04%—14.95%) 要多. 树皮炭的酸溶态和非生物利用态明显要高于枝条炭和叶片炭,证实树皮炭上Cd2+的矿物沉淀要明显比枝条炭要多.
-
不同部位梧桐生物质炭的脱附实验结果如图7所示. 3种生物质炭的脱附量在4 h后逐渐趋于平衡,其中脱附量最大的为叶片生物质炭,脱附量最小的是枝条生物质炭,这和萃取的结果相吻合 (叶片生物质炭的水溶性组分最大,枝条生物质炭的水溶性组分最小). 此外与其他生物质炭材料相比,梧桐树的生物质炭对于重金属Cd的吸附效果处于较高水平 (表5).
-
生物质炭原材料的差异会显著影响生物质炭对重金属的吸附固定效率. 通过分析3种部位梧桐生物质炭的原材料特性发现,树皮具备丰富的有机质和多种矿物元素,树叶的木质素含量较高,枝条的管状结构最为明显 (表1). 热解成生物质炭后叶片炭的比表面积最大,但是孔隙结构最差,孔隙体积最低 (表2),而SEM的结果进一步证实在热解过程中,木质素熔融堵塞孔隙,导致生物质炭孔隙结构,尤其是微孔结构变差[48],而枝条炭则出现较大的孔隙结构和总孔体积.比表面积的增加能够为Cd2+的吸附提供更多的接触面积,有利于生物质炭对Cd2+的吸附[49]. 生物质炭上含有的阳离子也是提高其吸附重金属的潜在机理之一[50],本研究结果表明树皮炭中含有多种元素 (K2+、Mg2+等,图1),有利于促进阳离子交换机制的形成. 此外,红外结果表明,树叶炭的官能团信号相对较弱,其原因是树叶原料中纤维素或木质素的羟基在热解过程断裂造成炭表面的酚羟基、醇羟基和烷基等基团消失[51]. 虽然树皮炭的比表面积相比其他两种炭不具备优势,但是其表面上丰富的阳离子和官能团有利于其对重金属Cd2+的去除修复作用[38].
-
本研究中梧桐不同部位生物质炭初始吸附速率均为枝条炭>树皮炭>树叶炭 (图2),造成这种现象的主要原因是这一阶段的吸附主要以生物质炭表面物理吸附为主,枝条炭的孔隙结构比树皮炭和树叶炭更好,有利于初期吸附速率的提升. 之后Cd2+进入生物质炭孔隙中,进一步与内部表面上的活性位点发生反应,这一过程进行速率相对较慢[52]. 树皮炭的平衡吸附量高于其他两种生物质炭,造成这个结果可能与比表面积、孔隙吸附、阳离子转换和化学沉淀等有关. 虽然比表面积和孔隙结构往往是影响生物质炭吸附的重要因素,但是在本研究中,比表面积的提升并不一定占据主导地位,吸附动力学结果表明叶片炭的吸附量低于树皮炭和枝条炭 (图2),而叶片炭的比表面积反而高于树皮炭和枝条炭,这与比表面积越高,吸附量越大的结果相反,因此证实存在另一种吸附机理占主导. 吸附动力学结果表明3种生物质炭的吸附过程与准二级动力学模型更加匹配,说明其吸附过程主要为化学吸附 (图2,表3). 而等温实验中Langmuir模型相关系数高于Freundlich模型,这表明梧桐不同部位生物质炭对Cd2+以表面单分子层吸附为主,因此说明整个吸附过程中物理吸附与化学吸附共存,这与前人的研究一致[53]. 而ATR和XRD均证实吸附后的生物质炭表面出现了明显的Cd矿物 (图5). 因此,阳离子转换和化学沉淀吸附才是梧桐生物质炭吸附Cd2+的主导因素.
-
生物质炭的孔隙结构改善提高了其对重金属吸附的能力,但是这种吸附往往是不稳定的. 萃取实验中水溶态的Cd2+十分少,证实孔隙吸附并不是梧桐生物质炭吸附Cd2+的主要机理. 而可交换态具备一定的比例,这可能是由于梧桐生物质炭中丰富的官能团和表面静电吸附作用等机理所致,可交换态的Cd2+具有一定的生物利用度,但是不够稳定,因此也可能会有环境污染的风险. 本研究多项结果证实化学沉淀是梧桐生物质炭吸附Cd2+的主要机理. 梧桐不同部位生物质炭吸附Cd2+后的ATR结果表明生物质炭表面有碳酸盐类生成,而XRD结果进一步证实其为CdCO3. 此外根据四步萃取法得出不同部位生物质炭中酸溶态的Cd比例较多,树皮炭 (47.01%)>枝条炭 (43.35%)>叶片炭 (38.61%),这也证实CdCO3在生物质炭吸附治理Cd的过程中起到主导作用,且碳酸盐矿物也比生物质炭自身孔隙吸附和离子交换等方式要更加稳固[54]. 这也和其他研究相吻合,如李瑞月等[55]研究表明化学沉淀是小麦/水稻秸秆生物质炭去除溶液中的重金属离子的主要机理. Xu等[56]的研究发现牛粪生物质炭对Cu2+、Zn2+、Cd2+的去除75%—100%取决于溶液中
$ {\rm{CO}}_3^{2 - }$ 和$ {\rm{PO}}_4^{3 - }$ 与金属离子产生的沉淀,其中90%的沉淀是形成碳酸盐沉淀. 而第四步萃取中的非生物利用态来自于Cd2+的表面络合吸附或沉淀,而此部分与不同部位生物质炭的pH和官能团等因素均有很大相关性,但所占比例不大,不足以起到影响生物质炭吸附Cd2+的主导作用.综上所述,在吸附固定水体中的Cd污染时,梧桐不同部位的初始性质对生物质炭吸附Cd2+具有明显的影响,梧桐皮具备更高的吸附量和重金属稳定形态. 梧桐枝条具备丰富的孔隙结构和快速的吸附速率. 梧桐叶片炭吸附效果均不如其他两种部位,且脱附率最高,但其具备较大的比表面积. 在环境中生物质炭对重金属的修复往往需要具备稳定和可持续性,而梧桐生物质炭对Cd2+的吸附效果比大部分生物质炭要好. 因此,从吸附效果和生产成本的角度,本研究建议以梧桐皮为主,枝条炭和叶片炭为辅的材料配比在相对较低制备温度下制备所得的生物质炭对重金属Cd进行修复固定.
-
(1) 3种不同部位的梧桐生物质炭对Cd2+有很好的吸附固定作用,其吸附效果依次为:梧桐皮>梧桐枝>梧桐叶. 而3种材料对Cd2+的吸附机理是物理吸附和化学沉淀共存,并且多项结果证实化学沉淀是梧桐生物质炭吸附Cd2+的主要机理.
(2) 本研究通过对比3种不同梧桐材料特性和吸附结果发现比表面积的提高不是梧桐生物质炭吸附Cd2+能力上升的主要优势因素. 重金属形态萃取、ATR和XRD的结果共同证实梧桐生物质炭对Cd2+的吸附固定主要是以Cd碳酸盐为主.
(3) 相对于梧桐树枝条炭和叶片炭而言,树皮炭的稳定性Cd矿物成分最高,不易对水体环境产生二次污染,是一种较为理想的吸附材料.
不同部位梧桐生物质炭对水溶液中镉吸附的机理
Mechanism of cadmium adsorption by biochar from different parts of platanus acerifolia in aqueous solution
-
摘要: 为了探究梧桐不同部位废弃物所制备的生物质炭 (皮、枝、叶) 对Cd2+的吸附效率和稳定修复的机理,以此为园林废弃物炭化利用在重金属污染修复方面的应用提供科学依据. 利用实验室模拟法,通过高温煅烧法制备梧桐不同部位生物质炭,采用元素分析仪、比表面积及孔隙分析 (BET)、X射线衍射仪 (XRD)、扫描电镜/能谱 (SEM/EDS) 及衰减全反射红外光谱 (ATR-IR) 等技术研究不同反应时间、重金属浓度和溶液初始pH条件下生物质炭对Cd2+吸附效果的影响,并运用四步萃取法和脱附实验分析生物质炭上Cd2+的吸附形态和稳定性. 3种生物质炭都在8 h左右达到吸附平衡,最终吸附量依次为树皮炭>枝条炭>叶片炭;溶液初始浓度为0.5—2 g·L−1时Cd2+的吸附量呈增长趋势,在2.5—3g·L−1时逐渐平缓;生物质炭Cd2+吸附量均随着pH的升高而升高,但在pH值为5—8时,吸附的趋势逐渐平稳;树皮炭的酸溶态和非生物利用态的稳定Cd形态要高于枝条炭和叶片炭;比表面积不是影响梧桐生物质炭吸附Cd2+的主要影响因素,吸附动力学,ATR,XRD和重金属形态萃取均证实Cd碳酸盐类矿物生成是主导吸附机理;3种生物质炭的脱附量在4 h后逐渐趋于平衡,其中脱附量最大为叶片炭,最小为树皮炭. 梧桐不同部位的初始性质对生物质炭吸附Cd2+具有明显的影响,其中梧桐皮具备更高的吸附量和重金属稳定形态,并且相比其他种类生物质炭有明显优势. 因此,从吸附效果和生产成本的角度,本研究建议以梧桐皮为主,枝条和叶片为辅的生物质炭对重金属Cd进行修复治理.Abstract: To explore the adsorption efficiency of Cd2+ by biochar (bark, branch and leaf) prepared from Wutong waste from different components and the mechanism of repair stability, and provide scientific basis for the application of carbonization of garden wastes in heavy metal pollution remediation. The biochar made from different platanus acerifolia components was prepared by means of laboratory simulation and calcined at high temperature. The effects of reaction time, heavy metal concentration and initial pH on the adsorption of Cd2+ by biochar were studied by elemental analyzer, specific surface area and pore analyzer (BET), X ray diffractometer (XRD), scanning electron microscopy/energy dispersive spectrometry (SEM/EDS) and attenuated total reflectance infrared spectroscopy (ATR-IR). Four step extraction method and desorption experiment were used to analyze the adsorption form and stability of Cd2+ on biochar. All the three biochar reached adsorption equilibrium at about 8 h and the final adsorption amount was bark biochar > branch biochar > leaf biochar; When the initial concentration of Cd2+ solution was 0.5—2 g·L−1, the adsorption amount of Cd2+ by biochar showed an increasing trend and gradually flattens out at 2.5—3 g·L−1; The adsorption of Cd2+ on biochar increased with the increase of pH, but the adsorption trend gradually stabilized at pH 5—8; The acid soluble and abiotic utilization of Cd speciation in bark biochar were more stable than that in branch biochar and leaf biochar. The specific surface area was not the main factor affecting the adsorption of platanus acerifolia biochar, adsorption kinetics, ATR, XRD and heavy metal speciation confirmed that the formation of Cd-carbonate minerals is the dominant adsorption mechanism. The desorption amount of the three biochar tended to balance gradually after 4 h. The maximum desorption amount was leaf biochar and the minimum was bark biochar. Different platanus acerifolia components had obvious effects on Cd2+ adsorption by biochar. The bark biochar had higher adsorption capacity and stable form of heavy metals and it had significant advantages over other biochar species. Therefore, from the perspective of adsorption effect and production cost, this study suggested that biochar should be prepared mainly from bark, supplemented by branches and leaves, and use it to repair and remediation Cd pollution.
-
Key words:
- sycamore waste /
- biochar /
- water body /
- cadmium /
- adsorption
-

-
表 1 生物质炭的工业分析和元素分析
Table 1. Industrial analysis and elemental analysis of biochar
生物质炭
Biochar工业分析/%
Proximate analysis元素分析/%
Elemental analysis产率/%
Productivity木质素含量/%
Lignin contents灰分 挥发分 固定碳 碳 (C) 氮 (N) 硫 (S) 其他 C/N 树皮炭 13.12 42.21 40.67 63.59 1.02 0.23 35.16 62.34 26.15 15.81 枝条炭 15.95 38.62 41.43 56.11 1.56 0.25 42.08 35.97 23.76 19.15 叶片炭 17.37 35.42 43.21 55.56 1.67 0.25 42.52 33.27 29.26 23.57 表 2 生物质炭孔隙结构特征
Table 2. Pore structure characteristics of biochar
生物质炭
Biochar比表面积/(m2 ·g−1)
Specific surface area平均孔径/nm
Mean pore size微孔体积/(cm3 ·g−1)
Micropore volume中孔体积/(cm3 ·g−1)
The pore volume总孔隙体积/(cm3 ·g−1)
Total pore volume树皮炭 295.7 2.6 0.022 0.038 0.060 枝条炭 305.5 2.5 0.083 0.111 0.194 叶片炭 312.3 2.9 0.010 0.020 0.030 表 3 不同生物质炭吸附Cd2+动力学参数
Table 3. Kinetic parameters of Cd2+ adsorption by different biochar
生物质炭
BiocharQe/(mg·g−1) k1/min−1 R2 准一级动力学模型 树皮炭 383.590 0.01163 0.97152 枝条炭 345.872 0.01812 0.89178 叶片炭 295.409 0.01470 0.87999 生物质炭
BiocharQe/(mg·g−1) k2/(g· (mg·min)−1) R2 准二级动力学模型 树皮炭 426.845 0.01544 0.99438 枝条炭 379.550 0.02460 0.94299 叶片炭 323.276 0.02103 0.96870 注:Qe为t时刻对应的吸附平衡时的吸附量,k1、k2为速率常数.
Note: Qe is the adsorption amount corresponding to the adsorption equilibrium at time t, k1, k2 are the rate constants.表 4 不同生物质炭吸附等温线参数
Table 4. Adsorption isotherm parameters of different biochar
生物质炭
BiocharLangmuir等温模型
Langmuir isotherm modelFreundlich等温模型
Freundlich isotherm modelQmax/(mg·g−1) b/(L·mg−1) R2 Kf /(mg1+n·g−1·L-n) 1/n R2 树皮炭 292.75 0.0976 0.9901 252.99 -0.763 0.6605 枝条炭 288.51 0.0793 0.9798 242.90 -0.792 0.6836 叶片炭 255.23 0.0777 0.9762 242.08 -0.103 0.6845 注:Qmax为最大吸附量,参数b、Kf分别和与吸附强度和吸附量有关.
Note: Qmax is the maximum adsorption capacity, the parameters b and Kf are respectively related to the adsorption strength and adsorption capacity.表 5 不同生物质炭对水体环境中重金属的去除作用
Table 5. Removal of heavy metals in water environment by different biochar
原材料
Raw
material比表面积 /
(m2 ·g−1 )
BET总孔隙体积/
(cm3 ·g−1)
Total pore volume化学沉淀
Chemical
precipitation制备条件/°C
Preparation
condition吸附量/
(mg·g−1)
Adsorbing capacity吸附动力学模型
Dynamic
adsorption model吸附等温线
Adsorption
isotherm文献
Literature梧桐皮 295.7 0.060 ${\rm{CO}}_3^{2 - }/{\rm{SiO}}_3^{2 - } $ 450 426.845 准二级 Langmuir 本研究 梧桐枝 305.5 0.194 ${\rm{CO}}_3^{2 - }/{\rm{SiO}}_3^{2 - } $ 450 379.550 准二级 Langmuir 本研究 梧桐叶 312.3 0.030 ${\rm{CO}}_3^{2 - }/{\rm{SiO}}_3^{2 - } $ 450 323.276 准二级 Langmuir 本研究 玉米秸秆 70.7 0.036 $ {\rm{CO}}_3^{2 - }$ 600 106.86 准二级 Langmuir [31] 稻杆 73.40 0.065 ${\rm{PO} }_4^{3 - }/{\rm{CO} }_3^{2 - }/{\rm{Si} }{ {\rm{O} }_4^{ - } }$ 700 60.61 准二级 Langmuir [32] 泥煤苔 - - - 800 39.8 准二级 Langmuir [33] 牧豆壳 3.11 - - 350 38.3 准二级 Langmuir [33] 水葫芦 - - ${\rm{PO}}_4^3/{\rm{CO}}_3^{2 - } $ 450 70.3 准二级 Langmuir [34] 牛粪 5.61 - ${\rm{PO} }_4^3/{\rm{CO} }_3^{2 }$ 200 54.6 准二级 Langmuir [35] 果穗 206.45 0.59 - 100 62.5 准二级 Langmuir [36] -
[1] KHAN M U, MALIK R N, MUHAMMAD S, et al. Human health risk from heavy metal via food crops consumption with wastewater irrigation practices in pakistan [J]. Chemosphere, 2013, 93(10): 2230-2238. doi: 10.1016/j.chemosphere.2013.07.067 [2] 吴晴雯, 孟梁, 张志豪, 等. 芦苇秸秆生物炭对水体中重金属Ni2+的吸附特性 [J]. 环境化学, 2015, 34(9): 1703-1709. doi: 10.7524/j.issn.0254-6108.2015.09.2015031108 WU Q W, MENG L, ZHANG Z H, et al. Adsorption behaviors of Ni2+ onto reed straw biochar in the aquatic solutions [J]. Environmental Chemistry, 2015, 34(9): 1703-1709(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.09.2015031108
[3] CHEN L, LIAN S, LIU M D, et al. Trans-provincial health impacts of atmospheric mercury emissions in China [J]. Nat Commun, 2019, 10(1): 1484. doi: 10.1038/s41467-019-09080-6 [4] ELAIGWU S E, ROCHER V, KYRIAKOU G, et al. Removal of Pb2+ and Cd2+ from aqueous solution using chars from pyrolysis and microwave-assisted hydrothermal carbonization of Prosopis africana shell [J]. Journal of Industrial and Engineering Chemistry, 2014, 20(5): 3467-3473. doi: 10.1016/j.jiec.2013.12.036 [5] FAN T, LIU Y G, FENG B Y, et al. Biosorption of Cadmium(Ⅱ), Zinc(Ⅱ) and Lead(Ⅱ) by Penicillium simplicissimum: Isotherms, kinetics and thermodynamics [J]. Journal of Hazardous Materials, 2008, 160(23): 655-661. [6] MEUNIER N, DROGUI P, MONTANE C, et al. Comparison between electrocoagulation and chemical precipitation for metals removal from acidic soil leachate [J]. Journal of Hazardous Materials, 2006, 137(1): 581-590. doi: 10.1016/j.jhazmat.2006.02.050 [7] MUTLU A, LEE B, PARK G, et al. Long-term concentrations of airborne cadmium in metropolitan cities in Korea and potential health risks [J]. Atmospheric Environment, 2012, 47: 164-173. doi: 10.1016/j.atmosenv.2011.11.019 [8] HE H J, XIANG Z H, CHEN X J, et al. Biosorption of Cd(Ⅱ) from synthetic wastewater using dry biofilms from biotrickling filters [J]. International Journal of Environmental Science and Technology, 2018, 15(7): 1491-1500. doi: 10.1007/s13762-017-1507-8 [9] 王璐, 赵保卫, 马锋锋, 等. 马铃薯秸秆生物炭对黄土吸附Cd(Ⅱ)的影响 [J]. 环境化学, 2016, 35(7): 1422-1430. doi: 10.7524/j.issn.0254-6108.2016.07.2015121103 WANG L, ZHAO B W, MA F F, et al. Effects of biochar derived from potato straw on adsorption of Cd(Ⅱ) onto loess [J]. Environmental Chemistry, 2016, 35(7): 1422-1430(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.07.2015121103
[10] BEESLEY L, MORENOJIMENEZ E, GOMEZEYLES J L, et al. A review of biochars' potential role in the remediation, revegetation and restoration of contaminated soils [J]. Environmental Pollution, 2011, 159(12): 3269-3282. doi: 10.1016/j.envpol.2011.07.023 [11] SHEN Z T, TIAN D, ZHANG X Y, et al. Mechanisms of biochar assisted immobilization of Pb2+ by bioapatite in aqueous solution [J]. Chemosphere, 2018, 190: 260-266. doi: 10.1016/j.chemosphere.2017.09.140 [12] XU X Y, CAO X D, ZHAO L, et al. Comparison of rice husk and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: role of mineral components in biochars [J]. Chemosphere, 2013, 92(8): 955-961. doi: 10.1016/j.chemosphere.2013.03.009 [13] SHEN Z T, JIN F, WANG F, et al. Sorption of lead by Salisbury biochar produced from British broadleaf hardwood [J]. Bioresource Technology, 2015, 193: 553-556. doi: 10.1016/j.biortech.2015.06.111 [14] SHEN Z T, HOU D Y, ZHAO B, et al. Stability of heavy metals in soil washing residue with and without biochar addition under accelerated ageing [J]. Science of The Total Environment, 2018, 619: 185-193. [15] DING W C, DONG X L, IME I M, et al. Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars [J]. Chemosphere, 2014, 105: 68-74. doi: 10.1016/j.chemosphere.2013.12.042 [16] PRADHAN P, ARORA A, MAHAJANI S M, et al. Pilot scale evaluation of fuel pellets production from garden waste biomass [J]. Energy for Sustainable Development, 2018, 43: 1-14. doi: 10.1016/j.esd.2017.11.005 [17] SHI Y, GE Y, CHANG J, et al. Garden waste biomass for renewable and sustainable energy production in China: Potential, challenges and development [J]. Renewable & Sustainable Energy Reviews, 2013, 22: 432-437. [18] GUPTA A, THENGANE S K, MAHAJANI S M, et al. CO2 gasification of char from lignocellulosic garden waste: Experimental and kinetic study [J]. Bioresource Technology, 2018, 263: 180-191. doi: 10.1016/j.biortech.2018.04.097 [19] IPPOLITO J A, BERRY C M, STRAWN D G, et al. Biochars reduce mine land soil bioavailable metals [J]. Journal of Environmental Quality, 2017, 46(2): 411-419. doi: 10.2134/jeq2016.10.0388 [20] 周润娟. 水葫芦生物炭对重金属竞争吸附特性及机制研究[D]. 芜湖: 安徽师范大学, 2019. ZHAO R J. Study on competitive adsorption characteristics and mechanisms of heavy metals by biochar derived from water hyacinth[D]. Wuhu: Anhui Normal University, 2019 (in Chinese).
[21] 汤嘉雯, 陈金焕, 王凯男, 等. 加拿大一枝黄花生物炭对Cd 2+ 的吸附特性及机理 [J]. 农业环境科学学报, 2019, 38(6): 1339-1348. doi: 10.11654/jaes.2018-1290 TANG J W, CHEN J H, WANG K N, et al. Characteristics and mechanism of cadmium adsorption by Solidago canadensis-derived biochar [J]. Journal of Agro-Environment Science, 2019, 38(6): 1339-1348(in Chinese). doi: 10.11654/jaes.2018-1290
[22] LIANG X Y, LIU S R, WANG H, et al. Variation of carbon and nitrogen stoichiometry along a chronosequence of natural temperate forest in northeastern China [J]. Journal of Plant Ecology, 2018, 11(3): 339-350. doi: 10.1093/jpe/rtx008 [23] FIDEL RIVKA. B, LAIRD DAVID. A, THOMPSON M L, et al. Characterization and quantification of biochar alkalinity [J]. Chemosphere, 2017, 167(1): 367-373. [24] 余贵芬, 青长乐, 牟树森, 等. 汞在腐殖酸上的吸附与解吸特征[J] 环境科学学报, 2001, 21(5): 601-606. YU G F, QING C L, MOU S S, et al. Characteristics of mercury adsorption and desorption on humic acids[J]. Acta Scientiae Circumstantiae, 2001, 21(5): 601-606 (in Chinese).
[25] CHENG Y, YANG C P, HE H J, et al. Biosorption of Pb(Ⅱ) ions from aqueous solutions by waste biomass from biotrickling filters: Kinetics, isotherms, and thermodynamics [J]. Journal of Environmental Engineering, 2016, 142(9): 689-695. [26] GADKIN J W, STEINER C, HARRIS K, et al. Effect of low-temperature pyrolysis conditions on biochar for agricultural use [J]. Transactions of the ASABE, 2008, 51(6): 2061-2069. doi: 10.13031/2013.25409 [27] SHEN Z T, ZHANG Y Y, JIN F, et al. Qualitative and quantitative characterisation of adsorption mechanisms of lead on four biochars [J]. Science of the Total Environment, 2017, 609: 1401-1410. doi: 10.1016/j.scitotenv.2017.08.008 [28] 毛凯, 陈颢明, 陈天民, 等. 不同粒径污泥生物质炭对水体中Zn污染的吸附效应研究 [J]. 环境科学学报, 2020, 40(2): 536-545. MAO K, CHEN H M, CHEN T N, et al. Adsorption effects of sludge biochar of different particie sizes on Zn contamination in water [J]. Acta Scientiae Circumstantiae, 2020, 40(2): 536-545(in Chinese).
[29] 高凯芳. 原材料和温度对生物炭的理化特性及镉吸附能力的影响研究[D]. 南昌: 江西师范大学, 2016: 16-17. GAO K F. Effects of raw materials and temperature on physical and chemical properties and cadmium adsorption capacity of biochar[D]. Nanchang Normal University, Jiangxi, 2016: 16-17 (in Chinese).
[30] 程德义, 杜超, 黄兆琴, 等. 巯基化稻壳炭吸附废水中 Zn(Ⅱ)试验研究 [J]. 环境工程技术学报, 2018, 25(5): 519-526. CHENG De Y, DU C, HUANG Z Q, et al. Adsorption of Zn (Ⅱ) from wastewater by sulfhydryl rice husk carbon [J]. Journal of Environmental Engineering Technology, 2018, 25(5): 519-526(in Chinese).
[31] SUN J, LIAN F, LIU Z, et al. Biochars derived from various crop straws: characterization and Cd(II) removal potential [J]. Ecotoxicology and Environmental Safety, 2014, 105: 226-231. [32] 戴静, 刘阳生. 四种原料热解产生的生物炭对Pb2+和Cd2+的吸附特性研究 [J]. 北京大学学报(自然科学版), 2013, 49(6): 1075-1082. DAI J, LIU Y S. Adsorption of Pb2+ and Cd2+ onto biochars derived from pyrolysis of four beikinds of biomasses [J]. Journal of Peking University, 2013, 49(6): 1075-1082(in Chinese).
[33] LEE S J, PARK J H, AHN Y, et al. Comparison of heavy metal adsorption by peat moss and peat moss-derived biochar produced under different carbonization conditions [J]. Water Air and Soil Pollution, 2015, 226(2): 9. doi: 10.1007/s11270-014-2275-4 [34] ZHANG F, WANG X, YIN D X, et al. Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes) [J]. Journal of Environmental Management, 2015, 153: 68-73. [35] XU X Y, CAO X D, ZHAO L, et al. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar [J]. Environmental Science and Pollution Research, 2013, 20(1): 358-368. doi: 10.1007/s11356-012-0873-5 [36] RUTHIRAAN M, MUBARAK N M, THINES R K, et al. Comparative kinetic study of functionalized carbon nanotubes and magnetic biochar for removal of Cd2+ ions from wastewater [J]. Korean Journal of Chemical Engineering, 2015, 32(3): 446-457. doi: 10.1007/s11814-014-0260-7 [37] 胡术刚, 栾小凯, 颜昌宙, 等. 改性生物炭的制备及其对水中镉离子的吸附试验 [J]. 环境工程, 2019, 37(5): 15-19,31. HU S G, LUAN X K, YAN C Z, et al. Preparation of modified biochar and its adsorption for cadmium ions in water [J]. Environmental. Engineering, 2019, 37(5): 15-19,31(in Chinese).
[38] 黄菲, 闫梦, 常建宁, 等. 不同菌糠生物炭对水体中Cu2+、Cd2+的吸附性能 [J]. 环境化学, 2020, 39(4): 1116-1128. doi: 10.7524/j.issn.0254-6108.2019091604 HUANG F, RUAN M, CHANG J N, et al. Adsorption performance of Cu2+ and Cd2+ in water by different biochars derived from spent mushroom substrate [J]. Environmental Chemistry, 2020, 39(4): 1116-1128(in Chinese). doi: 10.7524/j.issn.0254-6108.2019091604
[39] ZHAO S, JI Q, LI Z, et al. Characteristics and mineralization in soil of apple-derived biochar producecd at different temperatures [J]. Transactions of the Chinese Society for Agricultural Machinery, 2015, 46(6): 183-192. [40] KEILUWEIT M, NICO P S, JOHNSON M G, et al. Dynamic molecular structure of plant biomass-derived black carbon (biochar) [J]. Environmental Science & Technology, 2010, 44(4): 1247-1253. [41] WU W X, YANG M, FENG Q B, et al. Chemical characterization of rice straw-derived biochar for soil amendment [J]. Biomass & Bioenergy, 2012, 47: 268-276. [42] 张越, 林珈羽, 刘沅, 等. 改性生物炭对镉离子吸附性能研究 [J]. 武汉科技大学学报, 2016, 39(1): 48-52. ZHANG Y, LIN J Y, LIU Y, et al. Adsorption of cadmium ions by chemically modified biochar [J]. Journal of Wuhan University of Science and Technology, 2016, 39(1): 48-52(in Chinese).
[43] WANG C M, CHENG Y, WANG Y L, et al. Self-assembly of quasi-monocrystal CdCO3 nanorings [J]. Chinese Journal of Structural Chemistry, 2007, 26(7): 757-762. [44] SHAN R R, YAN L G, YANG K, et al. Adsorption of Cd(II) by Mg-Al-CO3- and magnetic Fe3O4/Mg-Al-CO3-layered double hydroxides: Kinetic, isothermal, thermodynamic and mechanistic studies [J]. Journal of Hazardous Materials, 2015, 299: 42-49. doi: 10.1016/j.jhazmat.2015.06.003 [45] ZHU X L, LV B X, SHANG X Q, et al. The immobilization effects on Pb, Cd and Cu by the inoculation of organic phosphorus-degrading bacteria (OPDB) with rapeseed dregs in acidic soil [J]. Geoderma, 2019, 350: 1-10. doi: 10.1016/j.geoderma.2019.04.015 [46] LUO N, MAO L, JIANG L, et al. Directly ultraviolet photochemical deposition of silver nanoparticles on silica spheres: Preparation and characterization [J]. Materials Letters, 2009, 63(1): 154-156. doi: 10.1016/j.matlet.2008.09.033 [47] ZHONG K F, JIN P, CHEN Q W, et al. Ni hollow nanospheres: Preparation and catalytic activity [J]. Journal of Nanomaterials, 2006, 2006(3): 499-502. [48] 罗凯, 陈汉平, 王贤华, 等. 生物质焦及其特性 [J]. 可再生能源, 2007, 25(1): 17-19. doi: 10.3969/j.issn.1671-5292.2007.01.005 LUO K, CHEN H P, WANG X H, et al. Characterization of biochar and its characteristics [J]. Renewable. Energy Resources, 2007, 25(1): 17-19(in Chinese). doi: 10.3969/j.issn.1671-5292.2007.01.005
[49] YUAN J H, XU R K, ZHANG H, et al. The forms of alkalis in the biochar produced from crop residues at different temperatures [J]. Bioresource Technology, 2011, 102(3): 3488-3497. doi: 10.1016/j.biortech.2010.11.018 [50] 李瑞月. 秸秆生物质炭及其改性炭对重金属的吸附性能及机理研究[D]. 南京: 南京农业大学, 2015: 27-28. LI R Y. The adsorption capacity and mechanism of residual-straw biochar and enhancedbiochar for heavy metal removal[D]. Nanjing: Agricultural University Jiangsu, 2015: 27-28 (in Chinese).
[51] 赵伟宁, 杨兴, 何丽芝, 等. 热解温度对典型南方木本园林废弃物生物质炭理化特性的影响 [J]. 浙江农林大学学报, 2018, 35(6): 24-33. ZHAO W N, YANG X, HE L Z, et al. Pyrolysis temperature with physicochemical properties of biochars derived from typical urban woody green wastes in southern China [J]. Journal of Zhejiang A&F University, 2018, 35(6): 24-33(in Chinese).
[52] 吴成, 张晓丽, 李关宾. 黑碳吸附汞砷铅镉离子的研究 [J]. 农业环境科学学报, 2007, 26(2): 770-774. doi: 10.3321/j.issn:1672-2043.2007.02.070 WU C, ZHANG X L, LI GB. Sorption of Hg2+, As3+, Pb2+and Cd2+by black carbon [J]. Journal of Agro-Environment Science, 2007, 26(2): 770-774(in Chinese). doi: 10.3321/j.issn:1672-2043.2007.02.070
[53] 温尔刚, 赵伟宁, 杨兴, 等. 法国梧桐叶片炭和枝条炭对水中Pb2+的吸附特性影响 [J]. 水土保持学报, 2019, 33(2): 311-318. WEN E G, ZHAO W N, YANG X, et al. Effect of biochars derived from platanus orientalis branches and leaves on the adsorption of Pb2+ in aqueous solution [J]. Journal of Soil and Water Conservation, 2019, 33(2): 311-318(in Chinese).
[54] CHEN H M, TANG L Y, WANG Z J, et al. Evaluating the protection of bacteria from extreme Cd (II) stress by P-enriched biochar [J]. Environmental Pollution, 2020, 263(7): 395-401. [55] 李瑞月, 陈德, 李恋卿, 等. 不同作物秸秆生物炭对溶液中Pb2+、Cd2+的吸附 [J]. 农业环境科学学报, 2015, 34(5): 1001-1008. doi: 10.11654/jaes.2015.05.025 LI R Y, CHEN D, LI L Q, et al. Adsorption of Pb2+ and Cd2+in aqueous solution by biochars derived from different crop residues [J]. journal of Agro-Environmengt Science, 2015, 34(5): 1001-1008(in Chinese). doi: 10.11654/jaes.2015.05.025
[56] XU X Y, CAO X, D ZHAO L, et al. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar [J]. Environmental Science & Pollution Research International, 2013, 20(1): 358-368. -




 下载:
下载: