|
[1]
|
王建宇, 李丽, 王蕴平, 等. 在线固相萃取-超高效液相色谱法检测水中15种多环芳烃类污染物 [J]. 环境化学, 2018, 37(12): 2832-2836.
WANG J Y, LI L, WANG Y P, et al. 15 kinds of polycyclic aromatic hydrocarbons in water were detected by solid phase extraction and ultra-high performance liquid chromatography [J]. Environmental Chemistry, 2018, 37(12): 2832-2836(in Chinese).
|
|
[2]
|
MUTER O, BARTKEVICS V. Advanced analytical techniques based on high resolution mass spectrometry for the detection of micropollutants and their toxicity in aquatic environments [J]. Current Opinion in Environmental Science & Health, 2020, 18: 1-6.
|
|
[3]
|
TUNÇELI A, ULAŞ A, ACAR O, et al. Solid phase extraction of cadmium and lead from water by amberlyst 15 and determination by flame atomic absorption spectrometry [J]. Bulletin of Environmental Contamination and Toxicology, 2019, 102(2): 297-302. doi: 10.1007/s00128-018-2498-y
|
|
[4]
|
QI X, WANG S, LI T, et al. An electroactive biofilm-based biosensor for water safety: Pollutants detection and early-warning [J]. Biosensors and Bioelectronics, 2021, 173: 112822. doi: 10.1016/j.bios.2020.112822
|
|
[5]
|
EJEIAN F, ETEDALI P, MANSOURI-TEHRANI H A, et al. Biosensors for wastewater monitoring: A review [J]. Biosensors and Bioelectronics, 2018, 118: 66-79.
|
|
[6]
|
宋丹, 杨荣, 罗强, 等. 核酸适体及其在环境检测中的应用 [J]. 环境污染与防治, 2018, 40(6): 717-722, 727.
SONG D, YANG R, LUO Q, et al. Aptamers and their applications in environmental detection [J]. Environmental Pollution and Control, 2018, 40(6): 717-722, 727(in Chinese).
|
|
[7]
|
NGUYEN V T, KWON Y S, GU M B. Aptamer-based environmental biosensors for small molecule contaminants [J]. Current Opinion in Biotechnology, 2017, 45: 15-23. doi: 10.1016/j.copbio.2016.11.020
|
|
[8]
|
穆晋, 杨巾栏, 张大伟, 等. 荧光金属纳米团簇的制备及其在环境污染物检测中的应用研究进展 [J]. 分析化学, 2021, 49(3): 319-329. doi: 10.1016/S1872-2040(21)60082-8
MU J, YANG J L, ZHANG D W, et al. Progress in preparation of metal nanoclusters and their application in detection of environmental pollutants [J]. Chinese Journal of Analytical Chemistry, 2021, 49(3): 319-329(in Chinese). doi: 10.1016/S1872-2040(21)60082-8
|
|
[9]
|
SHANG L, XU J, NIENHAUS G U. Recent advances in synthesizing metal nanocluster-based nanocomposites for application in sensing, imaging and catalysis [J]. Nano Today, 2019, 28: 100767. doi: 10.1016/j.nantod.2019.100767
|
|
[10]
|
QIAO Z J, ZHANG J, HAI X, et al. Recent advances in templated synthesis of metal nanoclusters and their applications in biosensing, bioimaging and theranostics [J]. Biosensors and Bioelectronics, 2021, 176: 112898.
|
|
[11]
|
XU J, ZHU X, ZHOU X, et al. Recent advances in the bioanalytical and biomedical applications of DNA-templated silver nanoclusters [J]. TrAC Trends in Analytical Chemistry, 2020, 124: 115786.
|
|
[12]
|
GUO Y H, PAN X Y, ZHANG W Y,, et al. Label-free probes using DNA-templated silver nanoclusters as versatile reporters [J]. Biosensors and Bioelectronics, 2020, 150: 111926.
|
|
[13]
|
贺锦灿, 李攻科, 胡玉玲. 基于DNA模板制备的金属纳米簇及其在分析检测中应用进展 [J]. 分析科学学报, 2018, 34(1): 127-133.
HE J C, LI G K, HU Y L. Application of DNA-templated metal nano-clusters on analysis and detection [J]. Journal of Analytical Science, 2018, 34(1): 127-133(in Chinese).
|
|
[14]
|
XU J N, WEI C Y. The aptamer DNA-templated fluorescence silver nanoclusters: ATP detection and preliminary mechanism investigation [J]. Biosensors and Bioelectronics, 2017, 87: 422-427.
|
|
[15]
|
ZHU J B, ZHANG L B, TENG Y, et al. G-quadruplex enhanced fluorescence of DNA-silver nanoclusters and their application in bioimaging [J]. Nanoscale, 2015, 7(31): 13224-13229. doi: 10.1039/C5NR03092G
|
|
[16]
|
ZHANG B Z, WEI C Y. An aptasensor for the label-free detection of thrombin based on turn-on fluorescent DNA-templated Cu/Ag nanoclusters [J]. RSC Advances, 2020, 10(58): 35374-35380. doi: 10.1039/D0RA04609D
|
|
[17]
|
SONG C X, XU J Y, CHEN Y, et al. DNA-templated fluorescent nanoclusters for metal ions detection [J]. Molecules, 2019, 24: 4189. doi: 10.3390/molecules24224189
|
|
[18]
|
GUO Y H, AMUNYELA H T N N, CHENG Y L, et al. Natural protein-templated fluorescent gold nanoclusters: Syntheses and applications [J]. Food Chemistry, 2021, 335: 127657.
|
|
[19]
|
OU G Z, ZHAO J, CHEN P, et al. Fabrication and application of noble metal nanoclusters as optical sensors for toxic metal ions [J]. Analytical and Bioanalytical Chemistry, 2018, 410(10): 2485-2498. doi: 10.1007/s00216-017-0808-6
|
|
[20]
|
CHEN Y X, PHIPPS M L, WERNER J H, et al. DNA templated metal nanoclusters: From emergent properties to unique applications [J]. Accounts of Chemical Research, 2018, 51(11): 2756-2763. doi: 10.1021/acs.accounts.8b00366
|
|
[21]
|
WANG B J, ZHAO M, MEHDI M, et al. Biomolecule-assisted synthesis and functionality of metal nanoclusters for biological sensing: A review [J]. Materials Chemistry Frontiers, 2019, 3(9): 1722-1735. doi: 10.1039/C9QM00165D
|
|
[22]
|
PANDYA A, LAD A N, SINGH S P, et al. DNA assembled metal nanoclusters: Synthesis to novel applications [J]. RSC Advances, 2016, 6(114): 113095-113114. doi: 10.1039/C6RA24098D
|
|
[23]
|
KENNEDY T A, MACLEAN J L, LIU J. Blue emitting gold nanoclusters templated by poly-cytosine DNA at low pH and poly-adenine DNA at neutral pH [J]. Chemical Communications, 2012, 48: 6845-6847. doi: 10.1039/c2cc32841k
|
|
[24]
|
LIU G Y, SHAO Y, MA K, et al. Synthesis of DNA-templated fluorescent gold nanoclusters [J]. Gold Bulletin, 2012, 45(2): 69-74. doi: 10.1007/s13404-012-0049-6
|
|
[25]
|
WANG H B, BAI H Y, DONG G L, et al. DNA-templated Au nanoclusters coupled with proximity-dependent hybridization and guanine-rich DNA induced quenching: A sensitive fluorescent biosensing platform for DNA detection [J]. Nanoscale Advances, 2019, 1(4): 1482-1488. doi: 10.1039/C8NA00278A
|
|
[26]
|
WANG Y Y, HU L H, LI L L, et al. Fluorescent gold nanoclusters: Promising fluorescent probes for sensors and bioimaging [J]. Journal of Analysis and Testing, 2017, 1(2): 1-19.
|
|
[27]
|
LI Z Y, WU Y T, TSENG W L. UV-light-induced improvement of fluorescence quantum yield of DNA-templated gold nanoclusters: Application to ratiometric fluorescent sensing of nucleic acids [J]. ACS Applied Materials & Interfaces, 2015, 7(42): 23708-23716.
|
|
[28]
|
PETTY J T, ZHENG J, HUD N V, et al. DNA-templated Ag nanocluster formation [J]. Journal of the American Chemical Society, 2004, 126(16): 5207-5212. doi: 10.1021/ja031931o
|
|
[29]
|
GWINN E G, O'NEILL P, GUERRERO A J, et al. Sequence-dependent fluorescence of DNA-hosted silver nanoclusters [J]. Advanced Materials, 2008, 20(2): 279-283. doi: 10.1002/adma.200702380
|
|
[30]
|
RICHARDS C I, CHOI S, HSIANG J C, et al. Oligonucleotide-stabilized Ag nanocluster fluorophores [J]. Journal of the American Chemical Society, 2008, 130(15): 5038-5039. doi: 10.1021/ja8005644
|
|
[31]
|
SHARMA J, YEH H C, YOO H, et al. A complementary palette of fluorescent silver nanoclusters [J]. Chemical Communications (Cambridge, England), 2010, 46(19): 3280-3282. doi: 10.1039/b927268b
|
|
[32]
|
LAN G Y, CHEN W Y, CHANG H T. Control of synthesis and optical properties of DNA templated silver nanoclusters by varying DNA length and sequence [J]. RSC Advances, 2011, 1(5): 802. doi: 10.1039/c1ra00181g
|
|
[33]
|
YU J, CHOI S, DICKSON R. Shuttle-based fluorogenic silver-cluster biolabels [J]. Angewandte Chemie, 2009, 121(2): 324-326. doi: 10.1002/ange.200804137
|
|
[34]
|
LI J J, ZHONG X Q, ZHANG H Q, et al. Binding-induced fluorescence turn-on assay using aptamer-functionalized silver nanocluster DNA probes [J]. Analytical Chemistry, 2012, 84(12): 5170-5174. doi: 10.1021/ac3006268
|
|
[35]
|
YEH H C, SHARMA J, HAN J J, et al. A DNA−Silver nanocluster probe that fluoresces upon hybridization [J]. Nano Letters, 2010, 10(8): 3106-3110. doi: 10.1021/nl101773c
|
|
[36]
|
YIN J J, HE X X, WANG K M, et al. Label-free and turn-on aptamer strategy for cancer cells detection based on a DNA-silver nanocluster fluorescence upon recognition-induced hybridization [J]. Analytical Chemistry, 2013, 85(24): 12011-12019. doi: 10.1021/ac402989u
|
|
[37]
|
GUO W W, YUAN J P, WANG E K. Oligonucleotide-stabilized Ag nanoclusters as novel fluorescence probes for the highly selective and sensitive detection of the Hg2+ ion [J]. Chemical Communications (Cambridge, England), 2009(23): 3395-3397. doi: 10.1039/b821518a
|
|
[38]
|
CHEN J Y, JI X H, TINNEFELD P, et al. Multifunctional dumbbell-shaped DNA-templated selective formation of fluorescent silver nanoclusters or copper nanoparticles for sensitive detection of biomolecules [J]. ACS Applied Materials & Interfaces, 2016, 8(3): 1786-1794.
|
|
[39]
|
LI C, WEI C. DNA-functionlized silver nanoclusters as label-free fluorescent probe for the highly sensitive detection of biothiols and acetylcholinesterase activity [J]. Sensors and Actuators B: Chemical,, 2017, 240: 451-458.
|
|
[40]
|
ROTARU A, DUTTA S, JENTZSCH E, et al. Selective dsDNA-templated formation of copper nanoparticles in solution [J]. Angewandte Chemie (International Ed. in English), 2010, 49(33): 5665-5667. doi: 10.1002/anie.200907256
|
|
[41]
|
QING Z, HE X, HE D, et al. Poly(thymine)-templated selective formation of fluorescent copper nanoparticles [J]. Angewandte Chemie International Edition, 2013, 52(37): 9901-9904.
|
|
[42]
|
LIU G Y, SHAO Y, PENG J, et al. Highly thymine-dependent formation of fluorescent copper nanoparticles templated by ss-DNA [J]. Nanotechnology, 2013, 24(34): 345502. doi: 10.1088/0957-4484/24/34/345502
|
|
[43]
|
QING T, QING Z, MAO Z, et al. dsDNA-templated fluorescent copper nanoparticles: Poly (AT-TA)-dependent formation [J]. RSC Advances, 2014, 4(105): 61092-61095. doi: 10.1039/C4RA11551A
|
|
[44]
|
ZHU H W, DAI W X, YU X D, et al. Poly thymine stabilized copper nanoclusters as a fluorescence probe for melamine sensing [J]. Talanta, 2015, 144: 642-647. doi: 10.1016/j.talanta.2015.07.022
|
|
[45]
|
OU L J, LI X Y, LIU H W, et al. Poly(thymine)-templated fluorescent copper nanoparticles for ultrasensitive label-free detection of Pb2+ ion [J]. Analytical Sciences, 2014, 30(7): 723-727. doi: 10.2116/analsci.30.723
|
|
[46]
|
HU Y H, WU Y M, CHEN T T, et al. Double-strand DNA-templated synthesis of copper nanoclusters as novel fluorescence probe for label-free detection of biothiols [J]. Analytical Methods, 2013, 5(14): 3577-3581. doi: 10.1039/c3ay40088c
|
|
[47]
|
WANG H B, LI Y, BAI H Y, et al. Fluorescent determination of dopamine using polythymine-templated copper nanoclusters [J]. Analytical Letters, 2018, 51(18): 2868-2877. doi: 10.1080/00032719.2018.1454457
|
|
[48]
|
李婷, 曹忠, 李盼盼, 等. 聚胸腺嘧啶单链DNA-CuNCs荧光增强法用于汞离子的高灵敏检测 [J]. 高等学校化学学报, 2016, 37(9): 1616-1621.
LI T, CAO Z, LI P P, et al. High-sensitive fluorescent enhancement detection of Hg(II) ions based on poly(thymine)-templated copper nanoclusters [J]. Chemical Journal of Chinese Universities, 2016, 37(9): 1616-1621(in Chinese).
|
|
[49]
|
LAN G Y, HUANG C C, CHANG H T. Silver nanoclusters as fluorescent probes for selective and sensitive detection of copper ions [J]. Chemical Communications (Cambridge, England), 2010, 46(8): 1257-1259. doi: 10.1039/b920783j
|
|
[50]
|
SU Y T, LAN G Y, CHEN W Y, et al. Detection of copper ions through recovery of the fluorescence of DNA-templated copper/silver nanoclusters in the presence of mercaptopropionic acid [J]. Analytical Chemistry, 2010, 82(20): 8566-8572. doi: 10.1021/ac101659d
|
|
[51]
|
AHN J K, KIM H Y, BAEK S, et al. A new s-adenosylhomocysteine hydrolase-linked method for adenosine detection based on DNA-templated fluorescent Cu/Ag nanoclusters [J]. Biosensors and Bioelectronics, 2017, 93: 330-334.
|
|
[52]
|
LAN G Y, CHEN W Y, CHANG H T. Characterization and application to the detection of single-stranded DNA binding protein of fluorescent DNA-templated copper/silver nanoclusters [J]. The Analyst, 2011, 136(18): 3623-3628. doi: 10.1039/c1an15258k
|
|
[53]
|
LI R D, WANG Q, YIN B C, et al. Enzyme-free detection of sequence-specific microRNAs based on nanoparticle-assisted signal amplification strategy [J]. Biosensors and Bioelectronics, 2016, 77: 995-1000.
|
|
[54]
|
DING Y J, LI X M, CHEN C, et al. A rapid evaluation of acute hydrogen sulfide poisoning in blood based on DNA-Cu/Ag nanocluster fluorescence probe [J]. Scientific Reports, 2017, 7(1): 9638. doi: 10.1038/s41598-017-09960-1
|
|
[55]
|
CHEN W Y, LAN G Y, CHANG H T. Use of fluorescent DNA-templated gold/silver nanoclusters for the detection of sulfide ions [J]. Analytical Chemistry, 2011, 83(24): 9450-9455. doi: 10.1021/ac202162u
|
|
[56]
|
ZHENG C, ZHENG A X, LIU B, et al. One-pot synthesized DNA-templated Ag/Pt bimetallic nanoclusters as peroxidase mimics for colorimetric detection of thrombin [J]. Chemical Communications, 2014, 50: 13103-13106.
|
|
[57]
|
TUERK C, GOLD L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase [J]. Science, 1990, 249(4968): 505-510. doi: 10.1126/science.2200121
|
|
[58]
|
ELLINGTON A D, SZOSTAK J W. In vitro selection of RNA molecules that bind specific ligands [J]. Nature, 1990, 346(6287): 818-822. doi: 10.1038/346818a0
|
|
[59]
|
KONG H Y, JONGHOE B. Nucleic Acid aptamers: new methods for selection, stabilization, and application in biomedical science [J]. Biomolecules & Therapeutics, 2013, 21(6): 423-434.
|
|
[60]
|
YUE F L, LI F L, KONG Q Q, et al. Recent advances in aptamer-based sensors for aminoglycoside antibiotics detection and their applications [J]. Science of The Total Environment, 2021, 762: 143129. doi: 10.1016/j.scitotenv.2020.143129
|
|
[61]
|
LI F Q, YU Z G, HAN X D, et al. Electrochemical aptamer-based sensors for food and water analysis: A review [J]. Analytica Chimica Acta, 2019, 1051: 1-23. doi: 10.1016/j.aca.2018.10.058
|
|
[62]
|
YANG Y B, YANG X D, YANG Y J, et al. Aptamer-functionalized carbon nanomaterials electrochemical sensors for detecting cancer relevant biomolecules [J]. Carbon, 2018, 129: 380-395. doi: 10.1016/j.carbon.2017.12.013
|
|
[63]
|
LI H H, AHMAD W, RONG Y W, et al. Designing an aptamer based magnetic and upconversion nanoparticles conjugated fluorescence sensor for screening Escherichia coli in food [J]. Food Control, 2020, 107: 106761. doi: 10.1016/j.foodcont.2019.106761
|
|
[64]
|
LIU F, DING A L, ZHENG J S, et al. A label-free aptasensor for ochratoxin a detection based on the structure switch of aptamer [J]. Sensors, 2018, 18(6): 1769. doi: 10.3390/s18061769
|
|
[65]
|
SOLHIA E, HASANZADEHA M. Critical role of biosensing on the efficient monitoring of cancer proteins/biomarkers using label-free aptamer based bioassay [J]. Biomedicine & Pharmacotherapy, 2020, 132: 110849.
|
|
[66]
|
FANG B Y, AN J, LIU B, et al. Hybridization induced fluorescence enhanced DNA-Ag nanocluster/aptamer probe for detection of prostate-specific antigen [J]. Colloids and Surfaces. B, Biointerfaces, 2019, 175: 358-364. doi: 10.1016/j.colsurfb.2018.12.013
|
|
[67]
|
ZHANG Z H, GUO C P, ZHANG S, et al. Carbon-based nanocomposites with aptamer-templated silver nanoclusters for the highly sensitive and selective detection of platelet-derived growth factor [J]. Biosensors and Bioelectronics, 2017, 89(Pt 2): 735-742.
|
|
[68]
|
LI J J, YOU J, ZHUANG Y P, et al. A “light-up” and “spectrum-shift” response of aptamer-functionalized silver nanoclusters for intracellular mRNA imaging [J]. Chemical Communications, 2014, 50(54): 7107-7110. doi: 10.1039/c4cc00160e
|
|
[69]
|
DING Y J, LI X M, GUO Y D, et al. Estimation of postmortem interval by vitreous potassium evaluation with a novel fluorescence aptasensor [J]. Scientific Reports, 2017, 7(1): 1868. doi: 10.1038/s41598-017-02027-1
|
|
[70]
|
WANG L, WANG M K, SHI F P, et al. Aptamer based fluorescence biosensor for protein kinase activity detection and inhibitor screening [J]. Sensors and Actuators B: Chemical, 2017, 252: 209-214.
|
|
[71]
|
LEE S T, BEAUMONT D, SU X D, et al. Formulation of DNA chimera templates: Effects on emission behavior of silver nanoclusters and sensing [J]. Analytica Chimica Acta, 2018, 1010: 62-68. doi: 10.1016/j.aca.2018.01.012
|
|
[72]
|
WANG Y Y, WANG S S, LU C S, et al. Three kinds of DNA-directed nanoclusters cooperating with graphene oxide for assaying mucin 1, carcinoembryonic antigen and cancer antigen 125 [J]. Sensors and Actuators B: Chemical, 2018, 262: 9-16. doi: 10.1016/j.snb.2018.01.235
|
|
[73]
|
ONO A, TOGASHI H. Highly selective oligonucleotide-based sensor for Mercury(Ⅱ) in aqueous solutions [J]. Angewandte Chemie (International ed. in English), 2004, 43(33): 4300-4302. doi: 10.1002/anie.200454172
|
|
[74]
|
ZHU S S, ZHUO Y, MIAO H, et al. Detection of mercury(II) by DNA templated gold nanoclusters based on forming thymidine-Hg(2+)-thymidine duplexes [J]. Luminescence, 2015, 30(5): 631-636. doi: 10.1002/bio.2797
|
|
[75]
|
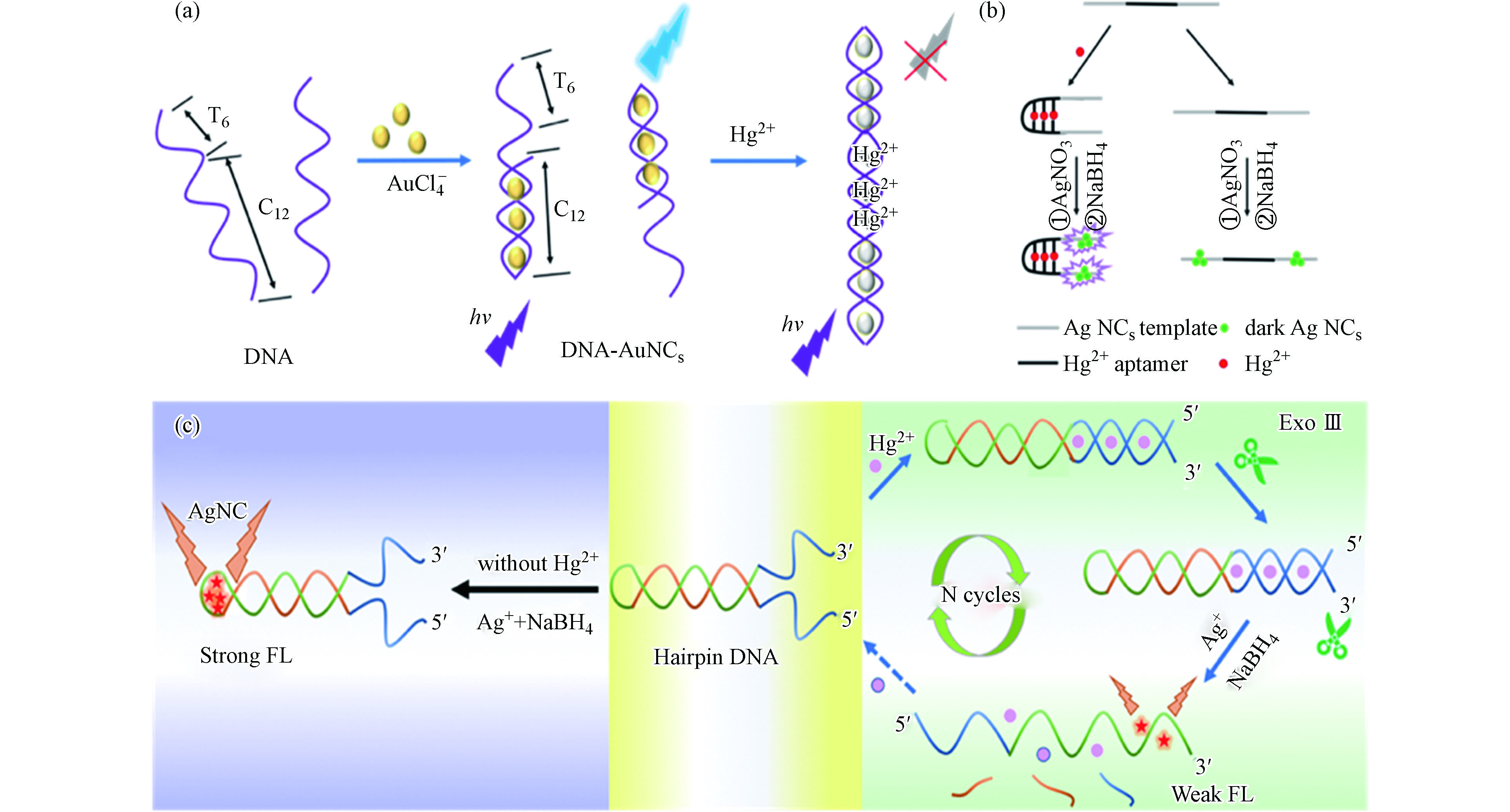
XU M D, GAO Z Q, WEI Q H, et al. Label-free hairpin DNA-scaffolded silver nanoclusters for fluorescent detection of Hg2+ using exonuclease III-assisted target recycling amplification [J]. Biosensors & Bioelectronics, 2016, 79: 411-415.
|
|
[76]
|
YIN J J, HE X X, JIA X K, et al. Highly sensitive label-free fluorescent detection of Hg2+ ions by DNA molecular machine-based Ag nanoclusters [J]. The Analyst, 2013, 138(8): 2350-2356. doi: 10.1039/c3an00029j
|
|
[77]
|
DENG L, ZHOU Z X, LI J, et al. Fluorescent silver nanoclusters in hybridized DNA duplexes for the turn-on detection of Hg2+ ions [J]. Chemical Communications (Cambridge, England), 2011, 47(39): 11065-11067. doi: 10.1039/c1cc14012d
|
|
[78]
|
ZHANG B Z, WEI C Y. Highly sensitive and selective fluorescence detection of Hg2+ based on turn-on aptamer DNA silver nanoclusters [J]. RSC Advances, 2017, 7(89): 56289-56295. doi: 10.1039/C7RA11566K
|
|
[79]
|
MA H Y, XUE N, WU S J, et al. Fluorometric determination of mercury(II) using positively charged gold nanoparticles, DNA-templated silver nanoclusters, T-Hg(II)-T interaction and exonuclease assisted signal amplification [J]. Microchimica Acta, 2019, 186: 307.
|
|
[80]
|
YIN B C, MA J L, LE H N, et al. A new mode to light up an adjacent DNA-scaffolded silver probe pair and its application for specific DNA detection [J]. Chemical Communications (Cambridge, England), 2014, 50(100): 15991-15994. doi: 10.1039/C4CC07209J
|
|
[81]
|
ZHANG B Z, WEI C Y. Highly sensitive and selective detection of Pb2+ using a turn-on fluorescent aptamer DNA silver nanoclusters sensor [J]. Talanta, 2018, 182: 125-130. doi: 10.1016/j.talanta.2018.01.061
|
|
[82]
|
WANG J, ZHANG Z Y, GAO X, et al. A single fluorophore ratiometric nanosensor based on dual-emission DNA-templated silver nanoclusters for ultrasensitive and selective Pb2+ detection [J]. Sensors and Actuators B: Chemical, 2019, 282: 712-718. doi: 10.1016/j.snb.2018.11.121
|
|
[83]
|
LI C Y, WEI C Y. DNA-templated silver nanocluster as a label-free fluorescent probe for the highly sensitive and selective detection of mercury ions [J]. Sensors and Actuators B: Chemical, 2017, 242: 563-568.
|
|
[84]
|
XIE J P, ZHENG Y G, YING J Y. Highly selective and ultrasensitive detection of Hg2+ based on fluorescence quenching of Au nanoclusters by Hg2+-Au+ interactions [J]. Chemical Communications, 2010, 46(6): 961-963. doi: 10.1039/B920748A
|
|
[85]
|
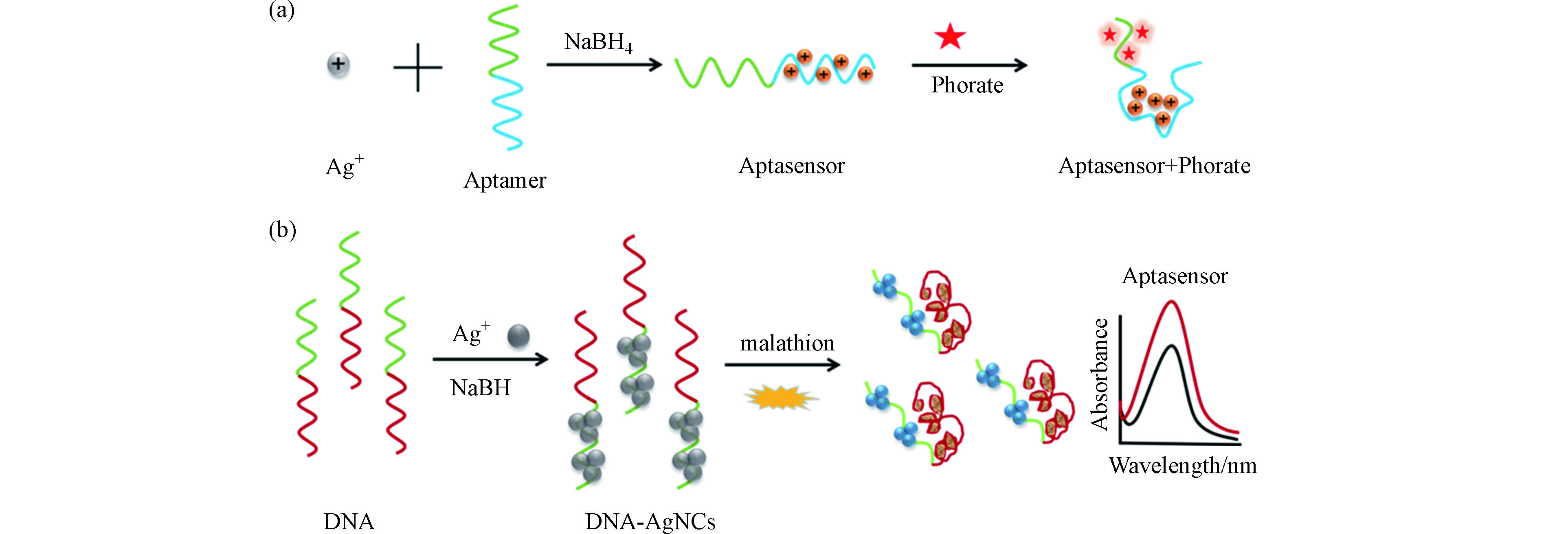
LI X M, SHI J, CHEN C, et al. One-step, visual and sensitive detection of phorate in blood based on a DNA-AgNC aptasensor [J]. New Journal of Chemistry, 2018, 42(8): 6293-6298. doi: 10.1039/C8NJ00958A
|
|
[86]
|
CHEN C, SHI J, GUO Y D, et al. A novel aptasensor for malathion blood samples detection based on DNA-silver nanocluster [J]. Analytical methods, 2018, 10(16): 1928-1934. doi: 10.1039/C8AY00428E
|
|
[87]
|
边孟孟, 李小青, 袁亚利. 基于DNA保护的银纳米簇荧光探针检测啶虫脒 [J]. 食品安全质量检测学报, 2019, 10(10): 2937-2941. doi: 10.3969/j.issn.2095-0381.2019.10.017
BIAN M M, LI X Q, YUAN Y L. Sensitive detection of acetamiprid silver nanoclusters fluorescent probes based on DNA protection [J]. Journal of Food Safety & Quality, 2019, 10(10): 2937-2941(in Chinese). doi: 10.3969/j.issn.2095-0381.2019.10.017
|
|
[88]
|
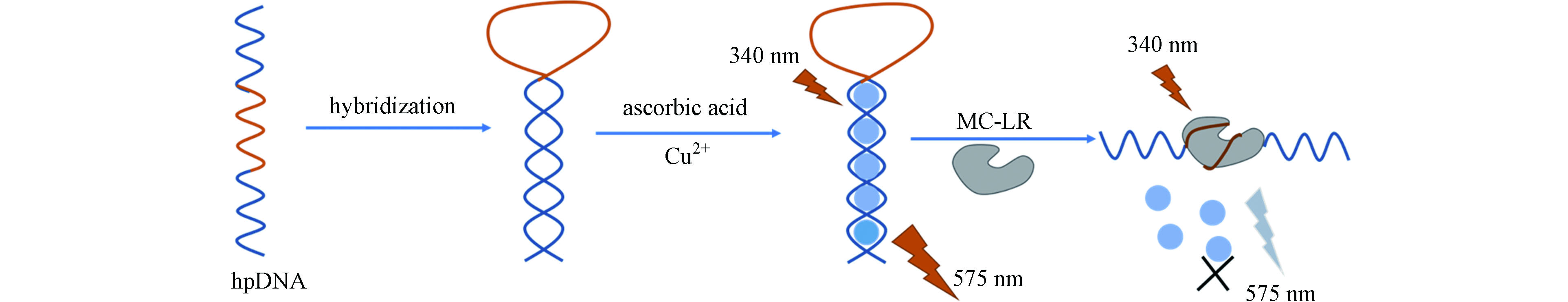
ZHANG Y L, ZHU Z Y, TENG X, et al. Enzyme-free fluorescent detection of microcystin-LR using hairpin DNA-templated copper nanoclusters as signal indicator [J]. Talanta, 2019, 202: 279-284. doi: 10.1016/j.talanta.2019.05.013
|
|
[89]
|
LI J X, JIANG D, SHAN X L, et al. An “off-on” electrochemiluminescence aptasensor for microcystin-LR assay based on the resonance energy transfer from PTCA/NH2-MIL-125(Ti) to gold nanoparticles [J]. Microchimica Acta, 2020, 187: 474.
|
|
[90]
|
LIU X Q, TANG Y F, LIU P P, et al. A highly sensitive electrochemical aptasensor for detection of microcystin-LR based on a dual signal amplification strategy [J]. Analyst, 2019, 144(5): 1671-1678. doi: 10.1039/C8AN01971A
|
|
[91]
|
LI X Y, CHENG R J, SHI H J, et al. A simple highly sensitive and selective aptamer-based colorimetric sensor for environmental toxins microcystin-LR in water samples [J]. Journal of Hazardous Materials, 2016, 304: 474-480.
|
|
[92]
|
HE D Y, WU Z Z, CUI B, et al. A novel SERS-based aptasensor for ultrasensitive sensing of microcystin-LR [J]. Food Chemistry, 2019, 278: 197-202. doi: 10.1016/j.foodchem.2018.11.071
|
|
[93]
|
TAGHDISI S M, DANESH N M, RAMEZANI M, et al. A novel fluorescent aptasensor for ultrasensitive detection of microcystin-LR based on single-walled carbon nanotubes and dapoxyl [J]. Talanta, 2017, 166: 187-192. doi: 10.1016/j.talanta.2017.01.053
|
|
[94]
|
PANG P F, TENG X, CHEN M, et al. Ultrasensitive enzyme-free electrochemical immunosensor for microcystin-LR using molybdenum disulfide/gold nanoclusters nanocomposites as platform and Au@Pt core-shell nanoparticles as signal enhancer [J]. Sensors and Actuators B: Chemical, 2018, 266: 400-407.
|
|
[95]
|
GAN C F, LING L, HE Z Y, et al. In-situ assembly of biocompatible core-shell hierarchical nanostructures sensitized immunosensor for microcystin-LR detection [J]. Biosensors and Bioelectronics, 2016, 78: 381-389.
|
|
[96]
|
ZANATO N, TALAMINI L, SILVA T R, et al. Microcystin-LR label-free immunosensor based on exfoliated graphite nanoplatelets and silver nanoparticles [J]. Talanta, 2017, 175: 38-45. doi: 10.1016/j.talanta.2017.07.021
|
|
[97]
|
XU Z L, YE S L, LUO L, et al. Fluorescent enzyme-linked immunoassay based on silane-doped carbon dots for sensitive detection of microcystin-LR in water and crucian samples [J]. Science of The Total Environment, 2020, 708: 134614.
|
|
[98]
|
沈强, 李嗣新, 胡俊. 微囊藻毒素HPLC快速检测方法研究 [J]. 环境科学与技术, 2017, 40(2): 103-106.
SHEN Q, LI S X, HU J. Study on rapid analysis of microcystins by HPLC [J]. Environmental Science & Technology, 2017, 40(2): 103-106(in Chinese).
|
|
[99]
|
KHAN I M, ZHAO S, NIAZI S, et al. Silver nanoclusters based FRET aptasensor for sensitive and selective fluorescent detection of T-2 toxin [J]. Sensors and Actuators B: Chemical, 2018, 277: 328-335. doi: 10.1016/j.snb.2018.09.021
|
|
[100]
|
ZHANG J, XIA Y K, CHEN M, et al. A fluorescent aptasensor based on DNA-scaffolded silver nanoclusters coupling with Zn(Ⅱ)-ion signal-enhancement for simultaneous detection of OTA and AFB1 [J]. Sensors and Actuators B: Chemical, 2016, 235: 79-85.
|
|
[101]
|
ZHOU Z X, DU Y, DONG S J. DNA-Ag nanoclusters as fluorescence probe for turn-on aptamer sensor of small molecules [J]. Biosensors and Bioelectronics, 2011, 28(1): 33-37.
|
|
[102]
|
SHARON E, ENKIN N, ALBADA B, et al. Aptasensors based on supramolecular structures of nucleic acid-stabilized Ag nanoclusters [J]. Chemical Communications, 2015, 51(6): 1100-1103. doi: 10.1039/C4CC08263J
|
|
[103]
|
ENKIN N, WANG F A, SHARON E, et al. Multiplexed analysis of genes using nucleic acid-stabilized silver-nanocluster quantum dots [J]. ACS Nano, 2014, 8(11): 11666-11673. doi: 10.1021/nn504983j
|
|
[104]
|
YAO Y Y, WANG X Z, DUAN W N, et al. A label-free, versatile and low-background chemiluminescence aptasensing strategy based on gold nanocluster catalysis combined with the separation of magnetic beads [J]. Analyst, 2018, 143: 709-714. doi: 10.1039/C7AN01765K
|



 下载:
下载: