-
水资源紧缺是全球面临的挑战,污水回用是缓解水资源紧缺的重要手段,而其中的关键问题是保障回用水质安全。污水回用主要包括生物处理工艺和深度处理工艺。生物处理主要去除氮、磷和有机物,常用的生物处理工艺包括厌氧好氧工艺(A/O),厌氧缺氧好氧工艺(A/A/O),膜生物反应器(MBR),UNITANK序批式活性污泥工艺和生物滤池等。深度处理工艺主要去除水中的微量有机污染物和杀灭水中的致病微生物,包括病原细菌、病毒及寄生虫卵等。目前污水回用中采用的深度处理工艺包括:氯、氯胺、二氧化氯、臭氧、紫外光照及紫外/过氧化氢等高级氧化(advanced oxidation process,AOP)工艺以及高铁酸盐、高锰酸盐等。
污水消毒是确保回用水水质安全的重要保障[1−2],在水消毒过程中,污水中含有的有机物,如腐殖酸、富里酸和微生物代谢物等与消毒剂发生氧化、加成、取代等反应生成消毒副产物,即DBPs[3−4]。研究表明,大部分DBPs具有潜在的致癌、致畸、致突变性[5−6],其中含氮DBPs具有比常规管制DBPs更高的毒性,目前新型含氮DBPs主要包括卤代乙腈、卤代硝基甲烷以及亚硝胺等[7-11]。
控制污水消毒过程中产生的DBPs是提高回用水水质安全的重要课题,目前对污水DBPs的控制手段主要包括前体物去除、消毒剂种类与投量控制,以及DBPs去除,本研究主要关注DBPs的前体物去除。污水中的有机物(effluent organic matters,EfOM)是DBPs的主要前体物,而因污水中的氨氮和EfOM含有的溶解性有机氮远远高于饮用水源,导致污水的消毒过程会产生大量的含氮的消毒副产物(N-DBPs)[12−13]。此外,污水中的无机离子,如溴离子在臭氧氧化过程中也会催化二甲基亚硝胺(N-Nitrosodimethylamine,NDMA)的生成[14]。
本研究旨在从污水回用角度出发,探究污水深度处理工艺对污水中消毒副产物及其前体物的去除效果。主要研究内容包括:(1)不同生物处理工艺(A/O,A/A/O,MBR,UNITANK,循环式活性污泥法(CAST), 氧化沟和生物滤池)对典型N-DBPs (包括亚硝胺类物质、卤乙腈和卤代硝基甲烷)的去除;(2)不同深度处理工艺(混凝沉淀,PAC,臭氧,二氧化氯,高铁酸盐氧化)对典型N-DBPs及其前体物的去除。
-
4种卤乙腈混合标样(haloacetonitrile,HANs)、三氯硝基甲烷(trichloronitromethane,TCNM)、1,2-二溴丙烷、二甲基亚硝胺(NDMA)、NDMA-d6内标、甲基叔丁基醚(MTBE)、甲醇(Methanol)、浓硫酸(98%H2SO4)、冰醋酸(CH3COOH)、次氯酸钠溶液(NaClO)、亚氯酸钠(NaClO2)、氯化铵(NH4Cl)、无水硫酸钠(Na2SO4)、抗坏血酸(Ascorbic acid)、N,N-二乙基-对苯二胺硫酸盐(DPD)、六水合硫酸亚铁铵(FAS)、碘单质(I2)、碘化钾(KI)、高铁酸钾(K2FeO4)、椰壳炭粉末(PAC)、无水磷酸氢二钠(Na2HPO4)、无水磷酸二氢钠(NaH2PO4)、氢氧化钾(KOH)、明矾。
二氧化氯储备液(ClO2)由亚氯酸钠与浓硫酸反应制备而成,其浓度用DPD/FAS比色法标定。一氯胺(NH2Cl)储备液(10000 mg·L−1,以氯(Cl2)计,全文中含氯浓度均以Cl2计)由氯化铵与次氯酸钠溶液依比例反应制备而成,现配现用。臭氧溶液(O3)使用臭氧发生装置产生臭氧,并溶解在4°C的 pH5磷酸盐缓冲液中,其浓度用靛蓝脱色比色法标定[15],现配现用。高铁酸钾溶液由高纯度(>90%)高铁酸钾固体溶于纯水中配制成,现配现用,由于购买的高铁酸钾固体纯度仅有约50%,需进一步采用有机溶剂洗涤与重结晶法纯化[16],简而言之,将低纯度的高铁酸钾固体用1 mol·L−1的KOH溶液洗涤抽滤,所得滤液与饱和KOH溶液混合置于冰水浴中析出晶体并过滤,所得晶体再依次用正己烷、戊烷、甲醇和乙醚冲洗以脱水脱碱,随后在烘箱中60 ℃干燥,真空干燥器中密封保存。
气相色谱仪(Agilent 7890A,美国安捷伦公司)、气相色谱质谱联用(Trace1300 ISQ,美国赛默飞公司)、氮氧化物分析仪(CLD-88,瑞士ECO Medics公司)、离子色谱仪(ICS-600,美国赛默飞公司)、紫外-可见分光光度计(UV-2700,日本岛津公司)、总有机碳测定仪(TOC-LCPH,日本岛津公司)、哈希水质检测仪(DR2800,美国哈希公司)pH计(S210,瑞士梅勒多-托利多公司)。
-
本研究选择8间污水处理厂的初沉池出水和生化反应池出水作为实验水样探究生物处理工艺对亚硝胺及N-DBPs前体物的影响,样品编号及水厂工艺见表1。由于A/A/O为国内污水处理厂常用的生物处理工艺,因此在探究深度处理工艺对亚硝胺及N-DBPs前体物的影响中,实验所用水样取自B污水厂的二级沉淀池出水,其出水特征更具有代表性。水样基本参数包括DOC、氨氮、UV254和无机离子浓度等均采用国标方法进行。
表2中汇总了各水样的基本参数,其中编号为A1—H1的水样为生物处理池进水,编号为A2—H2的水样为生物处理池出水,水样的DOC、氨氮和亚硝酸盐的浓度反映了含氮消毒副产物的部分前体物水平,这些参数对含氮消毒副产物的生成潜能有影响[17−20]。
-
混凝处理工艺:采用六位组合搅拌器进行混凝试验。在硅烷化玻璃烧杯中装2.1 L污水水样,开始快速搅拌的同时向水样中添加明矾(10 mg明矾·mg−1 TOC),搅拌条件为:100 r·min−1 快速搅拌1 min,30 r·min−1絮凝20 min,沉降时间60 min。沉淀后,收集和过滤上清液样品以进行亚硝胺和DBPs检测和模拟实际处理(uniform formation condition,UFC)试验。
PAC吸附工艺:用0.45 μm玻璃纤维滤膜过滤2.1 L污水样品,粉末活性炭使用前在超纯水中混合24 h。PAC投加量为10 mg·L−1和20 mg·L−1,反应器与混凝实验一致。恒速(100 r·min−1)搅拌4 h后沉降1 h,收集和过滤上清液样品以进行亚硝胺和DBPs检测和UFC试验。
氧化工艺所处理的污水水样均经0.45 µm玻璃纤维滤膜过滤,反应器均采用棕色玻璃瓶,可容纳 >2.1 L水。其中:①二氧化氯氧化工艺:向水样中投加 2 mg·L−1二氧化氯(按Cl2计算),顶空反应2、5、10、20、30 min后测定二氧化氯浓度,并用氮气吹脱剩余二氧化氯以终止反应,选取二氧化氯氧化30 min的水样进行亚硝胺和DBPs检测,所有投量的水样均进行UFC实验。②高铁酸盐氧化工艺:向水样中投加2.5、5、7.5、10 mg·L−1 (Fe)高铁酸钾,混合均匀后静置3 h,期间分别在1、2、3、5、7、10、15、30、60、90、120 min取样并用ABTS法测定高铁酸盐浓度[21],反应3 h后水样经0.45 µm玻璃纤维滤膜过滤后,投量为10 mg Fe·L−1的样品进行亚硝胺和DBPs检测,所有投量的样品均进行UFC实验。③臭氧氧化工艺:向水样中投加0.5、1、2、3 mg·L−1臭氧溶液并迅速摇匀,在15、30、40、60、90、120 s时取样进行臭氧浓度检测,以获得臭氧在水样中的衰减曲线。反应至水样中臭氧浓度低于检出限后,臭氧投量为3 mg ·L−1的样品亚硝胺和DBPs检测,所有投量的样品均进行UFC试验。所有实验均设置2组平行对照。
-
污水后氯胺化实验:采用一氯胺(pH 8.5)作为消毒剂,现配现用,向需要进行UFC实验的2.1 L样品中加入5 mg·L−1的一氯胺溶液,并在黑暗室温条件下维持3 d,保证反应后水样中的总氯含量大于1 mg·L−1,3 d后加入33 mg·L−1 抗坏血酸终止反应,并分装为500 mL、500 mL与30 mL分别进行NDMA、总亚硝胺(TONO)和卤代DBPs萃取检测。所有实验均设置2组平行对照。
-
水样的pH、UVA254、DOC分别采用pH计、UV-vis分光光度计、TOC测定仪。Cl−、Br−、NO3−和NO2−等阴离子采用配有Dionex IonPacTM AS19分析柱(4 mm× 250 mm)和Dionex IonPacTM AG保护柱(4 mm× 50 mm)的离子色谱仪定量,KOH淋洗液梯度程序为:初始KOH淋洗液浓度为10 mmol·L−1,保持10 min,然后以2 mmol·L−1·min−1升至20 mmol·L−1·min−1,保持5 min,淋洗液的流速为1 mL·min−1。氨氮采用纳氏试剂分光光度法测定。
-
样品经含有内标(1,2-二溴丙烷)的MTBE萃取后,依照美国环保署标准方法USEPA Method 551.1[22],采用气相色谱仪测定卤乙腈(HANs)和TCNM。采用Agilent DB-5 0.25 mm × 30 m分离柱,进样口温度为120 ℃,检测器温度为290 ℃。测定时进样体积均为2 μL,进样模式为不分流,氮气流速为1.0 mL·min–1。样品浓度定量采用内标法校正,回收率保证在80%–120%,每次检测前使用现配的标准溶液进行仪器状态检查校正。
-
样品的萃取参考Dai等研究中的固相萃取法[23]。NDMA浓度用GC-MS检测,采用Agilent DB-1701 0.25 mm × 60 m分离柱,载气为高纯氦气,流速为1.0 mL·min–1,升温程序为:37 ˚C维持4 min,以4 ˚C·min−1升至130 ˚C,10 ˚C·min−1升至160 ˚C,40 ˚C·min−1升至250 ˚C并维持2 min。MS端为PCI模式,扫描时间0.7 s,放射电流50 µA,溶剂延迟9.5 min,传输线温度230 ˚C,离子源温度150 ˚C,反应气为甲烷,样品浓度定量采用内标法校正,回收率保证在80%–120%,每次检测前使用现配的标准溶液进行仪器状态检查校正。TONO浓度用氮氧化物分析仪进行检测,样品中的亚硝胺在80 ℃下与I2-KI-醋酸溶液反应后生成一氧化氮气体(NO)进入氮氧化物分析仪,气体流速为200 mL·min−1。
-
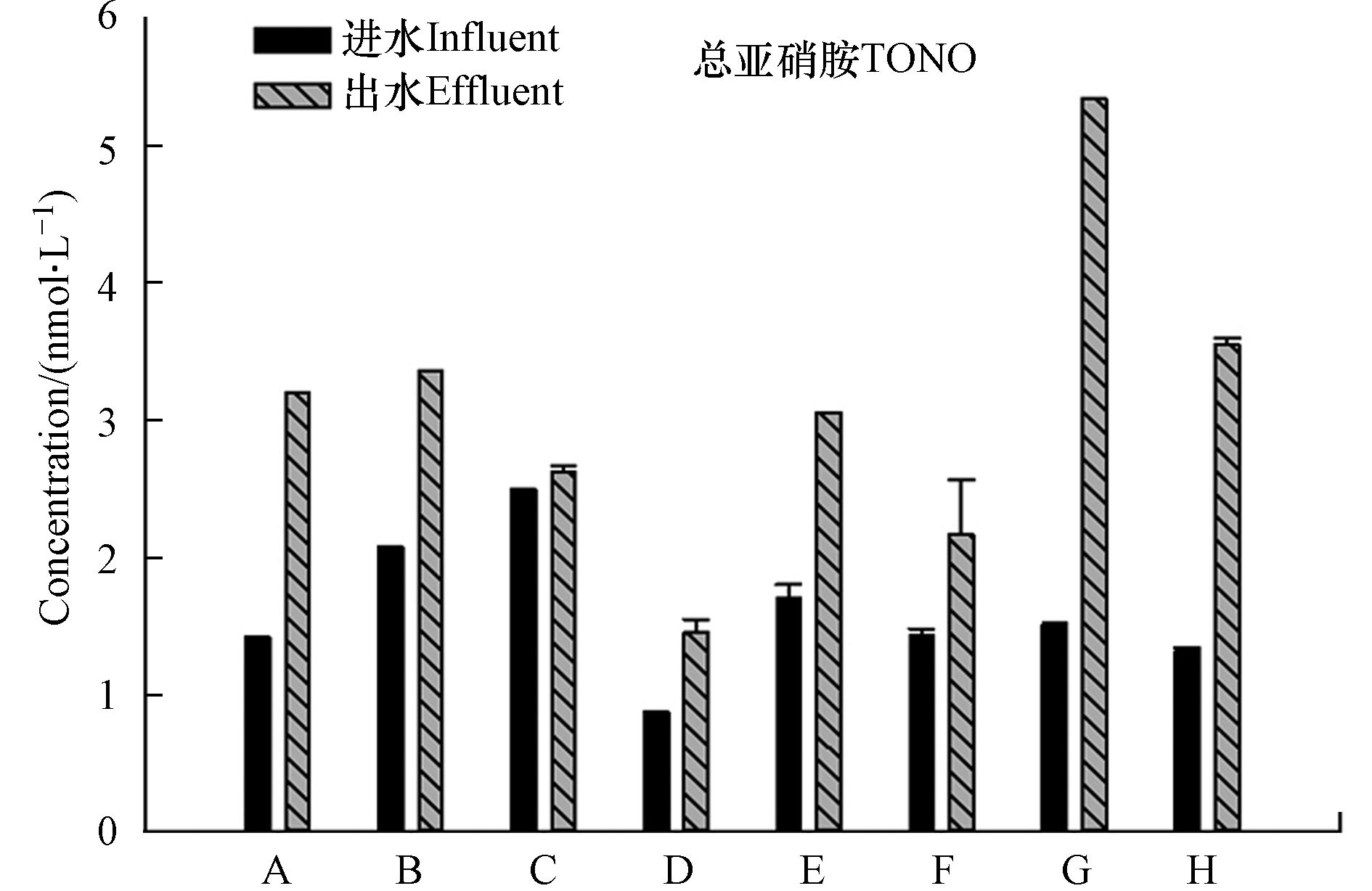
图1为A–H生化池进水与出水中总亚硝胺的存在水平,卤乙腈、三氯硝基甲烷未检出。进水中TONO的浓度范围为0.9—2.5 nmol·L−1,出水中的范围为1.4—5.3 nmol·L−1,TONO的浓度在经生物处理后均有不同程度的升高(5.0%—254.5%)。在生物处理的厌氧或缺氧阶段,污水中NO2−浓度在反硝化作用下几乎被完全去除[24],该阶段极少亚硝胺的生成,因此亚硝胺主要在生物处理的好氧阶段生成。在水厂G的进水中具有高浓度NO2−,且其出水中的NO2−浓度更高,在此过程中大量NO2−参与了亚硝胺的生成,导致TONO浓度大幅升高。污水生物处理过程中亚硝胺的降解途径主要是生物降解,生物降解效率与亚硝胺的官能团有直接关系,如亚硝基吗啉(N-nitrosomorpholine,NMOR)等不具有强供电子官能团的亚硝胺难以被生物降解[25−26]。亚硝胺也可能在生物处理过程中生成,主要依靠微生物作用,微生物细胞表面或细胞质膜上吸附的胺类有机物比游离态的胺的亚硝化作用强,且亚硝胺的生成速率由其前体物(EfOM与NO2−)浓度决定[27]。
-
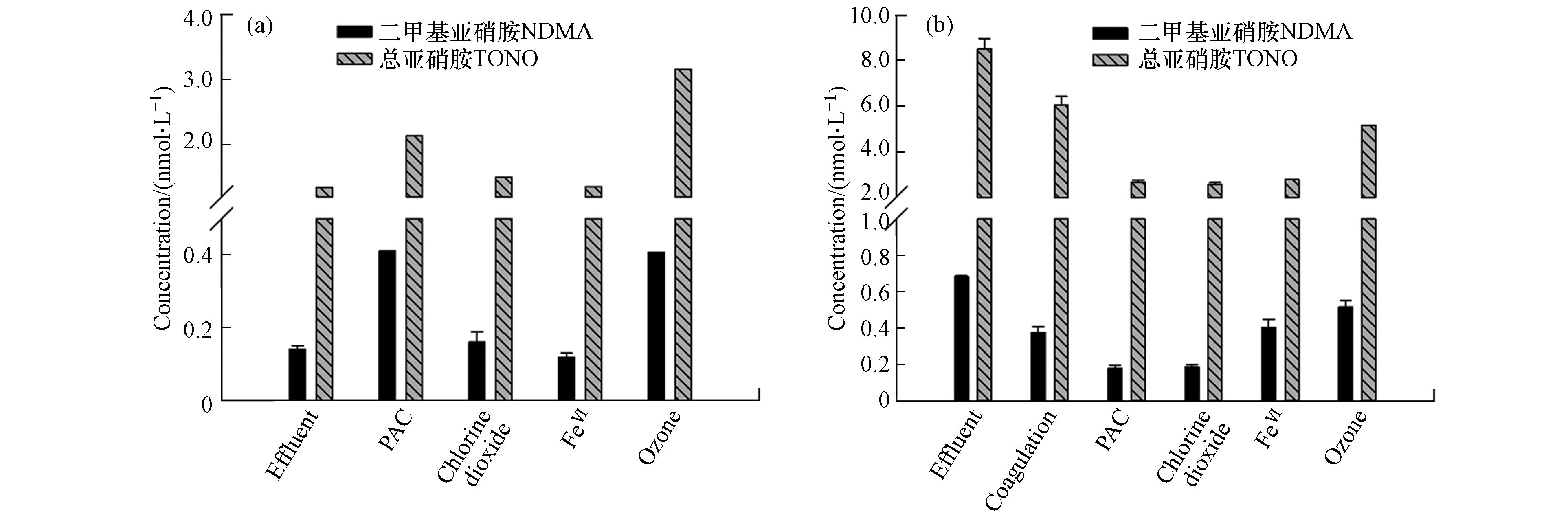
图2a为各深度处理工艺对污水中亚硝胺类物质的去除。原水B(图中标注为effluent)中含有微量的NDMA与TONO,浓度分别为0.14 nmol·L−1和1.35 nmol·L−1,实验结果表明二氧化氯与高铁酸盐对NDMA和TONO的浓度并没有显著影响,但PAC和臭氧处理均会导致NDMA和TONO的增加,PAC处理使NDMA和TONO分别增加了192%和61.5%,臭氧氧化则使NDMA和TONO分别增加了185%和138%。
二氧化氯可以与丁酰肼反应生成NDMA[28],其中一种中间产物为偏二甲肼(UDMH),此外与其他二级胺反应生成的NDMA量极低,对于本实验而言,30 min的二氧化氯氧化对污水中NDMA和TONO的浓度并无明显影响,推测污水中丁酰肼或UDMH的含量很低。
在经20 mg·L−1 PAC吸附处理后,NDMA的浓度增长至0.41 nmol·L−1,同时TONO的浓度增至2.14 nmol·L−1,活性炭在氧气存在的情况下可以催化水中的二级胺生成痕量的亚硝胺类物质,PAC表面的活性位点与氧分子反应生成活性氧(reactive oxygen species,ROS),进而促进PAC表面对氮分子的吸附,同时生成活性氮物质(reactive nitrogen species,RNS),如氧化亚氮和羟胺,进一步与吸附在PAC表面的二级胺反应生成亚硝胺[29−30]。本实验中采用的PAC为400目粉末活性炭,具有较高的比表面积,因此更容易产生亚硝胺。
臭氧(3 mg·L−1)处理的污水中NDMA与TONO浓度也显著提升,分别为0.41 nmol·L−1和3.16 nmol·L−1。臭氧氧化是水处理过程中NDMA生成的一种重要方式,臭氧与二甲胺(DMA)或N,N-二甲基磺胺反应会直接生成NDMA,同时臭氧与一些含氮类药物、染料以及二甲胺反应也会生成NDMA[31−33]。
经各种深度处理工艺后卤代消毒副产物的生成不明显,这是由于采用的深度处理中没有引入氯和氯胺,因此几乎没有卤代消毒副产物的生成。
-
为探究所选深度处理工艺对消毒副产物的前体物的去除效果,将经深度处理的污水再进行后氯胺化处理,并检测其中的各类消毒副产物的生成情况。结果表明,各深度处理工艺对亚硝胺类物质的前体物均有不同程度的去除(图2b),未经深度处理的原水经氯胺化后,NDMA浓度为0.69 nmol·L−1,而混凝、PAC吸附、二氧化氯、高铁酸钾和臭氧处理的污水经氯胺化后NDMA浓度分别为0.38、0.18、0.19、0.33、0.42 nmol·L−1,即NDMA前体物去除率顺序为:PAC(74%)≈二氧化氯(72%)>高铁酸钾(52%)>混凝(45%)>臭氧(39%)。同样的,混凝、PAC吸附、二氧化氯、高铁酸钾和臭氧处理的污水经氯胺化后TONO的浓度分别为6.06、2.67、2.55、2.65、4.50 nmol·L−1,均低于未经深度处理的污水后氯胺化样品(8.51 nmol·L−1),即TONO前体物去除率顺序为:二氧化氯(70%)≈高铁酸钾(69%)≈PAC(69%)>臭氧(47%)>混凝(29%)。
本实验中,采用10 mg·L−1明矾作为絮凝剂对NDMA的前体物去除达到40%,而TONO的前体物去除也低于30%,这可能是由于NDMA前体物的分子量较小(<1kDa),难以通过简单的混凝沉淀达到有效去除。由于亚硝胺类物质的亲水性强(如lg KowNDMA=-0.57)[34],PAC吸附无法对亚硝胺类物质本身起到良好去除效果,甚至可能催化NDMA的生成,但PAC对亚硝胺类的前体物具有吸附作用,能降低亚硝胺在后氯胺化过程中的生成。本实验采用10 mg·L−1的PAC处理污水厂二级出水4 h,对NDMA和TONO的前体物去除率分别达74%与69%。当投量为7—10 mg·L−1时,在饮用水厂采用PAC对进水进行吸附处理能去除约20%的NDMA前体物,且PAC对受轻度污染的水源的NDMA控制效果优于地表水[35],可推测从控制NDMA生成的角度来看,PAC更适用于污水回用。在前人对预氧化处理NDMA的前体物的研究中,采用氯、二氧化氯、臭氧、高铁酸盐和太阳光等对NDMA的前体物均有去除效果[36−37]。
为了比较二氧化氯、高铁酸盐和臭氧在不同的暴露剂量下对污水中含氮消毒副产物前体物的去除效果,将氧化工艺的暴露剂量与对应水样在后氯胺化时的消毒副产物生成量结合分析,见图3。
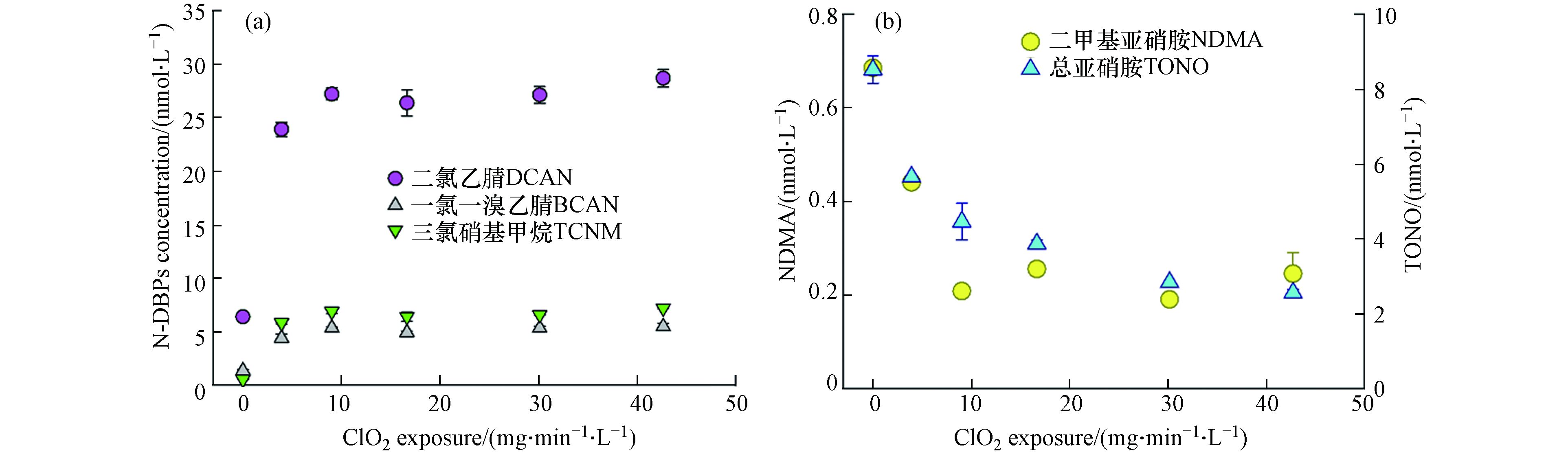
图3a表明,卤代N-DBPs的生成量会随着二氧化氯的暴露剂量增大而迅速增大,其中HANs的生成量增大了269%—346%,但当二氧化氯的暴露剂量超过9 mg·min−1·L−1时,卤代DBPs的浓度达到平衡,其中二氯乙腈(DCAN)从对照组的6.4 nmol·L−1迅速增至约27.2 nmol·L−1,增加了超过4倍,TCNM从1.4 nmol·L−1增至约5.3 nmol·L−1,BCAN从0.5 nmol·L−1增至约6.8 nmol·L−1。图3b表明,二氧化氯氧化对NDMA的前体物去除效果显著,随着二氧化氯暴露剂量从0 mg·min−1·L−1增至超过10 mg·min−1·L−1,氯胺化后的污水水样中NDMA浓度从0.68 nmol·L−1迅速减至约0.23 nmol·L−1,随后趋于稳定;而二氧化氯对TONO的前体物去除随二氧化氯暴露剂量的增大而逐渐增强,需达到30 mg·min−1·L−1的二氧化氯暴露剂量才趋于稳定(2.56 nmol·L−1)。可能的原因是NDMA的前体物分子量较小,更快被二氧化氯氧化,而二氧化氯氧化其他亚硝胺类的前体物则需更长的暴露时间。
HANs的前体物主要是水中的含氮有机物如氨基酸以及醛类物质,在之前的研究中发现,二氧化氯预氧化通常可降低HANs在后续消毒过程中的生成,而当后氯胺化时投加氯胺的量较低时(NH2Cl:precursor ≤ 10)时,采用二氧化氯预氧化游离酪氨酸会使DCAN的生成增加,这可能是由于游离酪氨酸中的α-氨基被二氧化氯氧化,从而增加了DCAN产率,而当采用高剂量氯胺处理时,二氧化氯的预氧化才能氧化HANs的前体物从而降低其在后消毒过程中的生成[38],推测本实验中的现象是由于实验采用模拟实际水处理的UFC过程,氯胺的浓度较低导致。经二氧化氯预氧化后,丁酰肼在氯胺化过程中的生成的NDMA随二氧化氯投量的增大而升高[28],在本实验中则呈现相反趋势,进一步说明污水中丁酰肼的含量极低。同时,二氧化氯能使一些NDMA的前体物如雷尼替丁、DMAI等快速氧化成DMA,但增加二氧化氯的暴露剂量并不能提高DMA的生成率,因此NDMA的生成控制在较低二氧化氯暴露水平下即达到平衡。而对于总亚硝胺而言,本实验首次发现其前体物的去除随二氧化氯的暴露增大而增大,在较高暴露水平(30 mg·min−1·L−1)才能达到较好去除。
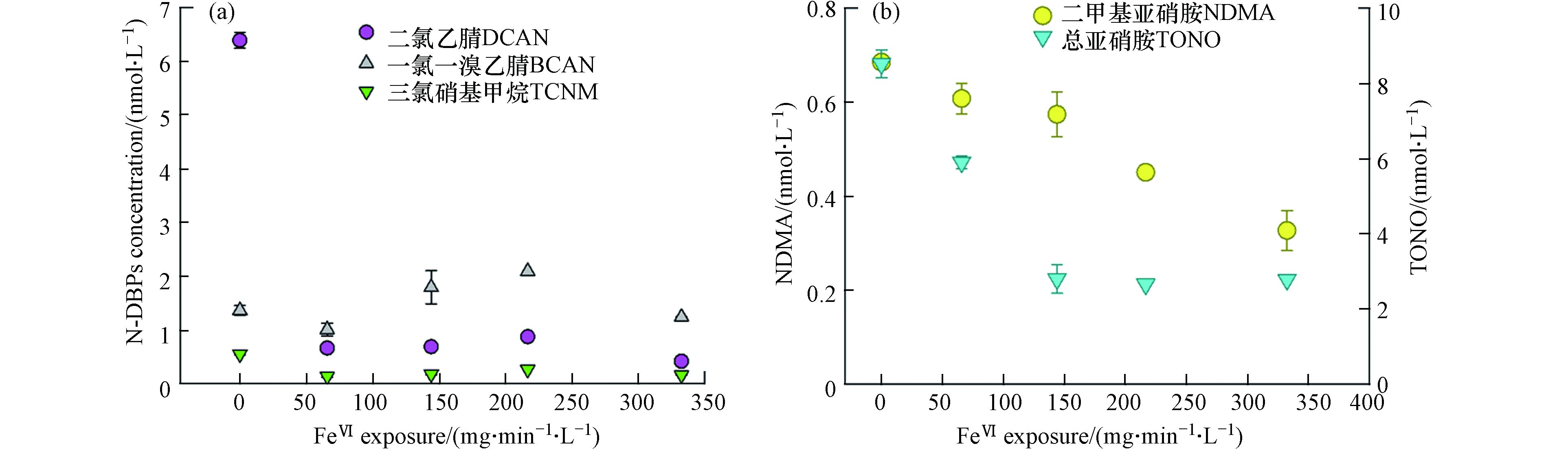
图4a表明,在模拟实际水处理的后氯胺化实验中,DCAN生成量会随着高铁酸盐的引入而迅速减少,从对照组的6.4 nmol·L−1迅速降至0.7 nmol·L−1,已接近检出限浓度(0.5 nmol·L−1),而BCAN和TCNM的浓度随高铁酸盐暴露剂量增大变化不明显,均接近检出限浓度(分别为0.9 nmol·L−1和0.3 nmol·L−1)。高铁酸盐能氧化氨基酸类和胺类物质,破坏C—N键,使含有—NH2官能团的物质如被破坏,而这类物质正是HANs的重要前体物[39−40]。图4b表明,氯胺化后的污水水样中NDMA浓度随高铁酸盐的暴露剂量从0 mg·min−1·L−1增至332 mg·min−1·L−1而从0.68 nmol·L−1逐步减至0.33 nmol·L−1,且尚未到达平衡;而高铁酸盐对TONO的前体物去除随高铁酸盐暴露剂量的增大而迅速增强,在143 mg·min−1·L−1的高铁酸盐暴露下TONO的浓度降至2.79 nmol·L−1,随后趋于稳定。
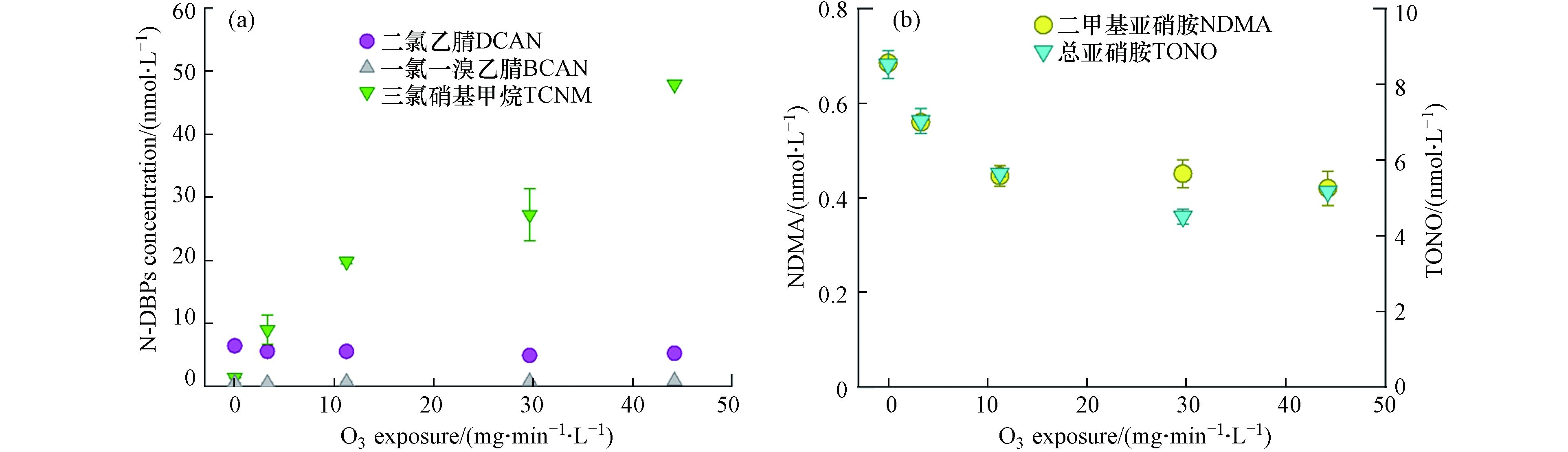
图5a表明,在模拟实际水处理的后氯胺化实验中,TCNM生成量会随臭氧浓度增大而迅速升高,从对照组的1.37 nmol·L−1以接近线性的趋势逐渐增至47.89 nmol·L−1,而DCAN和BCAN的浓度随臭氧暴露剂量增大变化不明显。图5b表明,氯胺化后的污水水样中,随臭氧的暴露剂量从0 mg·min−1·L−1增至0.19 mg·min−1·L−1,NDMA与TONO的浓度逐渐减至0.44 nmol·L−1和5.17 nmol·L−1,且到达平衡。臭氧氧化的污水会产生大量硝基甲烷,而硝基甲烷正是卤代硝基甲烷(以TCNM为主)的重要前体物[41],因此在本实验中TCNM在氯胺化过程中随前端臭氧暴露剂量增大而急剧增大的现象主要是由于臭氧氧化了污水中的有机物生成硝基甲烷,而N-烷基胺和一些含N-甲胺官能团的抗抑郁类药物在臭氧氧化后会普遍生成一级硝基烷烃,且转化率通常高于50%,说明本实验污水样品中可能含有大量含N-甲胺官能团的有机物。Lee等的研究表明[37],低剂量的臭氧暴露(0.2 mg·min−1·L−1)就能达到约78%的NDMA前体物去除,将臭氧暴露剂量增至1.0 mg·min−1·L−1对NDMA的生成控制并无明显影响,这与本实验中观测到的现象一致。
-
(1)在污水回用过程中,生物处理会促进生成亚硝胺(5.0%—254.5%)。本研究选用A/A/O生物处理后的污水出水进行深度处理,以探究生物处理与深度处理结合的工艺流程对含氮消毒副产物的控制。深度处理工艺中,粉末活性炭会促进亚硝胺的生成(61.5%),臭氧氧化则会直接生成亚硝胺(138%)。因此仅从亚硝胺的控制考虑,污水回用中采用二氧化氯或高铁酸盐氧化更能保障水质安全。
(2)选择的深度处理工艺对亚硝胺前体物均有去除,其中粉末活性炭和二氧化氯对NDMA和TONO的控制效果较好(约70.0%)。但氧化工艺对HANs和TCNM的前体物影响不一,高铁酸盐对两者的前体物均有显著去除,二氧化氯则增加了HANs的前体物(269%–346%),臭氧氧化则会增加TCNM的前体物。仅从典型含氮消毒副产物及其前体物的控制效果考虑,高铁酸盐氧化更适合在污水回用中作为深度处理工艺被应用,但高铁酸盐的大剂量制备工序繁琐复杂,在污水处理的实际应用中需考虑其便利性与实用性。
污水生物与深度处理技术对新型含氮消毒副产物及前体物的控制
Study on the control of emerging nitrogenous disinfection by-products and precursors by biological and advanced treatment for wastewater reclamation
-
摘要: 典型含氮消毒副产物(N-DBPs, 包括亚硝胺、卤乙腈和三氯硝基甲烷)的控制是污水回用过程中需关注的重点。本研究对采用不同生物处理工艺的污水厂进行生化池进出水中N-DBPs的存在水平分析,并选取其中一个污水厂的二级出水进行不同深度处理(粉末活性炭吸附、臭氧氧化、高铁酸盐氧化、二氧化氯氧化),研究其对N-DBPs及其前体物的去除。结果表明,生物处理过程会导致亚硝胺的生成。深度处理工艺中,粉末活性炭会催化亚硝胺的生成,臭氧氧化则会直接生成亚硝胺。选择的深度处理工艺对亚硝胺前体物均有去除,其中粉末活性炭和二氧化氯对二甲基亚硝胺和总亚硝胺的控制效果较好。但氧化工艺对卤乙腈(HANs)和三氯硝基甲烷(TCNM)的前体物影响不一,高铁酸盐对两者的前体物均有显著去除,二氧化氯会增加污水在低浓度氯胺处理时HANs的生成,臭氧氧化则会增加TCNM在后氯胺化过程中的生成。研究结果推动了污水深度处理技术的发展并为相关研究提供了理论指导。Abstract: The control of typical nitrogenous disinfection by-products (N-DBPs, including nitrosamines, haloacetonitriles and trichloronitromethane) is a major concern in wastewater reuse. In this study, the presence levels of N-DBPs in the influent and effluent of biochemical treatment in wastewater treatment plants (WWTPs) with different biological treatment processes were analyzed. The secondary effluent of one of the WWTPs was selected to investigate the removal of N-DBPs and their precursors in different advanced treatments (powdered activated carbon adsorption, ozone oxidation, ferrate oxidation, and chlorine dioxide oxidation). The results showed that the biological treatment process resulted in the generation of nitrosamines. For the advanced treatment processes, powdered activated carbon (PAC) catalyzed the generation of nitrosamines, while ozone oxidation formed nitrosamines directly. The selected advanced treatment processes showed different degrees of removal of nitrosamine precursors, with PAC and chlorine dioxide providing better control of N-nitrosodimethylamine and total nitrosamines. However, the oxidation processes had mixed effects on the precursors of haloacetonitriles (HANs) and trichloronitromethane (TCNM). Ferrate (FeVI) showed significant removal of precursors of both HANs and TCNM, while chlorine dioxide increased the formation of HANs in the effluent treated with low concentration of chloramine. Ozonation increased the generation of TCNM during post-chloramination. The results promote the development of advanced wastewater treatment technologies and provide theoretical guidance for related research.
-

-
表 1 样点污水处理厂基本信息
Table 1. Basic information of wastewater treatment plants in the research
污水厂编号
Number of wastewater
treatment plants污水处理能力(×104)/(m3·L−1)
Wastewater treatment
plant’s capacity采用的生物处理工艺
Biological treatment
processes生物处理类型
Type of biological
treatmentsA 10 A/O 完全硝化 B 10 A/A/O 完全硝化 C 20 Unitank 部分反硝化 D 10 A/A/O 弱硝化 E 10 CAST 完全硝化 F 5 Carrousel 2000氧化沟 完全反硝化 G 3 生物滤池 完全硝化 H 10 A/A/O 部分硝化 MBR 完全硝化 注Note:完全硝化Good nitrification:NH3 < 2 mg·L−1(按N算,下同), NO2− < 1 mg·L−1, NO3− > 10 mg·L−1。部分硝化Partial nitrification:NH3: 2—9 mg·L−1, NO3−: 2—10 mg·L−1。弱硝化Poor nitrification:NH3 > 9 mg·L−1, NO2− < 1 mg·L−1, NO3− < 2 mg·L−1。完全反硝化:NH3 < 2 mg·L−1, NO2− < 1 mg·L−1, NO3− < 5 mg·L−1。部分反硝化Partial denitrification:NH3 < 2 mg·L−1, NO3−: 5—10 mg·L−1. 表 2 8间水厂生物池进水与出水的基本水质参数
Table 2. Water parameters of influent and effluent of biological treatment processes in 8 WWTPs
编号
No.工艺
ProcesspH DOC/ (mg·L−1) NH3-N/(mg·L−1) Cl−/(µg·L−1) Br−/(µg·L−1) NO2−/(mg·L−1) NO3−/(mg·L−1) A1 A/O 7.3 14.5 19.8 21.0 196 0.0 4.4×10-4 A2 7.6 11.9 0.10 43.5 51.0 4.1×10-3 10 B1 A/A/O 7.2 28.0 15.7 11.8 61.6 0.0 5.2×10-4 B2 7.3 6.50 0.10 49.8 57.3 5.4×10-3 11 C1 UNITANK 7.4 11.2 10.3 27.9 48.0 7.5×10-2 6.4×10-3 C2 7.2 5.50 1.50 30.7 30.5 0.3 7.3 D1 A/A/O 7.3 16.8 21.7 63.0 50.7 3.1×10-3 0.0 D2 7.1 9.70 9.20 10.9 37.5 0.1 0.1 E1 CAST 7.3 23.5 14.5 28.0 45.6 3.8×10-3 0.0 E2 7.1 6.80 0.20 39.1 22.0 4.6×10-3 13 F1 Carrousel 2000 7.2 23.8 11.6 53.0 53.6 3.4×10-3 0.0 F2 7.4 8.40 0.20 44.3 30.1 1.9×10-3 3.0 G1 生物膜 7.5 9.50 6.20 34.0 56.1 0.3 6.6 G2 7.6 10.7 0.30 36.0 40.7 0.6 10 H1 MBR+
A/A/O7.8 22.7 23.6 47.1 60.9 8.3×10-3 5.3×10-4 H2 7.6 7.80 0.30 42.9 41.9 4.0×10-2 7.7 -
[1] LI Z G, LIU X Y, HUANG Z J, et al. Occurrence and ecological risk assessment of disinfection byproducts from chlorination of wastewater effluents in East China [J]. Water Research, 2019, 157: 247-257. doi: 10.1016/j.watres.2019.03.072 [2] LAZAROVA V, SAVOYE P, JANEX M L, et al. Advanced wastewater disinfection technologies: State of the art and perspectives [J]. Water Science and Technology, 1999, 40(4/5): 203-213. [3] MUNSON A E, SAIN L E, SANDERS V M, et al. Toxicology of organic drinking water contaminants: Trichloromethane, bromodichloromethane, dibromochloromethane and tribromomethane [J]. Environmental Health Perspectives, 1982, 46: 117-126. doi: 10.1289/ehp.8246117 [4] RICHARDSON S D. Disinfection by-products and other emerging contaminants in drinking water [J]. TrAC Trends in Analytical Chemistry, 2003, 22(10): 666-684. doi: 10.1016/S0165-9936(03)01003-3 [5] MILLS C J, BULL R J, CANTOR K P, et al. Workshop report. Health risks of drinking water chlorination by-products: Report of an expert working group [J]. Chronic Diseases in Canada, 1998, 19(3): 91-102. [6] SEDLAK D L, von GUNTEN U. Chemistry. The chlorine dilemma [J]. Science, 2011, 331(6013): 42-43. doi: 10.1126/science.1196397 [7] KIMURA S Y, KOMAKI Y, PLEWA M J, et al. Chloroacetonitrile and n, 2-dichloroacetamide formation from the reaction of chloroacetaldehyde and monochloramine in water [J]. Environmental Science & Technology, 2013, 47(21): 12382-12390. [8] CHUANG Y H, LIN A Y C, WANG X H, et al. The contribution of dissolved organic nitrogen and chloramines to nitrogenous disinfection byproduct formation from natural organic matter [J]. Water Research, 2013, 47(3): 1308-1316. doi: 10.1016/j.watres.2012.11.046 [9] PLEWA M J, WAGNER E D, MUELLNER M G, et al. Comparative mammalian cell toxicity of N-DBPs and C-DBPs[M]//ACS Symposium Series. Washington, DC: American Chemical Society, 2008: 36-50. [10] CHOI J, VALENTINE R L. Formation of N-nitrosodimethylamine (NDMA) from reaction of monochloramine: A new disinfection by-product [J]. Water Research, 2002, 36(4): 817-824. doi: 10.1016/S0043-1354(01)00303-7 [11] KRASNER S W, MCGUIRE M J, JACANGELO J G, et al. The occurrence of disinfection by-products in US drinking water [J]. Journal - American Water Works Association, 1989, 81(8): 41-53. doi: 10.1002/j.1551-8833.1989.tb03258.x [12] BARKER D J, STUCKEY D C. A review of soluble microbial products (SMP) in wastewater treatment systems [J]. Water Research, 1999, 33(14): 3063-3082. doi: 10.1016/S0043-1354(99)00022-6 [13] KRASNER S W, WESTERHOFF P, CHEN B Y, et al. Impact of wastewater treatment processes on organic carbon, organic nitrogen, and DBP precursors in effluent organic matter [J]. Environmental Science & Technology, 2009, 43(8): 2911-2918. [14] TROGOLO D, MISHRA B K, HEEB M B, et al. Molecular mechanism of NDMA formation from N, N-dimethylsulfamide during ozonation: Quantum chemical insights into a bromide-catalyzed pathway [J]. Environmental Science & Technology, 2015, 49(7): 4163-4175. [15] BADER H, HOIGNÉ J. Determination of ozone in water by the indigo method [J]. Water Research, 1981, 15(4): 449-456. doi: 10.1016/0043-1354(81)90054-3 [16] THOMPSON G W, OCKERMAN L T, SCHREYER J M. Preparation and purification of potassium ferrate. VI [J]. Journal of the American Chemical Society, 1951, 73(3): 1379-1381. [17] LIU P, FARRÉ M J, KELLER J, et al. Reducing natural organic matter and disinfection by-product precursors by alternating oxic and anoxic conditions during engineered short residence time riverbank filtration: A laboratory-scale column study [J]. Science of the Total Environment, 2016, 565: 616-625. doi: 10.1016/j.scitotenv.2016.05.061 [18] WU M R, LIANG Y M, PENG H L, et al. Bioavailability of soluble microbial products as the autochthonous precursors of disinfection by-products in aerobic and anoxic surface water [J]. Science of the Total Environment, 2019, 649: 960-968. doi: 10.1016/j.scitotenv.2018.08.354 [19] ZENG T, MITCH W A. Impact of nitrification on the formation of N-nitrosamines and halogenated disinfection byproducts within distribution system storage facilities [J]. Environmental Science & Technology, 2016, 50(6): 2964-2973. [20] SHAH A D, MITCH W A. Halonitroalkanes, halonitriles, haloamides, and N-nitrosamines: A critical review of nitrogenous disinfection byproduct formation pathways [J]. Environmental Science & Technology, 2012, 46(1): 119-131. [21] SHAO B B, DONG H Y, SUN B, et al. Role of ferrate(IV) and ferrate(V) in activating ferrate(VI) by calcium sulfite for enhanced oxidation of organic contaminants [J]. Environmental Science & Technology, 2019, 53(2): 894-902. [22] EPA U. National primary drinking water standards[S]. 2002 [23] DAI N, ZENG T, MITCH W A. Predicting N-nitrosamines: N-nitrosodiethanolamine as a significant component of total N-nitrosamines in recycled wastewater [J]. Environmental Science & Technology Letters, 2015, 2(3): 54-58. [24] PADHYE L, TEZEL U, MITCH W A, et al. Occurrence and fate of nitrosamines and their precursors in municipal sludge and anaerobic digestion systems [J]. Environmental Science & Technology, 2009, 43(9): 3087-3093. [25] WIJEKOON K C, FUJIOKA T, MCDONALD J A, et al. Removal of N-nitrosamines by an aerobic membrane bioreactor [J]. Bioresource Technology, 2013, 141: 41-45. doi: 10.1016/j.biortech.2013.01.057 [26] TADKAEW N, HAI F I, MCDONALD J A, et al. Removal of trace organics by MBR treatment: The role of molecular properties [J]. Water Research, 2011, 45(8): 2439-2451. doi: 10.1016/j.watres.2011.01.023 [27] RALT D, TANNENBAUM S R. The role of bacteria in nitrosamine formation[M]//ACS Symposium Series. WASHINGTON D C: AMERICAN Chemical Society, 1981: 157-164. [28] GAN W H, BOND T, YANG X, et al. Role of chlorine dioxide in N-nitrosodimethylamine formation from oxidation of model amines [J]. Environmental Science & Technology, 2015, 49(19): 11429-11437. [29] PADHYE L, WANG P, KARANFIL T, et al. Unexpected role of activated carbon in promoting transformation of secondary amines to N-nitrosamines [J]. Environmental Science & Technology, 2010, 44(11): 4161-4168. [30] PADHYE L P, HERTZBERG B, YUSHIN G, et al. N-nitrosamines formation from secondary amines by nitrogen fixation on the surface of activated carbon [J]. Environmental Science & Technology, 2011, 45(19): 8368-8376. [31] OYA M, KOSAKA K, ASAMI M, et al. Formation of N-nitrosodimethylamine (NDMA) by ozonation of dyes and related compounds [J]. Chemosphere, 2008, 73(11): 1724-1730. doi: 10.1016/j.chemosphere.2008.09.026 [32] SHEN R Q, ANDREWS S A. Demonstration of 20 pharmaceuticals and personal care products (PPCPs) as nitrosamine precursors during chloramine disinfection [J]. Water Research, 2011, 45(2): 944-952. doi: 10.1016/j.watres.2010.09.036 [33] SCHMIDT C K, BRAUCH H J. N, N-dimethylsulfamide as precursor for N-nitrosodimethylamine (NDMA) formation upon ozonation and its fate during drinking water treatment [J]. Environmental Science & Technology, 2008, 42(17): 6340-6346. [34] XU B, YE T, LI D P, et al. Measurement of dissolved organic nitrogen in a drinking water treatment plant: Size fraction, fate, and relation to water quality parameters [J]. Science of the Total Environment, 2011, 409(6): 1116-1122. doi: 10.1016/j.scitotenv.2010.12.016 [35] UZUN H, KIM D, KARANFIL T. The removal of N-nitrosodimethylamine formation potential in drinking water treatment plants [J]. Journal - American Water Works Association, 2017, 109(6): 15-28. [36] SELBES M, KIM D, KARANFIL T. The effect of pre-oxidation on NDMA formation and the influence of pH [J]. Water Research, 2014, 66: 169-179. doi: 10.1016/j.watres.2014.08.015 [37] LEE Y, von GUNTEN U. Oxidative transformation of micropollutants during municipal wastewater treatment: Comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrateVI, and ozone) and non-selective oxidants (hydroxyl radical) [J]. Water Research, 2010, 44(2): 555-566. doi: 10.1016/j.watres.2009.11.045 [38] YAO D C, CHU W H, BOND T, et al. Impact of ClO2 pre-oxidation on the formation of CX3R-type DBPs from tyrosine-based amino acid precursors during chlorination and chloramination [J]. Chemosphere, 2018, 196: 25-34. doi: 10.1016/j.chemosphere.2017.12.143 [39] YANG X, GUO W H, ZHANG X, et al. Formation of disinfection by-products after pre-oxidation with chlorine dioxide or ferrate [J]. Water Research, 2013, 47(15): 5856-5864. doi: 10.1016/j.watres.2013.07.010 [40] WANG A, LIN C S, SHEN Z, et al. Effects of pre-oxidation on haloacetonitrile and trichloronitromethane formation during subsequent chlorination of nitrogenous organic compounds [J]. International Journal of Environmental Research and Public Health, 2020, 17(3): 1046. doi: 10.3390/ijerph17031046 [41] SHI J L, MCCURRY D L. Transformation of N-methylamine drugs during wastewater ozonation: Formation of nitromethane, an efficient precursor to halonitromethanes [J]. Environmental Science & Technology, 2020, 54(4): 2182-2191. -



 下载:
下载: