-
印染废水是将棉,麻,毛,丝等印染后排放的废水,含有染料、酸碱、纤维杂质、无机盐等物质[1],染料化合物广泛应用于纺织、皮革、造纸、食品、光电学电池和染发剂等技术领域[2]. 然而,由于其高含水量,高染料浓度,不可生物降解性,强碱性,水质变化大,在大规模生产和广泛应用中,合成染料使自然环境被破坏,导致水的透明度降低,阻碍阳光进入水体,同时消耗水体中的溶解氧,破坏水生生态平衡,危及水生生物的生存[3]. 罗丹明B (rhodamine B,RhB)是应用最广泛的染料之一,即使是低浓度的RhB废水,由于其毒性,致癌性,诱变性和不可生物降解性[4],仍然对人类健康和水生生物有害,因此,寻求一种效率高且对环境友好的新工艺迫在眉睫. 吸附,氧化,微生物降解等是常见的去除废水中RhB的方法,然而却无法完全去除RhB[5]. 近年来,基于活化过一硫酸盐(peroxymonosulfate,PMS)产生SO4·−的高级氧化技术已相当成熟,通常以过渡金属离子,UV照射,加热和碳基材料对PMS进行活化,可生成SO4·−[6]. 高能量的要求限制了热活化和UV活化的工业应用,而金属离子的浸出带来二次污染的风险. 因此,开发高效,环保的PMS活化剂势在必行.
金属有机框架材料(MOFs)通过自组装与桥接有机配体连接,形成一种具有周期性网络结构的多孔晶体材料;金属有机框架衍生多孔碳作为一种很有前景的非金属催化材料,因其高孔隙率,高比表面积和高耐化学性而备受关注[7].MOFs足够大的内部反应空间和高密度的活性位点促使原料和催化剂充分反应. 而石墨N对PMS[8]的活化起着重要作用,因此使用含氮MOFs直接碳化可制备得到氮自掺杂多孔碳材料. 此外,杂原子掺杂可以改变sp2杂化碳骨架的电子性质,从而增加催化位点,有利于促进PMS活化和电子转移[9]. 硼原子与碳原子拥有相近的原子半径与电负性,硼掺杂可以增加碳材料的比表面积,同时可以与氮原子形成耦合效应,进一步提高PMS活化性能[10].
本研究以ZIF-67为前驱体,在高温下构建硼氮共掺杂多孔碳材料(B-NC)高效活化PMS,该催化剂在最适条件下对目标污染物罗丹明B有良好的降解效果. 对催化剂的形貌和结构进行了测试分析,并对B-NC活化PMS降解RhB的各种影响参数进行了优化. 通过猝灭实验和自由基捕获实验解析了B-NC活化PMS降解RhB的机理,本文为碳材料活化PMS降解污染物提供了新策略.
-
罗丹明B(RhB)购于福晨(天津)化学试剂有限公司,呋喃醇(FFA),L-组氨酸(C6H9N3O2),对苯醌(BQ),乙醇(CH3CH2OH),硫酸(H2SO4),硫代硫酸钠(Na2S2O3·5H2O)均购于成都科隆化学品股份有限公司. 过一硫酸盐(PMS),2-甲基咪唑,六水硝酸钴(Co(NO3)2·6H2O),硼酸,甲醇,丁醇(TBA),4-氨基-2,2,6,6-四甲基哌啶(TEMP),5,5-二甲基-1-吡啶N-氧化物(DMPO)和乙醇由中国上海阿拉丁生化科技有限公司提供. 所有其他化学品都是分析级的,并直接使用,没有任何进一步的净化.
-
紫外可见分光光度计(752N,上海仪电分析仪器有限公司),电子天平(ME203,梅特勒-托利多仪器有限公司),电热鼓风干燥箱(WGLL-125BE,天津市泰斯特仪器有限公司),电热恒温水浴锅(HHS,上海博讯实业有限公司医疗设备厂),集热式恒温加热磁力搅拌器(DF-101S,河南予华仪器有限责任公司),pH计(ZF-600,成都世纪方舟科技有限公司),医用离心机(TD-6,四川蜀科仪器有限公司).
-
称取1.455 g Co(NO3)2·6H2O和3.284 g 2-甲基咪唑于分别溶于70 mL CH3OH并混合,用保鲜膜封口,置于30 ℃恒温水浴锅中静置生长24 h后离心分离,甲醇清洗3次后置于80 ℃真空烘箱中烘干,得到ZIF-67. 将样品于 950 ℃管式炉中热解2 h后用3 mol·L−1 H2SO4和甲醇分别洗涤两次,抽滤后在80 ℃真空烘箱中烘干,得到氮掺杂多孔碳NC.
-
将0.2 g NC与0.5 g硼酸,20 mL甲醇混合并在25 ℃下搅拌24 h,于80 ℃烘箱中干燥以去除甲醇和未反应的硼酸. 烘干后的材料放入1100 ℃管式炉中热解2 h,用60 ℃热水洗涤去除未反应的硼酸并烘干,得到材料B-NC.在相同条件下取不同硼酸用量0、0.2、0.5、1、2、5 g制备一系列不同掺硼量的催化剂.
-
B-NC的形貌和晶格分别通过扫描电镜(SEM,FEI quanta250,美国)和透射电镜(TEM,FEI Tecnai G2F20,美国)测定,而催化剂的晶体构型由X射线衍射(XRD,Neo-Confucianism Rigaku Smartlab,日本)进行分析,通过电子顺磁共振技术检测确定反应过程中体系产生的活性物种(EPR,Bruker EMX nano,德国).
-
所有RhB降解实验均在250 mL玻璃烧杯中进行,并在室温下搅拌. 配制含RhB(初始浓度为30 mg·L−1)的150 mL溶液于烧杯中,在溶液中加入一定量的催化剂和PMS可立即引发反应. 在选定的时间点用移液枪取1 mL样,并用针头过滤器(0.22 μm)过滤. 用紫外-可见分光光度计(UV756)在554 nm处测定残留浓度. 用0.1 mol·L−1 H2SO4或NaOH调节RhB溶液的初始pH.根据预实验结果,在其余条件相同的条件下探究单因素变量对于RhB降解的影响(催化剂投加量0—30 mg,PMS投加量0—30 mg,初始RhB浓度20—100 mg·L−1,溶液初始pH为3—11,温度15—45 ℃).
为了鉴定反应中产生的活性物种,在 150 mL溶液中,pH 为 3,PMS 和B-NC的投加量为5 mg的条件下,分别加入一定量的甲醇(MeOH),叔丁醇(TBA),糠醇,对苯醌和L-组氨酸捕获活性物种.
-
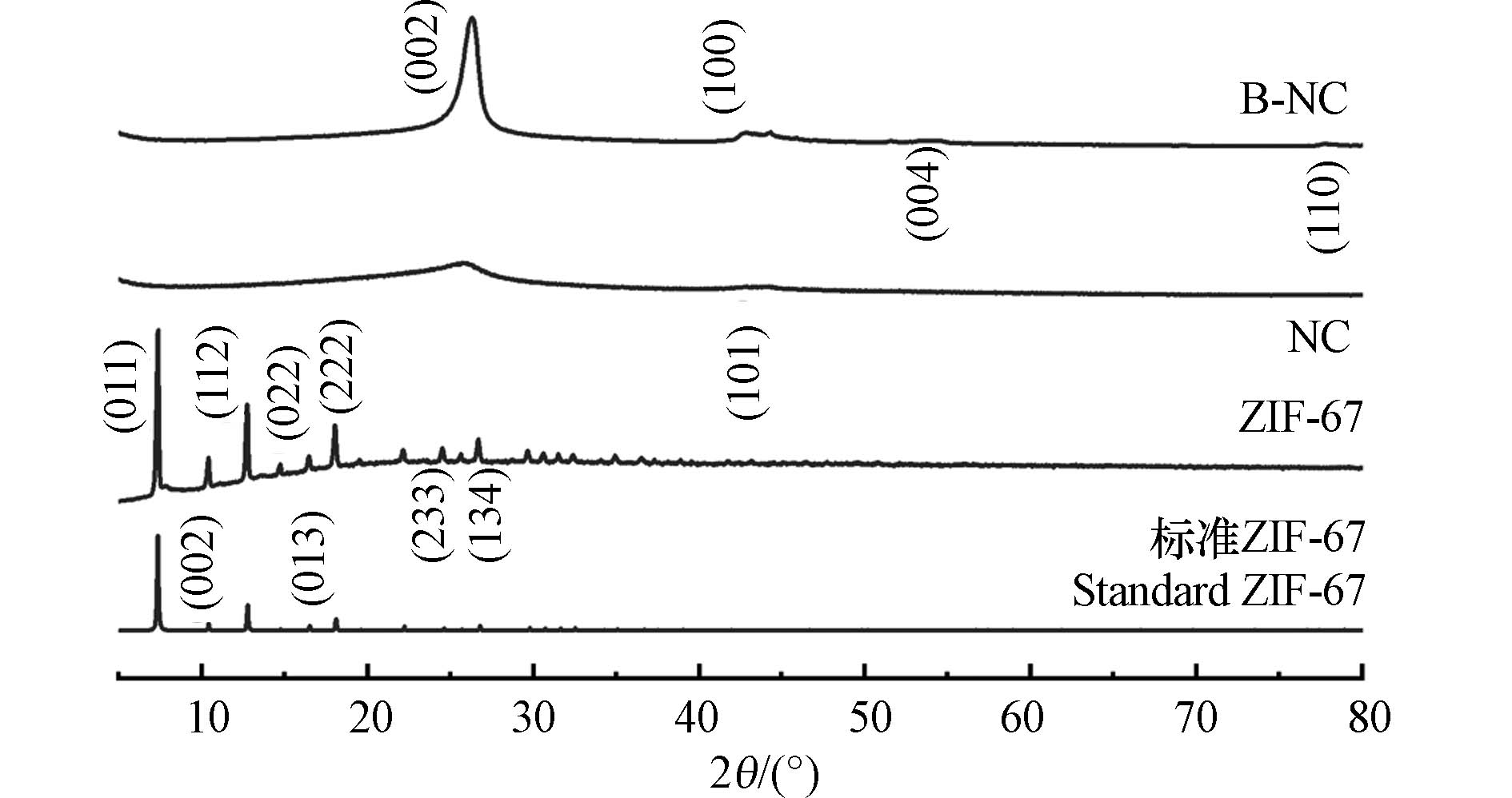
图1为制备的一系列材料的XRD图谱. 其中位于2θ = 10.36°、12.68°、14.70°、16.40°和17.98°的衍射峰分别对应ZIF-67特征峰的(002)、(112)、(022)、(013)和(222)晶面(分别与ZIF-67标准卡片模拟XRD图谱相吻合),且峰强较高,没有其他杂峰,说明ZIF-67材料合成成功[11].
ZIF-67直接碳化并用硫酸洗涤之后,骨架结构明显发生了变化, ZIF-67的特征衍射峰消失,进而出现了碳的(002)和(100)晶面高强度特征峰,但没有出现Co及其氧化物的特征峰,表明ZIF-67中的Co离子在高温碳化过程中暴露后被硫酸洗去. 从C晶面(002)的峰型可以看出,掺硼后得到的B-NC相对于掺硼前的NC拥有更好的晶型结构,B的掺杂提升了材料的石墨化程度.
-
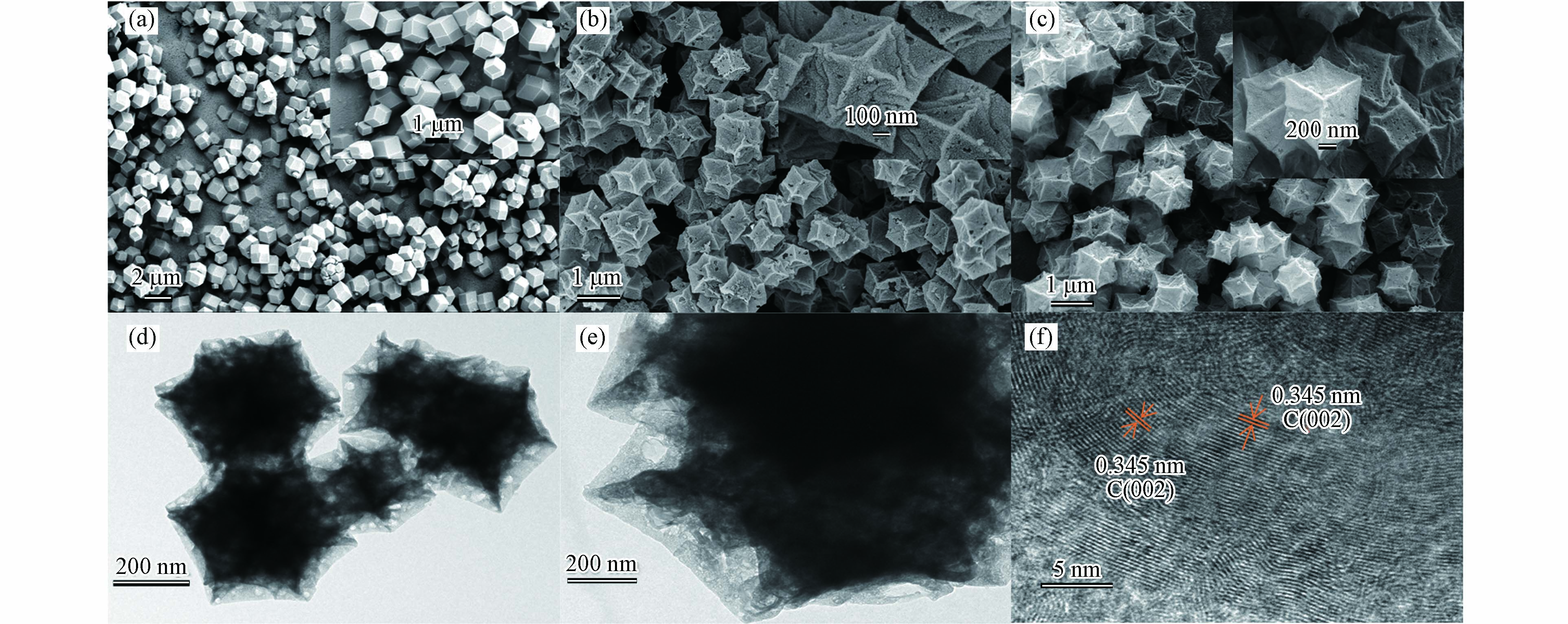
图2为ZIF-67及其系列衍生材料NC和B-NC的SEM图. 从图2a可以看出,ZIF-67呈光滑规则的菱形十二面体结构,颗粒分布均匀,界面清晰,平整光滑[12],在高温热解之后(图2b),得到的NC依然能保持十二面体的结构,但表面明显变得粗糙不平,并出现许多较大的孔隙,可能是金属离子暴露挥发后留下的缺陷.图2c表明掺B以后的催化剂同样可以保持ZIF-67的结构,并且许多缺陷处有了填补,TEM图(图2d,e)进一步证实了制备的B-NC具有规则的菱形十二面体形状,并且具有清晰有序的晶格条纹,晶格间距0.345 nm,表示为石墨C的 (002)晶面[13].
-
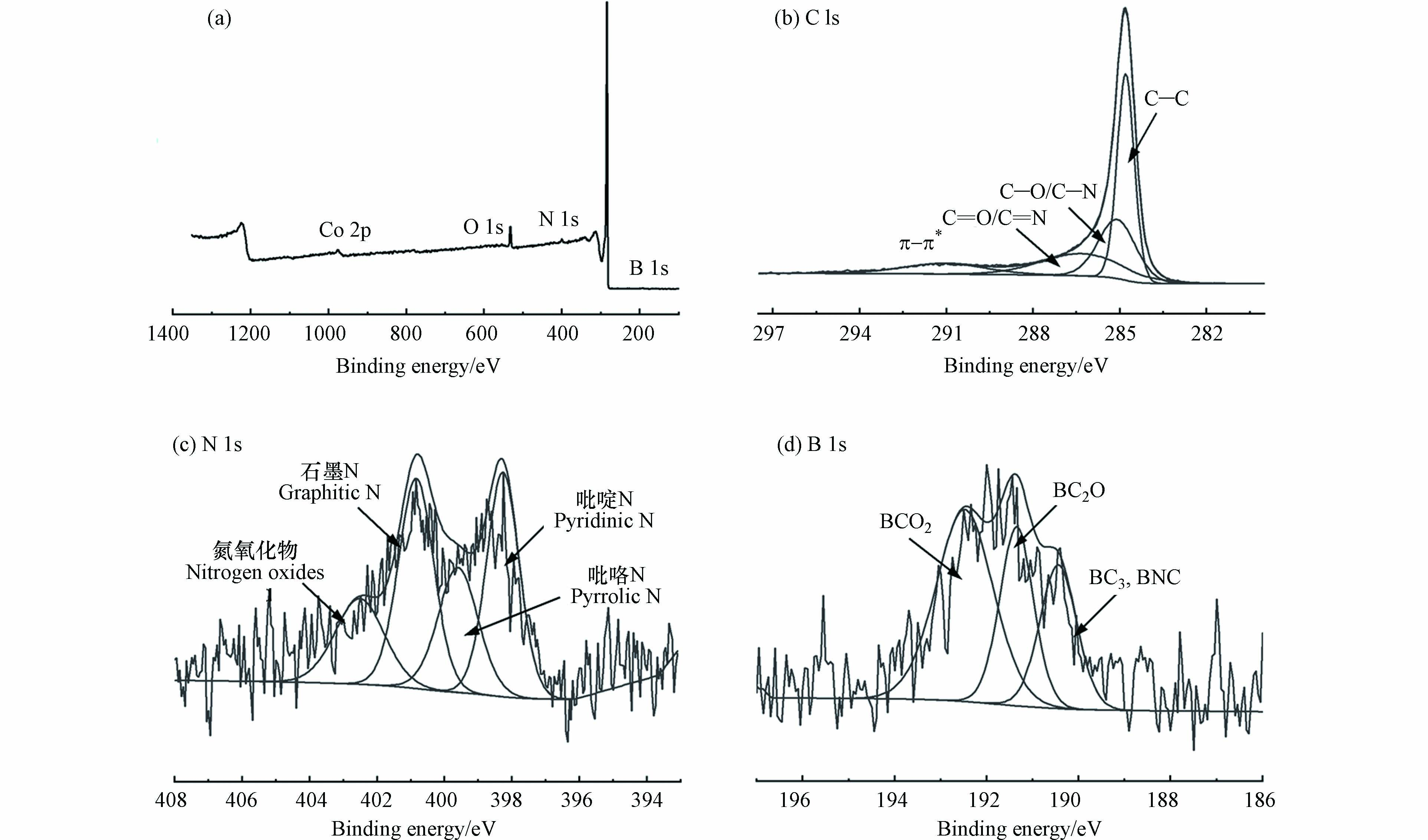
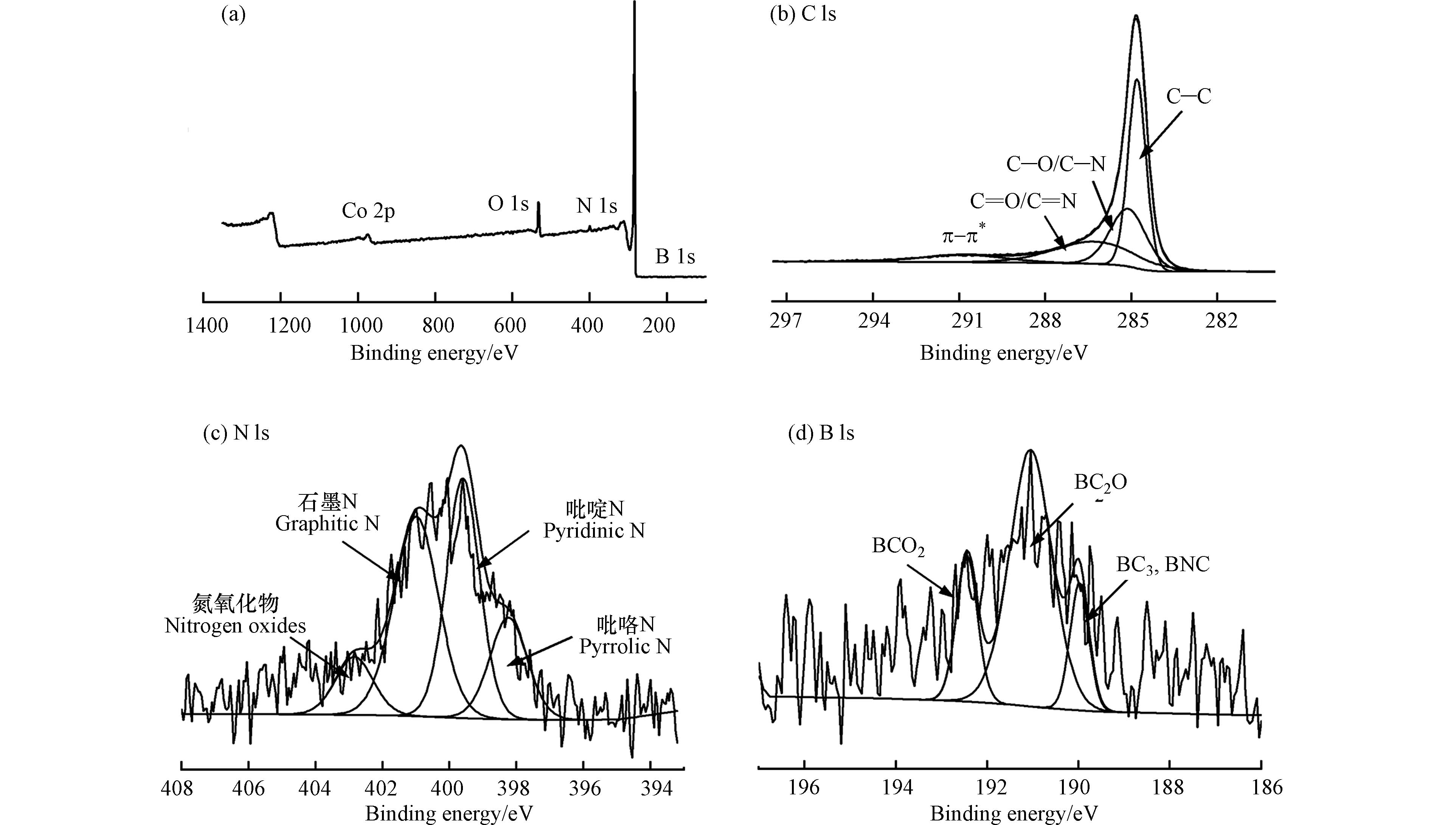
图3为B-NC的XPS图谱,用以研究其元素组成和化学构型. 从图3a中可以看出,B-NC的主要成分是C (284.8 eV)和O,其次是N和B以及极少量的Co,可以认为Co已基本去除干净. 其中图3b是B-NC的C 1s光谱,可以拟合出4个峰,分别为sp3-杂化碳(C—C)、C—O/C—N(285.09 eV)、C=O/C=N(286.31 eV)和π—π*震荡卫星峰(291.08 eV)[14],图3c为催化剂N1s的拟合谱,可以发现,氮的存在形态主要有吡咯氮(399.6 eV)、吡啶氮(边缘的6元杂合中的N, 398.27 eV)、石墨氮(sp2杂合N与3个sp2 C原子相邻,400.85 eV)和氮氧化物(402.52 eV),均是催化反应的催化活性位点[15]. 图3d为催化剂B 1s的拟合谱,在192.46 、191.43 、190.40 eV处有3个拟合峰,分别对应BCO2键、BC2O键和BC3,BNC键.B谱的存在表明硼已经被成功掺杂到多孔碳材料中,掺杂量为0.53%.
-
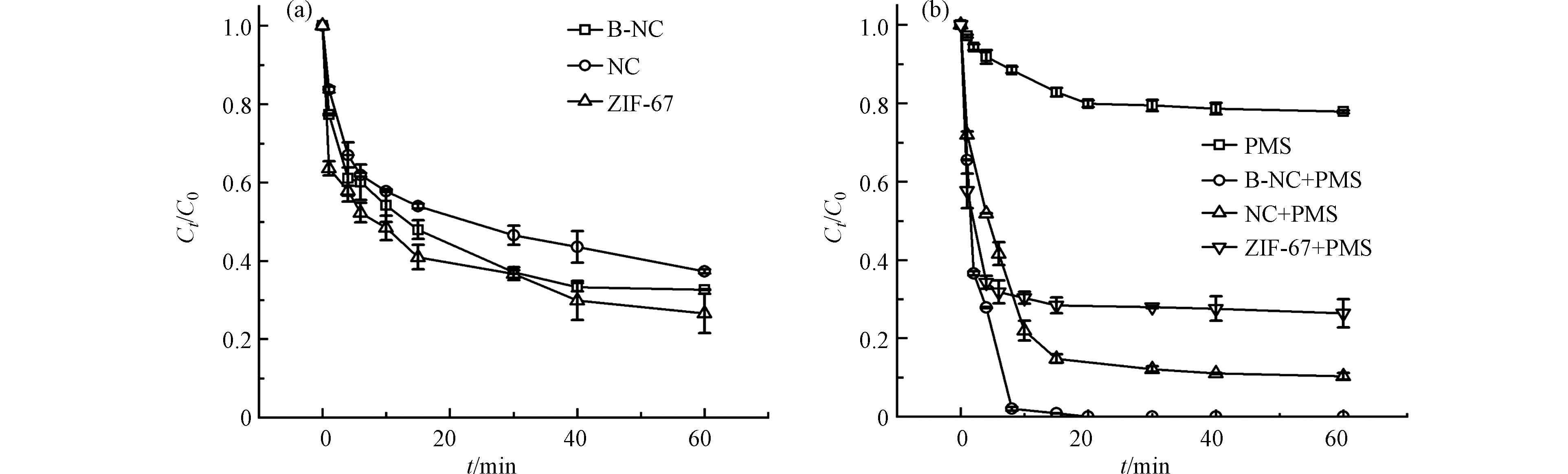
分别考察了ZIF-67,NC和B-NC3种材料的吸附性能以及在单独PMS,B-NC+PMS, NC+PMS和ZIF-67+PMS等4个体系下的RhB降解实验,其中RhB的浓度均为30 mg·L−1,结果如图4所示. 可以发现,所有催化剂都具有一定的吸附性能,其中ZIF-67吸附性能最好(k=0.0221),这是由MOF材料独有的大比表面积和孔结构决定的,而B-NC在60 min内的吸附量也可以达到为67.42%,k = 0.0186,NC的吸附量也有62.78%,k = 0.0444,这皆归因于高孔隙率对污染物的吸附,但无法有效去除RhB.加入PMS后,ZIF-67+PMS中RhB降解效果与吸附效果相差无几,在NC+PMS体系中,60 min后RhB降解率为90.00%左右,无论降解速率或降解率皆低于B-NC+PMS(10 min内即可降解99.18%). 说明了硼的掺杂可以有效提高NC的催化性能和催化效率. 单独5 mg PMS反应60 min后RhB降解率为22.15%,是因为PMS可以自分解与RhB直接反应,但PMS较为稳定,因此反应缓慢,降解效果较差. 单独PMS和单独B-NC的一级动力学常数分别为0.0108 min−1和0.0186 min−1,均远小于B-NC/PMS体系的0.3202 min−1,说明B-NC和PMS之间具有协同作用.
通过与其他不同碳材料进行性能对比(表1),充分显示了以ZIF-67作为前驱体制备的MOF衍生碳材料的优势,B-NC具有三维多孔十二面体结构,具有较高的比表面积和丰富的孔隙结构,B和N的双掺杂为PMS的活化提供了高密度的活性位点,多孔结构和非金属特性使得B-NC在多次回收后依然具有较好的PMS活化稳定性.
-
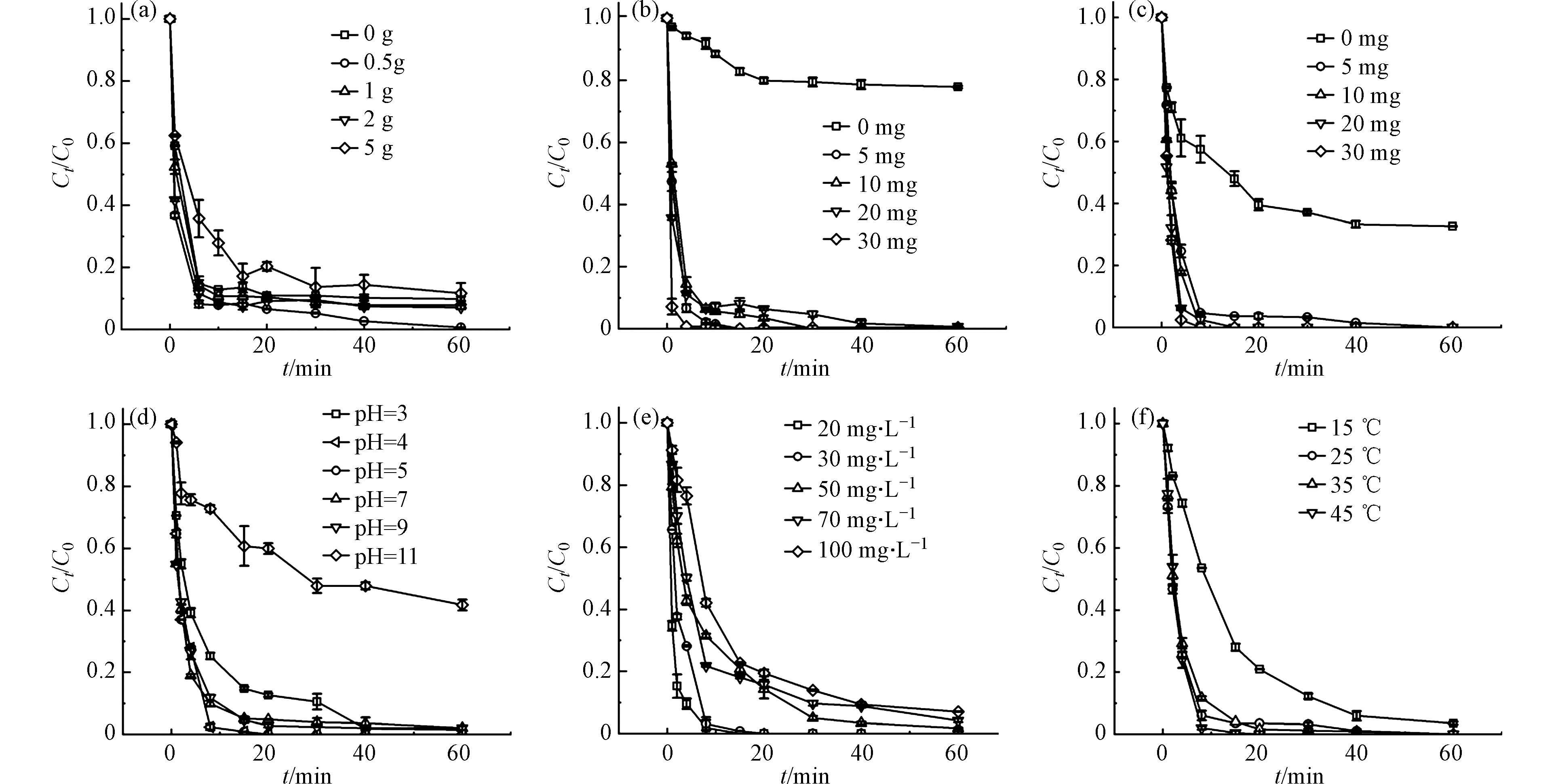
探究了硼含量对于RhB降解的影响,图5a为制备的一系列不同硼含量的催化剂降解RhB的性能比较,其中NC使用量为0.2 g. 结果表明,不掺硼时体系中RhB的降解率在2 min内仅为70%左右,而掺入适量硼后,2 min内RhB的降解率均可达到80%,说明掺硼能有效提高催化剂的催化性能. 当掺硼量为0.5—2 g时,RhB的降解率均可达到90%以上,且0.5 g的掺硼量可以在60 min内达到100%的降解率,考虑到经济性,后续实验使用0.5 g的掺硼量. 另外,RhB的降解率并没有持续随着掺硼量的增加而上升,可能是出现了解吸现象或过量硼堵塞了活性位点.
-
图5b为催化剂投加量对RhB降解的影响,当没有投加催化剂时,60 min后RhB降解率仅22.15%,B-NC投加量增加,RhB的降解速率随之加快,60 min后均可达到99%,这是由于PMS非常稳定,需要被激活从而产生活性氧物种,而催化剂的增加可以带来更多的活性位点从而使降解效率更高. 考虑到使用成本,后续实验皆投加5 mg催化剂.
-
设计了系列PMS投加量梯度对B-NC降解RhB的影响(图5c). 不投加PMS时,仅靠B-NC的吸附作用,60 min后仅有50%的降解率,投加PMS后,在8 min内RhB降解率皆可达到90%以上,这是由于更多的PMS可以被激活产生大量活性物种. 而当PMS投加量达到30 mg时,污染物在4 min时就已完全降解. 随PMS投加量增加,60 min后降解率的提高并不明显,这是因为过量的PMS会使得硫酸根自由基自猝灭而不能完全发挥催化作用[21]. 因此,根据经济成本分析选择5 mg的PMS作为最佳投加量.
-
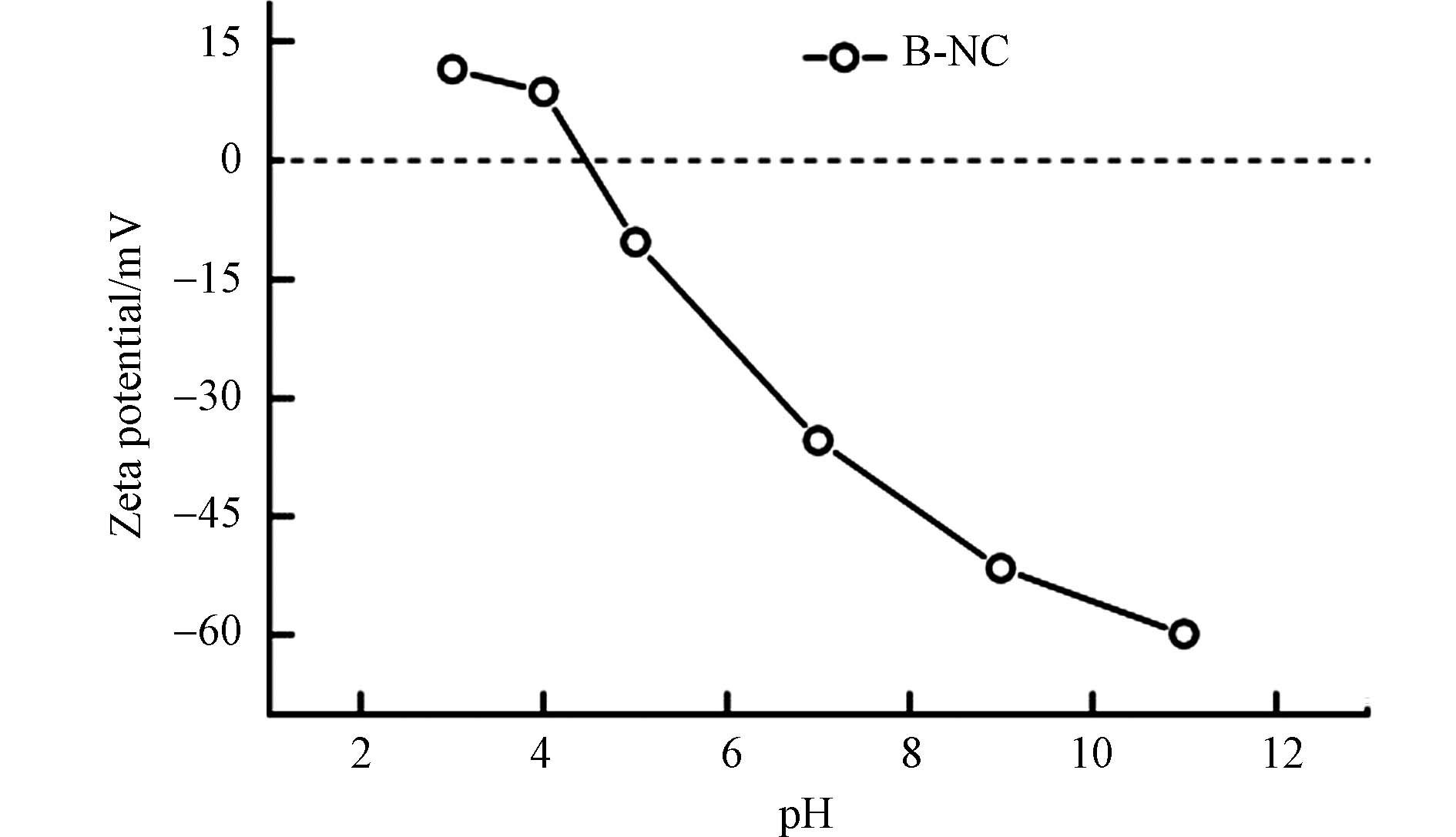
从图5d中可以观察到pH在B-NC降解RhB过程中的影响. 在pH为3—9之间时,RhB的降解率都可以达到95%以上,而在pH等于11时,60 min后降解率仅有不到60%,并且可以观察到不同条件下的溶液pH反应前后变化并不大(表2),B-NC/PMS/RhB体系在反应过程中基本处于和反应初始pH相近的环境下,说明该催化剂能在较宽的pH范围(3—9)内有较好的RhB催化降解性能. 过酸或过碱条件下降解率都有所下降,可能是碱性条件下PMS容易发生自溶,同时B-NC可能与OH-相互反应而使催化剂上的活性位点减少[10, 22]. 而溶液处于强酸性条件下时(pH为3),PMS较为稳定,一般以HSO5−为主,其O—O键与H+容易形成氢键,从而延缓与B-NC之间的相互作用[23]. 另外,在不同pH条件下测定了B-NC的Zeta电位,以此进一步阐明pH条件对RhB降解的影响,结果如图6所示,B-NC的等电点为4.46,当溶液处于酸性条件时(pH等于3、4时),带正电荷的B-NC和带负电荷的PMS之间存在静电吸引从而提高PMS的活化效率,使得RhB降解效率有效升高,而随着pH升高, B-NC表面负电荷增加,发生静电排斥作用,RhB的降解率随之降低[24-25]. 因此,在所有实验中选择pH为4作为最佳实验条件.
-
图5e显示了初始浓度对于RhB降解的影响. 在相同时间内,RhB初始浓度越高,其降解率越低. 在前10 min内,初始浓度为30 mg·L−1的RhB溶液降解率可达到99%,而初始浓度为50、70、100 mg·L−1的RhB降解率分别只有70.88%、77.59%、66.64%. 在其他条件相同的情况下,生成同样浓度的SO4·−,RhB浓度越高,降解速率就越慢.
-
图5f考察了在不同温度梯度下RhB的降解,加热是有效活化过硫酸盐的方法之一[26],反应温度对RhB的降解率影响不大. 该体系的催化活性对温度依赖性较弱,突出了B-NC固有的优良催化性能. 当体系在15 ℃到45 ℃条件下进行反应时,15 min内RhB的降解率从73%提高到95%以上,反应速率常数k从0.1059 min−1提高到0.3653 min−1,RhB完全去除的时间明显缩短. 这是因为升温容易克服反应活化能,活化PMS产生SO4·-[27],说明温度在激活PMS中起着重要作用. 考虑到实用性,经济性,实验皆在室温下进行.
-
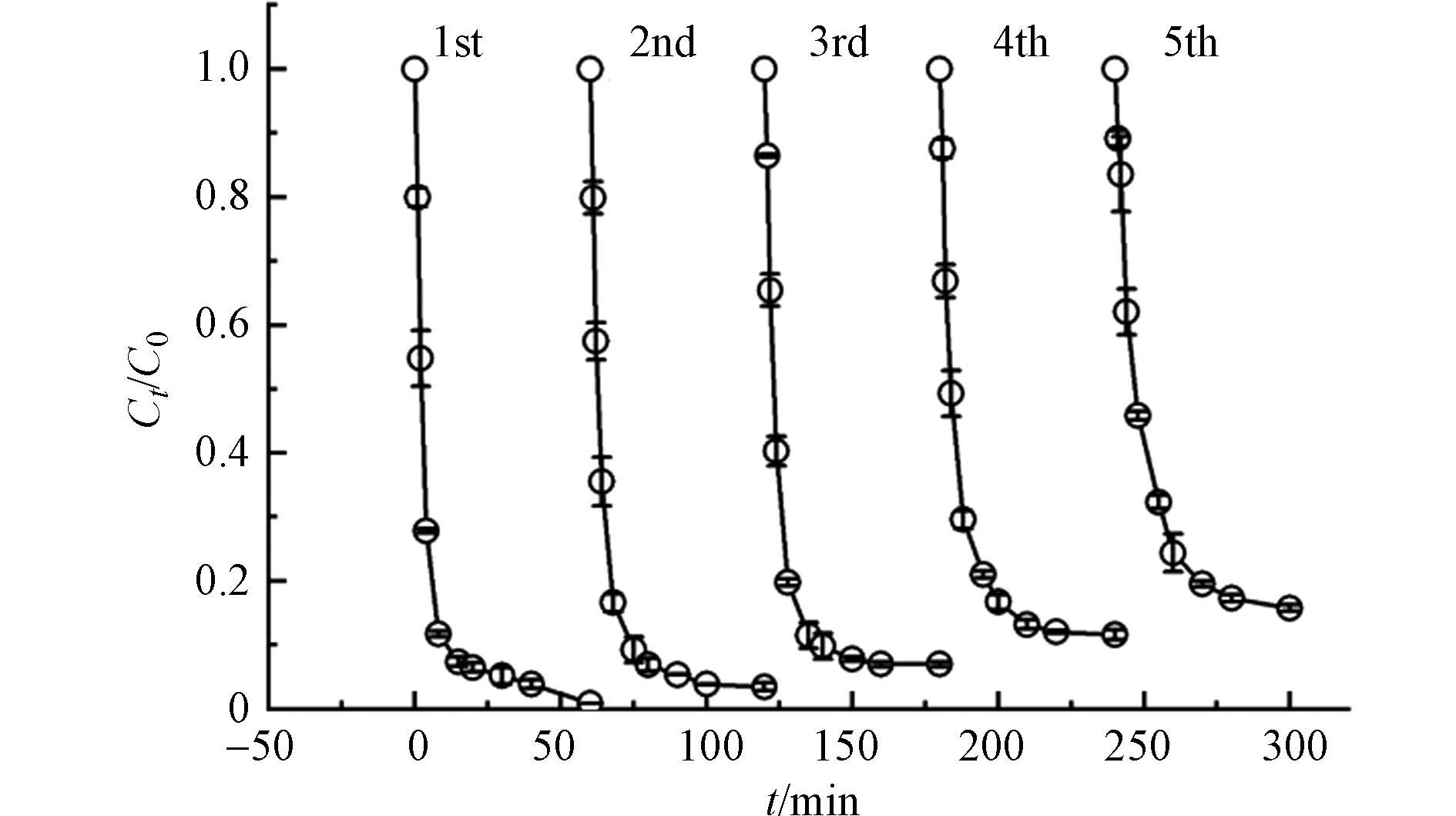
催化剂多次使用后的降解性能是其实际应用的重要指标. 将5 mg B-NC和5 mg PMS加入150 mL RhB溶液中反应1 h,反应结束后,将用过的B-NC过滤,用蒸馏水洗涤2次,最后在60 ℃真空烘箱中干燥12 h,进行后续循环实验,实验结果如图7所示. B-NC第五次使用后RhB的降解率依然可以达到84.21%,说明B-NC具有良好的可重复利用性能,稍有降低的降解率可能是由于降解产物对催化剂孔道的堵塞[28]. 通过抽滤干燥可以回收本研究的催化剂,回收率可以达到80%.
-
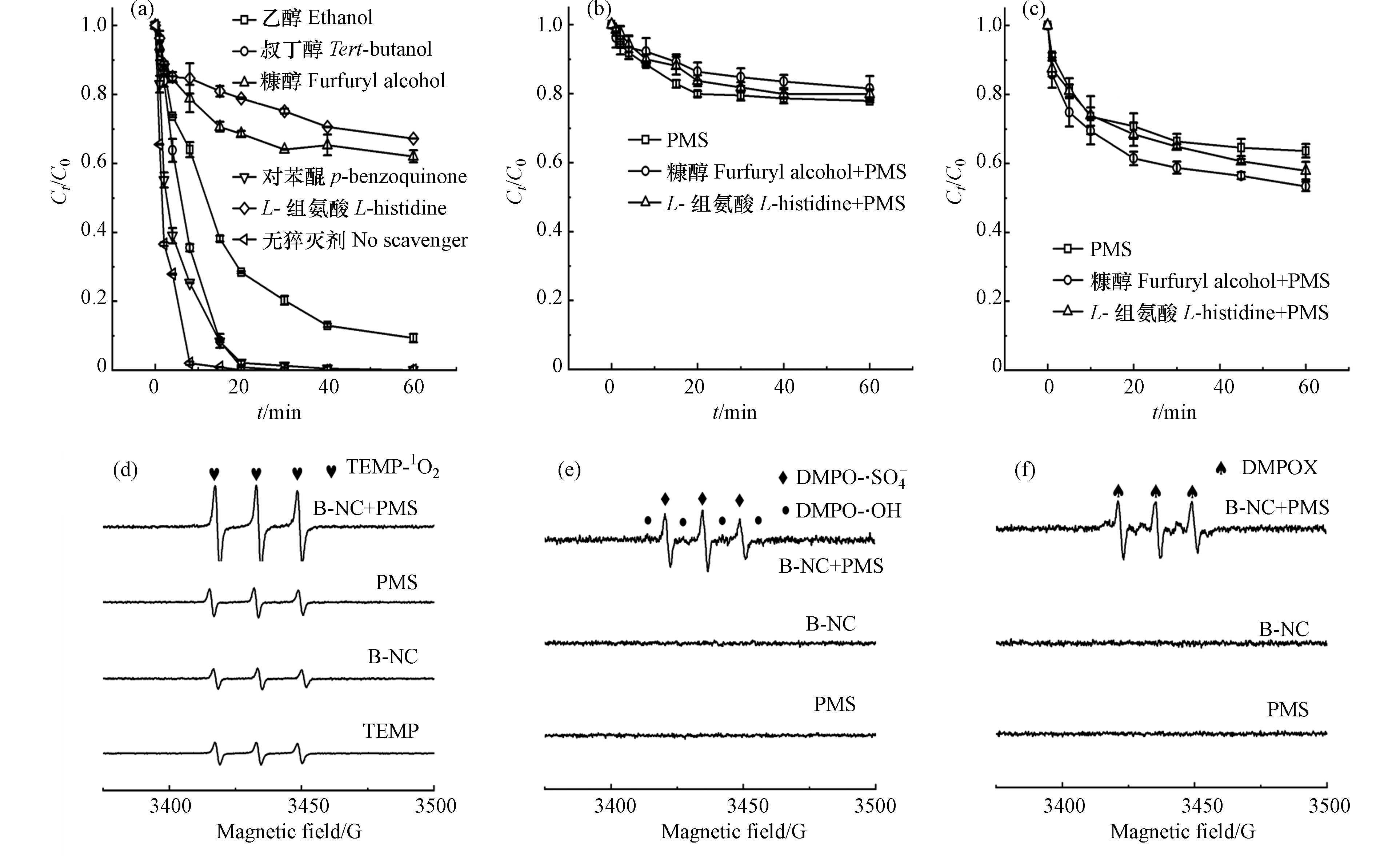
以乙醇、叔丁醇、糠醇、对苯醌以及L-组氨酸五种试剂作为自由基猝灭剂,对B-NC/PMS体系中产生的活性物种进行鉴定. 结果如图8a所示,未投加猝灭剂时,RhB降解率在10 min内即可达到99.18%,而加入L-组氨酸以及糠醇后,RhB降解率迅速下降到32.77%和36.68%,说明在此体系中存在单线态氧(1O2)并对RhB降解有明显抑制作用,单线态氧可能是该体系中发挥主要作用的活性物种. 此外,为测定糠醇与L-组氨酸在猝灭实验中是否与PMS反应,造成猝灭结果不准,将相同量的糠醇和L-组氨酸与5 mg的PMS单独反应降解RhB,并进行PMS消耗量的实验,实验结果如图8b所示,加入糠醇和L-组氨酸之后,与PMS单独降解RhB的降解率相差不大,而PMS消耗量实验(图8c)说明,糠醇和PMS的反应比L-组氨酸更强一些,但PMS并没有显著降低,进一步说明猝灭实验结果的准确性. 乙醇用于捕获SO4·−和·OH而叔丁醇仅用于鉴定·OH,加入乙醇后,RhB降解率下降到91.59%,而加入叔丁醇后降解率相差不大而降解速率明显下降,说明SO4·−和·OH在体系中皆有发挥一定作用,其中SO4·−作用更强而·OH的存在微弱. 作为超氧自由基(·O2−)的捕获剂,加入对苯醌后降解率没有明显变化,说明·O2−在体系中基本不存在. 综上所述,在B-NC/PMS/RhB体系中,自由基和非自由基共同作用,而1O2起主要作用.
为进一步阐明体系中不同活性物种的存在及确定优势物种,对反应体系进行了EPR测试, 用DMPO诱捕SO4·−,·OH,·O2−,TEMP捕获1O2[29],结果如图8(d—f)所示,使用TEMP捕获1O2可以发现单纯B-NC,PMS和B-NC+PMS体系图谱中均有有3个明显的特征峰,强度相等(图8 d),这是因为TEMP试剂本身发生了氧化造成的,而PMS和B-NC+PMS体系图谱的特征峰皆高于TEMP的背景峰,结合自由基猝灭实验可以得出1O2是反应体系中的主要活性物种之一, RhB的降解更依赖于非自由基方式[30],且PMS可以自分解产生1O2.
如图8e所示,B-NC+PMS体系图谱能观察到较弱的DMPO-SO4·−和DMPO-·OH的EPR特征峰,而单纯B-NC和PMS体系中没有峰出现,说明B-NC/PMS体系中同时产生了SO4·−和·OH,猝灭实验也表明相同的结果,而SO4·−在体系中比·OH更强,且两种自由基皆不起主要作用[20, 31]. 同时在系统中并未检测到·O2−信号,这与猝灭实验得到的结果一致. 此外,图8 f中存在3个特定的峰,可能是B-NC与PMS同时存在的条件下自旋降解产物的杂散自由基,也可能代表了DMPO-X加合物的信号(DMPO氧化形式),是过氧自由基(ROO·)产生的间接证据 (见式(1)—(3))[32, 33]. 其中,B-NC-OO·可能是DMPO氧化为DMPO-X的中间体.
此外,对使用过后的B-NC进行了XPS分析,以进一步阐述该体系降解RhB的机理. 如图9(a—d)所示,使用后的B-NC的活性位点如吡啶N、石墨N和BC3等仍然存在,且与新鲜B-NPC的活性位点基本相似,说明硼、氮共掺杂有利于提高稳定性. 从图9b发现,反应后表面C=O含量(21.46%—21.43%)下降,而C—O含量(21.91%—23.61%)增加,这是因为B-NC的C=O基团在反应过程中被氧化为电子转移的中间体或Lewis碱基上的孤对电子,可以有效增加相邻碳环的电子密度,引发氧化还原反应. 反应后B-NC的N1s高分辨率光谱显示(图9c),吡咯N从10.86%增加到50.41%,而吡啶N(41.65%—12.61%)、石墨氮(31.32%—30.53%)和氮氧化物(16.35%—6.45%)含量同时下降,表明吡啶氮、石墨氮和氮氧化物可能是PMS活化和RhB降解的潜在活性中心[14]. 研究报道,石墨N可以加速相邻碳原子之间的电子转移,破坏共轭石墨化碳网络的惯性,从而增加碳原子的正电荷,吡啶氮可贡献电子活化周围C原子,活化后的C原子转化为Lewis碱. 在图9d中,使用后的B-NC中BC2O含量 (34.54%—42.22%)和BCO2含量(26.17%—32.56%)皆有升高,而BC3,BNC含量从39.29%降低到25.21%,说明BC3,BNC也是该体系的活性位点,BC3可以通过改变碳基材料的价带结构和提高费米能级附近的密度来提高电子导电性[15],B掺杂剂同样可以从相邻的C原子中提取电子,形成带正电的碳中心,然后与PMS结合,通过非自由基途径生成1O2[30].
催化活化的实质是削弱和打破PMS中的O—O键以实现催化剂与PMS分子之间的电子转移,从而降解有机污染物. 研究报道,杂原子的掺杂可以有效提高碳材料催化剂催化PMS降解罗丹明B的能力,而N原子和硼原子被认为是氮掺杂碳质材料中PMS非自由基激活的主要活性位点[15, 34]. 本文制备的硼氮共掺杂催化剂中的硼原子和氮原子协同耦合可以打破sp2杂化碳网络的惰性,从相邻的碳原子中提取电子,诱导形成带正电的碳中心活性位点,再与亲核试剂(PMS)相结合生成活性物种,从而进行污染物的降解[35]. 另外,缺陷和亲电C=O基团有利于PMS吸附并改善污染物降解的速率. 基于以上的结果和分析,B-NC活化PMS降解RhB的机制可能如下(图10),首先,一些PMS分子与石墨氮以及BC3的协同作用诱导更多带正电的碳原子作为活性位点,可以通过非自由基途径迅速产生1O2 (见式(4)),同时通过自由基途径形成少量SO4·−和·OH (见式(5)—(6))[15],而吸附富集在活性位点周围的PMS可以自分解产生单线态氧(见式(7)). 同时,以·O2−为中间产物,经重组或与·OH发生反应生成1O2 (见式(8)—(9)),亲电含氧官能团C=O与吡啶氮皆可以增加碳原子的正电荷,通过电子重排削弱O—O键,形成表面亚稳PMS,或通过PMS与带正电的碳发生亲核加成反应生成反应性物质生成1O2 (见式(10)—(11)). 此外,吡啶氮有一部分电子转移到PMS中,导致O—O键断裂,产生少量SO4·−和·OH(见式(12))[34].
-
1)通过高温烧制,洗涤,离心,烘干等步骤成功制备B-NC催化剂,具有良好的PMS催化性能. 150 mL的RhB溶液中,在B与NC质量比5:2,PMS和催化剂投加5 mg的条件下,RhB在10 min内即可达到99.18%的降解率.
2)适当增大PMS与催化剂的投加量能提高RhB的降解率.RhB降解率随着其初始浓度的增大而减小. 适当升高温度对污染物的降解效率具有促进作用. 在较宽的pH范围(pH为3—9)内,体系对RhB的降解率都可达到95%以上,但在偏碱性(pH=11)条件下,污染物降解率下降至59.47%.
3)硼氮共掺杂为活化PMS提升更多活性位点,促进单线态氧的形成. 在B-NC/PMS体系中,主要通过非自由基(1O2)途径进行降解RhB,辅以自由基(SO4·−和·OH)降解RhB.
硼氮共掺杂多孔碳活化过硫酸盐降解罗丹明B性能和机制
Performance and mechanism of boron-nitrogen co-doped porous carbon as permonosulfate activator for Rhodamine B degradation
-
摘要: 工业废水中有大量的合成染料,排放到环境中造成严重的健康风险,引起人们广泛关注. 以过一硫酸盐(PMS)为基础的高级氧化法是一种有效的印染废水处理方法. 传统的过渡金属活化PMS降解染料的方法受到金属离子溶出的限制. 碳基材料的催化活化能力有待进一步提升. 而多孔碳材料有利于暴露更多活性位点,且杂原子掺杂可以使其表面的带电粒子的分布发生改变,有助于形成活性位点和促进电子传递. 因此,本文以金属-有机骨架材料(MOF)为前驱体,通过高温热解等方法合成硼,氮共掺杂多孔碳基材料B-NC,并以罗丹明B(RhB)为目标降解污染物,提出采用ZIF-67衍生材料活化PMS处理RhB染料,并探究了不同因素对降解的影响. 结果表明, 在中性室温条件下,150 mL初始浓度为30 mg·L−1的RhB溶液中,当催化剂和 PMS投加量为5 mg时,硼氮掺杂多孔碳材料活化PMS降解RhB的效率可达到100%. 进一步分析降解RhB废水的机理,自由基猝灭实验结果表明硼氮掺杂多孔碳材料活化PMS过程中1O2自由基起主导作用,硼氮共掺杂促进了1O2的形成. 研究结果表明基于金属有机框架衍生材料B-NC是一种环境友好,性能高效的PMS活化剂,这为高效处理染料废水提供了一种新策略,并降低了二次污染风险.Abstract: There are a large number of synthetic dyes in industrial wastewater, which cause considerable serious health risks to the environment, and have attracted widespread attention. Advanced oxidation based on permonosulfate (PMS) is an effective treatment method for printing and dyeing wastewater. Traditional transition metal-activated PMS for dye degradation is limited by metal ion dissolution. The catalytic activation ability of carbon-based materials needs to be further improved. Porous carbon materials are conducive to exposing more active sites, and heteroatom doping can change the distribution of charged particles on their surface, which is helpful to form active sites and promote electron transport. Therefore, in this paper, boron and nitrogen co-doped porous carbon matrix composite B-NC was synthesized by high-temperature pyrolysis with metal-organic framework materials (MOF) as the precursor, and Rhodamine B (RhB) was used as the target to degrade pollutants. ZIF-67 derived material was used to activate PMS to treat RhB dye, and the effects of different factors on degradation were investigated. The results showed that the degradation efficiency of RhB by BN-doped porous carbon material can reach 100% when the catalyst and PMS dosage was 5 mg in 150 mL RhB solution with an initial concentration of 30 mg·L−1 at neutral room temperature. The mechanism of degradation of RhB wastewater was further analyzed. The results of free radical quenching experiment showed that 1O2 radical played a leading role in the process of PMS activation by boron-nitrogen doped porous carbon materials, and the formation of 1O2 was promoted by boron-nitrogen co-doping. The results showed that metal-organic frame-derived material B-NC was an environmentally friendly and efficient PMS activator, which provided a new strategy for the efficient treatment of dye wastewater and reduces the risk of secondary pollution.
-
Key words:
- Peroxymonosulfate /
- Rhodamine B /
- Singlet oxygen /
- Metal-organic frameworks
-

-
表 1 非金属碳质材料及其PMS活化性能比较
Table 1. Comparison of metal-free carbonaceous materials and their PMS activation performance.
催化剂
Catalyst污染物
Pollutants去除效率
Efficiency of removal活性物种
Active species稳定性
Stability文献引用
Ref.NG-600/ PMS 10 mg·L−1 SAM 50 min后100% SO4·- 循环3次后50 min内降解率58% [16] N-IrGO/ PMS 5 mg·L−1 BP-1 60 min后100% 1O2 循环3次后60 min内降解率50% [17] NBC-900/ PDS 20 mg·L−1 SMX 45 min后100% 电子转移 循环3次后90 min内降解率42.51% [18] PGBF-N/ PMS 20 mg·L−1 TC 150 min后96.5% 1O2和电子转移 循环4次后150 min内降解率60% [19] PFSC-900/PMS 20 mg·L−1TC 120 min后90.91% 1O2主导 循环8次后120 min内降解率80% [14] CNTs/PS 20 mg·L−1RhB 150 min后100% SO4·-、·OH和电子转移 循环3次后210 min内降解率72.9% [20] B-NC/PMS 30 mg·L−1 RhB 60 min后100% 1O2、SO4·-、·OH 循环5次后60 min内降解率84.21% 本研究 表 2 反应前后pH的变化
Table 2. Change of solution pH before and after reaction
pH 反应前 3 4 5 7 9 11 反应后 2.87 3.58 3.82 6.80 7.90 10.64 -
[1] LIU Z C, KHAN T A, ISLAM M A, et al. A review on the treatment of dyes in printing and dyeing wastewater by plant biomass carbon [J]. Bioresource Technology, 2022, 354: 127168. doi: 10.1016/j.biortech.2022.127168 [2] WALKER G M, WEATHERLEY L R. Textile wastewater treatment using granular activated carbon adsorption in fixed beds [J]. Separation Science and Technology, 2000, 35(9): 1329-1341. doi: 10.1081/SS-100100227 [3] MARTÍNEZ-HUITLE C A, BRILLAS E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review [J]. Applied Catalysis B:Environmental, 2009, 87(3/4): 105-145. [4] RASALINGAM S, PENG R, KOODALI R T. An insight into the adsorption and photocatalytic degradation of rhodamine B in periodic mesoporous materials [J]. Applied Catalysis B:Environmental, 2015, 174/175: 49-59. doi: 10.1016/j.apcatb.2015.02.040 [5] SHARMA G, DIONYSIOU D D, SHARMA S, et al. Highly efficient Sr/Ce/activated carbon bimetallic nanocomposite for photoinduced degradation of rhodamine B [J]. Catalysis Today, 2019, 335: 437-451. doi: 10.1016/j.cattod.2019.03.063 [6] LIU N, DING F, WENG C H, et al. Minimizing the interference of carbonate ions on degradation of SRF3B dye by Fe0-aggregate-activated persulfate process [J]. Separation and Purification Technology, 2016, 169: 230-240. doi: 10.1016/j.seppur.2016.05.039 [7] ZHANG M, LUO R, WANG C H, et al. Confined pyrolysis of metal-organic frameworks to N-doped hierarchical carbon for non-radical dominated advanced oxidation processes [J]. Journal of Materials Chemistry A, 2019, 7(20): 12547-12555. doi: 10.1039/C9TA02931A [8] WANG G L, CHEN S, QUAN X, et al. Enhanced activation of peroxymonosulfate by nitrogen doped porous carbon for effective removal of organic pollutants [J]. Carbon, 2017, 115: 730-739. doi: 10.1016/j.carbon.2017.01.060 [9] XIE J L, LUO X, CHEN L, et al. ZIF-8 derived boron, nitrogen co-doped porous carbon as metal-free peroxymonosulfate activator for tetracycline hydrochloride degradation: Performance, mechanism and biotoxicity [J]. Chemical Engineering Journal, 2022, 440: 135760. doi: 10.1016/j.cej.2022.135760 [10] GAO S W, WANG Z, WANG H R, et al. Peroxydisulfate activation using B-doped biochar for the degradation of oxytetracycline in water [J]. Applied Surface Science, 2022, 599: 153917. doi: 10.1016/j.apsusc.2022.153917 [11] HOU W X, HUANG Y, LIU X. Highly efficient and recyclable ZIF-67 catalyst for the degradation of tetracycline [J]. Catalysis Letters, 2020, 150(10): 3017-3022. doi: 10.1007/s10562-020-03210-2 [12] GONG Y, ZHAO X, ZHANG H, et al. MOF-derived nitrogen doped carbon modified g-C3N4 heterostructure composite with enhanced photocatalytic activity for bisphenol A degradation with peroxymonosulfate under visible light irradiation [J]. Applied Catalysis B:Environmental, 2018, 233: 35-45. doi: 10.1016/j.apcatb.2018.03.077 [13] LONG Y H, LI S R, YANG P Z, et al. Synthesis of ZIF-67 derived honeycomb porous Co/NC catalyst for AO7 degradation via activation of peroxymonosulfate [J]. Separation and Purification Technology, 2022, 286: 120470. doi: 10.1016/j.seppur.2022.120470 [14] HU Y, CHEN D Z, ZHANG R, et al. Singlet oxygen-dominated activation of peroxymonosulfate by passion fruit shell derived biochar for catalytic degradation of tetracycline through a non-radical oxidation pathway [J]. Journal of Hazardous Materials, 2021, 419: 126495. doi: 10.1016/j.jhazmat.2021.126495 [15] LI X J, YE Z Y, XIE S H, et al. Insight into the performance and mechanism of peroxymonosulfate activation by B, N co-doped hierarchical porous carbon for phenol degradation [J]. Journal of Environmental Chemical Engineering, 2022, 10(5): 108264. doi: 10.1016/j.jece.2022.108264 [16] CHEN X, OH W D, HU Z T, et al. Enhancing sulfacetamide degradation by peroxymonosulfate activation with N-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes [J]. Applied Catalysis B:Environmental, 2018, 225: 243-257. doi: 10.1016/j.apcatb.2017.11.071 [17] SUN P, LIU H, ZHAI Z C, et al. Degradation of UV filter BP-1 with nitrogen-doped industrial graphene as a metal-free catalyst of peroxymonosulfate activation [J]. Chemical Engineering Journal, 2019, 356: 262-271. doi: 10.1016/j.cej.2018.09.023 [18] HO S H, CHEN Y D, LI R X, et al. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation [J]. Water Research, 2019, 159: 77-86. doi: 10.1016/j.watres.2019.05.008 [19] YE S J, ZENG G M, TAN X F, et al. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer [J]. Applied Catalysis B:Environmental, 2020, 269: 118850. doi: 10.1016/j.apcatb.2020.118850 [20] CHEN S H, MA L Y, DU Y G, et al. Highly efficient degradation of rhodamine B by carbon nanotubes-activated persulfate [J]. Separation and Purification Technology, 2021, 256: 117788. doi: 10.1016/j.seppur.2020.117788 [21] 杨珂, 唐琪, 杨晓丹, 等. 铁酸铜非均相活化过硫酸盐降解罗丹明B [J]. 中国环境科学, 2019, 39(9): 3761-3769. doi: 10.3969/j.issn.1000-6923.2019.09.020 YANG K, TANG Q, YANG X D, et al. Degradation of rhodamine B by heterogeneous activation of persulfate with copper ferrate [J]. China Environmental Science, 2019, 39(9): 3761-3769(in Chinese). doi: 10.3969/j.issn.1000-6923.2019.09.020
[22] 王渊源, 阎鑫, 艾涛, 等. 碳化泡沫负载Co3O4活化过硫酸盐降解罗丹明B [J]. 环境科学, 2022, 43(4): 2039-2046. WANG Y Y, YAN X, AI T, et al. Carbonized foam supported Co3O4 activated peroxymonosulfate towards rhodamine B degradation [J]. Environmental Science, 2022, 43(4): 2039-2046(in Chinese).
[23] WANG G L, LIU Y C, DONG X L, et al. Transforming radical to non-radical pathway in peroxymonosulfate activation on nitrogen doped carbon sphere for enhanced removal of organic pollutants: Combined effect of nitrogen species and carbon structure [J]. Journal of Hazardous Materials, 2022, 437: 129357. doi: 10.1016/j.jhazmat.2022.129357 [24] del RIO M, GRIMALT ESCARABAJAL J C, TURNES PALOMINO G, et al. Zinc/Iron mixed-metal MOF-74 derived magnetic carbon nanorods for the enhanced removal of organic pollutants from water [J]. Chemical Engineering Journal, 2022, 428: 131147. doi: 10.1016/j.cej.2021.131147 [25] SILVESTRI D, KRAWCZYK K, PAWLYTA M, et al. Influence of catalyst zeta potential on the activation of persulfate [J]. Chemical Communications (Cambridge, England), 2021, 57(63): 7814-7817. doi: 10.1039/D1CC01946E [26] 邓靖, 冯善方, 马晓雁, 等. 热活化过硫酸盐降解水中卡马西平 [J]. 化工学报, 2015, 66(1): 410-418. DENG J, FENG S F, MA X Y, et al. Degradation of carbamazepine in water by thermally activated persulfate [J]. CIESC Journal, 2015, 66(1): 410-418(in Chinese).
[27] 王莹, 魏成耀, 黄天寅, 等. 氮掺杂碳纳米管活化过一硫酸盐降解酸性橙AO7 [J]. 中国环境科学, 2017, 37(7): 2583-2590. WANG Y, WEI C Y, HUANG T Y, et al. Activation of peroxymonosulfate by nitrogen-doped carbon nanotubes to decolorize acid orange 7 [J]. China Environmental Science, 2017, 37(7): 2583-2590(in Chinese).
[28] ZHU K, SHEN Y Q, HOU J M, et al. One-step synthesis of nitrogen and sulfur co-doped mesoporous graphite-like carbon nanosheets as a bifunctional material for tetracycline removal via adsorption and catalytic degradation processes: Performance and mechanism [J]. Chemical Engineering Journal, 2021, 412: 128521. doi: 10.1016/j.cej.2021.128521 [29] LIU B H, GUO W Q, WANG H Z, et al. B-doped graphitic porous biochar with enhanced surface affinity and electron transfer for efficient peroxydisulfate activation [J]. Chemical Engineering Journal, 2020, 396: 125119. doi: 10.1016/j.cej.2020.125119 [30] XIE J L, CHEN L, LUO X, et al. Degradation of tetracycline hydrochloride through efficient peroxymonosulfate activation by B, N co-doped porous carbon materials derived from metal-organic frameworks: Nonradical pathway mechanism [J]. Separation and Purification Technology, 2022, 281: 119887. doi: 10.1016/j.seppur.2021.119887 [31] GUO Y X, YAN L G, LI X G, et al. Goethite/biochar-activated peroxymonosulfate enhances tetracycline degradation: Inherent roles of radical and non-radical processes [J]. Science of the Total Environment, 2021, 783: 147102. doi: 10.1016/j.scitotenv.2021.147102 [32] RINALDUCCI S, PEDERSEN J Z, ZOLLA L. Generation of reactive oxygen species upon strong visible light irradiation of isolated phycobilisomes from Synechocystis PCC 6803 [J]. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 2008, 1777(5): 417-424. doi: 10.1016/j.bbabio.2008.02.005 [33] JIANG L L, ZHANG Y, ZHOU M H, et al. Oxidation of Rhodamine B by persulfate activated with porous carbon aerogel through a non-radical mechanism [J]. Journal of Hazardous Materials, 2018, 358: 53-61. doi: 10.1016/j.jhazmat.2018.06.048 [34] LIU S Q, ZHANG Z C, HUANG F, et al. Carbonized polyaniline activated peroxymonosulfate (PMS) for phenol degradation: Role of PMS adsorption and singlet oxygen generation [J]. Applied Catalysis B:Environmental, 2021, 286: 119921. doi: 10.1016/j.apcatb.2021.119921 [35] CHOONG Z Y, LIN K Y A, LISAK G, et al. Multi-heteroatom-doped carbocatalyst as peroxymonosulfate and peroxydisulfate activator for water purification: A critical review [J]. Journal of Hazardous Materials, 2022, 426: 128077. doi: 10.1016/j.jhazmat.2021.128077 -



 下载:
下载: