-
目前,各个国家包括中国政府都对汞的开采、使用和人为排放制定了严格的环保法规,然而历史上因汞污染所造成的环境问题仍然存在。我国的PVC生产行业多采用电石法工艺,即氯化汞触媒生产氯乙烯单体(vinyl chloride monomer, VCM)[1],该行业存在一定的汞排放问题。有学者在武汉市某电石法PVC生产企业的退役场地0.8~1.0 m深度的土壤中检测到汞,含量有31~2 100 mg·kg−1[2]。受污染土壤中的汞主要源自生产过程中的无组织排放[3]。PVC行业VCM生产过程中氯化汞触媒的外泄和副产物单质汞的挥发和沉降是周边土壤汞污染的最主要来源[2]。由于长期粗放生产而导致的土壤汞污染问题亟待解决[3-6]。
汞污染土壤的治理是指采用各种手段将汞污染物从土壤中分离、去除,或将汞污染物固定在土壤中、将其转化为无害物质,使土壤环境得到恢复。现代土壤修复技术按原理可分为物理修复法、化学修复法和生物修复法[7-10]。物理法中的热解法能直接去除受污染土壤中的汞,修复效果最为直接,但工程化实施费用过高,阻碍了该方法的广泛应用[11-13]。化学固化/稳定化技术是指通过外加试剂,以胶结、固化或形成汞化合物的形式来限制汞的迁移,从而降低汞污染[14-16]。然而,成本高和处理后汞的长期稳定性问题使得该技术大规模使用受限。生物修复法包括植物修复法和微生物修复法,其成本较低,寻找或培养适合的植物、菌株是技术关键[17]。
本研究选取锦州市某PVC化工厂附近的汞污染土壤为样品,探索基于湿法冶金工艺原理的浸提脱汞处理方法,采用硫代硫酸盐对土壤中的汞进行络合浸提处理,考察温度、硫代硫酸钠浓度以及时间等因素对汞浸提效果的影响,并通过Förstner 7步法[18]对汞污染土壤和浸提处理后的土壤进行形态分析,再用光分解回收法对含汞浸提液进行处理,以期实现以硫化汞的形式回收汞,为土壤中汞的处理提供新的思路。
全文HTML
-
汞污染土壤样品取自于某PVC化工厂附近(简称“含汞土壤”)。待含汞土壤自然风干后,用偏心研磨机磨细,过160目筛,用四分法混匀。参照《土壤质量 总汞的测定 冷原子吸收分光光度法》(GB/T 17136-1997)定量测定含汞土壤样品中的总汞含量,所用试剂均为分析纯。
-
含汞土壤浸提实验在恒温水浴槽内进行。含汞土壤浸出液的光分解脱汞实验采用功率为20 W的254 nm紫外灯为光源,以直径20 mm、长度200 mm的石英管为光反应器。
-
浸提实验:含汞土壤的浸提实验以硫代硫酸钠溶液为浸出剂,用转速为200 r·min−1的磁力搅拌保持浆液颗粒悬浮;改变浸出温度、浸出剂浓度和浸出时间,以考察浸出条件对汞浸出率的影响规律;浸出实验结束后,对浸出浆液进行液固分离;用F732-VJ型测汞仪(上海华光仪器仪表厂)分别对浸出液和残渣进行冷原子吸收法分析(Cold-AAS)以确定汞含量,并计算汞浸出率。
形态测定:采用Förstner 7步法[18]对含汞土壤和浸提后土壤中的汞进行化学连续提取,以确定汞形态。
光分解实验:把含汞浸出液装入石英管,再将石英管环状排列于紫外灯管外约5 cm处,使其均匀接受紫外线辐照。按预定时间结束光分解反应后,测定浸出液中残留的汞浓度,并计算汞的分解率,同时检测该时刻溶液的pH。
1.1. 实验原料
1.2. 实验装置
1.3. 实验方法
-
含汞土壤采用XRF进行全元素半定量分析得出各种元素的比例为:Si 55.94%、Al 10.32%、Ca 10.06%、K 6.98%、Fe 6.57%、C 5.80%、Na 1.87%、Ti 0.68%、Hg 0.33%、S 0.27%、P 0.098%。由XRF分析结果可知,汞含量大概为数千mg·kg−1,说明样品受污染程度严重。该样品中S、C和P的存在,会导致土壤中的汞除了以物理吸附形式存在外,还会有相当一部分以更稳定的化学结合态存在。进一步对含汞土壤进行消解、Cold-AAS定量分析,重复3次测量取平均值,得到土壤样品中总汞含量为2 400 mg·kg−1,后续浸提实验用该数值作为含汞土壤的初始总汞含量。
-
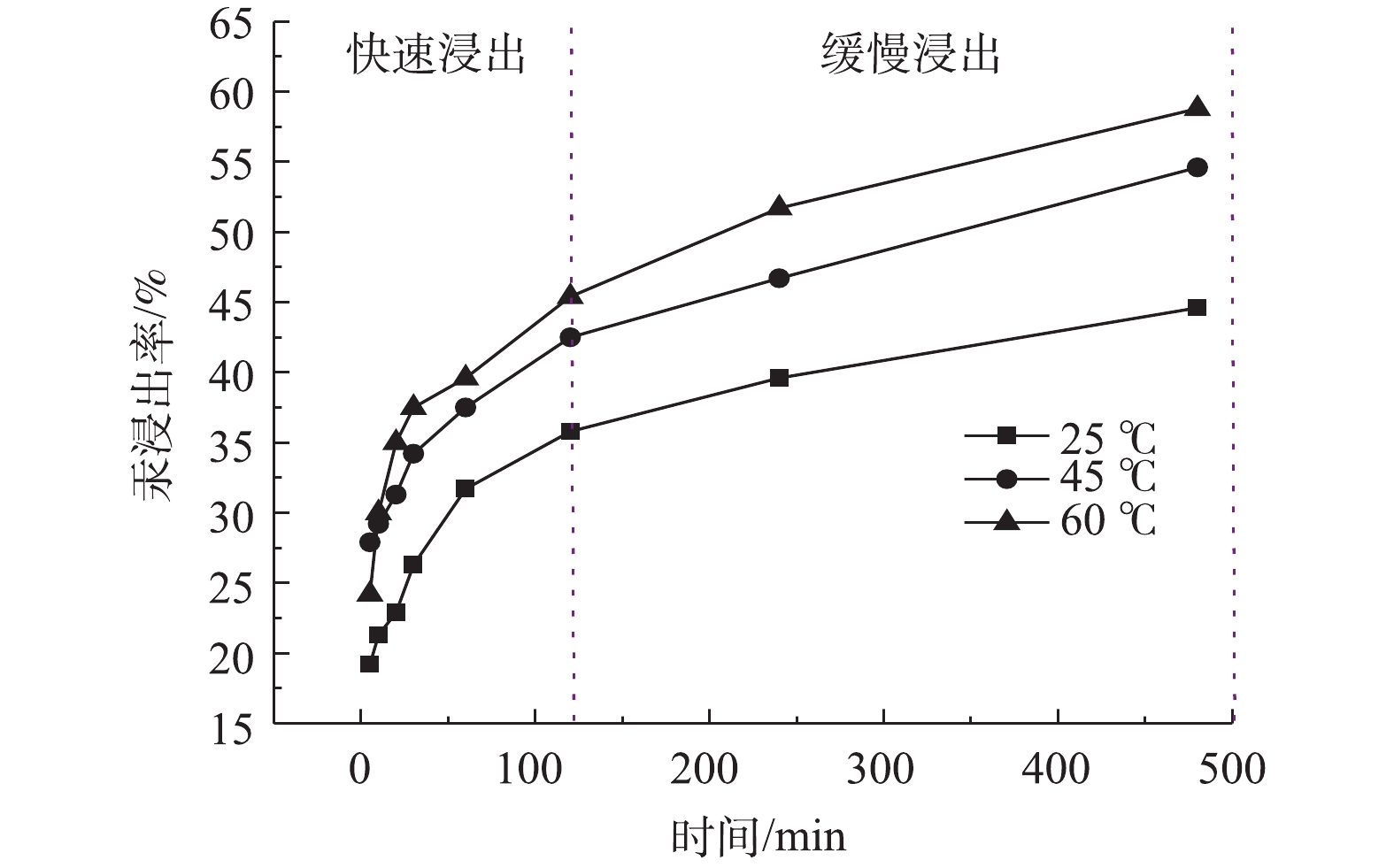
图1为温度对硫代硫酸钠溶液浸提含汞土壤的浸出效果影响。选取25、45和60 ℃ 3个温度条件,硫代硫酸钠浓度为0.01 mol·L−1,选取较大的液固比100∶1 (mL∶g)以确保浸出过程中游离浸出剂浓度保持恒定。由图1可知,在25 ℃下,浸出120 min后,汞的浸出率即可达到35.8%;继续延长浸出时间至480 min,汞的浸出率仅上升至44.6%。提高浸出温度,可明显增加汞的浸出速度。不同温度下,浸出曲线均呈现“快速”浸出和“慢速”浸出2个阶段。该现象与汞在含汞土壤中以不同赋存形态存在有关。考虑到浸提脱汞工艺的经济性,后续的浸出选择室温条件下进行。
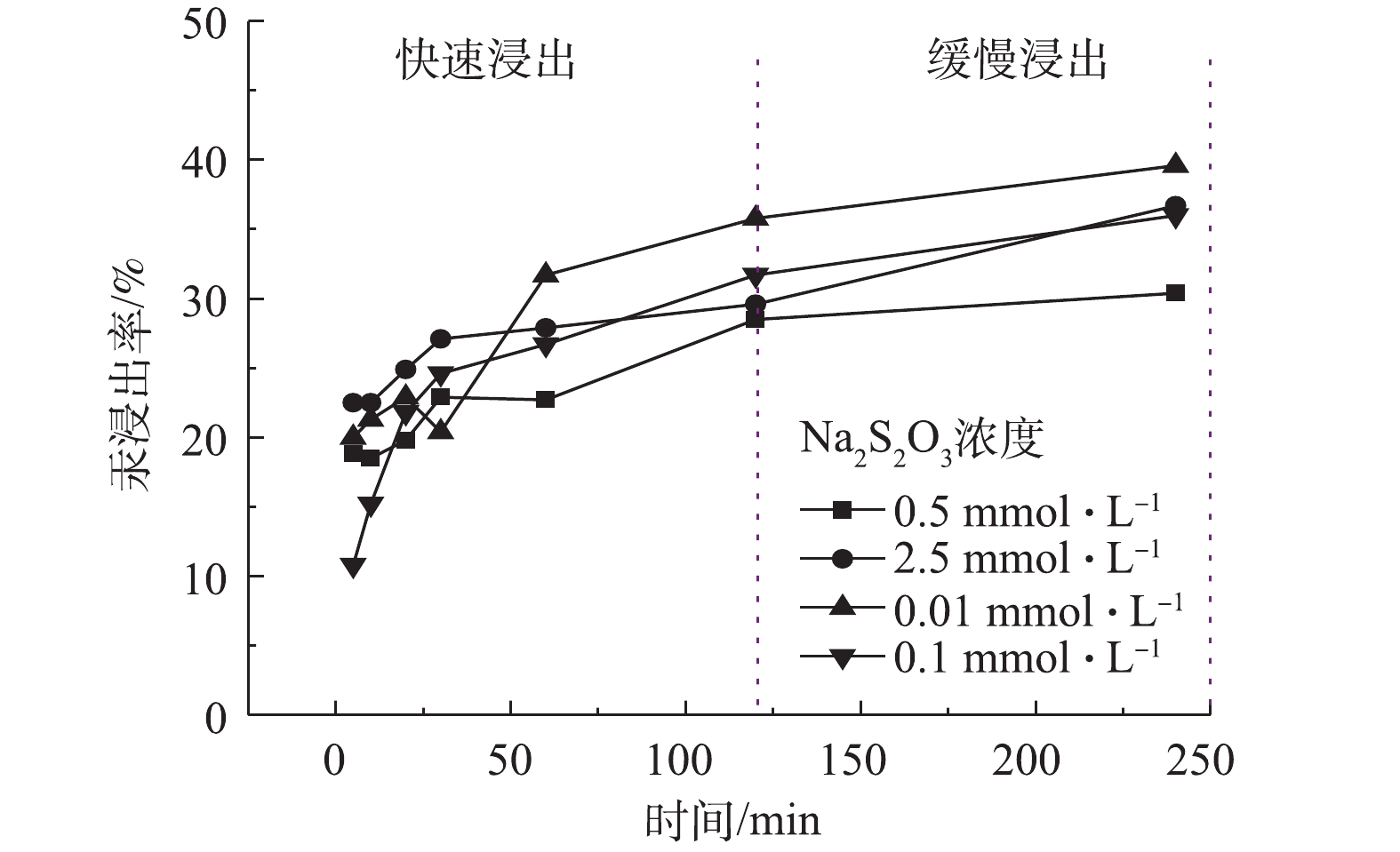
图2为室温条件下,不同浓度硫代硫酸钠溶液浸提含汞土壤的结果,浸出时仍采用100∶1 (mL∶g)的液固比,以维持浸出液中浸出剂浓度基本恒定。该结果同样体现浸出过程可大致分为“快”和“慢”2个阶段。以浸出时间120 min为界,继续延长浸出时间,汞的浸出率变化较小,仅有少量增幅。同时,无论浸出剂浓度大或小,浸出240 min后,汞浸出率均稳定在相近的数值,即30.4%~36.0%。当浸出剂浓度为0.01 mol·L−1时,汞的浸出率达到最高,在240 min时有39.6%的汞被浸提出来。该结果说明:一方面硫代硫酸盐是土壤中汞的有效浸出剂,在较低浓度下即可实现较好的浸出效果;另一方面说明硫代硫酸盐浸出剂浓度较高不必过高,浓度过高易导致硫代硫酸根的歧化反应分解和实际汞浸出率下降。
综上所述,硫代硫酸盐是一种有效的汞浸提剂。然而,室温下改变浸出剂浓度或提高浸出温度也不能将汞浸出率提升太高,在较长时间后,仅维持在45%~60%左右。因此,有必要对浸提前后汞的形态变化进行分析,以寻找提升浸出率的方法。
-
在温度为25 ℃时,选取液固比100∶1 (mL∶g),硫代硫酸钠浓度0.01 mol·L−1,浸提240 min后的土壤样品,按照Förstner 7步法的操作程序,对该样品Hg形态进行定量分析,结果见表1。由于分析误差的累积,7步法各形态汞的加和稍大于含汞样品初始总汞值。此处忽略累积分析误差,以各形态汞的定量总和为基准,计算各形态汞的相对比例。
在浸提实验前,含汞土壤样品质量为10.008 g;浸提之后,经离心液固分离、低温干燥,得到浸出后土壤样品质量为9.770 g;浸提后土壤样品仅有2.38%的质量减少。为便于数据分析,忽略浸提过程中土壤样品的质量变化,将Förstner 7步法得到浸提残渣中各形态Hg的实际含量,以浸出前含汞土壤样品中的初始总汞含量为基准进行折算,得到浸出残渣中各形态Hg占初始总汞含量的相对比例。
由表1可知,浸提前含汞土壤中的汞主要以盐酸溶态、硝酸溶态、有机质结合态和硫化态4类形态存在。前两者加和约占总Hg的38.0%(或加上水溶态和可交换态,为41.3%,可认为是易释放汞);有机结合态和硫化态的汞加和为58.1%,可认为是稳定态汞。经过硫代硫酸钠浸提后,土壤中以有机质结合态和硫化态存在的汞基本未发生变化,其相对比例之和仍占初始土壤总汞的56.8%,而以盐酸溶态和硝酸溶态存在的汞则显著减少。
以上结果可解释在较大的浸出剂浓度范围内进行浸提实验时,汞的最大浸出率在40%左右的原因。在室温下,硫代硫酸盐仅与含汞土壤中易释放态的汞作用,即能够有效浸出以水溶态、可交换态、盐酸溶态、硝酸溶态形式存在的汞,而对有机质结合态和硫化态汞无明显作用。
浸出温度升高可加速硫代硫酸盐与稳定态有机质结合汞、硫化态结合汞间的化学反应速度,从而在一定程度上提高汞浸出率。这类浸出率提高形式属于浸出过程化学反应动力学控制。虽然提高浸出温度对提高汞浸出率有一定促进,但从无害化工程处理角度考虑,加热成本势必会制约其应用。
综上所述,硫代硫酸钠是汞污染土壤中水溶态、可交换态、盐酸溶态、硝酸溶态汞的优良浸出剂,经硫代硫酸盐浸提处理后,土壤中残留汞基本以较稳定的有机质结合态和硫化态存在。赵婷等[13]也对贵州省清镇地区的高浓度汞污染土壤开展了低温热解工程修复研究。经热解修复后,残余汞基本以有机结合态和残渣态存在;且修复后残余汞对农耕作用的毒害作用明显下降。因此,硫代硫酸盐浸提含汞土壤,可有效减少易释放汞含量,从而实现含汞土壤的稳定化处理。
-
含汞土壤经过硫代硫酸钠浸提处理后,浸出液中含有以络合物形式Hg(S2O3)x2−2x存在的汞。浸提产生的含汞废液还需要低成本、简易可行的方法进行处理,否则会造成二次污染。本研究拟采用光分解法对含汞的硫代硫酸盐溶液进行处理,以分解汞的硫代硫酸盐络合物,使其以稳定的硫化汞形式沉淀,得以分离回收,实现浸提液再生。
图3为硫代硫酸盐溶液和含汞硫代硫酸盐溶液的UV-vis吸收光谱图对比。由谱图可知,硫代硫酸盐溶液仅在250 nm处有一个较窄的吸收峰;但当溶液中存在汞时,吸收峰的波峰移向长波方向,整个吸收峰展宽至230~300 nm。因此,当汞与过量的硫代硫酸根形成络合物后,络合离子对紫外线有强吸收效应。基于此,对含汞土壤实际浸出液进行了紫外光分解实验。
光分解浸出液是在液固比100∶1 (mL∶g)、硫代硫酸钠浓度0.01 mol·L−1、温度25 ℃、浸出含汞土壤240 min后得到,其含汞浓度为11.6 mg·L−1。在室温下,对浸出液进行了以254 nm紫外灯为光源的光分解处理,对汞的光分解率及溶液pH变化如图4所示。
汞-硫代硫酸盐络合物的光分解反应十分迅速且彻底。经过1 min紫外线照射,汞-硫代硫酸盐络合物的分解率即可达到95.7%;经5 min光分解,溶液中残留汞即可降至0.1 mg·L−1;汞-硫代硫酸盐络合物的分解率达到99.1%。在光分解过程中,随着反应的进行,溶液颜色由无色变为棕黑色(棕黑色为细小的沉淀颗粒,由收集到的沉淀物XRD分析,验证其为黑辰砂形式的硫化汞沉淀);且随着反应进行,浸出液pH逐渐降低。因此,可认为汞-硫代硫酸盐络合物在光分解过程中发生如式(1)和式(2) 2个反应。
光分解脱汞分为2步:汞-硫代硫酸盐的络合物捕获光子,本身跃迁至激发态;激发态的汞-硫代硫酸盐络合物分解为细小黑色硫化汞沉淀,同时有硫歧化为硫酸,后者使溶液pH降低。汞-硫代硫酸盐本身是热力学不稳定物质,但由于动力学因素限制,其在一定条件下无法发生歧化分解反应;引入紫外线照射后,对热力学上呈自发反应趋势的歧化分解反应提供了有效外界激励,使反应迅速进行。
2.1. 含汞土壤的表征
2.2. 含汞土壤的硫代硫酸钠浸提结果
2.3. 含汞土壤及浸提残渣中Hg的形态分析
2.4. 含汞浸出液的光分解脱Hg结果
-
1)本研究选取某PVC化工厂附近的土壤进行处理,土壤含Hg为2 400 mg·kg−1。经Förstner七步连续提取法分析,该样品中以水溶态、交换态、盐酸溶态和硝酸溶态等易释放态的Hg约占41.3%,而以有机质结合态和硫化态存在的Hg分别占33.9%和24.2%。
2)硫代硫酸钠是该含汞土壤中易释放态Hg的有效浸出剂,采用浓度为0.01 mol·L−1的硫代硫酸钠溶液在室温下浸出6 h,可浸出含汞土壤中39.6%的Hg。这部分汞约占含汞土壤初始易释放形态汞的95.8%。对浸出残渣进行汞形态分析,发现残余汞基本以有机质结合态和硫化态存在,即含汞土壤中可交换态、盐酸溶态、硝酸溶态的汞能够被硫代硫酸钠溶液有效浸出,有机质结合态和硫化态汞在此浸出条件下仍稳定存在于土壤中。
3)对含汞硫代硫酸盐浸出液进行了光分解处理。结果表明,汞硫代硫酸盐络合物因可有效吸收紫外光,而分解为硫化汞沉淀,经过5 min光分解反应,汞的分解沉淀率可达到99%以上。采用紫外线光分解是一种清洁、高效的汞沉淀分离回收方法。



 下载:
下载: