-
改革开放以来,我国工业建设和城市化进程取得巨大成就,但发展带来的大气环境问题日益严重[1-3]. 依赖化石燃料的重工业和交通业持续不断地向大气环境排放氮氧化物(NOx,x=1,2;NO占比约95%),不仅增加居民患呼吸道疾病的风险,还加剧光化学烟雾、酸雨和雾霾等污染事件的发生,严重威胁了居民健康和气候环境[4-9]. 面临严峻的大气环境污染形势,我国采取了一系列措施控制大气NOx的排放. 然而,在2011—2017年间,我国大气环境中NOx的浓度仍保持高位,NOx污染物的浓度只降了26%. 因此,开发高效净化氮氧化物技术迫在眉睫.
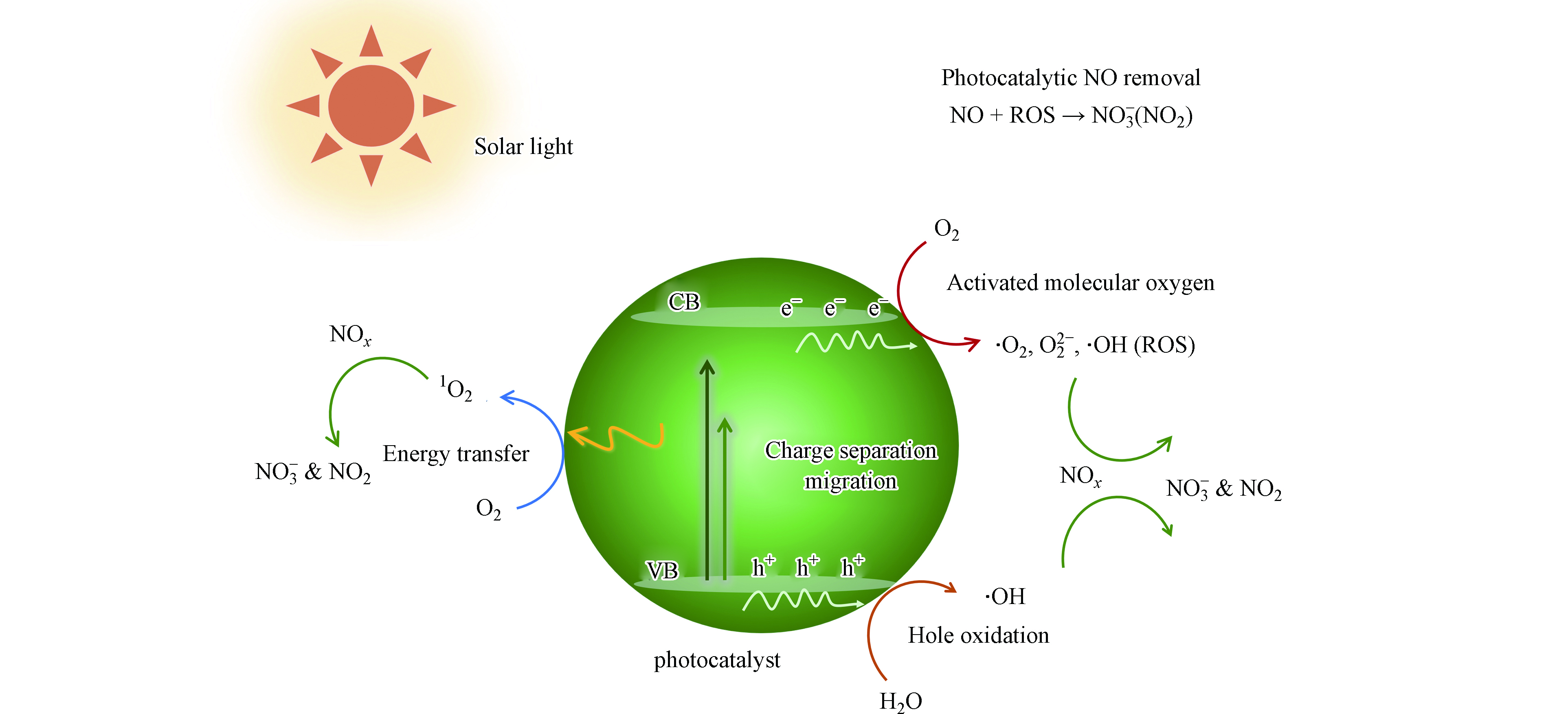
传统物理吸附和选择催化还原技术能有效减少工业废气和汽车尾气中高浓度的NO,但并不适用于净化大气和室内环境中低浓度的NO污染[10-12]. 与传统净化技术相比,光催化净化NO技术利用太阳能高效转化NO形成硝酸盐,在绿色治理NOx领域展现出广阔的应用前景[13-21]. 光催化去除NOx的基本步骤如图1所示,NO光催化剂在太阳能激发下产生大量光生电子和空穴. 激发态电子和空穴迅速迁移到半导体表面后,与表面吸附的分子反应产生新物种,例如光生电子还原O2生成·O2−,空穴氧化H2O形成·OH[22-29]. 这些活性氧物种均能够高效氧化NO形成硝酸盐或NO2(反应式1—8),有望实现太阳能绿色净化NO污染物. 然而,目前光催化消减NO技术的实际应用仍面临巨大挑战,催化剂的净化效率和选择性成为了限制其发展的重要因素.
众所周知,光催化去除NO效率与催化剂的物理电子结构紧密相关[30-34]. 可以通过改性策略改变催化剂的物理结构来提升催化剂的光吸收能力、载流子迁移效率或调控氧化还原电位,进而增强光催化去除NO的活性[35-39]. 遗憾的是,常规的改性策略一般无法实现选择性光催化去除NO形成硝酸盐,在反应过程中光生空穴和·OH仍可以氧化NO形成有毒中间产物NO2[35-36]. 因此,调节催化剂表界面反应产生的氧物种被认为是提升光催化去除NO选择性的有效策略,如光生电子还原O2产生的·O2−可定向氧化NO形成硝酸盐[37-39]. 然而,部分研究者发现·O2−也能氧化NO形成NO2[40]. 诚然,选择性光催化氧化NO反应的调控机制存在许多争议,这反映出我们对催化剂表界面氧化NO机理的认识不足.
鉴于此,本文综述了光催化去除NO的表界面反应研究进展,在介绍改性策略和关键化学过程的基础上,深刻讨论了影响光催化去除NO效率和选择性的关键因素. 最后,展望了光催化净化NO技术的发展和应用前景,旨在为设计高效净化NO的光催化剂及净化组件提供理论依据和实践参考.
-
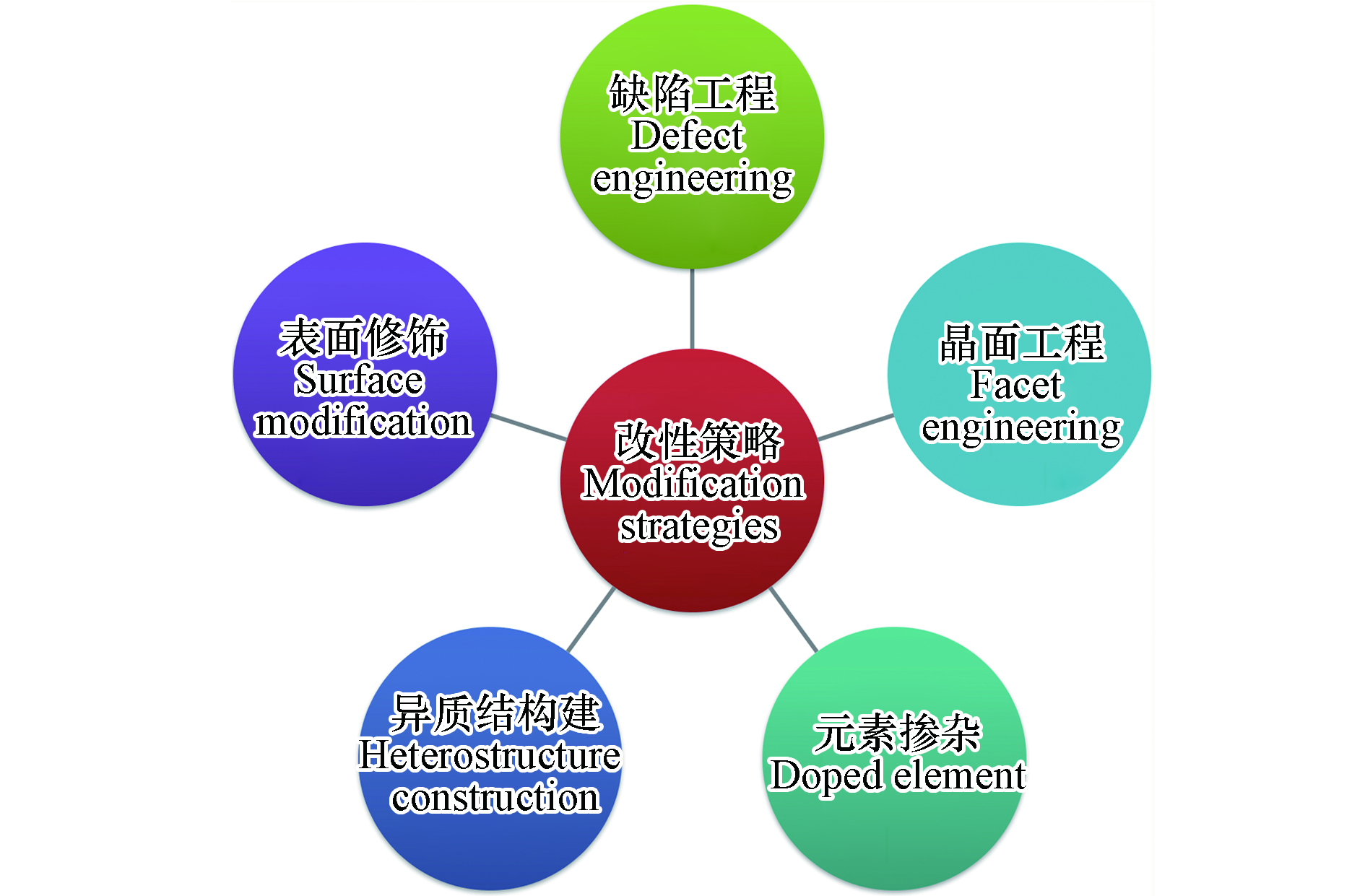
近年来,研究者设计开发了TiO2、铋系化合物、C3N4等消减NO的光催化剂,并通过调节半导体催化剂的物理电子结构发展了一系列提高光催化去除NO性能的策略. 如图2所示,典型的改性策略主要包括了构建缺陷位点、活性晶面暴露、元素掺杂、表面修饰和构筑异质结等.
-
构建表面缺陷是增强NO去除的重要改性策略(表1),它主要体现在两个方面:(1)调控半导体物理电子结构,增强太阳能吸收,促进载流子有效分离. (2)预先活化反应物,降低反应能垒,加速NO氧化反应进程.以半导体氧缺陷为例,它可以通过真空(气氛)煅烧和化学还原等方法实现. 在氧空位的形成过程中,表面氧原子会从晶格中脱离,氧原子留下的电子则会聚集在不饱和配位的金属或者非金属位点上;随后,这些局域在氧空位的电子会在催化剂导带和价带间形成一个新能级. 相关研究表明该缺陷能级不仅可以改变催化剂的费米能级位置和禁带宽度,拓宽太阳能吸收范围;它还可以捕获导带电子,有效避免导带电子与价带空穴的复合,促进载流子快速分离,最终实现高效催化去除NO[41-45]. 此外,负电性的氧空位可提供悬挂键吸附氧分子,促进氧分子活化产生活性氧物种,进而增强催化剂去除NO的性能[41-45]. 例如,Shang等[46]通过硼氢化钠还原法制备了含有氧缺陷的TiO2(TiO2-OV),与TiO2比较,TiO2-OV光催化去除NO的反应速率提升了2倍.
缺陷工程不仅有利于提升TiO2光催化去除NO的性能,在改性和提高二维层状铋系光催化剂的催化性能方面也非常有效. 由于铋系光催化剂内层间共价键和范德华力的相互作用会削弱晶格内的Bi—O键,所以铋系催化剂容易在外界因素的影响下断键形成氧空位.经过改性的铋系光催化剂通常具有较强的太阳光吸收能力、高效的载流子分离效率和更高的光催化净化NO活性. 此外,其他(非)金属缺陷的引入也能增强光催化去除NO活性[47-50]. 例如,Li等[49]在CO2气氛下煅烧g-C3N4制备了含有碳空位的g-C3N4,其可见光下催化去除NO的性能提升了2倍. Dong等[50]利用N缺陷也显著增强了g-C3N4光催化去除NO的效率. 虽然缺陷工程可显著提高光催化消减NO的性能,但是相关研究仍处于初级阶段,后期需要进一步完善表面缺陷结构对NO氧化反应的影响.
-
光催化去除NO是典型非均相氧化反应,催化剂吸附反应物O2和NO的能力对提升催化性能至关重要. 优先吸附的小分子会更容易得到催化剂的电子. 例如,优先吸附在催化剂表面的O2可获得光生电子,被还原为·O2−,进而实现光催化氧化NO形成硝酸盐或NO2. 事实上,反应物的吸附强弱取决于催化剂的表面结构和原子配位,这与光催化剂的暴露晶面密切相关. 调控不同的暴露晶面不仅可以实现定向活化反应物,还可以调控电子的传输路径,提升催化剂的性能(表1)[58, 73]. 2019年,Li等[53]通过水热法和紫外光照法合成了不同晶面暴露的缺陷态BiOCl纳米片. 与BiOCl(001)晶面相比,BiOCl(010)晶面具有更多的不饱和Bi位点和更高的电子利用率,在氧化去除NO方面表现出了显著优势.2019年,Xia等[52]成功制备了不同晶面暴露的Ag3PO4/Al2O3负载型催化剂. 在可见光激发下,Ag3PO4(111)四面体表现出更加优异的去除NO性能,在6 min内NO去除率达到100%. DFT计算结果表明高能晶面(111)更适合吸附O2和H2O,能够有效促进光生电子还原O2和H2O形成·O2−和·OH,进而提升了催化剂去除NO的效率. 利用晶面工程增强NO去除性能的研究不胜枚举,诸如铋系化合物Bi2O2CO3[74]、BiOBr[75]和TiO2[51]等. 这充分说明了光催化氧化去除NO反应具有晶面依赖性,如何调控晶面的物理结构提升NO的催化性能值得我们进一步思考和探究.
-
元素掺杂是通过引入外来非金属或者金属原子取代催化剂主体原子的方式增强光催化剂去除NO的性能(表1)[40, 59, 76-78]. 一方面,掺杂原子能影响催化剂的电子物理结构. 例如,掺杂原子轨道杂化形成的受主/施主能级可以缩小原催化剂的禁带宽度,扩宽太阳光吸收范围,进而增强催化剂可见光光催化NO去除的能力.另一方面,掺杂原子的嵌入会影响催化剂的界面电荷传输.比如,表面掺杂原子可导致催化剂界面电荷分布不均匀. 富集电荷的位点会成为吸附活化反应物的新位点,这使得催化剂更加有利于NO的氧化去除. 而进入催化剂层隙结构的掺杂原子可以通过改变催化剂电子传输通道,进一步抑制载流子复合,从而产生更多活性氧物种用于NO的氧化. Zhou等[60]通过Sr元素多位点掺杂g-C3N4实现了高效光催化去除NO,反应速率提升了1.5倍,中间有毒产物NO2的转化率从62.5%下降到了15.8%. Zhang等[79]设计合成的Mo掺杂C3N4与g-C3N4异质结证明了Mo原子不仅增加了催化剂的可见光响应,加速载流子的分离;Mo原子还为分子氧活化提供电子,促进催化剂产生活性氧物种氧化去除NO. 相关实验证明B元素和C元素掺杂都可提升g-C3N4催化去除NO的活性[80-81]. 此外,N元素掺杂的(BiO)2CO3[82-84]、Ce掺杂的SnO2[61]和La掺杂的ZnO[85]也被证实能通过元素掺杂增加光吸收和促进载流子分离两个途径提升催化剂光催化去除NO的效率. 诚然,元素掺杂被广泛应用于提升催化剂的活性,但是元素掺杂对催化剂的物理结构和化学性质产生的影响仍不清楚,后期需要加强微观界面反应机理的研究.
-
表面修饰是改变催化剂表面电子传输的重要策略(表1),一般分为等离子表面修饰和其他金属(无机盐、无机酸)表面修饰[62-65, 86-93]. 相对而言,等离子表面修饰在提升催化剂性能方面更具有优势. 如果纳米尺寸的贵金属与催化剂的能级匹配,催化剂表面沉积的贵金属颗粒在光激发下产生表面等离子共振效应. 不仅可以拓宽催化剂的光吸收范围,还可以成为电子(空穴)陷阱,阻碍载流子的复合,增加电子和空穴的利用率. 并且纳米颗粒物通常易于吸附小分子,其产生的热电子或者热空穴可进一步加速催化反应的进行. 例如,等离子体催化剂Ag/TiO2[64]、Ag/TiO2-x[62]、Ag/g-C3N4[91]和Au/g-C3N4[92]都利用贵金属诱导产生的等离子效应提高NO去除性能. 除了贵金属,廉价Bi纳米颗粒也被广泛应用于改性半导体催化剂. 2015年,Dong等[93]通过在g-C3N4表面原位生长Bi纳米球制备了新型的等离子体NO光催化剂. Bi的等离子体共振效应显著提高了Bi/g-C3N4催化剂吸收太阳光的能力和载流子的分离效率. 在可见光激发下,g-C3N4的光生电子可以转移到了金属Bi上,有效地抑制了载流子的复合,增强了Bi/g-C3N4可见光光催化去除NO的性能. 该研究表明价格低廉的Bi可能替代贵金属成为新型等离子体金属,其在提高光催化效率方面具有巨大的潜力. 受该研究的启发,后续研究者开发了许多Bi金属诱导的等离子体效应催化剂[94-99],但是,目前等离子体效应增强NO催化去除的作用机制仍不清楚,还需借助先进的现代表征技术探究界面上热电子和光生电子的传输路径.
-
光催化反应的效率通常受限于载流子的复合. 当太阳光驱动催化剂产生电子-空穴时,只有部分的载流子能够快速分离并迁移至催化剂表面发生光化学反应,剩余的载流子在分离和迁移过程中发生了复合,失去了参与界面反应的能力. 针对载流子复合的问题,设计合成异质结光催化剂成为了延长载流子寿命的重要改性手段(表1)[66-68, 100-113],主要表现形式为Z型异质结和p-n型异质结. 它们都通过构建新的内建电场调控反应界面电荷传输,促进载流子在两个功能界面之间快速分离,有效抑制载流子的复合,进而提升催化剂的催化性能. 2021年,Geng等[72]构建了Z型α-Fe2O3/g-C3N4光催化剂. 在可见光照射下,α-Fe2O3表面的激发态电子会通过静电吸引转移到g-C3N4的价带上. 虽然α-Fe2O3的光生电子与g-C3N4的价带空穴发生了复合反应,但是这样有效抑制了单一组分催化剂的载流子复合,保留了α-Fe2O3的空穴和g-C3N4的光生电子. 因此,激发态的电子和空穴分别被O2和H2O捕获形成活性·O2−和·OH,最终实现高效NO氧化. 与单组分催化剂相比,Z型异质结体系具有更强的氧化还原能力和更有效的载流子分离和迁移的能力,是提升催化剂性能的有效策略. 对于不具备可见光光催化活性的催化剂来说,p-n异质结更适用于扩大其光吸收范围. Huang等[69]制备的Bi2O2CO3/ZnFe2O4光催化剂具有广泛的可见光吸收能力和高效的载流子分离性能. 相对于单一组分,Bi2O2CO3/ZnFe2O4复合催化剂光催化去除NO的性能更佳,产生的副产物NO2更少. 虽然n型半导体Bi2O2CO3不能响应可见光,但是p型半导体ZnFe2O4(约1.9 eV)作为光敏化剂,能通过构建p-n异质结内电场将激发电子传递给Bi2O2CO3,使其展现可见光光催化氧化去除NO的性能. 该研究为设计高性能p-n异质结催化剂提供了新的思路. 但是,由于异质结催化剂的结构复杂,异质结界面处的电荷传导机制仍不清楚. 内建电场的结构性导向大多是基于实验现象推测而得,后期需要进一步完善异质结界面处的电荷传输途径及其催化氧化NO反应机理的研究.
-
为了满足绿色化学和环境友好型催化的需求,选择性光催化去除NO十分必要,即催化剂能有效利用太阳能激发产生的电子-空穴催化氧化NO和O2形成稳定的硝酸盐,并有效抑制有毒产物NO2的产生. 一般概括而言,光催化消减NO形成硝酸盐主要涉及表界面分子氧活化、NO活化和表面硝酸盐迁移等关键化学过程. 因此,可通过改性策略调控催化剂表界面的关键化学过程,实现高效、高选择性光催化去除NO.
-
O2是大气中最常见的绿色氧化剂. 在太阳光照射下,吸附态O2可被光生电子还原为亚稳态活性氧物种,比如·O2−、O22-、H2O2和·OH等[56, 114-117]. 这些氧物种能够直接参与NO的催化氧化过程,对高效高选择性光催化净化NO至关重要. 微观上,分子氧活化过程与催化剂的物理化学性质息息相关. 因此,可以通过改性策略调控催化剂的能带结构和表面配位环境来增强分子氧活化效率和提升催化剂氧化去除NO的能力,如扩大催化剂的光吸收范围、促进载流子的快速传输和增强催化剂对O2的吸附等. 除了提高分子氧活化效率以外,调控分子氧的活化构型也是提升NO氧化反应选择性的重要手段.
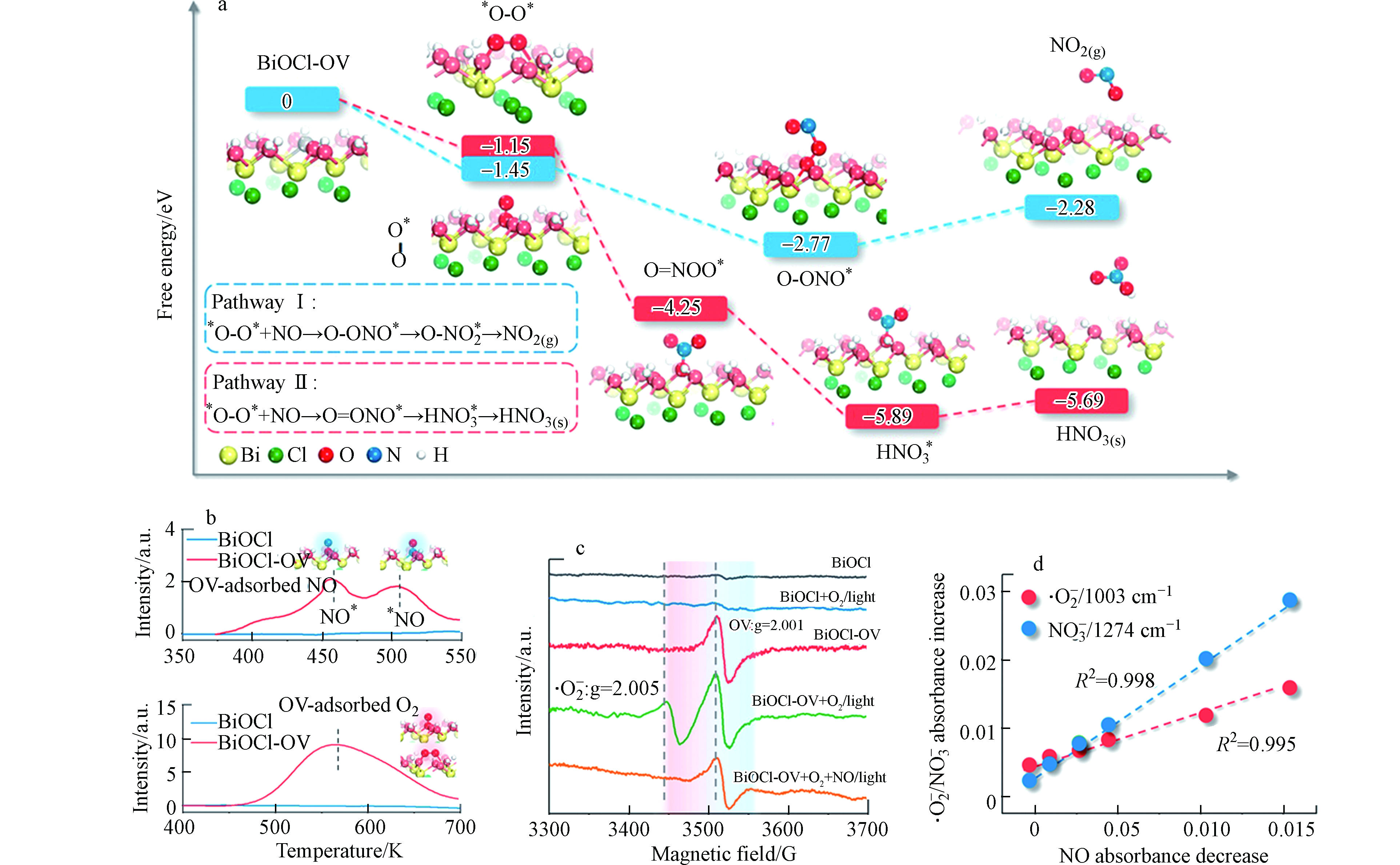
2018年,Li等[118]以缺陷态BiOCl为模型催化剂探究了分子氧活化过程对光催化NO反应的影响. 如图3所示,他们通过DFT模拟了缺陷态BiOCl(001)表面催化NO的表界面反应历程,发现氧空位是O2吸附的有利位点,其局域效应能够诱导O2活化生成结构特异性的·O2−. 相对于端位·O2−的氧化反应,桥连·O2−完全氧化NO生成硝酸盐的化学过程在热力学上更具有优势. 他们用溶剂热法合成了带有氧空位的BiOCl(001)催化剂,发现其在可见光下表现出了高效且高选择的NO消减性能,选择性超过99%. 原位TPD、ESR和原位红外光谱等一系列表征结果表明·O2−的生成量与NO氧化产物的累积量呈现正相关性,进一步佐证了·O2−对NO光催化消减的重要作用. 该工作提出了分子氧构型调控的新策略,为厘清半导体催化剂界面氧化去除NO机理提供了理论支撑.
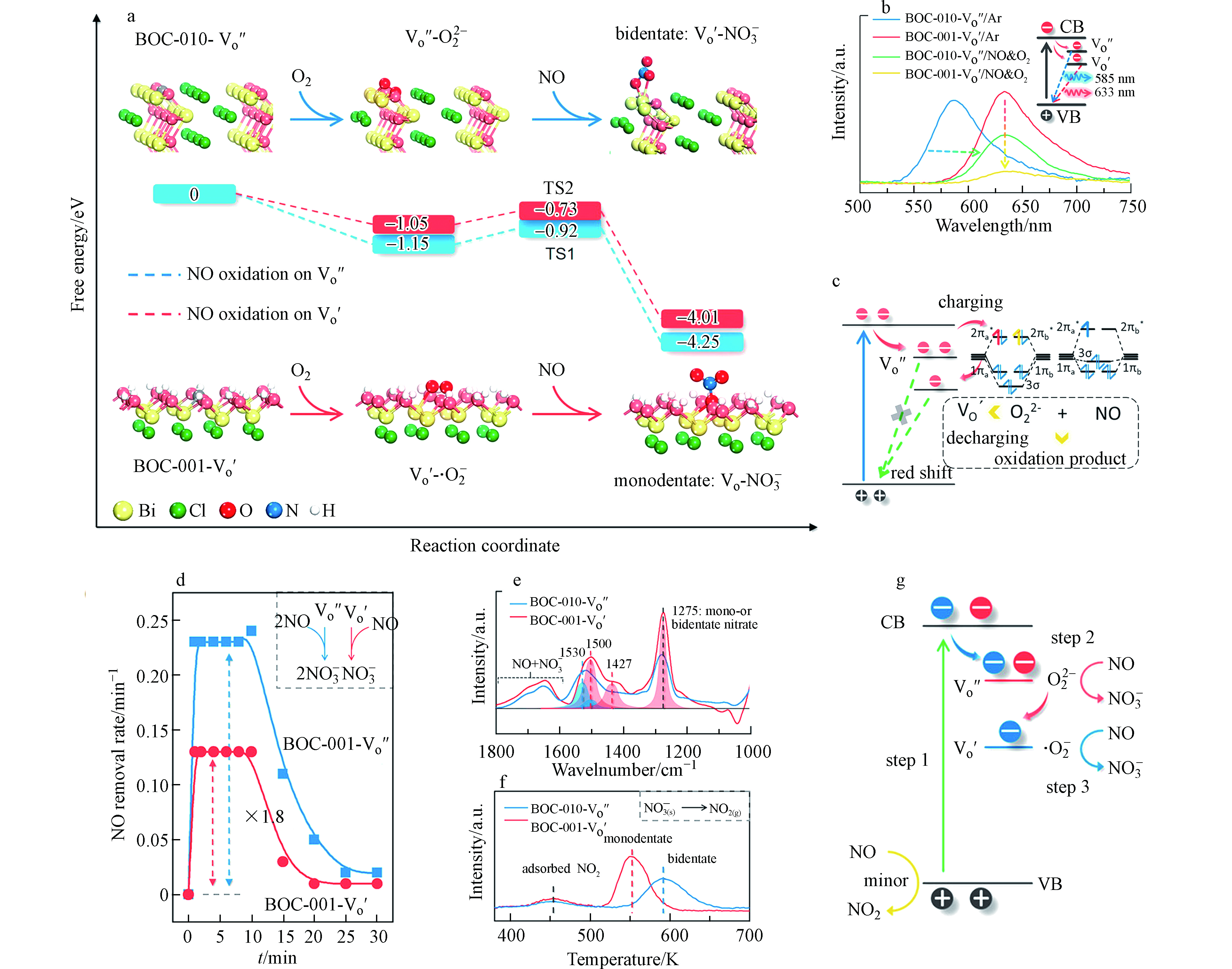
不同晶面暴露的缺陷态BiOCl具有不同结构的表面氧空位. 比如,BiOCl(001)的氧空位是单电子中心,所以其活化分子氧产生的活性物种是O2−;而BiOCl(010)的氧空位是双电子中心,对应的分子氧活化产物是O22-. 因此,BiOCl成为了研究不同种类活性氧物种氧化NO机理的天然模型催化剂. 在此基础上,Li等[53]利用不同晶面暴露的缺陷态BiOCl探究了O22-界面氧化NO的机理. 如图4所示,DFT计算表明缺陷态BiOCl(010)催化氧化NO形成硝酸盐的反应路径比(001)晶面更具有能量优势. 虽然缺陷态BiOCl(010)的氧空位处吸附了一分子硝酸盐,但是其仍保留了一个电子,该电子可进一步还原氧分子产生·O2−. 稳态荧光光谱表现出了两种特征峰(633 nm和585 nm),分别对应于单电子缺陷和双电子缺陷的荧光响应,这与DFT计算结果相吻合. 在可见光照射下,缺陷态BiOCl(010)氧化去除NO的最大速率是BiOCl(001)的1. 8倍,其中缺陷态BiOCl(010)表面氧化NO的产物为双齿硝酸盐,而缺陷态BiOCl(010)表面的氧化产物主要为单齿配位硝酸. 这说明(010)表面氧空位的电子传输途径更多,更加有利于氧气吸附和硝酸盐的形成. 因此,作者推测在O22-分级氧化NO过程中,缺陷态BiOCl(010)先经历单电子氧化NO途径产生硝酸根,剩余电子通过静电势吸引被氧空位回收后,含有单电子的氧空位可以进一步活化分子氧产生·O2−,继续氧化NO产生硝酸盐. 即一分子O22−可以氧化两分子NO,以此实现了NO高效氧化去除. 这项工作提出了多电子分级活化氧分子增强NO氧化机制,为发展高效NO净化催化剂和安全的净化组件提供了新思路. 除了典型的·O2−和O22−氧化剂以外,其他氧物种1O2、H2O2和·OH也在光催化氧化NO的反应中发挥了重要作用[119-120],但是这些活性物种主导的表界面氧化NO反应机制仍不清楚,需要进一步探究.
由上述活性氧物种主导的光催化氧化NO反应可知,表面缺陷是分子氧活化的重要活性位点. 特殊结构的氧空位能够选择性活化分子氧,这将显著提升光催化剂去除NO的选择性,避免二次产物NO2的产生. 除了活性氧物种氧化NO生成有害中间产物NO2,光生空穴氧化NO也是NO2产生的重要途径. Shang等[46]报道了缺陷态TiO2调控局域电子猝灭光生空穴增强NO氧化选择性的反应机制. DFT计算模拟了表面分子氧活化及其氧化NO的反应路径. TiO2表面氧空位能够定向活化分子氧产生桥连·O2−,随后桥连·O2−高选择氧化NO产生硝酸盐. 在光催化去除NO的过程中,缺陷态TiO2可选择性地氧化NO至硝酸盐,有效地避免了中间产物NO2的生成,反应的选择性高达99%. 荧光光谱监测了缺陷态TiO2内建电场载流子的迁移途径. 氧缺陷的局域电子不但可以诱导分子氧活化产生·O2−,还能与价带空穴复合,显著抑制了空穴氧化NO产生NO2的反应,最终实现了NO的高效高选择性去除. 后续Li等[121]利用多质子磷酸表面修饰策略在催化剂表面构建表面氢键,降低了氧空位的形成能,进一步拓展了表面缺陷工程,提升了NO氧化去除效率.
-
TiO2、铋系半导体和C3N4等光催化剂表面具有相对单一的反应位点,它们参与的表面光催化NO氧化反应通常经历Eley-Rideal(E-R)机制,即光催化去除NO是一种分子氧活化主导的反应. 一般而言,光催化剂的表面位点通常是电子聚集中心,所以表面反应位点倾向于将电子优先传递给电子轨道更加匹配的O2. 即在NO去除过程中,吸附在催化剂的表面缺陷或不饱和(金属、非金属)位点的O2将优先得到电子被还原成表面态的·O2−,随后·O2−捕获游离态NO反应生成硝酸盐. 这导致被电子活化的O2只能主动捕获空气中稀薄的NO污染物来完成光催化氧化NO反应,所以该途径主导的光催化去除低浓度NO效率普遍不高. 实际上,NO也可以被活化成高能状态,吸附态NO能够被光生空穴氧化形成NO+或与金属配位形成金属-NO中间体. 与未被活化的NO相比,NO+具有高能HOMO轨道,在热力学上更容易被活性氧物种氧化成硝酸盐. 并且,NO被吸附固定在催化剂表面还能够克服E-R机制对NO浓度的依赖性. 因此,双位点光催化剂能够同时吸附活化NO和O2,随后以Langmuir-Hinshelwood(L-H)机制氧化NO形成硝酸盐,更加有利于去除环境中低浓度的NO.
缺陷工程、表面修饰和构建异质结是建立双位点光催化去除NO体系的重要策略[122-124]. 例如,Liao等[122]利用氢气还原制备了氮缺陷态g-C3N4. 理论计算和原位红外光谱分析表明氮缺陷通过调控催化剂的禁带宽度加速光激发载流子的分离,使反应过程中产生更多的活性氧物种. 同时,氮缺陷位点还能氧化NO形成NO+,进一步促进NO氧化形成硝酸盐. 改性g-C3N4去除NO的效率提升了2.6倍. 如图5所示,Cui等[123]通过尿素和SrCO3热解反应制备了SrO表面修饰的非晶态氮化碳催化剂. 在光照条件下,该催化剂通过形成Sr-NOδ(+)中间产物显著促进了NO转化为硝酸盐. Wang等[124]通过构建SrCO3/BiOI核壳结构异质结增强了催化剂的可见光吸收和载流子的分离效率,进而增强了O2和NO的活化(·O2−、·OH和NO+),有效提升光催化去除NO的效率. O/Ba表面修饰的非晶态氮化碳[63]、氮掺杂的TiO2[125]、Bi-metal@Bi2GeO5[119]和(BiO)2CO3/g-C3N4[71]异质结等均证明双位点光催化活化O2和NO能提升催化剂光催化去除NO的性能,但是O2和NO在异质结材料界面反应机理研究尚未完善,仍需要进一步从分子层面探究O2和NO具体的反应位点.
2020年,Shang等[126]利用Au光激发产生的热空穴和CeO2表面天然的缺陷位点设计合成双位点Au/CeO2光催化剂,从微观层面上探究了O2和NO的吸附活化过程及硝酸盐形成机制. 如图6所示,DFT计算结果表明,Au/CeO2具有双活性中心,能有效地捕获和活化O2和NO,其中氧缺陷位点活化O2形成·O2−,Au位点的热空穴则预氧化NO形成NO+.
与单组分催化剂CeO2相比,预氧化形成的NO+更容易被·O2−氧化为硝酸盐,反应经历的L-H机制在热力学上比传统的E-R机制更具有能量优势. 去除NO的活性实验证明了DFT理论计算的猜想. 在可见光条件下,Au/CeO2氧化NO的速率是CeO2的3倍,去除效率也提升了15%. 经过5轮循环,Au/CeO2的活性依旧保持稳定,有毒中间产物NO2被抑制在较低水平. 单色光实验和荧光实验结果表明,Au产生的激发态电子倾向于向CeO2氧空位上迁移,空穴则会保留在Au表面,这为吸附氧化NO提供了有利条件. 并且,Au的局域电势促进了Au/CeO2载流子分离,延长了载流子寿命,有利于O2的活化. TPD测试进一步说明了Au/CeO2对O2和NO有较强的吸附能力,O2会优先吸附在氧空位处,NO吸附在Au颗粒上. 在NO催化反应中,红外光谱监测到了·O2−和产物硝酸盐的特征峰. 这些中间产物验证了DFT预测的双活性中心光催化NO氧化机理,证明了Au/CeO2表面通过E-R路径氧化NO产生硝酸盐. 该研究发展了双位点光催化去除NO的Au/CeO2催化剂,从分子层面阐明双活性中心光催化NO氧化机制,为开发高效NOx去除催化剂提供了新策略. 有趣的是,该研究选用氧化铈作为光催化去除NO的基底材料,为我国稀土资源的开发利用提供了新方向.
-
发展光催化剂的目的是净化大气环境或室内低浓度的NO污染气体. 在空气气氛下,光催化去除NO反应一般以氧化反应为主. NO会被活性氧物种氧化形成NO2、亚硝酸盐和硝酸盐等产物. 但是从环境效应和经济效应出发,NO净化系统不仅要避免有毒二次污染NO2的形成,还需要解决氧化产物硝酸盐占据光催化剂表面活性位点的问题,进而采取有效措施延长光催化剂的使用寿命.
2015年,Dong等[127]发现NO氧化产物硝酸盐沉积在BiOI催化剂表面会改变NO氧化去除机理. BiOI光催化氧化NO反应路径由非选择性氧化转变为选择性氧化,NO的氧化产物由硝酸盐转变为NO2. 捕获实验和红外实验表明由于硝酸盐占据了催化剂表面活性位点,抑制了分子氧活化产生·OH,所以,催化剂表面的空穴会直接氧化NO产生NO2. 遗憾的是,该研究没有考察硝酸盐对其他氧物种氧化NO的影响.
2019年,Shi等[70]继续探究催化剂表面硝酸盐对NO持续氧化的影响. 在反应初期,原位合成的缺陷态超薄BiOBr/BiOI催化剂表现出优异的光催化去除NO活性,其光催化氧化NO的产物为硝酸盐和NO2. 随着反应的进行,缺陷态超薄BiOBr/BiOI催化去除NO的效率保持稳定,但是它的副产物NO2产量不断增加. 这些现象表明硝酸盐的累积改变了NO氧化途径,该反应逐渐以选择性氧化形成NO2为主. 经过循环实验后的催化剂表面氧空位信号明显减弱,说明反应生成的硝酸盐占据了氧空位,改变了缺陷态超薄BiOBr/BiOI光催化NO的氧化路径. 但是,如果使用去离子水冲洗掉催化剂表面的硝酸盐,催化剂的催化性能恢复到反应初期,NO2的生成量也急剧下降. 所以作者推测硝酸盐是导致NO2浓度升高的主要原因. 通过进一步考察硝酸盐沉积对反应分子氧活化过程的影响,作者发现硝酸盐沉积削弱了分子氧活化效率,使得活性氧物种(·O2−和1O2)生产量显著减少,进而影响分子氧活化主导的NO氧化反应持续进行. 此外,表面沉积硝酸盐的催化剂主要起作用的活性物种由光生电子和·O2−转变为光生空穴和能量诱导产生的1O2. 以上实验结果表明,非选择性氧化产生的硝酸盐占据了超薄BiOBr/BiOI催化剂表面氧空位,抑制了表面分子氧活化反应,从而改变了光催化去除NO机理,即空穴选择性氧化NO形成NO2. 该研究阐明了NO氧化产物硝酸盐对光催化去除NO反应稳定性和选择性的影响,揭示了光催化去除NO催化剂的失活机制,为后期光催化技术的实际应用提供了新方向.
-
工业革命后,人类活动排放的NOx持续增加,严重威胁了生态环境. 如何高效治理大气环境中低浓度的NO污染成为亟待解决的环境科学问题. 光催化技术能有效氧化大气中低浓度NO产生硝酸盐,在深度净化大气NO污染领域具有广阔的应用前景. 在过去十几年里,针对NO光催化剂的效率、选择性等关键问题,科研工作者发展了许多新型高效光催化去除NO的催化剂,并利用DFT理论和原位红外等表征技术深入探究了光催化氧化去除NO的表界面反应机制,为定向开发高性能光催化消减NO催化剂奠定了坚实的基础. 然而光催化净化NO技术的实际应用依然面临很多挑战,亟待解决的问题如下:
(1)光催化消减NO催化剂的活性评判缺乏统一的标准,无法客观评价催化剂实际去除NO的性能.后期需要结合实际污染场景,建立科学的评价体系.
(2)需进一步考察大气中H2O、SO2、O3和VOCs(volatile organic compounds,挥发性有机物)等共存分子对光催化去除NO反应的影响,有针对性筛选出性能优异的消减NO光催化剂,拓展催化剂的适用性.
(3)目前报道的消减NO光催化剂涵盖了钛基材料、铋系层状材料和C3N4等.在实验室研究中,这些催化剂经过复杂的改性工艺后均能表现出优异的光催化去除NO性能. 但现阶段的光催化净化NO技术离实际应用还有很大距离,后期需要进一步优化催化剂的合成工艺和负载工艺,系统评估消减NO光催化剂的经济效益和环境效应,为高效光催化去除NO净化系统的大规模应用提供技术支撑.
光催化去除NO的研究进展
Research progress on photocatalytic NO removal
-
摘要: 光催化去除NO技术能利用太阳能转化大气环境中低浓度的NO为硝酸盐,在大气污染控制领域有广阔的应用前景. 本文综述了光催化去除NO的表界面反应研究进展,在介绍改性策略和关键化学过程的基础上,深刻讨论了影响光催化去除NO效率和选择性的关键因素. 最后,展望了光催化净化NO技术的发展和应用前景,为设计高效净化NO的光催化剂及净化组件提供理论依据和实践参考.Abstract: Photocatalytic removal of NO can convert low concentrations of NO pollutants to nitrate by utilizing solar energy in atmospheric environment, it has a broad application prospect in the field of air pollution control. In this paper, we specifically summarize the research progress in the surface-interface reaction of photocatalytic NO removal. Based on the introduction of the modification strategies and the key chemical processes, we highlight the key factors affecting the efficiency and selectivity of photocatalytic NO removal. Finally, we present the development and application prospect of the photocatalytic NO removal technology, as well as provide theoretical guidance and practical reference for the design of efficient NO photocatalysts and corresponding purification components.
-
Key words:
- photocatalysis /
- NO removal /
- environmental purification
-

-
图 4 (a)DFT模拟缺陷BiOCl(010)表面光催化氧化NO的化学过程,(b)稳态荧光光谱,(c)BiOCl(010)表面氧空位充放电电子传输示意图,(d)光催化去除NO性能图,(e)红外光谱分析,(f)NO2的TPD分析,(g)缺陷态BiOCl(010)光催化去除NO过程中电子传输过程[53]
Figure 4. (a)DFT calculation of photocatalytic NO oxidation on defective BiOCl(010) surface,(b)the steady-state fluorescence spectra,(c)schematic illustration of the charging-decharging scheme for BiOCl(010) surface with oxygen vacancies,(d)photocatalytic NO removal,(e)FTIR analysis,(f)NO2-TPD analysis,(g)schematic illustration of electronic excitation process for photocatalytic NO removal on defective BiOCl(010) surface[53]
图 6 (a)DFT计算模拟Au/CeO2和CeO2表面光催化NO氧化反应路径,(b)CeO2和Au/CeO2催化剂在可见光照射下光催化去除NO的性能,(c)对应的一级动力学常数,(d)O2和NO在Au/CeO2上吸附的示意图[126]
Figure 6. (a)DFT calculations for photocatalytic NO oxidation on the Au/CeO2 and CeO2,(b)photocatalytic NO removal performance of CeO2 and Au/CeO2 catalysts under the visible light irradiation,(c)the corresponding first-order kinetic constants,(d)illustration of O2 and NO adsorption on Au/CeO2[126]
表 1 NO光催化剂的改性策略
Table 1. Modification strategies of NO photocatalyst
年份Year 光催化剂Photocatalyst 改性策略Modified strategy 参考文献Reference 2017 TiO2(001) 晶面构建 [51] 2019 Ag3PO4@Al2O3 晶面构建 [52] 2019 BiOCl 晶面构建&表面缺陷 [53] 2018 Bi@BiOCl 表面缺陷&表面修饰 [54] 2018 Bi4MoO9@Bi metal core/shell heterostructure 表面缺陷&表面修饰 [55] 2018 Bi2O2CO3 表面缺陷 [44] 2018 SrFexTi1-xO3-δ 表面缺陷 [45] 2019 Bi@Bi2O2SiO3 表面缺陷&表面修饰 [56] 2019 BaSO4 (Ba vacancy) 表面缺陷 [47] 2019 g-C3N4 (N vacancy) 表面缺陷 [50] 2020 Bi@Bi2O2CO3 表面缺陷&表面修饰 [57] 2020 Bi2MoO6 表面缺陷 [58] 2016 Bi-doped BiMoO6 元素掺杂 [40] 2018 Sr-intercalated g-C3N4 元素掺杂 [59] 2018 Ca-intercalated g-C3N4 元素掺杂 [16] 2019 Sr multi-site doped C3N4 元素掺杂 [60] 2021 Ce-doped SnO2 元素掺杂 [61] 2020 group IIA element ion-doped g-C3N4 元素掺杂 [24] 2018 Bi/Bi2O3/Bi2WO6 表面修饰&异质结构建 [22] 2017 Ag@TiO2-x 表面修饰&表面缺陷 [62] 2017 monodisperse Bi@C3N4 表面修饰 [29] 2018 monodisperse Bi@graphene oxide 表面修饰&异质结构建 [17] 2018 O/Ba@C3N4 表面修饰 [63] 2018 Ag@TiO2 表面修饰 [64] 2019 Borate@polymer C3N4 表面修饰&表面缺陷 [65] 2021 single-atom Pd@g-C3N4 (C vacancy) 表面修饰&表面缺陷 [48] 2016 PdCl2/g-C3N4 异质结构建 [39] 2016 Bi2O2CO3/MoS2 异质结构建 [66] 2016 Bi2O2CO3/g-C3N4 异质结构建 [20] 2017 LaFeO3/SrTiO3 异质结构建 [67] 2017 Bi2O2CO3/MoS2/carbon nanofibers 异质结构建 [68] 2018 Bi2O2CO3/ZnFe2O4 异质结构建 [69] 2018 SrTiO3/SrCO3 异质结构建 [15] 2019 BiOBr/BiOI 异质结构建 [70] 2020 g-C3N4/(BiO)2CO3 异质结构建 [71] 2021 α-Fe2O3/g-C3N4 异质结构建 [72] -
[1] BRAUER M, AMANN M, BURNETT R T, et al. Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution [J]. Environmental Science & Technology, 2012, 46(2): 652-660. [2] BRAUER M, FREEDMAN G, FROSTAD J, et al. Ambient air pollution exposure estimation for the global burden of disease 2013 [J]. Environmental Science & Technology, 2016, 50(1): 79-88. [3] WEST J J, COHEN A, DENTENER F, et al. “What we breathe impacts our health: Improving understanding of the link between air pollution and health” [J]. Environmental Science & Technology, 2016, 50(10): 4895-4904. [4] KREUZER L B, PATEL C K N. Nitric oxide air pollution: Detection by optoacoustic spectroscopy [J]. Science, 1971, 173(3991): 45-47. doi: 10.1126/science.173.3991.45 [5] GÓMEZ ALVAREZ E, WORTHAM H, STREKOWSKI R, et al. Atmospheric photosensitized heterogeneous and multiphase reactions: From outdoors to indoors [J]. Environmental Science & Technology, 2012, 46(4): 1955-1963. [6] LIANG S, LIU Z, CRAWFORD-BROWN D, et al. Decoupling analysis and socioeconomic drivers of environmental pressure in China [J]. Environmental Science & Technology, 2014, 48(2): 1103-1113. [7] TIAN H Z, LIU K Y, HAO J M, et al. Nitrogen oxides emissions from thermal power plants in China: Current status and future predictions [J]. Environmental Science & Technology, 2013, 47(19): 11350-11357. [8] DAI W R, TAO Y, ZOU H H, et al. Gas-phase photoelectrocatalytic oxidation of NO via TiO2 nanorod array/FTO photoanodes [J]. Environmental Science & Technology, 2020, 54(9): 5902-5912. [9] XIAO S N, WAN Z, ZHOU J C, et al. Gas-phase photoelectrocatalysis for breaking down nitric oxide [J]. Environmental Science & Technology, 2019, 53(12): 7145-7154. [10] GRANGER P, PARVULESCU V I. Catalytic NOx abatement systems for mobile sources: From three-way to lean burn after-treatment technologies [J]. Chemical Reviews, 2011, 111(5): 3155-3207. doi: 10.1021/cr100168g [11] GUO Q B, SUN T H, WANG Y L, et al. Spray absorption and electrochemical reduction of nitrogen oxides from flue gas [J]. Environmental Science & Technology, 2013, 47(16): 9514-9522. [12] HEO I, KIM M K, SUNG S, et al. Combination of photocatalysis and HC/SCR for improved activity and durability of deNOx catalysts [J]. Environmental Science & Technology, 2013, 47(8): 3657-3664. [13] TONG H, OUYANG S X, BI Y P, et al. Nano-photocatalytic materials: Possibilities and challenges [J]. Advanced Materials, 2012, 24(2): 229-251. doi: 10.1002/adma.201102752 [14] CHEN X, CAI Y, LIANG R, et al. NH2-UiO-66(Zr) with fast electron transfer routes for breaking down nitric oxide via photocatalysis [J]. Applied Catalysis B:Environmental, 2020, 267: 118687. doi: 10.1016/j.apcatb.2020.118687 [15] JIN S, DONG G, LUO J, et al. Improved photocatalytic NO removal activity of SrTiO3 by using SrCO3 as a new co-catalyst [J]. Applied Catalysis B:Environmental, 2018, 227: 24-34. doi: 10.1016/j.apcatb.2018.01.020 [16] LI J, DONG X A, SUN Y, et al. Tailoring the rate-determining step in photocatalysis via localized excess electrons for efficient and safe air cleaning [J]. Applied Catalysis B:Environmental, 2018, 239: 187-195. doi: 10.1016/j.apcatb.2018.08.019 [17] LI X, ZHANG W, CUI W, et al. Bismuth spheres assembled on graphene oxide: Directional charge transfer enhances plasmonic photocatalysis and in situ DRIFTS studies [J]. Applied Catalysis B:Environmental, 2018, 221: 482-489. doi: 10.1016/j.apcatb.2017.09.046 [18] LUO J, DONG G, ZHU Y, et al. Switching of semiconducting behavior from n-type to p-type induced high photocatalytic NO removal activity in g-C3N4 [J]. Applied Catalysis B:Environmental, 2017, 214: 46-56. doi: 10.1016/j.apcatb.2017.05.016 [19] SUGRA EZ R, BALBUENA J, CRUZ-YUSTA M, et al. Efficient behaviour of hematite towards the photocatalytic degradation of NO gases [J]. Applied Catalysis B:Environmental, 2015, 165: 529-536. doi: 10.1016/j.apcatb.2014.10.025 [20] WANG Z, HUANG Y, HO W, et al. Fabrication of Bi2O2CO3/g-C3N4 heterojunctions for efficiently photocatalytic NO in air removal: In-situ self-sacrificial synthesis, characterizations and mechanistic study [J]. Applied Catalysis B:Environmental, 2016, 199: 123-133. doi: 10.1016/j.apcatb.2016.06.027 [21] ZHANG R Y, RAN T, CAO Y H, et al. Surface hydrogen atoms promote oxygen activation for solar light-driven NO oxidization over monolithic α-Ni(OH)2/Ni foam [J]. Environmental Science & Technology, 2020, 54(24): 16221-16230. [22] HE W, SUN Y, JIANG G, et al. Activation of amorphous Bi2WO6 with synchronous Bi metal and Bi2O3 coupling: Photocatalysis mechanism and reaction pathway [J]. Applied Catalysis B:Environmental, 2018, 232: 340-347. doi: 10.1016/j.apcatb.2018.03.047 [23] HUANG Y, GAO Y, ZHANG Q, et al. Biocompatible FeOOH-carbon quantum dots nanocomposites for gaseous NOx removal under visible light: Improved charge separation and high selectivity [J]. Journal of Hazardous Materials, 2018, 354: 54-62. doi: 10.1016/j.jhazmat.2018.04.071 [24] ZHOU M, DONG G, MA J, et al. Photocatalytic removal of NO by intercalated carbon nitride: The effect of group IIA element ions [J]. Applied Catalysis B:Environmental, 2020, 273: 119007. doi: 10.1016/j.apcatb.2020.119007 [25] CHEN X, ZHANG H, ZHANG D, et al. Controllable synthesis of mesoporous multi-shelled ZnO microspheres as efficient photocatalysts for NO oxidation [J]. Applied Surface Science, 2018, 435: 468-475. doi: 10.1016/j.apsusc.2017.11.045 [26] LI Y, SUN Y, HO W, et al. Highly enhanced visible-light photocatalytic NOx purification and conversion pathway on self-structurally modified g-C3N4 nanosheets [J]. Science Bulletin, 2018, 63(10): 609-620. doi: 10.1016/j.scib.2018.04.009 [27] HUO W C, DONG X A, LI J Y, et al. Synthesis of Bi2WO6 with gradient oxygen vacancies for highly photocatalytic NO oxidation and mechanism study [J]. Chemical Engineering Journal, 2019, 361: 129-138. doi: 10.1016/j.cej.2018.12.071 [28] LI J, CHEN R, CEN W, et al. Quantifying the activation energies of ROS-induced NOx conversion: Suppressed toxic intermediates generation and clarified reaction mechanism [J]. Chemical Engineering Journal, 2019, 375: 122026. doi: 10.1016/j.cej.2019.122026 [29] JIANG G, LI X, LAN M, et al. Monodisperse bismuth nanoparticles decorated graphitic carbon nitride: Enhanced visible-light-response photocatalytic NO removal and reaction pathway [J]. Applied Catalysis B:Environmental, 2017, 205: 532-540. doi: 10.1016/j.apcatb.2017.01.009 [30] CHEN M, HUANG Y, YAO J, et al. Visible-light-driven N-(BiO)2CO3/graphene oxide composites with improved photocatalytic activity and selectivity for NOx removal [J]. Applied Surface Science, 2018, 430: 137-144. doi: 10.1016/j.apsusc.2017.06.056 [31] CHEN M, LI X, HUANG Y, et al. Synthesis and characterization of Bi-BiPO4 nanocomposites as plasmonic photocatalysts for oxidative NO removal [J]. Applied Surface Science, 2020, 513: 145775. doi: 10.1016/j.apsusc.2020.145775 [32] HUANG Y, GAO Y, ZHANG Q, et al. Hierarchical porous ZnWO4 microspheres synthesized by ultrasonic spray pyrolysis: Characterization, mechanistic and photocatalytic NO removal studies [J]. Applied Catalysis A:General, 2016, 515: 170-178. doi: 10.1016/j.apcata.2016.02.007 [33] JIANG G, CAO J, CHEN M, et al. Photocatalytic NO oxidation on n-doped TiO2/g-C3N4 heterojunction: Enhanced efficiency, mechanism and reaction pathway [J]. Applied Surface Science, 2018, 458: 77-85. doi: 10.1016/j.apsusc.2018.07.087 [34] LI X, SHI H, WANG T, et al. Photocatalytic removal of NO by z-scheme mineral based heterojunction intermediated by carbon quantum dots [J]. Applied Surface Science, 2018, 456: 835-844. doi: 10.1016/j.apsusc.2018.06.133 [35] CUI W, LI J, CEN W, et al. Steering the interlayer energy barrier and charge flow via bioriented transportation channels in g-C3N4: Enhanced photocatalysis and reaction mechanism [J]. Journal of Catalysis, 2017, 352: 351-360. doi: 10.1016/j.jcat.2017.05.017 [36] UNER D, BAYAR I, TABARI T. The influence of relative humidity on photocatalytic oxidation of nitric oxide (NO) over TiO2 [J]. Applied Surface Science, 2015, 354: 260-266. doi: 10.1016/j.apsusc.2015.07.045 [37] ZOU Y, XIE Y, YU S, et al. SnO2 quantum dots anchored on g-C3N4 for enhanced visible-light photocatalytic removal of NO and toxic NO2 inhibition [J]. Applied Surface Science, 2019, 496: 143630. doi: 10.1016/j.apsusc.2019.143630 [38] DONG F, WANG Z Y, LI Y H, et al. Immobilization of polymeric g-C3N4 on structured ceramic foam for efficient visible light photocatalytic air purification with real indoor illumination [J]. Environmental Science & Technology, 2014, 48(17): 10345-10353. [39] ZHANG Z, XU M, HO W, et al. Simultaneous excitation of PdCl2 hybrid mesoporous g-C3N4 molecular/solid-state photocatalysts for enhancing the visible-light-induced oxidative removal of nitrogen oxides [J]. Applied Catalysis B:Environmental, 2016, 184: 174-181. doi: 10.1016/j.apcatb.2015.11.034 [40] DING X, HO W, SHANG J, et al. Self doping promoted photocatalytic removal of NO under visible light with Bi2MoO6: Indispensable role of superoxide ions [J]. Applied Catalysis B:Environmental, 2016, 182: 316-325. doi: 10.1016/j.apcatb.2015.09.046 [41] SHEN X, DONG G, WANG L, et al. Enhancing photocatalytic activity of NO removal through an in situ control of oxygen vacancies in growth of TiO2 [J]. Advanced Materials Interfaces, 2019, 6(19): 1901032. doi: 10.1002/admi.201901032 [42] MA J Z, WU H M, LIU Y C, et al. Photocatalytic removal of NOx over visible light responsive oxygen-deficient TiO2 [J]. The Journal of Physical Chemistry C, 2014, 118(14): 7434-7441. doi: 10.1021/jp500116n [43] SHAO M, LIU J J, DING W J, et al. Oxygen vacancy engineering of self-doped SnO2−x nanocrystals for ultrasensitive NO2 detection [J]. Journal of Materials Chemistry C, 2020, 8(2): 487-494. doi: 10.1039/C9TC05705F [44] YU S, ZHANG Y, DONG F, et al. Readily achieving concentration-tunable oxygen vacancies in Bi2O2CO3: Triple-functional role for efficient visible-light photocatalytic redox performance [J]. Applied Catalysis B:Environmental, 2018, 226: 441-450. doi: 10.1016/j.apcatb.2017.12.074 [45] ZHANG Q, HUANG Y, PENG S, et al. Synthesis of SrFexTi1-xO3-δ nanocubes with tunable oxygen vacancies for selective and efficient photocatalytic NO oxidation [J]. Applied Catalysis B:Environmental, 2018, 239: 1-9. doi: 10.1016/j.apcatb.2018.07.076 [46] SHANG H, LI M Q, LI H, et al. Oxygen vacancies promoted the selective photocatalytic removal of NO with blue TiO2 via simultaneous molecular oxygen activation and photogenerated hole annihilation [J]. Environmental Science & Technology, 2019, 53(11): 6444-6453. [47] CUI W, CHEN L, LI J, et al. Ba-vacancy induces semiconductor-like photocatalysis on insulator BaSO4 [J]. Applied Catalysis B:Environmental, 2019, 253: 293-299. doi: 10.1016/j.apcatb.2019.04.070 [48] LIU G, HUANG Y, LV H, et al. Confining single-atom Pd on g-C3N4 with carbon vacancies towards enhanced photocatalytic NO conversion [J]. Applied Catalysis B:Environmental, 2021, 284: 119683. doi: 10.1016/j.apcatb.2020.119683 [49] LI Y, HO W, LV K, et al. Carbon vacancy-induced enhancement of the visible light-driven photocatalytic oxidation of NO over g-C3N4 nanosheets [J]. Applied Surface Science, 2018, 430: 380-389. doi: 10.1016/j.apsusc.2017.06.054 [50] DONG G, ZHAO L, WU X, et al. Photocatalysis removing of NO based on modified carbon nitride: The effect of celestite mineral particles [J]. Applied Catalysis B:Environmental, 2019, 245: 459-468. doi: 10.1016/j.apcatb.2019.01.013 [51] YU J C C, NGUYEN V H, LASEK J, et al. Titania nanosheet photocatalysts with dominantly exposed (001) reactive facets for photocatalytic NOx abatement [J]. Applied Catalysis B:Environmental, 2017, 219: 391-400. doi: 10.1016/j.apcatb.2017.07.077 [52] XIA D, HU L, WANG Y, et al. Immobilization of facet-engineered Ag3PO4 on mesoporous Al2O3 for efficient industrial waste gas purification with indoor LED illumination [J]. Applied Catalysis B:Environmental, 2019, 256: 117811. doi: 10.1016/j.apcatb.2019.117811 [53] LI H, SHANG H, LI Y H, et al. Interfacial charging-decharging strategy for efficient and selective aerobic NO oxidation on oxygen vacancy [J]. Environmental Science & Technology, 2019, 53(12): 6964-6971. [54] WANG H, ZHANG W, LI X, et al. Highly enhanced visible light photocatalysis and in situ FT-IR studies on Bi metal@defective BiOCl hierarchical microspheres [J]. Applied Catalysis B:Environmental, 2018, 225: 218-227. doi: 10.1016/j.apcatb.2017.11.079 [55] HE W, SUN Y, JIANG G, et al. Defective Bi4MoO9/Bi metal core/shell heterostructure: Enhanced visible light photocatalysis and reaction mechanism [J]. Applied Catalysis B:Environmental, 2018, 239: 619-627. doi: 10.1016/j.apcatb.2018.08.064 [56] LI X, ZHANG W, LI J, et al. Transformation pathway and toxic intermediates inhibition of photocatalytic NO removal on designed Bi metal@defective Bi2O2SiO3 [J]. Applied Catalysis B:Environmental, 2019, 241: 187-195. doi: 10.1016/j.apcatb.2018.09.032 [57] CHEN P, LIU H, SUN Y, et al. Bi metal prevents the deactivation of oxygen vacancies in Bi2O2CO3 for stable and efficient photocatalytic NO abatement [J]. Applied Catalysis B:Environmental, 2020, 264: 118545. doi: 10.1016/j.apcatb.2019.118545 [58] WANG S, DING X, YANG N, et al. Insight into the effect of bromine on facet-dependent surface oxygen vacancies construction and stabilization of Bi2MoO6 for efficient photocatalytic NO removal [J]. Applied Catalysis B:Environmental, 2020, 265: 118585. doi: 10.1016/j.apcatb.2019.118585 [59] DONG X A, LI J, XING Q, et al. The activation of reactants and intermediates promotes the selective photocatalytic NO conversion on electron-localized Sr-intercalated g-C3N4 [J]. Applied Catalysis B:Environmental, 2018, 232: 69-76. doi: 10.1016/j.apcatb.2018.03.054 [60] ZHOU M, DONG G, YU F, et al. The deep oxidation of NO was realized by Sr multi-site doped g-C3N4 via photocatalytic method [J]. Applied Catalysis B:Environmental, 2019, 256: 117825. doi: 10.1016/j.apcatb.2019.117825 [61] SONG X, QIN G, CHENG G, et al. Oxygen defect-induced NO− intermediates promoting NO deep oxidation over Ce doped SnO2 under visible light [J]. Applied Catalysis B:Environmental, 2021, 284: 119761. doi: 10.1016/j.apcatb.2020.119761 [62] DUAN Y, ZHANG M, WANG L, et al. Plasmonic Ag-TiO2−x nanocomposites for the photocatalytic removal of NO under visible light with high selectivity: The role of oxygen vacancies [J]. Applied Catalysis B:Environmental, 2017, 204: 67-77. doi: 10.1016/j.apcatb.2016.11.023 [63] CUI W, LI J, SUN Y, et al. Enhancing ROS generation and suppressing toxic intermediate production in photocatalytic NO oxidation on O/Ba co-functionalized amorphous carbon nitride [J]. Applied Catalysis B:Environmental, 2018, 237: 938-946. doi: 10.1016/j.apcatb.2018.06.071 [64] DUAN Y, LUO J, ZHOU S, et al. TiO2-supported ag nanoclusters with enhanced visible light activity for the photocatalytic removal of NO [J]. Applied Catalysis B:Environmental, 2018, 234: 206-212. doi: 10.1016/j.apcatb.2018.04.041 [65] CAO J, ZHANG J, DONG X A, et al. Defective Borate-decorated polymer carbon nitride: Enhanced photocatalytic NO removal, synergy effect and reaction pathway [J]. Applied Catalysis B:Environmental, 2019, 249: 266-274. doi: 10.1016/j.apcatb.2019.03.012 [66] XIONG T, WEN M, DONG F, et al. Three dimensional z-scheme (BiO)2CO3/MoS2 with enhanced visible light photocatalytic NO removal [J]. Applied Catalysis B:Environmental, 2016, 199: 87-95. doi: 10.1016/j.apcatb.2016.06.032 [67] ZHANG Q, HUANG Y, PENG S, et al. Perovskite LaFeO3-SrTiO3 composite for synergistically enhanced NO removal under visible light excitation [J]. Applied Catalysis B:Environmental, 2017, 204: 346-357. doi: 10.1016/j.apcatb.2016.11.052 [68] HU J, CHEN D, LI N, et al. In situ fabrication of Bi2O2CO3/MoS2 on carbon nanofibers for efficient photocatalytic removal of NO under visible-light irradiation [J]. Applied Catalysis B:Environmental, 2017, 217: 224-231. doi: 10.1016/j.apcatb.2017.05.088 [69] HUANG Y, ZHU D, ZHANG Q, et al. Synthesis of a Bi2O2CO3/ZnFe2O4 heterojunction with enhanced photocatalytic activity for visible light irradiation-induced NO removal [J]. Applied Catalysis B:Environmental, 2018, 234: 70-78. doi: 10.1016/j.apcatb.2018.04.039 [70] SHI X, WANG P, LI W, et al. Change in photocatalytic NO removal mechanisms of ultrathin BiOBr/BiOI via NO3– adsorption [J]. Applied Catalysis B:Environmental, 2019, 243: 322-329. doi: 10.1016/j.apcatb.2018.10.037 [71] CUI W, CHEN L, SHENG J, et al. The pivotal roles of spatially separated charge localization centers on the molecules activation and photocatalysis mechanism [J]. Applied Catalysis B:Environmental, 2020, 262: 118251. doi: 10.1016/j.apcatb.2019.118251 [72] GENG Y, CHEN D, LI N, et al. Z-scheme 2D/2D α-Fe2O3/g-C3N4 heterojunction for photocatalytic oxidation of nitric oxide [J]. Applied Catalysis B:Environmental, 2021, 280: 119409. doi: 10.1016/j.apcatb.2020.119409 [73] DONG F, XIONG T, YAN S, et al. Facets and defects cooperatively promote visible light plasmonic photocatalysis with Bi nanowires@BiOCl nanosheets [J]. Journal of Catalysis, 2016, 344: 401-410. doi: 10.1016/j.jcat.2016.10.005 [74] CHEN P, SUN Y J, LIU H J, et al. Facet-dependent photocatalytic NO conversion pathways predetermined by adsorption activation patterns [J]. Nanoscale, 2019, 11(5): 2366-2373. doi: 10.1039/C8NR09147A [75] LIAO J, CHEN L, SUN M, et al. Improving visible-light-driven photocatalytic NO oxidation over BiOBr nanoplates through tunable oxygen vacancies [J]. Chinese Journal of Catalysis, 2018, 39(4): 779-789. doi: 10.1016/S1872-2067(18)63056-6 [76] RAMANA E V, PRASAD N V, TOBALDI D M, et al. Effect of samarium and vanadium Co-doping on structure, ferroelectric and photocatalytic properties of bismuth titanate [J]. RSC Advances, 2017, 7(16): 9680-9692. doi: 10.1039/C7RA00021A [77] YUAN C W, CHEN R M, WANG J D, et al. La-doping induced localized excess electrons on (BiO)2CO3 for efficient photocatalytic NO removal and toxic intermediates suppression [J]. Journal of hazardous materials, 2020, 400: 123174. doi: 10.1016/j.jhazmat.2020.123174 [78] CHANG L B, ZHU G Q, HASSAN Q U, et al. Synergetic effects of Pd(0) metal nanoparticles and Pd(2+) ions on enhanced photocatalytic activity of ZnWO4 nanorods for nitric oxide removal [J]. Langmuir:the ACS journal of surfaces and colloids, 2019, 35(35): 11265-11274. doi: 10.1021/acs.langmuir.9b01323 [79] ZHANG R, ZHANG A, CAO Y, et al. Mo-doped carbon nitride homojunction to promote oxygen activation for enhanced photocatalytic performance [J]. Chemical Engineering Journal, 2020, 401: 126028. doi: 10.1016/j.cej.2020.126028 [80] LI J R, RAN M X, CHEN P, et al. Controlling the secondary pollutant on B-doped g-C3N4 during photocatalytic NO removal: A combined DRIFTS and DFT investigation [J]. Catalysis Science & Technology, 2019, 9(17): 4531-4537. [81] RAN M, LI J, CUI W, et al. Efficient and stable photocatalytic NO removal on C self-doped g-C3N4: Electronic structure and reaction mechanism [J]. Catalysis Science & Technology, 2018, 8(13): 3387-3394. [82] JIN R, JIANG X, ZHOU Y, et al. Microspheres of graphene oxide coupled to n-doped Bi2O2CO3 for visible light photocatalysis [J]. Chinese Journal of Catalysis, 2016, 37(5): 760-768. doi: 10.1016/S1872-2067(15)61079-8 [83] ZHOU Y, ZHAO Z, WANG F, et al. Facile synthesis of surface n-doped Bi2O2CO3: Origin of visible light photocatalytic activity and in situ DRIFTS studies [J]. Journal of Hazardous Materials, 2016, 307: 163-172. doi: 10.1016/j.jhazmat.2015.12.072 [84] DONG X A, ZHANG W, CUI W, et al. Pt quantum dots deposited on n-doped (BiO)2CO3: Enhanced visible light photocatalytic NO removal and reaction pathway [J]. Catalysis Science & Technology, 2017, 7(6): 1324-1332. [85] YUAN C, CUI W, SUN Y, et al. Inhibition of the toxic byproduct during photocatalytic NO oxidation via La doping in ZnO [J]. Chinese Chemical Letters, 2020, 31(3): 751-754. doi: 10.1016/j.cclet.2019.09.033 [86] HUO W, XU W, CAO T, et al. Carbonate-intercalated defective bismuth tungstate for efficiently photocatalytic NO removal and promotion mechanism study [J]. Applied Catalysis B:Environmental, 2019, 254: 206-213. doi: 10.1016/j.apcatb.2019.04.099 [87] YI J, LIAO J, XIA K, et al. Integrating the merits of two-dimensional structure and heteroatom modification into semiconductor photocatalyst to boost NO removal [J]. Chemical Engineering Journal, 2019, 370: 944-951. doi: 10.1016/j.cej.2019.03.182 [88] DONG G H, YANG L P, WANG F, et al. Removal of nitric oxide through visible light photocatalysis by g-C3N4 modified with perylene imides [J]. ACS Catalysis, 2016, 6(10): 6511-6519. doi: 10.1021/acscatal.6b01657 [89] FENG X, ZHANG W, DENG H, et al. Efficient visible light photocatalytic NOx removal with cationic Ag clusters-grafted (BiO)2CO3 hierarchical superstructures [J]. Journal of Hazardous Materials, 2017, 322(PtA): 223-232. [90] HUO W, XU W, CAO T, et al. Carbonate doped Bi2MoO6 hierarchical nanostructure with enhanced transformation of active radicals for efficient photocatalytic removal of NO [J]. Journal of Colloid and Interface Science, 2019, 557: 816-824. doi: 10.1016/j.jcis.2019.09.089 [91] SUN Y, XIONG T, NI Z, et al. Improving g-C3N4 photocatalysis for NOx removal by Ag nanoparticles decoration [J]. Applied Surface Science, 2015, 358: 356-362. doi: 10.1016/j.apsusc.2015.07.071 [92] LI K, CUI W, LI J, et al. Tuning the reaction pathway of photocatalytic NO oxidation process to control the secondary pollution on monodisperse Au nanoparticles@g-C3N4 [J]. Chemical Engineering Journal, 2019, 378: 122184. doi: 10.1016/j.cej.2019.122184 [93] DONG F, ZHAO Z W, SUN Y J, et al. An advanced semimetal–organic Bi spheres–g-C3N4 nanohybrid with SPR-enhanced visible-light photocatalytic performance for NO purification [J]. Environmental Science & Technology, 2015, 49(20): 12432-12440. [94] LI X, SUN Y, XIONG T, et al. Activation of amorphous bismuth oxide via plasmonic Bi metal for efficient visible-light photocatalysis [J]. Journal of Catalysis, 2017, 352: 102-112. doi: 10.1016/j.jcat.2017.04.025 [95] LU Y, HUANG Y, ZHANG Y, et al. Effects of H2O2 generation over visible light-responsive Bi/Bi2O2CO3 nanosheets on their photocatalytic NO removal performance [J]. Chemical Engineering Journal, 2019, 363: 374-382. doi: 10.1016/j.cej.2019.01.172 [96] NI Z, ZHANG W, JIANG G, et al. Enhanced plasmonic photocatalysis by SiO2@Bi microspheres with hot-electron transportation channels via Bi–O–Si linkages [J]. Chinese Journal of Catalysis, 2017, 38(7): 1174-1183. doi: 10.1016/S1872-2067(17)62849-3 [97] SUN M, ZHANG W, SUN Y, et al. Synergistic integration of metallic Bi and defects on BiOI: Enhanced photocatalytic NO removal and conversion pathway [J]. Chinese Journal of Catalysis, 2019, 40(6): 826-836. doi: 10.1016/S1872-2067(18)63195-X [98] ZHANG L, YANG C, LV K, et al. SPR effect of bismuth enhanced visible photoreactivity of Bi2WO6 for NO abatement [J]. Chinese Journal of Catalysis, 2019, 40(5): 755-764. doi: 10.1016/S1872-2067(19)63320-6 [99] ZHU G, HOJAMBERDIEV M, ZHANG S, et al. Enhancing visible-light-induced photocatalytic activity of BiOI microspheres for NO removal by synchronous coupling with Bi metal and graphene [J]. Applied Surface Science, 2019, 467-468: 968-978. doi: 10.1016/j.apsusc.2018.10.246 [100] ZHAO Z, FAN J, LIU W, et al. In-situ hydrothermal synthesis of Ag3PO4/g-C3N4 composite and their photocatalytic decomposition of NOx [J]. Journal of Alloys and Compounds, 2017, 695: 2812-2819. doi: 10.1016/j.jallcom.2016.12.001 [101] ZHANG W, DONG X A, LIANG Y, et al. Ag/AgCl nanoparticles assembled on BiOCl/Bi12O17Cl2 nanosheets: Enhanced plasmonic visible light photocatalysis and in situ DRIFTS investigation [J]. Applied Surface Science, 2018, 455: 236-243. doi: 10.1016/j.apsusc.2018.05.171 [102] ZHANG W, DONG X A, JIA B, et al. 2D BiOCl/Bi12O17Cl2 nanojunction: Enhanced visible light photocatalytic NO removal and in situ DRIFTS investigation [J]. Applied Surface Science, 2018, 430: 571-577. doi: 10.1016/j.apsusc.2017.06.186 [103] ZHANG G P, ZHU X W, CHEN D Y, et al. Hierarchical z-scheme g-C3N4/Au/ZnIn2S4 photocatalyst for highly enhanced visible-light photocatalytic nitric oxide removal and carbon dioxide conversion [J]. Environmental Science:Nano, 2020, 7(2): 676-687. doi: 10.1039/C9EN01325C [104] WANG C, FU M, CAO J, et al. BaWO4/g-C3N4 heterostructure with excellent bifunctional photocatalytic performance [J]. Chemical Engineering Journal, 2020, 385: 123833. doi: 10.1016/j.cej.2019.123833 [105] LU Y, HUANG Y, CAO J-J, et al. Constructing z-scheme SnO2/N-doped carbon quantum dots/ZnSn(OH)6 nanohybrids with high redox ability for NOx removal under Vis-NIR light [J]. Journal of Materials Chemistry A, 2019, 7(26): 15782-15793. doi: 10.1039/C9TA03504D [106] LI Y, WU X, HO W, et al. Graphene-induced formation of visible-light-responsive SnO2-Zn2SnO4 z-scheme photocatalyst with surface vacancy for the enhanced photoreactivity towards NO and acetone oxidation [J]. Chemical Engineering Journal, 2018, 336: 200-210. doi: 10.1016/j.cej.2017.11.045 [107] KOU M, DENG Y, ZHANG R, et al. Molecular oxygen activation enhancement by BiOBr0.5I0.5/BiOI utilizing the synergistic effect of solid solution and heterojunctions for photocatalytic NO removal [J]. Chinese Journal of Catalysis, 2020, 41(10): 1480-1487. doi: 10.1016/S1872-2067(20)63607-5 [108] HUO W, CAO T, XU W, et al. Facile construction of Bi2MO3O12@Bi2O2CO3 heterojunctions for enhanced photocatalytic efficiency toward NO removal and study of the conversion process [J]. Chinese Journal of Catalysis, 2020, 41(2): 268-275. doi: 10.1016/S1872-2067(19)63460-1 [109] GUO Z, HUO W, CAO T, et al. Controllable synthesis of a 3D ZnS@MoO3 heterojunction via a hydrothermal method towards efficient NO purification under visible light [J]. CrystEngComm, 2020, 22(2): 257-266. doi: 10.1039/C9CE01375J [110] CHEN R, WANG H, WU H, et al. SrTiO3/BiOI heterostructure: Interfacial charge separation, enhanced photocatalytic activity, and reaction mechanism [J]. Chinese Journal of Catalysis, 2020, 41(4): 710-718. doi: 10.1016/S1872-2067(19)63472-8 [111] BALBUENA J, CARRARO G, CRUZ M, et al. Advances in photocatalytic NOx abatement through the use of Fe2O3/TiO2 nanocomposites [J]. RSC Advances, 2016, 6(78): 74878-74885. doi: 10.1039/C6RA15958C [112] WANG Z, HUANG Y, CHEN L, et al. In situ g-C3N4 self-sacrificial synthesis of a g-C3N4/LaCO3OH heterostructure with strong interfacial charge transfer and separation for photocatalytic NO removal [J]. Journal of Materials Chemistry A, 2018, 6(3): 972-981. doi: 10.1039/C7TA09132J [113] MENDOZA J A, LEE D H, KANG J H. Photocatalytic removal of gaseous nitrogen oxides using WO3/TiO2 particles under visible light irradiation: Effect of surface modification [J]. Chemosphere, 2017, 182: 539-546. doi: 10.1016/j.chemosphere.2017.05.069 [114] LI J, YAN P, LI K, et al. Generation and transformation of ROS on g-C3N4 for efficient photocatalytic NO removal: A combined in situ DRIFTS and DFT investigation [J]. Chinese Journal of Catalysis, 2018, 39(10): 1695-1703. doi: 10.1016/S1872-2067(18)63097-9 [115] NIE H Y, OU M, ZHONG Q, et al. Efficient visible-light photocatalytic oxidation of gaseous NO with graphitic carbon nitride (g-C3N4) activated by the alkaline hydrothermal treatment and mechanism analysis [J]. Journal of Hazardous Materials, 2015, 300: 598-606. doi: 10.1016/j.jhazmat.2015.07.066 [116] ZHU W, XIAO S N, ZHANG D Q, et al. Highly efficient and stable Au/CeO2-TiO2 photocatalyst for nitric oxide abatement: Potential application in flue gas treatment [J]. Langmuir:the ACS Journal of Surfaces and Colloids, 2015, 31(39): 10822-10830. doi: 10.1021/acs.langmuir.5b02232 [117] WANG H, HE W, DONG X A, et al. In situ DRIFT investigation on the photocatalytic NO oxidation mechanism with thermally exfoliated porous g-C3N4 nanosheets [J]. RSC Advances, 2017, 7(31): 19280-19287. doi: 10.1039/C7RA00879A [118] LI H, SHANG H, CAO X M, et al. Oxygen vacancies mediated complete visible light NO oxidation via side-on bridging superoxide radicals [J]. Environmental Science & Technology, 2018, 52(15): 8659-8665. [119] LI X, ZHANG W, CUI W, et al. Reactant activation and photocatalysis mechanisms on Bi-metal@Bi2GeO5 with oxygen vacancies: A combined experimental and theoretical investigation [J]. Chemical Engineering Journal, 2019, 370: 1366-1375. doi: 10.1016/j.cej.2019.04.003 [120] LI Y, GU M, ZHANG M, et al. C3N4 with engineered three coordinated (N3C) nitrogen vacancy boosts the production of 1O2 for efficient and stable NO photo-oxidation [J]. Chemical Engineering Journal, 2020, 389: 124421. doi: 10.1016/j.cej.2020.124421 [121] LI H, CHEN S, SHANG H, et al. Surface hydrogen bond network spatially confined BiOCl oxygen vacancy for photocatalysis [J]. Science Bulletin, 2020, 65(22): 1916-1923. doi: 10.1016/j.scib.2020.06.013 [122] LIAO J, CUI W, LI J, et al. Nitrogen defect structure and NO+ intermediate promoted photocatalytic NO removal on H2 treated g-C3N4 [J]. Chemical Engineering Journal, 2020, 379: 122282. doi: 10.1016/j.cej.2019.122282 [123] CUI W, LI J Y, DONG F, et al. Highly efficient performance and conversion pathway of photocatalytic NO oxidation on SrO-clusters@amorphous carbon nitride [J]. Environmental Science & Technology, 2017, 51(18): 10682-10690. [124] WANG H, SUN Y J, JIANG G M, et al. Unraveling the mechanisms of visible light photocatalytic NO purification on earth-abundant insulator-based core–shell heterojunctions [J]. Environmental Science & Technology, 2018, 52(3): 1479-1487. [125] TAN X F, QIN G D, CHENG G, et al. Oxygen vacancies enhance photocatalytic removal of NO over an n-doped TiO2 catalyst [J]. Catalysis Science & Technology, 2020, 10(20): 6923-6934. [126] SHANG H, HUANG S, LI H, et al. Dual-site activation enhanced photocatalytic removal of NO with Au/CeO2 [J]. Chemical Engineering Journal, 2020, 386: 124047. doi: 10.1016/j.cej.2020.124047 [127] DONG G, HO W, ZHANG L. Photocatalytic NO removal on BiOI surface: The change from nonselective oxidation to selective oxidation [J]. Applied Catalysis B:Environmental, 2015, 168-169: 490-496. doi: 10.1016/j.apcatb.2015.01.014 -



 下载:
下载: