-
燃煤烟气脱硫工艺分为湿法脱硫、干法脱硫和半干法脱硫3种[1-3]。其中,湿法脱硫工艺应用最广泛,SO2脱除率达95%以上。然而,该工艺存在设备腐蚀严重、易造成石膏雨,并产生大量废水等问题[4-6]。干法脱硫为气固反应,其工艺流程及装置较为简单,且具有能耗低、二次污染少等优点,但其脱硫效果较差、脱硫剂利用率较低,且系统运行可靠性不高[7]。半干法脱硫工艺为气、液、固三相反应,利用烟气显热可将湿浆料中的水分或喷入的增湿水加热蒸发以实现烟气增湿,其产物呈干燥态[8-9]。半干法脱硫工艺结合了湿法脱硫工艺和干法脱硫工艺的优势,且SO2脱除率可达90%以上[10-11],故备受关注。
半干法脱硫工艺采用Ca(OH)2为脱硫剂。在干燥条件下,Ca(OH)2几乎不与SO2反应,而在有水或水蒸气存在的条件下,Ca(OH)2与SO2具有很高的反应活性[12-15]。因此,探究水及其他因素对半干法脱硫效果的影响,对于其工况的选取及工艺的改进具有重要意义。式(1)、式(2)为有水及水蒸气存在的条件下Ca(OH)2与SO2的主要脱硫反应方程式。
本研究利用固定床反应器,以Ca(OH)2作为脱硫剂,考察反应温度、脱硫剂颗粒粒径、反应空速、模拟烟气中水蒸气含量对半干法脱硫反应的影响;同时,以脉冲增湿方法改善半干法工艺的脱硫效果,以期为钙基半干法脱硫工艺的改进提供参考。
-
所使用的二氧化硫气体(SO2,浓度1%,氮平衡)、空气(Air,21%O2/N2)、氮气(N2,纯度99.999%)均购自北京环宇京辉京城气体科技有限公司;氢氧化钙(Ca(OH)2,AR,≥95.0%)购自天津百伦斯生物技术有限公司;无水氯化钙(CaCl2,AR,≥96.0%)购自国药集团化学试剂有限公司。
-
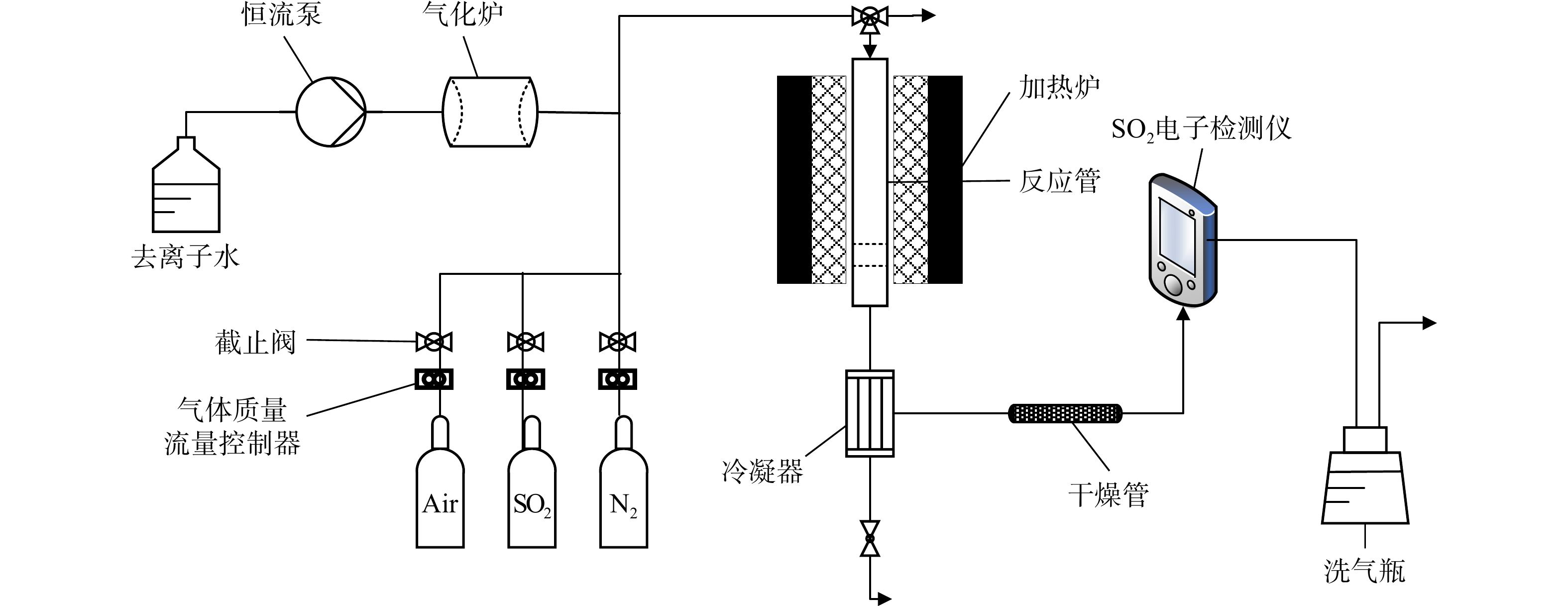
实验装置由配气系统、反应系统、出口气体检测系统、尾气处理系统4部分组成(见图1)。配气系统主要装置有恒流泵、气化炉、气瓶(SO2、N2、Air);反应系统主要有加热炉和管式固定床反应器;出口气体检测系统由冷凝器、干燥管、SO2检测仪组成;尾气处理系统为盛有NaOH水溶液的洗气瓶。
模拟烟气的SO2、N2及空气流量由质量流量控制器设定。SO2质量浓度为2 285.7 mg·Nm−3。去离子水经连接管路流入气化炉,其流量由恒流泵控制,在气化炉中产生水蒸气后再与反应气混合并通入固定床反应器中进行脱硫反应。
将200目的Ca(OH)2粉末加去离子水制浆后造粒,在120 ℃下干燥,再破碎、筛分,得到不同粒径的Ca(OH)2颗粒备用。固定床下部填充直径2 mm的刚玉球作为支撑,并在刚玉球上方装填Ca(OH)2颗粒。
反应管出口气体经过冷凝器、干燥管除水后,进入SO2检测仪进行分析,之后进入尾气处理系统经充分净化后外排至环境中。
-
采用SO2检测仪分析进出口SO2质量浓度。我国火电厂燃煤烟气中SO2的质量浓度排放标准为35 mg·Nm−3[16]。本研究将该质量浓度定义为SO2穿透浓度,将SO2出口质量浓度达到穿透浓度时的反应时间定义为穿透时间。
脱硫率(η)的计算方法式为式(3)。Ca(OH)2利用率(Ue)是评价脱硫效果及脱硫反应经济性的重要指标,其计算式为式(4)。Ca/S,即Ca(OH)2的使用量与SO2穿透时的SO2脱除量之比,其计算式为式(5)。
式中:Cin为反应气体中SO2进口质量浓度,mg·Nm−3;Cout为SO2出口质量浓度,mg·Nm−3;V为反应气体体积流量,mL·min−1;t1为脱硫反应的穿透时间,min;t2为脱硫反应的理论反应时间,min;
NCaOH2 为Ca(OH)2的使用量,mol;NSO2 为SO2穿透时的SO2脱除量,mol。 -
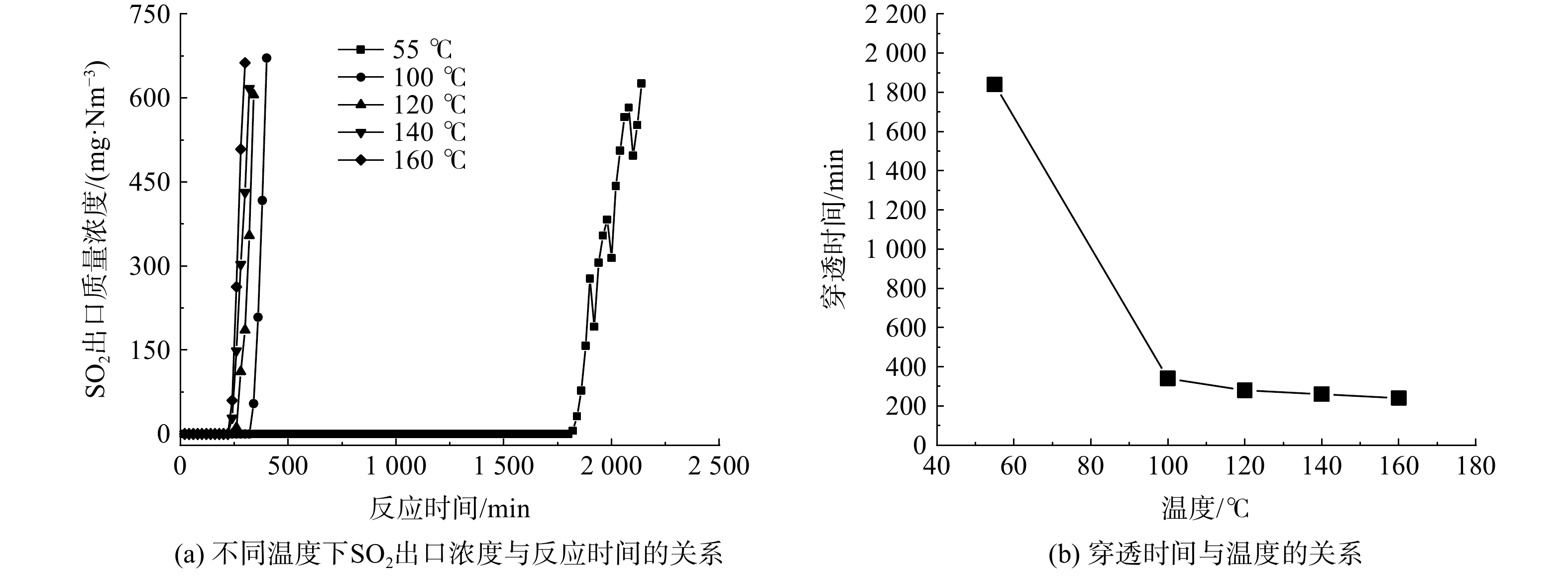
温度对Ca(OH)2与SO2的脱硫反应有一定影响。在55~160 ℃下,采用平均粒径0.58 mm的Ca(OH)2颗粒,在空速2 500 h−1、含硫气体中水蒸气体积分数5%的条件下进行脱硫实验,SO2出口质量浓度随反应时间的变化如图2(a)所示,穿透时间与反应温度的关系如图2(b)所示。穿透时间随温度的升高而降低,当温度由55 ℃逐渐升至160 ℃时,穿透时间由1 840 min降至240 min。此结果表明,在55~160 ℃下,Ca(OH)2的脱硫效果会随着反应温度的升高而下降,这与文献[17-18]的结论相吻合。
-
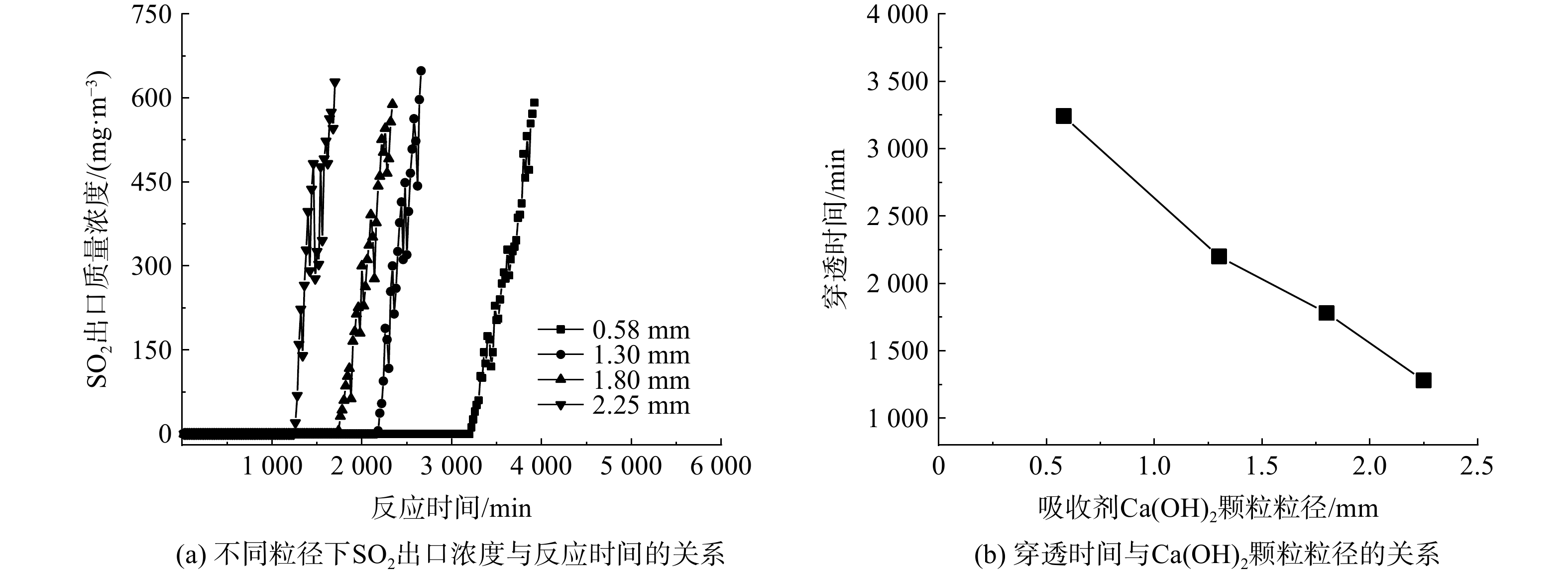
固定床反应器内填充的Ca(OH)2颗粒粒径越大,床层空隙率则越高。为避免空隙率过高影响实验结果,采用了40 mm直径的反应管。较大的管径比可有效减小壁面附近空隙增大时带来的壁面效应影响[19]。同时,Ca(OH)2颗粒粒径的增大还会造成SO2的内扩散阻力增大,不利于进行脱硫反应。图3反映了半干法脱硫在不同Ca(OH)2颗粒粒径条件下的实验结果,在55 ℃、2 500 h−1及水蒸气体积分数为12%的条件下,随着Ca(OH)2颗粒粒径的增大,脱硫反应的穿透时间不断缩短。
图4分别反映了Ca/S及Ca(OH)2利用率与Ca(OH)2颗粒粒径的变化关系。在Ca(OH)2颗粒的平均粒径为0.58 mm、反应温度为55 ℃、空速为2 500 h−1、水蒸气体积分数为12%的条件下,当出口SO2达到穿透浓度时,可获得本实验的最佳脱硫效果。根据式(4)和(5)计算出该条件下的Ca(OH)2利用率为71.3%、Ca/S为1.4。Ca/S随Ca(OH)2粒径的增加而不断增大,而Ca(OH)2利用率则不断降低。当Ca(OH)2颗粒的平均粒径由0.58 mm增至2.25 mm时,Ca/S由1.4增至3.6,Ca(OH)2的利用率则从71.3%降至27.8%。可见,Ca(OH)2颗粒的平均粒径增大不利于半干法脱硫效果的提升。这是由于:Ca(OH)2颗粒的粒径增大会造成颗粒的比表面积减小及SO2内扩散阻力的增加,导致颗粒内部大量Ca(OH)2未能与SO2发生反应,最终使得Ca(OH)2利用率降低,穿透时间随之缩短。
-
空速对脱硫效果的影响如图5所示。空速的提高使得脱硫反应的穿透时间缩短,在Ca(OH)2颗粒平均粒径为0.58 mm、水蒸气体积分数为12%、温度为55 ℃的条件下,当空速由2 500 h−1升至7 500 h−1时,穿透时间由3 240 min大幅降至260 min。
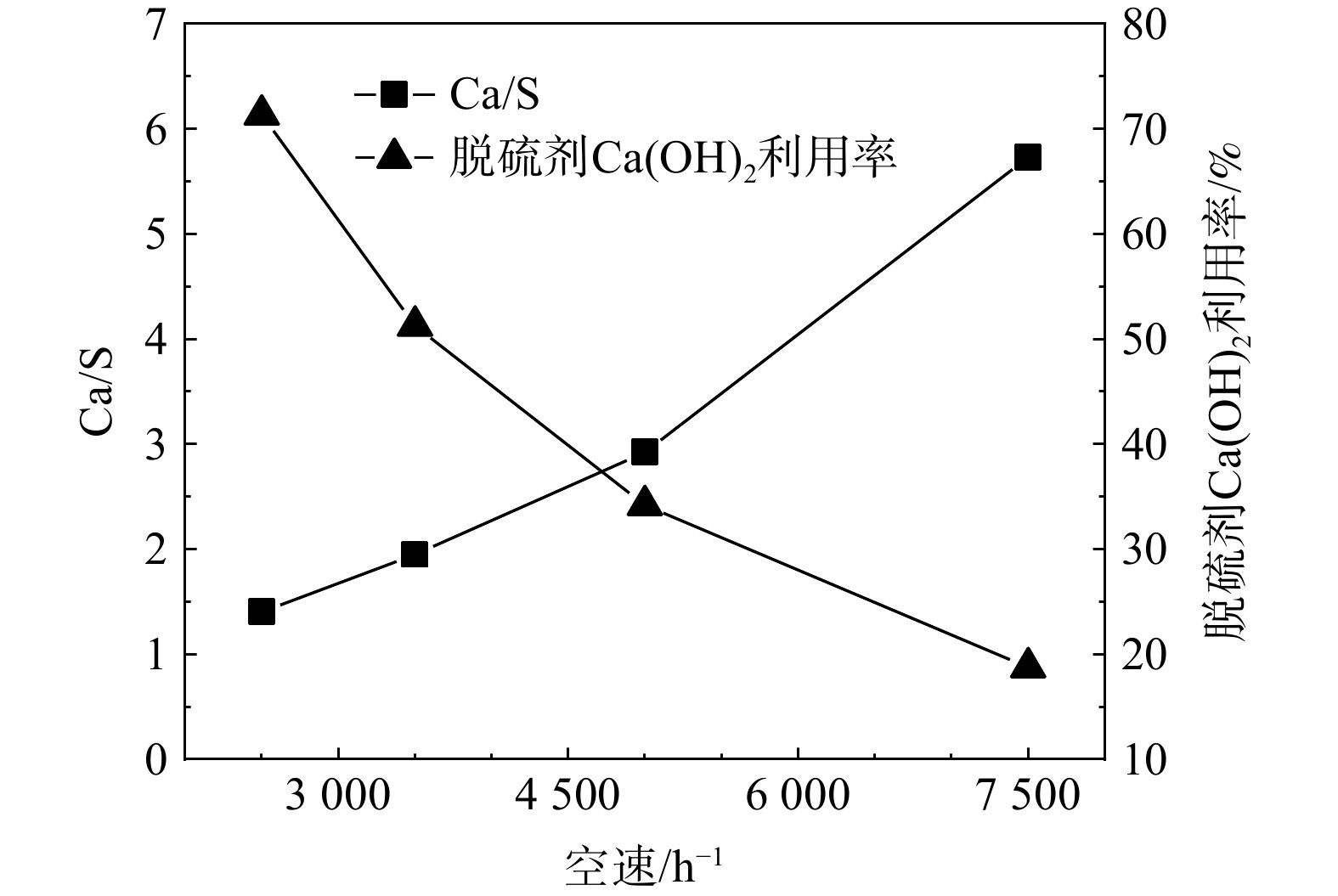
不同空速对Ca/S及脱硫剂Ca(OH)2利用率的影响如图6所示。随着反应空速的增大,Ca/S随之增大而Ca(OH)2利用率则随之降低。保持其他条件不变,当反应空速由2500 h−1增加到7500 h−1时,Ca/S由1.4提高为5.7,Ca(OH)2的利用率则由71.3%降低到18.8%。可见,空速的增大会导致脱硫效果急剧下降。在较高的空速下,烟气在床层的停留时间会缩短,脱硫反应速率则大幅提高。反应中会快速生成大量脱硫产物并覆盖至脱硫剂颗粒表面,所形成的致密产物层可阻止SO2与颗粒内部的Ca(OH)2继续反应,使得脱硫反应快速穿透。
-
在半干法脱硫工艺中,烟气中的水蒸气含量对脱硫效果影响显著[20-21]。图7反映了水蒸气体积分数对脱硫效果的影响规律。穿透时间随着反应气体中水蒸气含量的增加而增大,在Ca(OH)2颗粒平均粒径为0.58 mm、空速为2 500 h−1、温度为55 ℃的条件下,当水蒸气的体积分数由5%升至12%时,脱硫反应的穿透时间由1 840 min大幅增至3 240 min。
水蒸气体积分数对Ca/S及脱硫剂Ca(OH)2利用率的影响如图8所示。随着水蒸气含量的增加,Ca/S相应不断降低,Ca(OH)2利用率则不断提高。水蒸气的体积分数由5%增至12%时,Ca/S由2.5降至1.4,Ca(OH)2的利用率由40.9%增至71.3%。这表明水蒸气含量的增大有利于半干法脱硫反应的进行。实际上,烟气中水蒸气的体积分数越大,反应中Ca(OH)2颗粒的表面越容易润湿从而形成液膜,这使得Ca(OH)2可游离出部分OH−,进而与烟气中的SO2进行快速、充分的离子反应[22-23]。反之,当烟气中水蒸气含量太低时,水蒸气不足以使得Ca(OH)2颗粒表面润湿,SO2与Ca(OH)2的离子反应便难以进行。当烟气为不含水蒸气的极端干燥状态时,SO2与干燥的Ca(OH)2几乎不发生反应[24]。
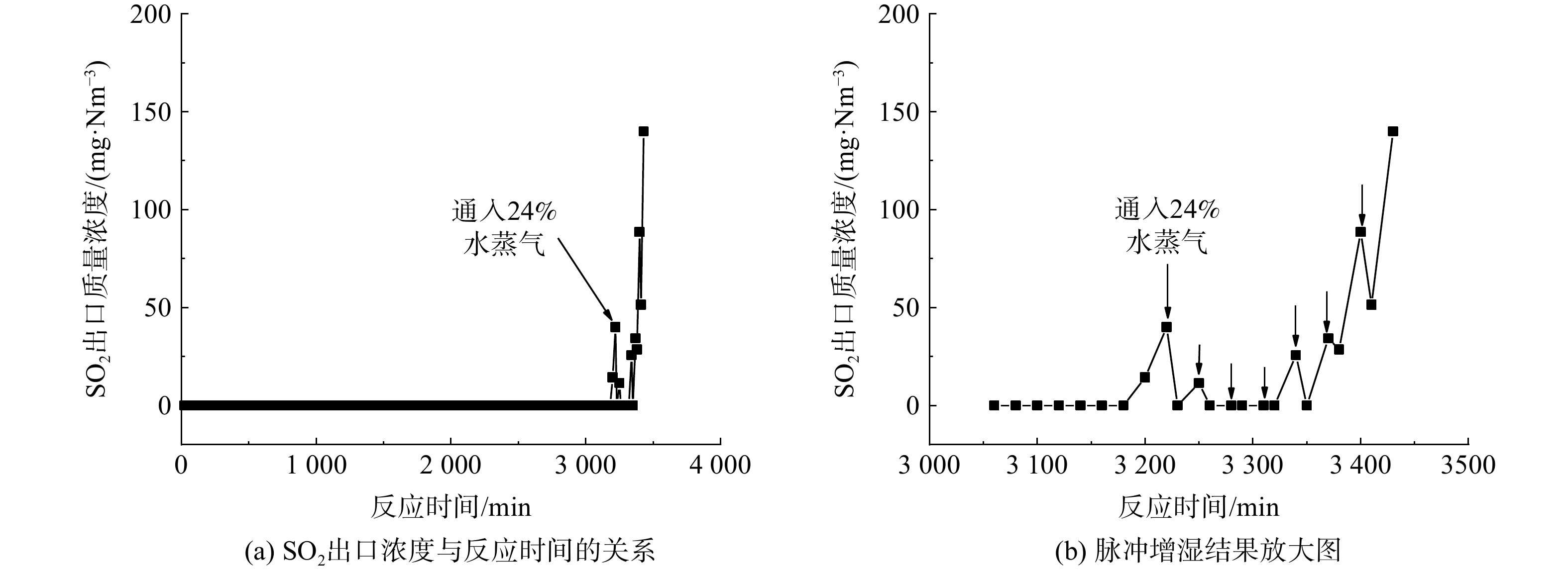
本研究提出了以脉冲式增湿方法对半干法脱硫工艺进行改进,故对水蒸气含量对半干法脱硫反应的影响进行了研究。当出口SO2质量浓度首次达到穿透浓度时,通过恒流泵调节水的流量,使反应气体中的水蒸气体积分数由12%增至24%并保持10 min。之后,将水蒸气体积分数调回原来的12%。20 min之后,再将反应气体中的水蒸气体积分数由12%增至24%并保持10 min。如此循环往复进行脉冲式的增湿,直至脱硫反应出口SO2质量浓度再次达到35 mg·Nm−3这一穿透浓度,结果如图9所示。图9(b)中的箭头为反应气体中的水蒸气体积分数由12%增至24%的时间节点。在反应3 220 min时,出口SO2质量浓度达到40 mg·Nm−3,即在水蒸气体积分数为12%条件下,脱硫反应首次穿透。而首次脉冲增湿后,出口SO2质量浓度降至0,接着水蒸气体积分数调回原先的12%并保持20 min。之后出口SO2质量浓度逐渐增至11 mg·Nm−3。这表明水蒸气含量的增加促进了脱硫,即使在反应穿透后,通过增加水蒸气含量仍可增加脱硫剂颗粒表面的润湿程度,使得SO2与颗粒内部未反应的Ca(OH)2得以继续反应。如此循环往复,直至第7次脉冲增湿时还有明显效果。在前6次脉冲增湿期间,出口SO2质量浓度一直保持低于穿透浓度。通过脉冲增湿,反应穿透时间延长了约160 min,Ca(OH)2利用率提高了4.4%,Ca/S由1.43降至1.36。
又研究了Ca(OH)2颗粒的平均粒径为2.25 mm时的脱硫反应当出口SO2浓度首次达到穿透浓度时脉冲增湿方法对脱硫效果的影响。实验结果如图10所示。结果表明,脉冲增湿使得该条件下的脱硫反应的穿透时间延长了约720 min,计算得到的Ca(OH)2利用率提高了15.6%,Ca/S由3.6降低到了2.3。2.25 mm的Ca(OH)2颗粒粒径条件下的脉冲增湿结果虽然仍与本实验最佳脱硫效果有较大差距,但脉冲增湿对脱硫效果的改善幅度较0.58 mm Ca(OH)2颗粒粒径的条件下有了较大提升。
分析脉冲式增湿方法对半干法脱硫效果的促进原因可发现,由于水蒸气体积分数为24%时,反应气体处于过饱和状态,在进入反应管后易凝结成为液态水并覆盖在Ca(OH)2颗粒表面形成液膜,使得脱硫剂表面及内部未反应的Ca(OH)2得以继续游离出部分OH−并与SO2发生反应,最终导致出口SO2质量浓度降低,穿透时间也相应延长。然而,长时间通入24%体积分数的水蒸气会发生Ca(OH)2颗粒团聚和床层板结,而以2:1的时长比循环通入水蒸气体积分数为12%和24%的脉冲增湿方法,则可保证在脱硫效果改善的同时有效避免上述不利情形发生。因此,采用脉冲增湿方法可优化半干法脱硫技术。
-
1)较低的反应温度、Ca(OH)2颗粒粒径的减小、反应空速的降低、烟气中水蒸气含量的提高均有利于半干法脱硫效果的提升,即有利于穿透时间的增加、脱硫剂利用率的提高及Ca/S的降低。当反应温度为55 ℃、Ca(OH)2颗粒平均粒径为0.58 mm、反应空速2 500 h−1、烟气中水蒸气体积分数12%时,可获得最佳脱硫效果。在SO2质量浓度为穿透浓度(35 mg·Nm−3)时,Ca(OH)2利用率达可到71.3%,Ca/S为1.4。
2)脉冲增湿方法可促进半干法脱硫工艺的脱硫效果。在上述最佳条件下,当反应器出口SO2达到穿透浓度时,通过实施脉冲增湿,即间歇提高反应气体中水蒸气体积分数可使穿透时间延长160 min,达到3 380 min,此时Ca(OH)2利用率提高了4.4%。采用脉冲增湿方法可优化半干法脱硫工艺。
钙基半干法燃煤烟气脱硫技术的影响因素
Influencing factors of coal-fired flue gas desulfurization by calcium-based semi-dry method
-
摘要: 半干法烟气脱硫技术因具有耗水量少、产物易处理等特点而备受关注。基于固定床反应器研究了反应温度、脱硫剂Ca(OH)2颗粒粒径、反应空速及烟气中水蒸气体积分数对Ca(OH)2脱硫性能的影响。结果表明,Ca(OH)2颗粒粒径的减小、反应空速的降低、烟气中水蒸气体积分数的提高及低反应温度等因素,有利于SO2的脱除、Ca(OH)2利用率的提高及Ca/S的降低。当Ca(OH)2颗粒的平均粒径为0.58 mm、反应空速为2 500 h−1、模拟烟气中水蒸气体积分数为12%、反应温度为55 ℃时,可获得最佳脱硫效果。在SO2穿透浓度35 mg·Nm−3下,Ca(OH)2的利用率可达到71.3%,此时Ca/S为1.4。在上述条件下,SO2达到穿透浓度时,通过实施脉冲增湿可使穿透时间延长、Ca(OH)2利用率进一步提升至75.7%。本研究结果可为半干法脱硫工艺的改进提供参考。
-
关键词:
- 烟气脱硫 /
- 固定床 /
- 半干法 /
- 脱硫剂Ca(OH)2 /
- 脉冲增湿
Abstract: Semi-dry flue gas desulfurization has attracted extensive attention because of its characteristics of low water consumption and easy treatment of products. The effects of reaction temperature, particle size of desulfurizer Ca(OH)2, reaction space velocity, and volume fraction of water vapor in flue gas on the desulfurization performance of Ca(OH)2 were researched in a fixed bed reactor. Results showed that the reduction of Ca(OH)2 particle size, the decrease of space velocity, the increase of water vapor volume fraction in flue gas and the low reaction temperature were conducive to the removal of SO2, Ca(OH)2 utilization and the reduction of Ca/S. The best desulfurization performance could be achieved when the average particle size of Ca(OH)2 was 0.58 mm, the space velocity was 2 500 h−1, the volume fraction of water vapor in the simulated flue gas was 12% and the reaction temperature was 55 ℃. When the SO2 penetration concentration reached 35 mg·Nm−3, the utilization rate of Ca(OH)2 was 71.3%, and the Ca/S was 1.4. Under the above conditions, when the content of SO2 reached penetration concentration, penetration time can be prolonged and Ca(OH)2 utilization rate can be further increased to 75.7% by implementing pulse humidification. The results of this study can provide reference for the improvement of semi-dry desulfurization process improvement. -
燃煤烟气脱硫工艺分为湿法脱硫、干法脱硫和半干法脱硫3种[1-3]。其中,湿法脱硫工艺应用最广泛,SO2脱除率达95%以上。然而,该工艺存在设备腐蚀严重、易造成石膏雨,并产生大量废水等问题[4-6]。干法脱硫为气固反应,其工艺流程及装置较为简单,且具有能耗低、二次污染少等优点,但其脱硫效果较差、脱硫剂利用率较低,且系统运行可靠性不高[7]。半干法脱硫工艺为气、液、固三相反应,利用烟气显热可将湿浆料中的水分或喷入的增湿水加热蒸发以实现烟气增湿,其产物呈干燥态[8-9]。半干法脱硫工艺结合了湿法脱硫工艺和干法脱硫工艺的优势,且SO2脱除率可达90%以上[10-11],故备受关注。
半干法脱硫工艺采用Ca(OH)2为脱硫剂。在干燥条件下,Ca(OH)2几乎不与SO2反应,而在有水或水蒸气存在的条件下,Ca(OH)2与SO2具有很高的反应活性[12-15]。因此,探究水及其他因素对半干法脱硫效果的影响,对于其工况的选取及工艺的改进具有重要意义。式(1)、式(2)为有水及水蒸气存在的条件下Ca(OH)2与SO2的主要脱硫反应方程式。
Ca(OH)2+SO2+H2O→CaSO3⋅2H2O (1) CaSO3⋅2H2O+1/2O2→CaSO4⋅2H2O (2) 本研究利用固定床反应器,以Ca(OH)2作为脱硫剂,考察反应温度、脱硫剂颗粒粒径、反应空速、模拟烟气中水蒸气含量对半干法脱硫反应的影响;同时,以脉冲增湿方法改善半干法工艺的脱硫效果,以期为钙基半干法脱硫工艺的改进提供参考。
1. 实验材料和方法
1.1 实验材料
所使用的二氧化硫气体(SO2,浓度1%,氮平衡)、空气(Air,21%O2/N2)、氮气(N2,纯度99.999%)均购自北京环宇京辉京城气体科技有限公司;氢氧化钙(Ca(OH)2,AR,≥95.0%)购自天津百伦斯生物技术有限公司;无水氯化钙(CaCl2,AR,≥96.0%)购自国药集团化学试剂有限公司。
1.2 实验装置和方法
实验装置由配气系统、反应系统、出口气体检测系统、尾气处理系统4部分组成(见图1)。配气系统主要装置有恒流泵、气化炉、气瓶(SO2、N2、Air);反应系统主要有加热炉和管式固定床反应器;出口气体检测系统由冷凝器、干燥管、SO2检测仪组成;尾气处理系统为盛有NaOH水溶液的洗气瓶。
模拟烟气的SO2、N2及空气流量由质量流量控制器设定。SO2质量浓度为2 285.7 mg·Nm−3。去离子水经连接管路流入气化炉,其流量由恒流泵控制,在气化炉中产生水蒸气后再与反应气混合并通入固定床反应器中进行脱硫反应。
将200目的Ca(OH)2粉末加去离子水制浆后造粒,在120 ℃下干燥,再破碎、筛分,得到不同粒径的Ca(OH)2颗粒备用。固定床下部填充直径2 mm的刚玉球作为支撑,并在刚玉球上方装填Ca(OH)2颗粒。
反应管出口气体经过冷凝器、干燥管除水后,进入SO2检测仪进行分析,之后进入尾气处理系统经充分净化后外排至环境中。
1.3 参数定义
采用SO2检测仪分析进出口SO2质量浓度。我国火电厂燃煤烟气中SO2的质量浓度排放标准为35 mg·Nm−3[16]。本研究将该质量浓度定义为SO2穿透浓度,将SO2出口质量浓度达到穿透浓度时的反应时间定义为穿透时间。
脱硫率(η)的计算方法式为式(3)。Ca(OH)2利用率(Ue)是评价脱硫效果及脱硫反应经济性的重要指标,其计算式为式(4)。Ca/S,即Ca(OH)2的使用量与SO2穿透时的SO2脱除量之比,其计算式为式(5)。
η(%)=Cin−CoutCin×100 (3) Ue=Vt1∫0(Cin−Cout)dt2285.7Vt2 (4) Ca/S=NCa(OH)2NSO2 (5) 式中:Cin为反应气体中SO2进口质量浓度,mg·Nm−3;Cout为SO2出口质量浓度,mg·Nm−3;V为反应气体体积流量,mL·min−1;t1为脱硫反应的穿透时间,min;t2为脱硫反应的理论反应时间,min;
NCaOH2 NSO2 2. 结果和讨论
2.1 反应温度对脱硫效果的影响
温度对Ca(OH)2与SO2的脱硫反应有一定影响。在55~160 ℃下,采用平均粒径0.58 mm的Ca(OH)2颗粒,在空速2 500 h−1、含硫气体中水蒸气体积分数5%的条件下进行脱硫实验,SO2出口质量浓度随反应时间的变化如图2(a)所示,穿透时间与反应温度的关系如图2(b)所示。穿透时间随温度的升高而降低,当温度由55 ℃逐渐升至160 ℃时,穿透时间由1 840 min降至240 min。此结果表明,在55~160 ℃下,Ca(OH)2的脱硫效果会随着反应温度的升高而下降,这与文献[17-18]的结论相吻合。
2.2 Ca(OH)2颗粒的粒径对脱硫效果的影响
固定床反应器内填充的Ca(OH)2颗粒粒径越大,床层空隙率则越高。为避免空隙率过高影响实验结果,采用了40 mm直径的反应管。较大的管径比可有效减小壁面附近空隙增大时带来的壁面效应影响[19]。同时,Ca(OH)2颗粒粒径的增大还会造成SO2的内扩散阻力增大,不利于进行脱硫反应。图3反映了半干法脱硫在不同Ca(OH)2颗粒粒径条件下的实验结果,在55 ℃、2 500 h−1及水蒸气体积分数为12%的条件下,随着Ca(OH)2颗粒粒径的增大,脱硫反应的穿透时间不断缩短。
图4分别反映了Ca/S及Ca(OH)2利用率与Ca(OH)2颗粒粒径的变化关系。在Ca(OH)2颗粒的平均粒径为0.58 mm、反应温度为55 ℃、空速为2 500 h−1、水蒸气体积分数为12%的条件下,当出口SO2达到穿透浓度时,可获得本实验的最佳脱硫效果。根据式(4)和(5)计算出该条件下的Ca(OH)2利用率为71.3%、Ca/S为1.4。Ca/S随Ca(OH)2粒径的增加而不断增大,而Ca(OH)2利用率则不断降低。当Ca(OH)2颗粒的平均粒径由0.58 mm增至2.25 mm时,Ca/S由1.4增至3.6,Ca(OH)2的利用率则从71.3%降至27.8%。可见,Ca(OH)2颗粒的平均粒径增大不利于半干法脱硫效果的提升。这是由于:Ca(OH)2颗粒的粒径增大会造成颗粒的比表面积减小及SO2内扩散阻力的增加,导致颗粒内部大量Ca(OH)2未能与SO2发生反应,最终使得Ca(OH)2利用率降低,穿透时间随之缩短。
2.3 空速对脱硫效果的影响
空速对脱硫效果的影响如图5所示。空速的提高使得脱硫反应的穿透时间缩短,在Ca(OH)2颗粒平均粒径为0.58 mm、水蒸气体积分数为12%、温度为55 ℃的条件下,当空速由2 500 h−1升至7 500 h−1时,穿透时间由3 240 min大幅降至260 min。
不同空速对Ca/S及脱硫剂Ca(OH)2利用率的影响如图6所示。随着反应空速的增大,Ca/S随之增大而Ca(OH)2利用率则随之降低。保持其他条件不变,当反应空速由2500 h−1增加到7500 h−1时,Ca/S由1.4提高为5.7,Ca(OH)2的利用率则由71.3%降低到18.8%。可见,空速的增大会导致脱硫效果急剧下降。在较高的空速下,烟气在床层的停留时间会缩短,脱硫反应速率则大幅提高。反应中会快速生成大量脱硫产物并覆盖至脱硫剂颗粒表面,所形成的致密产物层可阻止SO2与颗粒内部的Ca(OH)2继续反应,使得脱硫反应快速穿透。
2.4 反应气体中水蒸气含量对脱硫效果的影响
在半干法脱硫工艺中,烟气中的水蒸气含量对脱硫效果影响显著[20-21]。图7反映了水蒸气体积分数对脱硫效果的影响规律。穿透时间随着反应气体中水蒸气含量的增加而增大,在Ca(OH)2颗粒平均粒径为0.58 mm、空速为2 500 h−1、温度为55 ℃的条件下,当水蒸气的体积分数由5%升至12%时,脱硫反应的穿透时间由1 840 min大幅增至3 240 min。
水蒸气体积分数对Ca/S及脱硫剂Ca(OH)2利用率的影响如图8所示。随着水蒸气含量的增加,Ca/S相应不断降低,Ca(OH)2利用率则不断提高。水蒸气的体积分数由5%增至12%时,Ca/S由2.5降至1.4,Ca(OH)2的利用率由40.9%增至71.3%。这表明水蒸气含量的增大有利于半干法脱硫反应的进行。实际上,烟气中水蒸气的体积分数越大,反应中Ca(OH)2颗粒的表面越容易润湿从而形成液膜,这使得Ca(OH)2可游离出部分OH−,进而与烟气中的SO2进行快速、充分的离子反应[22-23]。反之,当烟气中水蒸气含量太低时,水蒸气不足以使得Ca(OH)2颗粒表面润湿,SO2与Ca(OH)2的离子反应便难以进行。当烟气为不含水蒸气的极端干燥状态时,SO2与干燥的Ca(OH)2几乎不发生反应[24]。
本研究提出了以脉冲式增湿方法对半干法脱硫工艺进行改进,故对水蒸气含量对半干法脱硫反应的影响进行了研究。当出口SO2质量浓度首次达到穿透浓度时,通过恒流泵调节水的流量,使反应气体中的水蒸气体积分数由12%增至24%并保持10 min。之后,将水蒸气体积分数调回原来的12%。20 min之后,再将反应气体中的水蒸气体积分数由12%增至24%并保持10 min。如此循环往复进行脉冲式的增湿,直至脱硫反应出口SO2质量浓度再次达到35 mg·Nm−3这一穿透浓度,结果如图9所示。图9(b)中的箭头为反应气体中的水蒸气体积分数由12%增至24%的时间节点。在反应3 220 min时,出口SO2质量浓度达到40 mg·Nm−3,即在水蒸气体积分数为12%条件下,脱硫反应首次穿透。而首次脉冲增湿后,出口SO2质量浓度降至0,接着水蒸气体积分数调回原先的12%并保持20 min。之后出口SO2质量浓度逐渐增至11 mg·Nm−3。这表明水蒸气含量的增加促进了脱硫,即使在反应穿透后,通过增加水蒸气含量仍可增加脱硫剂颗粒表面的润湿程度,使得SO2与颗粒内部未反应的Ca(OH)2得以继续反应。如此循环往复,直至第7次脉冲增湿时还有明显效果。在前6次脉冲增湿期间,出口SO2质量浓度一直保持低于穿透浓度。通过脉冲增湿,反应穿透时间延长了约160 min,Ca(OH)2利用率提高了4.4%,Ca/S由1.43降至1.36。
又研究了Ca(OH)2颗粒的平均粒径为2.25 mm时的脱硫反应当出口SO2浓度首次达到穿透浓度时脉冲增湿方法对脱硫效果的影响。实验结果如图10所示。结果表明,脉冲增湿使得该条件下的脱硫反应的穿透时间延长了约720 min,计算得到的Ca(OH)2利用率提高了15.6%,Ca/S由3.6降低到了2.3。2.25 mm的Ca(OH)2颗粒粒径条件下的脉冲增湿结果虽然仍与本实验最佳脱硫效果有较大差距,但脉冲增湿对脱硫效果的改善幅度较0.58 mm Ca(OH)2颗粒粒径的条件下有了较大提升。
分析脉冲式增湿方法对半干法脱硫效果的促进原因可发现,由于水蒸气体积分数为24%时,反应气体处于过饱和状态,在进入反应管后易凝结成为液态水并覆盖在Ca(OH)2颗粒表面形成液膜,使得脱硫剂表面及内部未反应的Ca(OH)2得以继续游离出部分OH−并与SO2发生反应,最终导致出口SO2质量浓度降低,穿透时间也相应延长。然而,长时间通入24%体积分数的水蒸气会发生Ca(OH)2颗粒团聚和床层板结,而以2:1的时长比循环通入水蒸气体积分数为12%和24%的脉冲增湿方法,则可保证在脱硫效果改善的同时有效避免上述不利情形发生。因此,采用脉冲增湿方法可优化半干法脱硫技术。
3. 结论
1)较低的反应温度、Ca(OH)2颗粒粒径的减小、反应空速的降低、烟气中水蒸气含量的提高均有利于半干法脱硫效果的提升,即有利于穿透时间的增加、脱硫剂利用率的提高及Ca/S的降低。当反应温度为55 ℃、Ca(OH)2颗粒平均粒径为0.58 mm、反应空速2 500 h−1、烟气中水蒸气体积分数12%时,可获得最佳脱硫效果。在SO2质量浓度为穿透浓度(35 mg·Nm−3)时,Ca(OH)2利用率达可到71.3%,Ca/S为1.4。
2)脉冲增湿方法可促进半干法脱硫工艺的脱硫效果。在上述最佳条件下,当反应器出口SO2达到穿透浓度时,通过实施脉冲增湿,即间歇提高反应气体中水蒸气体积分数可使穿透时间延长160 min,达到3 380 min,此时Ca(OH)2利用率提高了4.4%。采用脉冲增湿方法可优化半干法脱硫工艺。
-
-
[1] 吕清刚, 李诗媛, 黄粲然. 工业领域煤炭清洁高效燃烧利用技术现状与发展建议[J]. 中国科学院院刊, 2019, 34(4): 392-400. [2] ZHAO Z Y, ZHANG Y X, GAO W C, et al. Simulation of SO2 absorption and performance enhancement of wet flue gas desulfurization system[J]. Process Safety and Environmental Protection, 2021, 150: 453-463. doi: 10.1016/j.psep.2021.04.032 [3] ZHOU D, WEI R F, ZHU Y L, et al. Calcium sulfate whisker one-step preparation using semi-dry flue gas desulfurization ash and directional growth control[J]. Journal of Cleaner Production, 2021, 290: 125754. doi: 10.1016/j.jclepro.2020.125754 [4] ZHANG X P, WANG N H, Effect of Humidification water on semi-dry flue gas desulfurization[J]. Energy Procedia, 2012, 14: 1659-1664. [5] 张杨帆, 李定龙, 王晋. 我国烟气脱硫技术的发展现状与趋势[J]. 环境科学与管理, 2006, 31(4): 124-128. doi: 10.3969/j.issn.1673-1212.2006.04.040 [6] 刘海洋, 江澄宇, 谷小兵, 等. 燃煤电厂湿法脱硫废水零排放处理技术进展[J]. 环境工程, 2016, 34(4): 33-36,41. [7] ZHANGY Q, WANG Y X, LIU Y Q, et al. Experiments and simulation of varying parameters in cryogenic flue gas desulfurization process based on Aspen plus[J]. Separation and Purification Technology, 2021, 259: 118223. doi: 10.1016/j.seppur.2020.118223 [8] 崔名双. 半干法水合灰脱硫性能及机理研究[D]. 北京: 煤炭科学研究总院, 2019. [9] 韩松, 李雄飞, 靳虎, 等. 旋转喷雾干燥法烟气脱硫的工艺技术研究[J]. 中国环保产业, 2021(4): 50-53. doi: 10.3969/j.issn.1006-5377.2021.04.015 [10] LIU F G, CAI M Y, LIU X L, et al. O3 oxidation combined with semi-dry method for simultaneous desulfurization and denitrification of sintering/pelletizing flue gas[J]. Journal of Environmental Sciences, 2021, 104(6): 253-263. [11] 董献保. 烟气干法、湿法脱硫方案选择的探讨[J]. 当代化工研究, 2019(3): 52-53. doi: 10.3969/j.issn.1672-8114.2019.03.035 [12] GAREA A, HERRERA J L, MARQUES J A, et al. Kinetics of dry flue gas desulfurization at low temperatures using Ca(OH)2: competitive reactions of sulfation and carbonation[J]. Chemical Engineering Science, 2001, 56(4): 1387-1393. doi: 10.1016/S0009-2509(00)00362-6 [13] BERNARD L, FRECHE M, LACOUTJ. L, et al. Modeling of the dissolution of calcium hydroxide in the preparation of hydroxyapatite by neutralization[J]. Chemical Engineering Science, 2000, 55(23): 5683-5692. doi: 10.1016/S0009-2509(00)00205-0 [14] XU G W, GUO Q M, KANEKO T, et al. A new semi-dry desulfurization process using a powder-particle spouted bed[J]. Advances in Environmental Research, 2000, 4(1): 9-18. doi: 10.1016/S1093-0191(00)00003-4 [15] 佟会玲, 孙旭光, 李定凯, 等. 钙基湿法与半干法尾部烟气脱硫工艺耗水量的比较[J]. 电站系统工程, 2003, 19(6): 1-3,60. doi: 10.3969/j.issn.1005-006X.2003.06.001 [16] 《全面实施燃煤电厂超低排放和节能改造工作方案》[J]. 节能与环保, 2016(1): 32. [17] RUHLAND F, KIND R, WEISS S. The kinetics of the absorption of sulfur dioxide in calcium hydroxide suspensions[J]. Chemical Engineering Science, 1991, 46(4): 939-947. doi: 10.1016/0009-2509(91)85087-E [18] 杜佳, 齐晓辉. 影响燃煤电厂湿法烟气脱硫率的主要因素[J]. 化学工程与装备, 2019(3): 263-264. [19] 朱梓瑞, 刘雪东, 蒋良雄, 等. 管式固定床反应器柱状颗粒床层流体流动模拟与实验研究[J]. 过程工程学报, 2021, 21(9): 1022-1032. doi: 10.12034/j.issn.1009-606X.220288 [20] 尹水娥, 孙保民, 高旭东, 等. 介质阻挡放电中烟气相对湿度对脱硫脱硝的影响[J]. 动力工程学报, 2010, 30(1): 41-46. [21] WANG H, GUO S, YANG L, et al. Impacts of water vapor and AAEMs on limestone desulfurization during coal combustion in a bench-scale fluidized-bed combustor[J]. Fuel Processing Technology, 2017, 155: 134-143. doi: 10.1016/j.fuproc.2016.05.010 [22] ZHOU Y G, ZHU X, PENG J, et al. The effect of hydrogen peroxide solution on SO2 removal in the semidry flue gas desulfurization process[J]. Journal of Hazardous Materials, 2009, 170(1): 436-442. doi: 10.1016/j.jhazmat.2009.04.075 [23] WANG X, WANG S Y, WANG R C, et al. Numerical simulation of semi-dry desulfurization spouted bed using the discrete element method (DEM)[J]. Powder Technology, 2021, 378: 191-201. doi: 10.1016/j.powtec.2020.09.047 [24] 钱枫, 郑跃红, 张溱芳. 潮湿气氛下Ca(OH)2与SO2反应动力学研究[J]. 环境化学, 1999, 18(4): 315-320. -






 下载:
下载: