-
类金属砷是自然界(大气、土壤、水等环境介质)中普遍存在的元素,对人体有一定的毒性作用,易致癌[1]。自然条件下砷的释放很少达到对人体有害的程度,砷的释放、迁移和污染大多是由人类活动造成的,包括含砷矿物的开采、冶炼以及含砷物质的使用[2]。在我国,有超过15万km2的地区,近2 000万人生活在砷含量超过10 μg·L−1(WTO饮用水标准)水高砷污染区中;全世界约有2×109人受到高砷暴露的威胁[3]。因此,开发合理、高效的砷污染治理技术对保护人类健康、保障饮水安全具有重要意义。
砷在污染水体中主要形态为+3价(还原环境,亚砷酸根(AsO33−))和+5价(氧化环境,砷酸根(AsO43−)),其中,As(Ⅲ)毒性约为As(V)的25~60倍[4]。依据砷的不同形态及包括吸附、氧化-还原和混凝-沉淀等去除原理,目前治理砷污染水体的方法主要有离子交换和膜分离技术[5-6]、混凝沉淀法[7]、吸附法[8]和生物修复技术[9]。其中,离子交换法和膜分离技术是最有效的去除方法,但因使用价格昂贵,很难推广应用;混凝沉淀和吸附法,作为传统的水净化方法,需要消耗大量的化学制品,且因砷污染水体中含有复杂的有机物和竞争离子存在,使去除效果成为一项重大挑战;生物修复技术因其应用成本低和环境友好性等优势受到广泛关注,但因修复效率低,处理时间长和适宜生长环境控制较难的问题而受到很大限制。
近年来,基于铁碳微电解材料产生微原电池反应去除水体污染物的处理方法,受到越来越多研究者的关注[10]。该方法利用铁碳微电解材料兼具催化氧化、絮凝沉淀和氧化还原等污染物去除原理,具有效果稳定,应用范围广,操作方便等优点[11-12]。但传统铁碳微电解材料主要是将还原铁粉或废铁屑、工业碳粉和一定的粘结剂按照一定比例混匀由固定模具压制成型后通过高温烧结制备完成[13],需消耗大量碳粉和粘结剂等工业制成品,提升了制备成本。并且工业粘结剂在高温焙烧下易板结,不利于铁碳材料烧制过程中孔隙结构的产生,在砷污染水体的去除过程中,降低了Fe-C反应电极与含砷废水的接触面积,阻碍微原电池反应过程中的电子转移,去除效率不佳[14-15]。
鉴于此,在笔者所在团队前期研究基础上[16],提出了一种利用人工湿地芦苇制备得到的植物粉末取代工业碳粉,膨润土作为支撑剂和粘结剂,与还原铁粉(Fe0)按一定添加比例混匀,在一次性梯度升温条件下烧制成型的制备工艺。此外,有研究表明,植物基生物质材料在碳化后,具有较好稳定性和电化学性能,与还原性铁Fe0形成持续的Fe-C微原电池反应,而被当做良好的阴极材料[17]。但在一定的烧制温度下,碳基材料的性质如电子传递、稳定性和氧化还原能力,在很大程度上由其生物质前驱体的不同组分(半纤维素、纤维素和木质素)决定[18]。因此,本研究采用响应面法探究芦苇叶和芦苇秆植物基铁碳微电解材料最优制备条件,并且探讨了2种植物基中各主要生物质组分与制备条件相互作用对铁碳微电解材料除As(Ⅲ)效率与机理的影响,以期为铁碳微电解材料优化制备及其应用于含砷废水的处理提供参考。
-
还原铁粉(Fe0)、亚砷酸钠(NaAsO2)、氢氧化钠(NaOH)、盐酸(HCl)等均为分析纯(国药集团化学试剂有限公司);纤维素、半纤维素和木质素(阿拉丁试剂(上海)有限公司);钙基膨润土(辽宁省锦州市锦州金泰膨润土有限公司);实验用水均为去离子水。
实验过程所使用的仪器:DFT-100A型小型粉碎机(浙江大德仪器有限公司);TSQ-280型恒温振荡器(上海精宏有限公司);SXW-1200 ℃型马弗炉(上海实研有限公司)。
分析测试方法:铁碳微电解材料中铁含量采用《直接还原铁-金属铁含量的测定-三氯化铁分解重铬酸钾滴定法》(GB/T 38812.2-2020)方法进行测定[19];植物基原材料芦苇秆与芦苇叶中碳元素含量采用元素分析仪(Elementar Vario EL III,德国Elementar公司)测定;溶液中砷浓度采用电感耦合等离子体原子发射光谱(ICP-OES,Optima,美国PerkinElmer公司)测定;铁碳微电解材料中碳化铁(Fe3C)采用X射线衍射仪(XRD,D8 Advance,布鲁克AXS公司)进行物相分析[20];铁碳微电解材料的形貌采用场发射扫描电子显微镜(蔡司Gemini360/560场发射扫描电镜,德国蔡司公司)进行分析;铁碳微电解材料及纤维素、半纤维素及木质素在氮气保护下采用热重-差热综合热分析仪(TGA,Diamond TG/DTA,美国PerkinElmer公司)进行热稳定性分析(TG-DTG);铁碳微电解材料比表面积及孔隙度采用比表面积和孔隙度分析仪(TriStar II Plus Series,美国麦克仪器公司)测试。
-
选用西南科技大学流域生态环境与水污染控制实训中心人工湿地的芦苇为生物质原料,将其按芦苇秆和芦苇叶进行分选,去除杂质并洗净,置于烘箱中在80 ℃条件下烘至恒重后利用破碎机进行破碎,过200目筛,收集于棕色瓶中保存待用。将还原铁粉、植物粉末样品、膨润土、水按一定配比混匀加入造粒机中制备成直径大小为1~1.5 cm的球形颗粒,然后移入马弗炉烧制。烧制步骤为:先由室温升至设定碳化温度并恒温一段时间,进行绝氧热解碳化处理,在碳化过程结束后,继续升温至设定焙烧温度并恒温一段时间,进行绝氧焙烧成型处理,最后自然冷却得到植物基铁碳微电解材料。
-
制备植物基铁碳微电解材料的主要影响因子包括铁碳摩尔比(Fe/C)、碳化温度和焙烧温度。第一步根据单因素预备实验,确定各因子最佳水平的适当范围,后采用Box-Behnken模型进行实验设计。
1)单因素预备实验。取100 mL 10 mg·L−1的NaAsO2溶液于250 mL锥形瓶中,调节pH至3后,加入5 g植物基铁碳微电解材料,放入恒温摇床(25 ℃,100 r·min−1),避光振荡,计算反应48 h时间下的As(Ⅲ)去除率。铁碳微电解材料中砷去除率根据式(1)进行计算。
式中:R表示砷脱除率,%;A0表示铁碳微电解材料处理前溶液中砷的质量浓度,mg·L−1;Ai表示铁碳微电解材料处理后溶液中砷的质量浓度,mg·L−1。
铁碳摩尔比(Fe/C)最适条件范围。在铁碳微电解原材料配比中,铁与植物材料的添加量直接影响除砷过程中的“Fe-C原电池对”数量。根据芦苇秆与芦苇叶中的碳含量(表1),通过式(2)计算得到还原性铁添加量。
式中:M0为植物粉末质量,g;M1为还原铁粉(Fe0)质量,g;P为植物粉末中碳元素质量分数。
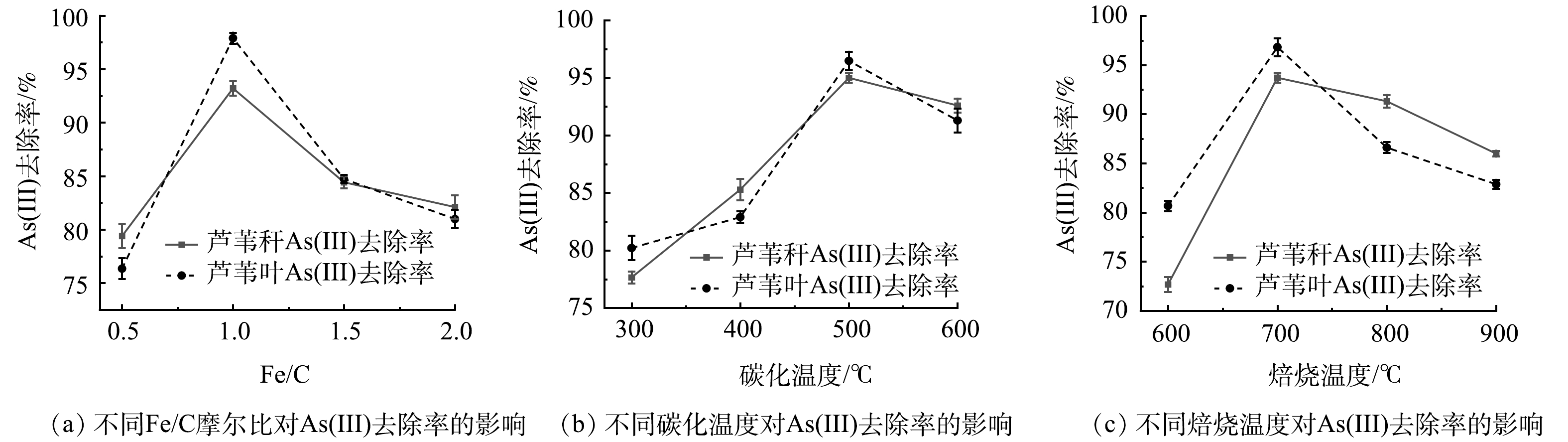
当碳化温度为500 ℃,恒温120 min,焙烧温度为700 ℃,恒温60 min时,不同Fe/C摩尔比条件下As(Ⅲ)的去除率如图1(a)所示。由图1(a)可知,As(Ⅲ)去除率随着Fe/C摩尔比的增加呈现先增加后下降的趋势,在Fe/C摩尔比为1时As(Ⅲ)去除效果最好。因此,选择Fe/C摩尔比为1进行后续实验。
当Fe/C摩尔比为1时,在碳化恒温120 min以及焙烧温度700 ℃、恒温60 min条件下,制得不同碳化温度条件下的铁碳微电解材料,As(Ⅲ)的去除率如图1(b)所示。由图1(b)可知,As(Ⅲ)去除率随着碳化温度的增加呈现逐渐增加再降低的趋势,在500 ℃去除效果最好。因此,选择碳化温度为500 ℃进行后实验。
当在Fe/C摩尔比为1,碳化温度为500 ℃时,在碳化恒温120 min和焙烧恒温60 min条件下,制得不同焙烧温度条件下的铁碳微电解材料,As(Ⅲ)的去除率如图1(c)所示。由图1(c)可知,以芦苇秆和芦苇叶为植物基材料制备的铁碳微电解材料对As(Ⅲ)去除率随着焙烧温度的升高,呈现先升后降的趋势,且在700 ℃时As(Ⅲ)的去除率最高。
2)响应面Box-Behnken设计。在前期单因素预备实验得到的各因子最佳水平的适当范围,运用Design Expert 11.1.0.1软件中的中心复合设计模块(Box-Behnken,BBD)[21]以3个因素作为自变量,砷去除率为响应值设计得到如表2所示的3因素3水平的响应曲面实验。
在响应曲面交互作用实验中,实验影响因素(自变量)与实验指数(因变量)之间的回归关系可以通过响应面方法(RSM)[22]进行分析,这种回归既可能是曲线关系,也可能是曲面关系,如式(3)所示。
式中:bi是线性效应;bii是第二次效应;bij是相互作用效应;A为铁炭摩尔比;B为碳化温度;C为焙烧温度。
-
根据Box-Behnken实验设计制备得到的铁碳微电解材料对As(Ⅲ)去除效率如表3所示。
将表3中的总砷去除率输入到Design Expert 11.1.0.1软件中进行数据分析和多元二次回归拟合,得到依照编码因数的二次响应曲面方程如式(4)和式(5)所示,实际因素的二次响应曲面方程如式(6)和式(7)所示。
为说明响应面模型及回归方程的有效性,对回归方程的可行性及相关性进行分析,得到分析结果如表4所示。R2均在95%置信区间内,同时校正系数与预测系数差值(Radj-RPred)均小于0.2,表明实测值与预测值之间偏差较小,拟合度好,可信度高;失拟影响因素项结果均不显著(P>0.05),代表失拟因素对于纯粹的误差可忽略不计,无失拟因数存在,3个自变量与响应值之间存在显著的相关性,误差小;精密度均大于4(一般认为较好拟合模型的精密度需大于4[23]),说明可实用性高,真实实验结果可用上述回归方程代替分析。同时从三因素间交互作用的相关系数可以看出:铁碳摩尔比(A)与焙烧温度(C)、碳化温度(B)与焙烧温度(C)之间的交互影响大于铁碳摩尔比(A)与碳化温度(B)间的交互作用,并表现为显著水平(P<0.05)。因此,本研究选择AC与BC因素之间的交互影响探究不同植物基生物质组分对铁碳微电解材料的除砷效率的影响。
-
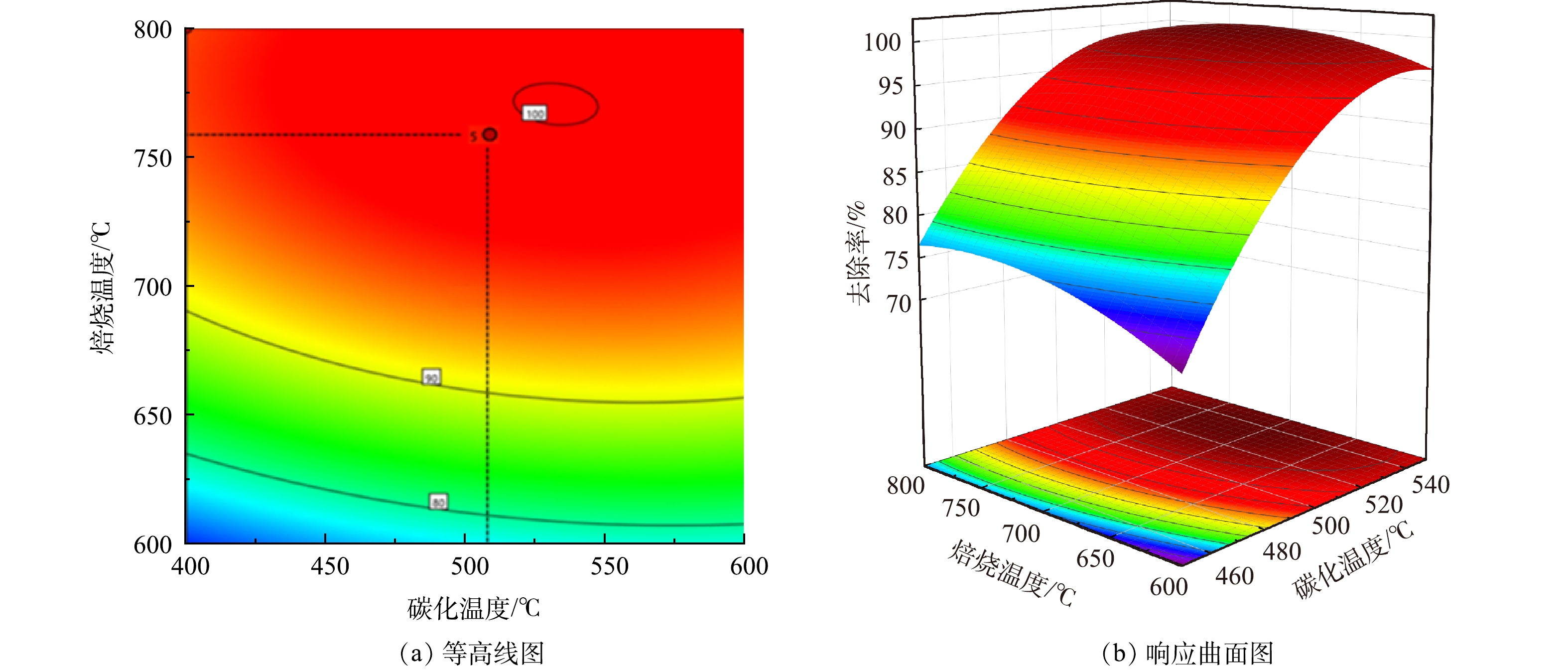
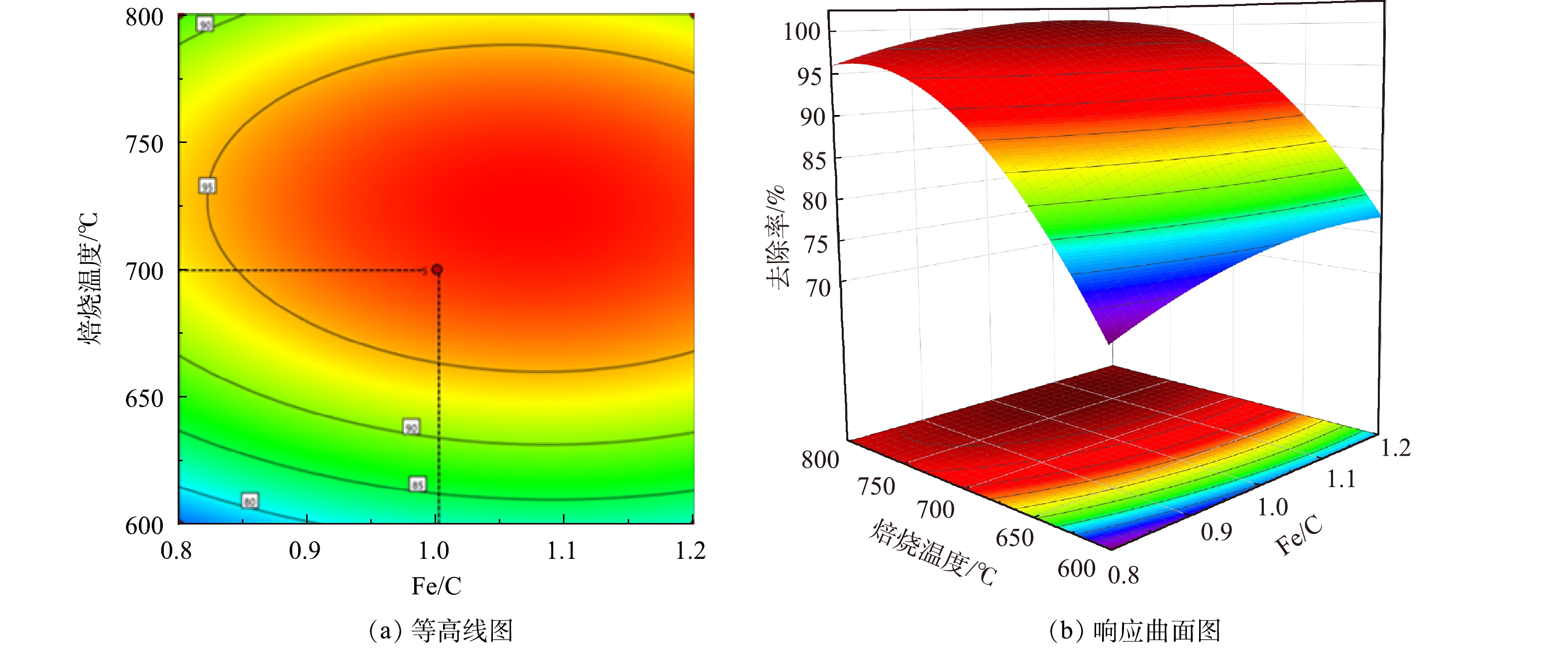
由响应面分析得到碳化温度和焙烧温度2个因素交互作用对总砷去除率的影响如图2和图3所示。在等高线图中,当中心图形呈现椭圆形时,交互作用强;而接近圆形时,交互作用弱。在响应曲面中,响应面坡度越大,自变量条件的改变对响应值的影响越大,反之影响越小。而当两者图形中的颜色越深代表响应值越大,效果越明显[24]。
由图2、图3中的等高线图看出,碳化温度与As(Ⅲ)的去除率呈正相关,焙烧温度与As(Ⅲ)去除率呈现先增后减的趋势,这与单因素实验结果一致。同时由等高线图也可以看出,沿C因素向峰值移动的等高线密度明显高于沿B因素向峰值移动的等高线密度,表明焙烧温度对响应值的贡献最大,这与方差分析得到的结果一致。响应面坡度陡峭,说明碳化温度与焙烧温度具有显著的交互作用,对As(Ⅲ)的去除影响较大。
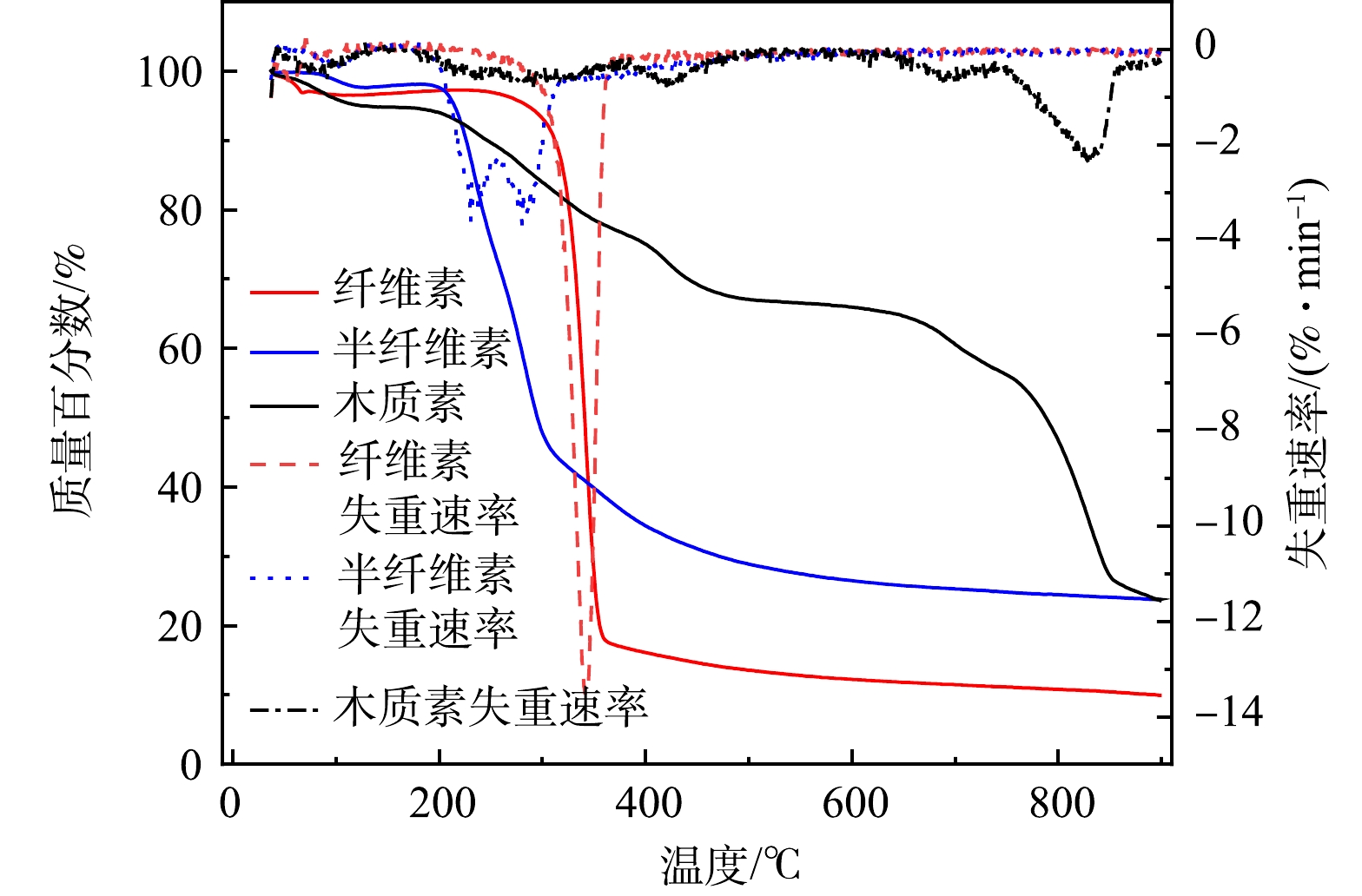
作为植物基铁碳微电解阴极材料与Fe0在水溶液中形成微原电池,发生原电池反应,其生物质组分主要由木质素、纤维素和半纤维素三种主要有机物构成,通过非共价键相互交联在一起构成植物骨架。在隔氧条件下进行梯度升温,会生成生物炭、生物油以及不可冷凝气体。生物炭具有稳定的芳香化、脂肪族链状结构及丰富的孔隙分布,具有良好的稳定性和吸附性[25],能提高植物基铁碳微电解材料对As(Ⅲ)的去除。生物炭产量、表面官能团的种类和数量以及碳化方式与温度紧密相关。目前,主要碳化方式有慢速热解、高温气化和水热碳化法[26]。与其它热解方法相比,慢速热解因其固相收率较高,气相产物释放较慢且均匀,更适于植物基材料碳化。在隔氧梯度升温过程中,植物基碳化为生物炭中碳含量增多,氧、氢及灰分比例下降。温度越高,碳化程度越高,生物炭越稳定,但生物炭产量与烧制温度成非线性关系,主要是由于不同湿地植物生物质组分中纤维素、半纤维素和木质素含量和稳定性的差异性造成的[27-28]。通过热重分析仪测试分析得到纤维素、半纤维素和木质素热稳定性如图4所示。
由图4中可以看出,当碳化温度超过400 ℃时,大部分半纤维素和纤维素被热解。木质素因高稳定性而最难分解,分解温度范围跨度大(160~900 ℃),能在超过700 ℃热解条件下使更多的碳基结构被保留了下来,成为植物基碳基成分中最主要的碳元素来源[29]。
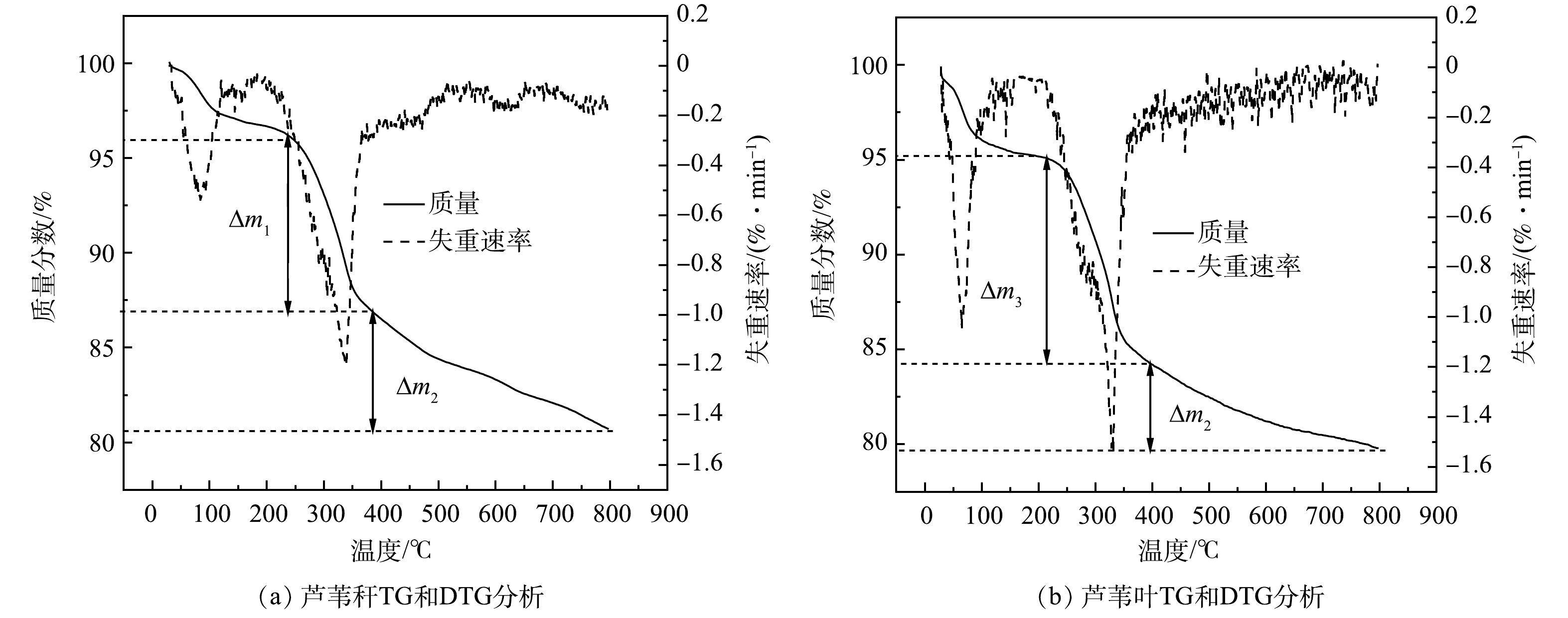
芦苇叶与芦苇秆植物基铁碳微电解材料的微商热重分析(DGT)与失重速率(TG)曲线如图5所示。由图5可以看出,在100 ℃左右时开始出现由铁碳微电解材料水分蒸发所导致的的失重峰。随着梯度升温至400 ℃左右时,植物基铁碳材料出现了不同程度的失重,其中芦苇叶植物基铁碳微电解材料的失重大于芦苇秆植物基铁碳微电解材料(△m1>△m3)。其原因可能是前者纤维素与半纤维素含量大于后者,在较低温度时更容易热解成生物炭,质量损失较快。但随着温度梯度的继续升高伴随着木质素的大量热解,含有更多木质素组分的芦苇秆植物基铁碳微电解材料迅速碳化,质量损失加快(△m2>△m4)。
因此,在相同Fe/C摩尔比和低于700 ℃焙烧温度下,芦苇叶植物基铁碳微电解材料对As(Ⅲ)去除率高于芦苇秆植物基铁碳微电解材料。分析认为,在低于700 ℃焙烧温度时,芦苇秆植物基有机成分中含有大量木质素,难以被完全碳化形成稳定的碳基材料,所以铁碳材料中有效碳元素比例较低;而芦苇叶植物基拥有大量能在较低温度下碳化的纤维素与半纤维素,碳化充分,有效碳元素含量多,能与Fe0形成大量的Fe0-C原电池参与反应,因此As(Ⅲ)去除率高。当焙烧温度超过700 ℃时,芦苇秆植物基中未被完全碳化的木质素被二次碳化,有效碳元素含量增加,Fe0-C原电池数量上升,As(Ⅲ)去除率提高。而芦苇叶植物基在第一次碳化过程中形成的碳基成分在二次碳化过程中灰分质量分数增加,有效碳元素降低,Fe0-C原电池数量下降,导致去除率下降。
-
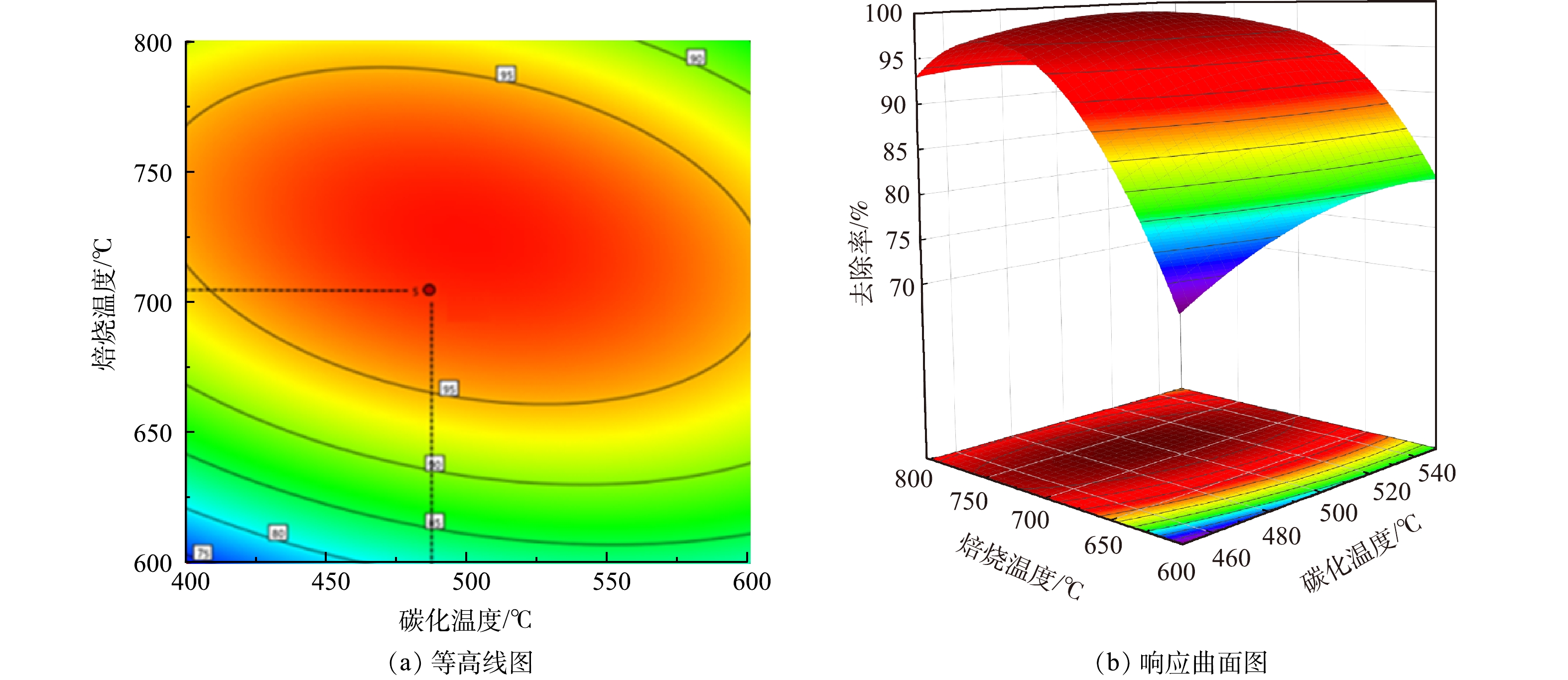
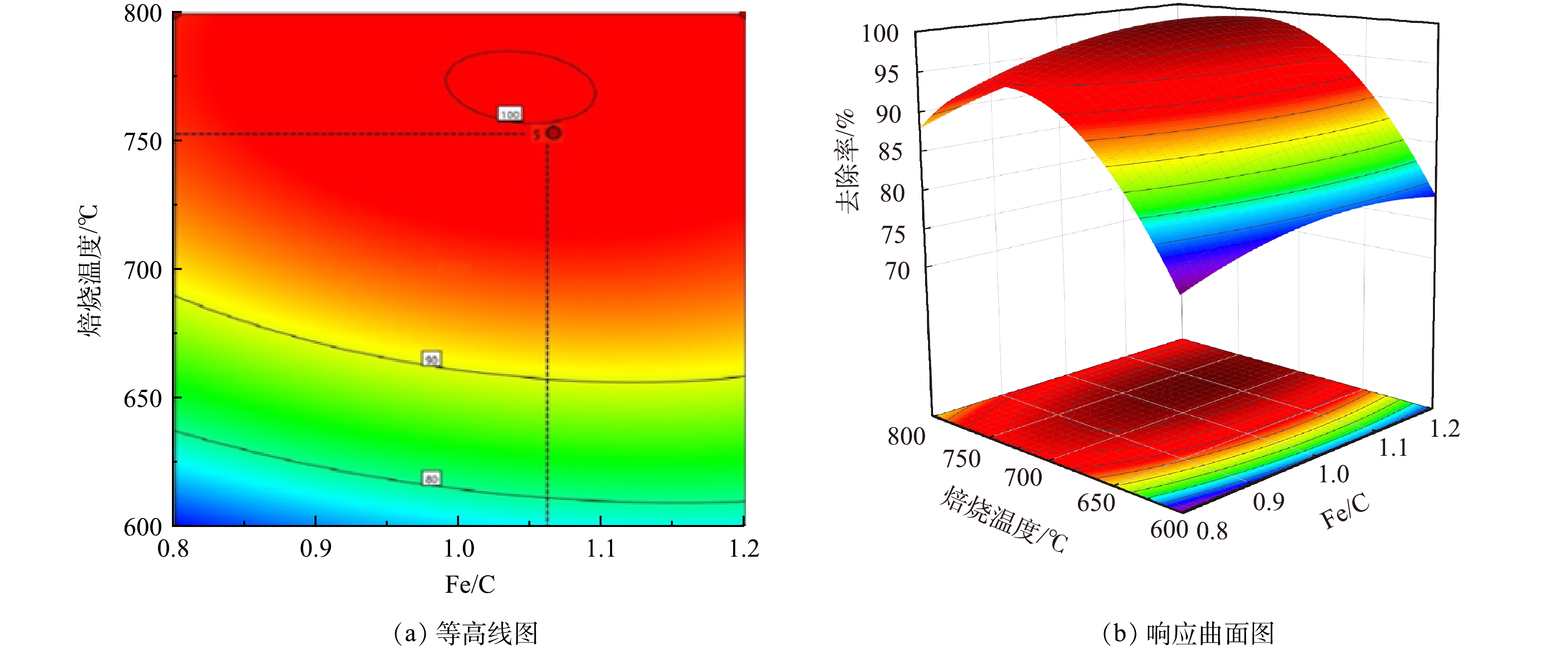
Fe/C摩尔比和焙烧温度2个因素交互作用对As(Ⅲ)去除率的影响如图6和图7所示。由响应面结果可以看出,并非碳含量比例越高,处理效果越明显。当Fe/C=0.8时,随着植物基生物质组分的不断碳化,碳含量线性增加,不仅导致Fe0的相对含量降低,微原电池数量的损失,而且过多的碳化成分在植物基铁碳微电解材料表面堆积,导致Fe0活性点位与As(Ⅲ)接触减少,导致去除率降低[30]。当Fe/C=1.2,即Fe0的含量过量时,随着焙烧温度上升,植物基的有效碳含量增加,微电解体系中阴极数量增多,配对原电池数量增加,电极反应加快。但过量Fe0和水中微电解产生的Fe2+使溶液体系保持为还原环境,抑制As(Ⅲ)向As(Ⅴ)的转化过程,降低铁盐对As(Ⅲ)的去除率。原因在于植物基铁碳材料中铁盐混凝作用去除水溶液中As(Ⅲ)主要是通过在微原电池反应中失去电子形成的Fe3+和Fe2+离子与As(Ⅲ)和As(Ⅴ)形成亚砷酸铁、砷酸铁等沉淀作用;以及Fe(OH)2及Fe(OH)3等沉淀对水相中的As(Ⅲ)和As(Ⅴ)的静电吸附作用[31]。主要的As(Ⅲ)化合物(AsO33−)具有中性电荷,As(V)化合物(H2AsO4−、HAsO42−)在4-10的pH范围内带负电荷。因此,对于铁盐的氢氧化物沉淀而言,对As(V)化合物静电引力更强;As(V)化合物更容易被扩散吸附到铁盐水解沉积物表面,从而被吸附去除。
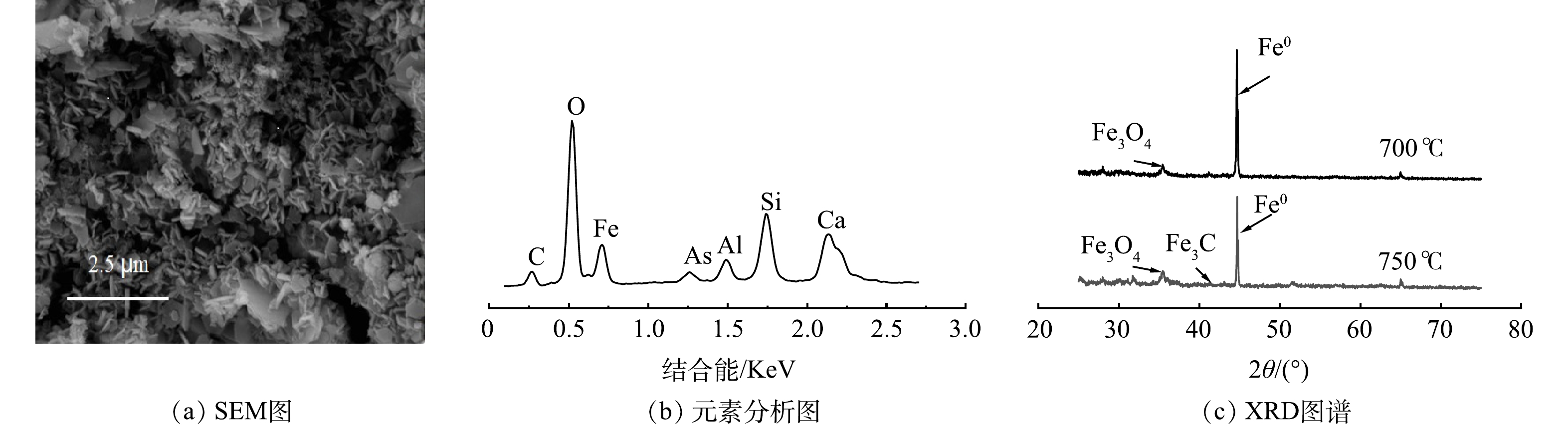
芦苇秆植物基铁碳微电解材料在Fe/C=1.06时,即Fe0过量6%左右时取得最大响应值。一方面,在铁碳微电解材料制备过程中,材料表面Fe0存在被氧化的风险,且温度越高,氧化越明显。因此,在高温烧制芦苇秆植物基铁碳微电解材料时,需投加过量Fe0来保证Fe0-C微原电池数量,同时不会对溶液氧化还原环境造成破坏,使反应持续进行。另一方面,在超过700 ℃的焙烧温度下,Fe0与植物基中碳化成分进一步碳化形成Fe3C[32],减少铁碳微电解材料表面积碳,释放Fe0活性位点,使得阴阳两电极之间电子转移速率提高。并且Fe3C可作为微原电池反应的阴极,能与Fe0形成Fe3C-Fe0微原电池反应[33],有效填补高温焙烧下,富含木质素芦苇秆植物基碳化成分中作为阴极碳元素含量的减少,使得Fe3C-Fe0和Fe0-C 2种微原电池能共同作用,提高溶液中的原电池数量。对于木质素含量不足的芦苇叶植物基,随着焙烧温度的提高,在接近750 ℃时,Fe3C作为微原电池阴极,不能够有效填补高温焙烧下,植物基碳化成分中作为阴极碳元素含量的减少,导致阴极数量减少,从而降低As(Ⅲ)的去除率,因此焙烧温度不宜超过700 ℃,在Fe/C=1.01时取得最佳制备条件。铁碳微电解表面材料的SEM和XRD分析表征结果如图8所示。可见,芦苇秆植物基铁碳微电解材料750 ℃出现类似鱼鳞状Fe3C特征物象及Fe3C特征峰[34],证实有Fe3C的生成。
-
芦苇叶和芦苇秆在300~800 ℃下制备的碳基材料的元素组成以及H/C、O/C和O+N/C比如表5所示。由表5可知,随着梯度升温的进行,在碳化过程中碳元素和灰分相对含量增加,氧元素和氢元素含量降低。这是由于温度升高,C—H、C—O键断裂,氢和氧元素在此过程中发生脱附丢失所致[35-36]。对于碳基材料,石墨晶格中存在的层状组成分子(LCM)更稳定。因此,在碳化过程中,随着热解温度的升高更多的杂原子丢失(O、N、S、P),碳原子趋向重排为六元环系统的大分子网络,以试图获得这种更稳定的类似石墨状态[37]的芳香结构。其中,由H/C原子比可知碳基材料芳香性的大小,H/C越低,芳香化程度越高。在植物基铁碳微电解材料中,高芳香性的碳基结构,具有更高的反应活性[38],有利于促进Fe0-C微原电池的电子转移,加快反应的进行。木质素因碳含量高和热稳定性强的因素,在高温条件下,生物质中木质素占比越高,H/C越低,碳基成分芳香性越强。因此,在高温焙烧条件下,生物质中木质素含量越高的植物基铁碳微电解材料,越有利于Fe-C微原电池反应的进行。
具有不同生物质组分的植物基材料其热解产物在氧化还原性质上也表现出较明显的差异[39]。热解产物氧化还原性质主要由有机官能团决定,如提供电子的羧基和酚羟基(还原剂)和接收电子的醌类和半醌类(氧化剂)等。随着热解的进行,有机成分中碳、氢和氧在热解过程中发生化学键的断裂,重组形成新的含氧和含碳官能团,从而表现出不同的氧化还原性质[25]。
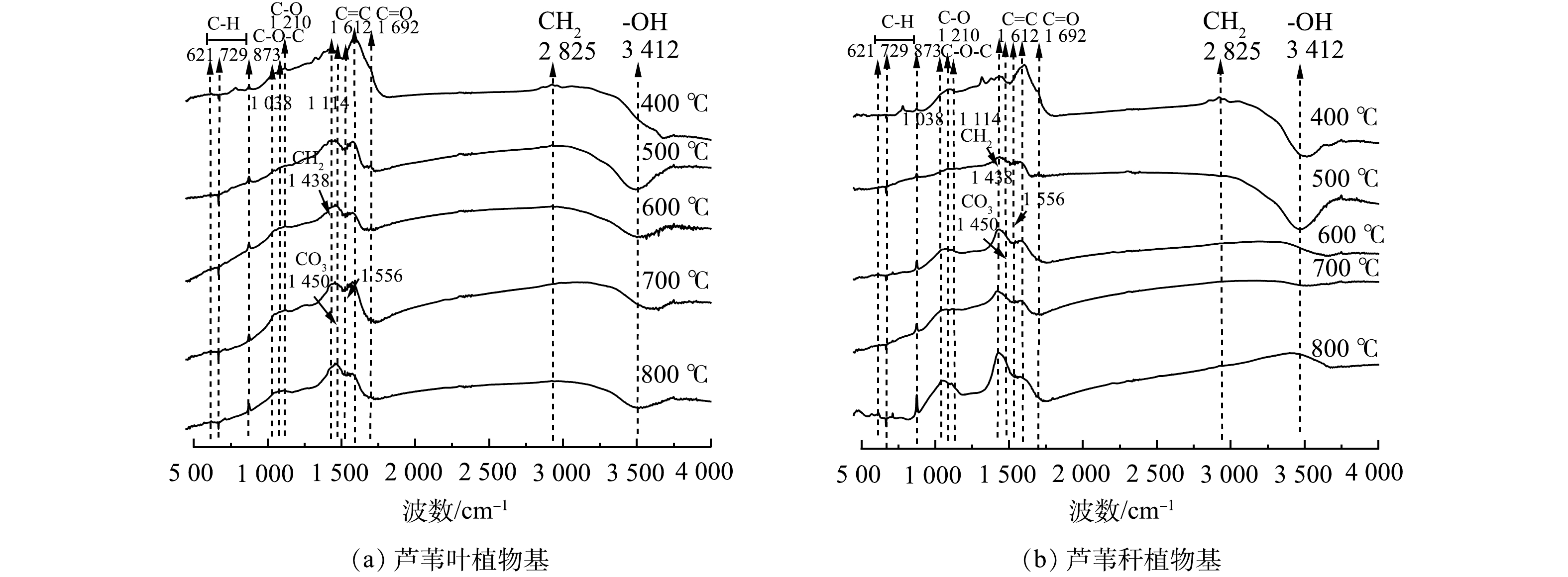
不同烧制温度下芦苇秆与芦苇叶植物基的傅里叶红外光谱图(FTIR)如图9所示。在低温热解过程中(200~500 ℃),植物有机质热解产生大量含氢和氧官能团,此时碳基材料官能团以羧基和酚羟基的供电子官能团为主,包括C—H(1 384~1 402 cm−1)、—COOH(1 692 cm−1)、CH2(2 825 cm−1)、O—H(3 200~3 400 cm−1)等。芦苇叶植物基因富含易热解的半纤维素和纤维素组分,在低温热解过程官能团演化更完全[40],此时有芦苇叶植物基还原能力强于芦苇秆植物基。随着热解温度的提高,—COOH、—CH2、C—H和O—H的伸缩振动的强度逐渐降低直至消失,原因在于随着温度的升高,大量C—H、C—O键断裂,氢和氧以水分子形式丢失,官能团转以氧原子为中心的向以碳原子为中心转移。此时—O—(1 114 cm−l)、C=O、C=C(1 612~1 692 cm−1)和C—O(1 210 cm−1)伸缩振动存在,碳基材料以羰基和醌基的得电子氧化官能团为主。木质素因结构刚性强,难以分解,所以在高热解温度下官能团演变更稳定[41],因此,芦苇秆植物基在1 612~1 692 cm−1处C=O、C=C和1 210 cm−1处C—O的峰值更强,氧化能力强于芦苇叶植物基。并在1 556 cm−1处出现新的特征峰,可能是由于木质类生物质在高温下芳香化,形成具有类石墨结构的Π共轭的晶格体系[37]。
-
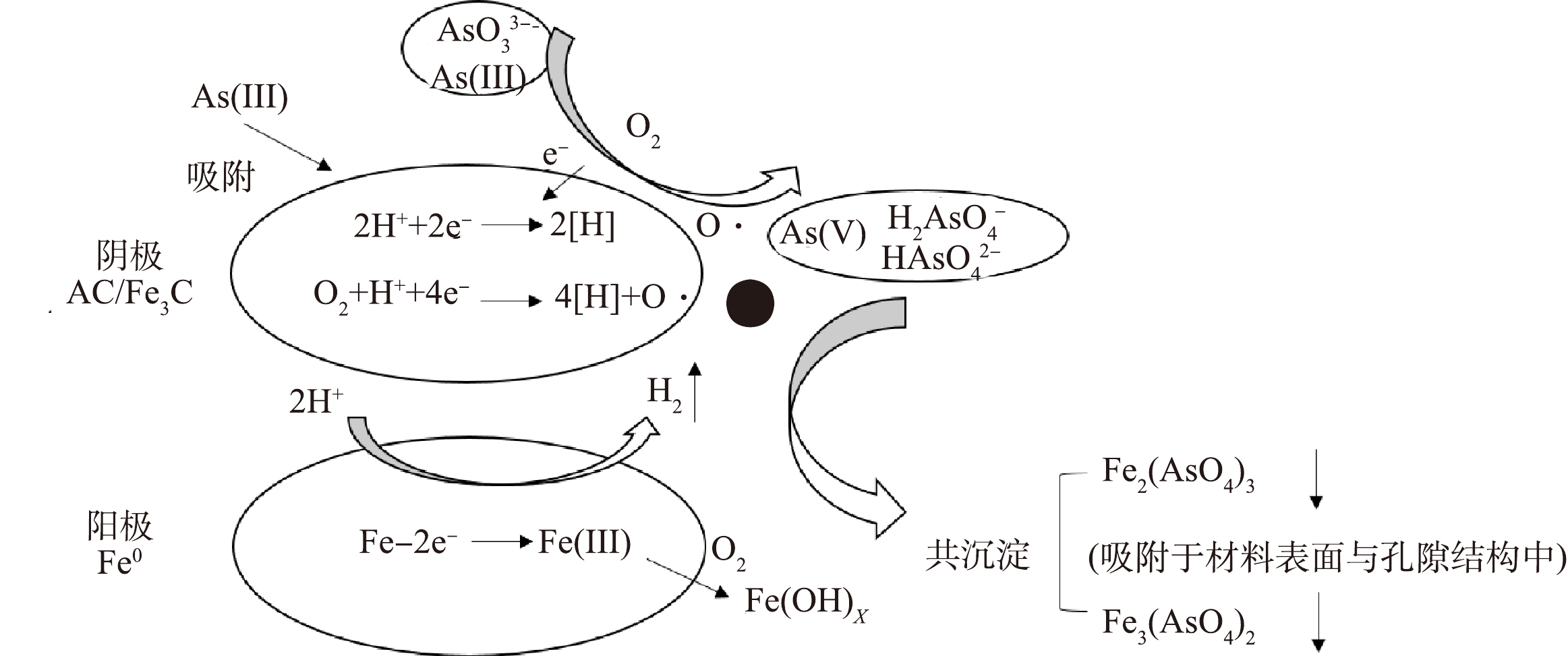
因植物基材料在热解过程中受热碳化并产生物油和不可冷凝气体,从而体积萎缩,使得植物基铁碳微电解材料形成连续贯通的多孔结构。本研究通过压汞法测得的植物基铁碳微电解材料的比表面积为1.1~1.2 m2·g−1、孔隙率为64.8%~65.32%,这有利于Fe-C微原电池反应的进行。植物基铁碳微电解材料对As(Ⅲ)去除机理可包括以下2条路径:一是在开始的反应过程中,As(Ⅲ)直接被植物基铁碳微电解材料吸附而去除;二是As(Ⅲ)被碳基材料中C=O、C=C和C—O等得电子官能团氧化为As(V),与在微原电池反应中失去电子形成的Fe3+和Fe2+共沉淀形成Fe2(AsO4)3、Fe3(AsO4)2,被吸附固定于铁碳微电解材料表面和多孔结构中,从而实现As(Ⅲ)的去除,去除机理如图10所示。
-
利用Design Expert 11.1.0.1软件对制备条件进行优化得到各植物基铁碳微电解材料最优预测模型,结果如表6所示。取100 mL 10 mg·L−1的NaAsO2溶液于250 mL锥形瓶中,调节pH至3后,加入根据最优制备条件分别制得的5 g植物基铁碳微电解材料,放入恒温摇床(25 ℃,100 r·min−1),避光振荡,计算得到反应48 h后芦苇杆和芦苇叶对As(Ⅲ)的去除率分别为98.12%和97.22%,与模型预测值的相对偏差分别为1.01%、1.46%,再次表明回归方程拟合度高,说明模型能够较好地适用于3个因数对芦苇叶和芦苇秆植物基铁碳微电解材料对砷去除率的影响。
-
1)在Fe/C=1.06、碳化温度为507.87 ℃、焙烧温度为751.92 ℃下制备的芦苇秆植物基铁碳微电解材料对As(Ⅲ)去除率最高;在Fe/C=1.01、碳化温度为498.65 ℃、焙烧温度为701.09 ℃条件下制备得到的芦苇叶植物基铁碳微电解材料对As(Ⅲ)去除率最高。5 g芦苇杆和芦苇叶对 10 mg·L−1 NaAsO2去除率分别为98.12%和97.22%。
2)植物基生物质的不同组分中,木质素含量占比越大,制备铁碳微电解材料烧制温度及还原性铁投加量越高;同时在超过700 ℃焙烧温度下有Fe3C生成,形成Fe3C-Fe微原电池反应,从而提高对As(Ⅲ)的去除效率。
3)当植物基生物质的不同组分中纤维素和半纤维素含量较高,木质素含量较低时,较低的碳化和焙烧温度能降低材料中灰分含量,提高有效碳质量分数,增加微原电池数量,从而提升对As(Ⅲ)的去除率;当植物基生物质组分中木质素含量较高时,提升焙烧温度,植物基氧化官能团增多并形成具有类石墨结构的Π共轭的晶格体系,使得植物基铁碳材料氧化及电子传递能力提高,从而可提高As(Ⅲ)的去除效率。
不同生物质组分对铁碳微电解材料制备及As(Ⅲ)去除效率的影响
Effects of different biomass components on the preparation of Fe-C microelectrolytic materials and As(III) removal efficiency
-
摘要: 以湿地植物芦苇、还原铁粉和膨润土为原材料,通过“均质化—碳化—焙烧”工艺,制备用于去除水体中As(Ⅲ)的植物基铁碳微电解材料,对其采用傅里叶红外光谱(FIR)、元素分析、X射线衍射分析及热重差热综合热分析(TGA)等方法进行系统表征,结合响应面优化研究对比得到不同植物基生物质前驱体(芦苇秆和芦苇叶)的不同组分(半纤维素、纤维素和木质素)对植物基铁碳微电解材料性能的影响。通过响应面模型获得的优化制备条件为:芦苇秆植物基铁碳微电解材料为Fe/C=1.06、碳化温度507.87 ℃、焙烧温度751.92 ℃,芦苇叶植物基铁碳微电解材料为Fe/C=1.01、碳化温度498.66 ℃、焙烧温度701.09 ℃,在此条件下5 g制备得到的材料对100 mL 10 mg·L−1 NaAsO2去除率分别为98.12%和97.22%。结果表明:在植物基生物质前驱体的不同组分中,纤维素和半纤维素含量越高,较低的碳化和焙烧温度能降低材料中灰分含量,提高有效碳元素质量分数,增加微原电池数量;木质素含量越高,高焙烧温度下,氧化及电子传递能力越强,有利于As(Ⅲ)转化为As(Ⅴ),从而提高As(Ⅲ)的去除效率。Abstract: A plant-based iron carbon microelectrolysis material for removing As(III) from water was prepared by using reed, reduced iron powder and bentonite as raw materials through a homogenization-carbonization-roasting process. It was characterized by Fourier transform infrared spectroscopy (FTIR), Elemental analysis, X-ray diffraction (XRD) and Thermogravimetric differential thermal synthesis (TGA). The effects of different components (hemicellulose, cellulose and lignin) of different plant-based biomass precursors (reed stalk and reed leaf) on the properties of plant-based Fe-C microelectrolytic materials were compared by response surface optimization. The optimal preparation conditions obtained by response surface model were as follows: Fe/C of 1.06, carbonization temperature of 507.87 ℃ and roasting temperature of 751.92 ℃; Fe/C of 1.01, carbonization temperature of 498.66 ℃ and roasting temperature of 701.09 ℃. The removal rates of 100 mL 10 mg·L−1 NaAsO2 by 5 g material based on above two conditions were 98.12% and 97.22%, respectively. The results showed that in different components of plant-based biomass precursors, the higher the content of cellulose and hemicellulose, the lower the carbonization and calcination temperature, as well as the lower the ash content, the higher the effective carbon content, and the higher the number of microgalvanic cells. The higher the lignin content is, the stronger the oxidation and electron transfer capacity is at high roasting temperature, which was conducive to the conversion of As (Ⅲ) to As (Ⅴ) and the improvement of As (Ⅲ) removal efficiency.
-
Key words:
- iron carbon microelectrolysis material /
- response surface method /
- As(Ⅲ) /
- reed /
- sintering temperature
-
类金属砷是自然界(大气、土壤、水等环境介质)中普遍存在的元素,对人体有一定的毒性作用,易致癌[1]。自然条件下砷的释放很少达到对人体有害的程度,砷的释放、迁移和污染大多是由人类活动造成的,包括含砷矿物的开采、冶炼以及含砷物质的使用[2]。在我国,有超过15万km2的地区,近2 000万人生活在砷含量超过10 μg·L−1(WTO饮用水标准)水高砷污染区中;全世界约有2×109人受到高砷暴露的威胁[3]。因此,开发合理、高效的砷污染治理技术对保护人类健康、保障饮水安全具有重要意义。
砷在污染水体中主要形态为+3价(还原环境,亚砷酸根(AsO33−))和+5价(氧化环境,砷酸根(AsO43−)),其中,As(Ⅲ)毒性约为As(V)的25~60倍[4]。依据砷的不同形态及包括吸附、氧化-还原和混凝-沉淀等去除原理,目前治理砷污染水体的方法主要有离子交换和膜分离技术[5-6]、混凝沉淀法[7]、吸附法[8]和生物修复技术[9]。其中,离子交换法和膜分离技术是最有效的去除方法,但因使用价格昂贵,很难推广应用;混凝沉淀和吸附法,作为传统的水净化方法,需要消耗大量的化学制品,且因砷污染水体中含有复杂的有机物和竞争离子存在,使去除效果成为一项重大挑战;生物修复技术因其应用成本低和环境友好性等优势受到广泛关注,但因修复效率低,处理时间长和适宜生长环境控制较难的问题而受到很大限制。
近年来,基于铁碳微电解材料产生微原电池反应去除水体污染物的处理方法,受到越来越多研究者的关注[10]。该方法利用铁碳微电解材料兼具催化氧化、絮凝沉淀和氧化还原等污染物去除原理,具有效果稳定,应用范围广,操作方便等优点[11-12]。但传统铁碳微电解材料主要是将还原铁粉或废铁屑、工业碳粉和一定的粘结剂按照一定比例混匀由固定模具压制成型后通过高温烧结制备完成[13],需消耗大量碳粉和粘结剂等工业制成品,提升了制备成本。并且工业粘结剂在高温焙烧下易板结,不利于铁碳材料烧制过程中孔隙结构的产生,在砷污染水体的去除过程中,降低了Fe-C反应电极与含砷废水的接触面积,阻碍微原电池反应过程中的电子转移,去除效率不佳[14-15]。
鉴于此,在笔者所在团队前期研究基础上[16],提出了一种利用人工湿地芦苇制备得到的植物粉末取代工业碳粉,膨润土作为支撑剂和粘结剂,与还原铁粉(Fe0)按一定添加比例混匀,在一次性梯度升温条件下烧制成型的制备工艺。此外,有研究表明,植物基生物质材料在碳化后,具有较好稳定性和电化学性能,与还原性铁Fe0形成持续的Fe-C微原电池反应,而被当做良好的阴极材料[17]。但在一定的烧制温度下,碳基材料的性质如电子传递、稳定性和氧化还原能力,在很大程度上由其生物质前驱体的不同组分(半纤维素、纤维素和木质素)决定[18]。因此,本研究采用响应面法探究芦苇叶和芦苇秆植物基铁碳微电解材料最优制备条件,并且探讨了2种植物基中各主要生物质组分与制备条件相互作用对铁碳微电解材料除As(Ⅲ)效率与机理的影响,以期为铁碳微电解材料优化制备及其应用于含砷废水的处理提供参考。
1. 材料与方法
1.1 试剂与仪器
还原铁粉(Fe0)、亚砷酸钠(NaAsO2)、氢氧化钠(NaOH)、盐酸(HCl)等均为分析纯(国药集团化学试剂有限公司);纤维素、半纤维素和木质素(阿拉丁试剂(上海)有限公司);钙基膨润土(辽宁省锦州市锦州金泰膨润土有限公司);实验用水均为去离子水。
实验过程所使用的仪器:DFT-100A型小型粉碎机(浙江大德仪器有限公司);TSQ-280型恒温振荡器(上海精宏有限公司);SXW-1200 ℃型马弗炉(上海实研有限公司)。
分析测试方法:铁碳微电解材料中铁含量采用《直接还原铁-金属铁含量的测定-三氯化铁分解重铬酸钾滴定法》(GB/T 38812.2-2020)方法进行测定[19];植物基原材料芦苇秆与芦苇叶中碳元素含量采用元素分析仪(Elementar Vario EL III,德国Elementar公司)测定;溶液中砷浓度采用电感耦合等离子体原子发射光谱(ICP-OES,Optima,美国PerkinElmer公司)测定;铁碳微电解材料中碳化铁(Fe3C)采用X射线衍射仪(XRD,D8 Advance,布鲁克AXS公司)进行物相分析[20];铁碳微电解材料的形貌采用场发射扫描电子显微镜(蔡司Gemini360/560场发射扫描电镜,德国蔡司公司)进行分析;铁碳微电解材料及纤维素、半纤维素及木质素在氮气保护下采用热重-差热综合热分析仪(TGA,Diamond TG/DTA,美国PerkinElmer公司)进行热稳定性分析(TG-DTG);铁碳微电解材料比表面积及孔隙度采用比表面积和孔隙度分析仪(TriStar II Plus Series,美国麦克仪器公司)测试。
1.2 植物基铁碳微电解材料的制备
选用西南科技大学流域生态环境与水污染控制实训中心人工湿地的芦苇为生物质原料,将其按芦苇秆和芦苇叶进行分选,去除杂质并洗净,置于烘箱中在80 ℃条件下烘至恒重后利用破碎机进行破碎,过200目筛,收集于棕色瓶中保存待用。将还原铁粉、植物粉末样品、膨润土、水按一定配比混匀加入造粒机中制备成直径大小为1~1.5 cm的球形颗粒,然后移入马弗炉烧制。烧制步骤为:先由室温升至设定碳化温度并恒温一段时间,进行绝氧热解碳化处理,在碳化过程结束后,继续升温至设定焙烧温度并恒温一段时间,进行绝氧焙烧成型处理,最后自然冷却得到植物基铁碳微电解材料。
2. 结果与讨论
2.1 响应面法研究优化制备条件
制备植物基铁碳微电解材料的主要影响因子包括铁碳摩尔比(Fe/C)、碳化温度和焙烧温度。第一步根据单因素预备实验,确定各因子最佳水平的适当范围,后采用Box-Behnken模型进行实验设计。
1)单因素预备实验。取100 mL 10 mg·L−1的NaAsO2溶液于250 mL锥形瓶中,调节pH至3后,加入5 g植物基铁碳微电解材料,放入恒温摇床(25 ℃,100 r·min−1),避光振荡,计算反应48 h时间下的As(Ⅲ)去除率。铁碳微电解材料中砷去除率根据式(1)进行计算。
R=(A0−AiA0)×100% (1) 式中:R表示砷脱除率,%;A0表示铁碳微电解材料处理前溶液中砷的质量浓度,mg·L−1;Ai表示铁碳微电解材料处理后溶液中砷的质量浓度,mg·L−1。
铁碳摩尔比(Fe/C)最适条件范围。在铁碳微电解原材料配比中,铁与植物材料的添加量直接影响除砷过程中的“Fe-C原电池对”数量。根据芦苇秆与芦苇叶中的碳含量(表1),通过式(2)计算得到还原性铁添加量。
表 1 湿地植物基材料的理化性质(基于干基计算)Table 1. Physicochemical properties of plant - based materials in wetland样品名称 元素组成% 原子比 灰分% C H N O S H/C O/C (O+N/C) 芦苇叶 39.42 3.65 1.05 49.49 0.12 1.11 0.94 0.96 8.37 芦苇秆 44.64 4.42 1.84 44.93 0.09 1.19 0.75 0.79 7.76 注:O%=100%-(C%-N%+H%+S%+灰分%) M1=56PM012 (2) 式中:M0为植物粉末质量,g;M1为还原铁粉(Fe0)质量,g;P为植物粉末中碳元素质量分数。
当碳化温度为500 ℃,恒温120 min,焙烧温度为700 ℃,恒温60 min时,不同Fe/C摩尔比条件下As(Ⅲ)的去除率如图1(a)所示。由图1(a)可知,As(Ⅲ)去除率随着Fe/C摩尔比的增加呈现先增加后下降的趋势,在Fe/C摩尔比为1时As(Ⅲ)去除效果最好。因此,选择Fe/C摩尔比为1进行后续实验。
当Fe/C摩尔比为1时,在碳化恒温120 min以及焙烧温度700 ℃、恒温60 min条件下,制得不同碳化温度条件下的铁碳微电解材料,As(Ⅲ)的去除率如图1(b)所示。由图1(b)可知,As(Ⅲ)去除率随着碳化温度的增加呈现逐渐增加再降低的趋势,在500 ℃去除效果最好。因此,选择碳化温度为500 ℃进行后实验。
当在Fe/C摩尔比为1,碳化温度为500 ℃时,在碳化恒温120 min和焙烧恒温60 min条件下,制得不同焙烧温度条件下的铁碳微电解材料,As(Ⅲ)的去除率如图1(c)所示。由图1(c)可知,以芦苇秆和芦苇叶为植物基材料制备的铁碳微电解材料对As(Ⅲ)去除率随着焙烧温度的升高,呈现先升后降的趋势,且在700 ℃时As(Ⅲ)的去除率最高。
2)响应面Box-Behnken设计。在前期单因素预备实验得到的各因子最佳水平的适当范围,运用Design Expert 11.1.0.1软件中的中心复合设计模块(Box-Behnken,BBD)[21]以3个因素作为自变量,砷去除率为响应值设计得到如表2所示的3因素3水平的响应曲面实验。
表 2 Box-Behnken 设计实验因素与水平Table 2. Factors and levels in Box-Behnken design水平 因素 A(铁碳摩尔比(Fe/C)) B(碳化温度/ ℃) C(焙烧温度/ ℃) −1(低水平) 0.8 450 600 0(中水平) 1.0 500 700 1(高水平) 1.2 550 800 在响应曲面交互作用实验中,实验影响因素(自变量)与实验指数(因变量)之间的回归关系可以通过响应面方法(RSM)[22]进行分析,这种回归既可能是曲线关系,也可能是曲面关系,如式(3)所示。
Y=b0+b1A+b2B+b3C+b12AB+b13AC+b23BC+b11A2+b22B2+33C2 (3) 式中:bi是线性效应;bii是第二次效应;bij是相互作用效应;A为铁炭摩尔比;B为碳化温度;C为焙烧温度。
2.2 响应面模型优化设计结果及模型检验
根据Box-Behnken实验设计制备得到的铁碳微电解材料对As(Ⅲ)去除效率如表3所示。
表 3 Box-Bennken响应面实验设计及结果Table 3. Box-Behnken response surface experiment design and results实验编号 因素 As(Ⅲ)去除率(芦苇秆)/% As(Ⅲ)去除率(芦苇叶)/% A B C 1 0.8 450 700 85.47 90.71 2 1.2 450 700 91.86 94.02 3 0.8 550 700 90.83 92.26 `4 1.2 550 700 95.36 96.26 5 0.8 500 600 70.36 74.71 6 1.2 500 600 76.39 78.44 7 0.8 500 800 96.49 87.78 8 1.2 500 800 99.26 93.26 9 1 450 600 70.18 72.43 10 1 550 600 76.68 80.36 11 1 450 800 96.14 93.25 12 1 550 800 97.48 87.58 13 1 500 700 95.53 98.33 14 1 500 700 96.05 98.36 15 1 500 700 95.81 99.12 16 1 500 700 96.42 98.92 17 1 500 700 96.63 98.56 将表3中的总砷去除率输入到Design Expert 11.1.0.1软件中进行数据分析和多元二次回归拟合,得到依照编码因数的二次响应曲面方程如式(4)和式(5)所示,实际因素的二次响应曲面方程如式(6)和式(7)所示。
Y(芦苇杆)=96.09+2.5A+2.09B+12.01C−0.47AB−0.74AC−1.29BC−2.31A2−2.89B2−8.07C2 (4) Y(芦苇叶)=96.66+2.06A+0.76B+6.99C+0.17AB+0.44AC−3.4BC−2.6A2−2.74B2−12.51C2 (5) Y(芦苇杆)=−903.68+177.36A+1.43B+1.42C−0.047AB−0.037AC−0.00026BC−57.85A2−0.00116B2−0.000807C2 (6) Y(芦苇叶)=−1134.61+116.46A+1.57B+2.14C+0.0173AB+0.0219AC−0.00068BC−65.04A2−0.0011B2−0.00125C2 (7) 为说明响应面模型及回归方程的有效性,对回归方程的可行性及相关性进行分析,得到分析结果如表4所示。R2均在95%置信区间内,同时校正系数与预测系数差值(Radj-RPred)均小于0.2,表明实测值与预测值之间偏差较小,拟合度好,可信度高;失拟影响因素项结果均不显著(P>0.05),代表失拟因素对于纯粹的误差可忽略不计,无失拟因数存在,3个自变量与响应值之间存在显著的相关性,误差小;精密度均大于4(一般认为较好拟合模型的精密度需大于4[23]),说明可实用性高,真实实验结果可用上述回归方程代替分析。同时从三因素间交互作用的相关系数可以看出:铁碳摩尔比(A)与焙烧温度(C)、碳化温度(B)与焙烧温度(C)之间的交互影响大于铁碳摩尔比(A)与碳化温度(B)间的交互作用,并表现为显著水平(P<0.05)。因此,本研究选择AC与BC因素之间的交互影响探究不同植物基生物质组分对铁碳微电解材料的除砷效率的影响。
表 4 回归方程可行性分析Table 4. Feasibility analysis of regression equation模型项 芦苇秆 芦苇叶 F值 P值 F值 P值 模型 582.18 <0.000 1 777.89 <0.000 1 A-Fe/C 163.52 <0.000 1 193.40 <0.000 1 B-碳化温度 113.79 0.001 7 25.94 0.001 4 C-焙烧温度 3 764.79 <0.000 1 2 216.75 <0.000 1 AB 2.82 0.136 8 0.674 8 0.438 5 AC 7.15 0.031 8 4.34 0.045 7 BC 21.73 0.002 3 262.14 <0.000 1 A2 73.59 <0.000 1 161.55 <0.000 1 B2 115.10 <0.000 1 179.73 <0.000 1 C2 895.90 <0.000 1 3 735.07 <0.000 1 失拟项 2.27 0.222 7 2.04 0.250 9 R2 0.998 7 0.999 0 Radj 0.997 0 0.997 7 RPred 0.985 8 0.989 7 精密度 70.043 1 81.958 7 注:P <0.05表示差异显著。 2.3 不同生物质组分对最适制备温度的影响
由响应面分析得到碳化温度和焙烧温度2个因素交互作用对总砷去除率的影响如图2和图3所示。在等高线图中,当中心图形呈现椭圆形时,交互作用强;而接近圆形时,交互作用弱。在响应曲面中,响应面坡度越大,自变量条件的改变对响应值的影响越大,反之影响越小。而当两者图形中的颜色越深代表响应值越大,效果越明显[24]。
由图2、图3中的等高线图看出,碳化温度与As(Ⅲ)的去除率呈正相关,焙烧温度与As(Ⅲ)去除率呈现先增后减的趋势,这与单因素实验结果一致。同时由等高线图也可以看出,沿C因素向峰值移动的等高线密度明显高于沿B因素向峰值移动的等高线密度,表明焙烧温度对响应值的贡献最大,这与方差分析得到的结果一致。响应面坡度陡峭,说明碳化温度与焙烧温度具有显著的交互作用,对As(Ⅲ)的去除影响较大。
作为植物基铁碳微电解阴极材料与Fe0在水溶液中形成微原电池,发生原电池反应,其生物质组分主要由木质素、纤维素和半纤维素三种主要有机物构成,通过非共价键相互交联在一起构成植物骨架。在隔氧条件下进行梯度升温,会生成生物炭、生物油以及不可冷凝气体。生物炭具有稳定的芳香化、脂肪族链状结构及丰富的孔隙分布,具有良好的稳定性和吸附性[25],能提高植物基铁碳微电解材料对As(Ⅲ)的去除。生物炭产量、表面官能团的种类和数量以及碳化方式与温度紧密相关。目前,主要碳化方式有慢速热解、高温气化和水热碳化法[26]。与其它热解方法相比,慢速热解因其固相收率较高,气相产物释放较慢且均匀,更适于植物基材料碳化。在隔氧梯度升温过程中,植物基碳化为生物炭中碳含量增多,氧、氢及灰分比例下降。温度越高,碳化程度越高,生物炭越稳定,但生物炭产量与烧制温度成非线性关系,主要是由于不同湿地植物生物质组分中纤维素、半纤维素和木质素含量和稳定性的差异性造成的[27-28]。通过热重分析仪测试分析得到纤维素、半纤维素和木质素热稳定性如图4所示。
由图4中可以看出,当碳化温度超过400 ℃时,大部分半纤维素和纤维素被热解。木质素因高稳定性而最难分解,分解温度范围跨度大(160~900 ℃),能在超过700 ℃热解条件下使更多的碳基结构被保留了下来,成为植物基碳基成分中最主要的碳元素来源[29]。
芦苇叶与芦苇秆植物基铁碳微电解材料的微商热重分析(DGT)与失重速率(TG)曲线如图5所示。由图5可以看出,在100 ℃左右时开始出现由铁碳微电解材料水分蒸发所导致的的失重峰。随着梯度升温至400 ℃左右时,植物基铁碳材料出现了不同程度的失重,其中芦苇叶植物基铁碳微电解材料的失重大于芦苇秆植物基铁碳微电解材料(△m1>△m3)。其原因可能是前者纤维素与半纤维素含量大于后者,在较低温度时更容易热解成生物炭,质量损失较快。但随着温度梯度的继续升高伴随着木质素的大量热解,含有更多木质素组分的芦苇秆植物基铁碳微电解材料迅速碳化,质量损失加快(△m2>△m4)。
因此,在相同Fe/C摩尔比和低于700 ℃焙烧温度下,芦苇叶植物基铁碳微电解材料对As(Ⅲ)去除率高于芦苇秆植物基铁碳微电解材料。分析认为,在低于700 ℃焙烧温度时,芦苇秆植物基有机成分中含有大量木质素,难以被完全碳化形成稳定的碳基材料,所以铁碳材料中有效碳元素比例较低;而芦苇叶植物基拥有大量能在较低温度下碳化的纤维素与半纤维素,碳化充分,有效碳元素含量多,能与Fe0形成大量的Fe0-C原电池参与反应,因此As(Ⅲ)去除率高。当焙烧温度超过700 ℃时,芦苇秆植物基中未被完全碳化的木质素被二次碳化,有效碳元素含量增加,Fe0-C原电池数量上升,As(Ⅲ)去除率提高。而芦苇叶植物基在第一次碳化过程中形成的碳基成分在二次碳化过程中灰分质量分数增加,有效碳元素降低,Fe0-C原电池数量下降,导致去除率下降。
2.4 不同生物质组成对还原铁粉(Fe0)投加量的影响
Fe/C摩尔比和焙烧温度2个因素交互作用对As(Ⅲ)去除率的影响如图6和图7所示。由响应面结果可以看出,并非碳含量比例越高,处理效果越明显。当Fe/C=0.8时,随着植物基生物质组分的不断碳化,碳含量线性增加,不仅导致Fe0的相对含量降低,微原电池数量的损失,而且过多的碳化成分在植物基铁碳微电解材料表面堆积,导致Fe0活性点位与As(Ⅲ)接触减少,导致去除率降低[30]。当Fe/C=1.2,即Fe0的含量过量时,随着焙烧温度上升,植物基的有效碳含量增加,微电解体系中阴极数量增多,配对原电池数量增加,电极反应加快。但过量Fe0和水中微电解产生的Fe2+使溶液体系保持为还原环境,抑制As(Ⅲ)向As(Ⅴ)的转化过程,降低铁盐对As(Ⅲ)的去除率。原因在于植物基铁碳材料中铁盐混凝作用去除水溶液中As(Ⅲ)主要是通过在微原电池反应中失去电子形成的Fe3+和Fe2+离子与As(Ⅲ)和As(Ⅴ)形成亚砷酸铁、砷酸铁等沉淀作用;以及Fe(OH)2及Fe(OH)3等沉淀对水相中的As(Ⅲ)和As(Ⅴ)的静电吸附作用[31]。主要的As(Ⅲ)化合物(AsO33−)具有中性电荷,As(V)化合物(H2AsO4−、HAsO42−)在4-10的pH范围内带负电荷。因此,对于铁盐的氢氧化物沉淀而言,对As(V)化合物静电引力更强;As(V)化合物更容易被扩散吸附到铁盐水解沉积物表面,从而被吸附去除。
芦苇秆植物基铁碳微电解材料在Fe/C=1.06时,即Fe0过量6%左右时取得最大响应值。一方面,在铁碳微电解材料制备过程中,材料表面Fe0存在被氧化的风险,且温度越高,氧化越明显。因此,在高温烧制芦苇秆植物基铁碳微电解材料时,需投加过量Fe0来保证Fe0-C微原电池数量,同时不会对溶液氧化还原环境造成破坏,使反应持续进行。另一方面,在超过700 ℃的焙烧温度下,Fe0与植物基中碳化成分进一步碳化形成Fe3C[32],减少铁碳微电解材料表面积碳,释放Fe0活性位点,使得阴阳两电极之间电子转移速率提高。并且Fe3C可作为微原电池反应的阴极,能与Fe0形成Fe3C-Fe0微原电池反应[33],有效填补高温焙烧下,富含木质素芦苇秆植物基碳化成分中作为阴极碳元素含量的减少,使得Fe3C-Fe0和Fe0-C 2种微原电池能共同作用,提高溶液中的原电池数量。对于木质素含量不足的芦苇叶植物基,随着焙烧温度的提高,在接近750 ℃时,Fe3C作为微原电池阴极,不能够有效填补高温焙烧下,植物基碳化成分中作为阴极碳元素含量的减少,导致阴极数量减少,从而降低As(Ⅲ)的去除率,因此焙烧温度不宜超过700 ℃,在Fe/C=1.01时取得最佳制备条件。铁碳微电解表面材料的SEM和XRD分析表征结果如图8所示。可见,芦苇秆植物基铁碳微电解材料750 ℃出现类似鱼鳞状Fe3C特征物象及Fe3C特征峰[34],证实有Fe3C的生成。
2.5 不同生物质组成对电子转移及氧化As(Ⅲ)能力的影响
芦苇叶和芦苇秆在300~800 ℃下制备的碳基材料的元素组成以及H/C、O/C和O+N/C比如表5所示。由表5可知,随着梯度升温的进行,在碳化过程中碳元素和灰分相对含量增加,氧元素和氢元素含量降低。这是由于温度升高,C—H、C—O键断裂,氢和氧元素在此过程中发生脱附丢失所致[35-36]。对于碳基材料,石墨晶格中存在的层状组成分子(LCM)更稳定。因此,在碳化过程中,随着热解温度的升高更多的杂原子丢失(O、N、S、P),碳原子趋向重排为六元环系统的大分子网络,以试图获得这种更稳定的类似石墨状态[37]的芳香结构。其中,由H/C原子比可知碳基材料芳香性的大小,H/C越低,芳香化程度越高。在植物基铁碳微电解材料中,高芳香性的碳基结构,具有更高的反应活性[38],有利于促进Fe0-C微原电池的电子转移,加快反应的进行。木质素因碳含量高和热稳定性强的因素,在高温条件下,生物质中木质素占比越高,H/C越低,碳基成分芳香性越强。因此,在高温焙烧条件下,生物质中木质素含量越高的植物基铁碳微电解材料,越有利于Fe-C微原电池反应的进行。
表 5 不同温度烧制下植物基碳化成分的元素组成和原子比Table 5. Elemental composition and atomic ratio of plant-based carbonization components fired at different temperatures样品 元素组成/% 原子比 灰分/% C H N O S H/C O/C (O+N/C) 芦苇叶300 45.12 3.25 1.52 36.76 0.16 0.86 0.63 0.64 16.23 芦苇叶400 50.76 3.12 2.46 26.07 0.17 0.74 0.39 0.43 22.34 芦苇叶500 48.15 2.19 1.92 21.16 0.16 0.55 0.33 0.36 30.26 芦苇叶600 46.12 2.04 1.76 15.02 0.19 0.53 0.24 0.28 38.39 芦苇叶700 44.23 1.63 1.71 9.24 0.32 0.44 0.16 0.19 46.29 芦苇叶800 41.23 1.14 1.29 7.37 0.17 0.33 0.13 0.16 51.38 芦苇秆300 51.58 4.23 1.77 35.06 0.54 0.98 0.51 0.54 10.36 芦苇秆400 57.21 3.02 2.42 24.82 1.05 0.63 0.33 0.36 16.32 芦苇秆500 59.39 2.27 2.13 14.49 0.95 0.46 0.18 0.21 25.03 芦苇秆600 61.54 1.45 1.94 7.02 1.26 0.28 0.09 0.58 30.67 芦苇秆700 63.25 1.13 2.61 5.36 0.83 0.21 0.07 0.10 32.04 芦苇秆800 52.49 1.09 1.98 4.75 0.75 0.25 0.07 0.10 43.28 注:O%=100%-(C%-N%+H%+S%+灰分%) 具有不同生物质组分的植物基材料其热解产物在氧化还原性质上也表现出较明显的差异[39]。热解产物氧化还原性质主要由有机官能团决定,如提供电子的羧基和酚羟基(还原剂)和接收电子的醌类和半醌类(氧化剂)等。随着热解的进行,有机成分中碳、氢和氧在热解过程中发生化学键的断裂,重组形成新的含氧和含碳官能团,从而表现出不同的氧化还原性质[25]。
不同烧制温度下芦苇秆与芦苇叶植物基的傅里叶红外光谱图(FTIR)如图9所示。在低温热解过程中(200~500 ℃),植物有机质热解产生大量含氢和氧官能团,此时碳基材料官能团以羧基和酚羟基的供电子官能团为主,包括C—H(1 384~1 402 cm−1)、—COOH(1 692 cm−1)、CH2(2 825 cm−1)、O—H(3 200~3 400 cm−1)等。芦苇叶植物基因富含易热解的半纤维素和纤维素组分,在低温热解过程官能团演化更完全[40],此时有芦苇叶植物基还原能力强于芦苇秆植物基。随着热解温度的提高,—COOH、—CH2、C—H和O—H的伸缩振动的强度逐渐降低直至消失,原因在于随着温度的升高,大量C—H、C—O键断裂,氢和氧以水分子形式丢失,官能团转以氧原子为中心的向以碳原子为中心转移。此时—O—(1 114 cm−l)、C=O、C=C(1 612~1 692 cm−1)和C—O(1 210 cm−1)伸缩振动存在,碳基材料以羰基和醌基的得电子氧化官能团为主。木质素因结构刚性强,难以分解,所以在高热解温度下官能团演变更稳定[41],因此,芦苇秆植物基在1 612~1 692 cm−1处C=O、C=C和1 210 cm−1处C—O的峰值更强,氧化能力强于芦苇叶植物基。并在1 556 cm−1处出现新的特征峰,可能是由于木质类生物质在高温下芳香化,形成具有类石墨结构的Π共轭的晶格体系[37]。
2.6 植物基铁碳微电解材料对As(Ⅲ)去除机理
因植物基材料在热解过程中受热碳化并产生物油和不可冷凝气体,从而体积萎缩,使得植物基铁碳微电解材料形成连续贯通的多孔结构。本研究通过压汞法测得的植物基铁碳微电解材料的比表面积为1.1~1.2 m2·g−1、孔隙率为64.8%~65.32%,这有利于Fe-C微原电池反应的进行。植物基铁碳微电解材料对As(Ⅲ)去除机理可包括以下2条路径:一是在开始的反应过程中,As(Ⅲ)直接被植物基铁碳微电解材料吸附而去除;二是As(Ⅲ)被碳基材料中C=O、C=C和C—O等得电子官能团氧化为As(V),与在微原电池反应中失去电子形成的Fe3+和Fe2+共沉淀形成Fe2(AsO4)3、Fe3(AsO4)2,被吸附固定于铁碳微电解材料表面和多孔结构中,从而实现As(Ⅲ)的去除,去除机理如图10所示。
3. 优化条件确定及模型
利用Design Expert 11.1.0.1软件对制备条件进行优化得到各植物基铁碳微电解材料最优预测模型,结果如表6所示。取100 mL 10 mg·L−1的NaAsO2溶液于250 mL锥形瓶中,调节pH至3后,加入根据最优制备条件分别制得的5 g植物基铁碳微电解材料,放入恒温摇床(25 ℃,100 r·min−1),避光振荡,计算得到反应48 h后芦苇杆和芦苇叶对As(Ⅲ)的去除率分别为98.12%和97.22%,与模型预测值的相对偏差分别为1.01%、1.46%,再次表明回归方程拟合度高,说明模型能够较好地适用于3个因数对芦苇叶和芦苇秆植物基铁碳微电解材料对砷去除率的影响。
表 6 各植物基铁碳微电解材料最优制备条件Table 6. The optimum preparation conditions of plant-based Fe-C microelectrolysis materials碳基材料 Fe/C摩尔比 碳化温度/℃ 焙烧温度/℃ As(Ⅲ)去除率/% 相对偏差/% 预测值 实验值 芦苇秆 1.06 507.87 751.92 99.19 98.12 1.07 芦苇叶 1.01 498.65 701.09 98.66 97.22 1.46 4. 结论
1)在Fe/C=1.06、碳化温度为507.87 ℃、焙烧温度为751.92 ℃下制备的芦苇秆植物基铁碳微电解材料对As(Ⅲ)去除率最高;在Fe/C=1.01、碳化温度为498.65 ℃、焙烧温度为701.09 ℃条件下制备得到的芦苇叶植物基铁碳微电解材料对As(Ⅲ)去除率最高。5 g芦苇杆和芦苇叶对 10 mg·L−1 NaAsO2去除率分别为98.12%和97.22%。
2)植物基生物质的不同组分中,木质素含量占比越大,制备铁碳微电解材料烧制温度及还原性铁投加量越高;同时在超过700 ℃焙烧温度下有Fe3C生成,形成Fe3C-Fe微原电池反应,从而提高对As(Ⅲ)的去除效率。
3)当植物基生物质的不同组分中纤维素和半纤维素含量较高,木质素含量较低时,较低的碳化和焙烧温度能降低材料中灰分含量,提高有效碳质量分数,增加微原电池数量,从而提升对As(Ⅲ)的去除率;当植物基生物质组分中木质素含量较高时,提升焙烧温度,植物基氧化官能团增多并形成具有类石墨结构的Π共轭的晶格体系,使得植物基铁碳材料氧化及电子传递能力提高,从而可提高As(Ⅲ)的去除效率。
-
表 1 湿地植物基材料的理化性质(基于干基计算)
Table 1. Physicochemical properties of plant - based materials in wetland
样品名称 元素组成% 原子比 灰分% C H N O S H/C O/C (O+N/C) 芦苇叶 39.42 3.65 1.05 49.49 0.12 1.11 0.94 0.96 8.37 芦苇秆 44.64 4.42 1.84 44.93 0.09 1.19 0.75 0.79 7.76 注:O%=100%-(C%-N%+H%+S%+灰分%) 表 2 Box-Behnken 设计实验因素与水平
Table 2. Factors and levels in Box-Behnken design
水平 因素 A(铁碳摩尔比(Fe/C)) B(碳化温度/ ℃) C(焙烧温度/ ℃) −1(低水平) 0.8 450 600 0(中水平) 1.0 500 700 1(高水平) 1.2 550 800 表 3 Box-Bennken响应面实验设计及结果
Table 3. Box-Behnken response surface experiment design and results
实验编号 因素 As(Ⅲ)去除率(芦苇秆)/% As(Ⅲ)去除率(芦苇叶)/% A B C 1 0.8 450 700 85.47 90.71 2 1.2 450 700 91.86 94.02 3 0.8 550 700 90.83 92.26 `4 1.2 550 700 95.36 96.26 5 0.8 500 600 70.36 74.71 6 1.2 500 600 76.39 78.44 7 0.8 500 800 96.49 87.78 8 1.2 500 800 99.26 93.26 9 1 450 600 70.18 72.43 10 1 550 600 76.68 80.36 11 1 450 800 96.14 93.25 12 1 550 800 97.48 87.58 13 1 500 700 95.53 98.33 14 1 500 700 96.05 98.36 15 1 500 700 95.81 99.12 16 1 500 700 96.42 98.92 17 1 500 700 96.63 98.56 表 4 回归方程可行性分析
Table 4. Feasibility analysis of regression equation
模型项 芦苇秆 芦苇叶 F值 P值 F值 P值 模型 582.18 <0.000 1 777.89 <0.000 1 A-Fe/C 163.52 <0.000 1 193.40 <0.000 1 B-碳化温度 113.79 0.001 7 25.94 0.001 4 C-焙烧温度 3 764.79 <0.000 1 2 216.75 <0.000 1 AB 2.82 0.136 8 0.674 8 0.438 5 AC 7.15 0.031 8 4.34 0.045 7 BC 21.73 0.002 3 262.14 <0.000 1 A2 73.59 <0.000 1 161.55 <0.000 1 B2 115.10 <0.000 1 179.73 <0.000 1 C2 895.90 <0.000 1 3 735.07 <0.000 1 失拟项 2.27 0.222 7 2.04 0.250 9 R2 0.998 7 0.999 0 Radj 0.997 0 0.997 7 RPred 0.985 8 0.989 7 精密度 70.043 1 81.958 7 注:P <0.05表示差异显著。 表 5 不同温度烧制下植物基碳化成分的元素组成和原子比
Table 5. Elemental composition and atomic ratio of plant-based carbonization components fired at different temperatures
样品 元素组成/% 原子比 灰分/% C H N O S H/C O/C (O+N/C) 芦苇叶300 45.12 3.25 1.52 36.76 0.16 0.86 0.63 0.64 16.23 芦苇叶400 50.76 3.12 2.46 26.07 0.17 0.74 0.39 0.43 22.34 芦苇叶500 48.15 2.19 1.92 21.16 0.16 0.55 0.33 0.36 30.26 芦苇叶600 46.12 2.04 1.76 15.02 0.19 0.53 0.24 0.28 38.39 芦苇叶700 44.23 1.63 1.71 9.24 0.32 0.44 0.16 0.19 46.29 芦苇叶800 41.23 1.14 1.29 7.37 0.17 0.33 0.13 0.16 51.38 芦苇秆300 51.58 4.23 1.77 35.06 0.54 0.98 0.51 0.54 10.36 芦苇秆400 57.21 3.02 2.42 24.82 1.05 0.63 0.33 0.36 16.32 芦苇秆500 59.39 2.27 2.13 14.49 0.95 0.46 0.18 0.21 25.03 芦苇秆600 61.54 1.45 1.94 7.02 1.26 0.28 0.09 0.58 30.67 芦苇秆700 63.25 1.13 2.61 5.36 0.83 0.21 0.07 0.10 32.04 芦苇秆800 52.49 1.09 1.98 4.75 0.75 0.25 0.07 0.10 43.28 注:O%=100%-(C%-N%+H%+S%+灰分%) 表 6 各植物基铁碳微电解材料最优制备条件
Table 6. The optimum preparation conditions of plant-based Fe-C microelectrolysis materials
碳基材料 Fe/C摩尔比 碳化温度/℃ 焙烧温度/℃ As(Ⅲ)去除率/% 相对偏差/% 预测值 实验值 芦苇秆 1.06 507.87 751.92 99.19 98.12 1.07 芦苇叶 1.01 498.65 701.09 98.66 97.22 1.46 -
[1] 张维, 齐丽娟, 宁钧宇, 等. 砷的健康危害评估[J]. 毒理学杂志, 2021, 35(5): 367-372. doi: 10.16421/j.cnki.1002-3127.2021.05.002 [2] ZONG L H, SEN L T, PING N. Adsorption of arsenate and arsenite from aqueous solutions by cerium-loaded cation exchange resin[J]. Journal of Rare Earths, 2012, 30(6): 563-572. doi: 10.1016/S1002-0721(12)60092-1 [3] BO Y, YAN L. Preparation and application of modified biochar for arsenic pollution remediation[J]. International Core Journal of Engineering, 2021, 7(11): 46-50. [4] 马杰. 砷在含水介质中迁移转化的胶体效应[D]. 北京: 中国地质大学, 2016. [5] RUI P L, JIU H Q, et al. Review on heterogeneous oxidation and adsorption for arsenic removal from drinking water[J]. Journal of Environmental Sciences, 2021, 110(12): 178-188. [6] 王钟辉, 张祥剑, 曾伍祥, 等. YT膜分离技术在有色冶炼行业脱砷中应用[C]//第四届全国膜分离技术在冶金工业中应用研讨会论文集. 2014: 44-47. [7] NING W, NAN N W, LI T, et al. Removal of aqueous As(III) Sb(III) by potassium ferrate (K2FeO4 ): The function of oxidation and flocculation[J]. Science of the Total Environment, 2020, 726: 138541. doi: 10.1016/j.scitotenv.2020.138541 [8] AYAN D, ABHIJT M. Hydrogeochemical characteristics and its role in controlling arsenic mobilization in a shallow aquifer[J]. Acta Geochimica, 2021, 40(6): 1-14. [9] LINA G. Research on bioremediation technology of soil heavy metal arsenic pollution[J]. International Core Journal of Engineering, 2021, 7(9): 74-76. [10] XIAO Y W, CHUN X L, SHOU F Y, MI L, et al. Uranium (VI) removal from aqueous solution using iron-carbon micro-electrolysis packing[J]. Separation and Purification Technology, 2020, 234: 116104. doi: 10.1016/j.seppur.2019.116104 [11] 吉冰冰, 于英潭, 肖玫, 等. 响应面分析法优化Fe(Ⅲ)-亚硫酸盐去除废水中的砷[J]. 环境工程学报, 2016, 10(9): 4803-4807. doi: 10.12030/j.cjee.201504141 [12] 杜利军, 付兴民, 惠贺龙, 等. 新型铁碳微电解填料制备与除磷性能评价[J]. 环境工程学报, 2020, 14(6): 1421-1427. doi: 10.12030/j.cjee.201903030 [13] 戚永洁, 张波, 蒋素英, 等. 铁碳微电解工艺中的填料板结钝化[J]. 印染, 2017, 43(4): 43-46. [14] 杜利军. 新型铁碳填料的制备与废水除磷性能研究[D]. 合肥: 中国科学院大学(中国科学院过程工程研究所), 2019. [15] ZHAO H D, NIE T, ZHAO H X, et al. Enhancement of Fe-C micro-electrolysis in water by magnetic field: Mechanism, influential factors and application effectiveness[J]. Journal of Hazardous Materials, 2020, 410: 124643. [16] 李密, 谌书, 王彬, 等. 人工湿地植物炭基材料特性及铁碳微电解填料制备[J]. 地球与环境, 2021, 49(1): 82-91. doi: 10.14050/j.cnki.1672-9250.2020.48.106 [17] LI X J, HAI L, QIANG K, et al. Interactions of high-rate nitrate reduction and heavy metal mitigation in iron-carbon-based constructed wetlands for purifying contaminated groundwater[J]. Water Research, 2020, 169(C): 127272. [18] MASUD H, YAN J L, RAVI N, et al. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis[J]. Science of the Total Environment, 2020, 744: 140714. doi: 10.1016/j.scitotenv.2020.140714 [19] 中华人民共和国国家市场监督管理总局, 中国国家标准化管理委员会. 直接还原铁 亚铁含量的测定 三氯化铁分解重铬酸钾滴定法: GB/T 38812.2-2020 [S]. 北京: 中国环境科学出版社, 2020. [20] 杨俊. 高温高压下Fe_3C状态方程研究[D]. 武汉: 武汉理工大学, 2019. [21] 陶厚永, 曹伟. 多项式回归与响应面分析的原理及应用[J]. 统计与决策, 2020, 36(8): 36-40. doi: 10.13546/j.cnki.tjyjc.2020.08.007 [22] 王永菲, 王成国. 响应面法的理论与应用[J]. 中央民族大学学报(自然科学版), 2005(3): 236-240. [23] ABDELKADER H, KARIM M, CHERIF B, et al. Comparison of artificial neural network (ANN) and response surface methodology (RSM) prediction in compressive strength of recycled concrete aggregates[J]. Construction and Building Materials, 2019, 209: 425-436. doi: 10.1016/j.conbuildmat.2019.03.119 [24] KUMAR S, MAZUMDER R. Development and optimization of venlafaxine hydrochloride floating microspheres using response surface plots[J]. Marmara Pharmaceutical Journal, 2018, 22(2): 277-285. doi: 10.12991/mpj.2018.65 [25] KLUPFEL L, KEILUWEIT M, KLEBER M, et al. Redox properties of plant biomass-derived black carbon (biochar).[J]. Environmental science & technology, 2014, 48(10): 5601-5611. [26] VIJAYARAGHAVN K. Recent advancements in biochar preparation, feedstocks, modification, characterization and future applications[J]. Environmental Technology Reviews, 2019, 8(1): 47-64. doi: 10.1080/21622515.2019.1631393 [27] OBEMAH D, BAOWEI Z, WEI Z. Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: An overview[J]. Advances in Materials Science and Engineering, 2014, 2014: 1-12. [28] 崔孝强. 水体修复植物基生物炭的环境应用及其机理研究[D]. 杭州: 浙江大学, 2018. [29] 张月. 生物炭的氧化还原机制及其环境应用[D]. 上海: 上海交通大学, 2019. [30] WEI W M, YU X H, CHUN Y X, et al. The mechanism of synergistic effect between iron-carbon microelectrolysis and biodegradation for strengthening phenols removal in coal gasification wastewater treatment[J]. Bioresource Technology, 2018, 271: 84-90. [31] RABIA A, HAMNA B, IRSHAD B, et al. A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions[J]. Chemical Engineering Journal, 2020, 396(C): 125195. [32] YU Q S, IRIS K M, DANIEL C, et al. Multifunctional iron-biochar composites for the removal of potentially toxic elements, inherent cations, and hetero-chloride from hydraulic fracturing wastewater[J]. Ecology Environment & Conservation, 2019, 124: 521-532. [33] 陈勇, 李义久, 唐文伟. 铁炭微电解法预处理富马酸有机废水的研究[J]. 工业用水与废水, 2003(6): 52-54. doi: 10.3969/j.issn.1009-2455.2003.06.016 [34] 张晓. Fe_3C基电极材料的制备及电催化性能的研究[D]. 西安: 陕西科技大学, 2021. [35] LING Z, XIN D C, ONDREJ M, et al. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures[J]. Journal of Hazardous Materials, 2013, 15(4): 256-257. [36] JUDY A L, KYOUNG S R, CLAUDIA K, et al. Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis[J]. Biofuels, 2011, 2(1): 71-106. [37] MARTIN P K, IGNACIO F C, AIDA N, et al. A multivariate curve resolution evaluation of an in-situ atr-ftir spectroscopy investigation of the electrochemical reduction of graphene oxide[J]. Electrochimica Acta, 2017, 255: 160-167. doi: 10.1016/j.electacta.2017.09.124 [38] JUAN H, YAO X, JING C T, HONG K C, et al. Persulfate activation with sawdust biochar in aqueous solution by enhanced electron donor-transfer effect[J]. Science of the Total Environment, 2019, 48(7): 768-777. [39] SHA S L, LIM I S, HUA Z, et al. Quantifying the contributions of surface area and redox-active moieties to electron exchange capacities of biochar[J]. Journal of Hazardous Materials, 2020, 394: 122541. doi: 10.1016/j.jhazmat.2020.122541 [40] LUISA B, JONAS M, THOMAS A, et al. The effect of the biomass components lignin, cellulose and hemicellulose on tga and fixed bed pyrolysis[J]. Journal of Analytical and Applied Pyrolysis, 2013, 12(1): 177184. [41] HAN P C, ZI H L, XU C, et al. Comparative pyrolysis behaviors of stalk, wood and shell biomass: Correlation of cellulose crystallinity and reaction kinetics[J]. Bioresource Technology, 2020, 310(C): 123498. -






 DownLoad:
DownLoad: