-
随着现代化工农业的发展,水体汞污染问题层出不穷,对生态环境和人类健康构成了威胁[1]。汞是一种具有高毒性、持久性和生物累积性的金属元素[2],由于工业生产和人为活动被排放到环境中,被生物有机体或组织吸收,经由食物链的转移和生物放大效应富集于体内,促使动物体中汞的浓度明显高于其生存环境。人体内积累大量的汞可导致胚胎死亡、细胞病变,甚至机体死亡[3]。因此,高效去除废水中的汞可以保护水资源及人类健康。目前,废水中去除汞离子的典型方法有化学沉淀法[4]、离子交换法[5]、电解法[6]、还原法[7]、溶剂萃取法[8]、吸附法[9]和微生物处理法[10]。其中吸附法作为一种处理重金属污染的高效方法,具有应用范围广,操作简单[11],可重复再生使用等优点。所以,开发新型吸附剂去除并回收废水中的汞具有重要的现实意义。
壳聚糖(chitosan,CS)是一种天然的碱性多糖生物聚合物,主要存在于虾蟹等动物的甲壳中,由壳质中的几丁质脱乙酰制成,在自然界中来源广泛,是一种良好的生物吸附剂[12]。CS分子中含有大量的氨基和羟基等配位基团,对很多重金属离子有良好的吸附作用,可用于化学改性。同时,由于其具有良好的生物相容、无毒害、可降解性、成本低、可持续性生产等特点,这使得CS在众多高分子材料中脱颖而出[13]。然而,CS在酸性介质中易溶解,具有稳定性差且选择性不强等缺点,极大地限制了其在处理废水中重金属方面的应用[14]。WANG[15]等通过戊二醛交联结合交替冻融制得壳聚糖-聚乙烯醇水凝胶,因静电吸引而仅仅获得了200.20 mg·g−1的吸附容量。
根据软硬酸碱理论,作为软碱的巯基(R-SH)可结合软酸Hg(Ⅱ),故很多研究利用巯基改性CS材料以提高其对Hg(Ⅱ)的去除性能和选择性[12,16]。GAVILAN[16]等以硫代氨基甲酰基衍生物改性壳聚糖,硫基的接枝扩展了材料应用的pH范围,在pH为2时,吸附能力高达450 mg·g−1,说明巯基的改性可以克服原始CS材料的缺点。为了克服这些问题,仅仅对CS分子链上丰富的氨基、羟基位点进行巯基改性是不够充分的,进行适当地交联以同时提高耐酸性和机械性能是另一种行之有效的途径。
在众多的含巯基改性剂中,巯基丙酸(3-Mercaptopropionic acid,MPA)具有低廉低毒、溶解性良好的特点。1-乙基-(3-二甲基氨基丙基)碳酰二亚胺(1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride,EDC)分子呈线性结构,作为活化剂已被广泛应用于多肽缩合反应,可将大量巯基接枝于CS上。故本研究基于氨基和羧基的酰胺化反应生成酰胺基,将MPA嫁接于CS上,并利用EDC交联,可增强CS的憎水性和氧阻隔能力[17],从而提高了吸附剂的耐酸性;大量的巯基负载于CS中,可提供丰富的活性位点,从而提高对Hg(Ⅱ)优异的吸附性能和选择性。而目前未有利用MPA进行巯基改性外加EDC交联的CS复合材料的报道。本研究首先使用扫描电镜(SEM)、红外光谱(FTIR)、X射线光电子能谱仪(XPS)、比表面积分析(BET)等方法表征了CS-MPA复合材料的表面形貌、化学官能团及结构组成特征;探讨了改性剂投加量、吸附时间、初始质量浓度等对吸附Hg(Ⅱ)效果的影响,并采用吸附动力学、等温线模型探明了其吸附特性;探究了pH和竞争离子对吸附性能的影响,考察了吸附剂的再生性能;最后揭示了改性壳聚糖材料与Hg(Ⅱ)的作用机理。
-
本研究中主要的实验试剂均为分析纯,其中壳聚糖(CS,脱乙酰度≥95%,黏度为100~200 mpa·s)来自上海阿拉丁有限公司;巯基丙酸(C3H6O2S,98%)来自上海阿拉丁试剂有限公司;1-乙基-3-(3-二甲基氨基丙基)碳二亚胺(EDC,97%)购买于阿达玛斯试剂有限公司;氢氧化钠(NaOH)、盐酸(HCl)、氯化汞(HgCl2)购买自国药集团化学试剂有限公司。
主要的大型仪器有傅里叶变换红外光谱仪(IRTracer-100型,日本岛津公司)、X射线光电子能谱仪(Thermo Scientific K-Alpha Nexsa 型,美国赛默飞世尔科技公司)、高分辨场发射扫描电镜(Sigma 300,德国蔡司公司)、氢化发生-原子荧光器(AFS-9230型,北京吉天仪器有限公司)。
-
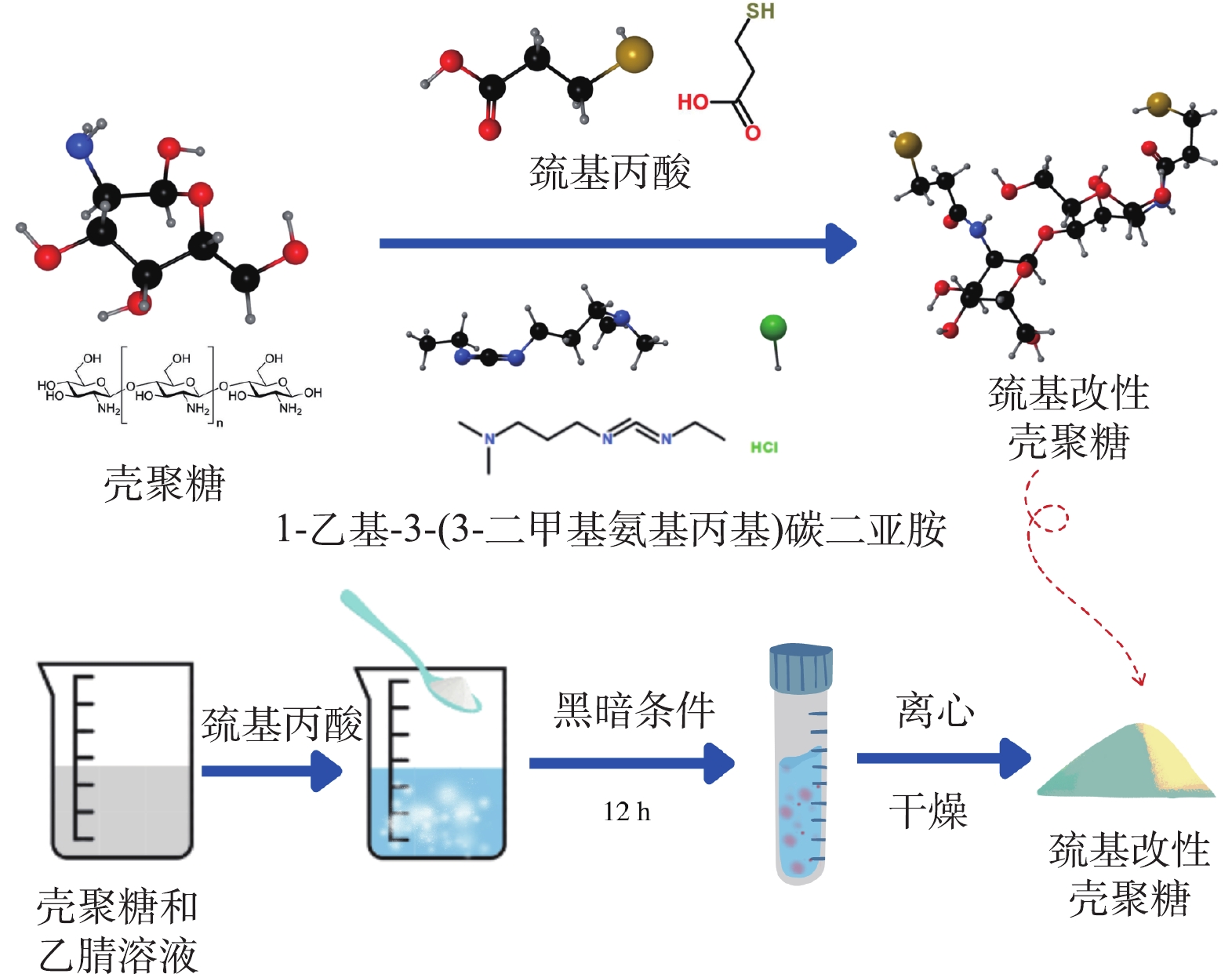
将1 g CS粉末加入30 mL乙腈和水的混合溶液中,磁力搅拌30 min将CS溶解。随后将1.073 g EDC和0.74 g巯基丙酸依次加入到混合溶液中,黑暗条件下反应12 h。使用超纯水和乙醇进行反复洗涤,直到确保洗涤液的pH约为7.0。将所得的粉末材料置于真空冷冻干燥机中干燥后得到最终产物CS-MPA。CS-MPA交联的化学反应如图1所示,首先,EDC与MPA上的羧基发生反应,形成氨基反应活性的中间体,然后再与CS上的氨基进行酰胺反应,链接MPA和CS,最终生成CS-MPA复合材料。
-
1) pH的影响。取50 mL含200 mg·L−1的Hg溶液中,使用0.1 mol·L−1的HCl和NaOH分别将pH调节至1~10,再取50 mg吸附剂加入到锥形瓶中,在25 ℃水浴振荡6 h,设置3个平行样,待吸附平衡后使用0.45 µm的滤膜迅速过滤,加入适量硝酸在60 ℃下消解12 h,随后使用原子荧光光度计测定初始、剩余Hg(II)的质量浓度。吸附容量和去除率分别根据式(1)和式(2)进行计算。
式中:Q为吸附容量,mg·g−1;Re为去除率,%;Co和Ce分别为初始和吸附平衡时Hg(Ⅱ)的质量浓度,mg·L−1;V为溶液体积,L;m为吸附剂的质量,g。
2)吸附动力学。准确称取1 g CS-MPA,加入1 L 200 mg·L−1 Hg(II)液中,25 ℃搅拌,于1~480 min内在不同时间抽取3个5 mL平行水样,过滤消解。后续测样及计算参考1)。采用伪一级(式(3))、伪二级动力学模型(式(4))和颗粒内扩散模型(式(5))拟合所得实验数据。
式中:Qe、Qt分别为平衡时、t时刻Hg(Ⅱ)的吸附量,mg·g−1;k1为伪一级模型,min−1;k2为伪二级模型的速率常数,g·(mg·min)−1;ki是内扩散速率常数,mg·g−1·min−0.5;qt是在t时刻的单位吸附量,mg·g−1。
3)吸附等温线。配置质量浓度梯度为100~1 000 mg·L−1的Hg(Ⅱ)溶液,调节pH为6,加入50 mg的吸附剂,振荡6 h后取出,过滤消解、测定Hg(Ⅱ)的质量浓度。使用Langmuir(式(6)) 等温模型和Freundlich(式(7)) 等温模型拟合相关数据。
式中:Qe、Qmax分别为平衡吸附量和理论最大吸附量,mg·g−1;Ce为Hg(Ⅱ)的平衡质量浓度,mg·L−1;KL是Langmuir模型的吸附常数,L·mg−1;KF是Freundlich模型的吸附常数,[(mg·g−1)·(L·mg−1)1/n)];n是Freundlich系数,与吸附强度有关;C0为Hg(Ⅱ)的初始质量浓度,mg·L−1;RL为分离常数。
4)竞争吸附实验。锥形瓶中分别加入50 mL 200 mg·L−1的汞、铜、铅、镉形成单一金属溶液,另再配制200 mg L−1的四种重金属的混合溶液,分别投加50 mg吸附剂,25 ℃吸附6 h,Hg(Ⅱ)质量浓度的测定参考1),用ICP-OES检测其他重金属的质量浓度。以选择性系数(KHg/M)量化复杂组分溶液中吸附剂对目标金属离子的选择性富集能力。
式中:KHg/M为选择性系数;Kd为金属离子的分配系数。
-
在pH=6,25 ℃下,将50 mg CS-MPA加入50 mL 200 mg·L−1的Hg(Ⅱ)溶液吸附平衡6 h,而后固液分离。因张保平等[18]发现盐酸硫脲解吸能力强,故本文以50 mL 1 mol·L−1的盐酸和硫脲混合溶液为洗脱液,在25 ℃下对吸附剂进行解吸12 h,用超纯水清洗吸附剂,干燥后的吸附剂可进行下1轮吸附循环,共重复5次循环吸附-解吸实验。
-
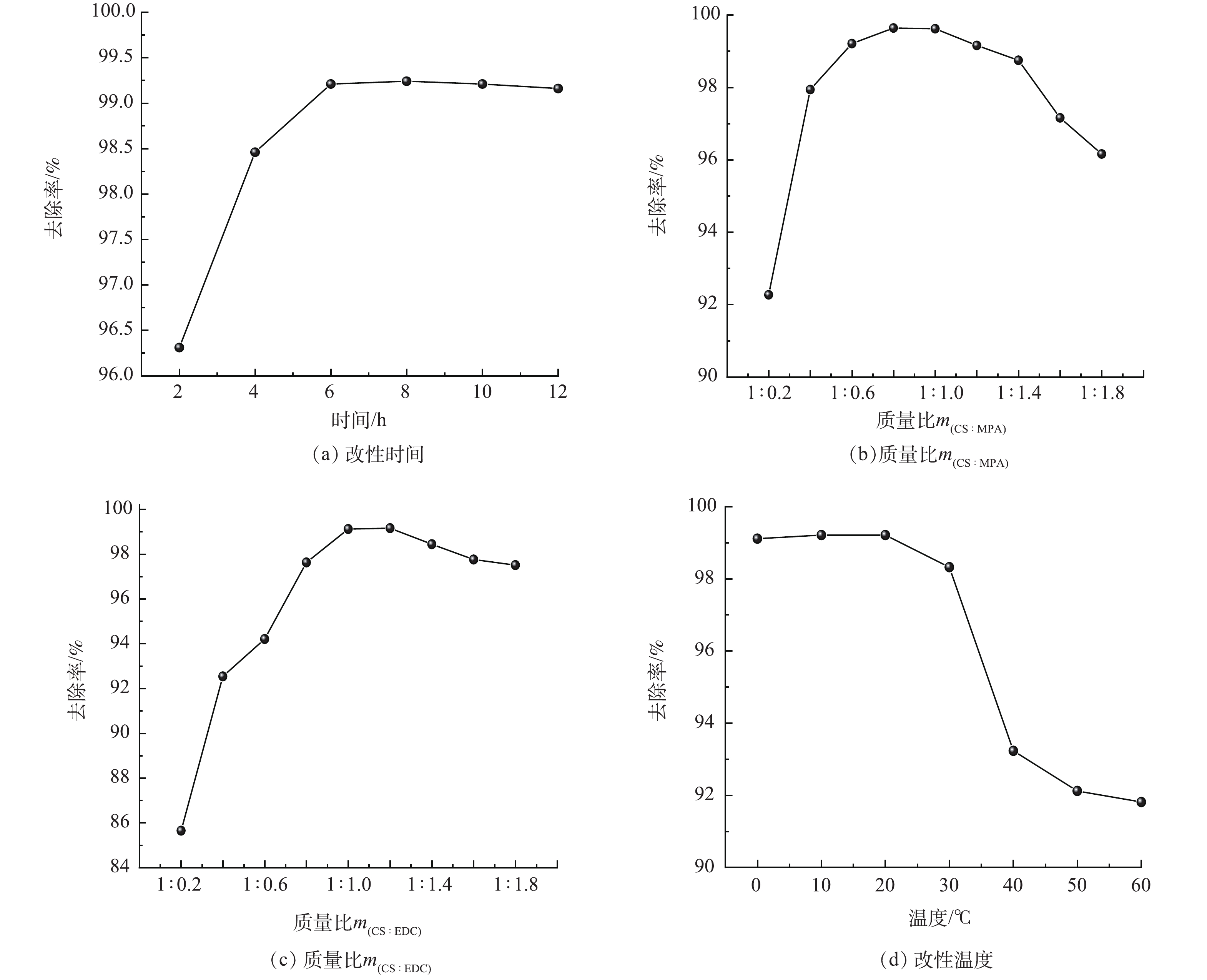
本研究采用单一变量法依次优化了制备过程中的改性时间、改性剂和CS的质量比(m(CS:MPA))、CS和EDC的质量比(m(CS:EDC))以及温度,制得的材料置于50 mL 200 mg·L−1的Hg溶液中25 ℃吸附6 h,结果如图2所示。在6 h内时,Hg(Ⅱ)的去除率随着时间的增加而增加,这一段时间巯基丙酸被不断接枝到CS长链上,吸附活性位点也随之不断增加;超过6 h后,去除率随着时间的延长并没有明显增加,说明延长时间已无法继续提高巯基的接枝量,分子内部参与反应的基团已饱和。故本研究选择6 h为最佳反应时间。
Hg(Ⅱ)的去除率随CS:MPA质量比的增加呈现出先增后减的趋势(图2(b))。当m(CS:MPA)从1∶0.2增加到1∶0.8时,去除率由92.27%显著地提升至99.64%。而随着摩尔比继续增加,去除率明显下降。这是由于MPA的用量在一定范围内的增加,会破坏CS的结构形态。天然CS颗粒结构疏松,能减少金属离子进入分子内部与活性部位螯合的空间阻力[19],提高羧基与CS上的氨基结合的可能性,使CS上的巯基负载量增加。但超过一定量后,接枝了巯基丙酸的CS分子,原来的三维排列方式改变了,再加上CS表面的氨基等可结合位点已经饱和,CS将不再接受MPA的负载。在同时考虑最大吸附容量与最低经济成本,选择m(CS:MPA)=1∶0.8作为最优的投加量。
此外,当m(CS:EDC)由1∶0.2增至1∶1时,Hg(Ⅱ)的去除率明显提高(图2(c));当继续增加至1∶1.2时,去除率变化不明显;继续增大至1∶1.8时,去除率呈下降趋势。出现此现象的原因是:在一定范围内,EDC作为羧基活化剂,不仅能够增强CS对巯基丙酸的吸附能力,还能够通过断裂CS分子中碳原子和杂原子之间的化学键来减少CS对MPA的分子结构阻力。当EDC的投加量超过一定范围后,高浓度的EDC阻碍了MPA与CS的有效接触,导致反应速率下降,反应程度降低。因此,选择m(CS:EDC)=1∶1为最佳的投加量。
当改性温度在0~20 ℃时,升高温度并没有明显提升去除率(图2(d));在20~60 ℃时,温度不断升高对去除率有明显的抑制作用。由此可推测,温度升高导致部分EDC失去活性,对羧基的活化性能下降,减少了MPA与CS反应的可能性,不利于酰胺化反应。因此,选择20 ℃为最佳反应温度。
-
1) FTIR表征及分析。图3(a)为CS、CS-MPA和负载Hg(Ⅱ)的CS-MPA的FTIR光谱。在CS光谱中,有1 157(C3-OH上的C—O 拉伸振动)、1 379(C—H弯曲振动)、1 593(—NH弯曲振动,酰胺Ⅱ)、1 653(C=O拉伸振动,酰胺Ⅰ)和3 444 cm−1(—OH拉伸振动)等特征吸收峰。相比CS谱图,在CS-MPA谱图中,1 082 cm−1附近出现的新峰为C=S的伸缩振动峰[20]。经对比发现,位于1 593 cm−1处(酰胺Ⅱ,—NH)和1 653 cm−1处的吸收峰(酰胺Ⅰ,C=O)轻微移动到1 550 cm−1和1 647 cm−1处。这是—COOH和酰胺中的羰基拉伸振动,这表明酰胺键的生成。此外,CS-MPA中位于1 406 cm−1处的新吸收峰为游离的—COO−[21],这也表明MPA通过酰胺反应成功被接枝到CS上。通过对CS-MPA的元素组成进行分析发现,氮和硫的质量百分比明显增加,进一步表明MPA通过氨基和羧基的酰胺反应成功接枝到CS材料上。当CS-MPA表面负载Hg(Ⅱ)时,一方面,NH2、C—O和C—N键的吸收峰强度显著降低,这可能是由于CS-MPA中的N和O元素与Hg(Ⅱ)的配位引起的。另一方面,在吸附Hg(Ⅱ)后,未观察到—OH峰(3 444 cm−1)的移动,证实Hg(Ⅱ)与羟基之间没有相互作用,同时,位于1 550 cm−1的酰胺Ⅱ峰(—NH弯曲振动)消失,位于1 406 cm−1的羰基拉伸振动减弱且发生一定的偏移,证实Hg(Ⅱ)与吸附剂上的酰胺基和氨基的相互作用。
2)比表面积BET分析。氮气吸附脱附法[22-23]测定得出改性前后的CS材料比表面积((图3(b)和图3(c))和孔径变化(图3(d)和图3(e))如图所示。可见,当相对压力低于0.1时,吸附量极低甚至接近零;当相对压力处于0.1~0.9时,样品的吸附相对比较平缓;当相对压力高于0.9时,毛细凝聚作用明显,吸附量迅速上升。这说明在改性材料中微孔与中孔较少。CS和CS-MPA复合材料的比表面积分别为0.338 m2·g−1和1.378 m2·g−1,孔体积分别为0.002 535 cm3·g−1和0.007 064 cm3·g−1。这些结果表明CS改性后比表面积和孔体积均有所增加,从而促进汞接近材料表面而与活性位点发生反应,增强材料表面的质量传输。与其他吸附剂相比,由于材料的比表面积和孔体积较小,故推测CS-MPA复合材料对汞的吸附主要发生在材料的表面。
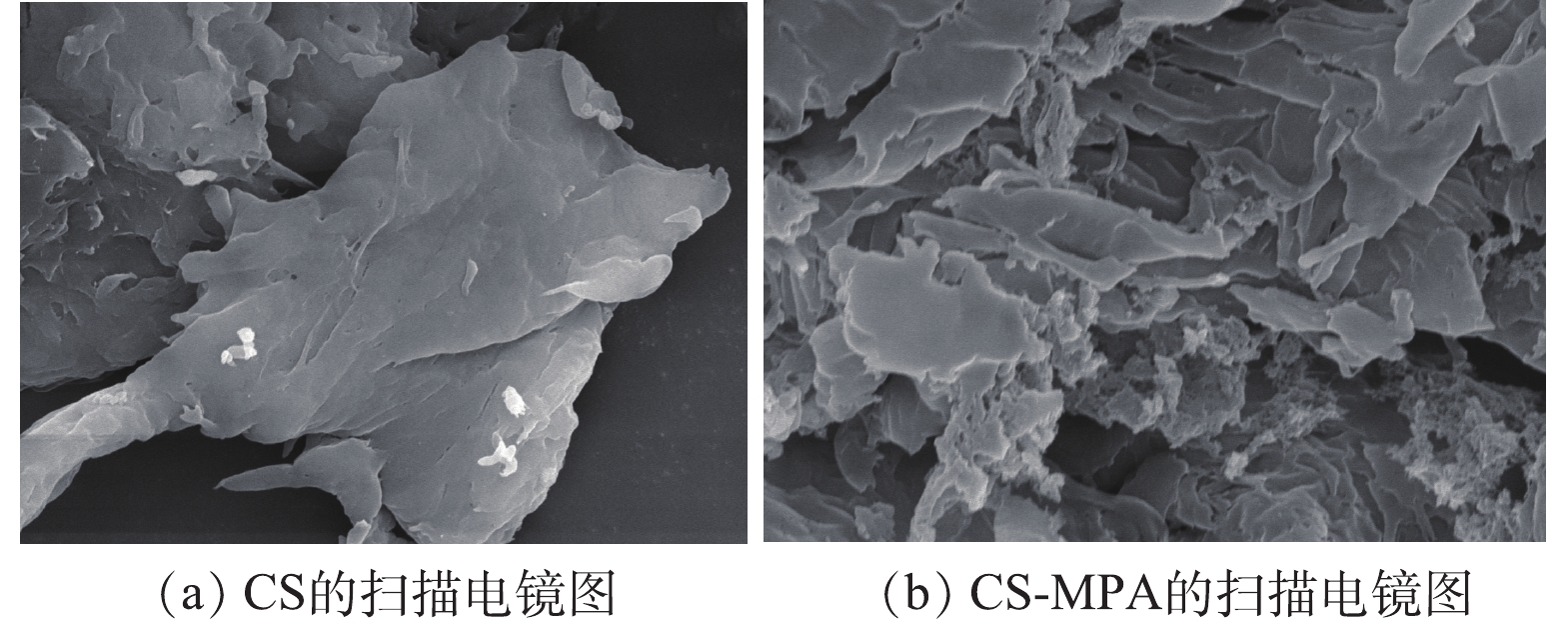
3) SEM和EDS表征及分析。由图4可见,改性前CS的表面(图4(a))相对光滑且致密,褶皱起伏小,为片状紧密结构;改性后CS的表面(图4(b))呈现出不规则形状的多层堆叠态,断裂程度虽不规整,但能看出其疏松多通道的结构。这表明CS改性后表面粗糙度增加,比表面积有所增大,较多的通道有利于材料进行吸附,提高膜吸附容量。
经EDS能谱仪分析可得,改性前的元素成分是碳(69.56%)、氧(26.70%)和氮(3.74%)。改性后的材料出现了S峰,占比3.32%,接枝率达到了理论接枝率的25%,且N的含量也增加到9.38%。这些结果均表明,巯基丙酸成功接枝到了CS上。
-
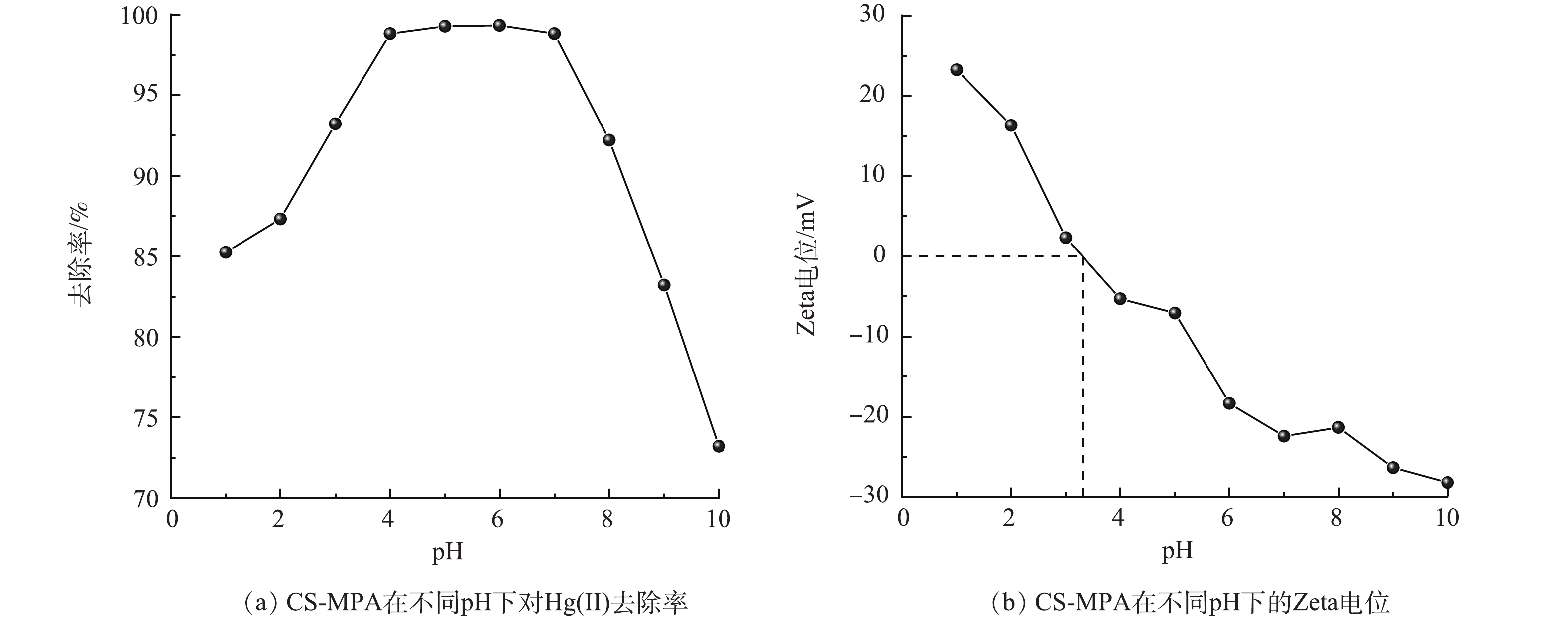
在众多的环境因素中,pH对吸附性能的影响最重要。本研究通过考察Zeta电位的变化探究了CS-MPA的表面化学性质。图5(a)和图5(b)反映了不同初始pH下CS-MPA复合材料对Hg(Ⅱ)去除率的变化和CS-MPA复合材料在不同pH的Zeta电位。在pH=1~4内,去除率随pH的增大也呈上升趋势;当pH=4~7时,去除率显著较为稳定在98.8%;随着pH的继续增加,去除效果则直线下降。由图5(b)可见,改性材料的零点电位在3.2附近,在pH<3.2时,CS-MPA复合材料表面电荷为正,这会排斥同样带正电荷的吸附质Hg(Ⅱ),导致其吸附容量低。当pH>3.2(pH在4~7)时,随着pH的增大,CS-MPA的表面电荷变为负值,表面负电逐渐增强,这增加其对阳离子Hg(Ⅱ)的吸引力,因此,随着pH的增加其吸附能力也随之提高。虽然CS-MPA复合材料的表面电荷在较高pH(7.0~10.0)下带有更多负电荷,但随OH−的增多,会促使Hg(Ⅱ)转化成Hg(OH)3-并占据主要形态,减弱了其与带负电材料的有效接触几率,导致材料表面的巯基对Hg(Ⅱ)的特征性吸附降低,从而降低了吸附容量。因此,后续的吸附实验中将初始浓度pH设置为6.0。
-
图6(a)为Hg(Ⅱ)吸附容量随时间的变化情况。CS-MPA复合材料上的褶皱、裂缝、疏松表面有利于汞快速地接近并结合吸附位点,吸附在最初的10 min速率较快。随着Hg(Ⅱ)对活性位点的逐渐占据,吸附速率逐渐减慢,直至吸附容量达到平衡。图6(b)~(d)为CS-MPA复合材料的动力学拟合线。通过伪一级和伪二级动力学模型[24]对所得实验数据进行拟合,伪二级动力学模型的可决系数(R2=0.999 9)高于伪一级的(R2=0.947),说明伪二级拟合程度更好;同时,伪二阶方程推算的qe值(200.00 mg·g−1)也更接近实验值(198.41 mg·g−1)。以上结果说明CS-MPA复合材料与Hg(Ⅱ)之间以化学吸附为主[25-26]。
此外,引入颗粒内扩散模型对数据进一步分析(图6(d)),结果表明,拟合线可分为不经过原点的斜率依次减小的3段直线,暗示着吸附反应并非为单一的过程[27],且颗粒内扩散不是CS-MPA吸附Hg(Ⅱ)的唯一限速步骤。吸附初始阶段,本体溶液与吸附剂间的浓度差而带来的传质动力促进了Hg(Ⅱ)迅速扩散(10 min内)到材料的外表面。随着吸附时间的延长,活性位点被占据,在膜扩散和内扩散共同影响下,内扩散阻力不断增大,传质动力减小,最后达到吸附平衡。
-
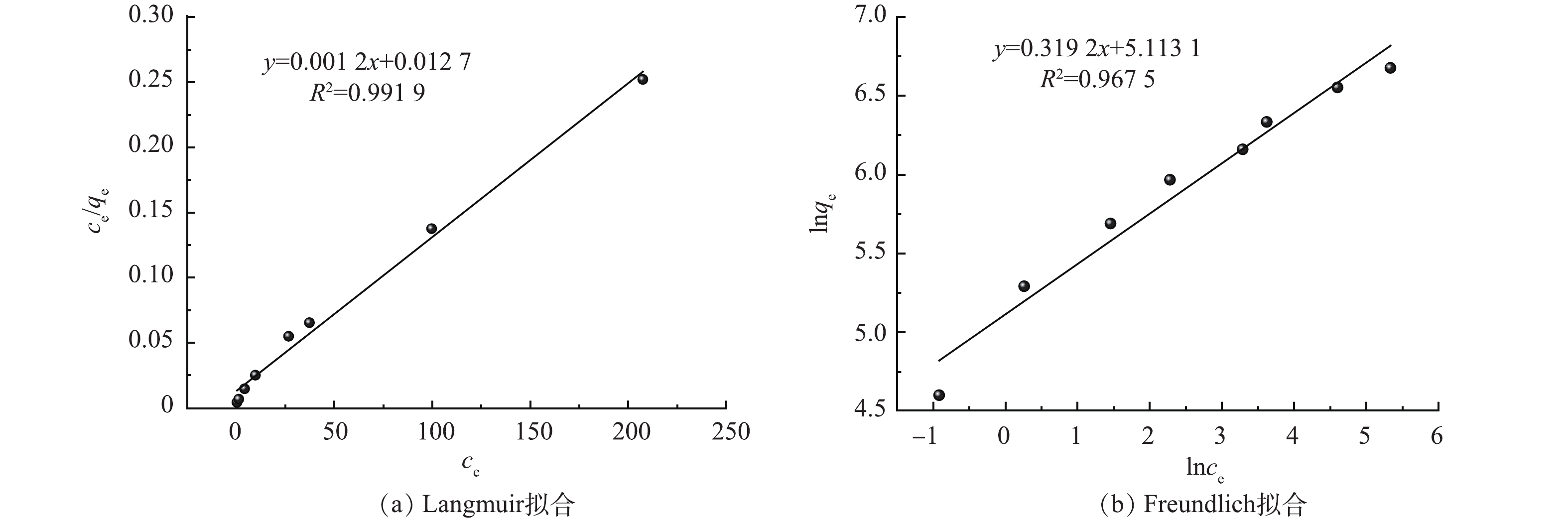
在pH=6.0、25 ℃的条件下,研究了初始Hg(Ⅱ)质量浓度(100~1 000 mg·L−1)对吸附效果的影响。结果显示随着汞离子浓度的增加CS-MPA复合材料吸附金属离子的能力也在持续增长,初始质量浓度较高时(>800 mg·L−1),材料逐渐达到其自身的最大吸附容量。图7中Langmuir和Freundlich吸附等温模型的拟合结果可以证明前者具有更好的线性,更适宜描述CS-MPA复合材料对汞的吸附行为,即吸附剂表面吸附了一层金属离子,一个吸附位点只吸附一个汞原子。由此推导得出CS-MPA复合材料的理论最大吸附容量为833.3 mg·g−1。值得一提的是,当初始汞质量浓度为50 mg·L−1时,吸附后剩余汞的质量浓度可满足《中华人民共和国污水综合排放标准》(GB 8978-1996)50 ug·L−1的要求。
将已报道的相关CS材料与本研究的CS-MPA复合材料相比(详见表1),本研究制备的CS基吸附剂具有优异的吸附容量,优于大多数类似改性材料,如三聚氰胺、聚乙烯基亚胺、乙二胺等接枝的CS,不过还是低于聚马来酸接枝的CS。
-
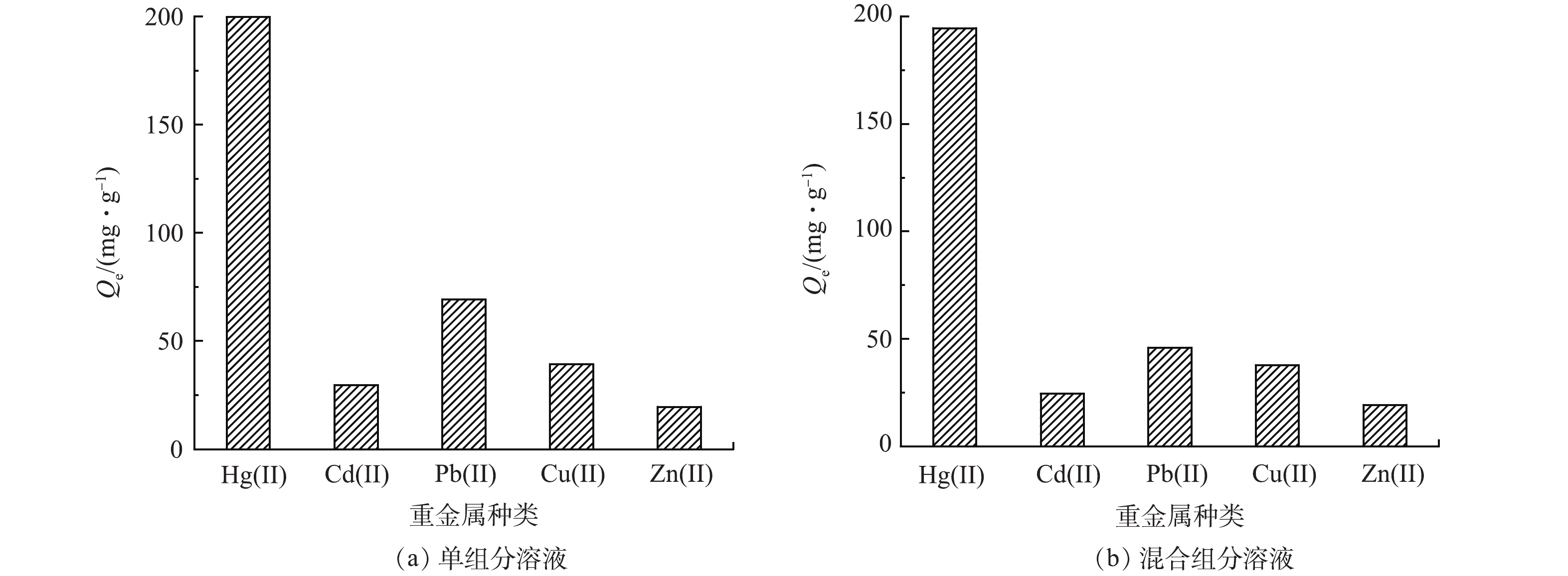
吸附选择性是评估吸附材料性能优劣的一项重要指标。在本研究选择Pb(Ⅱ)、Cu(Ⅱ)、Cd(Ⅱ)和Zn(Ⅱ)为竞争离子,图8(a)为CS-MPA复合材料在单组分溶液中对各离子的吸附容量,材料对Hg(Ⅱ)的吸附容量为199.74 mg·g−1,远高于其他金属离子;而且混合溶液中,Hg(Ⅱ)的吸附容量仍未下降,突出体现了CS-MPA复合材料可选择性地富集Hg(Ⅱ)。这一选择性通过KHg/M进行量化,从表2可得,CS-MPA复合材料可从混合溶液中提取约97.3%的Hg(Ⅱ),分配系数为3.6×104 mL·g−1,比其他离子的高2个数量级,KHg/M保持在119~334。这一结果直接证明了CS-MPA复合材料对Hg (Ⅱ)的高选择性。
-
提高吸附剂的循环利用性能可降低使用成本。经过5次吸附-解吸实验,CS-MPA对Hg(Ⅱ)的去除率分别为99.21%、92.56%、85.37%、84.63%、79.35%,去除率虽有略微下降但仍然维持在一个较高的水平。推测去除率下降的原因是:在解吸过程中,将CS-MPA处于1 mol·L−1 HCl和硫脲环境下进行洗脱时,未完全改性的CS会溶解在酸性溶剂中,导致可吸附位点减少;CS-MPA复合材料的巯基与汞的结合过于牢固以至于有部分汞并未解吸;酸性环境中多次洗脱导致原本CS-MPA复合材料结构发生变化,使得结构不够稳定。
-
如图9(a)所示,结合能在163.8 eV的S2p峰表明MPA被成功引入到CS上。Hg4f峰的出现,证实了Hg(Ⅱ)被吸附到CS-MPA复合材料上。在Hg4f的高分辨率光谱中,结合能隙为4.1 eV的2个典型强峰(0.5 eV和104.6 eV)分别对应于Hg4f7/2和Hg4f5/2。此结果证明汞仍以Hg(Ⅱ)的形态吸附到CS-MPA复合材料上,并未发生氧化还原等反应。此外由于本研究中溶液的pH<8,因此,Hg(Ⅱ)主要呈离子形式,并未以晶体等沉淀形式吸附在吸附剂表面。
CS-MPA复合材料吸附前后的C1s高分辨率谱图(图9(c)和图9(d))上没有发现明显的峰位移或强度降低,表明C原子不直接参与Hg(Ⅱ)的吸附。在O1s(图9(e)和9(f))的高分辨率光谱中,吸附后532.53 eV处的峰略微偏移至532.70 eV,表明酰胺基团参与了吸附反应。如图9(g)和图9(h)所示,N1s在399.66 eV (C=N)和400.99 eV (—NH2) 的2个峰分别移动到399.77 eV和402.11 eV,这是由于N和Hg(Ⅱ)之间的螯合反应[33-35],即前者向后者的空轨道提供了孤对电子。此外,在吸附前,在图9(i)和图9(j)中163.45 eV (S2p2/3)和164.69 eV (S2p1/2)的2个谱带归属于C-SH官能团。吸附后,这2个峰转移到162.37 eV和163.46 eV,表明C-SH和Hg(Ⅱ)的结合。
综上可知,Hg(Ⅱ)与吸附剂的结合机理可借助软硬酸碱理论来阐明,主要为改性材料中的O、N和S与Hg(Ⅱ)的相互作用,吸附机理如图9(k)所示。CS-MPA复合材料分子上的软碱,即巯基(—SH)和氨基(—NH2)与软酸Hg(Ⅱ)之间的螯合作用为主;此外,酰胺基也会和小部分的Hg(Ⅱ)发生结合。吸附主要以单层的化学吸附为主。
-
1)在质量比为m(CS:EDC:MPA)=1∶1∶0.8、反应温度为20 ℃时,反应6 h后制得的CS-MPA改性材料吸附效果最佳,此材料表面粗糙、具有丰富褶皱的片状结构,硫含量明显增加,比表面积略微提升。
2)在pH为1~10内,CS-MPA对Hg(Ⅱ)的去除率呈先升后降的趋势,在pH=4~7时去除率最高,稳定于98.8%。在200 mg L−1 Hg(Ⅱ)的溶液中加入CS-MPA,前10 min快速吸附,在360 min内可达到平衡,吸附行为主要为单层的化学反应,推算理论最大吸附容量为833.3 mg·g−1,优于大部分已报道的CS类吸附剂。
3) Hg(Ⅱ)与CS-MPA复合材料的结合可解释为软酸和软碱(丰富的巯基、氨基活性基团)的反应,巯基对汞的高亲和力体现为材料在复杂的溶液中也能高选择性地富集Hg(Ⅱ)。
4) CS-MPA具有较高的吸附容量、优异的选择性、良好的可回收性、结构稳定性等,可作为一种很有前景的废水处理生物吸附剂。
巯基改性壳聚糖复合材料的制备及其用于高容量去除水中Hg(Ⅱ)
A facile synthesis of thiol-modified chitosan composite for high-capacity capture of mercury from aqueous solution
-
摘要: 摘 要 壳聚糖(CS)因其无毒害、可降解性、成本低等特点而在众多高分子材料中脱颖而出,但稳定性和选择性较差,从而限制了其在重金属吸附中的应用。以巯基丙酸 (MPA) 为改性剂,以1-乙基-(3-二甲基氨基丙基)碳酰二亚胺(EDC) 为活化剂,制备了CS-MPA复合材料,使用单一变量法优化制备条件。结果表明,最优制备参数为:改性时间6 h,温度20 ℃,pH=6,m(CS:MPA:EDC)为1∶0.8∶1,制备得到的 CS-MPA在酸性条件下对Hg(II)依旧保持较高去除效率。在复合材料表面引入巯基后出现了多层堆叠的不规则形态,比表面积增大。CS-MPA对Hg(II)的吸附在 360 min 内可达到平衡,以单层吸热的化学反应为主,25 ℃时理论最大吸附容量推测为 833.3 mg·g−1。吸附机理主要是材料上的软碱(—SH和—NH2)与软酸 Hg(II)之间发生的螯合反应,其次为酰胺基的结合作用。CS-MPA对 Hg(II)有较高的吸附选择性;且经过5次的吸附-解吸循环后,去除率仍保持在较高水平。以上研究结果表明 CS-MPA对Hg(II)的去除效果较好,可为废水中Hg(II)的去除与回收提供参考。Abstract: Abstract Chitosan (CS) possesses prominent merits such as non-toxic, degradability, and low cost, which stands out from numerous materials, but its application in heavy metal adsorption was restricted by the poor stability and selectivity. In the study, a novel composite (thiol-modified chitosan, CS-MPA) was prepared with mercaptopropionic acid (MPA) as a modifier and 1-ethyl-(3-dimethylaminopropyl) carbodiimide (EDC) as an activator. The suitable preparation parameters were optimized by a single variable method. The results showed that the optimized preparation parameters were as follows: modifying time of 6h, 20 ℃, pH 6 and m(CS:MPA:EDC) of 1:0.8:1. The as-prepared material maintained high removal efficiency even at acidic pHs. After the grafting of sulfhydryl was introduced on the surface of the composite, the stacked irregular multilayer and the increased specific surface area occurred. The mercury adsorption onto the composite reached the equilibrium within 360 min, which was dominated by the endothermic monolayer chemical reaction; the theoretical maximum adsorption capacity was estimated as 833.3 mg·g−1 at 25 ℃. The adsorption mechanism was dominated by the chelation between the soft base groups(—SH and —NH2) and the soft acid of Hg(II), with an auxiliary function of acylamino binding. The CS-MPA possessed an outstanding selectivity to Hg(II) in the complex matrix. It maintained the good removal rates even after five adsorption-desorption cycles. The result proved the CS-MPA composite had a good Hg(II) removal performance and application potential, which can provide a reference for Hg(II) capture and recycling in the wastewater.
-
Key words:
- modified chitosan material /
- mercaptopropionic acid /
- mercury /
- adsorption
-

-
表 1 不同吸附材料对Hg(Ⅱ)吸附容量的比较
Table 1. Comparison of the adsorption capacity of Hg(Ⅱ) by different adsorption materials
表 2 CS-MPA在混合组分溶液中的选择性吸附能力
Table 2. Selective adsorption ability of CS-MPA in a mixed components solution.
金属离子 C0/(mg·L−1) Ce/(mg·L−1) Qe/(mg·g−1) R/% Kd/(mL·g−1) KHg/M Hg(Ⅱ) 200 5.47 194.53 97.27 35 563 Pb(Ⅱ) 200 154.06 45.94 22.97 298 119 Cu(Ⅱ) 200 162.28 37.72 18.86 232 153 Cd(Ⅱ) 200 175.48 24.52 12.26 140 255 Zn(Ⅱ) 200 180.74 19.26 9.63 107 334 -
[1] 杨英伟, 屈撑囤, 刘鲁珍. 水体汞污染的危害及其防治技术进展[J]. 石油化工应用, 2015, 34(6): 4-8. doi: 10.3969/j.issn.1673-5285.2015.06.002 [2] XUE Y B, DU P, SHENDI A A I, et al. Mercury bioremediation in aquatic environment by genetically modified bacteria with self-controlled biosecurity circuit[J]. Journal of Cleaner Production, 2022, 337: 130524. doi: 10.1016/j.jclepro.2022.130524 [3] 刘北辰. 从“水俣病”谈汞中毒[J]. 家庭医学, 2018, 591(7): 35. [4] 吴秀英, 吴农忠, 赵宏远, 等. 硫化钠处理含汞废水[J]. 中国环境科学, 1995, 15(2): 128-130. [5] 邹涛, 刘明远. 离子交换法处理工业废水中重金属的现状与发展[J]. 山东化工, 2017, 46(10): 190-192. [6] 付克明, 张勤善, 张蓓. 分子筛处理重金属离子污水的原理及应用[J]. 焦作大学学报, 2009, 23(4): 57-58. [7] ZADE P D, DHARMADHIKARI D M. Retrieval of mercury from wastewater as stable mercury ferrite[J]. Water Quality Research Journal of Canada, 2007, 42(4): 311-318. doi: 10.2166/wqrj.2007.033 [8] MELAMED R, DA LUZ A B. Efficiency of industrial minerals on the removal of mercury species from liquid effluents[J]. Science of the Total Environment, 2006, 368(1): 403-406. doi: 10.1016/j.scitotenv.2005.09.091 [9] SHAFIQUE A. Removal of toxic pollutants from aqueous medium through adsorption: A review[J]. Desalination and Water Treatment, 2021, 234: 38-57. doi: 10.5004/dwt.2021.27550 [10] WANG Q. Medical wastewater treatment system includes medical wastewater receiving tank, seven microbial treatment tanks, floating plant groove, pH change equipment, evaporative ammonia equipment, decomposition tank and nitrogen recovery equipment, CN105884115-A; CN105884115-B [P/OL]. [11] 牛耀岚, 吴曼菲, 胡湛波. 吸附法处理水体重金属污染的研究进展[J]. 华北水利水电大学学报(自然科学版), 2019, 40(2): 46-51. [12] 刘义, 张淑琴, 任大军, 等. 不同官能团改性壳聚糖吸附重金属的研究进展[J]. 化学试剂, 2022, 44(4): 495-503. doi: 10.13822/j.cnki.hxsj.2022008755 [13] ADNAN O, ABIDIN Z Z, IDRIS A, et al. A novel biocoagulant agent from mushroom chitosan as water and wastewater therapy[J]. Environmental Science and Pollution Research, 2017, 24(24): 20104-20112. doi: 10.1007/s11356-017-9560-x [14] 刘晓雯, 向海均, 苏碧森, 等. 改性壳聚糖对铅离子的吸附性能研究[J]. 山东化工, 2022, 51(13): 25-27. [15] WANG X, SUN R, WANG C. pH dependence and thermodynamics of Hg(II) adsorption onto chitosan-poly(vinyl alcohol) hydrogel adsorbent[J]. Colloids and Surfaces a-Physicochemical and Engineering Aspects, 2014, 441: 51-58. [16] GAVILAN K C, PESTOV A V, GARCIA H M, et al. Mercury sorption on a thiocarbamoyl derivative of chitosan[J]. Journal of Hazardous Materials, 2009, 165(1/2/3): 415-426. [17] CUI Z, BEACH E S, ANASTAS P T. Modification of chitosan films with environmentally benign reagents for increased water resistance[J]. Green Chemistry Letters and Reviews, 2011, 4(1): 35-40. doi: 10.1080/17518253.2010.500621 [18] 张保平, 王尹, 张恒, 等. 盐酸硫脲从负载钯吸附剂中分离钯[J]. 中国有色冶金, 2022, 51(5): 93-101. doi: 10.19612/j.cnki.cn11-5066/tf.2022.05.014 [19] GARCIA-CABEZON C, GODINHO V, SALVO-COMINO C, et al. Improved corrosion behavior and biocompatibility of porous titanium samples coated with bioactive chitosan-based nanocomposites[J]. Materials, 2021, 14(21): 6322. doi: 10.3390/ma14216322 [20] WU Q, HE H, ZHOU H, et al. Multiple active sites cellulose-based adsorbent for the removal of low-level Cu(II), Pb(II) and Cr(VI) via multiple cooperative mechanisms[J]. Carbohydrate Polymers, 2020, 233(19): 115860. [21] ZHANG Y, LIN S, QIAO J, et al. Malic acid-enhanced chitosan hydrogel beads (mCHBs) for the removal of Cr(VI) and Cu(II) from aqueous solution[J]. Chemical Engineering Journal, 2018, 353: 225-236. doi: 10.1016/j.cej.2018.06.143 [22] 陈小娟, 张伟庆, 余小岚, 等. 适用于本科教学的BET比表面测定实验[J]. 大学化学, 2017, 32(7): 60-67. [23] 高立新. 多孔材料物性表征之BET分析[J]. 广东化工, 2021, 48(13): 94-95. [24] SIMONIN J-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics[J]. Chemical Engineering Journal, 2016, 300: 254-263. doi: 10.1016/j.cej.2016.04.079 [25] ZENG H, WANG L, ZHANG D, et al. Amido-functionalized carboxymethyl chitosan/montmorillonite composite for highly efficient and cost-effective mercury removal from aqueous solution[J]. J Colloid Interface Science, 2019, 554: 479-487. doi: 10.1016/j.jcis.2019.07.029 [26] SHI Q, YANG X, ZHAO B, et al. Enhanced absorption of Hg2+ by a recyclable thiol-functionalized salix psammophila[J]. Water, Air, & Soil Pollution, 2021, 233(1): 13. [27] 刘婉霞. 壳聚糖基复合材料的制备及其吸附钴离子的研究[D]. 北京: 北京建筑大学, 2020 [28] GE H C, DU J. Selective adsorption of Pb(II) and Hg(II) on melamine-grafted chitosan[J]. International Journal of Biological Macromolecules, 2020, 162: 1880-1887. doi: 10.1016/j.ijbiomac.2020.08.070 [29] LIMIN Z, YIPING W, ZHIRONG L I U, et al. Adsorption of Hg2+and UO22+onto Fe3O4/modified chitosan microspheres[J]. Nuclear Techniques, 2007, 30(9): 768-772. [30] YANG Z K, YUAN Y, WANG Y T. Synthesis and evaluation of chitosan aryl azacrown ethers as adsorbents for metal ions[J]. Journal of Applied Polymer Science, 2000, 77(14): 3093-3098. doi: 10.1002/1097-4628(20000929)77:14<3093::AID-APP100>3.0.CO;2-2 [31] DODI G, HRITCU D, LISA G, et al. Core-shell magnetic chitosan particles functionalized by grafting: Synthesis and characterization[J]. Chemical Engineering Journal, 2012, 203: 130-141. doi: 10.1016/j.cej.2012.06.133 [32] GE H, HUA T. Synthesis and characterization of poly(maleic acid)-grafted crosslinked chitosan nanomaterial with high uptake and selectivity for Hg(II) sorption[J]. Carbohydrate Polymers, 2016, 153: 246-252. doi: 10.1016/j.carbpol.2016.07.110 [33] LI M, ZHANG Z, LI R, et al. Removal of Pb(II) and Cd(II) ions from aqueous solution by thiosemicarbazide modified chitosan[J]. International Journal of Biological Macromolecules, 2016, 8(6): 876-884. [34] LIANG W, LI M, JIANG S, et al. Polyamine-co-2, 6-diaminopyridine covalently bonded on chitosan for the adsorptive removal of Hg(II) ions from aqueous solution[J]. International Journal of Biological Macromolecules, 2019, 13(11): 853-862. [35] SITKO R, MUSIELAK M, SERDA M, et al. Thiosemicarbazide-grafted graphene oxide as superior adsorbent for highly efficient and selective removal of mercury ions from water[J]. Separation and Purification Technology, 2021, 2(5): 43-56. -



 下载:
下载: