-
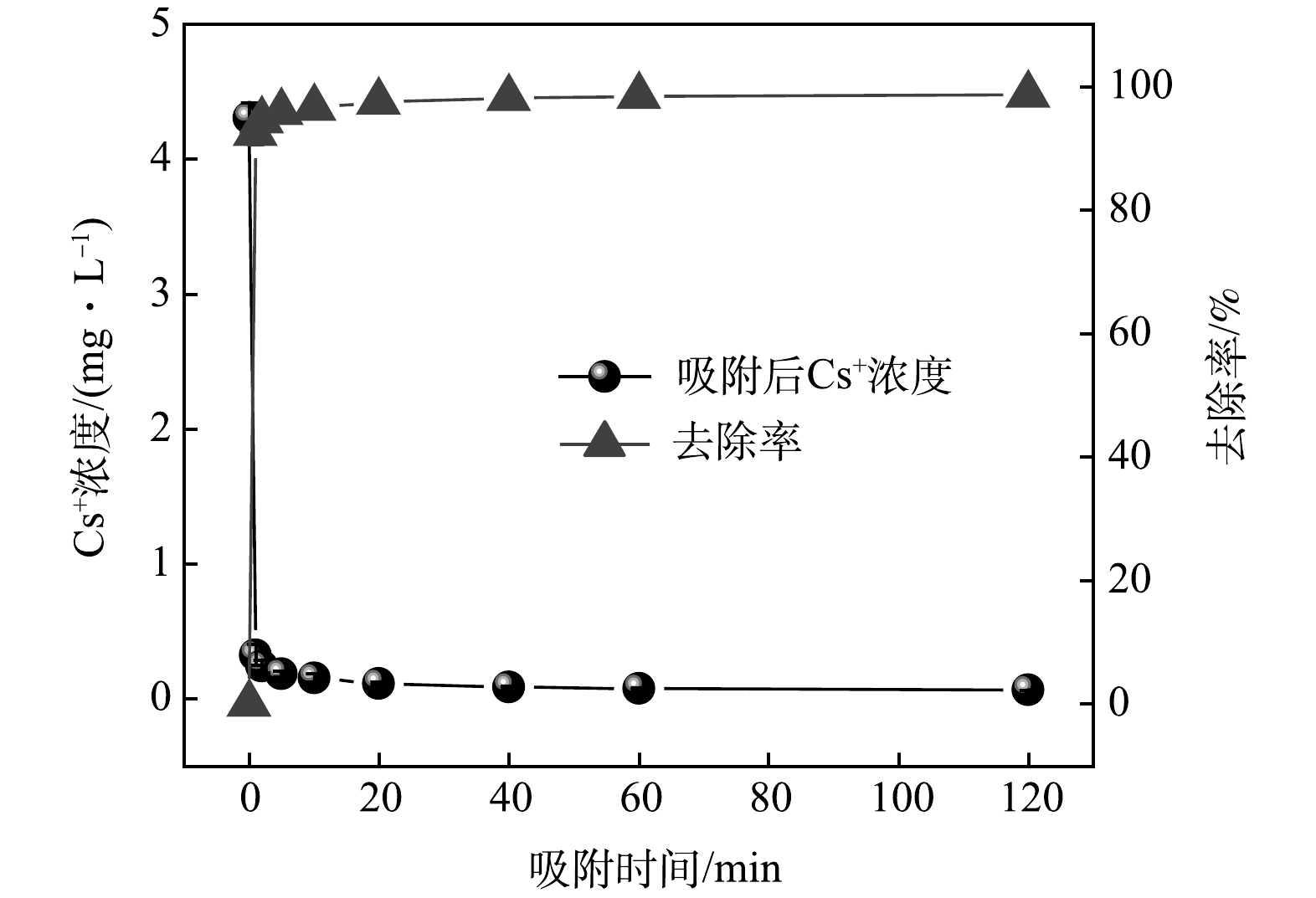
图 1 KZTS-NS吸附Cs+的吸附动力学曲线
Figure 1. Adsorption kinetics of KZTS-NS to Cs+ in aqueous solution
-
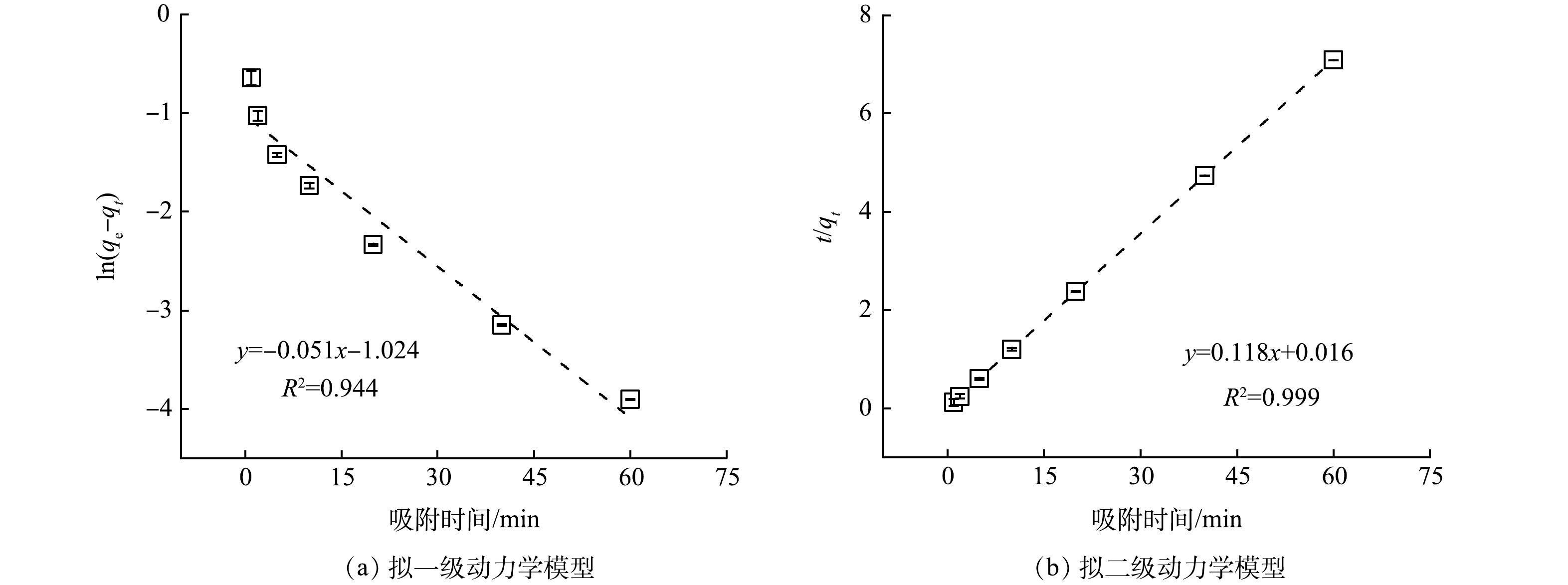
图 2 拟一级与拟二级动力学模型拟合
Figure 2. Fitting of pseudo-first-order and pseudo-second-order models
-
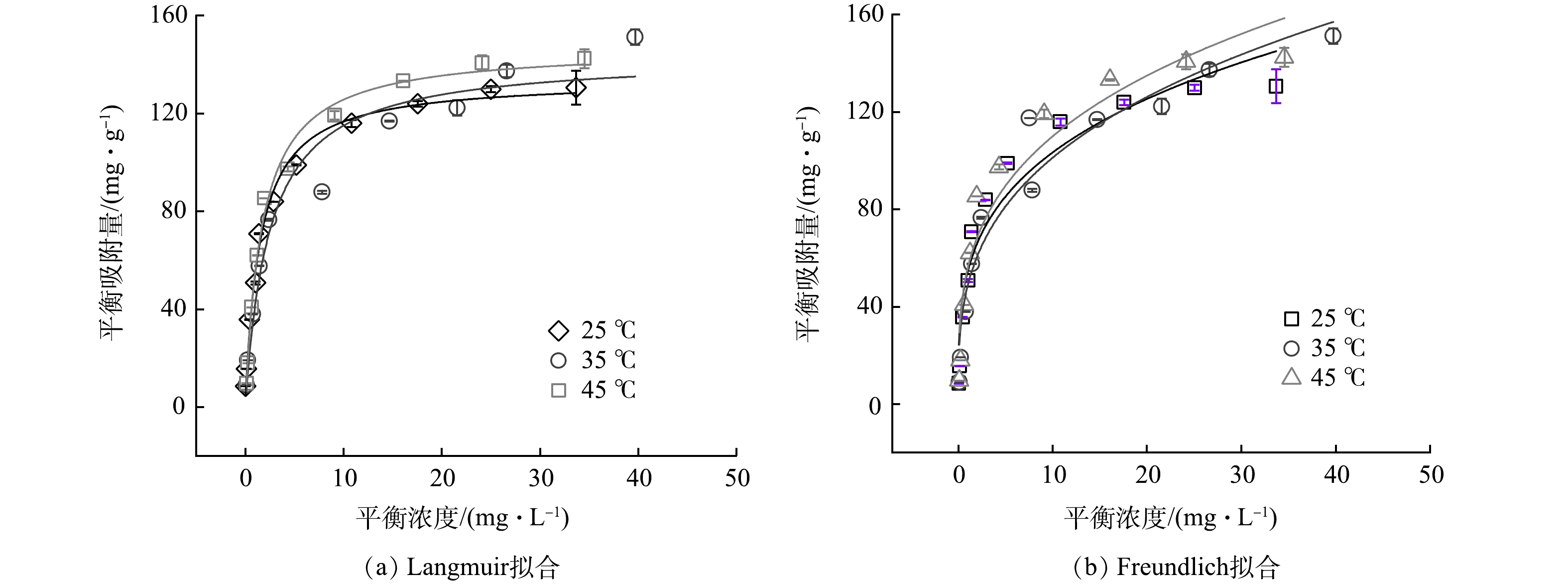
图 3 不同温度下KZTS-NS吸附Cs+的等温线以及Langmuir与Freundlich模型的数据拟合图
Figure 3. Adsorption isotherms of Cs+ onto KZTS-NS at varying temperature and the fitting curves of Langmuir and Freundlich models
-
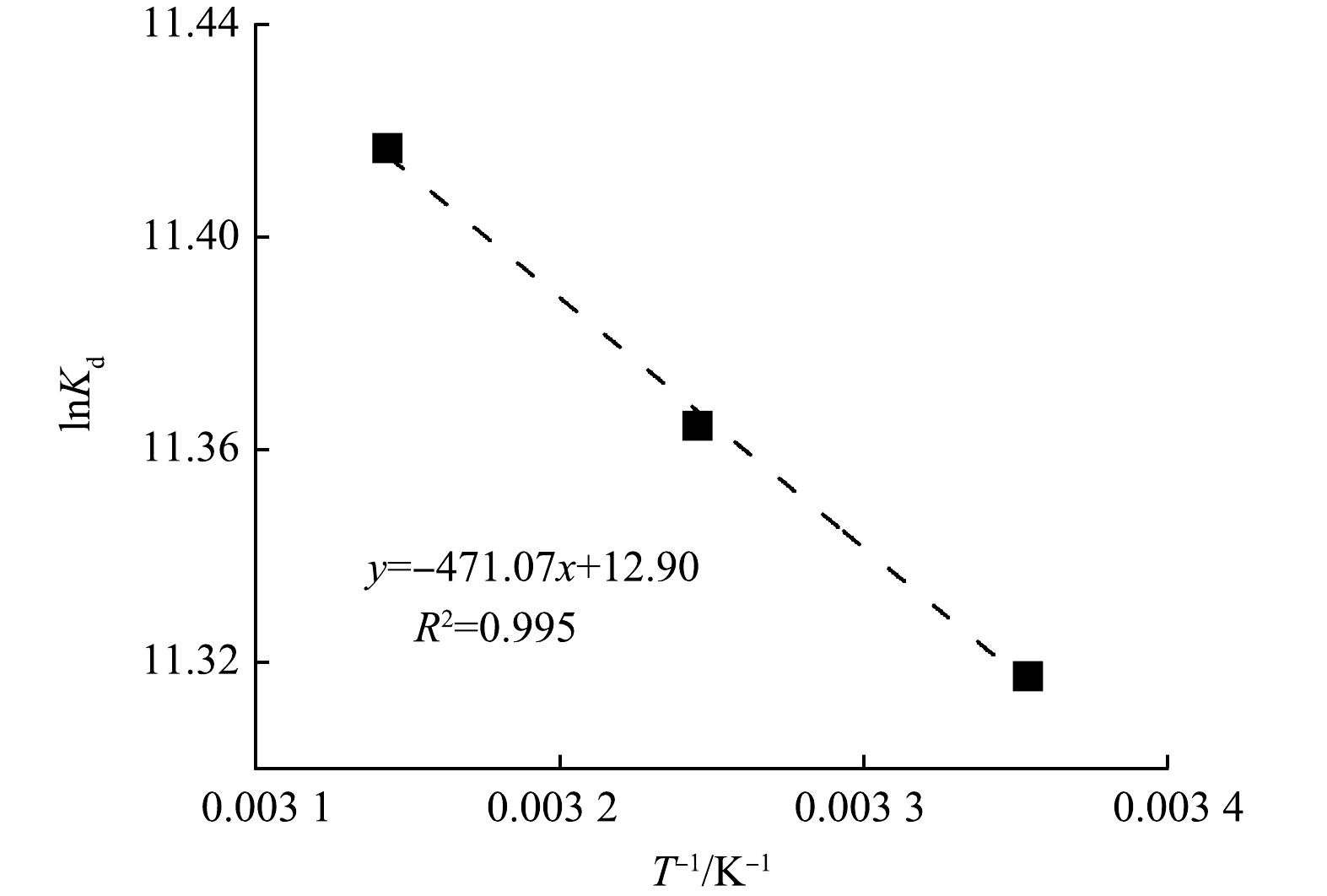
图 4 lnKd与T−1的关系图
Figure 4. Relationship between lnKd and T−1
-
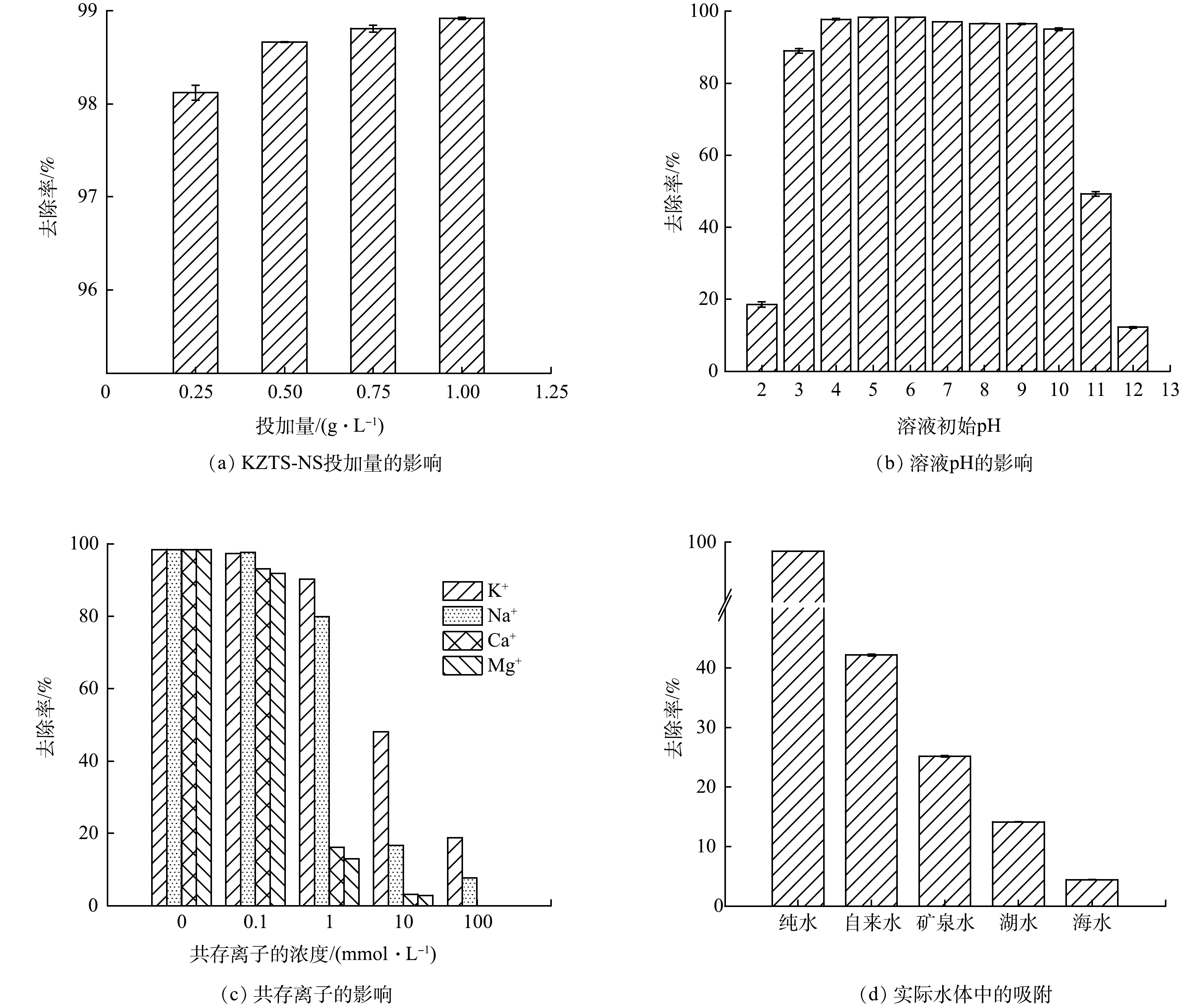
图 5 多种因素对KZTS-NS吸附Cs+的影响
Figure 5. Influence of various factors on the adsorption of Cs+ by KZTS-NS
-
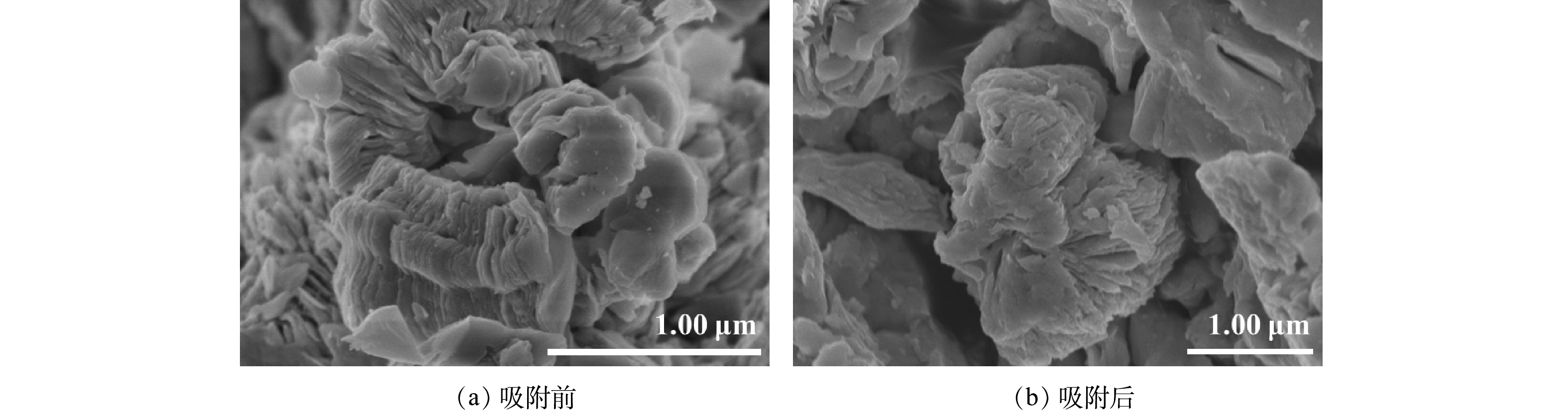
图 6 所合成的KZTS-NS吸附材料的SEM图
Figure 6. SEM images of the synthesized KZTS-NS adsorption material
-
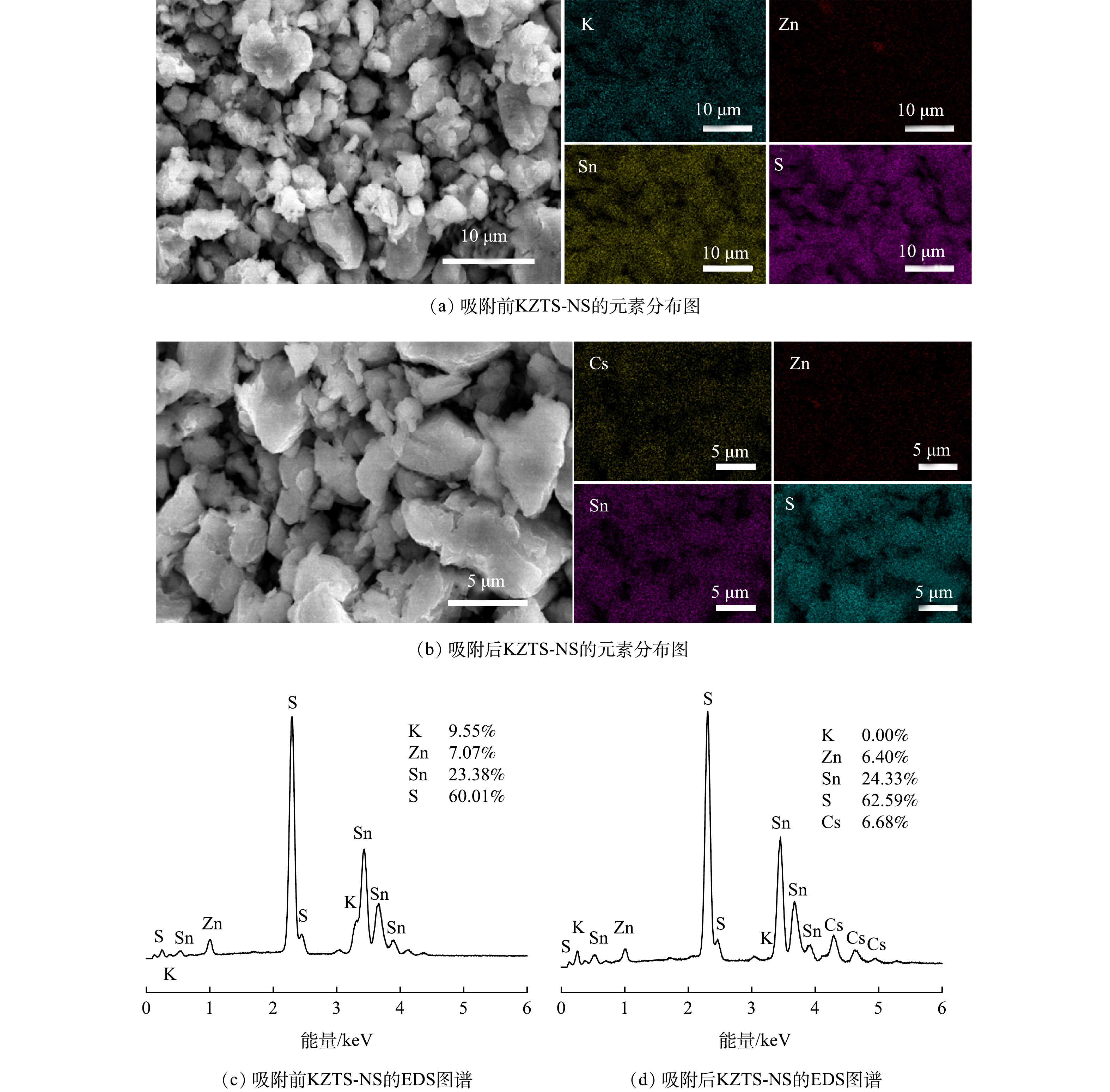
图 7 KZTS-NS吸附Cs+前后的元素分布图与EDS图谱
Figure 7. Elemental mapping images of KZTS-NS before and after adsorption of Cs+
-

图 8 吸附前后KZTS-NS的XRD谱图
Figure 8. XRD patterns of KZTS-NS before and after adsorption
-
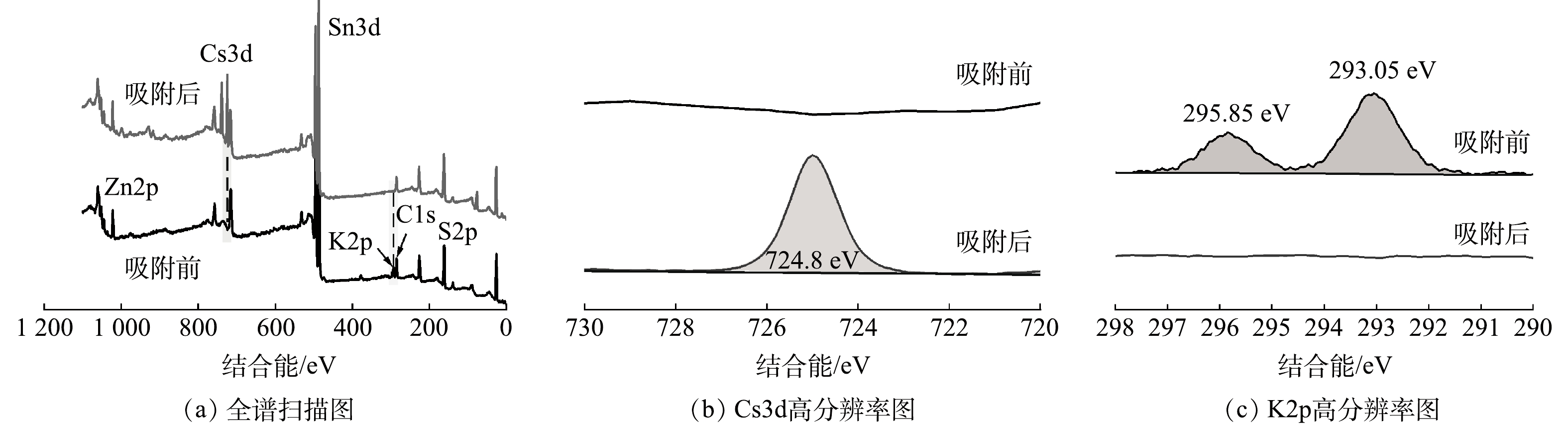
图 9 KZTS-NS吸附Cs+前后的XPS谱图
Figure 9. XPS spectra of KZTS-NS before and after Cs+ adsorption
-

图 10 不同温度下KZTS-NS吸附Cs+的量与脱附K+的量的关系图与KZTS-NS对Cs+的吸附机理图
Figure 10. Relationship between the absorption amount of Cs+ by KZTS-NS and the desorption amount of K+ at different temperatures and the adsorption mechanism of KZTS-NS for Cs+
Figure
10 ,Table
4 个