环境内分泌干扰物(endocrine-disrupting chemicals, EDCs)是指能够干扰生物体内源激素的合成、释放、转运、结合、作用或代谢,从而影响机体的内环境稳定、生殖、发育及行为的化学物质,主要指人类生产和生活活动中排放到环境中的有机污染物[1]。目前,环境中明确存在的EDCs有近百种,主要包括人工合成的激素类化合物(如乙烯雌酚、孕激素等)以及农业、工业生产中使用的化学物质或其降解产物(如有机氯农药、拟除虫菊酯、邻苯二甲酸酯、二噁英、多氯联苯和双酚类化合物等)。双酚类化合物(bisphenols, BPs)主要用于合成碳酸聚酯、环氧树脂、酚醛树脂和聚丙烯酸酯等高分子聚合材料,普遍应用于多种工业产品和日常生活消费品[2]。BPs经排放进入环境介质,污染环境的同时还通过食物链的放大作用在动物和人体内蓄积。目前,已经在水体、底泥以及动植物和人体内检测到多种双酚类化合物[3]。双酚A(bisphenol A, BPA)作为最典型且应用最广的BPs,早在20世纪30年代,就有研究发现BPA具有促进雌性小鼠生殖系统发育的效应,呈现出雌激素活性[4-5]。美国国家毒理学项目组也报告BPA能够影响胎儿、婴儿以及儿童的大脑发育、行为和前列腺发育[6]。因此,2008年,加拿大成为第一个在世界范围内禁止BPA用于婴儿食品容器的国家;随后,美国、欧盟以及中国也相继出台法律法规,禁止BPA用于婴儿奶瓶及其他儿童食品容器[7]。因而BPA替代品广泛应用于工业生产,如双酚B(bisphenol B, BPB)、双酚S(bisphenol S, BPS)、双酚F(bisphenol F, BPF)和双酚AF(bisphenol AF, BPAF)等。这些BPs同样具有一定的内分泌干扰效应和其他毒性作用,已经成为新的环境和健康风险因子。美国明尼苏达州已于2014年立法禁止BPA替代品使用,认为其可能干扰机体的内分泌系统从而导致生殖发育毒性、基因损伤、癌症以及其他系统的毒性作用[7]。BPs作为典型环境激素,对动物及人体健康存在潜在风险,已成为内分泌干扰领域研究热点之一。本文主要介绍了BPs种类、来源和污染现状,并阐述其多种内分泌干扰效应及相关作用机制,为进一步开展BPs风险评估和毒理学研究提供理论基础。
1 BPs的种类和应用(Types and applications of BPs)
BPs是一类分子中含有2~4个酚基的化合物(表1),主要包括BPA及其类似物。BPA是最典型的一种BPs,又称为2-(4,4’-二羟联苯)丙烷、2,2-二(4-羟基苯)丙烷、2,2-二(羟基苯)丙烷,分子式为C15H16O2,分子质量为228.28。BPA难溶于水,易溶于醇、醚、丙酮及碱性溶液,解离常数(pKa)为9.6~10.2,在碱性溶液中溶解度更高[8]。BPA广泛作为生产碳酸聚酯、环氧树脂以及阻燃剂和其他特殊产品的中间物使用,多种生活用品如粘合剂、罐头内壁、地板、指甲油、人造牙齿和食品包装材料等都含有BPA,因而,普通人群几乎无时无刻都在接触BPA[9]。随着BPA的逐步禁用,BPS、BPF、BPAF和BPB等多种BPs逐渐替代BPA用于工业制造[10]。
BPB是BPA中心碳原子上的一个甲基被乙烷基替代所形成的类似物,可替代BPA用于环氧树脂等聚合物的制造[11]。由于BPA的禁用,BPB的产量逐年增加,在工业和生活用品中的应用也越来越广泛,已经在罐头食品、饮料、海鲜和去皮西红柿,婴儿食品和商业牛奶等食品中检出,成为环境和食品中新的污染物[12-15]。BPS是2个苯环被一个磺基连接所形成的有机化合物,其酸性高于其他双酚类化合物,正辛醇-水分配系数低于其他BPs,亲水性更高,生物富集性较低,且具有耐高温和耐光照的特性[16]。BPS于1869年首次合成,主要用于生产染料,2 000年左右作为BPA的替代品用于合成环氧树脂等高分子聚合物。目前,BPS作为BPA的替代物主要用于生产热敏纸显影剂或婴儿奶瓶[17]。欧洲每年生产或进口的BPS达到1 000~10 000 t[18]。BPF是2个苯环经亚甲基连接形成的化合物,其结构与BPA相似。由于BPF具有比BPA环氧树脂更低的黏性和更强的耐受性[19-20],适用性更广泛。其产量在各国均持续增长,成为BPA主要的替代品之一[21]。BPAF是BPA的氟化衍生物,主要用作氟橡胶工业中的交联剂。此外,BPAF也是环氧树脂、碳酸聚酯和聚酰胺等聚合物的重要合成单体,广泛应用于特殊高分子聚合物、高温复合材料、电子材料和可透气性薄膜等的制造[22]。此外,双酚C(bisphenol C, BPC)、双酚E(bisphenol E, BPE)、双酚G(bisphenol G, BPG)、双酚P(bisphenol P, BPP)、双酚AP(bisphenol AP, BPAP)、双酚Z(bisphenol Z, BPZ)、双酚M(bisphenol M, BPM)、双酚芴(fluorene-9-bisphenol, BHPF)、双酚BP(bisphenol BP, BPBP)、双酚TMC(bisphenol TMC, BPTMC)和双酚PH(bisphenol PH, BPPH)等BPs也在工业生产中具有一定的应用,用量相对较少,但此类物质具有较高的正辛醇-水分配系数和生物富集因子,水溶性降低,脂溶性增加,更易在生物体内蓄积,可能会产生更强的毒性效应。
表1 双酚类化合物(BPs)的分子结构和基本信息
Table 1 The properties and molecular structures of bisphenols (BPs)
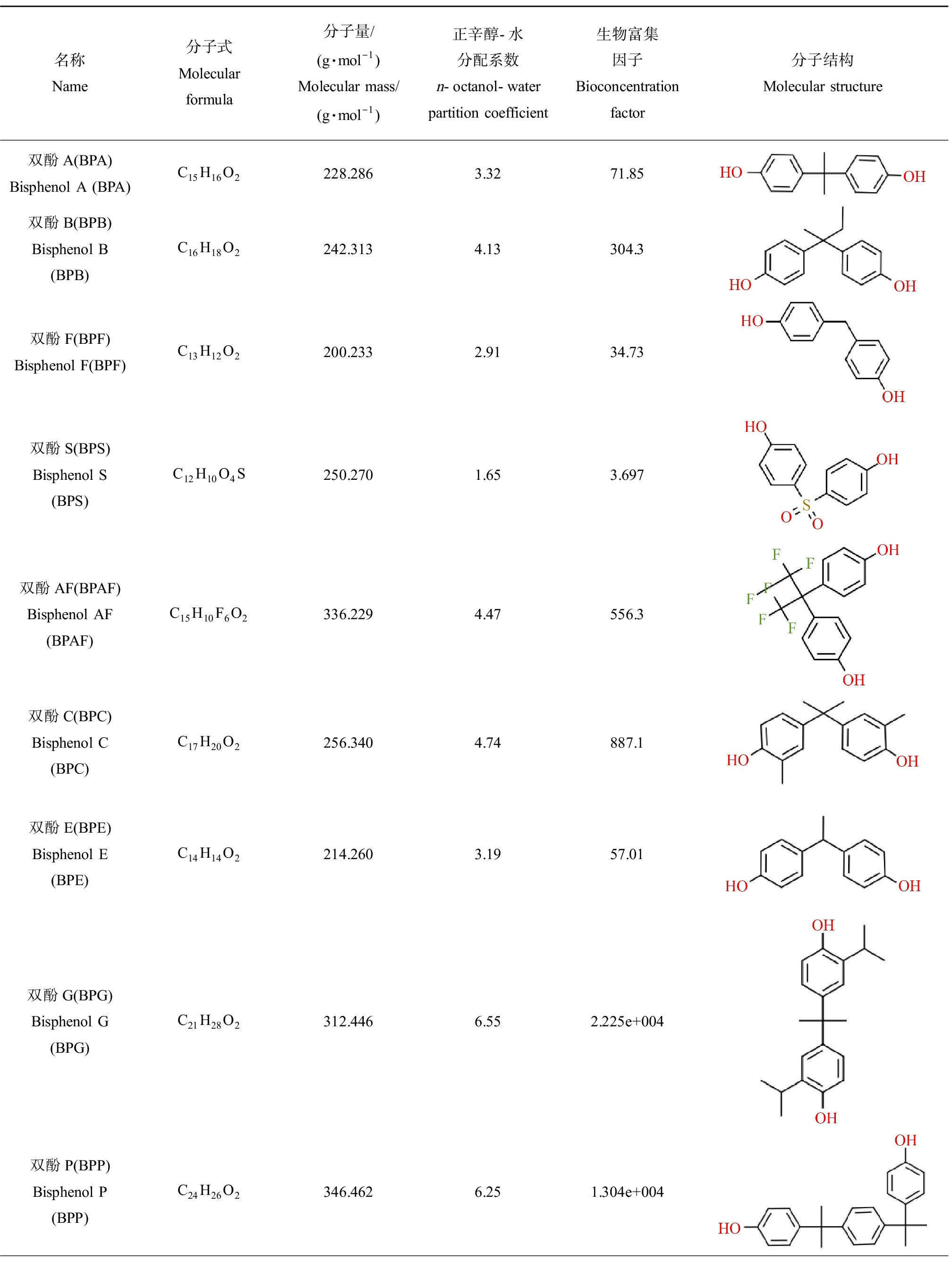
名称Name分子式Molecular formula分子量/(g·mol-1)Molecular mass/(g·mol-1)正辛醇-水分配系数n-octanol-water partition coefficient生物富集因子Bioconcentration factor分子结构Molecular structure双酚A(BPA)Bisphenol A (BPA)C15H16O2228.2863.3271.85双酚B(BPB) Bisphenol B(BPB)C16H18O2242.3134.13304.3双酚F(BPF)Bisphenol F(BPF)C13H12O2200.2332.9134.73双酚S(BPS)Bisphenol S(BPS)C12H10O4S250.2701.653.697双酚AF(BPAF)Bisphenol AF(BPAF)C15H10F6O2336.2294.47556.3双酚C(BPC)Bisphenol C(BPC)C17H20O2256.3404.74887.1双酚E(BPE)Bisphenol E(BPE)C14H14O2214.2603.1957.01双酚G(BPG)Bisphenol G(BPG)C21H28O2312.4466.552.225e+004双酚P(BPP)Bisphenol P(BPP)C24H26O2346.4626.251.304e+004
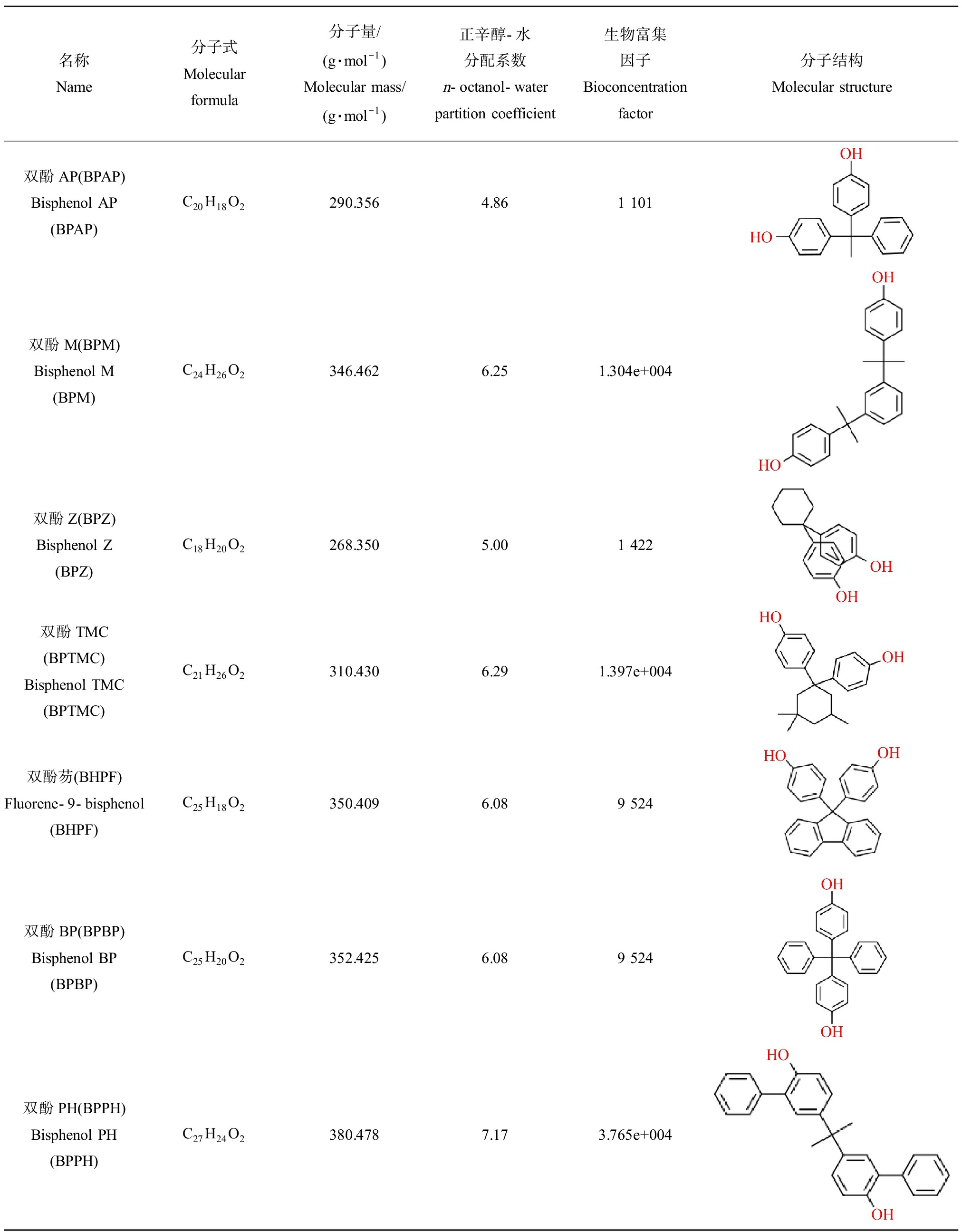
名称Name分子式Molecular formula分子量/(g·mol-1)Molecular mass/(g·mol-1)正辛醇-水分配系数n-octanol-water partition coefficient生物富集因子Bioconcentration factor分子结构Molecular structure双酚AP(BPAP)Bisphenol AP(BPAP)C20H18O2290.3564.861 101双酚M(BPM)Bisphenol M(BPM)C24H26O2346.4626.251.304e+004双酚Z(BPZ)Bisphenol Z(BPZ)C18H20O2268.3505.001 422双酚TMC(BPTMC)Bisphenol TMC(BPTMC)C21H26O2310.4306.291.397e+004双酚芴(BHPF)Fluorene-9-bisphenol(BHPF)C25H18O2350.4096.089 524双酚BP(BPBP)Bisphenol BP(BPBP)C25H20O2352.4256.089 524双酚PH(BPPH)Bisphenol PH(BPPH)C27H24O2380.4787.173.765e+004
2 BPs污染现状(Pollution status of BPs)
BPs经多种途径污染环境介质,并通过食物链在动物或人体内富集,威胁人体健康。尽管BPA已经在全球范围内禁止用于婴幼儿食品容器,但其还未在其他食品包装等生活用品或工业用品中禁用,仍有大量企业在使用BPA生产高分子聚合物。据统计,全球每年大约有500万t的BPA用于工业生产[23],并且从2013年至2019年期间,BPA需求量的年增长率达到4.6%[24]。此外,BPA替代品如BPS、BPF和BPAF的需求量也逐年增加。目前,已经在水体、底泥和室内灰尘等环境介质,罐头食品、罐装水、水果、蔬菜和畜禽肉等食品,人类尿液、母乳和血液中检出多种BPs[3]。BPs已经成为威胁人体健康和生命安全的重要风险因子。
2.1 环境介质暴露情况
BPs主要是在生产过程中经排放污染环境介质,包括水、沉积物、底泥、土壤和大气等(表2)。Jin和Zhu[25]在我国辽河流域的表层水中检出多种BPs,其中以BPA和BPS为主;Wan等[26]取我国长江流域中120份水样,均检测出BPS;Si等[27]在我国无锡太湖和滆湖中取水样,检测出BPA、BPF、BPS、BPAF和四溴双酚A(tetrabromobisphenol A,TBBPA)等5种BPs,其中,BPA、BPS、BPAF和BPF的检出率均较高;Zhang等[28]调查分析中国境内20处自来水处理厂源头水和饮用水中BPs的分布和含量,共检出6种BPs,分别是BPA、BPAF、BPB、BPE、BPF和BPS,检出率最高的是BPA、BPS和BPAF;Liu等[29]通过比较发现,2016年(200~950 ng·L-1)中国太湖流域表层水中BPs的暴露水平显著高于2013年(5.4~87 ng·L-1),但均以BPA、BPAF和BPF为主要暴露化合物。由此可见,BPA、BPS、BPAF和BPF等成为环境介质中最主要的BPs暴露物质,在环境介质中的暴露水平逐渐升高。自然条件下,天然胶体和悬浮颗粒物(suspended particulate matter, SPM)在水环境中无处不在,有超过50%的BPs在表层水中的分布与天然胶体有关[30]。BPs在水中的分布不再局限于溶解相中,胶体相和SPM表面也会有此类物质的残留。Zheng等[31]收集我国南京地区长江流域中的水样,并处理分成可溶解相、胶体相和悬浮颗粒相,在这3种介质中均检测出BPA、BPS、BPF、BPAF和BPE等5种BPs,但不同相中BPs的赋存水平不同,BPA和BPS倾向赋存于溶解相中,而BPAF更倾向持留于SPM上;Si等[27]也发现,BPAF主要存在于水样的胶体相中,其他BPs物质主要存在于溶解相中。由此可见,胶体相和SPM会进一步影响BPs在环境中的分布和迁移转化。
BPs污染呈全球性分布。Yu等[23]收集了美国35个州74个污水处理厂的76份废水污泥样品,检测出BPA、BPS、BPF、BPAF、BPP、BPB和BPZ等BPs,BPA、BPS、BPF和BPAF的检出率较高;Yamazaki等[32]分别从日本、韩国、中国和印度采集了不同河流中的表层水样,检测发现日本、韩国和中国的河流表层水样中BPF的暴露含量最大,甚至高于BPA的暴露量1倍~2倍,而印度河流的表层水样中BPA和BPS的暴露水平更高。Schmidt等[33]也在法国罗纳河表层水中检出了BPS。Liao等[34]分析美国、韩国和日本等国家河流底泥中BPs的暴露情况,结果显示,韩国底泥样品中BPs的暴露水平和检出率均显著高于从美国和日本收集的底泥样品,并检出多种BPA替代品尤其是BPF和BPS。而在我国浙江省采集的沉积物中,BPAF的暴露量显著高于BPA和其他BPs[35]。不同国家生产和使用BPs的情况不一致,可能是导致BPs在环境介质中的暴露特征出现差异的原因之一。一些BPA替代品如BPF、BPS和BPAF等已经成为许多国家环境介质中主要的双酚类污染物,暴露量呈上升趋势。此外,BPs还能附着在灰尘颗粒表面从而污染大气。目前,已经在室内灰尘中检出多种BPs。Wang等[36]采集12个国家的室内灰尘样品,均检测出BPA、BPS、BPF、BPAF、BPP、BPB、BPZ和BPAP等BPs,BPA、BPF、BPS和BPAF是最主要的贡献单体。希腊、美国和日本等国家室内灰尘样品中BPs总暴露量居于前列,不同国家室内灰尘样品中BPS总暴露量如表2所示。
2.2 人体暴露情况
含有BPs的日常消费品或工业用品广泛存在,人类接触和使用此类商品的频率很高,增加了人类暴露风险。BPA及其多种替代品可在人体体液(如尿液、血液和母乳)中检出。表3汇总了主要BPs在人体体液中的暴露水平。Ye等[38]对收集的2000—2014年间美国成年人尿液样品进行BPs暴露分析发现,BPA的暴露水平呈现下降趋势,而BPS和BPF则呈上升趋势。针对美国和7个亚洲国家人群尿液中BPS暴露情况的调查显示,日本和美国等国家人群尿液中BPS的暴露量较其他国家更高[39]。Liu等[40]调查中国南京高淳区学龄前儿童尿液中双酚类物质的含量,检测到BPA、BPF、BPS和BPAF这4种双酚类物质,以BPA和BPS的检出率和检测浓度居高。Xue等[41]也在印度儿童尿液中检出了BPA和BPS,暴露浓度均高于中国儿童尿液中BPA和BPS暴露量。此外,还能在尿液中检出BPB、BPP、BPZ、BPAF和BPAP等BPs[42]。Deceuninck等[43]也在母乳中检出BPA和BPS这2种BPs。还有研究发现BPA的代谢物和BPS能透过胎盘屏障从母体进入到胎儿的脐带血中,说明BPs可能会通过母婴传递进而威胁胎儿健康[44]。
表2 BPs在环境介质中的污染情况
Table 2 The contamination of BPs in environmental compartments
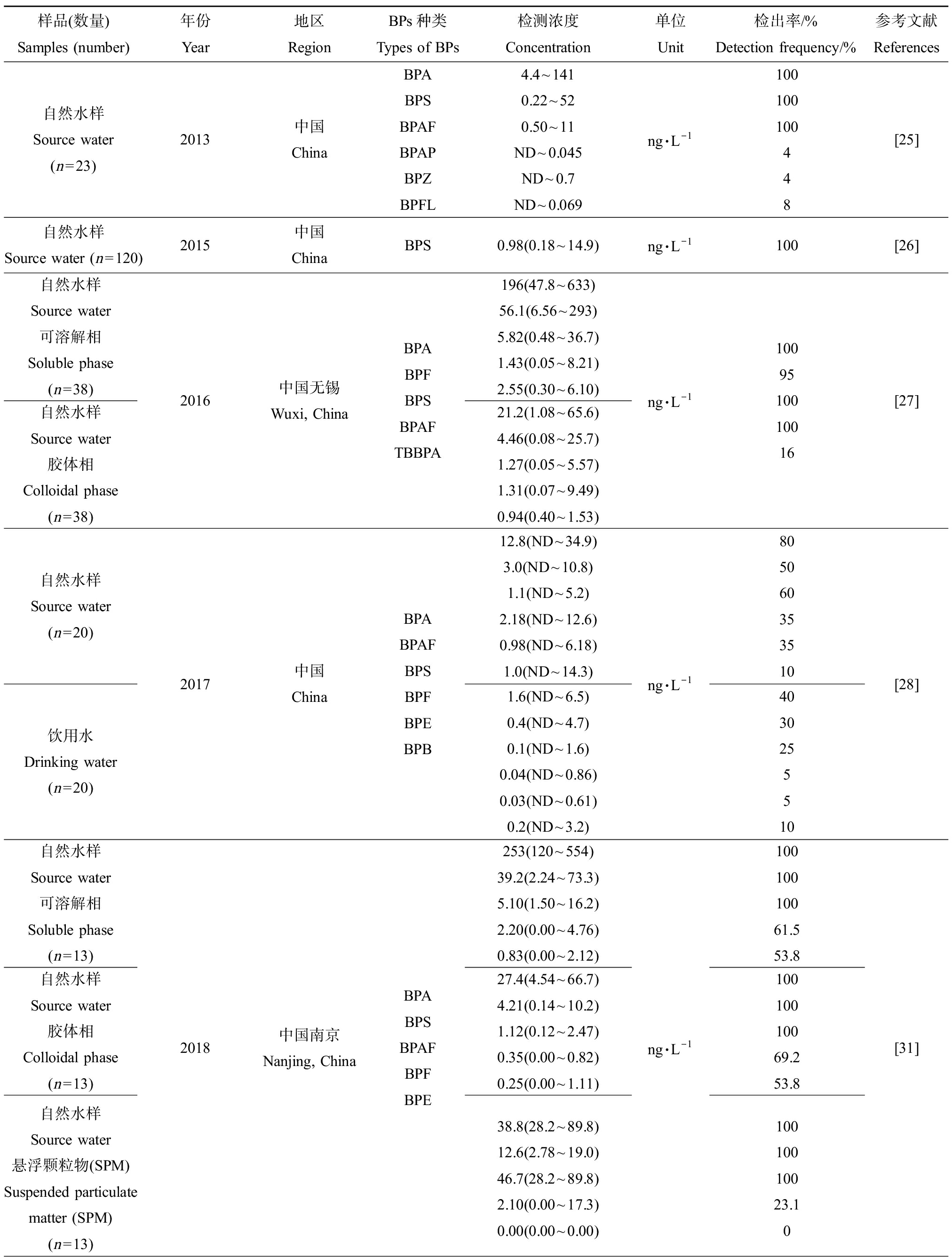
样品(数量)Samples (number)年份Year地区RegionBPs种类Types of BPs检测浓度Concentration单位Unit检出率/%Detection frequency/%参考文献References自然水样Source water(n=23)2013中国ChinaBPABPSBPAFBPAPBPZBPFL4.4~1410.22~520.50~11ND~0.045ND~0.7ND~0.069ng·L-1100100100448[25]自然水样Source water (n=120)2015中国ChinaBPS0.98(0.18~14.9)ng·L-1100[26]自然水样Source water可溶解相Soluble phase(n=38)自然水样Source water胶体相Colloidal phase(n=38)2016中国无锡Wuxi, ChinaBPABPFBPSBPAFTBBPA196(47.8~633)56.1(6.56~293)5.82(0.48~36.7)1.43(0.05~8.21)2.55(0.30~6.10)21.2(1.08~65.6)4.46(0.08~25.7)1.27(0.05~5.57)1.31(0.07~9.49)0.94(0.40~1.53)ng·L-11009510010016[27]自然水样Source water (n=20)饮用水Drinking water(n=20)2017中国ChinaBPABPAFBPSBPFBPEBPB12.8(ND~34.9)3.0(ND~10.8)1.1(ND~5.2)2.18(ND~12.6)0.98(ND~6.18)1.0(ND~14.3)1.6(ND~6.5)0.4(ND~4.7)0.1(ND~1.6)0.04(ND~0.86)0.03(ND~0.61)0.2(ND~3.2)ng·L-18050603535104030255510[28]自然水样Source water可溶解相Soluble phase(n=13)自然水样Source water胶体相Colloidal phase(n=13)自然水样Source water悬浮颗粒物(SPM)Suspended particulate matter (SPM)(n=13)2018中国南京Nanjing, ChinaBPABPSBPAFBPFBPE253(120~554)39.2(2.24~73.3)5.10(1.50~16.2)2.20(0.00~4.76)0.83(0.00~2.12)27.4(4.54~66.7)4.21(0.14~10.2)1.12(0.12~2.47)0.35(0.00~0.82)0.25(0.00~1.11)38.8(28.2~89.8)12.6(2.78~19.0)46.7(28.2~89.8)2.10(0.00~17.3)0.00(0.00~0.00)ng·L-110010010061.553.810010010069.253.810010010023.10[31]
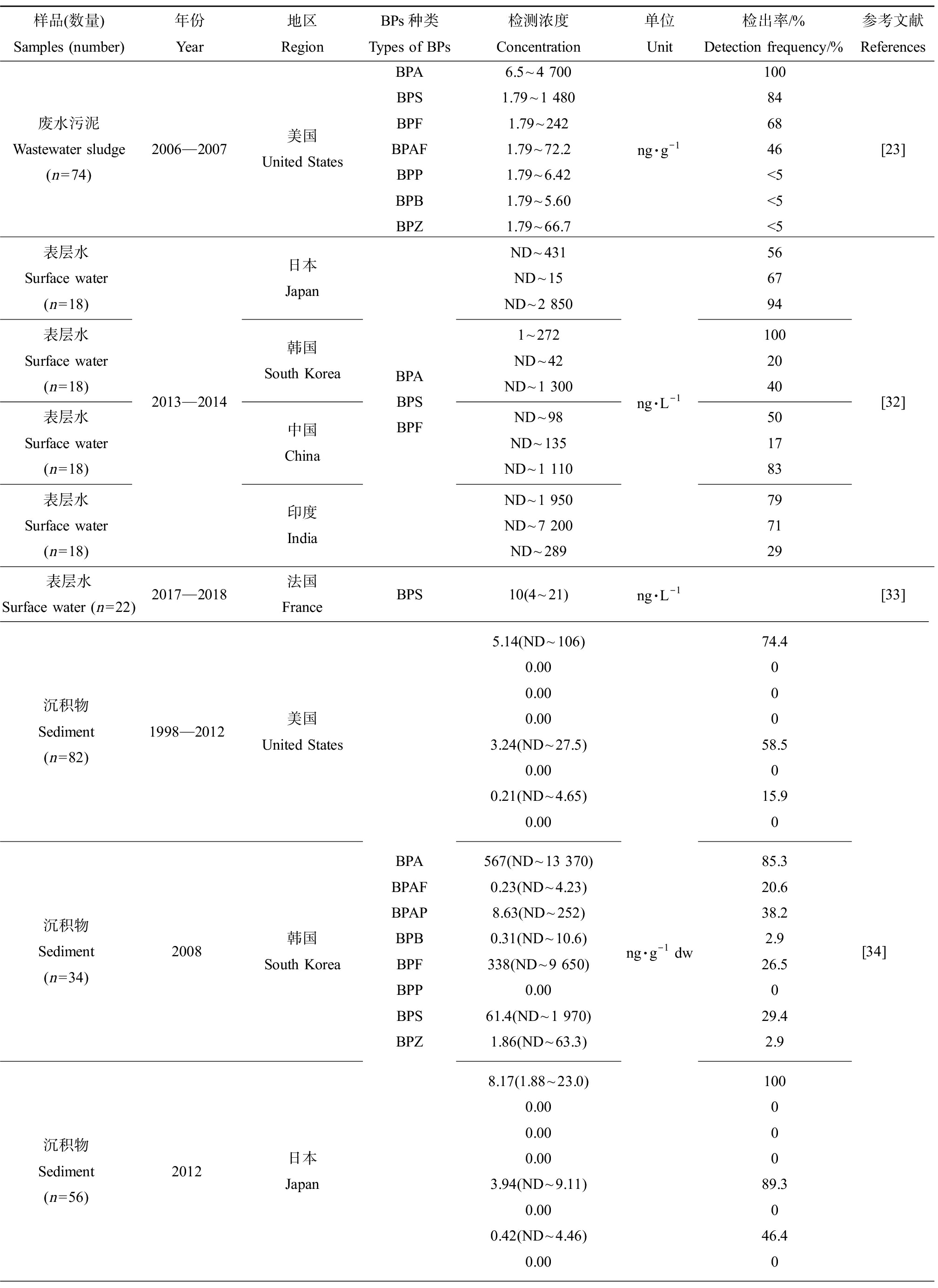
样品(数量)Samples (number)年份Year地区RegionBPs种类Types of BPs检测浓度Concentration单位Unit检出率/%Detection frequency/%参考文献References废水污泥Wastewater sludge(n=74)2006—2007美国United StatesBPABPSBPFBPAFBPPBPBBPZ6.5~4 7001.79~1 4801.79~2421.79~72.21.79~6.421.79~5.601.79~66.7ng·g-1100846846<5<5<5[23]表层水Surface water(n=18)表层水Surface water(n=18)表层水Surface water(n=18)表层水Surface water(n=18)2013—2014日本Japan韩国South Korea中国China印度IndiaBPABPSBPFND~431ND~15ND~2 8501~272ND~42ND~1 300ND~98ND~135ND~1 110ND~1 950ND~7 200ND~289ng·L-15667941002040501783797129[32]表层水Surface water (n=22)2017—2018法国FranceBPS10(4~21)ng·L-1[33]沉积物Sediment(n=82)1998—2012美国United States沉积物Sediment(n=34)2008韩国South Korea沉积物Sediment(n=56)2012日本JapanBPABPAFBPAPBPBBPFBPPBPSBPZ5.14(ND~106)0.000.000.003.24(ND~27.5)0.000.21(ND~4.65)0.00567(ND~13 370)0.23(ND~4.23)8.63(ND~252)0.31(ND~10.6)338(ND~9 650)0.0061.4(ND~1 970)1.86(ND~63.3)8.17(1.88~23.0)0.000.000.003.94(ND~9.11)0.000.42(ND~4.46)0.00ng·g-1 dw74.400058.5015.9085.320.638.22.926.5029.42.910000089.3046.40[34]
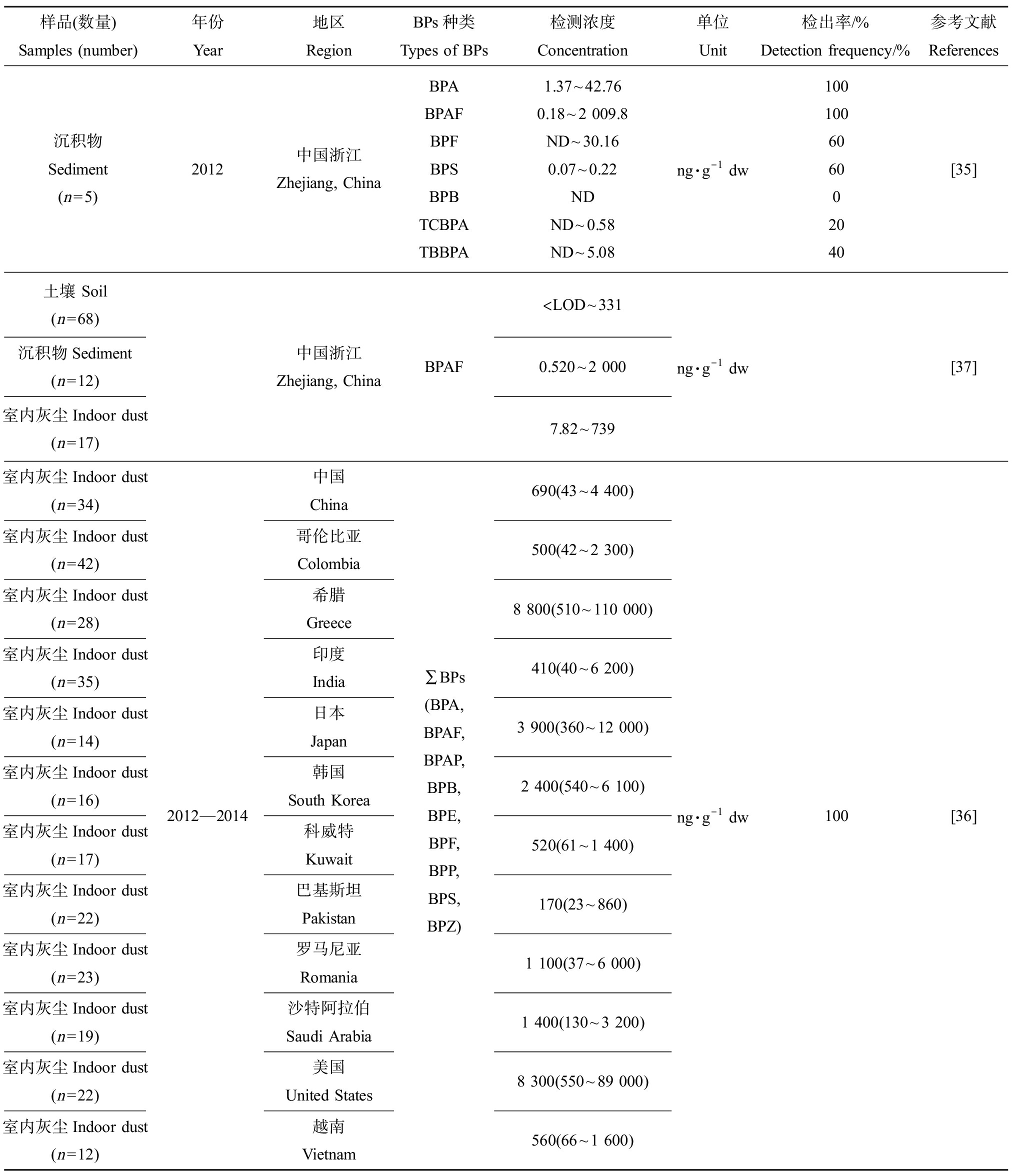
样品(数量)Samples (number)年份Year地区RegionBPs种类Types of BPs检测浓度Concentration单位Unit检出率/%Detection frequency/%参考文献References沉积物Sediment(n=5)2012中国浙江Zhejiang, ChinaBPABPAFBPFBPSBPBTCBPATBBPA1.37~42.760.18~2 009.8ND~30.160.07~0.22NDND~0.58ND~5.08ng·g-1 dw100100606002040[35]土壤 Soil (n=68)沉积物Sediment(n=12)室内灰尘Indoor dust (n=17)中国浙江Zhejiang, ChinaBPAF 注:ND表示未检出,LOD表示检测限,dw表示干质量;检测浓度结果显示为平均值(检测浓度范围);BPA表示双酚A,BPS表示双酚S,BPAF表示双酚AF,BPAP表示双酚AP,BPZ表示双酚Z,BPFL表示双酚芴,BPF表示双酚F,BPE表示双酚E,BPB表示双酚B,BPP表示双酚P,TCBPA表示四氯双酚A,TBBPA表示四溴双酚A。 Note: ND means not detected, LOD means limit of detection, and dw means dry weight; the result of concentrations were shown as mean (the range of detection concentration); BPA stands for bisphenol A, BPS stands for bisphenol S, BPAF stands for bisphenol AF, BPAP stands for bisphenol AP, BPZ stands for bisphenol Z, BPFL stands for fluorene-9-bisphenol, BPF stands for bisphenol F, BPE stands for bisphenol E, BPB stands for bisphenol B, BPP stands for bisphenol P, TCBPA stands for tetrachlorobisphenol A, and TBBPA stands for tetrabromobisphenol A.
表3 BPs化合物在人体体液中的含量
Table 3 The concentration of BPs in human body fluid
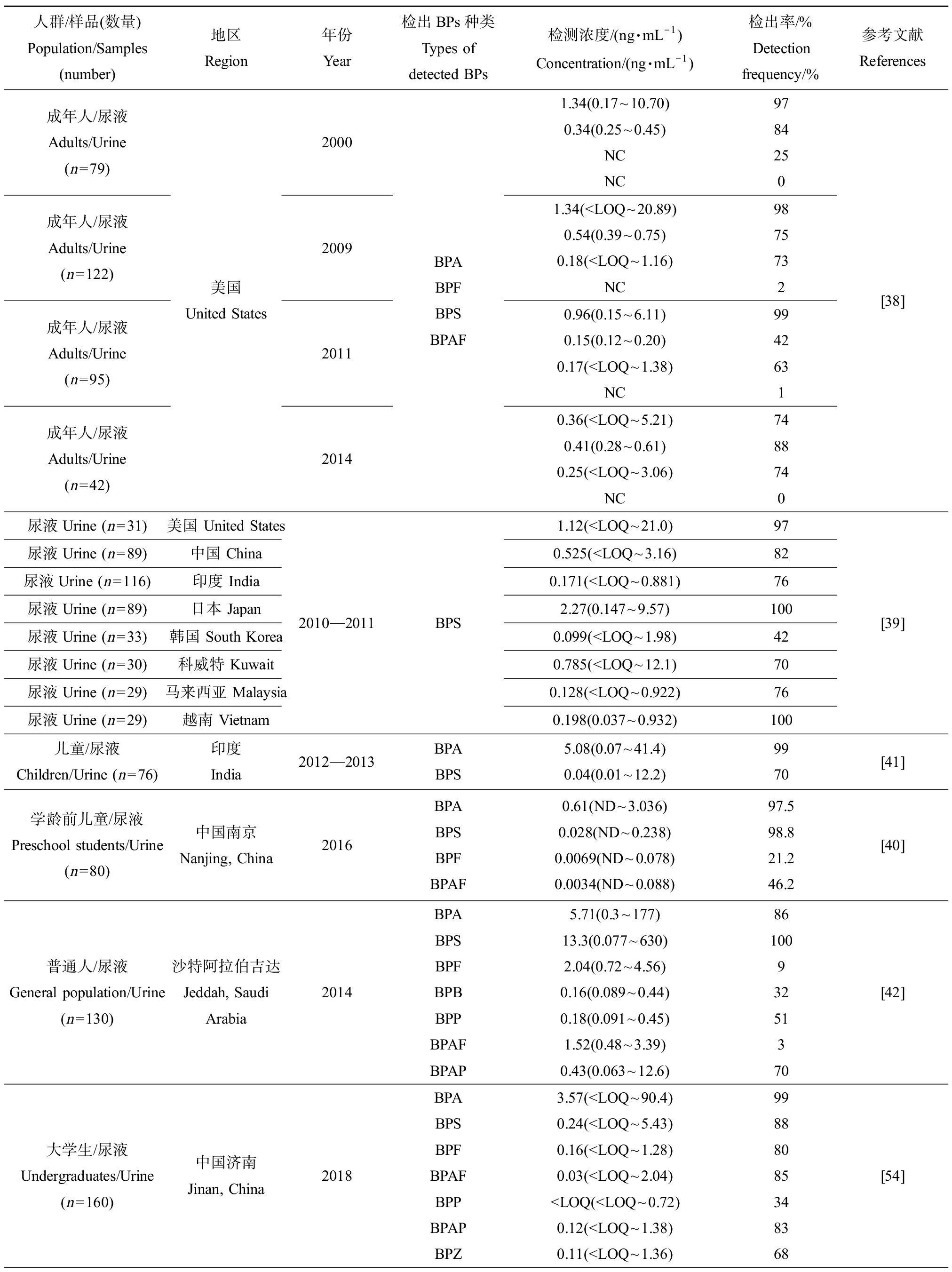
人群/样品(数量)Population/Samples (number)地区Region年份Year检出BPs种类Types of detected BPs检测浓度/(ng·mL-1)Concentration/(ng·mL-1)检出率/%Detection frequency/%参考文献References成年人/尿液Adults/Urine(n=79)成年人/尿液Adults/Urine(n=122)成年人/尿液Adults/Urine(n=95)成年人/尿液Adults/Urine(n=42)美国United States2000200920112014BPABPFBPS BPAF1.34(0.17~10.70)0.34(0.25~0.45)NCNC97842501.34(
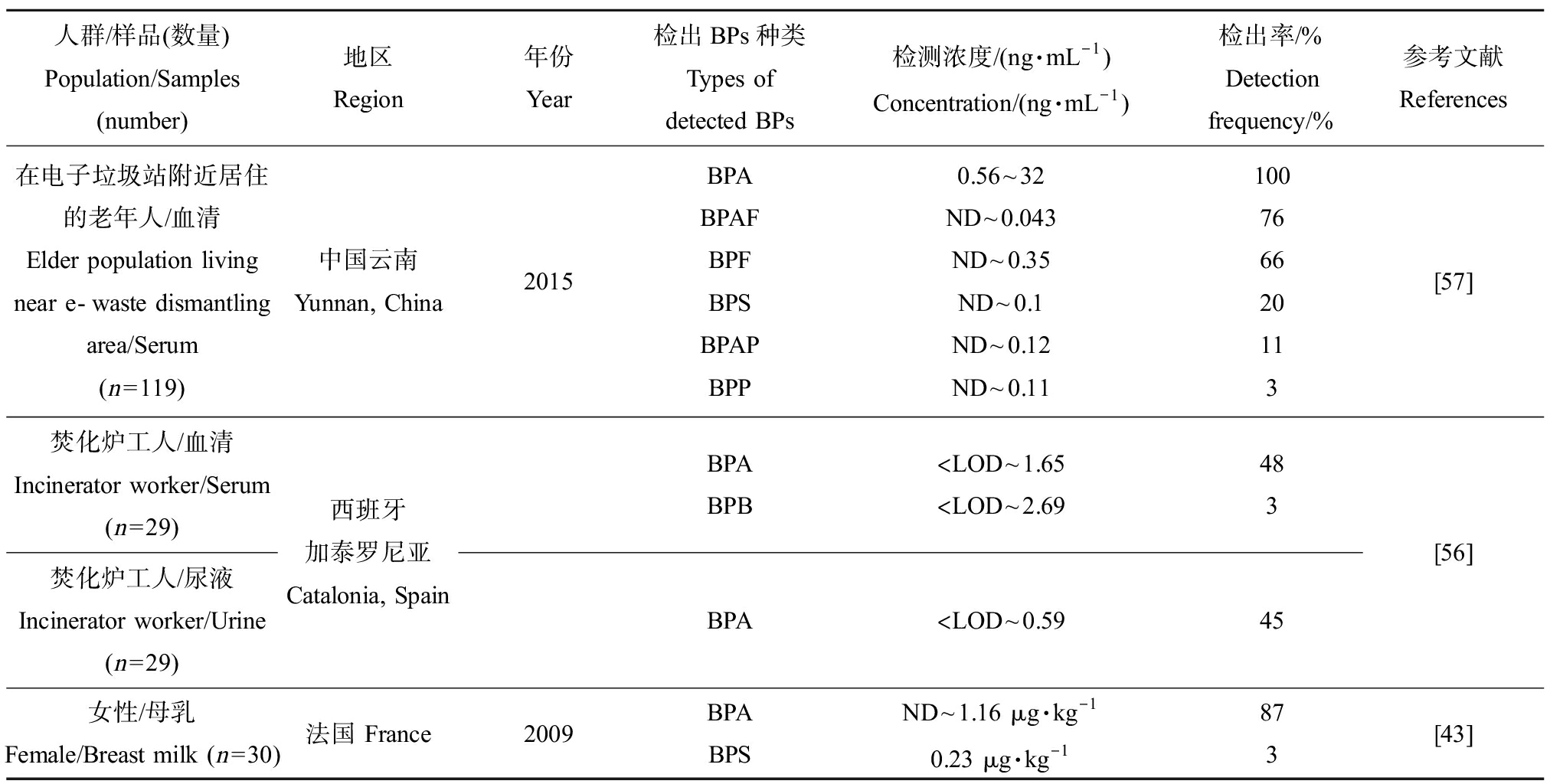
人群/样品(数量)Population/Samples (number)地区Region年份Year检出BPs种类Types of detected BPs检测浓度/(ng·mL-1)Concentration/(ng·mL-1)检出率/%Detection frequency/%参考文献References在电子垃圾站附近居住的老年人/血清Elder population living near e-waste dismantling area/Serum(n=119)中国云南Yunnan, China2015BPABPAFBPFBPSBPAPBPP0.56~32ND~0.043ND~0.35ND~0.1ND~0.12ND~0.11100766620113[57]焚化炉工人/血清Incinerator worker/Serum (n=29)焚化炉工人/尿液Incinerator worker/Urine (n=29)西班牙加泰罗尼亚Catalonia, SpainBPABPB 注: Note:
饮食是人类暴露BPs的最主要途径。一方面,塑料容器和罐头容器内壁涂层中的双酚类物质能够迁移到食品或其周围环境中。主要原因可能包括:(1)聚合物单体的不完全反应;(2)日常使用中接触酸性物质或者经过清洗和加热等操作;(3)高温灭菌、紫外线辐射、剧烈震动或使用不当引起的聚合材料的降解;(4)包装材料的自然老化[45-46]。BPs的迁移发生在其生产和应用的全过程,尤其是在食品再加工或储存的过程中,Zhou等[47]研究发现,一次性塑料饭盒中的BPs能够迁移至与其接触的水中,水浴加热或者微波加热均能够在较短时间内促进BPs的迁移。食品包装材料中的BPs迁移至食品中,经长时间的累积进而威胁食品安全和人体健康。已经有研究表明,美国和欧洲一些国家人体尿液中BPA的暴露与罐头食品的消费有显著的正向相关性[48-49];在加拿大的一项饮食研究中显示,罐头食品中BPA的暴露含量显著高于非罐头包装的食品[50],而孕妇的罐头食品消费活动与其BPA的暴露有关联性[51];Hartle等[49]在美国健康和营养检查调查项目中评估了罐头食品消费与消费者尿液中BPA浓度的相关性,发现食用罐头蔬菜、罐头意大利面和罐头汤与未食用相比,尿液中BPA浓度分别升高41%、70%和229%,罐头食品与尿液中BPA浓度有显著相关性。由此可见,罐头包装是BPs迁移至食品中的重要途径,也是人类暴露于BPs的途径之一。另一方面,BPs对水、土壤和大气环境的污染进一步使得农产品(如蔬菜、水果和畜禽产品等)受到BPs污染,已经成为威胁农产品质量安全的新风险因子。Zhou等[46]在中国浙江省2017—2018年度农产品风险监测项目中,检测了来自12大类农产品的379份实际样品,发现BPA是暴露水平最高的双酚类物质,其次是BPS、BPF和BPB。Xiong等[52]在中国兰州市采集的牛奶样品内检出了9种BPs。Cunha等[53]在海鱼、海藻和双壳类海鲜产品中检出BPA及其衍生物TBBPA。
吸入室内灰尘和长期接触含BPs的消费品也是人类BPs暴露的重要途径。Zhang等[54]调查中国大学生尿液样品及其所处室内灰尘中BPs的含量,结果显示BPA和BPS是最主要的BPs暴露物质,并且尿液中BPs的暴露总量与室内灰尘中BPs的暴露量呈现正相关性,表明非饮食暴露也是导致人体暴露BPs的途径之一,吸入含有BPs的室内灰尘也能导致BPs在人体内蓄积。Thayer等[55]研究发现,收银员使用热敏纸也是导致人体暴露于BPs(如BPA和BPS)的来源之一,所有在日常工作中接触热敏纸的工作人员都有暴露于双酚类化合物的风险。此外,González等[56]在从事危险废物焚化炉工作的工人血清中检出了BPA和BPB,BPA的暴露量和检出率均较高。Song等[57]检测居住于电子垃圾回收设施附近的老年人血清样品中BPs暴露含量,与参考地点人群血清中BPA相比较,居住于电子垃圾回收站附近的老人体内BPA的含量要明显高出参考地点,说明人体中BPA的暴露也与电子垃圾拆卸活动有一定的关联性,但BPAF和BPF等其他检出率较高的替代品未显示出相关性。BPS、BPF和BPAF是目前检出率仅次于BPA的BPs,虽然暴露水平还远低于BPA,但随着生产和使用量的不断增加,暴露水平逐渐上升。
3 BPs的内分泌干扰效应(Endocrine-disrupting effects of BPs)
BPA是一种典型的环境雌激素物质,在体内外对多种生物机体包括水生动物、啮齿类动物和哺乳动物均具有突出的内分泌干扰效应[58-60],因此,BPA逐渐被其他具有相似理化特性和商业价值的BPs替代。然而,越来越多的研究表明,BPA替代品同样具有突出的内分泌干扰效应,甚至比BPA更强,不仅干扰激素的合成和分泌,也能直接作用于激素受体而发挥毒性效应,导致生物体多种内分泌系统紊乱并进一步影响机体生殖功能、神经发育、新陈代谢以及激素依赖性疾病如乳腺癌的发生。
3.1 BPs的雌激素内分泌干扰效应
雌激素作为具有广泛生物活性的类固醇激素,主要以17β-雌二醇(E2)、雌素酮(E1)和雌酚等形成存在。E2是生物体内最为重要、活性最高的雌激素,其水平紊乱将干扰新陈代谢、影响性腺发育并与激素依赖性疾病密切相关。越来越多的研究发现,作为BPA替代品的多种BPs,如BPS、BPAF、BPB、BPF和BHPF等,对动物机体的内分泌干扰效应比BPA更强。能够干扰多种模式动物如斑马鱼、大鼠或小鼠体内类固醇激素合成信号通路上关键基因的表达,从而影响E2的分泌水平。此外,还能直接影响家畜动物包括猪、牛和羊等的生殖内分泌系统,影响卵巢的生长发育和生殖功能的发挥。表4总结了不同BPs在体内外实验中对不同种属动物雌激素内分泌系统的影响。
多种BPs能够影响雌激素受体(estrogen receptor,ER)活性,发挥雌激素干扰效应。ER属于核激素受体家族,包括ERα和ERβ这2种亚型。ER介导多种信号通路,调控很多生理活动如生殖发育、神经行为和脂肪代谢等,甚至参与外源性化合物对其他核激素受体的影响。已经有体内外试验证明BPs具有竞争和/或拮抗ERα和/或ERβ的效应,甚至介导ERα干扰其靶标基因如孕激素受体(progesteronereceptor, PR)、pS2、GREB1、SPUVE、WISP2和SDF-1的表达[71],这些基因与激素依赖性疾病如乳腺癌等密切相关。BPs与激素受体结合的特性是此类化合物发挥内分泌干扰效应并影响生物机体其他生理功能的直接作用机制。因此,广泛分析和评价BPs的激素受体活性是探究其内分泌干扰效应的重要环节。目前,基于内源性表达或重组ER以及荧光素酶报告基因的细胞模型构建了多种外源化合物的ER活性筛查方法。研究表明,BPs与ERα和ERβ的结合效力存在差异,并具有浓度依赖性。Li等[72]利用人宫颈癌细胞(HeLa)、人肝癌细胞(HepG2)和人子宫内膜癌细胞(Ishikawa)分析了BPA和BPAF的雌激素效应,研究发现较低浓度(≤10 nmol·L-1)BPA和BPAF对ERα和ERβ具有拮抗作用,而较高浓度(≥10 nmol·L-1)BPA和BPAF均对ERα和ERβ具有竞争结合的效应。值得关注的是,多种体外重组细胞模型检测发现BPAF、BPC、BPB和BPZ的ER竞争结合强度比BPA更强[73-78],显示出更强的内分泌干扰效应。表5列举了多种体外评估BPs雌激素干扰效应的动物细胞模型。虽然体外分析化合物激素受体活性的方法具有一定的优势,包括高通量检测、快速和可控等,但体外检测结果仍然与体内作用模式下的影响存在差异。因此,基于芳香化酶B和模式动物活体斑马鱼而开发的检测外源化合物激素受体活性的方法得到广泛应用。芳香化酶B是鱼类等水生动物脑组织中雌激素敏感分子,其编码基因cyp19a1b已经成为指示外源性化合物雌激素样活性的标志分子[79]。Moreman等[80]利用携带cyp19a1b基因和雌激素反应元件(estrogen response element, ERE)调控绿色荧光蛋白的转基因斑马鱼胚胎分析了BPA、BPAF、BPF和BPS的雌激素活性,结果显示4种BPs均能促进cyp19a1b基因的表达,雌激素效应大小是BPAF>BPA=BPF>BPS。体内实验进一步表明以BPAF为代表的BPA替代品同样具有雌激素干扰效应,甚至比BPA的雌激素受体干扰活性更强。
表4 BPs对雌激素内分泌系统的影响(体内外实验)
Table 4 Effects and outcomes of BPs on estrogen endocrine system in vivo and in vitro

BPs暴露动物/细胞Exposed animals/cells暴露周期Exposure times暴露浓度Exposure concentrations雌激素干扰效应Endocrine disrupting effects参考文献ReferencesBPS雌雄斑马鱼Female and male zebrafish 21d0.5, 5, 50 μg·L-1扰乱雌雄斑马鱼血浆雌二醇(E2)水平;性成熟指数和产卵量降低,子代斑马鱼畸形率增加Disruption in male and female zebrafish plasma estradiol (E2) levels; decreased sexual maturity in-dex and egg production, and the increased malfor-mation rate of progeny zebrafish[61]CF-1雌雄小鼠Female and male CF-1 mice 12 h1, 3, 9 mg·g-1雌雄小鼠尿液中E2水平降低Reduced levels of E2 in the urine of male and fe-male mice[62]猪卵巢颗粒细胞Swine granulosa cells48 h0.1, 1, 10 μmol·L-1抑制猪卵巢颗粒细胞中E2分泌;抑制细胞增殖,刺激超氧化物和一氧化氮生成,干扰细胞代谢功能Inhibition of E2 secretion in cultured swine granu-losa cells; inhibition of cell proliferation, stimula-tion of superoxide and nitric oxide production, and interference with cellular metabolism[63]牛卵巢颗粒细胞和卵泡膜细胞Bovine granulosa and theca cells6 d1 fmol·L-1~100 μmol·L-1刺激颗粒细胞中E2生成Stimulation of E2 production in bovine granulosa cells[64]妊娠羊Pregnant sheepGD30~GD1000.5 mg·kg-1绵羊胎盘内分泌功能障碍和融合型滋养层信号通路失调Endocrine dysfunction of sheep placenta and dys-regulation of fusion trophoblast signaling pathway[65]BPB雌雄斑马鱼Female and male zebrafish 21 d0.001, 0.01, 0.1, 1 mg·L-1干扰类固醇激素生物合成相关基因的表达;雄性斑马鱼E2分泌水平提高;鱼卵个数降低,孵化率和存活率降低,斑马鱼生殖功能受损Interference with the expression of steroidogenic genes; increased E2 secretion of male zebrafish; reduced numbers of eggs, decreased hatching rate and survival rate, and the damage on the repro-ductive function of zebrafish[66]BPAF雌雄斑马鱼Female and male zebrafish 28 d0.05, 0.25, 1 mg·L-1睾酮水平降低,E2水平升高(雄性斑马鱼);睾酮分泌水平升高(雌性斑马鱼)Decreased testosterone levels and increased E2 levels (male zebrafish); increased testosterone se-cretion (female zebrafish)[67]BPAFBPA小鼠(子宫内膜异位症)Mouse (endometriosis)6 weeks3, 30, 90 mg·kg-1干扰卵巢类固醇激素生成信号通路中相关基因表达;孕酮水平降低,闭锁卵泡数量增加;BPAF比BPA更能促进小鼠子宫内膜异位症的发展Interference with the expression of steroidogenic genes in the ovary; decreased progesterone levels and increased number of atretic follicles;BPAF promotes the development of endometriosis in mice more than BPA[68]

BPs暴露动物/细胞Exposed animals/cells暴露周期Exposure times暴露浓度Exposure concentrations雌激素干扰效应Endocrine disrupting effects参考文献ReferencesBHPF小鼠Mice猪卵母细胞Porcine oocyte10 d含BHPF的瓶装水Plastic bottled water containing BHPF子宫质量降低;雌激素应答相关基因sprr2a和sprr2b表达显著下降Decreased uterine weight; the down-regulation in the expression of estrogen response-related genes sprr2a and sprr2bPND24~PND600.4, 2, 10, 50 mg·kg-1子宫质量降低;子宫内膜萎缩;抗雌激素效应Reduced uterine weight; endometrial atrophy; an-tiestrogenic effects42~44 h25, 50, 75 μmol·L-1卵母细胞第1机体释放减少;纺锤体组装异常,三磷酸腺苷(ATP)水平降低,活性氧(ROS)积聚,诱导卵母细胞的早期凋亡;卵母细胞成熟紊乱Decreased oocyte first body release; abnormal spindle assembly, reduced adenosine triphosphate (ATP) levels, accumulation of reactive oxygen species (ROS), induction of early oocyte apopto-sis; disordered oocyte maturation[69][70]
注:GD表示妊娠期;PND表示围产期;BHPF表示双酚芴。
Note: GD means gestational day; PND means perinatal period; BHPF means fluorene-9-bisphenol.
表5 体外评估BPs雌激素受体活性的动物细胞模型
Table 5 Animal cell models for in vitro evaluating estrogen receptor activities of BPs
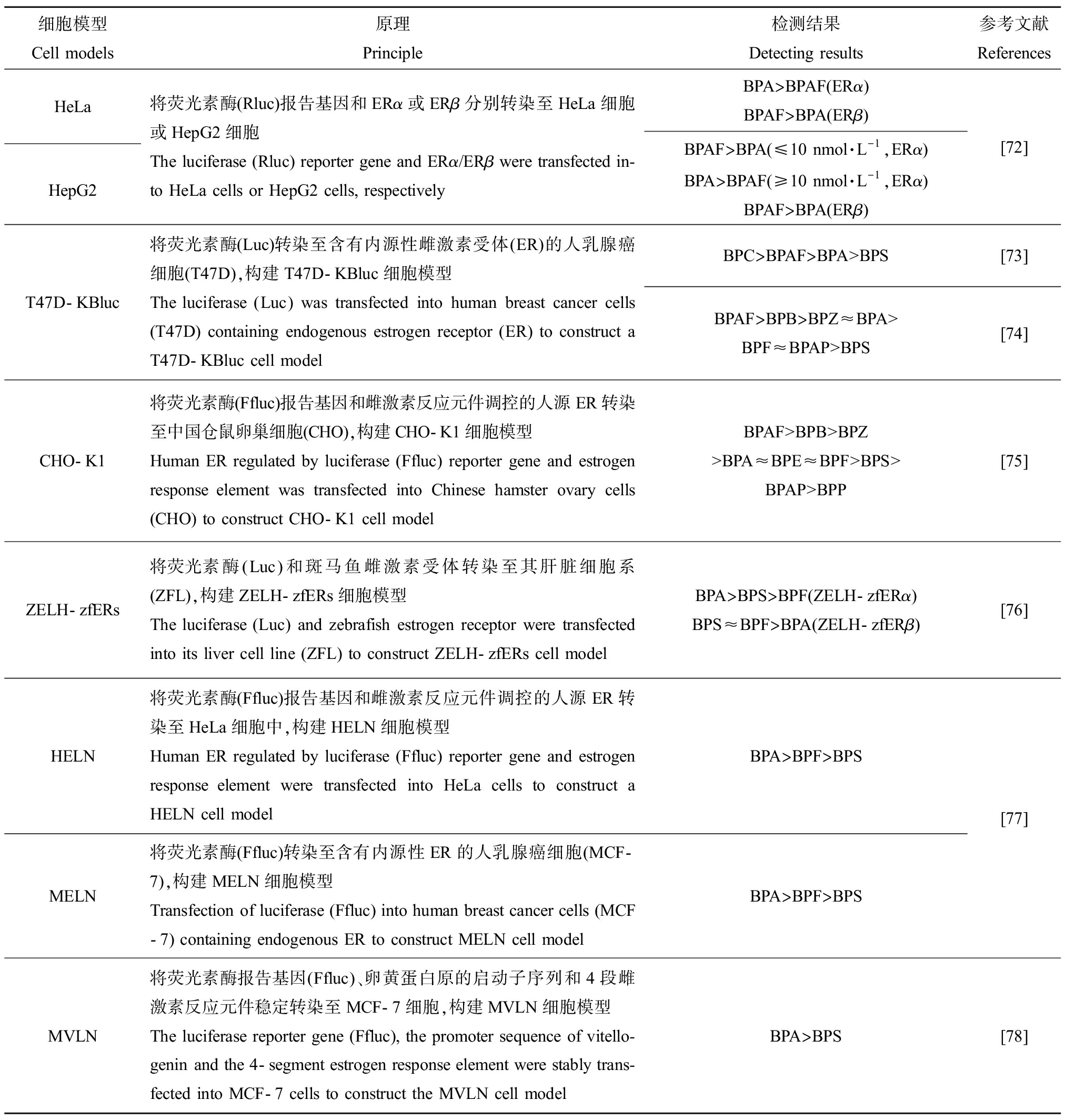
细胞模型Cell models原理Principle检测结果Detecting results参考文献ReferencesHeLaHepG2将荧光素酶(Rluc)报告基因和ERα或ERβ分别转染至HeLa细胞或HepG2细胞The luciferase (Rluc) reporter gene and ERα/ERβ were transfected in-to HeLa cells or HepG2 cells, respectivelyBPA>BPAF(ERα)BPAF>BPA(ERβ)BPAF>BPA(≤10 nmol·L-1,ERα)BPA>BPAF(≥10 nmol·L-1,ERα)BPAF>BPA(ERβ)[72]T47D-KBluc将荧光素酶(Luc)转染至含有内源性雌激素受体(ER)的人乳腺癌细胞(T47D),构建T47D-KBluc细胞模型The luciferase (Luc) was transfected into human breast cancer cells (T47D) containing endogenous estrogen receptor (ER) to construct a T47D-KBluc cell modelBPC>BPAF>BPA>BPS[73]BPAF>BPB>BPZ≈BPA>BPF≈BPAP>BPS[74]CHO-K1将荧光素酶(Ffluc)报告基因和雌激素反应元件调控的人源ER转染至中国仓鼠卵巢细胞(CHO),构建CHO-K1细胞模型Human ER regulated by luciferase (Ffluc) reporter gene and estrogen response element was transfected into Chinese hamster ovary cells (CHO) to construct CHO-K1 cell modelBPAF>BPB>BPZ>BPA≈BPE≈BPF>BPS>BPAP>BPP[75]ZELH-zfERs将荧光素酶(Luc)和斑马鱼雌激素受体转染至其肝脏细胞系(ZFL),构建ZELH-zfERs细胞模型The luciferase (Luc) and zebrafish estrogen receptor were transfected into its liver cell line (ZFL) to construct ZELH-zfERs cell modelBPA>BPS>BPF(ZELH-zfERα)BPS≈BPF>BPA(ZELH-zfERβ)[76]HELN将荧光素酶(Ffluc)报告基因和雌激素反应元件调控的人源ER转染至HeLa细胞中,构建HELN细胞模型Human ER regulated by luciferase (Ffluc) reporter gene and estrogen response element were transfected into HeLa cells to construct a HELN cell modelBPA>BPF>BPSMELN将荧光素酶(Ffluc)转染至含有内源性ER的人乳腺癌细胞(MCF-7),构建MELN细胞模型Transfection of luciferase (Ffluc) into human breast cancer cells (MCF-7) containing endogenous ER to construct MELN cell modelBPA>BPF>BPS[77]MVLN将荧光素酶报告基因(Ffluc)、卵黄蛋白原的启动子序列和4段雌激素反应元件稳定转染至MCF-7细胞,构建MVLN细胞模型The luciferase reporter gene (Ffluc), the promoter sequence of vitello-genin and the 4-segment estrogen response element were stably trans-fected into MCF-7 cells to construct the MVLN cell modelBPA>BPS[78]
注:BPC表示双酚C。
Note: BPC means bisphenol C.
3.2 BPs的雄激素干扰效应
雄激素是主要由睾丸合成和分泌的一种类固醇激素,与雄激素受体(androgen receptor, AR)结合,在维持雄性性腺发育和生殖功能的生理过程中发挥重要作用。研究表明,BPs能直接影响睾酮的生物合成和分泌,扰乱机体内雄激素水平。Feng等[81]发现高浓度BPAF短期暴露大鼠后,通过干扰睾酮生物合成途径中的基因和蛋白的表达而抑制睾酮的合成和分泌,显著降低血清中睾酮含量;小鼠在妊娠期和哺乳期暴露BPAF后,BPAF能够通过脐带血和乳汁从母体转移至子代的睾丸组织中,子代睾丸中睾酮的分泌量显著增加,但睾丸抑制素B的水平显著下降,睾丸抑制素B是评价雄性生精能力的重要分子指标,提示BPAF暴露能损伤雄性子代的生殖功能[82]。Ullah等[60]研究发现BPA及其替代品BPF、BPS和BPB暴露小鼠28 d后显著降低睾丸组织和血浆中的睾酮含量,并损伤了输精管及其间质、输精管直径和输精管上皮高度等睾丸和附睾的组织形态结构。此外,Ullah等[83]还开展了大鼠低剂量长期暴露BPF、BPS、BPB和BPA的试验,经48周饮水暴露后,改变了大鼠睾丸组织结构,干扰睾酮的生成和分泌,影响精子数量和质量,损伤雄性大鼠的生殖系统。
体外筛查分析表明,BPA及其替代品主要通过拮抗AR发挥雄激素干扰效应。不同BPs对AR的拮抗活性具有显著差异。其中,BPAF和BPA可以完全拮抗雄激素与AR的结合,而BPS仅具有微弱的拮抗活性。BPE、BPAF和BPB对AR的拮抗性比BPA更强,可能会进一步干扰AR介导的信号通路。表6列出了体外评估BPs的AR活性的动物细胞模型。
3.3 BPs的甲状腺素干扰效应
甲状腺激素是由甲状腺分泌的一种氨基酸衍生物,与甲状腺激素受体α(thyroid hormone receptor α, THRα)或β(thyroid hormonereceptor β,THRβ)结合发挥调控骨骼、大脑和生殖器官发育等重要生理功能。BPs能影响动物机体中甲状腺激素水平而发挥内分泌干扰效应。BPAF(50 μg·L-1)短期暴露(168 h)斑马鱼能显著降低甲状腺素的水平,同时影响了参与调控下丘脑-垂体-甲状腺(hypothalamic-pituitary-thyroid,HPT)轴中相关基因如促甲状腺激素β、甲状腺球蛋白、甲状腺素转运蛋白、thrα和thrβ等基因的转录活动,表现出显著的甲状腺内分泌干扰毒性[86];Kwon等[87]也发现BPAF(24.5 μg·L-1)低剂量长期暴露(21 d)斑马鱼,通过显著下调HPT轴中基因trh和tshβ的表达而促进体内甲状腺素分泌,提示BPAF通过干扰HPT轴的负反馈调控机制而影响甲状腺素的分泌,扰乱机体甲状腺激素稳态。此外,BPF(200 μg·L-1)短期暴露也能干扰斑马鱼体内三碘甲状腺原氨酸、甲状腺素和促甲状腺激素(thyroid-stimulating hormone,TSH)的生成和分泌,主要通过影响基因crh、nis、tg、ttr、dio2和ugt1ab的表达而实现,这些基因是参与调控甲状腺激素合成、分泌和代谢的关键信号分子[88]。值得注意的是,不同剂量和暴露时间会改变BPs对动物机体内分泌系统的终点效应,低剂量长期暴露仍然是需要重点关注的毒性效应评价模式。
研究发现,BPA及其替代品对甲状腺激素受体(thyroid hormonereceptor, THR)具有竞争结合和/或拮抗的活性,不同化合物在不同的暴露浓度下具有不同的THR活性。在荧光信号竞争结合试验中,BPA、BPS和BPF均可以竞争激活甲状腺素受体THRα和THRβ,结合能力大小是BPA>BPF>BPS,并且计算机分子对接模拟试验也证明这些BPs能与THR结合。但是,利用重组的GH3细胞模型检测发现,BPA和BPF在5~50 μmol·L-1浓度范围内以剂量-时间依赖性方式激活THR,但BPS暴露呈现倒“U”型效应关系,激活THR的效应较弱,且在50 μmol·L-1浓度下具有拮抗THR的作用[89]。Lu等[90]经酵母双杂交试验同样发现BPS及溴化衍生物四溴双酚S(tetrabromobisphenol S, TBBPS)在环境暴露相关浓度下均能够拮抗斑马鱼THRβ,并且能影响斑马鱼胚胎中THRβ mRNA的表达,具有一定的抗甲状腺激素效应。此外,GH3细胞也是评价污染物或化学品甲状腺内分泌活性的良好模型,主要通过目标化合物对细胞增殖的影响来衡量。Lee等[59]利用该细胞模型研究发现,单独暴露不同BPs均能以剂量-时间依赖性方式促进GH3细胞增殖,但分别与三碘甲状腺原氨酸(T3)共同暴露时,所有BPs均未加强T3诱导的细胞增殖效应,高浓度(10-6mol·L-1)的BPAF、BPA、BPB、BPF、BPS和BPZ反而拮抗T3诱导的细胞增殖效应。总体而言,BPAF、BPF、BPC和BPS的THR活性均比BPA强。表7列举了体外评估BPs甲状腺激素受体活性的方法。
3.4 BPs的神经内分泌干扰效应
研究表明,多种BPs暴露能影响神经内分泌系统并导致人或其他动物神经行为紊乱,包括认知缺陷、焦虑、抑郁和社交缺陷等,甚至与自闭症和多动症有一定相关性[92]。BPs对神经行为的影响,可能与扰乱神经分泌系统有关。此外,神经内分泌系统与性激素和甲状腺激素通过下丘脑-垂体-性腺(hypothalamic-pituitary-gonad, HPG)轴和HPT轴密切联系,下丘脑释放的促性腺激素释放激素(gonadotropin-releasing hormone,GnRHs)和促甲状腺激素释放激素(thyrotropin releasing hormone,TRH)能进一步刺激脑垂体分泌促性腺激素(gonadotropins,GtHs)和TSH,GtHs和TSH调控性腺中性激素以及甲状腺中甲状腺激素的合成和分泌,而性激素和甲状腺激素又可以通过负反馈机制调控GnRHs、GtHs、TRH和TSH的分泌。因此,BPs可能通过干扰性激素和甲状腺激素及其受体信号通路而影响神经内分泌系统,导致神经行为紊乱。Kinch等[92]研究发现低于人体暴露水平的BPA和BPS暴露斑马鱼胚胎后导致了下丘脑神经元的过早发育,AR信号通路参与了BPA和BPS影响神经发育的过程;Qiu等[93]还发现低浓度BPA和BPS暴露斑马鱼胚胎后增加了中枢和外周神经中枢GnRH3神经元的数量,同时延长了斑马鱼胚胎的孵育时间,kiss1/kiss1受体信号以及ER、THR和芳香化酶等信号分子也参与其中。也有研究报道,BHPF暴露干扰了雌雄斑马鱼在求爱行为上的偏好,诱发了焦虑和抑郁样症状,下调了雌激素受体、类固醇激素合成相关基因的表达[94],提示BHPF能同时对生殖和潜在行为产生不良效应。由此可见,BPs对内分泌系统的干扰效应不是单一存在的,神经内分泌、性激素内分泌和甲状腺素内分泌系统能同时受到BPs的影响。
表6 体外评估BPs雄激素受体活性的动物细胞模型
Table 6 Animal cell models for in vitro evaluating androgen receptor activities of BPs
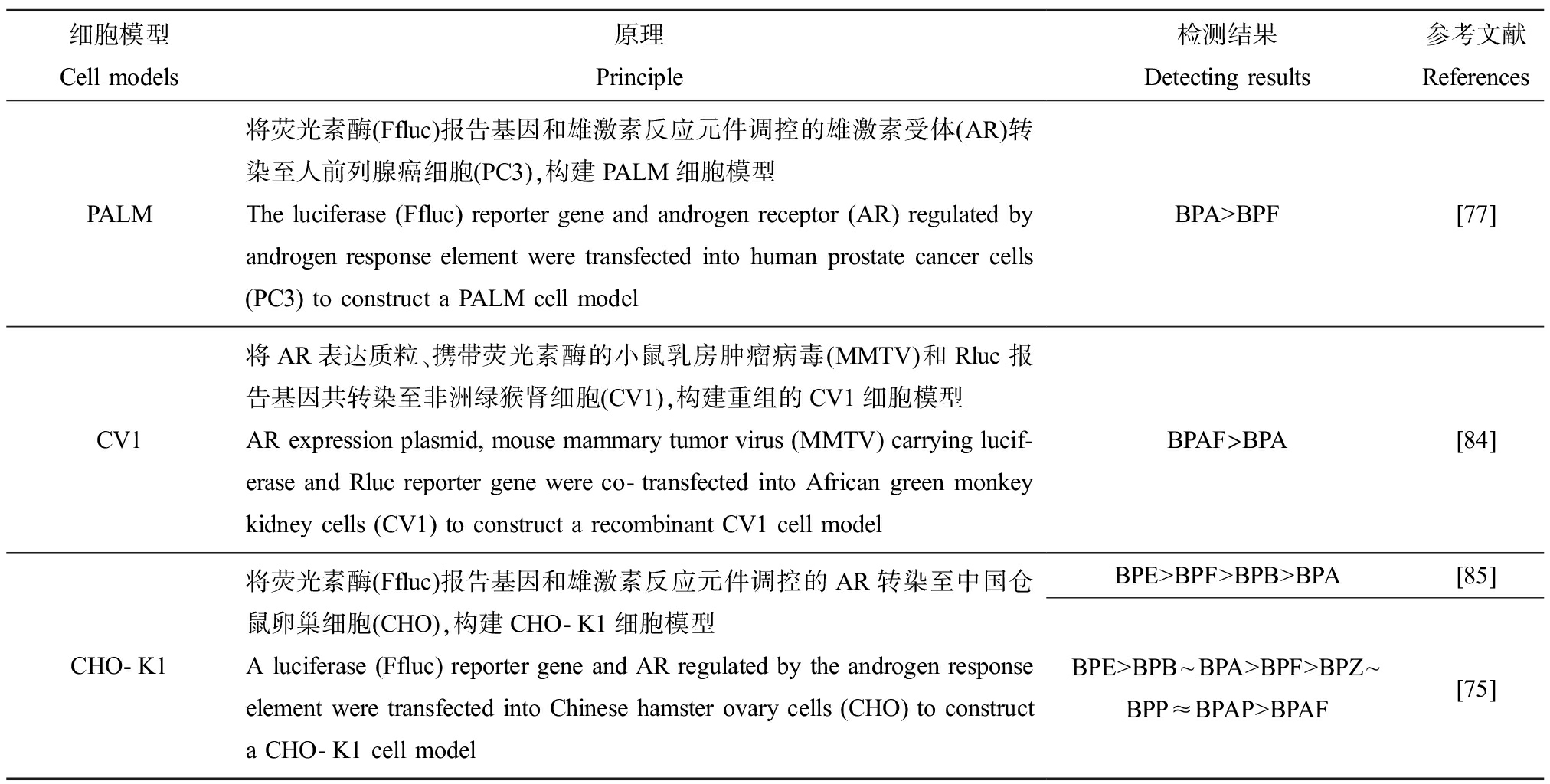
细胞模型Cell models原理Principle检测结果Detecting results参考文献ReferencesPALM将荧光素酶(Ffluc)报告基因和雄激素反应元件调控的雄激素受体(AR)转染至人前列腺癌细胞(PC3),构建PALM细胞模型The luciferase (Ffluc) reporter gene and androgen receptor (AR) regulated by androgen response element were transfected into human prostate cancer cells (PC3) to construct a PALM cell modelBPA>BPF[77]CV1将AR表达质粒、携带荧光素酶的小鼠乳房肿瘤病毒(MMTV)和Rluc报告基因共转染至非洲绿猴肾细胞(CV1),构建重组的CV1细胞模型AR expression plasmid, mouse mammary tumor virus (MMTV) carrying lucif-erase and Rluc reporter gene were co-transfected into African green monkey kidney cells (CV1) to construct a recombinant CV1 cell modelBPAF>BPA[84]CHO-K1将荧光素酶(Ffluc)报告基因和雄激素反应元件调控的AR转染至中国仓鼠卵巢细胞(CHO),构建CHO-K1细胞模型A luciferase (Ffluc) reporter gene and AR regulated by the androgen response element were transfected into Chinese hamster ovary cells (CHO) to construct a CHO-K1 cell modelBPE>BPF>BPB>BPA[85]BPE>BPB~BPA>BPF>BPZ~BPP≈BPAP>BPAF[75]
由于各内分泌系统之间存在相互作用,BPs对各内分泌系统的干扰效应强弱可能与生物机体的发育时期密切相关,在胚胎发育早期较为敏感。研究发现,孕期暴露BPAF后显著影响后代小鼠青春期时的情绪相关行为,并且雄性后代更易形成焦虑和抑郁样行为特征,长期记忆能力受到损伤[95];Ji等[96]也发现孕期和哺乳期暴露BHPF能诱导雄性后代而降低雌性后代焦虑或抑郁样的行为特征。因此,BPs孕期暴露不仅能影响亲代的神经行为特征,还能损伤子代的神经系统发育并导致神经行为紊乱。BPA能通过脐带血穿过胎盘屏障而在子代体内积聚[97],推测BPs可能直接在胚胎发育早期影响神经内分泌系统的发育,但其作用机制仍有待研究。BPA、BPF和BPS等双酚类化合物能干扰雌性大鼠脑组织中与多巴胺和5-羟色胺合成分泌相关基因的表达[98],多巴胺和5-羟色胺是调控大脑行为的重要神经递质分子,BPs对神经递质的影响是否与扰乱神经内分泌系统相关,两者在BPs导致神经行为紊乱过程中是否发挥协同作用也需要深入研究。
表7 体外评估BPs甲状腺激素受体活性的方法
Table 7 Methods for in vitro evaluating thyroid receptor activities of BPs
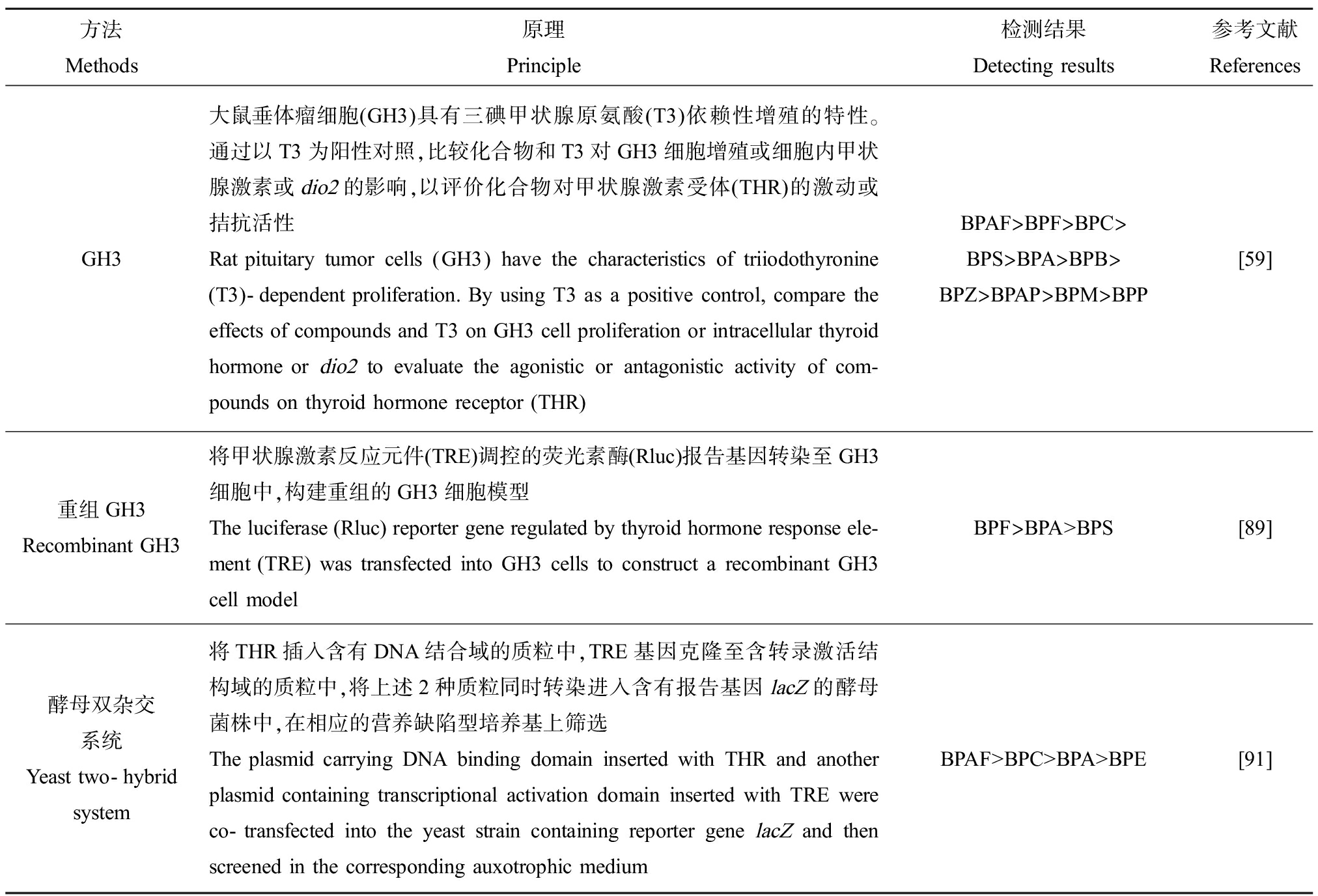
方法Methods原理Principle检测结果Detecting results参考文献ReferencesGH3大鼠垂体瘤细胞(GH3)具有三碘甲状腺原氨酸(T3)依赖性增殖的特性。通过以T3为阳性对照,比较化合物和T3对GH3细胞增殖或细胞内甲状腺激素或dio2的影响,以评价化合物对甲状腺激素受体(THR)的激动或拮抗活性Rat pituitary tumor cells (GH3) have the characteristics of triiodothyronine (T3)-dependent proliferation. By using T3 as a positive control, compare the effects of compounds and T3 on GH3 cell proliferation or intracellular thyroid hormone or dio2 to evaluate the agonistic or antagonistic activity of com-pounds on thyroid hormone receptor (THR)BPAF>BPF>BPC>BPS>BPA>BPB>BPZ>BPAP>BPM>BPP[59]重组GH3Recombinant GH3将甲状腺激素反应元件(TRE)调控的荧光素酶(Rluc)报告基因转染至GH3细胞中,构建重组的GH3细胞模型The luciferase (Rluc) reporter gene regulated by thyroid hormone response ele-ment (TRE) was transfected into GH3 cells to construct a recombinant GH3 cell modelBPF>BPA>BPS[89]酵母双杂交系统Yeast two-hybrid system将THR插入含有DNA结合域的质粒中,TRE基因克隆至含转录激活结构域的质粒中,将上述2种质粒同时转染进入含有报告基因lacZ的酵母菌株中,在相应的营养缺陷型培养基上筛选The plasmid carrying DNA binding domain inserted with THR and another plasmid containing transcriptional activation domain inserted with TRE were co-transfected into the yeast strain containing reporter gene lacZ and then screened in the corresponding auxotrophic mediumBPAF>BPC>BPA>BPE[91]
注:BPM表示双酚M。
Note: BPM means bisphenol M.
4 研究展望(Research prospect)
BPs是一类典型的环境内分泌干扰物,普遍存在于生态环境和日常消费品中,人类BPs暴露风险尤为突出,已经成为威胁人体健康的重要风险因子。针对BPA已经开展了较为全面的毒性研究和风险评估,但针对BPA替代品的毒性效应研究相对较少,尤其对内分泌干扰效应的研究仍然缺乏系统且全面的风险评估数据,存在大量值得探究的科学问题。未来研究需要关注以下几个方面。
(1)针对不断涌现的BPA替代品和相关衍生物的内分泌干扰效应进行评估。许多新型的BPA替代品或其衍生物常作为合成有机物的中间体而广泛应用,但仍然缺乏相应的毒性研究包括内分泌干扰效应的评价数据,需要针对此类BPs开展全面的内分泌干扰效应评估。
(2)建立易操作、高通量和高灵敏的外源性化合物内分泌干扰效应筛查技术。体外生物学分析方法是评估外源化合物内分泌干扰效应的理想模式,然而目前基于哺乳动物细胞或酵母构建的评价方法均存在一定的局限性,无法同时兼具高灵敏度、高稳定性和操作简便等需求。开发高通量、高灵敏和有效稳定的筛查BPs或其他化学物质内分泌干扰效应的生物分析方法,建立基于此的方法标准,将为EDCs内分泌干扰效应评估工作提供技术支撑。
(3)深入研究BPs内分泌干扰效应机制。首先,在目前对BPs内分泌干扰效应研究主要针对性激素和甲状腺素等内分泌系统基础上,加大对神经、肾上腺和胰腺等内分泌系统毒性效应与机制的研究,进一步明确BPs对这些内分泌系统的信号识别、传递和响应的调控机制,挖掘潜在生物标志物;其次,围绕“神经-内分泌”等调控网络,从整体角度理解BPs对机体内分泌的干扰效应与分子机制,分析BPs的内分泌干扰效应对机体其他功能的影响或对其他毒性机制的作用。
(4)BPs表观遗传效应及遗传毒性研究。已经有少量研究表明BPs能够通过表观遗传机制发挥内分泌干扰效应,表观遗传的改变可能产生跨代表型效应,是长远危害子代健康的潜在分子机制之一。但目前针对BPs表观遗传效应和相关机制的研究较少,BPs对表观遗传的影响与其发挥内分泌干扰效应有何内在联系,是否会遗传至子代并潜在影响子代的内分泌、神经和生殖发育等值得深入研究,将从新的角度和层次阐释BPs内分泌干扰效应的长期影响和机制。
(5)加大对多种BPs复合暴露的内分泌干扰效应和毒性机制研究。环境和人体中BPs污染和暴露数据显示,BPs的暴露呈现多种化合物共同存在的模式,其污染多为复合暴露,因而加强对BPs联合暴露效应与机制的研究,将有助于更真实地反映BPs的内分泌干扰效应及相关毒性反应终点,评估BPs暴露风险。
通讯作者简介:苏晓鸥(1963—),男,博士,研究员,主要研究方向为饲料质量安全和风险评估。
[1] Kavlock R J, Daston G P, DeRosa C, et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: A report of the US EPA-sponsored workshop [J]. Environmental Health Perspectives, 1996, 104(Suppl. 4): 715-740
[2] Welshons W V, Nagel S C, vom Saal F S. Large effects from small exposures. Ⅲ. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure [J]. Endocrinology, 2006, 147(6): s56-s69
[3] Chen D, Kannan K, Tan H L, et al. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity: A review [J]. Environmental Science & Technology, 2016, 50(11): 5438-5453
[4] Lee H R, Jeung E B, Cho M H, et al. Molecular mechanism(s) of endocrine-disrupting chemicals and their potent oestrogenicity in diverse cells and tissues that express oestrogen receptors [J]. Journal of Cellular and Molecular Medicine, 2013, 17(1): 1-11
[5] Costa E M F, Spritzer P M, Hohl A, et al. Effects of endocrine disruptors in the development of the female reproductive tract [J]. Arquivos Brasileiros de Endocrinologia e Metabologia, 2014, 58(2): 153-161
[6] National Toxicology Program (NTP). NTP Research Report on the CLARITY-BPA core study: A perinatal and chronic extended-dose-range study of bisphenol A in rats [R]. Washington DC: National Institute of Environmental Health Sciences, 2018
[7] Willhite C C, Daston G P. Bisphenol exposure, hazard and regulation [J]. Toxicology, 2019, 425: 152243
[8] 石珏. 双酚A暴露方式对线虫生长生殖发育及凋亡的影响[D]. 合肥: 中国科学技术大学, 2018: 1-4
Shi J. Effects of bisphenol A on the growth, reproductive development and apoptosis in Caenorhabditis elegans under different exposure ways [D]. Hefei: University of Science and Technology of China, 2018: 1-4 (in Chinese)
[9] 梁栋. 双酚类化合物的环境雌激素效应研究[D]. 北京: 中国科学院大学, 2014: 11
[10] Usman A, Ikhlas S, Ahmad M. Occurrence, toxicity and endocrine disrupting potential of bisphenol-B and bisphenol-F: A mini-review [J]. Toxicology Letters, 2019, 312: 222-227
[11] Cunha S C, Fernandes J O. Quantification of free and total bisphenol A and bisphenol B in human urine by dispersive liquid-liquid microextraction (DLLME) and heart-cutting multidimensional gas chromatography-mass spectrometry (MD-GC/MS) [J]. Talanta, 2010, 83(1): 117-125
[12] Alabi A, Caballero-Casero N, Rubio S. Quick and simple sample treatment for multiresidue analysis of bisphenols, bisphenol diglycidyl ethers and their derivatives in canned food prior to liquid chromatography and fluorescence detection [J]. Journal of Chromatography A, 2014, 1336: 23-33
[13] Cunha S C, Almeida C, Mendes E, et al. Simultaneous determination of bisphenol A and bisphenol B in beverages and powdered infant formula by dispersive liquid-liquid micro-extraction and heart-cutting multidimensional gas chromatography-mass spectrometry [J]. Food Additives & Contaminants: Part A, 2011, 28(4): 513-526
[14] Cunha S C, Cunha C, Ferreira A R, et al. Determination of bisphenol A and bisphenol B in canned seafood combining QuEChERS extraction with dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry [J]. Analytical and Bioanalytical Chemistry, 2012, 404(8): 2453-2463
[15] Grumetto L, Gennari O, Montesano D, et al. Determination of five bisphenols in commercial milk samples by liquid chromatography coupled to fluorescence detection [J]. Journal of Food Protection, 2013, 76(9): 1590-1596
[16] Kuruto-Niwa R, Nozawa R, Miyakoshi T, et al. Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system [J]. Environmental Toxicology and Pharmacology, 2005, 19(1): 121-130
[17] Simoneau C, Valzacchi S, Morkunas V, et al. Comparison of migration from polyethersulphone and polycarbonate baby bottles [J]. Food Additives & Contaminants: Part A, 2011, 28(12): 1763-1768
[18] Wu L H, Zhang X M, Wang F, et al. Occurrence of bisphenol S in the environment and implications for human exposure: A short review [J]. Science of the Total Environment, 2018, 615: 87-98
[19] Lu S Y, Yu Y L, Ren L, et al. Estimation of intake and uptake of bisphenols and triclosan from personal care products by dermal contact [J]. Science of the Total Environment, 2018, 621: 1389-1396
[20] Rochester J R, Bolden A L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes [J]. Environmental Health Perspectives, 2015, 123(7): 643-650
[21] Lee S, Liao C Y, Song G J, et al. Emission of bisphenol analogues including bisphenol A and bisphenol F from wastewater treatment plants in Korea [J]. Chemosphere, 2015, 119: 1000-1006
[22] Ji K, Choi K. Endocrine disruption potentials of bisphenol A alternatives: are bisphenol A alternatives safe from endocrine disruption? [J]. Korean Journal of Environmental Health Sciences, 2013, 39(1): 1-18
[23] Yu X H, Xue J C, Yao H, et al. Occurrence and estrogenic potency of eight bisphenol analogs in sewage sludge from the US EPA targeted national sewage sludge survey [J]. Journal of Hazardous Materials, 2015, 299: 733-739
[24] Im J, Löffler F E. Fate of bisphenol A in terrestrial and aquatic environments [J]. Environmental Science & Technology, 2016, 50(16): 8403-8416
[25] Jin H B, Zhu L Y. Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China [J]. Water Research, 2016, 103: 343-351
[26] Wan Y J, Xia W, Yang S Y, et al. Spatial distribution of bisphenol S in surface water and human serum from Yangtze River watershed, China: Implications for exposure through drinking water [J]. Chemosphere, 2018, 199: 595-602
[27] Si W, Cai Y F, Liu J C, et al. Investigating the role of colloids on the distribution of bisphenol analogues in surface water from an ecological demonstration area, China [J]. Science of the Total Environment, 2019, 673: 699-707
[28] Zhang H F, Zhang Y P, Li J B, et al. Occurrence and exposure assessment of bisphenol analogues in source water and drinking water in China [J]. Science of the Total Environment, 2019, 655: 607-613
[29] Liu Y H, Zhang S H, Song N H, et al. Occurrence, distribution and sources of bisphenol analogues in a shallow Chinese freshwater lake (Taihu Lake): Implications for ecological and human health risk [J]. Science of the Total Environment, 2017, 599-600: 1090-1098
[30] Wang Y X, Liu C, Shen Y, et al. Urinary levels of bisphenol A, F and S and markers of oxidative stress among healthy adult men: Variability and association analysis [J]. Environment International, 2019, 123: 301-309
[31] Zheng C Y, Liu J C, Ren J H, et al. Occurrence, distribution and ecological risk of bisphenol analogues in the surface water from a water diversion project in Nanjing, China [J]. International Journal of Environmental Research and Public Health, 2019, 16(18): 3296
[32] Yamazaki E, Yamashita N, Taniyasu S, et al. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India [J]. Ecotoxicology and Environmental Safety, 2015, 122: 565-572
[33] Schmidt N, Castro-Jiménez J, Fauvelle V, et al. Occurrence of organic plastic additives in surface waters of the Rh ne River (France) [J]. Environmental Pollution, 2020, 257: 113637
ne River (France) [J]. Environmental Pollution, 2020, 257: 113637
[34] Liao C Y, Liu F, Moon H B, et al. Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: Spatial and temporal distributions [J]. Environmental Science & Technology, 2012, 46(21): 11558-11565
[35] Yang Y J, Lu L B, Zhang J, et al. Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography-electrospray tandem mass spectrometry [J]. Journal of Chromatography A, 2014, 1328: 26-34
[36] Wang W, Abualnaja K O, Asimakopoulos A G, et al. A comparative assessment of human exposure to tetrabromobisphenol A and eight bisphenols including bisphenol A via indoor dust ingestion in twelve countries [J]. Environment International, 2015, 83: 183-191
[37] Song S J, Ruan T, Wang T, et al. Distribution and preliminary exposure assessment of bisphenol AF (BPAF) in various environmental matrices around a manufacturing plant in China [J]. Environmental Science & Technology, 2012, 46(24): 13136-13143
[38] Ye X Y, Wong L Y, Kramer J, et al. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of US adults during 2000-2014 [J]. Environmental Science & Technology, 2015, 49(19): 11834-11839
[39] Liao C Y, Liu F, Alomirah H, et al. Bisphenol S in urine from the United States and seven Asian countries: Occurrence and human exposures [J]. Environmental Science & Technology, 2012, 46(12): 6860-6866
[40] Liu Y H, Yan Z Y, Zhang Q, et al. Urinary levels, composition profile and cumulative risk of bisphenols in preschool-aged children from Nanjing suburb, China [J]. Ecotoxicology and Environmental Safety, 2019, 172: 444-450
[41] Xue J C, Wu Q, Sakthivel S, et al. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children [J]. Environmental Research, 2015, 137: 120-128
[42] Asimakopoulos A G, Xue J C, de Carvalho B P, et al. Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia [J]. Environmental Research, 2016, 150: 573-581
[43] Deceuninck Y, Bichon E, Marchand P, et al. Determination of bisphenol A and related substitutes/analogues in human breast milk using gas chromatography-tandem mass spectrometry [J]. Analytical and Bioanalytical Chemistry, 2015, 407(9): 2485-2497
[44] Liu J Y, Li J G, Wu Y N, et al. Bisphenol A metabolites and bisphenol S in paired maternal and cord serum [J]. Environmental Science & Technology, 2017, 51(4): 2456-2463
[45] Zhou J, Xu J J, Cong J M, et al. Optimization for quick, easy, cheap, effective, rugged and safe extraction of mycotoxins and veterinary drugs by response surface methodology for application to egg and milk [J]. Journal of Chromatography A, 2018, 1532: 20-29
[46] Zhou J, Chen X H, Pan S D, et al. Contamination status of bisphenol A and its analogues (bisphenol S, F and B) in foodstuffs and the implications for dietary exposure on adult residents in Zhejiang Province [J]. Food Chemistry, 2019, 294: 160-170
[47] Zhou Q H, Jin Z H, Li J, et al. A novel air-assisted liquid-liquid microextraction based on in situ phase separation for the HPLC determination of bisphenols migration from disposable lunch boxes to contacting water [J]. Talanta, 2018, 189: 116-121
[48] Covaci A, Hond E D, Geens T, et al. Urinary BPA measurements in children and mothers from six European member states: Overall results and determinants of exposure [J]. Environmental Research, 2015, 141: 77-85
[49] Hartle J C, Navas-Acien A, Lawrence R S. The consumption of canned food and beverages and urinary bisphenol A concentrations in NHANES 2003-2008 [J]. Environmental Research, 2016, 150: 375-382
[50] Cao X L, Perez-Locas C, Robichaud A, et al. Levels and temporal trend of bisphenol A in composite food samples from Canadian Total Diet Study 2008-2012 [J]. Food Additives & Contaminants: Part A, 2015, 32(12): 2154-2160
[51] Liu J Y, Wattar N, Field C J, et al. Exposure and dietary sources of bisphenol A (BPA) and BPA-alternatives among mothers in the APrON cohort study [J]. Environment International, 2018, 119: 319-326
[52] Xiong L, Yan P, Chu M, et al. A rapid and simple HPLC-FLD screening method with QuEChERS as the sample treatment for the simultaneous monitoring of nine bisphenols in milk [J]. Food Chemistry, 2018, 244: 371-377
[53] Cunha S C, Oliveira C, Fernandes J O. Development of QuEChERS-based extraction and liquid chromatography-tandem mass spectrometry method for simultaneous quantification of bisphenol A and tetrabromobisphenol A in seafood: Fish, bivalves, and seaweeds [J]. Analytical and Bioanalytical Chemistry, 2017, 409(1): 151-160
[54] Zhang H, Quan Q, Zhang M Y, et al. Occurrence of bisphenol A and its alternatives in paired urine and indoor dust from Chinese university students: Implications for human exposure [J]. Chemosphere, 2020, 247: 125987
[55] Thayer K A, Taylor K W, Garantziotis S, et al. Bisphenol A, bisphenol S, and 4-hydroxyphenyl 4-isopro oxyphenyl sulfone (BPSIP) in urine and blood of cashiers [J]. Environmental Health Perspectives, 2016, 124(4): 437-444
[56] González N, Cunha S C, Monteiro C, et al. Quantification of eight bisphenol analogues in blood and urine samples of workers in a hazardous waste incinerator [J]. Environmental Research, 2019, 176: 108576
[57] Song S M, Duan Y S, Zhang T, et al. Serum concentrations of bisphenol A and its alternatives in elderly population living around e-waste recycling facilities in China: Associations with fasting blood glucose [J]. Ecotoxicology and Environmental Safety, 2019, 169: 822-828
[58] Mu X Y, Huang Y, Li X X, et al. Developmental effects and estrogenicity of bisphenol A alternatives in a zebrafish embryo model [J]. Environmental Science & Technology, 2018, 52(5): 3222-3231
[59] Lee J Y, Kim S, Choi K, et al. Effects of bisphenol analogs on thyroid endocrine system and possible interaction with 17β-estradiol using GH3 cells [J]. Toxicology in Vitro, 2018, 53: 107-113
[60] Ullah A, Pirzada M, Jahan S, et al. Bisphenol A and its analogs bisphenol B, bisphenol F, and bisphenol S: Comparative in vitro and in vivo studies on the sperms and testicular tissues of rats [J]. Chemosphere, 2018, 209: 508-516
[61] Ji K, Hong S, Kho Y, et al. Effects of bisphenol S exposure on endocrine functions and reproduction of zebrafish [J]. Environmental Science & Technology, 2013, 47(15): 8793-8800
[62] Pollock T, Greville L J, Weaver R E, et al. Bisphenol S modulates concentrations of bisphenol A and oestradiol in female and male mice [J]. Xenobiotica, 2019, 49(5): 540-548
[63] Berni M, Gigante P, Bussolati S, et al. Bisphenol S, a bisphenol A alternative, impairs swine ovarian and adipose cell functions [J]. Domestic Animal Endocrinology, 2019, 66: 48-56
[64] Campen K A, Lavallee M, Combelles C. The impact of bisphenol S on bovine granulosa and theca cells [J]. Reproduction in Domestic Animals, 2018, 53(2): 450-457
[65] Gingrich J, Pu Y, Roberts J, et al. Gestational bisphenol S impairs placental endocrine function and the fusogenic trophoblast signaling pathway [J]. Archives of Toxicology, 2018, 92(5): 1861-1876
[66] Yang Q, Yang X H, Liu J N, et al. Exposure to bisphenol B disrupts steroid hormone homeostasis and gene expression in the hypothalamic-pituitary-gonadal axis of zebrafish [J]. Water, Air, & Soil Pollution, 2017, 228(3): 1-12
[67] Yang X X, Liu Y C, Li J, et al. Exposure to bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish [J]. Environmental Toxicology, 2016, 31(3): 285-294
[68] Jones R L, Lang S A, Kendziorski J A, et al. Use of a mouse model of experimentally induced endometriosis to evaluate and compare the effects of bisphenol A and bisphenol AF exposure [J]. Environmental Health Perspectives, 2018, 126(12): 127004
[69] Zhang Z B, Hu Y, Guo J L, et al. Fluorene-9-bisphenol is anti-oestrogenic and may cause adverse pregnancy outcomes in mice [J]. Nature Communications, 2017, 8: 14585
[70] Wang T, Han J, Duan X, et al. The toxic effects and possible mechanisms of bisphenol A on oocyte maturation of porcine in vitro [J]. Oncotarget, 2016, 7(22): 32554-32565
[71] Li Y, Luh C J, Burns K A, et al. Endocrine-disrupting chemicals (EDCs): in vitro mechanism of estrogenic activation and differential effects on ER target genes [J]. Environmental Health Perspectives, 2013, 121(4): 459-466
[72] Li Y, Burns K A, Arao Y, et al. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro [J]. Environmental Health Perspectives, 2012, 120(7): 1029-1035
[73] Conley J M, Hannas B R, Furr J R, et al. A demonstration of the uncertainty in predicting the estrogenic activity of individual chemicals and mixtures from an in vitro estrogen receptor transcriptional activation assay (T47D-KBluc) to the in vivo uterotrophic assay using oral exposure [J]. Toxicological Sciences, 2016, 153(2): 382-395
[74] Mesnage R, Phedonos A, Arno M, et al. Editor’s Highlight: Transcriptome Profiling Reveals Bisphenol A Alternatives Activate Estrogen Receptor Alpha in Human Breast Cancer Cells [J]. Toxicological Sciences: An Official Journal of the Society of Toxicology, 2017, 158(2): 431-443
[75] Kojima H, Takeuchi S, Sanoh S, et al. Profiling of bisphenol A and eight its analogues on transcriptional activity via human nuclear receptors[J]. Toxicology, 2019, 413: 48-55
[76] Le Fol V, Aït-Aïssa S, Sonavane M, et al. In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays [J]. Ecotoxicology and Environmental Safety, 2017, 142: 150-156
[77] Molina-Molina J M, Amaya E, Grimaldi M, et al. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors [J]. Toxicology and Applied Pharmacology, 2013, 272(1): 127-136
[78] Kang J S, Choi J S, Kim W K, et al. Estrogenic potency of bisphenol S, polyethersulfone and their metabolites generated by the rat liver S9 fractions on a MVLN cell using a luciferase reporter gene assay [J]. Reproductive Biology and Endocrinology, 2014, 12: 102
[79] Cano-Nicolau J, Vaillant C, Pellegrini E, et al. Estrogenic effects of several BPA analogs in the developing zebrafish brain [J]. Frontiers in Neuroscience, 2016, 10: 112
[80] Moreman J, Lee O, Trznadel M, et al. Acute toxicity, teratogenic, and estrogenic effects of bisphenol A and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in zebrafish embryo-larvae [J]. Environmental Science & Technology, 2017, 51(21): 12796-12805
[81] Feng Y X, Yin J, Jiao Z H, et al. Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats [J]. Toxicology Letters, 2012, 211(2): 201-209
[82] Li J, Sheng N, Cui R N, et al. Gestational and lactational exposure to bisphenol AF in maternal rats increases testosterone levels in 23-day-old male offspring [J]. Chemosphere, 2016, 163: 552-561
[83] Ullah A, Pirzada M, Jahan S, et al. Impact of low-dose chronic exposure to bisphenol A and its analogue bisphenol B, bisphenol F and bisphenol S on hypothalamo-pituitary-testicular activities in adult rats: A focus on the possible hormonal mode of action [J]. Food and Chemical Toxicology, 2018, 121: 24-36
[84] Teng C, Goodwin B, Shockley K, et al. Bisphenol A affects androgen receptor function via multiple mechanisms [J]. Chemico-Biological Interactions, 2013, 203(3): 556-564
[85] Rosenmai A K, Dybdahl M, Pedersen M, et al. Are structural analogues to bisphenol A safe alternatives? [J]. Toxicological Sciences, 2014, 139(1): 35-47
[86] Tang T L, Yang Y, Chen Y W, et al. Thyroid disruption in zebrafish larvae by short-term exposure to bisphenol AF [J]. International Journal of Environmental Research and Public Health, 2015, 12(10): 13069-13084
[87] Kwon B, Kho Y, Kim P G, et al. Thyroid endocrine disruption in male zebrafish following exposure to binary mixture of bisphenol AF and sulfamethoxazole [J]. Environmental Toxicology and Pharmacology, 2016, 48: 168-174
[88] Huang G M, Tian X F, Fang X D, et al. Waterborne exposure to bisphenol F causes thyroid endocrine disruption in zebrafish larvae [J]. Chemosphere, 2016, 147: 188-194
[89] Zhang Y F, Ren X M, Li Y Y, et al. Bisphenol A alternatives bisphenol S and bisphenol F interfere with thyroid hormone signaling pathway in vitro and in vivo [J]. Environmental Pollution, 2018, 237: 1072-1079
[90] Lu L P, Zhan T J, Ma M, et al. Thyroid disruption by bisphenol S analogues via thyroid hormone receptor β: in vitro, in vivo, and molecular dynamics simulation study [J]. Environmental Science & Technology, 2018, 52(11): 6617-6625
[91] Lei B, Xu J, Peng W, et al. In vitro profiling of toxicity and endocrine disrupting effects of bisphenol analogues by employing MCF-7 cells and two-hybrid yeast bioassay [J]. Environmental Toxicology, 2017, 32(1): 278-289
[92] Kinch C D, Ibhazehiebo K, Jeong J H, et al. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish [J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(5): 1475-1480
[93] Qiu W H, Zhao Y L, Yang M, et al. Actions of bisphenol A and bisphenol S on the reproductive neuroendocrine system during early development in zebrafish [J]. Endocrinology, 2016, 157(2): 636-647
[94] Mi P, Zhang Q P, Zhang S H, et al. The effects of fluorene-9-bisphenol on female zebrafish (Danio rerio) reproductive and exploratory behaviors [J]. Chemosphere, 2019, 228: 398-411
[95] Gong M, Huai Z Q, Song H, et al. Effects of maternal exposure to bisphenol AF on emotional behaviors in adolescent mice offspring [J]. Chemosphere, 2017, 187: 140-146
[96] Ji X M, Shi L L, Yin X, et al. Sex- and developmental stage-dependent effects of fluorene-9-bisphenol exposure on emotional behaviors in mice [J]. Chemosphere, 2019, 225: 890-896
[97] Kolatorova L, Vitku J, Hampl R, et al. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes [J]. Environmental Research, 2018, 163: 115-122
[98] Castro B, Sánchez P, Torres J M, et al. Bisphenol A, bisphenol F and bisphenol S affect differently 5α-reductase expression and dopamine-serotonin systems in the prefrontal cortex of juvenile female rats [J]. Environmental Research, 2015, 142: 281-287