粮食真菌毒素是粮食及其制品中的真菌分泌的次级代谢产物,可出现在“农田到餐桌”的各个环节,甚至可因粮食作物灌溉用水的排放进入水体或农产品精深加工的副产物(废弃物)而进入自然界,从而对生物体健康和环境构成威胁,需受到高度重视,环境中真菌毒素对世代暴露示意图如图1所示[1-3]。存在于粮食中的真菌毒素主要有黄曲霉毒素B1(aflatoxin B1, AFB1)、玉米赤霉烯酮(zearalenone, ZEN)、脱氧雪腐镰刀菌烯醇(deoxynivalenol, DON)、伏马菌素B1(fumonisin, FB1)以及赭曲霉毒素(ochratoxin, OTA)等,它们广泛存在于花生、玉米、小麦、燕麦、高粱和豆类等粮食作物及其制品中[4]。Biomin公司[5]在2019年对真菌毒素在亚洲地区的污染范围和危害程度进行统计,发现在所调查的1 231份小麦、玉米及其饲料制品中,OTA、ZEN、DON以及黄曲霉毒素(aflatoxins, AFs)等真菌毒素均有检出,其污染率分别为2%、75%、88%和31%,且对猪、牛、鸡、鱼以及虾类等动物均有风险。此外,除了传统粮食真菌毒素外,一些新兴的粮食真菌毒素已逐渐被检出,如恩镰孢菌素B1(enniatin B1, ENN B1)、白僵菌素(beauvericin, BEA)、交链孢酚(alternariol, AOH)等[6]。现已有大量研究表明,粮食真菌毒素具有细胞毒性、免疫毒性、生殖发育毒性、基因毒性、肝毒性、肾毒性以及致畸致癌等多种毒性作用[7-8]。此外,在实际生活中往往存在多种毒素同时污染的情况,对高/低等生物均可产生不同程度的联合毒性作用,其中以毒素间协同作用居多,可对人和动物造成严重影响[9-10]。值得一提的是,粮食真菌毒素不仅对亲本世代(parental generation, P0)具有毒性作用,甚至对后代(filial generation, F1-n),即对间接接触(子一代F1)或未接触毒素(子二代及以后F2-n)的子代个体仍具有毒害作用(图1)[11-12]。目前,有关粮食真菌毒素对P0代的生殖发育及遗传毒性已得到了广泛关注,但有关其对F1-n代个体产生的毒性作用研究还较为缺乏,或多局限于F1代个体,这可能受限于有关继代毒性实验动物模型的建立。因此,本文首先总结了常见粮食真菌毒素对P0代生殖系统的毒性作用,同时就其对子代(F1-n)发育的不良影响进行了归纳;随后对其诱导的生殖发育毒性作用机理进行了阐述;最后对粮食真菌毒素在未来的研究进行了展望,旨在为更加科学全面地评价粮食真菌毒素的毒性作用,为进一步开展相关毒作用风险评估,补充完善毒理学数据,合理制定相关限量标准与食品安全政策以及防治公共卫生健康提供重要的理论和参考依据。
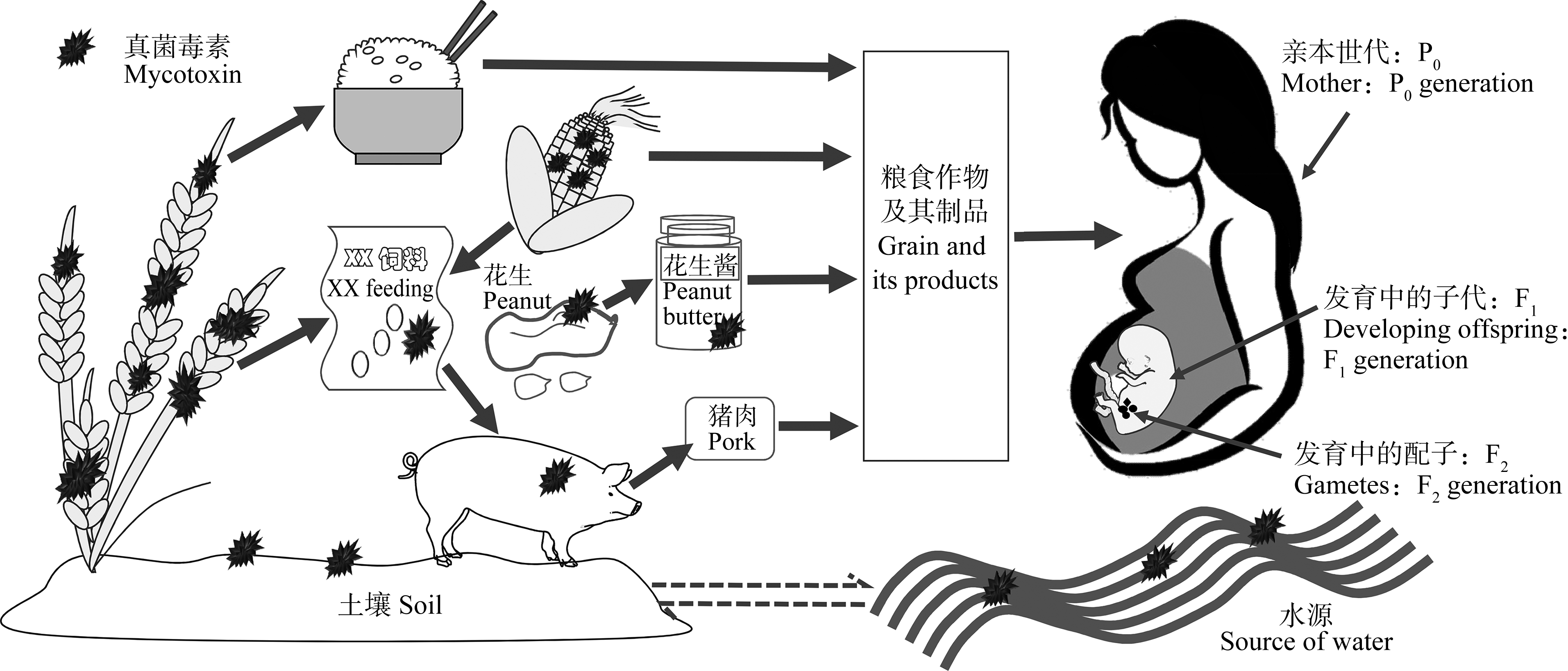
图1 环境中真菌毒素对亲本世代和子代暴露示意图[13]
Fig. 1 Schematic diagram of environmental mycotoxins exposure on parental and filial generation[13]
1 粮食真菌毒素对亲本世代的生殖发育与遗传毒性作用(Reproductive, developmental and genetic toxicities of grain mycotoxin on parental generation)
1.1 对雄性生殖系统的影响1.1.1 对雄性生殖器官的影响
Owumi等[14]研究发现,AFB1(75 μg·kg-1)可显著降低Wistar雄性大鼠睾丸和附睾的质量,而Gao等[15]则发现ZEN(20 mg·kg-1)可显著增加SD雄性成年大鼠的睾丸质量,降低精母细胞和成熟精子的数量,但对附睾质量无明显影响。ICR小鼠经单端孢霉烯(T-2)毒素处理56 d,睾丸的正常发育受阻,此不良影响会在小鼠发育至青春期后逐步减轻[16];但是,用T-2毒素对Pannon雄性白兔处理65 d,发现Pannon兔的睾丸、睾丸间细胞以及睾酮未产生明显不良变化[8],这一研究与ICR小鼠暴露于T-2毒素后所受的影响相反,可能是因为实验动物的物种间差异。此外,ZEN作用会抑制小鼠睾丸间质细胞(MLTC-1)与支持细胞(TM4)的增殖以及睾丸正常的细胞周期循环,造成睾丸间质细胞氧化损伤、线粒体膜电位改变以及DNA损伤[8,17-18]。这些研究均表明真菌毒素会对雄性动物生殖器官及其相关细胞的发育造成不同程度的损害。
1.1.2 对精子质量的影响
目前已有相关研究表明,粮食真菌毒素会影响雄性动物的精子质量。Zhou等[19]用AFB1对昆明雄性小鼠进行中长期低剂量(1 500、375和93.75 μg·kg-1·d-1,50 d)灌胃处理,发现AFB1会诱导小鼠的氧化应激,增加小鼠精子的畸形率,产生生殖毒性效应,并通过彗星试验发现其DNA受损、DNA与蛋白质之间发生交联,存在潜在的遗传毒性。Yang等[20]用DON(2 mg·kg-1和4 mg·kg-1)对40只BALB/c雄性小鼠进行灌胃,发现其精子畸形率显著上升并出现双头和双尾样畸形精子,精子活力及其运动能力也受到很大影响。与之相似,低浓度ZEN(20 μg·kg-1和40 μg·kg-1)灌胃处理CD1雄性小鼠,精子活力和浓度明显降低,精子质量下降[21]。此外,Eze等[22]用OTA、DON、ZEN、α-ZOL和β-ZOL(0.1、1和8 μmol·L-1)等毒素对MA-10睾丸间质细胞进行单独及与农药滴滴涕(DDT)联合染毒,均发现细胞睾酮分泌水平失调,而睾酮的分泌水平与精子成熟、精子发生以及精子的质量有关,从而导致雄性动物的正常生殖受到影响[23]。
精子发生是精原干细胞分裂分化为成熟精子的一个复杂过程,发生在睾丸的曲生精管中,其核心是减数分裂[24-25]。Pang等[21]和Men等[25]用ZEN(20μg·kg-1·d-1和40 μg·kg-1·d-1)分别经口灌喂CD1和ICR雄性小鼠,各自处理28 d或21 d后均发现小鼠精子质量下降、精子发生过程以及生精细胞的DNA双链受损,其受损程度与ZEN浓度和处理时间呈正相关。Jee等[26]利用较高浓度的ZEN(5 mg·kg-1)作用SD大鼠时也发现其精子发生受阻。上述几项研究表明,较高或较低浓度的ZEN对动物进行毒作用处理,均会影响机体的正常生殖能力。此外,由于精子发生与支持细胞和间质细胞有关,用OTA和ZEN对两者进行处理后可导致其细胞周期阻滞,细胞增殖受到抑制,死亡率增加,ZEN甚至会引起间质细胞的自噬作用[21, 27]。
总的来说,大量研究均表明,雄性动物短期或长期摄入粮食真菌毒素后,可引起精子形态的异常、精子质量、睾丸以及附睾质量的降低,影响精子成熟以及精子发生过程,并诱导DNA链断裂,对雄性动物产生一定的遗传毒性作用,故推测这些损伤最终会导致生殖能力的下降,影响后代的繁殖[12, 18, 28]。但也有相关研究指出小鼠生殖能力下降的结论不能仅仅通过小鼠精子数量和质量的下降而得出,需对其雄性个体在固定时间内生育后代的能力以及使用固定数量的精子进行人工受精等来做进一步的生育能力研究[7]。
1.2 对雌性生殖系统的影响1.2.1 对雌性生殖器官的影响
Li等[28]对昆明雌性小鼠进行AFB1毒作用处理,发现小鼠子宫组织中细胞间隙增大,微血管出血,炎细胞浸润,导致小鼠子宫受损。Wang等[29]和Ahmad等[30]分别用ZEN灌胃处理BALB/c雌性小鼠2周(10 mg·kg-1·d-1)和腹腔注射作用Parkes雌性小鼠30 d(2.5 mg·kg-1·d-1)后,均发现ZEN可导致雌性小鼠子宫质量增加,子宫和卵巢组织形态发生明显改变,生殖器官甚至生殖系统发生损伤。Jia等[31]用OTA通过腹腔注射对ICR雌性小鼠处理7 d后发现,小鼠卵巢出血量增多,组织结构发生损伤。同样地,用OTA(20 μmol·L-1和40 μmol·L-1)和DON(0.5、1和2 mg·L-1)分别对猪卵巢颗粒细胞进行染毒后发现,细胞增殖受到抑制,组织形态发生改变,正常细胞周期受阻[32-33]。不仅如此,Silva等[34]用低浓度的ZEN(1 μmol·L-1)作用体外培养的羊卵巢,发现羊卵泡的细胞增殖受阻,卵巢腔前卵泡正常发育受损,DNA双链发生断裂,表明ZEN对羊卵巢可能存在一定的遗传毒性作用。此外,Shi等[35]同时用ZEN(596.86 μg·kg-1,以单位饲料质量计)和DON(796 μg·kg-1,以单位饲料质量计)对未成熟的母猪染毒时发现,经染毒的母猪外阴尺寸明显增大,生殖器官质量显著降低,卵巢和子宫细胞发生凋亡。上述研究均表明,真菌毒素会对雌性动物生殖器官产生不良影响,使与之相关的生殖细胞发育受阻,组织结构产生异常,最终导致其生殖系统受到损伤。
1.2.2 对卵子质量的影响
粮食真菌毒素毒作用于雌性动物时会影响卵子的正常发育[36]。例如,Jia等[31]用OTA(7.5 μmol·L-1)对ICR雌性小鼠进行染毒,发现OTA会诱导体内活性氧积累、抑制卵子减数分裂过程、降低卵子质量、诱导卵母细胞凋亡并影响卵子成熟;Cheng等[37]用AFB1(10 μmol·L-1)处理体外成熟的猪卵母细胞,极大地损害了卵母细胞的细胞核并影响细胞质的成熟,损害程度与AFB1对卵母细胞的作用浓度和接触时间有关;Mastrorocco等[38]探究BEA(5 μmol·L-1)对幼羊卵母细胞的影响发现,BEA可抑制幼羊卵母细胞的减数分裂,促进未成熟卵母细胞凋亡,降低卵母细胞的成熟并使DNA链断裂。此外,Schoevers等[39]对猪卵母细胞复合物进行体外培养48 h后对其进行AOH(5、10和20 μmol·L-1)染毒,发现AOH会加速卵母细胞的退化并诱导成熟卵母细胞发生核畸变,但未抑制卵母细胞的成熟,这可能与AOH的浓度或其对卵母细胞的作用时间有关。由此可知,上述粮食真菌毒素作用于雌性动物会损害卵子的成熟和发育,从而对雌性动物的生殖系统和/或能力造成损害,并存在潜在的遗传毒性作用。
由此可见,大量研究已表明,粮食真菌毒素可导致雌性动物的子宫质量和体积下降,卵巢组织形态改变,卵子质量降低,并加速卵母细胞的退化及凋亡,损伤卵母细胞的遗传物质,影响卵母细胞的增殖及成熟过程,可推测最终可能损伤其生殖系统,导致生殖能力下降,甚至影响后代的生长发育[31, 39-43]。
2 粮食真菌毒素对子代的生殖发育与遗传毒性作用(Reproductive, developmental and genetic toxicities of grain mycotoxin on filial generation)
2.1 对胚胎发育的影响
胚胎发育是细胞和组织按照一定的顺序进行分化的过程,是子代个体生长发育的起点。现已有研究表明,粮食真菌毒素可对动物胚胎产生不同程度的毒性作用,影响胚胎的正常发育。Shin等[44]将猪胚胎在不同浓度的AFB1中毒作用7 d,当AFB1浓度为1 nmol·L-1时,便会显著抑制胚泡的形成,并诱导自噬作用及活性氧(reactive oxygen species, ROS)的产生,从而影响猪胚胎的正常生长发育。Huang等[45]通过静脉注射方式将ENN B1(1 mg·kg-1和3 mg·kg-1)注射进入ICR雌性小鼠胚胎,发现囊胚期胚胎凋亡,生长发育受损,体内胎儿质量下降。Wu等[46]发现受精6 h后的斑马鱼胚胎经不同浓度的OTA处理会出现脑出血及血管畸形等症状;当OTA浓度为0.5 μmol·L-1时,会使其中48%的胚胎出现脑出血。用HT-2毒素(5、10、20和50 nmol·L-1)、ZEN(5μmol·L-1和10 μmol·L-1)和BEA(0.5、1和3 μmol·L-1)分别对小鼠、猪和绵羊胚胎进行染毒,也得到了类似的结论,即胚胎发育速率降低以及胚泡质量受到损伤,从而导致胚胎的异常发育[47-48]。
2.2 对子代生长发育的影响
若动物体长期低剂量或短期高剂量摄入被粮食真菌毒素污染的食物,其子代的正常生长发育会受到不同程度的影响。例如,Bondy等[49]在Fischer雌性大鼠的繁殖、妊娠和哺乳整个时期用受OTA污染的饲料对其进行喂养,发现当OTA浓度达0.4 mg·kg-1时,其子代大鼠(产后21 d)的肾脏发生病变,病变程度与OTA浓度成正比,同时还影响子代大鼠的生长发育;当OTA浓度为1 mg·kg-1时,子代大鼠的体质量、肝脏和肾脏以及睾丸质量发生显著异常。与之相似,用受ZEN(40 mg·L-1)污染的饲料喂养小鼠,会导致其胎盘出血率增加,胎儿体质量降低,最终影响其生长发育[50]。此外,扁虫摄食受AFB1污染的食物后,其幼虫生长发育延迟,存活率降低,但这些不良影响会在青春期后逐步减轻[51];DON多世代连续处理秀丽隐杆线虫可使其子代对毒性的敏感性增强,更易受到毒害,转录组学表示DON主要通过影响泛酸盐和辅酶A生物合成通路对线虫产生多代毒性作用,影响子代线虫的生长发育[11]。
2.3 对子代生殖系统的影响
已有研究发现,ZEN(20 μg·kg-1和40 μg·kg-1)和T-2毒素(0.005 mg·kg-1和0.05 mg·kg-1)采用灌胃的方式对怀孕ICR小鼠进行染毒,可使其子代雄性小鼠精子质量下降,睾丸质量降低,精子发生受阻甚至睾酮合成受抑制[16, 25]。同样,T-2毒素对怀孕CD-1小鼠具有胚胎毒性,可对其子代小鼠的生殖系统造成损伤[52]。另外,对怀孕SD大鼠喂养含ZEN(10 mg·kg-1和20 mg·kg-1)的饲料后,其子代雌性大鼠的体质量与生存能力降低,卵泡中雌激素浓度升高,雌二醇降低,卵泡闭锁,子宫层变薄;而子代雄性大鼠睾丸质量增加,组织形态改变,精原细胞和精母细胞的正常周期受阻[15, 53]。类似地,用ZEN(0、2.5和5 mg·kg-1)对昆明小鼠进行皮下注射处理后,其子代小鼠精子质量、活性和成熟率均发生明显降低,DNA受损,生育能力及小鼠成年后睾酮分泌水平下降,表明ZEN会对小鼠产生继代毒性作用,并通过破坏小鼠的精原细胞,进而影响子代小鼠的生殖能力[54]。此外,周鸿媛[11]利用不同浓度DON(50、100、200和400 μg·mL-1)对秀丽隐杆线虫进行多代毒作用处理发现母体DON暴露会损害子代的生殖能力,其中子二代尤为明显。上述研究均表明粮食真菌毒素可能存在遗传毒性作用,并会对子代雌性和雄性生殖系统产生不同程度的影响。
综上所述,粮食真菌毒素会对动物子代的生长发育和生殖代谢等过程产生不同程度的生殖、发育及遗传毒性损伤。但鉴于目前研究粮食真菌毒素的继代毒性资料较少,P0代暴露会对F1-n代个体产生的毒性作用及其机理尚不明确,相关的推测及结论仍需进一步研究。
3 粮食真菌毒素生殖发育与遗传的毒作用机理(Mechanism of reproductive, developmental and genetic toxicities of grain mycotoxins)
3.1 氧化应激机制
氧化应激常被认为是引起机体受损的重要机制,机体在受到外界有害物质的刺激后,会引起体内相关活性分子水平的变化,氧化还原状态发生改变,从而影响机体的正常生长发育[55]。已有研究表明,动物体在摄入粮食真菌毒素后,会对机体内部造成一定的伤害。对雄性动物而言,抗氧化系统可有序调节精子发生和睾酮合成,在睾丸发育期间清除过量ROS,保证其正常发育。而当T-2毒素作用于(口服、腹腔注射)小鼠时,2种毒作用方式均会诱导小鼠体内氧化应激的产生,引起小鼠睾丸内部超氧化物歧化酶(superoxide dismutase, SOD)、过氧化氢酶(catalase, CAT)、谷胱甘肽过氧化物酶(glutathione peroxidase, GPX)以及ROS等水平发生变化,从而对小鼠睾丸发育,精子质量以及精子发生造成不良影响[16, 56]。在雌性动物体内,抗氧化系统可调节其卵母细胞的发育。AFB1在小鼠体内发挥毒作用时,会通过细胞色素P450代谢导致ROS的过度产生,诱导氧化应激,抑制减数分裂进程,损伤小鼠卵母细胞[37]。DON则通过降低SOD和谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-Px)活性,升高ROS和丙二醛(malondialdehyde,MDA)水平,诱导小鼠氧化应激的产生,并激活相关JNK/c-Jun的信号通路,促进细胞色素c从线粒体中释放等使小鼠精子受损[20]。正如诸多研究表明,粮食中的真菌毒素,如HT-2毒素、OTA以及ZEN分别对小鼠胚胎和猪胚胎进行毒作用时,会诱导胚胎发生氧化应激反应,ROS含量增加,导致线粒体功能障碍和DNA损伤,从而影响动物胚胎的正常发育[51, 57-59]。总之,多个研究结果表明,氧化应激是真菌毒素产生生殖发育与遗传毒性的一个重要机制,机体在摄入粮食真菌毒素后,体内ROS过量产生,氧化还原系统的平衡破坏,导致氧化应激反应的发生,甚至引起线粒体功能障碍、DNA损伤,进而影响机体的正常生长发育。
3.2 分子调控机制
基因及蛋白的正常表达是机体维持正常生命活动的必要条件,而食源性毒物会在不同程度上影响其表达水平,对机体产生毒性作用。现将主要粮食真菌毒素对动物体生殖发育与遗传方面的毒性作用及其分子调控机制整理如表1所示。
综上,主要粮食真菌毒素对动物体产生生殖发育及遗传毒性的分子调控机制涉及到的主要信号通路及基因/蛋白为:MAPK通路(p38/MAPK信号通路、JNK/MAPK信号通路、ERK1/2/MAPK信号通路)、PI3K/AKT通路及Nrf2通路、caspase 3、bax基因和蛋白等,将其分子机制图进行整理如图2所示。在机体内部可调控细胞自噬及凋亡、细胞增殖、代谢和炎症反应等过程,并在精原干细胞更新、精子成熟、精子发生及顶体反应等与雄性生殖相关的生理过程中也发挥重要作用。Huang等[60]在研究中发现AFB1可诱导小鼠氧化应激,进而抑制PI3K/AKT/mTOR信号通路、激活p38/MAPK信号通路,使与凋亡相关的蛋白表达上调(LC3、Beclin-1、Atg5、cyt-c、caspase 3和p62等),并改变血睾屏障相关蛋白的表达(Occludin和N-cadherin蛋白表达上调,Connexin 43表达下调),致使小鼠精子发生障碍、睾丸及血睾屏障受损[74];而Zhang等[75]则发现ZEN会降低p-PI3K/PI3K、p-AKT/AKT、p-mTOR/mTOR、p-ERK1/2/ERK1/2、p-JNK1/2/JNK1/2及p-P38/P38等蛋白的表达,通过激活PI3K/AKT/mTOR和MAPK信号通路诱导鸡颗粒细胞发生自噬及凋亡,这也说明不同的真菌毒素作用于不同的对象,其对PI3K/AKT/mTOR信号通路会产生不同的调节方式。另外,Bim、cytochrome c、caspase 3、caspase 8和caspase 9等蛋白表达上调及JNK/c-Jun信号通路发生变化,推测其可能是DON使小鼠精子受损、睾丸细胞凋亡的潜在机制[20]。此外,OTA可通过调节PI3K/Akt、ERK1/2和JNK信号通路来抑制小鼠睾丸细胞(TM3、TM4)的增殖,同时PI3K/Akt和ERK1/2信号通路的激活也与OTA的致癌性有关[72, 75]。此外,OTA也可通过激活PI3K/AKT信号通路及下调bax、caspase 9和Bcl2Ⅱ等凋亡相关基因抑制猪卵巢颗粒细胞(GCs)增殖并诱导其凋亡[74]。
表1 主要粮食真菌毒素的生殖发育与遗传毒性及其分子调控机制
Table 1 Reproductive, developmental and genetic toxicities and its molecular regulation mechanism of grain mycotoxins
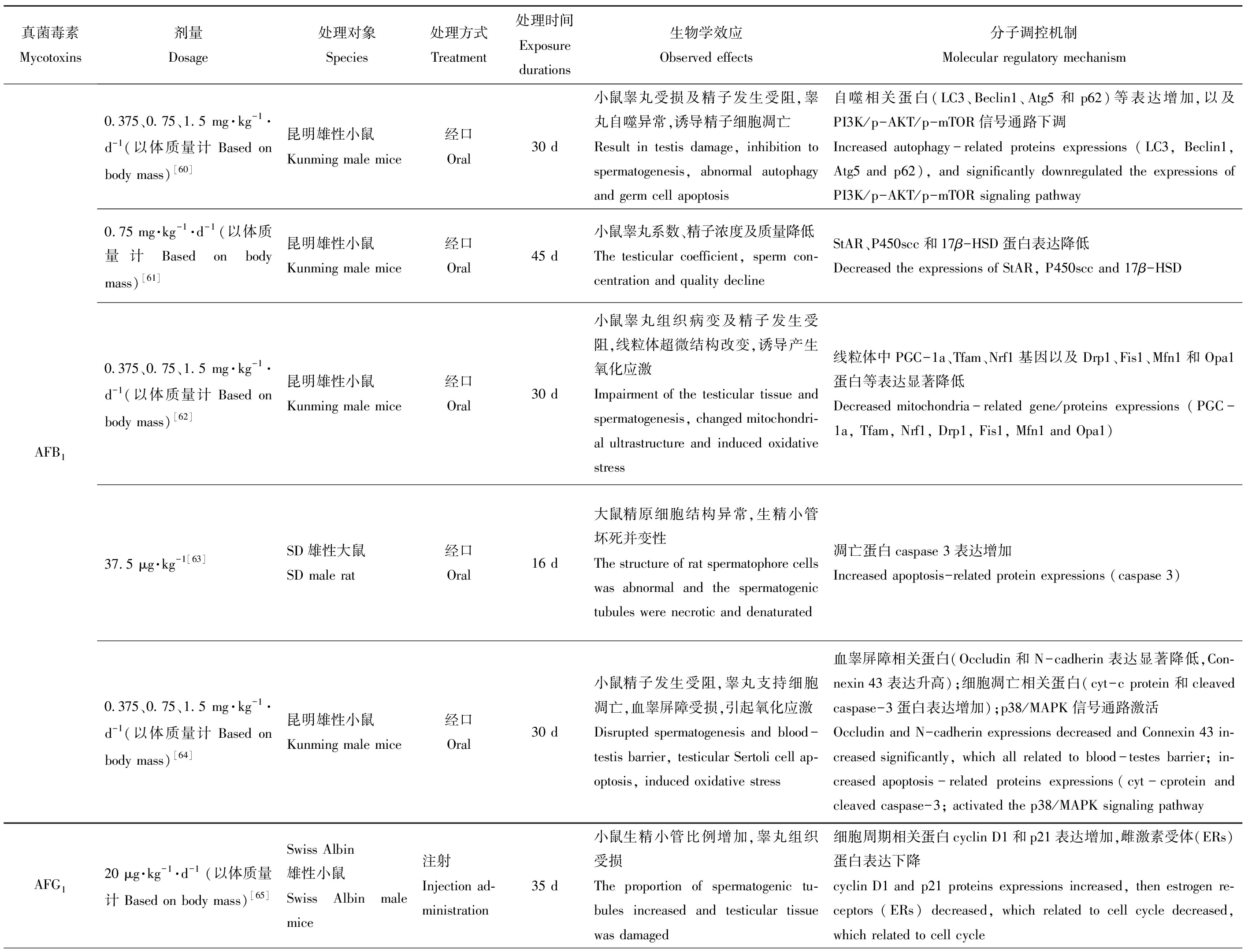
真菌毒素Mycotoxins剂量Dosage处理对象Species处理方式Treatment处理时间Exposure durations生物学效应Observed effects分子调控机制Molecular regulatory mechanismAFB10.375、0.75、1.5 mg·kg-1·d-1 (以体质量计 Based on body mass)[60]昆明雄性小鼠Kunming male mice经口Oral30 d小鼠睾丸受损及精子发生受阻,睾丸自噬异常,诱导精子细胞凋亡Result in testis damage, inhibition to spermatogenesis, abnormal autophagy and germ cell apoptosis自噬相关蛋白(LC3、Beclin1、Atg5和p62)等表达增加,以及PI3K/p-AKT/p-mTOR信号通路下调Increased autophagy-related proteins expressions (LC3, Beclin1, Atg5 and p62), and significantly downregulated the expressions of PI3K/p-AKT/p-mTOR signaling pathway0.75 mg·kg-1·d-1(以体质量计 Based on body mass)[61]昆明雄性小鼠Kunming male mice经口Oral45 d小鼠睾丸系数、精子浓度及质量降低The testicular coefficient, sperm con-centration and quality declineStAR、P450scc和17β-HSD蛋白表达降低Decreased the expressions of StAR, P450scc and 17β-HSD0.375、0.75、1.5 mg·kg-1·d-1 (以体质量计 Based on body mass)[62]昆明雄性小鼠Kunming male mice经口Oral30 d小鼠睾丸组织病变及精子发生受阻,线粒体超微结构改变,诱导产生氧化应激Impairment of the testicular tissue and spermatogenesis, changed mitochondri-al ultrastructure and induced oxidative stress线粒体中PGC-1a、Tfam、Nrf1基因以及Drp1、Fis1、Mfn1和Opa1蛋白等表达显著降低Decreased mitochondria-related gene/proteins expressions (PGC-1a, Tfam, Nrf1, Drp1, Fis1, Mfn1 and Opa1)37.5 μg·kg-1[63]SD雄性大鼠SD male rat经口Oral16 d大鼠精原细胞结构异常,生精小管坏死并变性The structure of rat spermatophore cells was abnormal and the spermatogenic tubules were necrotic and denaturated凋亡蛋白caspase 3表达增加Increased apoptosis-related protein expressions (caspase 3)0.375、0.75、1.5 mg·kg-1·d-1 (以体质量计 Based on body mass)[64]昆明雄性小鼠Kunming male mice经口Oral30 d小鼠精子发生受阻,睾丸支持细胞凋亡,血睾屏障受损,引起氧化应激Disrupted spermatogenesis and blood-testis barrier, testicular Sertoli cell ap-optosis, induced oxidative stress血睾屏障相关蛋白(Occludin和N-cadherin表达显著降低,Con-nexin 43表达升高);细胞凋亡相关蛋白(cyt-c protein和cleaved caspase-3蛋白表达增加);p38/MAPK信号通路激活Occludin and N-cadherin expressions decreased and Connexin 43 in-creased significantly, which all related to blood-testes barrier; in-creased apoptosis-related proteins expressions(cyt-cprotein and cleaved caspase-3; activated the p38/MAPK signaling pathwayAFG120 μg·kg-1·d-1(以体质量计 Based on body mass)[65]Swiss Albin雄性小鼠Swiss Albin male mice注射Injection ad-ministration35 d小鼠生精小管比例增加,睾丸组织受损The proportion of spermatogenic tu-bules increased and testicular tissue was damaged细胞周期相关蛋白cyclin D1和p21表达增加,雌激素受体(ERs)蛋白表达下降cyclin D1 and p21 proteins expressions increased, then estrogen re-ceptors (ERs) decreased, which related to cell cycle decreased, which related to cell cycle
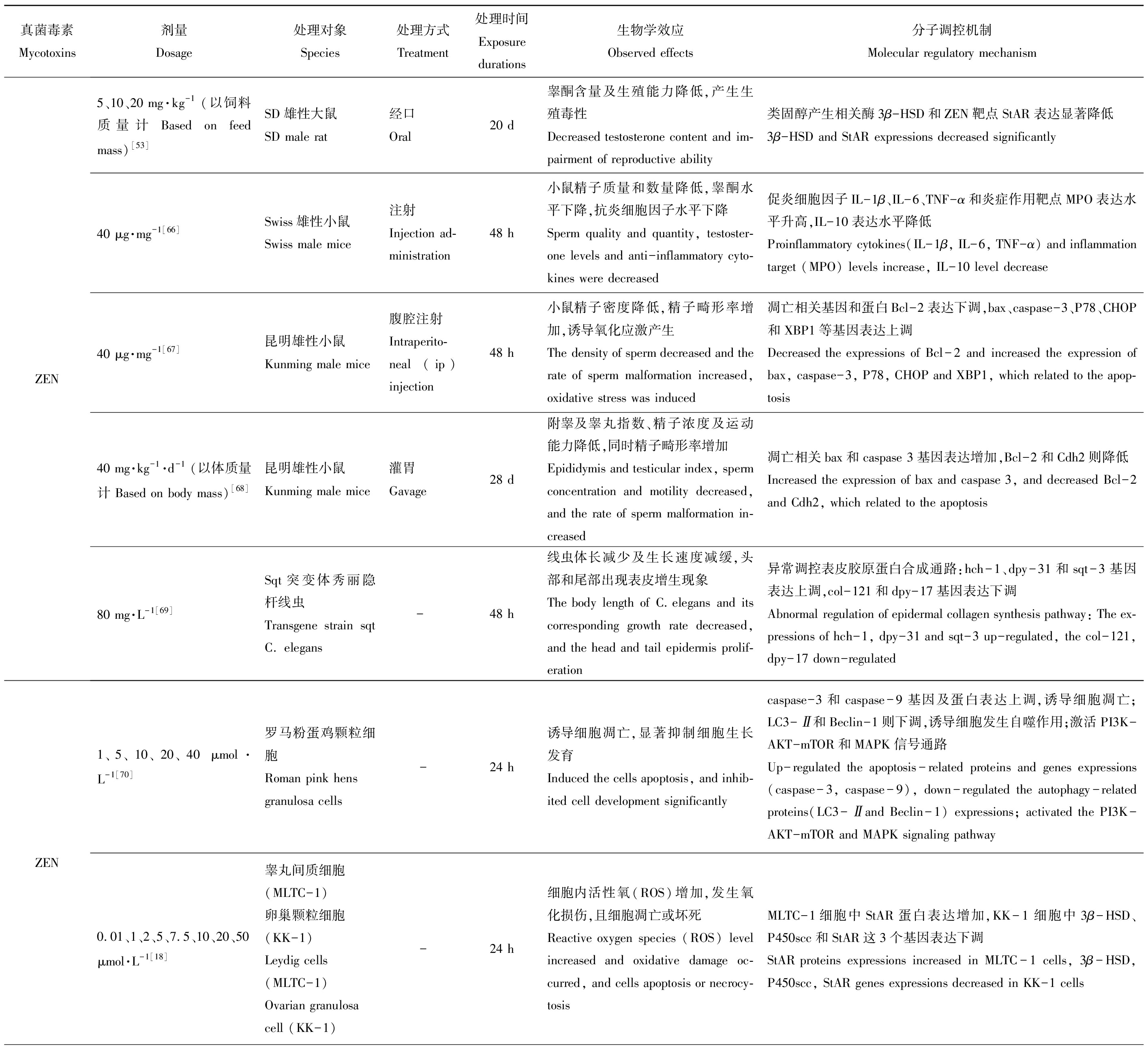
真菌毒素Mycotoxins剂量Dosage处理对象Species处理方式Treatment处理时间Exposure durations生物学效应Observed effects分子调控机制Molecular regulatory mechanismZEN5、10、20 mg·kg-1(以饲料质量计 Based on feed mass)[53]SD雄性大鼠SD male rat经口Oral20 d睾酮含量及生殖能力降低,产生生殖毒性Decreased testosterone content and im-pairment of reproductive ability类固醇产生相关酶3β-HSD和ZEN靶点StAR表达显著降低3β-HSD and StAR expressions decreased significantly40 μg·mg-1[66]Swiss雄性小鼠Swiss male mice注射Injection ad-ministration48 h小鼠精子质量和数量降低,睾酮水平下降,抗炎细胞因子水平下降Sperm quality and quantity, testoster-one levels and anti-inflammatory cyto-kines were decreased促炎细胞因子IL-1β、IL-6、TNF-α和炎症作用靶点MPO表达水平升高,IL-10表达水平降低Proinflammatory cytokines(IL-1β, IL-6, TNF-α) and inflammation target (MPO) levels increase, IL-10 level decrease40 μg·mg-1[67]昆明雄性小鼠Kunming male mice腹腔注射Intraperito-neal (ip) injection48 h小鼠精子密度降低,精子畸形率增加,诱导氧化应激产生The density of sperm decreased and the rate of sperm malformation increased, oxidative stress was induced凋亡相关基因和蛋白Bcl-2表达下调,bax、caspase-3、P78、CHOP和XBP1等基因表达上调Decreased the expressions of Bcl-2 and increased the expression of bax, caspase-3, P78, CHOP and XBP1, which related to the apop-tosis40 mg·kg-1·d-1(以体质量计 Based on body mass)[68]昆明雄性小鼠Kunming male mice灌胃Gavage28 d附睾及睾丸指数、精子浓度及运动能力降低,同时精子畸形率增加Epididymis and testicular index, sperm concentration and motility decreased, and the rate of sperm malformation in-creased凋亡相关bax和caspase 3基因表达增加,Bcl-2和Cdh2则降低Increased the expression of bax and caspase 3, and decreased Bcl-2 and Cdh2, which related to the apoptosis80 mg·L-1[69]Sqt突变体秀丽隐杆线虫Transgene strain sqt C. elegans -48 h线虫体长减少及生长速度减缓,头部和尾部出现表皮增生现象The body length of C.elegans and its corresponding growth rate decreased, and the head and tail epidermis prolif-eration异常调控表皮胶原蛋白合成通路:hch-1、dpy-31和sqt-3基因表达上调,col-121和dpy-17基因表达下调Abnormal regulation of epidermal collagen synthesis pathway: The ex-pressions of hch-1, dpy-31 and sqt-3 up-regulated, the col-121, dpy-17 down-regulatedZEN1、5、10、20、40 μmol·L-1[70]罗马粉蛋鸡颗粒细胞Roman pink hens granulosa cells-24 h诱导细胞凋亡,显著抑制细胞生长发育Induced the cells apoptosis, and inhib-ited cell development significantlycaspase-3和caspase-9基因及蛋白表达上调,诱导细胞凋亡;LC3-Ⅱ和Beclin-1则下调,诱导细胞发生自噬作用;激活PI3K-AKT-mTOR和MAPK信号通路Up-regulated the apoptosis-related proteins and genes expressions(caspase-3, caspase-9), down-regulated the autophagy-related proteins(LC3-Ⅱand Beclin-1) expressions; activated the PI3K-AKT-mTOR and MAPK signaling pathway0.01、1、2、5、7.5、10、20、50μmol·L-1[18]睾丸间质细胞(MLTC-1)卵巢颗粒细胞(KK-1)Leydig cells(MLTC-1)Ovarian granulosa cell (KK-1)-24 h细胞内活性氧(ROS)增加,发生氧化损伤,且细胞凋亡或坏死Reactive oxygen species (ROS) level increased and oxidative damage oc-curred, and cells apoptosis or necrocy-tosisMLTC-1细胞中StAR蛋白表达增加,KK-1细胞中3β-HSD、P450scc和StAR这3个基因表达下调StAR proteins expressions increased in MLTC-1 cells, 3β-HSD,P450scc, StAR genes expressions decreased in KK-1 cells
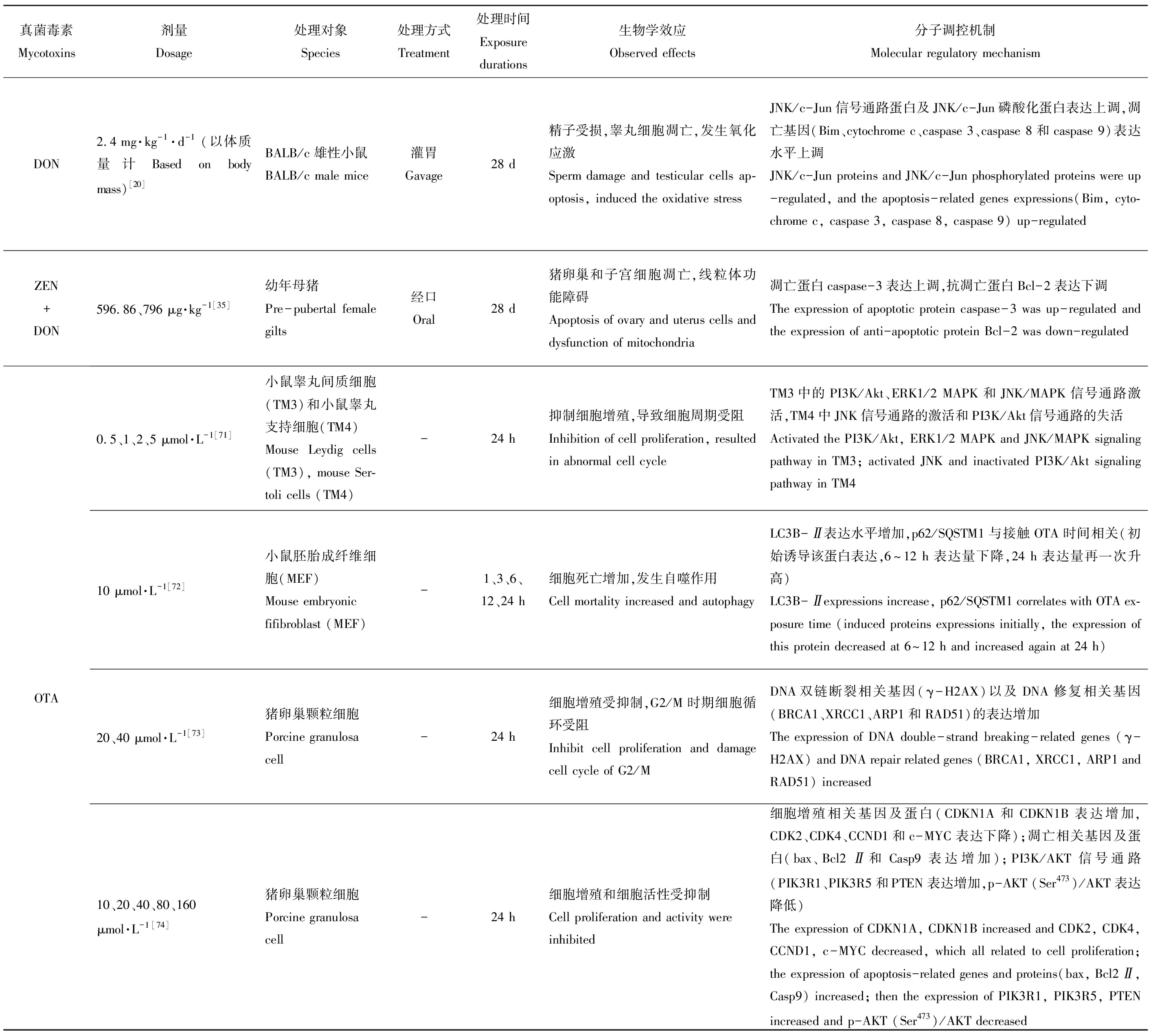
真菌毒素Mycotoxins剂量Dosage处理对象Species处理方式Treatment处理时间Exposure durations生物学效应Observed effects分子调控机制Molecular regulatory mechanismDON2.4 mg·kg-1·d-1(以体质量计 Based on body mass)[20]BALB/c雄性小鼠BALB/c male mice灌胃Gavage28 d精子受损,睾丸细胞凋亡,发生氧化应激Sperm damage and testicular cells ap-optosis, induced the oxidative stressJNK/c-Jun信号通路蛋白及JNK/c-Jun磷酸化蛋白表达上调,凋亡基因(Bim、cytochrome c、caspase 3、caspase 8和caspase 9)表达水平上调JNK/c-Jun proteins and JNK/c-Jun phosphorylated proteins were up-regulated, and the apoptosis-related genes expressions(Bim, cyto-chrome c, caspase 3, caspase 8, caspase 9) up-regulatedZEN+DON596.86、796 μg·kg-1[35]幼年母猪Pre-pubertal female gilts经口Oral28 d猪卵巢和子宫细胞凋亡,线粒体功能障碍Apoptosis of ovary and uterus cells and dysfunction of mitochondria凋亡蛋白caspase-3表达上调,抗凋亡蛋白Bcl-2表达下调The expression of apoptotic protein caspase-3 was up-regulated and the expression of anti-apoptotic protein Bcl-2 was down-regulatedOTA0.5、1、2、5 μmol·L-1[71]小鼠睾丸间质细胞(TM3)和小鼠睾丸支持细胞(TM4)Mouse Leydig cells(TM3), mouse Ser-toli cells (TM4)-24 h抑制细胞增殖,导致细胞周期受阻Inhibition of cell proliferation, resulted in abnormal cell cycleTM3中的PI3K/Akt、ERK1/2 MAPK和JNK/MAPK信号通路激活,TM4中JNK信号通路的激活和PI3K/Akt信号通路的失活Activated the PI3K/Akt, ERK1/2 MAPK and JNK/MAPK signaling pathway in TM3; activated JNK and inactivated PI3K/Akt signaling pathway in TM410 μmol·L-1[72]小鼠胚胎成纤维细胞(MEF)Mouse embryonic fifibroblast (MEF)-1、3、6、12、24 h细胞死亡增加,发生自噬作用Cell mortality increased and autophagyLC3B-Ⅱ表达水平增加,p62/SQSTM1与接触OTA时间相关(初始诱导该蛋白表达,6~12 h表达量下降,24 h表达量再一次升高)LC3B-Ⅱexpressions increase, p62/SQSTM1 correlates with OTA ex-posure time (induced proteins expressions initially, the expression of this protein decreased at 6~12 h and increased again at 24 h)20、40 μmol·L-1[73]猪卵巢颗粒细胞Porcine granulosa cell-24 h细胞增殖受抑制,G2/M时期细胞循环受阻Inhibit cell proliferation and damage cell cycle of G2/MDNA双链断裂相关基因(γ-H2AX)以及DNA修复相关基因(BRCA1、XRCC1、ARP1和RAD51)的表达增加The expression of DNA double-strand breaking-related genes (γ-H2AX) and DNA repair related genes (BRCA1, XRCC1, ARP1 and RAD51) increased10、20、40、80、160μmol·L-1[74]猪卵巢颗粒细胞Porcine granulosa cell-24 h细胞增殖和细胞活性受抑制Cell proliferation and activity were inhibited细胞增殖相关基因及蛋白(CDKN1A和CDKN1B表达增加,CDK2、CDK4、CCND1和c-MYC表达下降);凋亡相关基因及蛋白(bax、Bcl2Ⅱ和Casp9表达增加);PI3K/AKT信号通路(PIK3R1、PIK3R5和PTEN表达增加,p-AKT (Ser473)/AKT表达降低)The expression of CDKN1A, CDKN1B increased and CDK2, CDK4, CCND1, c-MYC decreased, which all related to cell proliferation; the expression of apoptosis-related genes and proteins(bax, Bcl2Ⅱ, Casp9) increased; then the expression of PIK3R1, PIK3R5, PTEN increased and p-AKT (Ser473)/AKT decreased
注:AFB1表示黄曲霉毒素B1;AFG1表示黄曲霉毒素G1;ZEN表示玉米赤霉烯酮;DON表示脱氧雪腐镰刀菌烯醇;OTA表示赭曲霉毒素。
Note: AFB1 is aflatoxin B1; AFG1 is aflatoxin G1;ZEN is zearalenone; DON is deoxynivalenol; OTA is ochratoxin.
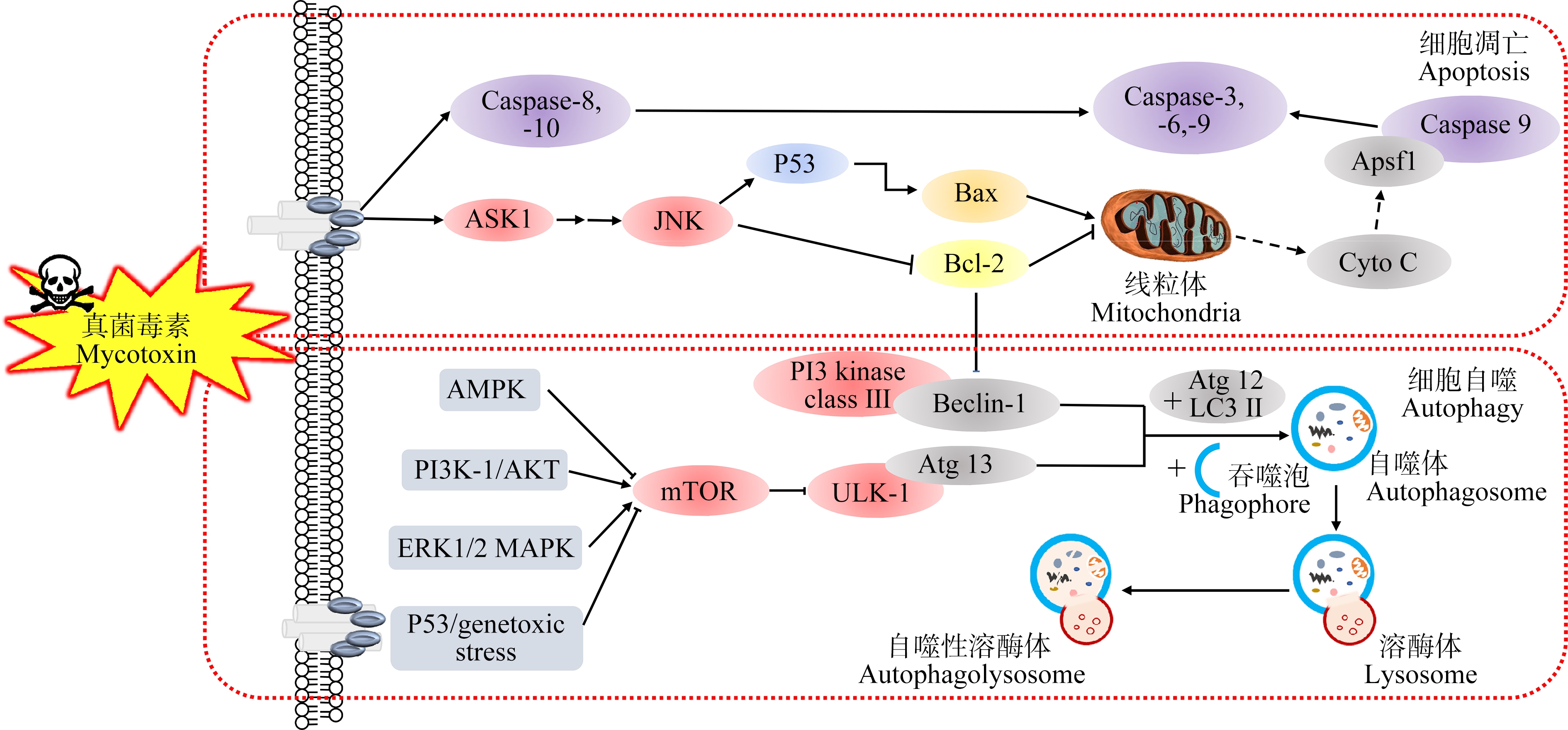
图2 主要粮食真菌毒素生长发育与遗传毒性分子机制图
Fig. 2 The molecular mechanism of reproductive, developmental and genetic toxicities of main grain mycotoxins
除上述主要信号通路外,粮食真菌毒素还可通过以下途径对机体产生毒性作用并进行分子调控。例如,AMPK和PTEN信号通路可影响精子活力[75];线粒体损伤相关基因(PGC-1α、Nrf1、Tfam、Drp1和Fis1等)和雄性激素相关基因(StAR、3β-HSD)的下调与雄性小鼠睾丸的发育、损伤及精子发生障碍有关[16, 62];先天免疫蛋白CXCL1、IL-1β和IL-8等的下调可导致小鼠胚胎的不正常发育[41];神经生长因子ngfa和神经元编码基因atp1b1b的下调则会影响斑马鱼胚胎早期的神经发育[76]。由此可见,不同信号通路间可形成复杂的互作网络,在机体受到粮食真菌毒素作用时可引起多条信号通路单独或共同参与相关基因和蛋白的调控,故还需对此进行更加广泛深入的研究,以便全面了解粮食真菌毒素的毒作用机制。
3.3 表观遗传机制
研究还发现,外源性有害物质是引起动物体表观遗传修饰的重要因素。人和动物在摄入粮食真菌毒素后,诱导机体出现不良反应,如内分泌紊乱、生殖系统DNA甲基化和诱发机体世代疾病等[77-78]。表观遗传虽不涉及DNA序列的改变,但其与DNA甲基化、组蛋白修饰以及染色质重塑等过程相关,是真菌毒素对机体产生毒作用的重要机制之一。已有研究表明,ZEN及其代谢产物α-ZOL作用于人体肝癌细胞会显著提高细胞DNA甲基化和组蛋白修饰水平(H3K27me3、H3K9me3和H3K9ac),通过表观遗传介导,影响细胞的脂代谢作用,从而影响细胞发育[77]。Men等[25]用ZEN对ICR小鼠进行处理,发现小鼠体内的H3K27、H3K9和5hmC的组蛋白甲基化标记物水平改变,DNA甲基化水平提高,引起表观遗传修饰,雄性小鼠的精子质量及其正常发育受损,而Zhu等[78]利用粮食真菌毒素(DON、AFs和FB)对ICR雌性小鼠染毒时得到类似的结论,且雌性小鼠卵母细胞的质量也受损。此外,也有研究发现表观遗传修饰可跨代遗传,如用环境中的污染物p,p’-DDE对SD大鼠进行世代染毒,发现F1、F2、F3代大鼠的精子质量下降、精子发生受阻并均产生DNA(H19和Gtl2)低甲基化作用,但F1、F2代中的变化是由于DNMT1和DNMT3A基因下调,F3代大鼠表型的变化是由于生殖系统中表观基因组的改变,而未直接引起其基因的改变,表明该环境污染物可以通过表观遗传修饰诱导使精子发生跨代损伤[79]。值得一提的是,粮食真菌毒素的毒性作用也会影响生物体亲本世代及子代的正常生长发育及生殖能力,而与此相关的研究还较为欠缺,因此亟需要对粮食真菌毒素在该方面进行更加全面、深入的研究。
综上所述,粮食真菌毒素造成动物体生长发育及生殖、遗传过程等受损的机制不尽相同。目前研究表明,其作用机制主要包括氧化应激、相关蛋白及基因表达的改变以及表观遗传等,但其他毒性作用机制仍需进行深入研究。
4 展望(Prospect)
本文通过综述粮食真菌毒素的生殖、发育与遗传毒性研究进展发现,相关研究多集中于粮食真菌毒素对动物体亲本个体(P0)或子一代(F1)的相关毒性研究,对其长期多世代个体F2-n接触粮食真菌毒素的研究还较为缺乏,其中与继代相关的靶标基因及其作用通路机理仍不明确,这可能是因为多世代毒理学研究存在所需实验动物数量多、实验周期长和操作繁琐等问题。因此,探索建立更多的动物实验模型,如利用秀丽隐杆线虫、斑马鱼等易于培养、生命周期短的模式动物对真菌毒素的继代毒性进行初步探究,并利用多组学技术、转基因或基因敲除等手段初步挖掘继代毒性的机理,为研究粮食真菌毒素对哺乳动物的世代毒性作用奠定基础。总之,深入研究粮食真菌毒素的继代毒性,全面解析真菌毒素的毒性,不仅有助于提供真菌毒素靶标性干预防治策略,对于制定更加科学客观的真菌毒素限量标准、对补充和完善相关毒理学资料也具有重要意义。
通讯作者简介:马良(1979—),女,博士,教授,主要研究方向为食品安全与质量控制。
共同通讯作者简介:周鸿媛(1989—),女,博士,讲师,主要研究方向为食品安全与质量控制。
[1] Zhou H Y, George S, Li C X, et al. Combined toxicity of prevalent mycotoxins studied in fish cell line and zebrafish larvae revealed that type of interactions is dose-dependent [J]. Aquatic Toxicology, 2017, 193: 60-71
[2] Luo S J, Du H L, Kebede H, et al. Contamination status of major mycotoxins in agricultural product and food stuff in Europe [J]. Food Control, 2021, 127: 108120
[3] Koletsi P, Schrama J W, Graat E A M, et al. The occurrence of mycotoxins in raw materials and fish feeds in Europe and the potential effects of deoxynivalenol (DON) on the health and growth of farmed fish species: A review[J]. Toxins, 2021, 13(6): 403
[4] Jin J, Beekmann K, Ringø E, et al. Interaction between food-borne mycotoxins and gut microbiota: A review [J]. Food Control, 2021, 126: 107998
[5] Biomin. World mycotoxin survey 2019[EB/OL]. [2021-07-20]. https://www.biomin.net/downloads/2019-biomin-world-mycotoxin-survey-report/
[6] 韩小敏, 李凤琴, 徐文静. 食品中白僵菌素和恩镰孢菌素的污染情况及分析方法研究进展[J]. 中国食品卫生杂志, 2017, 29(4): 508-513
Han X M, Li F Q, Xu W J. Research progress on the contamination of beauvericin and enniatins and the development of analytical method in food [J]. Chinese Journal of Food Hygiene, 2017, 29(4): 508-513 (in Chinese)
[7] Supriya C, Girish B P, Reddy P S. Aflatoxin B1-induced reproductive toxicity in male rats: Possible mechanism of action [J]. International Journal of Toxicology, 2014, 33(3): 155-161
[8] Kovács M, Tornyos G, Matics Z, et al. Effect of chronic T-2 toxin exposure in rabbit bucks, determination of the No Observed Adverse Effect Level (NOAEL) [J]. Animal Reproduction Science, 2013, 137(3-4): 245-252
[9] 周鸿媛, 唐莉莉, 路勇, 等. 脱氧雪腐镰刀菌烯醇、黄曲霉毒素B1和玉米赤霉烯酮对秀丽隐杆线虫的联合毒性研究[J]. 生态毒理学报, 2018, 13(3): 112-121
Zhou H Y, Tang L L, Lu Y, et al. Study on the combinatorial toxicity of deoxynivalenol, aflatoxin B1 and zearalenone on Caenorhabditis elegans [J]. Asian Journal of Ecotoxicology, 2018, 13(3): 112-121 (in Chinese)
[10] 陈铃霞. ZEN与DON对雄性小鼠生殖系统的联合毒性及茶多酚的保护作用研究[D]. 合肥: 安徽农业大学, 2019: 34-39
Chen L X. Combined toxicity of ZEN and DON to the reproductive system of male mice and the protective effect of tea polyphenols [D]. Hefei: Anhui Agricultural University, 2019: 34-39 (in Chinese)
[11] 周鸿媛. 脱氧雪腐镰刀菌烯醇(DON)的多代毒性及其联合毒性研究[D]. 无锡: 江南大学, 2018: 38-54
Zhou H Y. Study on the multi-generational and combined toxic effects of deoxynivalenol(DON) [D]. Wuxi: Jiangnan University, 2018: 38-54 (in Chinese)
[12] Dalla Bona M, Lizzi F, Borgato A, et al. Increasing toxicity of enrofloxacin over four generations of Daphnia magna [J]. Ecotoxicology and Environmental Safety, 2016, 132: 397-402
[13] Shaw J L A, Judy J D, Kumar A, et al. Incorporating transgenerational epigenetic inheritance into ecological risk assessment frameworks [J]. Environmental Science & Technology, 2017, 51(17): 9433-9445
[14] Owumi S E, Adedara I A, Akomolafe A P, et al. Gallic acid enhances reproductive function by modulating oxido-inflammatory and apoptosis mediators in rats exposed to aflatoxin-B1[J]. Experimental Biology and Medicine, 2020, 245(12): 1016-1028
[15] Gao X, Sun L H, Zhang N Y, et al. Gestational zearalenone exposure causes reproductive and developmental toxicity in pregnant rats and female offspring [J]. Toxins, 2017, 9(1): E21
[16] Shen J K, Perveen A, Kaka N, et al. Maternal exposure to T-2 toxin induces changes in antioxidant system and testosterone synthesis in the testes of mice offspring [J]. Animals: An Open Access Journal from MDPI, 2019, 10(1): E74
[17] 李雨哲. 玉米赤霉烯酮对生殖靶器官细胞毒性机制研究[D]. 北京: 中国农业大学, 2014: 12-68
Li Y Z. Investigation of the mechanism of the reproductive toxicity of zearalenone on cells from target organ [D]. Beijing: China Agriculture University, 2014: 12-68 (in Chinese)
[18] 李洪蛟. 丙酸睾酮对小鼠睾丸BNNF、NGF、NT-3和NT-4神经营养素表达的影响[D]. 长春: 吉林大学, 2016: 28-34
Li H J. Effects of testosterone propionate on the expression of BDNF, NGF, NT-3 and NT-4 neurotrophins in testes of mice [D]. Changchun: Jilin University, 2016: 28-34 (in Chinese)
[19] Zhou H Y, Wang J M, Ma L, et al. Oxidative DNA damage and multi-organ pathologies in male mice subchronically treated with aflatoxin B1[J]. Ecotoxicology and Environmental Safety, 2019, 186: 109697
[20] Yang J H, Wang J H, Guo W B, et al. Toxic effects and possible mechanisms of deoxynivalenol exposure on sperm and testicular damage in BALB/c mice [J]. Journal of Agricultural and Food Chemistry, 2019, 67(8): 2289-2295
[21] Pang J, Zhou Q S, Sun X F, et al. Effect of low-dose zearalenone exposure on reproductive capacity of male mice [J]. Toxicology and Applied Pharmacology, 2017, 333: 60-67
[22] Eze U A, Huntriss J D, Routledge M N, et al. In vitro effects of single and binary mixtures of regulated mycotoxins and persistent organochloride pesticides on steroid hormone production in MA-10 Leydig cell line [J]. Toxicology in Vitro, 2019, 60: 272-280
[23] 张新宇. 睾丸生殖功能调控的初步研究[D]. 北京: 北京协和医学院, 2013: 43-46
[24] Khoury D E, Fayjaloun S, Nassar M, et al. Updates on the effect of mycotoxins on male reproductive efficiency in mammals [J]. Toxins, 2019, 11(9): 515
[25] Men Y H, Zhao Y, Zhang P F, et al. Gestational exposure to low-dose zearalenone disrupting offspring spermatogenesis might be through epigenetic modifications[J]. Basic & Clinical Pharmacology & Toxicology, 2019, 125(4): 382-393
[26] Jee Y, Noh E M, Cho E S, et al. Involvement of the Fas and Fas ligand in testicular germ cell apoptosis by zearalenone in rat [J]. Journal of Veterinary Science, 2010, 11(2): 115-119
[27] Wang B J, Zheng W L, Feng N N, et al. The effects of autophagy and PI3K/AKT/m-TOR signaling pathway on the cell-cycle arrest of rats primary Sertoli cells induced by zearalenone [J]. Toxins, 2018, 10(10): E398
[28] Li Y Y, Huang P, Gao F Y, et al. Selenium ameliorates aflatoxin B1-induced uterine injury in female mice and necrosis of human endometrial microvascular endothelial cells [J]. Journal of Applied Toxicology, 2021, 41(5): 799-810
[29] Wang Y, Zhang J, Wang Y L, et al. Isolation and characterization of the Bacillus cereus BC7 strain, which is capable of zearalenone removal and intestinal flora modulation in mice [J]. Toxicon: Official Journal of the International Society on Toxinology, 2018, 155: 9-20
[30] Ahmad B, Shrivastava V K, Saleh R, et al. Protective effects of saffron against zearalenone-induced alterations in reproductive hormones in female mice (Mus musculus) [J]. Clinical and Experimental Reproductive Medicine, 2018, 45(4): 163-169
[31] Jia H Q, Jia C Q, An Q L, et al. Ochratoxin A exposure causes meiotic failure and oocyte deterioration in mice [J]. Theriogenology, 2020, 148: 236-248
[32] Yang M, Wu X D, Zhang W, et al. Transcriptional analysis of deoxynivalenol-induced apoptosis of sow ovarian granulosa cell [J]. Reproduction in Domestic Animals, 2020, 55(2): 217-228
[33] Zhang T Y, Kong L, Hao J X, et al. Effects of ochratoxin A exposure on DNA damage in porcine granulosa cells in vitro [J]. Toxicology Letters, 2020, 330: 167-175
[34] Silva T, de Brito D C C, de Sá N A R, et al. Equol: A microbiota metabolite able to alleviate the negative effects of zearalenone during in vitro culture of ovine preantral follicles [J]. Toxins, 2019, 11(11): 652
[35] Shi D H, Zhou J C, Zhao L H, et al. Alleviation of mycotoxin biodegradation agent on zearalenone and deoxynivalenol toxicosis in immature gilts [J]. Journal of Animal Science and Biotechnology, 2018, 9: 42
[36] 尹亚南. 金乌贼生殖系统结构特征和卵子发生的研究[D]. 上海: 上海海洋大学, 2018: 10
Ying Y N. Studies on structure of reproductive system and oogenesis in Sepia esculenta Hoyle [D]. Shanghai: Shanghai Ocean University, 2018: 10 (in Chinese)
[37] Cheng L H, Qin Y S, Hu X, et al. Melatonin protects in vitro matured porcine oocytes from toxicity of aflatoxin B1 [J]. Journal of Pineal Research, 2019, 66(4): e12543
[38] Mastrorocco A, Martino N A, Marzano G, et al. The mycotoxin beauvericin induces oocyte mitochondrial dysfunction and affects embryo development in the juvenile sheep [J]. Molecular Reproduction and Development, 2019, 86(10): 1430-1443
[39] Schoevers E J, Santos R R, Roelen B A J. Alternariol disturbs oocyte maturation and preimplantation development [J]. Mycotoxin Research, 2020, 36(1): 93-101
[40] Ye X Q, Andersen C, Zhao F. Effects of mycoestrogens on female reproduction [J]. Reproductive and Developmental Medicine, 2018, 2(1): 52
[41] 胡进. 玉米赤霉烯酮对小鼠子宫内膜基质细胞的毒性作用及机制研究[D]. 南京: 南京农业大学, 2016: 52-58
Hu J. Toxicity of zearalenone and its mechanism on mouse endometrial stromal cells [D]. Nanjing: Nanjing Agriculture University, 2016: 52-58 (in Chinese)
[42] 李佳. Hippo信号通路在调节卵巢生殖干细胞功能和延缓卵巢衰老中的作用[D]. 南昌: 南昌大学, 2016: 34-35
Li J. The roles of Hippo signaling pathway in regulating the function of ovarian germline stem cells and delaying ovarian aging [D]. Nanchang: Nanchang University, 2016: 34-35 (in Chinese)
[43] 王兰. 线粒体应激对卵子发育潜能及卵巢储备功能的影响[D]. 武汉: 华中科技大学, 2017: 42-49
Wang L. Impact of mitochondrial stress on oocyte competency and ovarian reserve [D]. Wuhan: Huazhong University of Science and Technology, 2017: 42-49 (in Chinese)
[44] Shin K T, Guo J, Niu Y J, et al. The toxic effect of aflatoxin B1 on early porcine embryonic development [J]. Theriogenology, 2018, 118: 157-163
[45] Huang C H, Wang F T, Chan W H. Enniatin B1 exerts embryotoxic effects on mouse blastocysts and induces oxidative stress and immunotoxicity during embryo development [J]. Environmental Toxicology, 2019, 34(1): 48-59
[46] Wu T S, Lin Y T, Huang Y T, et al. Ochratoxin A triggered intracerebral hemorrhage in embryonic zebrafish: Involvement of microRNA-731 and prolactin receptor [J]. Chemosphere, 2020, 242: 125143
[47] Zhang L P, Li L S, Xu J, et al. HT-2 toxin exposure induces mitochondria dysfunction and DNA damage during mouse early embryo development [J]. Reproductive Toxicology, 2019, 85: 104-109
[48] Xu Y, Zhang K H, Sun M H, et al. Protective effects of melatonin against zearalenone toxicity on porcine embryos in vitro [J]. Frontiers in Pharmacology, 2019, 10: 327
[49] Bondy G S, Coady L, Ross N, et al. A reproductive and developmental screening study of the fungal toxin ochratoxin A in Fischer rats [J]. Mycotoxin Research, 2018, 34(4): 241-255
[50] Li R, Andersen C L, Hu L M, et al. Dietary exposure to mycotoxin zearalenone (ZEA) during post-implantation adversely affects placental development in mice [J]. Reproductive Toxicology, 2019, 85: 42-50
[51] Zhao X X, Wang D X, Fields P G, et al. Effect of aflatoxin B1 on development, survival and fecundity of Ahasverus advena (Waltl) [J]. Journal of Stored Products Research, 2018, 77: 225-230
[52] Yang X, Liu P L, Cui Y L, et al. Review of the reproductive toxicity of T-2 toxin [J]. Journal of Agricultural and Food Chemistry, 2020, 68(3): 727-734
[53] Gao X, Xiao Z H, Li C, et al. Prenatal exposure to zearalenone disrupts reproductive potential and development via hormone-related genes in male rats [J]. Food and Chemical Toxicology, 2018, 116: 11-19
[54] Zhou Y W, Zhang D, Sun D H, et al. Zearalenone affects reproductive functions of male offspring via transgenerational cytotoxicity on spermatogonia in mouse [J]. Comparative Biochemistry and Physiology Toxicology & Pharmacology, 2020, 234: 108766
[55] 史雅凝. 鸡蛋膜蛋白酶解物的制备及其对肠道氧化应激和炎症的影响[D]. 无锡: 江南大学, 2015: 26-34
Shi Y N. Preparation of soluble eggshell membrane hydrolysate and its anti-oxidaitve stress and anti-inflammatory effects [D]. Wuxi: Jiangnan University, 2015: 26-34(in Chinese)
[56] Wu J, Yang C L, Liu J, et al. Betulinic acid attenuates T-2-toxin-induced testis oxidative damage through regulation of the JAK2/STAT3 signaling pathway in mice [J]. Biomolecules, 2019, 9(12): E787
[57] Huang C H, Wang F T, Chan W H. Prevention of ochratoxin A-induced oxidative stress-mediated apoptotic processes and impairment of embryonic development in mouse blastocysts by liquiritigenin [J]. Environmental Toxicology, 2019, 34(5): 573-584
[58] Szabó B, Kocsis R, Mézes M. Reproduction inhibiting effects of deoxynivalenol or T-2 toxin contaminated maize on Folsomia candida (Collembola) [J]. Acta Zoologica Academiae Scientiarum Hungaricae, 2019, 65(4): 323-334
[59] Xu Y, Zhang K H, Sun M H, et al. Protective effects of melatonin against zearalenone toxicity on porcine embryos in vitro [J]. Frontiers in Pharmacology, 2019, 10: 327
[60] Huang W Y, Cao Z, Zhang J, et al. Aflatoxin B1 promotes autophagy associated with oxidative stress-related PI3K/AKT/mTOR signaling pathway in mice testis [J]. Environmental Pollution, 2019, 255(Pt 2): 113317
[61] Cao Z, Shao B, Xu F B, et al. Protective effect of selenium on aflatoxin B1-induced testicular toxicity in mice [J]. Biological Trace Element Research, 2017, 180(2): 233-238
[62] Huang W Y, Cao Z, Yao Q C, et al. Mitochondrial damage are involved in aflatoxin B1-induced testicular damage and spermatogenesis disorder in mice [J]. The Science of the Total Environment, 2020, 701: 135077
[63] Omur A D, Yildirim B, Saglam Y S, et al. Activity of resveratrol on the influence of aflatoxin B1 on the testes of Sprague Dawley rats [J]. Polish Journal of Veterinary Sciences, 2019, 22(2): 313-320
[64] Huang W Y, Liu M L, Xiao B N, et al. Aflatoxin B1 disrupts blood-testis barrier integrity by reducing junction protein and promoting apoptosis in mice testes [J]. Food and Chemical Toxicology, 2021, 148: 111972
[65] Zamir-Nasta T, Pazhouhi M, Ghanbari A, et al. Expression of cyclin D1, p21, and estrogen receptor alpha in aflatoxin G1-induced disturbance in testicular tissue of albino mice [J]. Research in Pharmaceutical Sciences, 2021, 16(2): 182-192
[66] del Fabbro L, Jesse C R, de Gomes M G, et al. The flavonoid chrysin protects against zearalenone induced reproductive toxicity in male mice [J]. Toxicon, 2019, 165: 13-21
[67] Long M, Yang S H, Zhang Y, et al. Proanthocyanidin protects against acute zearalenone-induced testicular oxidative damage in male mice [J]. Environmental Science and Pollution Research International, 2017, 24(1): 938-946
[68] Long M, Yang S H, Wang Y, et al. The protective effect of selenium on chronic zearalenone-induced reproductive system damage in male mice [J]. Molecules, 2016, 21(12): E1687
[69] 杨振东. 模式生物秀丽隐杆线虫评价真菌毒素毒性毒理机制的研究[D]. 无锡: 江南大学, 2016: 61-69
Yang Z D. Study of mycotoxins toxic effects and mechanisms using a model organism nematode Caenorhabdites elegans [D]. Wuxi: Jiangnan University, 2016: 61-69(in Chinese)
[70] Zhu Y F, Wang H, Wang J P, et al. Zearalenone induces apoptosis and cytoprotective autophagy in chicken granulosa cells by PI3K-AKT-mTOR and MAPK signaling pathways [J]. Toxins, 2021, 13(3): 199
[71] Park H, Park H S, Lim W, et al. Ochratoxin A suppresses proliferation of Sertoli and Leydig cells in mice [J]. Medical Mycology, 2020, 58(1): 71-82
[72] Akpinar H A, Kahraman H, Yaman I. Ochratoxin A sequentially activates autophagy and the ubiquitin-proteasome system [J]. Toxins, 2019, 11(11): 615
[73] Zhang T Y, Kong L, Hao J X, et al. Effects of ochratoxin A exposure on DNA damage in porcine granulosa cells in vitro [J]. Toxicology Letters, 2020, 330: 167-175
[74] Zhang T Y, Sun X F, Li L, et al. Ochratoxin A exposure impairs porcine granulosa cell growth via the PI3K/AKT signaling pathway [J]. Journal of Agricultural and Food Chemistry, 2019, 67(9): 2679-2690
[75] Zhang T Y, Wu R Y, Zhao Y, et al. Ochratoxin A exposure decreased sperm motility via the AMPK and PTEN signaling pathways [J]. Toxicology and Applied Pharmacology, 2018, 340: 49-57
[76] Wu T S, Cheng Y C, Chen P J, et al. Exposure to aflatoxin B1 interferes with locomotion and neural development in zebrafish embryos and larvae [J]. Chemosphere, 2019, 217: 905-913
[77] Karaman E F, Zeybel M, Ozden S. Evaluation of the epigenetic alterations and gene expression levels of HepG2 cells exposed to zearalenone and α-zearalenol [J]. Toxicology Letters, 2020, 326: 52-60
[78] Zhu C C, Hou Y J, Han J, et al. Effect of mycotoxin-containing diets on epigenetic modifications of mouse oocytes by fluorescence microscopy analysis [J]. Microscopy and Microanalysis, 2014, 20(4): 1158-1166
[79] Song Y, Yang L. Transgenerational impaired spermatogenesis with sperm H19 and Gtl2 hypomethylation induced by the endocrine disruptor p,p’-DDE [J]. Toxicology Letters, 2018, 297: 34-41