邻苯二甲酸二(2-乙基己)酯(di-(2-ethylhexyl)phthalate, DEHP) 作为一种邻苯二甲酸酯类(phthalates, PAEs)增塑剂,在日常生活中最为常见。由于其本身可以聚合产品以增加塑料分子的柔韧性和延展性,因而被添加到许多食品及饮水的包装、玩具、医疗器械等产品中[1]。 但DEHP 与这些塑料制品是以非共价键范德华力结合,因此极易在加工、运输和使用过程中释放并进入环境介质之中[2-3],最终可通过不同途径,例如经口、皮肤和注射等进入机体,对人体和野生动物产生终生暴露的风险,这引起了人们对其安全性及对人体健康的潜在影响的担忧。
目前发现,水果蔬菜、乳制品、鱼和肉等食物中都含有DEHP[4]。 一般人群可以通过吸入空气、摄入食物和水接触DEHP,其中饮食是主要暴露途径[5]。除去非饮食摄入、医疗和职业暴露外,普通人群其他来源 DEHP 每日暴露值估计在 3 ~30 μg·kg-1[6]。儿童因吮吸或咀嚼塑料制品而接触DEHP 的每日暴露值可能达到85 μg·kg-1[7],此值已超过了美国环境保护局(US EPA)规定的供人类接触的参考剂量20 μg·kg-1,而婴幼儿和青少年大量食用肉类和奶制品而暴露的DEHP 每日可达90.6 μg·kg-1和21.6 μg·kg-1,也超过了这一阈值[8]。 此外,研究发现从医疗器械中迁移出的DEHP 对暴露患者具有潜在的生殖毒性影响,尤其是在敏感的亚群,如孕妇及婴幼儿等[9-10]。 重症监护中的新生儿每日DEHP 暴露量可达16 mg·kg-1,远高于估计的安全限值,可能通过多种机制促进炎症发展,导致大多数新生儿以炎症介导为主要特征的组织损伤[11]。
研究发现,DEHP 暴露可对人体内分泌[12]、生殖[13]、肝脏[14]、心脏[15]和神经[16]等产生毒性作用,但目前在神经方面的研究主要集中于孕妇产前DEHP接触对胎儿生长发育[17-18]以及儿童注意力表现的影响[19],但其具体机制尚不清楚,并且对成年人体健康风险的评估也相对较少。
新证据表明,邻苯二甲酸酯类暴露会加剧神经退行性疾病的患病风险[20]。 多项研究表明,DEHP暴露可以引起实验动物神经行为改变[21-23],但目前尚无对人类神经行为影响的研究报道。 DEHP 暴露可以导致大小鼠学习和记忆障碍[24-25]。 病例对照研究表明,产前和产后DEHP 暴露与注意缺陷多动障碍之间存在关联[26]。 最新研究发现DEHP 增加了自闭症儿童单核细胞的炎症转录因子以及炎性细胞因子的分子表达水平,揭示了其可能对自闭症相关的免疫功能障碍存在潜在影响[27]。 鉴于DEHP 在环境中普遍存在的潜在重大公共卫生影响,识别DEHP 的神经毒性作用及其相关机制,可为揭示DEHP 诱导的神经退行性疾病的可能机制提供重要的理论依据。
1 DEHP 进入脑的途径(How DEHP enters the brain)
已确定人类大脑有DEHP 暴露的风险,DEHP对大脑的影响,可能涉及以下途径(图1)。(1)胎盘屏障。 啮齿动物模型研究表明,DEHP 可以穿过大鼠的胎盘屏障进入胎鼠血液循环[28],破坏胎盘的正常发育,从而影响神经发育并导致畸胎。 妊娠期和产后DEHP 暴露可对大鼠大脑发育和功能产生有害影响[29]。(2)血脑屏障。 DEHP 易通过血脑屏障,并影响大脑发育和神经系统功能,医院来源的人体母体血浆样本中检出DEHP 水平为(1.15±0.81)μg·mL-1,新生儿脐带血浆中 DEHP 水平为(2.05±1.47) μg·mL-1,国内外的队列研究均表明孕妇产前DEHP 暴露与儿童不良的认知和行为结果相关[30-31]。(3)肠-脑途径。 在发育窗口期暴露于环境中的化学物质是神经发育障碍的成因[32-33],而这些障碍与微生物群失调相关。 研究表明,DEHP 可以直接改变肠道菌群,从而增加与行为异常有关的潜在神经毒性微生物代谢产物的产生[34]。
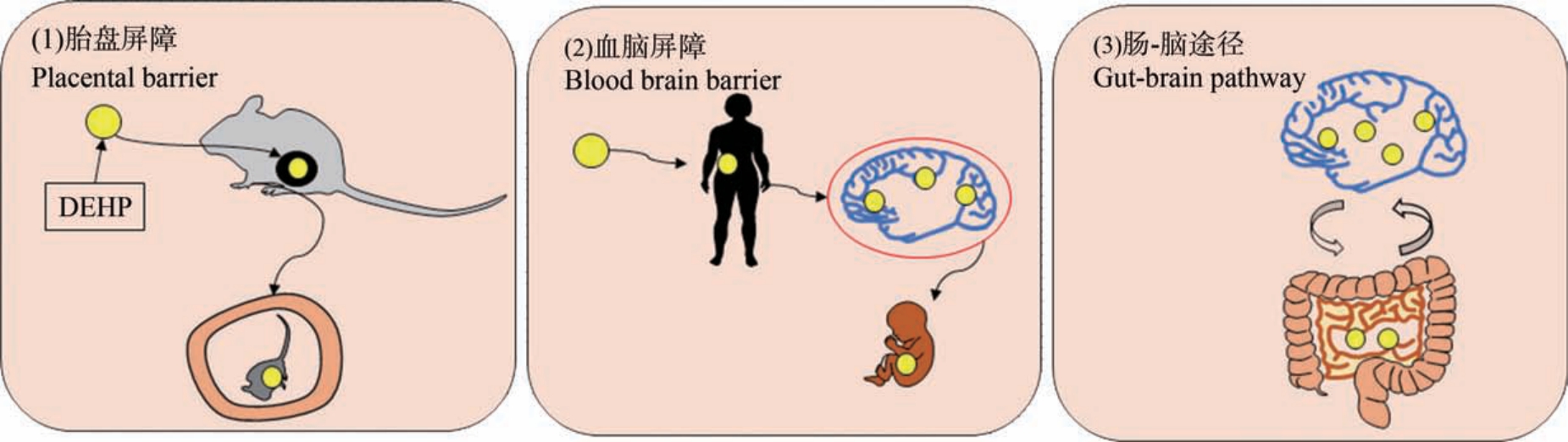
图1 DEHP 进入脑的途径
注:DEHP 表示邻苯二甲酸二(2-乙基己)酯。
Fig.1 The way that DEHP enters the brain
Note: DEHP stands for di-(2-ethylhexyl) phthalate.
2 DEHP 对机体神经毒性的表现(The neurotoxicity of DEHP)
2.1 对中枢神经系统的损伤(Damages to the central nervous system)
在胚胎期和幼儿期,由于血脑屏障尚未完全成熟,因此,DEHP 很容易通过血脑屏障进入到脑组织,进而产生毒性作用。 研究表明,暴露于 DEHP会损害胎盘的发育和功能[35],例如,母体DEHP 暴露后胎盘质量降低,胎盘迷宫层的血窦面积减少[36],会对胎儿的成长产生不利影响。 50 mg·kg-1 和200 mg·kg-1 DEHP 暴露可能会通过胎盘运输干扰甲状腺激素的水平,影响小鼠发育中的大脑神经细胞的增殖和迁移[37],还可以通过干扰胎盘甲状腺激素受体信号传导导致胎儿子宫内生长受限[38]。
DEHP 进入脑组织后不仅会损害神经元的结构,还会影响其功能。 例如,出生前 DEHP 暴露会影响大鼠大脑性分化区域的神经元,并随后导致神经变性[39]。 此外,出生后暴露会引起小鼠运动亢进和多巴胺能神经元数量的大大减少[40]。
DEHP 还会对脑组织的不同部位产生不同程度的损伤。 如产前暴露会损害小鼠新皮层的发育[41]。出生后16 d 到22 d 的雄性和雌性Long Evans 大鼠急性DEHP 暴露可以减少雄性大鼠海马CA3 区远端角质层中的轴突标记,降低齿状回和CA3 中未成熟和成熟神经元的细胞密度[42]。 母体DEHP 暴露后出生7、14 和21 d 的雄性幼鼠的海马CA1 亚区中基底树突的总长度和分支数减少[43]。 研究还发现,0.1 mol·L-1和 0.3 mol·L-1 的 DEHP 暴露还可抑制CA1 锥体细胞的电压门控钾通道,进而抑制神经元的兴奋性和突触可塑性,从而损害了实验动物的空间学习和记忆能力[25]。
越来越多的研究表明DEHP 暴露与神经元破坏之间存在关联。 DEHP 不仅会破坏神经细胞的完整性,使其活力下降,还会激活凋亡信号通路使神经细胞发生凋亡、抑制细胞增殖等。 研究表明,DEHP具有细胞毒性,并可诱导神经瘤母细胞(mouse neuroblastoma N2a cells, Neuro-2a)凋亡[44]。 10 μmol·L-1 DEHP 处理小鼠神经元和原代神经胶质细胞24 h 后,钙黄绿素AM 染色显示活神经元数目减少且神经胶质细胞数量增加[45],表明神经元-胶质细胞培养物由于暴露于DEHP 而受到损伤,为了维持神经元环境稳态并保护神经元免受有毒化学物质影响,胶质细胞增生机制被启动[46]。
2.2 对神经行为的损害(Damage to neurobehavior)
DEHP 作为一种环境内分泌干扰物,具有拟雌激素活性,早期暴露可能会破坏甲状腺功能,从而对儿童的神经发育产生不利影响[47]。 队列研究表明,孕期DEHP 暴露与儿童认知和神经行为改变有关,后代普遍表现出较低的认知得分,较差的内在(情绪、同伴关系等)和外在(多动/冲动等)行为;同时该研究还指出DEHP 暴露对认知、心理活动和行为发展的影响存在性别差异,DEHP 对男性后代神经行为的影响更为显著[48]。 另一项为期15年的随访出生队列研究结果表明,产前和儿童时期DEHP 暴露可能会影响8 ~14 岁儿童行为综合征的发展[49]。DEHP 影响神经系统发育可能与甲状腺激素的功能有关,而甲状腺激素在大脑发育和成熟的许多基本过程中起着必不可少的作用;DEHP 还可能通过干扰脂质信号传导途径改变大脑中脂质分布,进而影响大脑发育[46,50]。 此外,DEHP 的抗雄激素特性可能会影响性腺激素介导的神经发育过程,从而导致暴露结果的性别差异[51]。
3 神经毒性机制(Neurotoxicity mechanism)
3.1 细胞凋亡(Apoptosis)
DEHP 对神经毒性的研究目前主要集中在诱导神经细胞凋亡方面。 细胞凋亡是一种受基因调控的程序性死亡过程。 DEHP 以剂量依赖的方式显著抑制细胞活力从而引起神经细胞凋亡,还可引起神经细胞半胱天冬酶3(Caspase-3)蛋白表达水平显著增加。 Caspase-3 蛋白作为Caspase 家族的主要效应分子,在细胞凋亡过程中发挥重要作用,提示凋亡进入不可逆的阶段[52](图2)。 例如,在经DEHP 处理的小鼠海马神经元细胞系(hippocampal neuronal cell line,HT22)细胞中,与处理前对比,DEHP 以剂量依赖的方式显著上调细胞中的Caspase-8、Caspase-3 和促凋亡基因Bax 的蛋白含量水平,同时显著下调抗凋亡蛋白Bcl-2 的蛋白水平,这表明DEHP 可以诱导小鼠HT22 细胞凋亡[53]。 此外,长期暴露于0.1 ~100 μmol·L-1 DEHP,可以激活神经母细胞瘤细胞(SHSY5Y)中的Caspase-3,导致剂量依赖性细胞凋亡[54]。 经DEHP 处理的嗜铬细胞瘤细胞(PC12)出现p53 和Caspase-3 依赖性凋亡,同时Bax 蛋白表达增加且 Bcl-2 蛋白表达降低[55]。 除此之外,暴露于DEHP 还会诱导小鼠神经细胞瘤细胞系(N2a)细胞凋亡,并下调脑源性神经营养因子(brain derived neurotrophic factor, BDNF)、突触后致密蛋白 95(postsynaptic density protein 95, PSD95)和突触蛋白-1(synaptophysin 1, SYN1)的表达;降低环磷腺苷效应元件结合蛋白(cyclic adenosine phosphate response element binding protein, CREB)信号通路中蛋白激酶B(protein kinase B, Akt)的磷酸化水平,Ca2+/钙调蛋白依赖性蛋白激酶Ⅳ(Ca2+/calmodulin-dependent protein kinase Ⅳ, CaMKⅣ)、蛋白激酶 A(protein kinase A, PKA)和CREB 的催化亚基的表达水平[56]。
3.2 氧化损伤(Oxidative damage)
研究认为DEHP 可以通过激活生物体内的过氧化物酶体增生物激活受体(PPAR),引起编码过氧化氢酶的基因的选择性转录,使以活性氧为主的各种自由基含量增加,导致氧化应激状态[57-58],一旦活性氧清除系统的动态平衡遭到破坏后,就会造成严重的氧化损伤[59]。 DEHP 可以增加丙二醛的含量,降低谷胱甘肽过氧化物酶和超氧化物歧化酶的活性以及谷胱甘肽的含量。 研究发现,DEHP 以剂量依赖的方式显著抑制小鼠HT22 细胞活力并增加乳酸脱氢酶(lactatedehydrogenase, LDH)从细胞中的释放;而活性氧抑制剂乙酰半胱氨酸可以拮抗DEHP的作用[53]。 此外,DEHP 诱导的氧化应激还可以通过小鼠海马HT22 细胞中的线粒体裂变触发神经元细胞死亡。 过氧化物酶5(peroxiredoxin 5,Prx5)是一种抗氧化酶,可以作为H2O2 的传感器和收发器并通过细胞内氧化信号通路参与细胞信号传导[60],在此能通过抑制活性氧的产生来阻止DEHP 诱导的线粒体裂变;提示DEHP 暴露可能通过抑制Prx5 的活性进而影响活性氧的活性从而引起氧化损伤[61-62]。暴露于1 000 mg·kg-1的DEHP 还可通过调节线粒体动力学和生物发生激活转录因子NF-E2 相关因子2(Nrf2)介导的抗氧化防御反应,触发鹌鹑的脑线粒体功能障碍和氧化应激[63](图2)。 综上所述,DEHP 诱发氧化应激的主要机制可能与其影响线粒体的分裂和融合、干扰抗氧化酶基因的表达以及抑制抗氧化酶的活性有关(图3)。
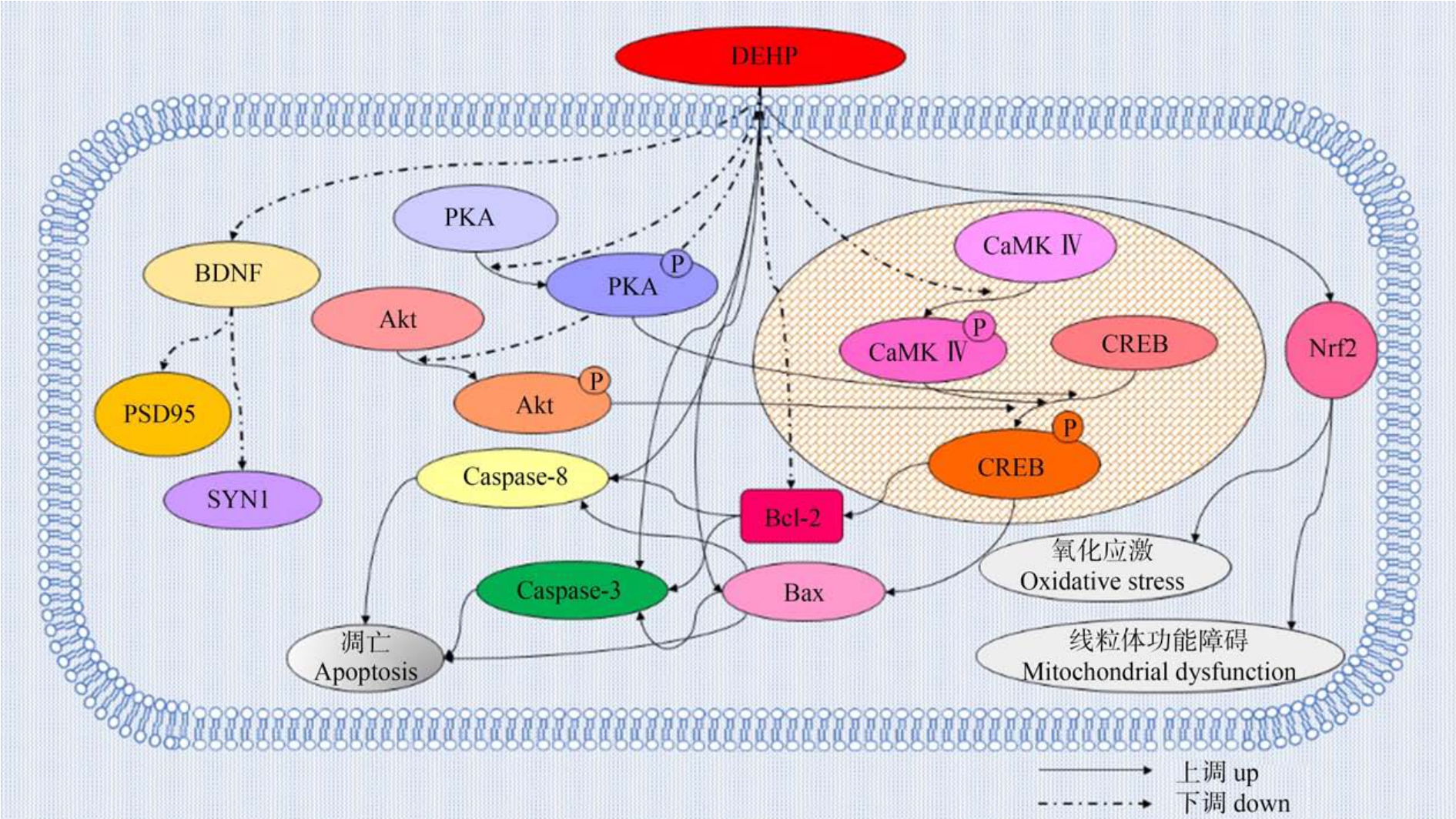
图2 DEHP 作用的潜在机制图
注:P 表示磷酸化;DEHP 表示邻苯二甲酸二(2-乙基己)酯;BDNF 表示脑源性神经营养因子;PSD95 表示突触后致密蛋白95;SYN1 表示突触蛋白-1;Akt 表示蛋白激酶B;PKA 表示蛋白激酶A;CREB 表示环磷腺苷效应元件结合蛋白;Caspase-3 表示半胱天冬酶3;Caspase-8 表示半胱天冬酶8;CaMKⅣ表示Ca2+/钙调蛋白依赖性蛋白激酶Ⅳ;Bax 表示促凋亡蛋白;Bcl-2 表示抗凋亡蛋白;Nrf2 表示转录因子NF-E2 相关因子2。
Fig.2 Diagram of the underlying mechanism of DEHP’s action
Note: P stands for phosphorylation; DEHP stands for di-(2-ethylhexyl) phthalate;BDNF stands for brain-derived neurotrophic factor; PSD95 stands for postsynaptic density protein 95; SYN1 stands for synapsin-1; Akt stands for protein kinase B; PKA stands for protein kinase A; CREB stands for cyclic adenosine phosphate response element binding protein; Caspase-3 stands for cysteinyl aspartate specific proteinase 3; Caspase-8 stands for cysteinyl aspartate specific proteinase 8; CaMKⅣstands for Ca2+/calmodulin-dependent protein kinase Ⅳ; Bax stands for pro-apoptotic gene; Bcl-2 stands for anti-apoptotic protein; Nrf2 stands for transcription factor NF-E2 related factor 2.
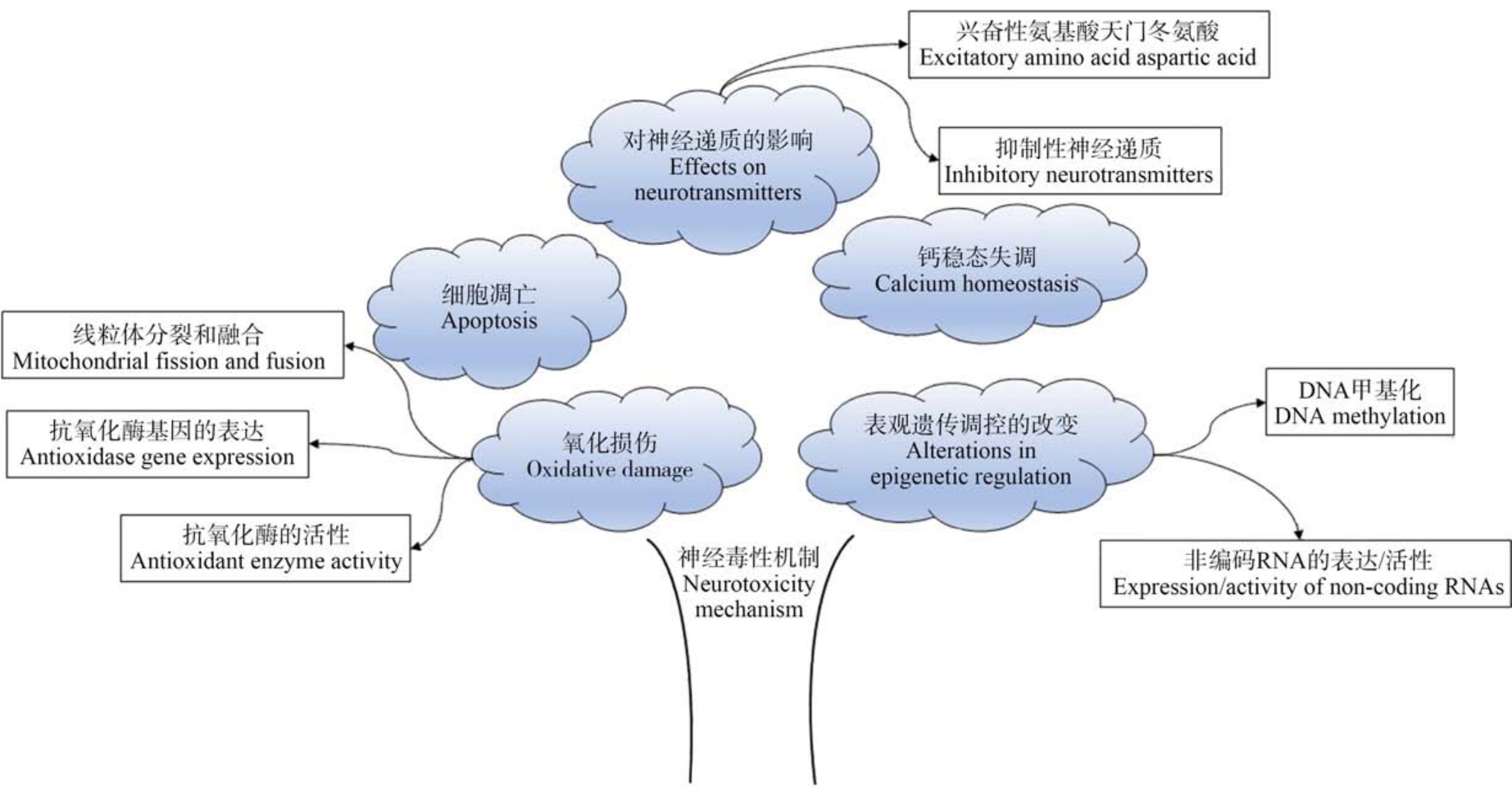
图3 DEHP 的神经毒性机制
Fig.3 The neurotoxic mechanism of DEHP
3.3 表观遗传调控的改变(Changes in epigenetic regulation)
研究表明,邻苯二甲酸酯可通过表观遗传学调控改变胎盘基因的表达,从而影响其正常活动[64]。 表观遗传调控机制主要包括DNA 甲基化、非编码RNA(包括 microRNA、lncRNA)的表达/活性失调等[65]。 在子宫内新生儿DEHP 暴露可能会导致基因启动子区域附近CpG 岛处的DNA 超甲基化/次甲基化、组蛋白修饰以及非编码RNA 的表达。 这些表观遗传标记可以诱导基因表达的变化,而这些变化可能持续一生,并可能导致不良的结局[66]。 microRNA 已证明在海马神经元的发育中起重要作用,例如miRNA 可以响应信号,调节海马神经元的形态和可塑性,还可调节神经突触的形成和成熟等[67]。 DEHP 暴露会损害雄性大鼠海马突触前和突触后元素的正常发育,但对雌性大鼠海马却没有影响。 结果表明,DEHP 暴露具有以性别特异性方式调节microRNA 的潜力,这可能会干扰雄性海马的正常发育[68]。 此外,因参与斑马鱼大脑发育过程而闻名的保守lncRNA Cyrano[69],用浓度为2 ng·L-1 DEHP 处理斑马鱼胚胎就能引起其表达发生变化,且当DEHP 达到一定浓度时就能引起神经发育相关基因的表达改变,这说明cyrano 参与了DEHP 引起的斑马鱼神经毒性效应。
3.4 钙稳态失调(Calcium homeostasis disorder)
神经元细胞内Ca2+稳态失调在许多神经毒性作用中发挥了关键作用,包括损害大脑发育和行为功能障碍[70-71]。 DEHP 可以诱导微粒体囊泡中Ca2+的释放,可能与Ryanodine 受体介导的作用机制相关[36]。 有研究发现,DEHP 暴露可导致离体培养的大鼠脑垂体神经末梢和嗜铬细胞瘤细胞内的Ca2+水平升高[72]。 离子通道研究表明 100 μmol·L-1 和300 μmol·L-1的 DEHP 可抑制钙通道的峰值电流幅度,且可通过抑制钙通道活性调节果蝇触角中投射神经元的胆碱能小突触传递[73]。 钙调蛋白(CaM)是钙调蛋白家族中分布最广泛且功能最全的蛋白质之一。 当Ca2+快速升高时,Ca2+/CaM 依赖性蛋白激酶Ⅱ(CaMKⅡ)可以被激活。 暴露于DEHP 后,青春期2 型糖尿病小鼠相较于青春期正常小鼠的CaM 和CaMKⅡ磷酸化水平显著增加,表明DEHP 可能导致Ca2+浓度升高,Ca2+与CaM 结合,进而导致下游CaMKⅡ磷酸化水平升高,这可能是DEHP 潜在的神经毒性机制之一[74]。 基于此,表明钙稳态失调在DEHP 介导的神经毒性中的潜在作用,可为进一步研究奠定基础。
3.5 对神经递质的影响(Effects on neurotransmitters)
神经递质在脑功能活动中起着重要的作用,DEHP 暴露可对脑内的递质产生影响。 5-羟色胺(5-hydroxytryptamine,5-HT),又称血清素,被认为是一种抑制性神经递质,可从位于脑干中缝核中的神经元中释放出来,并调节认知和情绪等[75]。 在中枢神经系统(central nervous system, CNS)中,γ-氨基丁酸(GABAergic, GABA)是主要的抑制性神经递质[76]。青春期雄性 ICR 小鼠经口 DEHP(0.18、1.8、18 和180 mg·kg-1·d-1)暴露 3 周,酶联免疫吸附试验和 Western blot 结果显示DEHP 显著降低了5-HT 和GABA的含量,表明DEHP 可以显著减少神经递质的释放[74]。 此外,自妊娠开始直至断奶,雌性大鼠口服暴露于剂量为30 mg·kg-1 d-1 的DEHP,其青春期雄性大鼠后代中,脑内兴奋性氨基酸天门冬氨酸的含量显著下降,而具有抑制特性的GABA 的含量则显著增加[77],此变化可能涉及生殖轴的神经内分泌调节从而影响神经系统的发育。 青春期前DEHP 暴露可导致多巴胺能系统的异常发育,从而影响青春期后大鼠的神经行为功能[78]。 慢性DEHP 产后暴露可引起年轻成年雄性大鼠睾丸轴神经内分泌调节的破坏,并且这种作用与焦虑样行为相关,由于GABA激动剂可以逆转这些作用,因此提示GABA 可能参与DEHP 诱导的生殖影响和行为改变[79]。
4 与神经退行性疾病的关系(Relationship with neurodegenerative diseases)
越来越多的研究表明环境因素可能是导致神经退行性疾病的主要原因之一[80-82]。 鉴于老年人中胰岛素抵抗与阿尔茨海默氏病(Alzheimer’s disease,AD)发病机理之间的潜在关系,有研究调查了围产期DEHP 暴露与阿尔茨海默症发病机理之间的关系。 结果表明,围产期暴露于DEHP 可能会影响海马中胰岛素的表达和胰岛素-Akt-糖原合成酶激酶3β(glycogen synthase kinase 3β, GSK-3β)信号通路[83]。 此外,在暴露于DEHP 的大鼠后代中还观察到认知能力受损和Tau 磷酸化水平升高[83],由此表明围产期暴露于DEHP 可能是与海马胰岛素抵抗和胰岛素代谢紊乱相关的阿尔茨海默症发病机制的潜在危险因素。 也有研究表明早期和长期暴露于DEHP 会增加秀丽隐杆线虫阿尔茨海默症模型的Aβ 毒性[84]。
进入肠道内的DEHP 会改变肠道微生物群落的多样性[85]、结构组成和代谢产物谱[34],随后可能改变其免疫反应等[86],最终进入血液循环干预大脑的功能。 最近的研究表明肠道菌群与神经退行性疾病有关。 目前,尽管没有直接证据表明DEHP 暴露与肠道菌群介导的神经退行性疾病相关。 但有研究表明肠道和大脑形成“微生物群-肠-脑轴”关联[87-88],肠道菌群参与肠道和大脑之间的双向通讯,表明肠道菌群的调节对神经元途径诱导有着有益作用,从而延迟阿尔茨海默症进展[89]。 因此,DEHP 暴露引起的肠道菌群改变可能通过肠-脑轴参与阿尔茨海默症的进展。
5 结语(Conclusion)
DEHP 已成为环境中普遍存在的污染物,越来越多的研究表明DEHP 暴露对人体和动物存在潜在的危害,不得不引起我们的重视。 目前,有关神经毒性机制的研究还有待完善,DEHP 与神经退行性疾病之间的关系仍缺乏相关理论与实验依据。 因此,加强DEHP 暴露的人群流行病学调查,继续深入研究DEHP 暴露的毒性效应及分子机制,以及与神经退行性疾病之间的联系和作用机制,可为疾病防治提供新的机遇,也将为今后制定更为合理的、针对不同暴露人群的安全限值和暴露限值提供理论参考。
[1]Schettler T. Human exposure to phthalates via consumer products [J].International Journal of Andrology,2006,29(1):134-139
[2]Ting K C, Gill M, Garbin O. GC/MS screening method for phthalate esters in children’ s toys [J]. Journal of AOAC International,2019,92(3):951-958
[3]Lai J P,Yang M L,Niessner R,et al.Molecularly imprinted microspheres and nanospheres for di(2-ethylhexyl)phthalate prepared by precipitation polymerization [J].Analytical and Bioanalytical Chemistry, 2007, 389(2):405-412
[4]Fierens T, Servaes K, Holderbeke M V, et al. Analysis of phthalates in food products and packaging materials sold on the Belgian market [J]. Food and Chemical Toxicology,2012,50(7):2575-2583
[5]Kavlock R, Boekelheide K, Chapin R, et al. NTP Center for the Evaluation of Risks to Human Reproduction:Phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate [J].Reproductive Toxicology,2002,16(5):529-653
[6]Doull J,Cattley R,Elcombe C,et al.A cancer risk assessment of di(2-ethylhexyl)phthalate: Application of the new US EPA risk assessment guidelines [J]. Regulatory Toxicology and Pharmacology,1999,29(3):327-357
[7]Steiner I,Scharf L,Fiala F,et al.Migration of di-(2-ethylhexyl) phthalate from PVC child articles into saliva and saliva simulant [J]. Food Additives & Contaminants,1998,15(7):812-817
[8]Serrano S E,Braun J,Trasande L,et al.Phthalates and diet: A review of the food monitoring and epidemiology data [J]. Environmental Health: A Global Access Science Source,2014,13(1):43
[9]den Braver-Sewradj S P, Piersma A, Hessel E V S. An update on the hazard of and exposure to diethyl hexyl phthalate (DEHP) alternatives used in medical devices [J].Critical Reviews in Toxicology,2020,50(8):650-672
[10]Villanger G D,Drover S S M,Nethery R C,et al.Associations between urine phthalate metabolites and thyroid function in pregnant women and the influence of iodine status [J]. Environment International,2020,137:105509
[11]Mallow E B, Fox M A. Phthalates and critically ill neonates: Device-related exposures and non-endocrine toxic risks [J]. Journal of Perinatology: Official Journal of the California Perinatal Association,2014,34(12):892-897
[12]Beg M A,Sheikh I A.Endocrine disruption:Structural interactions of androgen receptor against di(2-ethylhexyl)phthalate and its metabolites [J]. Toxics,2020,8(4):115
[13]Tassinari R, Tait S, Busani L, et al. Metabolic, reproductive and thyroid effects of bis(2-ethylhexyl) phthalate(DEHP) orally administered to male and female juvenile rats at dose levels derived from children biomonitoring study [J]. Toxicology,2021,449:152653
[14]Yu L L, Yang M, Cheng M, et al. Associations between urinary phthalate metabolite concentrations and markers of liver injury in the US adult population [J]. Environment International,2021,155:106608
[15]Ramadan M, Cooper B, Posnack N G. Bisphenols and phthalates: Plastic chemical exposures can contribute to adverse cardiovascular health outcomes [J]. Birth Defects Research,2020,112(17):1362-1385
[16]Gaynor J W,Ittenbach R F,Calafat A M,et al.Perioperative exposure to suspect neurotoxicants from medical devices in newborns with congenital heart defects [J]. The Annals of Thoracic Surgery,2019,107(2):567-572
[17]Li J F, Qian X, Zhou Y Q, et al. Trimester-specific and sex-specific effects of prenatal exposure to di(2-ethylhexyl) phthalate on fetal growth, birth size, and early-childhood growth: A longitudinal prospective cohort study [J].The Science of the Total Environment,2021,777:146146
[18]Al-Saleh I, Elkhatib R, Alrushud N, et al.Potential health risks of maternal phthalate exposure during the first trimester: The Saudi Early Autism and Environment Study(SEAES) [J].Environmental Research,2021,195:110882
[19]Watkins D J, Meeker J D, Tamayo-Ortiz M, et al. Gestational and peripubertal phthalate exposure in relation to attention performance in childhood and adolescence [J].Environmental Research,2021,196:110911
[20]Mesnil M, Defamie N, Naus C, et al. Brain disorders and chemical pollutants: A gap junction link? [J]. Biomolecules,2020,11(1):51
[21]Stroustrup A,Bragg J B,Andra S S,et al.Neonatal intensive care unit phthalate exposure and preterm infant neurobehavioral performance [J]. PLoS One, 2018, 13(3):e0193835
[22]Ahmadpour D, Mhaouty-Kodja S, Grange-Messent V.Disruption of the blood-brain barrier and its close environment following adult exposure to low doses of di(2-ethylhexyl) phthalate alone or in an environmental phthalate mixture in male mice [J]. Chemosphere, 2021,282:131013
[23]Choi G,Keil A P,Richardson D B,et al.Pregnancy exposure to organophosphate esters and the risk of attentiondeficit hyperactivity disorder in the Norwegian mother,father and child cohort study [J]. Environment International,2021,154:106549
[24]Dong J, Fu H, Fu Y Y, et al. Maternal exposure to di-(2-ethylhexyl) phthalate impairs hippocampal synaptic plasticity in male offspring: Involvement of damage to dendritic spine development [J]. ACS Chemical Neuroscience,2021,12(2):311-322
[25]Ran D Z, Luo Y, Gan Z J, et al. Neural mechanisms underlying the deficit of learning and memory by exposure to di(2-ethylhexyl) phthalate in rats [J]. Ecotoxicology and Environmental Safety,2019,174:58-65
[26]Minatoya M, Kishi R. A review of recent studies on bisphenol A and phthalate exposures and child neurodevelopment [J]. International Journal of Environmental Research and Public Health,2021,18(7):3585
[27]Nadeem A, Ahmad S F, Al-Harbi N O, et al. Ubiquitous plasticizer, di-(2-ethylhexyl) phthalate enhances existing inflammatory profile in monocytes of children with autism [J]. Toxicology,2020,446:152597
[28]Singh A R, Lawrence W H, Autianx J. Maternal-fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats [J]. Journal of Pharmaceutical Sciences,1975,64(8):1347-1350
[29]Lin H, Yuan K M, Li L Y, et al. In utero exposure to diethylhexyl phthalate affects rat brain development: A behavioral and genomic approach [J]. International Journal of Environmental Research and Public Health, 2015, 12(11):13696-13710
[30]Jankowska A,Polańska K,Koch H M,et al.Phthalate exposure and neurodevelopmental outcomes in early school age children from Poland [J]. Environmental Research,2019,179(Pt B):108829
[31]Qian X, Li J F, Xu S Q, et al. Prenatal exposure to phthalates and neurocognitive development in children at two years of age [J]. Environment International, 2019,131:105023
[32]Chakraborty R, Vijay Kumar M J, Clement J P. Critical aspects of neurodevelopment [J]. Neurobiology of Learning and Memory,2021,180:107415
[33]Liu S H, Bobb J F, Lee K H, et al. Lagged kernel machine regression for identifying time windows of susceptibility to exposures of complex mixtures [J]. Biostatistics,2018,19(3):325-341
[34]Lei M,Menon R,Manteiga S,et al.Environmental chemical diethylhexyl phthalate alters intestinal microbiota community structure and metabolite profile in mice [J].mSystems,2019,4(6): e00724-e00719
[35]Martínez-Razo L D, Martínez-Ibarra A, Vázquez-Martínez E R, et al. The impact of di-(2-ethylhexyl)phthalate and mono(2-ethylhexyl) phthalate in placental development, function, and pathophysiology [J]. Environment International,2021,146:106228
[36]Shen R, Zhao L L,Yu Z,et al.Maternal di-(2-ethylhexyl)phthalate exposure during pregnancy causes fetal growth restriction in a stage-specific but gender-independent manner [J]. Reproductive Toxicology,2017,67:117-124
[37]Luo B B, Feng Q W, Wu D J, et al. Experimental study on DEHP affect the neurodevelopment through interfering with placental thyroid hormones transport [J]. Chinese Journal of Industrial Hygiene and Occupational Diseases,2018,36(3):179-183
[38]Yu Z, Han Y, Shen R, et al. Gestational di-(2-ethylhexyl)phthalate exposure causes fetal intrauterine growth restriction through disturbing placental thyroid hormone receptor signaling [J]. Toxicology Letters,2018,294:1-10
[39]Dhanya C R, Indu A R, Deepadevi K V, et al. Inhibition of membrane Na(+)-K+ Atpase of the brain, liver and RBC in rats administered di(2-ethyl hexyl)phthalate(DEHP) a plasticizer used in polyvinyl chloride (PVC) blood storage bags [J]. Indian Journal of Experimental Biology,2003,41(8):814-820
[40]Tanida T, Warita K, Ishihara K, et al. Fetal and neonatal exposure to three typical environmental chemicals with different mechanisms of action: Mixed exposure to phenol, phthalate,and dioxin cancels the effects of sole exposure on mouse midbrain dopaminergic nuclei [J].Toxicology Letters,2009,189(1):40-47
[41]Komada M,Gendai Y,Kagawa N,et al.Prenatal exposure to di(2-ethylhexyl) phthalate impairs development of the mouse neocortex [J]. Toxicology Letters, 2016, 259: 69-79
[42]Smith C A,MacDonald A,Holahan M R.Acute postnatal exposure to di(2-ethylhexyl) phthalate adversely impacts hippocampal development in the male rat [J]. Neuroscience,2011,193:100-108
[43]You M D,Dong J,Fu Y Y,et al.Exposure to di-(2-ethylhexyl) phthalate during perinatal period gender-specifically impairs the dendritic growth of pyramidal neurons in rat offspring [J].Frontiers in Neuroscience,2018,12:444
[44]Aung K H,Win-Shwe T T,Kanaya M,et al.Involvement of hemeoxygenase-1 in di(2-ethylhexyl) phthalate (DEHP)-induced apoptosis of neuro-2a cells [J]. The Journal of Toxicological Sciences,2014,39(2):217-229
[45]Wójtowicz A K, Sitarz-Głownia A M, Szczęsna M, et al.The action of di-(2-ethylhexyl) phthalate (DEHP) in mouse cerebral cells involves an impairment in aryl hydrocarbon receptor (AhR) signaling [J].Neurotoxicity Research,2019,35(1):183-195
[46]Wu Y, Li K, Zuo H X, et al. Primary neuronal-astrocytic co-culture platform for neurotoxicity assessment of di-(2-ethylhexyl) phthalate [J]. Journal of Environmental Sciences,2014,26(5):1145-1153
[47]Li N, Papandonatos G D, Calafat A M, et al. Identifying periods of susceptibility to the impact of phthalates on children’s cognitive abilities [J].Environmental Research,2019,172:604-614
[48]Zhang Q, Chen X Z, Huang X, et al. The association be-tween prenatal exposure to phthalates and cognition and neurobehavior of children-evidence from birth cohorts [J].Neurotoxicology,2019,73:199-212
[49]Huang H B, Kuo P H, Su P H, et al. Prenatal and childhood exposure to phthalate diesters and neurobehavioral development in a 15-year follow-up birth cohort study[J]. Environmental Research,2019,172:569-577
[50]Xu Y, Agrawal S, Cook T J, et al. Di-(2-ethylhexyl)-phthalate affects lipid profiling in fetal rat brain upon maternal exposure [J]. Archives of Toxicology, 2007, 81(1):57-62
[51]Howdeshell K L, Wilson V S, Furr J, et al. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague-Dawley rat in a cumulative,dose-additive manner [J]. Toxicological Sciences: An Official Journal of the Society of Toxicology,2008,105(1):153-165
[52]García-Argüello S F, Lopez-Lorenzo B, Cornelissen B, et al. Development of[18F]ICMT-11 for imaging caspase-3/7 activity during therapy-induced apoptosis [J]. Cancers,2020,12(8): E2191
[53]Tu W, Li W F, Zhu X G, et al. Di-2-ethylhexyl phthalate(DEHP) induces apoptosis of mouse HT22 hippocampal neuronal cells via oxidative stress [J]. Toxicology and Industrial Health,2020,36(11):844-851
[54]Guida N,Laudati G,Galgani M,et al.Histone deacetylase 4 promotes ubiquitin-dependent proteasomal degradation of Sp3 in SH-SY5Y cells treated with di(2-ethylhexyl)phthalate (DEHP), determining neuronal death [J]. Toxicology and Applied Pharmacology,2014,280(1):190-198
[55]Amara I, Scuto M, Zappalà A, et al. Hericium erinaceus prevents DEHP-induced mitochondrial dysfunction and apoptosis in PC12 cells [J]. International Journal of Molecular Sciences,2020,21(6): E2138
[56]Qiu F, Zhou Y B, Deng Y K, et al. Knockdown of TNFAIP1 prevents di-(2-ethylhexyl)phthalate-induced neurotoxicity by activating CREB pathway [J]. Chemosphere,2020,241:125114
[57]胡存丽, 仲来福. 邻苯二甲酸(2-乙基已基)酯遗传毒性研究进展[J]. 大连医科大学学报,2007,29(2):185-190
Hu C L, Zhong L F.Genotoxic effects of di(2-ethylbexyl)phthalate in humans and animails [J]. Journal of Dalian Medical University,2007,29(2):185-190 (in Chinese)
[58]柯翔鸿. 邻苯二甲酸(2-乙基己基)酯对小鼠的氧化损伤和生殖毒性分子机制的研究[D]. 武汉: 华中师范大学,2008:11
Ke X H. Study on the oxidative damage and reproductive toxicity molecular mechanism of di-(2-ethylhexyl)phthalate in mice [D]. Wuhan: Central China Normal U-niversity,2008:11 (in Chinese)
[59]Andries A, Rozenski J, Vermeersch P, et al. Recent progress in the LC-MS/MS analysis of oxidative stress biomarkers [J]. Electrophoresis,2021,42(4):402-428
[60]Rhee S G. Overview on peroxiredoxin [J]. Molecules and Cells,2016,39(1):1-5
[61]Lee D G, Kim K M, Lee H S, et al.Peroxiredoxin 5 prevents diethylhexyl phthalate-induced neuronal cell death by inhibiting mitochondrial fission in mouse hippocampal HT-22 cells [J]. Neurotoxicology,2019,74:242-251
[62]Lu C J, Luo J J,Liu Y,et al.The oxidative stress responses caused by phthalate acid esters increases mRNA abundance of base excision repair(BER)genes in vivo and in vitro [J]. Ecotoxicology and Environmental Safety,2021,208:111525
[63]Luo Y,Li X N,Zhao Y,et al.DEHP triggers cerebral mitochondrial dysfunction and oxidative stress in quail(Coturnix japonica) via modulating mitochondrial dynamics and biogenesis and activating Nrf2-mediated defense response [J]. Chemosphere,2019,224:626-633
[64]Grindler N M, Vanderlinden L, Karthikraj R, et al. Exposure to phthalate, an endocrine disrupting chemical, alters the first trimester placental methylome and transcriptome in women [J]. Scientific Reports,2018,8(1):6086
[65]Saul D,Kosinsky R L.Epigenetics of aging and aging-associated diseases [J]. International Journal of Molecular Sciences,2021,22(1):401
[66]Singh S, Li S S L. Epigenetic effects of environmental chemicals bisphenol A and phthalates [J]. International Journal of Molecular Sciences,2012,13(8):10143-10153
[67]Stappert L, Klaus F, Brüstle O. microRNAs engage in complex circuits regulating adult neurogenesis [J]. Frontiers in Neuroscience,2018,12:707
[68]Luu B E,Green S R,Childers C L,et al.The roles of hippocampal microRNAs in response to acute postnatal exposure to di(2-ethylhexyl) phthalate in female and male rats [J]. Neurotoxicology,2017,59:98-104
[69]Sarangdhar M A,Chaubey D,Srikakulam N,et al.Parentally inherited long non-coding RNA Cyrano is involved in zebrafish neurodevelopment [J]. Nucleic Acids Research,2018,46(18):9726-9735
[70]Latoszek E, Czeredys M. Molecular components of storeoperated calcium channels in the regulation of neural stem cell physiology, neurogenesis, and the pathology of Huntington’s disease[J].Frontiers in Cell and Developmental Biology,2021,9:657337
[71]Kelemen K, Szilágyi T. New approach for untangling the role of uncommon calcium-binding proteins in the central nervous system [J]. Brain Sciences,2021,11(5):634
[72]Tully K, Kupfer D, Dopico A M, et al. A plasticizer released from Ⅳdrip Chambers elevates calcium levels in neurosecretory terminals [J]. Toxicology and Applied Pharmacology,2000,168(3):183-188
[73]Ran D Z, Cai S, Wu H L, et al. Di (2-ethylhexyl)phthalate modulates cholinergic mini-presynaptic transmission of projection neurons in Drosophila antennal lobe[J]. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association,2012,50(9):3291-3297
[74]Feng W W, Liu Y C, Ding Y Y, et al. Typical neurobehavioral methods and transcriptome analysis reveal the neurotoxicity and mechanisms of di (2-ethylhexyl)phthalate on pubertal male ICR mice with type 2 diabetes mellitus [J]. Archives of Toxicology, 2020, 94(4): 1279-1302
[75]Mattson M P,Maudsley S,Martin B.BDNF and 5-HT: A dynamic Duo in age-related neuronal plasticity and neurodegenerative disorders [J]. Trends in Neurosciences,2004,27(10):589-594
[76]Inoue T, Takamatsu Y, Okamura M, et al. Specific inhibition of α5 subunit-containing GABAA receptors enhances locomotor activity and neuronal activity in the motor cortex [J]. Biomedical Research,2021,42(3):103-108
[77]Carbone S,Szwarcfarb B,Ponzo O,et al.Impact of gestational and lactational phthalate exposure on hypothalamic content of amino acid neurotransmitters and FSH secretion in peripubertal male rats [J]. Neurotoxicology, 2010,31(6):747-751
[78]Holahan M R, Smith C A, Luu B E, et al. Preadolescent phthalate (DEHP) exposure is associated with elevated locomotor activity and reward-related behavior and a reduced number of tyrosine hydroxylase positive neurons in post-adolescent male and female rats [J]. Toxicological Sciences: An Official Journal of the Society of Toxicology,2018,165(2):512-530
[79]Carbone S, Ponzo O J, Gobetto N, et al. Effect of di(2-ethylhexyl) phthalate on the neuroendocrine regulation of reproduction in adult male rats and its relationship to anxiogenic behavior: Participation of GABAergic system [J].Human & Experimental Toxicology,2019,38(1):25-35
[80]Li Y N, Fang R Y, Liu Z H, et al. The association between toxic pesticide environmental exposure and Alzheimer’s disease: A scientometric and visualization analysis[J]. Chemosphere,2021,263:128238
[81]Rahman M A, Rahman M S, Uddin M J, et al. Emerging risk of environmental factors: Insight mechanisms of Alzheimer’ s diseases [J]. Environmental Science and Pollution Research International, 2020, 27(36): 44659-44672
[82]Mir R H, Sawhney G, Pottoo F H, et al.Role of environmental pollutants in Alzheimer’s disease: A review [J].Environmental Science and Pollution Research International,2020,27(36):44724-44742
[83]Sun W, Ban J B,Zhang N,et al.Perinatal exposure to di-(2-ethylhexyl)-phthalate leads to cognitive dysfunction and phospho-tau level increase in aged rats [J]. Environmental Toxicology,2014,29(5):596-603
[84]Yen P L, How C M, Hsiu-Chuan Liao V. Early-life and chronic exposure to di(2-ethylhexyl) phthalate enhances amyloid-β toxicity associated with an autophagy-related gene in Caenorhabditis elegans Alzheimer’ s disease models [J]. Chemosphere,2021,273:128594
[85]Yu Z, Shi Z H,Zheng Z Y,et al.DEHP induce cholesterol imbalance via disturbing bile acid metabolism by altering the composition of gut microbiota in rats [J].Chemosphere,2021,263:127959
[86]Yang Y N,Yang Y S H,Lin I H,et al.Phthalate exposure alters gut microbiota composition and IgM vaccine response in human newborns [J].Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 2019, 132:110700
[87]Javed I, Cui X J,Wang X Y,et al.Implications of the human gut-brain and gut-cancer axes for future nanomedicine [J]. ACS Nano,2020,14(11):14391-14416
[88]Needham B D, Kaddurah-Daouk R, Mazmanian S K.Gut microbial molecules in behavioural and neurodegenerative conditions [J]. Nature Reviews Neuroscience, 2020, 21(12):717-731
[89]Goyal D, Ali S A, Singh R K. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease [J].Progress in Neuro-Psychopharmacology and Biological Psychiatry,2021,106:110112