塑料及其制品的生产和使用给人类带来了极大的便利,但大量的废弃塑料难以回收利用而进入到大气、水体和土壤环境中,经过长期的物理、化学和生物降解作用会形成微小的塑料颗粒,当其粒径<5 mm时称为微塑料[1]。近期的研究发现,微塑料可以进一步破裂为粒径为纳米级别的塑料[2];纳米塑料还被应用于日化产品、药物输送、电子设备和医学诊断中,使其直接进入到环境中[3]。由于纳米塑料的尺寸极小,很容易被生物吞食或摄入。纳米塑料具有较大的比表面积导致其可以吸附其他污染物,呈现出对生物的复合效应,通过转运、富集等过程进行食物链传递,对生态环境和人类健康造成严重威胁[4-6]。
目前,关于纳米塑料对环境中生物的毒性效应研究已逐步开展起来,研究者利用环境生物学技术并将转录组学、代谢组学、蛋白质组学与基因编辑技术等结合起来,从细胞和分子层面上探讨纳米塑料对水生生物、陆生植物、无脊椎动物和微生物的毒性效应,但目前的相关研究报道只侧重在某一方面,并未进行系统的梳理和总结。因此,本文在归纳生物体对纳米塑料的摄取、富集和转运规律的基础上,论述了纳米塑料本身及其吸附的其他污染物对环境中生物的毒性效应(氧化应激、炎症、代谢紊乱和基因毒性等)及机制,并评估了纳米塑料对人类健康的潜在威胁,对未来需加强的研究方向进行了展望,为后期评估纳米塑料的生态毒性效应提供理论基础和科学依据。
1 生物体对纳米塑料的摄取、富集和转运(Uptake, enrichment and transport of nanoplastics by organisms)
纳米塑料的粒径非常小,容易被环境中的生物吞食和摄取而在体内富集,甚至还可以穿过肠道屏障进入到体液循环系统,在器官中积累或随粪便排出[3,7]。相关研究表明,水生生物可以吸附或摄取纳米塑料的粒径范围为30~1 000 nm[8]。Jeong等[9]研究发现,粒径为50 nm的聚苯乙烯可以穿过水蚤消化器官的细胞膜,并很快分散到全身;然而粒径为500 nm~6 μm的聚苯乙烯大部分被限制在消化器官内。不同粒径的纳米塑料在斑马鱼体内的积累也有差别,Lee等[10]通过扫描电镜观察发现,50 nm的聚苯乙烯作用下,绒毛膜表面粗糙,且孔道明显打开,这说明纳米塑料进入到斑马鱼体内并在组织和细胞中富集,例如神经系统、肌肉纤维和富含脂肪的区域等;200 nm和500 nm的聚苯乙烯主要在绒毛膜的表面聚集,并堵塞孔道。纳米塑料(100 nm)能够被蚕豆根尖吸收,并在细胞间隙中积累,可能会阻碍细胞壁孔对营养物质的运输[11]。纳米塑料会先聚集在黄瓜的根部系统,然后通过茎传输进入到叶子、花和果实中[12]。随着暴露浓度和时间的增加,纳米聚苯乙烯可以在粗梗水蕨孢子表面大量地吸附和积累,并抑制孢子的萌发和配子体的发育[13]。纳米塑料还可以吸附和聚集在小麦[14]、洋葱[15]的组织中。
此外,纳米塑料能够沿着食物链转移到更高营养级的生物,不断累积和富集。在水生生态系统中,浮游动物可以将纳米塑料携带至鱼类体内,Skjolding等[16]用暴露于纳米塑料中的卤虫喂食斑马鱼,在斑马鱼的胃肠道、头部和鳃部均发现了纳米塑料的积累。纳米塑料还可由浮游植物莱茵衣藻(生产者)传递给浮游动物大型蚤(初级消费者),再到小型鱼中国青鳉(次级消费者),最后进入到谈氏鳜鱼(第三级消费者)的体内[17]。目前还缺乏关于纳米塑料在陆地食物链中转移的报道,虽然科学家已在人类的粪便中检测到塑料颗粒的存在,但是纳米塑料在人类食物链中传递的研究还是空白。
2 纳米塑料对生物的毒性效应及作用机制(Toxic effects and mechanism of nanoplastics on organisms)
纳米塑料被环境中的生物吸附或吞食后,会对生物体产生一定的毒害作用[18],主要包括在生长和繁殖能力、死亡率、摄食率、细胞(氧化应激、炎症、线粒体和溶酶体功能障碍等)及分子水平(基因、蛋白质和代谢产物表达)等方面的效应等[19-29](图1)。由于具有巨大的比表面积和强疏水性,纳米塑料吸附环境中的其他污染物后,将改变污染物的生物可利用性,并使其毒性增加或削减[30-32]。目前该方面的研究主要集中在淡水/海洋生物和植物等,陆地生态系统中纳米塑料污染的研究也逐渐开展起来,但纳米塑料对动物及微生物的作用研究还较少。
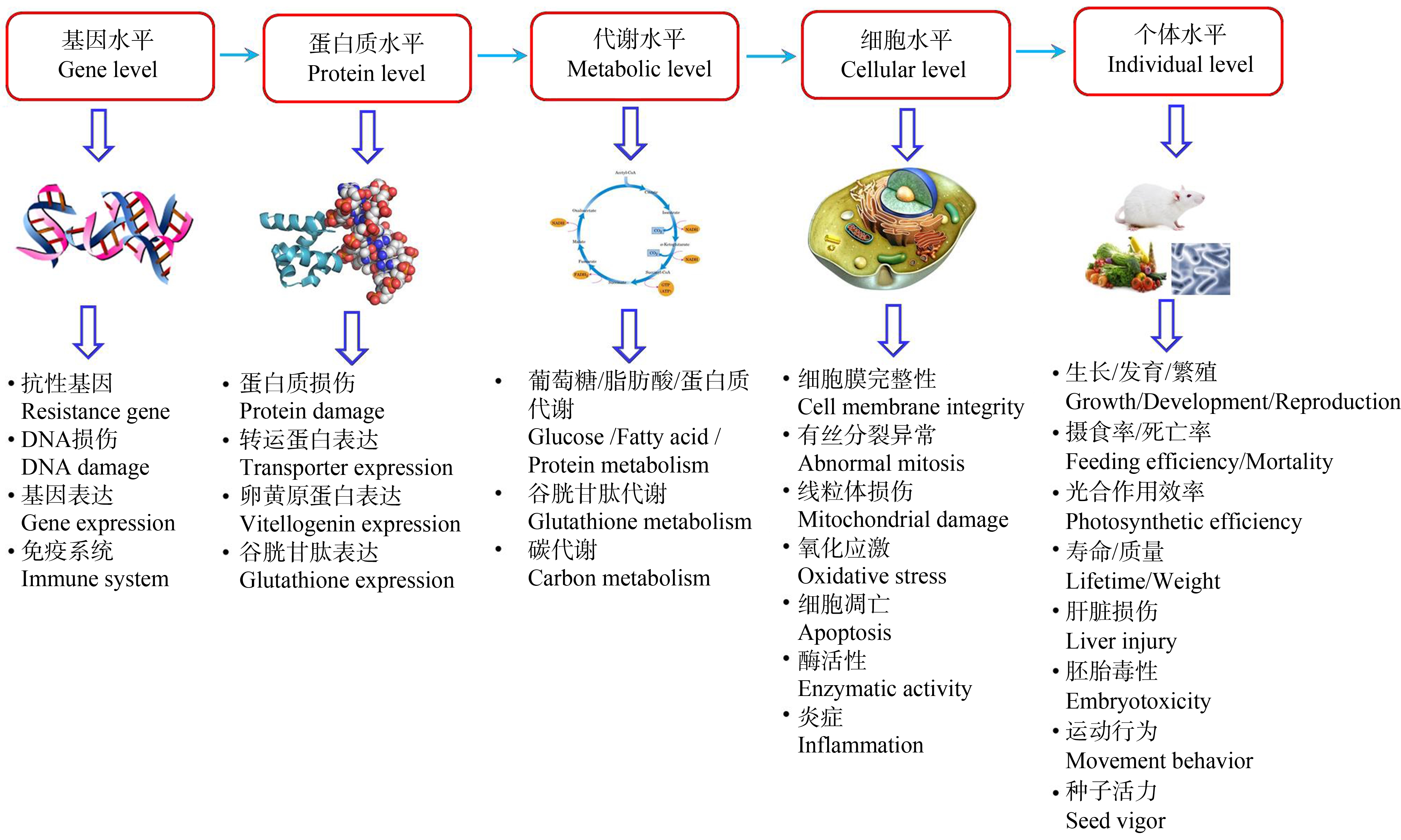
图1 纳米塑料及其吸附的污染物对环境中生物的毒性效应
Fig. 1 Toxic effects of nanoplastics and its adsorbed pollutants
2.1 水生生物
Peiponen等[33]的研究提供了大量的证据说明纳米塑料存在于水环境中。关于纳米塑料对水生生物的影响及机制的研究已引起广泛关注。纳米聚苯乙烯吸附在藻类表面通过阻碍光照强度、降低空气流动和叶绿素浓度来抑制光合作用,导致藻类发育障碍[19]。Ribeiro等[20]研究发现,纳米塑料可以进入到黑鲫的脑中,引起脑组织损伤和行为失常。纳米级聚苯乙烯和聚碳酸酯可以破坏肥头鲦鱼的抗氧化系统,引起氧化应激,并影响先天免疫系统[21];低浓度的纳米塑料能引起鱼脑中细胞活性显著降低,增加其氧化还原压力[22]。纳米聚苯乙烯可以在河蚬的外套膜、腮和内脏中蓄积,诱发肝脏损伤、神经毒性和肠道炎症等[23]。将牡蛎暴露于塑料颗粒中(50 nm),其受精成功率明显降低,部分幼虫的发育完全停止[34]。将20 nm的聚苯乙烯注射到斑马鱼胚胎后可以到达胚胎大脑,引起DNA氧化损伤[35];长时间暴露于纳米聚苯乙烯的大型蚤的寿命明显缩短[36]。Liu等[24,37]研究了添加和不添加纳米塑料的情况下淡水枝角水蚤蛋白质的表达差异,发现纳米塑料的加入使卵黄原蛋白和谷胱甘肽的表达发生上调,并引起氧化应激、信号转导和脂肪酸代谢、角质层和几丁质代谢途径的变化,从而抑制了淡水枝角水蚤的繁殖和生长;添加纳米塑料后增强了淡水枝角水蚤的糖代谢途径,促进了能量的产生,这可能是新生的水蚤抵抗纳米塑料毒性的方式之一。经纳米聚苯乙烯胁迫24 h后,三角褐指藻的光合作用系统受到损伤,并导致线粒体膜去极化和基因毒性[25],但Bergami等[26]研究发现,聚苯乙烯对杜氏藻的光合作用效率没有影响。
纳米塑料的生态毒性效应与其添加浓度/时间、粒径大小和表面电荷等有关[38]。(1)添加浓度与胁迫时间。随着纳米塑料浓度的增加,斜生栅藻中叶绿素a含量的降低速率加快[39]。低浓度的纳米聚苯乙烯(5 mg·L-1)增强了幼年日本沼虾的存活能力,高浓度时(10、20和40 mg·L-1)产生了抑制或毒性作用,这主要是通过调控与抗氧化作用和免疫防御相关的基因表达来完成[27]。随着添加浓度和暴露时间的增加,纳米塑料会对日本沼虾的葡萄糖代谢途径产生不利影响,因此抑制了糖酵解途径,与脂质代谢相关的基因表达发生下调,降低了相关酶的活性(例如己糖激酶、脂肪酶和乙酰辅酶A羧化酶等),导致日本沼虾幼体消化、转运和合成脂类的能力减弱,因此使其体内的脂肪含量降低[28]。(2)粒径。Singh等[40]研究发现,粒径为55 nm的聚苯乙烯对斑马鱼的基因毒性要>100 nm的,可能是因为粒径较大的纳米塑料被禁锢在消化道,粒径较小的可以透过器官而引起更高的毒性。轮虫在纳米聚苯乙烯的胁迫下呈现出粒径依赖方式的毒性作用,例如,MAPK信号通路和抗氧化酶被显著激活,生长速度和繁殖力降低,寿命缩短等[41]。(3)表面电荷。阳离子(—NH2)基团修饰的纳米塑料的毒性作用要大于阴离子(—COOH)的,两者对海胆胚胎和绿藻均导致严重的发育缺陷、生长抑制和致死效应[26,29]。阳离子修饰的纳米塑料对地中海贻贝具有显著的胚胎毒性,导致参与到早期壳形成的基因表达异常[42]。带正电荷(—NH2)的聚苯乙烯对大型蚤的毒性较大,可能是因为与大型蚤带负电荷的细胞膜发生了强烈的相互作用[43]。氨基改性的纳米聚苯乙烯(PS-NH2)抑制了微囊藻的光合效率,降低了有机物质合成,增强了氧化应激,通过提高转运蛋白的表达上调和破坏细胞膜完整性来促进微囊藻毒素向细胞外释放[38]。冯立娟[44]利用基因编辑技术和非靶向代谢组学方法研究纳米塑料的生物效应,发现PS-NH2通过破坏细胞膜的完整性和引起谷胱甘肽代谢途径的紊乱来对细长聚球藻产生毒性;带正电荷(—NH2)的聚苯乙烯显著刺激了铜绿微囊藻中微囊藻毒素和微囊藻毒素-亮氨酸-精氨酸的释放;带负电荷(—SO3H)的聚苯乙烯因为静电排斥作用,不容易与细胞膜接触,因此在短期内对微囊藻毒素的释放可以忽略不计。
由于纳米塑料巨大的比表面积和强疏水性,可以作为污染物的吸附载体而形成复合污染。环境中的生物暴露于复合污染物的毒性效应十分复杂。纳米聚苯乙烯促进了多环芳烃从暴露介质中吸附至纳米塑料上,进而降低了多环芳烃的生物利用性和生物积累[30]。纳米塑料的存在不仅改变了有机污染物诱发的生物体基因表达情况,也会引起代谢途径的变化。例如,纳米级聚苯乙烯的加入降低了卡马西平对紫贻贝的基因毒性,引起编码生物转化(cyp32)、解毒过程(gst)、DNA损伤(p53)、组织修复(hsp70)和免疫系统(lys)的基因表达下调[31]。Singh等[40]研究发现,纳米聚苯乙烯-荧蒽复合污染引起的DNA损伤程度要小于单一聚苯乙烯或荧蒽,这说明纳米塑料降低了荧蒽对斑马鱼的基因毒性,可能是因为纳米塑料吸附多环芳烃后降低了其生物可利用性。相反的,摄入纳米塑料会抑制轮虫的多异源抗药性,从而导致持久性有机污染物的毒性增强[32]。纳米塑料明显加剧了重金属对斑马鱼的毒性,增加了胚胎死亡率和畸形,降低了孵化率[10]。纳米聚苯乙烯的存在增强了磷酸三苯酯对斑马鱼的毒性,不仅引起斑马鱼的肝脏和生殖腺体积增大,还降低了受精率和卵孵化率,导致斑马鱼的繁殖性能下降[45]。在土壤中,草甘膦农药吸附在纳米塑料表面,抑制了铜绿微囊藻的生长[6]。
2.2 陆生植物
植物处于食物网的底端,是人类重要的食物来源。因此,纳米塑料对植物的毒性效应近年来受到广泛关注,例如洋葱、大葱、小麦、绿豆、蚕豆、大豆、生菜、莴苣和玉米等[46]。植物可以吸附或内化塑料颗粒,与纳米塑料的自身特性(粒径、浓度)、植物响应和环境介质等有关。研究发现吸附在植物表面的塑料主要有聚乙烯、聚丙烯和聚苯乙烯[46]。20 nm和100 nm的聚苯乙烯主要通过吸附于大豆种皮表面降低种子吸收水分的速度,从而抑制种子的活力和发芽率,20 nm的对根尖数量无显著影响,而100 nm的则表现出促进作用[47]。Giorgetti等[15]的报道指出,50 nm的聚苯乙烯能够内化于洋葱的根分生区细胞,抑制了根的延伸生长,引起氧化胁迫,产生细胞毒性(例如有丝分裂异常和基因毒性)。纳米塑料可以通过水通道蛋白进入到水稻的根部,从而抑制初生根的长度和质量,根的形态也会发生明显改变,与碳代谢相关的基因表达发生下调[48]。粒径为300 nm的聚苯乙烯增加了黄瓜根中丙二醛、脯氨酸和可溶性蛋白的含量及根活力,降低了钙、镁和铁的含量[11]。纳米聚苯乙烯可以进入种子内部,通过增强α-淀粉酶的活性而使淀粉颗粒的水解加速,进而产生更多的可溶性糖和能量用于小麦幼苗的生长[49]。聚苯乙烯颗粒对菜心幼苗的毒性作用呈现出明显的粒径效应,幼苗可以通过调节可溶性糖和可溶性蛋白的含量来保持细胞的渗透势,以应对聚苯乙烯的胁迫[50]。然而,目前还没有关于整株植物对纳米塑料的摄取、不同部位的转运和积累的报道,纳米塑料通过食物链传递及对食品安全造成威胁的研究也较为少见。
2.3 无脊椎动物及微生物
蚯蚓作为土壤食物链中的主要动物之一,在改善土壤肥力、维持土壤生态系统结构与功能等方面起到十分重要的作用[51]。纳米聚苯乙烯改变了土壤线蚓的肠道微生物组成,进而改变其摄食行为,影响线蚓的健康和生态功能(例如氮循环和污染物转化等)[52]。纳米塑料的添加增强了四环素在土壤线蚓组织中的积累,引起肠道中抗性基因丰度和多样性的增加[53]。此外,还有一些关于纳米塑料对模式生物毒性效应的研究,例如,Lei等[54]将线虫暴露于粒径为100 nm和500 nm的塑料颗粒后,线虫的身体弯曲、头部跳动频率和爬行速度加快,这说明纳米塑料可以改变线虫的运动行为。纳米聚苯乙烯通过线粒体损伤和降低神经元中多巴胺的含量引起秀丽隐杆线虫的神经毒性[55]。添加浓度为0.1 mg·L-1和1 mg·L-1的纳米聚苯乙烯后,抑制了秀丽隐杆线虫机体的免疫应答反应和线粒体未折叠蛋白反应,缩短了线虫的寿命[56]。聚苯乙烯诱导秀丽隐杆线虫中编码酪氨酸脱羧酶基因(tdc-1)的表达上调和编码谷氨酸转运体基因(eat-4)的表达下调,引起神经元毒性[57]。土壤中的跳虫通过创建生物孔隙来改良土壤系统,添加纳米聚苯乙烯后可以在几秒内进入到生物孔隙中,从而限制了跳虫的运动行为[58]。纳米聚苯乙烯可以进入到蜻蜓幼体细胞空腔内,引发不同组织的氧化应激反应,并对胆碱能神经系统产生不利影响,可能会导致神经功能和神经肌肉功能障碍[59]。
纳米塑料对土壤微生物群落具有显著的毒害作用,主要表现为菌群功能的改变和多样性的减少,酶活性的降低等[4]。细菌的个体非常小,只有粒径足够小的纳米塑料才可以进入到其细胞内[60]。50 nm的聚苯乙烯可以引起嗜碱盐单胞菌细胞膜的损伤,诱发显著的氧化还原压力并促进胞外聚合物的分泌[61]。虽然没有直接的证据说明纳米塑料可以嵌入到细胞膜而被菌体吸收,Rossi等[62]通过分子模拟研究发现,纳米塑料可以穿过脂质双分子层,改变细胞的功能,因此可以推测纳米塑料与细菌细胞膜之间的作用是其主要的毒性机制。纳米塑料可以通过与奥奈达希瓦氏菌细胞膜和胞外分泌物直接接触,改变其核黄素分泌[63]。Saygin和Baysal[64]的研究指出,由于细胞膜结构的不同,革兰氏阳性菌和阴性菌受纳米塑料影响的程度存在差异;Ustabasi和Baysal[65]探索了从牙膏中分离出的聚乙烯塑料对细菌的毒性作用,发现聚乙烯对革兰氏阳性菌(枯草芽孢杆菌)的损害程度要大于革兰氏阴性菌(铜绿假单胞菌)。带负电荷的纳米塑料在高浓度(200 mg·L-1)时,可以破坏细胞表面聚合物,降低水解酶活性和表面电荷,并且增加了活性氧的产生[66]。因此可以推测塑料颗粒的表面电荷、细菌的蛋白质代谢和脂质过氧化酶活性的变化是影响塑料与菌体之间相互作用的重要因素。
3 纳米塑料对人类健康的潜在影响(Potential effects of nanoplastics on human health)
农作物、鱼虾贝类等会对环境中的纳米颗粒进行吸收和转运,导致其在体内积累,并可通过食物链的营养转移造成农畜产品的纳米塑料污染,食品安全可能会受到影响,进而引发人类健康风险[67](图2)。但是食品中纳米塑料含量及随后的饮食暴露量的研究数据还十分欠缺,还无法评估存在于人类食物链中的纳米塑料对人体健康的潜在影响[44]。纳米塑料对人类健康可能存在的危害和风险已引起关注,纳米塑料进入人体的方式主要有经口摄取、吸入及皮肤接触,然后可能会沿着细胞间隙进行传输,或者通过肠黏膜细胞转运到毛细淋巴管,进入到淋巴组织,经血管到达多个器官[68-69]。纺织工人长期接触合成纤维,会出现呼吸系统疾病,症状主要包括咳嗽、呼吸障碍和肺活量减少等。可以推测,纳米塑料跟合成纤维相似,也可以通过空气或者食物携带进入到肺或胃肠系统[70]。
目前,尚无关于纳米塑料对人体的直接毒性效应的相关报道,主要通过体外实验探索纳米塑料对人体健康的间接影响,这些研究的对象局限在人类源细胞系和模型,产生的毒理效应主要包括诱发氧化应激、炎症、代谢紊乱和细胞毒性等[71]。纳米塑料对人类细胞的毒性与所选的细胞系有关,其对人单核巨噬细胞没有损害作用,但能引起与免疫相关的细胞系中活性氧产生和DNA损伤[71]。Gopinath等[72]进行体外人血细胞实验发现,纳米塑料进入血管后穿过肠绒毛,可以形成比原始纳米塑料具有更高的基因毒性和细胞毒性的蛋白质-塑料复合体,这可能是因为复合体可以逃离人体防御系统,在循环系统中存在时间较长。Domenech等[73]利用荧光探针技术研究发现,纳米聚苯乙烯可以穿过消化系统的上皮膜屏障,但对人结直肠癌和淋巴癌细胞膜渗透性和完整性并未产生影响。500 nm和60 nm的聚苯乙烯引起人胃癌(AGS)细胞的DNA断裂,使细胞活力和线粒体膜电位降低,从而诱发细胞凋亡[74]。人结直肠腺癌细胞暴露在500 nm的纳米塑料后,发生了线粒体去极化,可能是因为粒径稍微大一点的塑料进入细胞后可以逃离溶酶体,并对其他细胞器和蛋白质造成损害[75-76]。Xu等[77]的研究证明,粒径为25 nm的聚苯乙烯比70 nm的更容易内化进入肺癌人类肺泡基底上皮细胞质中,诱导与炎症相关的基因转录,引起细胞周期S期阻滞,触发了肿瘤坏死因子相关的凋亡途径,从而影响细胞活力。低浓度的纳米聚苯乙烯会引起支气管上皮细胞中与自噬和内质网应激相关的代谢发生变化,例如氨基酸和三羧酸循环过程中代谢产物的增加[78]。Hesler等[79]采用体外共培养模型进行研究发现,纳米塑料没有引起肠道和胎盘转运障碍,具有微弱的毒性作用。50 nm的塑料可以进入到肠基底外侧细胞和淋巴癌细胞的细胞核,因而引发基因毒性[73]。根据上述生物及人体细胞系对纳米塑料污染所做出的反应,可以推测人类为了应对纳米塑料的胁迫可能会出现基因组和行为的变化[71]。
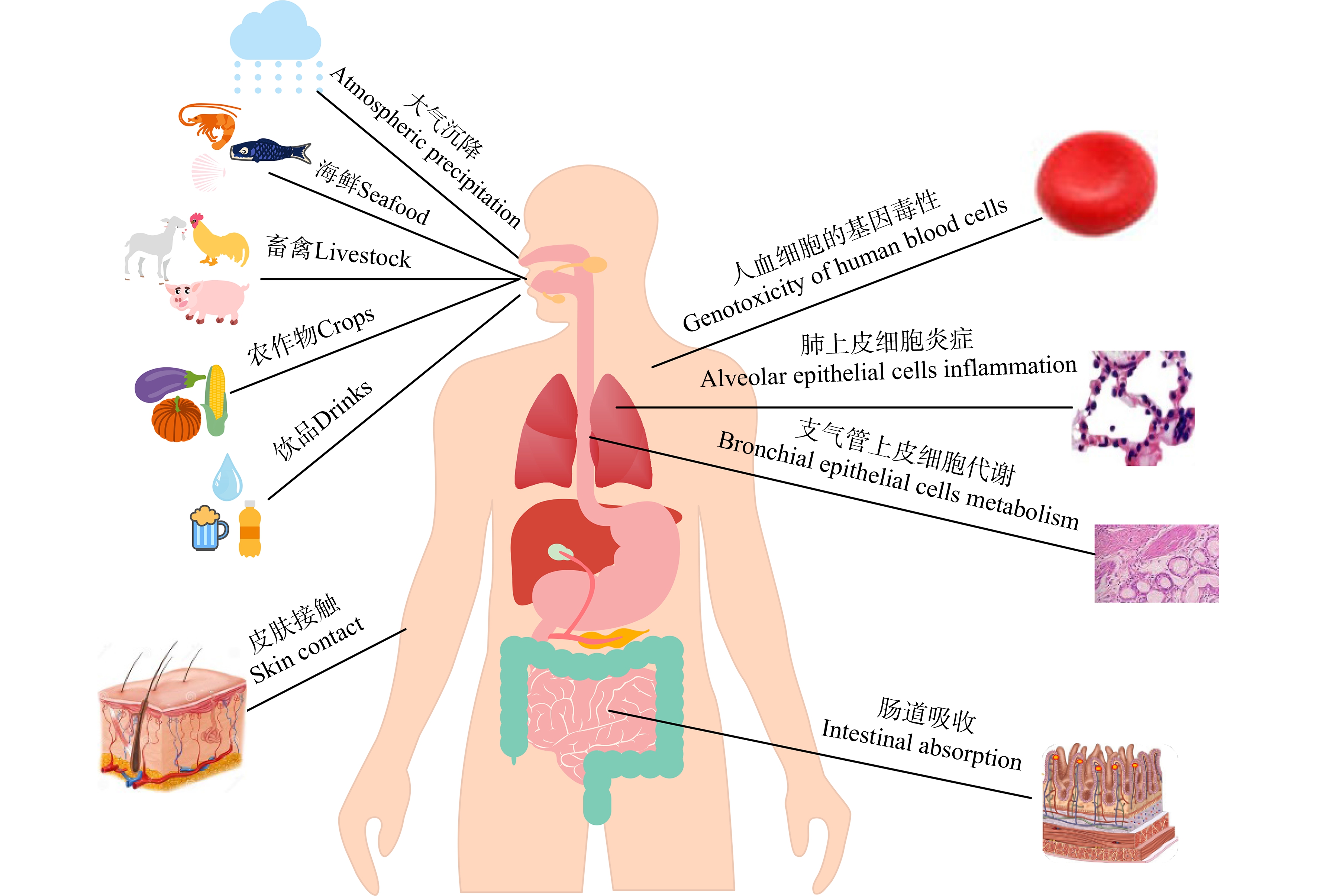
图2 纳米塑料的人体暴露风险[67]
Fig. 2 Human body exposure risks of nanoplastics[67]
4 展望(Expectation)
纳米塑料的长期存在会对环境中的水生生物、植物、无脊椎动物和微生物产生毒性作用,并可通过食物链转运富集对人类健康造成潜在威胁。然而作为一种新污染物,纳米塑料的生态毒理学效应及机制的研究亟待加强:
(1)研究中采用的纳米塑料的种类和形状相对单一,主要是关注了工程塑料的生态毒性,但对于环境中实际存在的纳米塑料的研究尚欠缺;
(2)纳米塑料难以溶于测试介质或体液中,在毒性暴露实验中还无法保证纳米塑料在溶液相中的准确浓度,目前是以单位体积/质量内颗粒物的量为计数方式,难以转化为生物体内残留量并表示为剂量-响应关系,需将更多的新技术和新方法应用到纳米塑料浓度标准化研究中;
(3)纳米塑料中含有的添加剂可能会不断释放出来,关于纳米塑料对生物体的毒性效应是由于其自身还是塑料添加剂的释放造成的,目前还没有明确的结论,还需做进一步的探索;
(4)目前没有足够的证据说明纳米塑料可以通过吞噬或穿透作用进入到细胞内,需结合同位素示踪与分子生物学新技术等深入探讨其引发的毒理学机制。
[1] Rillig M C. Microplastic in terrestrial ecosystems and the soil? [J]. Environmental Science & Technology, 2012, 46(12): 6453-6454
[2] 张晓菲, 汪磊. 环境中纳米塑料的分离与检测[J]. 环境化学, 2020, 39(1): 8-11
Zhang X F, Wang L. The separation and detection methods of nanoplastics in the environment [J]. Environmental Chemistry, 2020, 39(1): 8-11 (in Chinese)
[3] Gangadoo S, Owen S, Rajapaksha P, et al. Nano-plastics and their analytical characterisation and fate in the marine environment: From source to sea [J]. The Science of the Total Environment, 2020, 732: 138792
[4] Awet T T, Kohl Y, Meier F, et al. Effects of polystyrene nanoparticles on the microbiota and functional diversity of enzymes in soil [J]. Environmental Sciences Europe, 2018, 30(1): 11
[5] Lin W, Jiang R F, Hu S Z, et al. Investigating the toxicities of different functionalized polystyrene nanoplastics on Daphnia magna [J]. Ecotoxicology and Environmental Safety, 2019, 180: 509-516
[6] Zhang Q, Qu Q, Lu T, et al. The combined toxicity effect of nanoplastics and glyphosate on Microcystis aeruginosa growth [J]. Environmental Pollution, 2018, 243(Pt B): 1106-1112
[7] 陈璇, 章家恩, 危晖. 环境微塑料的迁移转化及生态毒理学研究进展[J]. 生态毒理学报, 2021, 16(6): 70-86
Chen X, Zhang J E, Wei H. Research progress and prospect on transportation, transformation and ecotoxicology of microplastics in environment [J]. Asian Journal of Ecotoxicology, 2021, 16(6): 70-86(in Chinese)
[8] Peng L C, Fu D D, Qi H Y, et al. Micro- and nano-plastics in marine environment: Source, distribution and threats: A review [J]. Science of the Total Environment, 2020, 698: 134254
[9] Jeong C B, Kang H M, Lee M C, et al. Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana [J]. Scientific Reports, 2017, 7: 41323
[10] Lee W S, Cho H J, Kim E, et al. Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos [J]. Nanoscale, 2019, 11(7): 3173-3185
[11] Jiang X F, Chen H, Liao Y C, et al. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba [J]. Environmental Pollution, 2019, 250: 831-838
[12] Li Z X, Li Q F, Li R J, et al. The distribution and impact of polystyrene nanoplastics on cucumber plants [J]. Environmental Science and Pollution Research International, 2021, 28(13): 16042-16053
[13] 苑文珂. 聚苯乙烯微/纳米塑料对重金属的吸附行为及其对两种典型水生生物的生态毒性研究[D]. 北京: 中国科学院大学, 2020: 37-48
Yuan W K. A study on the adsorption behaviors of micro/nano-plastics for heavy metals and their ecotoxicity toward two typical aquatic organisms [D]. Beijing: University of Chinese Academy of Sciences, 2020: 37-48 (in Chinese)
[14] Li L, Luo Y, Li R, et al. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode [J]. Nature Sustainability, 2020, 3: 929-937
[15] Giorgetti L, Spanò C, Muccifora S, et al. Exploring the interaction between polystyrene nanoplastics and Allium cepa during germination: Internalization in root cells, induction of toxicity and oxidative stress [J]. Plant Physiology and Biochemistry, 2020, 149: 170-177
[16] Skjolding L M, ![]() G, Jølck R I, et al. An assessment of the importance of exposure routes to the uptake and internal localisation of fluorescent nanoparticles in zebrafish (Danio rerio), using light sheet microscopy [J]. Nanotoxicology, 2017, 11(3): 351-359
G, Jølck R I, et al. An assessment of the importance of exposure routes to the uptake and internal localisation of fluorescent nanoparticles in zebrafish (Danio rerio), using light sheet microscopy [J]. Nanotoxicology, 2017, 11(3): 351-359
[17] Chae Y, Kim D, Kim S W, et al. Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain [J]. Scientific Reports, 2018, 8(1): 284
[18] Heddagaard F E, Møller P. Hazard assessment of small-size plastic particles: Is the conceptual framework of particle toxicology useful? [J]. Food and Chemical Toxicology, 2020, 136: 111106
[19] Besseling E, Wang B, Lürling M, et al. Nanoplastic affects growth of S. obliquus and reproduction of D. magna [J]. Environmental Science & Technology, 2014, 48(20): 12336-12343
[20] Ribeiro F, O’Brien J W, Galloway T, et al. Accumulation and fate of nano- and micro-plastics and associated contaminants in organisms [J]. Trends in Analytical Chemistry, 2019, 111: 139-147
[21] Greven A C, Merk T, Karagöz F, et al. Polycarbonate and polystyrene nanoplastic particles act as stressors to the innate immune system of fathead minnow (Pimephales promelas) [J]. Environmental Toxicology and Chemistry, 2016, 35(12): 3093-3100
[22] Ruiz-Palacios M, Almeida M, Martins M A, et al. Establishment of a brain cell line (FuB-1) from mummichog (Fundulus heteroclitus) and its application to fish virology, immunity and nanoplastics toxicology [J]. The Science of the Total Environment, 2020, 708: 134821
[23] Li Z L, Feng C H, Wu Y H, et al. Impacts of nanoplastics on bivalve: Fluorescence tracing of organ accumulation, oxidative stress and damage [J]. Journal of Hazardous Materials, 2020, 392: 122418
[24] Liu Z Q, Li Y M, Sepúlveda M S, et al. Development of an adverse outcome pathway for nanoplastic toxicity in Daphnia pulex using proteomics [J]. The Science of the Total Environment, 2021, 766: 144249
[25] Sendra M, Staffieri E, Yeste M P, et al. Are the primary characteristics of polystyrene nanoplastics responsible for toxicity and ad/absorption in the marine diatom Phaeodactylum tricornutum? [J]. Environmental Pollution, 2019, 249: 610-619
[26] Bergami E, Pugnalini S, Vannuccini M L, et al. Long-term toxicity of surface-charged polystyrene nanoplastics to marine planktonic species Dunaliella tertiolecta and Artemia franciscana [J]. Aquatic Toxicology, 2017, 189: 159-169
[27] Li Y M, Liu Z Q, Li M F, et al. Effects of nanoplastics on antioxidant and immune enzyme activities and related gene expression in juvenile Macrobrachium nipponense [J]. Journal of Hazardous Materials, 2020, 398: 122990
[28] Li Y M, Liu Z Q, Yang Y, et al. Effects of nanoplastics on energy metabolism in the oriental river prawn (Macrobrachium nipponense) [J]. Environmental Pollution, 2021, 268(Pt A): 115890
[29] Pinsino A, Bergami E, Della Torre C, et al. Amino-modified polystyrene nanoparticles affect signalling pathways of the sea urchin (Paracentrotus lividus) embryos [J]. Nanotoxicology, 2017, 11(2): 201-209
[30] Trevisan R, Voy C, Chen S X, et al. Nanoplastics decrease the toxicity of a complex PAH mixture but impair mitochondrial energy production in developing zebrafish [J]. Environmental Science & Technology, 2019, 53(14): 8405-8415
[31] Brandts I, Teles M, Gonçalves A P, et al. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine [J]. The Science of the Total Environment, 2018, 643: 775-784
[32] Jeong C B, Kang H M, Lee Y H, et al. Nanoplastic ingestion enhances toxicity of persistent organic pollutants (POPs) in the monogonont rotifer Brachionus koreanus via multixenobiotic resistance (MXR) disruption [J]. Environmental Science & Technology, 2018, 52(19): 11411-11418
[33] Peiponen K E, Räty J, Ishaq U, et al. Outlook on optical identification of micro- and nanoplastics in aquatic environments [J]. Chemosphere, 2019, 214: 424-429
[34] Tallec K, Huvet A, di Poi C, et al. Nanoplastics impaired oyster free living stages, gametes and embryos [J]. Environmental Pollution, 2018, 242(Pt B): 1226-1235
[35] Sökmen T Ö, Sulukan E, Tü![]() M, et al. Polystyrene nanoplastics (20 nm) are able to bioaccumulate and cause oxidative DNA damages in the brain tissue of zebrafish embryo (Danio rerio) [J]. Neurotoxicology, 2020, 77: 51-59
M, et al. Polystyrene nanoplastics (20 nm) are able to bioaccumulate and cause oxidative DNA damages in the brain tissue of zebrafish embryo (Danio rerio) [J]. Neurotoxicology, 2020, 77: 51-59
[36] Kelpsiene E, Torstensson O, Ekvall M T, et al. Long-term exposure to nanoplastics reduces life-time in Daphnia magna [J]. Scientific Reports, 2020, 10(1): 5979
[37] Liu Z Q, Li Y M, Pérez E, et al. Polystyrene nanoplastic induces oxidative stress, immune defense, and glycometabolism change in Daphnia pulex: Application of transcriptome profiling in risk assessment of nanoplastics [J]. Journal of Hazardous Materials, 2021, 402: 123778
[38] Feng L J, Sun X D, Zhu F P, et al. Nanoplastics promote microcystin synthesis and release from cyanobacterial Microcystis aeruginosa [J]. Environmental Science & Technology, 2020, 54(6): 3386-3394
[39] Zhao T, Tan L J, Huang W Q, et al. The interactions between micro polyvinyl chloride (mPVC) and marine dinoflagellate Karenia mikimotoi: The inhibition of growth, chlorophyll and photosynthetic efficiency [J]. Environmental Pollution, 2019, 247: 883-889
[40] Singh N, Bhagat J, Tiwari E, et al. Metal oxide nanoparticles and polycyclic aromatic hydrocarbons alter nanoplastic’s stability and toxicity to zebrafish [J]. Journal of Hazardous Materials, 2021, 407: 124382
[41] Jeong C B, Won E J, Kang H M, et al. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus) [J]. Environmental Science & Technology, 2016, 50(16): 8849-8857
[42] Canesi L, Ciacci C, Fabbri R, et al. Interactions of cationic polystyrene nanoparticles with marine bivalve hemocytes in a physiological environment: Role of soluble hemolymph proteins [J]. Environmental Research, 2016, 150: 73-81
[43] Nasser F, Lynch I. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna [J]. Journal of Proteomics, 2016, 137: 45-51
[44] 冯立娟. 纳米塑料对典型水生微生物的生物效应与作用机制[D]. 济南: 山东大学, 2020: 42-60
Feng L J. Biological effects and mechanisms of nanoplastics on typical aquatic microorganisms [D]. Jinan: Shandong University, 2020: 42-60 (in Chinese)
[45] He J Y, Yang X H, Liu H H. Enhanced toxicity of triphenyl phosphate to zebrafish in the presence of micro- and nano-plastics [J]. Science of the Total Environment, 2021, 756: 143986
[46] Mateos-Cárdenas A, van Pelt F N A M, O’Halloran J, et al. Adsorption, uptake and toxicity of micro- and nanoplastics: Effects on terrestrial plants and aquatic macrophytes [J]. Environmental Pollution, 2021, 284: 117183
[47] 吴佳妮, 杨天志, 连加攀, 等. 聚苯乙烯纳米塑料(PSNPs)对大豆(Glycine max)种子发芽和幼苗生长的影响[J]. 环境科学学报, 2020, 40(12): 4581-4589
Wu J N, Yang T Z, Lian J P, et al. Effects of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of soybean (Glycine max) [J]. Acta Scientiae Circumstantiae, 2020, 40(12): 4581-4589 (in Chinese)
[48] Zhou C Q, Lu C H, Mai L, et al. Response of rice (Oryza sativa L.) roots to nanoplastic treatment at seedling stage [J]. Journal of Hazardous Materials, 2021, 401: 123412
[49] 连加攀. 聚苯乙烯纳米塑料(PSNPs)对小麦单一及镉联合毒性研究[D]. 天津: 南开大学, 2020: 25-43
Lian J P. Single and combined toxicity of polystyrene nanoplastics (PSNPs) and cadmium to wheat (Triticum aestivum L.) [D]. Tianjin: Nankai University, 2020: 25-43 (in Chinese)
[50] 黄献培, 向垒, 郭静婕, 等. 聚苯乙烯微球对菜心种子及幼苗的毒性效应[J]. 农业环境科学学报, 2021, 40(5): 926-933
Huang X P, Xiang L, Guo J J, et al. Toxicity of polystyrene microplastics on seeds and seedlings of Brassica campestris L. [J]. Journal of Agro-Environment Science, 2021, 40(5): 926-933 (in Chinese)
[51] 薛颖昊, 黄宏坤, 靳拓, 等. 土壤微塑料和农药污染及其对土壤动物毒性效应的研究进展[J]. 农业环境科学学报, 2021, 40(2): 242-251
Xue Y H, Huang H K, Jin T, et al. Research progress on microplastic and pesticide pollutions and their toxic effects on soil organisms [J]. Journal of Agro-Environment Science, 2021, 40(2): 242-251 (in Chinese)
[52] Zhu B K, Fang Y M, Zhu D, et al. Exposure to nanoplastics disturbs the gut microbiome in the soil oligochaete Enchytraeus crypticus [J]. Environmental Pollution, 2018, 239: 408-415
[53] Ma J, Sheng G D, Chen Q L, et al. Do combined nanoscale polystyrene and tetracycline impact on the incidence of resistance genes and microbial community disturbance in Enchytraeus crypticus? [J]. Journal of Hazardous Materials, 2020, 387: 122012
[54] Lei L L, Liu M T, Song Y, et al. Polystyrene (nano)microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans [J]. Environmental Science: Nano, 2018, 5(8): 2009-2020
[55] Liu Q Y, Chen C X, Li M T, et al. Neurodevelopmental toxicity of polystyrene nanoplastics in Caenorhabditis elegans and the regulating effect of presenilin [J]. ACS Omega, 2020, 5(51): 33170-33177
[56] Qiu Y X, Luo L B, Yang Y H, et al. Potential toxicity of nanopolystyrene on lifespan and aging process of nematode Caenorhabditis elegans [J]. The Science of the Total Environment, 2020, 705: 135918
[57] Wang S T, Liu H L, Qu M, et al. Response of tyramine and glutamate related signals to nanoplastic exposure in Caenorhabditis elegans [J]. Ecotoxicology and Environmental Safety, 2021, 217: 112239
[58] Kim S W, An Y J. Soil microplastics inhibit the movement of springtail species [J]. Environment International, 2019, 126: 699-706
[59] Guimarães A T B, de Lima Rodrigues A S, Pereira P S, et al. Toxicity of polystyrene nanoplastics in dragonfly larvae: An insight on how these pollutants can affect bentonic macroinvertebrates [J]. The Science of the Total Environment, 2021, 752: 141936
[60] Matthews S, Mai L, Jeong C B, et al. Key mechanisms of micro- and nanoplastic (MNP) toxicity across taxonomic groups [J]. Comparative Biochemistry and Physiology Toxicology & Pharmacology, 2021, 247: 109056
[61] Sun X M, Chen B J, Li Q F, et al. Toxicities of polystyrene nano- and microplastics toward marine bacterium Halomonas alkaliphila [J]. The Science of the Total Environment, 2018, 642: 1378-1385
[62] Rossi G, Barnoud J, Monticelli L. Polystyrene nanoparticles perturb lipid membranes [J]. The Journal of Physical Chemistry Letters, 2014, 5(1): 241-246
[63] Fringer V S, Fawcett L P, Mitrano D M, et al. Impacts of nanoplastics on the viability and riboflavin secretion in the model bacteria Shewanella oneidensis [J]. Frontiers in Environmental Science, 2020, 8: 97
[64] Saygin H, Baysal A. Similarities and discrepancies between bio-based and conventional submicron-sized plastics: In relation to clinically important bacteria [J]. Bulletin of Environmental Contamination and Toxicology, 2020, 105(1): 26-35
[65] Ustabasi G S, Baysal A. Bacterial interactions of microplastics extracted from toothpaste under controlled conditions and the influence of seawater [J]. Science of the Total Environment, 2020, 703: 135024
[66] Chen W Y, Yuan D, Shan M, et al. Single and combined effects of amino polystyrene and perfluorooctane sulfonate on hydrogen-producing thermophilic bacteria and the interaction mechanisms [J]. The Science of the Total Environment, 2020, 703: 135015
[67] Kumar M, Chen H Y, Sarsaiya S, et al. Current research trends on micro- and nano-plastics as an emerging threat to global environment: A review [J]. Journal of Hazardous Materials, 2021, 409: 124967
[68] Lehner R, Weder C, Petri-Fink A, et al. Emergence of nanoplastic in the environment and possible impact on human health [J]. Environmental Science & Technology, 2019, 53(4): 1748-1765
[69] Teles M, Balasch J C, Oliveira M, et al. Insights into nanoplastics effects on human health [J]. Science Bulletin, 2020, 65(23): 1966-1969
[70] Daroowalla F, Wang M L, Piacitelli C, et al. Flock workers’ exposures and respiratory symptoms in five plants [J]. American Journal of Industrial Medicine, 2005, 47(2): 144-152
[71] Hoffman B U, Lumpkin E A. A gut feeling [J]. Science, 2018, 361(6408): 1203-1204
[72] Gopinath P M, Saranya V, Vijayakumar S, et al. Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics [J]. Scientific Reports, 2019, 9(1): 8860
[73] Domenech J, Hernández A, Rubio L, et al. Interactions of polystyrene nanoplastics with in vitro models of the human intestinal barrier [J]. Archives of Toxicology, 2020, 94(9): 2997-3012
[74] 闫协民. 聚苯乙烯微塑料/四环素复合污染物对AGS细胞损伤机理研究[D]. 湛江: 广东海洋大学, 2020: 16-31
Yan X M. A study on the damage mechanism of polystyrene microplastic/tetracycline complex on AGS cells [D]. Zhanjiang: Guangdong Ocean University, 2020: 16-31 (in Chinese)
[75] Li Y L, Hu Q, Miao G H, et al. Size-dependent mechanism of intracellular localization and cytotoxicity of mono-disperse spherical mesoporous nano- and micron-bioactive glass particles [J]. Journal of Biomedical Nanotechnology, 2016, 12(5): 863-877
[76] Wang Q Q, Bai J L, Ning B A, et al. Effects of bisphenol A and nanoscale and microscale polystyrene plastic exposure on particle uptake and toxicity in human Caco-2 cells [J]. Chemosphere, 2020, 254: 126788
[77] Xu M K, Halimu G, Zhang Q R, et al. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell [J]. The Science of the Total Environment, 2019, 694: 133794
[78] Lim S L, Ng C T, Zou L, et al. Targeted metabolomics reveals differential biological effects of nanoplastics and nanoZnO in human lung cells [J]. Nanotoxicology, 2019, 13(8): 1117-1132
[79] Hesler M, Aengenheister L, Ellinger B, et al. Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro [J]. Toxicology in Vitro: An International Journal Published in Association with BIBRA, 2019, 61: 104610