二甲基甲酰胺(N,N-dimethylformamide, DMF)因其理化性质优良而在工业生产和生活消费领域广泛应用,使用或生产过程中DMF可向土壤、水体和大气等环境介质扩散蓄积,并通过呼吸、皮肤或消化等途径进入机体[1]。代谢动力学实验表明DMF主要经肝细胞色素P450家族成员2E1代谢生成异氰酸甲酯等活性代谢产物,从而引起肝脏、肾脏和心脏等多系统毒性,其中尤以肝脏损害最为显著[2-4]。体内外实验均证实DMF可引起肝细胞凋亡、肝酶异常,还可增加机体对其他外源化合物的敏感性[5-7]。对此有研究归结于活性氧(ROS)有关的氧化应激级联反应,但同时也有研究通过基因敲除小鼠和人群丙二醛水平分析等结果推测,在DMF暴露中存在与氧化应激无关的损伤方式[7-9],但迄今为止DMF肝损害效应的具体途径和相关分子尚不明确。
Toll样受体4(toll-like receptors 4, TLR4)作为一种先天免疫因子,已被证明在汞、砷和环境有机污染物等多种外源化学物所致肝损伤中发挥作用,可在内、外源信号诱导下,通过宿主继发危险相关分子模式激活下游靶标如核因子κB(nuclear factor kappa-B, NF-κB)、蛋白激酶B(protein kinase B, Akt),诱发与肿瘤坏死因子α(tumor necrosis factor, TNF-α)、白介素1和白介素6等相关的炎性反应,还可经p38丝裂原活化蛋白激酶-自噬蛋白P62信号轴,通过c-jun氨基末端激酶(c-Jun N-terminal kinase, JNK)级联活化方式诱导肝细胞自噬和炎性反应,最终造成肝细胞损伤[10-12]。研究报道DMF暴露动物在出现消化道损害同时,可伴随S24-7、类杆菌科、理肯菌科和消化链球菌科等菌群数量改变,并推测此现象可能会间接影响Toll受体相关信号而诱导肝脑损害[13-14]。但截至目前,对于TLR4是否在DMF所致肝损伤中发挥作用及其涉及的具体分子尚不清楚。
鉴于以上原因,拟通过动物实验考察DMF对小鼠肝脏损害效应表现及对TLR4相关分子表达的影响,借此探讨DMF肝损伤的潜在机制与可能途径,为DMF生态毒理学研究提供基础数据。
1 材料与方法(Materials and methods)
1.1 试剂与仪器
试剂:DMF(Assay LC-MS 98%,Sigma-Aldrich公司,美国);RT-PCR试剂及引物(TaKaRa大连宝生物公司,中国);丙氨酸转氨酶试剂盒、谷草转氨酶试剂盒和碱性磷酸酶试剂盒(Applied Biosystems公司,美国);白细胞介素-1、白细胞介素-6和肿瘤坏死因子-α Elisa试剂盒(江苏酶免实业有限公司,中国);其他试剂均为国产分析纯。
仪器:5332型PCR仪(Eppendorf公司,德国)、MODEL550酶标仪(Bio-Rad公司,美国)、RM2126切片机(Leica公司,德国)、CK40显微镜(Olympus公司,日本)、CFI60数码相机(Nikon,日本)。
1.2 动物模型建立
雌雄各半ICR小鼠80只,雄性(23±2) g,雌性(20±2) g,上海西普尔必凯实验动物有限公司提供(SCXK沪2008-0016),实验室驯养1周后随机分为对照组(0 mg·kg-1·d-1, n=20)、低剂量组(350 mg·kg-1·d-1, n=20)、中剂量组(700 mg·kg-1·d-1, n=20)和高剂量组(1 400 mg·kg-1·d-1, n=20)。
1.3 指标检测
1.3.1 脏器系数
小鼠处死前称量质量,处死后用预冷生理盐水漂洗肝脏,滤纸吸干称取肝质量计算脏器系数:脏器系数=脏器质量(g)/小鼠体质量(g)×100%。
1.3.2 肝脏病理观察
肝脏组织用10%中性福尔马林溶液固定,常规脱水后石蜡包埋制备4 μm切片,苏木精-伊红(HE)、油红O染色后显微镜观察其病理学变化。
1.3.3 肝脏组织匀浆中指标的检测
按照测试盒说明书进行操作,测定肝脏组织匀浆中丙氨酸氨基转移酶(alanine aminotransferase, ALT)、谷草转氨酶(aspartate aminotransferase, AST)、碱性磷酸酶(alkaline phosphatase, ALP)活性以及总胆固醇(total cholesterol, TC)和甘油三酯(triglyceride, TG)含量。
1.3.4 肝脏蛋白质提取及蛋白印迹反应(Western blotting, WB)
称取约100 mg小鼠肝脏组织进行蛋白质提取,置于95 ℃水浴锅蛋白变性10 min,并于-20 ℃冰箱保存备用。根据实验目的,配制不同浓度的SDS-PAGE胶,80 V电压下分离30 min左右,直至marker颜色分开,切换至120 V电压直至结束,对蛋白质进行电泳分离。电泳结束后,在湿转转膜缓冲液中将SDS-PAGE胶上的蛋白转移至甲醇活化的PVDF膜上,100 V,湿转70 min。转膜结束后,加入适量含5%牛奶的TBST,室温轻摇封闭1 h,结束后一抗4 ℃孵育过夜。次日,一抗回收,TBST清洗(5 min×3次)。HRP偶联二抗室温孵育1 h,TBST清洗(5 min×3次)。用ECL发光液孵育约3 min,放入Tanon-5200全自动化学发光图像分析系统拍摄,后用Image J软件分析。
1.3.5 ELISA法测小鼠肝脏中炎性因子水平
称取小鼠肝脏组织约100 mg匀浆,用ELISA法测试小鼠肝脏中炎性因子白介素1(IL-1)、白介素6(IL-6)和肿瘤坏死因子α(TNF-α)的活性。将试剂盒在室温下放置20 min后,从铝箔袋中取出所需板条。在标准品孔、空白孔、样品孔按照说明书加入所需试剂及待测样品,将带有辣根过氧化物酶标记的抗体加入每个孔中,空白孔除外,然后用封板膜封住反应孔并于恒温箱温育60 min或水浴锅水浴37 ℃。弃去液体,残余水分拍干后加入洗涤液,放置1 min,弃去洗涤液,拍干,重复洗5次。在每个孔中加入A液和B液,37 ℃避光孵育15 min后,加入终止液,于15 min内在450 nm波长处测OD值。
1.4 统计学分析
实验数据用![]() 表示,用SPSS 24.0统计软件分析数据。采用单因素方差分析,方差齐性用Least-significant difference进行检验,组间比较用Student-Newman-Keuls法检验,P<0.05表示有显著性差异。
表示,用SPSS 24.0统计软件分析数据。采用单因素方差分析,方差齐性用Least-significant difference进行检验,组间比较用Student-Newman-Keuls法检验,P<0.05表示有显著性差异。
2 结果(Results)
2.1 DMF暴露对小鼠一般状况的影响
DMF暴露小鼠均出现不同程度活动迟缓,伴随毛发暗淡,脱毛。随暴露剂量及周期增长,出现明显易激惹,个别实验动物出现腹泻,鼻衄。
如表1所示,实验第5周时,高剂量组小鼠体质量(35.96±5.24) g与对照组(40.61±5.77) g相比开始出现显著降低,低、中剂量组小鼠虽也出现了体质量减少((40.06±5.55) g、(38.81±5.29) g),但与对照组相比差异并不显著。第12周时中、高剂量组小鼠体质量分别为(41.82±6.12) g和(39.62±6.19) g,相比对照组(46.87±6.90) g分别降低了约10.79%和15.48% (P<0.05)。此同时,DMF暴露造成小鼠肝质量的增加,不同剂量组小鼠肝脏系数与对照组相比均显著增加(P<0.05),如表2所示。
2.2 DMF暴露对小鼠肝脏形态影响
对各组小鼠肝脏形态进行比较,光镜下HE染色如图1所示,肝索排列整齐,细质均匀,细胞核着色清晰可见,DMF暴露组可见不同程度炎细胞浸润迁移,细胞空泡出现和细胞核偏移。油红O染色结果如图2所示,与对照组相比,暴露组小鼠细胞质及细胞间隙充斥着大量脂肪滴。
2.3 DMF暴露对小鼠肝脏代谢酶水平影响
对DMF暴露后小鼠肝脏匀浆中肝功能相关酶活性进行了检测,结果如表3所示,和对照组相比DMF暴露造成中、高剂量组ALT、AST和ALP含量显著上升(P<0.05),其中ALT和AST随暴露剂量增加而显著上升。对肝脏代谢产物的检测发现,TG和TC的水平都在DMF暴露组出现了增加,但TC仅在高剂量组出现显著增高,其中高剂量组和低剂量组相比也表现出显著的增加。
2.4 DMF暴露对小鼠肝脏中相关蛋白水平的影响
如图3所示,通过对DMF暴露小鼠TLR4相关通路蛋白质表达水平的检测发现,TLR4的表达随着DMF剂量增加显著上升,分别约是对照组的1.30倍、1.55倍和1.98倍,其下游分子Akt和NF-κB的磷酸化改变趋势与之类似,均随着DMF剂量的增加而显著增高(P<0.05)。
2.5 DMF暴露对小鼠肝脏中炎症因子的影响
如表4所示,ELISA结果显示小鼠肝脏中炎性因子IL-1、IL-6和TNF-α随着DMF暴露剂量的增加而增加。与对照组相比,IL-1水平在中、高剂量组出现显著升高,尤其高剂量组中达到最高,约为对照组4倍左右(P<0.05)。此外,和对照组相比,低剂量组、中剂量组和高剂量组IL-6活性分别升高约1.15倍、1.35倍和1.86倍,并有随着剂量在组间逐渐上升的趋势(P<0.05)。TNF-α水平在中、高剂量组相较对照组也分别升高约1.86倍和2.76倍(P<0.05)。
表1 二甲基甲酰胺(DMF)暴露对小鼠体质量的影响![]()
Table 1 Effect of N,N-dimethylformamide (DMF) exposure on body weight of mice ![]()
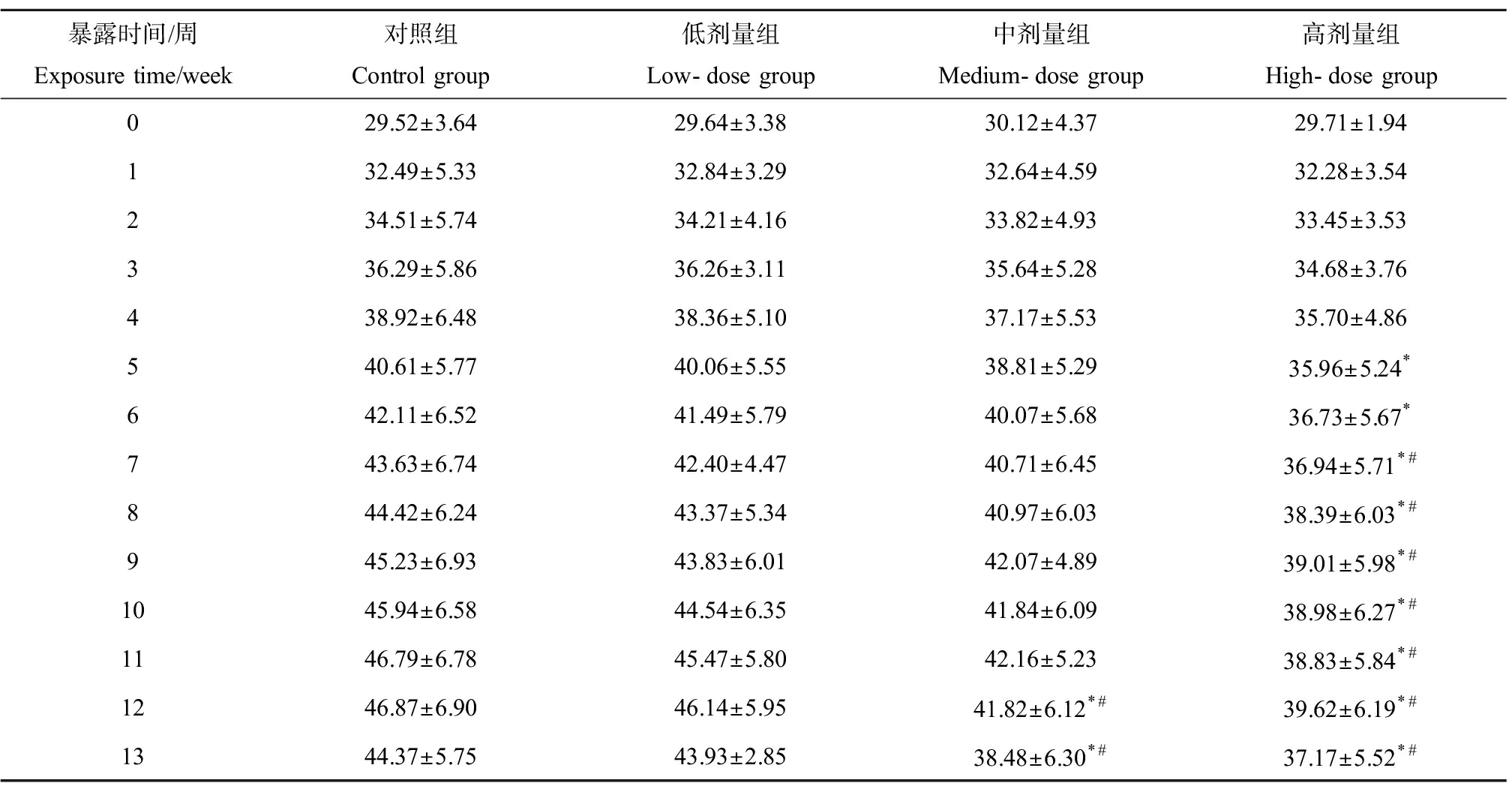
暴露时间/周Exposure time/week对照组Control group低剂量组Low-dose group中剂量组Medium-dose group高剂量组High-dose group029.52±3.6429.64±3.3830.12±4.3729.71±1.94132.49±5.3332.84±3.2932.64±4.5932.28±3.54234.51±5.7434.21±4.1633.82±4.9333.45±3.53336.29±5.8636.26±3.1135.64±5.2834.68±3.76438.92±6.4838.36±5.1037.17±5.5335.70±4.86540.61±5.7740.06±5.5538.81±5.2935.96±5.24*642.11±6.5241.49±5.7940.07±5.6836.73±5.67*743.63±6.7442.40±4.4740.71±6.4536.94±5.71*#844.42±6.2443.37±5.3440.97±6.0338.39±6.03*#945.23±6.9343.83±6.0142.07±4.8939.01±5.98*#1045.94±6.5844.54±6.3541.84±6.0938.98±6.27*#1146.79±6.7845.47±5.8042.16±5.2338.83±5.84*#1246.87±6.9046.14±5.9541.82±6.12*#39.62±6.19*#1344.37±5.7543.93±2.8538.48±6.30*#37.17±5.52*#
注:*与对照组相比,P<0.05;#与低剂量组相比,P<0.05。
Note: * indicates P <0.05 compared with control group; # indicates P<0.05 compared with low-dose group.
表2 DMF暴露对小鼠肝质量和脏器系数的影响![]() Table 2 Effect of DMF exposure on liver weight and organ index of mice
Table 2 Effect of DMF exposure on liver weight and organ index of mice ![]() n=20)
n=20)
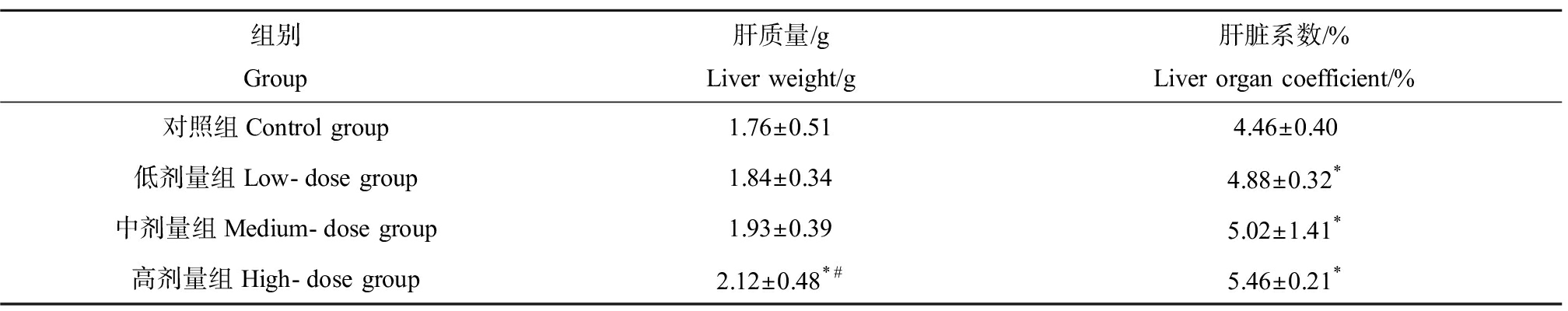
组别Group肝质量/gLiver weight/g肝脏系数/%Liver organ coefficient/%对照组Control group1.76±0.514.46±0.40低剂量组Low-dose group1.84±0.344.88±0.32*中剂量组Medium-dose group1.93±0.395.02±1.41*高剂量组High-dose group2.12±0.48*#5.46±0.21*
注:*与对照组相比,P<0.05;#与低剂量组相比,P<0.05。
Note: *indicates P<0.05 compared with control group; # indicates P<0.05 compared with low-dose group.

图1 DMF暴露后小鼠肝脏HE染色
注:(a)、(b)、(c)和(d)分别代表对照组、低剂量组、中剂量组和高剂量组(×400),n=20;黑色箭头表示空泡,红色箭头表示炎性细胞。
Fig. 1 HE staining of mouse liver after DMF exposure
Note: (a), (b), (c), and (d) represent the control group, low-dose group, medium-dose group, high-dose group (×400), n=20; the black arrows indicate vacuoles, and the red arrows indicate inflammatory cells.
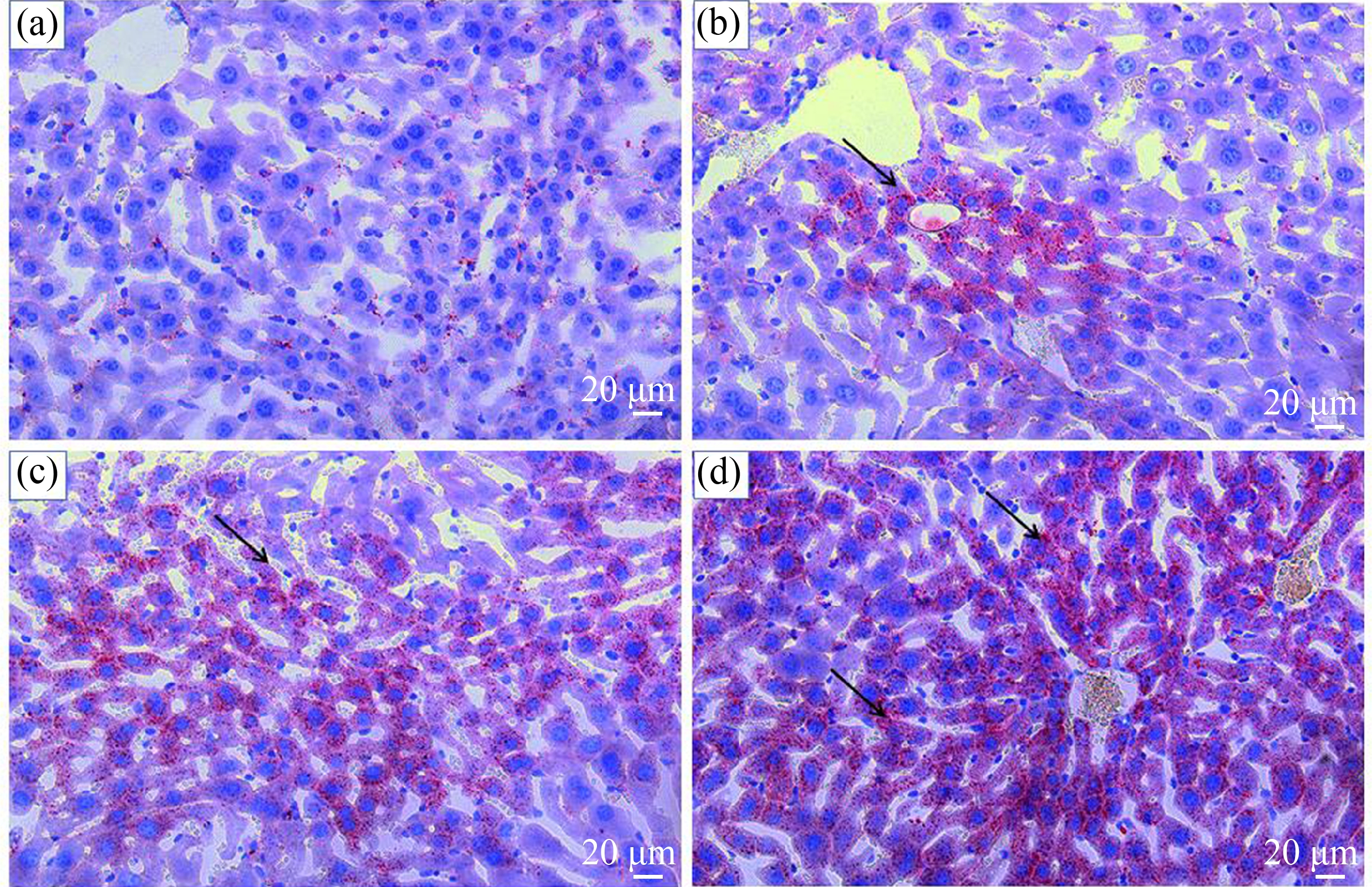
图2 DMF暴露后小鼠肝脏油红O染色
注:(a)、(b)、(c)和(d)分别代表对照组、低剂量组、中剂量组和高剂量组(×400),n=20;黑色箭头表示脂肪滴。
Fig. 2 Mice liver oil red O staining after DMF exposure
Note: (a), (b), (c), and (d) represent the control group, low-dose group, medium-dose group, high-dose group (×400), n=20; the black arrow shows the fat drop.
3 讨论(Discussion)
DMF属于人类健康领域优先研究的4种污染物之一,具有明显的肝脏毒性,但具体机制未明[15]。本次实验发现DMF能引起小鼠肝脏酶学及形态学的显著改变,伴随脂质代谢产物的水平升高,这与前人对不同途径接触DMF的啮齿类及人、兔等种属研究的结果类似[16-17],提示亚慢性DMF暴露可造成以肝脂代谢紊乱为特征的肝损伤表现。为进一步了解DMF毒效应机制,对TLR4及相关分子表达进行了检测。
TLR4属于宿主应答反应中的一种细胞表面受体分子,在天然免疫和炎症反应中具有中心枢纽作用[18]。除了其经典配体脂多糖(lipopolysaccharide, LPS)外,近年研究发现TLR4也可被包括环境内分泌干扰物在内的多种外源化合物所响应,通过识别病原相关分子模式及危险相关分子模式活化下游衔接分子如髓样分化因子88、β干扰素TIR结构域衔接蛋白等,最终调节信号依赖性转录因子活性,参与后续肝脏炎性损伤,被认为是急慢性肝损伤中重要一环[10-11, 19-21]。
TLR4的损伤效果与其对下游分子Akt的调控活化关系密切(图4)。TLR4可通过转录调节或诱导胞内磷脂酰肌醇激酶调节亚基P85活化来上调Akt磷酸化水平。活化的Akt通过上调固醇调节元件结合蛋白1c核积累、激活哺乳动物雷帕霉素靶蛋白受体、诱导肝X受体活化的方式上调脂肪酸合成酶和乙酰辅酶A羧化酶等表达促进脂质合成。研究发现野生型C3H/HeN小鼠和TLR4基因突变的C3H/HeJ小鼠相比具有更高的肝脏脂肪蓄积和炎性改变[22]。此外PM2.5暴露研究也发现TLR4可通过调节Akt反馈调节因子STAT3水平影响肝脏瘦素分泌,造成脂代谢异常[23-24]。反之,TLR4拮抗剂或敲除沉默均对缓解或阻断与下游Akt相关的脂毒性反应有效[25-26]。但同时作为增殖调控和糖脂代谢的交叉调节分子,细胞代谢失衡可促使Akt招募活化IκB激酶(IκB kinase, IKK)复合物并降解NF-κB抑制蛋白(inhibitor of nuclear factor kappa-B, IκB),使其转位入核调节下游靶基因转录,促进炎性免疫[27]。Shen等[28]通过药物抑制TLR4表达,发现能缓解Akt/NF-κB激活的炎性反应,从而降低大鼠卵巢细胞凋亡。此外TLR4下游分子MyD88的抑制剂可通过阻滞Akt磷酸化及下调IκB活性的方式减少NF-κB活化,抑制RAW 264.7细胞中LPS所引起的炎性损伤[29]。在本次实验中随着DMF暴露剂量的增加,TLR4表达水平出现明显增加,同时伴随下游Akt/NF-κB磷酸化水平的提升,与之同步的是血清中TG和TC的上升及肝细胞中脂滴数量的增加。由此推断,DMF首先通过激活TLR4/Akt造成了脂代谢通路的亢进,引起细胞中脂肪蓄积增多,正常情况下,肝系统通过加快转运和脂肪酸氧化来对抗脂肪合成,但随着血中游离脂肪酸水平及细胞脂变性加重超过机体代偿,又作为外源性诱因刺激Akt加快上调NF-κB,触发终末炎性反应回路。
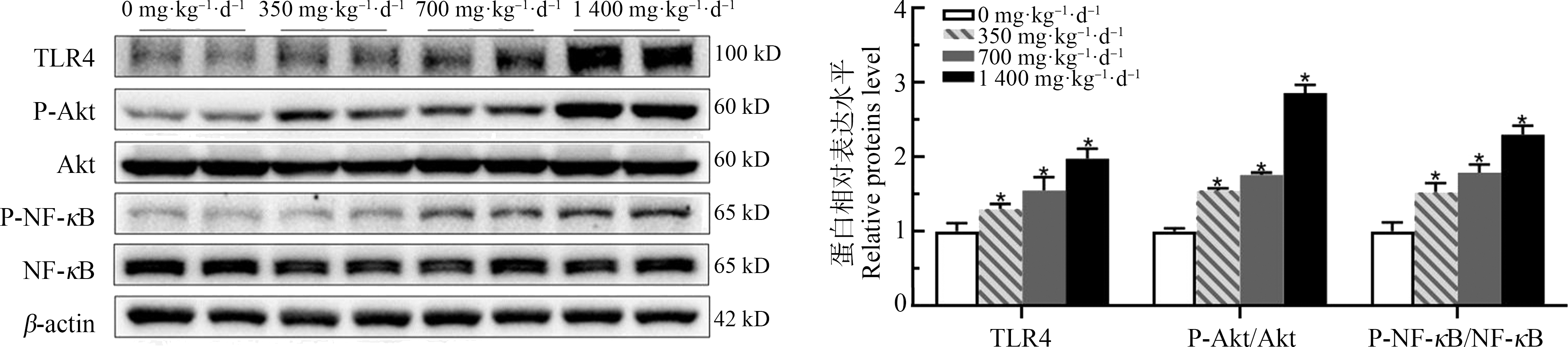
图3 DMF对小鼠蛋白表达水平的影响![]()
注:*与对照组相比,P<0.05。
Fig. 3 Effect of DMF on mouse protein expression level ![]() n=20)
n=20)
Note: *indicates P<0.05 compared with control group.
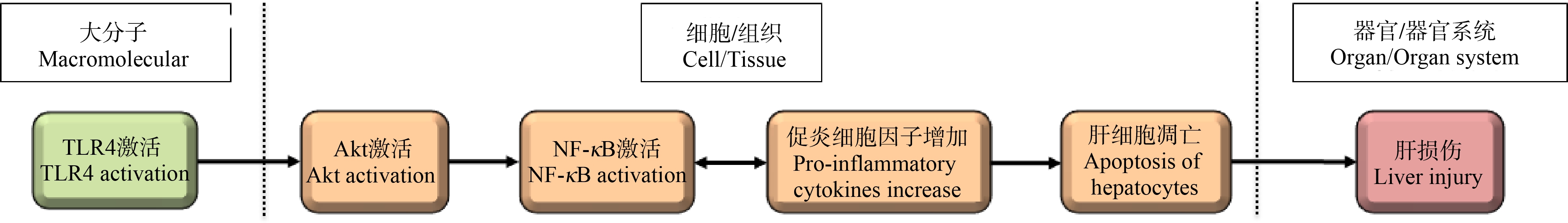
图4 TLR4相关分子在DMF致小鼠肝损伤中的作用方式
Fig. 4 TLR4 activation leading to liver injury with DMF exposure
表3 DMF暴露对小鼠血清肝代谢酶的影响![]() Table 3 Effect of DMF on liver metabolic enzyme activity in serum of mice
Table 3 Effect of DMF on liver metabolic enzyme activity in serum of mice ![]() n=20)
n=20)
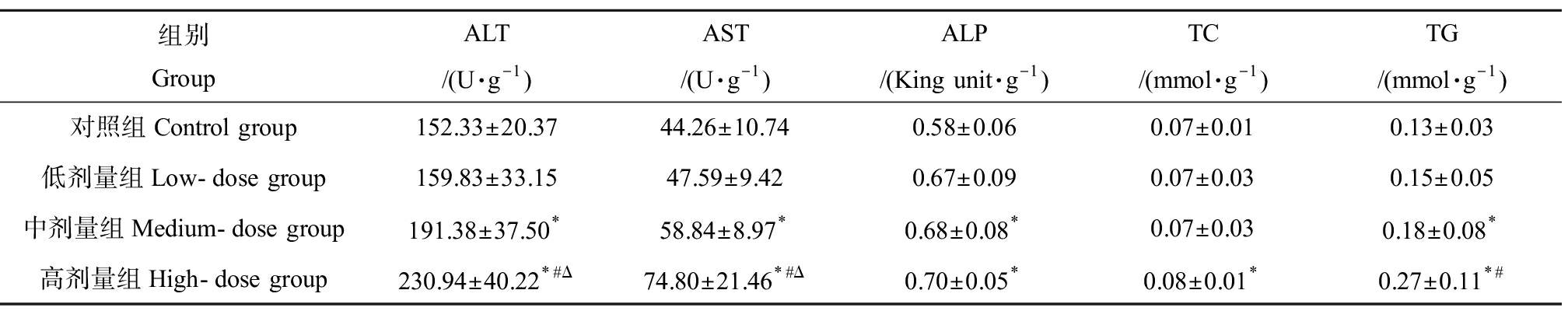
组别GroupALT/(U·g-1)AST/(U·g-1)ALP/(King unit·g-1)TC/(mmol·g-1)TG/(mmol·g-1)对照组Control group152.33±20.3744.26±10.740.58±0.060.07±0.010.13±0.03低剂量组Low-dose group159.83±33.1547.59±9.420.67±0.090.07±0.030.15±0.05中剂量组Medium-dose group191.38±37.50*58.84±8.97*0.68±0.08*0.07±0.030.18±0.08*高剂量组High-dose group230.94±40.22*#Δ74.80±21.46*#Δ0.70±0.05*0.08±0.01*0.27±0.11*#
注:*与对照组相比,P<0.05;#与低剂量组相比,P<0.05;Δ与中剂量组相比,P<0.05;ALT表示丙氨酸氨基转移酶,AST表示谷草转氨酶,ALP表示碱性磷酸酶,TC表示总胆固醇,TG表示甘油三酯。
Note: *indicates P<0.05 compared with control group, # indicates P<0.05 compared with low-dose group, Δ indicates P<0.05 compared with medium-dose group; ALT stands for alanine aminotransferase, AST stands for aspartate aminotransferase, ALP stands for alkaline phosphatase, TC stands for total cholesterol; TG stands for triglyceride.
表4 DMF对小鼠炎症因子活性的影响![]()
Table 4 Effect of DMF on the activity of inflammatory factors in mice ![]() n=20) (pg·mL-1)
n=20) (pg·mL-1)
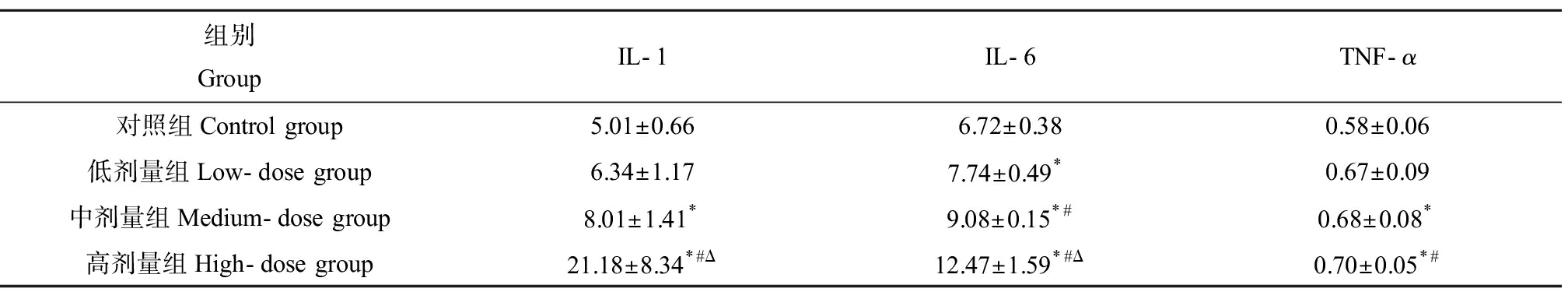
组别GroupIL-1IL-6TNF-α对照组Control group5.01±0.666.72±0.380.58±0.06低剂量组Low-dose group6.34±1.177.74±0.49*0.67±0.09中剂量组Medium-dose group8.01±1.41*9.08±0.15*#0.68±0.08*高剂量组High-dose group21.18±8.34*#Δ12.47±1.59*#Δ0.70±0.05*#
注:*与对照组相比,P<0.05;#与低剂量组相比,P<0.05;Δ与中剂量组相比,P<0.05。
Note: *indicates P <0.05 compared with control group; # indicates P <0.05 compared with low-dose group; Δ indicates P<0.05 compared with medium-dose group.
作为炎性网络的中心环节,NF-κB活化后将通过转录因子调控方式上调促炎细胞如TNF-α、IL-1、IL-6和IL-10等的表达。作为最早最重要的炎症介质,TNF-α能激活中性粒细胞和淋巴细胞促使白介素合成与释放,生成的IL-1和IL-6不仅刺激T细胞、巨噬细胞分泌各种趋化因子,还能增加肝细胞合成急性期蛋白引起肝细胞凋亡[30]。与此同时,以上促炎因子也能介由二酰基甘油和神经酰胺等第二信使作用激活丝裂原活化蛋白激酶通路,反向上调NF-κB并形成炎性环路[31]。在前人开展的体外实验中DMF可通过诱导高水平促炎因子如TNF-α、IL-1等分泌造成H9c2细胞凋亡,以往发现的DMF相关肝损伤模型中,同样存在TNF-α、IL-1等的显著上升[32-33]。据此有理由推断DMF有能力通过上调TLR4/Akt/NF-κB途径激活细胞炎性反应,并形成NF-κB与促炎因子的自调节反馈环路,造成炎性关系网的恶性往复,延长炎性反应时间,最终导致肝细胞凋亡和肝脏损伤的不良结局。
综上所述,通过本研究发现DMF亚慢性暴露可造成ICR小鼠出现肝脏脂代谢紊乱、肝细胞凋亡,最终出现肝损伤的不良结局。我们推测DMF诱导TLR4的表达上调是这一现象的开端,此后TLR4通过上调Akt活性影响下游NF-κB相关的炎性反应网络,造成肝脏免疫炎性调控失代偿而最终导致肝脏损伤的结局。
[1] Hu Z Y, Chang J, Guo F F, et al. The effects of dimethylformamide exposure on liver and kidney function in the elderly population: A cross-sectional study [J]. Medicine, 2020, 99(27): e20749
[2] Kim T H, Kim S G. Clinical outcomes of occupational exposure to N,N-dimethylformamide: Perspectives from experimental toxicology [J]. Safety and Health at Work, 2011, 2(2): 97-104
[3] Ding R, Chen D J, Yang Y J. Liver and heart toxicity due to 90-day oral exposure of ICR mice to N,N-dimethylformamide [J]. Environmental Toxicology and Pharmacology, 2011, 31(3): 357-363
[4] Zhao K, Wen L B. DMF attenuates cisplatin-induced kidney injury via activating Nrf2 signaling pathway and inhibiting NF-κB signaling pathway [J]. European Review for Medical and Pharmacological Sciences, 2018, 22(24): 8924-8931
[5] Zhang Z, Zhu W, Liu Z Q, et al. Aberrant expression of miRNA-192-5p contributes to N,N-dimethylformamide-induced hepatic apoptosis [J]. Journal of Applied Toxicology: JAT, 2020, 40(12): 1683-1693
[6] Kim T H, Kim Y W, Shin S M, et al. Synergistic hepatotoxicity of N,N-dimethylformamide with carbon tetrachloride in association with endoplasmic reticulum stress [J]. Chemico-Biological Interactions, 2010, 184(3): 492-501
[7] 李明君, 董婧, 曾涛. 氧化应激和枯否细胞介导的炎症在二甲基甲酰胺致小鼠急性肝损伤中的作用[C]// 中国毒理学会呼吸毒理专业委员会, 中华预防医学会卫生毒理分会. 2019全国呼吸毒理与卫生毒理学术研讨会论文集. 厦门: 中国毒理学会呼吸毒理专业委员会, 中华预防医学会卫生毒理分会, 2019: 43
[8] Liu Z Q, He Q M, Liu Y, et al. Hsa_circ_0005915 promotes N,N-dimethylformamide-induced oxidative stress in HL-7702 cells through NRF2/ARE axis [J]. Toxicology, 2021, 458: 152838
[9] Wu Z J, Liu Q, Wang L, et al. The essential role of CYP2E1 in metabolism and hepatotoxicity of N,N-dimethylformamide using a novel Cyp2e1 knockout mouse model and a population study [J]. Archives of Toxicology, 2019, 93(11): 3169-3181
[10] Dong L, Liu Y L, Wang D P, et al. Imbalanced inflammatory response in subchronic arsenic-induced liver injury and the protective effects of Ginkgo biloba extract in rats: Potential role of cytokines mediated cell-cell interactions [J]. Environmental Toxicology, 2021, 36(10): 2073-2092
[11] Jin R R, Liu L, Zhu W C, et al. Iron oxide nanoparticles promote macrophage autophagy and inflammatory response through activation of toll-like Receptor-4 signaling [J]. Biomaterials, 2019, 203: 23-30
[12] 张弦. TLR4介导的信号通路对肝细胞凋亡的影响及氧化苦参碱的干预机制研究[D]. 苏州: 苏州大学, 2016: 42-47
Zhang X. Effect of TLR4 mediated signal pathway on apoptosis of liver cells and intervention mechanism of oxymatrine [D]. Suzhou: Soochow University, 2016: 42-47 (in Chinese)
[13] Zhang M, Zheng M, Wu Z J, et al. Alteration of gut microbial community after N,N-dimethylformamide exposure [J]. The Journal of Toxicological Sciences, 2017, 42(2): 241-250
[14] 张蔓, 郑敏, 吴智君, 等. 二甲基甲酰胺暴露对大鼠肝脏损伤及肠道菌群的动态影响[C]//中国环境科学学会. 2017环境与公共健康学术会议暨中国环境科学学会环境医学与健康分会, 中国毒理学会生化与分子毒理专业委员会2017年年会论文集. 广州: 中国环境科学学会, 2017: 67
[15] Moorman W J, Ahlers H W, Chapin R E, et al. Prioritization of NTP reproductive toxicants for field studies [J]. Reproductive Toxicology (Elmsford, N Y), 2000, 14(4): 293-301
[16] Lynch D W, Placke M E, Persing R L, et al. Thirteen-week inhalation toxicity of N,N-dimethylformamide in F344/N rats and B6C3F1 mice [J]. Toxicological Sciences, 2003, 72(2): 347-358
[17] Kennedy G L. Acute and subchronic toxicity of dimethylformamide and dimethylacetamide following various routes of administration [J]. Drug and Chemical Toxicology, 1986, 9(2): 147-170
[18] Ciesielska A, Matyjek M, Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling [J]. Cellular and Molecular Life Sciences: CMLS, 2021, 78(4): 1233-1261
[19] Feng D, Zhang H M, Jiang X, et al. Bisphenol A exposure induces gut microbiota dysbiosis and consequent activation of gut-liver axis leading to hepatic steatosis in CD-1 mice [J]. Environmental Pollution, 2020, 265(Pt A): 114880
[20] 徐万鹏, 林军, 梁英琴, 等. 4-羟基-苯丙噁唑-2-酮对非酒精性脂肪肝病模型大鼠炎症和凋亡信号通路的影响[J]. 中国药房, 2021, 32(11): 1298-1303
Xu W P, Lin J, Liang Y Q, et al. Effects of 4-hydroxy-2(3H)-benzoxazolone on inflammatory and apoptosis signaling pathways in nonalcoholic fatty liver disease model rats [J]. China Pharmacy, 2021, 32(11): 1298-1303 (in Chinese)
[21] Kang H H, Kim I K, Lee H I, et al. Chronic intermittent hypoxia induces liver fibrosis in mice with diet-induced obesity via TLR4/MyD88/MAPK/NF-kB signaling pathways [J]. Biochemical and Biophysical Research Communications, 2017, 490(2): 349-355
[22] Uesugi T, Froh M, Arteel G E, et al. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice [J]. Hepatology, 2001, 34(1): 101-108
[23] Campolim C M, Weissmann L, Ferreira C K O, et al. Short-term exposure to air pollution (PM2.5) induces hypothalamic inflammation, and long-term leads to leptin resistance and obesity via Tlr4/Ikbke in mice [J]. Scientific Reports, 2020, 10(1): 10160
[24] Ya P, Xu H G, Ma Y M, et al. Liver injury induced in Balb/c mice by PM2.5 exposure and its alleviation by compound essential oils [J]. Biomedicine & Pharmacotherapy, 2018, 105: 590-598
[25] Jiang W P, Deng J S, Huang S S, et al. Sanghuangporus sanghuang mycelium prevents paracetamol-induced hepatotoxicity through regulating the MAPK/NF-κB, Keap1/Nrf2/HO-1, TLR4/PI3K/Akt, and CaMKKβ/LKB1/AMPK pathways and suppressing oxidative stress and inflammation [J]. Antioxidants, 2021, 10(6): 897
[26] Xu X L, Liu X D, Yang Y C, et al. Resveratrol exerts anti-osteoarthritic effect by inhibiting TLR4/NF-κB signaling pathway via the TLR4/Akt/FoxO1 axis in IL-1β-stimulated SW1353 cells [J]. Drug Design, Development and Therapy, 2020, 14: 2079-2090
[27] 瞿梅, 沈薇. PI3K/Akt对饱和脂肪酸诱导肝细胞脂变内质网应激及脂性凋亡的影响[J]. 中华肝脏病杂志, 2015(3): 194-199
Qu M, Shen W. Role of PI3K/Akt pathway in endoplasmic reticulum stress and apoptosis induced by saturated fatty acid in human steatotic hepatocytes [J]. Chinese Journal of Hepatology, 2015(3): 194-199 (in Chinese)
[28] Shen H R, Xu X, Li X L. Berberine exerts a protective effect on rats with polycystic ovary syndrome by inhibiting the inflammatory response and cell apoptosis [J]. Reproductive Biology and Endocrinology: RB&E, 2021, 19(1): 3
[29] Noman A S, Koide N, Hassan F, et al. Thalidomide inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production via down-regulation of MyD88 expression [J]. Innate Immunity, 2009, 15(1): 33-41
[30] Lv Y Y, Jin Y, Han G Z, et al. Ursolic acid suppresses IL-6 induced C-reactive protein expression in HepG2 and protects HUVECs from injury induced by CRP [J]. European Journal of Pharmaceutical Sciences, 2012, 45(1-2): 190-194
[31] Schütze S, Machleidt T, Krönke M. The role of diacylglycerol and ceramide in tumor necrosis factor and interleukin-1 signal transduction [J]. Journal of Leukocyte Biology, 1994, 56(5): 533-541
[32] 荣青秀, 杨永坚, 张婉婉, 等. 二甲基甲酰胺诱导H9c2心肌细胞凋亡机制的初步实验研究[C]. 哈尔滨: 中华预防医学会, 2016: 5-7
Rong Q X, Yang Y J, Zhang W W, et al. Preliminary experimental study on the mechanism of H9c2 cardiomyocyte apoptosis induced by dimethylformamide [C]. Harbin: Chinese Preventive Medicine Association, 2016: 5-7
[33] 张蔓, 郑敏, 吴智君, 等. 二甲基甲酰胺对大鼠肝脏抗氧化能力及PPAR mRNA的影响[J]. 卫生研究, 2018, 47(3): 352-357
Zhang M, Zheng M, Wu Z J, et al. Effects of N,N-dimethylformamide on hepatic antioxidant capacity and liver PPARs mRNA levels in rats [J]. Journal of Hygiene Research, 2018, 47(3): 352-357 (in Chinese)