市政污水来源广泛、成分复杂,且汇聚多种污染物。当前,用于市政污水处理效果评估及排放管控的指标主要是总氮、总磷和化学需氧量等常规参数,在此基础上,近年来逐步增加了常规和优控污染物的监测,然而在评估中依然缺乏生物毒性指标。我国水环境管理正在经历从总量控制、质量管理逐步转化为风险管控的过程,而风险评估需要同时考虑暴露和效应水平。常规理化指标或单纯污染物浓度难以反映污水的综合风险,导致出水生态风险评估不确定性高,难以精准有效地指导市政污水处理方法的发展,市政污水处理厂出水可能成为受纳水体污染的重要源头之一。研究显示,处理后的市政污水仍含有药物与个人护理品[1-3]、农药[4-5]和卤代阻燃剂[6]等多种污染物,随其排放进入受纳水体,这些污染物在水环境中发生降解转化,产生加和、协同和拮抗等复合毒性作用,对水生生物产生内分泌干扰[7]、发育毒性[8]、神经毒性[9]和遗传毒性[10]等不良效应。近期,Pereda等[11]指出,尽管经过三级处理和高度稀释,污水处理厂出水排放仍会危害受纳水环境的生态系统功能。低浓度、连续排放的出水会改变受纳水体的水质特征[12],造成复杂的复合污染状况。这些污染物及其转化产物的相互作用也将对水生生物形成复杂多变的暴露条件[2],可能破坏水生生物的生存环境,产生不良效应,影响水环境中鱼类及无脊椎动物的正常发育繁殖[13-15],从而改变受纳水生态系统的物种丰度[16]、群落组成和结构[17-18]及生态功能[11]。由此可见,有效管控污水处理厂出水及其受纳水体污染和风险对维护水生态健康尤为重要,但目前对出水受纳水体复合污染的风险评估研究仍较缺乏。
水生态风险评估包括暴露和效应评估,一般可通过模型估算或实际测量。当前常用的评估方法是采集环境样品,通过实验室化学分析获得样品中污染物浓度,进一步计算风险商;或通过实验室生物测试获得样品对受试生物的毒性效应。但样品采集和运输过程中污染物浓度、形态等会发生改变,且实验室生物测试较难反映野外真实暴露条件,这些差异都会降低风险评估的准确性[19]。近年发展起来的原位被动采样和生物暴露可有效降低实验室测试的误差,更加真实准确地反映暴露和效应的定量关系。
本综述在总结市政污水处理厂出水及受纳河流污染和风险评估研究现状的基础上,针对当前评估方法缺乏生物有效性和毒性识别的问题,提出利用原位被动采样-生物暴露联用技术追踪污水处理厂出水对受纳水体造成的污染暴露及效应变化,全面准确地评估受纳水体生态风险,为出水排放标准和风险管控提供数据支撑。
1 污水处理厂出水中污染物浓度和毒性(Concentrations and toxicity of contaminants in wastewater treatment plant effluents)
随着社会经济发展、人口增长及人类活动的加剧,近年来我国城镇和农村污水排放量均呈上升趋势。2020年全国城市污水排放量达571.4亿m3,比2010年增长了50.8%[20]。污水主要包括市政和工业污水,不同工业污水的成分差异较大,如纺织污水含大量有机染料,而石油化工污水含大量具有致癌性的烃类物质及其衍生物,但总体而言,其行业特征污染物一般较为清晰。针对各类工业污水的处理技术日益发展[21],污水处理设备不断升级,加之国家对工业污水的治理力度不断加大,工业污水污染得到较好的控制,排放量从2010年的237.5亿t下降到2016年的186.4亿t[22],并呈逐年下降趋势。相较而言,市政污水具有更大的排放量,来源广泛且成分更加复杂。各种传统和新污染物,如药物与个人护理品(pharmaceuticals and personal care products, PPCPs)、全氟化合物、农药和卤代/磷代阻燃剂等随着污水管网汇集到污水处理厂[3, 5-6, 23-24],进一步通过水解、光解和生物代谢等过程形成转化产物,不同污染物及其转化产物之间还会产生协同、加和、拮抗等复合毒性作用,导致严峻的复合污染问题。
当前污水处理厂最常用的处理技术是A2/O和氧化沟工艺,厌氧、好氧处理对氨氮、化学需氧量等常规污染物的处理效果很好,但传统的污水处理工艺难以去除不易生物降解的痕量污染物[4, 25],甚至出水中一些污染物浓度高于进水[26]。出水中仍有多种污染物被频繁检出(图1),包括PPCPs、杀虫剂、除草剂、性激素[27]和紫外吸收剂[28-29]等。其中,出水中多种PPCPs均有较高检出率,由于其潜在生物积累性和生物活性而受到广泛关注[13, 30]。例如,污水处理厂出水中12种典型PPCPs总浓度范围是242~16 561 ng·L-1[30-40],中国(5 541±5 145) ng·L-1高于美国(2 894±1 330) ng·L-1和欧洲(3 215±1 790) ng·L-1。不同PPCPs的污染特征也具有一定区域性,如出水中红霉素的浓度为98.1~14 400 ng·L-1((3 032±3 257) ng·L-1),显著高于除文拉法辛外的其他10种PPCPs(P<0.05)。中国污水处理厂出水中红霉素浓度(3 033±3 257) ng·L-1显著高于美国(145±118) ng·L-1和欧洲(398±269) ng·L-1;美国污水处理厂出水中磺胺甲恶唑的浓度则显著高于中国和欧洲;而欧洲地区卡马西平的浓度水平则显著高于中国(P<0.05,图2)。由此可见,污水处理厂出水中污染物的浓度和组成有较大的地域差异,可能与当地的生活习惯差异以及污水处理厂附近的工业污染相关。除PPCPs外,除草剂、杀虫剂和抗菌剂等多类新污染物也在出水中被广泛检出(图1(b)和1(c))。阿特拉津和异丙甲草胺的浓度分别是26~124 ng·L-1和75~471 ng·L-1[32, 35, 41]。德国污水处理厂出水检出新烟碱类杀虫剂吡虫啉的浓度高达237 ng·L-1[35]。抗菌剂三氯生在中国的检出浓度为14.7~226 ng·L-1[23, 37],与欧洲浓度水平相似(19~219 ng·L-1)[36, 42]。除此之外,人造甜味剂三氯蔗糖的出水浓度也较高,如中国污水处理厂出水浓度为1 370~5 490 ng·L-1[37]。大量研究表明,污水处理厂出水已成为水环境污染的重要点源之一[24, 43-45],对水生态系统的影响不容忽视。
然而,目前用于评估和监测出水水质的指标主要是氨氮、总磷和化学需氧量等常规指标,缺乏生物毒性指标,前期研究表明,污水处理厂出水仍会对水生生物产生毒性。如表1所示,Escher等[10]对澳大利亚2个市政污水处理厂出水和再生水进行了103种体外生物测试,结果表明内分泌干扰、遗传毒性等终点的阳性率高于63%;Gauthier和Vijayan[8]发现污水处理厂出水对斑马鱼胚胎造成发育延迟且对幼鱼造成代谢紊乱;Zhang等[47]利用斑马鱼死亡率表征出水急性毒性,同时进行微核试验和彗星实验检测遗传毒性,结果表明,出水的急性毒性被完全去除但遗传毒性没有降低,甚至有升高的趋势;Dépatie等[48]发现出水中溴代阻燃剂会干扰白斑狗鱼的脂质代谢功能;刘芸等[49]发现A2/O出水对大型溞的急性致死毒性显著降低,但出水中的内分泌干扰物仍然对大型溞具有明显生殖毒性。由此可见,经处理的污水处理厂出水排放可能威胁受纳水生态健康。
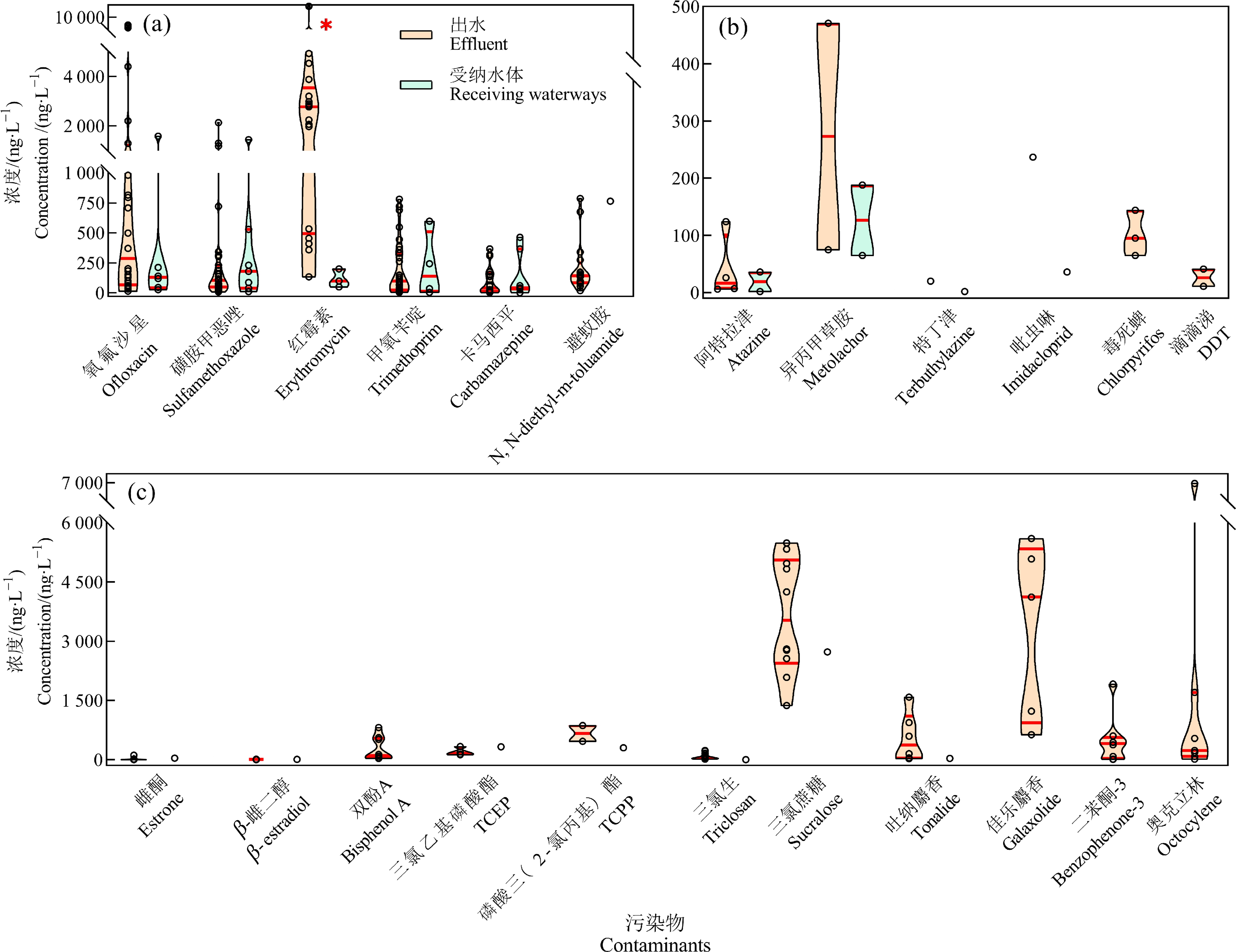
图1 全球出水[23, 27-42]及受纳水体[5, 12, 32, 34-35, 46]中典型污染物的浓度分布
注:(a)药物及个人护理品;(b)农药;(c)其他新污染物;3条红色实线分别为25%、50%和75%分位数;*表示浓度存在显著性差异(P<0.05);TCEP表示三(2-氯乙基)磷酸酯,TCPP表示磷酸三(1-氯-2-丙基)酯。
Fig. 1 Concentrations of typical contaminants in wastewater treatment plant effluent and the receiving waterways worldwide
Note: (a) Pharmaceutical and personal care products; (b) Pesticides; (c) Other emerging contaminants; the two red solid lines in the violins represent 25%, 50% and 75% of chemical concentrations; *indicates significant difference (P<0.05); TCEP means tris (2-chloroethyl) phosphate; TCPP means tris (1-chloro-2-propyl) phosphate.
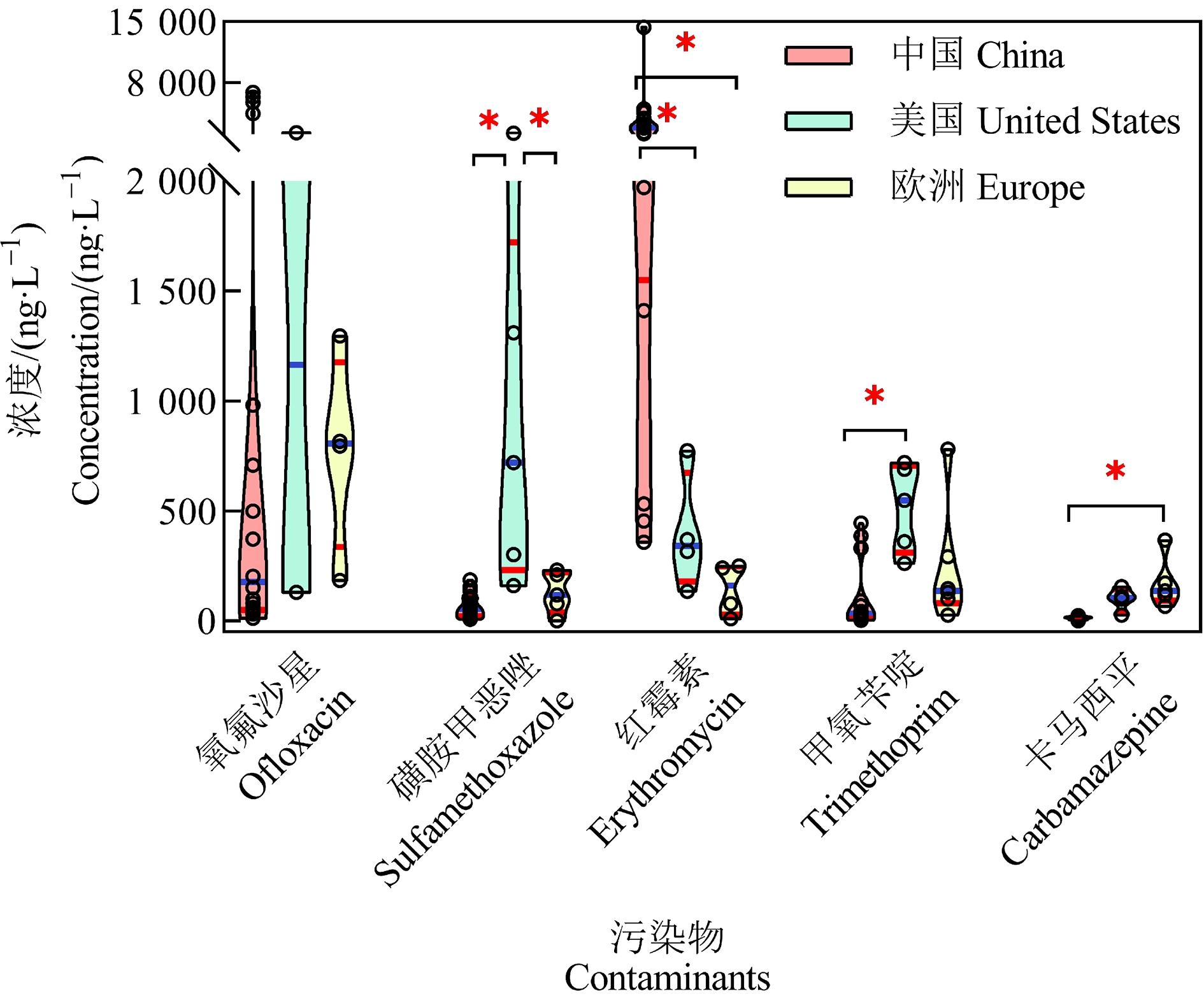
图2 污水处理厂出水中5个典型药物在中国[23, 31-33, 37, 39-40]、
美国[32-34]和欧洲[30, 35-36, 38, 42]的浓度水平
注:*表示浓度水平具有显著性差异(P<0.05)。
Fig. 2 Concentrations of five typical pharmaceuticals detected in wastewater treatment plant effluent in China, United States, and Europe
Note: *indicates significant difference (P<0.05).
2 污水处理厂出水受纳水体中污染物浓度和毒性(Concentrations and toxicity of contaminants in wastewater treatment plant effluent-receiving waterways)
截至2020年,中国城市污水处理厂达2 618座,污水处理率已达97.5%[20]。随着污水处理厂的扩增和污水处理率的上升,排放进入自然水体的出水也增加。一方面,受纳水体受到气候变化或人为影响而水量减少时,出水排放可补充水流量,一定程度上保护水生生物的栖息地[53];另一方面,出水排放导致的污染物输入又可能严重污染受纳水体(表 2),甚至破坏水生态结构和功能。以出水为主要水源的溪流与其上游或普通溪流相比,往往具有独特的物理和化学水质特征[54],连续的出水排放会改变受纳水体的溶解氧、温度、营养和化学成分负荷、毒性等[55]。有研究指出,出水口附近一般水温较高[56]而溶解氧水平较低[57],这可能会增加水体富营养化的风险并改变出水口附近的物种分布[53]。除此之外,污染物的输入也会对溪流生态系统造成严重影响。有研究显示,随出水进入受纳水体的污染物浓度与出水没有显著性差异,个别化合物的浓度甚至高于出水[34](图1)。出水中药物和个人护理品、农药、激素和微塑料等物质对受纳水体的生态影响已受到广泛的关注,研究发现受纳水体中的鱼类、蜗牛和钩虾等生物对药物、农药等物质具有一定的生物积累性[30, 58-59],药物的长期暴露对水生生物产生内分泌干扰效应[60]、生殖毒性[61],并改变鱼类肠道微生物群落变化[62],从而影响受纳水环境中鱼类健康(表1)。Liu等[52]将鲫鱼暴露在污水处理厂下游后分析其生物标志物变化,并结合实验室分析水样中药物活性物质的浓度,计算风险商,发现鲫鱼的综合生物标志物响应指数与药物活性物质的总风险商具有良好的相关性,说明出水中药物排放危害受纳水环境中鱼类健康。Münze等[5]发现出水输入显著增加了受纳水体中杀菌剂和杀虫剂的浓度,且出水是受纳水体中啶虫脒和吡虫啉的主要来源,增强河流毒性,影响无脊椎动物群落的组成和生态功能。Schmitz等[14]发现受纳水体促使鱼类代谢相关酶活性和氧化应激响应发生显著变化,长期暴露影响脂质代谢相关基因,进而影响其正常生长、繁殖[48]。
出水中污染物排放进入受纳河流后在水、颗粒物和沉积物等各介质间分配,并被生物累积(图3)。Schultz等[63]和Gibs等[1]在美国出水受纳河流的沉积物中检测到抗抑郁药、抗生素、三氯生和壬基酚等污染物。鱼类、水生和底栖无脊椎动物通过皮肤吸收和摄食颗粒物等途径累积污染物,有研究指出底栖贝类,如蛤蜊对抗抑郁药苯海拉明和乙酰氨基酚的生物累积系数已达美国环境保护局定义的中度生物累积水平[64]。可见,底栖无脊椎生物的生境也受到污水处理厂出水排放的影响,除了对底栖生物个体造成损伤外,还影响底栖无脊椎生物群落分布甚至生态功能[65]。
表1 污水处理厂出水及受纳水体生物测试方法及生物效应
Table 1 Bioassay approaches and biological effects of wastewater treatment plant effluent and the receiving waterways
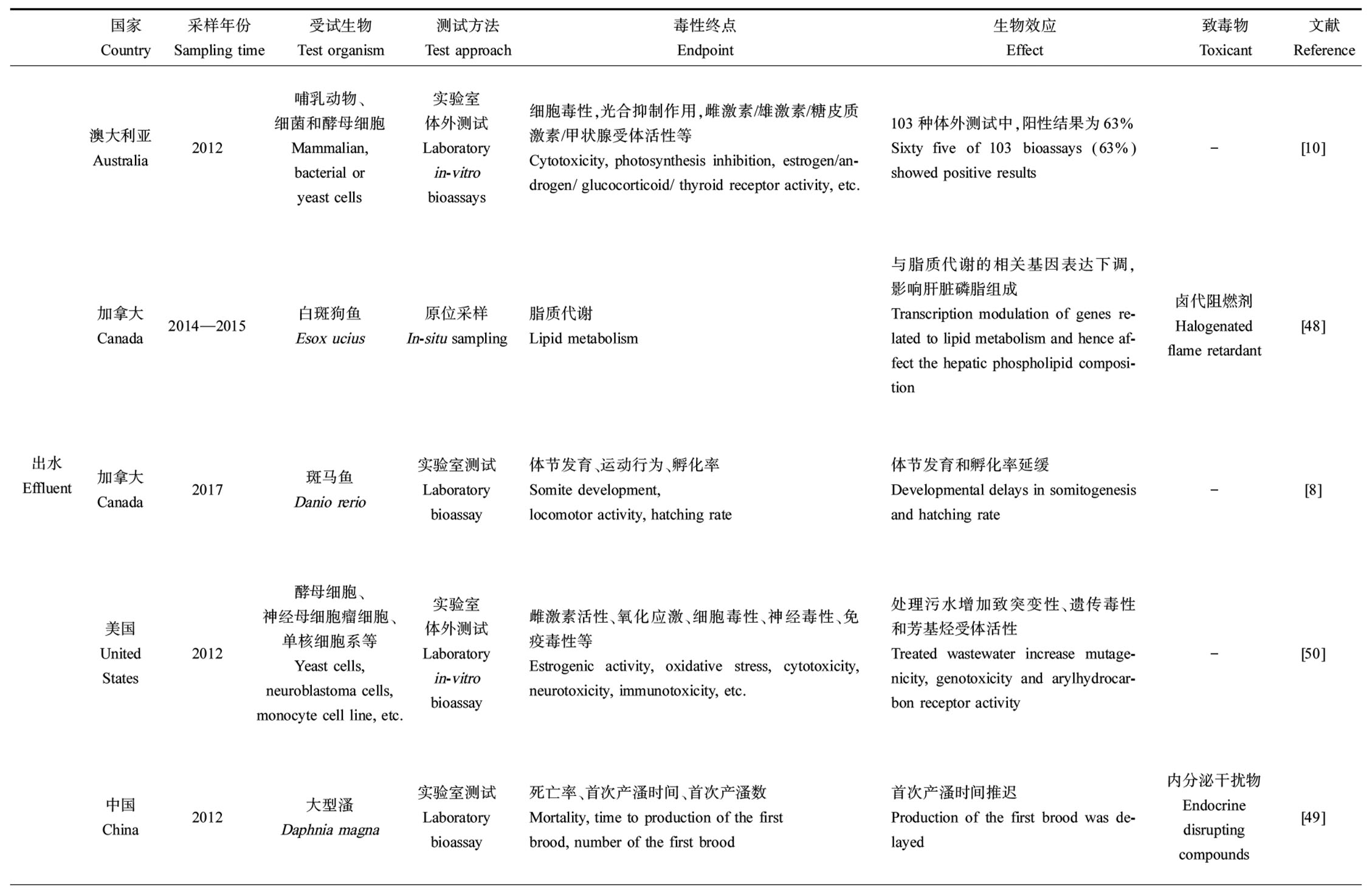
国家Country采样年份Sampling time受试生物Test organism测试方法Test approach毒性终点Endpoint生物效应Effect致毒物Toxicant文献Reference出水Effluent澳大利亚Australia2012哺乳动物、细菌和酵母细胞Mammalian, bacterial or yeast cells实验室体外测试Laboratory in-vitrobioassays细胞毒性,光合抑制作用,雌激素/雄激素/糖皮质激素/甲状腺受体活性等Cytotoxicity, photosynthesis inhibition, estrogen/an-drogen/ glucocorticoid/ thyroid receptor activity, etc.103种体外测试中,阳性结果为63%Sixty five of 103 bioassays (63%) showed positive results-[10]加拿大Canada2014—2015白斑狗鱼Esox ucius原位采样In-situ sampling脂质代谢Lipid metabolism与脂质代谢的相关基因表达下调,影响肝脏磷脂组成Transcription modulation of genes re-lated to lipid metabolism and hence af-fect the hepatic phospholipid composi-tion卤代阻燃剂Halogenated flame retardant[48]加拿大Canada2017斑马鱼Danio rerio实验室测试Laboratory bioassay体节发育、运动行为、孵化率Somite development, locomotor activity, hatching rate体节发育和孵化率延缓Developmental delays in somitogenesis and hatching rate-[8]美国United States2012酵母细胞、神经母细胞瘤细胞、单核细胞系等Yeast cells, neuroblastoma cells, monocyte cell line, etc.实验室体外测试Laboratoryin-vitrobioassay雌激素活性、氧化应激、细胞毒性、神经毒性、免疫毒性等Estrogenic activity, oxidative stress, cytotoxicity, neurotoxicity, immunotoxicity, etc.处理污水增加致突变性、遗传毒性和芳基烃受体活性Treated wastewater increase mutage-nicity, genotoxicity and arylhydrocar-bon receptor activity-[50]中国China2012大型溞Daphnia magna实验室测试Laboratory bioassay死亡率、首次产溞时间、首次产溞数Mortality, time to production of the first brood, number of the first brood首次产溞时间推迟Production of the first brood was de-layed内分泌干扰物Endocrine disrupting compounds[49]续表1国家Country采样年份Sampling time受试生物Test organism测试方法Test approach毒性终点Endpoint生物效应Effect致毒物Toxicant文献Reference
续表1
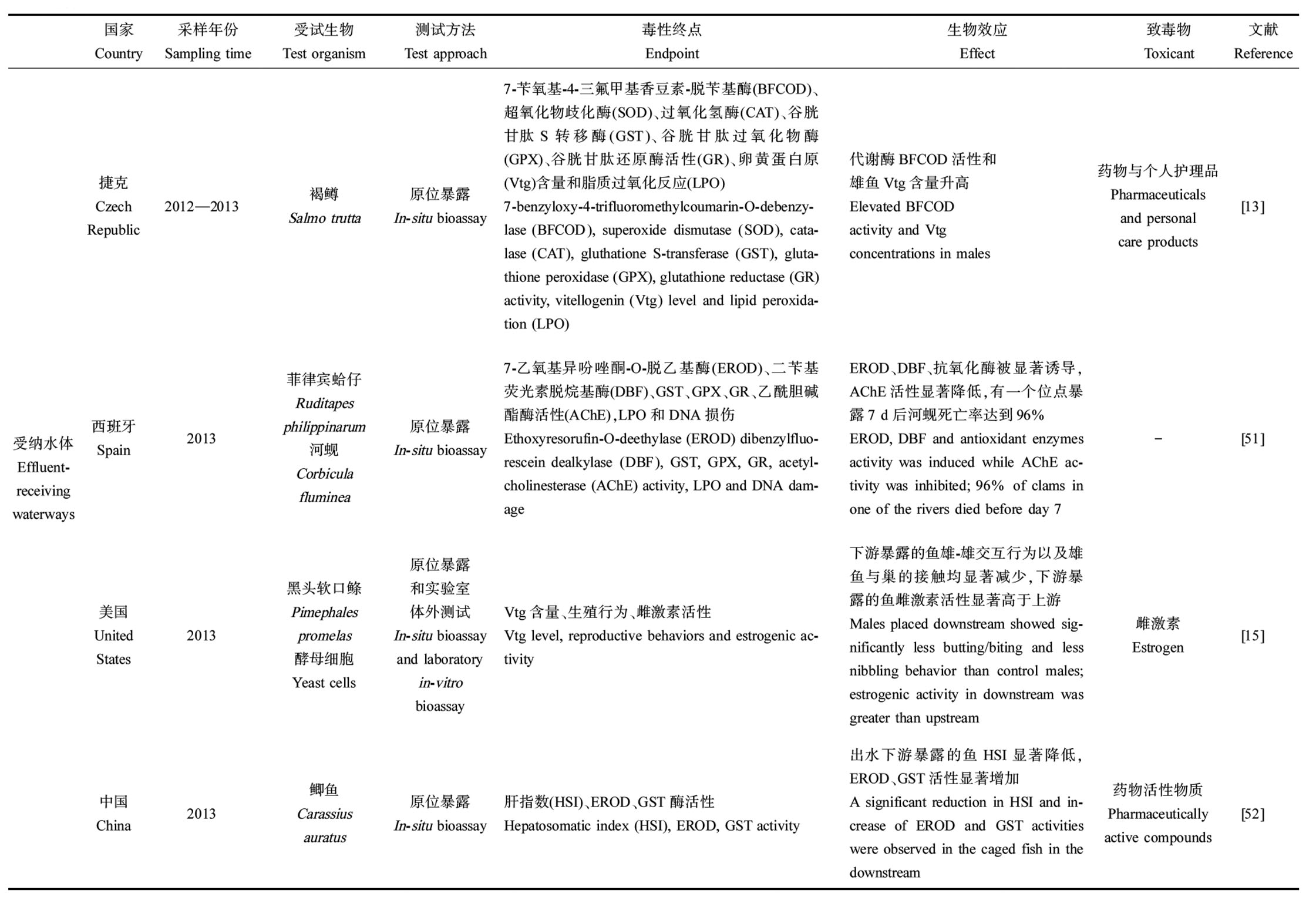
受纳水体Effluent-receiving waterways捷克Czech Republic2012—2013褐鳟Salmo trutta原位暴露In-situ bioassay7-苄氧基-4-三氟甲基香豆素-脱苄基酶(BF-COD)、超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、谷胱甘肽S转移酶(GST)、谷胱甘肽过氧化物酶(GPX)、谷胱甘肽还原酶活性(GR)、卵黄蛋白原(Vtg)含量和脂质过氧化反应(LPO)7-benzyloxy-4-trifluoromethylcoumarin-O-de-benzylase (BFCOD), superoxide dismutase (SOD), catalase (CAT), gluthatione S-transferase (GST), glutathione peroxidase (GPX), glutathione reductase (GR) activity, vitellogenin (Vtg) level and lipid per-oxidation (LPO)代谢酶BFCOD活性和雄鱼Vtg含量升高Elevated BFCOD activity and Vtg concentrations in males药物与个人护理品Pharmaceuticals and personal care products[13]西班牙Spain2013菲律宾蛤仔Ruditapes philippinarum河蚬Corbicula fluminea原位暴露In-situ bioassay7-乙氧基异吩唑酮-O-脱乙基酶(EROD)、二苄基荧光素脱烷基酶(DBF)、GST、GPX、GR、乙酰胆碱酯酶活性(AChE),LPO和DNA损伤Ethoxyresorufin-O-deethylase (EROD) dibenzylflu-orescein dealkylase (DBF), GST, GPX, GR, acetyl-cholinesterase (AChE) activity, LPO and DNA dam-ageEROD、DBF、抗氧化酶被显著诱导,AChE活性显著降低,有一个位点暴露7 d后河蚬死亡率达到96%EROD, DBF and antioxidant enzymes activity was induced while AChE ac-tivity was inhibited; 96% of clams in one of the rivers died before day 7-[51]美国United States2013黑头软口鲦Pimephales promelas酵母细胞Yeast cells原位暴露和实验室体外测试In-situ bioassay and laboratory in-vitrobioassayVtg含量、生殖行为、雌激素活性Vtg level, reproductive behaviors and estrogenic ac-tivity下游暴露的鱼雄-雄交互行为以及雄鱼与巢的接触均显著减少,下游暴露的鱼雌激素活性显著高于上游Males placed downstream showed sig-nificantly less butting/biting and less nibbling behavior than control males; estrogenic activity in downstream was greater than upstream雌激素Estrogen[15]中国China2013鲫鱼Carassius auratus原位暴露In-situ bioassay肝指数(HSI)、EROD、GST酶活性Hepatosomatic index (HSI), EROD, GST activity出水下游暴露的鱼HSI显著降低,EROD、GST活性显著增加A significant reduction in HSI and in-crease of EROD and GST activities were observed in the caged fish in the downstream药物活性物质Pharmaceutically active compounds[52]
表2 受纳水体中常见污染物种类和浓度水平
Table 2 Concentrations of contaminants in wastewater treatment plant effluent-receiving waterways
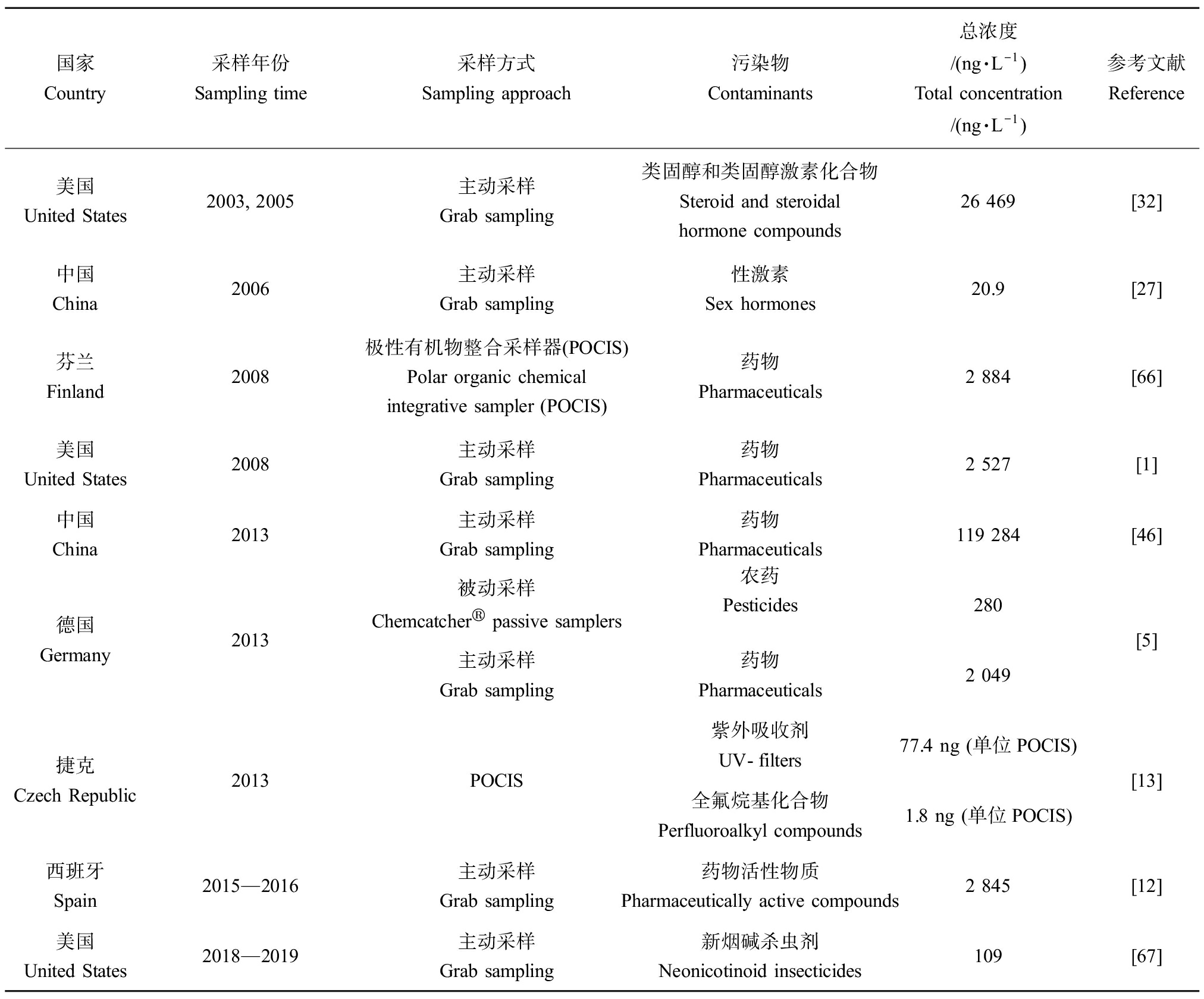
国家Country采样年份Sampling time采样方式Sampling approach污染物Contaminants总浓度/(ng·L-1)Total concentration/(ng·L-1)参考文献Reference美国United States2003, 2005主动采样Grab sampling类固醇和类固醇激素化合物Steroid and steroidal hormone compounds26 469[32]中国China2006主动采样Grab sampling性激素Sex hormones20.9[27]芬兰Finland2008极性有机物整合采样器(POCIS)Polar organic chemical integrative sampler (POCIS) 药物Pharmaceuticals2 884[66]美国United States2008主动采样Grab sampling药物Pharmaceuticals2 527[1]中国China2013主动采样Grab sampling药物Pharmaceuticals119 284[46]德国Germany2013被动采样Chemcatcher® passive samplers农药Pesticides280主动采样Grab sampling药物Pharmaceuticals2 049[5]捷克Czech Republic2013POCIS紫外吸收剂UV-filters77.4 ng (单位POCIS)全氟烷基化合物Perfluoroalkyl compounds1.8 ng (单位POCIS)[13]西班牙Spain2015—2016主动采样Grab sampling药物活性物质Pharmaceutically active compounds2 845[12]美国United States2018—2019主动采样Grab sampling 新烟碱杀虫剂Neonicotinoid insecticides109[67]
受纳水体水质和毒性水平随着水流传输而动态变化,上游河水的稀释作用、流速、流内衰减和季节变化等因素均会影响这一动态变化过程[55, 57, 68]。Barber等[32]对比了2个污水处理厂出水中多种有机污染物进入受纳河流后的迁移特征,发现流量、流速等水文条件明显影响污染物在受纳河流中的迁移过程,包括持久性和迁移距离,如雌激素、有机磷酸酯和药物等有机污染物在受纳河流中持久存在,迁移距离最高可达160 km,这无疑对下游水生健康造成威胁。Zhi等[2]量化了污水处理厂出水中109种药物及其代谢产物在受纳河流5 km内的衰减率,发现不同药物的衰减率差异很大,抗糖尿病药二甲双胍衰减率较低,在受纳河流中维持较高污染水平;而西酞普兰由于其容易被沉积物颗粒吸附而具有较高的衰减率,对于多数药物及其转化产物来说,沉积物吸附作用是它们在受纳河流中的主要衰减机制[69]。污染物在迁移过程中的动态衰减对水生生物形成复杂的暴露条件,进而影响受纳水生态健康。
污水处理厂出水中污染物在受纳水体中发生光降解、生物降解等转化过程进而生成各种转化产物,由于转化产物理化性质、环境行为和生物毒性均可能与母体有较大差异,使得受纳水体复合污染风险评估更加复杂。Yin等[70]开展了污水处理厂出水的光降解、生物降解及光-生物降解联合实验,结果表明光-生物降解联合可减少62.4%的溶解性有机碳,而光降解不仅减少了出水中芳香族化合物的数量,转化产物的分子量也明显变小,使得转化产物更易被生物利用。Webb等[67]发现受纳水体下游新烟碱农药吡虫啉的转化产物吡虫啉脲的浓度比出水高1.6倍~1.8倍,说明吡虫啉可能发生了河内转化;而吡虫啉脲是一种环境稳定性更高的转化产物[71-72],可能带来潜在的生态威胁。越来越多的研究表明,一些药物(如双氯芬酸、二甲双胍等)[73-74]和农药[75]的转化产物具有与母体相近甚至更高的水生毒性,因此,亟须将转化产物纳入监管范畴并评估其水生态风险[76]。
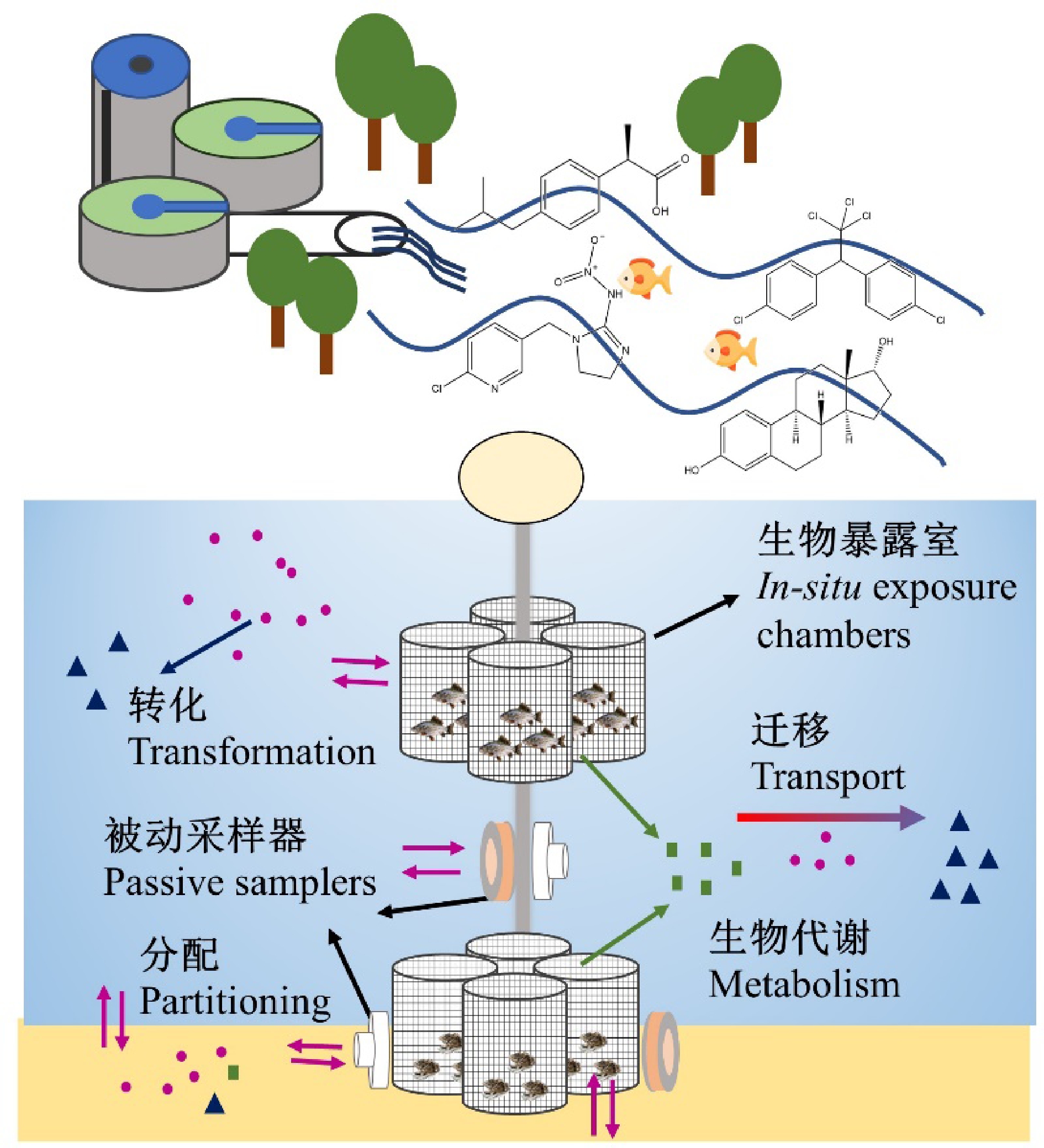
图3 利用原位被动采样-生物暴露联用方法追踪污水处理厂出水受纳水体生态风险示例
Fig. 3 Tracking ecological risk of effluent-receiving waterways using in-situ passive sampling and bioassays
3 追踪污水处理厂出水对受纳河流污染和毒性的方法(Approaches to track occurrence and toxicity of contaminants in effluent-receiving waterways)
鉴于污水处理厂出水对受纳水体的危害,一方面要强化污水处理技术,提高不同类型污染物的去除率。研究表明污水处理技术的提升能有效减少出水污染物的排放[77]并改善受纳水体的群落结构[78]。另一方面需准确追踪和评估出水中污染物在受纳水体中的污染状况、迁移转化等环境行为和毒性风险。目前,监测水环境污染水平的方法仍以主动采样为主,即采集野外环境样品带回实验室进行化学分析,对比毒性阈值,利用商值法评估生态风险。Jiang等[46]通过采集地表水、沉积物和地下水样品,分析了中国北方典型污水处理厂出水受纳水体中抗生素的分布情况,结果显示四环素、土霉素等是受纳水体中浓度较高的抗生素,通过风险商评估生态风险,发现受纳水体中抗生素对水生微生物造成较高风险。Zhi等[2]进行长达2年的野外采样,分析了温带地区污水处理厂出水受纳水体中药物及转化产物的分布差异,表明污染物在受纳水体中的迁移转化过程对水生生物造成复杂的复合暴露。Argolo等[79]利用细胞测试评估受纳水体中自由溶解态和颗粒结合态污染物的雌激素活性,发现颗粒相中雌激素活性与水相当甚至更高,呼吁关注受纳水体颗粒相中内分泌干扰物的生态风险。
主动采样在水生态风险评估中应用广泛,但存在一定局限性。通过主动采样获取的污染物浓度仅代表水环境中该污染物的瞬时浓度[80],而污水处理厂出水及其他输入源排放、流速和降雨等均会改变污染物的浓度。换言之,污染物在受纳水体中的分布水平随环境变化而发生时空动态变化,单次主动采样获得的瞬时数据并不具有代表性。另外,样品运输过程中污染物的浓度、形态、结构极可能发生改变,而且实验室恒定的温度、pH、溶解氧和光照等实验条件并不能真实反映野外实际暴露情况[81]。由此可见,利用主动采样获得的监测数据或风险评估结果存在一定的不确定性。相较而言,利用野外原位评估方法可以有效降低风险评估的不确定性。有研究显示,实验室和原位生物测试结果并不总是一致[19, 82],实验室和原位评估联用可获得更具说服力的结果,因此原位评估在生态风险评估中的应用极为重要。野外原位评估包括原位暴露评估、原位效应评估和原位暴露-效应联合评估等多种场景[83]。
原位暴露评估主要利用被动采样技术原位获得污染物的自由溶解态浓度,并通过商值法评估生态风险。原位被动采样获得基于生物有效性的时间加权浓度,相比主动采样的瞬时总浓度,能够更加真实有效地反映实际生物暴露水平[84]。常用的被动采样技术包括聚乙烯膜装置(polyethylene device, PED)、极性有机物整合采样器(polar organic chemical integrative sampler, POCIS)、半渗透膜装置(semi-permeable membrane device, SPMD)和薄膜梯度扩散装置 (diffusive gradients in thin films, DGT)等,用于监测水中的有机污染物、重金属及营养元素。Elkayar等[7]结合POCIS原位采样和实验室细菌毒性测试,发现污水处理厂出水受纳水体下游数千米处仍检出抗雄激素活性。Xie等[85]利用改良的POCIS监测污水处理厂进水、各处理阶段、出水及受纳水体污染水平,检出包括麝香、新烟碱类农药和抗生素等多种新污染物,结合斑马鱼胚胎测试,发现除污水处理厂出水排放外,城市受纳水体还受到非点源污染的影响。Chaudhary等[86]利用DGT测定出水受纳水体的水-沉积物界面处重金属的生物有效浓度并评估水生态风险,结果表明低浓度重金属造成的生态风险较低。该研究提出利用DGT测定水环境中重金属的生物有效浓度并评估其生态风险是一种行之有效的方法。
原位效应评估包括原位生物测试、采集野外生物进行效应评估和野外生态调查等方式。原位生物测试是通过将受试生物投放至野外环境进行暴露后,评估污染物在生物中的累积潜力及毒性[87]。原位生物测试已被广泛应用于水生态风险评估领域,受试生物涵盖了鱼类(如黑头软口鲦[88]、罗非鱼[89]等)、双壳贝类(如菲律宾蛤仔[90]、河蚬[51]等)和甲壳类等大型无脊椎动物(如钩虾[91]、大型溞[92]等)。暴露的环境介质可分为上层水体、底层水体、水-沉积物界面和沉积物孔隙水等。受试生物的毒性终点涵盖了从基因水平到个体水平,包括与内分泌、脂质代谢等相关的基因表达、代谢酶和抗氧化酶的活性、行为、生长速率和存活率等指标(表1)。一般根据研究目标和生物特征选择暴露装置、暴露形式和毒性终点。Skelton等[93]将黑头软口鲦原位暴露于受污水处理厂出水和其他非点源影响的河流中,4 d后对肝脏分别进行1H核磁共振和气相色谱分析其代谢组变化,发现出水及受纳水体暴露会扰乱黑头软口鲦的肝脏代谢。Aguirre-Martínez和Martín-Díaz[51]分别将菲律宾蛤仔和河蚬原位暴露于接受污水处理厂出水的海水和淡水中,结合DNA损伤、氧化应激、生物代谢和神经毒性相关的多种生物标志物评估受纳水环境沉积物对无脊椎动物的毒性特征,发现河蚬在出水中暴露7 d后死亡率高达96%,持续排放的高毒性出水严重威胁受纳淡水和海洋生物的生长繁殖。
采集野外生物分析其体内污染物浓度和毒性也是常用于评估受纳水体复合污染的重要方法。Munz等[59]采集了生活在污水处理厂出水受纳水体中的钩虾,分析其体内新烟碱类农药的浓度,并利用商值法估算毒性风险,发现基于内暴露浓度估算的风险高于基于外暴露的风险,呼吁在风险评估时需重视内暴露水平。除单独生物个体毒性外,还可通过测定不同营养级生物体内污染物浓度,探究污水处理厂出水中污染物在受纳水体中的生物放大效应[94]。此外,野外生态调查通过定量本土鱼类及无脊椎动物的种群丰度, 可直接反映生态系统的受损情况。Sánchez-Morales等[17]发现污水处理厂出水受纳水体下游多种大型甲壳类动物、水生昆虫幼虫的数量急剧下降,说明出水排放会影响受纳水体中生物种群的丰度,破坏水生态系统结构和功能。
相对于单独使用原位暴露或原位效应评估,原位暴露和效应的联合评估能够同时获得暴露与效应的关系(图3),使得评估结果更加全面和准确。然而,目前原位暴露和效应联合评估在污水处理厂出水受纳水体风险评估中的应用仍较缺乏。Lahti等[66]结合POCIS和原位生物暴露评估污水处理厂出水受纳水体中药物的生物暴露水平,虽然原位暴露后虹鳟鱼的7-乙氧基异吩唑酮-O-脱乙基酶(ethoxyresorufin-O-deethylase, EROD)活性和卵黄蛋白原含量无明显变化,但原位暴露生物表现出对药物的明显累积能力。柯润辉等[95]结合SPMD、体外细胞测试和原位生物暴露(鲫鱼)评估太湖水体的芳烃受体效应,SPMD定量的生物有效浓度与肝脏EROD活性具有良好相关性,表明原位暴露和效应数据具有较好的一致性。Burton等[96]研发了一套原位水-沉积物毒性评估装置,通过被动采样器SPMD和DGT获取污染物浓度水平,同时获得多种生物(水生、底表和底栖生物)的效应信息,并结合水质监测器获得的实时水文水质参数,综合评估上层水和沉积物的生态风险。综上所述,联合原位被动采样和生物暴露技术,建立污染物生物有效浓度和生物毒性间的定量关系,是综合评估污水处理厂出水中污染物对受纳水体风险的有效手段,评估结果可为有效管控出水受纳水体污染和风险提供基础数据。
4 展望(Perspectives)
针对污水处理厂出水造成的受纳水体复合污染问题,一方面要强化污水处理技术,提高不同类型污染物的去除率;另一方面还需加强对出水和受纳水体暴露和效应的联合评估。出水中含有多种污染物,是一个复杂的混合暴露体系,单纯通过污染物监测的暴露评估可能会低估出水的风险,除了要加强对新污染物及其转化产物的识别外,还需结合生物效应评估以准确甄别出水复合毒性的关键致毒物。污染物在受纳水体中的迁移转化过程可能会使毒性发生改变,包括污染物组成、联合毒性作用。因此,迫切需要追踪受纳水体的暴露和效应变化,利用原位被动采样-生物暴露联合技术,可更准确全面地追踪污水处理厂出水受纳水体的生态风险,为改善出水水质及管控出水受纳水生态风险提供基础数据。
[1] Gibs J, Heckathorn H A, Meyer M T, et al. Occurrence and partitioning of antibiotic compounds found in the water column and bottom sediments from a stream receiving two wastewater treatment plant effluents in northern New Jersey, 2008 [J]. The Science of the Total Environment, 2013, 458-460: 107-116
[2] Zhi H, Kolpin D W, Klaper R D, et al. Occurrence and spatiotemporal dynamics of pharmaceuticals in a temperate-region wastewater effluent-dominated stream: Variable inputs and differential attenuation yield evolving complex exposure mixtures [J]. Environmental Science & Technology, 2020, 54(20): 12967-12978
[3] Ramírez-Morales D, Masís-Mora M, Montiel-Mora J R, et al. Occurrence of pharmaceuticals, hazard assessment and ecotoxicological evaluation of wastewater treatment plants in Costa Rica [J]. The Science of the Total Environment, 2020, 746: 141200
[4] Munz N A, Burdon F J, de Zwart D, et al. Pesticides drive risk of micropollutants in wastewater-impacted streams during low flow conditions [J]. Water Research, 2017, 110: 366-377
[5] Münze R, Hannemann C, Orlinskiy P, et al. Pesticides from wastewater treatment plant effluents affect invertebrate communities [J]. The Science of the Total Environment, 2017, 599-600: 387-399
[6] Krzeminski P, Schwermer C, Wennberg A, et al. Occurrence of UV filters, fragrances and organophosphate flame retardants in municipal WWTP effluents and their removal during membrane post-treatment [J]. Journal of Hazardous Materials, 2017, 323: 166-176
[7] Elkayar K, Park J A, Pineda M, et al. Passive sampling and in vitro assays to monitor antiandrogens in a river affected by wastewater discharge [J]. The Science of the Total Environment, 2022, 804: 150067
[8] Gauthier P T, Vijayan M M. Municipal wastewater effluent exposure disrupts early development, larval behavior, and stress response in zebrafish [J]. Environmental Pollution, 2020, 259: 113757
[9] Silva S, Cravo A, Ferreira C, et al. Biomarker responses of the clam Ruditapes decussatus exposed to a complex mixture of environmental stressors under the influence of an urban wastewater-treatment plant [J]. Environmental Toxicology and Chemistry, 2021, 40(1): 272-283
[10] Escher B I, Allinson M, Altenburger R, et al. Benchmarking organic micropollutants in wastewater, recycled water and drinking water with in vitro bioassays [J]. Environmental Science & Technology, 2014, 48(3): 1940-1956
[11] Pereda O, Solagaistua L, Atristain M, et al. Impact of wastewater effluent pollution on stream functioning: A whole-ecosystem manipulation experiment [J]. Environmental Pollution, 2020, 258: 113719
[12] Mandaric L, Mor J R, Sabater S, et al. Impact of urban chemical pollution on water quality in small, rural and effluent-dominated Mediterranean streams and rivers [J]. The Science of the Total Environment, 2018, 613-614: 763-772
[13] Giang P T, Sakalli S, Fedorova G, et al. Biomarker response, health indicators, and intestinal microbiome composition in wild brown trout (Salmo trutta m. fario L.) exposed to a sewage treatment plant effluent-dominated stream [J]. The Science of the Total Environment, 2018, 625: 1494-1509
[14] Schmitz M, Deutschmann B, Markert N, et al. Demonstration of an aggregated biomarker response approach to assess the impact of point and diffuse contaminant sources in feral fish in a small river case study [J]. The Science of the Total Environment, 2022, 804: 150020
[15] Leese J M, McMahon J, Colosi J C. Effects of wastewater treatment plant effluent in a receiving stream on reproductive behavior of fathead minnows (Pimephales promelas) [J]. Fishes, 2021, 6(2): 14
[16] Millar E N, Surette M G, Kidd K A. Altered microbiomes of aquatic macroinvertebrates and riparian spiders downstream of municipal wastewater effluents [J]. Science of the Total Environment, 2022, 809: 151156
[17] Sánchez-Morales M, Sabater F, Mu oz I. Effects of urban wastewater on hyporheic habitat and invertebrates in Mediterranean streams [J]. Science of the Total Environment, 2018, 642: 937-945
oz I. Effects of urban wastewater on hyporheic habitat and invertebrates in Mediterranean streams [J]. Science of the Total Environment, 2018, 642: 937-945
[18] Pascual-Benito M, Ballesté E, Monleón-Getino T, et al. Impact of treated sewage effluent on the bacterial community composition in an intermittent Mediterranean stream [J]. Environmental Pollution, 2020, 266(Pt 1): 115254
[19] Smolders R, Bervoets L, Blust R. In situ and laboratory bioassays to evaluate the impact of effluent discharges on receiving aquatic ecosystems [J]. Environmental Pollution, 2004, 132(2): 231-243
[20] 中华人民共和国住房和城乡建设部. 2020年城乡建设统计年鉴全国历年城市排水和污水处理情况[R]. 北京: 中华人民共和国住房和城乡建设部, 2021
Ministry of Housing and Urban-Rural Development of the People’s Republic of China. National urban drainage and wastewater treatment in past years 2020 [R]. Beijing: Ministry of Housing and Urban-Rural Development of the People’s Republic of China, 2021 (in Chinese)
[21] 陈蕾, 王郑. 电化学高级氧化技术在工业废水处理中的应用[J]. 应用化工, 2019, 48(2): 434-437, 443
Chen L, Wang Z. Application of electrochemical advanced oxidation processes in industrial wastewater treatment [J]. Applied Chemical Industry, 2019, 48(2): 434-437, 443 (in Chinese)
[22] 中华人民共和国生态环境部. 2012-2017环境统计年报[R]. 北京: 中华人民共和国生态环境部, 2018
Ministry of Ecology and Environment of the People’s Republic of China. Annual statistic report on environment 2012-2017 [R]. Beijing: Ministry of Ecology and Environment of the People’s Republic of China, 2018 (in Chinese)
[23] Ben W W, Zhu B, Yuan X J, et al. Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China: Comparison of wastewater treatment processes [J]. Water Research, 2018, 130: 38-46
[24] Wang X B, Yu N Y, Qian Y L, et al. Non-target and suspect screening of per- and polyfluoroalkyl substances in Chinese municipal wastewater treatment plants [J]. Water Research, 2020, 183: 115989
[25] 柴玉峰, 张玉秀, 陈梅雪, 等. 冀西北典型北方小城镇污水处理厂中抗生素的分布和去除[J]. 环境科学, 2018, 39(6): 2724-2731
Chai Y F, Zhang Y X, Chen M X, et al. Distribution and treatment of antibiotics in typical WWTPs in small towns in China [J]. Environmental Science, 2018, 39(6): 2724-2731 (in Chinese)
[26] Liu S S, Cai Q S, Li C L, et al. In situ measurement of an emerging persistent, mobile and toxic (PMT) substance - Melamine and related triazines in waters by diffusive gradient in thin-films [J]. Water Research, 2021, 206: 117752
[27] Chang H, Wan Y, Wu S M, et al. Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: Comparison to estrogens [J]. Water Research, 2011, 45(2): 732-740
[28] Li W H, Ma Y M, Guo C S, et al. Occurrence and behavior of four of the most used sunscreen UV filters in a wastewater reclamation plant [J]. Water Research, 2007, 41(15): 3506-3512
[29] Langford K H, Reid M J, Fjeld E, et al. Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway [J]. Environment International, 2015, 80: 1-7
[30] Grabicova K, Grabic R, Fedorova G, et al. Bioaccumulation of psychoactive pharmaceuticals in fish in an effluent dominated stream [J]. Water Research, 2017, 124: 654-662
[31] Leung H W, Minh T B, Murphy M B, et al. Distribution, fate and risk assessment of antibiotics in sewage treatment plants in Hong Kong, South China [J]. Environment International, 2012, 42: 1-9
[32] Barber L B, Keefe S H, Brown G K, et al. Persistence and potential effects of complex organic contaminant mixtures in wastewater-impacted streams [J]. Environmental Science & Technology, 2013, 47(5): 2177-2188
[33] Arnnok P, Singh R R, Burakham R, et al. Selective uptake and bioaccumulation of antidepressants in fish from effluent-impacted Niagara River [J]. Environmental Science & Technology, 2017, 51(18): 10652-10662
[34] Dong B F, Kahl A, Cheng L, et al. Fate of trace organics in a wastewater effluent dependent stream [J]. The Science of the Total Environment, 2015, 518-519: 479-490
[35] Müller M E, Werneburg M, Glaser C, et al. Influence of emission sources and tributaries on the spatial and temporal patterns of micropollutant mixtures and associated effects in a small river [J]. Environmental Toxicology and Chemistry, 2020, 39(7): 1382-1391
[36] Díaz-Gardu o B, Pintado-Herrera M G, Biel-Maeso M, et al. Environmental risk assessment of effluents as a whole emerging contaminant: Efficiency of alternative tertiary treatments for wastewater depuration [J]. Water Research, 2017, 119: 136-149
o B, Pintado-Herrera M G, Biel-Maeso M, et al. Environmental risk assessment of effluents as a whole emerging contaminant: Efficiency of alternative tertiary treatments for wastewater depuration [J]. Water Research, 2017, 119: 136-149
[37] Yang Y Y, Liu W R, Liu Y S, et al. Suitability of pharmaceuticals and personal care products (PPCPs) and artificial sweeteners (ASs) as wastewater indicators in the Pearl River Delta, South China [J]. The Science of the Total Environment, 2017, 590-591: 611-619
![]() S, Senta I, Ahel M, et al. Occurrence and fate of emerging wastewater contaminants in Western Balkan Region [J]. The Science of the Total Environment, 2008, 399(1-3): 66-77
S, Senta I, Ahel M, et al. Occurrence and fate of emerging wastewater contaminants in Western Balkan Region [J]. The Science of the Total Environment, 2008, 399(1-3): 66-77
[39] Sun Q, Li M Y, Ma C, et al. Seasonal and spatial variations of PPCP occurrence, removal and mass loading in three wastewater treatment plants located in different urbanization areas in Xiamen, China [J]. Environmental Pollution, 2016, 208(Pt B): 371-381
[40] Sui Q, Huang J, Deng S B, et al. Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in different biological wastewater treatment processes [J]. Environmental Science & Technology, 2011, 45(8): 3341-3348
[41] Köck-Schulmeyer M, Villagrasa M, López de Alda M, et al. Occurrence and behavior of pesticides in wastewater treatment plants and their environmental impact [J]. The Science of the Total Environment, 2013, 458-460: 466-476
[42] Rosal R, Rodríguez A, Perdigón-Melón J A, et al. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation [J]. Water Research, 2010, 44(2): 578-588
[43] Coors A, Vollmar P, Sacher F, et al. Prospective environmental risk assessment of mixtures in wastewater treatment plant effluents - Theoretical considerations and experimental verification [J]. Water Research, 2018, 140: 56-66
[44] Lei K, Lin C Y, Zhu Y, et al. Estrogens in municipal wastewater and receiving waters in the Beijing-Tianjin-Hebei region, China: Occurrence and risk assessment of mixtures [J]. Journal of Hazardous Materials, 2020, 389: 121891
[45] Eggen R I, Hollender J, Joss A, et al. Reducing the discharge of micropollutants in the aquatic environment: The benefits of upgrading wastewater treatment plants [J]. Environmental Science & Technology, 2014, 48(14): 7683-7689
[46] Jiang Y H, Li M X, Guo C S, et al. Distribution and ecological risk of antibiotics in a typical effluent-receiving river (Wangyang River) in North China [J]. Chemosphere, 2014, 112: 267-274
[47] Zhang J, Zhang Y B, Liu W, et al. Evaluation of removal efficiency for acute toxicity and genotoxicity on zebrafish in anoxic-oxic process from selected municipal wastewater treatment plants [J]. Chemosphere, 2013, 90(11): 2662-2666
[48] Dépatie C, Houde M, Verreault J. Environmental exposure of northern Pike to a primary wastewater effluent: Impact on the lipidomic profile and lipid metabolism [J]. Aquatic Toxicology, 2020, 221: 105421
[49] 刘芸, 胡小英, 赵旭, 等. 广州市某A2/O工艺城镇污水处理厂出水对大型溞的生殖毒性作用[J]. 生态毒理学报, 2019, 14(5): 144-151
Liu Y, Hu X Y, Zhao X, et al. Reproductive toxicity of sewage treatment plant with A2/O process in Guangzhou on Daphnia magna [J]. Asian Journal of Ecotoxicology, 2019, 14(5): 144-151 (in Chinese)
[50] Jia A, Escher B I, Leusch F D, et al. In vitro bioassays to evaluate complex chemical mixtures in recycled water [J]. Water Research, 2015, 80: 1-11
[51] Aguirre-Martínez G V, Martín-Díaz M L. A multibiomarker approach to assess toxic effects of wastewater treatment plant effluents and activated defence mechanisms in marine (Ruditapes philippinarum) and fresh water (Corbicula fluminea) bivalve species [J]. Ecotoxicology, 2020, 29(7): 941-958
[52] Liu J C, Lu G H, Zhang Z H, et al. Biological effects and bioaccumulation of pharmaceutically active compounds in crucian carp caged near the outfall of a sewage treatment plant [J]. Environmental Science Processes & Impacts, 2015, 17(1): 54-61
[53] Hamdhani H, Eppehimer D E, Bogan M T. Release of treated effluent into streams: A global review of ecological impacts with a consideration of its potential use for environmental flows [J]. Freshwater Biology, 2020, 65(9): 1657-1670
[54] Brooks B W, Stanley J K, White J C, et al. Laboratory and field responses to cadmium: An experimental study in effluent-dominated stream mesocosms [J]. Environmental Toxicology and Chemistry, 2004, 23(4): 1057-1064
[55] Brooks B W, Riley T M, Taylor R D. Water quality of effluent-dominated ecosystems: Ecotoxicological, hydrological, and management considerations [J]. Hydrobiologia, 2006, 556(1): 365-379
[56] Canobbio S, Mezzanotte V, Sanfilippo U, et al. Effect of multiple stressors on water quality and macroinvertebrate assemblages in an effluent-dominated stream [J]. Water, Air, and Soil Pollution, 2009, 198(1): 359-371
[57] Matamoros V, Rodríguez Y. Influence of seasonality and vegetation on the attenuation of emerging contaminants in wastewater effluent-dominated streams. A preliminary study [J]. Chemosphere, 2017, 186: 269-277
[58] Du B W, Haddad S P, Scott W C, et al. Pharmaceutical bioaccumulation by periphyton and snails in an effluent-dependent stream during an extreme drought [J]. Chemosphere, 2015, 119: 927-934
[59] Munz N A, Fu Q G, Stamm C, et al. Internal concentrations in gammarids reveal increased risk of organic micropollutants in wastewater-impacted streams [J]. Environmental Science & Technology, 2018, 52(18): 10347-10358
[60] Niemuth N J, Klaper R D. Low-dose metformin exposure causes changes in expression of endocrine disruption-associated genes [J]. Aquatic Toxicology, 2018, 195: 33-40
[61] Niemuth N J, Klaper R D. Emerging wastewater contaminant metformin causes intersex and reduced fecundity in fish [J]. Chemosphere, 2015, 135: 38-45
[62] Restivo V E, Kidd K A, Surette M G, et al. Rainbow darter (Etheostoma caeruleum) from a river impacted by municipal wastewater effluents have altered gut content microbiomes [J]. The Science of the Total Environment, 2021, 751: 141724
[63] Schultz M M, Furlong E T, Kolpin D W, et al. Antidepressant pharmaceuticals in two US effluent-impacted streams: Occurrence and fate in water and sediment, and selective uptake in fish neural tissue [J]. Environmental Science & Technology, 2010, 44(6): 1918-1925
[64] Burket S R, Wright M V, Baker L F, et al. Periphyton, bivalves and fish differentially accumulate select pharmaceuticals in effluent-dependent stream mesocosms [J]. The Science of the Total Environment, 2020, 745: 140882
[65] Mor J R, Dolédec S, Acu a V, et al. Invertebrate community responses to urban wastewater effluent pollution under different hydro-morphological conditions [J]. Environmental Pollution, 2019, 252(Pt A): 483-492
a V, et al. Invertebrate community responses to urban wastewater effluent pollution under different hydro-morphological conditions [J]. Environmental Pollution, 2019, 252(Pt A): 483-492
[66] Lahti M, Brozinski J M, Segner H, et al. Bioavailability of pharmaceuticals in waters close to wastewater treatment plants: Use of fish bile for exposure assessment [J]. Environmental Toxicology and Chemistry, 2012, 31(8): 1831-1837
[67] Webb D T, Zhi H, Kolpin D W, et al. Emerging investigator series: Municipal wastewater as a year-round point source of neonicotinoid insecticides that persist in an effluent-dominated stream [J]. Environmental Science Processes & Impacts, 2021, 23(5): 678-688
[68] Gurr C J, Reinhard M. Harnessing natural attenuation of pharmaceuticals and hormones in rivers [J]. Environmental Science & Technology, 2006, 40(9): 2872-2876
[69] Zhi H, Mianecki A L, Kolpin D W, et al. Tandem field and laboratory approaches to quantify attenuation mechanisms of pharmaceutical and pharmaceutical transformation products in a wastewater effluent-dominated stream [J]. Water Research, 2021, 203: 117537
[70] Yin H L, Wang Y, Huang J S. Photodegradation-induced biological degradation of treated wastewater effluent organic matter in receiving waters [J]. Water Research, 2021, 204: 117567
[71] Thompson D A, Lehmler H J, Kolpin D W, et al. A critical review on the potential impacts of neonicotinoid insecticide use: Current knowledge of environmental fate, toxicity, and implications for human health [J]. Environmental Science Processes & Impacts, 2020, 22(6): 1315-1346
[72] Wong K K, Webb D T, Nagorzanski M R, et al. Chlorinated byproducts of neonicotinoids and their metabolites: An unrecognized human exposure potential? [J]. Environmental Science & Technology Letters, 2019, 6(2): 98-105
[73] Fu Q G, Fedrizzi D, Kosfeld V, et al. Biotransformation changes bioaccumulation and toxicity of diclofenac in aquatic organisms [J]. Environmental Science & Technology, 2020, 54(7): 4400-4408
[74] Maculewicz J, Kowalska D, ![]() K, et al. Transformation products of pharmaceuticals in the environment: Their fate, (eco)toxicity and bioaccumulation potential [J]. The Science of the Total Environment, 2022, 802: 149916
K, et al. Transformation products of pharmaceuticals in the environment: Their fate, (eco)toxicity and bioaccumulation potential [J]. The Science of the Total Environment, 2022, 802: 149916
[75] Sinclair C J, Boxall A B A. Assessing the ecotoxicity of pesticide transformation products [J]. Environmental Science & Technology, 2003, 37(20): 4617-4625
[76] Mahler B J, Nowell L H, Sandstrom M W, et al. Inclusion of pesticide transformation products is key to estimating pesticide exposures and effects in small US streams [J]. Environmental Science & Technology, 2021, 55(8): 4740-4752
[77] Su D, Ben W W, Strobel B W, et al. Impacts of wastewater treatment plant upgrades on the distribution and risks of pharmaceuticals in receiving rivers [J]. Journal of Hazardous Materials, 2021, 406: 124331
[78] Peschke K, Capowiez Y, Köhler H R, et al. Impact of a wastewater treatment plant upgrade on amphipods and other macroinvertebrates: Individual and community responses [J]. Frontiers in Environmental Science, 2019, 7: 64
[79] Argolo A D S, Gomes G, Bila D M. Insights into total estrogenic activity in a sewage-impacted urban stream assessed via ER transcriptional activation assay: Distribution between particulate and dissolved phases [J]. Ecotoxicology and Environmental Safety, 2021, 208: 111574
[80] Allan I J, Vrana B, Greenwood R, et al. A “toolbox” for biological and chemical monitoring requirements for the European Union’s Water Framework Directive [J]. Talanta, 2006, 69(2): 302-322
[81] Kahl M D, Villeneuve D L, Stevens K, et al. An inexpensive, temporally integrated system for monitoring occurrence and biological effects of aquatic contaminants in the field [J]. Environmental Toxicology and Chemistry, 2014, 33(7): 1584-1595
[82] Rosen G, Chadwick D B, Burton G A, et al. A sediment ecotoxicity assessment platform for in situ measures of chemistry, bioaccumulation and toxicity. Part 2: Integrated application to a shallow estuary [J]. Environmental Pollution, 2012, 162: 457-465
[83] 李慧珍, 裴媛媛, 游静. 流域水环境复合污染生态风险评估的研究进展[J]. 科学通报, 2019, 64(33): 3412-3428
Li H Z, Pei Y Y, You J. Ecological risk assessment of combined pollution in watersheds [J]. Chinese Science Bulletin, 2019, 64(33): 3412-3428 (in Chinese)
[84] Sodergren A. Solvent-filled dialysis membranes simulate uptake of pollutants by aquatic organisms [J]. Environmental Science and Technology, 1987, 21(9): 855-859
[85] Xie P H, Yan Q K, Xiong J J, et al. Point or non-point source: Toxicity evaluation using m-POCIS and zebrafish embryos in municipal sewage treatment plants and urban waterways [J]. Environmental Pollution, 2022, 292: 118307
[86] Chaudhary M, Quanz M, Williams J, et al. Assessment of metal(loid) concentrations using diffusive gradient thin (DGT) films in marine, freshwater and wetland aquatic ecosystems impacted by industrial effluents [J]. Case Studies in Chemical and Environmental Engineering, 2020, 2: 100041
[87] Burton G A Jr, Greenberg M S, Rowland C D, et al. In situ exposures using caged organisms: A multi-compartment approach to detect aquatic toxicity and bioaccumulation [J]. Environmental Pollution, 2005, 134(1): 133-144
[88] Perkins E J, Habib T, Escalon B L, et al. Prioritization of contaminants of emerging concern in wastewater treatment plant discharges using chemical: Gene interactions in caged fish [J]. Environmental Science & Technology, 2017, 51(15): 8701-8712
[89] Santana M S, Yamamoto F Y, Sandrini-Neto L, et al. Diffuse sources of contamination in freshwater fish: Detecting effects through active biomonitoring and multi-biomarker approaches [J]. Ecotoxicology and Environmental Safety, 2018, 149: 173-181
[90] Maranho L A, André C, DelValls T A, et al. In situ evaluation of wastewater discharges and the bioavailability of contaminants to marine biota [J]. The Science of the Total Environment, 2015, 538: 876-887
[91] Alric B, Geffard O, Chandesris A, et al. Multisubstance indicators based on caged Gammarus bioaccumulation reveal the influence of chemical contamination on stream macroinvertebrate abundances across France [J]. Environmental Science & Technology, 2019, 53(10): 5906-5915
[92] Cervi E C, Thiamkeelakul K, Hudson M, et al. Laboratory and field-based assessment of the effects of sediment capping materials on zinc flux, bioavailability, and toxicity [J]. Environmental Toxicology and Chemistry, 2020, 39(1): 240-249
[93] Skelton D M, Ekman D R, ![]() D, et al. Metabolomics for in situ environmental monitoring of surface waters impacted by contaminants from both point and nonpoint sources [J]. Environmental Science & Technology, 2014, 48(4): 2395-2403
D, et al. Metabolomics for in situ environmental monitoring of surface waters impacted by contaminants from both point and nonpoint sources [J]. Environmental Science & Technology, 2014, 48(4): 2395-2403
[94] Zhang Y, Luo X J, Wu J P, et al. Contaminant pattern and bioaccumulation of legacy and emerging organhalogen pollutants in the aquatic biota from an e-waste recycling region in South China [J]. Environmental Toxicology and Chemistry, 2010, 29(4): 852-859
[95] 柯润辉, 李剑, 许宜平, 等. 被动式采样器与原位鱼体暴露用于监测水体Ah受体效应的比较研究[J]. 环境科学, 2006, 27(11): 2309-2313
Ke R H, Li J, Xu Y P, et al. Comparison of the methods for assessing Ah effects in aquatic system by semipermeable membrane device and caged fish [J]. Environmental Science, 2006, 27(11): 2309-2313 (in Chinese)
[96] Burton G A Jr, Rosen G, Chadwick D B, et al. A sediment ecotoxicity assessment platform for in situ measures of chemistry, bioaccumulation and toxicity. Part 1: System description and proof of concept [J]. Environmental Pollution, 2012, 162: 449-456