环境内分泌干扰物(environmental endocrine disrupting chemicals, EDCs)是常见的有机污染物,通过干扰生物体内源激素产生、分布和代谢等发挥其作用[1]。EDCs化学性质稳定,可以被机体吸收且不易降解[2],通过食物链富集于鱼体,最终危害动物及人体健康。研究表明,EDCs可以干扰水生生物内分泌系统和免疫系统[3]。EDCs包括类雌激素和类雄激素,雄激素类物质可以引起哺乳动物出现雄性化特征[4],影响水生生物的组织器官正常功能,致使肝脏和性腺等器官出现多种病理变化[5]。
17α-甲基睾酮(17α-methyltestosterone, MT)具有合成简单、成本较低、效果明显等优点[6]。低浓度MT暴露可以诱导动物精子的发生及释放,促进雄性器官发育,而高浓度使用可能导致鱼类畸形或死亡[7]。MT添加投喂大口黑鲈(Micropterus salmoides),可以有效促进雌性幼鱼转变为雄性[8]。MT处理麦穗鱼(Pseudorasbora parva)7 d,肝脏出现空泡化,细胞核固缩等现象且随着MT浓度的升高和暴露时间的延长而加剧,性腺组织切片结果显示,雄鱼性腺出现精卵巢(testis-ova)现象[5,9]。
KISS/GPR54系统是由配体kisspeptin的编码基因kiss及其受体GPR54组成,鱼类kiss基因主要包括2个亚型kiss1和kiss2[10],GPR54基因也有GPR54α和GPR54β这2个亚型。KISS/GPR54系统通过下丘脑-垂体-性腺(hypothalamic-pituitary-gonad, HPG)轴调控促性腺激素释放激素(gonadotropin releasing hormone, GnRH)分泌和释放,从而调节下游促性腺激素,促进性腺发育成熟[11]。d’Anglemont de Tassigny等[12]和Funes等[13]分别敲除小鼠kiss和GPR54基因,均出现不育和促性腺激素功能减退症,对HPG轴功能造成严重影响。金鱼(Carassius auratus)成鱼注射1 μg·g-1(以体质量计)的KISS-1,血清中促黄体素(luteinizing hormone, LH)水平显著升高[14],为欧洲舌齿鲈(Dicentrarchus labrax)成鱼注射250 ng·g-1(以体质量计)的KISS-2,血清中LH和促卵泡素(follicle-stimulating hormone, FSH)水平显著升高[15]。
MicroRNAs(miRNAs)是一类长约22 nt的小分子非编码RNA,在细胞分化、增殖、生长、衰老、凋亡、器官发育、代谢调节和细胞信息传递等多种生物学过程中发挥作用[16],主要通过与靶基因3’非翻译区(3’-UTR)结合来影响基因或蛋白的表达,从而促进mRNA降解或阻止蛋白质翻译[17]。在多种鱼类中,miRNAs参与调控配子形成和性腺发育[16],测序数据显示,MT处理后稀有鮈鲫卵巢中存在3 949组miRNA-mRNA对[18]。目前许多研究涉及HPG轴相关miRNAs,其中miR-9和miR-200控制GnRH神经元的发育[19],miR-155和miR-200调控小鼠青春期前下丘脑GnRH的释放[20]。miR-25-3p和miR-92a-3p属于miR-25家族,可以与小鼠脑中kiss1基因的3’-UTR区结合,从而影响小鼠青春期的启动以及动情周期[21];中枢性性早熟女童血清中miR-137可以与kiss1基因的3’-UTR区结合发挥作用[22];过表达miR-199-3p导致细胞MAPK pathway活性降低从而抑制kiss1基因表达[23];miR-324-3p与kiss1基因的3’-UTR区结合抑制宫外孕早期kiss1基因的表达[24]。
稀有鮈鲫(Gobiocypris rarus)是我国极具代表性的鱼种,具有生活史周期短,方便饲养,温度、溶氧耐受范围广等优点,因此逐渐成为新型实验动物[25]。在毒理学、病理学、遗传学和基础生物学等关键学科领域均有使用稀有鮈鲫作为试验动物[26]。为探究MT干扰稀有鮈鲫生殖系统的作用机理,本研究采用0、25、50和100 ng·L-1的MT处理稀有鮈鲫7、14和21 d。qRT-PCR检测稀有鮈鲫脑中kiss和GPR54基因mRNA及其相关miRNAs的表达变化情况,为探究MT在稀有鮈鲫体内的作用机理提供可靠的理论基础,同时为进一步推进稀有鮈鲫成为我国特有水生模式生物提供依据。
1 材料与方法(Materials and methods)
1.1 试验动物
稀有鮈鲫选自山西农业大学水产科学系实验室同一批繁殖的8月龄成鱼。试验共设置4个组,对照组为0.001%无水乙醇,低、中、高MT处理组浓度分别设定为25、50和100 ng·L-1,雌雄分开饲养,每个试验组设置3个平行重复。本试验用水选用曝气24 h后的自来水,半净水暴露,pH范围7.6±0.2,水温范围(25±1) ℃,光/暗周期为14 h∶10 h。养殖鱼的密度为1 g·L-1,每天吸污(残饵和粪便)的同时换掉水族箱1/2的水,并加入等量的水和相应量的MT溶液,保证水族箱里MT浓度恒定。每天定时定量投喂红虫一次,投喂量为实验组内鱼总质量的3%,观察并记录各组试验鱼的健康状况。取样前1 d停止投饵,每组随机选取18尾稀有鮈鲫,MS222麻醉后取脑和性腺组织,脑组织置于Trizol中研磨后-80 ℃保存备用,性腺置于波恩氏液中固定24~48 h,用于石蜡组织切片观察。
1.2 石蜡组织切片制备
取出波恩氏液固定后的性腺组织,放置于包埋盒中,自来水缓冲12 h后酒精梯度脱水(50%、70%、80%、95%和100%乙醇中进行),二甲苯进行透明处理;将透明好的组织浸蜡包埋(多次熬制去除大部分杂质的石蜡),用镊子将组织放于石蜡模具中,去除气泡,放于65 ℃烘箱中透蜡30 min后取出,除去组织旁的气泡自然降温凝固,修整石蜡模块。使用切片机将组织石蜡切成6 μm厚的组织,H-E染色法染色,中性树脂胶封片处理,置于显微镜下观察性腺组织病理学变化。
1.3 总RNA提取及cDNA第一链的合成
取出超低温冰箱保存的稀有鮈鲫脑组织,Trizol一步法提取总RNA,1%琼脂糖凝胶电泳检测RNA完整性,分光光度计测定纯度和浓度。每份组织样本取5 μL,PrimeScriptTM RT Reagent Kit with gDNA Eraser(perfect real time)反转录合成cDNA第一链,M5 miRNA cDNA Synthesis Kit合成miRNA对应的第一链cDNA。
1.4 定量引物合成
参考已有的稀有鮈鲫kiss1、kiss2、GPR54α和GPR54β的引物序列[27],上海生工(Sangon Biotech)生物工程股份有限公司合成,普通PCR扩增,扩增产物1%琼脂糖凝胶电泳检测,凝胶电泳条带单一,清晰可见。采用实时荧光定量PCR(qRT-PCR)方法对稀有鮈鲫脑中4个基因的相对表达水平进行检测,每组检测6尾鱼。选择常用内参基因ef1a、β-actin、gapdh和tuba1作为候选,参考已有引物序列[28],筛选最佳内参基因。
参考实验室已有的稀有鮈鲫第二代测序数据,使用DNAman软件设计miR-25-3p、miR-92a-3p、miR-137-3p、miR-199-3p和miR-324-3p的引物,qRT-PCR检测稀有鮈鲫脑中5个miRNA的相对表达水平,每组检测6尾鱼,选择U6作为内参基因(表1)。
1.5 内参基因筛选
qRT-PCR方法检测β-actin、ef1a、gapdh和tuba1在对照组及MT处理组的表达量,使用GeNorm、BestKeeper和NormFinder[29]3种方法对4个候选基因表达稳定性进行评估,确定在MT暴露后仍然稳定的内参基因,保证实时荧光定量PCR结果的可信度。
1.6 实时荧光定量PCR
qRT-PCR检测稀有鮈鲫脑中kiss1、kiss2、GPR54α和GPR54β基因的相对表达量。采用TB Premix Ex TaqTM Ⅱ(Tli RNase H Plus)试剂盒(TaKaRa,大连)进行试验,每个组织设置3个重复,体系为20 μL(上下游引物各0.8 μL,cDNA模板2 μL(100 ng),荧光染料SYBR Green Ⅱ 10 μL,灭菌超纯水ddH2O 6.4 μL);采用M5 miRNA qPCR Assay Kit试剂盒(聚合美,北京)检测稀有鮈鲫脑中miR-25-3p、miR-92a-3p、miR-137-3p、miR-199-3p和miR-324-3p的相对表达量,每个组织设置3个重复,体系为20 μL(上下游引物各0.4 μL,miRNA第一链cDNA模板2 μL(100 ng),2× M5 miRNA qPCR Mixture (ROX) 10 μL,灭菌超纯水ddH2O 7.2 μL),混合均匀后于冰上快速加到八连管中。PCR程序为95 ℃预变性3 min,95 ℃变性5 s、60 ℃退火30 s、72 ℃复性30 s;40个循环;熔解曲线(95 ℃ 10 s,52 ℃ 5 s,95 ℃ 30 s)。
1.7 数据处理分析
采用F=2-ΔΔCq对获得的mRNA和miRNA表达量数据进行处理,RT-qPCR数据处理采用2-ΔΔCq,计算公式为F=2-ΔΔCq,ΔCq处理组=(Cq目的基因处理组平均值-Cq内参);ΔCq对照组=(Cq目的基因对照组平均值-Cq内参),ΔΔCq=ΔCq处理组-ΔCq对照组。运用SPSS 21.0进行统计学分析,采用单因素方差分析及Duncan’s多重比较检验法,P<0.05为差异显著,用不同小写字母表示。
表1 实时荧光定量PCR引物序列
Table 1 Primer sequences of qRT-PCR
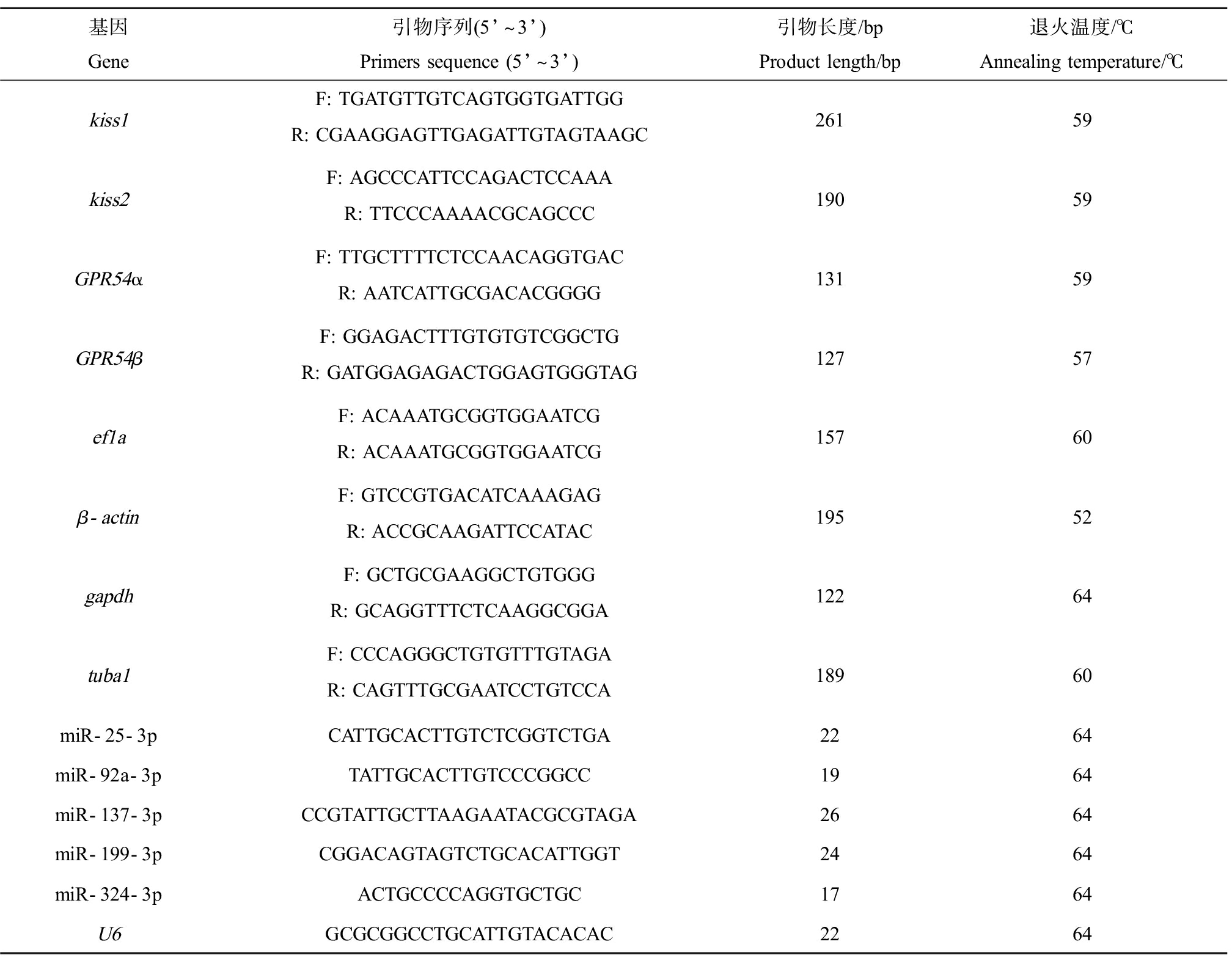
基因Gene引物序列(5’~3’)Primers sequence (5’~3’)引物长度/bpProduct length/bp退火温度/℃Annealing temperature/℃kiss1F: TGATGTTGTCAGTGGTGATTGGR: CGAAGGAGTTGAGATTGTAGTAAGC26159kiss2F: AGCCCATTCCAGACTCCAAAR: TTCCCAAAACGCAGCCC19059GPR54αF: TTGCTTTTCTCCAACAGGTGACR: AATCATTGCGACACGGGG13159GPR54βF: GGAGACTTTGTGTGTCGGCTGR: GATGGAGAGACTGGAGTGGGTAG12757ef1aF: ACAAATGCGGTGGAATCGR: ACAAATGCGGTGGAATCG15760β-actinF: GTCCGTGACATCAAAGAGR: ACCGCAAGATTCCATAC19552gapdhF: GCTGCGAAGGCTGTGGGR: GCAGGTTTCTCAAGGCGGA12264tuba1F: CCCAGGGCTGTGTTTGTAGAR: CAGTTTGCGAATCCTGTCCA18960miR-25-3pCATTGCACTTGTCTCGGTCTGA2264miR-92a-3pTATTGCACTTGTCCCGGCC1964miR-137-3pCCGTATTGCTTAAGAATACGCGTAGA2664miR-199-3pCGGACAGTAGTCTGCACATTGGT2464miR-324-3pACTGCCCCAGGTGCTGC1764U6GCGCGGCCTGCATTGTACACAC2264
2 结果(Results)
2.1 MT对稀有鮈鲫性腺组织学影响
25、50和100 ng·L-1的MT处理稀有鮈鲫7、14和21 d,雌雄鱼的性腺发育均受到了抑制,卵巢中的成熟卵细胞数量减少,精巢中的成熟精子比例降低。并且随着处理时间和浓度的变化,卵巢和精巢出现不同的变化。
2.1.1 MT对稀有鮈鲫雌鱼性腺组织学影响
石蜡切片结果如图1所示,MT处理稀有鮈鲫雌鱼7 d,对照组卵巢发育良好,成熟卵细胞(Voc)数量较多,初级卵母细胞(Poc)和次级卵母细胞(Coc)数目正常;低浓度处理组成熟卵细胞比例无明显变化;中浓度处理组和高浓度处理组成熟卵细胞比例明显降低,初级卵母细胞和次级卵母细胞所占比例增加。
MT处理稀有鮈鲫14 d,对照组卵巢发育正常;低浓度处理组未观察到成熟卵细胞,而未成熟卵细胞数量增多;中浓度处理组观察到初级卵母细胞数量增加,次级卵母细胞和成熟卵细胞数量减少;高浓度处理组卵巢中存在大量初级卵母细胞和少量次级卵母细胞,细胞个体变小。
MT处理稀有鮈鲫21 d,对照组卵巢发育正常,细胞比例未发生显著变化;低浓度和中浓度处理组均显示初级卵母细胞数量增加,未观察到成熟卵母细胞;高浓度处理组卵巢中存在大量发育初期的初级卵母细胞,未观察到成熟卵细胞和次级卵母细胞。
2.1.2 MT对稀有鮈鲫雄鱼性腺组织学影响
石蜡切片结果如图2所示,MT处理稀有鮈鲫雄鱼7 d,对照组精巢发育良好,精原细胞(Sg)、次级精母细胞(Sc)和成熟精子(Sz)所占比例正常;低浓度处理组未发生显著变化;中浓度处理组和高浓度处理组精巢中成熟精子数量减少。
MT处理稀有鮈鲫雄鱼14 d,对照组精巢发育良好,精原细胞、次级精母细胞和成熟精子所占比例正常;低浓度处理组和中浓度处理组未成熟的精母细胞和精原细胞的比例增加;高浓度处理组精巢中几乎观察不到成熟的精子,精原细胞和次级精母细胞数量增加。
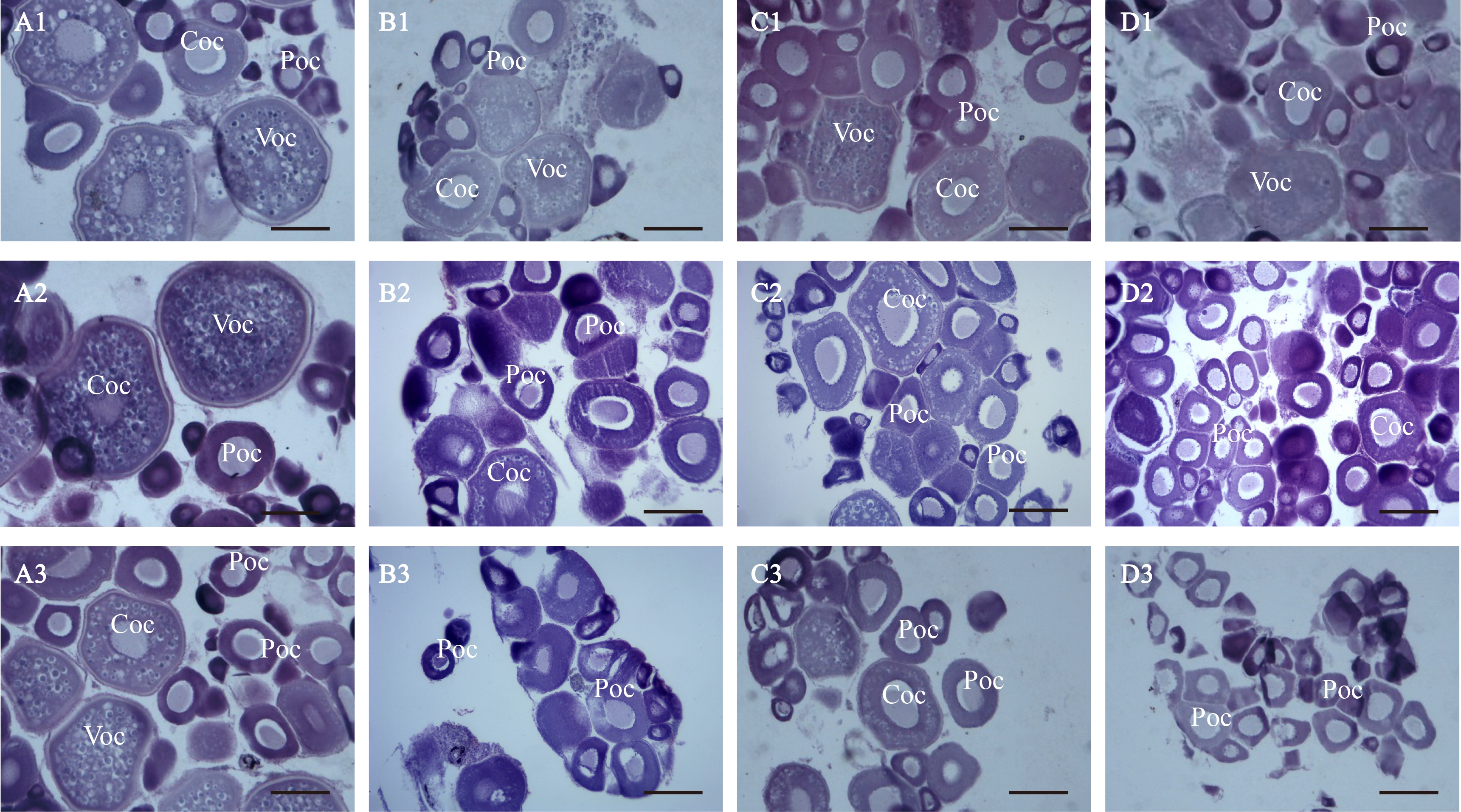
图1 17α-甲基睾酮(MT)对雌性稀有鮈鲫卵巢组织学影响(H-E染色,比例尺=200 μm)
注:A1、B1、C1和D1为MT(对照组、25、50和100 ng·L-1)处理雌鱼7 d后的卵巢组织;A2、B2、C2和D2为MT(对照组、25、50和100 ng·L-1)处理雌鱼14 d后的卵巢组织;A3、B3、C3和D3为MT(对照组、25、50和100 ng·L-1)处理雌鱼21 d后的卵巢组织;Voc为成熟卵细胞,Coc为次级卵母细胞,Poc为初级卵母细胞。
Fig. 1 Effects of 17α-methyltestosterone (MT) on ovary histopathology of female G. rarus (H-E stain, scale bars=200 μm)
Note: A1, B1, C1, and D1 respectively represent the ovary tissue changes of the control group, 25, 50, and 100 ng·L-1 MT treatment groups of female fish for 7 d; A2, B2, C2, and D2 respectively represent the ovary tissue changes of the control group, 25, 50 and 100 ng·L-1 MT treatment groups of female fish for 14 d; A3, B3, C3, and D3 respectively represent the ovary tissue changes of the control group, 25, 50 and 100 ng·L-1 MT treatment groups of female fish for 21 d; Voc means vitellogenic oocyte; Coc means cortical alveolus stage; Poc means perinucleolar oocyte.
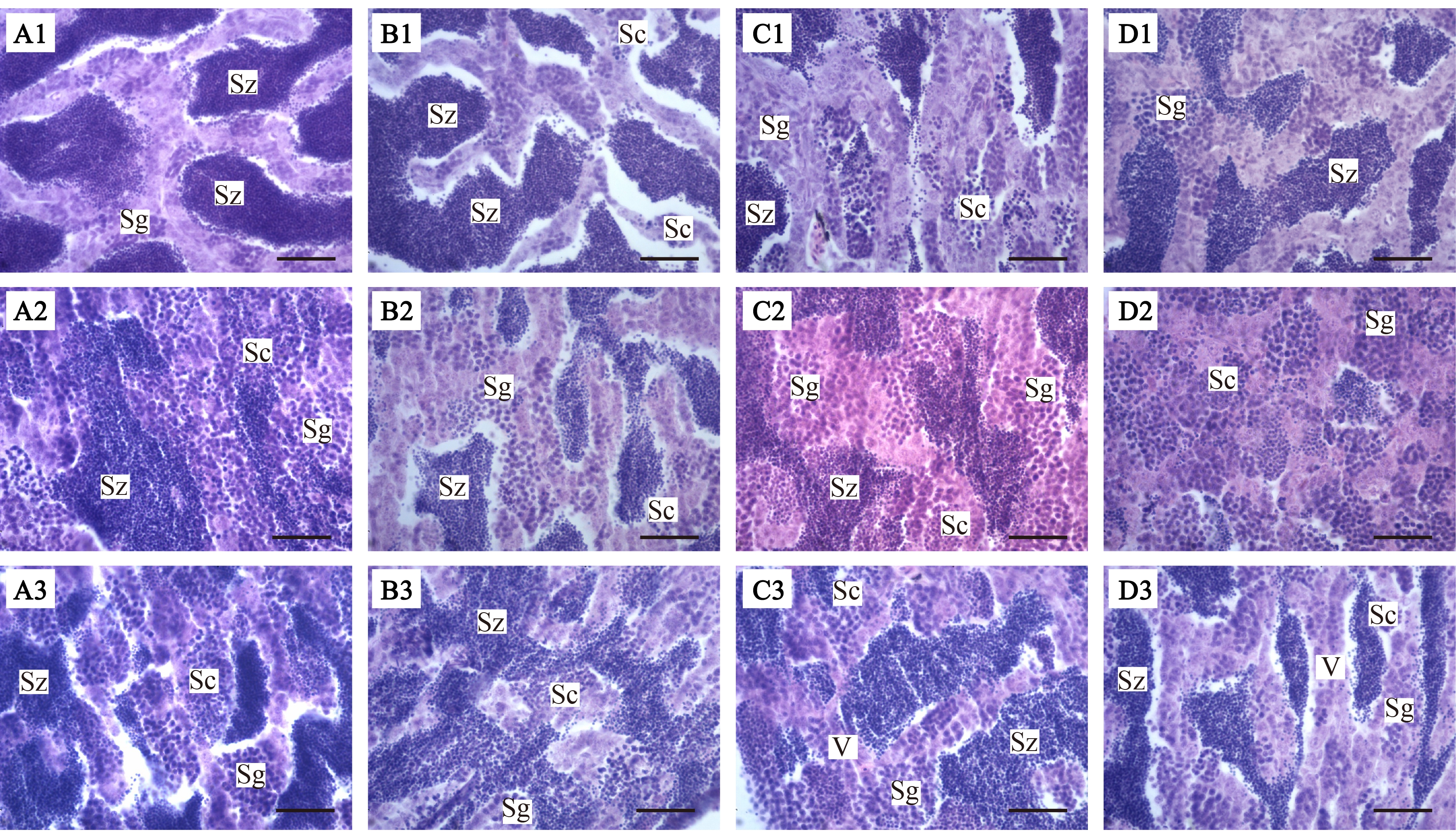
图2 MT对雄性稀有鮈鲫精巢组织学影响(H-E染色,比例尺=50 μm)
注:A1、B1、C1和D1为MT(对照组、25、50和100 ng·L-1)处理雄鱼7 d后的精巢组织;A2、B2、C2和D2为MT(对照组、25、50和100 ng·L-1)处理雄鱼14 d后的精巢组织;A3、B3、C3和D3为MT
(对照组、25、50和100 ng·L-1)处理雄鱼21 d后的精巢组织;Sz为成熟精子,Sc为次级精母细胞,Sg为精原细胞,V为空泡化。
Fig. 2 Effects of MT on testis histopathology of male G. rarus (H-E stain, scale bars=50 μm)
Note: A1, B1, C1, and D1 respectively represent the testis tissue changes of the control group, 25, 50, and 100 ng·L-1 MT treatment groups of male fish for 7 d; A2, B2, C2, and D2 respectively represent the testis tissue changes of the control group, 25, 50 and 100 ng·L-1 MT treatment groups of male fish for 14 d; A3, B3, C3, and D3 respectively represent the testis tissue changes of the control group, 25, 50 and 100 ng·L-1 MT treatment groups of male fish for 21 d; Sz means spermatozoo; Sc means spermatocyte; Sg means spermatogonium; V means vacuolation.
MT处理稀有鮈鲫雄鱼21 d,对照组精巢发育良好,各细胞比例正常;低浓度处理组和中浓度处理组成熟精子所占比例减少,出现少量细胞空泡化现象;高浓度处理组几乎观察不到成熟精子,未成熟的精母细胞和精原细胞比例增加,出现大量细胞空泡化现象。
2.2 内参基因稳定性结果
GeNorm分析内参基因稳定性结果显示,gapdh(2.02)>tuba1(1.22)>ef1a=β-actin(0.71),ef1a和β-actin的表达最稳定,gapdh的表达最不稳定(图3(a))。NormFinder分析内参基因稳定性结果显示,gapdh(1.85)>tuba1(0.84)>β-actin(0.78)>ef1a(0.25),ef1a的表达最稳定,gapdh的表达最不稳定(图3(b))。Bestkeeper分析内参基因的稳定性结果显示,gapdh(3.13)>β-actin(1.53)>tuba1(1.45)>ef1a(1.38),ef1a的表达最稳定,gapdh的表达最不稳定(图3(c))。
根据上述3种方法对所有内参基因的分析评估,结果均显示ef1a为MT暴露后稀有鮈鲫脑中最稳定的内参基因,因此本试验选用ef1a为最终内参基因。
2.3 MT对稀有鮈鲫脑中KISS/GPR54系统基因表达的影响
2.3.1 MT对稀有鮈鲫雌鱼脑中kiss与GPR54表达的影响
MT处理稀有鮈鲫雌鱼7、14和21 d,低浓度(25 ng·L-1)和高浓度(100 ng·L-1)MT显著降低脑中kiss1基因表达量。MT暴露14 d,中浓度(50 ng·L-1)组kiss1基因表达量显著升高(图4(a))。MT处理稀有鮈鲫雌鱼7 d,中浓度组kiss2基因表达量显著升高,而高浓度组表达量显著降低。延长处理至14 d,低浓度组kiss2基因表达量显著升高,为对照组的10倍,而高浓度组的表达量显著降低。延长处理至21 d,低浓度和高浓度组kiss2基因表达量显著升高(图4(b))。MT暴露稀有鮈鲫雌鱼7 d,低、中、高3个浓度组GPR54β基因表达量显著降低,延长处理至14 d,GPR54α和GPR54β基因表达量显著降低,延长处理至21 d,GPR54α表达量均降低(P<0.05,图4)。
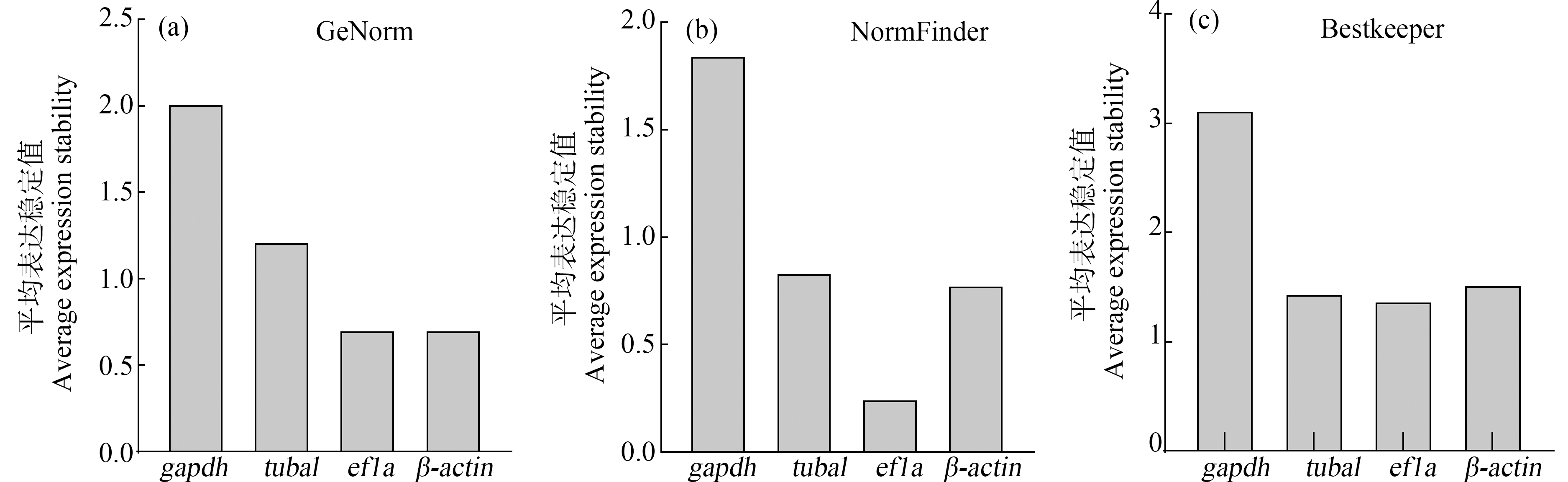
图3 MT诱导稀有鮈鲫脑内参基因的表达稳定值
Fig. 3 Stability of reference genes in brain of G. rarus exposed to MT
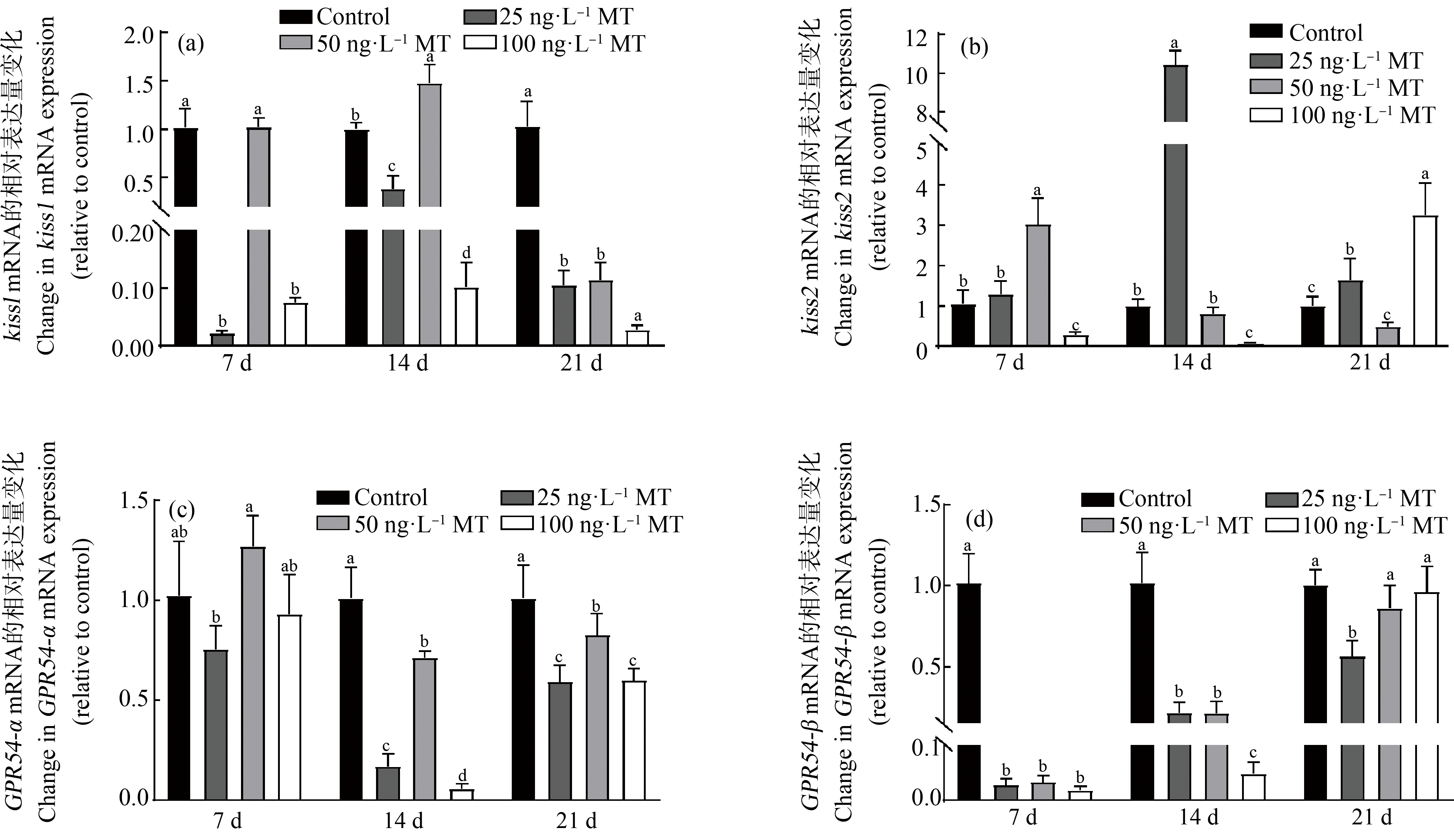
图4 MT对稀有鮈鲫雌鱼脑中kiss与GPR54基因表达量影响
注:试验结果表示为平均值±标准误;不同小写字母表示显著性水平(P<0.05)。
Fig. 4 Effect of MT on kiss and GPR54 genes in brain of female G. rarus
Note: The data were showed as mean±SEM; the different letters represented significant level (P<0.05).
2.3.2 MT对稀有鮈鲫雄鱼脑中kiss与GPR54表达的影响
MT处理稀有鮈鲫雄鱼7 d,中浓度组脑中kiss1基因表达量显著下降,而高浓度组表达量显著升高(图5(a))。低、中、高3个浓度组kiss2基因表达量显著升高,其中50 ng·L-1 MT对稀有鮈鲫kiss2基因表达的上调作用最为明显,是对照组的11倍(图5(b))。低浓度组脑中GPR54α基因表达量显著升高。低浓度和高浓度处理组GPR54β基因表达量显著升高。延长处理至14 d,中浓度组kiss1基因表达量显著升高,而高浓度组的表达量显著下降。低、中、高3个浓度组kiss2和GPR54α基因表达量显著降低,高浓度组GPR54β基因表达量显著升高。延长处理至21 d,低、中、高处理组kiss1基因表达量显著升高,而GPR54β基因表达量显著降低,中浓度处理组kiss2和GPR54α基因表达量显著升高(P<0.05,图5)。

图5 MT对稀有鮈鲫雄鱼脑中kiss与GPR54基因表达量影响
注:试验结果表示为平均值±标准误;不同小写字母表示显著性水平(P<0.05)。
Fig. 5 Effect of MT on kiss and GPR54 genes in brain of male G. rarus
Note: The data were showed as mean±SEM; the different letters represented significant level (P<0.05).
2.3.3 MT对稀有鮈鲫雌鱼脑中miRNAs表达的影响
MT处理稀有鮈鲫雌鱼7 d,低浓度组miR-25-3p、miR-92a-3p、miR-137-3p和miR-199-3p表达量均升高,而中浓度组和高浓度组表达量均降低,低浓度组miR-324-3p表达量显著降低。延长处理时间至14 d,低浓度组miR-25-3p和miR-92a-3p表达量升高,而中浓度组和高浓度组miR-25-3p、miR-92a-3p、miR-137-3p和miR-199-3p表达量均降低,miR-324-3p表达量无显著变化。延长暴露时间至21 d,低浓度组miR-25-3p、miR-92a-3p和miR-137-3p,中浓度处理组miR-137-3p和高浓度处理组miR-25-3p、miR-92a-3p和miR-137-3p表达量均显著下降,中浓度组miR-324-3p表达量显著升高(P<0.05,图6)。
2.3.4 MT对稀有鮈鲫雄鱼脑中miRNAs表达的影响
MT处理稀有鮈鲫雄鱼7 d,低浓度组miR-137-3p、miR-199-3p和miR-324-3p表达量显著升高,miR-25-3p表达量显著降低。中浓度和高浓度处理组miR-25-3p、miR-92a-3p、miR-137-3p和miR-199-3p表达量均降低。延长处理至14 d,miR-25-3p和miR-137-3p在低浓度和中浓度处理组均显著上升,miR-92a-3p和miR-199-3p均无显著变化,高浓度处理组4个miRNAs表达量均显著下降,miR-324-3p在低浓度和高浓度组中表达量显著升高。延长暴露时间至21 d,除高浓度处理组miR-199-3p无显著变化外,miR-25-3p、miR-92a-3p、miR-137-3p和miR-199-3p在低、中、高3个浓度处理组中均显著下降。中浓度处理组miR-324-3p表达量显著降低,而在高浓度处理组中显著升高(P<0.05,图7)。
3 讨论(Discussion)
3.1 MT对稀有鮈鲫性腺的影响
环境内分泌干扰物可以与性激素受体竞争性结合,妨碍内源性性激素与受体结合发挥作用,从而影响性激素正常生理功能[30]。本研究采用0、25、50和100 ng·L-1的MT暴露稀有鮈鲫7、14和21 d,随着处理浓度和处理时间的变化,MT对稀有鮈鲫性腺的发育产生不同程度的抑制作用。MT处理7、14和21 d后,稀有鮈鲫雌鱼卵巢都有不同程度的退化,且随着暴露时间的延长和暴露浓度的升高,卵巢退化的程度逐渐严重,成熟卵细胞比例逐渐降低。研究表明,50 mg·kg-1 MT投喂草鱼(Ctenopharyngodon idella)30 d时卵巢中出现精原细胞,30~150 d卵巢受到更加严重的抑制作用,卵母细胞的生成和成熟受到抑制[31]。暴露MT后黑头软口鲦(Pimephales promelas)和青鱂(Oryzias latipes)卵巢退化和发育迟缓,与本研究结果一致[9]。因此,本研究和前人的研究结果表明MT可以抑制鱼类卵巢的发育,阻碍生殖细胞的成熟。随着暴露时间的延长和暴露时间的增加,成熟的精子逐渐减少,精巢退化程度逐渐严重。研究表明,以含体质量0.5%的MT日粮喂养牙鲆(Paralichthys olivaceus)幼鱼,处理组的雄性率达到100%[32],50 ng·L-1 MT暴露麦穗鱼和380 ng·L-1 MT暴露青鱂雄鱼后处理组中雄鱼性腺均出现精卵巢(testis-ova)现象[9]。MT处理7 d后引起稀有鮈鲫雄鱼性腺指数显著降低,精巢发育受到抑制[33]。性成熟的锯盖鱼(Centropomus undecimalis)注射MT后,0.3~30 mg·kg-1浓度范围都可以促进精巢的生长和发育,加速精子的形成[34]。50 μg·L-1 MT处理雄性鳗鲡(Anguilla japonica)45 d,性腺指数显著升高,精巢发育受到促进,此时精巢中以精细胞为主[35]。因此,本研究和前人的研究结果都表明了MT可以影响鱼类性腺发育,高浓度MT可以抑制鱼类精巢的发育。

图6 MT对稀有鮈鲫雌鱼脑中kiss1相关miRNAs表达量影响
注:试验结果表示为平均值±标准误;不同小写字母表示显著性水平(P<0.05)。
Fig. 6 Effect of MT on kiss1 related miRNAs in brain of female G. rarus
Note: The data were showed as mean±SEM; the different letters represented significant level (P<0.05).

图7 MT对稀有鮈鲫雄鱼脑中kiss1相关miRNAs表达量影响
注:试验结果表示为平均值±标准误;不同小写字母表示显著性水平(P<0.05)。
Fig. 7 Effect of MT on kiss1 related miRNAs in brain of male G. rarus
Note: The data were showed as mean±SEM; the different letters represented significant level (P<0.05).
3.2 MT对稀有鮈鲫脑中KISS/GPR54系统的影响
KISS/GPR54系统在鱼类个体生殖和发育过程中发挥重要作用[36],在调控鱼类生殖内分泌中系统发挥重要作用,不仅参与调节鱼类青春期起始,参与调节鱼类繁殖行为,使其表现出不同的生物学活性,还可以调节鱼类季节性繁殖的生殖内分泌,调控鱼类性腺发育,促进分泌GnRH和促性腺激素[37]。研究结果显示,MT暴露稀有鮈鲫后,ef1a是脑中表达最稳定的内参基因,与双酚A暴露稀有鮈鲫脑中稳定表达基因一致[28]。MT处理雌鱼7 d和14 d,kiss2基因表达量均呈现先升高后降低的趋势,延长处理至21 d,kiss1与GPR54α表达量均降低。MT处理稀有鮈鲫雄鱼7 d,kiss1基因表达量先降低后升高,而kiss2基因表达量均显著升高,延长处理至14 d,kiss1基因表达量先升高后降低,kiss2基因表达量则降低,延长处理至21 d,kiss1表达量均升高,而GPR54β则降低。表明不同浓度的外源性雄激素MT能够改变稀有鮈鲫KISS/GPR54系统基因的表达模式,该系统在稀有鮈鲫个体生殖和发育过程中发挥重要的作用。大多数脊椎动物的研究报道指出KISS/GPR54系统结合HPG轴来调控GnRH的分泌和释放,从而控制下游的促性腺激素的释放和分泌[38-39]。在啮齿动物的研究中,KISS/GPR54系统通过应答由雌雄激素引起的正负反馈调节来调控下游促性腺激素的释放和分泌[40]。因此我们认为稀有鮈鲫HPG轴下游的调控机制与哺乳动物相似,KISS/GPR54系统发挥关键作用。
GPR54β可以直接在垂体水平或者通过性腺激素的反馈作用来调控促性腺激素的分泌。本试验结果显示,25、50或100 ng·L-1的MT处理7 d,可引起稀有鮈鲫雌鱼脑中GPR54β基因mRNA的表达量显著降低,我们推测MT通过抑制KISS与GPR54β的结合在垂体水平抑制下游激素的分泌,进而抑制稀有鮈鲫卵巢的发育,这与前人研究结果一致[27,39]。随着稀有鮈鲫雌鱼暴露时间延长至21 d,3个处理组与对照组表达量均无明显差异,MT的抑制作用逐渐减弱直至消失,这可能是MT干扰卵巢类固醇激素合成,从而引起神经内分泌系统HPG轴的负反馈调节,使GPR54β基因表达恢复正常水平,进而恢复其原有生理功能状态。这可能是机体在一定生理能力范围内,为了适应不同环境状态的一种生理补偿效应[40]。
在硬骨鱼类中,存在2种kiss基因分别为kiss1和kiss2以及2种GPR54基因分别为GPR54α(GPR54-2b)和GPR54β(GPR54-1b),且不同配体与受体之间能够相互作用,信号传导机制复杂多变,其不同的结合方式会导致其发挥不同的生理功能[41]。本研究发现,稀有鮈鲫雌鱼脑内kiss1与GPR54α基因的表达模式相同,在稀有鮈鲫雄鱼脑中kiss2与GPR54α的表达模式相同,因此,我们推测在雌鱼脑内kiss1与GPR54α基因可能对外源激素MT有相同的应答模式,也有可能是配体与受体特异性结合形成配体-受体对共同发挥作用。在稀有鮈鲫雄鱼脑中kiss2与GPR54α表达模式相同,可能对MT具有相同的响应模式,或者结合形成特异性配体-受体对。此结果与克氏双锯鱼(Amphiprion clarkii)中的研究结果不同,克氏双锯鱼脑和性腺中kiss1与GPR54-1b几乎具有相同的表达模式,说明kiss1可能通过GPR54-1b作为配体-受体对参与克氏双锯鱼的生理调节[42]。欧洲海鲈kiss1与GPR54α亲和性较高,而kiss2与GPR54β亲和性较高[43]。金鱼kiss1与GPR54β亲和性较高,而kiss2与GPR54α亲和性也较高[44]。因此我们推测kiss与GPR54基因的表达模式具相关性,但在不同鱼类中表达或调控方式不同,需要进一步深入研究KISS/GPR54系统参与鱼类生殖的调控机制。
本研究中,MT对稀有鮈鲫雌雄鱼脑中kiss1基因表达的上调作用分别为对照组为1.5倍和1.9倍,而kiss2基因表达的上调作用分别为对照组的10.4倍和11.0倍,因此,我们推测MT对稀有鮈鲫脑中kiss2基因表达的影响作用相对于kiss1基因影响较为明显,这可能预示着稀有鮈鲫的KISS-2神经元相较于KISS-1神经元对外源性雄激素作用更加敏感,这与雌二醇处理雌性稀有鮈鲫和斑马鱼幼鱼结果一致[27,45]。性成熟雌性斑马鱼(Danio rerio)和鲈鱼(Lateolabrax japonicus)研究中发现kiss2能在垂体水平较kiss1更显著地促进KISS/GPR54系统下游基因FSHβ和LHβ的表达,这说明kiss2可能是激活促性腺激素合成的关键因子[43,46]。对欧洲舌齿鲈分别注射KISS-1和KISS-2,注射KISS-2血清中LH升高4倍,FSH升高2倍,而注射KISS-1血清中LH仅为正常的2倍,而FSH则无显著变化[15]。上述试验结果表明,稀有鮈鲫暴露于外源性雄激素MT环境中,kiss2可能是性腺激素反馈调控过程中KISS/GPR54系统中的主要执行者。
3.3 miRNA介导MT影响稀有鮈鲫脑中kiss1基因的表达
本研究结果表明,低浓度MT处理稀有鮈鲫7 d,雌鱼脑中miR-25-3p、miR-92a-3p、miR-137-3p和miR-199-3p表达显著升高,kiss1基因表达显著降低。miR-25家族的miR-25-3p和miR-92a-3p可以与kiss1基因的3’-UTR区结合,从而影响小鼠青春期的启动以及动情周期[21]。在卵巢子宫内膜异位症中miR-25-3p直接靶向转录因子特异性蛋白(Sp1)发挥作用[14]。miR-92a-3p通过调控B细胞易位基因2(btg2)调控乳腺癌细胞增殖和转移[47],通过靶向大肿瘤抑制基因2(lats2)影响宫颈癌细胞的增殖、凋亡和侵袭[48]。中枢性性早熟女童血清中miR-137的表达与kiss1呈负相关,且与LH峰值,基础LH/FSH比值呈负相关[22],本研究结果也显示MT通过调控miR-137的表达进而影响kiss1的表达,进而影响稀有鮈鲫的性腺发育。
中浓度MT处理稀有鮈鲫7 d和高浓度MT处理稀有鮈鲫14 d,雄鱼脑中miR-324-3p表达显著升高,抑制kiss1基因表达,细胞学研究表明,miR-324-3p的过表达可以抑制kiss1的表达[49]。中浓度MT处理稀有鮈鲫21 d,雄鱼脑中miR-92a-3p、miR-137-3p和miR-199-3p表达显著降低,kiss1基因表达显著升高。将靶向miR-92a-3p的vivo-morpholinos(VMO)直接注射到斑马鱼卵巢中导致1细胞期胚胎中成熟miR-92a-3p的丰度显著降低,以及发育停滞的胚胎比例显著增加[50]。miR-199通过调控信号通路或靶向基因参与细胞增殖、分化和凋亡,miR-199-3p通过p38 MAPK途径调节kiss1基因的表达,从而调控大鼠青春期的起始[24]。因此我们推断中浓度MT处理稀有鮈鲫雄鱼21 d,降低miR-92a-3p、miR-137-3p和miR-199-3p的表达,进而显著升高kiss1的表达,从而干扰性腺发育。
综上所述,我们推测MT通过调控miR-25-3p、miR-92a-3p、miR-137-3p、miR-199-3p和miR-324-3p表达干扰kiss1基因的表达,进一步影响稀有鮈鲫性腺发育及卵细胞和精子的成熟。稀有鮈鲫kiss1基因表达受到miR-25-3p、miR-92a-3p、miR-137-3p、miR-199-3p和miR-324-3p等miRNAs的调控,有待进一步深入探究并验证其靶向关系。同时我们发现稀有鮈鲫kiss2基因对外源性雄激素更加敏感,可以作为监测环境中MT的生物标志物。
[1] 黄苑, 张维, 王瑞国, 等. 双酚类化合物污染现状和内分泌干扰效应研究进展[J]. 生态毒理学报, 2022, 17(1): 60-81
Huang Y, Zhang W, Wang R G, et al. Advances on pollution status and endocrine disrupting effects of bisphenols [J]. Asian Journal of Ecotoxicology, 2022, 17(1): 60-81 (in Chinese)
[2] Wang S, Zhu Z L, He J F, et al. Steroidal and phenolic endocrine disrupting chemicals (EDCs) in surface water of Bahe River, China: Distribution, bioaccumulation, risk assessment and estrogenic effect on Hemiculter leucisculus [J]. Environmental Pollution, 2018, 243(Pt A): 103-114
[3] Liu S, Chen H, Zhou G J, et al. Occurrence, source analysis and risk assessment of androgens, glucocorticoids and progestagens in the Hailing Bay region, South China Sea [J]. The Science of the Total Environment, 2015, 536: 99-107
[4] 王梦圆, 张龙飞, 汤云瑜, 等. 几种水生模式生物在持久性有机污染物毒理学评价中的研究进展[J]. 环境化学, 2021, 40(5): 1361-1378
Wang M Y, Zhang L F, Tang Y Y, et al. Research progress of several aquatic biological models in toxicological evaluation of persistent organic pollutants [J]. Environmental Chemistry, 2021, 40(5): 1361-1378 (in Chinese)
[5] 刘少贞, 杨琼, 周俊亮, 等. 17α-甲基睾酮对稀有鮈鲫肝脏脂质代谢的影响[J]. 生态毒理学报, 2021, 16(5): 87-101
Liu S Z, Yang Q, Zhou J L, et al. Effects of 17α-methyltestosterone on lipid metabolism in liver of Gobiocypris rarus [J]. Asian Journal of Ecotoxicology, 2021, 16(5): 87-101 (in Chinese)
[6] 侯丽萍, 何骏驹, 冯子懿, 等. 甲基睾酮对雌斑马鱼内分泌干扰效应的研究[J]. 湖南农业科学, 2017(3): 70-73
Hou L P, He J J, Feng Z Y, et al. Endocrine disruption of methyltestosterone on zebra fish [J]. Hunan Agricultural Sciences, 2017(3): 70-73 (in Chinese)
[7] Orn S, Svenson A, Viktor T, et al. Male-biased sex ratios and vitellogenin induction in zebrafish exposed to effluent water from a Swedish pulp mill [J]. Archives of Environmental Contamination and Toxicology, 2006, 51(3): 445-451
[8] 周家辉, 杜金星, 姜鹏, 等. 17α-甲基睾酮对大口黑鲈生长及性腺发育的影响[J]. 中国水产科学, 2021, 28(9): 1109-1117
Zhou J H, Du J X, Jiang P, et al. Effects of 17α-methyltestosterone on growth and sex differentiation in largemouth bass (Micropterus salmoides) [J]. Journal of Fishery Sciences of China, 2021, 28(9): 1109-1117 (in Chinese)
[9] 刘少贞, 朱玉婷, 赵凌瑞. 17α-甲基睾酮对麦穗鱼性腺组织学的影响[J]. 山西农业大学学报: 自然科学版, 2016, 36(2): 147-152
Liu S Z, Zhu Y T, Zhao L R. Effect of MT on gonadal histology of Pseudorasbora parva [J]. Journal of Shanxi Agricultural University: Natural Science Edition, 2016, 36(2): 147-152 (in Chinese)
[10] Shi Y, Zhang Y, Li S S, et al. Molecular identification of the Kiss2/Kiss1ra system and its potential function during 17alpha-methyltestosterone-induced sex reversal in the orange-spotted grouper, Epinephelus coioides [J]. Biology of Reproduction, 2010, 83(1): 63-74
[11] 甄婗, 吕拥芬, 李嫔. TTF1在雌鼠下丘脑的分布及其与KiSS1和GnRH表达的关系[J]. 上海交通大学学报: 医学版, 2018, 38(6): 598-604
Zhen N, Lv Y F, Li P. Expression of TTF1 in hypothalamus of female rats and its relationship with GnRH and KiSS1 [J]. Journal of Shanghai Jiao Tong University: Medical Science, 2018, 38(6): 598-604 (in Chinese)
[12] d’Anglemont de Tassigny X, Fagg L A, Dixon J P, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene [J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(25): 10714-10719
[13] Funes S, Hedrick J A, Vassileva G, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system [J]. Biochemical and Biophysical Research Communications, 2003, 312(4): 1357-1363
[14] Li S S, Zhang Y, Liu Y, et al. Structural and functional multiplicity of the kisspeptin/GPR54 system in goldfish (Carassius auratus) [J]. The Journal of Endocrinology, 2009, 201(3): 407-418
[15] Migaud H, Ismail R, Cowan M, et al. Kisspeptin and seasonal control of reproduction in male European sea bass (Dicentrarchus labrax) [J]. General and Comparative Endocrinology, 2012, 179(3): 384-399
[16] 李文笙, 王东方. 鱼类microRNA研究进展[J]. 水产学报, 2017, 41(4): 628-639
[17] Sayed D, Abdellatif M. microRNAs in development and disease [J]. Physiological Reviews, 2011, 91(3): 827-887
[18] Liu S Z, Yang Q, Chen Y, et al. Integrated analysis of mRNA- and miRNA-seq in the ovary of rare minnow Gobiocypris rarus in response to 17α-methyltestosterone [J]. Frontiers in Genetics, 2021, 12: 695699
[19] Garaffo G, Conte D, Provero P, et al. The Dlx5 and Foxg1 transcription factors, linked via miRNA-9 and-200, are required for the development of the olfactory and GnRH system [J]. Molecular and Cellular Neurosciences, 2015, 68: 103-119
[20] Messina A, Langlet F, Chachlaki K, et al. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty [J]. Nature Neuroscience, 2016, 19(6): 835-844
[21] 陈佳贤, 李晓宁, 王欣, 等. miR-92a-3p和miR-25-3p海绵上调Kiss1并影响雌性小鼠的青春期启动及动情周期[J]. 中国生物化学与分子生物学报, 2021, 37(4): 543-550
Chen J X, Li X N, Wang X, et al. miR-92a-3p and miR-25-3p sponges up-regulate Kiss1 and affect the onset of puberty and estrous cycle of female mice [J]. Chinese Journal of Biochemistry and Molecular Biology, 2021, 37(4): 543-550 (in Chinese)
[22] 唐家彦, 黄娟, 黄连红, 等. 血清miR-137在中枢性性早熟女童中的临床检测意义[J]. 实用医学杂志, 2016, 32(15): 2500-2503
Tang J Y, Huang J, Huang L H, et al. The clinical detection significance of serum miR-137 in central precocious puberty girls [J]. The Journal of Practical Medicine, 2016, 32(15): 2500-2503 (in Chinese)
[23] Li X N, Xiao J H, Li K, et al. miR-199-3p modulates the onset of puberty in rodents probably by regulating the expression of Kiss1 via the p38 MAPK pathway [J]. Molecular and Cellular Endocrinology, 2020, 518: 110994
[24] Romero-Ruiz A, Avenda o M S, Dominguez F, et al. Deregulation of miR-324/KISS1/kisspeptin in early ectopic pregnancy: Mechanistic findings with clinical and diagnostic implications [J]. American Journal of Obstetrics and Gynecology, 2019, 220(5): 480.e1-480480.e17
o M S, Dominguez F, et al. Deregulation of miR-324/KISS1/kisspeptin in early ectopic pregnancy: Mechanistic findings with clinical and diagnostic implications [J]. American Journal of Obstetrics and Gynecology, 2019, 220(5): 480.e1-480480.e17
[25] 王剑伟, 曹文宣. 中国本土鱼类模式生物稀有鮈鲫研究应用的历史与现状[J]. 生态毒理学报, 2017, 12(2): 20-33
Wang J W, Cao W X. Gobiocypris rarus as a Chinese native model organism: History and current situation [J]. Asian Journal of Ecotoxicology, 2017, 12(2): 20-33 (in Chinese)
[26] 王绿平, 张京佶, 赵华清. 稀有鮈鲫作为鱼类胚胎急性毒性试验受试鱼种的敏感性研究[J]. 生态毒理学报, 2021, 16(5): 102-112
Wang L P, Zhang J J, Zhao H Q. Sensitivity of Chinese rare minnows (Gobiocypris rarus) for fish embryo acute toxicity test [J]. Asian Journal of Ecotoxicology, 2021, 16(5): 102-112 (in Chinese)
[27] 杨彦平. 稀有鮈鲫Kiss/GPR54基因克隆及17α-乙炔雌二醇暴露对其表达的影响[D]. 杨凌: 西北农林科技大学, 2015: 20
Yang Y P. Molecular identification of Kiss/GPR54 and function analysis with mRNA expression profiles exposure to 17α-ethinylestradiol in rare minnow Gobiocypris rarus [D]. Yangling: Northwest A & F University, 2015: 20 (in Chinese)
[28] Qin F, Wang L H, Liu S Z, et al. Characterization of reference genes in rare minnow, Gobiocypris rarus (Actinopterygii: Cypriniformes: Cyprinidae), in early postembryonic development and in response to EDCs treatment [J]. Acta Ichthyologica et Piscatoria, 2013, 43(2): 127-138
[29] 吴建阳, 何冰, 杜玉洁, 等. 利用geNorm、NormFinder和BestKeeper软件进行内参基因稳定性分析的方法[J]. 现代农业科技, 2017(5): 278-281
Wu J Y, He B, Du Y J, et al. Analysis method of systematically evaluating stability of reference genes using geNorm, NormFinder and BestKeeper [J]. Modern Agricultural Science and Technology, 2017(5): 278-281 (in Chinese)
[30] 史熊杰, 刘春生, 余珂, 等. 环境内分泌干扰物毒理学研究[J]. 化学进展, 2009, 21(S1): 340-349
Shi X J, Liu C S, Yu K, et al. Toxicological research on environmental endocrine disruptors [J]. Progress in Chemistry, 2009, 21(S1): 340-349 (in Chinese)
[31] 姚汶励, 姜鹏, 白俊杰. 17α-甲基睾酮对草鱼性腺发育及性类固醇激素水平的影响[J]. 水产学报, 2019, 43(4): 801-806, 809, 807
Yao W L, Jiang P, Bai J J. Effects of 17α-methyltestosterone on gonadal development and hormone levels in grass carp (Ctenopharyngodon idella) [J]. Journal of Fisheries of China, 2019, 43(4): 801-806, 809, 807 (in Chinese)
[32] Zou Y X, Wu Z H, Fan Z F, et al. Analyses of mRNA-seq and miRNA-seq of the brain reveal the sex differences of gene expression and regulation before and during gonadal differentiation in 17β-estradiol or 17α-methyltestosterone-induced olive flounder (Paralichthys olivaceus) [J]. Molecular Reproduction and Development, 2020, 87(1): 78-90
[33] Liu S Z, Wang L H, Qin F, et al. Gonadal development and transcript profiling of steroidogenic enzymes in response to 17α-methyltestosterone in the rare minnow Gobiocypris rarus [J]. The Journal of Steroid Biochemistry and Molecular Biology, 2014, 143: 223-232
[34] Passini G, Sterzelecki F C, de Carvalho C V A, et al. 17α-methyltestosterone implants accelerate spermatogenesis in common snook, Centropomus undecimalis, during first sexual maturation [J]. Theriogenology, 2018, 106: 134-140
[35] 赖晓健, 彭帅, 张哲, 等. 甲基睾酮暴露对鳗鲡精巢发育的影响[J]. 水产科学, 2021, 40(6): 900-904
Lai X J, Peng S, Zhang Z, et al. Testis development induced in Japanese eel Anguilla japonica by 17α-methyltestosterone exposure [J]. Fisheries Science, 2021, 40(6): 900-904 (in Chinese)
[36] García-Galiano D, Pinilla L, Tena-Sempere M. Sex steroids and the control of the Kiss1 system: Developmental roles and major regulatory actions [J]. Journal of Neuroendocrinology, 2012, 24(1): 22-33
[37] 卓琦. Kisspeptin调控鱼类生殖内分泌的研究进展[J]. 动物学研究, 2013, 34(5): 519-530
Zhuo Q. Advances in the study of neuroendocrinological regulation of kisspeptin in fish reproduction [J]. Zoological Research, 2013, 34(5): 519-530 (in Chinese)
[38] Shahjahan M, Motohashi E, Doi H, et al. Elevation of Kiss2 and its receptor gene expression in the brain and pituitary of grass puffer during the spawning season [J]. General and Comparative Endocrinology, 2010, 169(1): 48-57
[39] Han S K, Gottsch M L, Lee K J, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty [J]. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 2005, 25(49): 11349-11356
[40] Costa D P, Sinervo B. Field physiology: Physiological insights from animals in nature [J]. Annual Review of Physiology, 2004, 66: 209-238
[41] 王滨, 柳学周, 徐永江, 等. Kisspeptin对鱼类生殖轴的调控机制研究[J]. 渔业科学进展, 2018, 39(4): 173-184
Wang B, Liu X Z, Xu Y J, et al. Regulatory mechanisms of kisspeptin on the reproductive axis in fish [J]. Progress in Fishery Sciences, 2018, 39(4): 173-184 (in Chinese)
[42] 张浩. 克氏双锯鱼kisspeptin/GPR54系统的分子鉴定和表达分析[D]. 海口: 海南大学, 2019: 51
Zhang H. Molecular characterization and expression analysis of kisspeptin/GPR54 system in anemonefish, Amphiprion clarkii [D]. Haikou: Hainan University, 2019: 51 (in Chinese)
[43] Felip A, Zanuy S, Pineda R, et al. Evidence for two distinct KiSS genes in non-placental vertebrates that encode kisspeptins with different gonadotropin-releasing activities in fish and mammals [J]. Molecular and Cellular Endocrinology, 2009, 312(1-2): 61-71
[44] Chang J P, Mar A, Wlasichuk M, et al. Kisspeptin-1 directly stimulates LH and GH secretion from goldfish pituitary cells in a Ca2+-dependent manner [J]. General and Comparative Endocrinology, 2012, 179(1): 38-46
[45] Servili A, Le Page Y, Leprince J, et al. Organization of two independent kisspeptin systems derived from evolutionary-ancient kiss genes in the brain of zebrafish [J]. Endocrinology, 2011, 152(4): 1527-1540
[46] Kitahashi T, Ogawa S, Parhar I S. Cloning and expression of kiss2 in the zebrafish and medaka [J]. Endocrinology, 2009, 150(2): 821-831
[47] Shen L C, Hong X X, Liu Y, et al. The miR-25-3p/Sp1 pathway is dysregulated in ovarian endometriosis [J]. The Journal of International Medical Research, 2020, 48(4): 300060520918437
[48] Huang J H, Zhou Q H, Chen C C, et al. microRNA miR-92a-3p regulates breast cancer cell proliferation and metastasis via regulating B-cell translocation gene 2 (BTG2) [J]. Bioengineered, 2021, 12(1): 2033-2044
[49] 孙丹, 周文婷, 吴绪峰, 等. miR-92a-3p靶向LATS2对宫颈癌细胞的增殖、凋亡和侵袭的影响和分子机制[J]. 广西医科大学学报, 2020, 37(10): 1784-1790
Sun D, Zhou W T, Wu X F, et al. Effect and molecular mechanism of miR-92a-3p targeting LATS2 on proliferation, apoptosis and invasion of cervical cancer cells [J]. Journal of Guangxi Medical University, 2020, 37(10): 1784-1790 (in Chinese)
[50] Presslauer C, Bizuayehu T T, Razmi K, et al. See-Thru-Gonad zebrafish line: Developmental and functional validation [J]. Reproduction, 2016, 152(5): 507-517