随着养殖业的快速发展,抗生素作为主要的饲料添加剂,在畜禽的疾病防治和促进生长等方面发挥了重要作用[1]。但抗生素的长期过度使用导致了严重的药物残留和细菌耐药性,引发了人畜食品安全和环境污染等诸多问题,造成严重的潜在人体健康风险[2]。早在2006年,欧盟就开始全面禁止在畜牧养殖中使用抗生素;紧随其后,美国、日本和韩国等国家也陆续出台了养殖中限用或禁用抗生素的相关法规;我国也在2019年7月10日发布了《中华人民共和国农业农村部第194号公告》,公告要求从2020年7月1日起,我国饲料生产企业要停止生产含有促生长类药物饲料添加剂的商品饲料,中药类除外[3]。在我国饲料行业进入全面禁抗的时代背景下,如何保证养殖业的生态健康和高产优质成为新的课题,因此,探寻更多的抗生素替代物逐渐成为研究热点。
藻类是植物系统中能进行光合作用,但根、茎、叶并没有真正分化的一类植物体,其种类繁多,适应能力较强,广泛分布于江河、湖泊和海洋等水体或潮湿处[4]。藻类生物质的组成中含有大量的矿物质元素,如碘、锌、铁、铜、钙、镁、钠和钾等,藻类中这些元素的含量远高于可食用陆地植物,部分甚至超过40%[5-6]。藻类会产生各种各样具有生物活性的代谢物包括多酚、多糖、多肽、脂肪酸、生物碱、内酯和色素等,其中众多物质卤化后可替代抗菌剂和抗生物被膜剂[7-8],或用来治疗各种疾病[9]。藻类和藻类代谢物含有丰富的蛋白质、维生素和矿物质,同时都表现出了很强的抗菌[10]、抗真菌[11]、抗癌[12]和抗疟疾活性[13],是天然的抗菌剂和抗氧化剂(图1),这一优势使得藻类非常有潜力成为合成抗生素的天然抗生素替代物,应用到畜禽养殖饲料添加剂中,从而减少抗生素的使用[14]。法国欧密斯集团和法国国家农业食品与环境研究院成功提取了海藻多糖,其能够影响猪小肠上皮细胞中的免疫介质转录,识别参与这种激活作用的代谢途径。体内研究证实海藻提取物具有免疫调节功能,可以提高猪体内单核细胞和异嗜白细胞的防御活性,促进乳源性免疫转移[15-16]。该研究证明了将藻类添加到动物饲料中,对病原微生物日益增长的抗生素耐药性的防控具有潜在的实用价值,是畜禽养殖替抗、减抗环节中一种新的解决方案。本文重点介绍藻类及其代谢物的抑菌机制和其在畜禽养殖业中的应用进展,为藻类替代抗生素的大规模应用奠定理论基础。
1 藻类代谢物的抑菌活性及机制(Types of algal metabolites with antibacterial activity and mechanism)
藻类的营养成分和代谢产物受种类、环境条件、地理位置、季节性、生长介质特性以及生长阶段等因素的影响[17],主要的生物活性化合物包括多酚、多糖、蛋白质和多肽、脂肪酸、甾醇、萜类化合物和色素等[18]。病原微生物对抗生素的抗药性已成为全球共同关注的公共卫生问题,探寻新的替代方案来解决这一问题已经刻不容缓。藻类产生的初生或次生代谢产物不仅有显著的生物活性,对包括多重耐药菌在内的多种耐药菌株也具有很强的抗菌作用[19]。目前对藻类代谢物抗菌潜力的研究在不断增加,期望开发出与传统合成抗生素相比,毒性更小、副作用更少、成本效益更高的新抗菌疗法。但将某一特定化合物的属性归因于一种活性是不够准确和全面的,在大多数情况下,抗菌效果可能是这些化合物协同作用的结果。本节将重点介绍藻类代谢物的抑菌活性及其机制。
1.1 多酚
多酚类化合物广泛存在于陆生和水生生物体内,具有很强的抗氧化性能,是动物和人类饮食中重要的组成部分。在藻类特别是褐藻中可以提取一种藻类特有的酚类化合物即邻氯单宁,可参与细胞壁的发育和生长[20]。藻类中多酚的含量相对高于其他陆地植物,有高抗氧化能力,且具有非常广泛的潜在细胞内靶标,对多种细菌均有抑制作用[21]。藻类多酚主要由乙酸丙二酸途径产生,存在于的岩藻糖颗粒中,通过与参与必需代谢途径和膜蛋白的细菌酶结合和直接抑制氧化磷酸化发挥抑菌作用。同时,多酚中含有大量羟基,可通过疏水相互作用和氢键与细菌蛋白质的酰胺基结合从而增强多酚的抑菌活性。从鼠尾藻(Sargassum thunbergii)中提取的低分子量邻氯单宁通过破坏细胞壁和细胞膜而影响副溶血弧菌的细胞形态,导致细胞质内容物外泄、膜通透性丧失和细菌细胞整体解体,从而发挥抗菌活性,这项研究表明了邻氯单宁在抑制食品致病菌传播方面的有效性[22]。从褐藻(Ecklonia Stolonifera)中分离得到的褐藻多酚在1/4最低抑菌浓度(MIC)(16 μg·mL-1)浓度下,使氨苄西林对耐甲氧西林金黄色葡萄球菌(MRSA)的MIC值由512 μg·mL-1降至0.5 μg·mL-1,说明褐藻多酚可明显降低MRSA对氨苄西林的高度耐药[23]。Nagayama等[24]证明来自褐藻(Ecklonia kurome)的多酚对MRSA和化脓性链球菌均具有杀菌活性,而且将褐藻多酚以最大日剂量1 500 mg·kg-1作用于小鼠身上,经过14 d观察没有发现任何紊乱症状或毒性效应。因此,褐藻多酚可作为治疗MRSA感染的有效药物进行进一步的研究。除此之外,藻类多酚还显示出其他潜在的特性,如抗菌、抗真菌、抗炎、抗疟和抗癌等[25]。
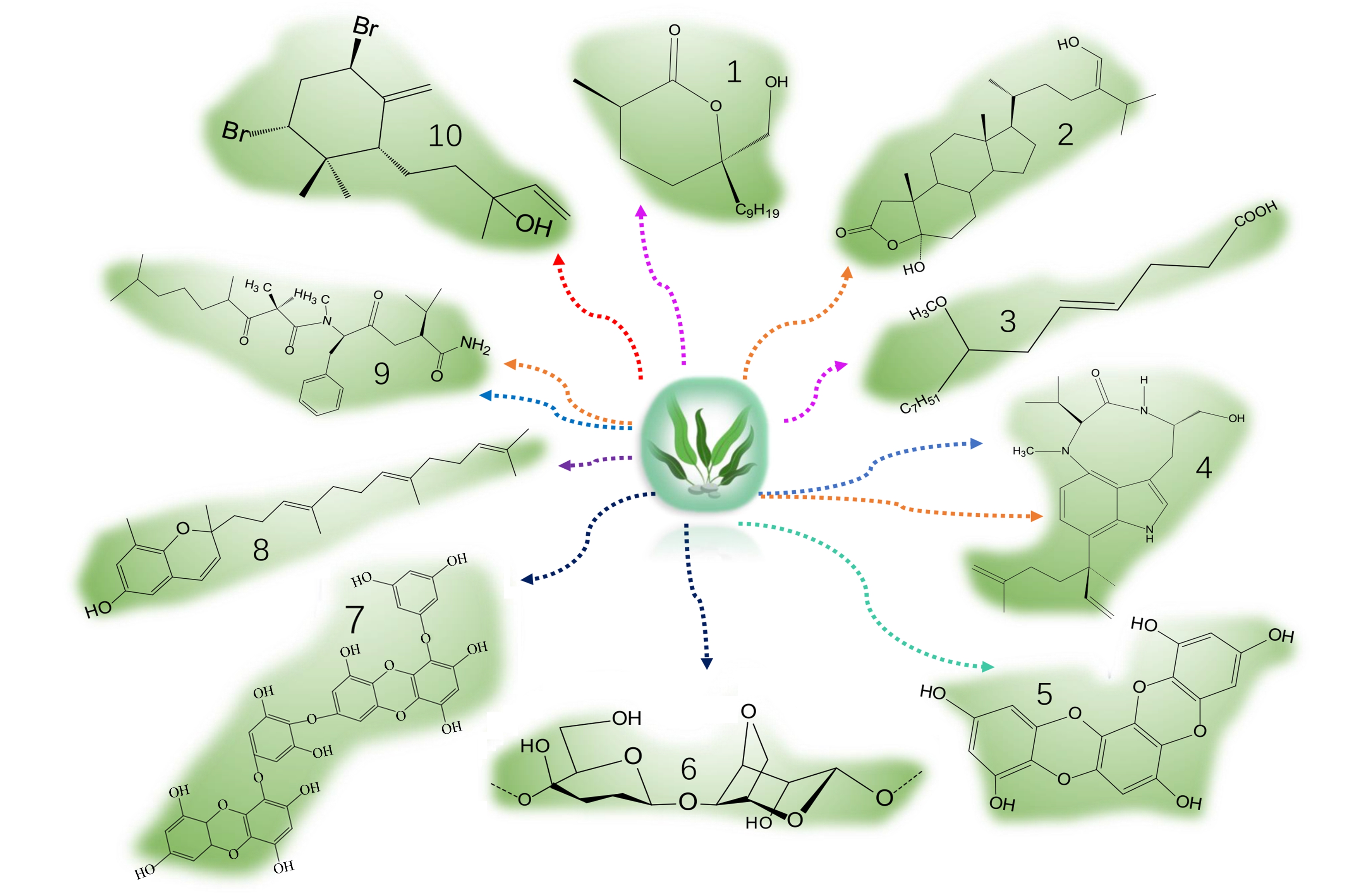
图1 典型藻类代谢物具有的生物活性
注:1.内酯,2.甾醇,3.脂肪酸,4.生物碱,5.和7.多酚,6.多糖,8.和10.萜类,9.多肽;不同颜色的线代表不同的抗菌活性,紫色为抗菌活性,
橙色为细胞毒性活性,蓝色为抗癌活性,深紫为抗真菌活性,红色为抗疟原活性,绿色为抗氧化活性,深蓝色为抗病毒活性。
Fig. 1 Biological activities of typical algal metabolites
Note: 1. Lactones, 2 Sterols, 3. Fatty acids, 4. Alkaloids, 5. and 7. Polyphenols, 6. Polysaccharides, 8. and 10. Terpenes, 9. Polypeptide; the lines of different colors represent different antibacterial activities; purple is antibacterial activity, orange is cytotoxic activity, blue is anticancer activity, dark purple is antifungal activity, red is antimalarial activity, green is antioxidant activity, and dark blue is antiviral activity.
除了天然产物的抗菌研究外,含有天然产物和抗生素的新型抗菌组合也成为新的研究重点[26]。研究发现多酚与抗生素有协同作用[27],藻类多酚可以改变细胞膜和细胞壁的通透性和完整性,这可以促进抗生素渗透到细菌的细胞质中。之后不同类型的抗生素,将对病原体重要功能(DNA的复制、转录或翻译)进行破坏,从而发挥抗菌作用[28],这可成为增强或恢复抗生素对多重耐药细菌(包括MRSA)引起的疾病的治疗,也可以作为应对当前抗生素耐药性的有效策略,同时也为我们开发新的、有潜在价值的抗生素替代物提供了新的思路。
1.2 多糖
多糖是藻类细胞壁主要的结构成分,其糖蛋白受体可以与细菌细胞壁、膜和核酸上的分子结合,导致膜结构紊乱、膜通透性丧失、蛋白质泄漏和细菌DNA功能障碍,从而发挥抗菌作用[29]。
从莱茵衣藻(Chlamydomonas reinhardtii)中提取的硫酸多糖主要通过抑制生物膜的形成或破坏已经形成的生物膜发挥抗菌作用,在4~8 mg·mL-1的浓度下,对枯草芽孢杆菌、粘膜奈瑟氏菌、大肠杆菌和链球菌等细菌生物被膜的抑制率为100%[30]。在冷热水中连续从褐海藻(Dictyopteris membranacea)和红海藻(Pterocladia capillacea)中提取出的多糖,可以抑制荧光假单胞菌、大肠杆菌、金黄色葡萄球菌和蜡状芽孢杆菌的生长,且多糖对金黄色葡萄球菌的抗菌活性比氨苄青霉素对金黄色葡萄球菌的抗菌活性高50%[31]。从厚叶凯氏藻属(Kjellmaniella crassifolia)、近缘多刺藻属(Polyope affinis)和冈村枝管藻(Cladosiphon okamuranus)等海藻中提取的多糖,具有抑制生物膜形成、粘附胃黏膜和表达感染过程中有关毒力因子的作用[32]。另外,多糖被证实可用来预防鲑鱼双歧杆菌感染,增强养殖鱼类的免疫功能。从藻类(Cystoseira barbata)中提取的海带多糖对大肠杆菌、肺炎克雷伯氏菌、肠炎沙门氏菌和铜绿假单胞菌等致病菌的生长有抑制作用,对铜绿假单胞菌的MIC在20~40 mg·mL-1之间。同时,这种多糖能有效促进大鼠皮肤创面的愈合、收缩和上皮细胞更新,使皮肤组织完全恢复[33]。此外,多糖还具有生物相容性,毒性可以忽略不计,可用于抑制感染和根除幽门螺杆菌[34]。
藻类多糖对多种细菌病原体具有广泛的抗菌活性,同时还具有抗肿瘤、抗氧化和抗真菌等多种有益活性,已显示出在开发有效药物系统方面的价值,可作为抗生素替代物用于畜禽养殖中,抑制抗生素耐药性的持续增长。
1.3 蛋白质和肽
藻类中含有丰富的蛋白质,其含量在不同的门之间有所不同,红藻约占干质量的10%~30%,褐藻约占干质量的5%~15%,绿藻约占干质量的3%~47%[26],在藻类中功能活跃的蛋白质主要分为2类,即凝集素和藻胆蛋白[35]。从红藻(Galaxaura argata和Eucheuma serra)中提取的凝集素对创伤弧菌的生长有明显的抑制作用。大部分藻类中几乎包含所有必需的氨基酸,且缬氨酸、蛋氨酸和亮氨酸的含量与卵蛋白相当。目前从简单的短链多肽和高度复杂的大蛋白衍生出来的几种抗菌肽(AMPs),显示出广泛的抗病原菌活性。由于它们具有两亲性,所以能够与细菌细胞膜上的非极性和极性位点相互作用,产生孔隙,进一步造成细菌细胞的泄漏和损伤[36],从而发挥抗菌作用。从钝顶螺旋藻(Spirulina platensis)中提取出的碱性蛋白酶和木瓜蛋白酶水解物对金黄色葡萄球菌和大肠杆菌均有良好的抗菌活性。随着研究的深入,藻类多肽的潜在抗菌作用也受到越来越多的关注,这些多肽通过修饰可以增强抗菌活性和其他生物化学特性,用于生物医学。近年来,从海藻中提取的多肽因其具有多种生物活性(血管紧张素转换酶抑制剂、抗高血压、抗氧化和抗糖尿病),而受到越来越多的关注[37]。此外,与具有特定靶点的抗生素不同,多肽会与细菌膜和其他通用成分相互作用,且多肽不会受由基因突变导致的微生物耐药性影响,在未来将多肽用于抵抗抗菌素耐药性环境有很高的利用价值[8]。
1.4 脂肪酸
脂肪酸几乎是所有生命形式的细胞膜中不可或缺的成分,是维持细胞膜完整性和细胞组织正常生命活动所必需的物质。当藻类受到病原体攻击时,会触发其防御机制,藻类细胞将会在细胞丧失完整性的特定条件下释放脂肪酸,使脂肪酸发挥抗菌作用。对于相同链长的脂肪酸,游离脂肪酸的抗菌能力更强,且会随着碳链不饱和度的增加而增加[38]。
藻类中富含游离脂肪酸,通过抑制电子传递链和氧化磷酸化,破坏养分吸收过程,影响酶活性和细胞产物的自身降解,最终导致细菌溶解,从而显示出抗菌活性。从羊栖菜(Sargassum fusiforme)和马尾藻(Sargassum vulgare)中经过有机溶剂和气质联用提取出脂肪酸,用脂肪酸对耐药金黄色葡萄球菌和肺炎克雷伯氏菌进行处理,通过透射电子显微镜分析细菌,发现细菌的形态发生了明显的变化:细胞壁变形、原生质体收缩、细胞质内容物泄漏、染色质解体,这表明脂肪酸对耐药金黄色葡萄球菌和肺炎克雷伯氏菌均有抗菌活性[39]。藻类脂肪酸中主要是游离脂肪酸发挥杀菌性能,迄今为止藻类脂肪酸尚未显示出对抗生素的抗药性。因为藻类脂肪酸的主要目标是细胞膜,受自发突变影响的可能性较小,利用这一特性藻类脂肪酸可作为经典抗生素的替代品。
1.5 其他
藻类代谢物还包括萜类、内酯类、色素、生物碱和甾醇等。藻类萜类化合物在结构上与植物提取物不同,其可作用于多个靶点,具有抗菌、抗癌、抗寄生虫等药理活性[40]。从藻类中提取的具有生物活性的次生代谢物内酯类化合物,可以抑制大肠杆菌和致病性铜绿假单胞菌的群体感应和生物膜的形成,是预防这些高致病性细菌的潜在化合物[41]。藻类中也含有不同种类的生物碱化合物,结瘤藻、发菜等蓝藻是生物碱良好的储存库,其中去甲肾上腺素吡啶对革兰氏阳性金黄色葡萄球菌、芽孢杆菌有较强的抑菌作用[42-44]。从藻类(Lessonia nigrescens)中分离得到的甾醇对结核分枝杆菌有较强的抑制作用,甾醇2个异构体的MIC分别为0.125 μg·mL-1和1 μg·mL-1[45]。甾醇对危及生命的细菌感染具有很高的抗菌活性,是一种有效的代谢物,可以作为抗生素的替代品引入体内治疗抗药性致病菌株。
不同藻类代谢物的作用机制不同,相当一部分代谢物直接给药时,对哺乳动物细胞没有毒性[46]。此外,经藻类代谢物修饰的纳米颗粒显示出更强的抗菌活性和更低的毒性。Gurusamy等[47]通过对石笋(Ulva lactuca)脂溶性提取物进行修饰,合成了对耐多药金黄色葡萄球菌、枯草芽孢杆菌、肺炎克雷伯菌和普通变形杆菌具有抗菌活性的AgNPs。对这些代谢物进行进一步的体内外研究,可以预防多重耐药病原体引起的致命感染,揭示它们在畜禽养殖中替代抗生素的潜力和应用价值。
2 藻类在畜禽养殖中的应用(Application of algae in livestock and poultry breeding)
2.1 藻类在家禽养殖中的应用
藻类已被用于家禽养殖中,以促进家禽生长、增加肠道微生物多样性、提高动物免疫力及肉和蛋的营养价值(图2)。全球养殖业在动物饲料中添加大量抗生素用于预防细菌性疾病、促进家禽生长,但也造成了严重的抗生素残留和细菌耐药性问题。目前,已有不少研究将藻类添加到家禽饲料中,分析藻类对家禽生长状况的影响(表1),以期减少或替代抗生素。Sugiharto等[48]在肉鸡0~21 d的基础日粮中添加1%的钝顶螺旋藻或抗生素,结果发现添加1%钝顶螺旋藻的肉鸡的生产性能、胴体性状和内脏器官发育等方面与添加抗生素显著相关(P<0.05),甚至更好,说明钝顶螺旋藻作为饲料添加剂比抗生素有更大的优势。在肉鸡日粮中添加3%的莴苣(U. lactuca),肉鸡的体质量、屠宰性能和胸肉率显著提高,其中含量较高的粗蛋白和氨基酸,尤其是蛋氨酸,发挥了主要作用[49]。藻类对鸡的免疫应答也有一定的促进作用。Kang等[50]在雏鸡的日粮中添加1%的新鲜小球藻(Chlorella),显著提高了雏鸡血液中白细胞和淋巴细胞的数量(P<0.05),免疫球蛋白(IgG、IgM和IgA)的水平也有所提高。这主要是因为小球藻内纤维、多糖(免疫球藻)、多肽和糖蛋白等物质对免疫应答有一定的刺激作用。另外,饲喂含0.5%小球藻的日粮能提高鸡的白细胞吞噬活性和吞噬能力并能够促进淋巴组织发育[51]。藻类也可促进肠道菌群的健康生态,减少微生物耐药性。Shanmugapriya和Babu[52]饲喂含有钝顶螺旋藻的鸡日粮显示鸡肠道乳酸菌数量增加,大肠杆菌减少。随后,Namra等[53]的研究结果表明,饲喂含0.9 g·kg-1和0.7 g·kg-1钝顶螺旋藻的日粮时,肉鸡肠道细菌总数和大肠杆菌总数显著下降,而乳杆菌总数显著增加(P<0.05)。然而,Sugiharto等[48]发现,用钝顶螺旋藻饲喂肉鸡不会影响回肠和盲肠乳杆菌的种群多样性。此外,藻类提取物也可显著提高鸡抗氧化活性,夏伦斌等[54]研究表明,饲料中添加藻类多糖可显著提高鸡血清中超氧化物歧化酶和谷胱甘肽过氧化物酶含量,降低丙二醛含量,从而提高鸡的抗氧化能力和存活率,且比添加抗生素更有优势。
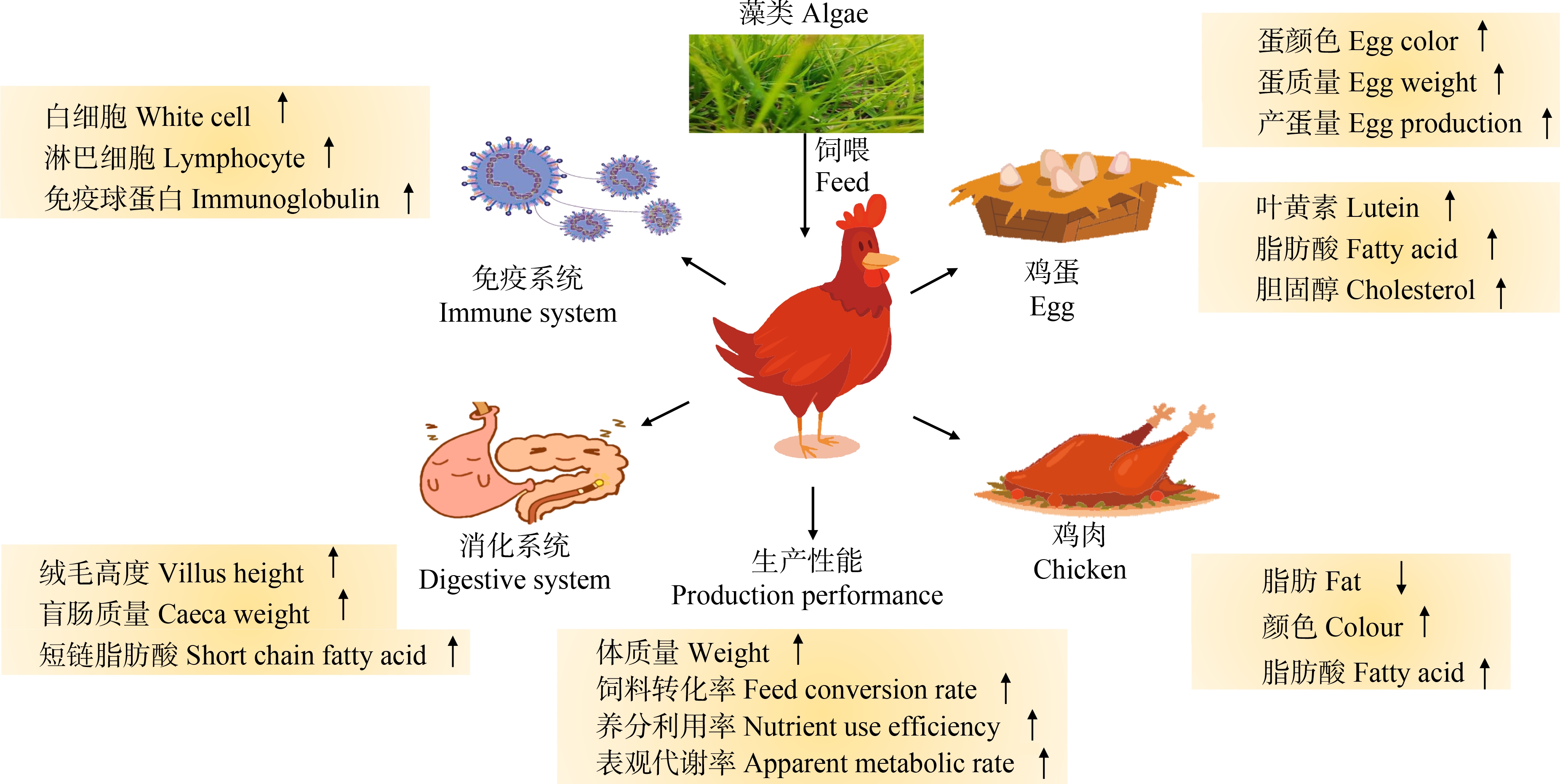
图2 饲喂藻类对鸡的影响
注:↑表示促进作用,↓表示抑制作用。
Fig. 2 Effects of feeding algae on chickens
Note: ↑promoting effect, ↓inhibitory effect.
将藻类添加到蛋鸡饲料中,可以提高鸡蛋的质量,减少鸡肠道中的致病菌,提高蛋鸡免疫能力。研究表明,在家禽饲料中添加海藻可以提高鸡蛋中维生素、矿物质和脂肪酸的水平,尤其可以显著提高ω-3脂肪酸含量[55-56]。添加1%~3%的石斛属海藻可以提高鸡蛋的产量和质量,增加质量、壳厚、蛋黄颜色和降低蛋黄胆固醇。海藻提取物还降低了粪便中大肠杆菌的含量,这表明动物的健康状况有所好转[14]。接下来仍需进一步的研究,以便获得海藻的生物利用度和最适浓度。肠炎沙门氏菌是一种可以通过卵巢和输卵管从蛋鸡垂直传播到鸡蛋和通过受污染的粪便而传播的致病菌。降低这种细菌的水平对于生产更安全的鸡蛋和减少沙门氏菌病向人类的传播至关重要。在含有4%的红海藻日粮中,肠炎沙门氏菌对蛋鸡生长和产蛋量的毒害效应有较大程度的降低。在饲料中添加藻类(Crispus)可以抑制沙门氏菌在排泄物和盲肠中的定植,这可能是由于其促进了乳酸菌的生长,增加了短链脂肪酸的浓度。添加这种饲料的禽类中免疫球蛋白(IgA)水平较高,表明海藻对体液免疫系统有一定的促进作用[57]。Kulshreshtha等[58]将红藻添加到家畜饲料中发现鸡肠道中的病原菌在减少,益生菌数量有所增加,绒毛高度得到改善,短链脂肪酸浓度增加。可能是由于红藻中含有的特定的生物活性化合物,如琼脂、卡拉胶、木聚糖和硫酸化半乳糖等。然而,还需要进一步的研究来确定这种机制。
以上结果表明,藻类及其代谢物可以起到增加家禽体质量和预防疾病的作用,是一种良好的抗生素替代物,可用于家禽饲料中,以减少抗生素的使用,抑制耐药菌的产生。除此之外,藻类及其代谢物还可以提供蛋白质、矿物质和维生素等营养物质,促进家禽的健康生长。
2.2 藻类在猪养殖中的应用
藻类及其代谢物可以提高饲料转化率促进猪生长,也可作用于免疫细胞或直接影响肠道上皮组织的炎症因子的表达,从而增强猪的免疫功能(表1)。在猪的养殖过程中,经常会发生感染梭状芽孢杆菌、大肠杆菌和沙门氏菌等问题,导致仔猪腹泻、成活率下降、生长受阻甚至死亡,产生极大的经济损失。因此,往往在猪饲料中添加抗生素,预防猪疾病感染,促进猪生长,但这也造成了严重的抗生素耐药性问题。为了解决这一问题,研究人员提出可将藻类及代谢物用于养猪场取代抗生素,从而减少抗生素的使用。藻类代谢物可通过提高肠道营养物质转运载体基因(SGLT1、GLUT2等)表达的和营养物质消化率改善断奶仔猪生长性能,同时也可以改善肠道形态,增加绒毛隐窝比值,提高营养物质的吸收面积[59]。在猪日粮中添加0.1%的发酵小球藻(Chlorella)可以改善仔猪的生长性能、养分消化率、粪便微生物(较低的大肠杆菌和较高的乳酸菌),并减少粪便有毒气体的排放[60]。结节藻也可以改善断奶仔猪胃肠健康状况,作为饲料添加剂在增加断奶仔猪肠道菌群多样性和维持肠道菌群的健康生态具有潜在的应用前景。研究表明,使用岩藻多糖和海带多糖提取物也可以改善仔猪的生产性能,缓解断奶后的常见问题。Dierick等[61]的实验结果表明,1%的海藻饲料对肠道菌群有抑制作用,尤其是大肠杆菌,同时提高了乳杆菌/大肠杆菌的比例,从而增强了对肠道疾病的抵抗力。然而,在消化率良好的仔猪日粮中添加0.25%、0.5%或1%的海藻粉并不能提高断奶仔猪的生产性能、肠道健康指标和改善血浆氧化状态,也不会改变前肠和盲肠中的微生物生态。推测是由于菌丝体中含有的邻氯单宁,抵消了其他化合物的益生作用,也可能是因为海藻百分含量太低,在氧化状态不变的情况下,饮食中的抗氧化维生素掩盖了海藻的抗氧化作用[62]。之后,Dierick等[63]进行了体外模拟活体实验,结果表明海藻对仔猪小肠和后肠菌群均有显著的抑制作用,其中对大肠杆菌的抑制作用最强,但发酵菌群(乳酸、挥发性脂肪酸)的活性也显著降低。另外,在日粮中添加巨藻和提取物对猪、肉鸡和鱼类等几种单胃动物的免疫状态和肠道健康也有积极影响[64]。许多藻类及其提取物都具有促进猪的生长和维持肠道菌群健康的作用,所以藻类或从中提取的生物活性成分,如海带多糖和岩藻多糖,可以作为猪饲料中抗生素的替代品,或环境友好的治疗剂替代品。
表1 藻类在畜禽养殖中的应用
Table 1 Application of algae in livestock and poultry breeding

畜禽Livestock种类Type剂量/%Dosage/%表现及影响Performance and effect参考文献Reference家禽Poultry肉鸡Broilers蛋鸡Laying hens石斛Ulva lactuca3增加体质量,提高屠宰率和胸肉率Increase weight, dressing percentage and breast meat percentage[49]小球藻Chlorella1增加白细胞和淋巴细胞数量Increase the number of white cells and lymphocytes[50]裙带菜U. pinnatifida4减少蛋白质分解Reduce protein decomposition[65]裂壶藻Schizochytrium sp.2降低胸肌率和饲料转化率,增加脂肪酸含量Decrease chest muscle rate and feed conversion rate and increase fatty acid content[66]钝顶螺旋藻Spirulina platensis0.2~0.8增加体质量,降低饲料转化率Increase weight and reduce feed conversion rate[67]结瘤蘑菇Ascophyllum nodosum1提高生长性能Improve growth performance[68]裂壶藻Schizochytrium spp.1.27~1.77提高蛋黄中多不饱和脂肪酸含量Increase the content of polyunsaturated fatty acids in egg yolk[69]绿藻E. prolifera1~3增加蛋质量、蛋壳厚度和蛋黄颜色,降低蛋黄中的胆固醇、粪便中的大肠杆菌含量和饲料转化率Increase egg weight, eggshell thickness and yolk color, decrease cholesterol in yolk, E. coli content in feces and feed conversion rate[70]浒苔Enteromorpha prolifera3增加蛋壳厚度和鸡蛋品质,降低鸡蛋胆固醇含量,改善免疫系统Increase eggshell thickness and egg quality, decreased egg cholesterol content, and improve immune system[70]褐藻Sargassum sp.3~6提高鸡蛋质量,降低蛋黄胆固醇、甘油三酯和ω-6脂肪酸,增加胡萝卜素和叶黄素含量Improve egg quality, reduce yolk cholesterol, triglyceride and omega-6 fatty acids, and increase carotene and lutein content[71]角叉菜和高氏红藻Chondrus crispus and Sarcodiotheca gaudichaudii2增加绒毛高度、盲肠质量、肠道益生菌浓度和鸡蛋中短链脂肪酸浓度Increase villus height, cecal weight, intestinal probiotic concentration and short-chain fatty acid concentration in eggs[58]马尾藻属Sargassum spp.0.2增加鸡蛋中蛋白和脂肪酸含量Increase the content of protein and fatty acids in eggs[72]裙带菜Undaria pinnatifida0.5提高产蛋量和蛋质量,增加血液中白蛋白含量Increase egg production and egg weight, increase the content of albumin in blood[73]
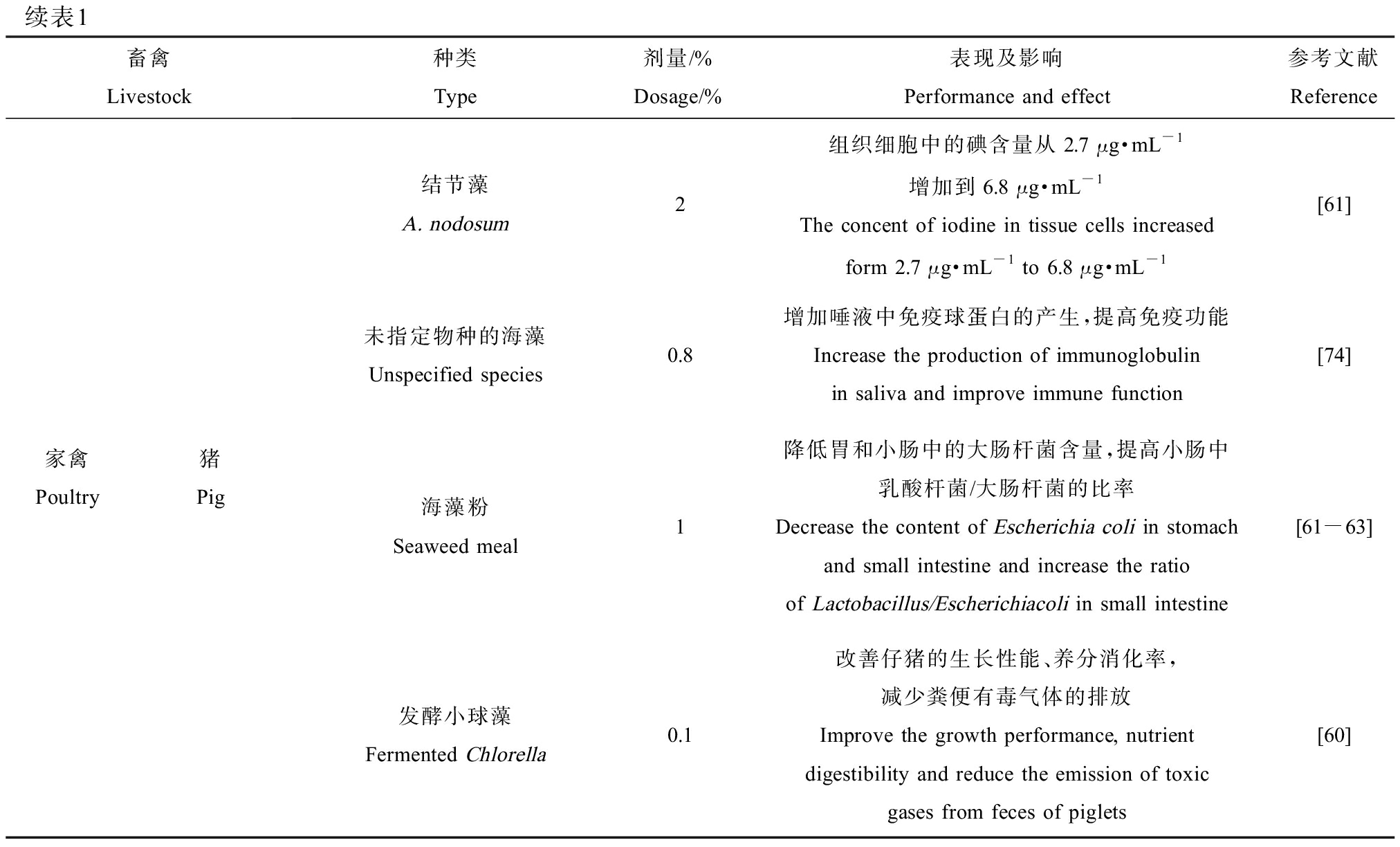
续表1畜禽Livestock种类Type剂量/%Dosage/%表现及影响Performance and effect参考文献Reference家禽Poultry猪Pig结节藻A. nodosum2组织细胞中的碘含量从2.7 μg·mL-1增加到6.8 μg·mL-1The concent of iodine in tissue cells increased form 2.7 μg·mL-1 to 6.8 μg·mL-1[61]未指定物种的海藻Unspecified species0.8增加唾液中免疫球蛋白的产生,提高免疫功能Increase the production of immunoglobulin in saliva and improve immune function[74]海藻粉Seaweed meal1降低胃和小肠中的大肠杆菌含量,提高小肠中乳酸杆菌/大肠杆菌的比率Decrease the content of Escherichia coli in stomach and small intestine and increase the ratio of Lactobacillus/Escherichiacoli in small intestine[61-63]发酵小球藻Fermented Chlorella0.1改善仔猪的生长性能、养分消化率,减少粪便有毒气体的排放Improve the growth performance, nutrient digestibility and reduce the emission of toxic gases from feces of piglets[60]
以上结果表明,藻类及其提取物可以通过改变猪的肠道微生物区系和调节免疫功能,改善营养物质的消化和吸收,增强肠道屏障功能,提高其生长性能,促进肠道健康。同时,其并没有表现出很强的耐药性,有比抗生素更好的生物稳定性能,在畜禽养殖中作为抗生素替代品使用有很大的应用前景。
3 结论与展望(Conclusion and prospect)
藻类富含多酚、多糖和脂肪酸等多种具有生物活性的代谢物,将藻类适量(10%以下)添加到畜禽饲料中,可以提高饲料的稳定性,改善动物的生产性能,提高肉和蛋的质量,对动物的肠道健康起积极作用,有望取代抗生素在养殖场中使用。藻类代谢物主要通过破坏细胞膜结构与功能、抑制细菌能量代谢、引起DNA损伤等发挥抑菌作用,而不会对宿主细胞产生实质性的不利影响。相较于利用单一的藻类代谢物,多种藻类代谢物之间及与各种抗生素相互联合,可以达到更为理想的抑菌效果,从而减少抗生素在畜禽养殖中的使用,以遏制不断增长的细菌耐药性。此外,藻类代谢物对多重耐药菌也表现出良好的抗菌能力,对这些代谢物进行详细的进一步研究以及体内实验,有望开发出新的抗菌化合物,用于治疗畜禽养殖甚至临床中由多重耐药菌引起的致命感染。
[1] 隋倩雯, 张俊亚, 魏源送, 等. 畜禽养殖过程抗生素使用与耐药病原菌及其抗性基因赋存的研究进展[J]. 生态毒理学报, 2015, 10(5): 20-34
Sui Q W, Zhang J Y, Wei Y S, et al. Veterinary antibiotics use, occurrence of antibiotic resistance pathogen and its antibiotic resistance genes in animal production: An overview [J]. Asian Journal of Ecotoxicology, 2015, 10(5): 20-34 (in Chinese)
[2] 李厚禹, 邵振鲁, 李碧菡, 等. 畜禽环境中抗生素的去除及其风险评估[J]. 生态毒理学报, 2020, 15(1): 79-93
Li H Y, Shao Z L, Li B H, et al. The removal and risk assessment of antibiotics in livestock environment [J]. Asian Journal of Ecotoxicology, 2020, 15(1): 79-93 (in Chinese)
[3] 田志梅, 崔艺燕, 杜宗亮, 等. 抗生素替代物在畜禽养殖中的研究及应用进展[J]. 动物营养学报, 2020, 32(4): 1516-1525
Tian Z M, Cui Y Y, Du Z L, et al. Advances in researches and applications of antibiotic alternatives in livestock breeding [J]. Chinese Journal of Animal Nutrition, 2020, 32(4): 1516-1525 (in Chinese)
[4] 宣雄智, 李文嘉, 李绍钰, 等. 藻类在猪和鸡养殖生产中的应用研究进展[J]. 中国畜牧兽医, 2019, 46(11): 3262-3269
Xuan X Z, Li W J, Li S Y, et al. Advances in the application of algae in pig and chicken production [J]. China Animal Husbandry & Veterinary Medicine, 2019, 46(11): 3262-3269 (in Chinese)
[5] Holdt S L, Kraan S. Bioactive compounds in seaweed: Functional food applications and legislation [J]. Journal of Applied Phycology, 2011, 23(3): 543-597
[6] Stengel D B, Connan S, Popper Z A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application [J]. Biotechnology Advances, 2011, 29(5): 483-501
[7] He Y, Yuan Q B, Mathieu J, et al. Antibiotic resistance genes from livestock waste: Occurrence, dissemination, and treatment [J]. NPJ Clean Water, 2020, 3: 4
[8] Bhowmick S, Mazumdar A, Moulick A, et al. Algal metabolites: An inevitable substitute for antibiotics [J]. Biotechnology Advances, 2020, 43: 107571
[9] Silva A, Silva S A, Lourenço-Lopes C, et al. Antibacterial use of macroalgae compounds against foodborne pathogens [J]. Antibiotics, 2020, 9(10): E712
[10] Kini S, Divyashree M, Mani M K, et al. Algae and Cyanobacteria as a Source of Novel Bioactive Compounds for Biomedical Applications [M]//Advances in Cyanobacterial Biology. Amsterdam: Elsevier, 2020: 173-194
[11] Blunt J W, Copp B R, Hu W P, et al. Marine natural products [J]. Natural Product Reports, 2007, 24(1): 31-86
[12] Lin Q, Sun H H, Yao K, et al. The prevalence, antibiotic resistance and biofilm formation of Staphylococcus aureus in bulk ready-to-eat foods [J]. Biomolecules, 2019, 9(10): E524
[13] Maisonneuve E, Gerdes K. Molecular mechanisms underlying bacterial persisters [J]. Cell, 2014, 157(3): 539-548
[14] Makkar H P S, Tran G, Heuzé V, et al. Seaweeds for livestock diets: A review [J]. Animal Feed Science and Technology, 2016, 212: 1-17
[15] 任淑静, 罗静如, Maria Garcia Suares. 海藻提取物有利于提高肉鸡生产性能[J]. 国外畜牧学(猪与禽), 2020, 40(11): 86-89
Ren S J, Luo J R, Suares M G. Patented seaweed technology helps immunity and production [J]. Animal Science Abroad (Pigs and Poultry), 2020, 40(11): 86-89 (in Chinese)
[16] 任淑静, Maria Garcia Suarez. 海洋巨藻多糖的免疫调节活性[J]. 国外畜牧学(猪与禽), 2020, 40(12): 70-72
Ren S J, Suarez M G. Immunomodulating activities of macroalgae [J]. Animal Science Abroad (Pigs and Poultry), 2020, 40(12): 70-72 (in Chinese)
[17] Vieira E F, Soares C, Machado S, et al. Seaweeds from the Portuguese coast as a source of proteinaceous material: Total and free amino acid composition profile [J]. Food Chemistry, 2018, 269: 264-275
[18] Cherry P, Yadav S, Strain C R, et al. Prebiotics from seaweeds: An ocean of opportunity? [J]. Marine Drugs, 2019, 17(6): E327
[19] Pérez M J, Falqué E, Domínguez H. Antimicrobial action of compounds from marine seaweed [J]. Marine Drugs, 2016, 14(3): 52
[20] Liu M, Hansen P E, Lin X K. Bromophenols in marine algae and their bioactivities [J]. Marine Drugs, 2011, 9(7): 1273-1292
[21] Kamei Y, Isnansetyo A. Lysis of methicillin-resistant Staphylococcus aureus by 2,4-diacetylphloroglucinol produced by Pseudomonas sp. AMSN isolated from a marine alga [J]. International Journal of Antimicrobial Agents, 2003, 21(1): 71-74
[22] Wei Y X, Liu Q, Xu C J, et al. Damage to the membrane permeability and cell death of Vibrio parahaemolyticus caused by phlorotannins with low molecular weight from Sargassum thunbergii [J]. Journal of Aquatic Food Product Technology, 2016, 25(3): 323-333
[23] Lee D S, Kang M S, Hwang H J, et al. Synergistic effect between dieckol from Ecklonia stolonifera and β-lactams against methicillin-resistant Staphylococcus aureus [J]. Biotechnology and Bioprocess Engineering, 2008, 13(6): 758-764
[24] Nagayama K, Iwamura Y, Shibata T, et al. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome [J]. The Journal of Antimicrobial Chemotherapy, 2002, 50(6): 889-893
[25] Thomas N V, Kim S K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae [J]. Environmental Toxicology and Pharmacology, 2011, 32(3): 325-335
[26] Hussain M A, Dawson C O. Economic impact of food safety outbreaks on food businesses [J]. Foods, 2013, 2(4): 585-589
[27] Bumunang E W, McAllister T A, Zaheer R, et al. Characterization of non-O157 Escherichia coli from cattle faecal samples in the north-west Province of South Africa [J]. Microorganisms, 2019, 7(8): E272
[28] Moraes J O, Cruz E A, Souza E G F, et al. Predicting adhesion and biofilm formation boundaries on stainless steel surfaces by five Salmonella enterica strains belonging to different serovars as a function of pH, temperature and NaCl concentration [J]. International Journal of Food Microbiology, 2018, 281: 90-100
[29] He F, Yang Y, Yang G, et al. Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03 [J]. Food Control, 2010, 21(9): 1257-1262
[30] Vishwakarma J, Vavilala S L. Evaluating the antibacterial and antibiofilm potential of sulphated polysaccharides extracted from green algae Chlamydomonas reinhardtii [J]. Journal of Applied Microbiology, 2019, 127(4): 1004-1017
[31] Abou Zeid A H, Aboutabl E A, Sleem A A, et al. Water soluble polysaccharides extracted from Pterocladia capillacea and Dictyopteris membranacea and their biological activities [J]. Carbohydrate Polymers, 2014, 113: 62-66
[32] Kadam S U, O’Donnell C P, Rai D K, et al. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity [J]. Marine Drugs, 2015, 13(7): 4270-4280
[33] Sellimi S, Maalej H, Rekik D M, et al. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed [J]. International Journal of Biological Macromolecules, 2018, 119: 633-644
[34] Besednova N N, Zaporozhets T S, Somova L M, et al. Review: Prospects for the use of extracts and polysaccharides from marine algae to prevent and treat the diseases caused by Helicobacter pylori [J]. Helicobacter, 2015, 20(2): 89-97
[35] Pangestuti R, Kim S K. Seaweed Proteins, Peptides, and Amino Acids [M]//Seaweed Sustainability. Amsterdam: Elsevier, 2015: 125-140
[36] Lordan S, Ross R P, Stanton C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases [J]. Marine Drugs, 2011, 9(6): 1056-1100
[37] Makkar H P S, Tran G, Heuzé V, et al. Seaweeds for livestock diets: A review [J]. Animal Feed Science and Technology, 2016, 212: 1-17
[38] Admassu H, Gasmalla M A A, Yang R J, et al. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties [J]. Journal of Food Science, 2018, 83(1): 6-16
[39] El Shafay S M, Ali S S, El-Sheekh M M. Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria [J]. The Egyptian Journal of Aquatic Research, 2016, 42(1): 65-74
[40] Anjali K P, Sangeetha B M, Devi G, et al. Bioprospecting of seaweeds (Ulva lactuca and Stoechospermum marginatum): The compound characterization and functional applications in medicine-a comparative study [J]. Journal of Photochemistry and Photobiology B, Biology, 2019, 200: 111622
[41] Ren D C, Bedzyk L A, Ye R W, et al. Differential gene expression shows natural brominated furanones interfere with the autoinducer-2 bacterial signaling system of Escherichia coli [J]. Biotechnology and Bioengineering, 2004, 88(5): 630-642
[42] Karpiński T M, Adamczak A. Fucoxanthin—An antibacterial carotenoid [J]. Antioxidants, 2019, 8(8): 239
[43] Volk R B, Furkert F H. Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth [J]. Microbiological Research, 2006, 161(2): 180-186
[44] Zheng L, Chen H M, Han X T, et al. Antimicrobial screening and active compound isolation from marine bacterium NJ6-3-1 associated with the sponge Hymeniacidon perleve [J]. World Journal of Microbiology and Biotechnology, 2005, 21(2): 201-206
[45] Wächter G A, Franzblau S G, Montenegro G, et al. Inhibition of Mycobacterium tuberculosis growth by saringosterol from Lessonia nigrescens [J]. Journal of Natural Products, 2001, 64(11): 1463-1464
[46] Morais T, Inácio A, Coutinho T, et al. Seaweed potential in the animal feed: A review [J]. Journal of Marine Science and Engineering, 2020, 8(8): 559
[47] Gurusamy S, Kulanthaisamy M R, Hari D G, et al. Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterilas for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens [J]. Journal of Photochemistry and Photobiology B, Biology, 2019, 193: 118-130
[48] Sugiharto S, Yudiarti T, Isroli I, et al. Effect of feeding duration of Spirulina platensis on growth performance, haematological parameters, intestinal microbial population and carcass traits of broiler chicks [J]. South African Journal of Animal Science, 2018, 48(1): 98
[49] El-Ghany W A A. Microalgae in poultry field: A comprehensive perspectives [J]. Advances in Animal and Veterinary Sciences, 2020, 8(9): 888-897
[50] Kang H K, Salim H M, Akter N, et al. Effect of various forms of dietary Chlorella supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens [J]. Journal of Applied Poultry Research, 2013, 22(1): 100-108
[51] Roque B M, Salwen J K, Kinley R, et al. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent [J]. Journal of Cleaner Production, 2019, 234: 132-138
[52] Shanmugapriya B, Babu S S. Research article dietary administration of Spirulina platensis as probiotics on growth performance and histopathology in broiler chicks [J]. International Journal of Recent Scientific Research, 2015, 6(2): 2650-2653
[53] Namra M M M, Ragab M S, Aly M M M, et al. Effect of dietary supplementation of algae meal (Spirulina platensis) as growth promoter on performance of broiler chickens [J]. Egyptian Poultry Science Journal, 2018, 38(2): 375-389
[54] 夏伦斌, 黄燕, 左瑞华, 等. 海藻多糖对肉鸡抗氧化性能及存活率的影响[J]. 畜牧与饲料科学, 2016, 37(4): 24-26
Xia L B, Huang Y, Zuo R H, et al. Effects of dietary supplementation of algal polysaccharide on antioxidant capacity and surviving rate of broilers [J]. Animal Husbandry and Feed Science, 2016, 37(4): 24-26 (in Chinese)
[55] Wang J, Yue H Y, Wu S G, et al. Nutritional modulation of health, egg quality and environmental pollution of the layers [J]. Animal Nutrition, 2017, 3(2): 91-96
[56] Ehr I J, Persia M E, Bobeck E A. Comparative omega-3 fatty acid enrichment of egg yolks from first-cycle laying hens fed flaxseed oil or ground flaxseed [J]. Poultry Science, 2017, 96(6): 1791-1799
[57] Tellez G, Pixley C, Wolfenden R E, et al. Probiotics/direct fed microbials for Salmonella control in poultry [J]. Food Research International, 2012, 45(2): 628-633
[58] Kulshreshtha G, Rathgeber B, Stratton G, et al. Feed supplementation with red seaweeds, Chondrus crispus and Sarcodiotheca gaudichaudii, affects performance, egg quality, and gut microbiota of layer hens [J]. Poultry Science, 2014, 93(12): 2991-3001
[59] McCauley J I, Labeeuw L, Jaramillo-Madrid A C, et al. Management of enteric methanogenesis in ruminants by algal-derived feed additives [J]. Current Pollution Reports, 2020, 6(3): 188-205
[60] Yan L, Lim S U, Kim I H. Effect of fermented chlorella supplementation on growth performance, nutrient digestibility, blood characteristics, fecal microbial and fecal noxious gas content in growing pigs [J]. Asian-Australasian Journal of Animal Sciences, 2012, 25(12): 1742-1747
[61] Dierick N, Ovyn A, de Smet S. Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs [J]. Journal of the Science of Food and Agriculture, 2009, 89(4): 584-594
[62] Michiels J, Skrivanova E, Missotten J, et al. Intact brown seaweed (Ascophyllum nodosum) in diets of weaned piglets: Effects on performance, gut bacteria and morphology and plasma oxidative status [J]. Journal of Animal Physiology and Animal Nutrition, 2012, 96(6): 1101-1111
[63] Dierick N, Ovyn A, de Smet S. In vitro assessment of the effect of intact marine brown macro-algae Ascophyllum nodosum on the gut flora of piglets [J]. Livestock Science, 2010, 133(1-3): 154-156
[64] Øverland M, Mydland L, Skrede A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals [J]. Journal of the Science of Food and Agriculture, 2018, 99: 13-24
[65] Morais T, Inácio A, Coutinho T, et al. Seaweed potential in the animal feed: A review [J]. Journal of Marine Science and Engineering, 2020, 8(8): 559
[66] Baeza E, Chartrin P, Lessire M, et al. Is it possible to increase n-3 fatty acid content of meat without affecting its technological and/or sensory quality and the growing performance of chickens? [J]. British Poultry Science, 2015, 56(5): 543-550
[67] Jamil A, Akanda M, Rahman M, et al. Prebiotic competence of spirulina on the production performance of broiler chickens [J]. Journal of Advanced Veterinary and Animal Research, 2015, 2(3): 304
[68] Evans F D, Critchley A T. Seaweeds for animal production use [J]. Journal of Applied Phycology, 2014, 26(2): 891-899
[69] Kostik V, Gjorgjeska B, Bauer B, et al. Production of shell eggs enriched with n-3 fatty acids [J]. IOSR Journal of Pharmacy, 2015, 5(8): 48-51
[70] 王述柏, 贾玉辉, 王利华, 等. 浒苔添加水平对蛋鸡产蛋性能、蛋品质、免疫功能及粪便微生物区系的影响[J]. 动物营养学报, 2013, 25(6): 1346-1352
Wang S B, Jia Y H, Wang L H, et al. Enteromorpha prolifera supplemental level: Effects on laying performance, egg quality, immune function and microflora in feces of laying hens [J]. Chinese Journal of Animal Nutrition, 2013, 25(6): 1346-1352 (in Chinese)
[71] Al-Harthi M A, El-Deek A A. Effect of different dietary concentrations of brown marine algae (Sargassum dentifebium) prepared by different methods on plasma and yolk lipid profiles, yolk total carotene and lutein plus zeaxanthin of laying hens [J]. Italian Journal of Animal Science, 2012, 11(4): e64
[72] Rizk Y S. Effect of dietary green tea and dried seaweed on productive and physiological performance of laying hens during late phase of production [J]. Egyptian Poultry Science Journal, 2017, 37(3): 685-706
[73] Choi Y, Lee E C, Na Y, et al. Effects of dietary supplementation with fermented and non-fermented brown algae by-products on laying performance, egg quality, and blood profile in laying hens [J]. Asian-Australasian Journal of Animal Sciences, 2018, 31(10): 1654-1659
[74] Katayama M, Fukuda T, Okamura T, et al. Effect of dietary addition of seaweed and licorice on the immune performance of pigs [J]. Nihon Chikusan Gakkaiho, 2011, 82(2): 274-281