中国是抗生素的生产和消费大国,据Zhang等[1]报道,2013年中国36种常用抗生素的总用量为92 700 t,估计有53 800 t会进入环境中。目前,地表水[2-6]、地下水[3-4]和土壤[7-11]中均有抗生素检出的相关报道。其中,抗生素会通过畜禽有机肥、农业灌溉等途径进入土壤,使土壤成为重要的汇。如Hu等[7]在中国北方有机蔬菜种植基地的土壤中检出抗生素浓度范围为0.1~2 683 μg·kg-1。土壤中的抗生素可以被植物吸收,进入食物链后会对人体产生被动暴露[12-14]。有研究表明,通过食物摄取可能引起抗生素的暴露剂量超过人体肠道菌群的最小选择剂量,促进抗性细菌的生长[15]。研究植物对抗生素的富集规律可以为评价人体健康风险提供参考。
前人研究表明,对于疏水的非解离有机污染物而言,亲脂性是影响其植物富集的重要因素,富集浓度与污染物亲脂性大致符合“钟形”模型[16]。然而,很多抗生素属于可解离有机污染物,在不同pH下能够呈现出不同的形态,包括阴离子、阳离子和中性形态,这导致抗生素的植物富集规律非常复杂。Kong等[17]发现可解离的土霉素在苜蓿中的吸收随pH改变有明显的差异。Wu等[18]研究发现7种呈中性形态的药物和个人护理用品的根部富集系数(logBCFroot)与其pH 6.5下的正辛醇-水分配系数(logDow)之间呈良好的线性关系,但包含了更多的离子态化合物后相关性显著下降,认为除了亲脂性,还有别的机制影响可解离药物在植物根部的富集。由此可见,形态对可解离化合物的植物富集具有重要的影响。即使都属于可解离化合物,不同抗生素的结构和性质存在显著的差异,形态对其植物吸收富集的影响也可能存在明显的区别,值得进一步研究。本文在pH 5~9下考察了形态对6种抗生素在小麦和生菜中富集的影响,并初步评估了其对人体的被动暴露,为进一步评估抗生素的人体健康风险奠定一定的基础。
1 材料与方法(Materials and methods)
1.1 实验材料
供试植物:小麦(wheat, Triticum aestivum L.)种子播撒于育苗盘中,避光发芽3 d后移入珍珠岩培养7 d,再进行水培适应,适应期间营养液由1/8霍兰格营养液逐渐过渡至1/2。生菜(lettuce, Lactuca sativa)种子播撒于营养土中育苗,生长约22 d后转移到1/2霍格兰营养液中,在营养液中适应14 d后用于暴露实验。植物培育均在人工气候箱中进行,条件设置为白天16 h,温度23 ℃,湿度60%,光照20 000 lx;黑夜8 h,温度12 ℃,湿度70%,光照为0 lx。
实验试剂:磺胺嘧啶(sulfadiazine, SDZ;纯度99%)、磺胺甲恶唑(sulfamethoxazole, SMX;纯度98%)、甲氧苄啶(trimethoprim, TRM;纯度98%)、克拉霉素(clarithromycin, CLA;纯度95%)、氯霉素(chloramphenicol, CAP;纯度98%)、恩诺沙星(enrofloxacin, ENR;纯度95%)以及内标物质克拉霉素-13C,D3(clarithromycin-13C, D3)、磺胺嘧啶-D4(sulfadiazine-D4)、磺胺甲恶唑-D4(sulfamethoxazole-D4)、甲氧苄啶-D3(trimethoprim-D3)、恩诺沙星-D5(enrofloxacin-D5)和氟苯尼考-D3(florfenicol-D3)均购自北京百灵威科技。甲醇(methanol;色谱纯)、乙腈(acetonitrile;色谱纯)、丙酮(acetone;色谱纯)和N-丙基二乙胺(primary secondary amine, PSA)均购于美国Sigma Aldrich;甲酸(formic acid;分析纯)和甲酸铵(ammonium formate;分析纯)均购于中国天津市科密欧化学试剂。
本研究所采用的主要仪器包括:高效液相色谱-三重四级杆质谱串联仪(UPLC I-Class-Waters Xevo TQS,Waters公司,美国)、精密pH计(FE28-Standard,梅特勒-托利多集团,瑞士)、人工气候箱(MGC350HP,上海一恒科学仪器有限公司,中国)、涡旋仪(M-02U,SlientShake,中国)、离心机(TDZ5-WS,湘仪离心机仪器有限公司,中国)、氮吹仪(MD200-2,杭州奥盛仪器有限公司,中国)和真空冷冻干燥机(FD-1A-50,北京博医康实验仪器有限公司,中国)。
1.2 实验方法
1.2.1 小麦暴露条件
在每个培养盆中倒入1 L 1/2霍兰格营养液,同时加入SDZ、SMX、TRM、CAP、CLA和ENR,使溶液中每种抗生素浓度达到50 μg·L-1,再使用HCl和NaOH溶液调节pH至6.5。选取长势相近且良好的小麦进行暴露,暴露时间为3 d,暴露期间每天调节一次pH。实验组和空白组均设置3个平行样品。暴露实验在人工气候箱中进行,具体培养条件见1.1。
1.2.2 生菜暴露条件
在每个培养盆中倒入1 L 1/2霍兰格营养液,同时加入SDZ、SMX、TRM、CAP、CLA和ENR,使溶液中每种抗生素浓度达到50 μg·L-1,分成5组,使用HCl和NaOH溶液调节pH分别至5、6、7、8和9。选取长势相近且良好的生菜进行暴露,暴露时间分别为1、3和9 d,暴露期间每天调节一次pH。实验组以及空白组均设置3个平行样品。人工气候箱的设置条件见1.1。
1.3 分析方法
1.3.1 植物样品处理
培养结束后,将植物的地上组织与根系分离,分别采用超纯水清洗3遍,然后在真空冷冻干燥机中冻干,采用破碎机研磨至粉状备用。称量300 mg地上组织冻干样品或100 mg根冻干样品,放入50 mL离心管中,加入50 ng混合内标物,避光放置20~30 min。添加一定量提取溶剂(V(乙腈)∶V(丙酮)=1∶1,乙腈用无水甲酸调节至pH=3),每次按照溶剂与植物样品的体积/质量比为16 mL·g-1的剂量添加。加入提取溶剂后涡旋1 min,超声15 min,在4 000 r·min-1下离心15 min,将上清液移取至另一样品管中,重复上述过程3次,混合3次提取的上清液。向上清液中按照25 mg·mL-1(上清液)添加PSA[19],然后涡旋1 min,避光放置30 min后取上清液氮吹近干。加入1 mL初始流动相(V(A)∶V(B)=95∶5,A为0.1%甲酸-甲酸铵溶液,B为体积比1∶1的甲醇-乙腈混合溶液)溶解抗生素,过0.2 μm的PTFE-Q滤膜,滤液转移到进样小瓶中待UPLC-MS/MS分析。
1.3.2 测试条件
色谱条件:使用C18色谱柱(2.1 mm×50 mm,1.7 μm),流动相A为甲酸(体积百分比0.1%)-甲酸铵(质量百分比0.1%)溶液,流动相B为乙腈-甲醇(1∶1 V/V)溶液。柱温40 ℃,流速为0.3 mL·min-1,进样量为5 μL。洗脱梯度为:0~2.5 min,95% A~90% A;2.5~7 min,90% A~10% A;7~8 min,10% A~95% A;8~10 min,95% A。
质谱条件:目标化合物采用电喷雾离子源正离子(ESI+)和负离子(ESI-)切换扫描的电离模式,在多反应监测模式(MRM)下进行数据采集。干燥气温度和流速分别为350 ℃和650 L·h-1,雾化气压力为700 kPa,毛细管电压为2.51 kV。具体质谱参数如表1所示。
1.3.3 质量控制
使用上述提取及检测方法获得的目标抗生素加标回收率如表2所示,地上组织中的加标回收率为95%~125%,根中的加标回收率为90%~120%。
1.3.4 数据处理
实验数据使用IBM SPSS Statistics Version 22进行显著性差异分析,Duncan检验的结果用不同小写字母标记,不同字母标记的组别表示在P<0.05下具有显著性差异。
2 结果与讨论(Results and discussion)
本研究选取4类共6种抗生素:磺胺类(SDZ和SMX)、氟喹诺酮类(ENR)、大环内酯类(CLA)和酰胺醇类(CAP),考察了形态对小麦和生菜吸收富集抗生素的影响。
2.1 抗生素在小麦中的富集
在pH 6.5下研究了6种抗生素在小麦中的富集情况,抗生素在根部和地上组织中的生物富集因子(bioconcentration factor, BCF)分别以BCFroot和BCFshoot表示。如图1所示,抗生素在小麦中的吸收表现为ENR>TRM>CAP>CLA>SMX>SDZ,地上组织中抗生素的浓度范围为8.8~318 μg·kg-1(干质量),根部浓度范围为132~7 248 μg·kg-1(干质量),BCFroot范围为2.6~145。由结果可知,ENR、TRM与SDZ、SMX、CAP、CLA在小麦根中的富集能力存在显著差异,可能与抗生素的结构和形态有关。考虑到小麦的完整生长周期较长,不便于在人工气候箱中培养,后续选取易于培养,生长周期较短,且食用量较大的生菜在不同pH下进一步考察形态对抗生素富集的影响。
表1 目标抗生素的质谱参数
Table 1 Mass spectrometer parameters of target antibiotics
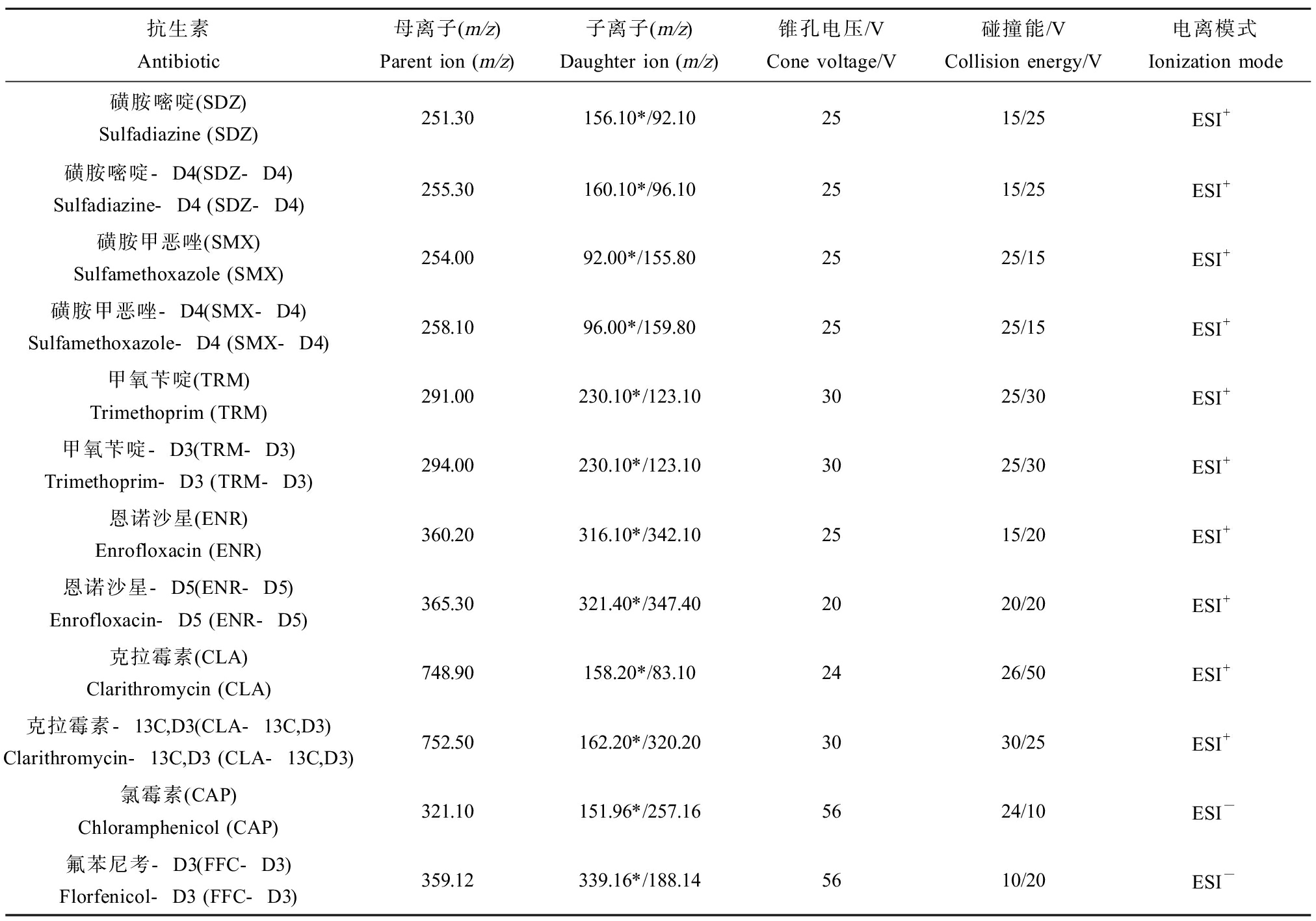
抗生素Antibiotic母离子(m/z)Parent ion (m/z)子离子(m/z)Daughter ion (m/z)锥孔电压/VCone voltage/V碰撞能/VCollision energy/V电离模式Ionization mode磺胺嘧啶(SDZ)Sulfadiazine (SDZ)251.30156.10*/92.102515/25ESI+磺胺嘧啶-D4(SDZ-D4)Sulfadiazine-D4 (SDZ-D4)255.30160.10*/96.102515/25ESI+磺胺甲恶唑(SMX)Sulfamethoxazole (SMX)254.0092.00*/155.802525/15ESI+磺胺甲恶唑-D4(SMX-D4)Sulfamethoxazole-D4 (SMX-D4)258.1096.00*/159.802525/15ESI+甲氧苄啶(TRM)Trimethoprim (TRM)291.00230.10*/123.103025/30ESI+甲氧苄啶-D3(TRM-D3)Trimethoprim-D3 (TRM-D3)294.00230.10*/123.103025/30ESI+恩诺沙星(ENR)Enrofloxacin (ENR)360.20316.10*/342.102515/20ESI+恩诺沙星-D5(ENR-D5)Enrofloxacin-D5 (ENR-D5)365.30321.40*/347.402020/20ESI+克拉霉素(CLA)Clarithromycin (CLA)748.90158.20*/83.102426/50ESI+克拉霉素-13C,D3(CLA-13C,D3)Clarithromycin-13C,D3 (CLA-13C,D3)752.50162.20*/320.203030/25ESI+氯霉素(CAP)Chloramphenicol (CAP)321.10151.96*/257.165624/10ESI-氟苯尼考-D3(FFC-D3)Florfenicol-D3 (FFC-D3)359.12339.16*/188.145610/20ESI-
注:*为定量离子。
Note: *is a quantitative ion.
表2 目标抗生素的加标回收率、相对标准偏差(RSD)、方法检出限(MLD)和定量限(MLQ)
Table 2 The spiked recoveries, relative standard deviation (RSD), method limit of detection (MLD) and method limit of quantification (MLQ) of target antibiotics
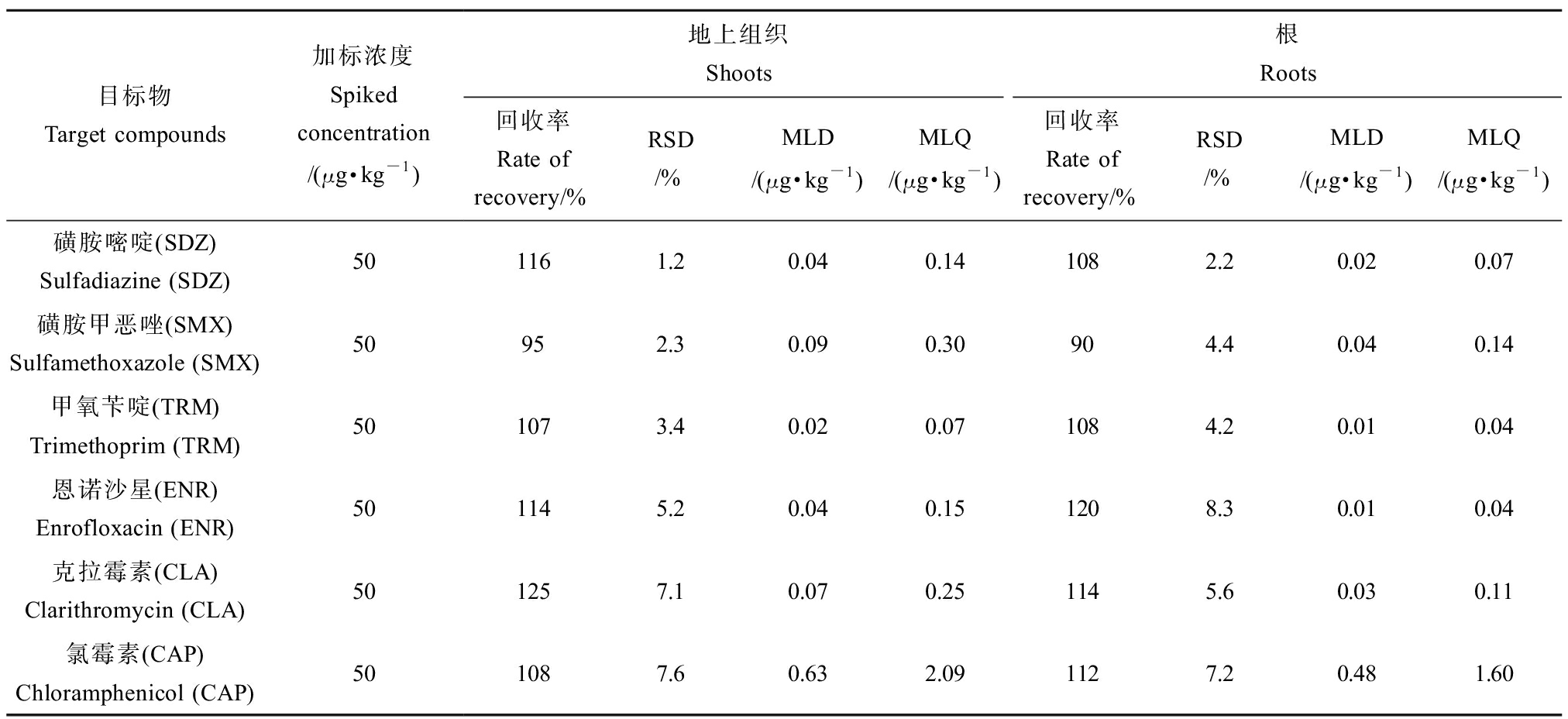
目标物Target compounds加标浓度Spiked concentration/(μg·kg-1)地上组织 Shoots根 Roots回收率Rate of recovery/%RSD/%MLD/(μg·kg-1)MLQ/(μg·kg-1)回收率Rate of recovery/%RSD/%MLD/(μg·kg-1)MLQ/(μg·kg-1)磺胺嘧啶(SDZ)Sulfadiazine (SDZ)501161.20.040.141082.20.020.07磺胺甲恶唑(SMX)Sulfamethoxazole (SMX)50952.30.090.30904.40.040.14甲氧苄啶(TRM)Trimethoprim (TRM)501073.40.020.071084.20.010.04恩诺沙星(ENR)Enrofloxacin (ENR)501145.20.040.151208.30.010.04克拉霉素(CLA)Clarithromycin (CLA)501257.10.070.251145.60.030.11氯霉素(CAP)Chloramphenicol (CAP)501087.60.632.091127.20.481.60
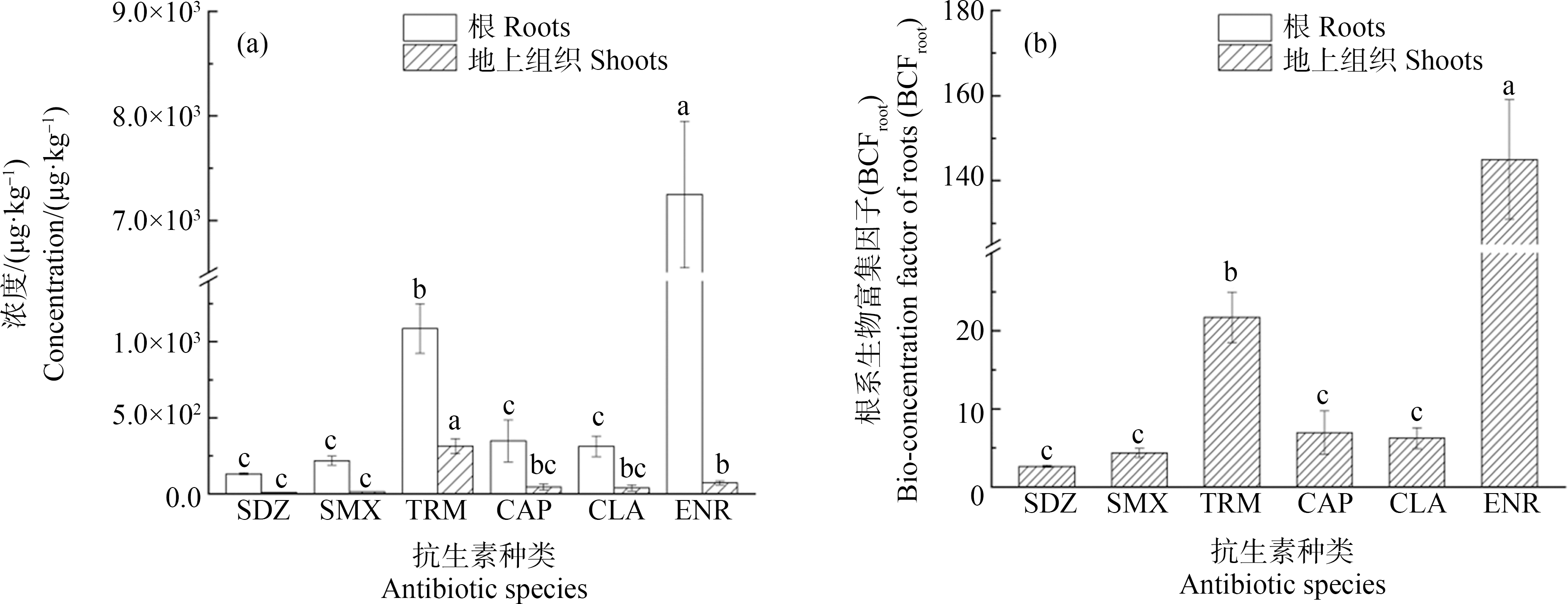
图1 6种抗生素在小麦根和地上组织中的蓄积浓度和根系富集因子
注:(a) 根部和地上组织中的浓度;(b) 根系生物富集因子。
Fig. 1 Accumulation concentration of six antibiotics in wheat roots and shoots, and root concentration factor in wheat
Note: (a) Concentration of roots and shoots; (b) Bioconcentration factor of roots.
2.2 抗生素在生菜中的富集
大部分抗生素属于可解离化合物,以图2的SMX为例,SMX具有2个可解离基团,在不同pH下以不同的形态存在。Li等[19]在土壤pH约为8.1的条件下研究了15种药物的植物富集,发现除了中性形态的药物之外,其他形态的药物对萝卜根部中的富集也具有重要贡献。本研究设置5、6、7、8和9的pH梯度,分1、3和9 d共3个暴露时间来考察形态对生菜富集抗生素的影响。
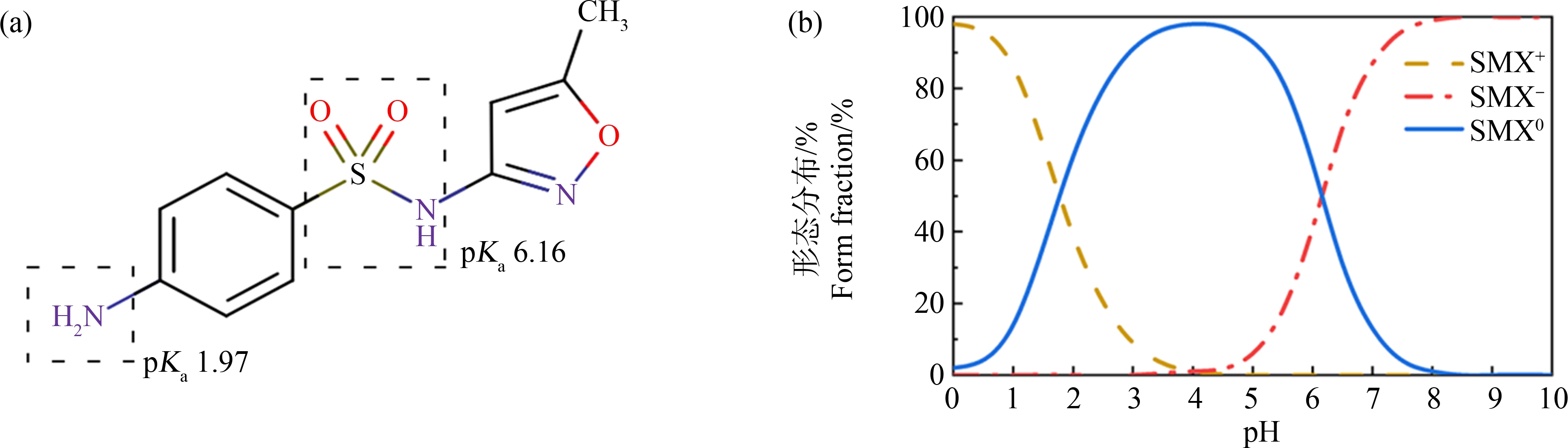
图2 SMX结构式(a)及其在不同pH下的形态分布(b)
Fig. 2 The structure of SMX (a) and its speciation distribution at different pH values (b)
2.2.1 暴露时间对富集的影响
由图3可知,根部和地上组织部分中抗生素的浓度分布在暴露1~9 d内基本一致,均大致表现为ENR>TRM>CAP>CLA>SMX>SDZ,与小麦中的富集情况相似,说明目标抗生素在这2种植物的吸收存在共同之处。除了CAP外,其他5种抗生素在根部和地上组织部分的富集浓度在暴露1~9 d内呈递增趋势。抗生素根部富集的浓度范围为37~45 916 μg·kg-1(干质量),地上组织部分的浓度范围为0.1~761 μg·kg-1(干质量)。以暴露第1天为例,抗生素的BCF数据列于表3。抗生素在生菜的BCFroot值在0.75~520之间,BCFshoot值在0.0009~1.7之间。其中ENR表现出最大的BCFroot值,比其他抗生素高约1~3个数量级。目标抗生素的BCFshoot均明显低于BCFroot,其中ENR和TRM拥有较高的BCFshoot值。
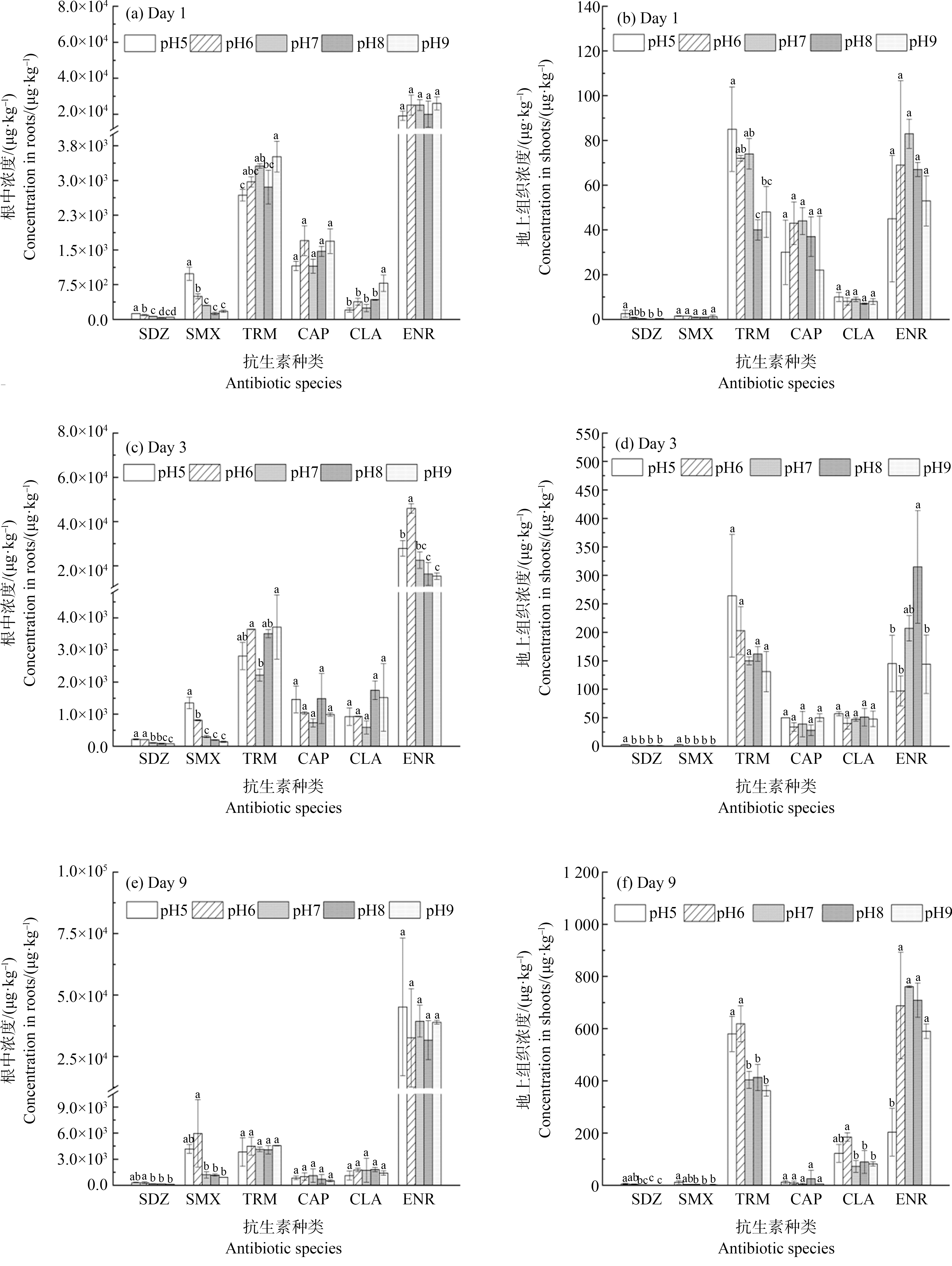
图3 暴露时间分别为1、3和9 d时6种抗生素在生菜根中和地上组织中的蓄积浓度
Fig. 3 Accumulation concentrations of 6 antibiotics in lettuce roots and shoots after exposure for 1, 3 and 9 d respectively
表3 暴露第1天时不同pH条件下抗生素在生菜中的生物富集因子(BCF)
Table 3 Bioconcentration factor (BCF) of antibiotics in lettuce under different pH conditions after one day of exposure
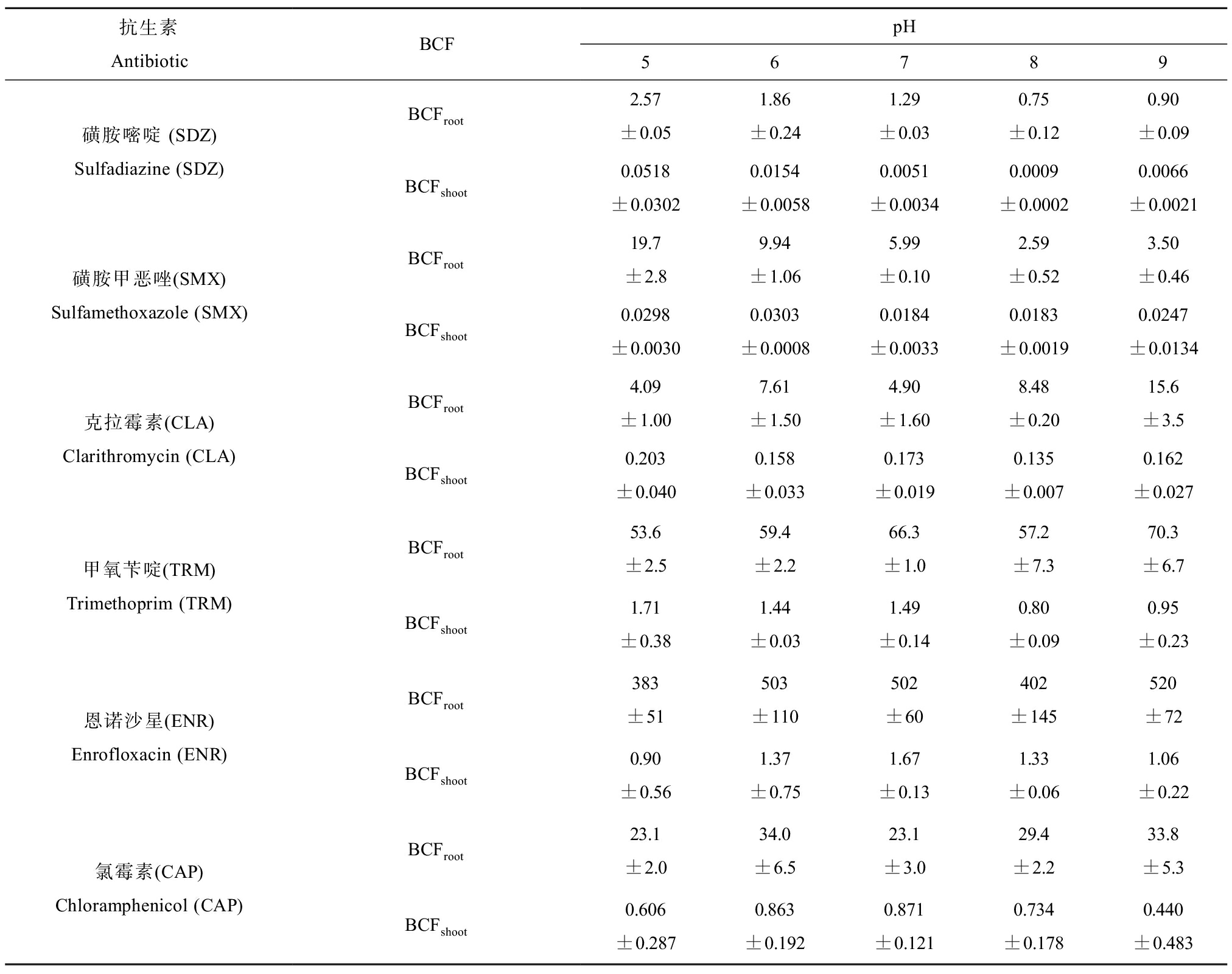
抗生素AntibioticBCFpH56789磺胺嘧啶 (SDZ)Sulfadiazine (SDZ)BCFroot2.57±0.051.86±0.241.29±0.030.75±0.120.90±0.09BCFshoot0.0518 ±0.03020.0154 ±0.00580.0051 ±0.00340.0009 ±0.00020.0066 ±0.0021磺胺甲恶唑(SMX)Sulfamethoxazole (SMX)BCFroot19.7±2.89.94±1.065.99±0.102.59±0.523.50±0.46BCFshoot0.0298±0.00300.0303±0.00080.0184±0.00330.0183±0.00190.0247±0.0134克拉霉素(CLA)Clarithromycin (CLA)BCFroot4.09±1.007.61±1.504.90±1.608.48±0.2015.6±3.5BCFshoot0.203±0.0400.158±0.0330.173±0.0190.135±0.0070.162±0.027甲氧苄啶(TRM)Trimethoprim (TRM)BCFroot53.6±2.559.4±2.266.3±1.057.2±7.370.3±6.7BCFshoot1.71±0.381.44±0.031.49±0.140.80±0.090.95±0.23恩诺沙星(ENR)Enrofloxacin (ENR)BCFroot383±51503±110502±60402±145520±72BCFshoot0.90±0.561.37±0.751.67±0.131.33±0.061.06±0.22氯霉素(CAP)Chloramphenicol (CAP)BCFroot23.1±2.034.0±6.523.1±3.029.4±2.233.8±5.3BCFshoot0.606±0.2870.863±0.1920.871±0.1210.734±0.1780.440±0.483
2.2.2 形态对抗生素富集的影响
目标抗生素的pKa如表4所示,在实验pH范围内计算所得的形态比例如表5所示,计算方法参考Wu等[18]的方法。目标抗生素在pH 5~9条件下的富集变化如图3所示。SDZ和SMX在pH 5~9下呈现出明显的富集差异,其富集浓度随pH升高呈现下降的趋势,其他4种抗生素的富集随pH变化没有明显的规律。分别对目标抗生素在pH 5~9下的logBCFroot与中性形态比例(A0)进行线性拟合。结果如图4所示,发现SDZ、SMX和CLA的logBCFroot与A0均表现出优良的正相关关系,其R2分别为0.931、0.926和0.751,说明SDZ、SMX和CLA的中性形态更容易被生菜吸收。有研究指出,化合物的阴离子和阳离子形态会受到植物细胞电排斥、电吸引、离子捕获等效应,从而影响其吸收富集,因此中性形态可能更容易被植物吸收富集[20-22]。TRM、CAP和ENR在生菜中的富集与中性形态之间没有明显的规律性。由于抗生素的结构和性质差异很大,其植物吸收可能存在多种不同机制。例如,Meng等[23]研究认为pH 5.5下呈现阳离子形态的环丙沙星可能是通过电化学质子梯度驱动被菜心根系吸收。因此,对于TRM、CAP和ENR的植物吸收规律还需要进一步研究。
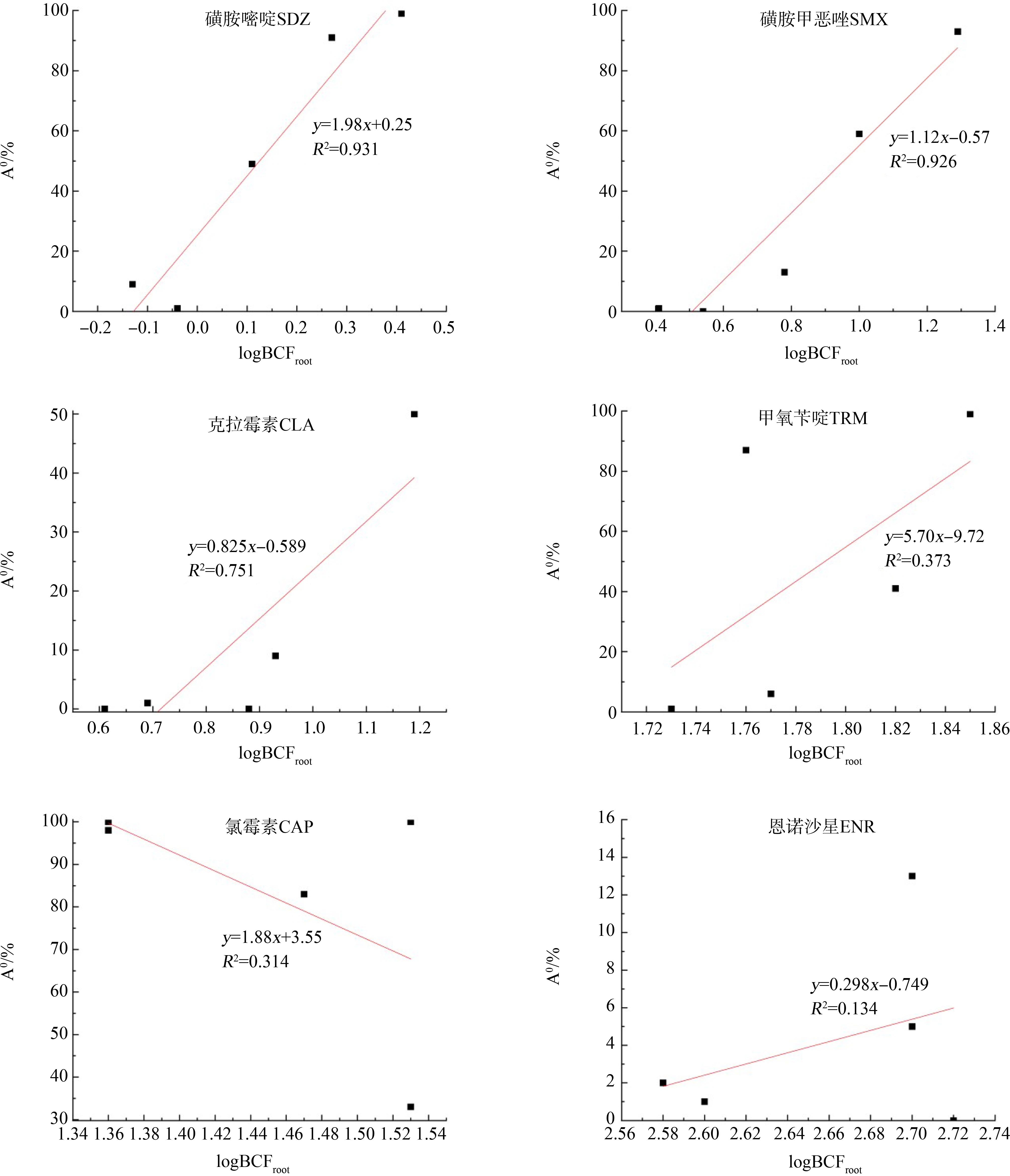
图4 暴露第1天时6种抗生素在生菜中logBCFroot和A0之间的线性关系
Fig. 4 Linear relationship between logBCFroot and A0 of 6 antibiotics in lettuce after one day of exposure
表4 目标抗生素的酸性解离常数(pKa)
Table 4 Acid dissociation constant (pKa) of target antibiotics
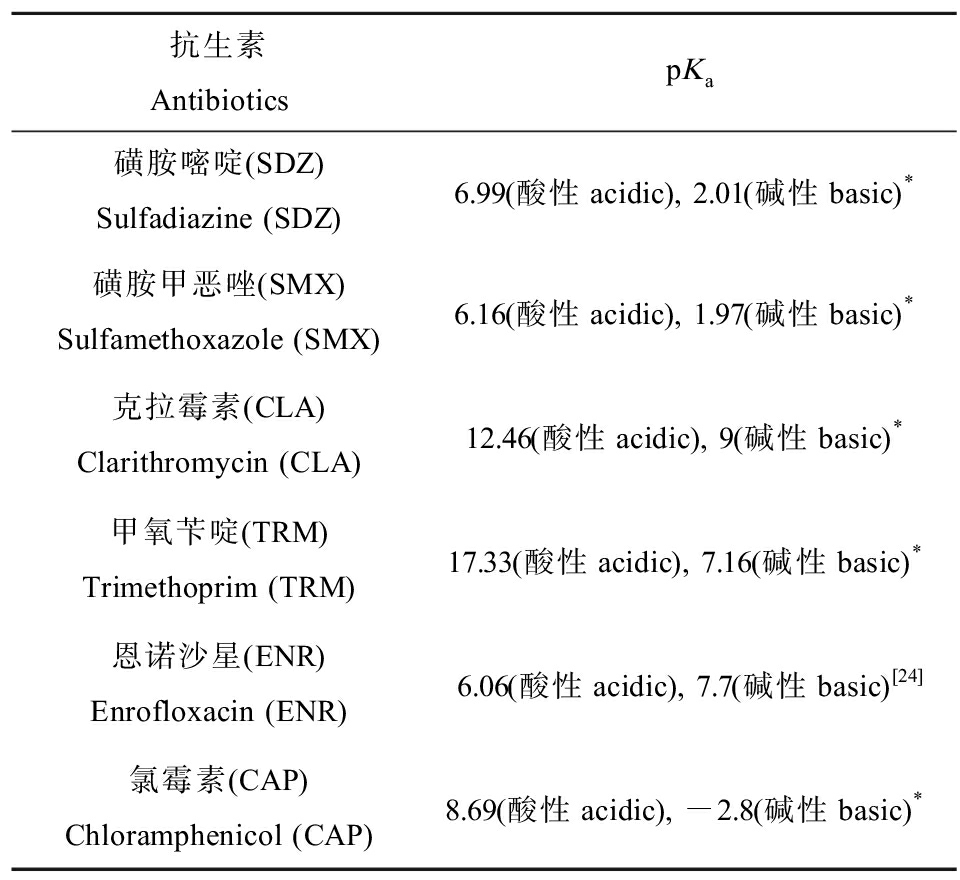
抗生素AntibioticspKa磺胺嘧啶(SDZ)Sulfadiazine (SDZ)6.99(酸性acidic), 2.01(碱性basic)*磺胺甲恶唑(SMX)Sulfamethoxazole (SMX)6.16(酸性acidic), 1.97(碱性basic)*克拉霉素(CLA)Clarithromycin (CLA)12.46(酸性acidic), 9(碱性basic)*甲氧苄啶(TRM)Trimethoprim (TRM)17.33(酸性acidic), 7.16(碱性basic)*恩诺沙星(ENR)Enrofloxacin (ENR)6.06(酸性acidic), 7.7(碱性basic)[24]氯霉素(CAP)Chloramphenicol (CAP)8.69(酸性acidic), -2.8(碱性basic)*
注:带*的数据来自DrugBank数据库(获取自http://www.drugbank.ca)。
Note: Data with *comes from DrugBank database (available at http://www.drugbank.ca).
表5 目标抗生素在不同pH条件下解离形态比例
Table 5 Proportion of dissociation form of target antibiotics under different pH conditions (%)
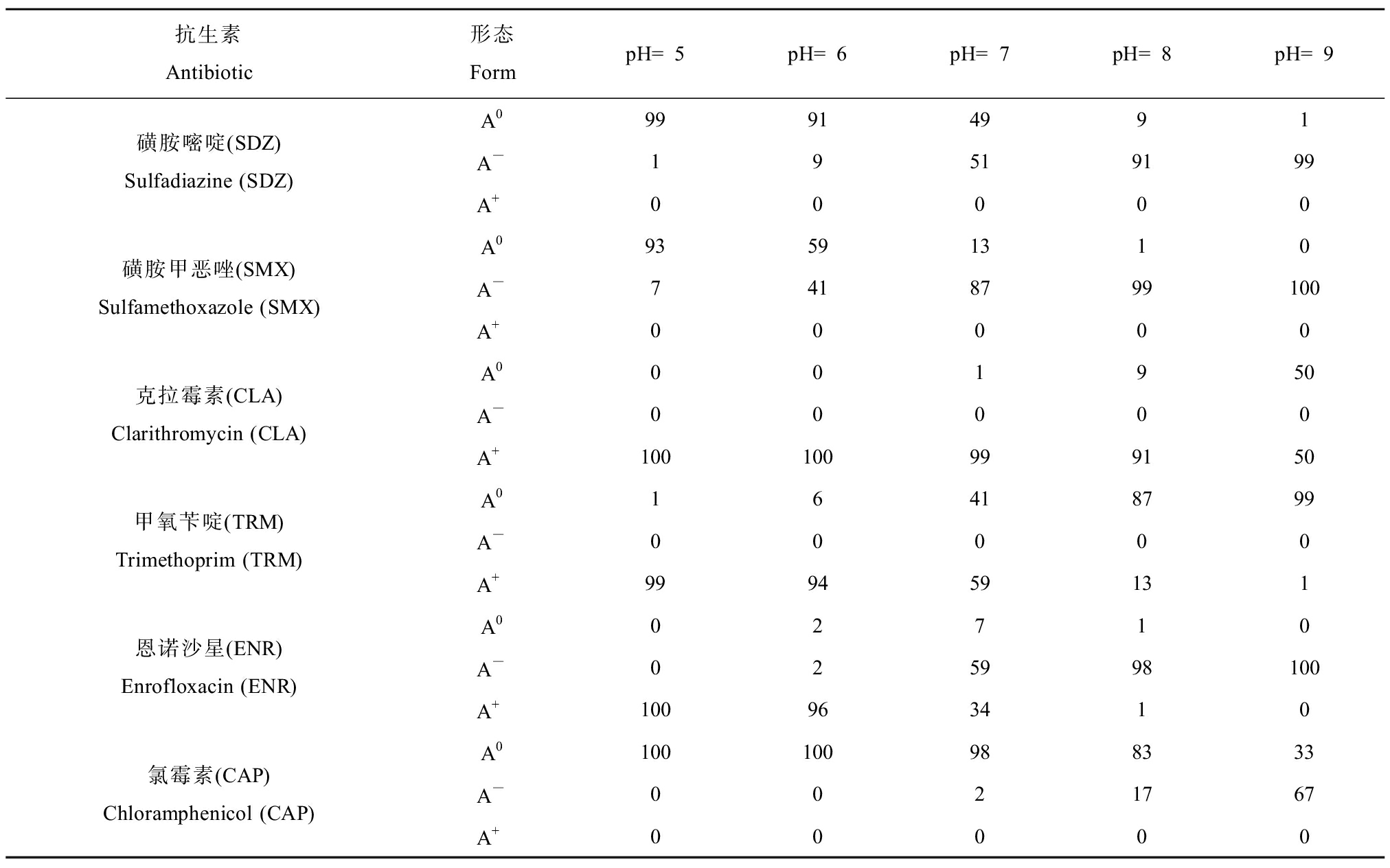
抗生素Antibiotic形态FormpH=5pH=6pH=7pH=8pH=9磺胺嘧啶(SDZ)Sulfadiazine (SDZ)A099914991A-19519199A+00000磺胺甲恶唑(SMX)Sulfamethoxazole (SMX)A093591310A-7418799100A+00000克拉霉素(CLA)Clarithromycin (CLA)A0001950A-00000A+100100999150甲氧苄啶(TRM)Trimethoprim (TRM)A016418799A-00000A+999459131恩诺沙星(ENR)Enrofloxacin (ENR)A002710A-025998100A+100963410氯霉素(CAP)Chloramphenicol (CAP)A0100100988333A-0021767A+00000
注:A0表示中性形态;A+表示阳离子形态;A-表示阴离子形态。
Note: A0 represents neutral form; A+ represents cationic form; A- represents anionic form.
2.3 对人体的暴露风险
抗生素被植物吸收后,其中一部分会进入植物可食部分,可能会引起人体的被动暴露。例如,Paltiel等[25]发现废水灌溉的农产品中抗癫痫药物卡马西平含量约为1 ng·g-1,志愿者食用农产品7 d后能在尿液中检测出20 ng·L-1的卡马西平及其代谢物,因此抗生素在植物中富集而产生的人体暴露风险仍不应忽视。以75 g·d-1的生菜食用量[26]来对每日预估摄入量(estimated daily intake, EDI)进行计算,结果如表6所示,发现在50 μg·L-1暴露溶液下培养9 d后,生菜中可食用部分中抗生素的EDI范围在0.006~0.951 μg·kg-1·d-1之间,均低于每日允许摄入量(acceptable daily intake, ADI)。
从暴露风险的角度考虑,虽然从表6的结果中可以看到所有目标抗生素的EDI均低于ADI,不同pH下某些抗生素的EDI有16倍的差距,这在一定程度上意味着不同pH土壤种植下植物富集产生的暴露风险也会存在显著差异。目前,对于长期摄入低剂量抗生素对人体影响的研究还比较缺乏[27-28]。Subirats等[15]研究发现,基于现有的ADI或者最大残留限量来进行每日食物摄取,肠道中的抗生素浓度可能会超出肠道微生物菌群最小选择剂量的10倍~1 000倍,从而促进耐药菌的生长。抗生素可能提高人类肠道中抗生素耐药性基因的多样性,从而导致人体生态失调[29],所以通过植物富集抗生素所产生的人体暴露风险仍需进一步考察。
综上,本研究发现SDZ和SMX在生菜中的富集会受到pH的显著影响,SDZ、SMX和CLA的富集浓度与中性形态比例呈现良好的正相关关系,但TRM、CAP和ENR的植物吸收规律还需要进一步研究。根据生菜可食用部分计算得到的EDI在pH 5~9范围内存在16倍的差距。本研究可以为评价抗生素通过植物富集产生的人体健康风险提供一定的参考。
表6 目标抗生素在不同pH下暴露9 d后的每日预估摄入量
Table 6 Estimated daily intake of target antibiotics after 9 d of exposure at different pH values
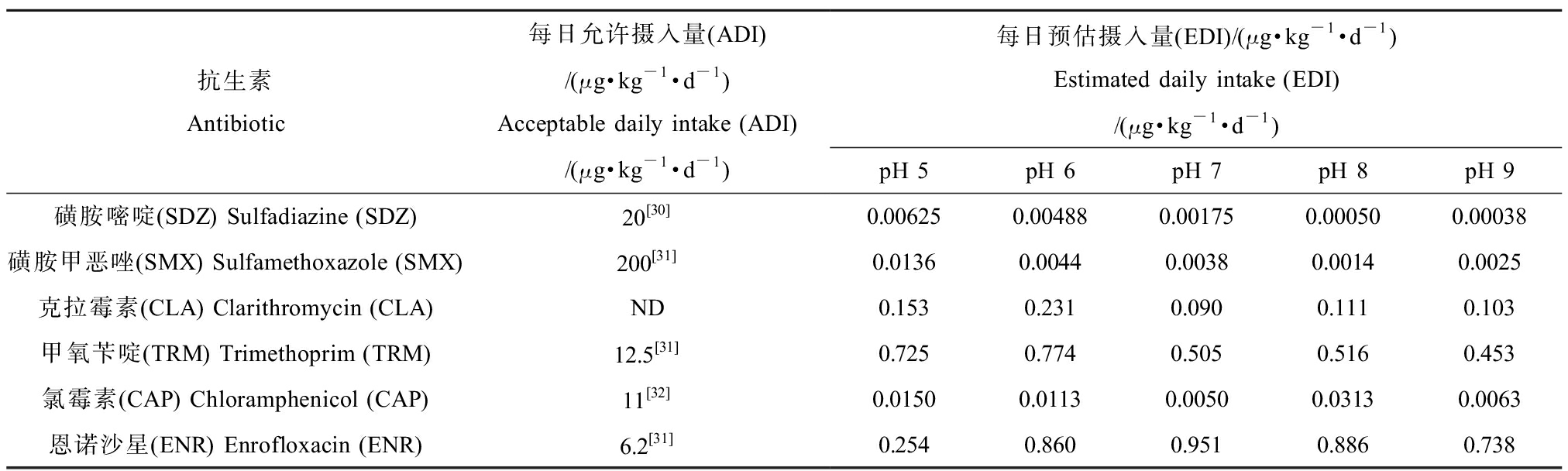
抗生素Antibiotic每日允许摄入量(ADI)/(μg·kg-1·d-1)Acceptable daily intake (ADI)/(μg·kg-1·d-1)每日预估摄入量(EDI)/(μg·kg-1·d-1)Estimated daily intake (EDI)/(μg·kg-1·d-1)pH 5pH 6pH 7pH 8pH 9磺胺嘧啶(SDZ) Sulfadiazine (SDZ)20[30]0.00625 0.004880.001750.000500.00038磺胺甲恶唑(SMX) Sulfamethoxazole (SMX)200[31]0.0136 0.00440.00380.00140.0025克拉霉素(CLA) Clarithromycin (CLA)ND0.153 0.2310.0900.1110.103甲氧苄啶(TRM) Trimethoprim (TRM)12.5[31]0.725 0.7740.5050.5160.453氯霉素(CAP) Chloramphenicol (CAP)11[32]0.0150 0.01130.00500.03130.0063恩诺沙星(ENR) Enrofloxacin (ENR)6.2[31]0.254 0.8600.9510.8860.738
注:ND表示未检索到对应数值。
Note: ND indicates no data available.
[1] Zhang Q Q, Ying G G, Pan C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance [J]. Environmental Science & Technology, 2015, 49(11): 6772-6782
[2] Fick J. Pharmaceuticals and personal care products in the environment contamination of surface, ground, and drinking water from pharmaceutical production [J]. Environmental Toxicology and Chemistry, 2009, 28(12): 2522-2527
[3] Yao L L, Wang Y X, Tong L, et al. Occurrence and risk assessment of antibiotics in surface water and groundwater from different depths of aquifers: A case study at Jianghan Plain, central China [J]. Ecotoxicology and Environmental Safety, 2017, 135: 236-242
[4] Jiang Y H, Li M X, Guo C S, et al. Distribution and ecological risk of antibiotics in a typical effluent-receiving river (Wangyang River) in North China [J]. Chemosphere, 2014, 112: 267-274
[5] Chen H Y, Jing L J, Teng Y G, et al. Characterization of antibiotics in a large-scale river system of China: Occurrence pattern, spatiotemporal distribution and environmental risks [J]. The Science of the Total Environment, 2018, 618: 409-418
[6] 刘锋, 陶然, 应光国, 等. 抗生素的环境归宿与生态效应研究进展[J]. 生态学报, 2010, 30(16): 4503-4511
Liu F, Tao R, Ying G G, et al. Advance in environmental fate and ecological effects of antibiotics [J]. Acta Ecologica Sinica, 2010, 30(16): 4503-4511 (in Chinese)
[7] Hu X G, Zhou Q X, Luo Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, Northern China [J]. Environmental Pollution, 2010, 158(9): 2992-2998
[8] Li C, Chen J Y, Wang J H, et al. Occurrence of antibiotics in soils and manures from greenhouse vegetable production bases of Beijing, China and an associated risk assessment [J]. The Science of the Total Environment, 2015, 521-522: 101-107
[9] Rutgersson C, Fick J, Marathe N, et al. Fluoroquinolones and qnr genes in sediment, water, soil, and human fecal flora in an environment polluted by manufacturing discharges [J]. Environmental Science & Technology, 2014, 48(14): 7825-7832
[10] 管荷兰, 于海凤, 王嘉宇. 氟喹诺酮类抗生素在土壤中的归趋及其生态毒性研究进展[J]. 生态学杂志, 2012, 31(12): 3228-3234
Guan H L, Yu H F, Wang J Y. Fate and ecological toxicity of fluoroquinolone antibiotics in soil: A review [J]. Chinese Journal of Ecology, 2012, 31(12): 3228-3234 (in Chinese)
[11] 王晓洁, 赵蔚, 张志超, 等. 兽用抗生素在土壤中的环境行为、生态毒性及危害调控[J]. 中国科学: 技术科学, 2021, 51(6): 615-636
Wang X J, Zhao W, Zhang Z C, et al. Veterinary antibiotics in soils: Environmental processes, ecotoxicity, and risk mitigation [J]. Scientia Sinica (Technologica), 2021, 51(6): 615-636 (in Chinese)
[12] Pan M, Chu L M. Fate of antibiotics in soil and their uptake by edible crops [J]. Science of the Total Environment, 2017, 599-600: 500-512
[13] 陈姗, 许凡, 张玮, 等. 磺胺类抗生素污染现状及其环境行为的研究进展[J]. 环境化学, 2019, 38(7): 1557-1569
Chen S, Xu F, Zhang W, et al. Research progress in pollution situation and environmental behavior of sulfonamides [J]. Environmental Chemistry, 2019, 38(7): 1557-1569 (in Chinese)
[14] 孔维栋, 朱永官. 抗生素类兽药对植物和土壤微生物的生态毒理学效应研究进展[J]. 生态毒理学报, 2007, 2(1): 1-9
Kong W D, Zhu Y G. A review on ecotoxicology of veterinary pharmaceuticals to plants and soil microbes [J]. Asian Journal of Ecotoxicology, 2007, 2(1): 1-9 (in Chinese)
[15] Subirats J, Domingues A, Topp E. Does dietary consumption of antibiotics by humans promote antibiotic resistance in the gut microbiome? [J]. Journal of Food Protection, 2019, 82(10): 1636-1642
[16] 陈秋方, Briggs G G, Evans A A. 水稻根系对非电离性农药的吸收、转移、分配与农药亲脂性的关系[J]. 核农学报, 1989, 3(1): 1-8
Chen Q F, Briggs G G, Evans A A. Relationships between lipophilicity and root uptake and translocation of non-ionised chemicals by rice [J]. Acta Agriculturae Nucleatae Sinica, 1989, 3(1): 1-8 (in Chinese)
[17] Kong W D, Zhu Y G, Liang Y C, et al. Uptake of oxytetracycline and its phytotoxicity to alfalfa (Medicago sativa L.) [J]. Environmental Pollution, 2007, 147(1): 187-193
[18] Wu X Q, Ernst F, Conkle J L, et al. Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables [J]. Environment International, 2013, 60: 15-22
[19] Li Y B, Sallach J B, Zhang W, et al. Insight into the distribution of pharmaceuticals in soil-water-plant systems [J]. Water Research, 2019, 152: 38-46
[20] Goldstein M, Shenker M, Chefetz B. Insights into the uptake processes of wastewater-borne pharmaceuticals by vegetables [J]. Environmental Science & Technology, 2014, 48(10): 5593-5600
[21] Trapp S. Bioaccumulation of Polar and Ionizable Compounds in Plants [M]//Ecotoxicology Modeling. Boston, MA: Springer US, 2009: 299-353
[22] Miller E L, Nason S L, Karthikeyan K G, et al. Root uptake of pharmaceuticals and personal care product ingredients [J]. Environmental Science & Technology, 2016, 50(2): 525-541
[23] Meng C, Huan D, Zhao H M, et al. Nitrate supply decreases uptake and accumulation of ciprofloxacin in Brassica parachinensis [J]. Journal of Hazardous Materials, 2021, 403: 123803
[24] Barbosa J, Barrón D, Jiménez-Lozano E, et al. Comparison between capillary electrophoresis, liquid chromatography, potentiometric and spectrophotometric techniques for evaluation of pKa values of zwitterionic drugs in acetonitrile-water mixtures [J]. Analytica Chimica Acta, 2001, 437(2): 309-321
[25] Paltiel O, Fedorova G, Tadmor G, et al. Human exposure to wastewater-derived pharmaceuticals in fresh produce: A randomized controlled trial focusing on carbamazepine [J]. Environmental Science & Technology, 2016, 50(8): 4476-4482
[26] 李丹, 高阳俊, 耿春女. 食物链途径人体健康风险评估的关键内容探讨[J]. 环境化学, 2015, 34(3): 431-441
Li D, Gao Y J, Geng C N. Discussions on the human health risk assessment by food-chain exposure pathways [J]. Environmental Chemistry, 2015, 34(3): 431-441 (in Chinese)
[27] 周启星, 罗义, 王美娥. 抗生素的环境残留、生态毒性及抗性基因污染[J]. 生态毒理学报, 2007, 2(3): 243-251
Zhou Q X, Luo Y, Wang M E. Environmental residues and ecotoxicity of antibiotics and their resistance gene pollution: A review [J]. Asian Journal of Ecotoxicology, 2007, 2(3): 243-251 (in Chinese)
[28] Gudda F O, Waigi M G, Odinga E S, et al. Antibiotic-contaminated wastewater irrigated vegetables pose resistance selection risks to the gut microbiome [J]. Environmental Pollution, 2020, 264: 114752
[29] Duan Y J, Chen Z Y, Tan L, et al. Gut resistomes, microbiota and antibiotic residues in Chinese patients undergoing antibiotic administration and healthy individuals [J]. The Science of the Total Environment, 2020, 705: 135674
[30] Boxall A B, Johnson P, Smith E J, et al. Uptake of veterinary medicines from soils into plants [J]. Journal of Agricultural and Food Chemistry, 2006, 54(6): 2288-2297
[31] Schwab B W, Hayes E P, Fiori J M, et al. Human pharmaceuticals in US surface waters: A human health risk assessment [J]. Regulatory Toxicology and Pharmacology, 2005, 42(3): 296-312
[32] Pan M, Chu L M. Transfer of antibiotics from wastewater or animal manure to soil and edible crops [J]. Environmental Pollution, 2017, 231: 829-836