卤代咔唑是一类杂环芳香烃化合物,根据取代元素不同,可以分为溴取代、氯取代、碘取代和混合取代等。卤代咔唑的主要来源是卤代靛蓝染料工业生产中的副产物[1]、光电材料的中间体[2]和除草剂[3],也有可能在自然条件下通过生物体内的氯过氧化物酶催化产生[4]。在北美洲和欧洲等地的土壤和水体底泥中较早检测到不同种类的溴代咔唑(bromocarbazoles, BCZs),例如五大湖中检测到了总量超过3 000 t的卤代咔唑,其中1,3,6,8-四溴咔唑(1,3,6,8-tetrabromo-9H-carbazole, 1368-BCZ)在美国密歇根湖中的含量高达112 ng·g-1[5];3,6-二溴咔唑(3,6-dibromo-9H-carbazole, 36-BCZ)在德国土壤中的浓度范围是0.2~19.8 ng·g-1[6]。我国的太湖底泥中也检测到多种BCZs,22个检测位点中的20个位点都检测到36-BCZ,另外,3-溴咔唑(3-bromo-9H-carbazole, 3-BCZ)、2,7-二溴咔唑(2,7-dibromo-9H-carbazole, 27-BCZ)和1,3,6-三溴咔唑(1,3,6-tribromo-9H-carbazole, 136-BCZ)也是较易检出的BCZs种类[7]。研究人员预测了具有代表性的卤代咔唑的理化性质,相较于其他种类的BCZs,1368-BCZ在土壤中的半衰期为360 d,在自然环境中具有较大的积累潜力,且具有较高的持久性和生物累积性,毒性方面也具有较强的水生生物毒性;36-BCZ也具有土壤中难生物降解的特性[8]。另外,在鱼类、海豹和鸟蛋中也检测到了不同浓度的BCZs[9-12],在鸬鹚蛋中,136-BCZ的含量最高[11]。由此可见,BCZs已经从环境进入到动物体内,并具有一定的生物积累效应,提示其可能具有潜在的人类暴露风险,因此近年来受到了许多关注。
研究人员对BCZs进行了不同生物体内和体外的相关毒性研究。研究表明1368-BCZ能对乙酰胆碱酯酶(acetylcholinesterase, AChE)表现出抑制作用,对蚯蚓产生神经毒性[13]。27-BCZ、36-BCZ和1368-BCZ都对斑马鱼产生了毒性效应:27-BCZ暴露后不仅对斑马鱼肌肉发育产生不良影响,也会导致胚胎脊柱弯曲,还展现出明显的心脏发育毒性等典型的二噁英毒性[14];36-BCZ暴露可以导致心脏畸形发育和血液阻塞[15];1368-BCZ暴露提高了斑马鱼胚胎畸形率,也会导致心脏轻微变形[14-15],还能促进活性氧的产生,诱导斑马鱼细胞凋亡[16]。可见,BCZs对神经系统、胚胎发育以及重要的生物过程都表现出类二噁英毒性。
BCZs的类二噁英毒性作用机制的相关研究表明,36-BCZ可以在大鼠肝癌细胞中通过乙氧基间苯二酚-o-脱乙基酶(ethoxyresorufin-o-deethylase, EROD)诱导类二噁英活性[6]。BCZs可以在稳定转染了含有二噁英反应元件(dioxin response element, DRE)质粒的小鼠的肝癌细胞CBG2.8D中激活芳香烃受体(aryl hydrocarbon receptor, AhR)通路[17],还可以在MDA-MB-468细胞中上调细胞色素P450酶1A1(cytochrome P450 1A1, cyp1a1)和细胞色素P450酶1B1(cytochrome P450 1B1, cyp1b1)的基因表达水平[18]。除AhR外,BCZs还可以作用于其他受体,例如27-BCZ和3-BCZ能够显著增加大鼠子宫质量和上皮细胞质量;同时对仓鼠卵巢细胞的雌激素受体(estrogen receptor, ER)通路具有一定的激活效应,表现出内分泌干扰效应[19]。因此,BCZs还可能通过ER通路影响某些生殖系统的疾病,这也是未来健康效应的研究方向。
乳腺癌是全世界各地女性最常见的生殖系统癌症之一。流行病学研究表明,终生暴露于某些雌激素是乳腺癌的已知风险因素[20]。AhR也已被报道可以在乳腺癌的发生中发挥作用,例如,乳腺癌细胞中敲除AhR可抑制MCF-7细胞增殖[21],大鼠乳腺癌细胞比正常乳腺组织表达更高水平的AhR[22]。可见,乳腺癌的发生发展与ER和AhR通路都有所联系。ER信号通路与AhR信号通路有许多相似之处,AhR可以通过多种机制与ER相互作用[23],例如AhR与ER靶基因的启动子结合可以削弱ER介导的转录[24];竞争共同的辅助因子[25];促进ER的降解、抑制17β-雌二醇(17β-estradiol, E2)合成[26];同时,AhR靶基因的激活也可以被雌激素和雌激素样化合物所抑制[27]。因此,细胞中AhR和ER之间的相互作用是复杂的。目前尚不清楚BCZs这种新型污染物是否可以在乳腺癌细胞中同时激活ER和AhR通路从而对乳腺癌产生影响,以及其中复杂的机制和相互作用关系。
BCZs作为一类新型的环境污染物,在底泥沉积物、土壤甚至动物体内均有检出,具有一定的持久性和生物累积能力;BCZs也被用于研究多卤代咔唑对不同生物的毒理学效应,因此探究BCZs的毒理学机制具有一定的意义,但目前还缺少系统全面的基于人群的毒理学数据,与人类相关疾病的研究尚不充分。因此,本实验选取5种BCZs进行研究,分别为3-BCZ、27-BCZ、36-BCZ、136-BCZ和1368-BCZ,借鉴不良结局途径(adverse outcome pathway, AOP)的思路[28-29],即从某些分子开始,研究关键事件、关键事件的关系以及造成的不良结局,来评估化学物质的效应和危害,对BCZs的毒理学机制进行研究。本实验以ER和AhR这2个通路为切入点,首先研究BCZs是否可以在ER阳性乳腺癌细胞MCF-7中激活ER和AhR通路,筛选出激活能力最强的BCZs;进一步分别干扰ahr或ER通路,研究具有共激活效应的BCZs与2个通路之间的交叉作用,明确BCZs对乳腺癌细胞的毒理学作用机制,补充BCZs的AOP相关数据,对于研究新型污染物的毒理效应具有一定的参考价值,也能为BCZs的毒性评估提供新的思路。
1 材料与方法(Materials and methods)
1.1 材料
人乳腺癌细胞株MCF-7购自中国医学科学院细胞资源中心。无酚红高糖DMEM培养基、无酚红胰酶、青霉素/链霉素混合物和减血清培养基(Gibco);活性炭处理的胎牛血清(Biological Industries);3-BCZ、27-BCZ、36-BCZ、136-BCZ、1368-BCZ和四氯二苯并-p-二噁英(tetrachlorodibenzo-p-dioxin, TCDD) (Wellington Laboratory);17β-雌二醇(17β-estradiol, E2) (Sigma)、二甲基亚砜(dimethyl sulfoxide, DMSO) (Sigma),pER-Luc Reporter (Yeasen),pGL4.43[luc2P/XRE/Hygro] Vector (Promega)。超微量分光光度计(Nanodrop 2000, Thermo),滤光片型多功能酶标仪(Infinite F200 Pro, Tecan),Glomax多功能检测系统(E7081, Promega),PCR仪(T100TM, BIO-RAD),qPCR仪(4485694, Life)。
1.2 方法1.2.1 细胞培养
MCF-7细胞所需的完全培养基为无酚红高糖DMEM培养基,添加10%活性炭处理的胎牛血清和1%青霉素/链霉素混合物。MCF-7细胞在37 ℃、5% CO2培养箱中培养,隔天换液一次,在细胞生长至80%~90%时用含量为0.25%无酚红胰酶消化后按照1∶3进行传代。
1.2.2 CCK-8法测细胞毒性
MCF-7细胞接种于96孔板中,贴壁培养24 h后,吸出原培养基,向孔中加入含有不同浓度BCZs的新培养基,同时设置空白组(只含有细胞和培养基)和对照组(含有细胞、培养基和0.1% DMSO)。Xu等[30]根据密歇根湖中1368-BCZ的浓度[5]经过换算得到浓度为10-7 mol·L-1,本实验依照该环境浓度进行设置,5种BCZs浓度分别为10-9 mol·L-1、10-8 mol·L-1和10-7 mol·L-1,每个浓度设置6个复孔。继续培养24 h后,每孔加入10 μL CCK-8试剂,继续在培养箱孵育1 h,用酶标仪测定在450 nm处的吸光值。
1.2.3 质粒转化、提取和转染
向感受态细胞中转化含有雌激素反应元件(estrogen response element, ERE)或二噁英反应元件(dioxin response element, DRE)序列的质粒,转化后的细胞进行扩增培养。质粒提取采用GeneJET Plasmid Maxiprep Kit (Thermo)试剂盒。质粒转染采用Lipofectamine LTX and PlusTM Reagent (Thermo)试剂盒,转染12 h。转染的质粒用于双荧光素酶报告基因检测中判定BCZs对ERE或DRE的激活作用。
1.2.4 双荧光素酶报告基因检测
双荧光素酶报告基因检测使用Dual-Luciferase® Reporter Assay System Kit (Promega)试剂盒。96孔板倒掉培养基,加入150 μL磷酸缓冲液淋洗,轻轻敲打。去除磷酸缓冲液后,用白色胶纸密封96孔板底部。每孔加入50 μL细胞裂解液(1×),放至振荡器上避光振荡15 min。使用Glomax多功能检测系统进行检测。
1.2.5 实时荧光定量PCR(qPCR)法检测基因表达水平
使用Gene JET RNA Purification Kit (Thermo)试剂盒提取总RNA。用超微量分光光度计测量总RNA浓度,计算所需总RNA体积。反转录过程按照RevertiAid First Strand cDNA Synthesis Kit (Thermo)试剂盒说明书进行。检测的基因分别为:三叶因子家族1(trefoil factor family 1, tff1)、芳香烃受体(aryl hydrocarbon receptor, ahr)、cyp1a1和cyp1b1。tff1是ER通路靶基因[31-32],cyp1a1和cyp1b1是AhR通路经典靶基因[33-34]。引物序列基于GenBank获得的序列,采用Primer-BLAST设计引物并进行验证,由生工生物科技公司合成,引物序列如表1所示。按照说明书在qPCR仪中设定程序进行实验。qPCR实验以甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase, gapdh)为内参,用2-ΔΔCT方法进行数据计算[35]。
1.2.6 siRNA干扰ahr表达
siRNA由SyngenTech公司(北京)设计合成,siRNA的终浓度为2×10-8 mol·L-1,序列如表2所示。按照RNAiMAX Transfection Reagent (Invitrogen)转染试剂盒说明书进行转染。BCZs的暴露浓度为10-7 mol·L-1,TCDD和E2暴露浓度为10-9 mol·L-1,暴露24 h后再测定ahr和tff1的基因表达水平。
表1 引物序列
Table 1 Primer sequences
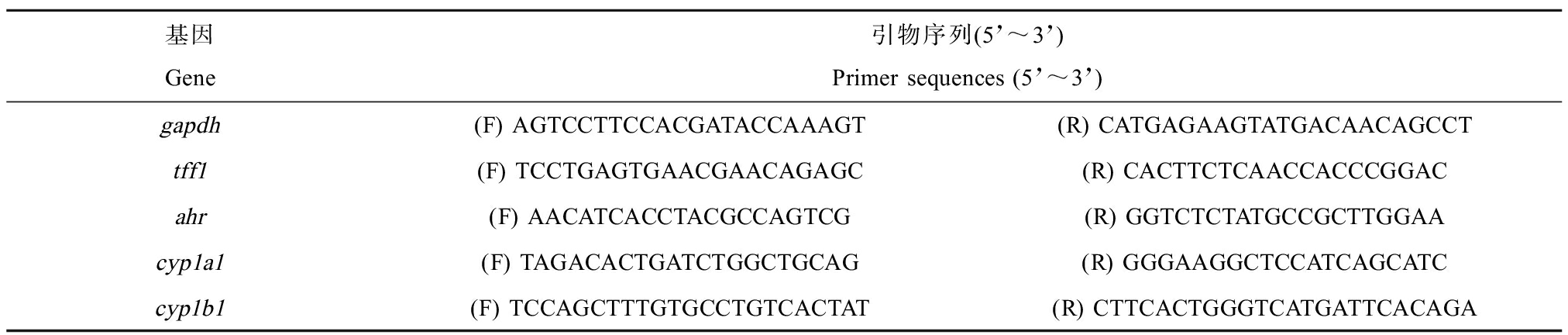
基因Gene引物序列(5’~3’)Primer sequences (5’~3’)gapdh(F) AGTCCTTCCACGATACCAAAGT(R) CATGAGAAGTATGACAACAGCCTtff1(F) TCCTGAGTGAACGAACAGAGC(R) CACTTCTCAACCACCCGGACahr(F) AACATCACCTACGCCAGTCG(R) GGTCTCTATGCCGCTTGGAAcyp1a1(F) TAGACACTGATCTGGCTGCAG(R) GGGAAGGCTCCATCAGCATCcyp1b1(F) TCCAGCTTTGTGCCTGTCACTAT(R) CTTCACTGGGTCATGATTCACAGA
表2 siRNA序列
Table 2 siRNA sequence
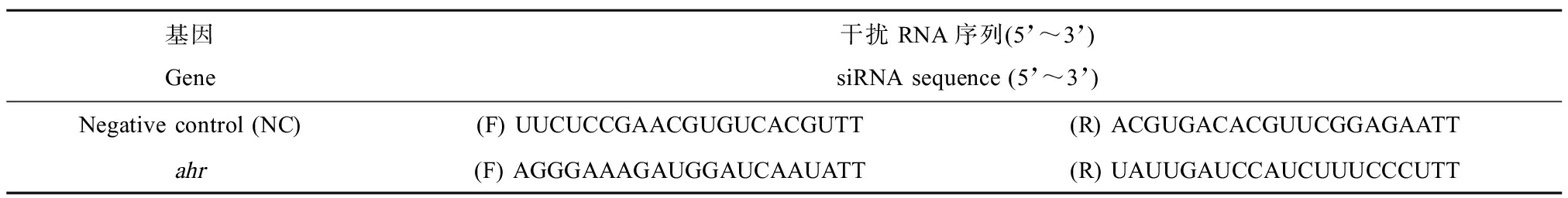
基因Gene干扰RNA序列(5’~3’)siRNA sequence (5’~3’)Negative control (NC)(F) UUCUCCGAACGUGUCACGUTT(R) ACGUGACACGUUCGGAGAATTahr(F) AGGGAAAGAUGGAUCAAUATT(R) UAUUGAUCCAUCUUUCCCUTT
1.2.7 ICI-182780(ICI)拮抗ER通路
实验组按照体积比1∶1 000用10-5 mol·L-1雌激素拮抗剂ICI预处理3 h,对照组用0.1% DMSO预处理3 h。BCZs暴露浓度为10-7 mol·L-1,TCDD为10-9 mol·L-1,按照体积比1∶1 000添加,暴露24 h后再测定AhR通路下游相关基因表达水平。
1.2.8 数据统计分析
数据统计和分析使用GraphPad prism软件(version 6, La Jolla, CA)。实验进行3次独立重复,实验结果表示为均值±SEM(n=3)。数据统计采用单向或双向方差分析,*表示P<0.05,**表示P<0.01,***表示P<0.001,****表示P<0.0001。
2 结果(Results)
2.1 细胞毒性检测
5种BCZs与TCDD的结构式如图1所示。采用CCK-8法检测BCZs暴露对MCF-7细胞毒性的影响。由图2可知,在3种浓度暴露24 h条件下,5种BCZs对MCF-7细胞均不产生细胞毒性,因此选取最高浓度10-7 mol·L-1作为后续实验的暴露浓度。
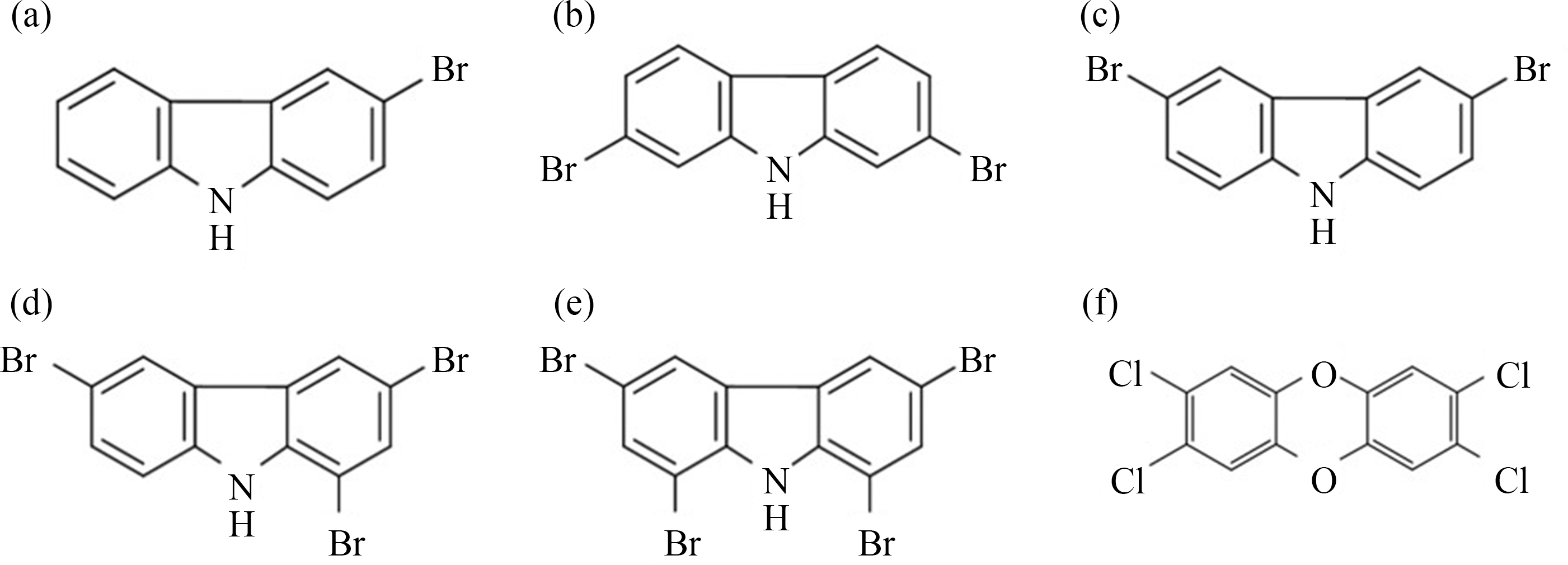
图1 BCZs和TCDD结构式
注:BCZs表示溴代咔唑,TCDD表示四氯二苯并-p-二噁英;(a) 3-BCZ,(b) 27-BCZ,(c) 36-BCZ,(d) 136-BCZ,(e) 1368-BCZ,(f) TCDD。
Fig. 1 Structure formulas of BCZs and TCDD
Note: BCZs stands for bromocarbazoles and TCDD stands for tetrachlorodibenzo-p-dioxin; (a) 3-BCZ, (b) 27-BCZ, (c) 36-BCZ, (d) 136-BCZ, (e) 1368-BCZ, (f) TCDD.
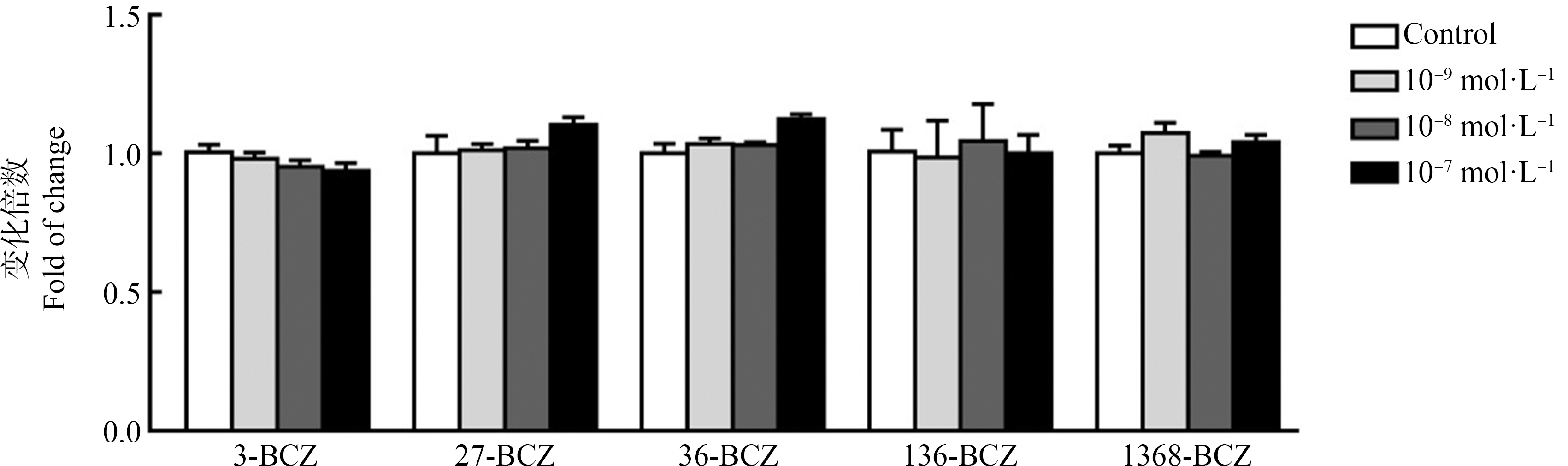
图2 BCZs的细胞毒性检测
注:对照组添加0.1% DMSO,DMSO表示二甲基亚砜。
Fig. 2 Cytotoxicity of BCZs
Note: Control group is 0.1% DMSO and DMSO stands for dimethyl sulfoxide.
2.2 BCZs对ER通路的激活作用
通过含ERE序列的双荧光素酶报告基因检测以及ER通路下游tff1基因表达水平,来评估MCF-7细胞中BCZs对ER通路的激活作用。5种BCZs中,1368-BCZ能够显著激活ERE(图3(a));36-BCZ、136-BCZ和1368-BCZ都能使tff1的基因表达水平显著升高,其中最为显著的是136-BCZ,其次为1368-BCZ(图3(b))。因此,对ER通路激活作用较强的是1368-BCZ。
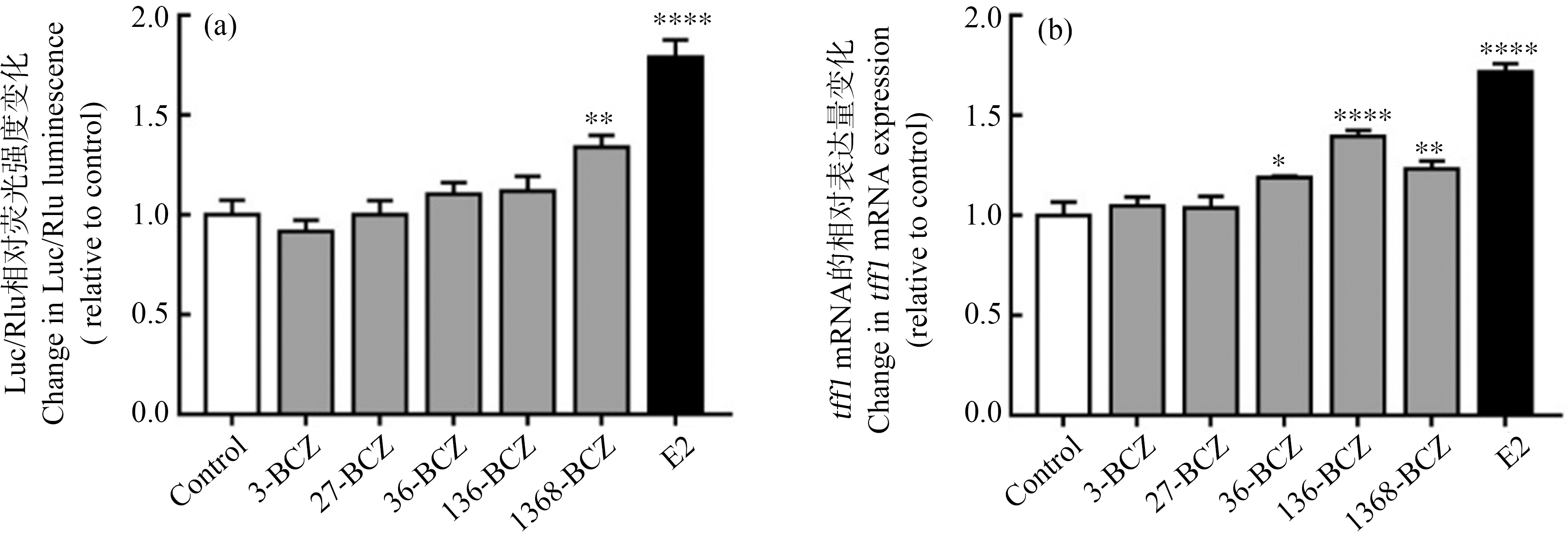
图3 BCZs对雌激素受体(ER)通路的激活
注:E2代表17β-雌二醇;(a) BCZs与E2对雌激素反应元件(ERE)的激活作用;(b) BCZs和E2诱导三叶因子家族1(tff1)基因表达水平的变化;BCZs浓度为10-7 mol·L-1,E2浓度为10-9 mol·L-1;qPCR检测以GAPDH为内参;与0.1% DMSO对照组相比,*表示P<0.05,**表示P<0.01,***表示P<0.001,****表示P<0.0001。
Fig. 3 Activation of estrogen receptor (ER) pathway induced by BCZs
Note: E2 stands for 17β-estradiol; (a) Activation of estrogen response element (ERE) by BCZs and E2; (b) Gene expression level change of trefoil factor family 1 (tff1) induced by BCZs and E2; the concentrations of BCZs and E2 were 10-7 mol·L-1 and 10-9 mol·L-1, respectively; GAPDH was used as internal control in qPCR; compared with 0.1% DMSO control group, *represents P<0.05, **represents P<0.01, ***represents P<0.001, and ****represents P<0.0001.
2.3 BCZs对AhR通路的激活作用
AhR信号通路的激活也是通过含DRE序列的双荧光素酶报告基因检测以及AhR通路下游经典靶基因cyp1a1和cyp1b1的表达来体现。5种BCZs中,对DRE激活能力最强的是1368-BCZ(图4(a));同样,1368-BCZ能使cyp1a1和cyp1b1的基因表达水平显著升高(图4(b)和4(c))。因此,对AhR通路激活作用最强的是1368-BCZ。
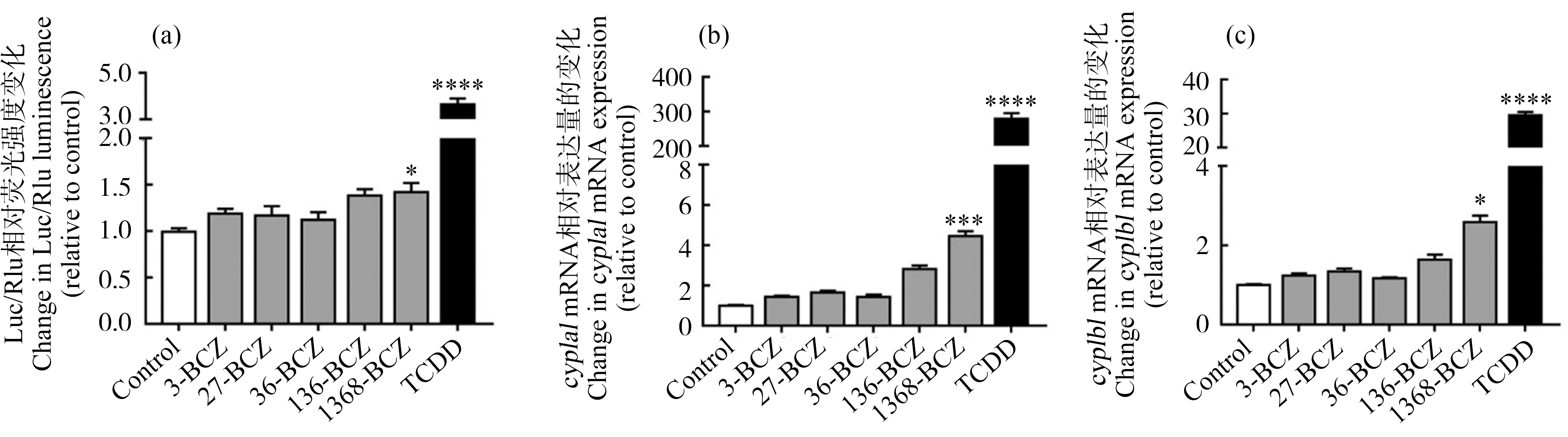
图4 BCZs对芳香烃受体(AhR)通路的激活
注:(a) BCZs与TCDD对二噁英反应元件(DRE)的激活作用;BCZs和TCDD诱导(b)细胞色素P450酶1A1(cyp1a1)和(c)细胞色素P450酶1B1(cyp1b1)基因的表达水平变化;BCZs浓度为10-7 mol·L-1,TCDD浓度为10-9 mol·L-1;qPCR检测以GAPDH为内参;与0.1% DMSO对照组相比,*表示P<0.05,**表示P<0.01,***表示P<0.001,****表示P<0.0001。
Fig. 4 Activation of AhR pathway induced by BCZs
Note: (a) Activation of dioxin response element (DRE) by BCZs and TCDD; gene expression level change of (b) cytochrome P450 1A1 (cyp1a1) and (c) cytochrome P450 1B1 (cyp1b1) induced by BCZs and TCDD; the concentrations of BCZs and TCDD were 10-7 mol·L-1 and 10-9 mol·L-1, respectively; GAPDH was used as internal control in qPCR; compared with 0.1% DMSO control group, *represents P<0.05, **represents P<0.01, ***represents P<0.001, and ****represents P<0.0001.
由此结果发现,1368-BCZ对ER和AhR通路均具有较强的激活作用。因此,以1368-BCZ为主要研究对象,探讨1368-BCZ激活的ER和AhR通路之间的相互作用。
2.4 ER通路与AhR通路的相互作用
转染ahr-siRNA后,ahr的表达显著下降,TCDD引起ahr表达下降了75.60%,1368-BCZ引起的ahr表达下降了74.35%(图5(a)),说明设计的siRNA可以有效干扰ahr表达。ahr被干扰后,E2对tff1的激活并没有受到显著影响,但是1368-BCZ激发的tff1表达相较NC对照组下降了21.25%,具有显著性差异(图5(b))。
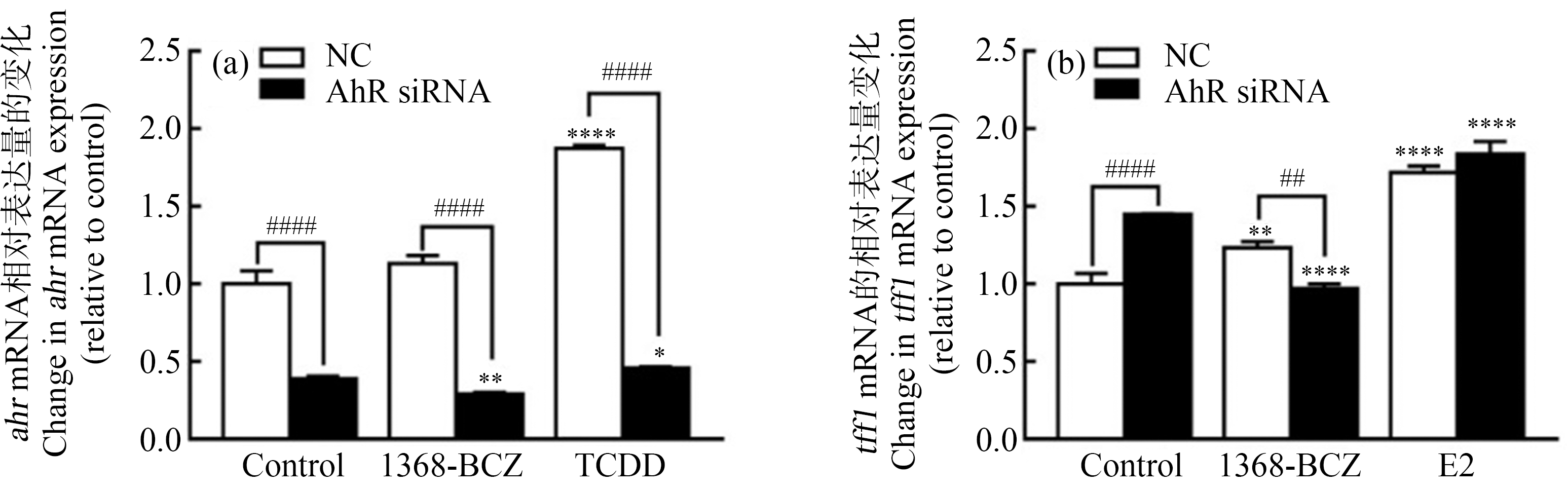
图5 ahr对ER通路的影响
注:siRNA处理后(a) ahr和(b) tff1基因表达的变化;1368-BCZ浓度为10-7 mol·L-1,TCDD和E2浓度为10-9 mol·L-1;qPCR检测以GAPDH为内参;与0.1% DMSO对照组相比,*表示P<0.05,**表示P<0.01,***表示P<0.001,****表示P<0.0001;与NC组相比,#表示P<0.05,##表示P<0.01,###表示P<0.001,####表示P<0.0001。
Fig. 5 Effect of ahr on ER pathway
Note: Gene expression level change of (a) ahr and (b) tff1 after treated by siRNA; the concentration of 1368-BCZ was 10-7 mol·L-1 and the concentrations of TCDD and E2 were 10-9 mol·L-1; GAPDH was used as internal control in qPCR; compared with 0.1% DMSO control group, *represents P<0.05, **represents P<0.01, ***represents P<0.001, and ****represents P<0.0001; compared with NC group, # represents P<0.05, ## represents P<0.01, ### represents P<0.001, and #### represents P<0.0001.
ICI处理后,1368-BCZ可以显著上调MCF-7细胞中cyp1a1(图6(a))和cyp1b1(图6(b))的表达水平,其中,cyp1a1表达提升至4.17倍,cyp1b1表达提升至1.40倍。另外,ICI拮抗后,ahr表达水平也所有提升,是原来的2.05倍(图6(c))。
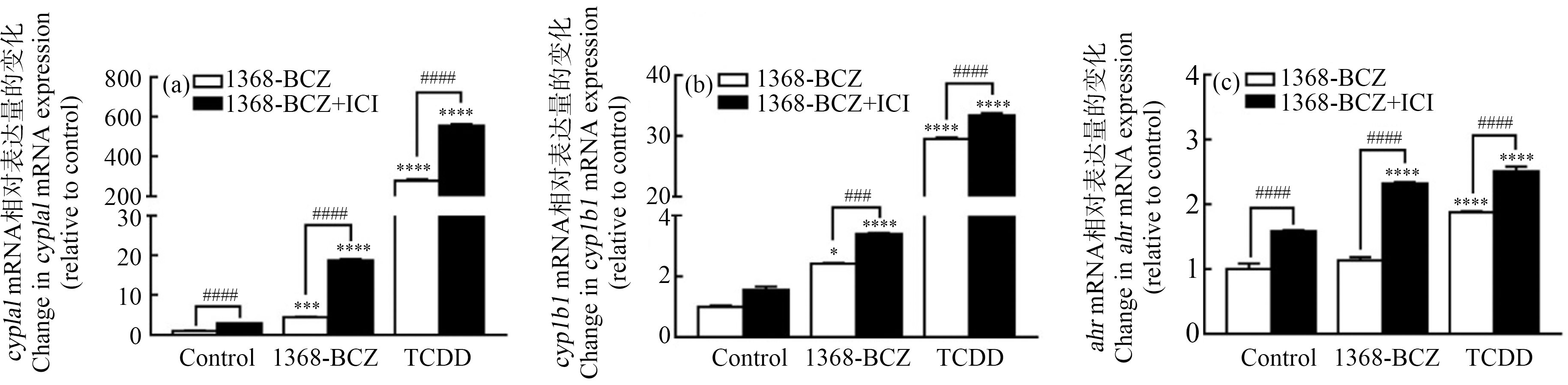
图6 ER通路对AhR通路的影响
注:ICI预处理后(a) cyp1a1、(b) cyp1b1和(c) ahr的基因表达水平变化;1368-BCZ浓度为10-7 mol·L-1,TCDD浓度为10-9 mol·L-1;qPCR检测以GAPDH为内参;与0.1% DMSO对照组相比,*表示P<0.05,**表示P<0.01,***表示P<0.001,****表示P<0.0001;ICI预处理组与DMSO预处理组对比,#表示P<0.05,##表示P<0.01,###表示P<0.001,####表示P<0.0001。
Fig. 6 Effect of ER pathway on AhR pathway
Note: Gene expression level change of (a) cyp1a1, (b) cyp1b1 and (c) ahr after treated by ICI; the concentrations of 1368-BCZ and TCDD were 10-7 mol·L-1 and 10-9 mol·L-1, respectively; GAPDH was used as internal control in qPCR; compared with 0.1% DMSO control group, *represents P<0.05, **represents P<0.01, ***represents P<0.001, and ****represents P<0.0001; compared between ICI and DMSO pre-treatment group, # represents P<0.05, ## represents P<0.01, ### represents P<0.001, and #### represents P<0.0001.
3 讨论(Discussion)
本实验以ER阳性乳腺癌细胞MCF-7为实验对象,选取5种BCZs在10-7 mol·L-1的浓度下暴露24 h,探究BCZs对ER和AhR通路的激活作用,并筛选激活作用最显著的BCZs探究通路间的相互作用。分析双荧光素酶报告基因检测结果(图3(a)和图4(a))以及ER通路下游tff1(图3(b))和AhR通路下游cyp1a1和cyp1b1基因的表达结果(图4(b)和图4(c)),1368-BCZ可以同时激活ER和AhR通路并上调2个通路下游基因的表达水平。BCZs在稳定转染了含有DRE序列的小鼠肝癌细胞CBG 2.8D中也可以激活AhR通路,Ma等[17]通过分子对接实验发现,BCZs均与小鼠AhR的配体结合域形成了稳定结合构象,表明BCZs也可能是AhR的一种配体。文献表明,内源或者外源AhR的配体也可以激活ER通路,在MCF-7细胞中表现出对2个通路的激活作用,外源配体如吲哚[3,2-b]咔唑[36]、6-甲基-1,3,8-三氯代二苯并呋喃、3,39-二吲哚甲烷、苯并[a]芘和多氯联苯[37];内源配体如植物激素染料木素、大豆黄酮、S-雌马酚和甘草素[38]。这些具有共激活能力的物质,对乳腺癌细胞的毒理学效应也不尽相同,例如染料木素、大豆黄酮和白杨素等植物雌激素可以抑制乳腺癌细胞的增殖[39];苯并[a]芘可以干扰DNA甲基化[40]。由于1368-BCZ对ER和AhR通路及下游基因都有显著的激活作用,也可以列为具有共激活能力的物质。这也为今后研究BCZs与这些化合物的共性和差异性,以及是否能产生类似的毒理学效应提供思路和方向。
1368-BCZ在MCF-7细胞中对ER和AhR通路的激活作用最为显著,可能与其卤族元素取代个数较多和本身理化性质较强有关。结构方面,1368-BCZ拥有4个卤素取代个数,Riddlell等[18]在三阴乳腺癌细胞MDA-MB-468中发现1368-BCZ和2367-CCZ比取代个数较少的卤代咔唑更能诱导cyp1a1和cyp1b1基因的表达。理化性质方面,根据Mumbo等[8]的预测结果,1368-BCZ相对于其他BCZs在自然环境中具有较高的持久性、生物累积性和水生生物毒性。其他毒理学研究也表明1368-BCZ具有较强的毒性,例如高胚胎畸形率[14],引起氧化应激反应以及DNA损伤,诱导斑马鱼细胞凋亡[16],还可以抑制AChE并对蚯蚓产生神经毒性[13]。我们的实验结果同样证明了1368-BCZ在MCF-7细胞中对ER和AhR通路的激活作用比其他低取代个数的BCZs效果更强,但是高取代咔唑可引起较强的毒理学效应的机制仍然不清楚。
ER与AhR的交叉作用十分复杂。接下来,本研究选取对2个通路均有显著激活作用的1368-BCZ进行后续ER和AhR通路交叉作用的研究。使用siRNA对MCF-7细胞中的ahr进行干扰后,1368-BCZ暴露后ER通路经典靶基因tff1的表达下降(图5(b)),说明1368-BCZ的雌激素效应部分依赖于ahr。相关研究表明,在T47D乳腺癌细胞中,AhR的异源二聚体芳香烃受体核转运因子(aryl hydrocarbon receptor nuclear translocator, ARNT)可以作为ER通路的辅助因子,结合在TFF1的启动子区域[41];但也有研究表明AhR可以从不同途径对ER产生抑制作用[23]。用ICI-182780拮抗ER通路后,1368-BCZ激活的AhR通路中,cyp1a1和cyp1b1的表达水平显著提高(图6(a)和6(b)),说明ER通路的激活对AhR通路相关基因的表达产生了一定的抑制。这种抑制作用可能是由于ER的激活抑制了ahr的表达,因为在ER被拮抗后,ahr的基因表达水平也显著提升(图6(c))。因此,1368-BCZ作为可以同时激活ER和AhR通路的一种污染物质,其激活的AhR通路对ER通路有一定促进作用,而激活的ER通路对AhR通路又有一定抑制作用。相关研究也有发现,共激活物质对ER通路的激活会抑制AhR通路下游基因的表达。当ERα被ICI抑制或被siRNA干扰时,染料木素、大豆黄酮、S-雌马酚和甘草素这些植物雌激素可以使cyp1a1和cyp1b1的表达水平增加[38]。对于这些共激活物质的研究大多是以ER对AhR通路的抑制作用为主,AhR对ER通路的影响研究相对较少,本实验补充了这一方面的相关研究结果。我们同样也发现,AhR通路对E2激活的ER通路没有显著影响(图5(b)),这可能由于E2是ER通路作用效果较为专一的强配体[38],ER通路对AhR通路的抑制作用占了主导,因此并没有表现出AhR通路对其的促进作用。与之类似的是,TCDD是AhR的强配体,具有极高的结合能力,只有当AhR表达缺失的时候,TCDD才可以表现出弱雌激素效应[42]。
综上所述,BCZs这类新型污染物在乳腺癌细胞MCF-7中可以同时激活ER和AhR通路,其中效应最为显著的是1368-BCZ,表明了1368-BCZ是最具有潜力的ER和AhR激动剂。1368-BCZ激活的AhR通路有助于ER通路靶基因tff1表达水平的提高,而1368-BCZ激活的ER通路可以抑制AhR通路靶基因cyp1a1和cyp1b1的表达,在ICI处理后,这种抑制作用被解除。ER和AhR通路都可以影响乳腺癌的发生和发展,雌激素可以通过ER通路促进ER阳性乳腺癌细胞的增殖,一些药物如他莫昔芬可以治疗ER阳性乳腺癌[43-44];MCF-7细胞中敲除AhR也会抑制细胞增殖[21]。本研究有助于人们更好地了解作为ER和AhR激动剂的BCZs的毒理学机制,为研究乳腺癌细胞中ER和AhR通路之间的交叉作用提供了数据,也为今后研究BCZs对乳腺癌细胞的毒理学效应打下基础。
[1] Parette R, McCrindle R, McMahon K S, et al. Halogenated indigo dyes: A likely source of 1,3,6,8-tetrabromocarbazole and some other halogenated carbazoles in the environment [J]. Chemosphere, 2015, 127: 18-26
[2] Karon K, Lapkowski M, Juozas G. Electrochemical and UV-Vis/ESR spectroelectrochemical properties of polymers obtained from isomeric 2,7- and 3,6- linked carbazole trimers; influence of the linking topology on polymers properties [J]. Electrochimica Acta, 2014, 123: 176-182
[3] Amine-Khodja A, Boulkamh A, Boule P. Photochemical behaviour of phenylurea herbicides [J]. Photochemical & Photobiological Sciences, 2004, 3(2): 145-156
[4] Mumbo J, Lenoir D, Henkelmann B, et al. Enzymatic synthesis of bromo- and chlorocarbazoles and elucidation of their structures by molecular modeling [J]. Environmental Science and Pollution Research International, 2013, 20(12): 8996-9005
[5] Guo J H, Li Z N, Ranasinghe P, et al. Spatial and temporal trends of polyhalogenated carbazoles in sediments of upper great lakes: Insights into their origin [J]. Environmental Science & Technology, 2017, 51(1): 89-97
[6] Mumbo J, Pandelova M, Mertes F, et al. The fingerprints of dioxin-like bromocarbazoles and chlorocarbazoles in selected forest soils in Germany [J]. Chemosphere, 2016, 162: 64-72
[7] Wu Y, Qiu Y L, Tan H L, et al. Polyhalogenated carbazoles in sediments from Lake Tai (China): Distribution, congener composition, and toxic equivalent evaluation [J]. Environmental Pollution, 2017, 220(Pt A): 142-149
[8] Mumbo J, Henkelmann B, Abdelaziz A, et al. Persistence and dioxin-like toxicity of carbazole and chlorocarbazoles in soil [J]. Environmental Science and Pollution Research International, 2015, 22(2): 1344-1356
[9] Kaehler S, Williams G A. Distribution of algae on tropical rocky Shores: Spatial and temporal patterns of non-coralline encrusting algae in Hong Kong [J]. Marine Biology, 1996, 125(1): 177-187
[10] Lee S C, Williams G A, Brown G D. Maculalactone L and three halogenated carbazole alkaloids from Kyrtuthrix maculans [J]. Phytochemistry, 1999, 52(3): 537-540
[11] Wu Y, Tan H L, Sutton R, et al. From sediment to top predators: Broad exposure of polyhalogenated carbazoles in San Francisco Bay (U.S.A.) [J]. Environmental Science & Technology, 2017, 51(4): 2038-2046
[12] Wu Y, Tan H L, Zhou C L, et al. Bioaccumulation and spatiotemporal trends of polyhalogenated carbazoles in Great Lakes fish from 2004 to 2016 [J]. Environmental Science & Technology, 2018, 52(8): 4536-4545
[13] 周音巧. 土壤中的1,3,6,8-四溴咔唑在蚯蚓体内的积累及生态毒性[D]. 杭州: 浙江工业大学, 2019: 37-38
Zhou Y Q. Accumulation and ecotoxicities of 1,3,6,8-tetrabromocarbazole in earthworms(Eisenia foetida)from spiked artificial soils [D]. Hangzhou: Zhejiang University of Technology, 2019: 37-38 (in Chinese)
[14] Ji C Y, Yan L, Chen Y C, et al. Evaluation of the developmental toxicity of 2,7-dibromocarbazole to zebrafish based on transcriptomics assay [J]. Journal of Hazardous Materials, 2019, 368: 514-522
[15] Fang M L, Guo J H, Chen D, et al. Halogenated carbazoles induce cardiotoxicity in developing zebrafish (Danio rerio) embryos [J]. Environmental Toxicology and Chemistry, 2016, 35(10): 2523-2529
[16] Zhang J W, Zhang C, Du Z K, et al. Emerging contaminant 1,3,6,8-tetrabromocarbazole induces oxidative damage and apoptosis during the embryonic development of zebrafish (Danio rerio) [J]. Science of the Total Environment, 2020, 743: 140753
[17] Ma D, Xie H Q, Zhang W L, et al. Aryl hydrocarbon receptor activity of polyhalogenated carbazoles and the molecular mechanism [J]. The Science of the Total Environment, 2019, 687: 516-526
[18] Riddell N, Jin U H, Safe S, et al. Characterization and biological potency of mono- to tetra-halogenated carbazoles [J]. Environmental Science & Technology, 2015, 49(17): 10658-10666
[19] Yue S Q, Zhang T, Shen Q Q, et al. Assessment of endocrine-disrupting effects of emerging polyhalogenated carbazoles (PHCZs): In vitro, in silico, and in vivo evidence [J]. Environment International, 2020, 140: 105729
[20] Hsieh C C, Lambe M, Trichopoulos D, et al. Early life exposure to oestrogen and testicular cancer risk: Evidence against an aetiological hypothesis [J]. British Journal of Cancer, 2002, 86(8): 1363-1364
[21] Salisbury T B, Morris G Z, Tomblin J K, et al. Aryl hydrocarbon receptor ligands inhibit igf-ii and adipokine stimulated breast cancer cell proliferation [J]. ISRN Endocrinology, 2013, 2013: 104850
[22] Trombino A F, Near R I, Matulka R A, et al. Expression of the aryl hydrocarbon receptor/transcription factor (AhR) and AhR-regulated CYP1 gene transcripts in a rat model of mammary tumorigenesis [J]. Breast Cancer Research and Treatment, 2000, 63(2): 117-131
[23] 谢昕岑, 于淼, 汝少国. 基于AhR信号通路的抗雌激素效应及其机制研究进展[J]. 生态毒理学报, 2021, 16(5): 148-159
Xie X C, Yu M, Ru S G. Research advances of anti-estrogenic effect based on AhR signaling pathway and its mechanism [J]. Asian Journal of Ecotoxicology, 2021, 16(5): 148-159 (in Chinese)
[24] Ahmed S, Valen E, Sandelin A, et al. Dioxin increases the interaction between aryl hydrocarbon receptor and estrogen receptor alpha at human promoters [J]. Toxicological Sciences, 2009, 111(2): 254-266
[25] Shanle E K, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: Identification and mechanisms of action [J]. Chemical Research in Toxicology, 2011, 24(1): 6-19
[26] Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action [J]. Chemical Research in Toxicology, 2003, 16(7): 807-816
[27] Beischlag T V, Luis Morales J, Hollingshead B D, et al. The aryl hydrocarbon receptor complex and the control of gene expression [J]. Critical Reviews in Eukaryotic Gene Expression, 2008, 18(3): 207-250
[28] 张婉君, 范瑞祺, 黄超, 等. 有害结局路径在农药风险评估及管理中应用的探讨[J]. 生态毒理学报, 2021, 16(6): 60-69
Zhang W J, Fan R Q, Huang C, et al. Discussion on application of adverse outcome pathway in pesticides risk assessment and management [J]. Asian Journal of Ecotoxicology, 2021, 16(6): 60-69 (in Chinese)
[29] 李子璇, 杨雪葳, 任利翔, 等. 有机氯农药介导的内分泌干扰相关不良结局通路(AOP)的研究进展[J]. 农药, 2021, 60(10): 703-711
Li Z X, Yang X W, Ren L X, et al. Advances in studies on endocrine disruption-related adverse outcome pathways (AOP) mediated by organochlorine pesticides [J]. Agrochemicals, 2021, 60(10): 703-711 (in Chinese)
[30] Xu T, Hu X X, Yang G L, et al. HIF-1alpha/VEGF pathway mediates 1,3,6,8-tetrabromo-9 H-carbazole-induced angiogenesis: A potential vascular toxicity of an emerging contaminant [J]. Journal of Hazardous Materials, 2022, 432: 128718
[31] Prest S J, May F E B, Westley B R. The estrogen-regulated protein, TFF1, stimulates migration of human breast cancer cells [J]. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 2002, 16(6): 592-594
[32] Pelden S, Insawang T, Thuwajit C, et al. The trefoil factor 1 (TFF1) protein involved in doxorubicin-induced apoptosis resistance is upregulated by estrogen in breast cancer cells [J]. Oncology Reports, 2013, 30(3): 1518-1526
[33] Do M T, Kim H G, Tran T T, et al. Metformin suppresses CYP1A1 and CYP1B1 expression in breast cancer cells by down-regulating aryl hydrocarbon receptor expression [J]. Toxicology and Applied Pharmacology, 2014, 280(1): 138-148
[34] Kerzee J K, Ramos K S. Constitutive and inducible expression of cyp1a1 and cyp1b1 in vascular smooth muscle cells [J]. Circulation Research, 2001, 89(7): 573-582
[35] Hindiyeh M Y, Moran-Gilad J, Manor Y, et al. Development and validation of a real time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) assay for investigation of wild poliovirus type 1-South Asian (SOAS) strain reintroduced into Israel, 2013 to 2014 [J]. Euro Surveillance, 2014, 19(7): 20710
[36] Liu H, Wormke M, Safe S H, et al. Indolo[3, 2-b]carbazole: A dietary-derived factor that exhibits both antiestrogenic and estrogenic activity [J]. Journal of the National Cancer Institute, 1994, 86(23): 1758-1765
[37] Liu S X, Abdelrahim M, Khan S, et al. Aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha in MCF-7 breast cancer cells [J]. Biological Chemistry, 2006, 387(9): 1209-1213
[38] Gong P, Madak-Erdogan Z, Flaws J A, et al. Estrogen receptor-α and aryl hydrocarbon receptor involvement in the actions of botanical estrogens in target cells [J]. Molecular and Cellular Endocrinology, 2016, 437: 190-200
[39] 孙佳琦, 张甘霖, 于明薇, 等. 植物雌激素与乳腺癌发病发展的研究进展[J]. 现代生物医学进展, 2016, 16(12): 2368-2371
Sun J Q, Zhang G L, Yu M W, et al. Research progress of phytoestrogen in incidence and development of breast cancer [J]. Progress in Modern Biomedicine, 2016, 16(12): 2368-2371 (in Chinese)
[40] Sadikovic B, Rodenhiser D I. Benzopyrene exposure disrupts DNA methylation and growth dynamics in breast cancer cells [J]. Toxicology and Applied Pharmacology, 2006, 216(3): 458-468
[41] Brunnberg S, Pettersson K, Rydin E, et al. The basic helix-loop-helix-PAS protein ARNT functions as a potent coactivator of estrogen receptor-dependent transcription [J]. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(11): 6517-6522
[42] Abdelrahim M, Smith R 3rd, Safe S. Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ah-responsiveness in MCF-7 and HepG2 cancer cells [J]. Molecular Pharmacology, 2003, 63(6): 1373-1381
[43] Yip C H, Rhodes A. Estrogen and progesterone receptors in breast cancer [J]. Future Oncology, 2014, 10(14): 2293-2301
[44] Saito R, Miki Y, Hata S, et al. Aryl hydrocarbon receptor induced intratumoral aromatase in breast cancer [J]. Breast Cancer Research and Treatment, 2017, 161(3): 399-407