为了预防水产动物病害,抗生素在集约化水产养殖过程中被普遍施用。养殖规模的增大也导致水产品中细菌性疾病尤其是在密集型的水产养殖体系中的发病率极高,由此导致了抗生素的过量使用,大部分抗生素流入河流湖泊等水体,最终汇入到中国近海。有研究表明,人类社会中大量使用抗生素和耐药菌的频繁出现之间存在明显的联系[1],例如随着抗结核杆菌药物的不断增多,耐药结核菌在世界范围内广泛传播[2]。长期在饲料中添加抗生素会导致养殖水产品体内诱导产生抗性菌株及抗生素抗性基因(antibiotic resistance genes, ARGs)。水产养殖环境已经是世界公认的ARGs储存库,水环境压力是一个特殊的环境特征,抗生素会随着水流流向各地,极大地促进ARGs的水平基因转移和物质交换。相比于环境中残留的抗生素,环境中ARGs的持续残留、菌群间的转移传播更会加重生态环境的污染。2013—2018年,我国22种常用的主要抗生素原料药产量维持在18~20万t,一半以上用于兽用饲料[3]。2019年,世界卫生组织将抗生素耐药性列为全球卫生十大威胁之一[4],并且预测,除非抗生素研究取得进展,否则到2050年,耐药性感染可能导致全球多达1 000万人死亡[5]。除了抗生素,重金属也具有抗菌特性,它们的抗菌效果使它们可以像抗生素一样发挥作用。因此,这些重金属元素也可能在选择耐药细菌中发挥直接或间接作用而导致类似的耐药问题。水产养殖环境中大量投放含重金属的促生长剂和杀菌剂已经导致大面积的水体污染,我国有研究人员调查了辽东湾[6]、北黄海[7]、杭州湾[8]和北部湾[9]水体重金属污染状况,分别发现铅、镉和汞等重金属含量超标严重。重金属具有高毒性、不可降解、易于生物积累等问题。沉积物是水体中重金属的主要存储介质,水环境中的重金属通过吸附和积累悬浮的细粒度颗粒而转移并聚集在沉积物中,造成严重污染。同时,重金属与抗生素也会产生复合耐药机制,驱动ARGs的产生和传播[10]。
基于此,本文介绍了水产养殖环境中重金属和抗生素的污染现状,比较了不同来源养殖水中ARGs的赋存情况,并且详细介绍了重金属对抗生素活性和细菌耐药性的影响,讨论了重金属和抗生素在这一过程中的一些潜在的共选择机制,以便更好地了解和评价水产养殖环境中ARGs的存在和散布风险。
1 水产养殖环境中抗生素、ARGs及重金属污染现状(Current situation of antibiotics, ARGs and heavy metals pollution in aquaculture environment)
抗生素是用于人类治疗的最成功的药物之一,由于它们可以抑制微生物种群生长,也被视为重要的污染物。除了用于人类治疗外,抗生素还广泛用于农林畜牧业和水产养殖业[11]。在渔业养殖过程中常使用的抗生素主要有五大类:磺胺类、四环素类、喹诺酮类、大环内脂类和β-内酰胺类药物[12]。抗生素在自然环境中释放的最明显的后果是耐药菌的出现[13]和抗性基因的传播,对于后者鉴于它们不是“可降解的污染物”,而是自动复制的元素,因此污染情况更为复杂。Chen等[14]调查了我国13个东南沿海养殖地区的抗生素、抗性基因污染现状及风险,结果显示海水和沉积物中存在11种常用抗生素和9种ARGs,其中磺胺类含量水平最高。有学者在西北地区最大的鲟鱼养殖场内陆湖瀛湖中检出8类共59种ARGs,并且养殖水域的ARGs相对丰度远高于上游非养殖型水域[15]。国内外研究发现,ARGs在全球范围内的水产品中广泛存在,由早期的四环素(TC)抗性基因(tetD、tetB、tetA、tetG)和氯霉素(CHL)抗性基因(catIV、catII)[16-18],到后期的的喹诺酮(CIP)类(qnrS5)和万古霉素(VAN)抗性基因(vanB)[19-20],ARGs种类不断增加,污染程度逐渐加重。表1统计了几个地区水产养殖环境中ARGs的污染情况,结果显示ARGs已具有一定的复杂性,不同地区ARGs赋存情况有很大差异且与水产品中ARGs息息相关。
表1 水产养殖环境沉积物、生物体及水体中抗生素抗性基因(ARGs)赋存情况
Table 1 Occurrence of antibiotic resistance genes (ARGs) in sediments, organisms
and water bodies in aquaculture environment
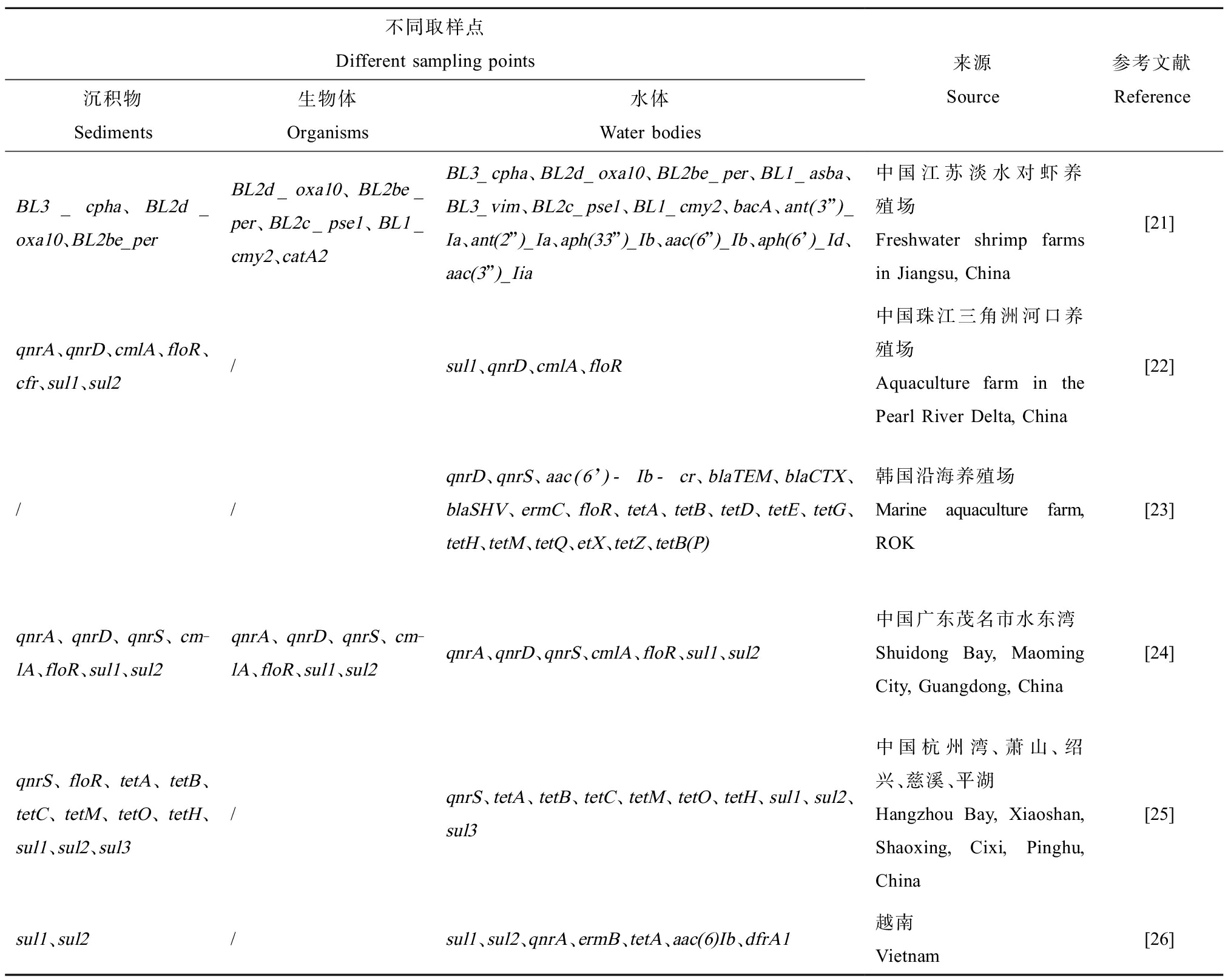
不同取样点Different sampling points沉积物Sediments生物体Organisms水体Water bodies来源Source参考文献ReferenceBL3_cpha、BL2d_oxa10、BL2be_perBL2d_oxa10、BL2be_per、BL2c_pse1、BL1_cmy2、catA2BL3_cpha、BL2d_oxa10、BL2be_per、BL1_asba、BL3_vim、BL2c_pse1、BL1_cmy2、bacA、ant(3”)_Ia、ant(2”)_Ia、aph(33”)_Ib、aac(6”)_Ib、aph(6’)_Id、aac(3”)_Iia中国江苏淡水对虾养殖场Freshwater shrimp farms in Jiangsu, China[21]qnrA、qnrD、cmlA、floR、cfr、sul1、sul2/sul1、qnrD、cmlA、floR中国珠江三角洲河口养殖场Aquaculture farm in the Pearl River Delta, China[22]//qnrD、qnrS、aac(6’)-Ib-cr、blaTEM、blaCTX、blaSHV、ermC、floR、tetA、tetB、tetD、tetE、tetG、tetH、tetM、tetQ、etX、tetZ、tetB(P)韩国沿海养殖场Marine aquaculture farm, ROK[23]qnrA、qnrD、qnrS、cm-lA、floR、sul1、sul2qnrA、qnrD、qnrS、cm-lA、floR、sul1、sul2qnrA、qnrD、qnrS、cmlA、floR、sul1、sul2中国广东茂名市水东湾Shuidong Bay, Maoming City, Guangdong, China[24]qnrS、floR、tetA、tetB、tetC、tetM、tetO、tetH、sul1、sul2、sul3/qnrS、tetA、tetB、tetC、tetM、tetO、tetH、sul1、sul2、sul3中国杭州湾、萧山、绍兴、慈溪、平湖Hangzhou Bay, Xiaoshan, Shaoxing, Cixi, Pinghu, China[25]sul1、sul2/sul1、sul2、qnrA、ermB、tetA、aac(6)Ib、dfrA1越南Vietnam[26]
和抗生素一样,重金属也广泛存在于不同生态系统中。且其化学性质稳定,不易降解,一些细菌可能因为重金属的毒性而死亡,同时有些细菌能够对重金属毒性产生耐受,并能够稳定遗传。它扰乱细菌生长代谢,激活菌体金属保护应激反应和生长状态,也可导致细菌对抗生素的耐药性。水产养殖水中的重金属主要由外源性污水引入以及内源性养殖方式引入两部分组成。外源性污水主要包括生活污水和工业污水两部分,动物粪便、农业径流或采矿副产品沉积物污染的土壤,都会随污水排入水产养殖区。内源性养殖方式引入方面,尽管抗生素制剂已经被用于养殖业,抗菌金属铜(Cu)、锌(Zn)、镉(Cd)和砷(As)等和其他无机化合物也依然在用于治疗水产动植物疾病。以海水养殖为例:2019年我国海水养殖产量为2 065.33万t,占全部养殖水产品产量的40.7%。其中大部分为滩涂或近海养殖[27]。而仅在2019年我国共向近海排放污水中就含六价铬(Cr6+)(2 217.3 kg)、铅(Pb2+)(6 910.5 kg)、汞(Hg2+)(270.2 kg)、镉(Cd2+)(339.2 kg)。其中,Cr6+、Pb2+在黄海海域排放最多,Hg2+在东海海域排放最多,Cd2+在南海海域排放最多。虽然测试结果受季节等因素变换有些许变化浮动,但也能部分反映这些海域重金属污染状况。重金属超标情况也在各个国家水产养殖领域中引起了极大的关注,例如,孟加拉国西南部沿海地区人工养殖虾池水和沉积物中总砷(总As)和硒(Se4+)检出浓度较高[28],而位于埃及的养殖水样中Cd2+、Cu2+和Zn2+重金属严重超标[29]。针对我国几个主要沿海地区水产品中重金属含量超标检出率情况的研究中[30-36],发现不同区域超标的重金属种类不同,Cr6+在广东沿海检出率较高,总As在杭州湾和渤海地区检出率较高,Pb2+在吉林和广东检出率较高,Cd2+则在吉林、山东和福建沿海检出率较高。有学者以山东、江苏、安徽、浙江、上海和福建等地区养殖池塘作为研究对象,发现有近一半的养殖池塘样点Cu2+和Pb2+超出我国国家标准,并且Cd2+污染最为严重,严重超出国家标准[37]。还有研究发现池塘养殖水Cu2+、Zn2+含量不仅存在安全风险,还会随着养殖时间的增加而增高[38]。
2 水产养殖环境中重金属对抗生素活性的影响(The effect of heavy metals on the activity of antibiotics in aquaculture environment)
重金属离子与有机物中含硫、氮和氧官能团的高亲和力结合会导致有机物的失活和损伤。大多数抗生素含有富电子基团,当其与重金属离子共存时,可作为电子供体与金属离子络合形成络合物,这可能会阻碍或增强抗生素的活性。重金属和抗生素络合形成的复杂化合物和叠加效应可能会影响水生环境中的生物。CIP[39]和四环素类[40](TCs),氯四环素(CTC)和氧四环素(OTC)抗生素含有羧基、碳基或哌嗪基(图1)。它们充当潜在的电子供体来协调金属。这些配体抗生素可以通过络合物结合多种金属。这种相互作用虽然减少了环境中抗生素和重金属的总量,但形成了被认为是新兴污染物的复合物。抗生素和重金属的复合物经常出现在天然水中被检测到[41]。
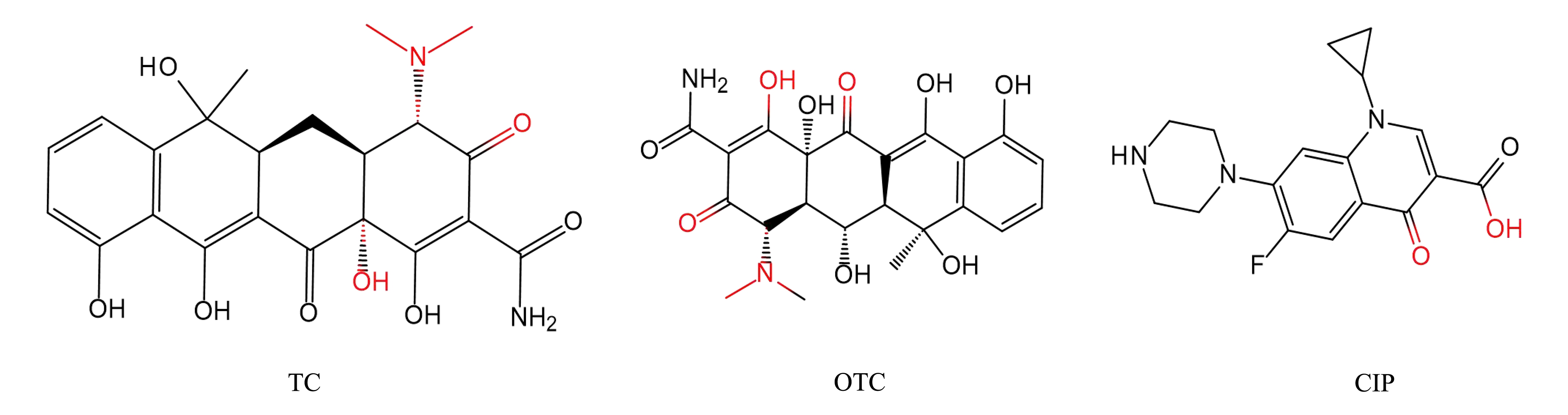
图1 不同抗生素与重金属的常见络合位点(红色基团)
注:TC表示四环素,OTC表示氧四环素,CIP表示环丙沙星。
Fig. 1 Common complexing sites of different antibiotics and heavy metals (red group)
Note: TC is tetracycline, OTC is oxytetracycline, and CIP is ciprofloxacin.
重金属和抗生素形成的络合物一般有1∶1、1∶2和1∶3共3种。一些重金属很容易与CIP和OTC[42]形成稳定的络合物。Zn2+可以与VAN[43]、CIP[44]以及β-内酰胺类[45]抗生素结合并且促进其水解失活。金属离子和抗生素形成的络合物一般和抗生素官能团,金属离子类型和含量以及水环境的影响有关。Cu2+和Al3+都能形成与TC的络合物,并且当Cu/TC物质的量比增加到1以上时,TC与Cu2+的络合位点将由BCD环转变为A环[46]。Zn2+还可以与VAN形成络合物,降低环境中Zn2+含量。Brillault等[47]测定了CIP与金属离子的表观,金属得电子能力顺序与金属的络合能力基本一致[48]。其中,Zn2+和Al3+的不一致可能与其离子半径有关,由于两者的得电子能力相差不大,而Al3+较小的半径减少了其络合时的空间位阻,导致其具有更大络合能力。除此之外,在不同pH的水环境条件下,Cu2+可与CIP形成不同的络合物。这些络合物通常比单独的金属离子或抗生素具有更高的毒性,对藻类的生长有更强的抑制作用,例如Cu2+和CTC的共存[49]就可以形成对蛋白核小球藻有毒性的复合体。
除了络合作用外,重金属作为有毒实体,也可以帮助抗生素杀死细菌,抗生素和重金属的叠加效应也对水环境中的生物产生影响。此外,金属的存在增加了喹诺酮和四环素抗生素与RNA靶点的亲和力,从而提高抗生素活性[50]。OTC和Cu2+的同时存在[51]对土壤微生物群落的功能多样性有显著的负面影响。当Pb2+与磺胺类抗生素共存时,对发光菌的急性毒性急剧上升[52]。全霉素显示强烈的锌依赖活性,只有在Zn2+存在的环境中才能发挥最大作用[53]。另外,Zn2+与妥布霉素(TO)、头孢他啶(CAZ)或CIP这3种抗生素共存时,可以增强抗生素的抗菌作用[54]。因此,虽然环境中的重金属可以对抗生素的活性和细菌对抗生素的敏感性产生不利的影响,但同时这些金属也可以对抗生素产生协同增效的作用。
3 水产养殖环境中重金属对细菌耐药性的影响(The effect of heavy metals on bacterial antibiotic resistance in aquaculture environment)
低浓度的金属阳离子作为某些金属蛋白的必需成分,虽然对正常细菌细胞功能至关重要,但在高水平时是有毒的。甚至,在更高的水平上,这些金属为耐药性提供了选择压力,这反过来又会由于金属之间的遗传和生理联系而驱动对抗生素的耐药性。关于重金属及其耐药性产生的情况在某些方面与抗生素污染相似[55]。此外,对有毒重金属和金属类物质的抗性基因(metal resistance genes, MRGs)也被认为是很古老的[56]。
重金属与环境细菌中抗生素耐药性的发展密切相关。许多研究表明环境中重金属的存在,会导致ARGs和耐药菌的丰度增加。Zhang等[57]的研究表明,0.2~1 mg·L-1三价砷作用6 h后,耐药菌即可快速实现共选择,同时耐药基因和可移动遗传元件(mobile genetic elements, MGEs)的相对丰度增加。Zhou等[58]通过对广东省3个鸭鱼养殖场的土壤、水和沉积物样本进行分析,发现Cu2+和Zn2+与多种ARGs类型有显著相关性,并证明了Cu2+和Zn2+与特定的ARGs的丰度呈现正相关。Li等[59]通过试验表明,重金属富集对微生物群落、ARGs和MRGs有显著影响。重金属也可以促进抗性质粒的水平转移而促进ARGs扩散,Lu等[60]通过实验证明,Ag+促进了质粒携带的ARGs在细菌属间的偶联转移,并且在浓度为1 μg·L-1和10 μg·L-1时的转移频率最大,不是完全的正相关关系。Devarajan等[61]在日内瓦湖不同地区的沉积物剖面中,使用定量PCR对样品中ARGs(blaTEM、blaSHV、blaCTX-M、blaNDM和aadA)进行鉴定同时发现粪便指示菌和假单胞菌属等指示细菌的丰度与沉积物中有机物和金属浓度密切相关,并且在湖泊富营养化后受污水处理厂污染的沉积物中,鉴定出丰富的ARGs。也有研究表明,即使环境中不含抗生素,依然存在ARGs的产生和转移,这与重金属有着密切联系[62],这也可以解释细菌耐药表型与基因型大部分情况下不对应的现象。此外,由重金属促进的细菌总生理状态的变化而导致抗生素耐药性的现象也已被发现。如图2所示,生物膜是将细菌嵌入在聚合物基质中表面的附着结构,细菌产生生物膜后会引起对抗生素抗性的增强[63],例如Cd2+可以增加链球菌生物膜形成能力,从而增强耐药性[64]。此外,双组分信号系统是一种跨膜信号转导系统,与多种细菌耐药性的形成密切相关[65]。在重金属刺激下,它可以通过介导细菌体内各种生理途径适应环境、感应体内外变化,从而在调控细菌耐药方面发挥重要作用[66]。
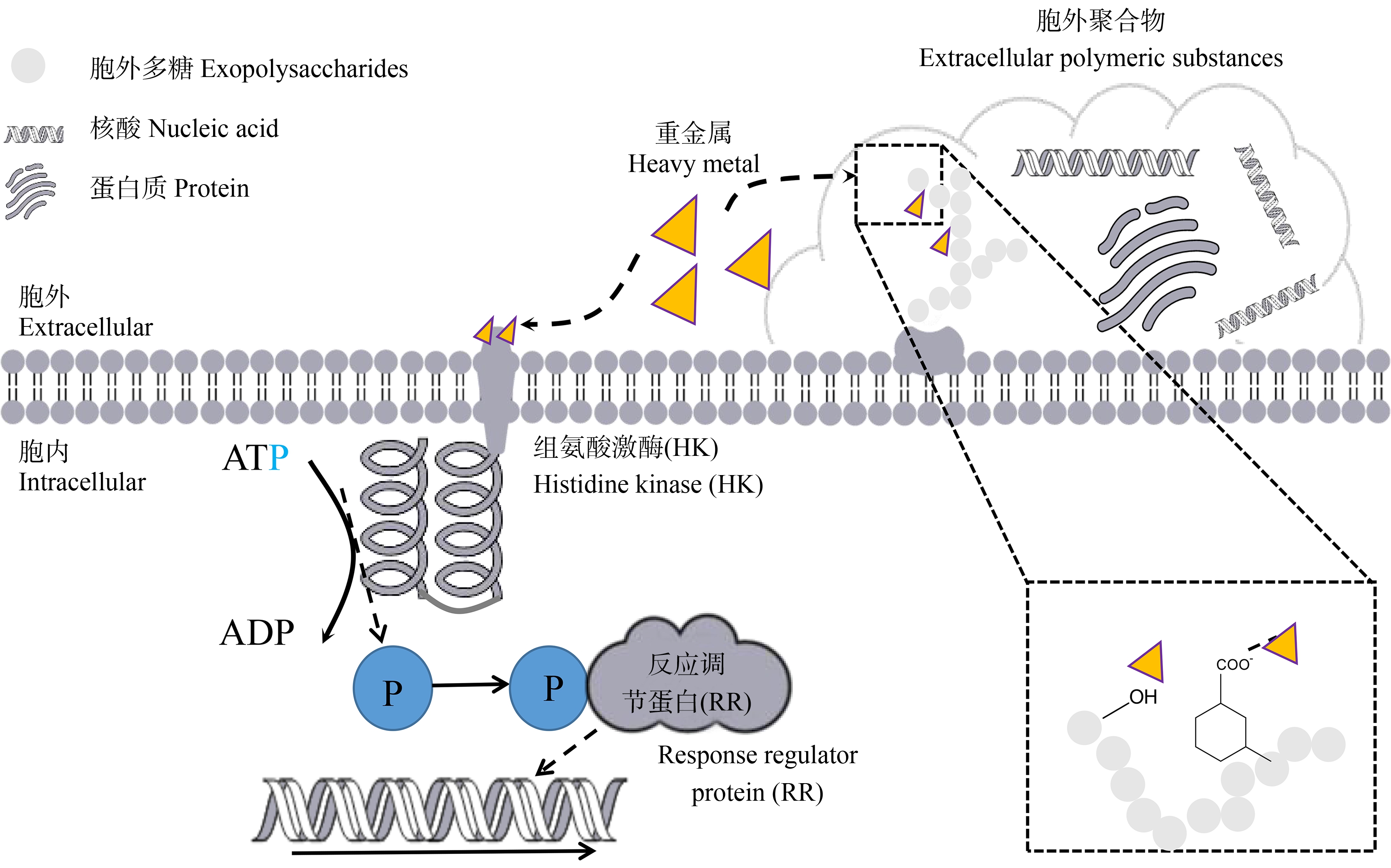
图2 双组分系统和生物膜示意图
Fig. 2 Schematic diagram of two-component system and biofilm
总之,环境中的重金属是常见的细菌应激源,它们为环境菌株的抗生素耐药性提供选择性压力。并且,重金属胁迫会以多种方式影响基因表达和细菌细胞生理结构,从而增强抗生素耐药性。严重的水体污染让水产品逐渐成为ARGs的储存库和传播扩散的载体,以至于严重威胁了人类健康以及妨碍了相关疾病的治疗。同样,有机体存在的环境反过来也可以影响抗生素的活性和细菌对抗生素的敏感性,了解环境因素的影响也是预测生物体内抗生素效果的关键。水环境由于其促进适应性和保护反应,通过改变细菌细胞生理条件也会影响细菌对抗生素的敏感性[67]。已经有研究表明,营养物质、重金属和细菌群落都可能直接或间接地促进了海水养殖区中ARGs的传播[68]。
4 水产养殖环境中重金属与抗生素耐药性的共选择(Co-selection of resistance to heavy metals and antibiotics in aquaculture environment)
细菌产生耐药性的方式主要有固有抗性和获得抗性2种。固有抗性是指细菌本身就对抗生素具有一定的抗性,其染色体上含有抗性基因,并可以在亲子代之间进行遗传。获得抗性是指细菌本身对某些抗生素敏感,但是经过某些环境因素的选择,使细菌获得了含ARGs的质粒等MGEs,或者直接诱导了基因突变、沉默基因表达,从而导致该菌在外形结构和生理、生化等方面发生了变化。同样重金属耐药性是许多暴露于金属环境中的微生物的常见表型。重金属污染的类型和水平与抗生素耐药性的特定模式之间的联系表明,这种共同选择过程背后有多种机制。这些共选择机制基本分为3种:协同抗性、交叉抗性和协同调控[69],如图3所示[70]。
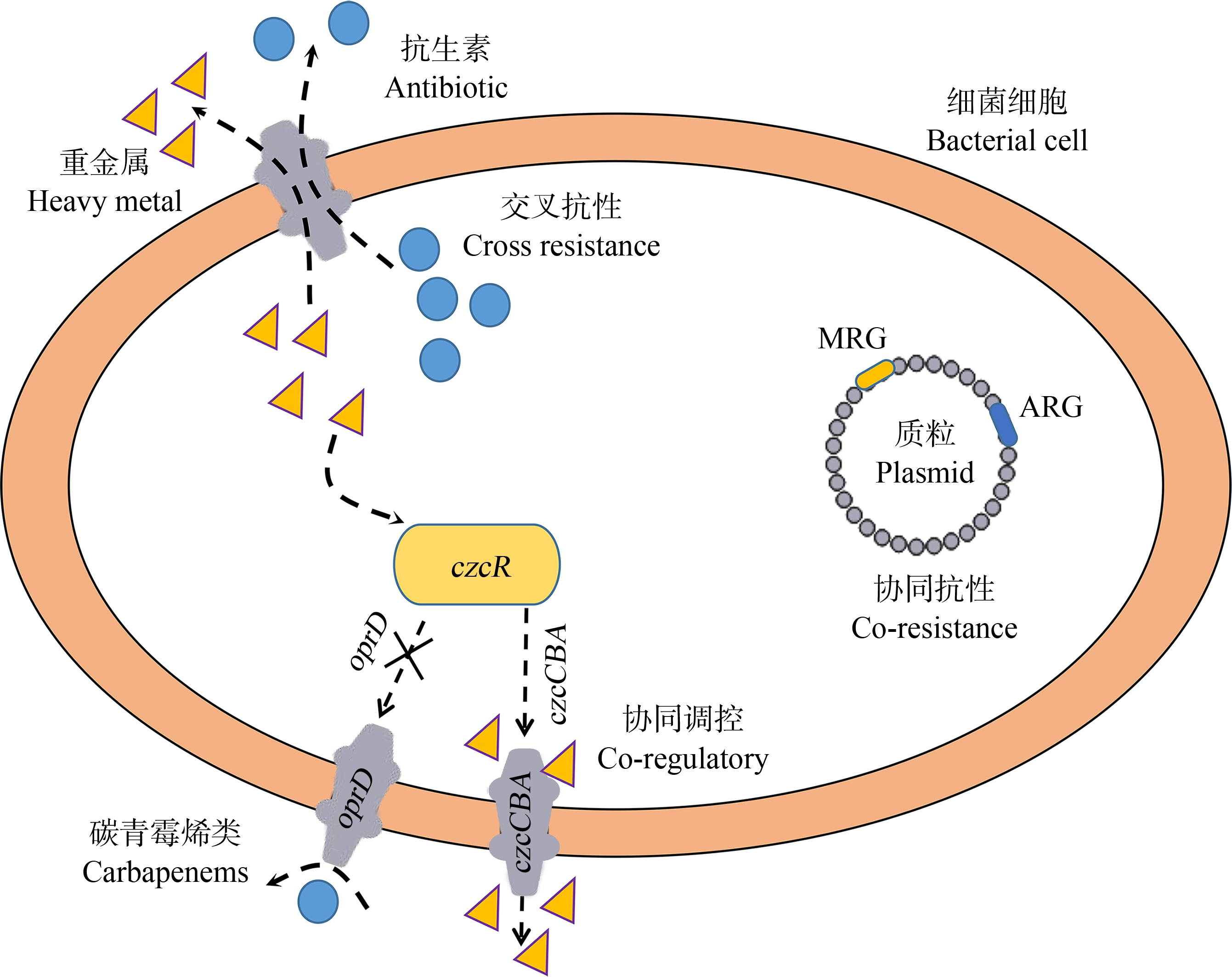
图3 重金属和抗生素耐药的共选择机制[70]
注:MRG表示金属类物质抗性基因,ARG表示抗生素抗性基因。
Fig. 3 Co-selection mechanism of heavy metal and antibiotic resistance[70]
Note: MRG represents metal resistance genes, and ARG represents antibiotic resistance genes.
协同抗性,是多个不同的抗性基因位于同一遗传元件上。在重金属和抗生素污染的环境中耐药菌的富集是通过选择在染色体或质粒上携带的MRGs和ARGs高表达来实现的。黄河底泥中得到的金黄色葡萄球菌,有一存在于质粒上的emrA/B基因同时对氨苄西林(AMP)和Cr6+具有抗性[71]。Hasman和Aarestrup[72]在屎肠杆菌(E. faecium)的同一质粒中发现,Cu2+抗性基因tcrB与编码大环内酯类(erm(B)和糖肽(vanA)的抗性基因相连。同样,对质粒和细菌基因组序列数据库的详细分析揭示了许多MRGs和ARGs共存于一个MGEs上或物理连接在染色体上的实例(表2)。除了质粒之外,其他MGEs,例如整合子和噬菌体[73]也会同时携带MRGs和ARGs。di Cesare等[74]在3个污水处理厂中进行了研究调查,发现了1类整合子与重金属抗性基因和抗生素抗性基因的丰度相关。
表2 细菌中金属和抗生素耐药性的协同抗性
Table 2 Co-resistance of metal and antibiotic resistance in bacteria
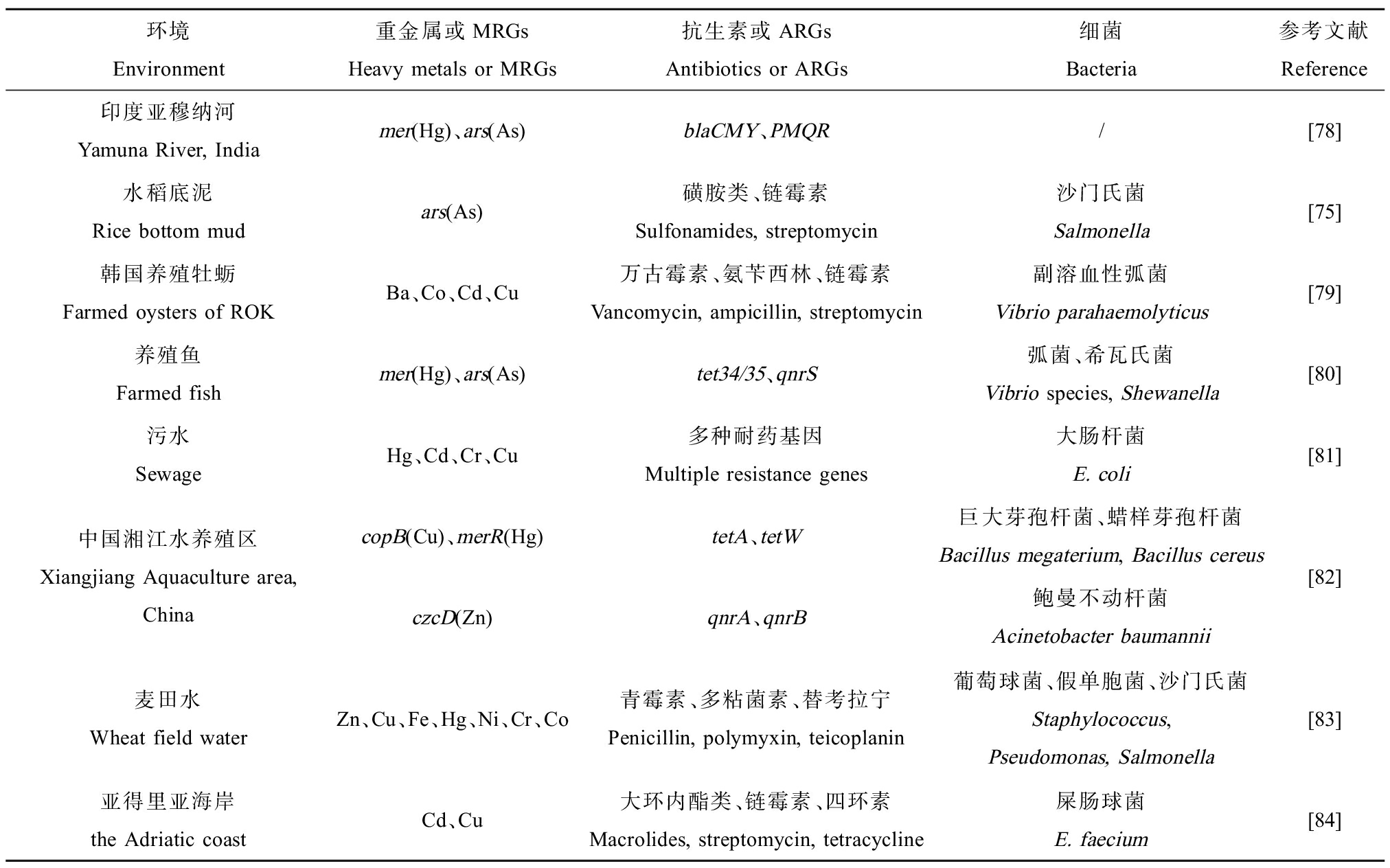
环境Environment重金属或MRGs Heavy metals or MRGs抗生素或ARGsAntibiotics or ARGs细菌Bacteria参考文献Reference印度亚穆纳河Yamuna River, Indiamer(Hg)、ars(As)blaCMY、PMQR/[78]水稻底泥Rice bottom mudars(As)磺胺类、链霉素Sulfonamides, streptomycin沙门氏菌Salmonella[75]韩国养殖牡蛎Farmed oysters of ROKBa、Co、Cd、Cu万古霉素、氨苄西林、链霉素Vancomycin, ampicillin, streptomycin副溶血性弧菌Vibrio parahaemolyticus[79]养殖鱼Farmed fishmer(Hg)、ars(As)tet34/35、qnrS弧菌、希瓦氏菌Vibrio species, Shewanella[80]污水SewageHg、Cd、Cr、Cu多种耐药基因Multiple resistance genes大肠杆菌E. coli[81]中国湘江水养殖区Xiangjiang Aquaculture area, ChinacopB(Cu)、merR(Hg)tetA、tetW巨大芽孢杆菌、蜡样芽孢杆菌Bacillus megaterium, Bacillus cereusczcD(Zn)qnrA、qnrB鲍曼不动杆菌Acinetobacter baumannii[82]麦田水Wheat field waterZn、Cu、Fe、Hg、Ni、Cr、Co青霉素、多粘菌素、替考拉宁Penicillin, polymyxin, teicoplanin葡萄球菌、假单胞菌、沙门氏菌Staphylococcus, Pseudomonas, Salmonella[83]亚得里亚海岸the Adriatic coastCd、Cu大环内酯类、链霉素、四环素Macrolides, streptomycin, tetracycline屎肠球菌E. faecium[84]
当一个遗传决定因素能够同时导致抗生素和重金属耐药性时,就会发生交叉耐药性。例如,tetL、merE和oprD基因对重金属和抗生素同时有耐药性。外排泵系统是常见的交叉抗性系统,但目前除外排泵之外,关于交叉抗性的研究还较少。
协同调控被定义为对细菌对重金属和抗生素暴露形成协调的反应,细菌通过转录翻译等协同调控机制调控ARGs的表达,同时增强菌株的耐药性。Cao等[75]为了验证重金属和抗生素的共同抗性机制比较了在As3+胁迫和非As3+胁迫下的细菌细胞的转录活性。结果显示,在As3+胁迫下,抗生素抗性基因arsD、arsB、arsA和arsC等均有所上调。这些结果表明抗生素抗性基因和抗生素可以被As3+共调控。Berg等[76]研究发现耐铜分离株对CHL和TC的耐药率明显较高。Jo等[77]研究发现,经过重金属(Co2+和Cu2+)预处理后,有7个菌株对卡那霉素(K)、链霉素(S)、TC和庆大霉素(CN)的抗性增强。显然,抗生素和重金属之间存在一个复杂的动态过程。
5 总结与展望(Summary and perspective)
在水产养殖环境中,有机和无机成分的复合污染是普遍共存的。目前抗生素与重金属复合污染问题日益严重,人们对复合污染逐渐重视,然而现在的大多数调查仍然停留在相关污染的研究调查阶段及抗性基因的定性定量分析阶段,目前关于两者的复合污染对细菌耐药的影响及防控研究并不多,需要更多的研究来评估复合污染物对环境中ARGs增殖和传播的影响。例如,目前大多数研究采用的重金属和抗生素浓度均为亚致死浓度和最小抑菌浓度,而抗生素和重金属胁迫浓度持续增加对于ARGs增殖和传播的影响则鲜有研究。另外,其他有毒物质也可能与抗生素耐药性的共同选择和传播有关,这进一步模糊了重金属的作用,因此,需要对金属和抗生素之间复杂的相互关系进行详细的研究,更好地解释复合污染的污染特性和污染机理,以便对抗生素耐药性的持续和扩散有一个连贯和严格的理解。此外,目前对于重金属与抗生素污染物的去除有较多的研究,而复合污染形成的络合物研究较少,复合污染物的处理比单一化学污染物的处理更为困难,未来还应进一步开展针对络合物去除技术的研究,挖掘不同技术对抗生素和重金属复合污染去除的综合手段。
[1] Zhu D, An X L, Chen Q L, et al. Antibiotics disturb the microbiome and increase the incidence of resistance genes in the gut of a common soil collembolan [J]. Environmental Science & Technology, 2018, 52(5): 3081-3090
[2] Shah I, Poojari V, Meshram H. Multi-drug resistant and extensively-drug resistant tuberculosis [J]. Indian Journal of Pediatrics, 2020, 87(10): 833-839
[3] 罗长健, 栗帅, 王琳. 基于WoS文献计量分析的抗生素污染研究进展[J]. 中国社会医学杂志, 2020, 37(4): 433-437
Luo C J, Li S, Wang L. Research progress of antibiotics pollution based on WoS bibliometric analysis [J]. Chinese Journal of Social Medicine, 2020, 37(4): 433-437 (in Chinese)
[4] Roberts S C, Zembower T R. Global increases in antibiotic consumption: A concerning trend for WHO targets [J]. The Lancet Infectious Diseases, 2021, 21(1): 10-11
[5] Clancy C J, Nguyen M H. Buying time: The AMR action fund and the state of antibiotic development in the United States 2020 [J]. Open Forum Infectious Diseases, 2020, 7(11): 464
[6] 宋永刚, 吴金浩, 邵泽伟, 等. 辽东湾近岸表层海水重金属污染分析与评价[J]. 渔业科学进展, 2016, 37(3): 14-19
Song Y G, Wu J H, Shao Z W, et al. Evaluation of heavy metal pollution in the offshore surface seawater of the Liaodong Bay [J]. Progress in Fishery Sciences, 2016, 37(3): 14-19 (in Chinese)
[7] 田琳, 陈洪涛, 杜俊涛, 等. 北黄海表层海水溶解态重金属的分布特征及其影响因素[J]. 中国海洋大学学报(自然科学版), 2009, 39(4): 617-621
Tian L, Chen H T, Du J T, et al. Factors influencing distribution of soluble heavy metals in north Yellow Sea surface seawaters [J]. Periodical of Ocean University of China, 2009, 39(4): 617-621 (in Chinese)
[8] 李娟英, 崔昱, 范清平, 等. 洋山港海域表层海水中重金属及石油烃污染周年监测与评价[J]. 上海海洋大学学报, 2014, 23(1): 95-101
Li J Y, Cui Y, Fan Q P, et al. Annual monitoring and analysis of pollution characters of heavy metal and petroleum hydrocarbon in surface seawater from Yangshan Port [J]. Journal of Shanghai Ocean University, 2014, 23(1): 95-101 (in Chinese)
[9] 莫小荣, 管超毅, 罗良娟, 等. 广西沿海牡蛎养殖区及牡蛎重金属污染研究进展[J]. 钦州学院学报, 2019, 34(3): 8-12, 60
Mo X R, Guan C Y, Luo L J, et al. A study of heavy metal pollution in oyster culture areas and oysters Guangxi [J]. Journal of Qinzhou University, 2019, 34(3): 8-12, 60 (in Chinese)
[10] Li L G, Xia Y, Zhang T. Co-occurrence of antibiotic and metal resistance genes revealed in complete genome collection [J]. The ISME Journal, 2017, 11(3): 651-662
[11] Acharya K P, Wilson R T. Antimicrobial resistance in Nepal [J]. Frontiers in Medicine, 2019, 6((4): 105
[12] 李兆新, 董晓, 孙晓杰, 等. 渔业养殖环境中抗生素残留检测与控制技术研究进展[J]. 食品安全质量检测学报, 2017, 8(7): 2678-2686
Li Z X, Dong X, Sun X J, et al. Advances in the detection and control of antibiotic residues in aquaculture environments [J]. Journal of Food Safety & Quality, 2017, 8(7): 2678-2686 (in Chinese)
[13] Kraemer S A, Ramachandran A, Perron G G. Antibiotic pollution in the environment: From microbial ecology to public policy [J]. Microorganisms, 2019, 7(6): 180
[14] Chen C Q, Zheng L, Zhou J L, et al. Persistence and risk of antibiotic residues and antibiotic resistance genes in major mariculture sites in Southeast China [J]. The Science of the Total Environment, 2017, 580: 1175-1184
[15] Gu J, Zhang L, Wang X J, et al. High-throughput analysis of the effects of different fish culture methods on antibiotic resistance gene abundances in a lake [J]. Environmental Science and Pollution Research International, 2019, 26(6): 5445-5453
[16] Han J E, Kim J H, Cheresca C H Jr, et al. First description of the qnrS-like (qnrS5) gene and analysis of quinolone resistance-determining regions in motile Aeromonas spp. from diseased fish and water [J]. Research in Microbiology, 2012, 163(1): 73-79
[17] Kim M, Kwon T H, Jung S M, et al. Antibiotic resistance of bacteria isolated from the internal organs of edible snow crabs [J]. PLoS One, 2013, 8(8): e70887
[18] 娄阳, 张昭寰, 肖莉莉, 等. 食品源抗生素抗性基因的来源与分布状况研究进展[J]. 食品工业科技, 2015, 36(12): 368-374
Lou Y, Zhang Z H, Xiao L L, et al. Research progress of source and distribution of antibiotic resistance genes in food [J]. Science and Technology of Food Industry, 2015, 36(12): 368-374 (in Chinese)
[19] Gao P P, Mao D, Luo Y, et al. Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment [J]. Water Research, 2012, 46(7): 2355-2364
[20] Hoa P T P, Managaki S, Nakada N, et al. Antibiotic contamination and occurrence of antibiotic-resistant bacteria in aquatic environments of northern Vietnam [J]. Science of the Total Environment, 2011, 409(15): 2894-2901
[21] Zhao Y T, Zhang X, Zhao Z, et al. Metagenomic analysis revealed the prevalence of antibiotic resistance genes in the gut and living environment of freshwater shrimp [J]. Journal of Hazardous Materials, 2018, 350: 10-18
[22] Su H C, Liu S, Hu X J, et al. Occurrence and temporal variation of antibiotic resistance genes (ARGs) in shrimp aquaculture: ARGs dissemination from farming source to reared organisms [J]. The Science of the Total Environment, 2017, 607-608: 357-366
[23] Jang H M, Kim Y B, Choi S, et al. Prevalence of antibiotic resistance genes from effluent of coastal aquaculture, South Korea [J]. Environmental Pollution, 2018, 233: 1049-1057
[24] Su H C, Hu X J, Wang L L, et al. Contamination of antibiotic resistance genes (ARGs) in a typical marine aquaculture farm: Source tracking of ARGs in reared aquatic organisms [J]. Journal of Environmental Science and Health Part B, Pesticides, Food Contaminants, and Agricultural Wastes, 2020, 55(3): 220-229
[25] Yuan J L, Ni M, Liu M, et al. Occurrence of antibiotics and antibiotic resistance genes in a typical estuary aquaculture region of Hangzhou Bay, China [J]. Marine Pollution Bulletin, 2019, 138: 376-384
[26] Pham T T H, Rossi R, Dinh H D K, et al. Analysis of antibiotic multi-resistant bacteria and resistance genes in the effluent of an intensive shrimp farm (Long An, Vietnam) [J]. Journal of Environmental Management, 2018, 214: 149-156
[27] 中国农业农村部渔业渔政管理局, 中国全国水产技术推广总站, 中国水产学会编制. 中国渔业统计年鉴-2020[M]. 北京: 中国农业出版社, 2020: 21-23
[28] Dietrich M, Ayers J. Geochemical partitioning and possible heavy metal(loid) bioaccumulation within aquaculture shrimp ponds [J]. The Science of the Total Environment, 2021, 788: 147777
[29] El Bahgy H E K, Elabd H, Elkorashey R M. Heavy metals bioaccumulation in marine cultured fish and its probabilistic health hazard [J]. Environmental Science and Pollution Research, 2021, 28(30): 41431-41438
[30] 吴烨飞, 陈火荣, 吴镇, 等. 福建省中北部海域捕捞水产品中4种重金属含量与风险评价[J]. 渔业研究, 2018, 40(6): 478-489
Wu Y F, Chen H R, Wu Z, et al. Concentrations and risk evaluation of four kinds of heavy metals in fishing aquatic products of the central and northern sea areas of Fujian Province [J]. Journal of Fisheries Research, 2018, 40(6): 478-489 (in Chinese)
[31] 李响. 吉林省4个城市市售海产品中重金属含量的调查研究[D]. 长春: 吉林大学, 2019: 18-27
Li X. Investigation and study on the content of heavy metals in commercial products of four cities in Jilin Province [D]. Changchun: Jilin University, 2019: 18-27 (in Chinese)
[32] 施沁璇. 钱塘江流域杭州段水产动物中重金属分布特征及安全性评价[D]. 杭州: 浙江大学, 2017: 23-31
[33] 荣飚, 洪华荣. 厦门市售水产品中重金属污染分析与评价[J]. 海峡预防医学杂志, 2015, 21(3): 52-54
[34] 姜兆刚, 闫兆凤. 威海市售海产品重金属污染情况及食用安全性评价[J]. 中国卫生产业, 2019, 16(20): 11-13
Jiang Z G, Yan Z F. Heavy metal pollution and food safety evaluation of seafood sold in Weihai [J]. China Health Industry, 2019, 16(20): 11-13 (in Chinese)
[35] 霍苗苗. 沿海地区居民摄入水产品中重金属安全风险评估[D]. 天津: 天津科技大学, 2016: 17-29
Huo M M. Risk assessment of heavy metals in aquatic products consumption by coastal areas residents [D]. Tianjin: Tianjin University of Science & Technology, 2016: 17-29 (in Chinese)
[36] 谢文平, 陈昆慈, 朱新平, 等. 珠江三角洲河网区水体及鱼体内重金属含量分析与评价[J]. 农业环境科学学报, 2010, 29(10): 1917-1923
Xie W P, Chen K C, Zhu X P, et al. Evaluation on heavy metal contents in water and fishes collected from the waterway in the Pearl River Delta, South China [J]. Journal of Agro-Environment Science, 2010, 29(10): 1917-1923 (in Chinese)
[37] 和庆. 长三角地区池塘养殖水产品重金属和多环芳烃污染评价及其生物有效性研究[D]. 上海: 上海海洋大学, 2018: 24-29
He Q. Pollution evaluation and bioavailability of heavy metals and polycyclic aromatic hydrocarbons in pond aquaculture products in the Yangtze River Delta [D]. Shanghai: Shanghai Ocean University, 2018: 24-29 (in Chinese)
[38] 庞洋洋, 罗伟, 李文红, 等. 淡水养殖池塘表层沉积物中重金属Cu、Zn含量积累及污染分析[J]. 水产科技情报, 2016, 43(1): 45-49
[39] Ferreira M, Gameiro P. Ciprofloxacin metalloantibiotic: An effective antibiotic with an influx route strongly dependent on lipid interaction? [J]. The Journal of Membrane Biology, 2015, 248(1): 125-136
[40] Ma Y, Zhou Q, Zhou S, et al. A bifunctional adsorbent with high surface area and cation exchange property for synergistic removal of tetracycline and Cu2+ [J]. Chemical Engineering Journal, 2014, 258: 26-33
[41] Lu L H, Liu J, Li Z, et al. Antibiotic resistance gene abundances associated with heavy metals and antibiotics in the sediments of Changshou Lake in the three Gorges Reservoir area, China [J]. Ecological Indicators, 2020, 113: 106275
[42] Zhang Y, Cai X, Lang X, et al. Insights into aquatic toxicities of the antibiotics oxytetracycline and ciprofloxacin in the presence of metal: Complexation versus mixture [J]. Environmental Pollution, 2012, 166: 48-56
[43] Zarkan A, Macklyne H R, Truman A W, et al. The frontline antibiotic vancomycin induces a zinc starvation response in bacteria by binding to Zn(Ⅱ) [J]. Scientific Reports, 2016, 6: 19602
[44] Uivarosi V. Metal complexes of quinolone antibiotics and their applications: An update [J]. Molecules, 2013, 18(9): 11153-11197
[45] El-Gamel N E A. Metal chelates of ampicillin versus amoxicillin: Synthesis, structural investigation, and biological studies [J]. Journal of Coordination Chemistry, 2010, 63(3): 534-543
[46] Hsu L C, Liu Y T, Syu C H, et al. Adsorption of tetracycline on Fe (hydr)oxides: Effects of pH and metal cation (Cu2+, Zn2+ and Al3+) addition in various molar ratios [J]. Royal Society Open Science, 2018, 5(3): 171941
[47] Brillault J, Tewes F, Couet W, et al. In vitro biopharmaceutical evaluation of ciprofloxacin/metal cation complexes for pulmonary administration [J]. European Journal of Pharmaceutical Sciences, 2017, 97: 92-98
[48] Pulicharla R, Das R K, Brar S K, et al. Toxicity of chlortetracycline and its metal complexes to model microorganisms in wastewater sludge [J]. The Science of the Total Environment, 2015, 532: 669-675
[49] Lu L, Wu Y X, Ding H J, et al. The combined and second exposure effect of copper (Ⅱ) and chlortetracycline on fresh water algae, Chlorella pyrenoidosa and Microcystis aeruginosa [J]. Environmental Toxicology and Pharmacology, 2015, 40(1): 140-148
[50] 汪晨. 水中典型药物与重金属的络合行为[D]. 南京: 东南大学, 2016: 2-9
Wang C. Complexation behavior of typical pharmaceutical antibiotics and heavy metals in water [D]. Nanjing: Southeast University, 2016: 2-9 (in Chinese)
[51] Jin X, Qiu S S, Wu K, et al. The effect of Cu2+ chelation on the direct photolysis of oxytetracycline: A study assisted by spectroscopy analysis and DFT calculation [J]. Environmental Pollution, 2016, 214: 831-839
[52] 李孟涵, 贺子琪, 苗家赫, 等. 重金属Pb与抗生素对发光菌的联合毒性研究[J]. 农业环境科学学报, 2020, 39(9): 1925-1936
Li M H, He Z Q, Miao J H, et al. Joint toxicity of heavy metal Pb and antibiotics to photobacterium [J]. Journal of Agro-Environment Science, 2020, 39(9): 1925-1936 (in Chinese)
[53] Chan A N, Shiver A L, Wever W J, et al. Role for dithiolopyrrolones in disrupting bacterial metal homeostasis [J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(10): 2717-2722
[54] Bayroodi E, Jalal R. Modulation of antibiotic resistance in Pseudomonas aeruginosa by ZnO nanoparticles [J]. Iranian Journal of Microbiology, 2016, 8(2): 85-92
[55] 邱文婕, 秦艳, 高品. 环境中重金属暴露对抗生素抗性基因演变影响研究进展[J]. 环境工程技术学报, 2021, 11(6): 1226-1231
Qiu W J, Qin Y, Gao P. Research progress on the effect of heavy metal exposure on the evolution of antibiotic resistance genes in the environment [J]. Journal of Environmental Engineering Technology, 2021, 11(6): 1226-1231 (in Chinese)
[56] 王小垒, 李凤姿, 何家乐, 等. 环境微生物抗生素与重金属抗性研究进展[J]. 环境科技, 2019, 32(1): 59-62
Wang X L, Li F Z, He J L, et al. The research progress of environmental microbial antibiotic and heavy metal resistance [J]. Environmental Science and Technology, 2019, 32(1): 59-62 (in Chinese)
[57] Zhang M L, Wan K, Zeng J, et al. Co-selection and stability of bacterial antibiotic resistance by arsenic pollution accidents in source water [J]. Environment International, 2020, 135: 105351
[58] Zhou Q, Wang M Z, Zhong X X, et al. Dissemination of resistance genes in duck/fish polyculture ponds in Guangdong Province: Correlations between Cu and Zn and antibiotic resistance genes [J]. Environmental Science and Pollution Research International, 2019, 26(8): 8182-8193
[59] Li Y Z, Chen H Y, Song L T, et al. Effects on microbiomes and resistomes and the source-specific ecological risks of heavy metals in the sediments of an urban river [J]. Journal of Hazardous Materials, 2021, 409: 124472
[60] Lu J, Wang Y, Jin M, et al. Both silver ions and silver nanoparticles facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes [J]. Water Research, 2020, 169: 115229
[61] Devarajan N, Laffite A, Graham N D, et al. Accumulation of clinically relevant antibiotic-resistance genes, bacterial load, and metals in freshwater lake sediments in Central Europe [J]. Environmental Science & Technology, 2015, 49(11): 6528-6537
[62] Zhong Q M, Cruz-Paredes C, Zhang S R, et al. Can heavy metal pollution induce bacterial resistance to heavy metals and antibiotics in soils from an ancient land-mine? [J]. Journal of Hazardous Materials, 2021, 411: 124962
[63] Kurniawan A, Sukandar, Satriya C, et al. Biofilm as a bioindicator of Cr Ⅵ pollution in the lotic ecosystems [J]. IOP Conference Series: Earth and Environmental Science, 2018, 137: 012062
[64] Xu S Z, Xing Y H, Liu S, et al. Co-effect of minerals and Cd(Ⅱ) promoted the formation of bacterial biofilm and consequently enhanced the sorption of Cd(Ⅱ) [J]. Environmental Pollution, 2020, 258: 113774
[65] 陈朝彦, 秦志丹, 蒋良艳, 等. 双组分系统调控肺炎克雷伯菌碳青霉烯耐药机制的研究进展[J]. 中华危重病急救医学, 2021(6): 761-764
Chen Z Y, Qin Z D, Jiang L Y, et al. Research progress on the mechanism of two-component systems in regulating carbapenem resistance of Klebsiella pneumonia [J]. Chinese Critical Care Medicine, 2021(6): 761-764 (in Chinese)
[66] 张佳奇, 徐艳, 罗义, 等. 重金属协同选择环境细菌抗生素抗性及其机制研究进展[J]. 农业环境科学学报, 2016, 35(3): 409-418
Zhang J Q, Xu Y, Luo Y, et al. Co-selection mechanisms of bacterial resistance to heavy metals and antibiotics [J]. Journal of Agro-Environment Science, 2016, 35(3): 409-418 (in Chinese)
[67] Crabbé A, Ostyn L, Staelens S, et al. Host metabolites stimulate the bacterial proton motive force to enhance the activity of aminoglycoside antibiotics [J]. PLoS Pathogens, 2019, 15(4): e1007697
[68] 朱光平, 薛晨晨, 范宏亮, 等. 环境中抗生素抗性基因研究进展[J]. 预防医学, 2020, 32(11): 1121-1125, 1129
Zhu G P, Xue C C, Fan H L, et al. Research progress of antibiotic resistance genes in environment [J]. Preventive Medicine, 2020, 32(11): 1121-1125, 1129 (in Chinese)
[69] Baker-Austin C, Wright M S, Stepanauskas R, et al. Co-selection of antibiotic and metal resistance [J]. Trends in Microbiology, 2006, 14(4): 176-182
[70] Wang D, Chen W Z, Huang S Q, et al. Structural basis of Zn(Ⅱ) induced metal detoxification and antibiotic resistance by histidine kinase CzcS in Pseudomonas aeruginosa [J]. PLoS Pathogens, 2017, 13(7): e1006533
[71] 张赫. 一株分离自黄河污泥的金黄色葡萄球菌菌株LZ-01的抗生素和重金属共抗性研究[D]. 兰州: 兰州大学, 2016: 8-14
Zhang H. Co-resistance study of a Staphylococcus aureus strain LZ-01 isolated from contaminated sludge of the Yellow River to antibiotics and heavy metals [D]. Lanzhou: Lanzhou University, 2016: 8-14 (in Chinese)
[72] Hasman H, Aarestrup F M. tcrB, a gene conferring transferable copper resistance in Enterococcus faecium: Occurrence, transferability, and linkage to macrolide and glycopeptide resistance [J]. Antimicrobial Agents and Chemotherapy, 2002, 46(5): 1410-1416
[73] Calero-Cáceres W, Méndez J, Martín-Díaz J, et al. The occurrence of antibiotic resistance genes in a Mediterranean River and their persistence in the riverbed sediment [J]. Environmental Pollution, 2017, 223: 384-394
[74] di Cesare A, Eckert E M, D’Urso S, et al. Co-occurrence of integrase 1, antibiotic and heavy metal resistance genes in municipal wastewater treatment plants [J]. Water Research, 2016, 94: 208-214
[75] Cao J Y, Yang G Q, Mai Q J, et al. Co-selection of antibiotic-resistant bacteria in a paddy soil exposed to As(Ⅲ) contamination with an emphasis on potential pathogens [J]. The Science of the Total Environment, 2020, 725: 138367
[76] Berg J, Thorsen M K, Holm P E, et al. Cu exposure under field conditions coselects for antibiotic resistance as determined by a novel cultivation-independent bacterial community tolerance assay [J]. Environmental Science & Technology, 2010, 44(22): 8724-8728
[77] Jo S, Shin C, Shin Y, et al. Heavy metal and antibiotic co-resistance in Vibrio parahaemolyticus isolated from shellfish [J]. Marine Pollution Bulletin, 2020, 156: 111246
[78] Siddiqui M T, Mondal A H, Gogry F A, et al. Plasmid-mediated ampicillin, quinolone, and heavy metal Co-resistance among ESBL-producing isolates from the Yamuna River, New Delhi, India [J]. Antibiotics, 2020, 9(11): 826
[79] Kang C H, Shin Y, Yu H, et al. Antibiotic and heavy-metal resistance of Vibrio parahaemolyticus isolated from oysters in Korea [J]. Marine Pollution Bulletin, 2018, 135: 69-74
[80] Lloyd N A, Nazaret S, Barkay T. Whole genome sequences to assess the link between antibiotic and metal resistance in three coastal marine bacteria isolated from the mummichog gastrointestinal tract [J]. Marine Pollution Bulletin, 2018, 135: 514-520
[81] Mandal M, Das S N, Mandal S. Principal component analysis exploring the association between antibiotic resistance and heavy metal tolerance of plasmid-bearing sewage wastewater bacteria of clinical relevance [J]. Access Microbiology, 2020, 2(3): 95
[82] Xu Y, Xu J, Mao D, et al. Effect of the selective pressure of sub-lethal level of heavy metals on the fate and distribution of ARGs in the catchment scale [J]. Environmental Pollution, 2017, 220: 900-908
[83] Ali N M, Mazhar S A, Mazhar B, et al. Antibacterial activity of different plant extracts and antibiotics on pathogenic bacterial isolates from wheat field water [J]. Pakistan Journal of Pharmaceutical Sciences, 2017, 30(4): 1321-1325
[84] Vignaroli C, Pasquaroli S, Citterio B, et al. Antibiotic and heavy metal resistance in enterococci from coastal marine sediment [J]. Environmental Pollution, 2018, 237: 406-413