自20世纪50年代以来,化学合成农药在全世界农业生产中广泛使用,全球农药产量以每年约11%的速度增长[1],目前在土壤、沉积物、地表水、地下水和海水等环境介质和生物体内均有检出农药残留。越来越多的研究表明环境中的农药污染与生物脂代谢紊乱密切相关[2],脂质在鱼类生长发育中发挥着重要作用,涉及机体的能量供应和储存、生物膜组成等重要的生命过程[3],因而农药类污染物对脂代谢稳态的干扰作用可能是其影响鱼类生存、生长和繁殖的重要原因。本文综述了近10年农药类化合物对鱼类脂代谢的干扰作用及机制,为揭示农药导致脂代谢紊乱的效应及机制提供依据。
1 农药类化合物污染现状(Pollution status of pesticide compounds)
农药按其防治目标生物不同,可以分为杀虫剂(如滴滴涕、六六六、敌百虫和久效磷等)、除草剂(如草甘膦、阿特拉津和扑草净等)、杀菌剂(如三氯生、苯醚甲环唑和溴代吡咯腈等)、杀螺剂(如螺螨酯、螺甲螨酯和螺虫乙酯等)、杀线虫剂(如霜霉威、毒死蜱等)、杀真菌剂(如多菌灵等)和植物生长调节剂等[4]。一般农药施用后直接作用于目标生物的有效利用率很低,约99%的农药会渗透进入环境中[5]。近年来农药类化合物在地表水、地下水、海水和沉积物等各种环境介质中频繁检出,例如,在中国洞庭湖区域的22个地表水样品中,检出滴滴涕的平均浓度为14.20ng·L-1,六六六的平均浓度为26.70 ng·L-1[6];Sumon等[7]对孟加拉国西北部水体的农药残留调查发现,地表水中毒死蜱的检出浓度高达9.10×103ng·L-1。在我国松嫩平原典型农业区的30个地下水样本中,呋喃丹的检出浓度约为1 972 ng·L-1[8]。调查发现西班牙农村和城市地区地下水中的除草剂残留浓度较高,检出阿特拉津的平均浓度为3.45×103ng·L-1,西马嗪的平均浓度为1.69×103ng·L-1,扑草净的平均浓度高于0.5×103ng·L-1[9]。此外,通过土地开垦、土壤侵蚀、暴雨径流、河流输入和沉积,农药污染物最终会进入海洋,而沉积物则成为污染物的最终蓄库[10]。在中国北江水系沉积物中,三氯生的平均检出浓度为7.01 ng·g-1[11];Li等[12]对中国珠江三角洲地区城市水道中沉积物的农药残留进行分析发现,珠江三角洲地区氯氰菊酯的检出浓度最高,为1.44~219 ng·g-1。Yang等[13]对中国黄海和渤海的64个监测站采集的样本进行了除草剂浓度测定,其中阿特拉津、扑草净和特丁净,莠灭津是表层海水中含量最高的4种除草剂,其浓度分别高达720、748.88、296.64和132.48 ng·L-1。表1中总结了下文所涉及的主要农药种类在不同环境介质中的检出浓度。
表1 不同环境介质中农药类化合物的残留水平
Table 1 Residual levels of pesticides in different environmental media
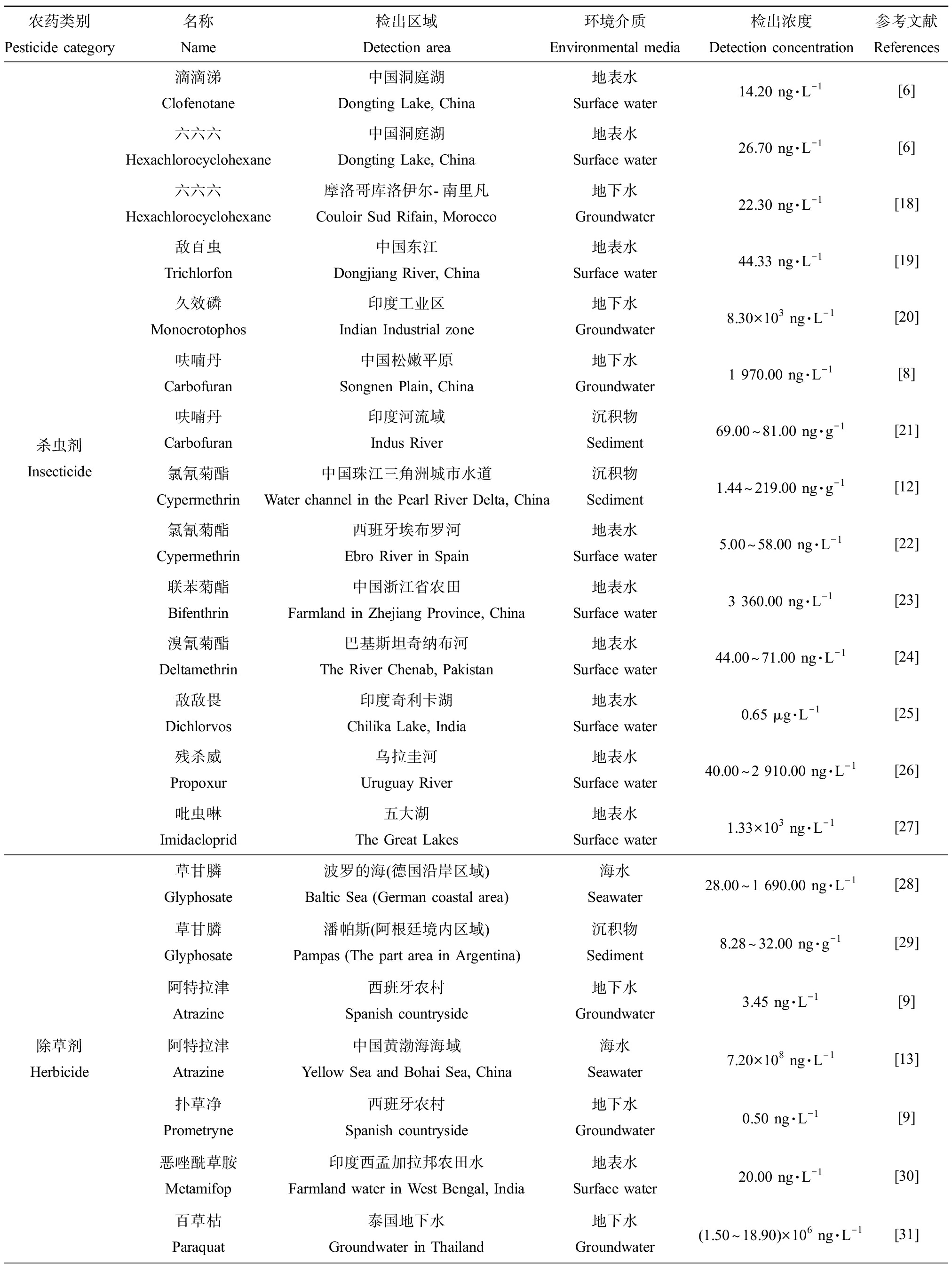
农药类别Pesticide category名称Name检出区域Detection area环境介质Environmental media检出浓度Detection concentration参考文献References杀虫剂Insecticide滴滴涕Clofenotane中国洞庭湖Dongting Lake, China地表水Surface water14.20 ng·L-1[6]六六六Hexachlorocyclohexane中国洞庭湖Dongting Lake, China地表水Surface water26.70 ng·L-1[6]六六六Hexachlorocyclohexane摩洛哥库洛伊尔-南里凡Couloir Sud Rifain, Morocco地下水Groundwater22.30 ng·L-1[18]敌百虫Trichlorfon中国东江Dongjiang River, China地表水Surface water44.33 ng·L-1[19]久效磷Monocrotophos印度工业区Indian Industrial zone地下水Groundwater8.30×103 ng·L-1[20]呋喃丹Carbofuran中国松嫩平原Songnen Plain, China地下水Groundwater1 970.00 ng·L-1[8]呋喃丹Carbofuran印度河流域Indus River沉积物Sediment69.00~81.00 ng·g-1[21]氯氰菊酯Cypermethrin中国珠江三角洲城市水道Water channel in the Pearl River Delta, China沉积物Sediment1.44~219.00 ng·g-1[12]氯氰菊酯Cypermethrin西班牙埃布罗河Ebro River in Spain地表水Surface water5.00~58.00 ng·L-1[22]联苯菊酯Bifenthrin中国浙江省农田Farmland in Zhejiang Province, China地表水Surface water3 360.00 ng·L-1[23]溴氰菊酯Deltamethrin巴基斯坦奇纳布河The River Chenab, Pakistan地表水Surface water44.00~71.00 ng·L-1[24]敌敌畏Dichlorvos印度奇利卡湖Chilika Lake, India地表水Surface water0.65 μg·L-1[25]残杀威Propoxur乌拉圭河Uruguay River地表水Surface water40.00~2 910.00 ng·L-1[26]吡虫啉Imidacloprid五大湖The Great Lakes地表水Surface water1.33×103 ng·L-1[27]除草剂Herbicide草甘膦Glyphosate波罗的海(德国沿岸区域)Baltic Sea (German coastal area)海水Seawater28.00~1 690.00 ng·L-1[28]草甘膦Glyphosate潘帕斯(阿根廷境内区域)Pampas (The part area in Argentina)沉积物Sediment8.28~32.00 ng·g-1[29]阿特拉津Atrazine西班牙农村Spanish countryside地下水Groundwater3.45 ng·L-1[9]阿特拉津Atrazine中国黄渤海海域Yellow Sea and Bohai Sea, China海水Seawater7.20×108 ng·L-1[13]扑草净Prometryne西班牙农村Spanish countryside地下水Groundwater0.50 ng·L-1[9]恶唑酰草胺Metamifop印度西孟加拉邦农田水Farmland water in West Bengal, India地表水Surface water20.00 ng·L-1[30]百草枯Paraquat泰国地下水Groundwater in Thailand地下水Groundwater(1.50~18.90)×106 ng·L-1[31]

续表1农药类别Pesticide category名称Name检出区域Detection area环境介质Environmental media检出浓度Detection concentration参考文献References除草剂Herbicide乙草胺Acetochlor中国广西甘蔗产区Guangxi Sugarcane Production Area, China地表水Surface water88.00 ng·L-1[32]敌草隆Diuron日本獭户内海Seto Inland Sea, Japan海水Seawater65.00 ng·L-1[33]西马津Simazine中国辽东半岛海域Liaodong Peninsula Sea Area, China海水Seawater1.40~5.30 ng·L-1[34]杀菌剂Bactericide三氯生Triclosan中国北江Beijiang River, China沉积物Sediment7.01 ng·g-1[11]苯醚甲环唑Difenoconazole中国九龙河流域Jiulong River, China地表水Surface water3 904.00 ng·L-1[35]溴代吡咯腈Tralopyril葡萄牙阿维罗港Porto Avero, Portugal海水Seawater0.70 ng·L-1[36]丙环唑Propiconazole哥斯达黎加Costa Rica地表水Surface water125.00 ng·L-1[37]三环唑Tricyclazole中国武汉Wuhan, China地表水Surface water4.21~67.90 ng·L-1[38]腐霉利Procymidone中国九龙河流域Jiulong River, China地表水Surface water(0.15~13.00)×103 ng·L-1[39]氟酰胺Flutolanil日本南部区域Southern Japan地表水Surface water30.00~1 640.00 ng·L-1[40]啶酰菌胺Boscalid美国加利福尼亚沿海河口Estuaries off the coast of California, USA沉积物Sediment0.70~88.80 ng·g-1[41]三唑醇Triadimenol中国辽东半岛海域Liaodong Peninsula Sea Area, China海水Seawater4.30~20.80 ng·g-1[34]杀螺剂Molluscide螺螨酯Spirodiclofen萨瓦河沉积物Sava River sediments沉积物Sediment881.00 ng·g-1[42]螺甲螨酯Spiromesifen萨瓦河沉积物Sava River sediments沉积物Sediment1 750.00 ng·g-1[42]螺虫乙酯Spirotetramat萨瓦河沉积物Sava River sediments沉积物Sediment431.00 ng·g-1[42]氯柳硝胺Chlorosalanide中国鄱阳湖地区南麂山岛Nanjishan Island, Poyang Lake Region, China地表水Surface water(1.00~38.00)×103 ng·L-1[43]杀线虫剂Nematicide霜霉威Propamocarb西班牙阿尔梅里亚Almeria, Spain地表水Surface water3.80×106 ng·L-1[44]毒死蜱Chlorpyrifos孟加拉国西北部地区Northwest Bangladesh地表水Surface water9.10×103 ng·L-1[7]杀真菌剂Fungicide多菌灵Carbendazim略布列加河Llobregat River地表水Surface water10.82~697.39 ng·L-1[45]
由于其化学稳定性和亲脂性,农药类化合物往往会在生物组织中积累,并在食物链中逐级放大。据报道,在埃塞俄比亚科卡湖的4种鱼类体内检出滴滴涕、硫丹和毒死蜱等农药,其中滴滴涕的检出浓度最高,为0.05~72.53 ng·g-1[14]。我国学者在一些海洋动物中也检出了三嗪类除草剂,如青岛胶州湾海域无脊椎动物中阿特拉津的平均浓度为0.31 ng·g-1,鱼类中阿特拉津的平均浓度为0.3 ng·g-1[15]。在印度托尔萨河流域的鱼类样本中检测到三氯生的残留,其检出范围为91.1~589 ng·g-1,其中厚唇新条鳅(Neonemacheilus)样本中的检出浓度最低,为91.1 ng·g-1,印度大帆鲫(Oreichthys cosuatis)的检出浓度最高,为589 ng·g-1[16]。Riaz等[17]检测了巴基斯坦奇纳布河中美洲鳗鲡(Anguilla rostrata)体内拟除虫菊酯类杀虫剂的浓度,检出氯氰菊酯、溴氰菊酯、氯菊酯和联苯菊酯的平均浓度分别为472、459、278和251 ng·g-1。
2 农药类化合物对鱼类的脂代谢影响及作用机制(Effects and mechanism of pesticide chemicals on lipid metabolism in fish)
脂质作为重要的营养、能源和结构物质,在生物体内的代谢具有重要意义。脂质是脂肪和类脂的总称,其中脂肪也称甘油三酯(triglyceride, TG),而类脂主要包括磷脂和胆固醇等多种脂质。鱼类的脂代谢稳态是一个复杂的调控过程,源自食物的脂质进入鱼体内,经消化后被肠吸收,在血液中以脂蛋白的形式运输,进而在肝脏等组织中进行代谢,而脂肪组织是脂质蓄积和储存的主要场所[46]。研究发现,很多农药类化合物能够通过干扰鱼体内脂质的消化吸收、合成、分解和转运等一个或多个脂代谢调控过程,进而导致鱼体内脂代谢紊乱、TG等脂质水平异常。表2中总结了下文所涉及的农药类污染物干扰鱼类脂质代谢的机制。
表2 农药类污染物干扰鱼类脂质代谢的机制
Table 2 Mechanisms underlying the disruption of lipid metabolism induced by pesticides in fish
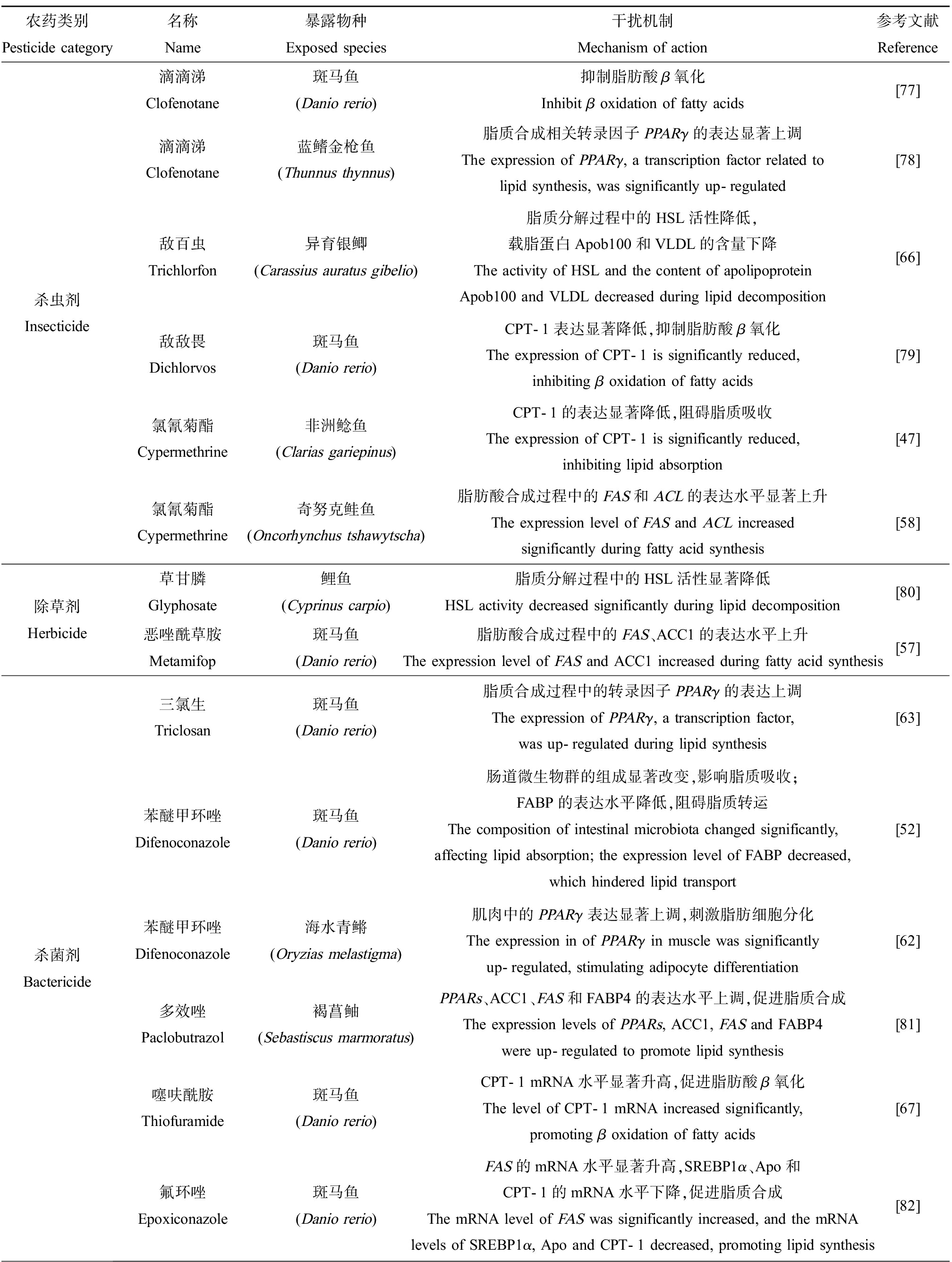
农药类别Pesticide category名称Name暴露物种Exposed species干扰机制Mechanism of action参考文献Reference杀虫剂Insecticide滴滴涕Clofenotane斑马鱼(Danio rerio)抑制脂肪酸β氧化Inhibit β oxidation of fatty acids[77]滴滴涕Clofenotane蓝鳍金枪鱼(Thunnus thynnus)脂质合成相关转录因子PPARγ的表达显著上调The expression of PPARγ, a transcription factor related to lipid synthesis, was significantly up-regulated[78]敌百虫Trichlorfon异育银鲫(Carassius auratus gibelio)脂质分解过程中的HSL活性降低,载脂蛋白Apob100和VLDL的含量下降The activity of HSL and the content of apolipoprotein Apob100 and VLDL decreased during lipid decomposition[66]敌敌畏Dichlorvos斑马鱼(Danio rerio)CPT-1表达显著降低,抑制脂肪酸β氧化The expression of CPT-1 is significantly reduced, inhibiting β oxidation of fatty acids[79]氯氰菊酯Cypermethrine非洲鲶鱼(Clarias gariepinus)CPT-1的表达显著降低,阻碍脂质吸收The expression of CPT-1 is significantly reduced, inhibiting lipid absorption[47]氯氰菊酯Cypermethrine奇努克鲑鱼(Oncorhynchus tshawytscha)脂肪酸合成过程中的FAS和ACL的表达水平显著上升The expression level of FAS and ACL increased significantly during fatty acid synthesis[58]除草剂Herbicide草甘膦Glyphosate鲤鱼(Cyprinus carpio)脂质分解过程中的HSL活性显著降低HSL activity decreased significantly during lipid decomposition[80]恶唑酰草胺Metamifop斑马鱼(Danio rerio)脂肪酸合成过程中的FAS、ACC1的表达水平上升The expression level of FAS and ACC1 increased during fatty acid synthesis[57]杀菌剂Bactericide三氯生Triclosan斑马鱼(Danio rerio)脂质合成过程中的转录因子PPARγ的表达上调The expression of PPARγ, a transcription factor, was up-regulated during lipid synthesis[63]苯醚甲环唑Difenoconazole斑马鱼(Danio rerio)肠道微生物群的组成显著改变,影响脂质吸收;FABP的表达水平降低,阻碍脂质转运The composition of intestinal microbiota changed significantly, affecting lipid absorption; the expression level of FABP decreased, which hindered lipid transport[52]苯醚甲环唑Difenoconazole海水青鳉(Oryzias melastigma)肌肉中的PPARγ表达显著上调,刺激脂肪细胞分化The expression in of PPARγ in muscle was significantly up-regulated, stimulating adipocyte differentiation[62]多效唑Paclobutrazol褐菖鲉(Sebastiscus marmoratus)PPARs、ACC1、FAS和FABP4的表达水平上调,促进脂质合成The expression levels of PPARs, ACC1, FAS and FABP4 were up-regulated to promote lipid synthesis[81]噻呋酰胺Thiofuramide斑马鱼(Danio rerio)CPT-1 mRNA水平显著升高,促进脂肪酸β氧化The level of CPT-1 mRNA increased significantly, promoting β oxidation of fatty acids[67]氟环唑Epoxiconazole斑马鱼(Danio rerio)FAS的mRNA水平显著升高,SREBP1α、Apo和CPT-1的mRNA水平下降,促进脂质合成The mRNA level of FAS was significantly increased, and the mRNA levels of SREBP1α, Apo and CPT-1 decreased, promoting lipid synthesis[82]
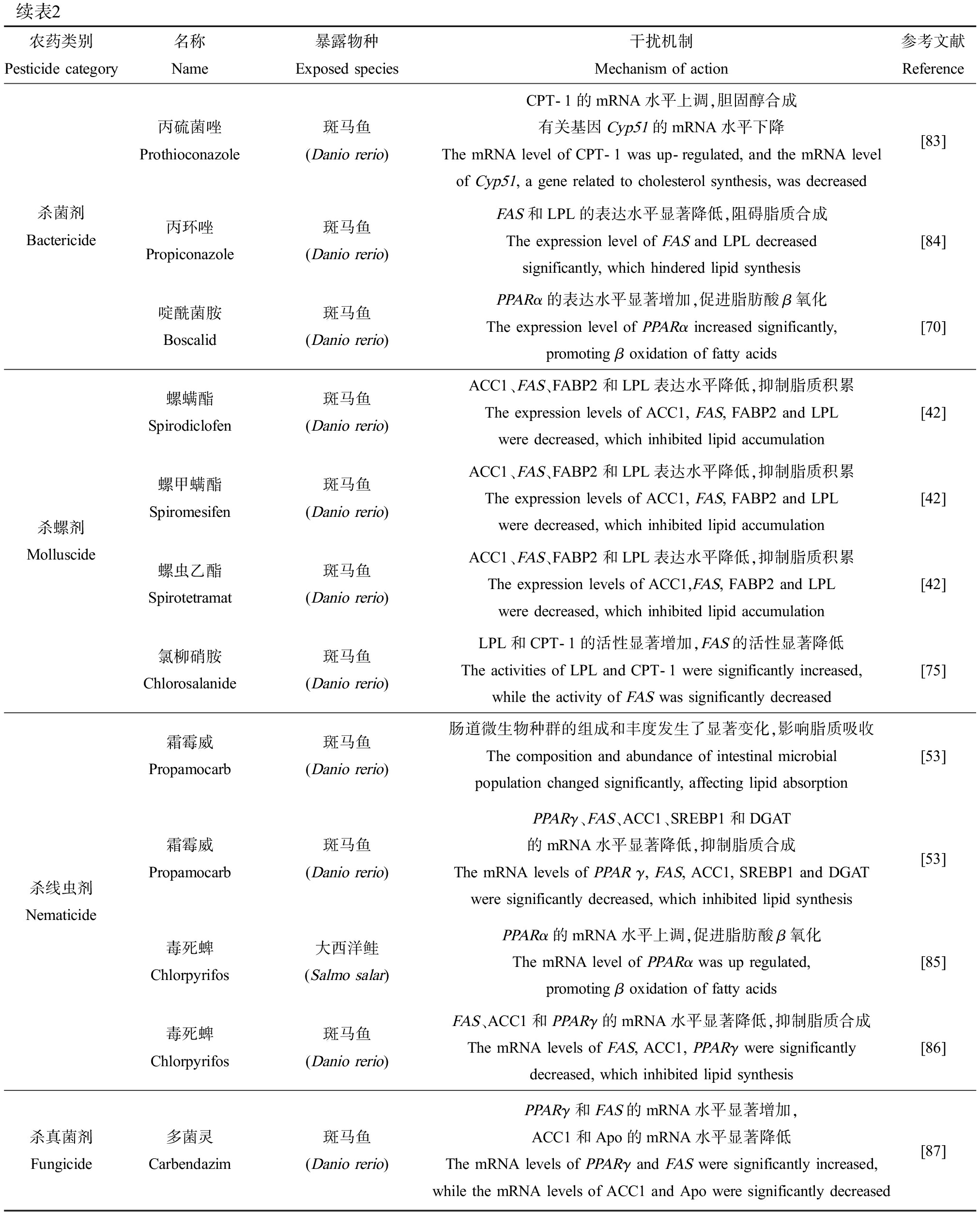
续表2农药类别Pesticide category名称Name暴露物种Exposed species干扰机制Mechanism of action参考文献Reference杀菌剂Bactericide丙硫菌唑Prothioconazole斑马鱼(Danio rerio)CPT-1的mRNA水平上调,胆固醇合成有关基因Cyp51的mRNA水平下降The mRNA level of CPT-1 was up-regulated, and the mRNA level of Cyp51, a gene related to cholesterol synthesis, was decreased[83]丙环唑Propiconazole斑马鱼(Danio rerio)FAS和LPL的表达水平显著降低,阻碍脂质合成The expression level of FAS and LPL decreased significantly, which hindered lipid synthesis[84]啶酰菌胺Boscalid斑马鱼(Danio rerio)PPARα的表达水平显著增加,促进脂肪酸β氧化The expression level of PPARα increased significantly, promoting β oxidation of fatty acids[70]杀螺剂Molluscide螺螨酯Spirodiclofen斑马鱼(Danio rerio)ACC1、FAS、FABP2和LPL表达水平降低,抑制脂质积累The expression levels of ACC1, FAS, FABP2 and LPL were decreased, which inhibited lipid accumulation[42]螺甲螨酯Spiromesifen斑马鱼(Danio rerio)ACC1、FAS、FABP2和LPL表达水平降低,抑制脂质积累The expression levels of ACC1, FAS, FABP2 and LPL were decreased, which inhibited lipid accumulation[42]螺虫乙酯Spirotetramat斑马鱼(Danio rerio)ACC1、FAS、FABP2和LPL表达水平降低,抑制脂质积累The expression levels of ACC1,FAS, FABP2 and LPL were decreased, which inhibited lipid accumulation[42]氯柳硝胺Chlorosalanide斑马鱼(Danio rerio)LPL和CPT-1的活性显著增加,FAS的活性显著降低The activities of LPL and CPT-1 were significantly increased, while the activity of FAS was significantly decreased[75]杀线虫剂Nematicide霜霉威Propamocarb斑马鱼(Danio rerio)肠道微生物种群的组成和丰度发生了显著变化,影响脂质吸收The composition and abundance of intestinal microbial population changed significantly, affecting lipid absorption[53]霜霉威Propamocarb斑马鱼(Danio rerio)PPARγ、FAS、ACC1、SREBP1和DGAT的mRNA水平显著降低,抑制脂质合成The mRNA levels of PPAR γ, FAS, ACC1, SREBP1 and DGAT were significantly decreased, which inhibited lipid synthesis[53]毒死蜱Chlorpyrifos大西洋鲑(Salmo salar)PPARα的mRNA水平上调,促进脂肪酸β氧化The mRNA level of PPARα was up regulated, promoting β oxidation of fatty acids[85]毒死蜱Chlorpyrifos斑马鱼(Danio rerio)FAS、ACC1和PPARγ的mRNA水平显著降低,抑制脂质合成The mRNA levels of FAS, ACC1, PPARγ were significantly decreased, which inhibited lipid synthesis[86]杀真菌剂Fungicide多菌灵Carbendazim斑马鱼(Danio rerio)PPARγ和FAS的mRNA水平显著增加,ACC1和Apo的mRNA水平显著降低The mRNA levels of PPARγ and FAS were significantly increased, while the mRNA levels of ACC1 and Apo were significantly decreased[87]
2.1 干扰鱼类脂质的消化吸收(Disruption of the lipid digestion and absorption in fish)
鱼类消化和吸收脂质的主要场所为肠道。首先,膳食脂质在肠腔内经胰脂肪酶等消化酶水解为游离脂肪酸、单酰甘油和二酰甘油等,然后被胆汁酸盐溶解、乳化形成微胶团(micelle),再被肠上皮细胞吸收。肠道摄取后,长链脂肪酸被重新酯化,然后在脂蛋白的作用下形成乳糜微粒(chylomicron, CM),经由淋巴进入血液循环系统并用于脂质的储存、β氧化或其他机体代谢过程。中短链脂肪酸直接通过门静脉进入血液并参与β氧化,或被转运至其他组织或细胞中发挥相应生理功能。
肠道消化与吸收脂质主要是依靠肠道内的各种分泌物、消化酶等,尤其是肠道消化酶的活性能够直接反映鱼类对脂质的消化吸收水平及利用能力。许多研究表明农药类化合物可以通过影响肠道内消化酶的活性干扰脂质的消化吸收。非洲鲶鱼(Clarias gariepinus)和斑马鱼(Danio rerio)分别暴露于氯氰菊酯(50 μg·L-1和100 μg·L-1)和亚致死浓度(0.025、0.10和0.40 mg·L-1)的恶唑酰草胺后,其肠道内脂肪酶的活性均显著降低,使得脂肪吸收能力受损[47-48]。亚致死浓度(0.75 μg·L-1和7.5 μg·L-1)的三丁基锡暴露幼年鲤鱼(Cyprinus carpio)60 d后,也显著抑制其肠道内的消化酶(胰蛋白酶、脂肪酶)活性[49]。由此可知多种农药类化合物可以通过抑制肠道内消化酶的活性,从而阻碍脂质的消化与吸收过程,进而降低机体内脂质含量。
此外,农药也可以通过影响鱼类的肠道菌群丰度及组成来干扰脂质吸收。有研究表明肠道微生物群与脂质代谢之间存在密切关系,厚壁菌等微生物群可增加肠上皮中的脂质,并影响脂肪吸收[50]。例如,Semova等[51]通过在无菌斑马鱼肠道内定植菌群,发现肠道细菌能够增加宿主肠上皮细胞对脂肪酸的吸收和脂滴数量。肠道菌群的组成受饮食、外部环境以及肠道内环境的影响,肠道菌群组成变化会影响脂肪分布、胰岛素敏感度、能量与脂质代谢等生理功能。Jiang等[52]研究发现,苯醚甲环唑(0.4 mg·L-1)暴露斑马鱼21 d后,斑马鱼肠道微生物群的组成显著改变,西杆菌的相对丰度降低,厚壁菌、肠杆菌和拟杆菌的丰度增加,肝脏内脂质蓄积明显。雄性成年斑马鱼暴露于霜霉威(1 000 μg·L-1)7 d后,其肝脏TG含量显著降低,同时肠道微生物种群的组成和丰度发生了显著变化,在门水平上,α-变形菌、γ-变形菌、拟杆菌和厚壁菌的丰度显著增加,在属水平上,鲸杆菌和希瓦氏菌显著减少,黄杆菌、不动杆菌、巨单胞菌和沉积物杆状菌的丰度显著增加。这些分类群的变化可以减少短链脂肪酸和抗菌肽的产生,从而间接影响肝脏功能和脂质代谢[53]。三苯基锡(100 ng·L-1)暴露海水青鳉(Oryzias melastigma)21 d后,显著改变海水青鳉的肠道微生物群丰度,从而使其肠道微生物多样性显著下降。对暴露组海水青鳉肠道进行代谢组学分析发现其代谢谱发生显著变化,并且差异代谢物主要集中在脂质代谢途径中[54]。因而,农药类化合物可以通过干扰鱼类肠道内菌群的种类与含量进而影响肝脏内中性脂质与脂肪酸的含量。
2.2 干扰鱼类的脂质合成代谢(Disruption of the lipid anabolism in fish)
鱼体内的脂肪来源主要分为2种,一是从食物中摄入的外源性途径,二是在肝脏、脂肪组织中进行的从头合成,也就是内源性合成。脂质合成的主要途径是脂肪酸合成。脂肪酸合成的前体是乙酰辅酶A,首先在乙酰辅酶A羧化酶(acetyl-CoA carboxylase, ACC)的催化作用下羧化生成丙二酰辅酶A,然后乙酰辅酶A和丙二酰辅酶在脂肪酸合成酶(fatty acid synthase, FAS)的多次催化下生成十六碳的饱和脂肪酸——棕榈酸。棕榈酸可在动物肝细胞中的内质网和线粒体内在脂肪酸去饱和酶的作用下进行碳链延长与脂肪酸的脱饱和,从而得到更长碳链的脂肪酸或不饱和脂肪酸。
鱼类的脂质合成过程由多个酶促反应组成,因而研究发现农药类污染物干扰脂质合成过程中FAS、ACC等关键酶或基因的表达是其导致TG等脂质含量异常的重要原因。FAS在长链脂肪酸的从头合成中发挥关键作用,是脂肪合成的关键基因之一,其表达量的升高会促进生物体内脂肪的合成[55]。而ACC主要在脂肪组织中表达,是催化长链脂肪酸合成过程中的限速酶[56]。除草剂恶唑酰草胺(0.40 mg·L-1)暴露成年斑马鱼21 d后,脂质合成相关基因FAS和ACC1的转录水平上调,促进脂质合成,使得肝脏内TG和游离脂肪酸水平显著增加[57]。幼年奇努克鲑鱼(Oncorhynchus tshawytscha)暴露于氯氰菊酯21 d后,通过上调肝脏中FAS和ATP柠檬酸裂解酶(ATP-citrate lyase, ACL)的表达水平,促进脂质合成,进而导致鲑鱼体内脂质蓄积[58]。
同时,农药类污染物还可以通过干扰调控鱼类脂代谢过程的转录因子的表达而干扰脂质的合成代谢。已知固醇调节元件结合蛋白(sterol regulatory element binding protein, SREBP)是维持细胞内脂肪稳态的重要核转录因子,SREBP1参与调节生物体内大多数脂肪生成相关基因的转录,与脂质合成密切相关[59]。研究报道,吡噻菌胺(1.20 mg·L-1)暴露斑马鱼胚胎以及霜霉威(1 000 μg·L-1)暴露成年斑马鱼7 d后,均会影响鱼类体内SREBP1的表达,进而调控FAS、ACC1的活性从而引起其TG含量改变[60,53]。过氧化物酶体增殖物激活受体家族(peroxisome proliferators-activated receptors, PPARs)是一类由配体激活的转录因子,在脂肪细胞分化及脂肪代谢方面发挥关键作用。PPARγ能够调控脂肪细胞的分化与成熟,诱导脂肪细胞相关基因的表达。其表达升高会促进机体对脂质的吸收和脂肪的沉积[61]。转录组分析表明,三苯基锡(1、10和100 ng·L-1)暴露海水青鳉21 d后能够激活PPAR信号通路,其中PPARγ基因表达量显著上调,刺激脂肪细胞分化,导致机体肥胖[54]。研究发现,苯醚甲环唑(1000ng·L-1)暴露海水青鳉6个月以及亚致死剂量的三氯生(200 μg·L-1)急性暴露斑马鱼仔鱼后均会上调其PPARγ的表达,从而刺激脂肪细胞分化,诱导其脂质积聚[62-63]。PPAR基因表达水平的改变可能与农药类化合物干扰了其转录调控机制有关,例如,双(2-乙基己基)-2,3,4,5-四溴邻苯二甲酸酯(1 412 μg·L-1)暴露斑马鱼胚胎14 d后,通过显著上调DNA羟化酶(ten-eleven translocation, TET)表达量,促进了PPARγ启动子的去甲基化,进而导致该基因转录上调,最终使得斑马鱼幼鱼体内TG水平发生改变[64]。
2.3 干扰鱼类的脂质分解代谢(Disruption of the lipid catabolism in fish)
脂质的分解主要分为两部分即TG的水解和脂肪酸的β氧化。与脂质合成相似,脂质分解过程中也有许多催化酶起到重要的调控作用。激素敏感脂肪酶(hormone-sensitive triglyceride lipase, HSL)可以将TG分解为脂肪酸和甘油并释放进入血液供其他组织氧化利用,是TG分解过程中的限速酶。此外肉碱棕榈酰转移酶1(carnitine palmitoyl transterase-1, CPT-1)将脂肪酸的活化产物脂酰辅酶A转移进入线粒体从而进行β氧化过程,是脂肪酸β氧化过程的主要限速酶。农药类化合物可以通过影响TG水解和/或脂肪酸氧化过程中这些关键酶的表达、活性而干扰鱼类的脂质代谢。研究报道,草甘膦(5 mg·L-1和50 mg·L-1)暴露幼年鲤鱼45 d、敌百虫(2.0 mg·L-1)暴露异育银鲫(Carassius auratus gibelio)30 d后,均能显著降低肝脏内HSL活性,抑制脂质分解,从而导致其体内脂质含量增加[65-66]。斑马鱼暴露于噻呋酰胺(0.19 mg·L-1和1.90 mg·L-1)28 d后,其体内脂质分解相关基因CPT-1活性显著升高,促进脂肪酸的β氧化,进而导致TG和总胆固醇(total cholesterol, TC)含量显著降低[67]。
另外,农药化合物也可以通过干扰脂质分解代谢相关转录因子的表达干扰鱼类的脂质代谢过程。PPARs家族中的PPARα通过调控酰基辅酶A中脂肪酸转化、脂肪酸进入线粒体和线粒体脂肪酸分解代谢酶的表达,在脂质分解代谢中发挥重要作用[68]。研究表明,PPARα的激活能够显著提升其肝脏脂肪酸的β氧化效率及CPT-1活性,并降低草鱼血浆中TG和TC水平[69]。而杀菌剂啶酰菌胺(1.0 mg·L-1)能够通过提高斑马鱼肝脏内PPARα的表达水平上调CPT-1的表达,进而促进脂肪酸β氧化,最终促使斑马鱼肝脏中TG、TC含量显著降低[70]。
2.4 干扰鱼类的脂质转运过程(Disruption of the lipid transport in fish)
在鱼体中,脂质的运输由内源性运输和外源性运输组成。在外源性途径中,从食物中摄取的脂肪经消化后分解为长链脂肪酸和一酰甘油,再由肠上皮细胞吸收后转化成TG,然后与载脂蛋白(apolipoprotein, APO)、胆固醇等结合形成CM释放到血液中。在内源性转运系统中,肝脏中合成的长链脂肪酸酯化成TG后与载脂蛋白、胆固醇等形成极低密度脂蛋白(very low-density lipoprotein, VLDL)运输到肝外组织中储存或利用。脂蛋白脂肪酶(lipoproteinlipase, LPL)是控制脂质运输的关键酶,它的作用是从CM和VLDL中水解TG,将TG衍生的脂肪酸输送到外周组织以供利用和储存[71]。研究表明,机体内脂肪酸跨膜运输是被动扩散或由蛋白介导的过程,脂肪酸被组织摄取涉及多种蛋白的参与,包括脂肪酸转运蛋白(fatty acid transport protein, FATP)、脂肪酸结合蛋白(fatty acid binding protein, FABP)等。
农药类化合物可以通过影响载脂蛋白、VLDL、LPL和脂肪酸结合蛋白的活性从而破坏鱼类的脂代谢稳态。例如,敌百虫(2.0 mg·L-1)暴露异育银鲫30 d导致其肝脏内VLDL和载脂蛋白Apob100的含量显著下降,从而不能将肝脏内的脂质转运至外周组织,致使肝脏内脂肪沉积[66]。Zhang等[72]发现3种杀螨剂螺螨酯、螺甲螨酯和螺虫乙酯暴露斑马鱼胚胎8 d后,显著抑制了FABP2和LPL的活性,胚胎内总胆固醇含量显著降低。苯醚甲环唑(2.0 mg·L-1)暴露斑马鱼幼鱼120 h后,脂肪酸含量和FABP的表达水平降低,阻碍脂质转运,使得幼鱼体内TG水平升高[52]。
除了影响上述的脂代谢过程之外,农药类化合物还可以通过其他的途径干扰鱼类的脂质代谢,比如,通过诱导线粒体的功能障碍从而影响鱼类的脂代谢,线粒体在以三磷酸腺苷(adenosine triphosphate, ATP)的形式从营养物质中产生能量方面起着核心作用,通过线粒体的脂质氧化增加可能会减少脂质积累[73]。有研究报道,氯硝柳胺暴露会增加生物体内二磷酸腺苷(adenosine diphosphate, ADP)/ATP比率,随后增加脂质和葡萄糖氧化,最终抑制肝脏中TG的合成和糖异生[74]。Zhu等[75]发现斑马鱼暴露于氯硝柳胺(40 mg·L-1)120 h后,其ATP含量显著下降,从而抑制TG和TC合成。此外,农药类化合物还可以通过干扰鱼类的糖代谢从而间接影响鱼类的脂代谢,甲基硫菌灵(12.5 mg·L-1和25 mg·L-1)暴露斑马鱼28 d后,斑马鱼肝脏中糖原和多糖物质的积累减少,参与糖酵解的关键酶乳酸脱氢酶以及戊糖磷酸途径的限速酶6-磷酸葡萄糖酸脱氢酶的活性升高,肝脏转化或储存糖原的能力降低致使糖代谢不能提供足够的能量来支撑机体,因此TG被转化以满足机体的能量需求,从而斑马鱼肝脏内TG含量降低[76]。
3 总结与展望(Summary and prospect)
综上所述,目前农药类化合物的使用已经对水生环境造成严重污染,并且诱导鱼类脂代谢紊乱的发生。农药可以通过干扰脂质消化吸收、合成、分解和转运等过程中关键酶、基因和转录因子的表达及线粒体功能受损和干扰糖代谢等脂质代谢过程,导致脂肪酸、TG等脂质生化指标的异常。目前关于农药的应用及污染现状已经得到较为深入的研究,但是关于农药对鱼类等水生生物脂代谢毒性机制的研究还存在许多有待解决的问题。
首先,目前关于农药类化合物对鱼类脂质代谢的影响研究大多关注中性脂质与胆固醇代谢,对磷脂的研究较少。磷脂是一种极性脂质,具有两亲性,常与胆固醇、糖脂和蛋白质等大分子共同构成生物体的细胞膜,不仅有助于膳食脂质在肠道内的消化吸收[88],同时也是鱼类体内参与脂质转运的载脂蛋白的重要组成部分[89]。现有的相关研究主要报道了农药化合物暴露对鱼体内磷脂含量的影响,例如,Sarma等[90]将蓝点石斑(Channa punctatus)暴露于亚致死浓度的硫丹(8.1 μg·L-1)96 h后,发现蓝点石斑肝脏和肌肉内的总脂质、胆固醇和脂肪酸含量显著降低,肝脏内的磷脂水平也显著下降;在胡鲶(Clarias batrachus)生殖周期的卵黄形成阶段,将其暴露于亚致死浓度的杀虫剂马拉硫磷(4 mg·L-1)和六六六(8 mg·L-1)4周后,发现马拉硫磷能够抑制其肝脏磷脂向性腺的转运,而六六六不仅阻碍肝脏磷脂向性腺的转运,而且减少其在肝脏中的合成[91]。磷脂作为鱼类生殖前期的必需营养素,可作为底物参与卵黄蛋白原的合成,并能加速肝脏中的脂质经血液转运至卵巢中,促进卵巢卵黄形成[92]。而卵黄积累不仅可以促进卵母细胞的发育,同时也为胚胎的生长发育提供必需的营养物质和能量。因此农药类化合物对鱼类肝脏和/或性腺内磷脂的影响可能会进而干扰鱼类的繁殖及子代的生长发育,但目前鲜有研究关注这一方面,因此今后可以深入研究农药类污染物对鱼类中磷脂代谢的干扰作用机制,尤其是在不同生殖周期探究农药对磷脂的影响可为解析其对鱼类卵巢功能及子代生长发育的干扰机制提供新的视角。
其次,目前已有的研究大多是关于单一农药对水生生物的脂代谢毒性效应,但是在实际应用过程中,常常是多种农药或者农药与其他污染物联合使用从而达到更好的防治效果,这些污染物彼此之间可能存在拮抗或者协同等多种作用关系。因此在今后的研究中应该更加重视农药与其他污染物联合暴露对鱼类及其他水生生物脂代谢的影响,例如,有文献研究三氯生与双酚A共同暴露诱导斑马鱼脂质代谢紊乱的差异机制[93],从而为农药的水生生态毒性和环境风险评估提供更加充分的科学依据。
此外,目前用于农药脂代谢毒性作用研究的水生生物种类较为局限,但是不同种类的水生生物对农药的敏感性存在较大的差异,因此今后可以进一步加强农药对多种水生生物或者不同营养级水生生物的脂代谢毒性效应研究。而且农药对鱼类脂质代谢的影响并非仅仅影响脂质代谢本身,而更有可能由脂质代谢异常进一步影响鱼体的生殖、发育以及抗病免疫过程等。然而,这些相关生理过程的关键连接点、作用靶位点以及作用机制均尚未阐明。因此,在今后的研究中,应加强利用现代生物学技术,深入研究农药影响脂质代谢及其关联生理过程的分子机制,这不仅有利于更深入地阐明鱼类代谢性疾病的发生原因,也为保障水产品的绿色安全提供重要的参考依据。
[1] Damalas C A, Eleftherohorinos I G.Pesticide exposure, safety issues, and risk assessment indicators[J].International Journal of Environmental Research and Public Health, 2011, 8(5): 1402-1419
[2] Kopp R, Martínez I O, Legradi J, et al.Exposure to endocrine disrupting chemicals perturbs lipid metabolism and circadian rhythms[J].Journal of Environmental Sciences(China), 2017, 62: 133-137
[3] Fraher D, Sanigorski A, Mellett N A, et al.Zebrafish embryonic lipidomic analysis reveals that the yolk cell is metabolically active in processing lipid[J].Cell Reports, 2016, 14(6): 1317-1329
[4] Mnif W, Hassine A I H, Bouaziz A, et al.Effect of endocrine disruptor pesticides: A review[J].International Journal of Environmental Research and Public Health, 2011, 8(6): 2265-2303
[5] Hernández A F, Gil F, Lacasa a M, et al.Pesticide exposure and genetic variation in xenobiotic-metabolizing enzymes interact to induce biochemical liver damage[J].Food and Chemical Toxicology, 2013, 61: 144-151
a M, et al.Pesticide exposure and genetic variation in xenobiotic-metabolizing enzymes interact to induce biochemical liver damage[J].Food and Chemical Toxicology, 2013, 61: 144-151
[6] Cao F M, Li Z Z, He Q, et al.Occurrence, spatial distribution, source, and ecological risk assessment of organochlorine pesticides in Dongting Lake, China[J].Environmental Science and Pollution Research, 2021, 28(24): 30841-30857
[7] Sumon K A, Rashid H, Peeters E T H M, et al.Environmental monitoring and risk assessment of organophosphate pesticides in aquatic ecosystems of north-west Bangladesh[J].Chemosphere, 2018, 206: 92-100
[8] Huang F Y, Li Z Y, Zhang C, et al.Pesticides in the typical agricultural groundwater in Songnen plain, northeast China: Occurrence, spatial distribution and health risks[J].Environmental Geochemistry and Health, 2019, 41(6): 2681-2695
[9] Jurado A, Vàzquez-Su é E, Carrera J, et al.Emerging organic contaminants in groundwater in Spain: A review of sources, recent occurrence and fate in a European context[J].Science of the Total Environment, 2012, 440: 82-94
é E, Carrera J, et al.Emerging organic contaminants in groundwater in Spain: A review of sources, recent occurrence and fate in a European context[J].Science of the Total Environment, 2012, 440: 82-94
[10] Huang Y M, Zhang R J, Li K C, et al.Experimental study on the role of sedimentation and degradation processes on atmospheric deposition of persistent organic pollutants in a subtropical water column[J].Environmental Science &Technology, 2017, 51(8): 4424-4433
[11] Chen Z F, Wen H B, Dai X X, et al.Contamination and risk profiles of triclosan and triclocarban in sediments from a less urbanized region in China[J].Journal of Hazardous Materials, 2018, 357: 376-383
[12] Li H Z, Tyler Mehler W, Lydy M J, et al.Occurrence and distribution of sediment-associated insecticides in urban waterways in the Pearl River Delta, China[J].Chemosphere, 2011, 82(10): 1373-1379
[13] Yang L Q, Li H M, Zhang Y Y, et al.Environmental risk assessment of triazine herbicides in the Bohai Sea and the Yellow Sea and their toxicity to phytoplankton at environmental concentrations[J].Environment International, 2019, 133: 105175
[14] Deribe E, Rosseland B O, Borgstrøm R, et al.Bioaccumulation of persistent organic pollutants(POPs)in fish species from Lake Koka, Ethiopia: The influence of lipid content and trophic position[J].The Science of the Total Environment, 2011, 410-411: 136-145
[15] Supe Tulcan R X, Ouyang W, Gu X, et al.Typical herbicide residues, trophic transfer, bioconcentration, and health risk of marine organisms[J].Environment International, 2021, 152: 106500
[16] Das Sarkar S, Nag S K, Kumari K, et al.Occurrence and safety evaluation of antimicrobial compounds triclosan and triclocarban in water and fishes of the multitrophic niche of River Torsa, India[J].Archives of Environmental Contamination and Toxicology, 2020, 79(4): 488-499
[17] Riaz G, Tabinda A B, Kashif M, et al.Monitoring and spatiotemporal variations of pyrethroid insecticides in surface water, sediment, and fish of the River Chenab Pakistan[J].Environmental Science and Pollution Research International, 2018, 25(23): 22584-22597
[18] Benaabidate L, Fryar A E.Controls on ground water chemistry in the central Couloir Sud Rifain, Morocco[J].Ground Water, 2010, 48(2): 306-319
[19] Xu M J, Huang H T, Li N, et al.Occurrence and ecological risk of pharmaceuticals and personal care products(PPCPs)and pesticides in typical surface watersheds, China[J].Ecotoxicology and Environmental Safety, 2019, 175: 289-298
[20] AnjumR, Malik A.Evaluation of mutagenicity of wastewater in the vicinity of pesticide industry[J].Environmental Toxicology and Pharmacology, 2013, 35(2): 284-291
[21] Jabeen F, Chaudhry A S, Manzoor S, et al.Examining pyrethroids, carbamates and neonicotenoids in fish, water and sediments from the Indus River for potential health risks[J].Environmental Monitoring and Assessment, 2015, 187(2): 29
[22] Feo M L, Eljarrat E, Barceló D.A rapid and sensitive analytical method for the determination of 14 pyrethroids in water samples[J].Journal of Chromatography A, 2010, 1217(15): 2248-2253
[23] Bennett E R, Moore M T, Cooper C M, et al.Vegetated agricultural drainage ditches for the mitigation of pyrethroid-associated runoff[J].Environmental Toxicology and Chemistry, 2005, 24(9): 2121-2127
[24] Riaz G, Tabinda A B, Kashif M, et al.Monitoring and spatiotemporal variations of pyrethroid insecticides in surface water, sediment, and fish of the River Chenab Pakistan[J].Environmental Science and Pollution Research International, 2018, 25(23): 22584-22597
[25] Nag S K, Saha K, Bandopadhyay S, et al.Status of pesticide residues in water, sediment, and fishes of Chilika Lake, India[J].Environmental Monitoring and Assessment, 2020, 192(2): 122
[26] Gonçalves C, Marins A T, do Amaral A M B, et al.Ecological impacts of pesticides on Astyanax jacuhiensis(Characiformes: Characidae)from the Uruguay River, Brazil[J].Ecotoxicology and Environmental Safety, 2020, 205: 111314
[27] Metcalfe C D, Helm P, Paterson G, et al.Pesticides related to land use in watersheds of the Great Lakes Basin[J].The Science of the Total Environment, 2019, 648: 681-692
[28] Skeff W, Neumann C, Schulz-Bull D E.Glyphosate and AMPA in the estuaries of the Baltic Sea method optimization and field study[J].Marine Pollution Bulletin, 2015, 100(1): 577-585
[29] Pérez D J, Iturburu F G, Calderon G, et al.Ecological risk assessment of current-use pesticides and biocides in soils, sediments and surface water of a mixed land-use basin of the Pampas region, Argentina[J].Chemosphere, 2021, 263: 128061
[30] Barik S R, Ganguly P, Patra S, et al.Persistence behavior of metamifop and its metabolite in rice ecosystem[J].Chemosphere, 2018, 193: 875-882
[31] Amondham W, Parkpian P, Polprasert C, et al.Paraquat adsorption, degradation, and remobilization in tropical soils of Thailand[J].Journal of Environmental Science and Health, Part B, 2006, 41(5): 485-507
[32] Li H H, Feng Y J, Li X S, et al.Analytical confirmation of various herbicides in drinking water resources in sugarcane production regions of Guangxi, China[J].Bulletin of Environmental Contamination and Toxicology, 2018, 100(6): 815-820
[33] Chidya R, Derbalah A, Abdel-Dayem S, et al.Contamination, dynamics, and health risk assessment of pesticides in seawater and marine samples from the Seto Inland Sea, Japan[J].Environmental Science and Pollution Research International, 2022, 29(45): 67894-67907
[34] Xie H J, Wang X P, Chen J W, et al.Occurrence, distribution and ecological risks of antibiotics and pesticides in coastal waters around Liaodong Peninsula, China[J].Science of the Total Environment, 2019, 656: 946-951
[35] Zheng S, Chen B, Qiu X Y, et al.Distribution and risk assessment of 82 pesticides in Jiulong River and estuary in South China[J].Chemosphere, 2016, 144: 1177-1192
[36] Oliveira I B, Beiras R, Thomas K V, et al.Acute toxicity of tralopyril, capsaicin and triphenylborane pyridine to marine invertebrates[J].Ecotoxicology, 2014, 23(7): 1336-1344
[37] Castillo L E, Martínez E, Ruepert C, et al.Water quality and macroinvertebrate community response following pesticide applications in a banana plantation, Limon, Costa Rica[J].The Science of the Total Environment, 2006, 367(1): 418-432
[38] Jurado A, Vàzquez-Su é E, Carrera J, et al.Emerging organic contaminants in groundwater in Spain: A review of sources, recent occurrence and fate in a European context[J].The Science of the Total Environment, 2012, 440: 82-94
é E, Carrera J, et al.Emerging organic contaminants in groundwater in Spain: A review of sources, recent occurrence and fate in a European context[J].The Science of the Total Environment, 2012, 440: 82-94
[39] Zheng S, Chen B, Qiu X Y, et al.Distribution and risk assessment of 82 pesticides in Jiulong River and estuary in South China[J].Chemosphere, 2016, 144: 1177-1192
[40] A asco N, Uno S, Koyama J, et al.Assessment of pesticide residues in freshwater areas affected by rice paddy effluents in Southern Japan[J].Environmental Monitoring and Assessment, 2010, 160(1-4): 371-383
asco N, Uno S, Koyama J, et al.Assessment of pesticide residues in freshwater areas affected by rice paddy effluents in Southern Japan[J].Environmental Monitoring and Assessment, 2010, 160(1-4): 371-383
[41] Smalling K L, Kuivila K M, Orlando J L, et al.Environmental fate of fungicides and other current-use pesticides in a central California Estuary[J].Marine Pollution Bulletin, 2013, 73(1): 144-153
![]() D, et al.Assessment of river sediment toxicity: Combining empirical zebrafish embryotoxicity testing with in silicotoxicity characterization[J].Science of the Total Environment, 2018, 643: 435-450
D, et al.Assessment of river sediment toxicity: Combining empirical zebrafish embryotoxicity testing with in silicotoxicity characterization[J].Science of the Total Environment, 2018, 643: 435-450
[43] Huang D G, Zhen J, Quan S Q, et al.Risk assessment for niclosamide residues in water and sediments from Nan Ji Shan Island within Poyang Lake Region, China[J].Advanced Materials Research, 2013, 721: 608-612
[44] López-Ruiz R, Romero-González R, Garrido Frenich A.Dissipation kinetics of fenamidone, propamocarb and their metabolites in ambient soil and water samples and unknown screening of metabolites[J].Journal of Environmental Management, 2020, 254: 109818
[45] Masiá, Campo J, Navarro-Ortega A, et al.Pesticide monitoring in the basin of Llobregat River(Catalonia, Spain)and comparison with historical data[J].The Science of the Total Environment, 2015, 503-504: 58-68
[46] Mansbach C M 2nd, Gorelick F.Development and physiological regulation of intestinal lipid absorption.Ⅱ.Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons[J].American Journal of Physiology Gastrointestinal and Liver Physiology, 2007, 293(4): G645-G650
[47] Adeyemi J A, Olise C C, Bamidele O S, et al.Effects of ultraviolet photooxidation of cypermethrin on the activities of phosphatases and digestive enzymes, and intestinal histopathology in African catfish, Clarias gariepinus(Burchell, 1822)[J].Journal of Experimental Zoology Part A, Ecological and Integrative Physiology, 2020, 333(8): 543-549
[48] Zhao F, Guo M Y, Zhang M N, et al.Sub-lethal concentration of metamifop exposure impair gut health of zebrafish(Danio rerio)[J].Chemosphere, 2022, 303: 135081
[49] Li Z H, Li P, Shi Z C.Molecular responses in digestive tract of juvenile common carp after chronic exposure to sublethal tributyltin[J].Ecotoxicology and Environmental Safety, 2014, 109: 10-14
[50] Sheng Y, Ren H, Limbu S M, et al.The presence or absence of intestinal microbiota affects lipid deposition and related genes expression in zebrafish(Danio rerio)[J].Frontiers in Microbiology, 2018, 9: 1124
[51] Semova I, Carten J D, Stombaugh J, et al.Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish[J].Cell Host &Microbe, 2012, 12(3): 277-288
[52] Jiang J H, Chen L Z, Wu S G, et al.Effects of difenoconazole on hepatotoxicity, lipid metabolism and gut microbiota in zebrafish(Danio rerio)[J].Environmental Pollution, 2020, 265: 114844
[53] Zhang R, Pan Z H, Wang X Y, et al.Short-term propamocarb exposure induces hepatic metabolism disorder associated with gut microbiota dysbiosis in adult male zebrafish[J].Acta Biochimica et Biophysica Sinica, 2019, 51(1): 88-96
[54] He S W, Yu D D, Li P, et al.Triphenyltin exposure causes changes in health-associated gut microbiome and metabolites in marine medaka[J].Environmental Pollution, 2021, 288: 117751
[55] 马慧敏, 刘昌奇.脂肪酸合成酶(FAS)基因的研究进展以及日粮成分对其表达的调控[J].饲料工业, 2007, 28(22): 59-64
[56] Tong L.Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery[J].Cellular and Molecular Life Sciences, 2005, 62(16): 1784-1803
[57] Zhao F, Zhang M N, Guo M Y, et al.Effects of sublethal concentration of metamifop on hepatic lipid metabolism in adult zebrafish(Danio rerio)[J].Aquatic Toxicology, 2021, 238: 105938
[58] Fuller N, Magnuson J T, Huff Hartz K E, et al.Effects of dietary cypermethrin exposure on swimming performance and expression of lipid homeostatic genes in livers of juvenile Chinook salmon, Oncorhynchus tshawytscha[J].Ecotoxicology, 2021, 30(2): 257-267
[59] Xu Y H, Tan X Y, Xu Y C, et al.Novel insights for SREBP-1 as a key transcription factor in regulating lipogenesis in a freshwater teleost, grass carp Ctenopharyngodon idella[J].The British Journal of Nutrition, 2019, 122(11): 1201-1211
[60] Qian L, Qi S Z, Cao F J, et al.Effects of penthiopyrad on the development and behaviour of zebrafish in early-life stages[J].Chemosphere, 2019, 214: 184-194
[61] Yu Y H, Wu S C, Cheng W T, et al.The function of porcine PPARγand dietary fish oil effect on the expression of lipid and glucose metabolism related genes[J].The Journal of Nutritional Biochemistry, 2011, 22(2): 179-186
[62] Dong X C, Li Y, Zhang L M, et al.Influence of difenoconazole on lipid metabolism in marine medaka(Oryzias melastigma)[J].Ecotoxicology, 2016, 25(5): 982-990
[63] 王杨, 吴国辉, 钱秋慧, 等.三氯生对斑马鱼发育和脂质代谢的影响[J].中国环境科学, 2022, 42(3): 1394-1400
Wang Y, Wu G H, Qian Q H, et al.Effects of triclosan environmental exposure on zebrafish development and lipid metabolism[J].China Environmental Science, 2022, 42(3): 1394-1400(in Chinese)
[64] Guo W, Han J, Wu S, et al.Bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate affects lipid metabolism in zebrafish larvae via DNA methylation modification[J].Environmental Science &Technology, 2020, 54(1): 355-363
[65] Liu J B, Dong C Y, Zhai Z Z, et al.Glyphosate-induced lipid metabolism disorder contributes to hepatotoxicity in juvenile common carp[J].Environmental Pollution, 2021, 269: 116186
[66] Xu W N, Liu W B, Shao X P, et al.Effect of trichlorfon on hepatic lipid accumulation in crucian carp Carassius auratus gibelio[J].Journal of Aquatic Animal Health, 2012, 24(3): 185-194
[67] Yang Y, Dong F S, Liu X G, et al.Thifluzamide affects lipid metabolism in zebrafish(Danio reio)[J].The Science of the Total Environment, 2018, 633: 1227-1236
[68] Gervois P, Torra I P, Fruchart J C, et al.Regulation of lipid and lipoprotein metabolism by PPAR activators[J].Clinical Chemistry and Laboratory Medicine, 2000, 38(1): 3-11
[69] Du Z Y, Clouet P, Degrace P, et al.Hypolipidaemic effects of fenofibrate and fasting in the herbivorous grass carp(Ctenopharyngodon idella)fed a high-fat diet[J].The British Journal of Nutrition, 2008, 100(6): 1200-1212
[70] Qian L, Zhang J, Chen X G, et al.Toxic effects of boscalid in adult zebrafish(Danio rerio)on carbohydrate and lipid metabolism[J].Environmental Pollution, 2019, 247: 775-782
[71] Wang H, Eckel R.Regulation of lipid and lipoprotein metabolism by PPAR activators[J].Cytogenetics &Cell Genetics, 2009, 297(2): 271
[72] Zhang J, Qian L, Teng M M, et al.The lipid metabolism alteration of three spirocyclic tetramic acids on zebrafish(Danio rerio)embryos[J].Environmental Pollution, 2019, 248: 715-725
[73] Chen Z, Tian R F, She Z G, et al.Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease[J].Free Radical Biology and Medicine, 2020, 152: 116-141
[74] Hardie D G.AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function[J].Genes &Development, 2011, 25(18): 1895-1908
[75] Zhu B R, He W, Yang F, et al.High-throughput transcriptome sequencing reveals the developmental toxicity mechanisms of niclosamide in zebrafish embryo[J].Chemosphere, 2020, 244: 125468
[76] Jia K, Cheng B, Huang L R, et al.Thiophanate-methyl induces severe hepatotoxicity in zebrafish[J].Chemosphere, 2020, 248: 125941
[77] Zhong H Y, Dong L J, Dong Q J, et al.Quantitative analysis of aberrant fatty acid composition of zebrafish hepatic lipids induced by organochlorine pesticide using stable isotope-coded transmethylation and gas chromatography-mass spectrometry[J].Analytical and Bioanalytical Chemistry, 2012, 404(1): 207-216
[78] Maisano M, Cappello T, Oliva S, et al.PCB and OCP accumulation and evidence of hepatic alteration in the Atlantic bluefin tuna, T. thynnus, from the Mediterranean Sea[J].Marine Environmental Research, 2016, 121: 40-48
[79] Bui-Nguyen T M, Baer C E, Lewis J A, et al.Dichlorvos exposure results in large scale disruption of energy metabolism in the liver of the zebrafish, Danio rerio[J].BMC Genomics, 2015, 16: 853
[80] Liu J B, Dong C Y, Zhai Z Z, et al.Glyphosate-induced lipid metabolism disorder contributes to hepatotoxicity in juvenile common carp[J].Environmental Pollution, 2021, 269: 116186
[81] Sun L B, Li J S, Zuo Z H, et al.Chronic exposure to paclobutrazol causes hepatic steatosis in male rockfish Sebastiscus marmoratusand the mechanism involved[J].Aquatic Toxicology, 2013, 126: 148-153
[82] Weng Y, Huang Z Z, Wu A Y, et al.Embryonic toxicity of epoxiconazole exposure to the early life stage of zebrafish[J].The Science of the Total Environment, 2021, 778: 146407
[83] Tian S N, Teng M M, Meng Z Y, et al.Toxicity effects in zebrafish embryos(Danio rerio)induced by prothioconazole[J].Environmental Pollution, 2019, 255(Pt 2): 113269
[84] Teng M M, Zhao F, Zhou Y M, et al.Effect of propiconazole on the lipid metabolism of zebrafish embryos(Danio rerio)[J].Journal of Agricultural and Food Chemistry, 2019, 67(16): 4623-4631
[85] Olsvik P A, Hammer S K, Sanden M, et al.Chlorpyrifos-induced dysfunction of lipid metabolism is not restored by supplementation of polyunsaturated fatty acids EPA and ARA in Atlantic salmon liver cells[J].Toxicology in Vitro, 2019, 61: 104655
[86] Wang X Y, Shen M L, Zhou J J, et al.Chlorpyrifos disturbs hepatic metabolism associated with oxidative stress and gut microbiota dysbiosis in adult zebrafish[J].Comparative Biochemistry and Physiology Part C: Toxicology &Pharmacology, 2019, 216: 19-28
[87] Bao Z W, Zhao Y, Wu A Y, et al.Sub-chronic carbendazim exposure induces hepatic glycolipid metabolism disorder accompanied by gut microbiota dysbiosis in adult zebrafish(Daino rerio)[J].The Science of the Total Environment, 2020, 739: 140081
[88] 曾端, 叶元土.鱼类食性与消化系统结构的研究[J].西南农业大学学报, 1998, 20(4): 361-364
Zeng D, Ye Y T.Studies on digestive system and different feeding habits of some fishes in freshwater[J].Journal of Southwest Agricultural University, 1998, 20(4): 361-364(in Chinese)
[89] Tocher D R.Glycerophospholipid metabolism[J].Biochemistry and Molecular Biology of Fishes, 1995, 4: 119-157
[90] Sarma K, Pal A K, Grinson-George, et al.Effect of sub-lethal concentration of endosulfan on lipid and fatty acid metabolism of spotted murrel, Channa punctatus[J].Journal of Environmental Biology, 2015, 36(2): 451-454
[91] Lal B, Singh T P.Impact of pesticides on lipid metabolism in the freshwater catfish, Clarias batrachus, during the vitellogenic phase of its annual reproductive cycle[J].Ecotoxicology and Environmental Safety, 1987, 13(1): 13-23
[92] Sui L Y, Wu X G, Wille M, et al.Effect of dietary soybean lecithin on reproductive performance of Chinese mitten crab Eriocheir sinensis[J].Aquaculture International, 2009, 17(1): 45-56
[93] Sun L M, Ling Y H, Jiang J H, et al.Differential mechanisms regarding triclosan vs.bisphenol A and fluorene-9-bisphenol induced zebrafish lipid-metabolism disorders by RNA-Seq[J].Chemosphere, 2020, 251: 126318