包括神经系统疾病在内的许多疾病都与环境因素密切相关。大量研究证实,暴露于环境污染物会导致神经炎症、氧化应激、线粒体功能障碍和神经元凋亡等,从而诱发一系列神经毒性和疾病。例如,大鼠长期暴露于铅(Pb)后,其海马中白细胞介素(IL)-1β和肿瘤坏死因子-α水平上升,同时慢性胶质细胞活化并伴有炎症和神经退行性特征[1]。斑马鱼在双酚A(BPA)处理后,焦虑和恐惧反应异常,而这些行为改变可能源于其中枢神经系统中参与抗氧化防御机制的基因表达失调[2]。此外,低剂量甲基汞可通过改变线粒体功能和引起mtDNA的氧化损伤诱导人类神经祖细胞凋亡[3];而大鼠肾上腺髓质嗜铬瘤分化细胞(PC12)在经过Mn3O4纳米颗粒暴露后,细胞活力降低,并通过引发氧化应激诱导细胞发生凋亡[4]。
一般认为,大多数环境污染物主要改变生物体的表观基因组而非直接改变其DNA序列。表观遗传学是研究在不改变DNA序列情况下的基因表达的可遗传变化,其研究对象是表观基因组。表观基因组相对于基因组而言,不仅序列包含遗传信息,同时它们的修饰也记载遗传信息。表观基因组记录生物体DNA和组蛋白的一些改变或修饰,同时这些变化或修饰可以从亲本传给子代。表观遗传修饰包含DNA甲基化(DNA methylation)、组蛋白修饰(histone modification)和非编码RNA(non-coding RNA, ncRNA)等,它们可以在外界环境的影响下改变基因组功能。
DNA甲基化是DNA化学修饰的一种形式,指在DNA甲基化转移酶(如Dnmt1、Dnmt2、Dnmt3a和Dnmt3b等)的作用下,将一个甲基加到胞嘧啶上,并转化为5-甲基胞嘧啶的一种修饰[5]。这一过程通常是发生在CpG岛上,对生物的基因表达调控具有重要意义。DNA甲基化参与调控许多细胞过程,包含染色质结构、染色质重塑、X染色体失活、基因组印记、染色体稳定性以及基因转录等等。一般情况下,基因启动子的高甲基化通常被认为可导致该基因的表达水平降低。相反,基因启动子的低甲基化则可激活基因表达。
组蛋白修饰主要是指发生在核心组蛋白N端氨基酸残基上的甲基化、乙酰化、磷酸化和泛素化等共价修饰[6]。组蛋白修饰可以通过影响相邻核小体中不同组蛋白的接触或组蛋白与DNA的相互作用影响高阶染色质结构,使之变得松散或紧密,从而影响基因的转录水平[7]。其中,组蛋白高乙酰化能够使染色质结构变松散,与转录激活有关。组蛋白乙酰化酶和组蛋白去乙酰化酶的作用维持了组蛋白乙酰化的动态平衡。但当外界环境发生改变时,这种稳态便会被破坏,从而使基因表达水平发生变化。组蛋白甲基化则是另一种重要的组蛋白修饰,在基因表达中同时具有激活和抑制的作用。组蛋白中不同位点赖氨酸残基上发生的甲基化具有不同的生物学功能。比如,组蛋白H3K9的甲基化通常导致基因沉默,而H3K4甲基化则与基因表达激活有关[8]。
非编码RNA也在基因表达调控中发挥重要作用。根据非编码RNA的大小可以分为长链非编码RNA(lncRNA)和短链非编码RNA(sncRNA),常见的短链非编码RNA包括小干扰RNA(siRNA)、微小RNA(miRNA)等[9]。其中miRNA可通过导致靶标mRNA的降解或翻译沉默调控基因表达,因而一般可使基因表达沉默。
大多数人类中枢神经系统疾病都与表观基因组的扰动有关[10]。表观遗传修饰最典型的作用主要表现在神经细胞命运决定、突触、神经网络连接和可塑性以及跨代遗传等。例如,有研究表明,缺乏Dnmt1和Dnmt3a的小鼠DNA甲基化水平降低,海马CA1区域的可塑性发生改变,导致学习记忆功能受损[11]。死亡域相关蛋白是一组组蛋白伴侣,它通过促进H3.3加载和转录结合到神经元基因相关的染色质中响应神经元去极化,从而导致基因转录激活[12]。miRNAs、Piwi互作RNA(piRNAs)和lncRNA都参与调节突触的形成和功能。miR-339-5p靶向调节BACE1,导致BACE1在阿尔茨海默症(AD)患者脑中的表达水平降低[13]。此外,许多研究已经聚焦于不同的表观遗传因子如何协调神经干细胞的自我更新和维持、谱系限制及神经元和胶质成熟等方面[14]。
目前关于环境污染物诱导神经毒性的机制研究也逐渐深入。随着表观遗传学研究技术的不断发展,表观遗传修饰在环境污染物暴露导致神经毒性中的作用机制研究日益受到重视[15-16]。本文主要综述了典型环境污染物(金属、有机污染物和空气颗粒物等)暴露诱导神经系统毒性的表观遗传学机制(DNA甲基化、组蛋白修饰和非编码RNA)相关研究。
1 环境污染物暴露诱导神经毒性的表观遗传机制(Epigenetic mechanism of neurotoxicity induced by environmental pollutants exposure)
1.1 重金属
1.1.1 铝(Al)
有研究探讨了Al电解工人认知功能与DNA甲基化的关系。结果表明,简易精神状态检查得分和全基因组DNA甲基化水平随血清Al浓度的升高而降低,轻度认知障碍(MCI)发病风险增加,并且MCI与DNA甲基化显著负相关[17]。另一项研究表明,淀粉样前体蛋白(APP)基因启动子DNA甲基化水平的降低可能与工人血清Al水平的升高有关[18]。此外,在动物模型中也有相同的发现,雄性大鼠暴露于AlCl3后,海马组织中APP启动子DNA甲基化水平降低,APP、Aβ含量升高[19],从而增加AD患病风险。小鼠在AlCl3暴露后,海马中MBDs、DnMTs和MeCPb表达水平降低导致APP基因的低甲基化和Aβ沉积[20],成为AD发展的潜在风险因素。报道显示,大鼠暴露于麦芽酸铝后,脑组织的全基因组DNA甲基化水平降低,并导致学习记忆功能障碍[21]。
组蛋白修饰也与Al诱导的神经毒性密切相关。有研究表明,职业Al暴露可通过提高组蛋白H3的甲基化修饰(尤其H3K27me3和H3K9me2)水平,抑制学习记忆相关蛋白BDNF和EGR1的表达,从而影响学习记忆功能[22]。此外,Al暴露可上调组蛋白去乙酰化酶6(HDAC6)的表达,下调BDNF启动子的H3K9和H4K12乙酰化,最终抑制BDNF表达,导致大鼠空间学习记忆能力减弱[23]。低强度脉冲超声(LIPUS)则可通过恢复组蛋白乙酰化和BDNF表达,使受损的认知功能得到改善[23]。
1.1.2 砷(As)
As暴露和表观遗传修饰之间的一个可能联系在于其生物转化,即通过共用染色质重塑所需的甲基供体S-腺苷甲硫氨酸(S-adenosyl methionine, SAM)对DNA甲基化产生影响[24]。随着大鼠脑皮质和海马组织中总As浓度增加,DNA甲基转移酶(DNMTs)和去甲基化酶(TETs)表达下调,导致脑组织中DNA甲基化/去甲基化过程显著受到抑制,且这种变化呈剂量依赖性[25]。同时,As可以破坏氧化/抗氧化平衡,并通过TCA循环和α-酮戊二酸(α-KG)途径进一步抑制TETs的表达,从而导致DNA甲基化/去甲基化破坏,诱导大鼠学习记忆功能损伤[25]。
发育阶段暴露于低水平As可导致组蛋白H3K9ac和H3K4me3在成年雌性小鼠中水平下降,但在雄性小鼠中上升,最终导致小鼠齿状回海马神经发生障碍,并出现抑郁样症状[26]。母鼠在妊娠期持续As暴露后,子代小鼠的空间、情景记忆功能受到损害,这可能是由于小鼠皮质和海马组织H3K9的低乙酰化[27]。
流行病学调查和实验研究表明,miRNA在As暴露中也发挥着重要作用,同时可能对认知功能产生影响。As可通过上调miR-219,靶向降低CaMKⅡ水平,诱导学习和记忆障碍[28]。同时NMDA受体亚基2(NR2)和记忆相关蛋白c-Fos和c-Jun可通过抑制miR-219而上调,从而改善As诱导的海马结构损害和学习记忆损伤[28]。
1.1.3 锰(Mn)
有研究测定了201名电焊工人血液中的NOS2外显子1的DNA甲基化水平,发现该位点的低甲基化促进了该基因的表达,从而诱导神经炎症的发生,增加帕金森病(PD)的发病风险[29]。人神经母细胞瘤细胞(SH-SY5Y)经慢性Mn暴露后,Parkin和PINK1这2个基因均发生高甲基化,基因活性降低,也可能加剧PD发病风险[30]。酪氨酸羟化酶(TH)在多巴胺的生物合成中起着至关重要的作用。Mn暴露可导致TH的DNA甲基化水平升高,基因表达水平下降,从而抑制多巴胺生物合成[30]。此外,SH-SY5Y细胞在慢性Mn暴露后,通过诱导高甲基化下调了PINK1-PARK2表达,表明Mn可能通过表观遗传调控导致神经细胞线粒体功能障碍[30]。由于表观遗传修饰变化是可遗传的,小鼠在Mn暴露后,其子代海马中一些参与分化过程的基因如Mid1、Nr2f1和Atp1a3等发生高甲基化,致使多巴胺能中间神经元数量减少,海马神经发生持续中断[31]。还有研究表明,产前Mn暴露可能通过表观遗传机制影响胎儿神经发育。分析胎盘的全基因组甲基化,发现713个CpG位点甲基化与Mn暴露有关,其中5个差异甲基化位点位于神经发育相关基因中[32]。
PC12和SH-SY5Y细胞暴露于MnCl2后,HDAC3和HDAC4表达水平显著增加,同时HAT水平下降,导致H3和H4乙酰化水平下调,从而诱导细胞凋亡,而HAT抑制剂处理后则可以缓解Mn诱导的细胞活力下降以及凋亡[33]。
1.1.4 铅(Pb)
研究发现,雌性小鼠在孕前、孕中和哺乳期持续Pb暴露后,其子代小鼠海马体中MECP2水平显著下降,DNMT1水平显著增加,同时在皮质中发现DNMT1、DNMT3a和MECP2的表达显著增加,此外,子代雌鼠皮质中的糖皮质激素受体基因(Nr3c1)甲基化也发生显著改变,这可能会给小鼠海马和皮质功能以及认知功能造成不利影响[34]。有趣的是,围产期Pb暴露与出生后早期Pb暴露对小鼠海马中DNA甲基化相关酶的影响有所不同。与对照组相比,Dnmt1只有在出生后早期Pb暴露的雄鼠存在显著差异,Dnmt3a在围产期暴露雄鼠和出生后早期暴露雌鼠都显著降低,雌鼠MeCP2表达水平显著降低,可能会导致多种认知障碍疾病[35]。此外,Pb暴露还可能通过DNA高甲基化下调神经分化相关基因表达,从而促进神经退行性过程[36]。早期Pb暴露(出生1~21 d)会导致DNMT1活性降低,改变参与AD通路的APP和β分泌酶1(bace1)基因的甲基化水平,导致APP的过表达,以及生命后期Aβ水平的增加,增加晚年AD发病风险[37]。
小鼠早期Pb暴露后,大脑皮层中与基因激活相关的组蛋白H3K9ac和H3K4me2水平下降,而与基因活性抑制相关的H3K27me3蛋白水平上升[38],这可能增加神经退行性疾病的患病风险。
靶向AD相关蛋白的miRNA也会受到Pb的影响。生命早期Pb暴露显著影响海马中6个靶向神经毒性蛋白的miRNA表达。其中,miR-106b(靶向APP mRNA)和miR-124(靶向SP1 mRNA)减少,这可能会导致晚年神经毒性蛋白的过度表达,增加AD的发病风险[39]。
1.1.5 汞(Hg)
有研究表明,产前Hg暴露水平与PON1基因的低甲基化水平相关,这可能导致了儿童期男孩的认知功能障碍[40]。除此之外,Hg暴露后,斑马鱼中长链非编码RNA Malat1可被特异性上调10倍以上,Malat1在斑马鱼胚胎的脑区、眼睛和脊索中高度表达,并改变神经发育相关基因表达模式,导致斑马鱼幼体的神经行为障碍[41]。
由于大脑对甲基汞(MeHg)具有很强的亲和力,它可以通过中性氨基酸转运系统Ⅰ与L-半胱氨酸的复合体穿过血脑屏障并分布到大脑的各个区域[42],使大脑功能受到不同程度的损害。
越来越多的研究表明,MeHg诱导的神经系统疾病与DNA甲基化有关。大鼠胚胎皮质神经干细胞(NSCs)暴露于低剂量MeHg后,整体DNA甲基化水平下降,细胞周期调节因子p16和p21表达上升,导致细胞周期阻滞,并且这些变化在无MeHg暴露的传代细胞中也可观察到[43]。
胎儿脑源性永生细胞(LUHMES)暴露于MeHg后,TH基因启动子的组蛋白H3K27me3显著增加,TH水平降低,从而抑制多巴胺的生物合成[44]。此外,MeHg显著下调了小鼠海马中BDNF启动子的H3乙酰化并上调H3K27me3,同时DNA甲基化也显著上调,导致BDNF基因表达抑制,小鼠出现抑郁行为[45]。
人类神经祖细胞ReNcell CX对MeHg高度敏感。研究结果显示,低剂量MeHg可通过降低miR-25的水平、上调p53以及线粒体发生相关基因的表达,进而可能损害细胞线粒体功能[46]。
1.1.6 其他重金属
铜(Cu)暴露与神经系统中DNA甲基化、组蛋白修饰之间的联系尚未被发现。但有研究发现,Cu暴露可能会导致某些miRNA水平的变化。miR-187、miR-128、miR-138、miR-183和miR-7a已经被证明在嗅觉系统中特异性表达,特别是miR-183家族成员在斑马鱼的神经感觉器官中大量表达。有研究结果显示,斑马鱼经Cu暴露后,其侧线嗅觉感觉细胞中miR-183表达水平显著下降,嗅觉上皮细胞凋亡增加,感觉神经元丢失,最终可能导致斑马鱼嗅觉障碍[47]。
镍(Ni)处理的原代培养海马神经元中可以观察到细胞树突的复杂性降低,同时组蛋白乙酰化受到了抑制[48]。此外,Ni暴露后的小鼠海马组织组蛋白低乙酰化,海马树突复杂性下降,并且学习记忆能力受损[48]。神经细胞(Neuro-2a)在Ni暴露下,miR-210过表达并导致ISCU1/2表达水平下调, miR-210抑制剂则可缓解Ni诱导的ISCU1/2下调[49]。ISCU1/2负责线粒体呼吸和能量产生[50],其表达下调可能影响细胞的线粒体功能。
铅和镉联合暴露则通过上调组蛋白去乙酰化酶HDAC2表达,降低大鼠海马组织的组蛋白乙酰化水平,最终降低大鼠海马树突棘密度,损害大鼠的学习记忆能力[51]。
1.2 有机物
1.2.1 多环芳烃(PAHs)
一项流行病学研究发现,产前PAHs暴露与LINE1 DNA甲基化负相关,而LINE1甲基化与儿童智商显著正相关,但LINE1并未直接介导PAHs暴露与智商之间的关联[52]。
苯并(a)芘(B[a]P)是一种常见的PAHs。由于其亲脂性,B[a]P及其代谢产物可以穿过血脑屏障,到达脑组织,引起中枢神经系统损伤[53]。斑马鱼暴露于B[a]P后,DNA甲基转移酶表达普遍降低,整体DNA甲基化水平下降,斑马鱼现表出社会焦虑样行为,并且F2代成年斑马鱼也存在此现象[54],这可能与表观遗传修饰的跨代遗传有关。此外,B[a]P暴露后,大鼠海马中miRNA水平下降,而DNA甲基化和lncRNA水平显著上调,其中差异甲基化基因涉及通路大部分与学习记忆相关,最终导致大鼠的学习记忆功能障碍[55]。而小鼠暴露于B[a]P后,可通过DNA高甲基化下调NR2B[56]、BDNF[57]基因的转录水平,致使小鼠出现短期记忆缺陷、焦虑样行为及认知和行为障碍。在体外实验中也发现,海马神经元细胞HT22经B[a]P暴露后,BDNF基因启动子的DNA甲基化水平上升,基因转录水平下降,从而诱导细胞肿胀及炎症发生[57]。
1.2.2 双酚A(BPA)
有研究观察到,产前BPA暴露后,低APGAR(神经发育疾病风险增加的预测因子)与BPA诱导的Grin2b高甲基化有关[58]。此外,BPA暴露会导致雌性大鼠后代海马体中的BDNF基因启动子高度甲基化,下调其基因转录水平,从而影响大鼠的空间学习记忆功能[59]。另一研究发现,子代大鼠海马组织中的Fkbp5基因高甲基化也与母本BPA暴露有关,并因此对子代大鼠的应激反应产生不良影响[60]。
围产期的小鼠暴露于BPA后,其子代雄鼠的空间记忆能力受到损伤,同时伴随着大脑皮质和海马的DNA甲基化减少以及组蛋白H3乙酰化增加[61]。氯化钾协同转运蛋白2(KCC2)参与维持细胞内氯化物的平衡,负责从成熟神经元中转运出氯化物[62]。有研究发现,经BPA暴露后,小鼠大脑皮层神经元中组蛋白乙酰化水平下降,Kcc2基因的表达受到了抑制和延迟,从而导致皮质神经网络受损[63]。
1.2.3 农药
Neuro-2a细胞用百草枯处理后,通过ROS上调DNA甲基化,降低与细胞增殖、迁移与凋亡相关的miR-17-5p表达水平,进而促进细胞凋亡[64]。此外,N27多巴胺能神经元细胞暴露于百草枯后,PKCδ蛋白水解增强,同时细胞中HDAC蛋白水平显著下降、组蛋白H3乙酰化增加[65]。PKCδ蛋白水解活化是细胞死亡的关键标志之一。因此,百草枯可通过提高组蛋白乙酰化水平,增强PKCδ水解活化,从而诱导神经元细胞凋亡[65]。
研究表明,氯菊酯暴露导致斑马鱼整体游泳活动迟发性降低、焦虑,这可能是由于DNA高甲基化诱导的谷氨酸活性相关基因(如fmr1、pnocb等)的下调所导致,并且该表观遗传变化所导致的神经行为改变具有跨代遗传效应[66]。
C57BL/6小鼠发育过程中暴露于狄氏剂,可导致其脑黑质中Nr4a2和Lmx1b等多巴胺能神经元发育相关基因的DNA甲基化水平改变,从而导致多巴胺能神经元进行性退化,增加PD发病风险[67]。组蛋白修饰在狄氏剂诱导的神经细胞死亡中发挥重要作用。中脑多巴胺能神经元细胞暴露于狄氏剂,可诱导组蛋白H3和H4的高度乙酰化,并导致cAMP反应元件结合蛋白的积累和蛋白激酶PKCδ的水解活化,进而促进细胞凋亡[68]。
体外实验发现,分化前阿特拉津暴露会诱发SH-SY5Y细胞一系列的表型改变,例如突触数量以及长度发生变化,同时伴随着表观遗传基因组的变化,如DNA甲基化增加,H3K9me3和H3K27me3的减少。这些表观遗传修饰变化可能破坏了组成性异染色质的形成,并导致SNCA表达上调,增加PD风险[69]。
1.2.4 其他有机物
多氯联苯暴露后,大鼠小脑和大脑皮层甲基化水平下调,并诱导小脑和大脑皮层发生DNA损伤[70]。SK-N-SH细胞经全氟辛烷磺酸(PFOS)暴露后,BDNF基因启动子的DNA甲基化水平及miRNA-16、miRNA-22和miRNA-30a-5p均上升,BDNF转录水平下降,最终导致细胞缩小、活力下降[71]。
另外,挥发性有机物(VOCs)复合暴露导致MALAT1 lncRNA显著减少,同时8个基因因DNA超甲基化而显著下调,其中包含CNTNAP3、SULT4A1、CLIP2、CACNG8、WNT7B、GLS2、TP73和FMR1等基因,这些基因的下调可能导致突触、树突棘的数量和密度降低,以及运动、学习记忆能力受损[72]。
1.3 空气颗粒物
少量研究表明,表观遗传调控在空气颗粒物暴露诱导的神经毒性中发挥重要作用。人体和动物模型研究发现,空气颗粒物暴露引起的脑组织中H3K9me2/3降低可能导致基因组不稳定、DNA损伤及APP基因的转录上调,从而增加AD发病风险[73]。在另一研究中也发现,空气细颗粒物可通过提高SH-SY5Y细胞的DNA甲基化水平,抑制突触相关基因的转录表达,增加自闭症的发病几率[74]。焊接烟雾暴露后,大鼠全脑组织的DNA甲基化水平升高,端粒长度增加,神经退化标志蛋白表达上调,提示表观遗传变化、端粒长度与神经退行性改变之间的可能联系[75]。
2 结论与展望(Conclusion and prospect)
环境污染物可通过引起各种表观遗传修饰的改变而诱导神经毒性(表1)。基于现有的研究结果,我们发现环境污染物可以直接或通过氧化胁迫等其他机制间接引起表观遗传机制改变,进而导致神经系统功能相关基因表达失调,最终诱导神经毒性(图1)。然而,目前该领域研究仍存在一定的局限性。
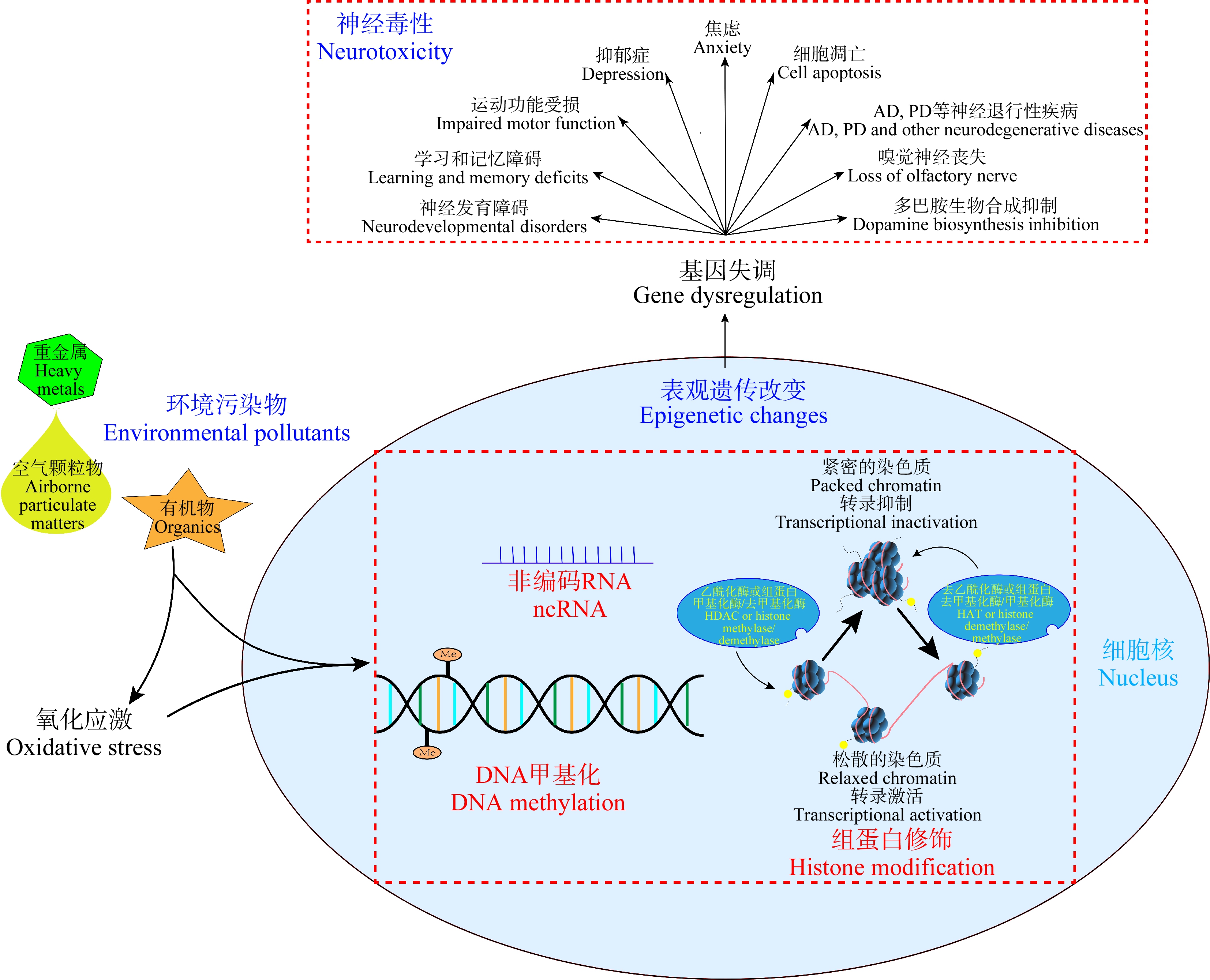
图1 环境污染物诱导神经毒性的表观遗传机制示意图
注:AD表示阿尔茨海默病,PD表示帕金森病。
Fig.1 Proposed epigenetic mechanism of neurotoxicity induced by environmental pollutants
Note: AD stands for Alzheimer Disease, and PD stands for Parkinson Disease.
表1 环境污染物对表观遗传修饰的影响及其神经毒性效应
Table 1 Effects of environmental pollutants on epigenetic modifications and their neurotoxic effects
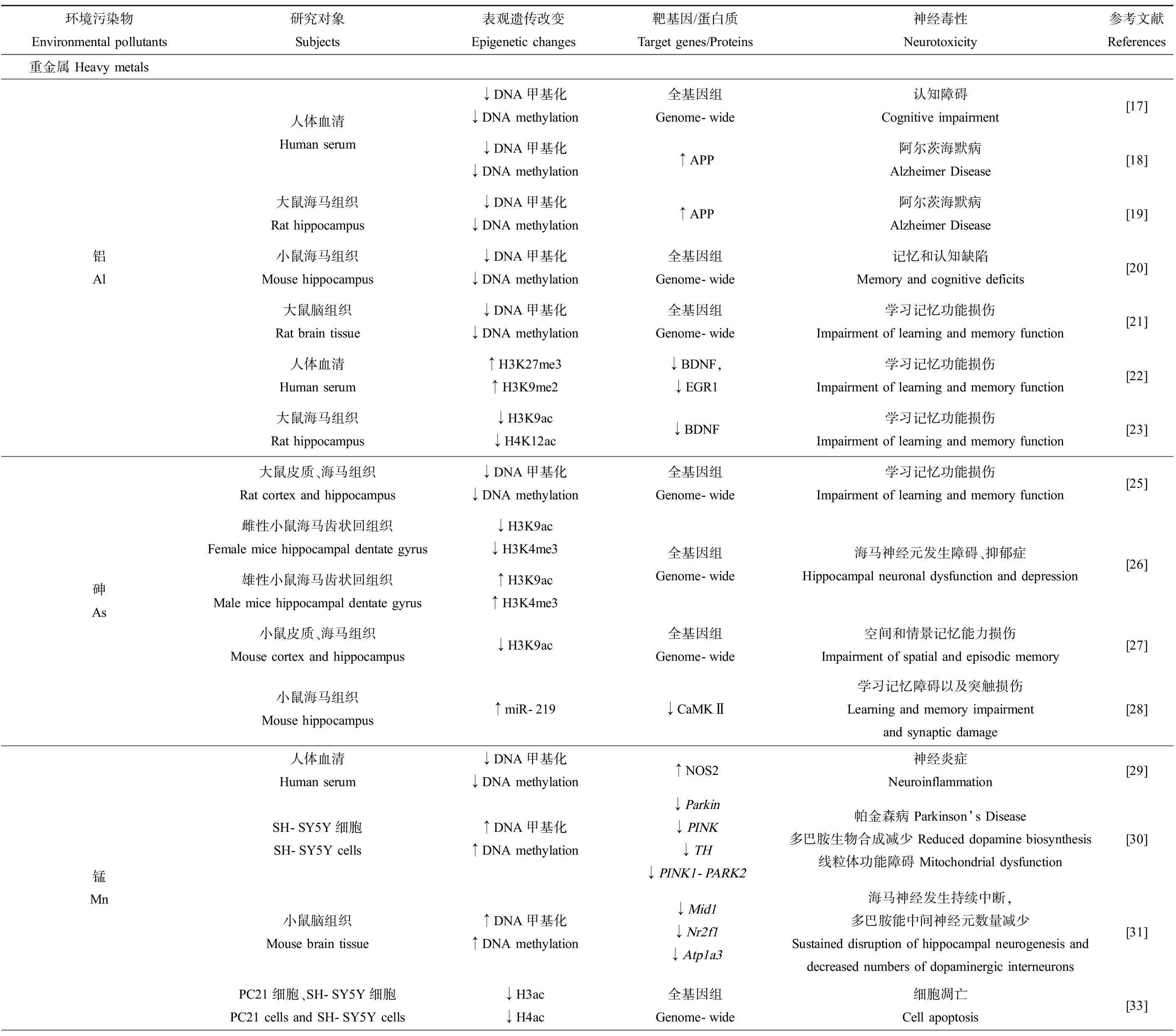
环境污染物Environmental pollutants研究对象Subjects表观遗传改变Epigenetic changes靶基因/蛋白质Target genes/Proteins神经毒性Neurotoxicity参考文献References重金属 Heavy metals铝 Al人体血清Human serum大鼠海马组织Rat hippocampus小鼠海马组织Mouse hippocampus大鼠脑组织Rat brain tissue人体血清Human serum大鼠海马组织Rat hippocampus↓DNA甲基化↓DNA methylation全基因组Genome-wide认知障碍Cognitive impairment[17]↓DNA甲基化↓DNA methylation↑APP阿尔茨海默病Alzheimer Disease[18]↓DNA甲基化↓DNA methylation↑APP阿尔茨海默病Alzheimer Disease[19]↓DNA甲基化↓DNA methylation全基因组Genome-wide记忆和认知缺陷Memory and cognitive deficits[20]↓DNA甲基化↓DNA methylation全基因组Genome-wide学习记忆功能损伤Impairment of learning and memory function[21]↑H3K27me3↑H3K9me2↓BDNF,↓EGR1学习记忆功能损伤Impairment of learning and memory function[22]↓H3K9ac↓H4K12ac↓BDNF学习记忆功能损伤Impairment of learning and memory function[23]砷 As大鼠皮质、海马组织Rat cortex and hippocampus↓DNA甲基化↓DNA methylation雌性小鼠海马齿状回组织Female mice hippocampal dentate gyrus↓H3K9ac ↓H3K4me3雄性小鼠海马齿状回组织Male mice hippocampal dentate gyrus↑H3K9ac↑H3K4me3小鼠皮质、海马组织Mouse cortex and hippocampus↓H3K9ac小鼠海马组织Mouse hippocampus↑miR-219全基因组Genome-wide学习记忆功能损伤Impairment of learning and memory function[25]全基因组Genome-wide海马神经元发生障碍、抑郁症Hippocampal neuronal dysfunction and depression[26]全基因组Genome-wide空间和情景记忆能力损伤Impairment of spatial and episodic memory[27]↓CaMKⅡ学习记忆障碍以及突触损伤Learning and memory impairment and synaptic damage[28]锰 Mn人体血清Human serum↓DNA甲基化↓DNA methylation↑NOS2神经炎症Neuroinflammation[29]SH-SY5Y细胞SH-SY5Y cells↑DNA甲基化↑DNA methylation↓Parkin ↓PINK↓TH ↓PINK1-PARK2帕金森病 Parkinson’s Disease多巴胺生物合成减少 Reduced dopamine biosynthesis线粒体功能障碍 Mitochondrial dysfunction[30]小鼠脑组织Mouse brain tissue↑DNA甲基化↑DNA methylation↓Mid1↓Nr2f1↓Atp1a3海马神经发生持续中断,多巴胺能中间神经元数量减少Sustained disruption of hippocampal neurogenesis and decreased numbers of dopaminergic interneurons[31]PC21细胞、SH-SY5Y细胞PC21 cells and SH-SY5Y cells↓H3ac↓H4ac全基因组Genome-wide细胞凋亡Cell apoptosis[33]
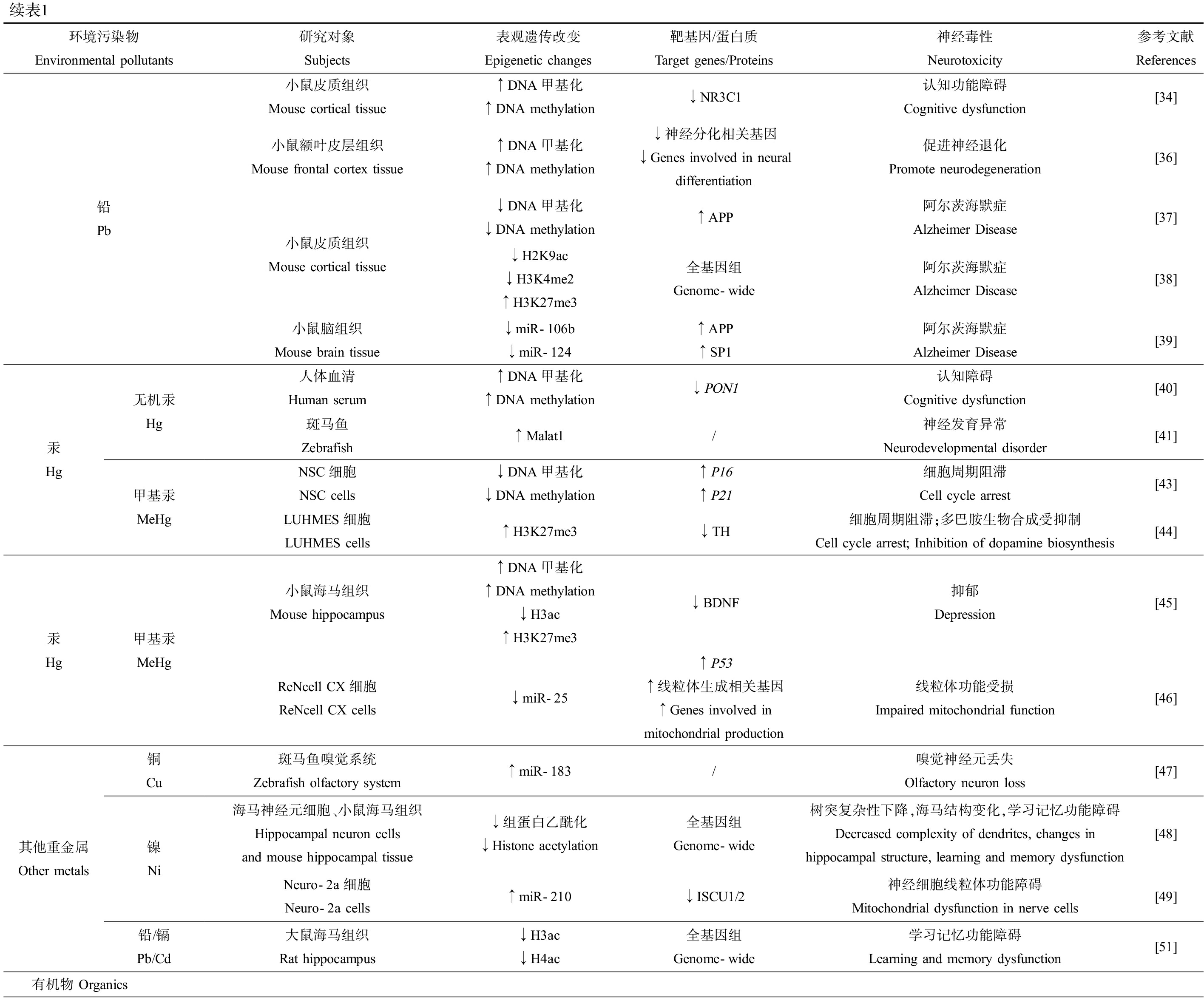
续表1环境污染物Environmental pollutants研究对象Subjects表观遗传改变Epigenetic changes靶基因/蛋白质Target genes/Proteins神经毒性Neurotoxicity参考文献References铅 Pb小鼠皮质组织Mouse cortical tissue小鼠额叶皮层组织Mouse frontal cortex tissue小鼠皮质组织Mouse cortical tissue小鼠脑组织Mouse brain tissue↑DNA甲基化↑DNA methylation↓NR3C1认知功能障碍Cognitive dysfunction[34]↑DNA甲基化↑DNA methylation↓神经分化相关基因↓Genes involved in neural differentiation促进神经退化Promote neurodegeneration[36]↓DNA甲基化↓DNA methylation↑APP阿尔茨海默症Alzheimer Disease[37]↓H2K9ac↓H3K4me2↑H3K27me3全基因组Genome-wide阿尔茨海默症Alzheimer Disease[38]↓miR-106b↓miR-124↑APP↑SP1阿尔茨海默症Alzheimer Disease[39]汞 Hg无机汞 Hg甲基汞MeHg人体血清Human serum↑DNA甲基化↑DNA methylation↓PON1认知障碍Cognitive dysfunction[40]斑马鱼Zebrafish↑Malat1/神经发育异常Neurodevelopmental disorder[41]NSC细胞NSC cells↓DNA甲基化↓DNA methylation↑P16↑P21细胞周期阻滞Cell cycle arrest[43]LUHMES细胞LUHMES cells↑H3K27me3↓TH细胞周期阻滞;多巴胺生物合成受抑制Cell cycle arrest; Inhibition of dopamine biosynthesis[44]汞 Hg甲基汞MeHg小鼠海马组织Mouse hippocampus↑DNA甲基化 ↑DNA methylation↓H3ac ↑H3K27me3↓BDNF抑郁Depression[45]ReNcell CX细胞ReNcell CX cells↓miR-25↑P53↑线粒体生成相关基因↑Genes involved in mitochondrial production线粒体功能受损Impaired mitochondrial function[46]其他重金属Other metals铜 Cu镍 Ni铅/镉 Pb/Cd斑马鱼嗅觉系统Zebrafish olfactory system↑miR-183/嗅觉神经元丢失Olfactory neuron loss[47]海马神经元细胞、小鼠海马组织Hippocampal neuron cells and mouse hippocampal tissue↓组蛋白乙酰化↓Histone acetylation全基因组Genome-wide树突复杂性下降,海马结构变化,学习记忆功能障碍Decreased complexity of dendrites, changes in hippocampal structure, learning and memory dysfunction[48]Neuro-2a细胞Neuro-2a cells↑miR-210↓ISCU1/2神经细胞线粒体功能障碍Mitochondrial dysfunction in nerve cells[49]大鼠海马组织Rat hippocampus↓H3ac↓H4ac全基因组Genome-wide学习记忆功能障碍Learning and memory dysfunction[51]有机物 Organics

续表1环境污染物Environmental pollutants研究对象Subjects表观遗传改变Epigenetic changes靶基因/蛋白质Target genes/Proteins神经毒性Neurotoxicity参考文献References多环芳烃PAHs多环芳烃PAHs苯并(a)芘B[a]P脐带血Cord blood↑DNA甲基化↑DNA methylation全基因组Genome-wide儿童神经发育异常Neurodevelopmental abnormalities in children[52]斑马鱼及其子代Zebrafish and their offspring↓DNA甲基化↓DNA methylation全基因组Genome-wide焦虑Anxiety[54]大鼠海马组织Rat hippocampus↓miRNA↑DNA甲基化↑DNA methylation↑lncRNA/学习记忆功能受损Impaired learning and memory function[55]小鼠皮质、海马组织Mouse cortex and hippocampus↑DNA甲基化↑DNA methylation↓NR2B短期记忆缺陷和焦虑样行为Short-term memory deficits and anxiety-like behaviors[56]小鼠脑组织Mouse brain tissue↑DNA甲基化↑DNA methylation↓BDNF认知和行为障碍Cognitive and behavioral disorders[57]HT22细胞HT22 cells↑DNA甲基化↑DNA methylation↓BDNF细胞肿胀和炎症Cell swelling and inflammation[57]双酚ABPA尿液Urine大鼠海马组织Rat hippocampus小鼠皮质、海马组织Mouse cortex and hippocampus小鼠皮质组织Mouse cortical tissue↑DNA甲基化↑DNA methylation↓GRIN2B神经发育疾病风险增加Increased risk of neurodevelopmental disorders[58]↑DNA甲基化↑DNA methylation↓BDNF学习记忆功能障碍Learning and memory dysfunction[59]↑DNA甲基化↑DNA methylation↓Fkbp5应激反应障碍Stress response disorder[60]↓DNA甲基化↓DNA methylation↑H3ac全基因组Genome-wide记忆损伤Memory impairment[61]↓H3ac↓H4ac↓Kcc2皮质神经网络受损Cortical neural networks were damaged[63]农药Pesticides百草枯Paraquat氯菊酯Permethrin狄氏剂Dieldrin阿拉特津AtrazineNeuro-2a细胞Neuro-2a cells↑DNA甲基化↑DNA methylation↓miR-17-5pN27细胞N27 cells↑H3ac↑PKCδ水解活化↑PKC δ hydrolytic activation斑马鱼及其子代Zebrafish and their offspring↑DNA甲基化↑DNA methylation↓fmr1↓pnocb雌性小鼠中脑黑质组织Female mice midbrain nigra organization↓DNA甲基化↓DNA methylation↑Nr4a2雄性小鼠中脑黑质组织Male mice midbrain nigra organization↑DNA甲基化↑DNA methylation↓Lmx1bPC12细胞PC12 cells↑H3ac↑H4ac ↑PKCδ水解活化↑PKC δ hydrolytic activationSH-SY5Y细胞SH-SY5Y cells↑DNA甲基化↑DNA methylation↓H3K9me3↓H3K27me3全基因组Genome-wide细胞凋亡Cell apoptosis[64]细胞凋亡Cell apoptosis[65]运动缺陷,焦虑Motor deficits and anxiety[66]多巴胺能神经元进行性退化,PDProgressive degeneration of dopaminergic neurons, PD[67]细胞凋亡Cell apoptosis[68]突触数量和长度变化,PD Changes in number and length of synapses and PD[69]
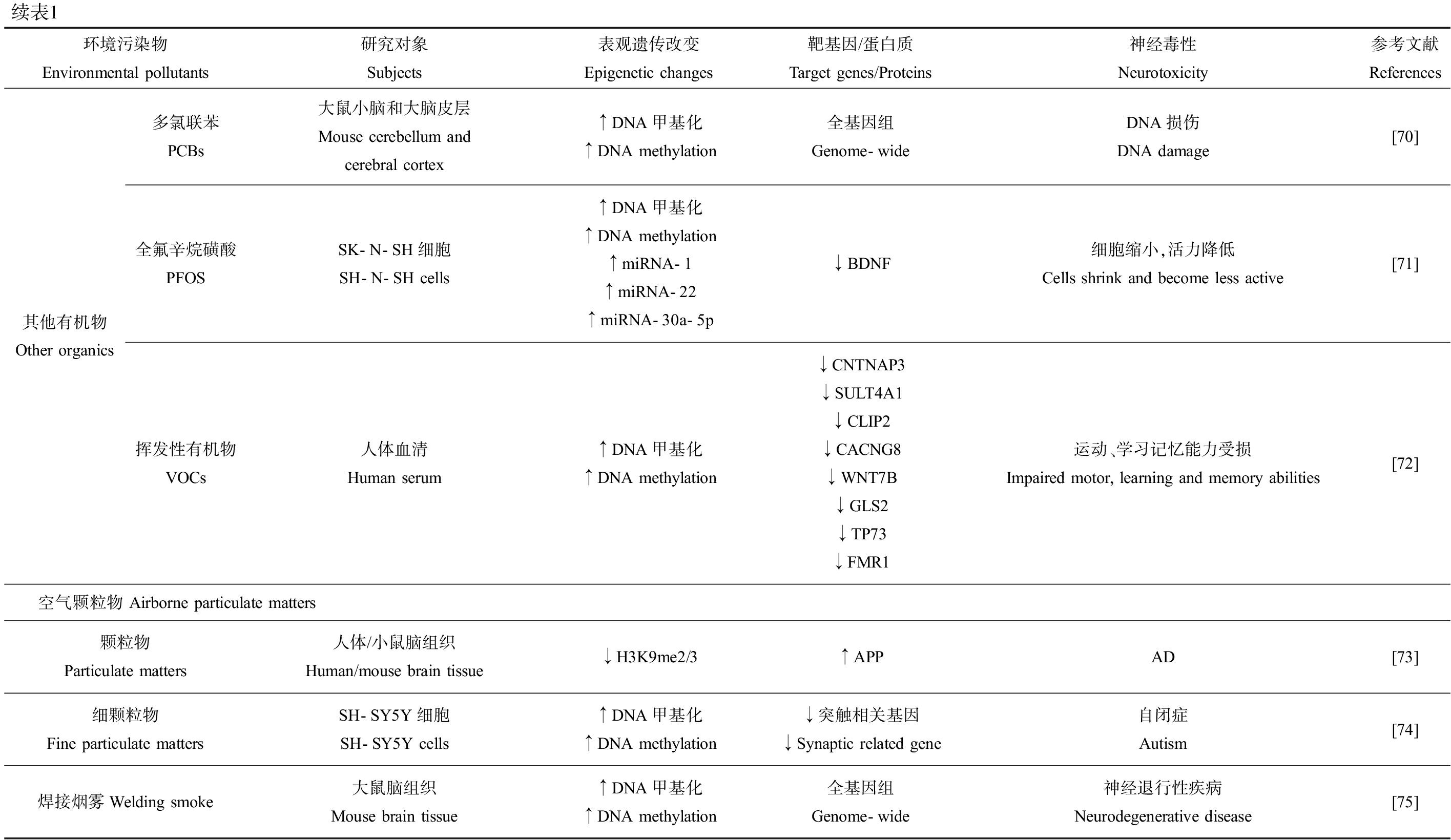
续表1环境污染物Environmental pollutants研究对象Subjects表观遗传改变Epigenetic changes靶基因/蛋白质Target genes/Proteins神经毒性Neurotoxicity参考文献References其他有机物Other organics多氯联苯PCBs大鼠小脑和大脑皮层Mouse cerebellum and cerebral cortex↑DNA甲基化↑DNA methylation全基因组Genome-wideDNA损伤DNA damage[70]全氟辛烷磺酸PFOSSK-N-SH细胞SH-N-SH cells↑DNA甲基化↑DNA methylation↑miRNA-1↑miRNA-22↑miRNA-30a-5p↓BDNF细胞缩小,活力降低Cells shrink and become less active[71]挥发性有机物VOCs人体血清Human serum↑DNA甲基化↑DNA methylation↓CNTNAP3 ↓SULT4A1↓CLIP2 ↓CACNG8↓WNT7B ↓GLS2↓TP73 ↓FMR1运动、学习记忆能力受损Impaired motor, learning and memory abilities[72]空气颗粒物 Airborne particulate matters颗粒物Particulate matters人体/小鼠脑组织Human/mouse brain tissue↓H3K9me2/3↑APPAD[73]细颗粒物Fine particulate mattersSH-SY5Y细胞SH-SY5Y cells↑DNA甲基化↑DNA methylation↓突触相关基因↓Synaptic related gene自闭症Autism[74]焊接烟雾Welding smoke大鼠脑组织Mouse brain tissue↑DNA甲基化↑DNA methylation全基因组Genome-wide神经退行性疾病Neurodegenerative disease[75]
其一,目前大多数研究都主要集中于DNA甲基化在环境污染物诱导神经毒性中的作用,而组蛋白修饰和ncRNA的相关研究还较少。因此,进一步探究这2种修饰在环境污染物暴露导致神经毒性中的作用,可加深我们对于相关表观遗传机制的理解。此外,目前的研究更多的是关注污染物对单一修饰的影响,而对于不同类型表观遗传机制之间的相互作用值得进一步研究。其二,人类处于一个复杂的环境中,同时暴露于各种污染物且浓度不一。但大多数研究均着眼于单一污染物通过表观遗传机制诱导神经毒性,极少关注混合暴露的毒性机制。因此,污染物联合暴露引起神经毒性的表观遗传机制研究亟待开展。其三,越来越多的研究证据表明,环境污染物可通过诱导神经炎症、氧化应激、内质网应激、线粒体功能障碍、髓鞘破坏和血脑屏障结构改变等途径引起神经毒性[76]。因此,进一步探讨这些生理变化与表观遗传信息改变之间的关系有望为环境污染物的神经毒性机制研究提供新思路。最后,年龄和性别似乎是环境污染物诱导神经毒性的易感窗口。例如,相较于青壮年,幼儿或老年这2个阶段似乎更容易受到外界环境压力干扰[77-78];此外,环境污染物的神经毒性作用可能存在性别特异性[79-81]。因此,从表观遗传的角度解释环境污染物诱导神经毒性的年龄易感性以及性别特异性也是未来的研究新方向。
[1] Struzynska L, Dabrowska-Bouta B, Koza K, et al.Inflammation-like glial response in lead-exposed immature rat brain[J].Toxicological Sciences, 2007, 95(1): 156-162
[2] Salahinejad A, Attaran A, Naderi M, et al.Chronic exposure to bisphenol S induces oxidative stress, abnormal anxiety, and fear responses in adult zebrafish(Danio rerio)[J].The Science of the Total Environment, 2021, 750: 141633
[3] Wang X J, Yan M L, Zhao L N, et al.Low-dose methylmercury-induced apoptosis and mitochondrial DNA mutation in human embryonic neural progenitor cells[J].Oxidative Medicine and Cellular Longevity, 2016, 2016: 5137042
[4] ChenX, Wu G Z, Zhang Z, et al.Neurotoxicity of Mn3O4nanoparticles: Apoptosis and dopaminergic neurons damage pathway[J].Ecotoxicology and Environmental Safety, 2020, 188: 109909
[5] Qureshi I A, Mehler M F.Epigenetic mechanisms underlying nervous system diseases[J].Handbook of Clinical Neurology, 2018, 147: 43-58
[6] Lennartsson A, Ekwall K.Histone modification patterns and epigenetic codes[J].Biochimica et Biophysica Acta, 2009, 1790(9): 863-868
[7] Strahl B D, David Allis C.The language of covalent histone modifications[J].Nature, 2000, 403(6765): 41-45
[8] Zhang Y J, Sun Z X, Jia J Q, et al.Overview of histone modification[J].Advances in Experimental Medicine and Biology, 2021, 1283: 1-16
[9] Mattick J S, Makunin I V.Non-coding RNA[J].Human Molecular Genetics, 2006, 15(1): R17-R29
[10] Qureshi I A, Mehler M F.Impact of nuclear organization and dynamics on epigenetic regulation in the central nervous system: Implications for neurological disease states[J].Annals of the New York Academy of Sciences, 2010, 1204(Suppl.): E20-E37
[11] Feng J, Zhou Y, Campbell S L, et al.Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons[J].Nature Neuroscience, 2010, 13(4): 423-430
[12] Michod D, Bartesaghi S, Khelifi A, et al.Calcium-dependent dephosphorylation of the histone chaperone DAXX regulates H3.3 loading and transcription upon neuronal activation[J].Neuron, 2012, 74(1): 122-135
[13] Long J M, Ray B, Lahiri D K.MicroRNA-339-5p down-regulates protein expression of β-site amyloid precursor protein-cleaving enzyme 1(BACE1)in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects[J].The Journal of Biological Chemistry, 2014, 289(8): 5184-5198
[14] MuhChyi C, Juliandi B, Matsuda T, et al.Epigenetic regulation of neural stem cell fate during corticogenesis[J].International Journal of Developmental Neuroscience, 2013, 31(6): 424-433
[15] Ijomone O M, Ijomone O K, Iroegbu J D, et al.Epigenetic influence of environmentally neurotoxic metals[J].Neurotoxicology, 2020, 81: 51-65
[16] Yu G X, Guo Z K, Li H Y.Analyses of epigenetic modification in environmental pollutants-induced neurotoxicity[J].Methods in Molecular Biology, 2021, 2326: 123-141
[17] Yang X J, Yuan Y Z, Lu X T, et al.The relationship between cognitive impairment and global DNA methylation decrease among aluminum potroom workers[J].Journal of Occupational and Environmental Medicine, 2015, 57(7): 713-717
[18] Yang X J, Yuan Y Z, Niu Q.Association between serum aluminium level and methylation of amyloid precursor protein gene in workers engaged in aluminium electrolysis[J].Chinese Journal of Industrial Hygiene and Occupational Diseases, 2016, 34(4): 255-258
[19] Yang X J, Yuan Y Z, Niu Q.Effects of aluminium chloride on the methylation of app in hippocampal of rats[J].Journal of Hygiene Research, 2016, 45(3): 345-349, 355
[20] Ikram M F, Farhat S M, Mahboob A, et al.Expression of DnMTs and MBDs in AlCl3-induced neurotoxicity mouse model[J].Biological Trace Element Research, 2021, 199(9): 3433-3444
[21] Yuan Y Z, Yang X J, Ren P, et al.Research of aluminum to the cognitive ability and genome-wide methylation in rats[J].Journal of Hygiene Research, 2015, 44(3): 359-363
[22] Pan B L, Zhou Y, Li H, et al.Relationship between occupational aluminium exposure and histone lysine modification through methylation[J].Journal of Trace Elements in Medicine and Biology: Organ of the Society for Minerals and Trace Elements(GMS), 2020, 61: 126551
[23] Li J, Zhang D D, Wang C Q, et al.Protective effects of low-intensity pulsed ultrasound on aluminum overload-induced cerebral damage through epigenetic regulation of brain-derived neurotrophic factor expression[J].Bioscience Reports, 2019, 39(1): BSR20181185
[24] Lin S, Shi Q, Nix F B, et al.A novel S-adenosyl-L-methionine: Arsenic(Ⅲ)methyltransferase from rat liver cytosol[J].The Journal of Biological Chemistry, 2002, 277(13): 10795-10803
[25] Du X Y, Tian M P, Wang X X, et al.Cortex and hippocampus DNA epigenetic response to a long-term arsenic exposure via drinking water[J].Environmental Pollution, 2018, 234: 590-600
[26] Tyler C R, Hafez A K, Solomon E R, et al.Developmental exposure to 50 parts-per-billion arsenic influences histone modifications and associated epigenetic machinery in a region-and sex-specific manner in the adult mouse brain[J].Toxicology and Applied Pharmacology, 2015, 288(1): 40-51
[27] Cronican A A, Fitz N F, Carter A, et al.Genome-wide alteration of histone H3K9 acetylation pattern in mouse offspring prenatally exposed to arsenic[J].PLoS One, 2013, 8(2): e53478
[28] Wang D J, Wang X D, Liu X F, et al.Inhibition of miR-219 alleviates arsenic-induced learning and memory impairments and synaptic damage through up-regulating CaMKⅡ in the hippocampus[J].Neurochemical Research, 2018, 43(4): 948-958
[29] Searles Nielsen S, Checkoway H, Criswell S R, et al.Inducible nitric oxide synthase gene methylation and Parkinsonism in manganese-exposed welders[J].Parkinsonism &Related Disorders, 2015, 21(4): 355-360
[30] Tarale P, Sivanesan S, Daiwile A P, et al.Global DNA methylation profiling of manganese-exposed human neuroblastoma SH-SY5Y cells reveals epigenetic alterations in Parkinson’s disease-associated genes[J].Archives of Toxicology, 2017, 91(7): 2629-2641
[31] Wang L Y, Shiraki A, Itahashi M, et al.Aberration in epigenetic gene regulation in hippocampal neurogenesis by developmental exposure to manganese chloride in mice[J].Toxicological Sciences, 2013, 136(1): 154-165
[32] Maccani J Z J, Koestler D C, Houseman E A, et al.DNA methylation changes in the placenta are associated with fetal manganese exposure[J].Reproductive Toxicology, 2015, 57: 43-49
[33] Guo Z K, Zhang Z P, Wang Q Q, et al.Manganese chloride induces histone acetylation changes in neuronal cells: Its role in manganese-induced damage[J].Neurotoxicology, 2018, 65: 255-263
[34] Sobolewski M, Varma G, Adams B, et al.Developmental lead exposure and prenatal stress result in sex-specific reprograming of adult stress physiology and epigenetic profiles in brain[J].Toxicological Sciences, 2018, 163(2): 478-489
[35] Schneider J S, Kidd S K, Anderson D W.Influence of developmental lead exposure on expression of DNA methyltransferases and methyl cytosine-binding proteins in hippocampus[J].Toxicology Letters, 2013, 217(1): 75-81
[36] Dosunmu R, Alashwal H, Zawia N H.Genome-wide expression and methylation profiling in the aged rodent brain due to early-life Pb exposure and its relevance to aging[J].Mechanisms of Ageing and Development, 2012, 133(6): 435-443
[37] Basha M R, Wei W, Bakheet S A, et al.The fetal basis of amyloidogenesis: Exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain[J].The Journal of Neuroscience, 2005, 25(4): 823-829
[38] Eid A, Bihaqi S W, Renehan W E, et al.Developmental lead exposure and lifespan alterations in epigenetic regulators and their correspondence to biomarkers of Alzheimer’s disease[J].Alzheimer’s &Dementia, 2016, 2: 123-131
[39] Masoud A M, Bihaqi S W, Machan J T, et al.Early-life exposure to lead(Pb)alters the expression of microRNA that target proteins associated with Alzheimer’S disease[J].Journal of Alzheimer’s Disease, 2016, 51(4): 1257-1264
[40] Cardenas A, Rifas-Shiman S L, Agha G, et al.Persistent DNA methylation changes associated with prenatal mercury exposure and cognitive performance during childhood[J].Scientific Reports, 2017, 7(1): 288
[41] Cao M X, Song F, Yang X, et al.Identification of potential long noncoding RNA biomarker of mercury compounds in zebrafish embryos[J].Chemical Research in Toxicology, 2019, 32(5): 878-886
[42] Ke T, Gonçalves F M, Gonçalves C L, et al.Post-translational modifications in MeHg-induced neurotoxicity[J].Biochimica et Biophysica Acta Molecular Basis of Disease, 2019, 1865(8): 2068-2081
[43] Bose R, Onishchenko N, Edoff K, et al.Inherited effects of low-dose exposure to methylmercury in neural stem cells[J].Toxicological Sciences, 2012, 130(2): 383-390
[44] Go S, Kurita H, Matsumoto K, et al.Methylmercury causes epigenetic suppression of the tyrosine hydroxylase gene in an in vitroneuronal differentiation model[J].Biochemical and Biophysical Research Communications, 2018, 502(4): 435-441
[45] Onishchenko N, Karpova N, Sabri F, et al.Long-lasting depression-like behavior and epigenetic changes of BDNFgene expression induced by perinatal exposure to methylmercury[J].Journal of Neurochemistry, 2008, 106(3): 1378-1387
[46] Wang X J, Yan M L, Zhao L N, et al.Low-dose methylmercury-induced genes regulate mitochondrial biogenesis via miR-25 in immortalized human embryonic neural progenitor cells[J].International Journal of Molecular Sciences, 2016, 17(12): 2058
[47] Wang L, Bammler T K, Beyer R P, et al.Copper-induced deregulation of microRNA expression in the zebrafish olfactory system[J].Environmental Science &Technology, 2013, 47(13): 7466-7474
[48] Zhou C, Liu M Y, Mei X, et al.Histone hypoacetylation contributes to neurotoxicity induced by chronic nickel exposure in vivoand in vitro[J].Science of the Total Environment, 2021, 783: 147014
[49] He M, Lu Y, Xu S, et al.MiRNA-210 modulates a nickel-induced cellular energy metabolism shift by repressing the iron-sulfur cluster assembly proteins ISCU1/2in Neuro-2a cells[J].Cell Death &Disease, 2014, 5(2): e1090
[50] Tong W H, Rouault T A.Functions of mitochondrial ISCU and cytosolic ISCU in mammalian iron-sulfur cluster biogenesis and iron homeostasis[J].Cell Metabolism, 2006, 3(3): 199-210
[51] Zhou R Q, Zhao J, Li D Y, et al.Combined exposure of lead and cadmium leads to the aggravated neurotoxicity through regulating the expression of histone deacetylase 2[J].Chemosphere, 2020, 252: 126589
[52] Lee J, Kalia V, Perera F, et al.Prenatal airborne polycyclic aromatic hydrocarbon exposure, LINE1 methylation and child development in a Chinese cohort[J].Environment International, 2017, 99: 315-320
[53] Chepelev N L, Moffat I D, Bowers W J, et al.Neurotoxicity may be an overlooked consequence of benzo[a]pyrene exposure that is relevant to human health risk assessment[J].Mutation Research Reviews in Mutation Research, 2015, 764: 64-89
[54] Knecht A L, Truong L, Marvel S W, et al.Transgenerational inheritance of neurobehavioral and physiological deficits from developmental exposure to benzo[a]pyrene in zebrafish[J].Toxicology and Applied Pharmacology, 2017, 329: 148-157
[55] Wang J, Li C L, Tu B J, et al.Integrated epigenetics, transcriptomics, and metabolomics to analyze the mechanisms of benzo[a]pyrene neurotoxicity in the hippocampus[J].Toxicological Sciences, 2018, 166(1): 65-81
[56] Zhang W P, Tian F J, Zheng J P, et al.Chronic administration of benzo(a)pyrene induces memory impairment and anxiety-like behavior and increases of NR2B DNA methylation[J].PLoS One, 2016, 11(2): e0149574
[57] Li Y Y, Cao J J, Hao Z S, et al.Aspirin ameliorates the cognition impairment in mice following benzo[a]pyrene treatment via down-regulating BDNF IV methylation[J].Neurotoxicology, 2022, 89: 20-30
[58] Alavian-Ghavanini A, Lin P I, Lind P M, et al.Prenatal bisphenol A exposure is linked to epigenetic changes in glutamate receptor subunit gene Grin2b in female rats and humans[J].Scientific Reports, 2018, 8(1): 11315
[59] Cheong A, Johnson S A, Howald E C, et al.Gene expression and DNA methylation changes in the hypothalamus and hippocampus of adult rats developmentally exposed to bisphenol A or ethinyl estradiol: A CLARITY-BPA consortium study[J].Epigenetics, 2018, 13(7): 704-720
[60] Kitraki E, Nalvarte I, Alavian-Ghavanini A, et al.Developmental exposure to bisphenol A alters expression and DNA methylation of Fkbp5, an important regulator of the stress response[J].Molecular and Cellular Endocrinology, 2015, 417: 191-199
[61] Kumar D, Thakur M K.Effect of perinatal exposure to bisphenol-A on DNA methylation and histone acetylation in cerebral cortex and hippocampus of postnatal male mice[J].The Journal of Toxicological Sciences, 2017, 42(3): 281-289
[62] Bortone D, Polleux F.KCC2expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner[J].Neuron, 2009, 62(1): 53-71
[63] Yeo M, Berglund K, Hanna M, et al.Bisphenol A delays the perinatal chloride shift in cortical neurons by epigenetic effects on the Kcc2 promoter[J].Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(11): 4315-4320
[64] Zhan Y T, Guo Z K, Zheng F L, et al.Reactive oxygen species regulate miR-17-5p expression via DNA methylation in paraquat-induced nerve cell damage[J].Environmental Toxicology, 2020, 35(12): 1364-1373
[65] Song C, Kanthasamy A, Jin H, et al.Paraquat induces epigenetic changes by promoting histone acetylation in cell culture models of dopaminergic degeneration[J].Neurotoxicology, 2011, 32(5): 586-595
[66] Blanc M, Antczak P, Cousin X, et al.The insecticide permethrin induces transgenerational behavioral changes linked to transcriptomic and epigenetic alterations in zebrafish(Danio rerio)[J].The Science of the Total Environment, 2021, 779: 146404
[67] Kochmanski J, VanOeveren S E, Patterson J R, et al.Developmental dieldrin exposure alters DNA methylation at genes related to dopaminergic neuron development and Parkinson’s disease in mouse midbrain[J].Toxicological Sciences, 2019, 169(2): 593-607
[68] Song C, Kanthasamy A, Anantharam V, et al.Environmental neurotoxic pesticide increases histone acetylation to promote apoptosis in dopaminergic neuronal cells: Relevance to epigenetic mechanisms of neurodegeneration[J].Molecular Pharmacology, 2010, 77(4): 621-632
[69] Xie J K, Lin L, Sánchez O F, et al.Pre-differentiation exposure to low-dose of atrazine results in persistent phenotypic changes in human neuronal cell lines[J].Environmental Pollution, 2021, 271: 116379
![]() M E.Assessment of epigenetic changes and oxidative DNA damage in rat pups exposed to polychlorinated biphenyls and the protective effect of curcumin in the prenatal period[J].Journal of Basic and Clinical Physiology and Pharmacology, 2019, 30(3).doi: 10.1515/jbcpp-2018-0182
M E.Assessment of epigenetic changes and oxidative DNA damage in rat pups exposed to polychlorinated biphenyls and the protective effect of curcumin in the prenatal period[J].Journal of Basic and Clinical Physiology and Pharmacology, 2019, 30(3).doi: 10.1515/jbcpp-2018-0182
[71] Guo X X, He Q Z, Li W, et al.Brain-derived neurotrophic factor mediated perfluorooctane sulfonate induced-neurotoxicity via epigenetics regulation in SK-N-SH cells[J].International Journal of Molecular Sciences, 2017, 18(4): 893
[72] Yu S Y, Koh E J, Kim S H, et al.Integrated analysis of multi-omics data on epigenetic changes caused by combined exposure to environmental hazards[J].Environmental Toxicology, 2021, 36(6): 1001-1010
[73] Calderón-Garcidue as L, Herrera-Soto A, Jury N, et al.Reduced repressive epigenetic marks, increased DNA damage and Alzheimer’s disease hallmarks in the brain of humans and mice exposed to particulate urban air pollution[J].Environmental Research, 2020, 183: 109226
as L, Herrera-Soto A, Jury N, et al.Reduced repressive epigenetic marks, increased DNA damage and Alzheimer’s disease hallmarks in the brain of humans and mice exposed to particulate urban air pollution[J].Environmental Research, 2020, 183: 109226
[74] Wei H Y, Liang F, Meng G, et al.Redox/methylation mediated abnormal DNA methylation as regulators of ambient fine particulate matter-induced neurodevelopment related impairment in human neuronal cells[J].Scientific Reports, 2016, 6: 33402
[75] Shoeb M, Mustafa G M, Kodali V K, et al.A possible relationship between telomere length and markers of neurodegeneration in rat brain after welding fume inhalation exposure[J].Environmental Research, 2020, 180: 108900
[76] Iqubal A, Ahmed M, Ahmad S, et al.Environmental neurotoxic pollutants: Review[J].Environmental Science and Pollution Research, 2020, 27(33): 41175-41198
[77] Oh J, Schmidt R J, Tancredi D, et al.Prenatal exposure to per-and polyfluoroalkyl substances and cognitive development in infancy and toddlerhood[J].Environmental Research, 2021, 196: 110939
[78] Przybyla J, Houseman E A, Smit E, et al.A path analysis of multiple neurotoxic chemicals and cognitive functioning in older US adults(NHANES 1999-2002)[J].Environmental Health, 2017, 16(1): 19
[79] Gade M, Comfort N, Re D B.Sex-specific neurotoxic effects of heavy metal pollutants: Epidemiological, experimental evidence and candidate mechanisms[J].Environmental Research, 2021, 201: 111558
[80] Venerosi A, Ricceri L, Tait S, et al.Sex dimorphic behaviors as markers of neuroendocrine disruption by environmental chemicals: The case of chlorpyrifos[J].Neurotoxicology, 2012, 33(6): 1420-1426
[81] Zhang L B, Liu Y, Chen H G, et al.Transcriptome analysis reveals sex-specific alterations in gonads of green mussel exposed to organophosphorus insecticide triazophos[J].Comparative Biochemistry and Physiology Part C: Toxicology &Pharmacology, 2022, 257: 109333