海参属于无脊椎动物棘皮动物纲,是一种常见的海洋无脊椎动物[1]。刺参(Apostichopus japonicas)是海参的一种,富含氨基酸、脂肪酸、胶原蛋白和皂甙等生物活性成分[2],具有较高食用和药用价值,是中国最具养殖优势和经济价值的物种之一。近年来,在市场需求的推动下,中国的海参养殖业发展迅速[3]。不过,海参养殖业的健康发展受到养殖海水质量的极大影响,一旦养殖海水受到环境污染物例如人类用药的污染,则会对海参养殖业造成潜在的健康影响。金刚烷胺是一种用于治疗帕金森病的运动障碍和流感预防的药物[4-5],因其价格低廉且能够有效抑制流感病毒活性等特点,曾被用于畜禽养殖中流感病毒的防治。但流感病毒的肆虐和部分养殖户的非法使用,加剧了环境中金刚烷胺的积累,过量使用的金刚烷胺进入水体,从而对水体生态环境产生破坏[6-7]。研究证实,我国部分海域已受到金刚烷胺污染,且金刚烷胺能够在生物体内富集[8-10]。中国农业部发布560号公告禁止金刚烷胺类人用抗病毒药移植兽用,但研究发现海水和刺参中仍能检出金刚烷胺,刺参中检出量为4.3 μg·kg-1[11],而部分养殖海水中最高可达140 ng·L-1[12]。
现如今,关于金刚烷胺的研究主要为建立畜禽产品中金刚烷胺的检测方法[13-15],水产品中金刚烷胺的研究较少[16-18],金刚烷胺胁迫后刺参的毒性效应研究仍处于相对空白的阶段。因此,有必要对金刚烷胺胁迫后刺参的响应机制和生物效应进行表征。代谢组学是一种新兴的组学技术,是系统生物学的重要组成部分。通过筛选实验组与对照组的差异代谢物,可以探究细胞、组织、器官甚至整个生物体的生理、发育或病理状态的变化[19]。通过代谢组学技术筛选获得的特定生物标志物,能够深入了解金刚烷胺胁迫后刺参的响应调控机制。
1 材料与方法(Materials and methods)
1.1 实验动物、试剂
本研究所用刺参为山东安源种业科技有限公司提供的人工繁育刺参,平均体质量(50±3.0) g。实验前选择健康、活力好的刺参,于实验室60 L长方体玻璃水槽中暂养7 d,连续充氧,水温13~15 ℃,每24 h换水1次,清理刺参粪便,换水量为1/2,暂养和后续实验中均禁食,实验用海水均为新鲜、清洁海水。
金刚烷胺(上海麦克林生化科技有限公司),纯度≥98%。甲醇、甲酸和醋酸铵(纯度为LC-MS Grade)均购自美国Thermo公司,质谱级水购自德国Merck公司。
1.2 仪器和设备
质谱仪(型号Q ExactiveTM HF,德国Thermo);色谱仪(型号Vanquish UHPLC,德国Thermo);色谱柱(型号Hypesil Gold column,美国Thermo);低温离心机(型号D3024R,美国Scilogex)
1.3 实验方法
1.3.1 动物实验及模型建立
将金刚烷胺配制成100 mg·L-1的储备液,置于4 ℃冰箱保存。实验水缸中每缸加入30 L海水,加入金刚烷胺储备液,使缸内金刚烷胺浓度达到100 μg·L-1。本次实验共2组,分别为对照组和实验组,每组4个平行,每个平行投放15只左右刺参,同时用正常海水养殖组作为对照组。暂养结束后,挑选健康、活力好的刺参随机投入,以便进行后续实验,实验共持续96 h。
1.3.2 样品采集
暴露实验结束后,随机抽取4个平行组的6只刺参,将每只刺参的肠道一分为六,对照组和实验组分别设置6个平行样本,放置于2 mL冻存管中,取样结束后,液氮速冻,然后置于-80 ℃的冰箱中待检。
1.3.3 代谢物提取
取100 mg液氮研磨的刺参肠道样本,置于EP管中,加500 μL的80%甲醇水溶液;涡旋震荡后冰浴静置5 min,15 000 g条件下4 ℃离心20 min;取部分上清液加质谱级水稀释至甲醇含量为53%;15 000 g条件下4 ℃离心20 min,收集上清,进样供LC-MS/MS分析[20]。
1.3.4 LC-MS检测
色谱条件:选择Hypesil Gold column(C18)色谱柱,柱温为40 ℃,流速为0.2 mL·min-1。正离子模式下,流动相A为0.1%甲酸,流动相B为甲醇;负离子模式下,流动相A为5 mmol·L-1醋酸铵,pH 9.0,流动相B为甲醇。梯度洗脱程序见表1。
表1 流动相梯度洗脱程序
Table 1 Gradient elution program
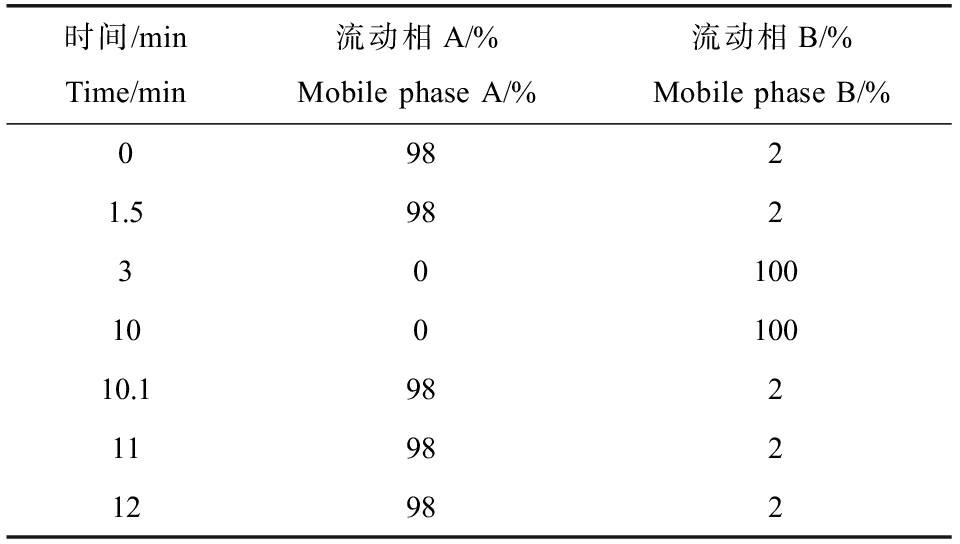
时间/minTime/min流动相A/%Mobile phase A/%流动相B/%Mobile phase B/%09821.59823010010010010.19821198212982
质谱条件:扫描范围选择m/z 100~1 500;ESI源的设置为喷雾电压(spray voltage) 3.5 kV,鞘气流速(sheath gas flow rate) 244 976.7 Pa,辅助气流速(aux gas flow rate) 10 L·min-1,离子传输管温度(capillary temp) 320 ℃,离子导入射频电平(S-lens RF level) 60,辅助气加热器温度(aux gas heater temp) 350 ℃,极性(polarity)为positive, negative,MS/MS二级扫描为数据依赖性扫描(data-dependent scans)。
1.4 数据处理
原始数据文件使用CD3.1(Thermo Fisher)进行处理,对每种代谢物进行峰对准、峰选择和定量。将峰强度归一化为总光谱强度,然后用于基于加性离子、分子离子峰和片段离子的分子式预测。然后将峰值与mzCloud、mzVault和MassList数据库进行匹配,以获得准确的定性和相对定量结果。
使用KEGG数据库(https://www.genome.jp/kegg)和HMDB数据库(https://hmdb.ca/metabolites)对鉴定的代谢物进行注释。使用MetaX软件通过主成分分析(PCA)和偏最小二乘判别分析(PLS-DA)评估处理后的数据。通过排列试验验证了PLS-DA模型,以R2和Q2值作为拟合优劣的参数。根据变量投影重要度VIP>1,且t检验P<0.05,利用差异倍数(fold-change)FC>1.2筛选表达量显著增加的代谢物,FC<0.833筛选表达量显著下降的代谢物。最后,使用KEGG数据库对差异表达的代谢产物进行富集通路分析。
2 结果(Results)
2.1 PCA分析和PLS-DA分析
在本研究PCA的得分图中,每个点代表一个独立的样品,剔除异常样本后重新进行PCA分析后结果如图1所示,对照组和实验组样品具有明显的分离,2组样品都分布在置信区间(95%)内,说明2组样品之间的代谢产物存在明显差异。为更充分筛选2组样品间的差异代谢物,进一步对刺参肠道组织样本采用PLS-DA数据分析。结果如图2所示,实验组和对照组的样品点置于95%的置信区间内,但分布在不同的区域且明显不相交,2组样品实现了完全分离,表明金刚烷胺胁迫后的海参肠道组织与对照组中的代谢物具有明显的差异。为进一步验证该模型是否过拟合,对模型进行排序验证,结果显示,R2数据大于Q2数据且Q2回归线与Y轴截距<0(R2=0.81,Q2=-1.54),说明预测能力较好,没有出现过拟合现象,可用于后续分析。

图1 金刚烷胺在刺参实验组与对照组对比的PCA得分图
注:CGM表示对照组;TGM表示实验组。
Fig. 1 PCA score chart of amantadine in the exposure group and control group of A. japonicus
Note: CGM denotes control group; TGM denotes experimental group.
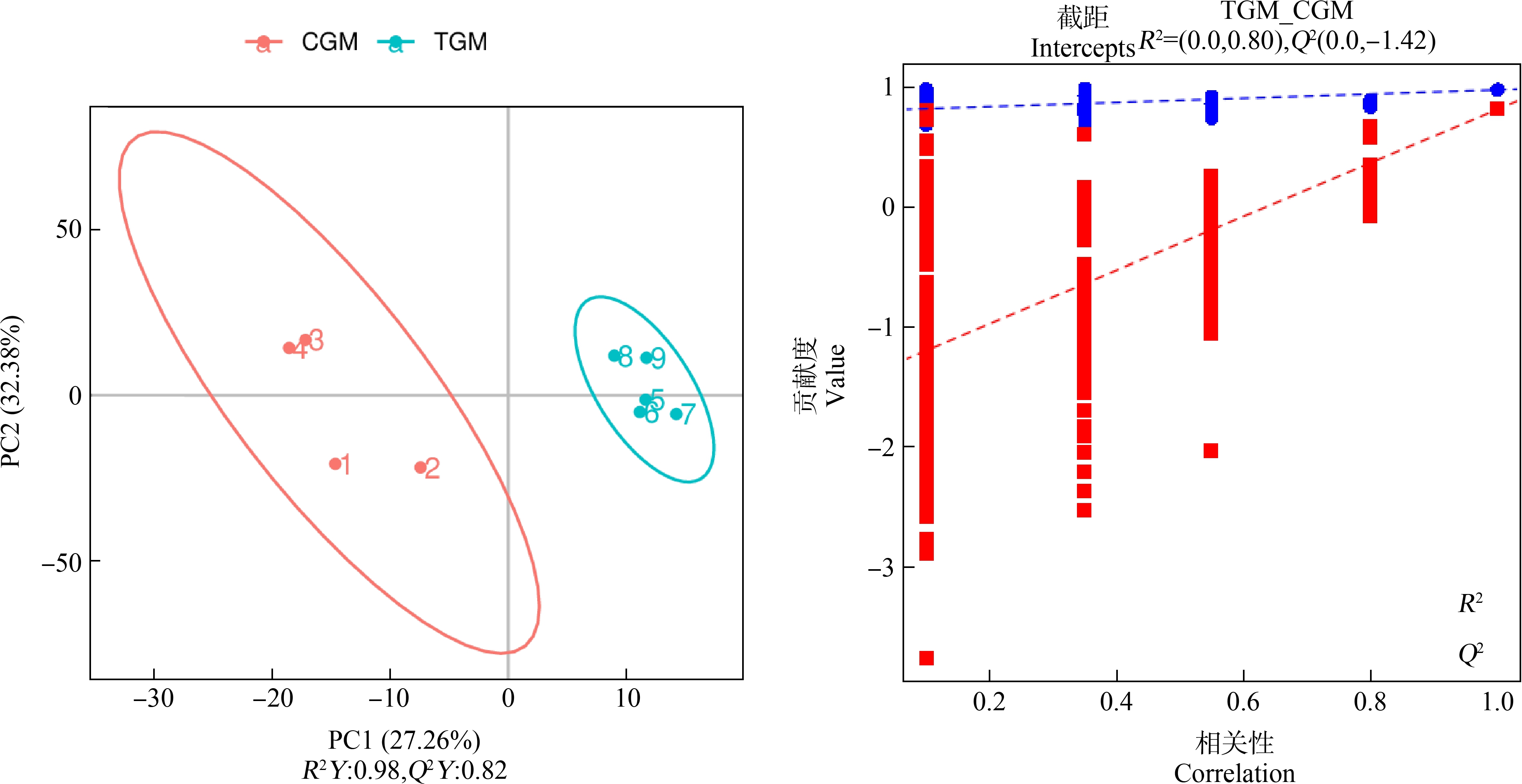
图2 金刚烷胺在刺参实验组与对照组对比的PLS-DA得分图
Fig. 2 PLS-DA score chart of amantadine in the exposure group and control group of A. japonicus
2.2 差异代谢物筛选
从PLS-DA模型中获得投影中的变量重要性(VIP)值,并选择VIP>1、P<0.05和FC>1.2,或FC<0.833的代谢物作为显著不同的代谢物。2组样品总共筛选出了115个差异代谢物,并将这些差异代谢物以可视化的火山图进行展示,得到实验组和对照组的差异代谢物如图3所示,其中上调表达的差异代谢物有72个,下调表达有43个。

图3 金刚烷胺在刺参实验组与对照组的差异代谢物火山图
Fig. 3 Volcano mapof differential metabolites of amantadine in the exposure group and the control group of A. japonicus
为了更全面、直观地展示样品之间的关系,对获得的各组差异代谢物进行层次聚类分析,得出同一比较对2组之间和组内代谢表达模式的差异情况,如图4所示。来自同一组的样本聚集在一个簇中,表明它们的代谢物具有相似的表达模式,可能具有相似的功能或者可能共享相同的代谢过程。聚类热图显示,实验组和对照组的差异代谢物分为2个主要聚类,表明本研究筛选的差异代谢物可以显著区分。
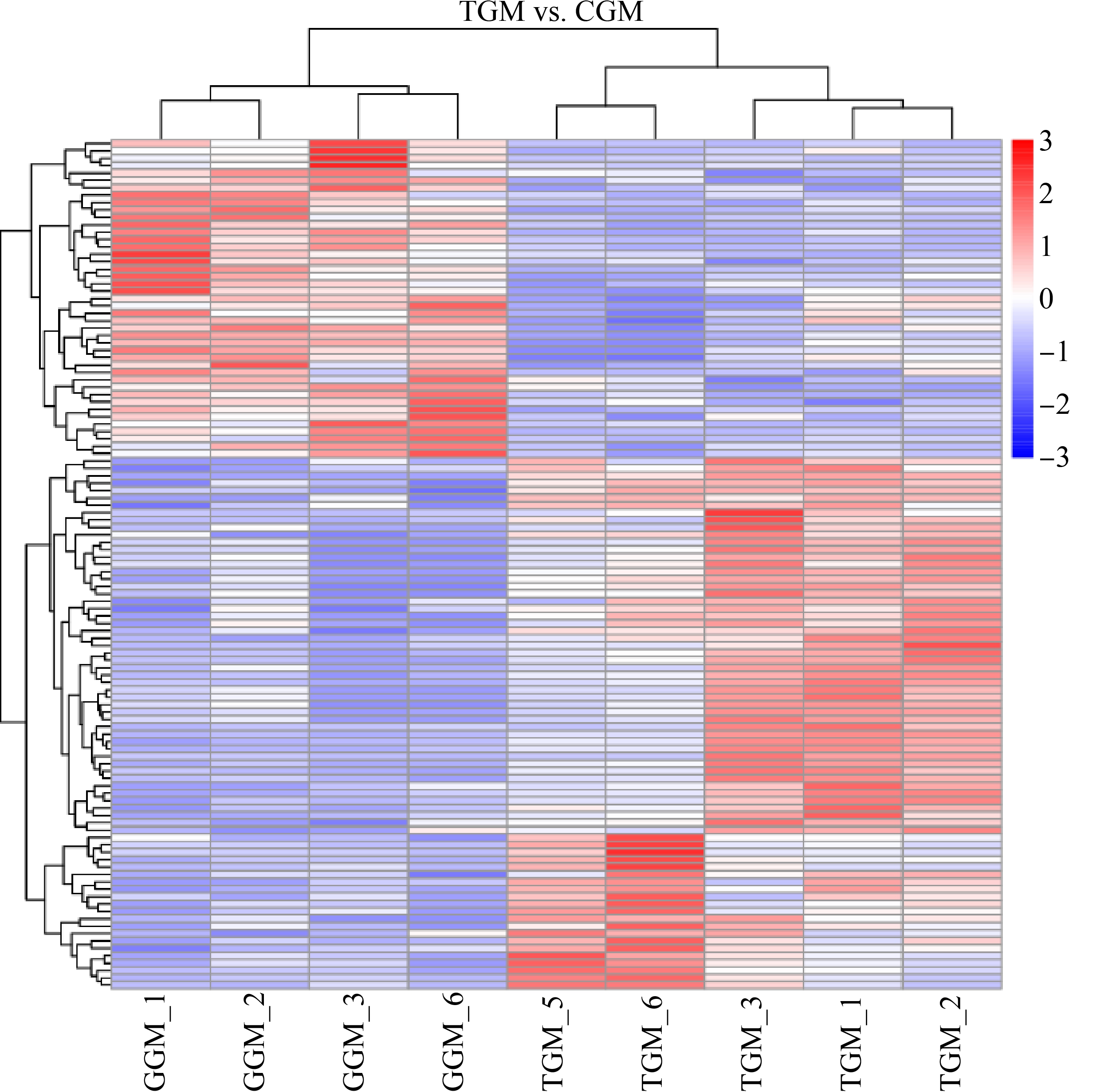
图4 金刚烷胺在刺参实验组与对照组差异代谢物聚类热图
Fig. 4 Heat map of differential metabolites of amantadine in the exposure group and the control group of A. japonicus
将主要差异代谢物在HMDB数据库中进行比对分析,注释到37个差异代谢物,这些代谢物主要为有机酸和衍生物(缬氨酸-丝氨酸(Val-Ser)、N-甘氨酰-L-脯氨酸(N-glycyl-L-proline)和酪氨酰丙氨酸(tyrosylalanine)等)、脂类和类脂分子(睾酮(testosterone)、甲基睾酮(methyltestosterone)和牛磺鹅去氧胆酸(taurochenodeoxycholic acid (TCDCA))等)以及核苷、核苷酸和类似物(腺苷(adenosine)、单磷酸鸟苷(guanosine monophosphate (GMP))和单磷酸腺苷(adenosine 5’-monophosphate (AMP))等(图5)。
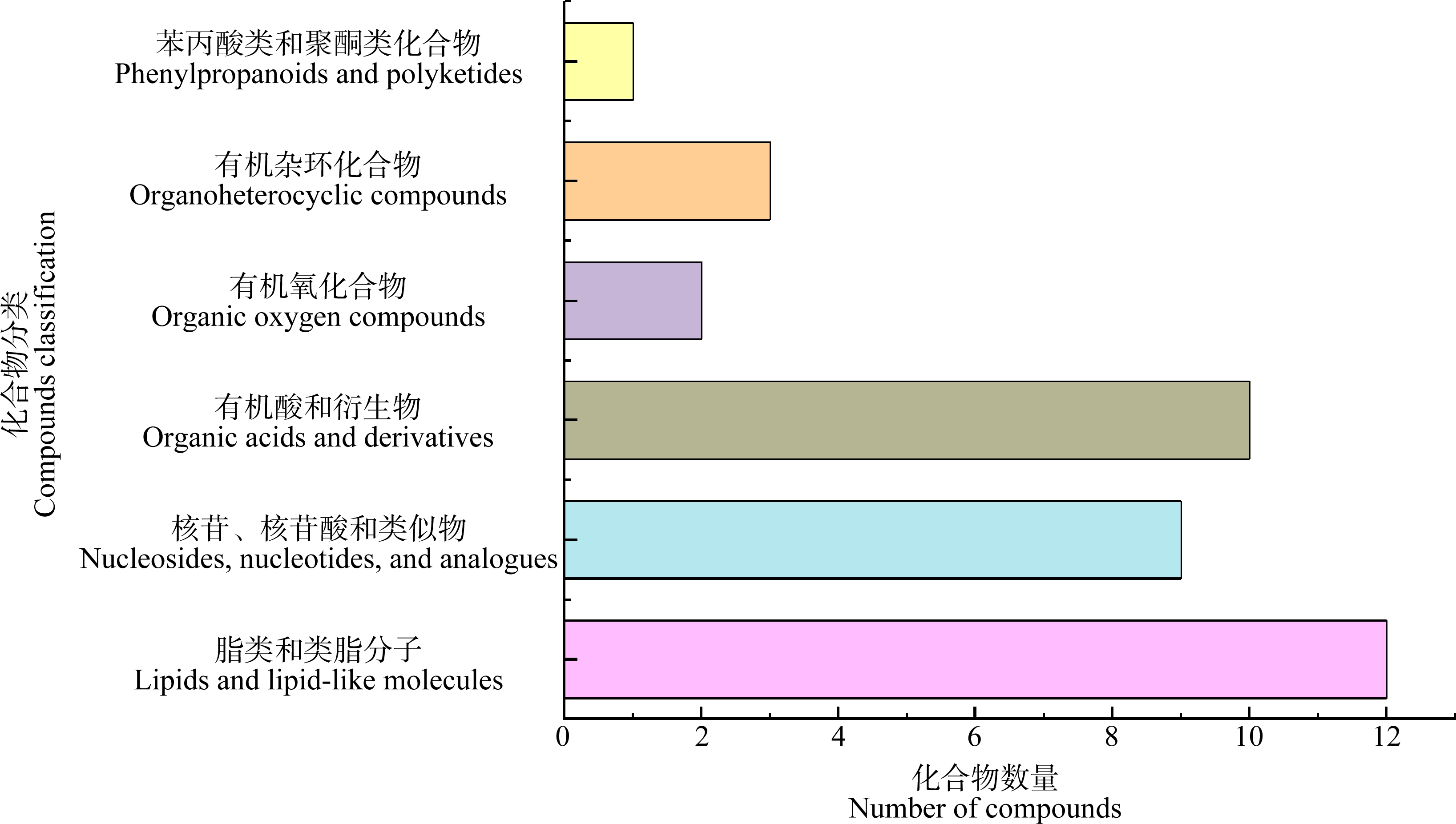
图5 金刚烷胺在刺参实验组与对照组差异代谢物化合物分类图
Fig. 5 Compound classification chart of different metabolites of amantadine in the exposure group and control group of A. japonicus
2.3 差异代谢通路分析
为进一步探究金刚烷胺对海参肠道的影响,将筛选出的差异代谢进行KEGG功能通路注释分析,金刚烷胺导致刺参肠道损伤的作用机制可能与2类(新陈代谢、有机体系统)8条代谢通路有关,主要有氨基酸代谢、核苷酸代谢、类脂物代谢作用、消化系统、内分泌系统和感觉系统通路等(图6)。
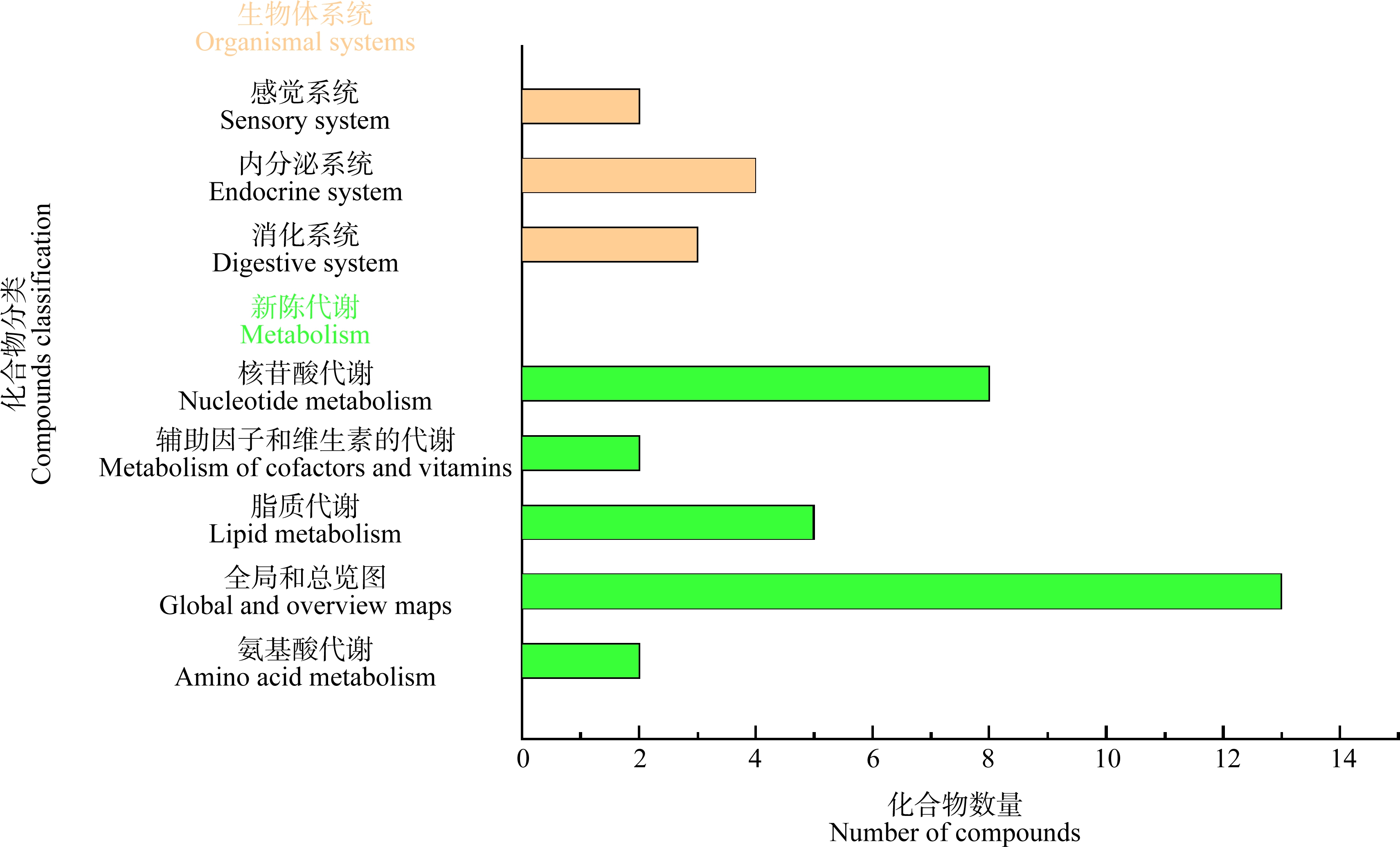
图6 金刚烷胺在刺参实验组与对照组差异代谢物的KEGG功能通路统计图
Fig. 6 KEGG functional pathway statistic chart of different metabolites of amantadine in the exposure group and control group of A. japonicus
3 讨论(Discussion)
嘌呤和嘧啶作为核酸(即DNA、RNA)的必需成分,能够调节细胞代谢,参与脂质和碳水化合物代谢以及信号转导[21-22]。研究发现,单磷酸鸟苷(GMP)、单磷酸腺苷(AMP)和腺苷含量下降,将会对cAMP、cGMP-PKG、mTOR和PI3K-Akt等信号通路产生影响。胞外核苷酸酶将血浆中的AMP转化为腺苷,腺苷激活腺苷环化酶(AC),AC促进ATP向cAMP的分裂[23-24]。NO可以通过鸟苷酸环化酶起作用以诱导cGMP的合成。受cGMP和cAMP调控的cGMP-PKG和cAMP信号通路与钙离子的稳态相关,钙浓度增加可以干扰细胞内信号转导,以触发神经酰胺等细胞死亡效应物,从而导致线粒体和内质网的不可逆损伤。PKG磷酸化细胞蛋白上的丝氨酸和苏氨酸残基,激活后丝氨酸和苏氨酸能够激活AMPK信号通路,加速细胞内ATP的消耗[25]。因此,我们推测金刚烷胺可能通过影响刺参cGMP-PKG信号通路和AMPK信号通路导致刺参能量代谢紊乱。
类固醇激素能够维持生物的正常生理功能、生殖、新陈代谢和免疫功能[26-27]。类固醇性激素包最重要的衍生物分别是人体和动物体内的睾酮、雌二醇和孕酮[28]。雄性和雌性动物中类固醇性激素(包括睾酮、雌二醇和黄体酮)的合成和分泌能够影响其生殖能力,而甲基睾酮能够抑制内源性睾酮产生。因此,金刚烷胺可能通过增加甲基睾酮含量,来抑制睾酮的产生,进而影响内分泌抵抗通路和类固醇降解通路[29],扰乱刺参的生殖能力。牛磺鹅去氧胆酸(TCDCA)是胆汁酸的主要有效成分之一,在本研究中,TCDCA对原发性胆汁酸生物合成、二级胆汁酸生物合成和胆固醇代谢产生影响[30-31]。胆汁酸合成与胆固醇相关,同时,研究发现cAMP水平的降低可能影响胆固醇的产生[32],胆固醇是构成细胞膜的重要组成成分,而金刚烷胺能够影响细胞膜形态[33-34],因此,金刚烷胺可能通过影响cGMP-PKG信号通路和降低TCDCA含量减少胆固醇含量从而造成细胞膜损伤。
游离氨基酸及其分解代谢物能够平衡海洋软体动物以及其他海洋无脊椎动物细胞内渗透压[35]。研究显示,实验组中的含甘氨酸、脯氨酸和酪氨酸等二肽含量发生改变。胶原蛋白主要由成纤维细胞通过蛋白质合成途径由化、肽形成的起始、肽延伸、终止和翻译后修饰,其中,含甘氨酸和脯氨酸的二肽含量上升,说明实验组刺参的胶原蛋白可能发生降解[36-37]。此外,甘氨酸与GSH代谢通路有关,能够调控金刚烷胺胁迫引起的氧化应激反应[38]。正常生理条件下,苯丙氨酸被羟基化为酪氨酸,酪氨酸可以通过酶促快速修复受损细胞。本研究中含酪氨酸的二肽含量减少且苯丙氨酸的代谢通路受到影响,表明苯丙氨酸向酪氨酸的转化可能受到抑制[39-40]。这些研究表明,金刚烷胺可能影响蛋白质合成,从而导致刺参的胶原蛋白降解,同时,还将通过苯丙氨酸途径减少酪氨酸的合成,从而加重刺参的细胞受损。
本研究通过金刚烷胺胁迫刺参,实验组和对照组研究结果显示,通过PLS-DA分析(VIP>1)、t检验(P<0.05)和FC>1.2,或FC<0.833筛选了115个差异代谢物。其中,腺苷和AMP等核苷和核苷酸含量下调,睾酮和TCDCA含量下调,含甘氨酸、脯氨酸二肽含量上调。
对代谢通路进行分析后发现,金刚烷胺可能通过降低TCDCA含量影响胆固醇代谢,从而改变细胞膜形态。细胞膜形态发生改变,抑制氨基酸的摄取。腺苷和AMP等核苷酸含量降低导致刺参的信号转导和能量代谢通路异常。甘氨酸和脯氨酸能够合成胶原蛋白,细胞膜发生形变后,外部摄入的甘氨酸和脯氨酸减少和金刚烷胺胁迫后,可能导致刺参内部胶原蛋白降解,从而使甘氨酸和脯氨酸的含量升高。本研究讨论了刺参在这些代谢水平上对金刚烷胺应激的毒理学反应和其他响应调控机制,并为这些潜在机制提供了新的见解。但仍有必要进行进一步的研究,以验证分离的差异代谢物,并阐明金刚烷胺对刺参的毒性机制和其他响应调控。
[1] Byrne M, Rowe F, Uthicke S. Molecular taxonomy, phylogeny and evolution in the family Stichopodidae (Aspidochirotida: Holothuroidea) based on COI and 16S mitochondrial DNA [J]. Molecular Phylogenetics and Evolution, 2010, 56(3): 1068-1081
[2] Li Y L, Wang R J, Xun X G, et al. Sea cucumber genome provides insights into saponin biosynthesis and aestivation regulation [J]. Cell Discovery, 2018, 4(1): 1-17
[3] Yang H S, Hamel J, Mercier A. The Sea Cucumber Apostichopus japonicus: History, Biology and Aquaculture [M]. New York: American: Academic Press, 2015: 25-35
[4] Alves Galvão M G, Rocha Crispino Santos M A, Alves da Cunha A J. Amantadine and rimantadine for influenza A in children and the elderly [J]. The Cochrane Database of Systematic Reviews, 2014, 2014(11): CD002745
[5] Lang G, Narayan O, Rouse B T. Prevention of malignant avian influenza by 1-adamantanamine hydrochloride [J]. Archiv Für Die Gesamte Virusforschung, 1970, 32(2): 171-184
[6] Peng Q C, Song J M, Li X G, et al. Biogeochemical characteristics and ecological risk assessment of pharmaceutically active compounds (PhACs) in the surface seawaters of Jiaozhou Bay, North China [J]. Environmental Pollution, 2019, 255(Pt 1): 113247
[7] Zhang R H, Du J, Dong X B, et al. Occurrence and ecological risks of 156 pharmaceuticals and 296 pesticides in seawater from mariculture areas of Northeast China [J]. The Science of the Total Environment, 2021, 792: 148375
[8] Xu Y J, Ren C B, Han D F, et al. Analysis of amantadine in Laminaria japonica and seawater of Daqin Island by ultra high performance liquid chromatography with positive electrospray ionization tandem mass spectrometry [J]. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 2019, 1126-1127: 121697
[9] 齐刚, 张秀芹, 刘婷, 等. 超高效液相色谱串联质谱法检测鸡蛋中的金刚烷胺和金刚乙胺[J]. 现代畜牧科技, 2017(1): 4-5, 7
Qi G, Zhang X Q, Liu T, et al. The amantadine and rimantadine detection from eggs with ultra high performance liquid chromatography tandem mass spectrometry [J]. Modern Animal Husbandry Science &Technology, 2017(1): 4-5, 7 (in Chinese)
[10] 郑伟云. 金刚烷胺与藻类质量安全相关性研究[D]. 烟台: 烟台大学, 2019: 29-33
[11] 任传博, 王倩, 高永刚, 等. 响应面法优化刺参(Apostichopus japonicus)中金刚烷胺检测[J]. 食品安全质量检测学报, 2019, 10(10): 2967-2975
Ren C B, Wang Q, Gao Y G, et al. Optimization the detection of amantadine from Apostichopus japonicus by response surface methodology [J]. Journal of Food Safety &Quality, 2019, 10(10): 2967-2975 (in Chinese)
[12] Peng Q C, Song J M, Li X G, et al. Biogeochemical characteristics and ecological risk assessment of pharmaceutically active compounds (PhACs) in the surface seawaters of Jiaozhou Bay, North China [J]. Environmental Pollution, 2019, 255(Pt 1): 113247
[13] Wu Y L, Chen R X, Xue Y, et al. Simultaneous determination of amantadine, rimantadine and memantine in chicken muscle using multi-walled carbon nanotubes as a reversed-dispersive solid phase extraction sorbent [J]. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 2014, 965: 197-205
[14] Pan Y T, Wang Z P, Duan C F, et al. Comparison of two fluorescence quantitative immunochromatographic assays for the detection of amantadine in chicken muscle [J]. Food Chemistry, 2022, 377: 131931
[15] Zhang T, Zhang L, Liu J X, et al. Development of a molecularly imprinted microspheres-based microplate fluorescence method for detection of amantadine and rimantadine in chicken [J]. Food Additives &Contaminants Part A, Chemistry, Analysis, Control, Exposure &Risk Assessment, 2021, 38(7): 1136-1147
[16] 李诗言, 王扬, 周凡, 等. 高效液相色谱-串联质谱法快速测定乌鳢肌肉中金刚烷胺、金刚乙胺和美金刚残留[J]. 中国渔业质量与标准, 2018, 8(5): 64-72
Li S Y, Wang Y, Zhou F, et al. Rapid and simultaneous determination of amantadine, rimantadine and memantine in Ophiocephalus argus Cantor meat by high performance liquid chromatography-tandem mass spectrometry [J]. Chinese Fishery Quality and Standards, 2018, 8(5): 64-72 (in Chinese)
[17] 郑伟云, 孙利芹, 田秀慧, 等. 金刚烷胺在菊花江蓠体内的富集和消除规律研究[J]. 食品安全质量检测学报, 2019, 10(1): 226-233
Zheng W Y, Sun L Q, Tian X H, et al. Rules of accumulation and elimination of amantadine residue in Gracilaria lichenoides [J]. Journal of Food Safety &Quality, 2019, 10(1): 226-233 (in Chinese)
[18] Scalzo F, Boisvert M, Wang W Q. Amantadine-induced structural and motor effects in developing zebrafish [J]. Neurotoxicology and Teratology, 2011, 33(4): 510
[19] Cappello T, de Marco G, Oliveri Conti G, et al. Time-dependent metabolic disorders induced by short-term exposure to polystyrene microplastics in the Mediterranean mussel Mytilus galloprovincialis [J]. Ecotoxicology and Environmental Safety, 2021, 209: 111780
[20] Want E J, Masson P, Michopoulos F, et al. Global metabolic profiling of animal and human tissues via UPLC-MS [J]. Nature Protocols, 2013, 8(1): 17-32
[21] Zhao H, French J B, Fang Y, et al. The purinosome, a multi-protein complex involved in the de novo biosynthesis of purines in humans [J]. Chemical Communications, 2013, 49(40): 4444-4452
[22] Stasolla C, Katahira R, Thorpe T A, et al. Purine and pyrimidine nucleotide metabolism in higher plants [J]. Journal of Plant Physiology, 2003, 160(11): 1271-1295
[23] Liu J H, Zhao Y, Ding Z, et al. Iron accumulation with age alters metabolic pattern and circadian clock gene expression through the reduction of AMP-modulated histone methylation [J]. The Journal of Biological Chemistry, 2022, 298(6): 101968
[24] Olabiyi A A, Carvalho F B, Bottari N B, et al. Tiger nut and walnut extracts modulate extracellular metabolism of ATP and adenosine through the NOS/cGMP/PKG signalling pathway in kidney slices [J]. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 2018, 43: 140-149
[25] Wan X X, Belanger K, Widen S G, et al. Genes of the cGMP-PKG-Ca2+ signaling pathway are alternatively spliced in cardiomyopathy: Role of RBFOX2 [J]. Biochimica et Biophysica Acta Molecular Basis of Disease, 2020, 1866(3): 165620
[26] Noppe H, Le Bizec B, Verheyden K, et al. Novel analytical methods for the determination of steroid hormones in edible matrices [J]. Analytica Chimica Acta, 2008, 611(1): 1-16
[27] Streck G. Chemical and biological analysis of estrogenic, progestagenic and androgenic steroids in the environment [J]. Trends in Analytical Chemistry, 2009, 28(6): 635-652
[28] Guedes-Alonso R, Montesdeoca-Esponda S, Sosa-Ferrera Z, et al. Liquid chromatography methodologies for the determination of steroid hormones in aquatic environmental systems [J]. Trends in Environmental Analytical Chemistry, 2014, 3-4: 14-27
[29] Zheng W L, Feng N N, Wang Y, et al. Effects of zearalenone and its derivatives on the synthesis and secretion of mammalian sex steroid hormones: A review [J]. Food and Chemical Toxicology, 2019, 126: 262-276
[30] Qi Y C, Duan G Z, Mao W, et al. Taurochenodeoxycholic acid mediates cAMP-PKA-CREB signaling pathway [J]. Chinese Journal of Natural Medicines, 2020, 18(12): 898-906
[31] Charkoftaki G, Tan W Y, Berrios-Carcamo P, et al. Liver metabolomics identifies bile acid profile changes at early stages of alcoholic liver disease in mice [J]. Chemico-Biological Interactions, 2022, 360: 109931
[32] Sokanovic S J, Baburski A Z, Janjic M M, et al. The opposing roles of nitric oxide and cGMP in the age-associated decline in rat testicular steroidogenesis [J]. Endocrinology, 2013, 154(10): 3914-3924
[33] Augusti-Tocco G, De Petrocellis B, Scarano E. Effects of amantadine on the early embryonic development of the sea urchin [J]. Developmental Biology, 1969, 19(4): 341-357
[34] Suwalsky M, Jemiola-Rzeminska M, Altamirano M, et al. Interactions of the antiviral and antiparkinson agent amantadine with lipid membranes and human erythrocytes [J]. Biophysical Chemistry, 2015, 202: 13-20
[35] Yancey P H, Clark M E, Hand S C, et al. Living with water stress: Evolution of osmolyte systems [J]. Science, 1982, 217(4566): 1214-1222
[36] Nguyen T V, Ragg N L C, Alfaro A C, et al. Physiological stress associated with mechanical harvesting and transport of cultured mussels (Perna canaliculus): A metabolomics approach [J]. Aquaculture, 2020, 529: 735657
[37] Sun X J, Tu K, Li L, et al. Integrated transcriptome and metabolome analysis reveals molecular responses of the clams to acute hypoxia [J]. Marine Environmental Research, 2021, 168: 105317
[38] Xu Y J, Liu H, Han D F, et al. Metabolomic alterations in the digestive system of the Mantis shrimp Oratosquilla oratoria following short-term exposure to cadmium [J]. Frontiers in Physiology, 2021, 12: 706579
[39] Xu D F, Zheng X X, Li C H, et al. Insights into the response mechanism of Litopenaeus vannamei exposed to cold stress during live transport combining untargeted metabolomics and biochemical assays [J]. Journal of Thermal Biology, 2022, 104: 103200
[40] Li L, Chen M Y, Storey K B. Metabolic response of longitudinal muscles to acute hypoxia in sea cucumber Apostichopus japonicus (Selenka): A metabolome integrated analysis [J]. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 2019, 29: 235-244