饮用水消毒是指灭活水中病原微生物的工艺过程,该工艺极大减少了包括伤寒和霍乱在内各种水传染病的传播,在保障饮用水安全方面有重要意义[1]。常见的消毒方式包括氯气、氯胺、二氧化氯、臭氧和紫外线消毒等。随着技术水平提高,新的消毒剂和消毒方法不断出现[1]。然而,在降低生物风险的同时,消毒剂常与水中某些物质反应,产生消毒副产物(disinfection byproducts, DBPs),并引发公共健康问题。因此,饮用水中DBPs受到广泛关注。
1974年,Rook J. J.首先发现氯气消毒中存在的次氯酸和次溴酸会与水体中天然有机物(natural organic matter, NOM)发生反应,产生4种三卤甲烷(trihalomethanes, THMs),这也是第一次正式提出DBPs的概念。此后,美国国家癌症研究所发现饮用水中氯仿对小鼠具有致癌作用[2],THMs还可能会危害动物生殖健康,DBPs毒性研究成为热点。另一类典型DBPs卤代乙酸(haloacetic acids, HAAs)也被证明具有毒性和致癌性。这2类DBPs已被世界卫生组织(World Health Organization, WHO)、美国环境保护局(United States Environmental Protection Agency, US EPA)和中国卫生部纳入到饮用水的卫生标准中[3]。
随着DBPs分析方法和检测技术的快速发展,越来越多新兴DBPs被鉴别出来。从1974年起,众多种类的DBPs被逐渐发现,如图1所示。1984年亚氯酸盐被发现,1986年卤乙腈(haloacetonitrile, HANs)被检出,1989年卤乙酸(haloacetic acid, HAAs)被检出,之后又发现了芳香族、醛类、卤代硝基甲烷(halonitromethanes, HNMs)、卤代酮(haloketones, HKs)、卤代呋喃酮和亚硝胺(N-nitrosamines, NAs)等DBPs,1993年溴化DBPs被发现,如溴酸盐![]() 从2003年开始,碘化DBPs开始受到关注。目前,关于DBPs的毒性研究工作仍在快速发展,预计未来还会有更多新兴DBPs被检出。
从2003年开始,碘化DBPs开始受到关注。目前,关于DBPs的毒性研究工作仍在快速发展,预计未来还会有更多新兴DBPs被检出。

图1 消毒副产物(DBPs)历史回顾[4]
Fig. 1 The historical review of disinfection byproducts (DBPs)[4]
关于饮用水DBPs,我们课题组已有相关的研究和梳理[4]。饮用水消毒副产物研究主要包含:(1)DBPs前体物分析[5-7];(2)消毒方式对DBPs的影响;(3)DBPs的检测与分析方法[8-9];(4)DBPs毒性机制与识别[10-11];(5)DBPs的控制等。其中,已有从DBPs的检测方法、来源和控制三方面进行总结的综述文章[4, 11-12]。DBPs作为有潜在危害的一大类化合物,其性质由化学结构决定。目前,仍然缺乏从副产物化学特性出发,梳理其化学组成、生物毒性和生成转化的研究。
本文从DBPs的化学特性出发,综述国内、外饮用水中已有限定标准和典型新兴DBPs的研究。重点解析典型DBPs的毒性效应和潜在危害;分析其化学组成、生成规律和转化路径;并梳理和总结其主要的前体物。在此基础上,对饮用水中DBPs的研究发展趋势进行展望,以期为充分发现和全面认识DBPs提供参考。
1 主要消毒副产物种类与限值(Principal kinds of DBPs)
DBPs种类繁多,图2显示了主要DBPs的研究情![]() 芳香族DBPs、NAs、HNMs、亚氯酸盐、氯酸盐和醛类等为重点关注的典型DBPs。
芳香族DBPs、NAs、HNMs、亚氯酸盐、氯酸盐和醛类等为重点关注的典型DBPs。

图2 1974—2018年各种消毒副产物的研究分布情况[4]
Fig. 2 Distribution of different kinds of DBPs from 1974 to 2018 [4]
饮用水中被检出的DBPs已受到广泛重视。我国新修订的《生活饮用水卫生标准》(GB 5749—2022)中对卤乙烷![]() 芳香族化合物、亚氯酸盐、氯酸盐和三氯乙醛等主要DBPs的含量制定了浓度限值,提高了部分DBPs前体物的要求。各DBPs在饮用水中限值如表1所示。
芳香族化合物、亚氯酸盐、氯酸盐和三氯乙醛等主要DBPs的含量制定了浓度限值,提高了部分DBPs前体物的要求。各DBPs在饮用水中限值如表1所示。
表1 生活饮用水标准内主要DBPs及新兴DBPs种类与限值
Table 1 DBPs in standards for drinking water quality and emerging DBPs, and their limits
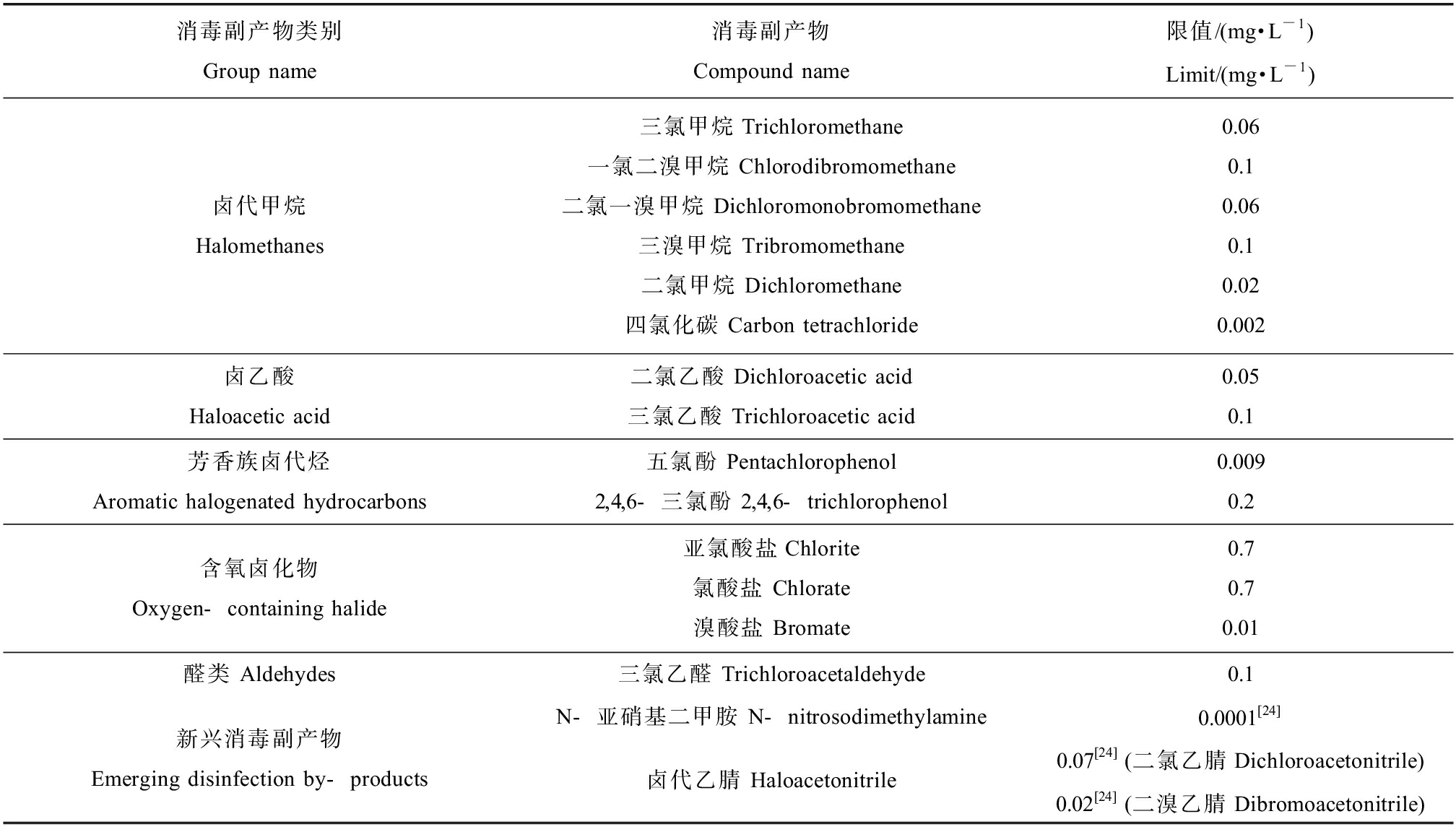
消毒副产物类别Group name消毒副产物Compound name限值/(mg·L-1)Limit/(mg·L-1)卤代甲烷Halomethanes三氯甲烷Trichloromethane一氯二溴甲烷 Chlorodibromomethane 二氯一溴甲烷 Dichloromonobromomethane三溴甲烷 Tribromomethane 二氯甲烷 Dichloromethane 四氯化碳 Carbon tetrachloride 0.06 0.1 0.060.10.020.002卤乙酸Haloacetic acid二氯乙酸 Dichloroacetic acid 三氯乙酸 Trichloroacetic acid0.050.1芳香族卤代烃Aromatic halogenated hydrocarbons五氯酚 Pentachlorophenol2,4,6-三氯酚 2,4,6-trichlorophenol 0.0090.2含氧卤化物Oxygen-containing halide亚氯酸盐Chlorite氯酸盐 Chlorate 溴酸盐 Bromate 0.70.70.01醛类 Aldehydes三氯乙醛 Trichloroacetaldehyde 0.1新兴消毒副产物Emerging disinfection by-productsN-亚硝基二甲胺 N-nitrosodimethylamine0.0001[24]卤代乙腈 Haloacetonitrile0.07[24] (二氯乙腈Dichloroacetonitrile)0.02[24] (二溴乙腈Dibromoacetonitrile)
目前,另一些新兴DBPs由于其具有更高的毒性正逐渐引起人们的重视[13-14],如HKs[15]、HNMs[16-17]、NAs[18-19]、HANs[20-21]和卤代乙酰胺(haloacetamides, HAcAms)[22-23]等。这些DBPs虽未被列入中国饮用水标准,但由于其高风险,国际上一些国家或地区已经开始对其最大浓度进行限定。WHO在2022年3月颁布的Guidelines for Drinking-water Quality: Fourth Edition Incorporating the First and Second Addenda设定饮用水中N-亚硝基二甲胺(N-nitrosodimethylamine, NDMA)的浓度限值为0.0001 mg·L-1,二氯乙腈(dichloroacetonitrile, DCAN)和二溴乙腈(dibromoacetonitrile, DBAN)的推荐值为0.07 mg·L-1和0.02 mg·L-1[24];美国将亚硝胺、卤代酮、卤代醛、二氯乙腈和二溴乙腈、溴化硝基甲烷均纳入条例规定的范畴内[25]。
2 《生活饮用水卫生标准》(GB 5749—2022)内消毒副产物(DBPs in standards for drinking water quality (GB 5749—2022))
2.1 三卤甲烷
2.1.1 三卤甲烷及其毒性
THMs是最早发现的DBPs,由1个H原子和3个卤原子包围中心的碳原子形成。4种最常见的三卤甲烷为氯仿(CHCl3)、一溴二氯甲烷(CHCl2Br)、一氯二溴甲烷(CHClBr2)和溴仿(CHBr3)[26]。接触THMs会对人体健康产生不良影响,长期暴露于THMs会增加患膀胱癌、直肠癌和结肠癌等风险[27]。THMs毒性强弱受中心碳原子周围的3个卤原子的种类影响,大部分溴化或碘化的卤代甲烷比其氯代副产物具有更高的细胞毒性和基因毒性[28],应该受到特别关注。
2.1.2 生成及转化
THMs主要在氯化消毒过程中产生,由卤原子取代碳原子上的氢形成的卤代甲烷。以卤原子与甲基酮类化合物之间的反应为例,反应机理为:氯或溴的吸电子效应使ClCH2-完全形成三卤甲基酮的结构,碱对酮的加成后C—C键断裂,分离出去的碳负离子在3个卤原子的吸电子效应下保持相对稳定,最终生成三卤甲烷[1]。
饮用水中THMs具有种类繁多的前体物,主要是NOM[29]。NOM是一类复杂的有机混合物,其分子量和化学结构等性质与THMs的生成量密切相关。分子量<1 000 Da的溶解性有机物有较高的THMs生成潜能[30]。高氧碳比的多环芳烃化合物、多酚化合物、富含氧官能团的酚和不饱和脂肪族化合物等以及含有—OH、—S—和—NH2等给电子官能团的化合物更易在氯化过程中生成THMs[9]。因此,从结构上看,不饱和键或给电子官能团会消耗卤素,从而生成THMs。根据THMs的生成机制,可以通过优化消毒方式和工艺条件,有效降低THMs的生成。
2.2 卤代乙酸
2.2.1 卤代乙酸及其毒性
HAAs是由卤原子在乙酸的甲基上发生取代而形成,其极性高且挥发性小。HAAs在高于23 ℃的温度下会分解,生成THMs[31]。HAAs紫外光光降解度会随着卤化度及卤素的分子量的增加而增加,如三卤乙酸(TXAA)>二卤代乙酸(DXAA)>单卤代乙酸(MXAA)。
氯代乙酸的致癌潜力与取代的氯原子数有关,研究表明,一氯乙酸对小鼠无致癌性,但二氯乙酸和三氯乙酸会增加小鼠肝肿瘤的患病率[32]。研究发现,一氯乙酸、一溴乙酸、二溴乙酸和三溴乙酸等4种卤代乙酸均具有致突变性和遗传毒性,其中一溴乙酸毒性最强,二溴乙酸会对小鼠的胸腺和脾脏产生明显的免疫毒性[33]。另一项研究评估了氯代、溴代和碘代乙酸的体外致癌性,碘代乙酸对人膀胱细胞具有很强的细胞毒性[34]。HAAs能够与人体内雄激素受体结合,改变基因转录,当体内的修复机制无法修复其造成的改变时,人的内分泌系统将受到干扰[35]。
2.2.2 生成及转化
HAAs的生成与消毒方式、工艺条件及原水水质密切相关。HAAs通常在氯气、氯胺和二氧化氯消毒过程中产生,氯气消毒最易生成。当采用预臭氧工艺时,氯化消毒还会使HAAs的形成潜力进一步增加,且其形成潜力与氯投加量和pH值有关[36]。在消毒过程中,游离氯会与前体物发生亲电子加成反应,随着氯投加量的升高,反应从取代反应变为氧化反应,而HAAs主要是通过氧化形成,因此有效氯浓度越高,HAAs的生成潜能越大。
HAAs的前体物主要来源于腐殖酸、富里酸等腐殖类有机物,以及亲水酸、聚糖、氨基酸、蛋白质和烃类化合物等非腐殖类有机物。不同组成和结构的前体物表现出不同的反应性质,HAAs的生成潜能主要来自于疏水性组分,其中疏水中性物质占较大比例。水中含有溴离子时,氯消毒会生成比氯化HAAs毒性更强的溴化HAAs,溴离子浓度和亲水性有机物比例越高,生成溴化HAAs水平越高[37]。当原水含有碘化有机物时,存在生成碘化HAAs的风险,因其高毒性而备受关注。
2.3 溴酸盐
2.3.1 溴酸盐及其毒性
溴酸盐是由1个溴原子与3个氧原子组成的具有三角锥形结构的化合物,其氧化活性被认为是毒性作用机制的一个重要因![]() 会造成细胞DNA的氧化损伤,对动物和人类具有潜在的致癌
会造成细胞DNA的氧化损伤,对动物和人类具有潜在的致癌![]() 对肝细胞具有遗传毒性,在肾脏中会引起氧化损伤和染色体突变等问题[39]。
对肝细胞具有遗传毒性,在肾脏中会引起氧化损伤和染色体突变等问题[39]。
2.3.2 生成及转化
![]() 一般在含有溴化物的原水,臭氧消毒过程中产生,当溴化物浓度超过50 μ g·L-1时,在臭氧或羟基自由基的氧化下形成
一般在含有溴化物的原水,臭氧消毒过程中产生,当溴化物浓度超过50 μ g·L-1时,在臭氧或羟基自由基的氧化下形成![]() 由图3可知,Br-先被氧化为HOBr,再进一步反应生成
由图3可知,Br-先被氧化为HOBr,再进一步反应生成![]() 和
和![]() 再被氧化为
再被氧化为![]() 与羟基自由基的反应中,Br-先转化为BrBrO·,再分别生成HOBr和BrOBrO·,BrOBrO·最终转化为
与羟基自由基的反应中,Br-先转化为BrBrO·,再分别生成HOBr和BrOBrO·,BrOBrO·最终转化为![]() 和
和![]() 其中HOBr是反应过程中的重要中间产物,与最终
其中HOBr是反应过程中的重要中间产物,与最终![]() 的生成量密切相关。当水中含有Cl-和
的生成量密切相关。当水中含有Cl-和![]() 时,
时,![]() 的生成会受到明显抑制,而
的生成会受到明显抑制,而![]() 的存在则会促进
的存在则会促进![]() 的生成[41]。原水水质和运行因素都会影响
的生成[41]。原水水质和运行因素都会影响![]() 的生成。
的生成。
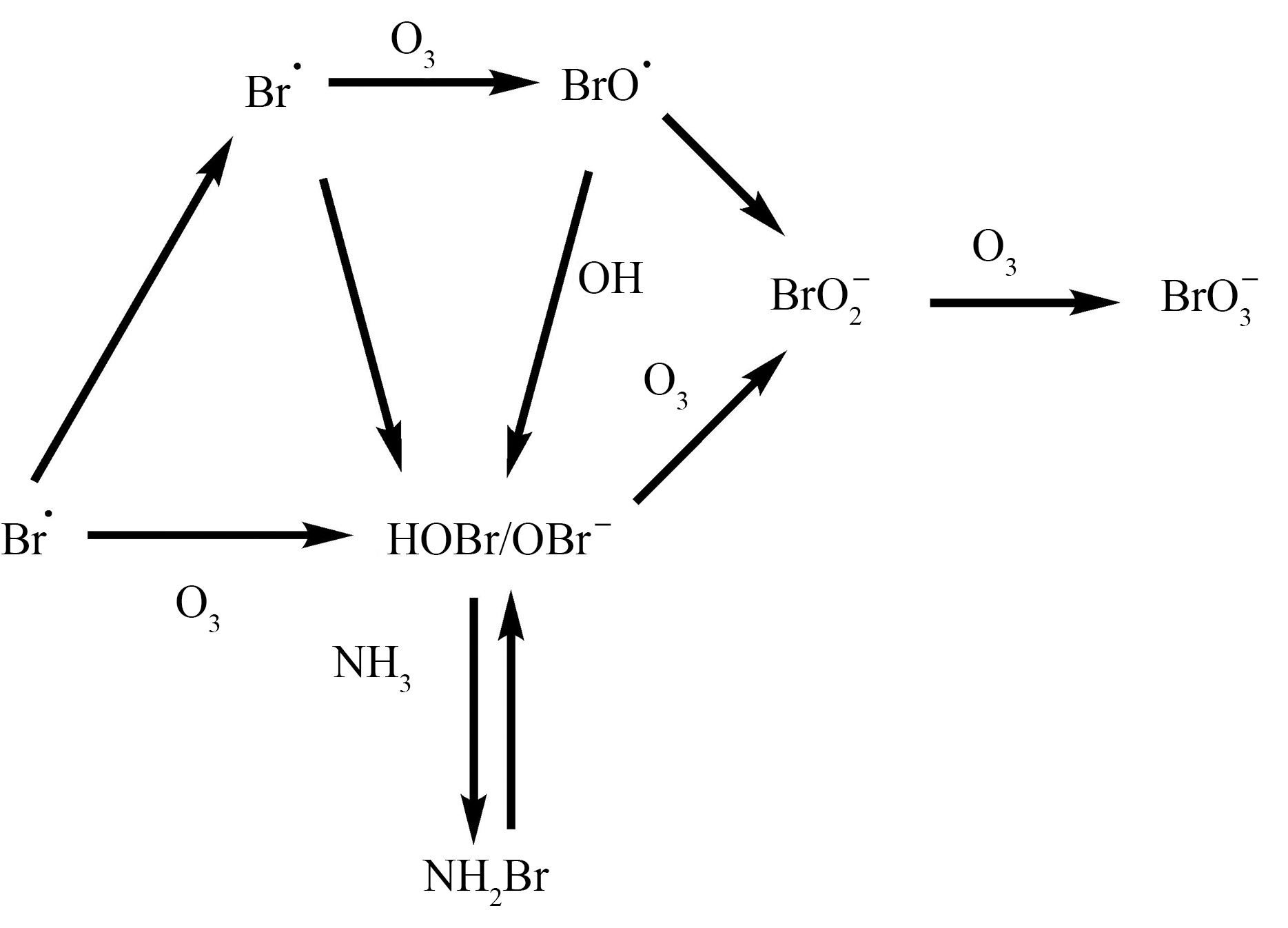
图3 溴酸盐生成机理[41]
Fig. 3 Bromate formation mechanism[41]
2.4 醛类消毒副产物
2.4.1 醛类消毒副产物及其毒性
醛类DBPs结构中含有一个醛基,此外,卤代醛类DBPs中与C原子相连的H原子被卤原子所取代,使得醛类DBPs具有毒性。甲醛和三氯乙醛是生活饮用水卫生标准中的2项水质指标。甲醛会损害细胞DNA,具有遗传毒性,对人体生殖系统和免疫系统也存在危害,而且具有致癌性,已被国际癌症研究中心定为1A类致癌物。三氯乙醛属于卤代醛类DBPs,会对DNA产生损伤,具有基因毒性和致癌性。
2.4.2 生成及转化
甲醛主要在臭氧消毒过程中产生。水中的不饱和有机物,如腐殖酸、疏水酸性和亲水中性有机污染物,在臭氧作用下形成羰基化合物和含氧酸类等物质,水解后不饱和键断裂,最终生成甲醛。含有芳香族官能团和双键的物质,也容易受到臭氧攻击而形成羰基。三氯乙醛主要在氯化过程中产生,一些小分子酸(苹果酸)、氨基酸(天冬酰胺和天冬氨酸等)、酚类化合物(间苯二酚和2,4,6-三氯酚等)和蛋白质等有机物是三氯乙醛的重要前体物,主要通过一系列的氯代、脱羧和水解等反应生成[42]。以天冬酰胺为例,三氯乙醛的生成转化途径如图4所示。

图4 三氯乙醛生成及转化途径[42]
Fig. 4 Production and conversion pathways of trichloroacetaldehyde[42]
2.5 芳香族类消毒副产物
2.5.1 芳香族类消毒副产物及其毒性
五氯酚和2,4,6-三氯苯酚由于其毒性和致癌性而成为最常研究的氯酚[43],饮用水中发现的芳香族类DBPs主要包括卤代酚(HP)、卤代羟基苯甲醛(HBAD)和卤代羟基苯甲酸(HBAC)。通常芳香族DBPs含有苯基或杂环结构,这种特殊结构使其具有更高的稳定性,亲电子取代反应更容易发生。
芳香族DBPs比脂肪族DBPs具有更大的毒性,原因在于具有苯基或杂环的特殊结构使其更易发生亲电子反应,被卤原子取代,增强了毒性。五氯酚能够通过皮肤、呼吸道或食物链进入人体并富集在体内,对人体产生危害,是一种难降解的持久性有机污染物。2,4,6-三氯酚也具有很强的生物毒性,当2,4,6-三氯酚浓度达到4 mg·L-1时,会显著降低鲫鱼的胚胎孵化率,浓度在0.5 mg·L-1时也会导致幼苗存活率明显下降[44]。此外,2,4,6-三氯酚还具有致癌性、致畸性和致突变性,其所含氯原子会对生物的裂解酶造成破坏。长期接触被2,4,6-三氯酚和五氯酚污染的饮用水会增加消化道感染、哮喘和抑郁等疾病的风险[45]。
2.5.2 生成及转化
氯代酚一般是酚类在氯气消毒过程中产生的副产物,2,4,6-三氯酚是较为常见的一种。消毒过程中,次氯酸带正电的氯原子依次进攻壬基酚分子中与羟基邻位的2个碳原子,生成一氯壬基酚和二氯壬基酚,然后次氯酸中的部分羟基进攻二氯壬基酚侧链中带正电的α-C,造成碳链的断裂,带正电的氯原子再进攻苯环上的部分电荷为带负电的碳原子,生成2,4,6-三氯酚[46]。壬基酚和次氯酸的反应转化详细途径如图5所示。
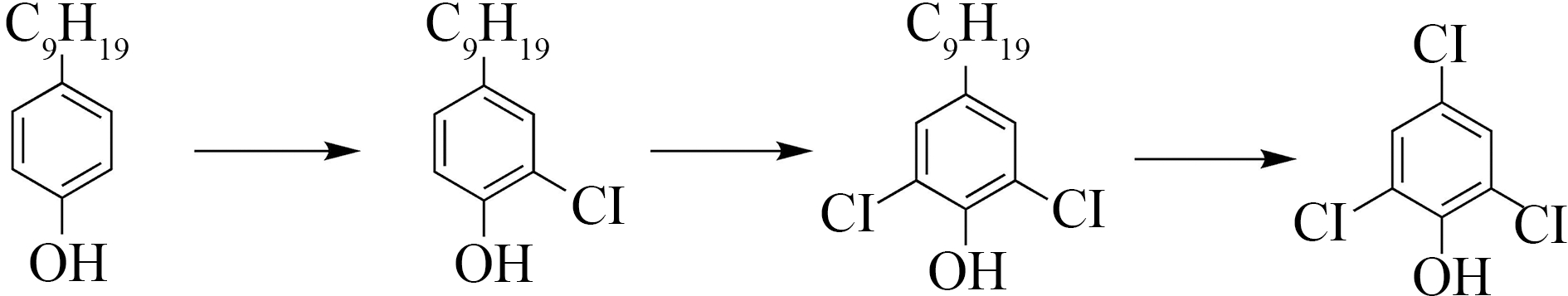
图5 壬基酚生成2,4,6-三氯苯酚的转化途径[46]
Fig. 5 Conversion pathway of nonylphenol to 2,4,6-trichlorophenol [46]
3 新兴消毒副产物(Emerging DBPs)
3.1 亚硝胺
3.1.1 亚硝胺及其毒性
在饮用水中亚硝胺类DBPs具有很大风险。饮用水中检出的常见亚硝胺类DBPs包括N-二甲基亚硝胺、N-亚硝基二乙胺(NDEA)、N-亚硝基二正丁胺(NDBA)、N-亚硝基吗啉(NMor)、N-亚硝基甲基乙胺(NMEA)和N-亚硝基二苯胺(NDPhA)等[47],它们是由亚硝基的N原子与氨基中的N原子连接而成的化合物,这一特殊结构使得这类化合物具有强毒性。
N-二甲基亚硝胺具有致癌性、致突变性和致畸性,美国加利福尼亚州卫生服务部将饮用水中N-二甲基亚硝胺的允许浓度控制为1 ng·L-1[48]。
3.1.2 生成及转化
臭氧、氯、氯胺和二氧化氯消毒都会产生NAs[19]。水厂氯化过程中加入亚硝酸盐也会与二甲胺产生NAs,并随着次氯酸根增加,产率也会增加[49]。
NDMA是最常检出的亚硝胺类DBPs,但其前体物的鉴定难度大。最初学者们发现二甲胺(DMA)是亚硝胺类DBPs的前体物[19]。但是DMA在自然水体或城市污水中的浓度很低,被认为是水体中占比很小的NDMA前体物[50]。含有DMA官能团的叔胺和季胺也可以产生NAs[51],包括药品[50, 52]、个人护理品[53]、农药中的杀菌剂或除草剂[54]。水处理中添加和使用的含氨基的聚合物混凝剂[55]、含有氮表面基团的碳纳米管[56]和具有季胺结构的阴离子交换树脂[57]等都会在消毒处理饮用水中引入NAs前体物,导致水中NAs产生。
前体物的不同化学结构导致NDMA生成率不同。例如,不同叔胺结构的氯胺消毒产生NDMA的转化率完全不同,Diuron作为前体物只能生成0.15%的NDMA,而雷尼替丁作为前体物转化率则可以达到60%[58]。前体物中存在给电子基团可以增加氮原子上的电子密度,促进与氯的反应,导致NDMA的高生成率[53]。现今对于各类水体中NAs前体物的鉴别不够充分,进一步解析NAs前体物的化学结构非常必要。
NDMA产生率与原水来源、水质及NOM密切相关。水体有机物中的疏水性酸组分是NDMA的主要来源,样品中272 nm紫外吸收值在氧化过程中的改变值与NDMA的生成量线性相关[59]。因此,可以通过分析前体物的特性评估NDMA的生成率。
消毒剂与氮含量比值,pH和溶解氧都会影响NDMA的生成。高氯胺比有利于产生更多的二氯胺[60],叔胺能转化成更多的NAs[53]。在pH=8时NDMA转化率最高,且受溶解氧的影响[50]。水中存在的无机氮(NO2-、NH4+)也会显著增加氯胺消毒过程中亚硝胺生成量[50]。
图6显示了NDMA的生成机理,已有NDMA的生成机理包括:(a)甲胺形成UMDH/UMDH-Cl中间体再生成NDMA;(b)自由基偶联反应。在氯胺消毒过程中,一氯胺与二甲胺发生亲核取代反应,从而形成不对称的二甲基肼中间体(UDMH)或UDMH-Cl,之后被一氯胺氧化生成NDMA[61]。
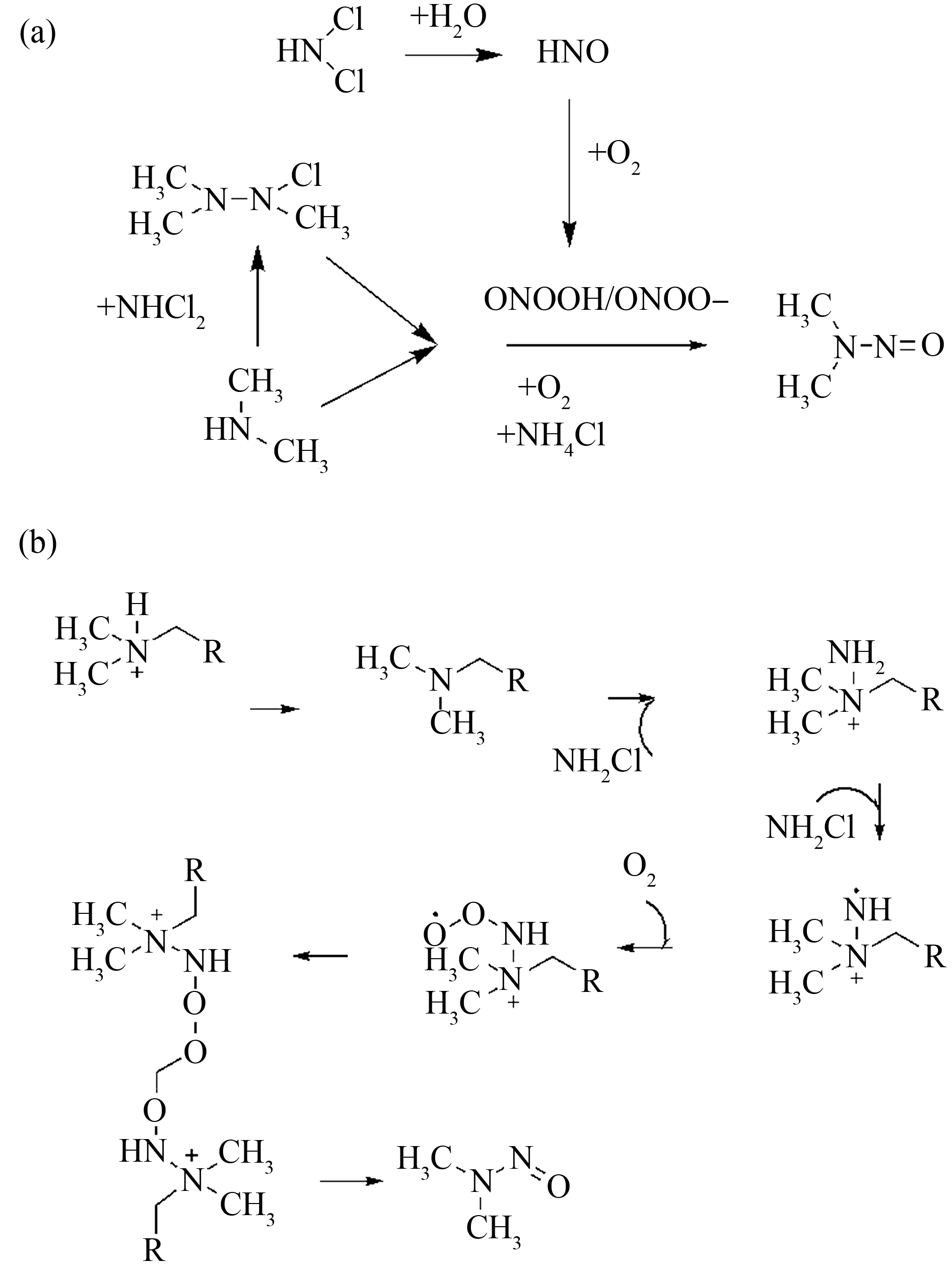
图6 N-亚硝基二甲胺(NDMA)生成机理[62-63]
注:(a) 偏二甲肼(UDMH)形成机理;(b) 自由基偶联反应。
Fig. 6 N-nitrosodimethylamine (NDMA) formation mechanism[62-63]
Note: (a) Unsymmetrical dimethylhydrazine (UDMH) formation mechanism; (b) Free radical coupling reaction.
溶解氧没有形成独立的自由基,而是发生了结合反应,在芳香环上形成了N—O—O·中间体,叔胺在生成NDMA时经过了一系列的氯化取代、电子转移、氧化及自由基偶联反应,最终生成了NDMA[62-63]。
3.2 卤代乙腈
3.2.1 卤代乙腈及其毒性
HANs在与腈键相连的α碳上最多包含3个卤素,二卤代乙腈是在氯化水中形成的最丰富的HANs,其生成潜能仅次于THMs和氯代HAAs。饮用水中普遍存在HANs污染[45]。
HANs是一种高度致癌的含氮消毒副产物,其遗传毒性和细胞毒性比卤代乙酸高1~2个数量级[64]。HANs会引起小鼠急性基因组DNA损伤并诱导皮肤肿瘤,其细胞毒性顺序为一溴乙腈>二溴乙腈>一氯乙腈>三氯乙腈>二氯乙腈。与三氯乙腈、一溴乙腈和二溴乙腈相比,一氯乙腈和三氯乙腈表现出更强的遗传毒性[64]。
3.2.2 生成及转化
目前水中含氮有机物如氨基酸、蛋白质、氮杂环芳香族化合物和腐殖质、胺类、嘧啶、嘌呤及二肽都可以生成HANs[13, 65]。且氯胺消毒会生成比氯消毒更多的卤代腈,因为氯胺提供了更多的氮源和可与醛发生加成反应的一氯胺(NH2Cl),并减少腈类物质的水解。溴代卤代腈也被观察到在水体消毒中生成,生成腈的量通常与水中的含氮量有关系,有研究指出胶体类含量多的水体容易生成更多的腈[66]。
目前普遍认为,腈的生成途径主要包含以下2种:(1)醛途径[13, 67];(2)脱羧途径[13, 68],如图7所示。在醛途径过程中,醛发生亲电加成后生成氯氨基醇,之后缓慢地分解生成氯亚胺,其可以迅速失去HCl生成腈[67]。在脱羧途径中,氨基酸先发生氯代反应生成二氯代氨基酸,再发生脱HCl,形成胺基羧酸盐中间体,继续发生协同脱羧作用,脱去Cl-和CO2生成腈[36]。这个反应过程会与生成亚胺和醛形成竞争反应[36];但也有研究指出氯胺会先脱CO2,产生亚胺,之后再脱去HCl形成相应的卤代腈,且氯代氨基酸的分解是限速步骤[68]。
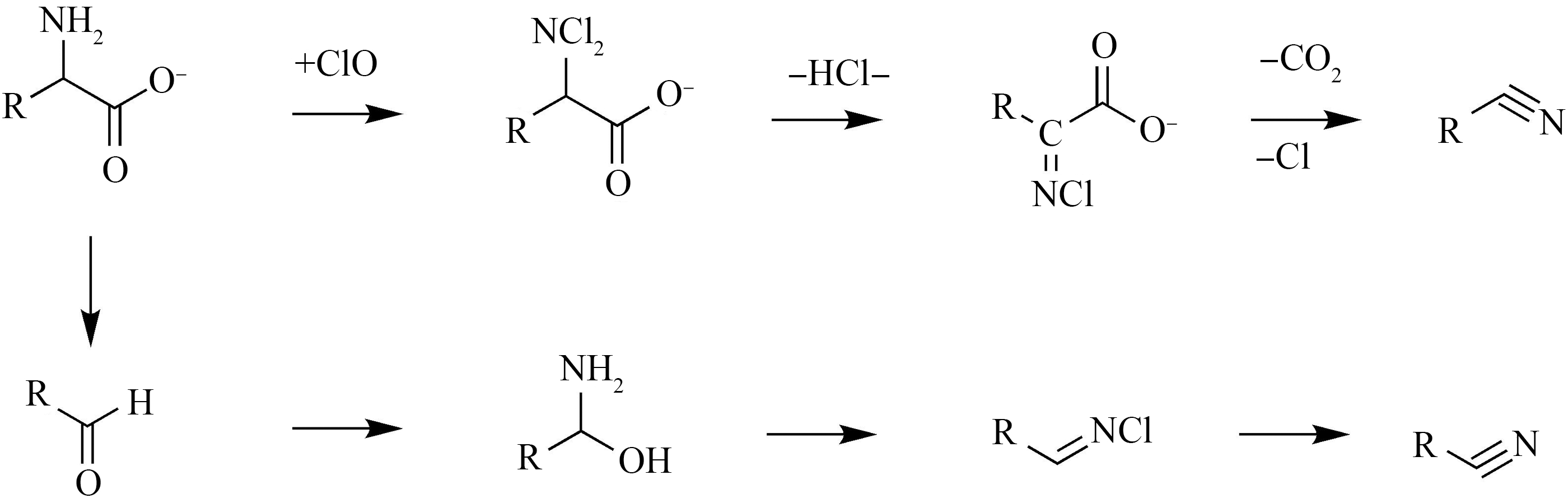
图7 卤乙腈生成机理:脱羧途径与醛途径[13, 67]
Fig. 7 The formation mechanism of haloacetonitrile: Decarboxylation pathway and aldehyde pathway[13, 67]
反应的过程受pH和Cl/N比影响。pH越高,生成腈的含量越高[68]。高pH有助于氯酸根以HClO的形式存在,有助于氯代胺的氯代反应。低pH时,次氯酸盐会加强腈的水解,降低卤代腈的转化率[68]。高Cl/N比则有助于氨基酸物质形成二氯代物质[13]。该反应与生成醛的反应同时存在并互相竞争,在低Cl/N的情况下,氨基酸消毒主要产物为卤代醛和氯亚胺,而在高Cl/N的情况下生成产物以腈和氯胺为主[69]。
3.3 卤代硝基甲烷
3.3.1 卤代硝基甲烷及其毒性
HNMs是指含有一个硝基,α碳上的H原子被卤素取代的一类化合物。饮用水中常见的4种HNMs包括二氯硝基甲烷(dichloronitromethane, DCNM)、二溴硝基甲烷(dibromonitromethane, DBNM)、三氯硝基甲烷(trichloronitromethane, TCNM)和三溴硝基甲烷(tribromonitromethane, TBNM)。
HNMs通常在使用臭氧-氯气、氯气或氯胺处理的水中被检测到,含量一般在10 μg·L-1 以下,主要由消毒剂氧化和卤素原子取代形成[12, 70]。US EPA关于HNMs的浓度调查结果显示,HNMs的浓度介于0.1~5 μg·L-1之间,其中TCNM的浓度水平较高[71]。中国的70多个自来水厂中也都检测到TCNM的存在[64]。
HNMs的α碳上连接的硝基使其有更高的遗传毒性和细胞毒性。此外,HNMs毒性强弱还与卤原子的种类和数目相关,例如,溴代硝基甲烷的毒性高于氯代硝基甲烷。有研究调查了9种卤代硝基甲烷的诱变能力,包括一氯硝基甲烷、二氯硝基甲烷、三氯硝基甲烷、一溴硝基甲烷、二溴硝基甲烷、三溴硝基甲烷、溴氯硝基甲烷、一溴二氯硝基甲烷和二溴一氯硝基甲烷,结果均可在沙门氏菌中致突变[72]。
3.3.2 生成及转化
HNMs的前体物包括天然水体中的硝基甲烷、氨基酸、氨基糖、硝基酚、藻类有机物(AOM)及无机硝酸盐污染物等[73]。苏氨酸和色氨酸的TCNM形成势最高,甘氨酸也能形成大量HNMs[57]。不同氨基酸生成HNMs潜能不同,可能是由氨基酸的R基团引起的。
TCNM作为最常检测到也是含量最高的一种HNMs,对于其生成途径,第一种解释是含有胺基的物质,发生氯代后,氯胺基被氧化生成硝基烷,之后甲烷基发生氯代反应生成硝基甲烷,如图8所示[74]。
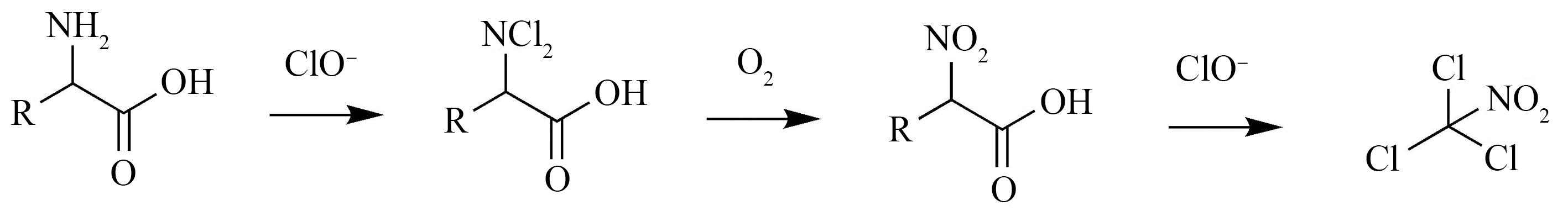
图8 三氯硝基甲烷生成的卤代、氧化和脱羧过程[74]
Fig. 8 Halogenation, oxidation and decarboxylation process of trichloronitromethane [74]
同时,TCNM的生成途径还包括:(1)亚硝酸盐和次氯酸通过2步反应分别生成硝化产物ClNO2和N2O4,氯化反应后苯环裂解,形成TCNM;(2)UV作为预处理使亚硝酸盐与硝酸盐分解产生NO、NO·和ONOOH等,与NOM发生硝化反应后再进行氯化反应生成TCNM[75]。
影响氯化过程中HNMs形成的因素包括溶解有机氮、溴化物、亚硝酸盐和溶解有机碳浓度[76]。另外,在亚硝酸盐存在的情况下,TCNM的生成量与前体物的芳香性以及苯环上羟基的位置和数量有关,且腐殖质比非腐殖质更易形成TCNM[77]。同时,pH也会影响TCNM的生成量,学者指出氯胺在酸性条件下更易水解生成自由氯,而水解生成的自由氯对TCNM及其他HNMs的生成起到了促进作用[78]。
4 消毒副产物毒理学方法研究进展(Research progress on toxicological methods of DBPs)
近年来,对DBPs毒理学的研究不断深入,研究的主要方法包括毒性测试及非实验手段,非实验手段中的定量结构-活性关系(简称构效关系;quantilative structure-activily relationship, QSAR)是毒性预测研究中的热点。
4.1 毒性测试
消毒副产物的毒性测试,最早选用一些细菌作为模式生物,如沙门氏菌或大肠杆菌[79-80]。主要的测试实验有Ames变异反应突变实验、SOS显色反应和单细胞凝胶溶胶电泳等。这些方法首先测试细菌暴露于DBPs中的基因突变及DNA损伤,然后针对哺乳动物进行简单相关分析或外推。CHO(Chinese hamster ovary)细胞是评价细胞毒性最常用的一种细胞[81],此外,Hep G2(human hepatoma cells)细胞也可用于评价芳香族DBPs的细胞毒性[81-82]。表2归纳了《生活饮用水卫生标准》(GB 5749—2022)内主要消毒副产物及新兴消毒副产物的毒性特征。
表2 生活饮用水标准内主要DBPs及新兴DBPs毒性
Table 2 Toxicity summary of DBPs in standards for drinking water quality and emerging DBPs
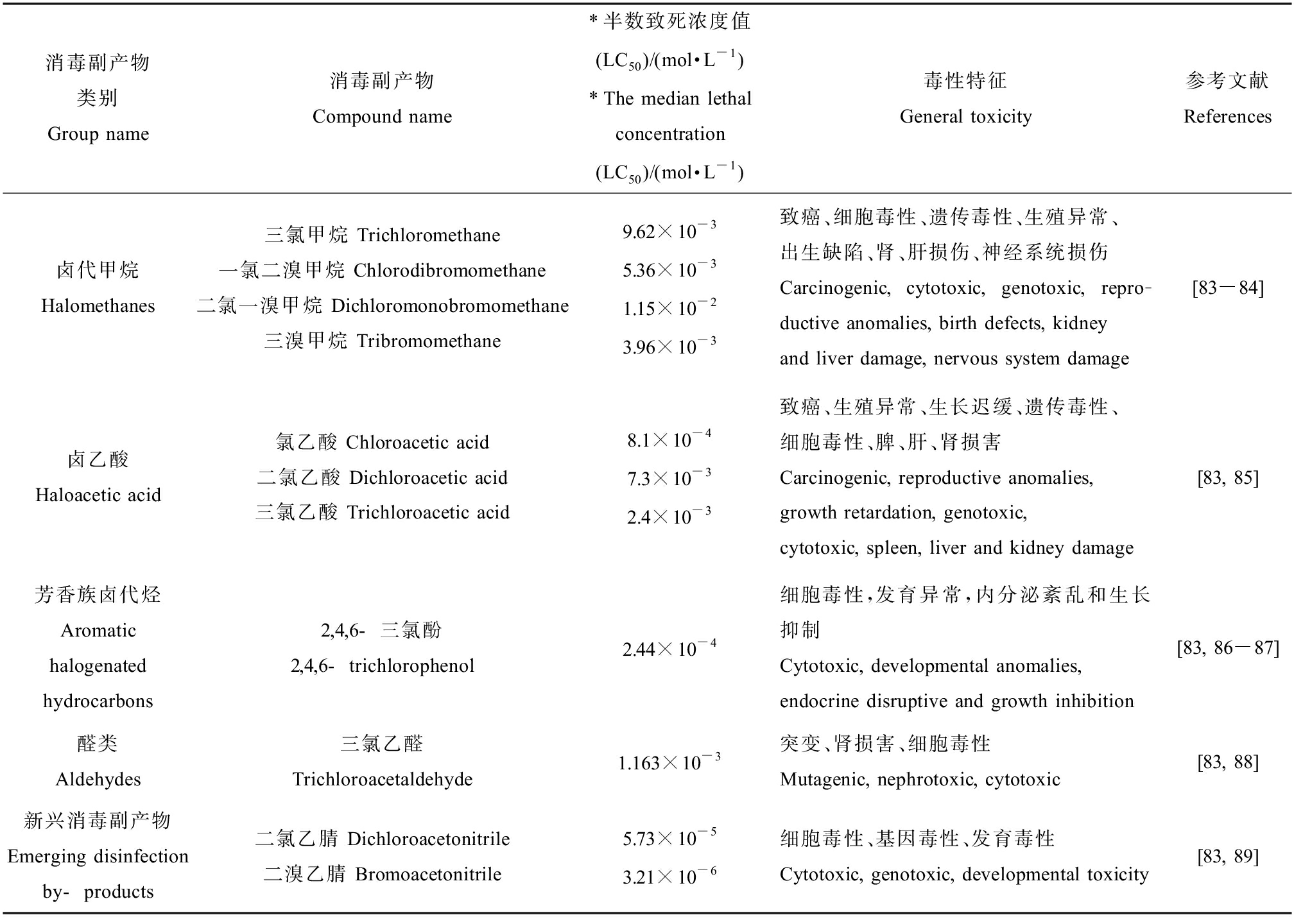
消毒副产物类别Group name消毒副产物Compound name*半数致死浓度值(LC50)/(mol·L-1)*The median lethal concentration(LC50)/(mol·L-1)毒性特征General toxicity参考文献References卤代甲烷Halomethanes三氯甲烷 Trichloromethane一氯二溴甲烷 Chlorodibromomethane二氯一溴甲烷 Dichloromonobromomethane三溴甲烷 Tribromomethane9.62×10-35.36×10-31.15×10-23.96×10-3致癌、细胞毒性、遗传毒性、生殖异常、出生缺陷、肾、肝损伤、神经系统损伤Carcinogenic, cytotoxic, genotoxic, repro-ductive anomalies, birth defects, kidney and liver damage, nervous system damage[83-84]卤乙酸Haloacetic acid氯乙酸 Chloroacetic acid二氯乙酸 Dichloroacetic acid三氯乙酸 Trichloroacetic acid8.1×10-47.3×10-32.4×10-3致癌、生殖异常、生长迟缓、遗传毒性、细胞毒性、脾、肝、肾损害Carcinogenic, reproductive anomalies, growth retardation, genotoxic, cytotoxic, spleen, liver and kidney damage[83, 85]芳香族卤代烃Aromatic halogenated hydrocarbons2,4,6-三氯酚 2,4,6-trichlorophenol2.44×10-4细胞毒性,发育异常,内分泌紊乱和生长抑制Cytotoxic, developmental anomalies, endocrine disruptive and growth inhibition[83, 86-87]醛类Aldehydes三氯乙醛Trichloroacetaldehyde1.163×10-3突变、肾损害、细胞毒性Mutagenic, nephrotoxic, cytotoxic[83, 88]新兴消毒副产物Emerging disinfection by-products二氯乙腈 Dichloroacetonitrile二溴乙腈 Bromoacetonitrile5.73×10-53.21×10-6细胞毒性、基因毒性、发育毒性Cytotoxic, genotoxic, developmental toxicity[83, 89]
注:*LC50代表暴露时间为72 h,CHO细胞的半数致死浓度。
Note: *LC50 values included that inducing a cell density of 50% as compared to the concurrent negative controls by Chinese hamster ovary cells bioassay with the exposure time of 72 h.
尽管这些检测手段可以比较准确地比较各类DBPs的毒性,但是其实验过程相对复杂,检测过程需要耗费大量人力物力。
4.2 定量结构-活性关系模型
科学技术的进步与人们日益增长的美好生活需要成为新兴化合物合成的基础,同时也进一步加大了水体中消毒副产物的检测难度。利用非实验手段预测消毒副产物毒性,QSAR模型是其中的研究重点。
在DBPs毒性预测的研究中,QSAR借助数学方法建立DBPs分子结构与毒性之间的量化模型,通过对已知毒性的DBPs建立模型,找到其结构与毒性之间的定量关系,进而预测未知DBPs的毒性或对DBPs的毒性机制进行研究。Hansch等于1962年提出构效关系,运用了多元线性回归法、偏最小二乘法、人工神经网络和支持向量机等数学方法,对上万种化合物建立了QSAR模型。1975年,Veith和Konasewic正式将QSAR引入到环境研究领域。1991年,Tuppurainen等[90]首次将QSAR应用到消毒副产物的毒性研究中。
近年来,QSAR也被逐渐应用到DBPs毒性研究中。Wu等[91]基于Hep G2细胞体外毒性试验和斑马鱼细胞体内毒性试验的EC50值建立了2个QSAR模型,以进一步研究卤代环状DBPs和脂肪族DBPs的毒性机制。对于不同的毒性终点,DBPs的毒性机制由不同的物理化学性质反映,从而由不同分子描述符表示。体外毒性预测模型从17个候选描述符中筛选出了最低空轨道能(energy of the lowest unoccupied molecular orbital, ELUMO)和价度之和(sum of valence degrees, SVDe)2个描述符,体内毒性预测模型由总价连通性(total valence connectivity, TVCon)、最高占有轨道能(energy of the highest occupied molecular orbital, EHOMO)和ELUMO这3个分子描述符组成。结果表明,模型预测的EC50值与实验值基本吻合。其中,体外毒性预测模型和体内毒性预测模型的相关系数(r2)分别为0.707和0.702,模型显示出较好的预测能力。Zhang等[86]为研究卤代芳香族DBPs对人体健康的危害,比较了隶属于4个亚群共15种卤代芳香族DBPs的对CHO细胞的毒性,基于观察到的细胞毒性数据和筛选描述符并建立QSAR模型。模型表明,芳香族DBPs对CAT受体的相互作用与DBPs的亲电/亲核反应在DBPs的毒性机制研究中是至关重要的。模型相关系数(r2)为0.844,对卤代芳香DBPs的毒性显示出较好的预测能力。Qin等[92]建立了基于X-Microtox、GSH+、GSH-、DNA+和DNA-等5项生物测试方法,预测50多种DBPs毒性的QSAR模型。类似的,QSAR在预测多种卤代DBPs的水解动力学的相关研究也有开展[93]。
QSAR可以建立预测DBPs毒性等各类理化性质和研究毒性机制的理论模型,在分子水平上诠释物质结构对性质的决定性作用,拓展QSAR在环境中的应用范围。不过由于水体体系复杂,要找到合适的分子结构描述符,构建合理的QSAR模型,依然任重道远。
5 结语与展望(Conclusion and outlook)
(1)国家新的《生活饮用水卫生标准》(GB 5749—2022)提高了部分DBPs前体物的要求,新兴消毒副产物NAs、HAAs和HNMs应逐渐受到重视。深入解析消毒剂与前体物之间发生的氯代、氧化、脱羧和水解等主要反应,不断识别新兴消毒副产物至关重要。
(2)DBPs的生成量与前体物种类、分子量和化学结构密切相关。受制于水源水质与前体物不同种类,不同DBPs之间可以互相转化,通过寻找前体物共同的化学结构来评估不同种类DBPs的形成十分重要。
(3)DBPs毒性识别方法有实验手段和非实验手段2种,寻找合适的分子结构描述符,对于将非实验手段中QSAR模型应用到DBPs的毒性预测与机制研究中十分重要。
[1] Kruithof J C, Kamp P C, Martijn B J. UV/H2O2 treatment: A practical solution for organic contaminant control and primary disinfection [J]. Ozone: Science &Engineering, 2007, 29(4): 273-280
[2] Keysser C. National cancer institute carcinogenesis technical report series [J]. Toxicologic Pathology, 1976, 4: 17-19
[3] Dalvi A G I, Al-Rasheed R, Javeed M A. Haloacetic acids (HAAs) formation in desalination processes from disinfectants [J]. Desalination, 2000, 129(3): 261-271
[4] Tang Y L, Long X, Wu M Y, et al. Bibliometric review of research trends on disinfection by-products in drinking water during 1975-2018 [J]. Separation and Purification Technology, 2020, 241: 116741
[5] Liu S G, Li Z L, Dong H Y, et al. Formation of iodo-trihalomethanes, iodo-acetic acids, and iodo-acetamides during chloramination of iodide-containing waters: Factors influencing formation and reaction pathways [J]. Journal of Hazardous Materials, 2017, 321: 28-36
[6] Guan C T, Jiang J, Pang S Y, et al. Effect of iodide on transformation of phenolic compounds by nonradical activation of peroxydisulfate in the presence of carbon nanotube: Kinetics, impacting factors, and formation of iodinated aromatic products [J]. Chemosphere, 2018, 208: 559-568
[7] Pan Y, Li W B, Li A M, et al. A new group of disinfection byproducts in drinking water: Trihalo-hydroxy-cyclopentene-diones [J]. Environmental Science &Technology, 2016, 50(14): 7344-7352
[8] Zhang H F, Zhang Y H, Shi Q, et al. Characterization of unknown brominated disinfection byproducts during chlorination using ultrahigh resolution mass spectrometry [J]. Environmental Science &Technology, 2014, 48(6): 3112-3119
[9] Zhang H F, Zhang Y H, Shi Q, et al. Characterization of low molecular weight dissolved natural organic matter along the treatment trait of a waterworks using Fourier transform ion cyclotron resonance mass spectrometry [J]. Water Research, 2012, 46(16): 5197-5204
[10] Zhou W J, Lou L J, Zhu L F, et al. Formation and cytotoxicity of a new disinfection by-product (DBP) phenazine by chloramination of water containing diphenylamine [J]. Journal of Environmental Sciences (China), 2012, 24(7): 1217-1224
[11] Chaukura N, Marais S, Moyo W, et al. Contemporary issues on the occurrence and removal of disinfection byproducts in drinking water—A review [J]. Journal of Environmental Chemical Engineering, 2020, 8(2): 103659
[12] Sun G Y, Yuan J, Zhang Z W, et al. Research progress on the precursors and formation mechanisms of typical N-DBPs in drinking water [J]. Desalination and Water Treatment, 2018, 129: 325-331
[13] Yang X, Fan C, Shang C, et al. Nitrogenous disinfection byproducts formation and nitrogen origin exploration during chloramination of nitrogenous organic compounds [J]. Water Research, 2010, 44(9): 2691-2702
[14] Pontie M, Buisson H, Diawara C K, et al. Studies of halide ions mass transfer in nanofiltration—Application to selective defluorination of brackish drinking water [J]. Desalination, 2003, 157(1-3): 127-134
[15] Westerhoff P, Mash H. Dissolved organic nitrogen in drinking water supplies: A review [J]. Journal of Water Supply: Research and Technology - AQUA, 2002, 51(8): 415-448
[16] Ye Z, Liu W J, Sun W J, et al. Role of ammonia on haloacetonitriles and halonitromethanes formation during ultraviolet irradiation followed by chlorination/chloramination [J]. Chemical Engineering Journal, 2018, 337: 275-281
[17] Shan J H, Hu J, Kaplan-Bekaroglu S S, et al. The effects of pH, bromide and nitrite on halonitromethane and trihalomethane formation from amino acids and amino sugars [J]. Chemosphere, 2012, 86(4): 323-328
[18] Choi J H, Valentine R L. N-nitrosodimethylamine formation hy free-chlorine-enhanced nitrosation of dimethylamine [J]. Environmental Science &Technology, 2003, 37(21): 4871-4876
[19] Choi J, Valentine R L. Formation of N-nitrosodimethylamine (NDMA) from reaction of monochloramine: A new disinfection by-product [J]. Water Research, 2002, 36(4): 817-824
[20] Kanan A, Karanfil T. Estimation of haloacetonitriles formation in water: Uniform formation conditions versus formation potential tests [J]. The Science of the Total Environment, 2020, 744: 140987
[21] Zhang Y, Han X M, Niu Z G. Health risk assessment of haloacetonitriles in drinking water based on internal dose [J]. Environmental Pollution, 2018, 236: 899-906
[22] Kosaka K, Ohkubo K, Akiba M. Occurrence and formation of haloacetamides from chlorination at water purification plants across Japan [J]. Water Research, 2016, 106: 470-476
[23] Chu W H, Gao N Y, Deng Y. Formation of haloacetamides during chlorination of dissolved organic nitrogen aspartic acid [J]. Journal of Hazardous Materials, 2010, 173(1-3): 82-86
[24] World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda [R]. Geneva: World Health Organization, 2022
[25] Woo Y T, Lai D, McLain J, et al. Use of mechanism-based structure-activity relationships analysis in carcinogenic potential ranking for drinking water disinfection by-products [J]. Environmental Health Perspectives, 2002, 110(Suppl 1): 75-87
[26] Bellar T A, Lichtenberg J J, Kroner R C. The occurrence of organohalides in chlorinated drinking waters [J]. Journal - American Water Works Association, 1974, 66(12): 703-706
[27] Rahman M B, Cowie C, Driscoll T, et al. Colon and rectal cancer incidence and water trihalomethane concentrations in New South Wales, Australia [J]. BMC Cancer, 2014, 14: 445
[28] Wagner E D, Plewa M J. CHO cell cytotoxicity and genotoxicity analyses of disinfection by-products: An updated review [J]. Journal of Environmental Sciences, 2017, 58: 64-76
[29] Bond T, Goslan E H, Parsons S A, et al. Treatment of disinfection by-product precursors [J]. Environmental Technology, 2011, 32(1-2): 1-25
[30] 李大鹏, 黄强, 朱贺振. 黄河原水中消毒副产物前体物的组成特征及其化学预氧化处理效果对比[J]. 环境科学学报, 2016, 36(3): 827-833
Li D P, Huang Q, Zhu H Z. Composition of disinfection byproducts precursors in the Yellow River raw water and comparison of different pre-oxidation treatments [J]. Acta Scientiae Circumstantiae, 2016, 36(3): 827-833 (in Chinese)
[31] Zhang X R, Minear R A. Decomposition of trihaloacetic acids and formation of the corresponding trihalomethanes in drinking water [J]. Water Research, 2002, 36(14): 3665-3673
[32] Ge R, Yang S, Kramer P M, et al. The effect of dichloroacetic acid and trichloroacetic acid on DNA methylation and cell proliferation in B6C3F1 mice [J]. Journal of Biochemical and Molecular Toxicology, 2001, 15(2): 100-106
[33] Ghadimkhani A, Zhang W, Marhaba T. Ceramic membrane defouling (cleaning) by air nano bubbles [J]. Chemosphere, 2016, 146: 379-384
[34] Marsà A, Cortés C, Hernández A, et al. Hazard assessment of three haloacetic acids, as byproducts of water disinfection, in human urothelial cells [J]. Toxicology and Applied Pharmacology, 2018, 347: 70-78
[35] Holmes B E, Smeester L, Fry R C, et al. Identification of endocrine active disinfection by-products (DBPs) that bind to the androgen receptor [J]. Chemosphere, 2017, 187: 114-122
[36] Qi W X, Zhang H, Hu C Z, et al. Effect of ozonation on the characteristics of effluent organic matter fractions and subsequent associations with disinfection by-products formation [J]. The Science of the Total Environment, 2018, 610-611: 1057-1064
[37] 毛玉琴, 应海儿, 杨宏伟. 不同净水工艺对含溴水体消毒副产物生成势的影响[J]. 中国给水排水, 2020, 36(5): 1-6
Mao Y Q, Ying H E, Yang H W. Influence of different water purification processes on disinfection by-products formation potential of bromide-containing water [J]. China Water &Wastewater, 2020, 36(5): 1-6 (in Chinese)
[38] Priestley C C, Green R M, Fellows M D, et al. Anomalous genotoxic responses induced in mouse lymphoma L5178Y cells by potassium bromate [J]. Toxicology, 2010, 267(1-3): 45-53
[39] Liu D M, Wang Z W, Zhu Q, et al. Drinking water toxicity study of the environmental contaminant--Bromate [J]. Regulatory Toxicology and Pharmacology, 2015, 73(3): 802-810
[40] Von G U. Ozonation of drinking water: Part Ⅱ. Disinfection and by-product formation in presence of bromide, iodide or chlorine [J]. Water Research, 2003, 37(7): 1469-1487
[41] 吴悦, 吴纯德, 刘吕刚, 等. 含溴水臭氧化过程阴离子对溴酸盐生成的影响[J]. 环境科学, 2015, 36(9): 3292-3297
Wu Y, Wu C D, Liu L G, et al. Effects of anions on bromate formation during ozonation of bromide-containing water [J]. Environmental Science, 2015, 36(9): 3292-3297 (in Chinese)
[42] 张维清, 邹惠仙. 三氯乙醛前驱物的筛选及其生成影响因素探讨[J]. 环境科学与技术, 2005, 28(2): 29-31, 116
Zhang W Q, Zou H X. Screening and generation of 3-chloroacetaldehyde precursor [J]. Environmental Science and Technology, 2005, 28(2): 29-31, 116 (in Chinese)
[43] Hebert A, Forestier D, Lenes D, et al. Innovative method for prioritizing emerging disinfection by-products (DBPs) in drinking water on the basis of their potential impact on public health [J]. Water Research, 2010, 44(10): 3147-3165
[44] Yin D Q, Hu S Q, Jin H J, et al. Deriving freshwater quality criteria for 2,4,6-trichlorophenol for protection of aquatic life in China [J]. Chemosphere, 2003, 52(1): 67-73
[45] Tang Y L, Long X, Wu M Y, et al. Bibliometric review of research trends on disinfection by-products in drinking water during 1975-2018 [J]. Separation and Purification Technology, 2020, 241: 116741
[46] 胡建英, 谢国红, 相泽贵子. 4-壬基酚在氯消毒过程中的氧化途径[J]. 环境化学, 2002, 21(3): 254-258
Hu J Y, Xie G H, Aizawa T. Aqueous chlorination pathways of 4-nonylphenol [J]. Environmental Chemistry, 2002, 21(3): 254-258 (in Chinese)
[47] Wang W F, Ren S Y, Zhang H F, et al. Occurrence of nine nitrosamines and secondary amines in source water and drinking water: Potential of secondary amines as nitrosamine precursors [J]. Water Research, 2011, 45(16): 4930-4938
[48] Chung J, Ahn C H, Chen Z, et al. Bio-reduction of N-nitrosodimethylamine (NDMA) using a hydrogen-based membrane biofilm reactor [J]. Chemosphere, 2008, 70(3): 516-520
[49] Schreiber I M, Mitch W A. Influence of the order of reagent addition on NDMA formation during chloramination [J]. Environmental Science &Technology, 2005, 39(10): 3811-3818
[50] Le Roux J, Gallard H, Croué J P. Chloramination of nitrogenous contaminants (pharmaceuticals and pesticides): NDMA and halogenated DBPs formation [J]. Water Research, 2011, 45(10): 3164-3174
[51] Liu X K, Lin Y F, Ruan T, et al. Identification of N-nitrosamines and nitrogenous heterocyclic byproducts during chloramination of aromatic secondary amine precursors [J]. Environmental Science &Technology, 2020, 54(20): 12949-12958
[52] Chen W H, Wang Y H, Hsu T H. The competitive effect of different chlorination disinfection methods and additional inorganic nitrogen on nitrosamine formation from aromatic and heterocyclic amine-containing pharmaceuticals [J]. Chemosphere, 2021, 267: 128922
[53] Shen R Q, Andrews S A. Demonstration of 20 pharmaceuticals and personal care products (PPCPs) as nitrosamine precursors during chloramine disinfection [J]. Water Research, 2011, 45(2): 944-952
[54] Chen W H, Yang Y C, Wang Y H, et al. Effect of molecular characteristics on the formation of nitrosamines during chlor(am)ination of phenylurea herbicides [J]. Environmental Science Processes &Impacts, 2015, 17(12): 2092-2100
[55] Mitch W A, Sedlak D L. Characterization and fate of N-nitrosodimethylamine precursors in municipal wastewater treatment plants [J]. Environmental Science &Technology, 2004, 38(5): 1445-1454
[56] Verdugo E M, Krause C, Genskow K, et al. N-functionalized carbon nanotubes as a source and precursor of N-nitrosodimethylamine: Implications for environmental fate, transport, and toxicity [J]. Environmental Science &Technology, 2014, 48(16): 9279-9287
[57] Shah A D, Krasner S W, Lee C F, et al. Trade-offs in disinfection byproduct formation associated with precursor preoxidation for control of N-nitrosodimethylamine formation [J]. Environmental Science &Technology, 2012, 46(9): 4809-4818
[58] Liu Y D, Selbes M, Zeng C C, et al. Formation mechanism of NDMA from ranitidine, trimethylamine, and other tertiary amines during chloramination: A computational study [J]. Environmental Science &Technology, 2014, 48(15): 8653-8663
[59] Chen Z, Valentine R L. Formation of N-nitrosodimethylamine (NDMA) from humic substances in natural water [J]. Environmental Science &Technology, 2007, 41(17): 6059-6065
[60] Charrois J W A, Hrudey S E. Breakpoint chlorination and free-chlorine contact time: Implications for drinking water N-nitrosodimethylamine concentrations [J]. Water Research, 2007, 41(3): 674-682
[61] Mitch W A, Sedlak D L. Formation of N-nitrosodimethylamine (NDMA) from dimethylamine during chlorination [J]. Environmental Science &Technology, 2002, 36(4): 588-595
[62] Spahr S, von Gunten U, Hofstetter T B. Carbon, hydrogen, and nitrogen isotope fractionation trends in N-nitrosodimethylamine reflect the formation pathway during chloramination of tertiary amines [J]. Environmental Science &Technology, 2017, 51(22): 13170-13179
[63] Spahr S, Cirpka O, Gunten U, et al. Formation of N-nitrosodimethylamine during chloramination of secondary and tertiary amines: Role of molecular oxygen and radical intermediates [J]. Environmental Science and Technology, 2016, 51(1): 280-290
[64] Muellner M G, Wagner E D, McCalla K, et al. Haloacetonitriles vs. regulated haloacetic acids: Are nitrogen-containing DBPs more toxic? [J]. Environmental Science &Technology, 2007, 41(2): 645-651
[65] Lee J H, Na C Z, Ramirez R L, et al. Cyanogen chloride precursor analysis in chlorinated river water [J]. Environmental Science &Technology, 2006, 40(5): 1478-1484
[66] Reckhow D, Singer P, Malcolm R. Chlorination of humic materials: Byproduct formation and chemical interpretations [J]. Environmental Science &Technology, 1990, 24: 1655-1664
[67] Pedersen E J, Urbansky E, Mari as B, et al. Formation of cyanogen chloride from the reaction of monochloramine with formaldehyde [J]. Environmental Science &Technology, 1999, 33(23): 4239-4249
as B, et al. Formation of cyanogen chloride from the reaction of monochloramine with formaldehyde [J]. Environmental Science &Technology, 1999, 33(23): 4239-4249
[68] Na C Z, Olson T M. Mechanism and kinetics of cyanogen chloride formation from the chlorination of glycine [J]. Environmental Science &Technology, 2006, 40(5): 1469-1477
[69] Barbara C, Scully Frank E. Chloramines V: Products and implications of the chlorination of lysine in municipal wastewaters [J]. Environmental Science &Technology, 1997, 31(6): 1680-1685
[70] Fu J, Wang X M, Bai W L, et al. Azo compound degradation kinetics and halonitromethane formation kinetics during chlorination [J]. Chemosphere, 2017, 174: 110-116
[71] Richardson S D, Plewa M J, Wagner E D, et al. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research [J]. Mutation Research, 2007, 636(1-3): 178-242
[72] Kundu B, Richardson S D, Granville C A, et al. Comparative mutagenicity of halomethanes and halonitromethanes in Salmonella TA100: Structure-activity analysis and mutation spectra [J]. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 2004, 554(1-2): 335-350
[73] Buerge I J, Kahle M, Buser H R, et al. Nicotine derivatives in wastewater and surface waters: Application as chemical markers for domestic wastewater [J]. Environmental Science &Technology, 2008, 42(17): 6354-6360
[74] Deng L, Wen L J, Dai W J, et al. Impact of tryptophan on the formation of TCNM in the process of UV/chlorine disinfection [J]. Environmental Science and Pollution Research International, 2018, 25(23): 23227-23235
[75] 高乃云, 楚文海, 徐斌. 从生成机制谈饮用水中新型消毒副产物的控制策略[J]. 给水排水, 2017, 53(2): 1-5
[76] Hong H C, Qian L Y, Xiong Y J, et al. Use of multiple regression models to evaluate the formation of halonitromethane via chlorination/chloramination of water from Tai Lake and the Qiantang River, China [J]. Chemosphere, 2015, 119: 540-546
[77] Gan G J, Qiu L, Wu H, et al. Effect of nitrite on the formation of trichloronitromethane (TCNM) during chlorination of polyhydroxy-phenols and sugars [J]. Water, Air, &Soil Pollution, 2017, 228(6): 208
[78] Zhou X L, Zheng L L, Chen S Y, et al. Factors influencing DBPs occurrence in tap water of Jinhua Region in Zhejiang Province, China [J]. Ecotoxicology and Environmental Safety, 2019, 171: 813-822
[79] DeMarini D M, Shelton M L, Warren S H, et al. Glutathione S-transferase-mediated induction of GC→AT transitions by halomethanes in Salmonella [J]. Environmental and Molecular Mutagenesis, 1997, 30(4): 440-447
[80] Giller S, Le Curieux F, Erb F, et al. Comparative genotoxicity of halogenated acetic acids found in drinking water [J]. Mutagenesis, 1997, 12(5): 321-328
[81] Liu X Y, Chen L, Yang M T, et al. The occurrence, characteristics, transformation and control of aromatic disinfection by-products: A review [J]. Water Research, 2020, 184: 116076
[82] Hu S Y, Gong T T, Xian Q M, et al. Formation of iodinated trihalomethanes and haloacetic acids from aromatic iodinated disinfection byproducts during chloramination [J]. Water Research, 2018, 147: 254-263
[83] Kali S, Khan M, Ghaffar M S, et al. Occurrence, influencing factors, toxicity, regulations, and abatement approaches for disinfection by-products in chlorinated drinking water: A comprehensive review [J]. Environmental Pollution, 2021, 281: 116950
[84] Ewaid S H, Rabee A M, Al-Naseri S K. Carcinogenic risk assessment of trihalomethanes in major drinking water sources of Baghdad City [J]. Water Resources, 2018, 45(5): 803-812
[85] Mompremier R, Mariles  , Bravo J E, et al. Study of the variation of haloacetic acids in a simulated water distribution network [J]. Water Supply, 2019, 19(1): 88-96
, Bravo J E, et al. Study of the variation of haloacetic acids in a simulated water distribution network [J]. Water Supply, 2019, 19(1): 88-96
[86] Zhang Z X, Zhu Q Y, Huang C, et al. Comparative cytotoxicity of halogenated aromatic DBPs and implications of the corresponding developed QSAR model to toxicity mechanisms of those DBPs: Binding interactions between aromatic DBPs and catalase play an important role [J]. Water Research, 2020, 170: 115283
[87] Liu J Q, Zhang X R, Li Y, et al. Phototransformation of halophenolic disinfection byproducts in receiving seawater: Kinetics, products, and toxicity [J]. Water Research, 2019, 150: 68-76
[88] Li Y, Jiang J Y, Li W X, et al. Volatile DBPs contributed marginally to the developmental toxicity of drinking water DBP mixtures against Platynereis dumerilii [J]. Chemosphere, 2020, 252: 126611
[89] Zhang Y M, Chu W H, Yao D C, et al. Control of aliphatic halogenated DBP precursors with multiple drinking water treatment processes: Formation potential and integrated toxicity [J]. Journal of Environmental Sciences (China), 2017, 58: 322-330
[90] Tuppurainen K, Lötjönen S, Laatikainen R, et al. About the mutagenicity of chlorine-substituted furanones and halopropenals. A QSAR study using molecular orbital indices [J]. Mutation Research, 1991, 247(1): 97-102
[91] Wu Y, Wei W Z, Luo J Y, et al. Comparative toxicity analyses from different endpoints: Are new cyclic disinfection byproducts (DBPs) more toxic than common aliphatic DBPs? [J]. Environmental Science &Technology, 2022, 56(1): 194-207
[92] Qin L T, Zhang X, Chen Y H, et al. Predictive QSAR models for the toxicity of disinfection byproducts [J]. Molecules, 2017, 22(10): 1671
[93] Wang L, Chen B Y, Zhang T. Predicting hydrolysis kinetics for multiple types of halogenated disinfection byproducts via QSAR models [J]. Chemical Engineering Journal, 2018, 342: 372-385