Daughton和Ternes[1]于1999年首次提出药物与个人护理品(pharmaceuticals and personal care products, PPCPs)概念,主要包括:人用和兽用药物,例如抗生素、类固醇、止痛药和降压药等;以及化妆品、防晒霜、洗发水和染发剂等个人护理品。作为一类与人类日常生活密切相关的化学物质,PPCPs生产和使用量巨大,并在生产、使用过程中不断地向环境介质中释放。研究发现,PPCPs在废水[2]、表层水[3]、地下水[4]、土壤[5]和饮用水[6]等多种环境介质中广泛检出。大部分PPCPs的化学结构稳定,进入受纳环境后长期残留,其在水环境中的检出浓度通常介于ng·L-1到μg·L-1水平,土壤或沉积物中检出浓度一般在ng·g-1水平。环境中残留的PPCPs,不仅会对生态系统造成潜在的风险,还会影响人类健康,如抗生素污染会诱导耐药菌和耐药基因的传播扩散,对人类健康造成潜在威胁[7]。最近Science杂志的封面论文研究发现典型紫外吸收剂羟苯甲酮会在珊瑚体内富集和代谢,产生危害珊瑚生存的光毒素,加速濒危生态系统的消失[8]。另外,还有研究发现典型杀生剂三氯生对鱼类具有发育毒性和神经毒性[9]。因此,PPCPs的污染防控引起国内外广泛关注,我国也提出将抗生素等新污染物作为重点管控新污染物进行系统监测和污染防控。
现阶段针对PPCPs的常用去除方法包括物理法(吸附法、膜分离法)、化学氧化法(辐射分解、芬顿氧化法、臭氧氧化法、电化学氧化和过硫酸盐氧化等)、微生物降解法(好氧降解、厌氧降解)以及物化法-生物法组合技术等[10]。其中,微生物降解法因其环境友好、成本低等优势而受到广泛关注。PPCPs好氧微生物降解因其条件易于控制,目前已有大量相关研究。表1中列举了药物、苯并三唑、激素和抗生素等4类常见的PPCPs,研究发现这些PPCPs如萘普生、5-氯代苯并三氮唑、磺胺甲噁唑的好氧微生物降解效率和降解半衰期显著低于厌氧微生物降解(表1)[11-15]。通常疏水性的PPCPs进入受纳环境后,倾向于分布在厌氧或者缺氧环境中,厌氧微生物降解可能是其主要的消解方式,而目前针对PPCPs厌氧微生物降解机制研究仍很有限。因此,深入开展PPCPs的厌氧降解研究具有重要意义,了解其在环境中的厌氧降解转化过程、影响因素与制约因子,有助于系统认识PPCPs的污染过程与环境归趋,从而针对性地研发污染阻断和控制技术。本研究以典型PPCPs为例介绍了其在污水处理厂中的厌氧去除情况,并总结了PPCPs厌氧降解的主要影响因素,重点归纳了磺胺甲噁唑、苯并三唑和三氯生等3种典型PPCPs类化合物的厌氧降解转化途径,以期为PPCPs的污染防治提供科学依据。
表1 典型药物与个人护理品(PPCPs)的好氧与厌氧降解效率差异
Table 1 Difference between aerobic and anaerobic degradation of typical pharmaceuticals and personal care products (PPCPs)
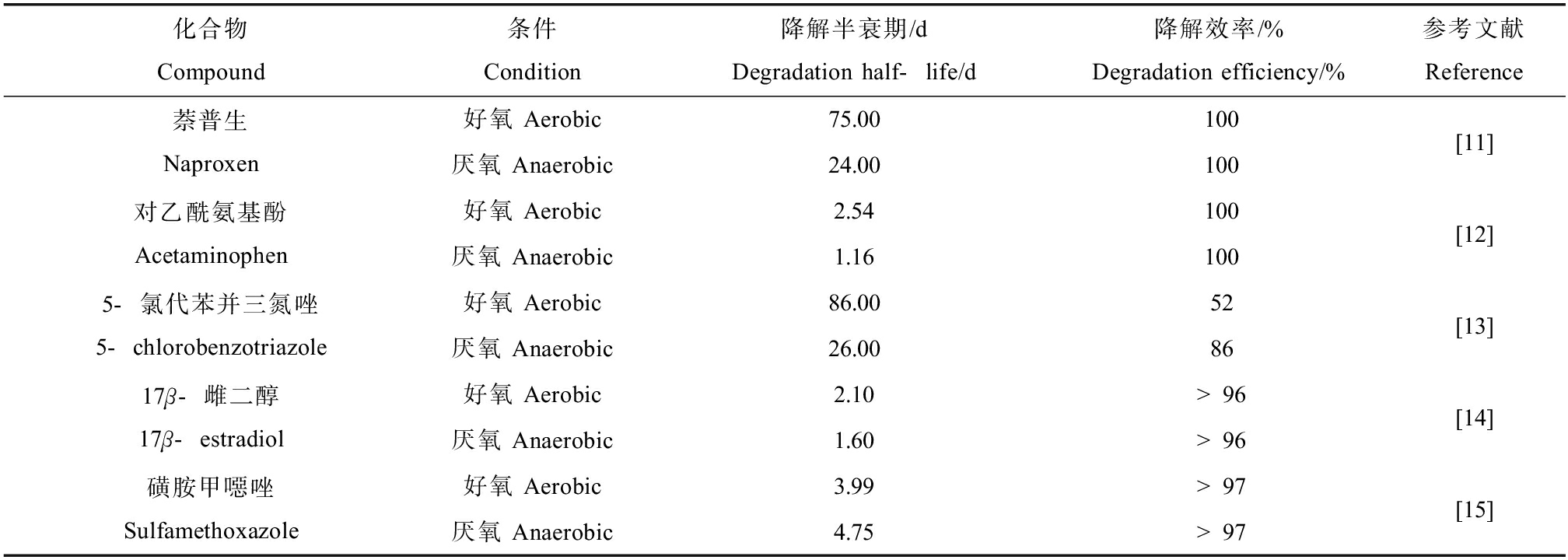
化合物Compound条件Condition降解半衰期/dDegradation half-life/d降解效率/%Degradation efficiency/%参考文献Reference萘普生Naproxen好氧 Aerobic75.00100厌氧 Anaerobic24.00100[11]对乙酰氨基酚Acetaminophen好氧 Aerobic2.54100厌氧 Anaerobic1.16100[12]5-氯代苯并三氮唑5-chlorobenzotriazole好氧 Aerobic86.0052厌氧 Anaerobic26.0086[13]17β-雌二醇17β-estradiol好氧 Aerobic2.10>96厌氧 Anaerobic1.60>96[14]磺胺甲噁唑Sulfamethoxazole好氧 Aerobic3.99>97厌氧 Anaerobic4.75>97[15]
1 城市污水处理厂中PPCPs的厌氧去除途径(Anaerobic removal pathways of PPCPs in urban sewage treatment plants)
疏水性PPCPs受辛醇/水分配系数(Kow)、解离常数(pKa)等物化性质,环境温度、pH值等外界因素影响,容易吸附于污泥中[16]。如三氯生、舍曲林、奥克立林和UV-326等化合物因Kow值较高,在污泥相的占比分别可达41%、90%、92%和54%(图1)[17-19]。此外,还有研究发现污泥吸附也是部分亲水性PPCPs在污水处理厂中的主要去除途径。依诺沙星、诺氟沙星、环丙沙星和恩诺沙星等Kow在-0.2~0.46之间的氟喹诺酮类抗生素,在污水处理厂或实验室模拟污水处理实验中高达73.9%~86.2%可吸附到污泥相(图1)[20-25]。污泥对氟喹诺酮类抗生素的强吸附作用可能是由带电的化合物基团与微生物及惰性颗粒表面发生的静电作用所引起[26]。而污泥对四环素、土霉素等低Kow的四环素类抗生素的吸附作用则与阳离子交换机制密切相关。污泥相通常处于厌氧或缺氧环境,吸附在污泥中的PPCPs会进一步发生厌氧降解转化。Martín等[27]研究发现低浓度对乙酰氨基酚、双氯芬酸、水杨酸、苯扎贝特和氟喹诺酮类抗生素等PPCPs在厌氧消化工艺中的去除率显著高于好氧处理工艺。此外,文拉法辛、泛影酸和曲马多等在好氧工艺过程中去除效率极低的化合物,进入厌氧工艺后其去除率有显著提升[28-29]。相比于好氧工艺,厌氧消化工艺已经成为污水处理厂去除一些难降解PPCPs的有效手段。
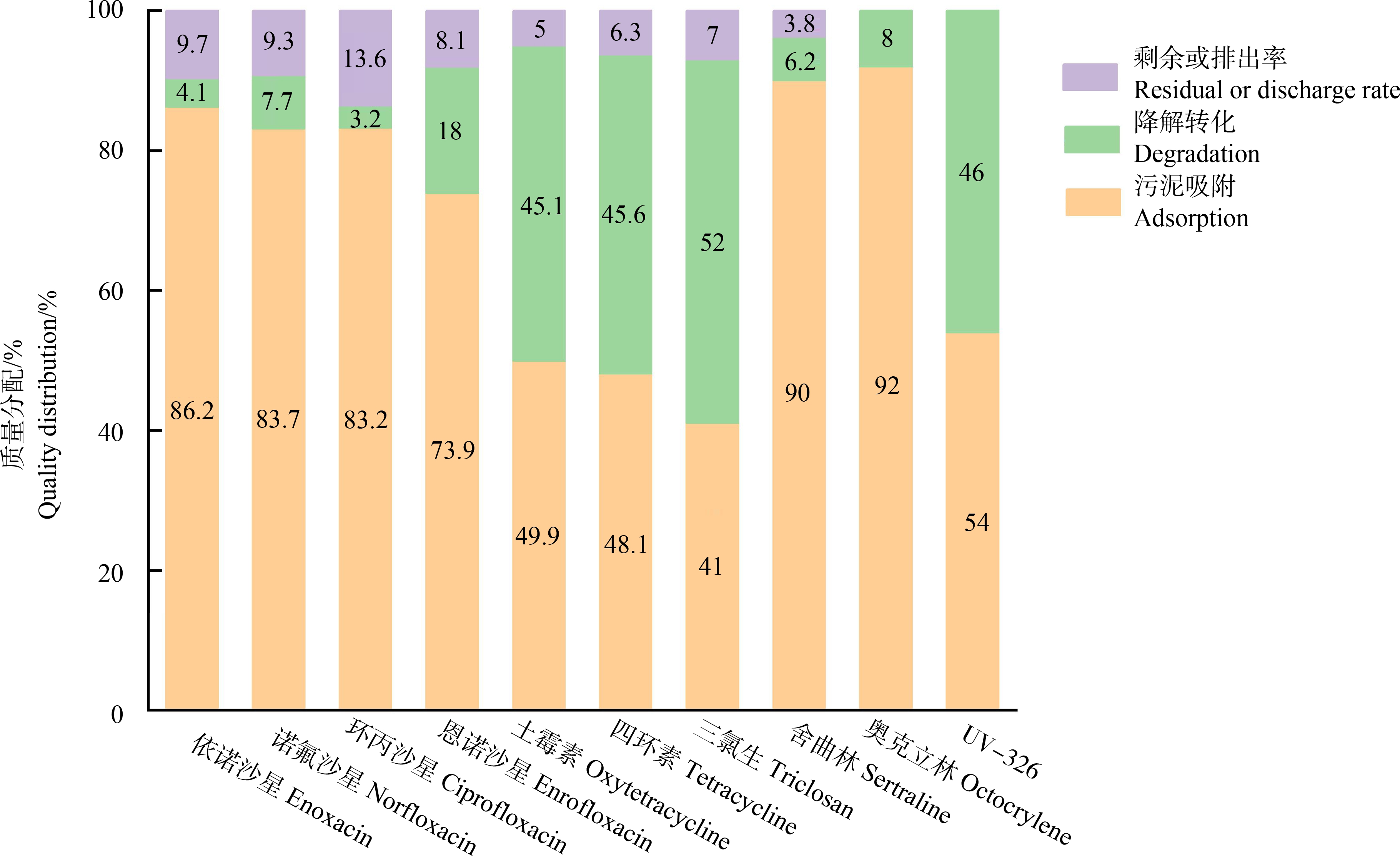
图1 典型PPCPs在城市污水厂中的主要去除途径及贡献[17-25]
Fig. 1 Main removal pathways and contributions of typical PPCPs in municipal sewage treatment plants[17-25]
2 PPCPs厌氧降解转化的主要影响因素(Main influence factors of anaerobic biodegradation of PPCPs)
2.1 化学结构(Chemical structure)
PPCPs的化学结构决定了其理化性质和生物可降解性,官能团会影响PPCPs的生物可降解性[30-35]。对于含有苯环的化合物,苯环上连接的—COOH、—OH等官能团能够促进其羟基化并增加生物可降解性,而卤素则会降低化合物的生物可降解性[35]。Musson等[32]对比研究了4种化学结构不同PPCPs的厌氧降解效率,发现乙酰水杨酸>酒石酸美托洛尔>对乙酰氨基酚>布洛芬。乙酰水杨酸因苯环上含—COOH、—OH官能团,呈现出较高的生物可降解性;而酒石酸美托洛尔苯环上含有醚键和氨基、对乙酰氨基酚苯环上侧链分支含酰胺键、布洛芬侧链高度分支,这些特征官能团可能在一定程度上影响了3种化合物的生物可降解性,导致其厌氧降解效率显著低于乙酰水杨酸[32]。
2.2 微生物(Microorganisms)
微生物是PPCPs厌氧降解转化的主要执行者,其组成、稳定性和功能微生物丰度等均会影响PPCPs的厌氧降解[36]。Sella等[37]研究发现3种不同污泥中磺胺甲噁唑的降解效果有差异,微生物群落结构相对稳定的污泥体系中磺胺甲噁唑降解效果最好。Wolfson等[38]对比研究发现添加目标化合物苯海拉明后,丛毛单胞菌科、共生细菌科和厌氧绳菌科细菌明显富集,说明该类细菌可能是苯海拉明降解功能菌群。大量研究证实,厌氧降解功能单菌的筛选可有效促进特定污染物的降解效果。Chopra和Kumar[39]从印度哈里亚纳邦污水中分离获得厌氧菌株德伦特杆菌S1(Bacillus drentensis strain S1),其对乙酰氨基酚(初始浓度为300 mg·L-1)的厌氧降解效率高达93%。Ouyang等[40]在初始浓度100 μmol·L-1磺胺甲噁唑的硫酸盐还原体系中加入富集培养的希尔登伯勒普通脱硫弧菌(Desulfovibrio vulgaris Hildenborough),磺胺甲噁唑的降解效果高达90%。吴丹等[41]从某市政污水处理厂厌氧消化污泥中分离得到柠檬酸盐杆菌属BP3-1(Citrobacter amalonaticus strain BP3-1),对BP-3(2 mg·L-1)的降解效率高达98.3%。
此外,研究还发现温度、pH和腐殖酸等多种环境因素会影响微生物活性,进而影响PPCPs的厌氧降解转化效率。在一定温度范围内,微生物活性随温度改变而变化,影响PPCPs的厌氧降解速率[42]。然而,也有研究发现温度对部分PPCPs的厌氧降解无明显影响。Carballa等[43-44]研究发现除了布洛芬和罗红霉素外,佳乐麝香、吐纳麝香、卡马西平、地西泮、萘普生、双氯芬酸、碘普罗胺、磺胺甲噁唑、雌酮、17β-雌二醇和17α-乙炔雌二醇等11种PPCPs的厌氧降解均不受温度的影响。另一方面,不同微生物的最适生长pH不同,通常当微生物处于最适生长pH值时,其对PPCPs的厌氧降解效率较高。Mao等[45]研究发现,不同的pH条件下希瓦氏菌属对磺胺吡啶和磺胺甲噁唑的降解效率有显著差异,当pH值为7~8时2种抗生素的降解效率均最高。
腐殖酸物质(humic substances, HS)作为自然环境中存在的动物、植物和微生物残骸及其降解产物中经过高度转化的而成的有机物质[46],能够影响多种PPCPs的厌氧生物降解,研究发现五氯苯酚的微生物还原性脱氯受其影响显著[47-49];此外,多种HS可作为氧化还原中间介质影响2,4-二氯苯氧乙酸的厌氧微生物降解[50]。HS影响PPCPs厌氧微生物降解的方式可能存在多种:(1)HS可作为电子供体或者受体,在HS还原微生物和一些不溶的电子受体(如三价铁氧化物)之间穿梭传递电子,通过影响微生物的无氧呼吸改变微生物对PPCPs的降解活性[51];(2)HS能够改变微生物的生长活性;(3)HS可将部分PPCPs转化为低毒产物或充当疏水性化合物的表面活性剂以增加PPCPs的生物可利用性。然而,目前关于HS对PPCPs厌氧微生物降解的影响机制尚未明确,有待进一步研究。
2.3 外加条件(Additional conditions)
2.3.1 碳源(Carbon source)
通过添加外源碳源,促进PPCPs的厌氧共代谢转化已被广泛报道,其共代谢降解机理主要包括:(1)降解菌更易于利用外源碳源,释放酶非特异性作用于PPCPs促进其降解;(2)降解菌利用外源碳源促进其自身生长,提高特定降解酶的分泌从而促进PPCPs降解。不同碳源对PPCPs的厌氧共代谢降解转化效果影响差异显著。控制外加碳源为单一变量,当蛋白胨作为共代谢碳源时,磺胺甲噁唑的厌氧降解半衰期为4.53 d,而可溶淀粉作为共代谢碳源时,磺胺甲噁唑的厌氧降解半衰期缩短至1.61 d[52]。然而,Baquero等[53]研究发现当醋酸钠作为共代谢碳源时,卡马西平和双氯芬酸的去除效率降低。添加合适的碳源构建共代谢体系可有效提高PPCPs的厌氧降解效率,而不同碳源对其厌氧降解的影响机制仍有待深入研究。
2.3.2 氧化还原电位(Redox potential)
氧化还原条件也会对PPCPs的厌氧降解产生影响,部分难降解化合物只能在特定的条件下有效降解。由表2可知,咖啡因、萘普生、阿替洛尔、普萘洛尔、苯并三唑、5-甲基苯并三唑、5-氯代苯并三唑、美托洛尔和环丙沙星在不同氧化还原条件下降解效率差异显著[11,13,54-56]。如萘普生在硝酸盐还原条件下难降解,在硫酸盐还原条件下降解效率较高;而普萘洛尔则呈现出相反的降解规律。咖啡因在产甲烷条件下降解效率较低,而在硫酸盐还原和硝酸盐还原条件下均呈现较高的降解效率[55]。
表2 PPCPs在不同厌氧降解条件下的降解情况
Table 2 Degradation of PPCPs under different anaerobic condition

化合物Compound条件Condition降解半衰期/dDegradation half-life/d降解效率/%Degradation efficiency/%参考文献Reference咖啡因Caffeine硫酸盐还原 Sulfate-reducing-a100硝酸盐还原 Nitrate-reducing-a100产甲烷 Methanogenic- a<20[54]萘普生Naproxen阿替洛尔Atenolol普萘洛尔Propranolol锰还原 Manganese-reducing66100铁还原 Iron-reducing24100硫酸盐还原 Sulfate-reducing87100硝酸盐还原 Nitrate-reducingn.d.b-2c锰还原 Manganese-reducingn.d.b18铁还原 Iron-reducing38152硫酸盐还原 Sulfate-reducingn.d. b19硝酸盐还原 Nitrate-reducing192100锰还原 Manganese-reducingn.d. b20铁还原 Iron-reducingn.d. b12硫酸盐还原 Sulfate-reducingn.d.b-2c硝酸盐还原 Nitrate-reducing25866[11]苯并三唑Benzotriazole5-甲基苯并三唑5-methylbenzotriazole5-氯代苯并三唑5-chlorobenzotriazole厌氧对照 Anaerobic control14436铁还原 Iron-reducing23931硫酸盐还原 Sulfate-reducing16518硝酸盐还原 Nitrate-reducing31524厌氧对照 Anaerobic control5761铁还原 Iron-reducing4176硫酸盐还原 Sulfate-reducing8847硝酸盐还原 Nitrate-reducing12835厌氧对照 Anaerobic control4471铁还原 Iron-reducing2686硫酸盐还原 Sulfate-reducing9645硝酸盐还原 Nitrate-reducing7853[13]美托洛尔Metoprolol硫酸盐还原 Sulfate-reducing-a56硝酸盐还原 Nitrate-reducing- a-9c产甲烷 Methanogenic- a52[55]环丙沙星Ciprofloxacin发酵 Fermentation- an.d. b硫酸盐还原 Sulfate-reducing-a80硝酸盐还原 Nitrate-reducing-a82[56]
注:a “-”表示文献中未给出数据;b “n.d.”表示在实验周期内未被检测到;c 降解效率为负数可能是由于污泥解吸附作用或是较小的分析偏差导致。
Note: a “-” indicated that no data was given in the reference; b “n.d. ” indicated that it was not detected during the experimental period; c The negative degradation efficiency may be caused by sludge desorption or small analysis deviation.
目前,关于PPCPs厌氧降解效率的影响因素研究普遍以控制单一变量为主,然而不同因素之间具有相互作用。如不同的氧化还原条件会影响PPCPs降解菌群的组成[56],温度变化对降解菌生理活性产生影响进而导致降解菌最适pH值发生改变等。因此,未来开展PPCPs厌氧微生物降解研究时应尽可能模拟复杂自然环境体系,考虑多因素相互作用对其降解转化规律的影响。
3 典型PPCPs的厌氧降解转化途径(Anaerobic biodegradation pathways of typical PPCPs)
本文以使用量大、检出率高、具有潜在生态毒性和健康风险的磺胺甲噁唑、苯并三唑以及三氯生3种典型的PPCPs为例,归纳总结了其在不同氧化还原条件下的厌氧降解转化途径。
3.1 磺胺甲噁唑(Sulfamethoxazole)
磺胺甲噁唑(sulfamethoxazole, SMX)是一种磺胺类的抗生素,由氨基取代的苯环通过磺酰胺与甲基化异噁唑环相连组成(图2),常用于人类和动物疾病预防和治疗,使用量大,且被人和动物服用以后不能完全代谢,其在环境中广泛分布,对人、动物和环境造成潜在危害。相比于传统的去除工艺,研究发现厌氧序批生物膜反应器、横流式厌氧固定化生物量反应器等厌氧工艺对SMX的去除效果较好[57]。目前,针对SMX在硫酸盐还原条件和铁还原条件下的厌氧降解转化机理已展开了一系列深入的研究[23,40,58-59],普遍认为SMX的厌氧降解途径起始于异噁唑环O—N键断裂。在铁还原条件下,铁循环功能微生物将Fe(Ⅲ)还原为Fe(Ⅱ),进而Fe(Ⅱ)诱导SMX中的异噁唑环O—N键断裂[59]。而SMX并不与硫化物反应,推测SMX在硫酸盐还原条件和铁还原条件下的异噁唑环O—N键断键机制不同。Jia等[23]研究进一步发现SMX在硫酸盐还原条件下异噁唑环O—N键断裂很可能是由DNHP依赖性还原酶(NADH-dependent reductases)所诱导,例如亚硫酸盐还原酶的催化反应。此外,学者研究发现SMX在硫酸盐还原条件发生降解,而其在氧化还原电位较高的硝酸盐还原条件下并未发生降解[40,60],可能是由于低氧化还原电位蛋白酶在高氧化还原电位下被抑制导致。虽然SMX在硫酸盐还原条件和在硝酸盐还原条件下断键机制不同,但其在不同氧化还原条件下的厌氧降解均与DNHP依赖性还原酶等酶催化作用有密切关系。这类酶可能属于低氧化还原点位还原酶,在低氧化还原条件下起作用,而在高氧化还原电位下被抑制。总之,SMX在不同氧化还原条件下的厌氧降解途径差异较大,涉及的机理也相对复杂,有待进一步研究。

图2 磺胺甲噁唑(SMX)在不同氧化还原条件下可能的厌氧降解途径 [23,40,58,59]
Fig. 2 Possible anaerobic degradation pathways of sulfamethoxazole (SMX) under different redox condition [23,40,58,59]
3.2 苯并三唑(1H-benzotriazole)
苯并三唑类是生产量和使用量最大的一类紫外吸收剂,广泛添加于防晒霜、杀菌剂、洗涤剂、药物、轮胎橡胶防腐蚀剂和制冷剂等产品中[61]。苯并三唑(1H-benzotriazole, BT)结构如图3所示,由苯环和三唑环连接组成。伴随着BT的大规模使用,其在地下水[62]、城市雨水径流[63],甚至人体尿液[64]中均有检出。已有研究表明,BT会造成鱼类内分泌系统絮乱,存在潜在环境风险[65]。目前针对BT的厌氧降解转化研究并不多,有限的研究发现BT的微生物降解与氧化还原条件相关,且其在不同的氧化还原条件下的降解途径不尽相同(图3):硝酸盐还原条件下,BT仅发生甲基化形成1-甲基苯并三唑(B);硫酸盐和铁还原条件下,BT能够发生N—N键的断裂,紧接着甲基化形成二甲基苄胺(C)或聚合形成咔唑(D)[13,66-67]。在不同氧化还原条件下,BT的降解转化产物均有苯酚生成,但其降解途径不同。硝酸盐还原条件下,BT首先发生甲基取代反应,而硫酸盐和铁还原条件下经由N—N键断裂后开环。分析其可能的原因,不同氧化还原条件下发挥作用的酶不同,进而导致BT的降解转化途径不同。

图3 苯并三唑(BT)在不同氧化还原条件下可能的厌氧降解途径 [13,66,67]
Fig. 3 Possible anaerobic degradation pathways of 1H-benzotriazole (BT) under different redox condition [13,66,67]
3.3 三氯生(Triclosan)
三氯生(triclosan, TCS)是一种人工合成的广谱类杀菌剂,由一个间位被2个氯元素取代的苯环和一个间位分别被氯元素、羟基取代的苯环通过醚键相连接(图4)。TCS具有较好的杀菌作用,在生产生活中广泛应用于牙膏、肥皂和洗发水等个人护理品。TCS因具有生物累积性、生物毒性及环境毒性[68],其去除研究受到广泛关注。虽然有研究认为TCS在厌氧条件下一般难以降解[69],但Gangadharan Puthiya Veetil等[70]研究发现其在硫酸盐还原条件和产甲烷条件下均会发生厌氧降解。在低氧化还原电位的硫酸盐还原条件和产甲烷条件下,TCS能够发生二苯醚键断裂和还原性脱氯反应,这可能是由低氧化还原电位酶介导。而Ying等[69]发现TCS在厌氧土壤中难以降解,可能是因为样品氧化还原电位较高抑制了低氧化还原电位酶发挥作用。

图4 三氯生(TCS)在不同氧化还原条件下可能的厌氧降解途径 [69-70]
Fig. 4 Possible anaerobic degradation pathways of triclosan (TCS) under different redox condition [69-70]
综合上述典型PPCPs降解途径发现:不同氧化还原条件对PPCPs的厌氧降解途径存在显著影响,酶是PPCPs厌氧降解中的重要参与者[71],而不同的氧化还原电位会促进或抑制酶活性,进而影响PPCPs的降解转化途径。
4 总结与展望(Conclusion and prospect)
PPCPs是一类具有生态毒性、可在环境介质中迁移转化的新污染物,其在受纳环境中的分配和降解转化决定其环境持久性和影响。探究PPCPs在环境中的降解转化过程、影响因素和制约因子等,可为针对性地研发PPCPs污染阻断和管控技术提供科学依据和技术指导。多种PPCPs属于疏水性化合物,进入受纳环境后,往往分布在厌氧或者缺氧环境,厌氧微生物降解是其主要的降解转化方式。近年来,针对PPCPs的厌氧降解研究取得了一定的进展,揭示了化学结构、微生物、外加条件如碳源和氧化还原条件等是其重要影响因素,也发现了厌氧降解过程中可能涉及一些还原酶活性的变化,并筛获了一些特定的降解微生物。然而,自然环境中PPCPs的厌氧微生物降解是一个复杂体系,现阶段对于严格厌氧条件的模拟、多因素条件组合筛查、降解功能菌群的相互作用机理等方面仍存在许多挑战,需要进一步研究。
(1)强化PPCPs的有机质-厌氧微生物共代谢降解机制研究。前期研究多以单一环境因素下的模拟厌氧降解研究为主,而自然环境因子复杂多样,其中有机质-厌氧共代谢是PPCPs非常重要的厌氧降解过程,未来有必要深入研究有机质-厌氧微生物降解协同作用机理,可通过理论计算和高分辨质谱技术等手段准确筛查PPCPs厌氧降解中间产物,并结合基因组学、蛋白组学和代谢组学等技术,从多水平分析有机质-厌氧微生物共代谢机制。
(2)聚焦PPCPs厌氧降解菌群筛选及其功能研究,前期研究虽已筛获特定的降解菌株,但针对其功能基因相关研究较少,降解作用机理尚不清楚。有鉴于此,通过基因组学和转录组学等多组学手段揭示降解功能基因,为后续PPCPs特定厌氧降解工程菌研制做储备。另一方面,在实际环境中,PPCPs的厌氧降解转化通常是依赖功能菌群间的相互作用完成。因此,有必要加强对PPCPs具有高效降解能力的功能菌群评价与筛选研究。
(3)突破厌氧降解菌群培养体系构建和原位厌氧降解研究。模拟自然环境中的厌氧条件、厌氧培养基的单一性和选择性、厌氧培养过程中极易发生污染等是制约厌氧降解研究突破的重要因素。开发和模拟自然环境中的厌氧条件将有助于揭示污染物的厌氧降解转化过程;而研发广谱的厌氧微生物培养基则有助于降解功能菌的筛选。因此,未来有必要进一步开展污染场地原位的厌氧降解实验和修复验证实验研究。
[1] Daughton C G, Ternes T A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? [J]. Environmental Health Perspectives, 1999, 107(Suppl 6): 907-938
[2] Wang Y F, Huang H O, Wei X M. Influence of wastewater precoagulation on adsorptive filtration of pharmaceutical and personal care products by carbon nanotube membranes [J]. Chemical Engineering Journal, 2018, 333: 66-75
[3] Yu X, Sui Q, Lyu S G, et al. Do high levels of PPCPs in landfill leachates influence the water environment in the vicinity of landfills? A case study of the largest landfill in China [J]. Environment International, 2020, 135: 105404
[4] Kibuye F A, Gall H E, Elkin K R, et al. Fate of pharmaceuticals in a spray-irrigation system: From wastewater to groundwater [J]. Science of the Total Environment, 2019, 654: 197-208
[5] Papaioannou D, Koukoulakis P H, Papageorgiou M, et al. Investigation of pharmaceutical and personal care product interactions of soil and beets (Beta vulgaris L.) under the effect of wastewater reuse [J]. Chemosphere, 2020, 238: 124553
[6] Kim H, Homan M. Evaluation of pharmaceuticals and personal care products (PPCPs) in drinking water originating from Lake Erie [J]. Journal of Great Lakes Research, 2020, 46: 1321-1330
[7] Lu W W, Wang M, Wu J Q, et al. Spread of chloramphenicol and tetracycline resistance genes by plasmid mobilization in agricultural soil [J]. Environmental Pollution, 2020, 260: 113998
[8] Vuckovic D, Tinoco A I, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals [J]. Science, 2022, 376(6593): 644-648
[9] Liu F, Zhang Y, Wang F. Environmental relevant concentrations of triclosan affected developmental toxicity, oxidative stress, and apoptosis in zebrafish embryos [J]. Environmental Toxicology, 2022, 37(4): 848-857
[10] 王建龙. 废水中药品及个人护理用品(PPCPs)的去除技术研究进展[J]. 四川师范大学学报(自然科学版), 2020, 43(2): 143-172, 140
Wang J L. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review [J]. Journal of Sichuan Normal University (Natural Science), 2020, 43(2): 143-172, 140 (in Chinese)
[11] Schmidt N, Page D, Tiehm A. Biodegradation of pharmaceuticals and endocrine disruptors with oxygen, nitrate, manganese (Ⅳ), iron (Ⅲ) and sulfate as electron acceptors [J]. Journal of Contaminant Hydrology, 2017, 203: 62-69
[12] Yang C W, Chen Y E, Chang B. Microbial communities associated with acetaminophen biodegradation from mangrove sediment [J]. Sustainability, 2020, 12(13): 5410
[13] Liu Y S, Ying G G, Shareef A, et al. Biodegradation of three selected benzotriazoles under aerobic and anaerobic conditions [J]. Water Research, 2011, 45(16): 5005-5014
[14] Carr D L, Morse A N, Zak J C, et al. Microbially mediated degradation of common pharmaceuticals and personal care products in soil under aerobic and reduced oxygen conditions [J]. Water, Air, &Soil Pollution, 2011, 216(1): 633-642
[15] Chang B, Chao W, Yeh S, et al. Biodegradation of sulfamethoxazole in milkfish (Chanos chanos) pond sediments [J]. Applied Sciences, 2019, 9(19): 4000
[16] Li Y D, Bi E P, Chen H H. Sorption behavior of ofloxacin to kaolinite: Effects of pH, ionic strength, and Cu(Ⅱ) [J]. Water, Air, &Soil Pollution, 2017, 228(1): 46
[17] Samaras V G, Stasinakis A S, Mamais D, et al. Fate of selected pharmaceuticals and synthetic endocrine disrupting compounds during wastewater treatment and sludge anaerobic digestion [J]. Journal of Hazardous Materials, 2013, 244-245: 259-267
[18] Gornik T, Kovacic A, Heath E, et al. Biotransformation study of antidepressant sertraline and its removal during biological wastewater treatment [J]. Water Research, 2020, 181: 115864
[19] Liu Y S, Ying G G, Shareef A, et al. Occurrence and removal of benzotriazoles and ultraviolet filters in a municipal wastewater treatment plant [J]. Environmental Pollution, 2012, 165: 225-232
[20] Polesel F, Andersen H R, Trapp S, et al. Removal of antibiotics in biological wastewater treatment systems—A critical assessment using the activated sludge modeling framework for xenobiotics (ASM-X) [J]. Environmental Science &Technology, 2016, 50(19): 10316-10334
[21] Wang L, Qiang Z M, Li Y G, et al. An insight into the removal of fluoroquinolones in activated sludge process: Sorption and biodegradation characteristics [J]. Journal of Environmental Sciences, 2017, 56: 263-271
[22] Zhang H Q, Jia Y Y, Khanal S K, et al. Understanding the role of extracellular polymeric substances on ciprofloxacin adsorption in aerobic sludge, anaerobic sludge, and sulfate-reducing bacteria sludge systems [J]. Environmental Science &Technology, 2018, 52(11): 6476-6486
[23] Jia Y Y, Zhang H Q, Khanal S K, et al. Insights into pharmaceuticals removal in an anaerobic sulfate-reducing bacteria sludge system [J]. Water Research, 2019, 161: 191-201
[24] Ashfaq M, Li Y, Wang Y W, et al. Occurrence, fate, and mass balance of different classes of pharmaceuticals and personal care products in an anaerobic-anoxic-oxic wastewater treatment plant in Xiamen, China [J]. Water Research, 2017, 123: 655-667
[25] Hou J, Chen Z Y, Gao J, et al. Simultaneous removal of antibiotics and antibiotic resistance genes from pharmaceutical wastewater using the combinations of up-flow anaerobic sludge bed, anoxic-oxic tank, and advanced oxidation technologies [J]. Water Research, 2019, 159: 511-520
[26] Vieno N, Tuhkanen T, Kronberg L. Elimination of pharmaceuticals in sewage treatment plants in Finland [J]. Water Research, 2007, 41(5): 1001-1012
[27] Martín J, Santos J L, Aparicio I, et al. Pharmaceutically active compounds in sludge stabilization treatments: Anaerobic and aerobic digestion, wastewater stabilization ponds and composting [J]. The Science of the Total Environment, 2015, 503-504: 97-104
[28] Falås P, Wick A, Castronovo S, et al. Tracing the limits of organic micropollutant removal in biological wastewater treatment [J]. Water Research, 2016, 95: 240-249
[29] Yang J X, Luo Y J, Fu X H, et al. Unexpected degradation and deiodination of diatrizoate by the Cu(Ⅱ)/S(Ⅳ) system under anaerobic conditions [J]. Water Research, 2021, 198: 117137
[30] Cheng S S, Ho C Y, Wu J H. Pilot study of UASB process treating PTA manufacturing wastewater [J]. Water Science and Technology, 1997, 36(6-7): 73-82
[31] Liu S M, Wu C H, Huang H J. Toxicity and anaerobic biodegradability of pyridine and its derivatives under sulfidogenic conditions [J]. Chemosphere, 1998, 36(10): 2345-2357
[32] Musson S E, Campo P, Tolaymat T, et al. Assessment of the anaerobic degradation of six active pharmaceutical ingredients [J]. The Science of the Total Environment, 2010, 408(9): 2068-2074
[33] Okey R W, Stensel H D. A QSAR-based biodegradability model—A QSBR [J]. Water Research, 1996, 30(9): 2206-2214
[34] Zhang A Q, Han S K, Ma J, et al. Aerobic microbial degradation of aromatic sulfur-containing compounds and effect of chemical structures [J]. Chemosphere, 1998, 36(15): 3033-3041
[35] Shin M, Duncan B, Seto P, et al. Dynamics of selected pre-existing polybrominated diphenylethers (PBDEs) in municipal wastewater sludge under anaerobic conditions [J]. Chemosphere, 2010, 78(10): 1220-1224
[36] Kim S, Rossmassler K, Broeckling C D, et al. Impact of inoculum sources on biotransformation of pharmaceuticals and personal care products [J]. Water Research, 2017, 125: 227-236
[37] Sella C F, Carneiro R B, Sabatini C A, et al. Can different inoculum sources influence the biodegradation of sulfamethoxazole antibiotic during anaerobic digestion? [J]. Brazilian Journal of Chemical Engineering, 2022, 39(1): 35-46
[38] Wolfson S J, Porter A W, Villani T S, et al. The antihistamine diphenhydramine is demethylated by anaerobic wastewater microorganisms [J]. Chemosphere, 2018, 202: 460-466
[39] Chopra S, Kumar D. Characterization, optimization and kinetics study of acetaminophen degradation by Bacillus drentensis strain S1 and waste water degradation analysis [J]. Bioresources and Bioprocessing, 2020, 7(14): 113-120
[40] Ouyang W Y, Birkigt J, Richnow H H, et al. Anaerobic transformation and detoxification of sulfamethoxazole by sulfate-reducing enrichments and Desulfovibrio vulgaris [J]. Environmental Science &Technology, 2021, 55(1): 271-282
[41] 吴丹, 孙悦宏, 李浩, 等. 有机紫外吸收剂BP-3的厌氧污泥降解特性[J]. 环境科学学报, 2022, 42(10): 254-263
Wu D, Sun Y H, Li H, et al. Anaerobic biodegradation characteristics of organic UV filter BP-3 in sludge [J]. Acta Scientiae Circumstantiae, 2022, 42(10): 254-263 (in Chinese)
[42] Hart O E, Halden R U. Modeling wastewater temperature and attenuation of sewage-borne biomarkers globally [J]. Water Research, 2020, 172: 115473
[43] Carballa M, Omil F, Alder A C, et al. Comparison between the conventional anaerobic digestion of sewage sludge and its combination with a chemical or thermal pre-treatment concerning the removal of pharmaceuticals and personal care products [J]. Water Science and Technology: A Journal of the International Association on Water Pollution Research, 2006, 53(8): 109-117
[44] Carballa M, Omil F, Ternes T, et al. Fate of pharmaceutical and personal care products (PPCPs) during anaerobic digestion of sewage sludge [J]. Water Research, 2007, 41(10): 2139-2150
[45] Mao F, Liu X H, Wu K, et al. Biodegradation of sulfonamides by Shewanella oneidensis MR-1 and Shewanella sp. strain MR-4 [J]. Biodegradation, 2018, 29(2): 129-140
[46] Lützow M V, Kögel-Knabner I, Ekschmitt K, et al. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review [J]. European Journal of Soil Science, 2006, 57(4): 426-445
[47] Zhang C F, Zhang D D, Xiao Z X, et al. Characterization of humins from different natural sources and the effect on microbial reductive dechlorination of pentachlorophenol [J]. Chemosphere, 2015, 131: 110-116
[48] Zhang D D, Zhang C F, Li Z L, et al. Electrochemical stimulation of microbial reductive dechlorination of pentachlorophenol using solid-state redox mediator (humin) immobilization [J]. Bioresource Technology, 2014, 164: 232-240
[49] Zhang D D, Zhang C F, Xiao Z X, et al. Humin as an electron donor for enhancement of multiple microbial reduction reactions with different redox potentials in a consortium [J]. Journal of Bioscience and Bioengineering, 2015, 119(2): 188-194
[50] Wu C Y, Zhuang L, Zhou S G, et al. Humic substance-mediated reduction of iron(Ⅲ) oxides and degradation of 2,4-D by an alkaliphilic bacterium, Corynebacterium humireducens MFC-5 [J]. Microbial Biotechnology, 2013, 6(2): 141-149
[51] Lau M P, Sander M, Gelbrecht J, et al. Solid phases as important electron acceptors in freshwater organic sediments [J]. Biogeochemistry, 2015, 123(1): 49-61
[52] He K, Yin Q D, Liu A K, et al. Enhanced anaerobic degradation of amide pharmaceuticals by dosing ferroferric oxide or anthraquinone-2,6-disulfonate [J]. Journal of Water Process Engineering, 2017, 18: 192-197
[53] Baquero E S, Rodríguez D C, Pe uela G A. Individual and synergic effect of carbamazepine and diclofenac in the removal of organic matter from an expanded granular bed anaerobic reactor [J]. Water Science and Technology: A Journal of the International Association on Water Pollution Research, 2022, 85(5): 1620-1635
uela G A. Individual and synergic effect of carbamazepine and diclofenac in the removal of organic matter from an expanded granular bed anaerobic reactor [J]. Water Science and Technology: A Journal of the International Association on Water Pollution Research, 2022, 85(5): 1620-1635
[54] He Y J, Sutton N B, Rijnaarts H H M, et al. Pharmaceutical biodegradation under three anaerobic redox conditions evaluated by chemical and toxicological analyses [J]. The Science of the Total Environment, 2018, 618: 658-664
[55] de Wilt A, He Y J, Sutton N, et al. Sorption and biodegradation of six pharmaceutically active compounds under four different redox conditions [J]. Chemosphere, 2018, 193: 811-819
[56] Martins M, Sanches S, Pereira I A C. Anaerobic biodegradation of pharmaceutical compounds: New insights into the pharmaceutical-degrading bacteria [J]. Journal of Hazardous Materials, 2018, 357: 289-297
[57] Carneiro R B, Sabatini C A, Santos-Neto  J, et al. Feasibility of anaerobic packed and structured-bed reactors for sulfamethoxazole and ciprofloxacin removal from domestic sewage [J]. The Science of the Total Environment, 2019, 678: 419-429
J, et al. Feasibility of anaerobic packed and structured-bed reactors for sulfamethoxazole and ciprofloxacin removal from domestic sewage [J]. The Science of the Total Environment, 2019, 678: 419-429
[58] Jia Y Y, Khanal S K, Zhang H Q, et al. Sulfamethoxazole degradation in anaerobic sulfate-reducing bacteria sludge system [J]. Water Research, 2017, 119: 12-20
[59] Mohatt J L, Hu L H, Finneran K T, et al. Microbially mediated abiotic transformation of the antimicrobial agent sulfamethoxazole under iron-reducing soil conditions [J]. Environmental Science &Technology, 2011, 45(11): 4793-4801
[60] Barbieri M, Carrera J, Sanchez-Vila X, et al. Microcosm experiments to control anaerobic redox conditions when studying the fate of organic micropollutants in aquifer material [J]. Journal of Contaminant Hydrology, 2011, 126(3-4): 330-345
[61] Struk-Soko owska J, Kotowska U, Piekutin J, et al. Analysis of 1H-benzotriazole removal efficiency from wastewater in individual process phases of a sequencing batch reactor SBR [J]. Water Resources and Industry, 2022, 28: 100182
owska J, Kotowska U, Piekutin J, et al. Analysis of 1H-benzotriazole removal efficiency from wastewater in individual process phases of a sequencing batch reactor SBR [J]. Water Resources and Industry, 2022, 28: 100182
[62] Loos R, Locoro G, Comero S, et al. Pan-European survey on the occurrence of selected polar organic persistent pollutants in ground water [J]. Water Research, 2010, 44(14): 4115-4126
[63] Parajulee A, Lei Y D, De Silva A O, et al. Assessing the source-to-stream transport of benzotriazoles during rainfall and snowmelt in urban and agricultural watersheds [J]. Environmental Science &Technology, 2017, 51(8): 4191-4198
[64] Asimakopoulos A G, Bletsou A A, Wu Q, et al. Determination of benzotriazoles and benzothiazoles in human urine by liquid chromatography-tandem mass spectrometry [J]. Analytical Chemistry, 2013, 85(1): 441-448
[65] Liang X F, Wang M, Chen X, et al. Endocrine disrupting effects of benzotriazole in rare minnow (Gobiocypris rarus) in a sex-dependent manner [J]. Chemosphere, 2014, 112: 154-162
[66] Liu Y S, Ying G G, Shareef A, et al. Biodegradation of three selected benzotriazoles in aquifer materials under aerobic and anaerobic conditions [J]. Journal of Contaminant Hydrology, 2013, 151: 131-139
[67] Alotaibi M D, Patterson B M, McKinley A J, et al. Fate of benzotriazole and 5-methylbenzotriazole in recycled water recharged into an anaerobic aquifer: Column studies [J]. Water Research, 2015, 70: 184-195
[68] Ding T D, Lin K D, Bao L J, et al. Biouptake, toxicity and biotransformation of triclosan in diatom Cyclotella sp. and the influence of humic acid [J]. Environmental Pollution, 2018, 234: 231-242
[69] Ying G G, Yu X Y, Kookana R S. Biological degradation of triclocarban and triclosan in a soil under aerobic and anaerobic conditions and comparison with environmental fate modelling [J]. Environmental Pollution, 2007, 150(3): 300-305
[70] Gangadharan Puthiya Veetil P, Vijaya Nadaraja A, Bhasi A, et al. Degradation of triclosan under aerobic, anoxic, and anaerobic conditions [J]. Applied Biochemistry and Biotechnology, 2012, 167(6): 1603-1612
[71] Gonzalez-Gil L, Carballa M, Lema J M. Cometabolic enzymatic transformation of organic micropollutants under methanogenic conditions [J]. Environmental Science &Technology, 2017, 51(5): 2963-2971