全氟辛烷磺酸(perfluorooctane sulfonate, PFOS)是一种典型的全氟烷基化合物(per uoroalkyl substances, PFASs)。自3M公司于1949年将其用于疏水性增强剂添加至防污产品中以来,PFOS及其相关化合物已广泛用于纸张、消防泡沫和润滑剂等家用及工业消费品中[1-2]。PFOS在自然环境和生物体内均不易降解,使其成为一种具有高生物蓄积性的持久性有机污染物[3]。我国也于2021年将PFOS列入重点管控的新污染物清单中[4]。目前,流行病学研究已发现PFOS暴露与多种不良健康结局相关,包括发育缺陷、流产、新生儿死亡率、肝功能、内分泌干扰、肿瘤发生等疾病[5-7]。
uoroalkyl substances, PFASs)。自3M公司于1949年将其用于疏水性增强剂添加至防污产品中以来,PFOS及其相关化合物已广泛用于纸张、消防泡沫和润滑剂等家用及工业消费品中[1-2]。PFOS在自然环境和生物体内均不易降解,使其成为一种具有高生物蓄积性的持久性有机污染物[3]。我国也于2021年将PFOS列入重点管控的新污染物清单中[4]。目前,流行病学研究已发现PFOS暴露与多种不良健康结局相关,包括发育缺陷、流产、新生儿死亡率、肝功能、内分泌干扰、肿瘤发生等疾病[5-7]。
代谢物是生物体新陈代谢产生的各类小分子物质,可反映机体对环境或疾病压力的生理响应。流行病学研究发现PFOS暴露与人群代谢综合征之间存在一定的相关性[8-9]。人群血清中PFOS的浓度与脂质代谢、葡萄糖代谢、钙代谢紊乱有显著的相关性[10-12]。有研究表明20 μmol·L-1 PFOS暴露(24 h或48 h)后,人肝细胞中的胆汁酸代谢发生紊乱[13]。此外,低剂量(1 mg·kg-1)PFOS暴露28 d可影响成年和子代斑马鱼的脂质代谢[14-15]。但上述研究仅探讨PFOS对特定类型代谢物的影响,机体对于PFOS暴露的整体代谢响应还鲜有报道。
代谢组学(metabolomics)是一种定量测定生物体内所有代谢物(代谢组)水平的化学表型分析方法。与单一毒理学终点检测相比,代谢组学技术可更全面、系统地分析机体内的整体代谢变化,因而是研究个体对环境暴露的毒理响应的强有力工具。目前,基于代谢组学技术的PFOS暴露毒性研究,大多集中于黑头软口鲦、虹鳟鱼、斑马鱼、蜥蜴、淡水龟等非哺乳动物,较少以哺乳动物为实验模型,并且主要关注急性/亚急性PFOS暴露(1~21 d)对个体代谢的影响[16-23]。然而,由于与人类的物种差异较大,上述非哺乳动物实验结果难以外推到人类。此外,研究发现自然环境中PFOS的水平相对较低(土壤浓度范围为0.4~10.4 ng·g-1,水体浓度范围为0.1~14.1 ng·L-1)[24-25],而普通人群血清中PFOS的平均浓度可达1.7~73.2 ng·mL-1[26],这可能是长期暴露于环境中的PFOS并在人体中蓄积导致的。因此,长期低剂量暴露更符合现实中人群PFOS的暴露情况。
机体各器官组织的代谢物可被吸收进入血液循环。因此,血液中的代谢物被称为组织的“代谢足迹”,其水平变化可综合反映机体的生理功能改变[27]。由于其高灵敏性和定量重现性,血清代谢组学技术已作为一种发现目的标志物的有效策略得到广泛应用[28-30]。因此,本研究旨在利用代谢组学技术分析长期暴露于低剂量PFOS下大鼠血清中代谢物水平及代谢通路的变化,从而揭示PFOS的代谢毒性效应,并筛选响应PFOS暴露的代谢标志物,为PFOS暴露的健康风险评估提供科学依据。
1 材料与方法(Materials and methods)
1.1 仪器与试剂
Speedva SPD131DDA冷冻干燥机(美国Thermo公司);Eppendrof 5810R高速离心机(美国Thermo公司);Waters ACQUITY UPLC I-Class超高效液相色谱仪(美国Waters公司);Q Exactive组合型四极杆OrbitrapTM质谱仪(美国Thermo公司);ACQUITY UPLC BEH C18色谱柱(1.7 μm,100 mm×2.1 mm i.d.,美国Waters公司);PFOS(美国Sigma-Aldrich公司,纯度98%)。
1.2 大鼠PFOS暴露
40只雄性Wistar大鼠(10周龄)购自厦门大学实验动物中心。实验期间,所有大鼠均在(23±2) ℃、(45±10)%的相对湿度、12 h/12 h的光/暗循环条件下饲养,并自由摄取食物和饮水。适应性饲养一周后,随机将其分为4组(每组n=10)包括对照组和3个暴露组。暴露组大鼠通过灌胃给予0.015、0.15、1.5 mg·kg-1·d-1的PFOS,对照组以玉米油灌胃,连续暴露60 d。数据显示,世界普通人群血清中PFOS的平均浓度范围为1.7~73.2 ng·mL-1 (相当于1.7~73.2 μg·kg-1)[26]。根据大鼠与人类的暴露剂量转换方法(体表面积比)[31],本研究中大鼠的PFOS暴露剂量0.015 mg·kg-1·d-1和0.15 mg·kg-1·d-1相当于人类的2.5 μg·kg-1和25 μg·kg-1,均处于普通人群的PFOS暴露水平范围内,因此被认为是环境相关剂量。暴露结束后,处死大鼠,收集血清并储存于液氮中。本动物实验方案得到中国科学院城市环境研究所伦理审查委员会批准。
1.3 血清PFOS含量分析
200 μL大鼠血清样品加入内标后在室温下平衡过夜。继而加入0.5 mol·L-1四丁基硫酸氢铵(TBA)、0.25 mol·L-1 Na2CO3和100%甲基叔丁基醚(MTBE)混合均匀。样品经3 000 r·min-1离心15 min后,收集上清液。利用MTBE重复上述提取过程2次后,将上清液合并、干燥,使用50%甲醇重新溶解后进行LC-MS/MS分析,并利用同样的方法分析质量控制样品和标准样品[32]。
1.4 代谢组学样品制备
取大鼠血清100 μL,加入300 μL冷甲醇以沉淀蛋白质。超声处理后(每次5 min,共3次),16 000 g离心15 min后取上清液。于离心浓缩仪蒸干后,用100 μL 50%甲醇复溶,并从每个样本中取等量混合作为质量控制(QC)样品。
1.5 UPLC-MS分析
色谱(UPLC)条件:柱温40 ℃;流动相A为甲醇(含0.1%甲酸),流动相B为H2O(含0.1%甲酸);梯度洗脱程序为0 min,0% A;2~17 min,A由0%线性增至100%;22 min,0% A,并保持3 min;流速为0.4 mL·min-1,进样量为5 μL。质谱(MS)条件:电喷雾离子源(ESI),分别采用正、负离子模式采集数据,Full MS分辨率70 000,扫描范围(m/z)为100~1 000;正、负离子模式的喷针电压分别设置为3 500 V和2 500 V;探针加热器温度设置为420 ℃,毛细管温度设置为260 ℃;载气采用氮气,鞘气、辅助气和扫气的流速分别为50、10和1 L·min-1。样本采用随机进样,每10个样本加入一次质控样本检测。Full MS/dd-MS2模式用于获得代谢物的二级结构信息,NCE设为30%。
1.6 数据处理与分析
LC-MS离子信息通过Compounds Discovery软件(美国Thermo公司)实现包括峰提取、峰对齐、未知物检测、未知物组成预测及数据库匹配。QC校正后,对获得的离子信息进行求和、归一化,导入SIMCA-P软件进行正交偏最小二乘判别分析(orthogonal partial least squares-discriminant analysis, OPLS-DA),并采用7折交叉验证对模型拟合程度和预测能力进行检验。根据VIP(variable importance in projection)值和丰度均值比较结果筛选差异代谢物,利用HMDB数据库(http://www.hmdb.ca)进行代谢物鉴定,MetaboAnalyst软件(http://www.metaboanalyst.ca)和KEGG数据库(https://www.kegg.jp)分析代谢通路。
采用SPSS(v22.0)软件进行统计学分析。使用单因素方差分析(ANOVA)和LSD检验比较代谢物的组间差异。使用受试者工作曲线(receiver operating characteristic curve, ROC)评价代谢标志物的诊断性能。所有结果均以均值±标准差(mean±SD)表示,P<0.05为差异有统计学意义。*表示P<0.05,**表示P<0.01,***表示P<0.001。
2 结果(Results)
2.1 大鼠血清PFOS浓度
采用液相色谱-质谱联用技术测定大鼠血清中的PFOS浓度。结果显示,随着暴露剂量的升高,大鼠血清中PFOS浓度分别为0.022、3.774、13.584、69.75 ng·μL-1(图1)。与对照组相比,暴露后大鼠血清中PFOS浓度显著升高,并呈现良好的剂量依赖效应。该结果表明PFOS在大鼠体内具有明显的蓄积作用,并可能因此产生系列毒性效应。
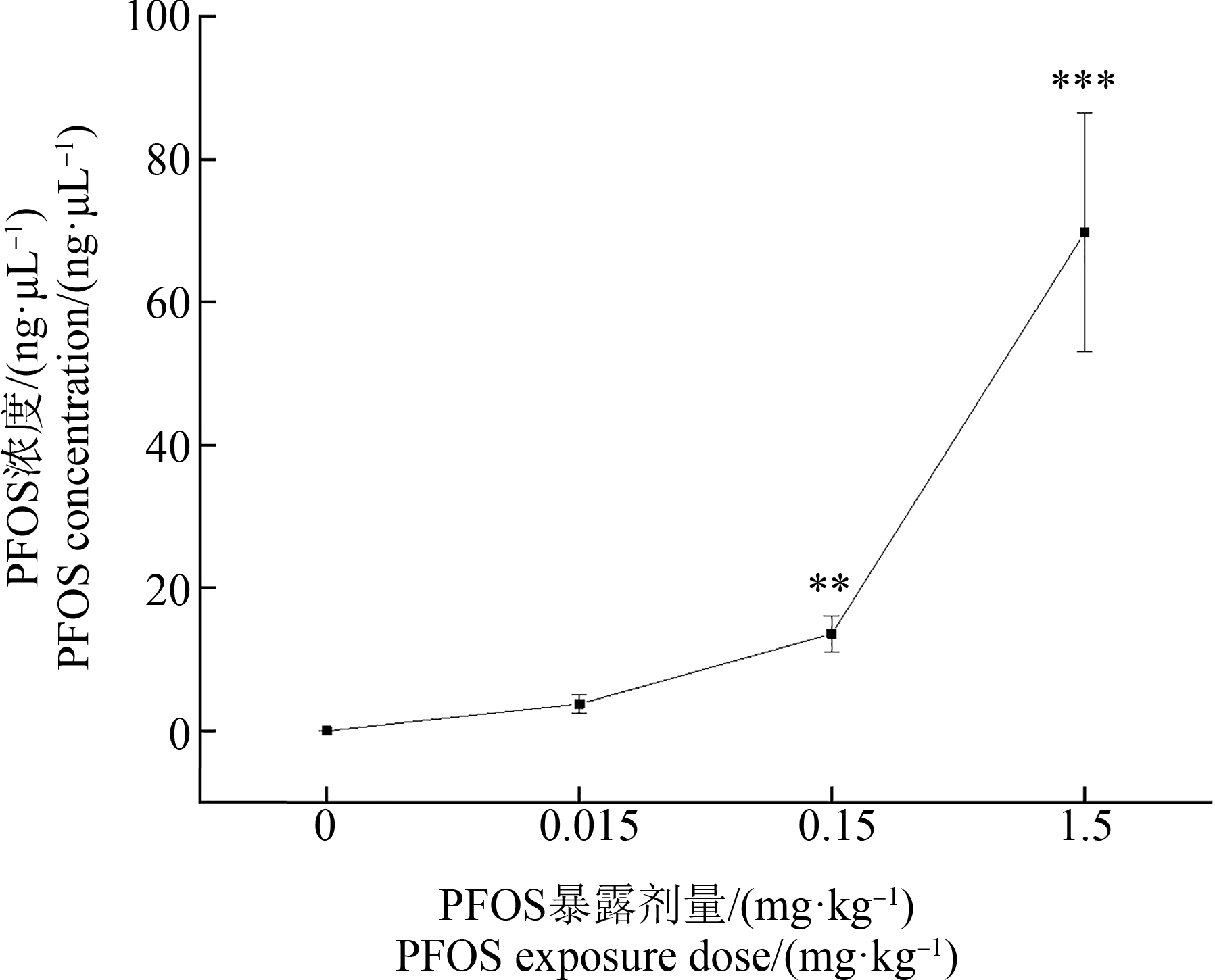
图1 不同剂量全氟辛烷磺酸(PFOS)暴露后大鼠血清中的PFOS浓度
注:与对照组相比,*P<0.05,**P<0.01,***P<0.001。
Fig. 1 Serum perfluorooctane sulfonate (PFOS) concentration in rats exposed to different doses of PFOS
Note: Compared with the control, *P<0.05, **P<0.01, ***P<0.001.
2.2 大鼠血清代谢组分析
采用OPLS-DA模型逐一分析对照组和各个PFOS暴露组的代谢组差异。由图2(a), (c), (e)可见,各暴露组和对照组的代谢轮廓均明显分离(0.015 mg·kg-1:R2Y=0.992, Q2=0.91;0.15 mg·kg-1:R2Y=0.993, Q2=0.931;1.5 mg·kg-1:R2Y=0.998, Q2=0.939),表明PFOS暴露导致大鼠血清代谢发生了显著变化。进一步对OPLS-DA模型进行999次permutation验证,结果显示3个模型均未存在过拟合现象(图2(b), (d), (f)),说明模型稳健可靠,可用于进一步分析。
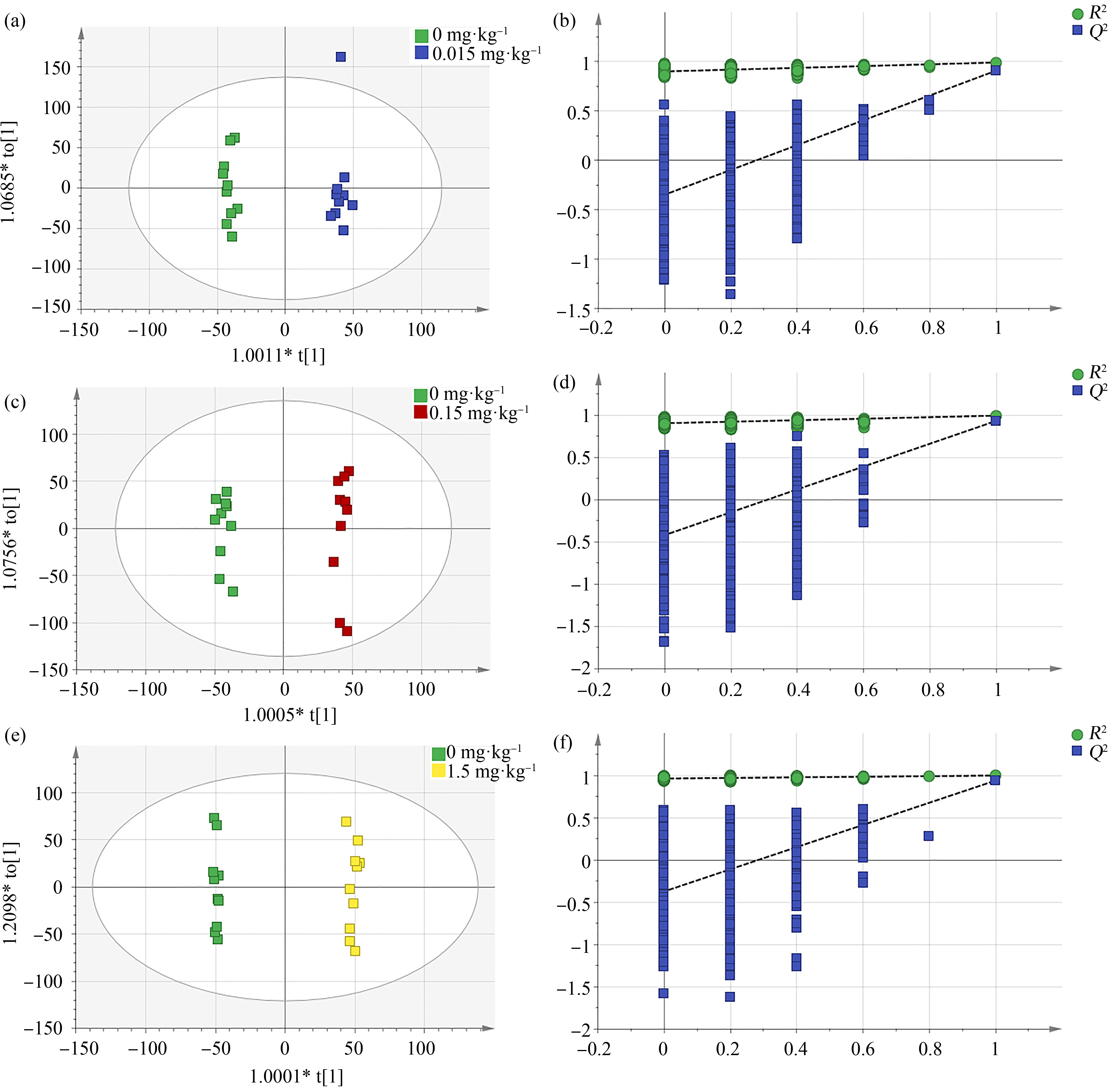
图2 各暴露组与对照组大鼠血清代谢组的OPLS-DA得分图
Fig. 2 OPLS-DA score plots of rat serum metabolome for each PFOS exposure group and control group
2.3 血清代谢标志物及代谢通路
通过代谢组分析,在大鼠血清中共检出5 823种代谢物。进而根据以下标准筛选PFOS暴露后的差异代谢物:(1)VIP值>1.0;(2)jack-knifing置信区间>0;(3)暴露组的代谢物丰度(相对质谱强度)与对照组相比具有显著差异(P<0.05, Mann-Whitney U检验)。最终,各剂量组分别筛选出130(图3(a))、92(图3(b))、120(图3(c))种代谢标志物,且3组共有的代谢物为9种(图3(d)),其中5种在PFOS暴露后显著降低,4种显著升高,但这些变化均没有明显的剂量效应关系(表1)。
表1 不同剂量PFOS暴露组中共同的血清代谢标志物
Table 1 Common serum metabolic markers identified in different doses of PFOS exposure groups

代谢物名称Metabolites变化趋势Change变化倍数(暴露组/对照组)Fold change (Treatment/Control)0.015 mg·kg-10.15 mg·kg-11.5 mg·kg-1代谢通路Metabolic pathway神经酸Nervonic acid↑2.65***1.56*1.81***白三烯A4Leukotriene A4↓0.47***0.59***0.47***前列腺素A2Prostaglandin A2↑3.47***2.33**3.29***11,14,15-三羟基二十碳三烯酸11,14,15-THETA↓0.49***0.79*0.58**脂肪酸代谢Fatty acid metabolism溶血磷脂酸LysoPA(18:3(9Z,12Z,15Z)/0:0)↑2.15***1.78***1.98***胆甾酮Cholestenone↑7.21***7.31***25.74***脂质代谢Lipid metabolism肌苷Inosine↓0.41***0.41***0.59***嘌呤Purine↓0.53***0.62***0.62***嘌呤代谢Purine metabolism全反式视黄酸all-trans-Retinoic acid↓0.59***0.69***0.48***视黄醇代谢Retinol metabolism
注:*P<0.05,**P<0.01,***P<0.001。
Note: *P<0.05, **P<0.01, ***P<0.001.

图3 差异代谢标志物的筛选
注:(a)为0.015 mg·kg-1组;(b)为0.15 mg·kg-1组;(c)为1.5 mg·kg-1组;(d)为Venn图;FC表示变化倍数。
Fig. 3 Identification of differential metabolic markers
Note: (a) means 0.015 mg·kg-1 group; (b) means 0.15 mg·kg-1 group; (c) means 1.5 mg·kg-1 group; (d) means Venn diagram; FC means fold change.
利用MetaboAnalyst软件和KEGG数据库对筛查出的差异代谢标志物进行代谢通路分析。由表1可见,在3个剂量组中均有显著变化的9种代谢物主要涉及脂质/脂肪酸代谢(6种)、嘌呤代谢(2种)及视黄醇代谢(1种)。以上结果表明,PFOS暴露主要影响了大鼠体内的脂质、脂肪酸和嘌呤代谢等过程。
2.4 ROC曲线
在生物标志物的筛查中,受试者工作曲线(receiver operating characteristic curve, ROC)被广泛应用于评价生物标志物的诊断能力,代谢物的曲线下面积(area under curve, AUC)越接近1其诊断性能越好。将筛查出的9种共同血清代谢标志物进行ROC曲线分析,结果显示单一标志物的AUC都在0.8~1.0的范围内(图4(a), (b)),说明这些标志物均具有较高的PFOS暴露判别能力。多种标志物组合模型比单一标志物的判别性能更佳。当超过7种标志物组合在一起时,其AUC=1(图4(c)),表明这些标志物组合是PFOS暴露的最佳判别模型。

图4 血清代谢标志物的ROC
注:a表示下调的代谢标志物;b表示上调的代谢标志物;c表示多种代谢标志物组合模型。
Fig. 4 ROC for serum metabolic markers
Note: a means decreased metabolic markers; b means increased metabolic markers; c means multiple metabolic marker models.
3 讨论(Discussion)
本研究聚焦于新污染物PFOS长期低剂量暴露后大鼠血清中代谢物的变化,探讨其代谢毒性通路,并筛选可表征PFOS暴露效应的代谢标志物。已有研究证实,血清中的白蛋白是PFOS的主要载体蛋白之一[33],并且PFOS易在血液中蓄积,其在血液中的半衰期可达5.4年[34]。本研究结果也表明,暴露后PFOS可在大鼠血清中显著蓄积。其中最低剂量组(0.015 mg·kg-1)大鼠血清中PFOS的浓度(3.774 μg·mL-1)与职业暴露人群血清中的PFOS水平(0.14~2.44 μg·mL-1)相当[26],说明即使是低剂量暴露,PFOS也可能通过生物累积达到较高浓度,从而产生代谢毒性效应。其他研究同样发现PFOS在大鼠体内的蓄积效应[35-37]。代谢组学分析结果显示,PFOS暴露可显著改变大鼠血清中大量代谢物的水平,并且主要导致脂质和脂肪酸代谢、嘌呤代谢、视黄醇代谢等代谢途径发生紊乱。此外,又以脂质和脂肪酸代谢为主要富集通路,说明该代谢通路可能是PFOS毒性作用的主要靶标。流行病学研究同样发现PFAS暴露会引起脂质代谢和嘌呤代谢紊乱[38-40]。
ROC曲线分析是定义生物标志物诊断性能的有效方法[41]。一般来说,AUC>0.7的生物标志物被认为可应用于大多数的临床诊断[42]。本研究中,在不同剂量PFOS暴露后筛选出的9种共同差异代谢物的AUC均>0.8,表明它们对于PFOS暴露具有较高的预测能力。此外,标志物组合的ROC分析结果显示当7种代谢物组合时,其AUC可达到1,提示7种以上(含7种)标志物组合具有更强的判别性能。总之,以上结果表明这9种代谢物具有作为生物标志物的潜力,应用于PFOS暴露的健康风险评估。
脂类是身体储能和供能的重要物质,也是生物膜的重要结构成分,脂质代谢在机体代谢中占据着极其重要的位置。本研究中,PFOS暴露的大鼠血清中多种脂质和脂肪酸代谢相关的代谢物含量发生改变。其中,白三烯A4(leukotriene A4, LTA4)、前列腺素A2(prostaglandin A2, PGA2)、11,14,15-三羟基二十碳三烯酸(11,14,15-trihydroxyeicosatrienoic acid, 11,14,15-THETA)是不饱和脂肪酸花生四烯酸(arachidonic acid, AA)的3种代谢产物;而神经酸(nervonic acid)、LysoPA(18:3(9Z,12Z,15Z)/0:0)、胆甾酮(cholestenone)分别属于长链不饱和脂肪酸、溶血磷脂酸和胆固醇衍生物。许多流行病学研究发现血清中总胆固醇(total cholesterol, TC)、甘油三酯(triglyceride, TG)与PFOS水平存在关联;动物实验研究也证实PFOS暴露可导致大鼠肝脏脂质含量的增加,这些结果均表明PFOS暴露会引起机体的脂质代谢紊乱[43-47]。此外,PFOS暴露会引起脂质转运相关基因表达的改变,并影响酰基辅酶A氧化酶、脱氢酶等脂质代谢相关酶的活性[48-49]。许多研究还认为PFOS可通过影响PPARα、PXR和CAR等核受体导致脂质代谢紊乱[50]。结合本研究结果,大鼠血清中多种脂质和脂肪酸含量发生显著变化,表明脂质代谢紊乱可能是长期低剂量PFOS暴露的主要毒性效应之一。
除了脂质代谢之外,PFOS暴露还干扰了大鼠的嘌呤代谢和视黄醇代谢。嘌呤(purine)也称为嘌呤碱或1 H-嘌呤,肌苷(inosine)是嘌呤和嘌呤核苷降解为尿酸的中间体[51-52]。本研究结果显示,PFOS暴露后大鼠血清中的嘌呤和肌苷水平均显著下降。有体外研究发现,暴露于PFOS(10 μmol·L-1)24 h后大鼠肾小球系膜细胞中的肌苷含量发生改变,提示嘌呤代谢发生紊乱[53];另一种全氟化合物十一氟己烷磺酸(PFHxA)同样可干扰小鼠肝脏中的嘌呤代谢[54]。全反式视黄酸(all-trans-retinoic acid)是视黄酸的一种异构体,是视黄醇(维生素A)的氧化形式,在细胞增殖、分化、凋亡和胚胎发育中发挥重要作用[55]。人群研究发现视黄醇代谢与血清中的总PFAS和PFOA水平具有显著的正相关关系[56]。
本研究利用基于UPLC-MS的代谢组学技术研究了长期低剂量PFOS暴露后大鼠血清中的代谢物水平变化,筛选出在3个不同剂量暴露下均发生显著改变的9种代谢标志物。其中,LysoPA、肌苷和嘌呤在其他研究中也被发现与PFOS暴露有关,提示它们具有作为表征PFOS毒性的代谢标志物的潜力[23,53,57]。分析这些代谢物的生物学功能,发现它们涉及脂质代谢、嘌呤代谢等代谢途径(图5),并提出脂质代谢紊乱是PFOS的主要毒性效应。本研究结果有望从代谢的角度增进我们对于低剂量PFOS毒性效应的认识,然而PFOS干扰机体代谢的内在机制还需进一步深入研究。

图5 差异代谢标志物所涉及的代谢通路网络
注:橙色表示代谢物在血清中含量升高,绿色表示含量降低。
Fig. 5 Schematic overview of the metabolic pathway network involved in the identified metabolic markers
Note: Orange indicates the increased metabolites, while green indicates the decreased ones.
[1] Boyles A L, Blain R B, Rochester J R, et al. Systematic review of community health impacts of mountaintop removal mining [J]. Environment International, 2017, 107: 163-172
[2] Paul A G, Jones K C, Sweetman A J. A first global production, emission, and environmental inventory for perfluorooctane sulfonate [J]. Environmental Science &Technology, 2009, 43(2): 386-392
[3] Wang T, Wang Y W, Liao C Y, et al. Perspectives on the inclusion of perfluorooctane sulfonate into the Stockholm Convention on persistent organic pollutants [J]. Environmental Science &Technology, 2009, 43(14): 5171-5175
[4] 中华人民共和国生态环境部. 重点管控新污染物清单(2021年版) [R]. 北京: 中华人民共和国生态环境部, 2021
[5] Peden-Adams M M, Stuckey J E, Gaworecki K M, et al. Developmental toxicity in white leghorn chickens following in ovo exposure to perfluorooctane sulfonate (PFOS) [J]. Reproductive Toxicology, 2009, 27(3-4): 307-318
[6] Kennedy G L Jr, Butenhoff J L, Olsen G W, et al. The toxicology of perfluorooctanoate [J]. Critical Reviews in Toxicology, 2004, 34(4): 351-384
[7] Betts K S. Perfluoroalkyl acids: What is the evidence telling us? [J]. Environmental Health Perspectives, 2007, 115(5): A250-A256
[8] Lin C Y, Chen P C, Lin Y C, et al. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults [J]. Diabetes Care, 2009, 32(4): 702-707
[9] Liu H S, Wen L L, Chu P L, et al. Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013-2014 [J]. Environmental Pollution, 2018, 232: 73-79
[10] Cardenas A, Gold D R, Hauser R, et al. Plasma concentrations of per- and polyfluoroalkyl substances at baseline and associations with glycemic indicators and diabetes incidence among high-risk adults in the diabetes prevention program trial [J]. Environmental Health Perspectives, 2017, 125(10): 107001
[11] Cakmak S, Lukina A, Karthikeyan S, et al. The association between blood PFAS concentrations and clinical biochemical measures of organ function and metabolism in participants of the Canadian Health Measures Survey (CHMS) [J]. The Science of the Total Environment, 2022, 827: 153900
[12] Chen A M, Jandarov R, Zhou L, et al. Association of perfluoroalkyl substances exposure with cardiometabolic traits in an island population of the eastern Adriatic Coast of Croatia [J]. The Science of the Total Environment, 2019, 683: 29-36
[13] Behr A C, Kwiatkowski A, Ståhlman M, et al. Impairment of bile acid metabolism by perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in human HepaRG hepatoma cells [J]. Archives of Toxicology, 2020, 94(5): 1673-1686
[14] Cui Y, Lv S, Liu J, et al. Chronic perfluorooctanesulfonic acid exposure disrupts lipid metabolism in zebrafish [J]. Human &Experimental Toxicology, 2017, 36(3): 207-217
[15] Yi S J, Chen P Y, Yang L P, et al. Probing the hepatotoxicity mechanisms of novel chlorinated polyfluoroalkyl sulfonates to zebrafish larvae: Implication of structural specificity [J]. Environment International, 2019, 133(Pt B): 105262
[16] Johnson C H, Ivanisevic J, Siuzdak G. Metabolomics: Beyond biomarkers and towards mechanisms [J]. Nature Reviews Molecular Cell Biology, 2016, 17(7): 451-459
[17] Zhang L B, Sun W, Chen H G, et al. Transcriptome analysis of acute exposure of the Manila clam, Ruditapes philippinarum to perfluorooctane sulfonate (PFOS) [J]. Comparative Biochemistry and Physiology Toxicology &Pharmacology, 2020, 231: 108736
[18] Ortiz-Villanueva E, Jaumot J, Martínez R, et al. Assessment of endocrine disruptors effects on zebrafish (Danio rerio) embryos by untargeted LC-HRMS metabolomic analysis [J]. The Science of the Total Environment, 2018, 635: 156-166
[19] Beale D J, Hillyer K, Nilsson S, et al. Bioaccumulation and metabolic response of PFAS mixtures in wild-caught freshwater turtles (Emydura macquarii macquarii) using omics-based ecosurveillance techniques [J]. The Science of the Total Environment, 2022, 806(Pt 3): 151264
[20] Oakes K D, Sibley P K, Martin J W, et al. Short-term exposures of fish to perfluorooctane sulfonate: Acute effects on fatty acyl-coa oxidase activity, oxidative stress, and circulating sex steroids [J]. Environmental Toxicology and Chemistry, 2005, 24(5): 1172-1181
[21] Zhang L M, Rimal B, Nichols R G, et al. Perfluorooctane sulfonate alters gut microbiota-host metabolic homeostasis in mice [J]. Toxicology, 2020, 431: 152365
[22] Deng P, Durham J, Liu J P, et al. Metabolomic, lipidomic, transcriptomic, and metagenomic analyses in mice exposed to PFOS and fed soluble and insoluble dietary fibers [J]. Environmental Health Perspectives, 2022, 130(11): 117003
[23] Li Z J, Lin Z Y, Ji S Q, et al. Perfluorooctanesulfonic acid exposure altered hypothalamic metabolism and disturbed male fecundity [J]. The Science of the Total Environment, 2022, 844: 156881
[24] Jin Y H, Liu W, Sato I, et al. PFOS and PFOA in environmental and tap water in China [J]. Chemosphere, 2009, 77(5): 605-611
[25] Zareitalabad P, Siemens J, Hamer M, et al. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater - A review on concentrations and distribution coefficients [J]. Chemosphere, 2013, 91(6): 725-732
[26] Benford D, Boer J, Carere A, et al. Opinion of the Scientific Panel on contaminants in the food chain on perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts [J]. EFSA Journal, 2008, 653: 1-131
[27] Kell D B, Brown M, Davey H M, et al. Metabolic footprinting and systems biology: The medium is the message [J]. Nature Reviews Microbiology, 2005, 3(7): 557-565
[28] Wang X J, Sun H, Zhang A H, et al. Potential role of metabolomics apporoaches in the area of traditional Chinese medicine: As pillars of the bridge between Chinese and Western medicine [J]. Journal of Pharmaceutical and Biomedical Analysis, 2011, 55(5): 859-868
[29] Winder C L, Cornmell R, Schuler S, et al. Metabolic fingerprinting as a tool to monitor whole-cell biotransformations [J]. Analytical and Bioanalytical Chemistry, 2011, 399(1): 387-401
[30] Zhang A H, Sun H, Wang P, et al. Future perspectives of personalized medicine in traditional Chinese medicine: A systems biology approach [J]. Complementary Therapies in Medicine, 2012, 20(1-2): 93-99
[31] Nair A B, Jacob S. A simple practice guide for dose conversion between animals and human [J]. Journal of Basic and Clinical Pharmacy, 2016, 7(2): 27-31
[32] Alam M N, Han X, Nan B R, et al. Chronic low-level perfluorooctane sulfonate (PFOS) exposure promotes testicular steroidogenesis through enhanced histone acetylation [J]. Environmental Pollution, 2021, 284: 117518
[33] Forsthuber M, Kaiser A M, Granitzer S, et al. Albumin is the major carrier protein for PFOS, PFOA, PFHxS, PFNA and PFDA in human plasma [J]. Environment International, 2020, 137: 105324
[34] Olsen G W, Burris J M, Ehresman D J, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers [J]. Environmental Health Perspectives, 2007, 115(9): 1298-1305
[35] Austin M E, Kasturi B S, Barber M, et al. Neuroendocrine effects of perfluorooctane sulfonate in rats [J]. Environmental Health Perspectives, 2003, 111(12): 1485-1489
[36] Chang S C, Thibodeaux J R, Eastvold M L, et al. Negative bias from analog methods used in the analysis of free thyroxine in rat serum containing perfluorooctanesulfonate (PFOS) [J]. Toxicology, 2007, 234(1-2): 21-33
[37] Conley J M, Lambright C S, Evans N, et al. Developmental toxicity of Nafion byproduct 2 (NBP2) in the Sprague-Dawley rat with comparisons to hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) and perfluorooctane sulfonate (PFOS) [J]. Environment International, 2022, 160: 107056
[38] Jin R, McConnell R, Catherine C, et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in children: An untargeted metabolomics approach [J]. Environment International, 2020, 134: 105220
[39] Hu X, Li S Z, Cirillo P M, et al. Reprint of “metabolome wide association study of serum poly and perfluoroalkyl substances (PFASs) in pregnancy and early postpartum” [J]. Reproductive Toxicology, 2020, 92: 120-128
[40] Alderete T L, Jin R, Walker D I, et al. Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: A proof-of-concept analysis [J]. Environment International, 2019, 126: 445-453
[41] Huang Q Y, Hu D Y, Wang X F, et al. The modification of indoor PM2.5 exposure to chronic obstructive pulmonary disease in Chinese elderly people: A meet-in-metabolite analysis [J]. Environment International, 2018, 121(Pt 2): 1243-1252
[42] Zhang J, Mu X L, Xia Y K, et al. Metabolomic analysis reveals a unique urinary pattern in normozoospermic infertile men [J]. Journal of Proteome Research, 2014, 13(6): 3088-3099
[43] Steenland K, Tinker S, Frisbee S, et al. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant [J]. American Journal of Epidemiology, 2009, 170(10): 1268-1278
[44] Wan H T, Zhao Y G, Wei X, et al. PFOS-induced hepatic steatosis, the mechanistic actions on β-oxidation and lipid transport [J]. Biochimica et Biophysica Acta, 2012, 1820(7): 1092-1101
[45] Imes C C, Austin M A. Low-density lipoprotein cholesterol, apolipoprotein B, and risk of coronary heart disease: From familial hyperlipidemia to genomics [J]. Biological Research for Nursing, 2013, 15(3): 292-308
[46] Li Y, Barregard L, Xu Y Y, et al. Associations between perfluoroalkyl substances and serum lipids in a Swedish adult population with contaminated drinking water [J]. Environmental Health: A Global Access Science Source, 2020, 19(1): 33
[47] Bijland S, Rensen P C, Pieterman E J, et al. Perfluoroalkyl sulfonates cause alkyl chain length-dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3-Leiden CETP mice [J]. Toxicological Sciences: An Official Journal of the Society of Toxicology, 2011, 123(1): 290-303
[48] Cheng J F, Lv S P, Nie S F, et al. Chronic perfluorooctane sulfonate (PFOS) exposure induces hepatic steatosis in zebrafish [J]. Aquatic Toxicology, 2016, 176: 45-52
[49] Martínez R, Navarro-Martín L, Luccarelli C, et al. Unravelling the mechanisms of PFOS toxicity by combining morphological and transcriptomic analyses in zebrafish embryos [J]. The Science of the Total Environment, 2019, 674: 462-471
[50] Fragki S, Dirven H, Fletcher T, et al. Systemic PFOS and PFOA exposure and disturbed lipid homeostasis in humans: What do we know and what not? [J]. Critical Reviews in Toxicology, 2021, 51(2): 141-164
[51] Chen P, Goldberg D E, Kolb B, et al. Inosine induces axonal rewiring and improves behavioral outcome after stroke [J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(13): 9031-9036
[52] Yegutkin G G, Samburski S S, Jalkanen S. Soluble purine-converting enzymes circulate in human blood and regulate extracellular ATP level via counteracting pyrophosphatase and phosphotransfer reactions [J]. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 2003, 17(10): 1328-1330
[53] Gong X, Yang C X, Hong Y J, et al. PFOA and PFOS promote diabetic renal injury in vitro by impairing the metabolisms of amino acids and purines [J]. The Science of the Total Environment, 2019, 676: 72-86
[54] Jiang L L, Hong Y J, Xie G S, et al. Comprehensive multi-omics approaches reveal the hepatotoxic mechanism of perfluorohexanoic acid (PFHxA) in mice [J]. The Science of the Total Environment, 2021, 790: 148160
[55] Ni X L, Hu G H, Cai X. The success and the challenge of all-trans retinoic acid in the treatment of cancer [J]. Critical Reviews in Food Science and Nutrition, 2019, 59(sup1): S71-S80
[56] Li Y Q, Lu X Y, Yu N Y, et al. Exposure to legacy and novel perfluoroalkyl substance disturbs the metabolic homeostasis in pregnant women and fetuses: A metabolome-wide association study [J]. Environment International, 2021, 156: 106627
[57] Li C H, Jiang L D, Qi Y, et al. Integration of metabolomics and proteomics reveals the underlying hepatotoxic mechanism of perfluorooctane sulfonate (PFOS) and 6∶2 chlorinated polyfluoroalkyl ether sulfonic acid (6∶2 Cl-PFESA) in primary human hepatocytes [J]. Ecotoxicology and Environmental Safety, 2023, 249: 114361