类固醇激素因其在ng·L-1浓度范围内即可对生物体造成损害而受到广泛关注[1]。睾酮作为最主要的类固醇激素之一[2],能够在多种环境介质中被检出,在地表水中睾酮的检出范围在0.6~6.0 ng·L-1[3],而在牧场径流水中其检出水平能达到μg·L-1的级别[4]。环境中的睾酮主要来源于人类、牲畜排泄物及医院和家庭废水,其中成年男性排泄物中雄激素水平远高于妇女、儿童[5],虽然禽畜废水排放量低于家庭废水,但随着集中式动物饲养场(concentrated animal feeding operations, CAFO)规模扩大,且牲畜排泄物中雄激素水平较人类排泄物更高[6],因此,来源于牲畜排泄物的雄激素所致的生态破坏也不容小觑。有研究表明,环境雄激素暴露会对野生水生生物产生许多不利影响[7],能够引起鱼类性腺发育异常且出现雄性化特征[8],长期暴露于睾酮会影响大型水蚤生殖能力,导致其产卵率下降[9]。目前仅有研究证据表明高浓度的睾酮暴露会影响水生生物的生殖能力,而对于环境相关浓度睾酮产生的影响并未阐明,亟待进一步研究。
代谢组学研究主要通过对生物体内所有低分子量代谢物进行定性和定量分析,观察内源性代谢产物的变化情况,以小分子代谢物为切入点,探讨体内外刺激因素对机体产生的影响[10]。相较于基因组学、转录组学及蛋白组学,代谢组学能真实反映出机体已经发生的改变[11],并且鉴于代谢组学技术应用于环境污染物的毒性效应评估时速度快、灵敏度高,对于低剂量或环境剂量污染物的毒性效应评估方面优势明显[12]。Lasso回归能够通过线性模型拟合对变量进行筛选,达到降低模型复杂度的目的,另外Lasso回归建模对于变量类型的要求比较低,无论变量连续或是离散均能使用此方法建立模型,因此,在本研究运用Lasso回归方法对于介导睾酮暴露所致相关代谢扰动的重要差异代谢物进行筛选。秀丽隐杆线虫(Caenorhabditis elegans, C. elegans)因其具备体积小、易于培养、繁殖速度快、通体透明易于观察等特点,且与人类基因组具有高度同源性,已经成为模式生物[13]。许多研究表明,秀丽隐杆线虫是代谢组学研究的理想生物模型,已被用于研究不同化学物质暴露对于发育、寿命、脂质代谢等方面的影响[14]。
本研究利用代谢组学技术,在已有环境相关浓度基础上进行有限外推确定染毒浓度,研究环境相关浓度睾酮暴露对秀丽隐杆线虫代谢谱的影响,揭示秀丽隐杆线虫体内可能受到影响的代谢物及代谢通路,以期为进一步研究环境相关浓度睾酮对机体的毒作用机制提供科学线索。
1 材料与方法(Materials and methods)
1.1 实验仪器与试剂
高分辨串联质谱TripleTOF® 5600+ LC-MS/MS系统(美国AB Sciex),ACQUITY UPLC BEH C18色谱柱(1.7 μm,2.15 mm)(美国Waters),Milli-Q Advantage A10超纯水系统(美国Millipore),KQ-500B型超声波清洗仪(昆山超声仪器有限公司,中国),BSA124S-CW型万分之一电子分析天平(德国Sartorius),0.22 μm有机相滤器(南京泰普瑞,中国);甲醇、乙腈(德国Merck),甲酸(美国赛默飞),溶剂均为LC-MS级。
1.2 染毒方法
将睾酮溶解于DMSO中,配制成l g·L-1的储备液,然后用无菌M9溶液稀释成所需染毒浓度,根据文献中显示的睾酮在地表水[3]和牧场径流水[15]中的浓度,在环境浓度基础上做有限外推,设定了环境相关浓度,浓度组分别为0.1、1、10 μg·L-1,并以M9溶液为对照组。染毒培养皿(规格35 mm)中提前一天滴加40 μL OP50菌液并涂抹均匀,过夜培养。加入200 μL染毒液,于超净工作台中晾干。将同步化后孵化的L1幼虫虫液3 000 r·min-1离心1 min,每个染毒培养皿加入10 μL虫液染毒48 h。
1.3 样品前处理
称取收集的线虫样本60 mg,加入200 μL水匀浆,向匀浆液中加入800 μL甲醇∶乙腈(1∶1,V∶V),用冰水浴超声提取10 min。-20 ℃冰箱放置1 h,4 ℃下12 000 r·min-1离心15 min后,取上清氮气吹干,质谱检测前加入100 μL乙腈∶水(1∶1,V∶V)复溶,冰水浴超声5 min,4 ℃ 12 000 r·min-1离心15 min,取上清液进样分析。质量控制(QC)样品是所有样品的上清液的平等混合物。
1.4 超高效液相色谱串联质谱(UPLC-MS/MS)上样条件
分析在UPLCHSST3柱(2.1 mm×100 mm,1.8 μm)上进行,流动相为正向模式的0.1%甲酸水溶液、负向模式的5 mmol·L-1乙酸铵水溶液(A)和乙腈(B),流速为0.5 mL·min-1,柱温40 ℃,进样为3 μL。梯度洗脱方式的详细过程如下:0~1.0 min,1% B;1.0~8.0 min,1%~99% B;8.0~10.0 min,99% B;10.0~10.1 min,99%~1% B;10.1~12 min,1% B。
大气压化学电离源(APCI)分别采用正离子和负离子扫描方式对样品进行质谱信号采集,每批分析前均检测3次超纯水作为空白样品。为避免系统误差,样品以随机顺序进行分析,同时,队列期间每6个样品设置1个QC样品。在整个分析过程中样品置于4 ℃的自动进样器中。
1.5 代谢组数据分析
通过ProteoWizard 3.020315将原始数据转换为“mzML”格式。使用MSDIAL ver.4.80对数据进行峰值检测、提取、排列和注释。对注释的结果进行整合,而后用MetaboAnalyst 5.0通过偏最小二乘判别分析(partial least squared discriminant analysis, PLS-DA)对峰面积进行统计分析。应用PLS模型的变量意义(VIP)得分和方差分析来确定暴露组和对照组之间的差异代谢物。VIP>1且P<0.05作为显著的差异代谢物,然后将筛选出的共同差异性代谢物在两者之间进行比较,以确定具有剂量反应关系的代谢物。同时用MetaboAnalyst 5.0 (http://www.metaboanalyst.ca/)对确定的生物标志物进行分析,用KEGG数据库(https://www.kegg.jp/kegg/pathway.html)对得出的差异代谢物进行通路富集以推测出在睾酮暴露下可能发生显著变化的代谢通路。
1.6 Lasso回归模型
利用“Glmnet”包(2.0-16版)来拟合Lasso回归。选择对睾酮影响代谢扰动过程中有价值的差异代谢物,根据研究中的最低标准,使用10倍交叉验证调整Lasso模型中最低标准的选择。Lasso通过调节Lambda,使得自变量维度得到压缩。基于惩罚系数对样本数量进行选择,对原有的数据进行压缩,将非显著的变量相对系数压缩至0。
2 结果(Results)
2.1 环境相关浓度睾酮暴露后秀丽隐杆线虫代谢物谱图分析
睾酮暴露48 h后,阴阳离子模式下样本的总离子流图如图1(a)和(b)所示,将0.1、1和10 μg·L-1暴露组与对照组进行PLS-DA分析。PLS-DA(图1(c))分析结果显示,Accuracy=0.92,Q2=0.51,R2=0.99,在该模型中Q2反映模型的预测能力,R2代表模型累计方差值,反映模型拟合能力,通常情况下Q2及R2高于0.5表明结果较好,越接近1表明模型的拟合准确性越好,因此可知结果具有较好的数据真实再现力及解释能力,各暴露组均能与对照组明显分离,表明环境相关浓度睾酮暴露能扰动秀丽隐杆线虫代谢水平。随后对各暴露组数据进一步分析,结果显示,Q2=0.95,R2=0.97(图1(d));Q2=0.97,R2=0.98(图1(e));Q2=0.98,R2=0.99(图1(f)),PLS-DA状态下模型拟合及预测能力皆良好,对照组与各暴露组在秀丽隐杆线虫代谢谱中均区分明显。

图1 环境相关浓度睾酮暴露后阴阳离子模式下总离子流图以及PLS-DA得分图
注:(a)、(b)分别代表阳离子及阴离子模式下总离子流图,(c)为对照组与所有实验组间PLS-DA得分图,(d)、(e)、(f)分别代表0.1、1和10 μg·L-1实验组与对照组之间的PLS-DA得分图。
Fig. 1 Total ion current in cationic and anion mode after exposure to testosterone at ambient concentrations and PLS-DA score diagram
Note: (a) and (b) represent total ion current diagrams in cationic and anion modes, respectively; (c) PLS-DA score chart between control group and all experimental groups; (d), (e) and (f) represent PLS-DA scores of 0.1, 1 and 10 μg·L-1 experimental groups and control groups, respectively.
2.2 环境相关浓度睾酮暴露后秀丽隐杆线虫差异代谢物筛选
鉴定了6 422种代谢物,其中正离子模式下有4 320种代谢物,负离子模式下有2 102种代谢物。通过对不同组别进行差异代谢物分析,分别得到409种(control vs. 0.1 μg·L-1)、219种(control vs. 1 μg·L-1)、697种(control vs. 10 μg·L-1)差异代谢物(图2(a))。0.1 μg·L-1剂量组与对照组相比(图2(b)),有180种物质显著上调,227种物质显著下调,能够识别的物质有28种,表1列举了其中具有代表性的部分物质,主要包括鞘氨醇(sphingosine, Sph)、脱磷酸辅酶A等脂类及能量代谢类物质;1 μg·L-1剂量组与对照组相比(图2(c)),有74种物质显著上调,82种物质显著下调,能够识别的物质有23种,表2列举了其中具有代表性的部分物质,主要包括辅酶A(coenzyme A, CoA)、鸟苷(guanosine)、L-精氨酸(L-arginine)等能量、嘌呤代谢及氨基酸类物质;10 μg·L-1剂量组与对照组相比(图2(d)),有265种物质显著上调,228种物质显著下调,能够识别的物质有66种,表3列举了其中具有代表性的部分物质,主要包括CoA、鸟嘌呤、神经酰胺(ceramide, Cer)、半胱氨酸(S-adenosyl-L-homocysteine, SAH)等脂类、氨基酸类以及嘌呤和能量代谢类物质。随后对各组能识别的差异代谢物进行聚类分析,各组结果区分皆较为明显(图2(e), (f), (g)),进一步证明环境相关浓度睾酮暴露对秀丽隐杆线虫代谢谱的影响。
表1 暴露于0.1 μg·L-1睾酮后能够识别的代表性差异代谢物
Table 1 Representative differential metabolites identified after exposure to 0.1 μg·L-1 testosterone

代谢物名称保留时间/min质荷比变量投影重要度P值log2 (FC)MetabolitesRetention time/minm/zVIPP valueControl0.1 μg·L-1IDN8-乙酰亚精胺N8-acetylspermidine 16.56548188.998766.37140.00240161-4.8511C01029乳酸 Lactose 5.891113.926221.11380.00245131-1.0748C01970草酸 Shikimic acid 3.474115.003331.45870.00255661-1.3407C00493甲酰基-N-乙酰基-5-甲氧基犬尿胺Acetyl-N-formyl-5-methoxykynurenamine 15.85692.927531.9920.00310941-2.0229C05642脱磷酸辅酶A Dephospho-CoA14.776112.936731.84240.000260111.9357HMDB0001373甲氧基查尔酮4’-methoxychalcone 0.5488.040372.50130.00261181-3.865HMDB00325852-(4-烯丙基-2,6-二甲氧基苯氧基)-1-(3,4,5-三甲氧基苯基)-1-丙醇2-(4-allyl-2,6-dimethoxyphenoxy)-1-(3,4,5-trimethoxyphenyl)-1-propanol 13.3689.023792.64160.00239761-2.0449HMDB00308286-β-D-吡喃葡萄糖基-8-β-D-吡喃核糖苷元6-beta-D-glucopyranosyl-8-beta-D-ribopyranosylapigenin 15.30294.981391.28160.00280131-1.3932HMDB00335714-羟基肉桂酸 4-hydroxycinnamic acid 11.00478.958922.5310.00285041-3.9895C00811鞘氨醇 Sphingosine 7.06299.92641.77130.00013871-2.008HMDB0000252油酸 Oleic acid 17.842104.953361.38080.00715091-1.4331C00712

图2 环境相关浓度睾酮暴露后差异代谢物结果图
注:(a)差异代谢物韦恩图;(b)、(c)、(d)分别代表0.1、1和10 μg·L-1实验组与对照组之间的差异代谢物火山图;(e)、(f)、(g)分别代表0.1、1和10 μg·L-1实验组与对照组之间的差异代谢物热图;红色和蓝色分别代表上调和下调。
Fig. 2 Graphs of differential metabolite results after exposure to environmentally relevant concentrations of testosterone
Note: (a) Venn diagram of differential metabolites; (b), (c) and (d) represent the volcanoes of differential metabolites between 0.1, 1 and 10 μg·L-1 exposure groups and control groups, respectively; (e), (f) and (g) represent heat maps of differential metabolites between 0.1, 1 and 10 μg·L-1 exposure groups and control groups, respectively; red and blue represent up-regulated and down-regulated.
表2 暴露于1 μg·L-1睾酮后能够识别的代表性差异代谢物
Table 2 Representative differential metabolites identified after exposure to 1 μg·L-1 testosterone

代谢物名称保留时间/min质荷比变量投影重要度P值log2 (FC)MetabolitesRetention time/minm/zVIPP valueControl1 μg·L-1ID胆酸 Cholic acid 15.08380.963691.25040.00159141C00695 N8-乙酰亚精胺N8-acetylspermidine 16.56548188.998766.37147.60E-071-4.2828C01029甲酰基-N-乙酰基-5-甲氧基犬尿胺Acetyl-N-formyl-5-methoxykynurenamine 0.76465189.12311.93730.00310941-1.3572C05642甲氧基查尔酮4’-methoxychalcone 0.5488.040373.01011.30E-061-4.5619HMDB00325852-(4-烯丙基-2,6-二甲氧基苯氧基)-1-(3,4,5-三甲氧基苯基)-1-丙醇2-(4-allyl-2,6-dimethoxyphenoxy)-1-(3,4,5-trimethoxyphenyl)-1-propanol 13.3689.023792.64160.00239761-1.8755HMDB0030828脱氧胆酸 Deoxycholic acid 15.08992.927533.25670.00229761-4.6207C044836-β-D-吡喃葡萄糖基-8-β-D-吡喃核糖苷元6-beta-D-glucopyranosyl-8-beta-D-ribopyranosylapigenin 15.30294.981391.28160.00280131HMDB0033571鸟苷 Guanosine 17.98296.95921.1310.000022111.9701C00387L-精氨酸 L-arginine 16.55996.95955.21820.000055413.4066C00062腺苷 Adenosine 0.17996.959711.22920.000076511.8326C00212咪唑乙酸 Imidazoleacetic acid 14.048108.019931.76730.00001131-1.8723C02835辅酶 A Coenzyme A 0.497109.011253.69120.00008851-1.5922 C00010
表3 暴露于10 μg·L-1睾酮后能够识别的代表性差异代谢物
Table 3 Representative differential metabolites identified after exposure to 10 μg·L-1 testosterone
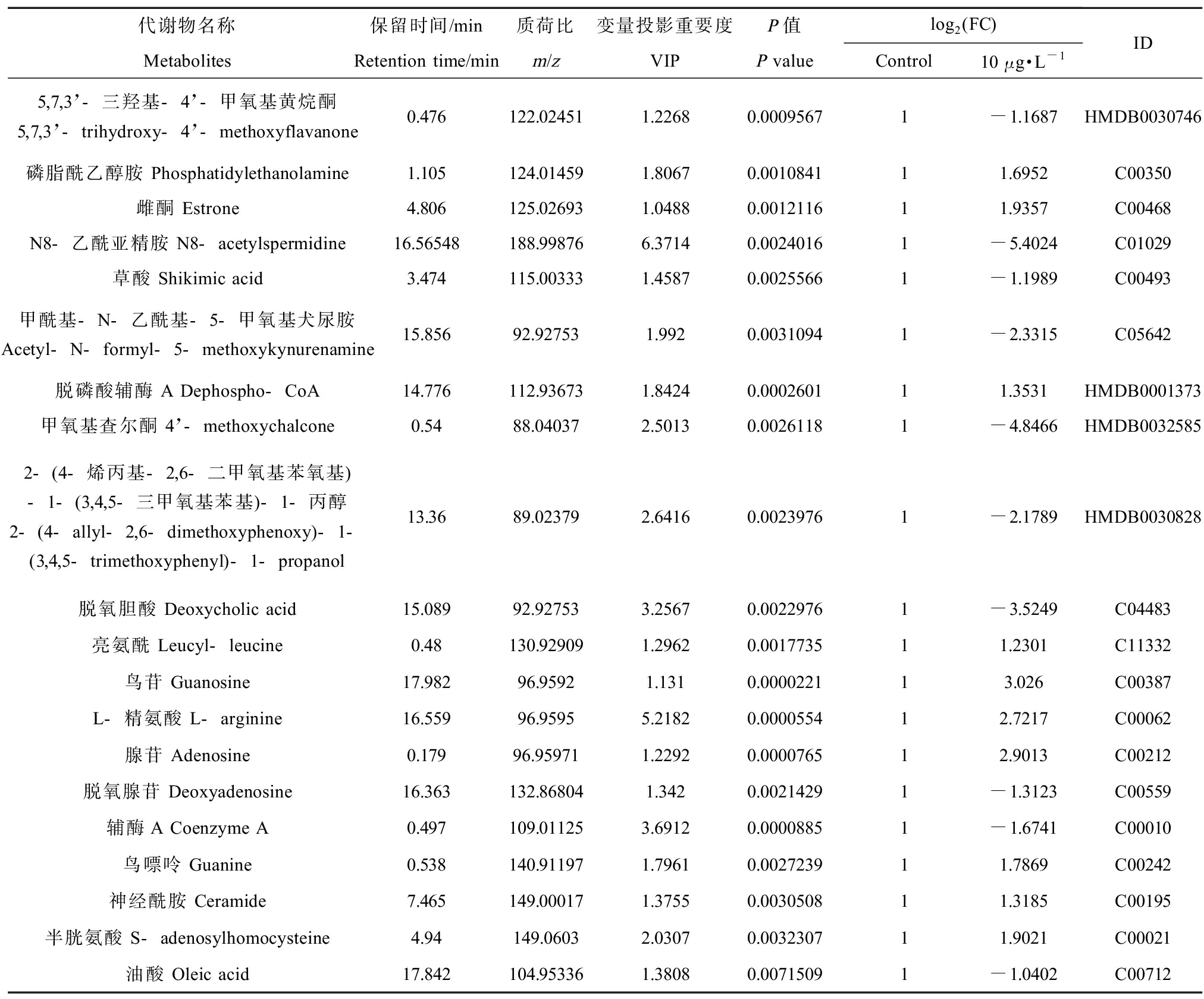
代谢物名称保留时间/min质荷比变量投影重要度P值log2 (FC)MetabolitesRetention time/minm/zVIPP valueControl10 μg·L-1ID5,7,3’-三羟基-4’-甲氧基黄烷酮5,7,3’-trihydroxy-4’-methoxyflavanone 0.476122.024511.22680.00095671-1.1687HMDB0030746磷脂酰乙醇胺 Phosphatidylethanolamine 1.105124.014591.80670.001084111.6952C00350雌酮 Estrone 4.806125.026931.04880.001211611.9357C00468N8-乙酰亚精胺 N8-acetylspermidine16.56548188.998766.37140.00240161-5.4024C01029草酸 Shikimic acid 3.474115.003331.45870.00255661-1.1989C00493甲酰基-N-乙酰基-5-甲氧基犬尿胺Acetyl-N-formyl-5-methoxykynurenamine 15.85692.927531.9920.00310941-2.3315C05642脱磷酸辅酶 A Dephospho-CoA14.776112.936731.84240.000260111.3531HMDB0001373甲氧基查尔酮 4’-methoxychalcone 0.5488.040372.50130.00261181-4.8466HMDB00325852-(4-烯丙基-2,6-二甲氧基苯氧基)-1-(3,4,5-三甲氧基苯基)-1-丙醇2-(4-allyl-2,6-dimethoxyphenoxy)-1-(3,4,5-trimethoxyphenyl)-1-propanol 13.3689.023792.64160.00239761-2.1789HMDB0030828脱氧胆酸 Deoxycholic acid 15.08992.927533.25670.00229761-3.5249C04483亮氨酰 Leucyl-leucine 0.48130.929091.29620.001773511.2301C11332鸟苷 Guanosine 17.98296.95921.1310.000022113.026C00387L-精氨酸 L-arginine 16.55996.95955.21820.000055412.7217C00062腺苷 Adenosine 0.17996.959711.22920.000076512.9013C00212脱氧腺苷 Deoxyadenosine 16.363132.868041.3420.00214291-1.3123C00559辅酶A Coenzyme A 0.497109.011253.69120.00008851-1.6741C00010 鸟嘌呤 Guanine0.538140.911971.79610.002723911.7869C00242神经酰胺 Ceramide 7.465149.000171.37550.003050811.3185C00195半胱氨酸 S-adenosylhomocysteine 4.94149.06032.03070.003230711.9021C00021油酸 Oleic acid 17.842104.953361.38080.00715091-1.0402C00712
2.3 环境相关浓度睾酮暴露后秀丽隐杆线虫差异代谢物通路富集
对各剂量组差异代谢物进行通路富集,3组共有13种物质与KEGG代谢途径相匹配(表4)。0.1 μg·L-1睾酮暴露后能与KEGG代谢途径相匹配的物质包括:Sph、N8-乙酰半胺(N8-acetylspermidine)、甲酰基-N-乙酰基-5-甲氧基犬尿胺(acetyl-N-formyl-5-methoxykynurenamine, AFMK),此3种差异代谢物的含量均显著降低;1 μg·L-1睾酮暴露后能与KEGG代谢途径相匹配的物质包括:咪唑-4-乙酸酯(imidazole-4-acetic acid, IAA)、CoA、N8-乙酰半胺、AFMK、鸟嘌呤、腺苷(adenosine)、L-精氨酸,其中前4种差异代谢物的含量显著降低,后3种显著升高;10 μg·L-1睾酮暴露后能与KEGG代谢途径相匹配的物质包括:脱氧腺苷(deoxyadenosine)、CoA、N8-乙酰半胺、AFMK、鸟嘌呤、腺苷、鸟苷(guanosine)、磷脂酰乙醇胺(phosphatidylethanolamine, PE)、Cer、L-精氨酸、SAH,其中前4种差异代谢物的含量显著降低,后7种显著升高。与低浓度睾酮暴露相比,暴露于更高浓度的睾酮会导致更多差异代谢物的显著变化,这些差异代谢物主要包括脂类、嘌呤、氨基酸及能量相关物质,表明受睾酮浓度的升高的影响,秀丽隐杆线虫代谢谱的扰动加剧。
表4 环境相关浓度睾酮暴露后各剂量组与KEGG代谢途径相匹配的差异代谢物变化情况
Table 4 Changes of differential metabolites matching KEGG metabolic pathways in each dose group after exposure to environmentally relevant concentrations of testosterone

睾酮浓度/(μg·L-1)Testosterone concentrations/(μg·L-1)代谢物名称Metabolites保留时间/minRetention time/min质荷比m/z变量投影重要度VIPP值P valuelog2 (FC)ControlExposedID0.1鞘氨醇Sphingosine 9.667300.289891.32240.000023451-2.008C00319N8-乙酰半胺N8-acetylspermidine 16.56548188.998761.9920.00159141-4.8511C01029N-甲酰基-N-乙酰基-5-甲氧基犬尿胺Acetyl-N-formyl-5-methoxykynurenamine 0.76465189.12313.89150.00310941-2.0229C056421鸟嘌呤Guanosine 15.883150.043091.9310.0008692811.9701C00387腺苷Adenosine 3.464266.089261.22927.60E-0711.8326C00212咪唑-4-乙酸酯Imidazole-4-acetate 17.692125.031641.76730.000012331-1.8723C02835辅酶A Coenzyme A 1.942766.103391.14033.37E-091-1.5922C00010L-精氨酸L-arginine 0.633173.104282.96750.00001371113.4066C00062N8-乙酰半胺N8-acetylspermidine 16.56548188.998761.9920.00159141-4.2828C01029N-甲酰基-N-乙酰基-5-甲氧基犬尿胺Acetyl-N-formyl-5-methoxykynurenamine 0.76465189.12313.89150.00310941-1.3572C0564210鸟苷Guanosine 2.008282.084081.1310.000111213.026C00387腺苷Adenosine 3.464266.089261.22927.60E-0712.9013C00212脱氧腺苷Deoxyadenosine 1.136252.109091.3420.000077341-1.3123C00559鸟嘌呤Guanine 15.883150.043091.9310.0008692811.7869C00387辅酶A Coenzyme A 1.942766.103391.14033.37E-091-1.6741C00010磷脂酰乙醇胺Phosphatidylethanolamine 6.651734.442691.80670.0000438211.6952C00350 L-精氨酸L-arginine0.633173.104282.96750.00001371112.7217C00062神经酰胺Ceramide 13.766538.518431.37550.0000777611.3185C00195半胱氨酸S-adenosylhomocysteine 2.529383.113012.03070.0000987611.9021C00021N8-乙酰半胺N8-acetylspermidine 16.56548188.998761.9920.00159141-5.4024C01029N-甲酰基-N-乙酰基-5-甲氧基犬尿胺Acetyl-N-formyl-5-methoxykynurenamine 0.76465189.12313.89150.00310941-2.3315C05642
在图3中总结了受差异代谢物影响的相关代谢途径,0.1 μg·L-1睾酮暴露后受干扰的差异代谢途径主要包括:鞘脂代谢及色氨酸代谢;1 μg·L-1睾酮暴露后受干扰的差异代谢途径主要包括:嘌呤代谢、组氨酸代谢、脂肪酸降解、泛酸与辅酶A的生物合成、精氨酸与脯氨酸代谢以及色氨酸代谢;而10 μg·L-1睾酮暴露后受干扰的差异代谢途径主要包括:嘌呤代谢、鞘脂代谢、甘油磷脂代谢、脂肪酸降解、泛酸与辅酶A的生物合成、精氨酸与脯氨酸代谢、色氨酸代谢以及半胱氨酸和蛋氨酸代谢。我们发现相较于低浓度睾酮,暴露于更高浓度的睾酮对脂类代谢、嘌呤代谢、氨基酸代谢及能量代谢途径的扰动更明显,其中色氨酸代谢途径在低、中、高3个剂量组中均受到干扰。

图3 环境相关浓度睾酮暴露后差异代谢通路整体视图
注:红色和绿色分别代表环境相关浓度睾酮暴露后上调和下调的差异代谢物。
Fig. 3 Overall view of differential metabolic pathways after exposure to environmentally relevant concentrations of testosterone
Note: Red and green represent differential metabolites up-regulated and down-regulated, respectively, after exposure to environmentally relevant concentrations of testosterone.
2.4 环境相关浓度睾酮暴露后秀丽隐杆线虫差异代谢物的Lasso回归分析
利用Lasso回归建立模型,筛选介导环境相关浓度睾酮暴露与代谢扰动关联的重要差异代谢物。结果显示(图4),λ=8时选取8个变量,Lasso回归中包含的8个变量在不同惩罚参数值下的结果及其对应系数如图5所示,其中有3个物质:AFMK(coefficient=-0.002833)、三乙醇胺(coefficient=-0.00011)以及L-精氨酸(coefficient=0.000852)为内源性代谢物,在睾酮引起代谢扰动的过程中可能发挥着较为重要的作用,AFMK为3个剂量组共有的差异代谢物,能够与色氨酸代谢途径相匹配,提示AFMK可能作为睾酮暴露后引起秀丽隐杆线虫代谢异常过程中的潜在生物标志物。

图4 Lasso回归交叉验证结果
注:2条虚线中间的值是log(λ)的正负标准差的范围,左边的虚线表示模型误差最小时的谐波参数log(λ)的,λ=8时选取8个变量。
Fig. 4 Cross-validation results of Lasso regression
Note: The value in the middle of the two dashed lines is the range of positive and negative standard deviation of log(λ); the dashed line on the left represents the harmonic parameter log(λ) with the smallest error of the model; 8 variables are selected when λ=8.

图5 剔除无效变量后Lasso系数分布图
注:18代表三乙醇胺,30代表N-甲酰基-N-乙酰基-5-甲氧基犬尿胺,35代表2-(4-烯丙基-2,6-二甲氧基苯氧基)-1-(3,4,5-三甲氧基苯基)-1-丙醇,42代表川陈皮素,47代表L-精氨酸,65代表加巴喷丁,70代表间苯异戊酮,86代表茉莉酸。
Fig. 5 Distribution of Lasso coefficient after removing invalid variables
Note: 18 means triethanolamine, 30 means acetyl-N-formyl-5-methoxykynurenamine, 35 means 2-(4-allyl-2,6-dimethoxyphenoxy)-1-(3,4,5-trimethoxyphenyl)-1-propanol, 42 means nobiletin, 47 means L-arginine, 65 means gabapentin, 70 means phlorisovalerophenone, and 86 means jasmonic acid.
3 讨论(Discussion)
睾酮作为一种重要的雄激素广泛存在于生存环境中,目前相关毒性研究仅限于较高水平的睾酮暴露,对于环境相关暴露水平的研究有限。本研究运用非靶向代谢组学,探讨环境相关浓度睾酮暴露后对秀丽隐杆线虫代谢谱的干扰,进而阐明其潜在的毒作用机制。研究结果显示,低剂量睾酮暴露首先引起秀丽隐杆线虫脂质及氨基酸代谢异常,随着暴露浓度的升高进一步对嘌呤及能量代谢途径产生干扰。
脂质代谢紊乱出现在睾酮暴露的较早时期,在低剂量(0.1 μg·L-1)睾酮暴露下首先引起鞘脂代谢异常。鞘脂是一种具有重要作用的脂质,在秀丽隐杆线虫的卵母细胞形成,早期胚胎分裂等过程中发挥重要作用[16]。在本研究中,暴露于0.1 μg·L-1睾酮后Sph显著升高。Gao等[17]研究发现,睾酮处理后的线虫体内活性氧水平剧增,进而导致氧化损伤及细胞凋亡,并且随着活性氧的积累,许多线虫的生殖区消失,严重抑制了秀丽隐杆线虫的繁殖。随着暴露浓度的升高,暴露于高浓度(10 μg·L-1)睾酮引起的脂质代谢异常更为显著,不仅对鞘脂代谢途径产生干扰,同时也会影响甘油磷脂代谢过程。Cer在10 μg·L-1睾酮暴露组中显著升高,作为鞘脂代谢的核心成分,Cer在ATP过量合成时积累,Sph由Cer降解生成[18]。Cer作为诱导细胞凋亡的第二信使,能够通过促进活性氧的产生以及线粒体功能障碍诱导生殖细胞凋亡[19],除此以外,神经酰胺的异常形成还会阻断胰岛素信号转导并促进脂肪炎症[20]。甘油磷脂是生物膜的重要组成成分,也是秀丽隐杆线虫中最主要的脂类[21],与氧化磷酸化[22]、线粒体稳定性等密切相关[23]。Cappello等[24]研究发现,暴露于合成孕激素类物质屈螺酮后贻贝的能量代谢及甘油磷脂代谢过程受到明显干扰。PE属于甘油磷脂的一种,在本研究中显著升高,作为线粒体内膜的主要组成成分之一,线粒体形态及功能的异常与PE水平的异常改变密切相关[25]。另外,由于PE中含有大量不饱和脂肪酸,易受氧化影响且易导致质膜脂质过氧化,进而对精子运动性及活力产生不利影响[26]。众所周知,环境性激素类物质诱导的生殖损伤与活性氧积累所致的氧化损伤及线粒体功能障碍有关[27],我们先前的实验结果显示,暴露于环境相关浓度睾酮会导致秀丽隐杆线虫后代数目及生殖细胞数目显著下降。这些结果表明,暴露于环境相关浓度睾酮所致秀丽隐杆线虫的生殖损伤可能与脂质代谢紊乱引起的活性氧水平升高以及线粒体功能障碍有关,同时可能对正常的内分泌过程产生影响。
与脂质代谢紊乱一致,氨基酸代谢紊乱也在较低水平睾酮暴露时被观察到,不同的是,我们在低、中、高3个剂量组均能观察到氨基酸代谢异常。AFMK作为3个剂量组共有的差异代谢物与色氨酸代谢途径相匹配,其水平在3个剂量暴露组中均显著下降,表明色氨酸代谢途径在3个暴露组均受到明显干扰。Lasso回归的结果表明,AFMK可能在睾酮引起代谢紊乱的过程中起关键作用,表明AFMK可能作为睾酮引起机体代谢紊乱的潜在生物标志物。已知AFMK是褪黑素在体内的主要代谢产物,和褪黑素一样可以作为活性氧清除剂[28],通过增加AFMK能够有效防止自由基损伤引起的脂质过氧化[29]。有研究表明,在哺乳动物中褪黑素调节精子发生、保护精子免受热应激损伤[30],并且褪黑素能够与精子中Ca2+功能的细胞内调节剂钙调蛋白相互作用从而提高冷冻后解冻精子的运动性及活力[31]。另一方面,褪黑素通过活性氧的清除以及调节BAX/BCL-2的平衡以维持线粒体膜的通透性,而正常的线粒体功能所产生的ATP是精子活动所必需的[32]。因此,我们推测AFMK含量降低可能与机体活性氧水平升高有关,这种改变可能与精子功能损伤密切相关,然而其确切原因及调节机制需待进一步研究。IAA在1 μg·L-1睾酮暴露组中显著下降,提示组氨酸代谢异常。IAA作为一种组胺氧化代谢物,具有阵痛、镇静、催眠等作用[33],还需要更多的研究来进一步了解组氨酸代谢睾酮暴露后的作用机制。随暴露浓度进一步提升,暴露于10 μg·L-1睾酮后氨基酸类物质(L-精氨酸、SAH)显著升高。其中,L-精氨酸作为一氧化氮的前体之一,对于维持细胞中正常的一氧化氮水平十分重要,而一氧化氮参与调节线粒体的生物发生、呼吸链的调节以及氧化应激[34]。L-精氨酸的显著上升表明环境相关浓度的睾酮暴露可能影响正常的线粒体功能以及体内活性氧水平。SAH是蛋氨酸循环的代谢物,作为同型半胱氨酸的前体,SAH水平的升高能引起多种类型的细胞凋亡[35],另外,细胞内SAH升高能够诱导脂肪分解以及脂肪因子分泌失调[36],目前关于睾酮在精氨酸代谢及蛋氨酸代谢过程中的作用机制研究有限,亟待进一步研究。这些结果表明,暴露于环境相关浓度睾酮可能通过扰乱氨基酸代谢对正常的线粒体功能、活性氧水平以及内分泌功能产生影响。
随着暴露浓度的升高,观察到嘌呤代谢的异常,嘌呤代谢合成并分解嘌呤,在许多细胞的生长增殖过程中都需要嘌呤代谢的参与,嘌呤代谢的异常会导致机体氧化应激水平升高[37]。鸟苷和腺苷对于氧化应激十分敏感,腺苷能够介导多种激素的作用,并且腺苷本身也有其自分泌、旁分泌和全身作用[38],在一项针对患有不育症的男性煤矿作业工人中观察到精子DNA碱基的氧化增加伴随着氧鸟嘌呤水平迅速升高[39],作为鸟嘌呤的一种致突变性氧化损伤产物,其水平升高表明精子DNA氧化受损严重。已有研究表明,包括睾酮在内的类固醇激素暴露会增加机体的氧化应激水平[40],并且睾酮暴露后精细胞数量、精子数量及活力均明显下降[41],有证据表明,活性氧水平过高不仅能通过脂质过氧化及DNA损伤还能通过诱导精子中酶的失活以及蛋白质氧化引起男性不育[42]。因此,暴露于较高浓度的睾酮可能通过扰乱嘌呤代谢,增加机体氧化应激水平进而诱导雄性生殖功能损伤。
最后,在中、高剂量组中观察到能量代谢的异常。辅酶A是生物体内许多代谢过程中必不可少的辅助因子,它通过自身接受和释放巯基,起着转移酰基的作用,在糖代谢、脂代谢、三羧酸循环(tricarboxylic acid cycle, TCA)等过程中均发挥重要作用[43-45]。TCA是体内产生ATP的主要途径,辅酶A对于TCA中的关键酶α-酮戊二酸及丙酮酸脱氢酶复合体的合成十分重要[45],TCA活性的降低会导致线粒体能量输出减少,进而促使氧化应激及细胞凋亡[46]。暴露于1 μg·L-1和10 μg·L-1睾酮后辅酶A水平的显著下降表明秀丽隐杆线虫的能量稳态被破坏,这种能量代谢的紊乱会对繁殖、发育等机体其他生物功能造成影响[47]。
低、中、高3个剂量组差异代谢物的鉴定及通路富集结果表明,暴露于环境相关浓度睾酮后首先会引起秀丽隐杆线虫脂质及氨基酸代谢异常,随着暴露浓度的升高进一步影响嘌呤及能量代谢途径。这些代谢过程的异常均与氧化应激水平的升高相关,提示暴露于环境相关浓度的睾酮后,首先会引起机体氧化应激水平的升高,进而诱导线粒体功能损伤及能量代谢障碍,最终影响精子的正常功能,导致雄性生殖损伤。结合代谢组学及Lasso回归分析结果,AFMK可能作为睾酮引起机体代谢紊乱的潜在生物标志物,在3个剂量组中均显著降低。其作为褪黑素在体内的主要代谢产物,具有高水平的活性氧清除功效,提示睾酮暴露后及时补充适量褪黑素可能会对睾酮暴露所引起的代谢紊乱及活性氧水平升高起到一定改善作用。
本研究对不同环境相关浓度睾酮暴露48 h后的秀丽隐杆线虫中的代谢物进行测定,发现环境相关浓度睾酮能引起秀丽隐杆线虫代谢谱的显著变化,代谢产物的改变主要涉及的代谢途径包括:鞘脂代谢、甘油磷脂代谢、色氨酸代谢、脂肪酸降解、泛酸与辅酶A的生物合成、半胱氨酸和蛋氨酸代谢、组氨酸代谢、精氨酸和脯氨酸代谢以及嘌呤代谢途径,这些变化可能是机体氧化应激及线粒体功能障碍的信号,相较于低浓度暴露组,脂质、嘌呤、氨基酸及能量代谢在高浓度睾酮暴露下扰动更为明显,提示睾酮浓度升高加剧机体生殖功能损伤。Lasso回归的结果显示,AFMK可能作为睾酮暴露造成秀丽隐杆线虫代谢异常的潜在生物标志物在睾酮影响代谢扰动的过程中发挥重要作用。本研究通过对暴露于环境相关浓度睾酮后秀丽隐杆线虫代谢特征的初步分析,帮助我们了解睾酮暴露后激活的相关途径,为研究睾酮潜在毒性机制提供理论依据并为睾酮的安全性评估提供潜在的生物标志物。
[1] Adeel M, Song X M, Wang Y Y, et al. Environmental impact of estrogens on human, animal and plant life: A critical review [J]. Environment International, 2017, 99: 107-119
[2] Arnon S, Dahan O, Elhanany S, et al. Transport of testosterone and estrogen from dairy-farm waste lagoons to groundwater [J]. Environmental Science &Technology, 2008, 42(15): 5521-5526
[3] Vulliet E, Wiest L, Baudot R, et al. Multi-residue analysis of steroids at sub-ng/L levels in surface and ground-waters using liquid chromatography coupled to tandem mass spectrometry [J]. Journal of Chromatography A, 2008, 1210(1): 84-91
[4] Finlay-Moore O, Hartel P G, Cabrera M L. 17β-estradiol and testosterone in soil and runoff from grasslands amended with broiler litter [J]. Journal of Environmental Quality, 2000, 29(5): 1604-1611
[5] Liu Z H, Kanjo Y, Mizutani S. Urinary excretion rates of natural estrogens and androgens from humans, and their occurrence and fate in the environment: A review [J]. Science of the Total Environment, 2009, 407(18): 4975-4985
[6] Liu X W, Shi J H, Zhang H, et al. Estimating estrogen release and load from humans and livestock in Shanghai, China [J]. Journal of Environmental Quality, 2014, 43(2): 568-577
[7] Tomkins P, Saaristo M, Bertram M G, et al. An endocrine-disrupting agricultural contaminant impacts sequential female mate choice in fish [J]. Environmental Pollution, 2018, 237: 103-110
[8] Morthorst J E, Holbech H, Bjerregaard P. Trenbolone causes irreversible masculinization of zebrafish at environmentally relevant concentrations [J]. Aquatic Toxicology, 2010, 98(4): 336-343
[9] Barbosa I R, Nogueira A J A, Soares A M V M. Acute and chronic effects of testosterone and 4-hydroxyandrostenedione to the crustacean Daphnia magna [J]. Ecotoxicology and Environmental Safety, 2008, 71(3): 757-764
[10] Nicholson J K, Connelly J, Lindon J C, et al. Metabonomics: A platform for studying drug toxicity and gene function [J]. Nature Reviews Drug Discovery, 2002, 1(2): 153-161
[11] 李英帅. 应用代谢组学技术进行中医药研究探讨[J]. 安徽中医学院学报, 2008, 27(6): 1-5
[12] 耿柠波, 张海军, 王菲迪, 等. 代谢组学技术在环境毒理学研究中的应用[J]. 生态毒理学报, 2016, 11(3): 26-35
Geng N B, Zhang H J, Wang F D, et al. A review on the application of metabonomic approaches in environmental toxicology [J]. Asian Journal of Ecotoxicology, 2016, 11(3): 26-35 (in Chinese)
[13] Kaletta T, Hengartner M O. Finding function in novel targets: C. elegans as a model organism [J]. Nature Reviews Drug Discovery, 2006, 5(5): 387-399
[14] Salzer L, Witting M. Quo vadis Caenorhabditis elegans metabolomics-A review of current methods and applications to explore metabolism in the nematode [J]. Metabolites, 2021, 11(5): 284
[15] Jenkins M B, Endale D M, Schomberg H H, et al. 17beta-estradiol and testosterone in drainage and runoff from poultry litter applications to tilled and no-till crop land under irrigation [J]. Journal of Environmental Management, 2009, 90(8): 2659-2664
[16] Hänel V, Pendleton C, Witting M. The sphingolipidome of the model organism Caenorhabditis elegans [J]. Chemistry and Physics of Lipids, 2019, 222: 15-22
[17] Gao H J, Qi G F, Yin R, et al. Bacillus cereus strain S2 shows high nematicidal activity against Meloidogyne incognita by producing sphingosine [J]. Scientific Reports, 2016, 6: 28756
[18] Green C D, Maceyka M, Cowart L A, et al. Sphingolipids in metabolic disease: The good, the bad, and the unknown [J]. Cell Metabolism, 2021, 33(7): 1293-1306
[19] Lee S, Kang H G, Jeong P S, et al. Heat stress impairs oocyte maturation through ceramide-mediated apoptosis in pigs [J]. The Science of the Total Environment, 2021, 755(Pt 1): 144144
[20] Lazúrová Z, Mitro P. Adenosine - A mediator with multisystemic effects (or a hormone?) [J]. Vnitrni Lekarstvi, 2017, 63(9): 617-623
[21] Witting M, Schmitt-Kopplin P. The Caenorhabditis elegans lipidome: A primer for lipid analysis in Caenorhabditis elegans [J]. Archives of Biochemistry and Biophysics, 2016, 589: 27-37
[22] Böttinger L, Horvath S E, Kleinschroth T, et al. Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain super complexes [J]. Journal of Molecular Biology, 2012, 423(5): 677-686
[23] Birner R, Bürgermeister M, Schneiter R, et al. Roles of phosphatidylethanolamine and of its several biosynthetic pathways in Saccharomyces cerevisiae [J]. Molecular Biology of the Cell, 2001, 12(4): 997-1007
[24] Cappello T, Fernandes D, Maisano M, et al. Sex steroids and metabolic responses in mussels Mytilus galloprovincialis exposed to drospirenone [J]. Ecotoxicology and Environmental Safety, 2017, 143: 166-172
[25] Mukherjee I, Ghosh M, Meinecke M. MICOS and the mitochondrial inner membrane morphology - When things get out of shape [J]. FEBS Letters, 2021, 595(8): 1159-1183
[26] Sun Y, Sun X P, Zhao L M, et al. DJ-1 deficiency causes metabolic abnormality in ornidazole-induced asthenozoospermia [J]. Reproduction, 2020, 160(6): 931-941
[27] Bauer M A, Carmona-Gutiérrez D, Ruckenstuhl C, et al. Spermidine promotes mating and fertilization efficiency in model organisms [J]. Cell Cycle, 2013, 12(2): 346-352
[28] Rong K, Zheng H, Yang R B, et al. Melatonin and its metabolite N(1)-acetyl-N(1)-formyl-5-methoxykynuramine improve learning and memory impairment related to Alzheimer’s Disease in rats [J]. Journal of Biochemical and Molecular Toxicology, 2020, 34(2): e22430
[29] Tan D X, Manchester L C, Burkhardt S, et al. N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant [J]. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 2001, 15(12): 2294-2296
[30] Yang M H, Guan S Y, Tao J L, et al. Melatonin promotes male reproductive performance and increases testosterone synthesis in mammalian Leydig cells [J]. Biology of Reproduction, 2021, 104(6): 1322-1336
[31] Ofosu J, Qazi I H, Fang Y, et al. Use of melatonin in sperm cryopreservation of farm animals: A brief review [J]. Animal Reproduction Science, 2021, 233: 106850
[32] ChaithraShree A R, Ingole S D, Dighe V D, et al. Effect of melatonin on bovine sperm characteristics and ultrastructure changes following cryopreservation [J]. Veterinary Medicine and Science, 2020, 6(2): 177-186
[33] Zhao W R, Ding H R, Hu S, et al. An efficient biocatalytic synthesis of imidazole-4-acetic acid [J]. Biotechnology Letters, 2018, 40(7): 1049-1055
[34] Barros C D S, Livramento J B, Mouro M G, et al. L-arginine reduces nitro-oxidative stress in cultured cells with mitochondrial deficiency [J]. Nutrients, 2021, 13(2): 534
[35] Kharbanda K K, Rogers D D, Mailliard M E, et al. Role of elevated S-adenosylhomocysteine in rat hepatocyte apoptosis: Protection by betaine [J]. Biochemical Pharmacology, 2005, 70(12): 1883-1890
[36] Arumugam M K, Chava S, Rasineni K, et al. Elevated S-adenosylhomocysteine induces adipocyte dysfunction to promote alcohol-associated liver steatosis [J]. Scientific Reports, 2021, 11(1): 14693
[37] Tian R, Yang C, Chai S M, et al. Evolutionary impacts of purine metabolism genes on mammalian oxidative stress adaptation [J]. Zoological Research, 2022, 43(2): 241-254
[38] Fang Z J, Pyne S, Pyne N J. Ceramide and sphingosine 1-phosphate in adipose dysfunction [J]. Progress in Lipid Research, 2019, 74: 145-159
[39] Rashki Ghaleno L, Alizadeh A, Drevet J R, et al. Oxidation of sperm DNA and male infertility [J]. Antioxidants, 2021, 10(1): 97
[40] Emer E, Yildiz O, Seyrek M, et al. High-dose testosterone and dehydroepiandrosterone induce cardiotoxicity in rats: Assessment of echocardiographic, morphologic, and oxidative stress parameters [J]. Human &Experimental Toxicology, 2016, 35(5): 562-572
[41] Ramezani Tehrani F, Noroozzadeh M, Zahediasl S, et al. Prenatal testosterone exposure worsen the reproductive performance of male rat at adulthood [J]. PLoS One, 2013, 8(8): e71705
[42] Barati E, Nikzad H, Karimian M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management [J]. Cellular and Molecular Life Sciences, 2020, 77(1): 93-113
[43] Leonardi R, Zhang Y M, Rock C O, et al. Coenzyme A: Back in action [J]. Progress in Lipid Research, 2005, 44(2-3): 125-153
[44] Tahiliani A G, Beinlich C J. Pantothenic acid in health and disease [J]. Vitamins and Hormones, 1991, 46: 165-228
[45] Ma T, Liu T H, Xie P F, et al. UPLC-MS-based urine nontargeted metabolic profiling identifies dysregulation of pantothenate and CoA biosynthesis pathway in diabetic kidney disease [J]. Life Sciences, 2020, 258: 118160
[46] Yin L J, Luo M, Wang R, et al. Mitochondria in sex hormone-induced disorder of energy metabolism in males and females [J]. Frontiers in Endocrinology, 2021, 12: 749451
[47] Sokolova I M, Frederich M, Bagwe R, et al. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates [J]. Marine Environmental Research, 2012, 79: 1-15