轮胎磨损颗粒是轮胎胎面与路面滚动剪切时产生的一种新型微塑料[1],也是环境中微塑料的主要来源之一[2]。据报道,欧盟轮胎磨损颗粒每年的排放量可达1 327 000 t,美国每年的排放量可达1 120 000 t;德国每年的排放量可达12 200 t;其中,约有11 000 t的轮胎磨损颗粒会进入地表水中[3]。这些颗粒的可浸出物质会迁移到水环境中,并对水生生物产生不利影响。已有研究表明,轮胎浸出液会影响黑头鲦鱼(Pimephales promelas)胚胎的生长发育,导致胚胎孵化率下降,畸形率上升,眼睛和身体的黑色素减少[4]。Tian等[5]也发现轮胎浸出液对银大马哈鱼(Oncorhynchus kisutch)有剧毒,可在短时间内导致银大马哈鱼的大量死亡。
已有文献指出,轮胎浸出液中含有重金属、多环芳烃、挥发性有机化合物及半挥发性有机化合物等有毒物质[6]。1,3-二苯胍(1,3-diphenyl guanidine, DPG)是橡胶生产过程中添加的硫化促进剂,用于促进橡胶的生产[7]。研究表明,DPG是轮胎浸出液的主要成分之一[8],并且在世界范围内的水环境中广泛存在。加拿大多伦多地区的地表水样和污水处理厂中均检测到了DPG的存在,其峰值浓度达0.52 μg·L-1 [9];德国、西班牙及荷兰的水样也检测到了浓度达100 ng·L-1的DPG[10];DPG在欧洲的莱茵河水样中的检出频率高达91%,最大浓度达140 ng·L-1 [11];日本的荒川河中检测到浓度为467 ng·L-1的DPG[12];美国华盛顿州的水域中也检测到浓度高达540 ng·L-1 DPG[13]。DPG还可从高密度聚乙烯管道中迁移,在中国长沙自来水中曾检测到高达230~560 μg·L-1的DPG[14]。DPG在加拿大安大略省4个污水处理厂的进水口处的浓度分别为(47.7±0.6)、(57.5±9.9)、(77.9±8.1)及(141.6±16.4) ng·POCIS-1(POCIS是广泛采用的极地有机化学综合采样器,因暂无DPG的采样率,故未能换算为ng·L-1),出水口处的浓度分别为(35.8±4.7)、(228.1±8.1)、(124.1±0.7)及(143.4±18.4) ng·POCIS-1 [15]。DPG在中国珠江三角洲3个污水处理厂进水口处的浓度分别为(3 570±2 580)、(137±10.6)及(505±38.8) ng·L-1,在出水口处的浓度为(116±6.95)、(158±7.8)及(58.2±10.1) ng·L-1,去除率分别为96.8%、-15.2%、88.5% [16]。根据Scheurer等[11]通过固定生物床反应器模拟生物降解过程,发现DPG的生物半衰期<10 d;而Zahn等[8]通过活性污泥模拟生物转化过程,发现DPG不可被生物转化。因此,综合比较国内外污水处理厂进出水口处检测到的DPG浓度,表明DPG在污水处理过程中的去除率可能和污水处理工艺相关。DPG不仅广泛存在于环境样本中,在人体血清中也检测到浓度达1.7 ng·mL-1的DPG,并且DPG可以透过胎盘屏障,进而可能对胎儿的生长发育造成不利的影响[17] 。
目前有关DPG的毒性报道较少,将DPG以4 mg·kg-1·d-1和8 mg·kg-1·d-1的剂量暴露于小鼠,会导致小鼠生成形态异常的精子,并使精子数量及睾丸质量显著减少,此外,DPG还可诱导小鼠生成不规则形状的生精小管,从而降低小鼠的生育指数[18]。因此认为DPG具有潜在的生殖毒性,可能会影响小鼠的生殖能力。然而作为水环境中的一种污染物,目前DPG对水生生物的生殖发育毒性及其机制尚不清楚,其对轮胎磨损颗粒的水生生物毒性贡献仍未可知。
鱼类的生殖过程是由下丘脑-垂体-性腺(hypothalamic-pituitary-gonadal, HPG)轴介导,并受到类固醇激素的调控[19]。因此类固醇激素水平变化及性腺指数异常是鱼类生殖毒性的主要特征[20]。例如,暴露于阿特拉津后,斑马鱼出现性别分化偏向雌性、产卵减少及精子缺陷[20];双酚F会导致斑马鱼的性腺凋亡及性腺指数的下降,并诱导斑马鱼生殖系统的氧化应激和凋亡[21];三氯生会影响斑马鱼体内的性激素水平,进而影响斑马鱼的精子发生和卵子发生,从而损害其生殖能力[22]。这些指标的改变对化合物生殖发育毒性具有很好的指示作用。
斑马鱼(Danio rerio, zebrafish)作为一种水生模式生物,具有很多优势:成鱼个体小、易于养殖、产卵周期短且产卵量大、胚胎透明且发育迅速、遗传学背景清楚且与人类基因组同源性高[23-25]。因此,在急性毒性、神经毒性、遗传毒性、慢性毒性、生殖毒性、内分泌干扰效应和发育毒性等生态毒理学研究中得到广泛的应用[26-30]。
基于以上研究背景,本研究以斑马鱼为受试模型生物,将不同浓度的DPG暴露于斑马鱼胚胎及仔鱼,通过转录组测序、基因表达水平定量分析及对斑马鱼仔鱼体内的性激素水平测定,来揭示DPG对斑马鱼的生殖发育毒性及机制。本研究为轮胎磨损颗粒的毒性研究提供了新的线索。
1 材料与方法(Materials and methods)
1.1 化学品和试剂
DPG(纯度≥99.5%;分子量:211.268;CAS号:102-06-7)购自上海阿拉丁生化科技股份有限公司。甲醇采用色谱纯等级,购自迪码科技有限公司(北京),为助溶剂[31]。
暴露液使用标准稀释水进行稀释。标准稀释水的配制参考《化学品 鱼类急性毒性试验》(GB/T 27861—2011)标准[32](包含294.00 mg·L-1 CaCl2·2H2O,123.35 mg·L-1 MgSO4·7H2O,64.75 mg·L-1 NaHCO3及5.75 mg·L-1 KCl)。配制标准稀释水的化学品均采用分析纯等级,购自迪码科技有限公司(北京)。
1.2 斑马鱼饲养
亲本野生AB型斑马鱼购于中国科学院武汉水生生物所,养殖于自控循环集中养殖系统(北京爱生科技发展有限公司,中国)。培养条件:温度为(28.0±2.0) ℃,pH为7.2±0.2,电导率为450~500 μS·cm-1,光暗循环为14 h∶10 h,每日早晚各喂食丰年虾一次。产卵前一天,将雌雄鱼以1∶1的比例置于产卵缸中,使用挡板将雌雄鱼分开,于第2天一早抽掉隔板,使雌雄鱼自由交配,30 min后收集受精卵。
1.3 斑马鱼胚胎暴露
斑马鱼胚胎及仔鱼急性毒性实验的设计参考《鱼类胚胎急性毒性测试(OECD TG236)》[33]。使用前将DPG用甲醇配制成高浓度的储备液,急性毒性实验中所用相应浓度的暴露液由高浓度储备液经标准稀释水稀释而得,并且保证最高浓度暴露液中甲醇的含量不超过暴露溶液体积的1%(V∶V)。根据Maes等[34]的研究,将1.5%(V∶V)的甲醇暴露于斑马鱼胚胎及仔鱼168 h,该浓度对斑马鱼胚胎及仔鱼没有任何明显的不良反应。因此,根据DPG在甲醇中的溶解度,本文将含1%(V∶V)甲醇的最高浓度组作为最大浓度来研究DPG对斑马鱼胚胎及仔鱼的急性毒性,故暴露浓度分别设置为0.0000、1.5625、3.1250、6.2500、12.5000、25.0000 mg·L-1,溶剂对照组为1%(V∶V)的甲醇溶液,同时设置空白对照组(标准稀释水),且空白对照组与溶剂对照组在死亡率、孵化率及体长等生理指标的差异无统计学意义。使用六孔板作为暴露容器,每孔放置20枚受精正常的胚胎(4 hpf; hours post fertilization, hpf),并加入10 mL的暴露液,每个浓度设置3个生物学重复。暴露时间为120 h,期间每隔24 h更换新的暴露液,并于24、48、72、96、120 h记录胚胎的死亡数,每日仔细观察胚胎的死亡、孵化及畸形情况,并及时移除死亡胚胎。
斑马鱼胚胎及仔鱼暴露实验中所用的相应浓度的暴露液由高浓度储备液经标准稀释水稀释而得,并且保证最高浓度暴露液中甲醇的含量不超过暴露溶液体积的0.1%(V∶V)。根据我国自来水厂管道中DPG的浓度(230~560 μg·L-1),将低、中、高3个浓度暴露组的浓度分别设置为30、100和300 μg·L-1,来研究DPG潜在的毒性效应,溶剂对照组为0.1%(V∶V)的甲醇溶液。使用六孔板作为暴露容器,每孔放置50枚受精正常的胚胎(4 hpf),并加入10 mL的暴露液,每个浓度设置12个生物学重复。暴露时间为120 h,期间每隔24 h更换新的暴露液。每日仔细观察胚胎的死亡、孵化及畸形情况,并及时移除死亡胚胎。暴露结束后,收集120 hpf的斑马鱼仔鱼,除水后置于液氮中冷冻,并于-80 ℃冰箱中保存,用于后续的转录组测序、生殖细胞发育及类固醇合成通路相关基因的表达水平分析及性激素水平测定。
1.4 总RNA提取和cDNA合成
使用TRNzol A+试剂(北京天根生化科技有限公司)从斑马鱼仔鱼中提取总RNA,并使用NanoDrop-2000分光光度计(Thermo Fisher Scientific,美国)测定提取的总RNA浓度,并通过A260/A280的比值对提取的总RNA质量进行监测。
使用One-step gDNA Removal and cDNA Synthesis SuperMix Kit试剂盒(北京全式金生物技术有限公司)将提取的总RNA反转录成cDNA,反应体系为20 μL,于42 ℃孵育15 min,85 ℃加热失活5 s,得到的cDNA保存在-20 ℃冰箱中。
1.5 转录组测序与分析
将收集的高浓度DPG暴露组的斑马鱼仔鱼用于转录组测序。转录组测序工作由上海中科新生命生物科技有限公司完成。获得原始数据后,去除带有接头和低质量的reads,以确保测序质量并获得clean reads。并与NCBI网站上的斑马鱼参考基因组(https://www.ncbi.nlm.nih.gov/genome/?term=Danio+rerio)进行比对。本次测序共注释到31 949个基因,通过R包对注释到clusterProfiler的所有基因基于GO数据库及KEGG数据库分别进行基因集富集分析(Gene Set Enrichment Analysis, GSEA),将富集到的Padjust<0.05的生物学过程定义为显著富集的生物学过程;通过DEseq2 R包对注释到的基因进行差异基因分析,并将Padjust<0.05且|log2 (fold change)|>1的基因定义为差异基因(differential expressed genes, DEGs),其中将Padjust<0.05且log2 (fold change)>1的基因定义为上调基因,Padjust<0.05且log2 (fold change)<-1的基因定义为下调基因;将得到的差异基因通过clusterProfiler R包基于GO数据库进行GO富集分析,基于KEGG数据库进行KEGG富集分析,将富集到的Padjust<0.05的生物学过程定义为显著富集的生物学过程。
1.6 实时荧光定量PCR(RT-qPCR)
使用TransStart Top Green qPCR SuperMix试剂盒(北京全式金生物技术有限公司)将反转录合成的cDNA在Bio-Rad CFX(Bio-Rad,美国)实时荧光定量PCR仪中进行RT-qPCR分析。热循环程序如下:94 ℃ 30 s,开始40个循环,94 ℃ 5 s,53 ℃ 15 s和72 ℃ 10 s。
引物通过Primer Primer5软件自行进行设计,并由生工生物工程(上海)股份有限公司进行合成。实验结果使用2-ΔΔCt方法进行相关定量,以斑马鱼的rpl13a基因作为管家基因,选择与生殖细胞发育相关的SRY盒转录因子(sox9b)、种质(buc)、透明带糖蛋白(zp3)、双性别和mab-3相关转录因子(dmrt1)基因及促卵泡激素亚基β(fshb)基因,与类固醇合成通路相关的细胞色素P450酶(cyp11a1、cyp17a1、cyp19a1a)、3-β-羟基类固醇脱氢酶(hsd3b1)、17-β-羟基类固醇脱氢酶(hsd17b1)、类固醇激素受体(ar、esr1)、尿苷二磷酸葡萄糖醛酸转移酶(ugt1a5)及磺基转移酶(sult1st7)基因进行表达水平分析,引物序列见表1。
表1 用于RT-qPCR的引物序列
Table 1 Primer sequences used for RT-qPCR
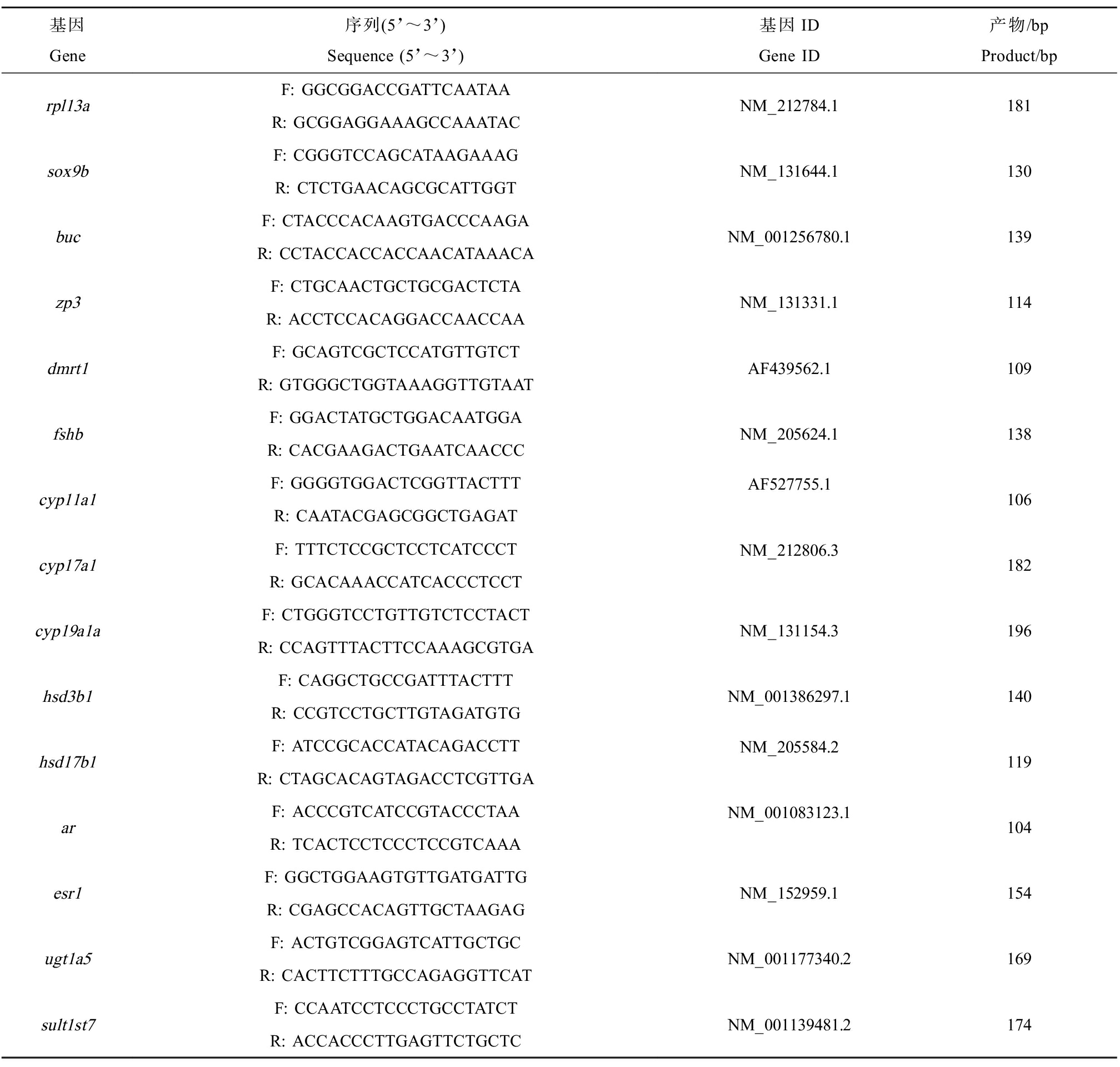
基因Gene序列(5’~3’)Sequence (5’~3’)基因IDGene ID产物/bpProduct/bprpl13aF: GGCGGACCGATTCAATAAR: GCGGAGGAAAGCCAAATACNM_212784.1181sox9bF: CGGGTCCAGCATAAGAAAGR: CTCTGAACAGCGCATTGGTNM_131644.1130bucF: CTACCCACAAGTGACCCAAGAR: CCTACCACCACCAACATAAACANM_001256780.1139zp3F: CTGCAACTGCTGCGACTCTAR: ACCTCCACAGGACCAACCAANM_131331.1114dmrt1F: GCAGTCGCTCCATGTTGTCTR: GTGGGCTGGTAAAGGTTGTAATAF439562.1109fshbF: GGACTATGCTGGACAATGGAR: CACGAAGACTGAATCAACCCNM_205624.1138cyp11a1F: GGGGTGGACTCGGTTACTTTR: CAATACGAGCGGCTGAGATAF527755.1106cyp17a1F: TTTCTCCGCTCCTCATCCCTR: GCACAAACCATCACCCTCCTNM_212806.3182cyp19a1aF: CTGGGTCCTGTTGTCTCCTACTR: CCAGTTTACTTCCAAAGCGTGANM_131154.3196hsd3b1F: CAGGCTGCCGATTTACTTTR: CCGTCCTGCTTGTAGATGTGNM_001386297.1140hsd17b1F: ATCCGCACCATACAGACCTTR: CTAGCACAGTAGACCTCGTTGANM_205584.2119arF: ACCCGTCATCCGTACCCTAAR: TCACTCCTCCCTCCGTCAAANM_001083123.1104esr1F: GGCTGGAAGTGTTGATGATTGR: CGAGCCACAGTTGCTAAGAGNM_152959.1154ugt1a5F: ACTGTCGGAGTCATTGCTGCR: CACTTCTTTGCCAGAGGTTCATNM_001177340.2169sult1st7F: CCAATCCTCCCTGCCTATCTR: ACCACCCTTGAGTTCTGCTCNM_001139481.2174
1.7 激素的测定
将收集到的斑马鱼仔鱼按比例加入预冷的PBS进行匀浆处理,并于4 ℃、5 000 r·min-1下离心7 min。取上清液根据斑马鱼ELISA试剂盒(上海酶联生物科技有限公司,中国)说明书对斑马鱼仔鱼体内的性激素(雌二醇E2和睾酮T)水平进行测定,同时使用BCA蛋白浓度测定试剂盒(增强型) (碧云天生物技术有限公司,上海)测定总蛋白浓度用于归一化处理。数据结果以总激素含量与总蛋白浓度的比值表示。
1.8 数据统计和分析
使用SPSS软件对实验数据进行统计分析,所有数据均以平均值±标准差(mean±standard deviation)进行表示。多个均值之间使用单因素方差分析进行比较,并进行最小显著差异法(least significant difference, LSD)检验或Dunnett’s T3(不假设等方差)事后检验。P<0.05被认为具有显著的统计学差异。
2 结果(Results)
2.1 斑马鱼胚胎及仔鱼的急性毒性
根据Zahn等[8]的研究,DPG可在水溶液中稳定存在。将0.0000、1.5625、3.1250、6.2500、12.5000、25.0000 mg·L-1的DPG暴露于斑马鱼胚胎进行急性毒性实验,溶剂对照组与空白对照组在孵化率、存活率及体长等生理指标上无显著性差异。与溶剂对照组相比,DPG的所有暴露浓度对斑马鱼胚胎及仔鱼的孵化率(72 h)、存活率(120 h)及体长(120 h)均没有任何显著影响,急性毒性为低毒(表2)。
表2 1,3-二苯胍(DPG)对斑马鱼仔鱼生理参数的影响
Table 2 Effects of 1,3-diphenyl guanidine (DPG) on physiological parameters of zebrafish larvae
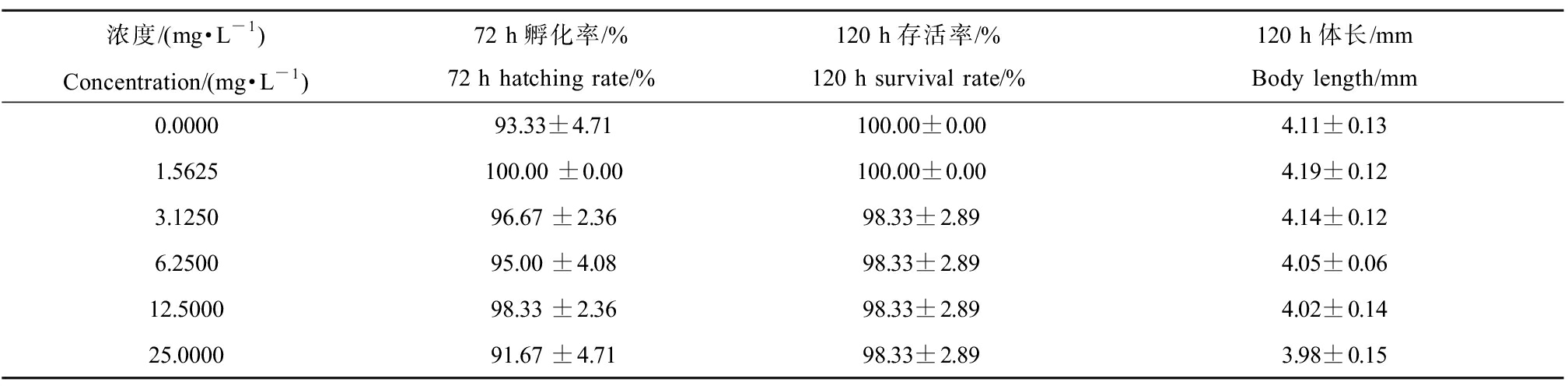
浓度/(mg·L-1)Concentration/(mg·L-1)72 h孵化率/%72 h hatching rate/%120 h存活率/%120 h survival rate/% 120 h体长/mmBody length/mm0.000093.33±4.71100.00±0.004.11±0.131.5625100.00 ±0.00100.00±0.004.19±0.123.125096.67 ±2.3698.33±2.894.14±0.126.250095.00 ±4.0898.33±2.894.05±0.0612.500098.33 ±2.3698.33±2.894.02±0.1425.000091.67 ±4.7198.33±2.893.98±0.15
注:含1% CH3OH(V∶V)的溶剂对照设为浓度0.0000 mg·L-1。
Note: The solvent control containing 1% CH3OH (V∶V) was set as a concentration of 0.0000 mg·L-1.
2.2 转录组数据分析
通过转录组数据分析发现,300 μg·L-1 DPG暴露组与溶剂对照组相比,有4 797个差异基因,其中包含2 985个上调基因和1 812个下调基因(图1)。GSEA的GO分析结果表明,有18个与生殖发育相关的生物学过程被显著富集,主要分为三大类。(1)与性别分化相关的生物学过程,包括生殖结构发育(GO:0048608)、生殖系统发育(GO:0061458)、初级性特征的发育(GO:0045137)、性腺发育(GO:0008406)、雌性主要性征的发育(GO:0046545)、雌性性腺的发育(GO:0008585)及性别决定(GO:0007530);(2)与精卵识别相关的生物学过程,包括:精子与透明带的结合(GO:0007339)、精卵识别(GO:0035036)、细胞识别>(GO:0009988)及生殖过程的调控(GO:2000241);(3)与生殖细胞发育相关的生物学过程,包括:雌性配子产生(GO:0007292)、参与多细胞生物生殖的细胞过程(GO:0022412)、卵子发生(GO:0048477)、种质(GO:0060293)、生殖细胞发育(GO:0007281)、极质(GO:0045495)及多细胞生物生殖过程(GO:0048609)(表3)。进一步对差异基因进行GO富集分析发现,有17个与生殖发育相关的生物学过程被显著富集,主要分为两大类,一类是与精卵识别相关的生物学过程,包括:精子与透明带的结合(GO:0007339)、精卵识别(GO:0035036)、细胞识别(GO:0009988)、顶体反应的调节(GO:0060046)、顶体反应的正向调节(GO:2000344)及顶体酶结合(GO:0032190);另一类是生殖细胞发育相关的生物学过程,包括:与生殖有关的发育过程(GO:0003006)、参与多细胞生物生殖的细胞过程(GO:0022412)、雌性配子产生(GO:0007292)、多细胞生物生殖(GO:0032504)、卵壳形成(GO:0035803)、配子产生(GO:0007276)、卵子发生(GO:0048477)、生殖细胞发育(GO:0007281)、生殖过程(GO:0022414)、生殖(GO:0000003)及有性生殖(GO:0019953)(表4)。综合比较GSEA的GO富集分析及差异基因的GO富集分析发现,均富集到了7个相同的与生殖发育相关的生物学过程,包括:精卵识别(GO:0035036)、卵子发生(GO:0048477)、生殖细胞发育(GO:0007281)、雌性配子产生(GO:0007292)、参与多细胞生物生殖过程中的细胞过程(GO:0022412)、细胞识别(GO:0009988)及精子与透明带的结合(GO:0007339)(图2)。
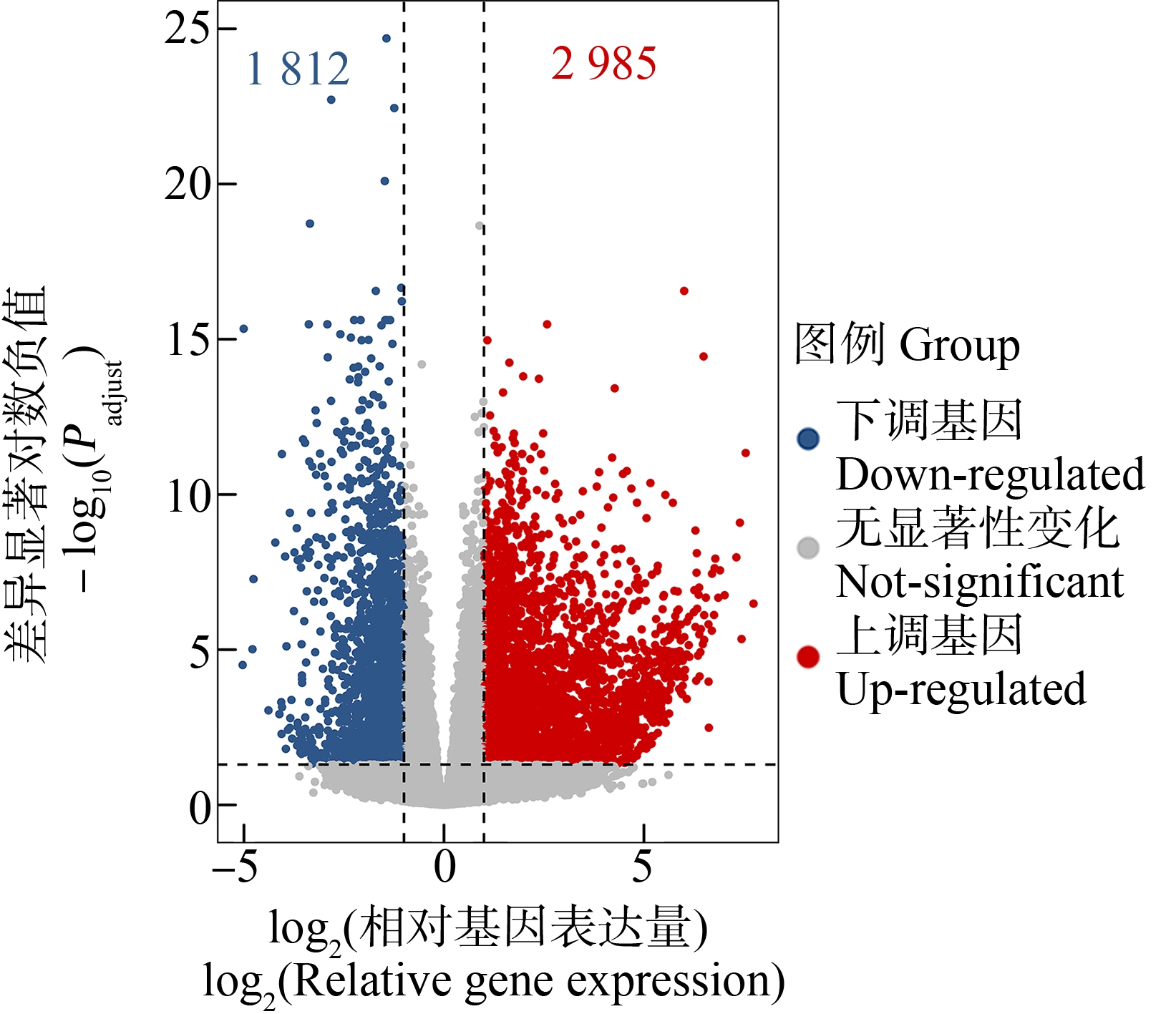
图1 300 μg·L-1 1,3-二苯胍(DPG)暴露组与溶剂对照组的差异基因火山图
注:红色的点表示上调基因,蓝色的点表示下调基因,灰色的点表示没有显著性变化的基因。
Fig. 1 Volcano plot of differentially expressed genes between the 300 μg·L-1 1,3-diphenyl guanidine (DPG) exposure group and the solvent control group
Note: Red dots represent up-regulated genes, blue dots represent down-regulated genes, and grey dots represent genes with no significant change.
表3 300 μg·L-1 DPG暴露组GSEA的GO富集分析
Table 3 GO enrichment analysis of GSEA in 300 μg·L-1 DPG-treated group

类别Category生物学过程Term描述Description校正的P值PadjustBPGO:0007292雌性配子产生 Female gamete generation0.0072 BPGO:0022412参与多细胞生物生殖的细胞过程 Cellular process involved in reproduction in multicellular organism0.0087 BPGO:0007339精子与透明带的结合 Binding of sperm to zona pellucida 0.0093 BPGO:0048608生殖结构发育 Reproductive structure development0.0119 BPGO:0061458生殖系统发育 Reproductive system development0.0119 BPGO:0045137初级性特征的发育 Development of primary sexual characteristics0.0132 BPGO:0048477卵子发生 Oogenesis0.0144 BPGO:0035036精卵识别 Sperm-egg recognition0.0146 BPGO:0009988细胞识别 Cell-cell recognition0.0190BPGO:2000241生殖过程调控 Regulation of reproductive process0.0199 BPGO:0008406性腺发育 Gonad development0.0206 CCGO:0060293种质 Germ plasm0.0301 BPGO:0007281生殖细胞发育 Germ cell development0.0314 BPGO:0046545雌性主要性征的发育 Development of primary female sexual characteristics0.0348 CCGO:0045495极质 Pole plasm0.0390 BPGO:0008585雌性性腺发育 Female gonad development0.0391 BPGO:0048609多细胞生物生殖过程 Multicellular organismal reproductive process0.0462 BPGO:0007530性别决定 Sex determination0.0499
注:BP是指生物学过程(biological process),CC是指细胞成分(cellular component)。
Note: BP refers to biological process and CC refers to cellular component.
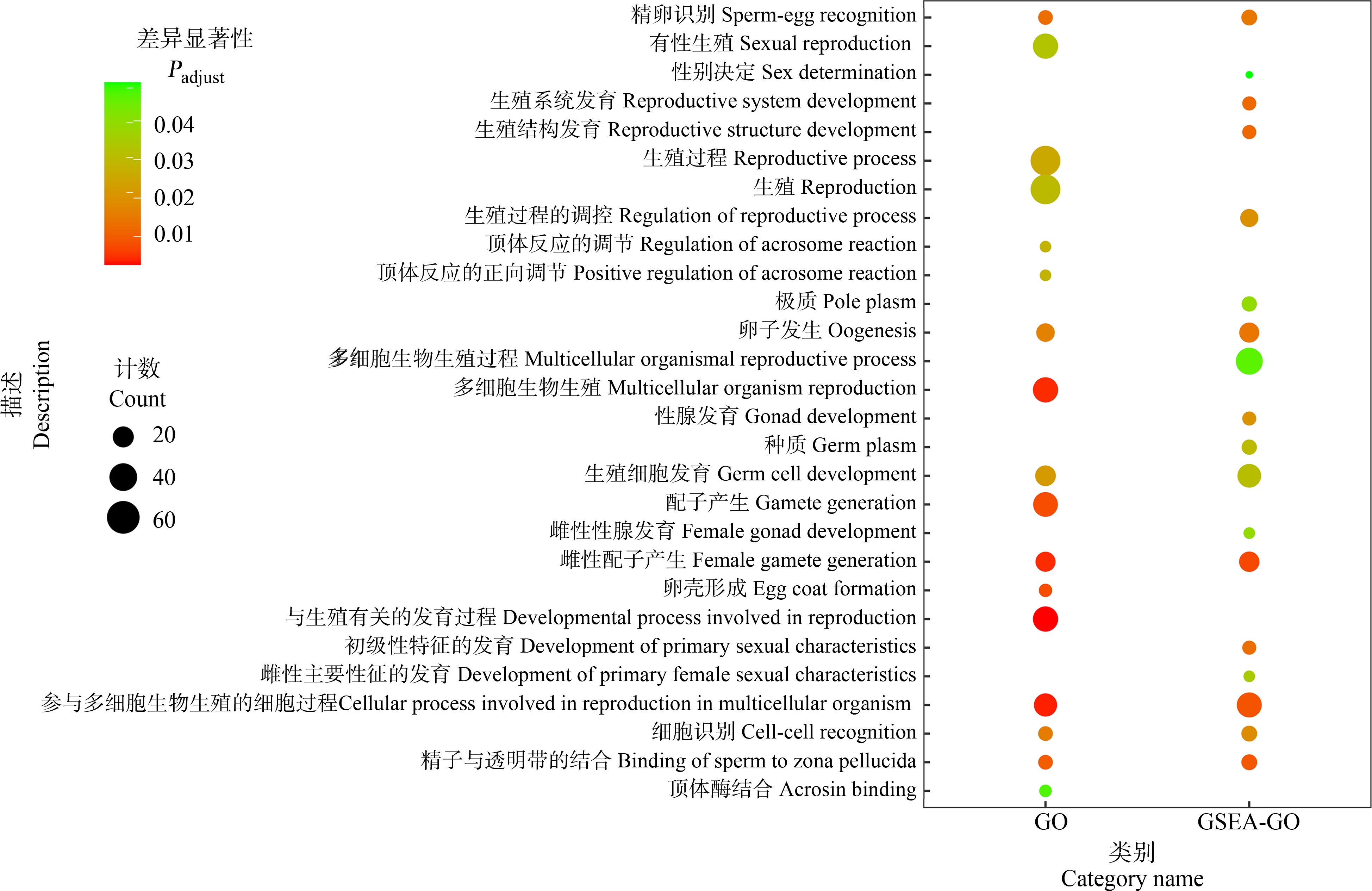
图2 差异基因的GO富集分析与GSEA的GO富集分析结果对比(Padjust<0.05)
注:点的大小和该点富集到的基因数目成正比,点的颜色与差异显著性成正比,颜色越红代表与溶剂对照组差异越显著。
Fig. 2 The contrast of GO enrichment analysis between DEGs and GSEA (Padjust<0.05)
Note: The size of the dots was proportional to the number of genes enriched at the point, and the color of the dots was proportional to the difference significance; the reder the color, the more significant the difference from the solvent control group.
表4 300 μg·L-1 DPG暴露组差异基因的GO富集分析
Table 4 GO enrichment analysis of differentially expressed gene in 300 μg·L-1 DPG-treated group
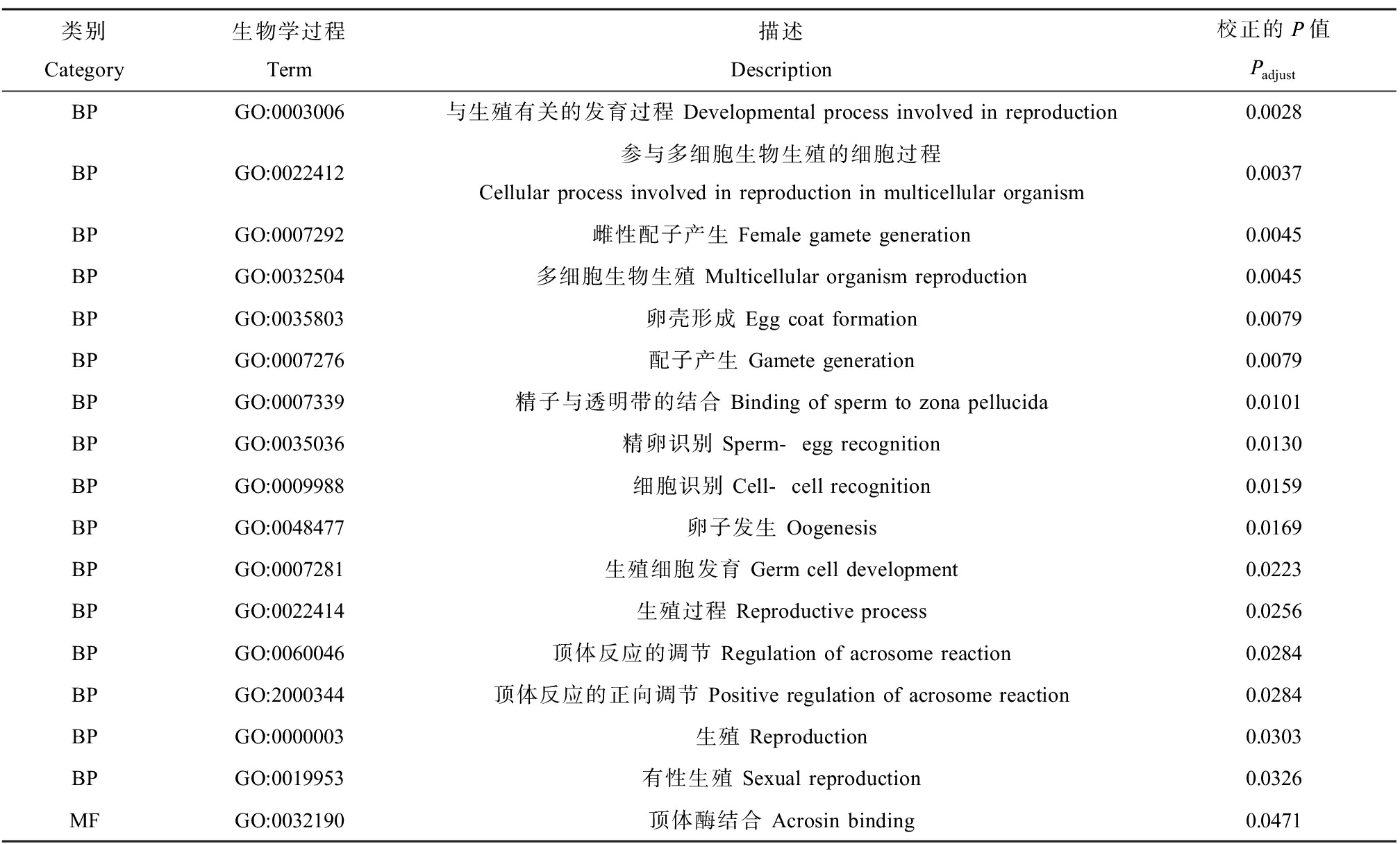
类别Category生物学过程Term描述Description校正的P值PadjustBPGO:0003006与生殖有关的发育过程 Developmental process involved in reproduction0.0028BPGO:0022412参与多细胞生物生殖的细胞过程 Cellular process involved in reproduction in multicellular organism0.0037BPGO:0007292雌性配子产生 Female gamete generation0.0045BPGO:0032504多细胞生物生殖 Multicellular organism reproduction0.0045BPGO:0035803卵壳形成 Egg coat formation0.0079BPGO:0007276配子产生 Gamete generation0.0079BPGO:0007339精子与透明带的结合 Binding of sperm to zona pellucida 0.0101BPGO:0035036精卵识别 Sperm-egg recognition0.0130BPGO:0009988细胞识别 Cell-cell recognition0.0159BPGO:0048477卵子发生 Oogenesis0.0169BPGO:0007281生殖细胞发育 Germ cell development0.0223BPGO:0022414生殖过程 Reproductive process0.0256BPGO:0060046顶体反应的调节 Regulation of acrosome reaction0.0284BPGO:2000344顶体反应的正向调节 Positive regulation of acrosome reaction 0.0284BPGO:0000003生殖 Reproduction0.0303BPGO:0019953有性生殖 Sexual reproduction0.0326MFGO:0032190顶体酶结合 Acrosin binding0.0471
注:BP是指生物学过程(biological process);MF是指细胞成分(molecular function)。
Note: BP refers to biological process and MF refers to molecular function.
2.3 与生殖细胞发育相关基因的表达
在GSEA的GO富集分析及差异基因的GO富集分析中均富集到了与生殖细胞发育相关的生物学过程,因此选择了与生殖细胞发育相关的基因进行表达水平分析。通过3个暴露组的qPCR结果发现低浓度组sox9b (P<0.01)、zp3 (P<0.001)、dmrt1 (P<0.05)基因的表达水平显著下调,buc (P<0.05)基因的表达水平显著上调;中浓度组zp3 (P<0.001)、dmrt1 (P<0.01)基因的表达水平显著下调;高浓度组zp3 (P<0.001)、dmrt1 (P<0.05)基因的表达水平显著下调,buc (P<0.05)及fshb (P<0.05)基因的表达水平显著上调;且高浓度组sox9b、buc及fshb基因的表达水平与RNA-seq结果一致(图3)。
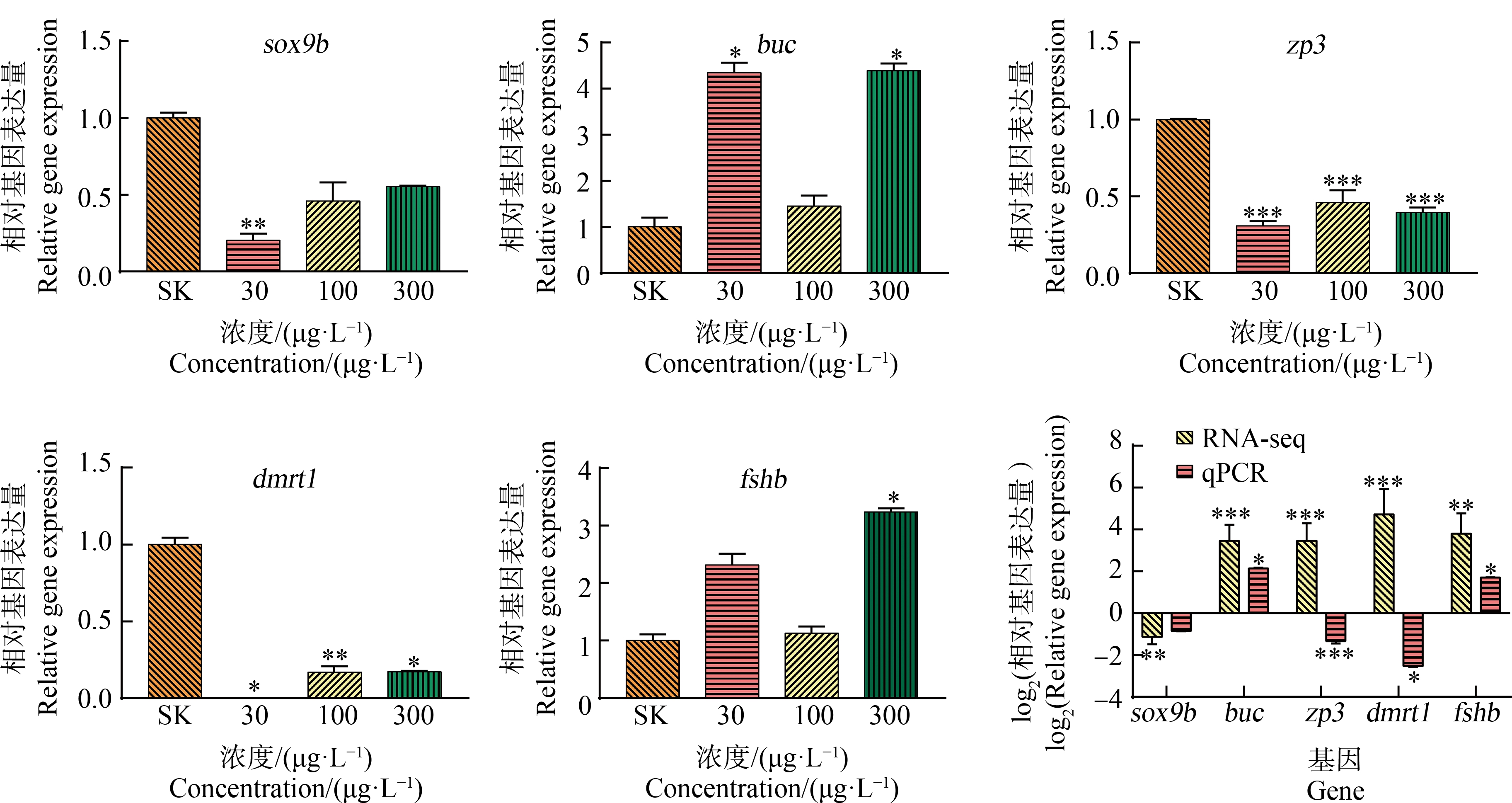
图3 120 hpf斑马鱼仔鱼体内与生殖细胞发育相关基因的表达
注:SK表示含0.1% CH3OH(V∶V)的溶剂对照,*表示P<0.05,**表示P<0.01,***表示P<0.001。
Fig. 3 Expression of genes involved in germ cell development in larval zebrafish at 120 hpf
Note: SK stands for solvent control containing 0.1% CH3OH (V∶V); *represents P<0.05, **represents P<0.01, and ***represents P<0.001.
2.4 类固醇生物合成相关基因的表达
类固醇激素在调控鱼类的生殖过程中有重要的作用[19],故选择了类固醇合成通路的相关基因进行表达水平分析。与溶剂对照组相比,低浓度组cyp17a1 (P<0.01)、ar (P<0.001)、esr1 (P<0.05)及sul1st7 (P<0.001)基因表达水平显著下调;中浓度组ar (P<0.05)、esr1 (P<0.05)及sult1st7 (P<0.01)基因表达水平显著下调,cyp19a1a (P<0.05)、ugt1a5 (P<0.05)基因表达水平显著上调;高浓度组sul1st7 (P<0.05)基因表达水平显著下调,cyp11a1 (P<0.01)、cyp17a1 (P<0.001)、cyp19a1a (P<0.01)、hsd17b1 (P<0.001)及ar (P<0.01)基因表达水平显著上调;且cyp19a1a基因的表达水平随着暴露剂量的增加而增加;在3个暴露浓度下,hsd3b1的表达水平均无明显变化(图4)。
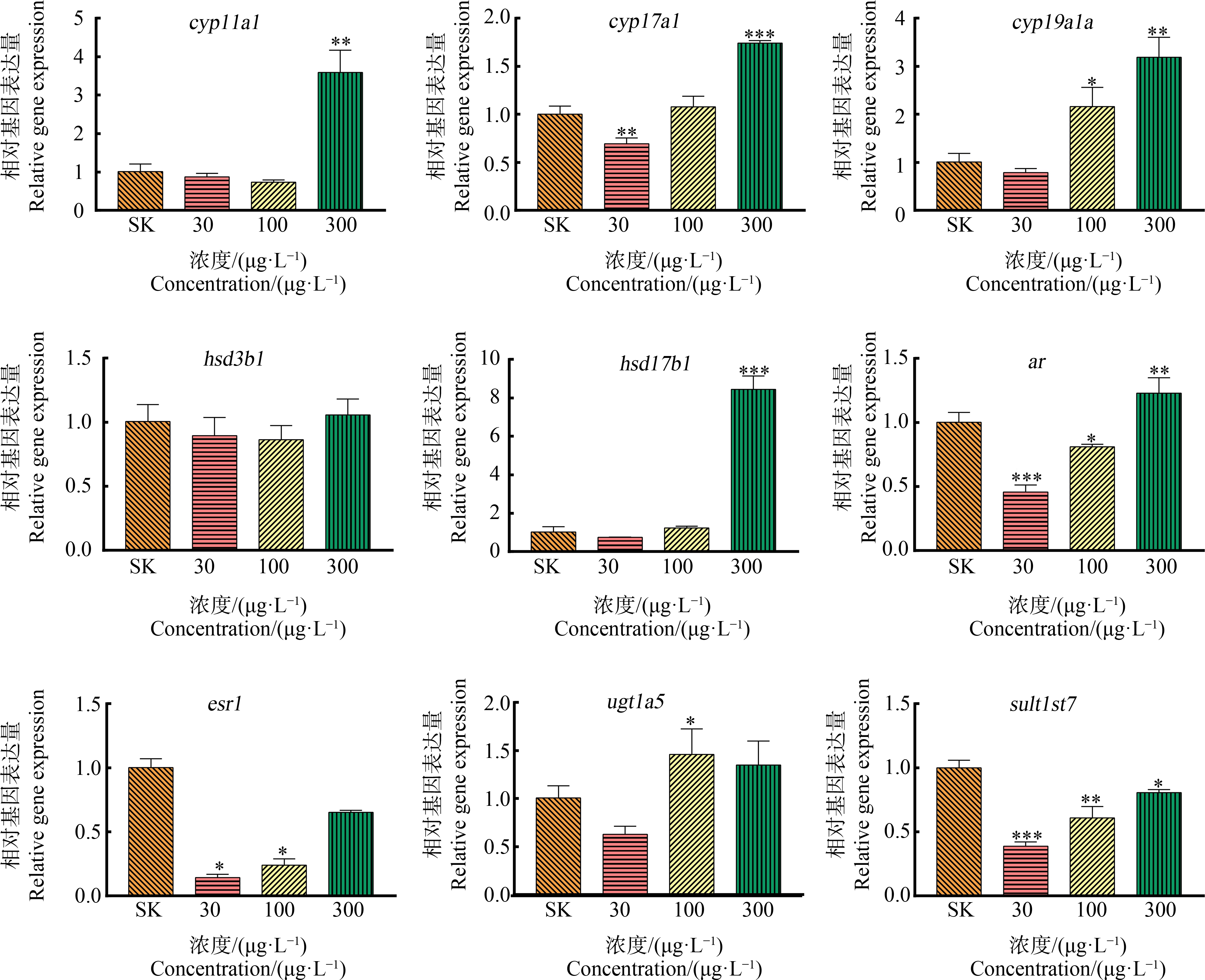
图4 120 hpf斑马鱼仔鱼体内类固醇生物合成通路相关基因的表达
注:SK表示含0.1% CH3OH(V∶V)的溶剂对照,*表示P<0.05,**表示P<0.01,***表示P<0.001。
Fig. 4 Expression of genes involved in steroid biosynthesis pathway in larval zebrafish at 120 hpf
Note: SK stands for solvent control containing 0.1% CH3OH (V∶V); *represents P<0.05, **represents P<0.01, and ***represents P<0.001.
将高浓度组的类固醇合成通路相关基因的RNA-seq和qPCR结果进行对比,发现hsd17b1、cyp11a1、cyp17a1、cyp19a1a、ar及ugt1a5基因的表达水平与RNA-seq结果一致(图5)。
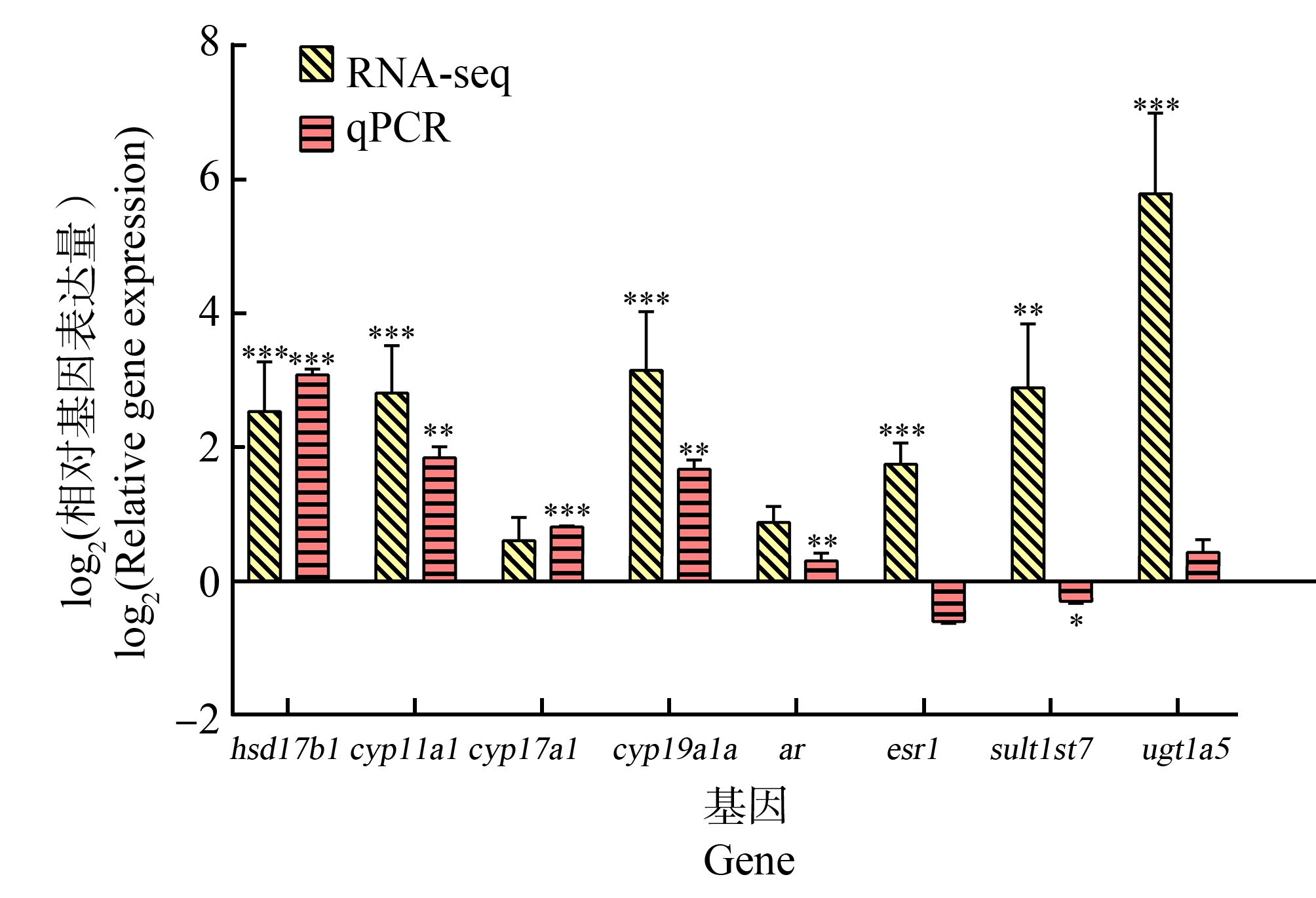
图5 通过RNA测序和qPCR检测DPG暴露后斑马鱼仔鱼中类固醇生物合成通路相关基因的mRNA水平
注:*表示P<0.05,**表示P<0.01,***表示P<0.001。
Fig. 5 The mRNA levels of genes involved in steroid biosynthesis pathway in zebrafish larvae determined using RNA sequencing and qPCR after DPG exposure
Note: *represents P<0.05, **represents P<0.01, and ***represents P<0.001.
2.5 性激素水平测定
通过性激素水平测定进一步验证DPG的暴露对斑马鱼生殖发育的影响。与溶剂对照组相比,3个浓度组的斑马鱼仔鱼体内的雌二醇水平均出现了显著上升(低浓度组P<0.05,中浓度组及高浓度组P<0.01),而睾酮水平无明显变化,高浓度组雌二醇与睾酮的比值显著增加(P<0.05),且雌二醇与睾酮的比值与暴露浓度之间存在剂量响应关系,即雌二醇与睾酮的比值随着暴露浓度的增加而增加(图6)。
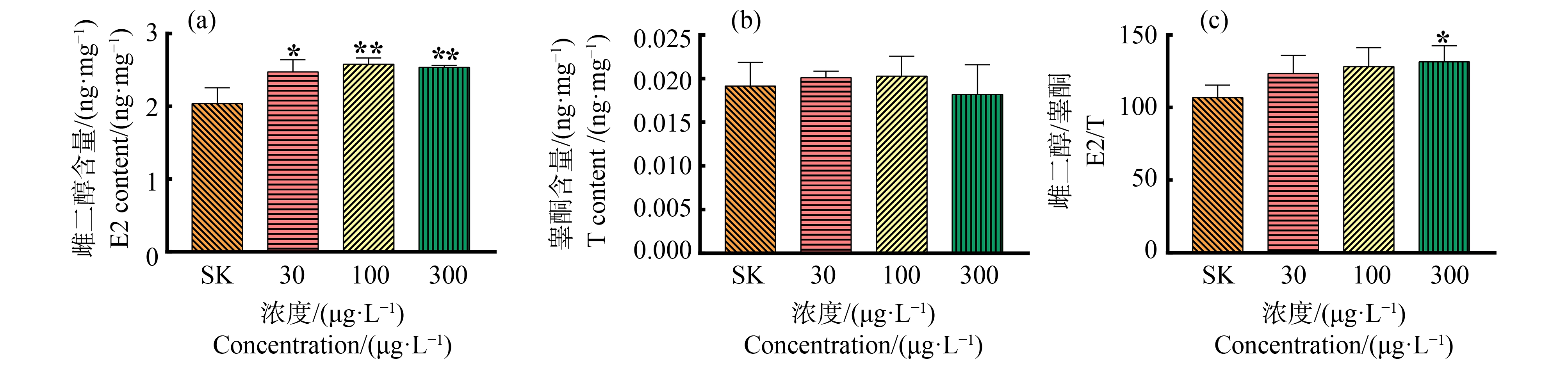
图6 斑马鱼体内的雌二醇水平(a)、睾酮水平(b)和斑马鱼体内雌二醇与睾酮的比值(c)
注:SK表示含0.1% CH3OH(V∶V)的溶剂对照,*表示P<0.05,**表示P<0.01。
Fig. 6 Estradiol levels (a), testosterone levels (b) and the ratio of estradiol to testosterone (c) in zebrafish
Note: SK stands for solvent control containing 0.1% CH3OH(V∶V); *represents P<0.05, and **represents P<0.01.
3 讨论(Discussion)
在DPG对斑马鱼胚胎及仔鱼的急性毒性实验中,与溶剂对照组相比,在设计的最高浓度25 mg·L-1下,DPG对斑马鱼胚胎及仔鱼的孵化率(72 h)、存活率(120 h)及体长(120 h)均没有显著影响,表明DPG对斑马鱼胚胎及仔鱼的120 h-LC50>25 mg·L-1。参照我国《危险化学品鱼类急性毒性分级试验方法》(GB/T 21281—2007)[35],当10.0 mg·L-1≤96 h-LC50≤100.0 mg·L-1为急性毒性三级,推测DPG对斑马鱼的急性毒性为低毒。
进一步对高浓度暴露组的斑马鱼仔鱼进行转录组测序分析,从分子水平来揭示其潜在的毒性作用机制。从转录组测序结果分析,DPG高浓度暴露组使与生殖发育相关的生物学过程被显著富集,包括:精卵识别(GO:0035036)、卵子发生(GO:0048477)、生殖细胞发育(GO:0007281)、雌性配子产生(GO:0007292)、参与多细胞生物生殖过程中的细胞过程(GO:0022412)、细胞识别(GO:0009988)及精子与透明带的结合(GO:0007339) (图2)。这表明DPG的暴露会影响斑马鱼的生殖发育。将10 mg·L-1降解较差的医用口罩(PDM)暴露于斑马鱼胚胎及仔鱼10 d,发现PDM可显著富集斑马鱼仔鱼中与生殖相关的生物学过程:精子与透明带的结合、顶体反应的正向调节和卵壳形成;而将同等浓度高度降解的医用口罩(HDM)暴露于斑马鱼胚胎及仔鱼10 d,发现HDM也可显著富集斑马鱼仔鱼中与生殖相关的生物学过程:极粒组织、参与配子生成的DNA甲基化、卵壳形成、顶体反应的正向调节、精子与透明带的结合、配子生成、减数分裂的核分裂、卵子发生、生殖细胞发育、精子细胞发育、减数分裂细胞周期和精子发生[36]。这与本研究结果类似。
虽然转录组测序结果是可靠的,但是因其在数据采集过程中固有的随机抽样过程导致转录组数据具有测量噪声[37],并且由于转录组测序和qPCR在检测方法及基因表达定量上的不同,因此可能会出现转录组测序结果与qPCR结果不一致的情况。相比较而言,针对特定基因的qPCR的结果具有更高的准确性。因此,转录组测序结果需要qPCR进一步验证。通过转录组分析结果,选择与生殖细胞发育相关的基因(sox9b、buc、zp3、dmrt1及fshb)进行表达水平定量分析。生殖细胞在斑马鱼性别分化中具有重要作用,生殖细胞缺陷会导致第二性征的性别逆转[38-39]。sox9是睾丸决定的重要调节因子,在斑马鱼中,sox9分为sox9a和sox9b这2个亚型,sox9a在发育和成熟的睾丸中特异性表达,而sox9b参与原始卵巢卵泡的发育[40],并且sox9b对日本青鳉生殖细胞增殖具有重要作用[41];buc通过调节种质mRNA的表达来参与斑马鱼早期发育过程中的种质组装[42];dmrt1在脊椎动物的性别决定和性腺性别分化中起着至关重要的作用,对斑马鱼睾丸小管的正常发育和精子发生至关重要[43];zp3蛋白在斑马鱼卵母细胞中特异性表达,是主要精子受体并能够诱导顶体反应[44-45];fshb与斑马鱼卵泡的发育相关,缺少fshb会降低雌性斑马鱼的生殖能力[46]。在本研究中,DPG的暴露导致zp3及dmrt1基因表达的显著下调,buc及fshb基因表达的显著上调。而cyp19a1a可通过抑制dmrt1基因的表达来诱导性腺分化成卵巢[47],表明DPG可通过促进cyp19a1a基因的表达及抑制dmrt1基因的表达。类固醇可通过作用于下丘脑或垂体来发挥正反馈或者负反馈作用,并且雌二醇的暴露可以显著增加斑马鱼中fshb基因的表达水平[48],这与本研究结果一致。
转录组数据表明,DPG的暴露会影响斑马鱼的生殖发育过程,而鱼类的生殖发育过程受到类固醇激素的调节[19],因此,我们选择了类固醇合成通路相关基因(cyp11a1、cyp17a1、cyp19a1a、hsd3b1、hsd17b1、ar、esr1、ugt1a5、sult1st7)进行表达水平定量分析。细胞色素P450侧链切割酶(cyp11a1)将胆固醇转化为孕烯醇酮是类固醇生物合成的第一步[49]。cyp17a1是负责雄激素合成的关键酶[50],敲除cyp17a1基因导致雄性斑马鱼的雄激素水平减少,并损伤其交配行为[51]。17β-羟基类固醇脱氢酶Ⅲ型(hsd17b3)可将雄烯二酮转化为睾酮以及将11-酮雄烯二酮转化为11-酮睾酮,而17β-hsd Ⅰ型(hsd17b1)可催化雌酮转化为雌二醇[52-53]。细胞色素P450芳香化酶(cyp19)是类固醇生成途径中的末端酶,可将雄激素转化为雌激素。在硬骨鱼中,cyp19a基因分为cyp19a1a和cyp19a1b这2个亚型,cyp19a1a在卵巢中表达,cyp19a1b在大脑中表达[54-55],cyp19a1a基因是斑马鱼性别分化中不可或缺的基因[56]。雄激素受体(ar)主要促进雄性生殖器官的发育和精子发生,而雌激素受体(esr1)对于成年斑马鱼的性腺发育和卵巢维持必不可少[57-59]。qPCR结果表明DPG的暴露导致高浓度组的cyp11a1、cyp17a1、cyp19a1a、hsd17b1及ar基因表达水平的显著上调,因此,DPG的暴露会显著影响类固醇合成通路相关基因的表达。为了进一步验证该结果,我们对斑马鱼仔鱼体内的性激素水平(雌二醇和睾酮)也进行了测定。雌二醇是硬骨鱼体内的主要雌激素[60]。雌二醇在斑马鱼卵巢的分化中具有重要作用,在胚胎仔鱼阶段暴露于雌二醇会导致雌性比例增加[61],从而使斑马鱼的性别比例失衡。根据性激素测定结果,发现DPG的暴露会导致斑马鱼仔鱼体内雌二醇水平的升高。通过转录组测序、qPCR及性激素水平测定结果,推测DPG可通过影响类固醇合成通路相关基因的表达最终导致斑马鱼仔鱼体内雌二醇水平的升高(图7)。DPG通过促进cyp11a1、cyp17a1及hsd17b1基因的表达,加快了胆固醇合成睾酮的生物过程;通过促进cyp19a1a基因的表达,提高睾酮转化为雌二醇的效率,因此导致斑马鱼仔鱼体内雌二醇含量的增加及雌二醇与睾酮比值的升高。在哺乳动物中,雌二醇水平的调控也与此一致。围产期暴露于氯氰菊酯,导致C57BL雄性小鼠睾丸中cyp19a1基因表达的增加,雌二醇水平的升高及雌二醇含量与睾酮比值的升高[62]。
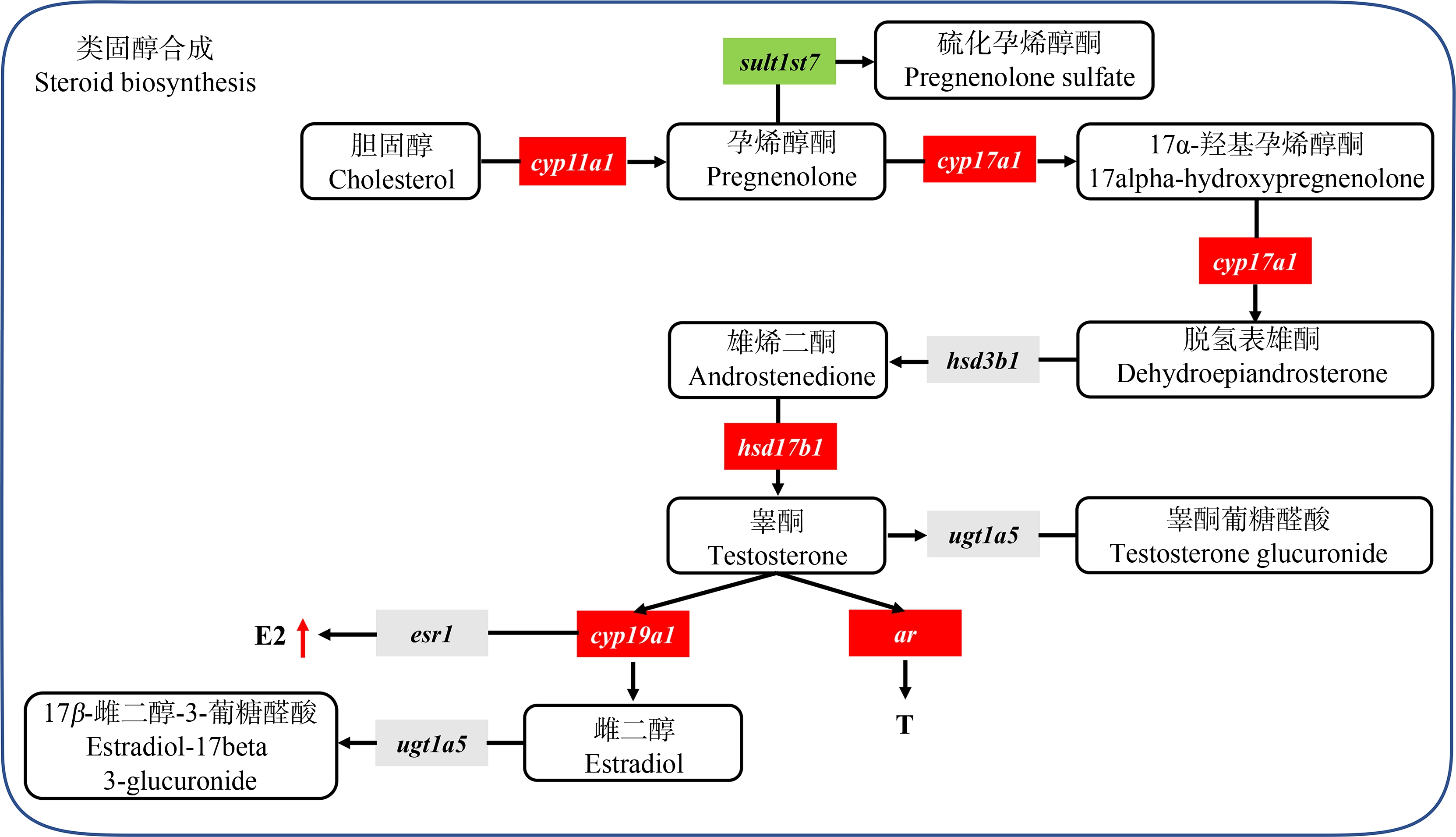
图7 DPG暴露后斑马鱼仔鱼类固醇生物合成调控机制图
注:红色表示上调基因,绿色表示下调基因,灰色为无显著变化的基因,红色箭头表示含量增加。
Fig. 7 The regulatory mechanism of steroid biosynthesis in zebrafish larvae after DPG exposure
Note: Up-regulated genes are shown in red, down-regulated genes are shown in green, genes with no significant changes are shown in gray, and red arrows indicate increased content.
值得注意的是,本文发现在分子水平上引起鱼类生殖毒性的DPG浓度为300 μg·L-1,而我国长沙自来水中曾检测到DPG浓度高达560 μg·L-1[14],其是否会引起公共卫生问题,需要进行更多的研究。同时,也很有必要找到DPG对哺乳动物的无可观察不良效应的浓度(no observed adverse effect level, NOAEL),以确定每日可接受摄入量(acceptable daily intake, ADI),保证居民的饮水安全。
通过以上研究,我们得出结论,DPG对斑马鱼胚胎及仔鱼的急性毒性低,但从转录水平上发现DPG暴露后导致与精卵识别及生殖细胞发育相关的生物学过程发生显著变化,并通过影响类固醇合成通路相关基因的表达水平导致斑马鱼仔鱼体内雌二醇水平的升高。以上研究结果表明,在分子水平上,DPG能够影响斑马鱼胚胎及仔鱼的发育,但是在个体水平上仍然缺乏直接的证据揭示DPG暴露对斑马鱼生殖和胚胎发育的不良结局。作为轮胎磨损颗粒主要成分之一的DPG(在环境相关浓度下会引起斑马鱼的毒性反应)很可能也是轮胎磨损颗粒毒性主要贡献者之一。根据本研究结果,推断DPG很可能不是轮胎磨损颗粒对水生生物急性毒性的贡献者,但可能是轮胎磨损颗粒对鱼类生殖毒性的贡献者。
[1] Rogge W F, Hildemann L M, Mazurek M A, et al. Sources of fine organic aerosol. 3. Road dust, tire debris, and organometallic brake lining dust: Roads as sources and sinks [J]. Environmental Science &Technology, 1993, 27(9): 1892-1904
[2] Knight L J, Parker-Jurd F N F, Al-Sid-Cheikh M, et al. Tyre wear particles: An abundant yet widely unreported microplastic? [J]. Environmental Science and Pollution Research International, 2020, 27(15): 18345-18354
[3] Wagner S, Hüffer T, Klöckner P, et al. Tire wear particles in the aquatic environment - A review on generation, analysis, occurrence, fate and effects [J]. Water Research, 2018, 139: 83-100
[4] Chibwe L, Parrott J L, Shires K, et al. A deep dive into the complex chemical mixture and toxicity of tire wear particle leachate in fathead minnow [J]. Environmental Toxicology and Chemistry, 2022, 41(5): 1144-1153
[5] Tian Z Y, Zhao H Q, Peter K T, et al. A ubiquitous tire rubber-derived chemical induces acute mortality in coho salmon [J]. Science, 2021, 371(6525): 185-189
[6] Mohajerani A, Kurmus H, Conti D, et al. Environmental impacts and leachate analysis of waste rubber incorporated in construction and road materials: A review [J]. The Science of the Total Environment, 2022, 835: 155269
[7] Jin J, van Swaaij A P J, Noordermeer J W M, et al. On the various roles of 1,3-diphenyl guanidine in silica/silane reinforced sbr/br blends [J]. Polymer Testing, 2021, 93: 106858
[8] Zahn D, Mucha P, Zilles V, et al. Identification of potentially mobile and persistent transformation products of REACH-registered chemicals and their occurrence in surface waters [J]. Water Research, 2019, 150: 86-96
[9] Johannessen C, Helm P, Lashuk B, et al. The tire wear compounds 6PPD-quinone and 1,3-diphenylguanidine in an urban watershed [J]. Archives of Environmental Contamination and Toxicology, 2022, 82(2): 171-179
[10] Schulze S, Zahn D, Montes R, et al. Occurrence of emerging persistent and mobile organic contaminants in European water samples [J]. Water Research, 2019, 153: 80-90
[11] Scheurer M, Sandholzer A, Schnabel T, et al. Persistent and mobile organic chemicals in water resources: Occurrence and removal options for water utilities [J]. Water Supply, 2022, 22(2): 1575-1592
[12] Xie L, Nakajima F, Kasuga I, et al. Simultaneous screening for chemically diverse micropollutants in public water bodies in Japan by high-performance liquid chromatography-Orbitrap mass spectrometry [J]. Chemosphere, 2020, 1: 128524
[13] Hou F, Tian Z Y, Peter K T, et al. Quantification of organic contaminants in urban stormwater by isotope dilution and liquid chromatography-tandem mass spectrometry [J]. Analytical and Bioanalytical Chemistry, 2019, 411(29): 7791-7806
[14] Tang J, Tang L, Zhang C, et al. Different senescent HDPE pipe-risk: Brief field investigation from source water to tap water in China (Changsha City) [J]. Environmental Science and Pollution Research International, 2015, 22(20): 16210-16214
[15] Johannessen C, Metcalfe C D. The occurrence of tire wear compounds and their transformation products in municipal wastewater and drinking water treatment plants [J]. Environmental Monitoring and Assessment, 2022, 194(10): 731
[16] Zhang H Y, Huang Z, Liu Y H, et al. Occurrence and risks of 23 tire additives and their transformation products in an urban water system [J]. Environment International, 2023, 171: 107715
[17] Tang S Q, Sun X F, Qiao X H, et al. Prenatal exposure to emerging plasticizers and synthetic antioxidants and their potency to cross human placenta [J]. Environmental Science &Technology, 2022, 56(12): 8507-8517
[18] Bempong M A, Hall E V. Reproductive toxicology of 1,3-diphenylguanidine: Analysis of induced sperm abnormalities in mice and hamsters and reproductive consequences in mice [J]. Journal of Toxicology and Environmental Health, 1983, 11(4-6): 869-878
[19] Ma Y B, Han J, Guo Y Y, et al. Disruption of endocrine function in in vitro H295R cell-based and in in vivo assay in zebrafish by 2,4-dichlorophenol [J]. Aquatic Toxicology, 2012, 106-107: 173-181
[20] Yang C, Lim W, Song G. Reproductive toxicity due to herbicide exposure in freshwater organisms [J]. Comparative Biochemistry and Physiology Part C: Toxicology &Pharmacology, 2021, 248: 109103
[21] Gu J, Li L Z, Yin X G, et al. Long-term exposure of zebrafish to bisphenol F: Adverse effects on parental reproduction and offspring neurodevelopment [J]. Aquatic Toxicology, 2022, 248: 106190
[22] Qiao Y J, He J Y, Han P, et al. Long-term exposure to environmental relevant triclosan induces reproductive toxicity on adult zebrafish and its potential mechanism [J]. The Science of the Total Environment, 2022, 826: 154026
[23] Howe K, Clark M D, Torroja C F, et al. The zebrafish reference genome sequence and its relationship to the human genome [J]. Nature, 2013, 496(7446): 498-503
[24] Spence R, Gerlach G, Lawrence C, et al. The behaviour and ecology of the zebrafish, Danio rerio [J]. Biological Reviews of the Cambridge Philosophical Society, 2008, 83(1): 13-34
[25] Segner H. Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption [J]. Comparative Biochemistry and Physiology Toxicology &Pharmacology, 2009, 149(2): 187-195
[26] Scholz S, Fischer S, Gündel U, et al. The zebrafish embryo model in environmental risk assessment—Applications beyond acute toxicity testing [J]. Environmental Science and Pollution Research, 2008, 15(5): 394-404
[27] Fraysse B, Mons R, Garric J. Development of a zebrafish 4-day embryo-larval bioassay to assess toxicity of chemicals [J]. Ecotoxicology and Environmental Safety, 2006, 63(2): 253-267
[28] Kalueff A V, Echevarria D J, Homechaudhuri S, et al. Zebrafish neurobehavioral phenomics for aquatic neuropharmacology and toxicology research [J]. Aquatic Toxicology, 2016, 170: 297-309
[29] Huang T, Zhao Y H, He J, et al. Endocrine disruption by azole fungicides in fish: A review of the evidence [J]. Science of the Total Environment, 2022, 822: 153412
[30] He J H, Gao J M, Huang C J, et al. Zebrafish models for assessing developmental and reproductive toxicity [J]. Neurotoxicology and Teratology, 2014, 42: 35-42
[31] David R M, Jones H S, Panter G H, et al. Interference with xenobiotic metabolic activity by the commonly used vehicle solvents dimethylsulfoxide and methanol in zebrafish (Danio rerio) larvae but not Daphnia magna [J]. Chemosphere, 2012, 88(8): 912-917
[32] 国家质量监督检验检疫总局, 中国国家标准化管理委员会. 化学品 鱼类急性毒性试验: GB/T 27861—2011[S]. 北京: 中国标准出版社, 2012
[33] Organisation for Economic Co-operation and Development (OECD). Test No. 236: Fish embryo acute toxicity (FET) test [R]. Paris: OECD, 2013
[34] Maes J, Verlooy L, Buenafe O E, et al. Evaluation of 14 organic solvents and carriers for screening applications in zebrafish embryos and larvae [J]. PLoS One, 2012, 7(10): e43850
[35] 国家质量监督检验检疫总局, 中国国家标准化管理委员会. 危险化学品鱼类急性毒性分级试验方法: GB/T 21281—2007[S]. 北京: 中国标准出版社, 2008
[36] Sendra M, Pereiro P, Yeste M P, et al. Surgical face masks as a source of emergent pollutants in aquatic systems: Analysis of their degradation product effects in Danio rerio through RNA-Seq [J]. Journal of Hazardous Materials, 2022, 428: 128186
[37] Su Z Q,  abaj P P, Li S, et al. A comprehensive assessment of RNA-seq accuracy, reproducibility and information content by the Sequencing Quality Control Consortium [J]. Nature Biotechnology, 2014, 32(9): 903-914
abaj P P, Li S, et al. A comprehensive assessment of RNA-seq accuracy, reproducibility and information content by the Sequencing Quality Control Consortium [J]. Nature Biotechnology, 2014, 32(9): 903-914
[38] Kikuchi M, Nishimura T, Ishishita S, et al. foxl3, a sexual switch in germ cells, initiates two independent molecular pathways for commitment to oogenesis in medaka [J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(22): 12174-12181
[39] Kurokawa H, Saito D, Nakamura S, et al. Germ cells are essential for sexual dimorphism in the medaka gonad [J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(43): 16958-16963
[40] Yokoi H, Yan Y L, Miller M R, et al. Expression profiling of zebrafish sox9 mutants reveals that Sox9 is required for retinal differentiation [J]. Developmental Biology, 2009, 329(1): 1-15
[41] Nakamura S, Watakabe I, Nishimura T, et al. Analysis of medaka sox9 orthologue reveals a conserved role in germ cell maintenance [J]. PLoS One, 2012, 7(1): e29982
[42] Ren F, Miao R, Xiao R, et al. m6A reader Igf2bp3 enables germ plasm assembly by m6A-dependent regulation of gene expression in zebrafish [J]. Science Bulletin, 2021, 66(11): 1119-1128
[43] Webster K A, Schach U, Ordaz A, et al. Dmrt1 is necessary for male sexual development in zebrafish [J]. Developmental Biology, 2017, 422(1): 33-46
[44] Liu X J, Wang H, Gong Z Y. Tandem-repeated zebrafish zp3 genes possess oocyte-specific promoters and are insensitive to estrogen induction [J]. Biology of Reproduction, 2006, 74(6): 1016-1025
[45] Mold D E, Dinitz A E, Sambandan D R. Regulation of zebrafish zona pellucida gene activity in developing oocytes [J]. Biology of Reproduction, 2009, 81(1): 101-110
[46] Chu L H, Li J Z, Liu Y, et al. Gonadotropin signaling in zebrafish ovary and testis development: Insights from gene knockout study [J]. Molecular Endocrinology, 2015, 29(12): 1743-1758
[47] Wu K, Song W Y, Zhang Z W, et al. Disruption of dmrt1 rescues the all-male phenotype of the cyp19a1a mutant in zebrafish - a novel insight into the roles of aromatase/estrogens in gonadal differentiation and early folliculogenesis [J]. Development, 2020, 147(4): dev182758
[48] Lin S W, Ge W. Differential regulation of gonadotropins (FSH and LH) and growth hormone (GH) by neuroendocrine, endocrine, and paracrine factors in the zebrafish—An in vitro approach [J]. General and Comparative Endocrinology, 2009, 160(2): 183-193
[49] Miller W L, Auchus R J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders [J]. Endocrine Reviews, 2011, 32(1): 81-151
[50] Zhai G, Shu T T, Xia Y G, et al. Androgen signaling regulates the transcription of anti-Müllerian hormone via synergy with SRY-related protein SOX9A [J]. Science Bulletin, 2017, 62(3): 197-203
[51] Shu T T, Zhai G, Pradhan A, et al. Zebrafish cyp17a1 knockout reveals that androgen-mediated signaling is important for male brain sex differentiation [J]. General and Comparative Endocrinology, 2020, 295: 113490
[52] Mindnich R, Deluca D, Adamski J. Identification and characterization of 17 beta-hydroxysteroid dehydrogenases in the zebrafish, Danio rerio [J]. Molecular and Cellular Endocrinology, 2004, 215(1-2): 19-30
[53] Tokarz J, Möller G, de Angelis M H, et al. Zebrafish and steroids: What do we know and what do we need to know? [J]. The Journal of Steroid Biochemistry and Molecular Biology, 2013, 137: 165-173
[54] Trant J M, Gavasso S, Ackers J, et al. Developmental expression of cytochrome P450 aromatase genes (CYP19a and CYP19b) in zebrafish fry (Danio rerio) [J]. The Journal of Experimental Zoology, 2001, 290(5): 475-483
[55] Chiang E F, Yan Y L, Tong S K, et al. Characterization of duplicated zebrafish cyp19 genes [J]. The Journal of Experimental Zoology, 2001, 290(7): 709-714
[56] Tang H P, Chen Y, Liu Y, et al. New insights into the role of estrogens in male fertility based on findings in aromatase-deficient zebrafish [J]. Endocrinology, 2017, 158(9): 3042-3054
[57] Hossain M S, Larsson A, Scherbak N, et al. Zebrafish androgen receptor: Isolation, molecular, and biochemical characterization [J]. Biology of Reproduction, 2008, 78(2): 361-369
[58] Crowder C M, Lassiter C S, Gorelick D A. Nuclear androgen receptor regulates testes organization and oocyte maturation in zebrafish [J]. Endocrinology, 2018, 159(2): 980-993
[59] Chen Y, Tang H P, Wang L, et al. Fertility enhancement but premature ovarian failure in esr1-deficient female zebrafish [J]. Frontiers in Endocrinology, 2018, 9: 567
[60] Hou L P, Shu H, Lin L L, et al. Modulation of transcription of genes related to the hypothalamic-pituitary-gonadal and the hypothalamic-pituitary-adrenal axes in zebrafish (Danio rerio) embryos/larvae by androstenedione [J]. Ecotoxicology and Environmental Safety, 2018, 156: 403-408
[61] Brion F, Tyler C R, Palazzi X, et al. Impacts of 17beta-estradiol, including environmentally relevant concentrations, on reproduction after exposure during embryo-larval-, juvenile- and adult-life stages in zebrafish (Danio rerio) [J]. Aquatic Toxicology, 2004, 68(3): 193-217
[62] Huang C B, Li X D. Maternal cypermethrin exposure during the perinatal period impairs testicular development in C57BL male offspring [J]. PLoS One, 2014, 9(5): e96781