2-乙基己基磷酸二苯酯(2-ethylhexyl diphenyl phosphate, EHDPP)是一种具有磷酸酯主链结构的有机磷阻燃剂,被广泛用于照相胶片、黏合剂、油漆、橡胶和食品包装等各种产品中[1]。作为聚合物的添加剂,EHDPP不与产品化学结合,因此在产品制造、使用和处置过程中很容易释放到环境中[2]。由于EHDPP的高消耗量和易从相关产品中释放,其在各种环境样本中普遍存在,例如水、空气、灰尘、沉积物、生物体、食物甚至人体中[3-9]。我国吉林省松花江的地表水中EHDPP浓度为94 ng·L-1[10]。Liu等[11]和Xing等[12]对中国太湖地表水检测发现,EHDPP检出率分别为88%和100%,平均浓度为1.9 ng·L-1和2.8 ng·L-1。中国辽河表层沉积物中EHDPP检出率为100%,浓度为3.35 ng·g-1(以单位干质量计),且是引起该地区生态风险的主要贡献者[6]。瑞典野生鱼类中EHDPP浓度高达14 000 ng·g-1(以单位脂质量计) [13]。我国北京市50名孕妇的蜕膜和绒毛膜样品中,EHDPP检出率分别为100%与96%,平均浓度分别为18.0 ng·g-1(以单位干质量计)和44.4 ng·g-1(以单位干质量计)[14]。
毒理学研究表明,EHDPP暴露会在各种生物体或细胞系中引起神经毒性[15-16]、生殖毒性[17-18]及内分泌干扰效应[19-20]。最近,体外研究发现EHDPP可能通过干扰甲状腺激素与转运蛋白结合影响甲状腺激素稳态。Zhao等[21]使用荧光分析法发现EHDPP和甲状腺激素转运蛋白有明显结合能力(IC50为8 340 nmol·L-1);另有研究表明,0.1、1和10 μmol·L-1 EHDPP暴露鸡胚肝细胞36 h,甲状腺激素转运蛋白基因(transthyretin, ttr)浓度依赖式显著下调[14]。此外,环境中检出率较高的其他有机磷阻燃剂,如磷酸三(1,3-二氯异丙基)酯(tris(1,3-dichloro-2-propyl)phosphate, TDCPP)[22]、磷酸三苯酯(triphenyl phosphate, TPHP)[23]、磷酸三(2-丁氧基乙基)酯(tris (2-butoxyethyl) phosphate, TBOEP)[24]、磷酸三(2-氯丙基)酯(tris (1-chloro-2-propyl) phosphate, TCIPP)[25]已被证实会抑制斑马鱼生长,干扰甲状腺激素生成,引起甲状腺功能紊乱,具有内分泌干扰效应。但是目前还没有EHDPP对甲状腺激素影响的体内研究。此外,大量研究报道了母源性EHDPP暴露会导致子代发育毒性。如雌性日本青鳉暴露于环境浓度EHDPP(405 ng·L-1和1 161 ng·L-1)后,在子代胚胎中检测出高于母体肝、脑、肌肉和卵巢组织的EHDPP含量,并且子代仔鱼眼部发育异常[26]。Yan等[27]研究发现围产期小鼠灌喂EHDPP(300 μg·kg-1·d-1)导致子代雄性体质量下降、糖耐量紊乱,梭菌丰度增加,糖脂代谢通路相关基因下调。然而,甲状腺功能紊乱是否可以通过母体EHDPP暴露转移到后代仍不清楚。
甲状腺激素(thyroid hormones, TH)包括2种形式,甲状腺素(thyroxin, T4)和具有生物活性的三碘甲状腺原氨酸(triiodothyronin, T3),其是影响多种生理过程的关键激素,包括神经发育、代谢、生殖、体温调节、心脏功能和组织修复[28]。在鱼类中,垂体分泌的促甲状腺激素(hyroid-stimulating hormone, TSH)刺激甲状腺激素的合成和释放,TSH主要由下丘脑通过促肾上腺皮质激素释放激素(corticotropin-releasing hormone, CRH)调控[29]。下丘脑-垂体-甲状腺(hypothalamic-pituitary-thyroid, HPT)轴的一些重要基因调控着甲状腺激素分泌、合成、转运、代谢和循环[30]。甲状腺激素的生物合成主要发生在甲状腺滤泡细胞中,在TSH的刺激下,甲状腺滤泡上皮细胞膜上的钠碘转运体(sodium/iodide symporter, NIS)从血液中摄取I-,继而I-被甲状腺过氧化物酶(thyroid peroxidase, TPO)氧化成活性碘;在甲状腺滤泡腔中,活性碘与甲状腺球蛋白(thyroglobulin, TG)酪氨酸残基结合后通过缩合反应形成T3和T4;进入血液的T3和T4经甲状腺激素转运蛋白运送至外周组织;在外周组织中,T4在脱碘酶(deiodinase 1, DIO1和deiodinase 2, DIO2)外环脱碘作用下转换成更具活性的T3;T3与甲状腺激素受体(hyroid receptor α, TRα和thyroid receptor β, TRβ)结合,激活相应的生物学效应;除脱碘酶转化代谢外,甲状腺激素还可以在肝脏中通过硫酸化和葡萄糖醛酸化被降解[31]。
本研究中,将斑马鱼从卵裂期(2 hpf)至120 d暴露于环境相关浓度(0、1和10 μg·L-1) EHDPP,通过检测亲代斑马鱼(F0)血清中TH水平和HPT轴基因表达情况,以及F1代仔鱼体内TH水平和HPT轴相关基因表达情况,初步探究了环境浓度EHDPP长期暴露对成鱼甲状腺内分泌干扰效应及传递毒性。研究结果为全面评估EHDPP的甲状腺内分泌干扰效应提供了理论依据,对其他有机磷阻燃剂导致甲状腺激素紊乱的机制研究也有参考意义。
1 材料与方法(Materials and methods)
1.1 实验材料
2-乙基己基二苯基磷酸酯(EHDPP)纯度>90%,购自梯希爱化成工业发展有限公司(上海,中国)。用于稀释和储备EHDPP的二甲基亚砜(dimethyl sulfoxide, DMSO)纯度>99%,购自Sigma-Aldrich公司(密苏里州圣路易斯,美国)。Trizol试剂、PrimeScriPt®RT reagent试剂盒、SYBR®Real-Time PCR Master购自Takara公司(大连,中国)。甲状腺素(T4)检测试剂盒和三碘甲状腺原氨酸(T3)检测试剂盒购自武汉云克隆科技有限公司(武汉,中国),基因引物购自中国上海生工公司(上海,中国),丰年虫购自中国天津红太阳水产养殖股份有限公司(天津,中国),其他化学试剂均为分析纯级别。
1.2 斑马鱼的饲养及暴露实验
实验过程符合斑马鱼的伦理规范,按照丁希胜等[32]的方法进行标准饲养和暴露实验。斑马鱼胚胎(野生型AB品系)购于国家斑马鱼中心。基于之前的研究,5 μg·L-1 EHDPP暴露成年斑马鱼21 d对生长没有产生显著影响[33],选择同一数量级的1 μg·L-1作为EHDPP最低暴露浓度。选取发育正常的卵裂期胚胎(2 hpf),随机分配至含有0、1和10 μg·L-1 EHDPP暴露液的培养皿中,每个皿放置100枚胚胎,每个浓度设置3个平行,所有暴露组DMSO浓度为0.001% (V/V)。将培养皿置于光照培养箱中,5 d后将孵化出的仔鱼转移到25 L的鱼缸内,每24 h全部更换一次暴露液。光暗周期为14 h∶10 h,水温稳定在(28±0.5) ℃,每日投喂2次丰年虫。
暴露120 d后,随机选择4尾雌鱼,4尾雄鱼,以1∶1的比例将其混在一起产卵,将发育正常的胚胎转移到直径90 mm的培养皿中,每个培养皿放置50枚胚胎,清水培养至120 hpf。每天清理卵膜及死掉的胚胎,统计120 hpf存活率,每个浓度随机选取30尾仔鱼分别在72 hpf和120 hpf统计心率和体长。
测量成鱼体长、体质量后,用0.03%间氨基苯甲酸乙酯甲磺酸盐(0.03% MS-222)将其麻醉,断尾取血,收集血清检测甲状腺激素;解剖斑马鱼取组织样品(脑、肝和性腺),称量质量计算脑体质常数(brain size index, BSI)、肝体质常数(hepatosomatic index, HSI)和性腺体质常数(gonadosomatic index, GSI)。
1.3 成鱼和仔鱼甲状腺激素的检测
暴露结束后,收集成鱼的血于200 μL离心管中,静置5 min后置于离心机,5 000×g,离心5 min,同一性别5尾鱼的血清合计10 μL为一个样品,-80 ℃保存。收集200尾仔鱼作为一个平行样本,每个样本加入0.25 mL的样品稀释液后用组织匀浆破碎仪匀浆。冰上超声破碎5 min,随后4 ℃,5 000×g,离心10 min,取上清于-80 ℃备用。成鱼血清和仔鱼体内甲状腺激素的检测严格按照酶联免疫吸附试验法(ELISA)说明书进行操作。T3和T4的检测限分别为50.5 pg·mL-1和1.29 ng·mL-1,组内和组间检测变异系数分别低于10%和12%。
1.4 实时荧光定量PCR (qRT-PCR)
斑马鱼暴露120 d后,收集F0代肝脏、脑(包括下丘脑和垂体)(同一性别3个肝脏或脑作为1个样本)和F1代仔鱼(30尾作为一个样本)测定基因表达,每个样本3个重复。RNA提取、纯化、cDNA合成,实时荧光定量PCR以及引物序列等参考试剂盒说明书和Yu等[34]的文章,引物序列见表1。
表1 下丘脑-垂体-甲状腺(HPT)轴相关基因的引物序列信息
Table 1 Primers sequences for tested genes along hypothalamic-pituitary-thyroid (HPT) axis

基因名称Gene name正义链序列(5’~3’)Sense strand (5’~3’)反义链序列(5’~3’)Antisense strand (5’~3’)基因登录号Gene IDgapdhCTGGTGACCCGTGCTGCTTTTTGCCGCCTTCTGCCTTANM_001115114tgCCAGCCGAAAGGATAGAGTTGATACTGCCGTGGAATAGGAXM_001335283nisGGTGGCATGAAGGCTGTAATGATACGGGATCCATTGTTGGNM_001089391crhTTCGGGAAGTAACCACAAGCCTGCACTCTATTCGCCTTCCNM_001007379tshβGCAGATCCTCACTTCACCTACCGCACAGGTTTGGAGCATCTCAAY135147nkx2.1AGGACGGTAAACCGTGTCAGCACCATGCTGCTCGTGTACTNM_131589pax8GAAGATCGCGGAGTACAAGCCTGCACTTTAGTGCGGATGAAF072549trαCTATGAACAGCACATCCGACAAGAGCACACCACACACGGCTCATCNM_131396trβTGGGAGATGATACGGGTTGTATAGGTGCCGATCCAATGTCNM_131340ttrCGGGTGGAGTTTGACACTTTGCTCAGAAGGAGAGCCAGTABC081488dio1GTTCAAACAGCTTGTCAAGGACTAGCAAGCCTCTCCTCCAAGTTBC076008dio2GCATAGGCAGTCGCTCATTTTGTGGTCTCTCATCCAACCANM_212789ugt1abCCACCAAGTCTTTCCGTGTTGCAGTCCTTCACAGGCTTTCNM_213422
1.5 数据处理与分析
所有实验组设置3个平行,数据分析使用SPSS Statistics 20.0软件(IBM CorP., Armonk,NY, USA)。实验数据分别使用Kolmogorov-Smirnov和Levene’s检验方法进行正态分布检验和方差齐性检验。通过单因素方差分析(one-way analysis of variance, ANOVA)评估对照组和实验组间的差异,采用Tukey’s比较法后检验,数据表示为平均值±标准误(Mean±SEM),P<0.05为显著性差异。
2 结果(Results)
2.1 F0代和F1代生长发育终点指标
EHDPP暴露120 d对F0代孵化率和存活率的影响如表2所示。与对照组相比,10 μg·L-1 EHDPP暴露组孵化率显著降低8.84%(P<0.05)。在1 μg·L-1和10 μg·L-1 EHDPP暴露组,存活率相较于对照组分别显著降低15.04%和19.91%(P<0.05,P<0.01)。
表2 0、1、10 μg·L-1 2-乙基己基磷酸二苯酯(EHDPP)暴露120 d F0代斑马鱼孵化率和存活率
Table 2 Hatching rate and survival rate in F0 zebrafish exposed to 0, 1 and 10 μg·L-1 2-ethylhexyl diphenyl phosphate (EHDPP) for 120 d
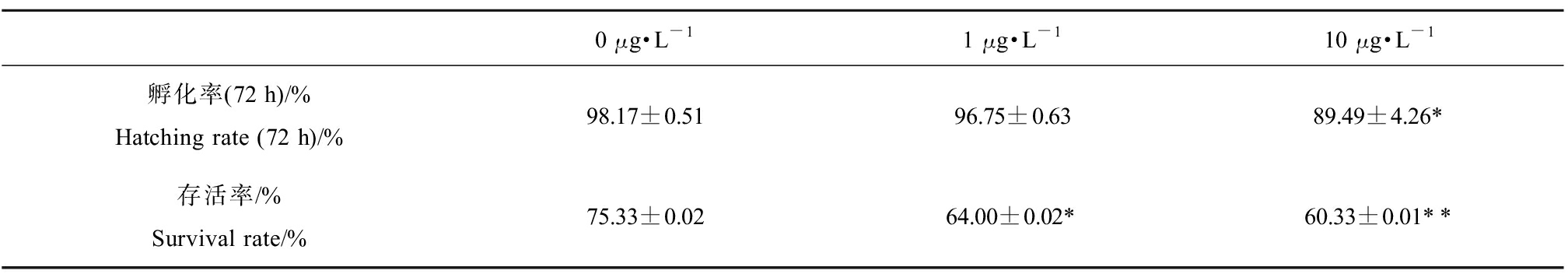
0 μg·L-11 μg·L-110 μg·L-1孵化率(72 h)/%Hatching rate (72 h)/%98.17±0.5196.75±0.6389.49±4.26*存活率/%Survival rate/%75.33±0.0264.00±0.02*60.33±0.01**
注:每个浓度3个平行,每个平行100枚胚胎;实验数据以平均值±标准误表示(mean±SEM);*表示暴露组与对照组之间具有显著性差异(*P<0.05、**P<0.01)。
Note: There were three replicates and each group contained 100 embryos; experimental data expressed as mean±standard error of mean (mean±SEM); asterisk indicated significant difference between exposed group and control group (*P<0.05, **P<0.01).
EHDPP对斑马鱼成鱼生长发育的影响如表3所示。与对照组相比,雌鱼体长无显著变化,10 μg·L-1 EHDPP暴露组雄鱼体长显著降低6.68%(P<0.05);雌鱼体质量在10 μg·L-1 EHDPP暴露组显著下降21.21%(P<0.05),雄鱼体质量在1 μg·L-1和10 μg·L-1 EHDPP暴露组分别显著降低20.59%和26.47%(P<0.05,P<0.01)。在雌鱼中,与对照组相比,BSI无显著变化,HSI和GSI在10 μg·L-1 EHDPP暴露组显著降低(20.00%和33.97%)(P<0.05,P<0.01)。在雄鱼中,与对照组相比,BSI在1 μg·L-1和10 μg·L-1 EHDPP暴露组分别显著降低16.81%和18.14%(P<0.05,P<0.05),HSI在1 μg·L-1和10 μg·L-1 EHDPP暴露组分别显著降低29.89%和38.04%(P<0.05,P<0.05),GSI在1 μg·L-1和10 μg·L-1 EHDPP暴露组分别显著降低37.39%和48.70%(P<0.01,P<0.01)。
表3 EHDPP(0、1、10 μg·L-1)暴露120 d对F0代斑马鱼体质指数的影响
Table 3 Somatic indices in F0 zebrafish after exposure to EHDPP (0, 1, 10 μg·L-1) for 120 d
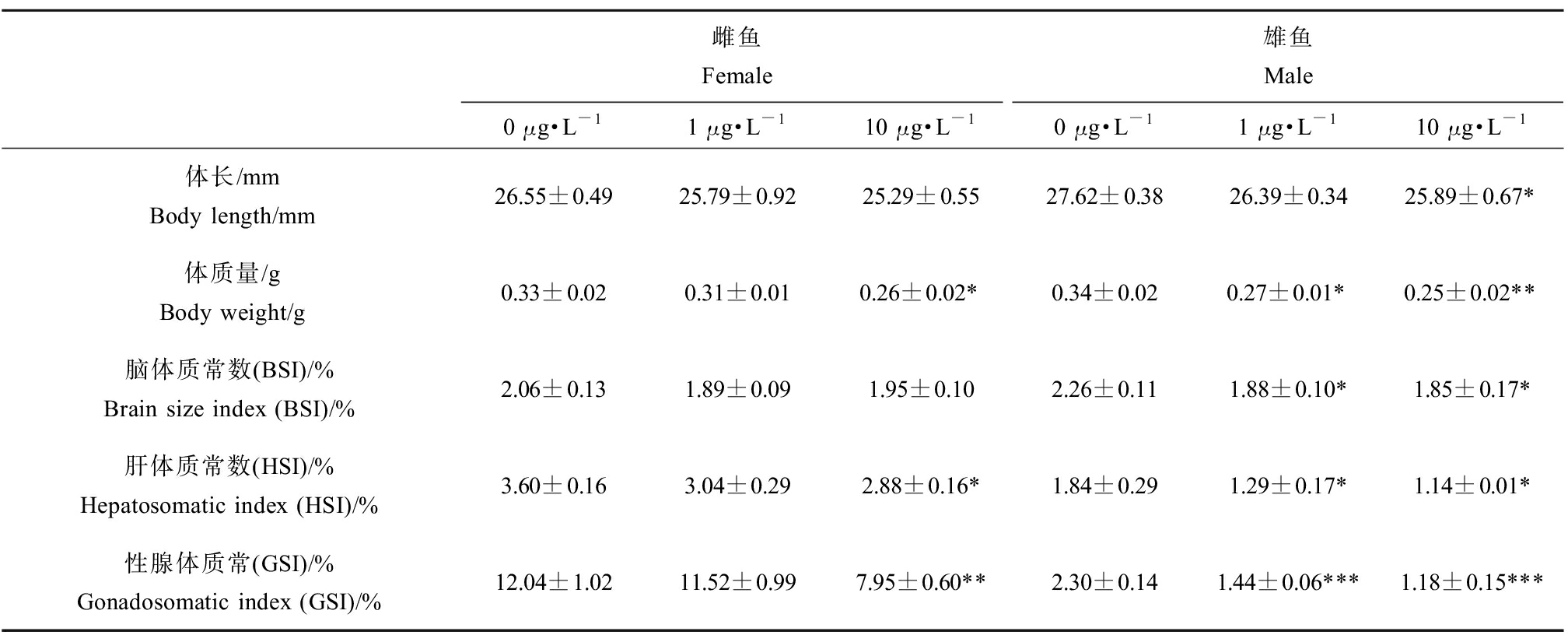
雌鱼Female雄鱼Male0 μg·L-11 μg·L-110 μg·L-10 μg·L-11 μg·L-110 μg·L-1体长/mmBody length/mm26.55±0.4925.79±0.9225.29±0.5527.62±0.3826.39±0.3425.89±0.67*体质量/gBody weight/g0.33±0.020.31±0.010.26±0.02*0.34±0.020.27±0.01*0.25±0.02**脑体质常数(BSI)/%Brain size index (BSI)/%2.06±0.131.89±0.091.95±0.102.26±0.111.88±0.10*1.85±0.17*肝体质常数(HSI)/%Hepatosomatic index (HSI)/%3.60±0.163.04±0.292.88±0.16*1.84±0.291.29±0.17*1.14±0.01*性腺体质常(GSI)/%Gonadosomatic index (GSI)/%12.04±1.0211.52±0.997.95±0.60**2.30±0.141.44±0.06***1.18±0.15***
注:BSI=100%×脑质量/体质量,HSI=100%×肝脏质量/体质量,GSI=100%×性腺质量/体质量;每个浓度3个平行,每个平行至少10个数据;实验数据以平均值±标准误表示(mean±SEM);*表示暴露组与对照组之间具有显著性差异(*P<0.05、**P<0.01、***P<0.001)。
Note: BSI=100%×brain weight/body weight, HSI=100%×liver weight/body weight, GSI=100%×gonad weight/body weight; three replicates per concentration, with at least 10 data per replicates; experimental data expressed as mean±standard error of mean (mean±SEM); asterisk indicated significant difference between exposed group and control group (*P<0.05, **P<0.01, ***P<0.001).
EHDPP对F1代仔鱼生长发育的影响如表4所示,与对照组相比,F1代仔鱼心率、体长和存活率无显著差异。
表4 母源性EHDPP(0、1、10 μg·L-1)暴露120 d对F1代仔鱼心率、体长和存活率的影响
Table 4 Heart rate, body length and survival rate in F1 larvae derived from parental exposure to EHDPP (0, 1, 10 μg·L-1) for 120 d
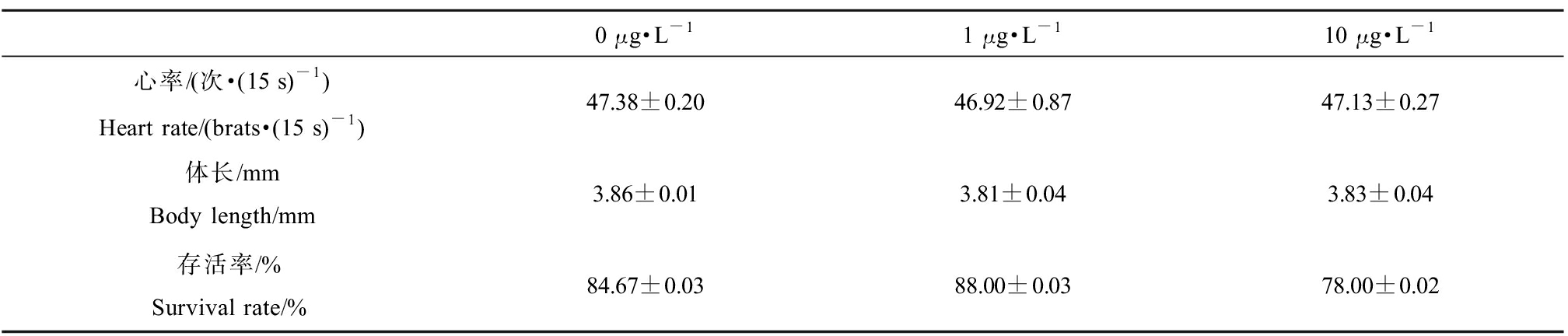
0 μg·L-11 μg·L-110 μg·L-1心率/(次·(15 s)-1)Heart rate/(brats·(15 s)-1)47.38±0.2046.92±0.8747.13±0.27体长/mmBody length/mm3.86±0.013.81±0.043.83±0.04存活率/%Survival rate/%84.67±0.0388.00±0.0378.00±0.02
注:每个浓度3个平行,心率与体长每个平行10尾鱼数据,存活率每个平行共50尾仔鱼;实验数据以平均值±标准误表示(mean±SEM)。
Note: Three replicates per concentration, 10 larvae per replicate for heart rate and body length, and a total of 50 larvae per replicate for survival rate; experimental data expressed as mean±standard error of mean (mean±SEM).
2.2 F0代和F1代甲状腺激素含量变化
如表5所示,在F0代斑马鱼中,与对照组相比,雌鱼血清中总T4水平在10 μg·L-1 EHDPP暴露组显著降低22.30%(P<0.05),雄鱼血清中总T4水平在1 μg·L-1 EHDPP暴露组显著降低34.31%(P<0.05);雌鱼、雄鱼血清中总T3水平相较于对照组均无显著差异。F1代仔鱼中,10 μg·L-1 EHDPP亲代暴露组总T4水平与对照组相比显著降低37.52%(P<0.05),1 μg·L-1 EHDPP亲代暴露组总T3水平与对照组相比显著降低16.57%(P<0.05)。
表5 EHDPP(0、1、10 μg·L-1)暴露120 d对F0成年斑马鱼和未暴露F1代仔鱼总甲状腺素(T4)和三碘甲状腺原氨酸(T3)水平的影响
Table 5 Total thyroxine (T4) and triiodothyronin (T3) levels in F0 adult zebrafish after exposed to EHDPP (0, 1, 10 μg·L-1) for 120 d and in the F1 larvae derived from parental zebrafish exposed to EHDPP
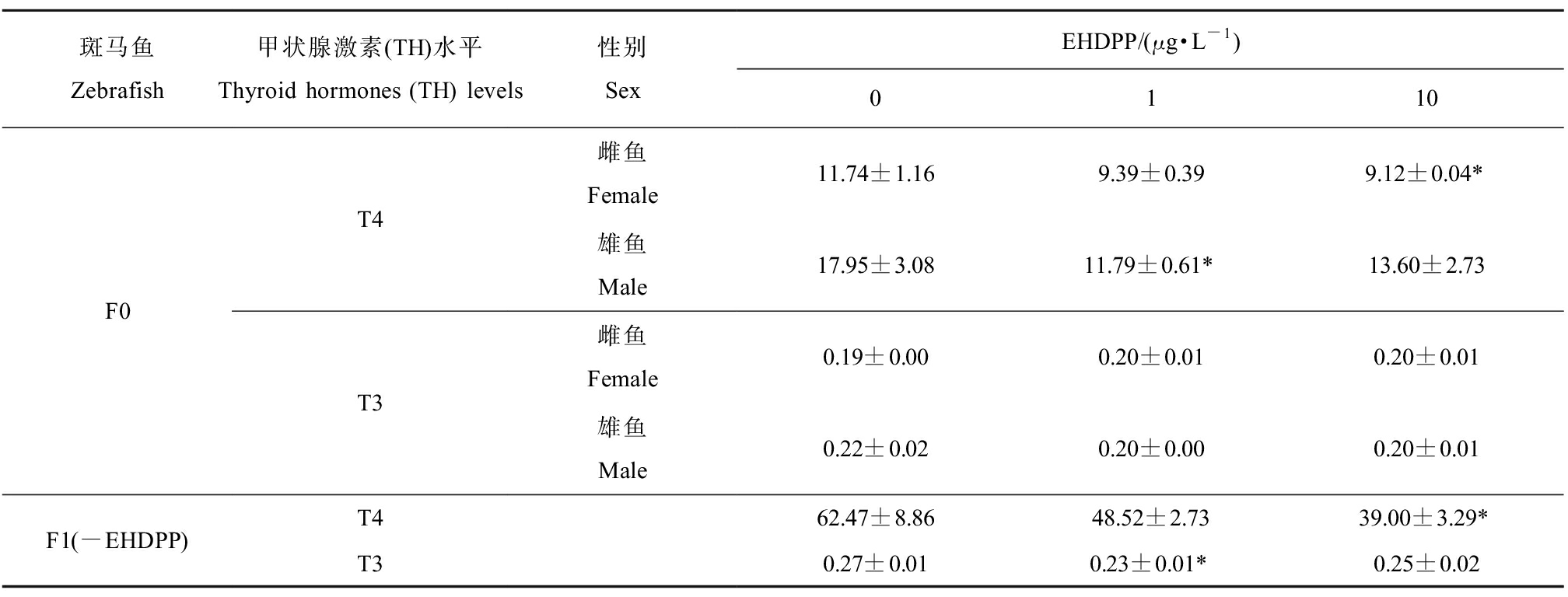
斑马鱼Zebrafish甲状腺激素(TH)水平Thyroid hormones (TH) levels性别SexEHDPP/(μg·L-1)0110F0T4T3雌鱼Female11.74±1.169.39±0.399.12±0.04*雄鱼Male17.95±3.0811.79±0.61*13.60±2.73雌鱼Female0.19±0.000.20±0.010.20±0.01雄鱼Male0.22±0.020.20±0.000.20±0.01F1(-EHDPP)T462.47±8.8648.52±2.7339.00±3.29*T30.27±0.010.23±0.01*0.25±0.02
注:T4和T3水平单位为ng·mL-1;对于成年斑马鱼,同一性别5尾鱼的血清为一个样本,每个浓度3个平行;对于F1代仔鱼,200尾仔鱼为一个样本,每个浓度3个平行;每个样本做2个技术重复;实验数据以平均值±标准误表示(mean±SEM);*表示暴露组与对照组之间具有显著性差异(*P<0.05)。
Note: The T4 and T3 levels were expressed as ng·mL-1; for the adult zebrafish, serum samples from five individual fish were pooled and measured with 3 replicates; for the F1 generation, 200 larvae were measured with 3 replicates; two technique duplications were performed for each sample; experimental data expressed as mean±standard error of mean (mean±SEM); asterisk indicated significant difference between exposed group and control group (*P<0.05).
2.3 F0代和F1代HPT轴基因表达量
如图1(a)所示,在雌鱼脑中,与对照组相比,crh基因在1 μg·L-1和10 μg·L-1 EHDPP暴露组显著下调,分别下调3.77倍和7.58倍;tshβ基因在10 μg·L-1 EHDPP暴露组显著上调(2.33倍)。如图1(b)所示,在雄鱼脑中,crh基因和tshβ基因表达水平相较于对照组无显著差异。
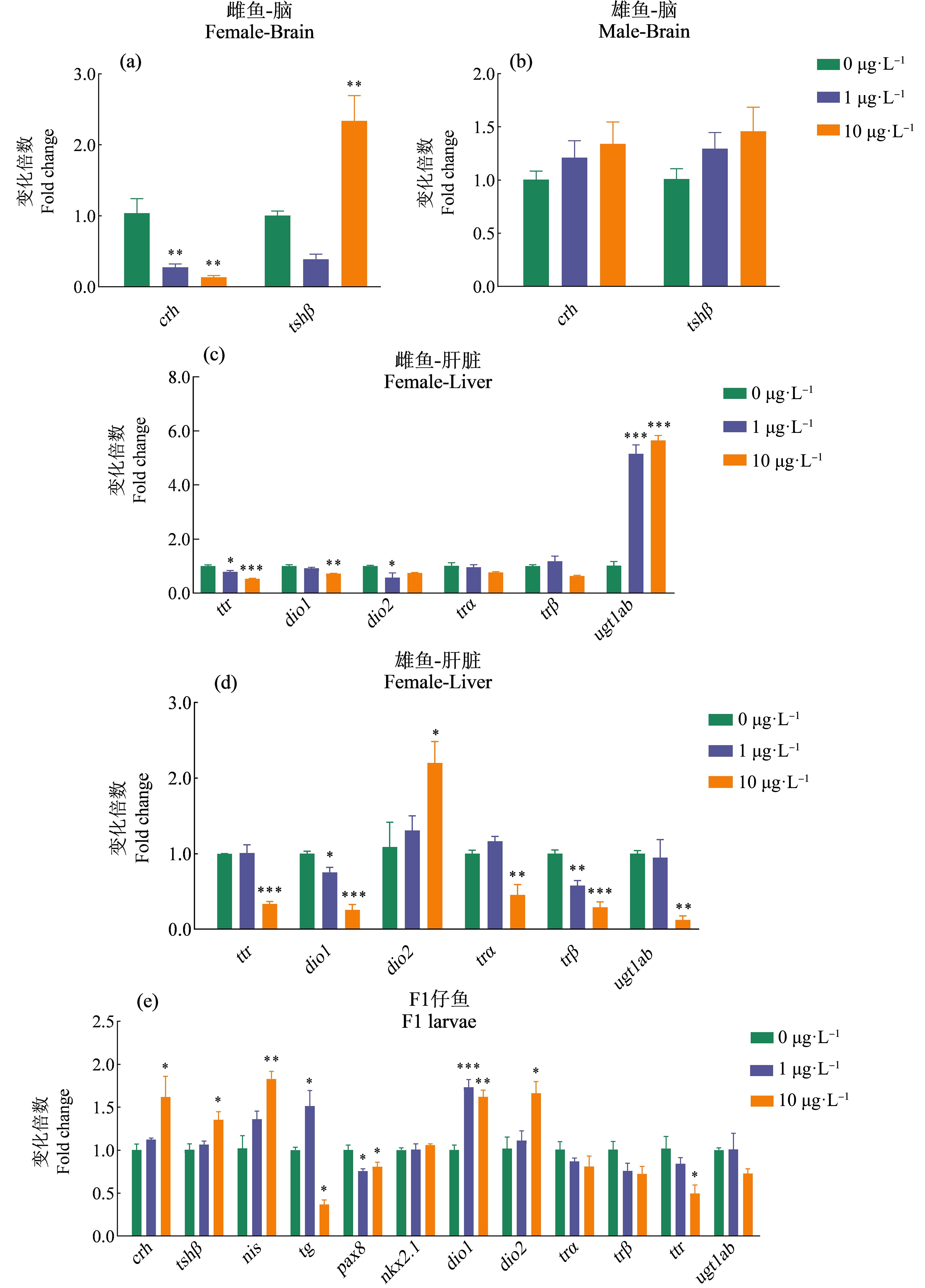
图1 EHDPP(0、1、10 μg·L-1)暴露120 d对F0成年斑马鱼和未暴露F1代仔鱼HPT轴基因表达的影响
注:对于成年斑马鱼,同一性别的3个脑或肝为一个样本,每个浓度3个平行;对于F1代仔鱼,30尾仔鱼为一个样本,每个浓度3个平行;实验数据以平均值±标准误表示(mean±SEM);*表示暴露组与对照组之间具有显著性差异(*P<0.05、**P<0.01、***P<0.001)。
Fig. 1 Gene transcription of HPT axis in F0 adult zebrafish after exposed to EHDPP (0, 1, 10 μg·L-1) for 120 d and in the F1 larvae derived from parental zebrafish exposed to EHDPP
Note: For the adult zebrafish, brain or liver from three individual fish were pooled and measured with 3 replicates; for the F1 larvae, 30 larvae were measured with 3 replicates; experimental data expressed as mean±standard error of mean (mean±SEM); asterisk indicated significant difference between exposed group and control group (*P<0.05, **P<0.01, ***P<0.001).
如图1(c)所示,在雌鱼肝脏中,与对照组相比,ttr基因在1 μg·L-1和10 μg·L-1 EHDPP暴露组分别显著下调1.27倍和1.88倍;dio1基因在10 μg·L-1 EHDPP暴露组显著下调1.39倍,dio2基因在1 μg·L-1 EHDPP暴露组显著下调1.75倍;trα基因和trβ基因表达量无显著变化;ugt1ab基因在1 μg·L-1和10 μg·L-1 EHDPP暴露组分别显著上调至对照组的5.04倍和5.03倍。
如图1(d)所示,在雄鱼肝脏中,与对照组相比,ttr基因在10 μg·L-1 EHDPP暴露组显著下调(2.97倍);dio1基因在1 μg·L-1和10 μg·L-1 EHDPP暴露
组分别显著下调1.33倍和3.91倍,dio2基因在10 μg·L-1 EHDPP暴露组显著上调2.02倍;trα基因在10 μg·L-1 EHDPP暴露组显著下调2.21倍,trβ基因在1 μg·L-1和10 μg·L-1 EHDPP暴露组均显著下调(1.73倍和3.46倍),ugt1ab基因在10 μg·L-1 EHDPP暴露组显著下调8.07倍。
如图1(e)所示,在F1代仔鱼中,与对照组相比,crh基因和tshβ基因在10 μg·L-1 EHDPP亲代暴露组均显著上调(1.61倍和1.35倍);nis基因在10 μg·L-1 EHDPP亲代暴露组显著上调1.79倍;tg基因在1 μg·L-1 EHDPP亲代暴露组显著上调1.51倍,在10 μg·L-1 EHDPP亲代暴露组显著下调2.73倍;pax8基因在所有EHDPP亲代暴露组均显著下调,分别是对照组的1.33倍和1.24倍;nkx2.1基因相对于对照组无差异;dio1基因在所有EHDPP亲代暴露组均显著上调(1.73倍和1.62倍);dio2基因在10 μg·L-1 EHDPP亲代暴露组显著上调1.64倍;ttr基因在10 μg·L-1 EHDPP亲代暴露组显著下调至对照的2.05倍;ugt1ab基因相较于对照组无显著变化。
3 讨论(Discussion)
EHDPP作为一种应用广泛的有机磷阻燃剂,在各种环境样本中普遍存在。体外实验表明EHDPP显著降低鸡胚肝细胞中甲状腺激素转运相关基因表达[21],但是目前尚未有体内实验表明EHDPP具有甲状腺干扰效应。此外,大量研究发现EHDPP具有影响后代的传递毒性,比如EHDPP能够在青鳉子代胚胎中蓄积,使子代仔鱼眼部发育异常;围产期小鼠暴露EHDPP导致子代雄性体质量降低,诱发糖脂代谢紊乱[26-27],表明了评估EHDPP传递毒性的重要性。因此,本研究将斑马鱼胚胎暴露于环境浓度EHDPP中至性成熟,探究了F0代成鱼以及F1代幼鱼甲状腺激素的变化以及HPT轴基因表达情况,系统评估了长期暴露对鱼类及其子代的甲状腺干扰效应。
EHDPP暴露后,F0代斑马鱼存活率显著降低,雌鱼体质量,雄鱼体长、体质量显著降低,表明EHDPP暴露对斑马鱼产生了显著的生长发育毒性。这和之前阻燃剂的研究结果相似,Wang等[35]研究发现,从2 hpf开始暴露于4、20和100 μg·L-1 TDCPP 6个月,斑马鱼生长受到显著抑制,体质量显著降低。环境相关浓度磷酸二苯酯(diphenyl phosphate, DPhP)长期暴露斑马鱼,雄鱼体质量、体长显著降低[36]。此外,本研究中,EHDPP对斑马鱼的生长发育毒性还体现在体质常数上,雌鱼HSI、GSI,雄鱼BSI、HSI和GSI呈现浓度依赖性显著降低。这与之前关于TDCPP的研究结果类似,0.5 mg·L-1 TDCPP暴露120 d导致雌雄斑马鱼HSI和GSI显著降低[37]。
EHDPP暴露导致斑马鱼雌鱼和雄鱼血清中总T4水平显著降低,T3水平无显著变化,首次在活体实验中表明该化合物具有显著的甲状腺干扰效应。有机磷阻燃剂暴露导致鱼类甲状腺激素降低在之前的研究中也有报道。比如40 μg·L-1 磷酸三(2-氯乙基)酯(tris (2-chloroethyl) phosphate, TCEP)急性暴露导致麦瑞加拉鲮鱼T4和T3显著降低[38]。100 μg·L-1 TDCPP暴露斑马鱼胚胎180 d导致成年雌鱼体内T4和T3显著降低[39]。本研究中,与T3相比,成鱼T4对于EHDPP暴露更敏感。之前的研究也报道了10 μg·L-1 TBOEP暴露10日龄斑马鱼60 d,T4水平显著降低,T3水平无显著变化[40]。此外,一些内分泌干扰化学物质暴露鱼类后对T4水平的影响也远大于T3[41-42]。也有研究表明暴露于有机磷阻燃剂后鱼类TH显著上调或者呈现性别依赖式变化。比如,斑马鱼仔鱼暴露于40 μg·L-1 TPHP 7 d,T4和T3水平显著升高[23]。成年斑马鱼暴露于1 mg·L-1 TDCPP 14 d,雄鱼血浆中T4和T3水平显著降低,雌鱼血浆中T4和T3水平显著升高[43]。因此,TH的变化可能与化合物暴露的方式、时间、浓度和物种的性别有关。甲状腺激素在鱼类正常的生长发育过程中起着至关重要的作用[44]。据报道,暴露于600 μg·L-1 TDCPP显著降低斑马鱼T4和T3水平,并影响其早期生长发育[45]。与TDCPP的研究结果相似,本研究中观察到成鱼明显的生长发育受阻,可能与EHDPP显著降低T4水平有关。
大量的研究发现,甲状腺内分泌紊乱与HPT轴基因异常表达密切相关[46-48]。为探究EHDPP甲状腺干扰效应机制,分析了斑马鱼HPT轴关键基因表达情况。斑马鱼中下丘脑分泌的CRH和垂体分泌的TSH调控着甲状腺滤泡对TH合成与释放,同时,TH对CRH和TSH也有着负反馈调节机制[29]。本研究中,雌鱼crh基因表达显著下调可能使T4分泌受到影响进而造成T4水平下降。雌鱼tshβ基因表达显著上调可能是T4水平降低所产生的负反馈调节,先前的研究发现T4水平降低伴随着tshβ基因表达上调[49]。在雄鱼中,T4水平降低,crh和tshβ基因没有显著变化,暗示EHDPP作用于雄鱼甲状腺内分泌系统的分子机制可能与雌鱼不同。TTR作为一种主要的转运蛋白,可以结合TH并将其转移到靶组织,对维持靶组织中TH平衡起关键作用[50]。本研究中,EHDPP暴露抑制雌鱼ttr基因表达。这一现象在其他阻燃剂中也有被报道,50 μg·L-1 2,2’,4’,4-五溴联苯醚(2,2’,4,4’-pentabromodiphenyl ether, BDE-99)暴露4月龄斑马鱼导致雌鱼T4水平显著降低,ttr基因表达显著下调[51]。有学者提出,ttr基因表达下调可能会引起与T4结合的ttr数量减少,从而导致更多的未结合的T4,这些未结合T4可能更容易被肝脏分解代谢,从而造成斑马鱼体内T4减少[52]。因此,EHDPP可能通过下调ttr基因表达,扰乱T4与TTR的结合从而导致雌鱼T4水平降低。然而,ttr基因显著下调并没有改变雄鱼T4水平,可能的原因是与TH水平相比,基因转录对环境变化更为敏感。类似的,0.08 mg·L-1和0.38 mg·L-1十溴二苯醚(decabrominated diphenyl ethers, BDE-209)暴露斑马鱼胚胎/仔鱼14 d后ttr基因显著下调,T4水平无显著变化[53]。在斑马鱼中,dio1基因对碘的恢复和甲状腺激素的降解有重要的影响,dio2基因催化T4外环脱碘生成生物活性更高的T3[54]。本研究中,dio1基因在雌雄鱼中表达显著下调,dio2基因表达在雌鱼中显著下调而在雄鱼中显著上调。dio1基因表达下调反映出成鱼由于T4含量降低所启动的调节机制[55],通过减少甲状腺激素降解来避免其含量进一步降低。类似的,Zhao等[56]的研究表明,暴露于2,2’,4’,4-四溴二苯醚(2,2’,4,4’-tetrabromodiphenyl ether, BDE-47)后斑马鱼dio1基因表达下调,T4水平降低。事实上,暴露于环境污染物后,斑马鱼脱碘酶基因表达与甲状腺激素含量的作用关系有很大差异。例如,关于邻苯二甲酸单乙基己基酯(mono-(2-ethylhexyl) phthalate, MEHP)的研究结果表明,dio1和dio2基因上调,T4水平降低[57]。另外一项2 000 μg·L-1 TBOEP对斑马鱼的暴露数据显示,dio1和dio2基因显著下调,T4水平显著升高[58]。因此,脱碘酶和甲状腺激素之间的作用关系还需要进一步研究。TH通过与甲状腺激素受体(TRs)结合发挥其生物活性,TRs有2种受体亚型,trα和trβ。本研究的数据显示,1 μg·L-1 EHDPP暴露引起雄鱼T4含量显著降低,trβ基因显著下调,trα基因表达水平无明显改变,表明雄鱼trβ基因比trα基因更有效地响应TH含量变化。10 μg·L-1 EHDPP暴露后,雄鱼TH无显著变化,trα和trβ基因转录水平显著下调,可能是trα和trβ基因水平比激素水平对EHDPP暴露更为敏感,这与100 μg·L-1 2,4,6-三溴苯酚(2,4,6-tribromophel, TBP)急性暴露对斑马鱼仔鱼trα和trβ基因的影响一致[59]。Trα和trβ基因下调可能导致TH无法结合,使下游级联反应激活失败,较少的TH能发挥作用进而抑制雄鱼的生长。研究表明,尿苷二磷酸葡萄糖醛酸转移酶(uridine diphosphate glucuronosyl transferase, UGTs)可能通过增加共轭激素胆汁排泄,催化T4葡萄糖醛酸化,从而部分解释T4水平的降低[60]。据报道,200 μg·L-1 TCEP暴露导致斑马鱼T4显著减少伴随着ugt1ab基因过度表达[61]。与之前的报道一致,本研究也观察到雌鱼ugt1ab基因表达显著上调,这可能会催化T4葡萄糖醛酸化,导致T4水平下降。而雄鱼中,ugt1ab基因在最高浓度组显著降低,TH无显著变化。以前的研究表明,仍然需要对ugt1ab进行功能鉴定,以确定其在斑马鱼TH代谢中的潜在作用[62]。因此,在斑马鱼中观察到的ugt1ab表达和TH含量之间的关联机制需要在未来的研究中加以阐明。
斑马鱼胚胎的特点是体外受精和发育,它们通过卵黄囊接收来自母体的甲状腺激素。孵化后,卵黄囊逐渐被吸收,这一过程在120 dpf前完成,仔鱼从72 hpf开始自身持续合成甲状腺激素,来弥补母源性甲状腺激素的减少[63]。本研究中,与来自对照组成鱼的仔鱼相比,来自长期暴露于EHDPP亲鱼的F1仔鱼中T4和T3含量均显著降低,表明仔鱼甲状腺内分泌系统受到干扰,这可能与EHDPP暴露引起母体甲状腺内分泌紊乱有关,直接影响了仔鱼的甲状腺功能。越来越多的研究证实,传递毒性可能不仅来自转移的污染物,也与一些生理指标的变化有关,比如甲状腺激素[64-66]。有机磷阻燃剂引起亲代TH显著变化导致子代甲状腺内分泌紊乱的研究时有报道。Wang等[67]发现100 μg·L-1 TDCPP母体暴露3个月,F0代雌性斑马鱼T4水平降低,未暴露F1代仔鱼T4水平降低。环境相关浓度TCEP暴露斑马鱼胚胎120 d导致成鱼、子代鱼卵、仔鱼T4水平均显著降低[68]。类似的,最近的一项研究也发现,30 μg·L-1 TBP暴露斑马鱼胚胎60 d导致成鱼及子代仔鱼甲状腺激素水平均显著升高[69]。本研究发现亲代斑马鱼T4水平和F1代仔鱼T4和T3水平均显著降低。综合以上报道,笔者推测EHDPP暴露诱发母体甲状腺内分泌紊乱,从而干扰F1代仔鱼的甲状腺内分泌系统。
大量研究表明,母体污染物暴露降低斑马鱼子代甲状腺激素水平,并改变HPT轴基因表达,检测HPT轴基因表达可以潜在地用于评估污染物对子代THs动态的影响[70-71]。因此,本研究中检测了Fl代仔鱼HPT轴相关基因的表达情况。crh和tshβ基因表达上调可能是T4水平下降对下丘脑和垂体的负反馈。此前也有报道称,300 nmol·L-1 BDE-209降低斑马鱼体内T4水平,上调crh和tshβ基因表达[72]。nis在tsh刺激下通过主动运输将血液中的碘离子转移到甲状腺滤泡细胞[73]。本研究中tshβ基因上调可能刺激了nis基因表达上调,以补偿T4和T3的降低。TG是甲状腺激素的蛋白质前体,在甲状腺激素合成途径中发挥重要作用[74],F1代仔鱼tg基因表达下调提示仔鱼甲状腺激素合成可能受到干扰造成T4减少。对甲状腺发育(nkx2.1和pax8)密切相关基因进行了检测。pax8基因表达在F1代仔鱼中显著降低,表明甲状腺的发育可能被抑制导致T4和T3水平降低。在F1代仔鱼观察到dio1和dio2基因表达显著上调,这与之前的报道一致,即dio1和dio2基因表达增加与斑马鱼仔鱼甲状腺功能的减退有关[75]。dio2基因表达上调催化了T4向T3转化,导致T4水平降低,而增加的dio1基因可能有助于降低增加的T3水平。此外,与本研究中成鱼结果一致,F1仔鱼ttr基因表达被抑制,这可能会增加机体对未结合TH的清除,从而导致仔鱼体内TH水平下降。
综上所述,环境浓度EHDPP长期暴露具有显著的生长发育毒性,表现为存活率、体质量、HSI和GSI均显著性下降。进一步研究发现,EHDPP降低成鱼T4水平,表明EHDPP长期暴露对成鱼产生甲状腺内分泌干扰效应,这可能在一定程度上抑制成鱼生长。此外,F1代仔鱼T3和T4水平显著降低,说明母源性EHDPP暴露可导致仔鱼甲状腺内分泌紊乱,具有母体传递毒性。EHDPP对成鱼及仔鱼的甲状腺内分泌干扰效应可能与HPT轴基因异常表达有关。雌鱼甲状腺激素分泌相关基因(crh)显著下调、代谢基因(ugt1ab)显著上调,提示EHDPP可能抑制T4分泌,催化T4葡萄糖醛酸化,降低雌鱼T4水平;甲状腺激素转运相关基因(ttr)显著下调表明EHDPP可能通过干扰T4与TTR结合导致T4降低。雄鱼甲状腺激素受体(trα和trβ)显著下调,表明EHDPP可能降低甲状腺激素受体活性,较少的甲状腺激素能结合受体发挥作用从而抑制雄鱼生长;F1代仔鱼甲状腺激素降低可能不仅仅是甲状腺激素合成相关基因(tg)和早期甲状腺发育基因(pax8)显著下调,脱碘酶基因(deiodinase 1, dio1和deiodinase 2, dio2)表达显著上调的结果,还可能与亲代甲状腺内分泌紊乱有直接关系。本研究首次在活体实验中表明EHDPP具有显著的甲状腺干扰效应,尤为值得注意的是,长期暴露于EHDPP会对未暴露子代产生甲状腺内分泌干扰效应,研究结果对加强EHDPP毒理学认识提供了数据补充。在后续的研究中,我们将从表观遗传学角度和亲本暴露化学品传递角度深入揭示母源性EHDPP暴露对子代甲状腺干扰的机制。
[1] Brooke D N, Crookes M J, Quarterman P, et al. Environmental risk evaluation report: 2-ethylhexyl diphenyl phosphate (CAS No. 1241-94-7) [R]. Bristol: Environment Agency, 2009: 97
[2] Wong F, de Wit C A, Newton S R. Concentrations and variability of organophosphate esters, halogenated flame retardants, and polybrominated diphenyl ethers in indoor and outdoor air in Stockholm, Sweden [J]. Environmental Pollution, 2018, 240: 514-522
[3] Cristale J, García Vázquez A, Barata C, et al. Priority and emerging flame retardants in rivers: Occurrence in water and sediment, Daphnia magna toxicity and risk assessment [J]. Environment International, 2013, 59: 232-243
[4] Christia C, Poma G, Besis A, et al. Legacy and emerging organophosphοrus flame retardants in car dust from Greece: Implications for human exposure [J]. Chemosphere, 2018, 196: 231-239
[5] Yadav I C, Devi N L, Singh V K, et al. Measurement of legacy and emerging flame retardants in indoor dust from a rural village (Kopawa) in Nepal: Implication for source apportionment and health risk assessment [J]. Ecotoxicology and Environmental Safety, 2019, 168: 304-314
[6] Luo Q, Gu L Y, Wu Z P, et al. Distribution, source apportionment and ecological risks of organophosphate esters in surface sediments from the Liao River, Northeast China [J]. Chemosphere, 2020, 250: 126297
[7] Hallanger I G, Sagerup K, Evenset A, et al. Organophosphorous flame retardants in biota from Svalbard, Norway [J]. Marine Pollution Bulletin, 2015, 101(1): 442-447
[8] Zhao L M, Jian K, Su H J, et al. Organophosphate esters (OPEs) in Chinese foodstuffs: Dietary intake estimation via a market basket method, and suspect screening using high-resolution mass spectrometry [J]. Environment International, 2019, 128: 343-352
[9] Zhao F R, Wan Y, Zhao H Q, et al. Levels of blood organophosphorus flame retardants and association with changes in human sphingolipid homeostasis [J]. Environmental Science &Technology, 2016, 50(16): 8896-8903
[10] Wang X W, Liu J F, Yin Y G. Development of an ultra-high-performance liquid chromatography-tandem mass spectrometry method for high throughput determination of organophosphorus flame retardants in environmental water [J]. Journal of Chromatography A, 2011, 1218(38): 6705-6711
[11] Liu Y H, Song N H, Guo R X, et al. Occurrence and partitioning behavior of organophosphate esters in surface water and sediment of a shallow Chinese freshwater lake (Taihu Lake): Implication for eco-toxicity risk [J]. Chemosphere, 2018, 202: 255-263
[12] Xing L Q, Zhang Q, Sun X, et al. Occurrence, distribution and risk assessment of organophosphate esters in surface water and sediment from a shallow freshwater Lake, China [J]. Science of the Total Environment, 2018, 636: 632-640
[13] Sundkvist A M, Olofsson U, Haglund P. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk [J]. Journal of Environmental Monitoring, 2010, 12(4): 943-951
[14] Shen J Y, Zhang Y Y, Yu N Y, et al. Organophosphate ester, 2-ethylhexyl diphenyl phosphate (EHDPP), elicits cytotoxic and transcriptomic effects in chicken embryonic hepatocytes and its biotransformation profile compared to humans [J]. Environmental Science &Technology, 2019, 53(4): 2151-2160
[15] Alzualde A, Behl M, Sipes N S, et al. Toxicity profiling of flame retardants in zebrafish embryos using a battery of assays for developmental toxicity, neurotoxicity, cardiotoxicity and hepatotoxicity toward human relevance [J]. Neurotoxicology and Teratology, 2018, 70: 40-50
[16] 顾杰, 吴江, 王宏烨, 等. 有机磷酸酯对斑马鱼的早期神经毒性作用研究[J]. 生态毒理学报, 2019, 14(5): 152-158
Gu J, Wu J, Wang H Y, et al. Neurotoxicity of organophosphate esters on the early life stages of zebrafish [J]. Asian Journal of Ecotoxicology, 2019, 14(5): 152-158 (in Chinese)
[17] Hu W X, Gao F M, Zhang H, et al. Activation of peroxisome proliferator-activated receptor gamma and disruption of progesterone synthesis of 2-ethylhexyl diphenyl phosphate in human placental choriocarcinoma cells: Comparison with triphenyl phosphate [J]. Environmental Science &Technology, 2017, 51(7): 4061-4068
[18] Li Y, Kang Q Y, Chen R C, et al. 2-ethylhexyl diphenyl phosphate and its hydroxylated metabolites are anti-androgenic and cause adverse reproductive outcomes in male Japanese medaka (Oryzias latipes) [J]. Environmental Science &Technology, 2020, 54(14): 8919-8925
[19] Schang G, Robaire B, Hales B F. Organophosphate flame retardants act as endocrine-disrupting chemicals in MA-10 mouse tumor leydig cells [J]. Toxicological Sciences, 2016, 150(2): 499-509
[20] Cao L Y, Ren X M, Li C H, et al. Organophosphate esters bind to and inhibit estrogen-related receptorγ in cells [J]. Environmental Science &Technology Letters, 2018, 5(2): 68-73
[21] Zhao F R, Chen M, Gao F M, et al. Organophosphorus flame retardants in pregnant women and their transfer to chorionic villi [J]. Environmental Science &Technology, 2017, 51(11): 6489-6497
[22] Li R W, Yang L H, Han J, et al. Early-life exposure to tris (1,3-dichloro-2-propyl) phosphate caused multigenerational neurodevelopmental toxicity in zebrafish via altering maternal thyroid hormones transfer and epigenetic modifications [J]. Environmental Pollution, 2021, 285: 117471
[23] Kim S, Jung J, Lee I, et al. Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines [J]. Aquatic Toxicology, 2015, 160: 188-196
[24] Ma Z Y, Tang S, Su G Y, et al. Effects of tris (2-butoxyethyl) phosphate (TBOEP) on endocrine axes during development of early life stages of zebrafish (Danio rerio) [J]. Chemosphere, 2016, 144: 1920-1927
[25] Yan Z F, Feng C L, Jin X W, et al. Organophosphate esters cause thyroid dysfunction via multiple signaling pathways in zebrafish brain [J]. Environmental Science and Ecotechnology, 2022, 12: 100198
[26] Li Y, Ma H J, Chen R C, et al. Maternal transfer of 2-ethylhexyl diphenyl phosphate leads to developmental toxicity possibly by blocking the retinoic acid receptor and retinoic X receptor in Japanese medaka (Oryzias latipes) [J]. Environmental Science &Technology, 2021, 55(8): 5056-5064
[27] Yan S, Wang D Z, Teng M M, et al. Perinatal exposure to 2-ethylhexyl diphenyl phosphate (EHDPHP) affected the metabolic homeostasis of male mouse offspring: Unexpected findings help to explain dose- and diet- specific phenomena [J]. Journal of Hazardous Materials, 2020, 388: 122034
[28] Porterfield S P, Hendrich C E. The role of thyroid hormones in prenatal and neonatal neurological development—Current perspectives [J]. Endocrine Reviews, 1993, 14(1): 94-106
[29] DeGroef B, Van der Geyten S, Darras V M, et al. Role of corticotropin-releasing hormone as a thyrotropin-releasing factor in non-mammalian vertebrates [J]. General and Comparative Endocrinology, 2006, 146(1): 62-68
[30] Ortiga-Carvalho T M, Chiamolera M I, Pazos-Moura C C, et al. Hypothalamus-pituitary-thyroid axis [J]. Comprehensive Physiology, 2016, 6(3): 1387-1428
[31] Blanton M L, Specker J L. The hypothalamic-pituitary-thyroid (HPT) axis in fish and its role in fish development and reproduction [J]. Critical Reviews in Toxicology, 2007, 37(1-2): 97-115
[32] 丁希胜, 马徐发, 余丽琴. 环境剂量磷酸三(1,3-二氯异丙基)酯多代暴露对斑马鱼子代仔鱼的神经发育毒性[J]. 生态毒理学报, 2020, 15(2): 50-60
Ding X S, Ma X F, Yu L Q. Neurodevelopmental toxicity of zebrafish offspring after multigenerational exposure to tris(1,3-dichloro-2-propyl) phosphate at environmental concentrations [J]. Asian Journal of Ecotoxicology, 2020, 15(2): 50-60 (in Chinese)
[33] Yang R Y, Wang X, Wang J W, et al. Insights into the sex-dependent reproductive toxicity of 2-ethylhexyl diphenyl phosphate on zebrafish (Danio rerio) [J]. Environment International, 2022, 158: 106928
[34] Yu L Q, Deng J, Shi X J, et al. Exposure to DE-71 alters thyroid hormone levels and gene transcription in the hypothalamic-pituitary-thyroid axis of zebrafish larvae [J]. Aquatic Toxicology, 2010, 97(3): 226-233
[35] Wang Q W, Lam J C, Man Y C, et al. Bioconcentration, metabolism and neurotoxicity of theorganophorous flame retardant 1,3-dichloro 2-propyl phosphate (TDCPP) to zebrafish [J]. Aquatic Toxicology, 2015, 158: 108-115
[36] Chen Q L, Lian X L, An J J, et al. Life cycle exposure to environmentally relevant concentrations of diphenyl phosphate (DPhP) inhibits growth and energy metabolism of zebrafish in a sex-specific manner [J]. Environmental Science &Technology, 2021, 55(19): 13122-13131
[37] Liu X S, Lu X X, Hong J B, et al. Effects of long-term exposure to TDCPP in zebrafish (Danio rerio)—Alternations of hormone balance and gene transcriptions along hypothalamus-pituitary axes [J]. Animal Models and Experimental Medicine, 2022, 5(3): 239-247
[38] Sutha J, Anila P A, Umamaheswari S, et al. Biochemical responses of a freshwater fish Cirrhinus mrigala exposed to tris(2-chloroethyl) phosphate (TCEP) [J]. Environmental Science and Pollution Research International, 2020, 27(27): 34369-34387
[39] Xu T, Wang Q W, Shi Q P, et al. Bioconcentration, metabolism and alterations of thyroid hormones of tris(1,3-dichloro-2-propyl) phosphate (TDCPP) in zebrafish [J]. Environmental Toxicology and Pharmacology, 2015, 40(2): 581-586
[40] Zeng X Y, Sun H, Huang Y Y, et al. Effects of environmentally relevant concentrations of tris (2-butoxyethyl) phosphate on growth and transcription of genes involved in the GH/IGF and HPT axes in zebrafish (Danio rerio) [J]. Chemosphere, 2018, 212: 376-384
[41] Yu L Q, Lam J C W, Guo Y Y, et al. Parental transfer of polybrominated diphenyl ethers (PBDEs) and thyroid endocrine disruption in zebrafish [J]. Environmental Science &Technology, 2011, 45(24): 10652-10659
[42] Liang X, Yu L, Gui W J, et al. Exposure to difenoconazole causes changes of thyroid hormone and gene expression levels in zebrafish larvae [J]. Environmental Toxicology and Pharmacology, 2015, 40(3): 983-987
[43] Liu X S, Cai Y, Wang Y, et al. Effects of tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and triphenyl phosphate (TPP) on sex-dependent alterations of thyroid hormones in adult zebrafish [J]. Ecotoxicology and Environmental Safety, 2019, 170: 25-32
[44] Brown D D. The role of thyroid hormone in zebrafish and axolotl development [J]. Proceedings of the National Academy of Sciences of the United States of America, 1997, 94(24): 13011-13016
[45] Wang Q W, Liang K, Liu J F, et al. Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic-pituitary-thyroid axis [J]. Aquatic Toxicology, 2013, 126: 207-213
[46] Liu S Y, Chang J H, Zhao Y, et al. Changes of thyroid hormone levels and related gene expression in zebrafish on early life stage exposure to triadimefon [J]. Environmental Toxicology and Pharmacology, 2011, 32(3): 472-477
[47] Tu W Q, Xu C, Lu B, et al. Acute exposure to synthetic pyrethroids causes bioconcentration and disruption of the hypothalamus-pituitary-thyroid axis in zebrafish embryos [J]. The Science of the Total Environment, 2016, 542(Pt A): 876-885
[48] Kim H, Ji K. Exposure to humidifier disinfectants induces developmental effects and disrupts thyroid endocrine systems in zebrafish larvae [J]. Ecotoxicology and Environmental Safety, 2019, 184: 109663
[49] Yao F, Li Y F, Ru H J, et al. Thyroid disruption and developmental toxicity caused by triphenyltin (TPT) in zebrafish embryos/larvae [J]. Toxicology and Applied Pharmacology, 2020, 394: 114957
[50] Morgado I, Santos C R, Jacinto R, et al. Regulation of transthyretin by thyroid hormones in fish [J]. General and Comparative Endocrinology, 2007, 152(2-3): 189-197
[51] Wu L Y, Li Y F, Ru H J, et al. Parental exposure to 2,2’,4,4’5-pentain polybrominated diphenyl ethers (BDE-99) causes thyroid disruption and developmental toxicity in zebrafish [J]. Toxicology and Applied Pharmacology, 2019, 372: 11-18
[52] Fernie K J, Shutt J L, Mayne G, et al. Exposure to polybrominated diphenyl ethers (PBDEs): Changes in thyroid, vitamin A, glutathione homeostasis, and oxidative stress in American kestrels (Falco sparverius) [J]. Toxicological Sciences: An Official Journal of the Society of Toxicology, 2005, 88(2): 375-383
[53] Chen Q, Yu L Q, Yang L H, et al. Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae [J]. Aquatic Toxicology, 2012, 110-111: 141-148
[54] Orozco A, Valverde-R C. Thyroid hormone deiodination in fish [J]. Thyroid: Official Journal of the American Thyroid Association, 2005, 15(8): 799-813
[55] Li P, Li Z H, Zhong L Q. Effects of low concentrations of triphenyltin on neurobehavior and the thyroid endocrine system in zebrafish [J]. Ecotoxicology and Environmental Safety, 2019, 186: 109776
[56] Zhao X S, Wang S T, Li D M, et al. Effects of perchlorate on BDE-47-induced alteration thyroid hormone and gene expression of in the hypothalamus-pituitary-thyroid axis in zebrafish larvae [J]. Environmental Toxicology and Pharmacology, 2013, 36(3): 1176-1185
[57] Zhai W H, Huang Z G, Chen L, et al. Thyroid endocrine disruption in zebrafish larvae after exposure to mono-(2-ethylhexyl) phthalate (MEHP) [J]. PLoS One, 2014, 9(3): e92465
[58] Liu Y R, Wu D, Xu Q L, et al. Acute exposure to tris (2-butoxyethyl) phosphate (TBOEP) affects growth and development of embryo-larval zebrafish [J]. Aquatic Toxicology, 2017, 191: 17-24
[59] Fu J J, Guo Y Y, Wang M, et al. Bioconcentration of 2,4,6-tribromophenol (TBP) and thyroid endocrine disruption in zebrafish larvae [J]. Ecotoxicology and Environmental Safety, 2020, 206: 111207
[60] Hood A, Klaassen C D. Differential effects of microsomal enzyme inducers on in vitro thyroxine (T4) and triiodothyronine (T3) glucuronidation [J]. Toxicological Sciences, 2000, 55(1): 78-84
[61] Hu F X, Zhao Y X, Yuan Y, et al. Effects of environmentally relevant concentrations of tris (2-chloroethyl) phosphate (TCEP) on early life stages of zebrafish (Danio rerio) [J]. Environmental Toxicology and Pharmacology, 2021, 83: 103600
[62] Raldúa D, Thienpont B, Babin P J. Zebrafish eleutheroembryos as an alternative system for screening chemicals disrupting the mammalian thyroid gland morphogenesis and function [J]. Reproductive Toxicology, 2012, 33(2): 188-197
[63] Porazzi P, Calebiro D, Benato F, et al. Thyroid gland development and function in the zebrafish model [J]. Molecular and Cellular Endocrinology, 2009, 312(1-2): 14-23
[64] Si W R, Zhao M J, Che H M, et al. Microcystin-LR induced transgenerational effects of thyroid disruption in zebrafish offspring by endoplasmic reticulum stress-mediated thyroglobulin accumulation and apoptosis [J]. Environmental Pollution, 2023, 322: 121117
[65] Zhao X S, Ren X, Ren B X, et al. Life-cycle exposure to BDE-47 results in thyroid endocrine disruption to adults and offsprings of zebrafish (Danio rerio) [J]. Environmental Toxicology and Pharmacology, 2016, 48: 157-167
[66] Li S Y, Wu Q, Sun Q Q, et al. Parental exposure to tebuconazole causes thyroid endocrine disruption in zebrafish and developmental toxicity in offspring [J]. Aquatic Toxicology, 2019, 211: 116-123
[67] Wang Q W, Lai N L, Wang X F, et al. Bioconcentration and transfer of the organophorous flame retardant 1,3-dichloro-2-propyl phosphate causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish larvae [J]. Environmental Science &Technology, 2015, 49(8): 5123-5132
[68] Wang H K, Jing C, Peng H K, et al. Parental whole life-cycle exposure to tris (2-chloroethyl) phosphate (TCEP) disrupts embryonic development and thyroid system in zebrafish offspring [J]. Ecotoxicology and Environmental Safety, 2022, 248: 114313
[69] Zhou Y X, Fu J J, Wang M, et al. Parental and transgenerational impairments of thyroid endocrine system in zebrafish by 2,4,6-tribromophenol [J]. Journal of Environmental Sciences (China), 2023, 124: 291-299
[70] Cheng H C, Yan W, Wu Q, et al. Parental exposure to microcystin-LR induced thyroid endocrine disruption in zebrafish offspring, a transgenerational toxicity [J]. Environmental Pollution, 2017, 230: 981-988
[71] Han Z H, Li Y F, Zhang S H, et al. Prenatal transfer of decabromodiphenyl ether (BDE-209) results in disruption of the thyroid system and developmental toxicity in zebrafish offspring [J]. Aquatic Toxicology, 2017, 190: 46-52
[72] Wang X C, Ling S Y, Guan K L, et al. Bioconcentration, biotransformation, and thyroid endocrine disruption of decabromodiphenyl ethane (DBDPE), a novel brominated flame retardant, in zebrafish larvae [J]. Environmental Science &Technology, 2019, 53(14): 8437-8446
[73] Dohán O, Carrasco N. Advances in Na(+)/I(-) symporter (NIS) research in the thyroid and beyond [J]. Molecular and Cellular Endocrinology, 2003, 213(1): 59-70
[74] Noguchi Y, Harii N, Giuliani C, et al. Thyroglobulin (Tg) induces thyroid cell growth in a concentration-specific manner by a mechanism other than thyrotropin/cAMP stimulation [J]. Biochemical and Biophysical Research Communications, 2010, 391(1): 890-894
[75] Zhang S N, Guo X C, Lu S Y, et al. Exposure to PFDoA causes disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae [J]. Environmental Pollution, 2018, 235: 974-982