污染物的毒性表现在许多方面。近年来,越来越多的研究发现,肌肉是污染物运动神经系统毒性的重要靶点[1]。了解污染物影响肌发生过程的毒理机制在评估污染物的潜在毒理风险和人类避免污染物伤害方面有重要意义。由于二噁英暴露可造成唇腭裂畸形,其肌肉发育毒性逐渐受到关注[2-3]。细胞学实验发现,10-10 mol·L-1的2,3,7,8-四氯二苯并-对-二噁英(2,3,7,8-tetrachlorodibenzo-p-dioxin,TCDD)处理可通过干扰融合过程和抑制肌管结构蛋白表达来抑制C2C12细胞的肌管形成[4]。但对具有一定类二噁英毒性的卤代咔唑(polyhalogenated carbazoles, PHCZs)的肌发育毒性研究还十分有限。2,7-二溴咔唑(2,7-dibromo-9H-carbazole, 27-BCZ)是近年来检出频次较高一种卤代咔唑(polyhalogenated carbazoles, PHCZs)[5-6],其在土壤中的半衰期为120 d,在环境中具有累积性[7]。目前,27-BCZ的部分毒理学效应已被阐明,包括胚胎发育毒性[8]、心脏毒性[9]、内分泌干扰毒性[10]等。有研究采用野生型斑马鱼胚胎探究了多种PHCZs的急性毒性,发现其中只有27-BCZ表现出强毒性,可能会对胚胎早期发育构成重大风险[11],因此,27-BCZ的环境健康风险值得关注。另有研究发现,27-BCZ具有心脏致畸效应,转录组学分析发现暴露于27-BCZ的斑马鱼有90个基因表达改变,且许多通路与芳香烃受体(aryl hydrocarbon receptor, AhR)的激活有关[11]。Fang等[9]发现在0.03 mmol·L-1 27-BCZ暴露后,斑马鱼胚胎开始显示严重的心脏畸形,且微摩尔数量级的PHCZs浓度暴露即可诱导心脏细胞色素P450酶1A1(cytochrome P450 1A1,cyp1a1)基因表达和蛋白合成,推测27-BCZ可能具有类二噁英的毒性效应。以上研究表明,27-BCZ暴露可能影响生物体正常发育,其毒性效应可能与其类似二噁英的化学结构以及AhR信号通路有关,因此27-BCZ的健康风险值得我们关注。
肌肉发育过程的调控机制严格而复杂,受到多种调控因素和多种信号的共同作用[12]。本文首先从细胞层面探究27-BCZ是否对肌源性分化有TCDD样的干扰作用;并进一步从分子层面探究27-BCZ在分化过程中对发育效应分子Myh3和Myh4表达及分化调控分子肌生成调节因子家族(myogenic regulatory factors, MRFs)的影响,旨在明确新型污染物27-BCZ的肌肉发育毒性效应,并为PHCZs类污染物的风险评估提供理论依据。
1 材料与方法(Materials and methods)
1.1 材料和仪器
小鼠成肌细胞C2C12购于北京协和细胞资源中心。27-BCZ(CAS: 136630-39-2)购自Wellington Laboratory公司(加拿大)。二甲基亚砜(dimethyl sulfoxide, DMSO)购自Sigma公司(美国)。磷酸缓冲液(PBS)、Cell Counting Kit-8(CCK-8)、H&E染色试剂盒购自Solarbio公司(中国)。青霉素/链霉素(P/S)、胰蛋白酶、Dulbecco’s Modified Eagles (DMEM)培养基、马血清(HS)购自Gibco公司(美国)。胎牛血清(FBS)购自Biological Industries公司(美国)。GeneJET® RNA Purification Kit、RevertAid First Strand cDNA Synthesis Kit购自Thermo Fisher Scientific公司(美国);GoTaq® qPCR Master Mix Kit购自Promega公司(美国)。以上试剂均为分析纯。
细胞培养箱(Thermo Fisher Scientific, Thermo 311,美国);光学显微镜(中国上海光学仪器厂,OLYMPUS CKX41,日本);显微镜照相机(Canon,DS12631,日本);超净工作台(上海力康,HFsafe-1200,中国);超微量分光光度计(Thermo, Nanodrop 2000,美国);普通PCR仪(BIO-RAD, BIO-RAD T100TM Thermal Cycler,美国);滤光片型多功能酶标仪(Tecan,Infinite F200 Pro,瑞士);实时荧光定量PCR仪(Thermo Fisher Scientific, Roche Light Cycle 480,美国)。
1.2 方法
1.2.1 细胞培养
本实验基于小鼠成肌细胞C2C12,采用逐级血清饥饿法,诱导C2C12细胞融合分化成梭形肌管,构建肌肉分化发育模型[4]。C2C12细胞采用含20% FBS和1% P/S的DMEM培养基(Growth Medium, GM)进行培养。细胞正常传代3代以上,观察到梭形的细胞均匀贴壁生长,无大面积成团且生长速度稳定,此时将细胞接种到6孔板中准备诱导分化,细胞密度为105个·mL-1,每孔2 mL培养基。当细胞在GM中生长到密度为80%左右时,更换为含10% FBS和1% P/S的DMEM培养基,以控制细胞的增殖速度,使细胞充分融合,防止因增长过快导致细胞成团贴壁生长从而影响分化。当细胞达到完全融合状态时,改用含2% HS和1% P/S的DMEM培养基(Differentiation Medium, DM)进行诱导分化[13]。培养条件为37 ℃、5% CO2。之后每天换液,在DM中生长到第2天时应观察到细胞更紧密整齐地规则排列,并且有已经融合的较短的梭形肌管出现;在第3天时应明显观察到大部分肌管发生融合,此时肌管生长旺盛;到第6天时大多数细胞分化为成熟的梭形肌管,肌管形成基本完毕。
本研究选择分化过程3个有代表性的时间节点:第1天(day1),代表分化前期(尚未开始分化,绝大部分细胞为单核圆形);第3天(day3),代表分化中期(大部分单核细胞融合成多核的梭形肌管);第6天(day6),代表分化末期(肌管成熟)进行实验研究。
1.2.2 化合物暴露
本实验所用27-BCZ以DMSO为溶剂,母液浓度为10-4 mol·L-1。CCK-8测增殖毒性实验中27-BCZ处理浓度为10-14~10-7 mol·L-1,以DMSO(0.1%)为溶剂对照,从分化第0天(day0)开始连续27-BCZ处理,测定处理24、48、144 h后的细胞活力。在形态学实验和基因表达测定中,采用4种27-BCZ处理浓度,分别为10-10、10-9、10-8、10-7 mol·L-1,从分化day0开始持续处理细胞,收集分化day1、day3、day6的细胞进行后续实验。
1.2.3 CCK-8法测定细胞毒性效应
参考CCK-8试剂盒说明书测定27-BCZ对细胞增殖的影响。细胞按照每孔104个的密度接种在96孔板中,37 ℃培养24 h后去除原培养基进行化合物暴露。27-BCZ的浓度为10-14~10-7 mol·L-1,每组6个平行复孔。分别暴露24 h(day1)、48 h(day2)、144 h(day6)后,每孔加入10 μL CCK-8试剂,在培养箱中孵育1~4 h,使用酶标仪(Infinite F200 Pro,Tecan)测定在450 nm波长下的吸光度。
细胞活力计算公式为:细胞存活率=(A处理组-A空白对照)/(A阴性对照-A空白对照)
式中:处理组指溶剂对照组和27-BCZ处理组;空白对照指培养基(DM)对照;阴性对照指不加任何化合物下正常生长的细胞。
1.2.4 细胞苏木素-伊红染色(Hematoxylin-Eosin staining,H&E染色)
本研究采用H&E染色对分化的细胞进行形态学分析。C2C12细胞以每孔106个细胞的密度接种于6孔板后,取分化day1、day3和day6的细胞进行H&E染色。具体步骤参考试剂盒说明。染色结束后显微镜下观察并拍照:6孔板中每个孔随机取3个视野进行拍照记录,结果使用ImageJ 1.51k软件(Media Cybernetics, USA)进行分析,记录每条肌管内细胞核数、视野内细胞核总数。
本研究使用2个评价指标来评估肌发育过程:细胞融合指数和单条肌管内细胞核数。细胞融合指数为融合进肌管内的细胞核总数比视野内总细胞核数的比值,反映了C2C12参与分化的效率。单条肌管内细胞核数为每个处理组中,每条肌管内的细胞核的数量,反映了肌管成熟的程度。单条肌管内细胞核数越多,代表该条肌管发育越成熟。使用小提琴图来呈现单条肌管内细胞核数的分布情况,以此评估分化效果。小提琴图中,对应Y轴的范围表示肌管内细胞核数量的分布的范围,每个小提琴最宽处对应的Y轴值代表该处理组中绝大多数肌管内细胞核数目。
1.2.5 实时荧光定量PCR(qRT-PCR)法检测基因表达水平
C2C12细胞接种于6孔板中,接种密度为每孔106个。收集诱导分化day1、day3和day6的细胞。吸去培养基,1×PBS冲洗2遍。使用GeneJET®RNA纯化试剂盒(Thermo)提取细胞总RNA。利用RevertAid First Strand cDNA Synthesis Kit(Thermo)将RNA逆转录为cDNA。然后,使用QuantStudionTM 6 Flex实时PCR系统(Thermo)以及GoTaq®qPCR Master Mix (Promega, Madison, WI, USA)进行实时荧光定量PCR检测。所选基因包括MyoD、Myogenin、Mrf4、Myh3、Myh4、cyp1a1、cyp1b1、甘油醛-3-磷酸脱氢酶(gapdh)。引物由Primer Premier 6 (Premier, Biosoft)设计(序列见表1),由生工生物技术(上海,中国)合成。所有样本均进行3次独立重复实验,并采用2-ΔΔCT方法[14]分析mRNA表达水平。
表1 引物序列
Table 1 Primer sequences used in qRT-PCR study

基因Gene基因IDGene ID特异性引物序列(5’~3’)Specific primer sequence (5’~3’)目的片段长度/bpDestination fragment length/bpMyoD17927(F) AGCACTACAGTGGCGACTCA(R) GCTCCACTATGCTGGACAGG201Myogenin17928(F) AGGCTGGGTGTGCATGTGA(R) TTAAAAGCCCCCTGCTACAGAAG70Mrf417878(F) GCTAAGGAAGGAGGAGCAAA(R) GAAGAAAGGCGCTGAAGACT62Myh317883(F) AAGGCCAAAAAGGCCATC(R) TCTTCTGCTCCCCTTCCA235Myh417884(F) TTGAAAAGACGAAGCAGCGAC(R) AGAGAGCGGGACTCCTCCTG190cyp1a113076(F) GACCCTTACAAGTATTTGGTCGT(R) GGTATCCAGAGCCAGTAACCT145cyp1b113078(F) TCCTCTTTACCAGATACCCGGAT(R) AAAAGCTGGAGAATCGCATT153gapdh14433(F) AGCTCACTGGCATGGCCTTC(R) ACGCCTGCTTCACCACCTTC117
1.2.6 数据统计分析
数据统计和分析使用GraphPad Prism软件(版本6, La Jolla, CA,美国)进行统计分析和绘制图形。实验结果表示为均值±标准差(n=3),进行3次独立重复实验。统计学检验采用单因素方差分析或双因素方差分析,采用Bonferroni方法进行多重比较校正。P<0.05表示差异有统计学意义。
2 结果(Results)
2.1 27-BCZ对细胞增殖的影响
首先采用CCK-8实验明确27-BCZ处理对细胞活力的影响。结果表明,经过24 h(图1(a))或48 h 27-BCZ(图1(b))处理后,细胞活力与对照组相比无明显变化,说明10-10~10-7mol·L-1浓度范围内的27-BCZ无增殖毒性。但10-8mol·L-1和10-7 mol·L-1的27-BCZ处理144 h(图1(c))后,细胞活力与对照组相比增加,且差异有统计学意义(P<0.05),提示高浓度27-BCZ可能对细胞增殖有促进作用。
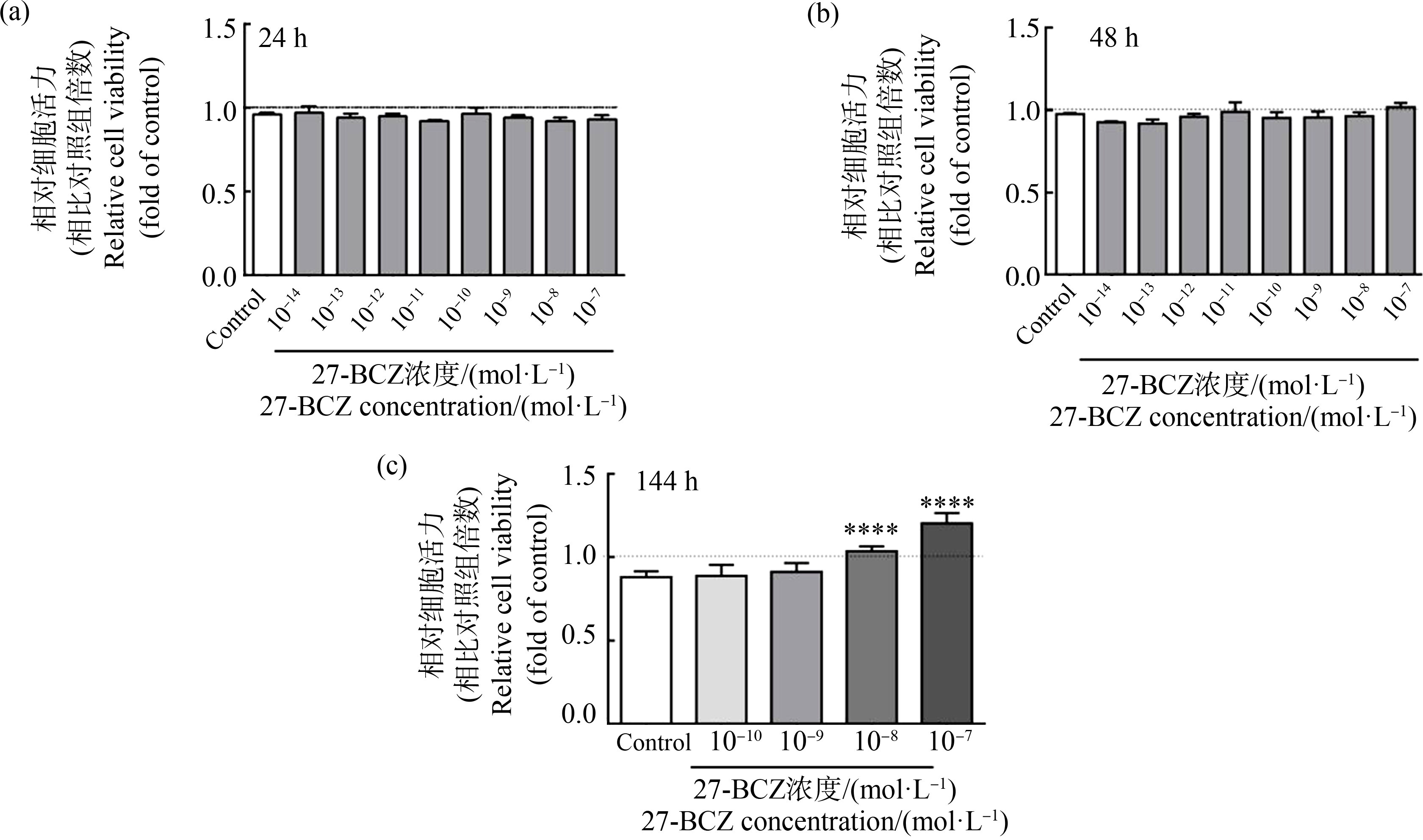
图1 27-BCZ对细胞活力的影响
注:27-BCZ处理浓度为10-14~10-7 mol·L-1,DMSO(0.1%)用作溶剂对照;27-BCZ从第0天开始持续处理;处理24 h(a)、48 h(b)和144 h(c)的细胞通过CCK-8法测定细胞活力;具体实验步骤见“材料与方法”的相关内容;数据为对照组的倍数,用平均值±标准差(n=3)表示,进行3次独立重复实验;统计分析方法为单因素方差分析和Bonferroni多重比较( *表示27-BCZ与对照组之间差异有统计学意义,显著性水平设为P<0.05,****代表P<0.0001)。
Fig. 1 Effect of 27-BCZ on C2C12 cell viability during differentiation
Note: The treatment concentration of 27-BCZ were 10-14~10-7 mol·L-1; DMSO (0.1%) served as the solvent control; 27-BCZ was continuously treated from day0; cells treated for 24 h (a), 48 h (b) and 144 h (c) were subjected to determine cell viability by CCK-8 method; see the relevant contents of “Materials and Methods” for specific experimental steps; the data are folds of control group and expressed as mean±SD (n=3); all samples were measured in three independent experiments; statistical analysis methods were one-way ANOVA and Bonferroni multiple comparison ( *indicates a statistically significant difference between the 27-BCZ treatment and control; the level of significance was set at P<0.05; ****represents P<0.0001).
2.2 27-BCZ抑制C2C12细胞分化过程中肌管的形成
为探究27-BCZ暴露对肌发育的影响,选择对细胞增殖无毒性的浓度范围(10-10~10-7mol·L-1)的27-BCZ连续处理C2C12细胞,并选择肌管形成的中期(day3)和肌管形成末期(day6)作为时间节点,通过H&E染色,观察27-BCZ对肌管形成产生的影响。10-7 mol·L-1 27-BCZ处理对分化的抑制作用在day3较为明显(图2(a))。从肌管day3的H&E染色图观察到,10-7 mol·L-1 27-BCZ处理组中,分化的C2C12细胞的数量明显少于溶剂对照组,并且每个肌管中的细胞核数量较少(图2(a))。从小提琴图中观察到,10-7 mol·L-1 27-BCZ处理组day3的细胞核数量大部分约为5个,处理组单条肌管内融合的细胞核平均数量比对照组少3个,小提琴形状扁平,表明数据集中分布在较低水平(图2(b))。在分化末期day6,从染色结果可以观察到,随着处理浓度升高,形成的肌管形状更细,肌管内细胞核数略有降低(图2(a)),但单条肌管内融合的细胞核平均数量与对照组相比差异无统计学意义(图2(b))。

图2 27-BCZ处理后C2C12的形态学变化
注:27-BCZ的处理浓度为10-10~10-7mol·L-1,DMSO的处理浓度为0.1%,连续27-BCZ处理从第0天开始;收集在分化day3和day6的细胞进行H&E染色;每个处理浓度选取3个随机视野(n=3),统计肌管内细胞核数;(a)每个时间点的不同浓度处理组选取的代表性图片,比例尺=50 μm;(b) 27-BCZ处理后肌管中细胞数量的小提琴图;统计分析方法为单因素方差分析和Bonferroni多重比较(*表示27-BCZ处理组与对照组(day3和day6)的平均值的差异有统计学意义;显著性水平设定为P<0.05;*代表P<0.05,****代表P<0.0001)。
Fig. 2 Morphological changes after 27-BCZ treatment
Note: The treatment concentrations of 27-BCZ were 10-10~10-7mol·L-1, and that of DMSO solvent was 0.1%; the continuous 27-BCZ treatment started from day0; cells were collected on differentiation day3 and day6 for H&E staining; select three random fields (n=3) for each treatment concentration and count the number of nuclei in the myotubes; (a) One of the representative pictures of each treatment group at each time point is shown; scale bar=50 μm; (b) Violin plot of the number of nuclei in myotubes after treatment with 27-BCZ; statistical analysis methods were one-way ANOVA and Bonferroni multiple comparison (*indicates a statistically significant difference between the 27-BCZ treatment and control (day3 and day6) in the mean value of nuclei in myotubes; the level of significance was set at P<0.05; *represents P<0.05, ****represents P<0.0001).
为了进一步表征C2C12细胞肌管形成的效率,我们通过计算融合肌管中的细胞核总数(肌管内细胞核不少于2个)与在每个选定视野中观察到的细胞核总数的比率来量化融合指数。结果表明,27-BCZ处理组的融合指数以浓度依赖的方式降低(图3)。结果表明,在分化中期day3,高浓度27-BCZ不仅降低肌管的细胞核容量(图2(b)),肌管形成的效率下降,最高浓度处理组的细胞融合指数下降33.97%(图3),且与对照组相比差异有统计学意义(P<0.05)。在分化末期day6,27-BCZ 10-7 mol·L-1处理组细胞融合指数与对照组相比降低32.55%(图3)。结合肌管内细胞核数和融合指数,我们发现在分化末期,虽然27-BCZ对肌管内细胞核容量与对照组相比差异无统计学意义(P>0.05),但是C2C12细胞参与融合形成多核肌管的效率变低(图2(b)和图3)。
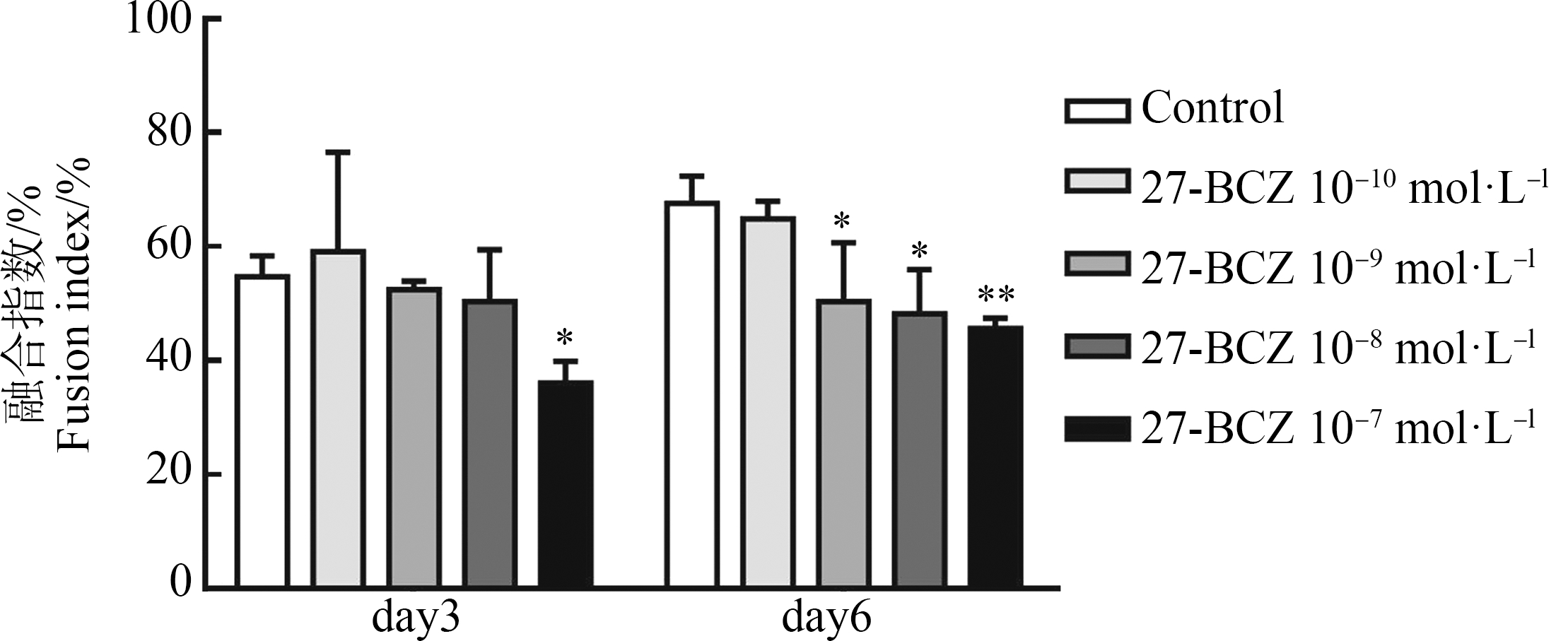
图3 27-BCZ暴露抑制分化到day3和day6的细胞融合指数
注:将C2C12细胞接种到6个孔板中以诱导分化,收集分化到day3和day6的细胞进行H&E染色统计;27-BCZ的处理浓度为10-10~10-7 mol·L-1,DMSO(0.1%)用作溶剂对照;融合指数,代表参与融合形成肌管的细胞核数与细胞核总数之比;具体实验步骤见“材料与方法”的相关内容;数据为平均值±标准差(n=3),进行3次独立重复实验;统计分析方法为单因素方差分析和Bonferroni多重比较( *表示27-BCZ处理组与对照组之间差异有统计学意义;显著性水平设置为P<0.05,*代表P<0.05,**代表P<0.01)。
Fig. 3 27-BCZ reduced the fusion index in day3 and day6 of C2C12 differentiation
Note: C2C12 cells were seeded into six well plates to induce differentiation, and cells were collected at day3 and day6 of differentiation for H&E staining; the treatment concentration of 27-BCZ were 10-10~10-7 mol·L-1; DMSO (0.1%) served as the solvent control; fusion index, representing the proportion of total nuclei found in myotubes; see the relevant contents of “Materials and Methods” for specific experimental steps; the data is mean±SD (n=3); all samples were measured in three independent experiments; statistical analysis methods were two-way ANOVA and Bonferroni multiple comparison ( *indicates a statistically significant difference between the 27-BCZ treatment and control; the level of significance was set at P<0.05; *represents P<0.05, **represents P<0.01).
2.3 27-BCZ降低肌管成熟标记基因Myh3和Myh4的表达
肌球蛋白重链(myosin heavy chain,MyHC)是肌管的重要结构蛋白,本研究使用其编码基因Myh3和Myh4作为分化成熟的标志分子。27-BCZ处理抑制了肌管形成中期和末期C2C12细胞中Myh3和Myh4的表达,且大多数抑制作用呈浓度依赖性(图4(a)和图4(b))。如图4所示,在对照组中,Myh3基因的表达在day3已经达到较高水平,day6基本没有明显的变化(图4(a))。27-BCZ对Myh3的抑制效果在分化中期day3开始体现,在day3时,10-9 mol·L-1和10-7 mol·L-1 27-BCZ处理组的Myh3比对照组分别下降了34.16%和38.88%;在day6时10-7 mol·L-1 27-BCZ处理组Myh3比对照组下降了37.97%(图4(a))。
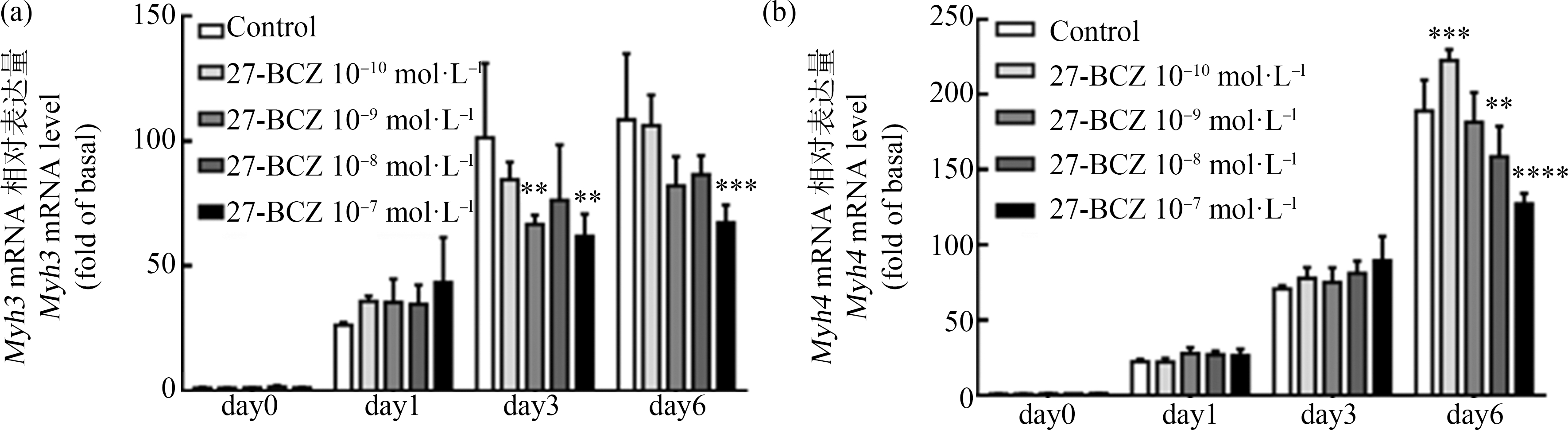
图4 27-BCZ对Myh3和Myh4基因表达的影响
注:从day0到day6,用10-10~10-7 mol·L-1的27-BCZ或溶剂对照(DMSO, 0.1%)连续处理C2C12细胞,并在day1、day3和day6收集;通过qRT-PCR测定Myh3(a)和Myh4(b)的mRNA表达水平,通过gapdh进行定量标准化;数值为相比于day0对照组表达量倍数,并表示为平均值±标准差(n=3),进行3次独立重复实验;统计分析方法为双因素方差分析和Bonferroni多重比较( *表示在同一时间点与对照组相比差异有统计学意义;显著性水平设置为P<0.05;**代表P<0.01,***代表P<0.001,****代表P<0.0001)。
Fig. 4 Effect of 27-BCZ on gene expression of Myh3 and Myh4
Note: C2C12 cells were continuously treated with 27-BCZ at 10-10 to 10-7 mol·L-1, or solvent control (DMSO, 0.1%) from day0 to day6, and collected on day1, day3 and day6; the mRNA expression levels of Myh3 (a) and Myh4 (b) were determined by qRT-PCR, quantified by normalization to internal control gapdh; values are fold of basal level obtained on day0, and expressed as mean±SD (n=3); each independent sample was tested in triplicate; statistical analysis methods were two-way ANOVA and Bonferroni multiple comparison (*indicates statistically significant difference compared with the control group in the same time point; the level of significance was set at P<0.05; **represents P<0.01, ***represents P<0.001, ****represents P<0.0001).
正常成肌分化(对照组)状态下,Myh4的表达量随时间持续增加,在day6时达到最高值(图4(b))。
在分化前期和中期,即day1和day3,27-BCZ暴露浓度增加对Myh4的表达量具有一定促进作用,但与对照组相比差异无统计学意义(P>0.05)(图4(b))。在day6,与对照组相比,10-10 mol·L-1 27-BCZ处理组中Myh4的表达量提高17.75%,10-8 mol·L-1和10-7 mol·L-1 27-BCZ处理组中Myh4的表达量分别降低了16.12%和32.69%,表达量与对照组相比,差异有统计学意义(P<0.05)(图4(b))。在day6,随27-BCZ暴露浓度增加,Myh4表达量呈现低浓度促进表达和高浓度抑制表达的结果(图4(b))。
2.4 27-BCZ暴露干扰肌发育调节基因MRFs的表达
MyoD和Myogenin被认为是肌生成的2个最终分化驱动因素[15]。在对照组中,MyoD的mRNA表达在分化期间先增加,然后减少,在day3达到最高表达水平(图5(a))。27-BCZ处理存在时间和浓度依赖性抑制MyoD的mRNA表达(图5(a))。最高浓度的27-BCZ处理后,MyoD基因的表达从分化day1到day6与对照组相比降低,且差异有统计学意义(P<0.05),在day6达到最高抑制率(34.64%);而对于10-8 mol·L-1处理组,在后期(day3和day6)观察到显著的影响;然而,在10-10 mol·L-1和10-9 mol·L-1 27-BCZ处理组中均未检测到显著影响(图5(a))。作为三者中低剂量即产生效应的最敏感的MRF,与对照组相比,MyoD基因表达随时间变化的曲线也发生了变化,即在10-8 mol·L-1处理时,表达稍早达到峰值水平,在10-7 mol·L-1处理6 d后,与对照组相比表达急剧下降(降低34.64%)(图5(a))。关于另一种早中期的MRF,Myogenin仅在第3天以10-9 mol·L-1处理后与对照组相比差异才有统计学意义(P<0.05),整个分化期的表达谱没有变化(图5(c))。如图5(b)所示,分化前期和中期Mrf4表达水平较低,直到day6,所有处理组才观察到Mrf4的mRNA表达,与day6的对照组相比,在10-9 mol·L-1和10-7 mol·L-1的27-BCZ处理后,Mrf4表达受到抑制,分别降低了12.40%和34.71%,且差异有统计学意义。
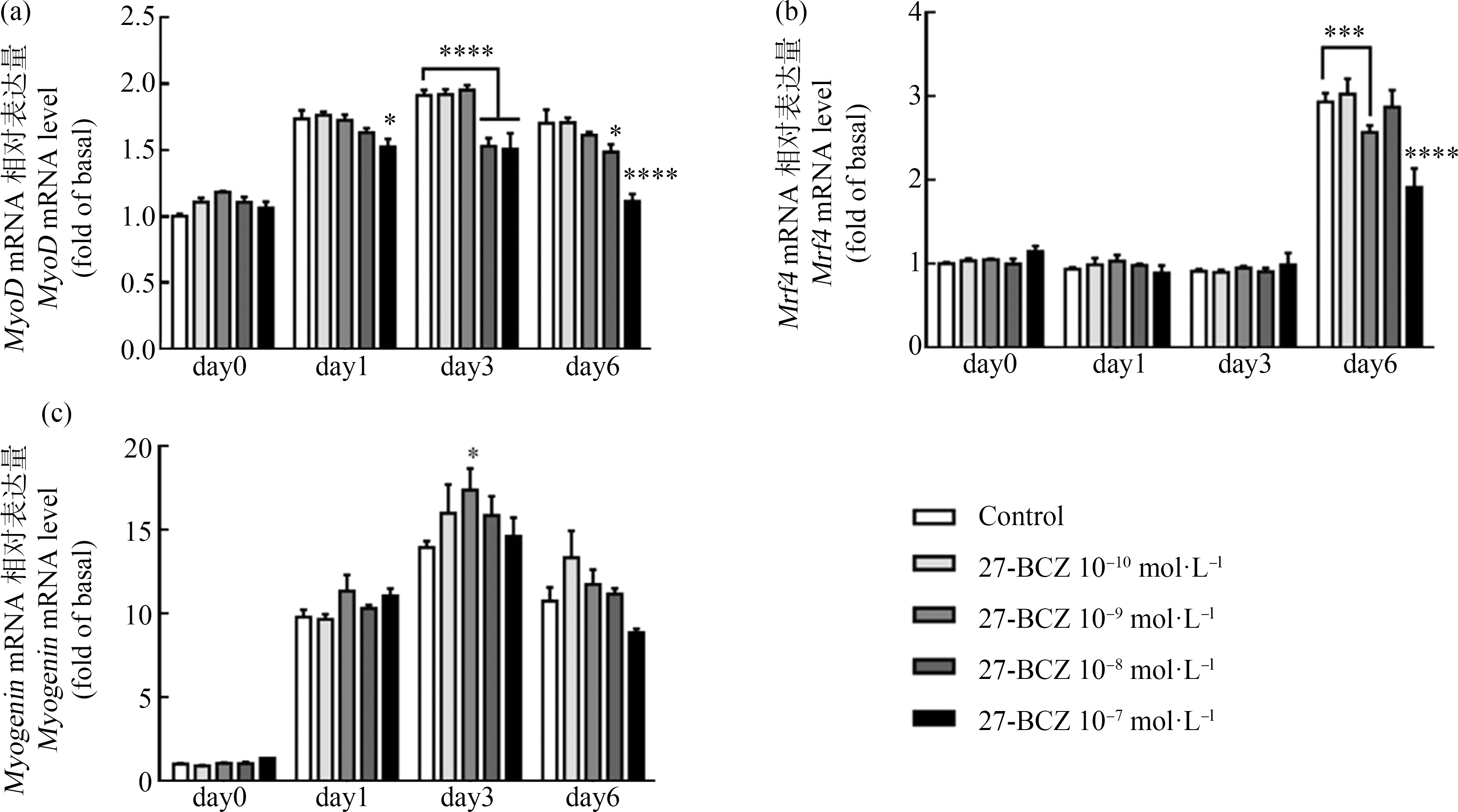
图5 27-BCZ对MRF基因表达的影响
注:从day0到day6,用10-10~10-7 mol·L-1的27-BCZ或溶剂对照(DMSO, 0.1%)连续处理C2C12细胞,并在day1、day3和day6收集;通过qRT-PCR测定(a)MyoD、(b)Myogenin、(c)Mrf4的mRNA表达水平,通过管家基因gapdh进行定量;数值为第0天获得的基础水平的倍数,并表示为平均值±标准差(n=3),进行3次独立重复实验;统计分析方法为双因素方差分析和Bonferroni多重比较(*表示在同一时间点与对照组相比差异有统计学意义;显著性水平设置为P<0.05,*代表P<0.05,***代表P<0.001,****代表P<0.0001)。
Fig. 5 Effects of 27-BCZ on the gene expression of MRFs
Note: C2C12 cells were continuously treated with 27-BCZ at 10-10 to 10-7 mol·L-1, or solvent control (DMSO, 0.1%) from day0 to day6, and collected on day1, day3 and day6; the mRNA expression levels of (a) MyoD, (b) Myogenin, (c) Mrf4 were determined by qRT-PCR, quantified by normalization to internal control gapdh; values are fold of basal level obtained on day0, and expressed as mean±SD (n=3); each independent sample was tested in triplicate; statistical analysis methods were two-way ANOVA and Bonferroni multiple comparison (*indicates a statistically significant difference compared with the control group in the same time point; the level of significance was set at P<0.05; *represents P<0.05, ***represents P<0.001, ****represents P<0.0001).
2.5 27-BCZ诱导cyp1a1和cyp1b1基因表达
cyp1a1和cyp1b1是AhR通路2个经典的下游靶基因。由于先前已经报道,27-BCZ在小鼠肝癌细胞中激活AhR效率相对较高[7],我们认为27-BCZ在C2C12细胞中可能具有类似的AhR激活能力。因此,我们研究了27-BCZ对AhR下游2个基因表达的影响,以明确其在我们的研究模型中对激活AhR的作用。结果显示,27-BCZ以时间和浓度依赖的方式诱导cyp1a1和cyp1b1的mRNA表达(图6)。且cyp1a1基因表达诱导比cyp1b1更有效,cyp1a1和cyp1b1的最大诱导都出现在分化末期day6,与day0对照组相比最高激活倍数分别为9.4倍和6.2倍(图6)。
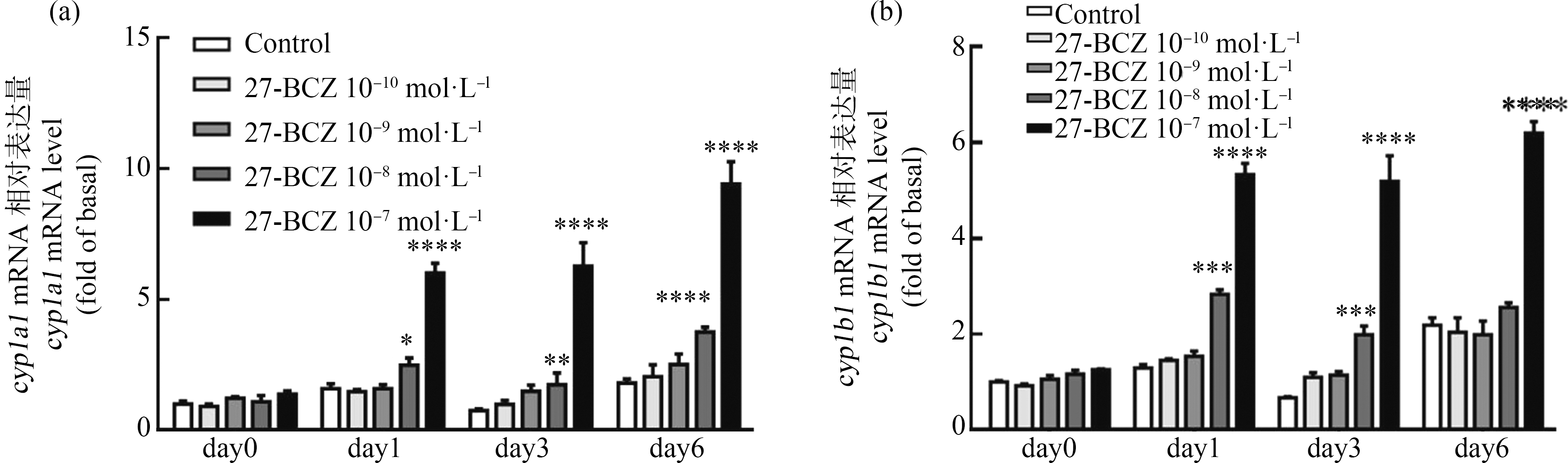
图6 27-BCZ对AhR通路下游基因表达的影响
注:从day0到day6,用10-10~10-7 mol·L-1的27-BCZ或溶剂对照(DMSO, 0.1%)连续处理C2C12细胞,并在day1、day3和day6收集;通过qRT-PCR测定cyp1a1(a)和cyp1b1(b)的mRNA表达水平,通过管家基因gapdh进行定量;数值为第0天获得的基础水平的倍数,并表示为平均值±标准差(n=3),进行3次独立重复实验;统计分析方法为双因素方差分析和Bonferroni多重比较( *表示在同一时间点与对照组相比差异有统计学意义;显著性水平设置为P<0.05,*代表P<0.05,**代表P<0.01,***代表P<0.001,****代表P<0.0001)
Fig. 6 Effect of 27-BCZ on the expression of downstream genes of AhR pathway
Note: C2C12 cells were continuously treated with 27-BCZ at 10-10 to 10-7 mol·L-1, or solvent control (DMSO, 0.1%) from day0 to day6, and collected on day1, day3 and day6; the mRNA expression levels of cyp1a1 (a) and cyp1b1 (b) were determined by qRT-PCR, quantified by normalization to internal control gapdh; values are fold of basal level obtained on day0, and expressed as mean±SD (n=3); each independent sample was tested in triplicate; statistical analysis methods were two-way ANOVA and Bonferroni multiple comparison (*indicates statistically significant difference compared with the control group in the same time point; the level of significance was set at P<0.05; *represents P<0.05, **represents P<0.01, ***represents P<0.001, ****represents P<0.0001).
3 讨论(Discussion)
本研究检测27-BCZ暴露后C2C12细胞肌发育形态学和肌生成标志分子和调节分子的变化,实验数据显示27-BCZ具有与TCDD类似的肌发育干扰效应。27-BCZ可以在细胞和分子水平上引起肌源性分化的紊乱,影响肌管的形成和成熟,且抑制效应呈剂量、时间依赖性。在分子水平上,我们发现27-BCZ暴露影响肌分化调节因子MRFs的表达,并抑制分化成熟标志分子Myh3和Myh4的表达,与形态学上抑制肌分化表现出一致性。另外,27-BCZ在影响肌发生的同时,还能够激活AhR下游靶基因cyp1a1和cyp1b1,表现出与二噁英类似的AhR活性,为进一步机制研究提供方向。
我们统计细胞融合指数和单条肌管内细胞核数这2个评估分化效果的参数指标的变化,分析27-BCZ暴露后对肌管形态学的影响。结果显示,27-BCZ在分化中期(day3)可以抑制肌管的形成,表现为细胞融合效率的降低和肌管内细胞核容量的减少。而在分化末期day6,27-BCZ对分化的影响主要表现在降低细胞融合效率。且27-BCZ暴露时间越长,处理浓度越高,对细胞融合效率和肌管内细胞容量的抑制作用越显著。这与此前文献报道的TCDD抑制肌管生成而导致肌发育毒性结果类似[4]。说明27-BCZ是一种具有类似二噁英肌发育毒性的污染物。
在CCK-8实验中我们发现在诱导分化的条件下,分化末期高浓度27-BCZ处理能使细胞活力上升,提示高浓度27-BCZ可能促进细胞的增殖。这与此前报道的肌肉发育过程中,增殖和分化呈现负相关的结果相一致。有研究报道肌肉发育是一个高时序性并且不可逆的过程。肌发育早期,首先检测到Myogenin的表达升高,但此时细胞DNA复制过程继续进行,肌母细胞增殖,为分化做准备。此后,细胞周期抑制蛋白p21高水平表达,细胞退出细胞周期,细胞增殖受抑制,分化过程开启。最后,p21(+)单核成肌细胞激活肌肉结构蛋白MyHC表达,促使单核肌细胞融合形成多核肌管[12, 16]。因此,细胞的增殖和分化是互斥过程。细胞的增殖情况可以间接表明其分化效果。本研究发现27-BCZ在分化末期对增殖有促进作用,间接说明了27-BCZ具有抑制肌肉分化的效果,提示27-BCZ可能是通过减缓或推后细胞周期退出,导致细胞不断增殖,来抑制分化,从而造成发育异常。
分子水平上,MyHC是肌纤维结构蛋白肌球蛋白的关键亚基,MyHC不同亚型,分别由MYH基因家族不同成员编码,在不同类型的肌肉中差异表达[17]。本研究选用了成年骨骼肌中表达量最高的MyHCⅡb的编码基因Myh4和胚胎型MyHC的编码基因Myh3,来研究27-BCZ对肌分化成熟的标志分子MyHC的影响。在分化day3,高浓度27-BCZ处理对Myh3就开始产生影响。在分化day6,27-BCZ抑制分化末期标志分子Myh3和Myh4的表达,说明27-BCZ显著抑制分化成熟,从分子层面证实了其肌发育毒性效应,与形态学结果相一致。此外,我们发现暴露27-BCZ会干扰调节因子MRFs的表达。据报道,转化生长因子-β(TGF-β)信号通路与唇腭裂等许多发育疾病密切相关[18]。而TGF-β家族配体与磷酸化的跨膜受体结合并激活Smad2和Smad3,从而与Smad4形成复合物,这种复合物易位到细胞核以调节靶基因MRFs转录因子MyoD和Myogenin的转录[19]。MRFs家族(MyoD[20]、Myf5[21]、Mrf4[22]、Myogenin[23])作为肌分化重要的调节因子,在调控C2C12退出细胞周期、促进细胞融合,以及诱导肌管进一步发育成肌纤维过程发挥着重要作用[24]。因此,本研究中27-BCZ对C2C12细胞肌发育的干扰作用可能是通过影响MRFs的表达而产生的。
另外,AhR参与调节信号转导、细胞分化、细胞凋亡等重要的生理过程。它能探测出物质侵入,并通过调节下游靶基因产生应激反应,分解有毒物质,同时介导污染物的毒性作用[25]。cyp1a1和cyp1b1是AhR信号通路重要的下游靶基因。cyp1a1是一种异型生物物质代谢变化的酶,其表达会导致生物的畸形生长、免疫抑制和促肿瘤生长[26]。Ma等[7]通过CBG2.8D检测系统和分子对接数据发现多种卤代咔唑具有芳香烃受体生物活性,本研究在肌细胞中同样发现10-8mol·L-1和10-7mol·L-1的27-BCZ处理能显著激活AhR下游的靶基因cyp1a1和cyp1b1表达,提示AhR可能在27-BCZ的肌肉发育毒性中发挥作用。
本研究使用C2C12肌发育模型,探究了27-BCZ对肌发育的影响。结果表明27-BCZ暴露后,C2C12细胞在细胞和分子水平都表现出肌发育毒性效应,并能激活AhR下游靶基因cyp1a1和cyp1b1的表达。肌发育过程相关分子机制极为复杂,可能受多种信号通路交互作用,27-BCZ暴露导致肌发生的病理学改变并不清楚,但我们的研究为新型污染物卤代咔唑的风险评估以及污染物相关肌发育疾病研究提供了可参考的前瞻性实验结果。
[1] Lam G, Juricek L, Dayal H, et al. Toxicological effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the skeletal muscle of mice during the perinatal period: A metabolomics study [J]. Environmental Sciences Europe, 2022, 34(1): 1-12
[2] Yamada T, Mishima K, Fujiwara K, et al. Cleft lip and palate in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin: A morphological in vivo study [J]. Congenital Anomalies, 2006, 46(1): 21-25
[3] Tao Y C, Liu X Z, Cui L L, et al. Oct4 plays a role in 2,3,7,8 - tetrachlorobenzo-p-dioxin (TCDD) inducing cleft palate and inhibiting mesenchymal proliferation [J]. Toxicology, 2020, 438: 152444
[4] Xie H Q, Xia Y J, Xu T, et al. 2,3,7,8-tetrachlorodibenzo-p-dioxin induces alterations in myogenic differentiation of C2C12 cells [J]. Environmental Pollution, 2018, 235: 965-973
[5] Zhu H H, Zheng M G, Zheng L, et al. Distribution and ecotoxicological effects of polyhalogenated carbazoles in sediments from Jiaozhou Bay wetland [J]. Marine Pollution Bulletin, 2019, 146: 393-398
[6] Wang G W, Jiang T M, Li S, et al. Occurrence and exposure risk evaluation of polyhalogenated carbazoles (PHCZs) in drinking water [J]. The Science of the Total Environment, 2021, 750: 141615
[7] Ma D, Xie H Q, Zhang W L, et al. Aryl hydrocarbon receptor activity of polyhalogenated carbazoles and the molecular mechanism [J]. The Science of the Total Environment, 2019, 687: 516-526
[8] Zhang J W, Zhang C, Du Z K, et al. Emerging contaminant 1,3,6,8-tetrabromocarbazole induces oxidative damage and apoptosis during the embryonic development of zebrafish (Danio rerio) [J]. The Science of the Total Environment, 2020, 743: 140753
[9] Fang M L, Guo J H, Chen D, et al. Halogenated carbazoles induce cardiotoxicity in developing zebrafish (Danio rerio) embryos [J]. Environmental Toxicology and Chemistry, 2016, 35(10): 2523-2529
[10] Yue S Q, Zhang T, Shen Q Q, et al. Assessment of endocrine-disrupting effects of emerging polyhalogenated carbazoles (PHCZs): In vitro, in silico, and in vivo evidence [J]. Environment International, 2020, 140: 105729
[11] Ji C Y, Yan L, Chen Y C, et al. Evaluation of the developmental toxicity of 2,7-dibromocarbazole to zebrafish based on transcriptomics assay [J]. Journal of Hazardous Materials, 2019, 368: 514-522
[12] Hernández-Hernández J M, García-González E G, Brun C E, et al. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration [J]. Seminars in Cell &Developmental Biology, 2017, 72: 10-18
[13] Bajaj P, Reddy B, Millet L, et al. Patterning the differentiation of C2C12 skeletal myoblasts [J]. Integrative Biology, 2011, 3(9): 897-909
[14] Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method [J]. Methods, 2001, 25(4): 402-408
[15] Hasty P, Bradley A, Morris J H, et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene [J]. Nature, 1993, 364(6437): 501-506
[16] Andrés V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis [J]. The Journal of Cell Biology, 1996, 132(4): 657-666
[17] Agarwal M, Sharma A, Kumar P, et al. Myosin heavy chain-embryonic regulates skeletal muscle differentiation during mammalian development [J]. Development, 2020, 147(7): dev184507
[18] Wei Z X, Sakamuru S, Zhang L, et al. Identification and profiling of environmental chemicals that inhibit the TGFβ/SMAD signaling pathway [J]. Chemical Research in Toxicology, 2019, 32(12): 2433-2444
[19] Langley B, Thomas M, Bishop A, et al. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression [J]. The Journal of Biological Chemistry, 2002, 277(51): 49831-49840
[20] Wang R T, Chen F L, Chen Q, et al. MyoD is a 3D genome structure organizer for muscle cell identity [J]. Nature Communications, 2022, 13: 205
[21] Guo R C, You X, Meng K, et al. Single-cell RNA sequencing reveals heterogeneity of Myf5-derived cells and altered myogenic fate in the absence of SRSF2 [J]. Advanced Science, 2022, 9(18): e2105775
[22] Hinits Y, Osborn D P, Carvajal J J, et al. Mrf4 (myf6) is dynamically expressed in differentiated zebrafish skeletal muscle [J]. Gene Expression Patterns, 2007, 7(7): 738-745
[23] Ganassi M, Badodi S, Ortuste Quiroga H P, et al. Myogenin promotes myocyte fusion to balance fibre number and size [J]. Nature Communications, 2018, 9(1): 4232
[24] Shirakawa T, Toyono T, Inoue A, et al. Factors regulating or regulated by myogenic regulatory factors in skeletal muscle stem cells [J]. Cells, 2022, 11(9): 1493
[25] Sorg O. AhR signalling and dioxin toxicity [J]. Toxicology Letters, 2014, 230(2): 225-233
[26] Sul D, Kim H S, Cho E K, et al. 2,3,7,8-TCDD neurotoxicity in neuroblastoma cells is caused by increased oxidative stress, intracellular calcium levels, and tau phosphorylation [J]. Toxicology, 2009, 255(1-2): 65-71