抗生素的发现给医疗带来划时代的飞跃,在临床上被广泛使用。世界上,每年抗生素的使用量已经超过了10万t[1]。然而抗生素的大量生产及滥用,导致地表水和土壤抗生素的大量残留(表1)。在韩国仁川农田土壤表层检测到四环素高达17.74 mg·kg-1[2],我国珠江三角洲和珠江口流域的8个监测点均检测到大环内酯类、氟喹诺酮类和磺酰胺类抗生素,残留浓度为0.7~127 ng·L-1[3]。在我国江苏某畜禽养殖场周围地表水中共检测出了9种抗生素,其中最多的是磺胺二甲嘧啶,检测的最大浓度为211 μg·L-1[4]。另外,Sim等[5]在牲畜废水处理厂进水口则检测到了浓度高达3 005 μg·L-1的林可霉素,在制药污水处理厂的出水口残留的林可霉素浓度甚至达到了43 909 μg·L-1。正常情况下,废水处理厂的能力有限,大多数具有一定浓度的抗生素被持续和大量的输入水体中,并且有些抗生素的半衰期较长,水体的流动性较差,导致抗生素残留浓度极高。同时也导致了环境中残留的抗生素浓度跨度极大[5-6]。环境中残留的抗生素可通过食物链进入人体,造成潜在的危害,尤其是对孕妇和婴幼儿造成不利影响。
表1 抗生素在环境中残留的浓度同斑马鱼实验浓度联系
Table 1 The concentration of antibiotic residues in the environment is related to the experimental concentration of zebrafish
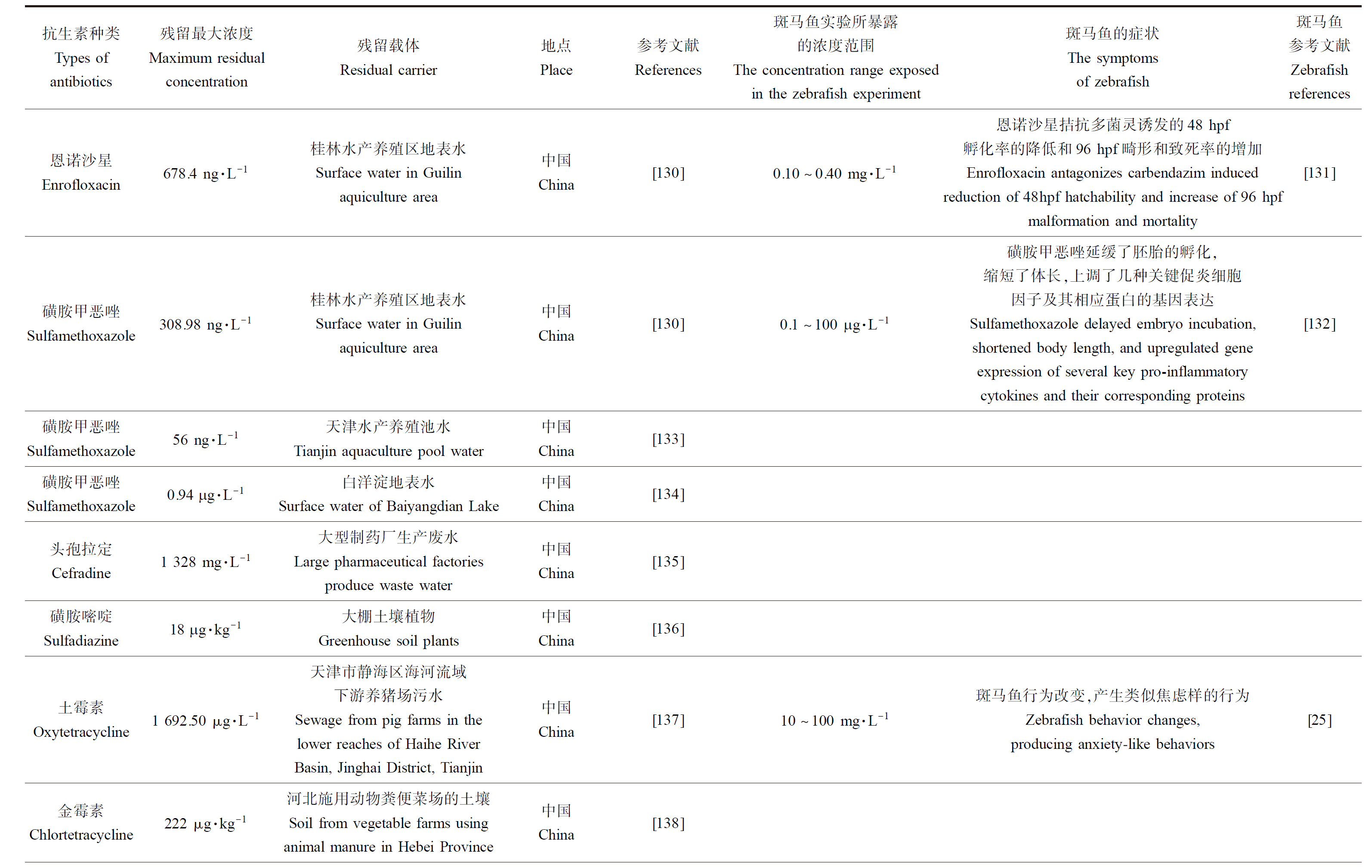
续表1
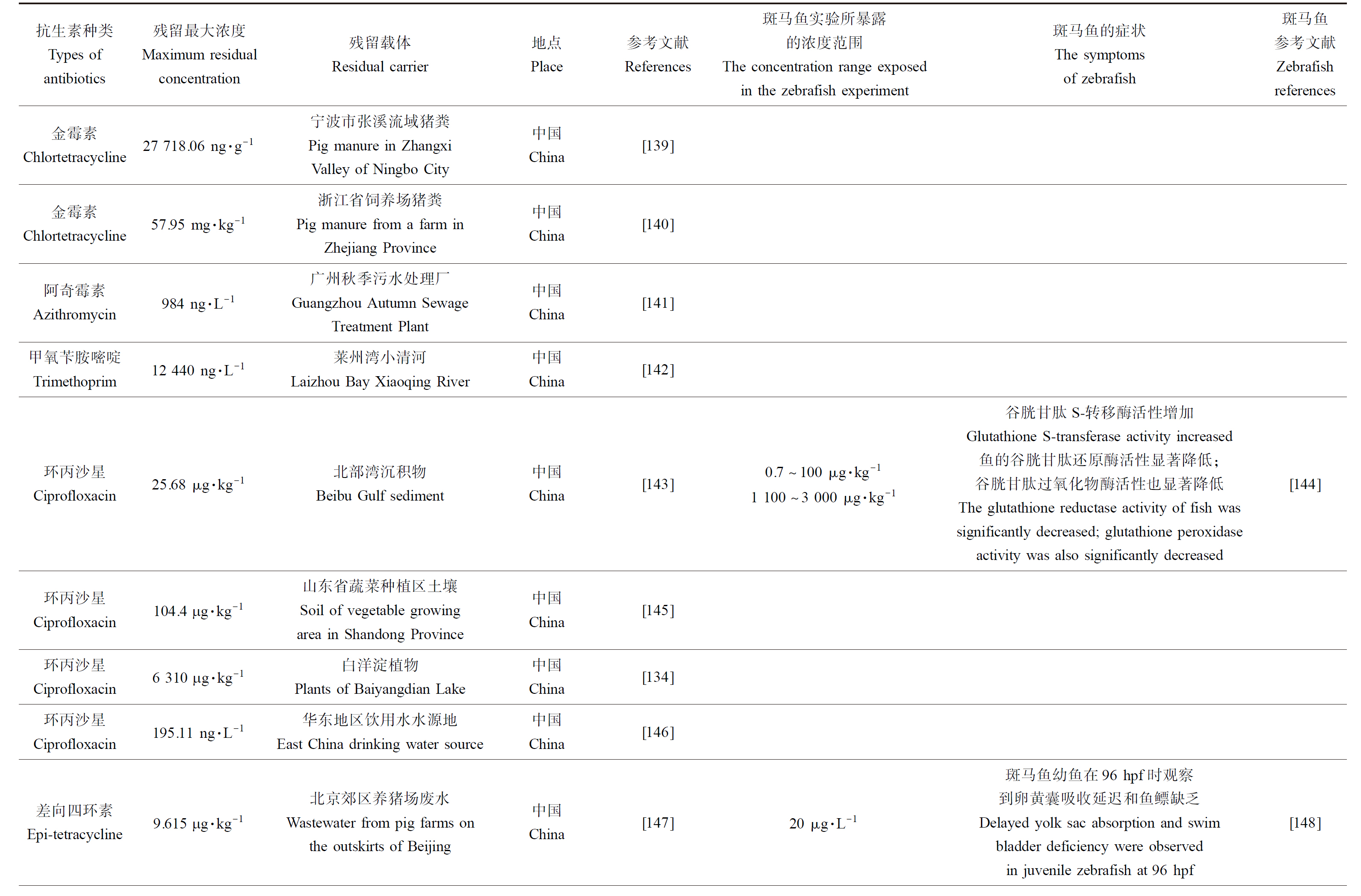
续表1

斑马鱼(Danio rerio)的行为变化与环境联系密切,环境抗生素可引起斑马鱼的焦虑/抑郁样行为。斑马鱼产生焦虑/抑郁样行为时,其行为表现与啮齿类动物相似,如斑马鱼对刺激较为敏感,在水中会更趋向于水底和黑暗环境、游泳速度不稳定,有突然转弯或者静止不动的行为[7-10]。由于斑马鱼具有产卵数量多、孵化快、价格低廉、对药物和污染物敏感等诸多的优点[11]。并且斑马鱼的基因和人类具有87%的同源性,所以实验结果对人类疾病研究有一定的参考意义[12-14]。此外,斑马鱼神经系统具有神经递质,例如多巴胺、五羟色胺、乙酰胆碱等,是进行神经系统研究的良好模型[15]。斑马鱼肠道结构也与人类较为相似,分为上皮层、黏膜层、肌肉层和浆膜层,具有生物屏障、黏液屏障和上皮屏障。研究表明,斑马鱼焦虑/抑郁行为与肠-脑途径密切相关[16-17]。抗生素可通过影响斑马鱼肠道微生物及其代谢产物组成,后经由多种途径联系大脑,传递信息,最终诱发焦虑/抑郁,引起斑马鱼行为的改变。本文对抗生素引起不同年龄阶段斑马鱼焦虑/抑郁样行为进行了总结,并从”肠-脑”途径机制方面进行分析。以期为抗生素和斑马鱼神经行为机制方面研究提供一定的参考。
1 抗生素对斑马鱼焦虑/抑郁样行为的影响(Effect of antibiotics on anxiety/depression behavior of zebrafish)
1.1 抗生素对幼鱼/青年斑马鱼(0~5月龄)焦虑/抑郁样行为影响(Effects of antibiotics on anxiety/depression-like behavior in juvenile/young zebrafish (0~5 months of age))
高浓度抗生素可造成斑马鱼焦虑/抑郁样行为(表2)。研究表明,抗生素单一和联合暴露均会降低幼龄斑马鱼的平均游泳速度,增加移动距离。Gonçalves等[18]将斑马鱼暴露于浓度为100 mg·L-1的阿莫西林溶液中7 d后,通过分析新型水槽试验结果发现阿莫西林暴露显著抑制斑马鱼平均游泳速度,延长其静止不动的时间。Cheng等[19]将6 hpf(hour post fertilization, hpf)的斑马鱼卵暴露在不同浓度林可霉素溶液(15、30、60 μg·mL-1)中,暴露至6 dpf(day post fertilization, dpf),发现随着林可霉素浓度的增加,斑马鱼的移动距离和平均速度减小。Han等[20]将5 dpf的斑马鱼暴露于新霉素125 μmol·L-1 1 h后,新霉素组在黑暗中游泳的总距离显著下降,平均速度降低。Zhang等[21]将氟喹诺酮类和四环素类抗生素联合暴露于新生斑马鱼胚胎至96 hpf后,最终18.75 mg·L-1联合暴露组平均速度显著低于对照组,而4.69 mg·L-1和9.38 mg·L-1处理组平均速度高于对照组。在低浓度的红霉素(1 μg·L-1和10 μg·L-1)染毒中也发现斑马鱼幼鱼的移动距离较多的现象[22]。
表2 抗生素对斑马鱼行为的影响
Table 2 Summary of the effects of antibiotics on zebrafish behavior

续表2

低浓度抗生素造成斑马鱼(3~5月龄)焦虑样行为(表2)。在3个月的持续暴露实验中,Wang等[23]将6 hpf的斑马鱼卵暴露在β-二酮抗生素(氧氟沙星、环丙沙星、恩诺沙星、强力霉素、金霉素和土霉素等浓度等体积和等质量混合物)中,发现低浓度的β-二酮抗生素(6.25 mg·L-1)会导致斑马鱼在上部停留的时间高于对照组40%,运动距离也增加15%(新型水槽试验),而高浓度的β-二酮抗生素(25 mg·L-1)会导致斑马鱼在上部停留的时间同对照组相比减少29%,产生焦虑样行为。此外,Qiu等[24]将120 dpf斑马鱼暴露于环境浓度(5 μg·L-1)土霉素(oxytetracycline, OTC)中1个月后,在黑白箱测试实验中发现斑马鱼的总游泳距离增加。
1.2 抗生素对成年斑马鱼(5月龄以上)焦虑/抑郁样行为影响(Effects of antibiotics on anxiety/depression related behaviors of adult zebrafish (5 months and older))
抗生素可引起斑马鱼(5月龄以上)产生焦虑样相关行为(表2)。土霉素会引起成年斑马鱼的焦虑样行为,暴露于在OTC浓度为10、20和100 mg·L-1时,新型水槽试验中斑马鱼在顶部停留的时间均少于对照组,说明斑马鱼有焦虑倾向[25-26]。与此相似,氧氟沙星、磺胺甲恶唑(sulfamethoxazole, SMX)会缩短其在顶部停留的时间,显著增加在底部区域的时间[27]。Almeida等[28]将斑马鱼成鱼暴露于0、0.1、10、10 000 mg·L-1的OTC中2个月后,在新型水槽试验中发现暴露于OTC组别的斑马鱼在底部的停留时间随OTC浓度升高有上升的趋势,而0.1 mg·L-1和10 000 mg·L-1的OTC还增加了斑马鱼游动的距离(增加了35.1%和30.4%)。Petersen等[29]发现25 mg·L-1环丙沙星、12.5 mg·L-1和25 mg·L-1头孢他啶和12.5 mg·L-1金霉素组暴露也增加了成年斑马鱼的游泳距离(暴露96 h)。Suryanto等[30]将成年斑马鱼分别暴露在100 μg·L-1的20种抗生素溶液中,通过3D运动测试的方法,发现红霉素、庆大霉素、磺胺嘧啶、四环素显著增加斑马鱼在水缸顶部的时间,并表现出抗焦虑行为。
2 抗生素通过肠-脑有关途径影响斑马鱼的焦虑/抑郁样行为(Antibiotics affect anxiety/depression-like behavior in zebrafish through gut-brain pathways)
抗生素引起斑马鱼肠道菌群的失衡,肠道菌群的变化通过肠-脑有关途径引发斑马鱼的焦虑/抑郁样行为。
2.1 抗生素能够引起斑马鱼肠道菌群及其代谢物质组成和水平的改变(Antibiotics can cause changes in the intestinal flora of zebrafish)
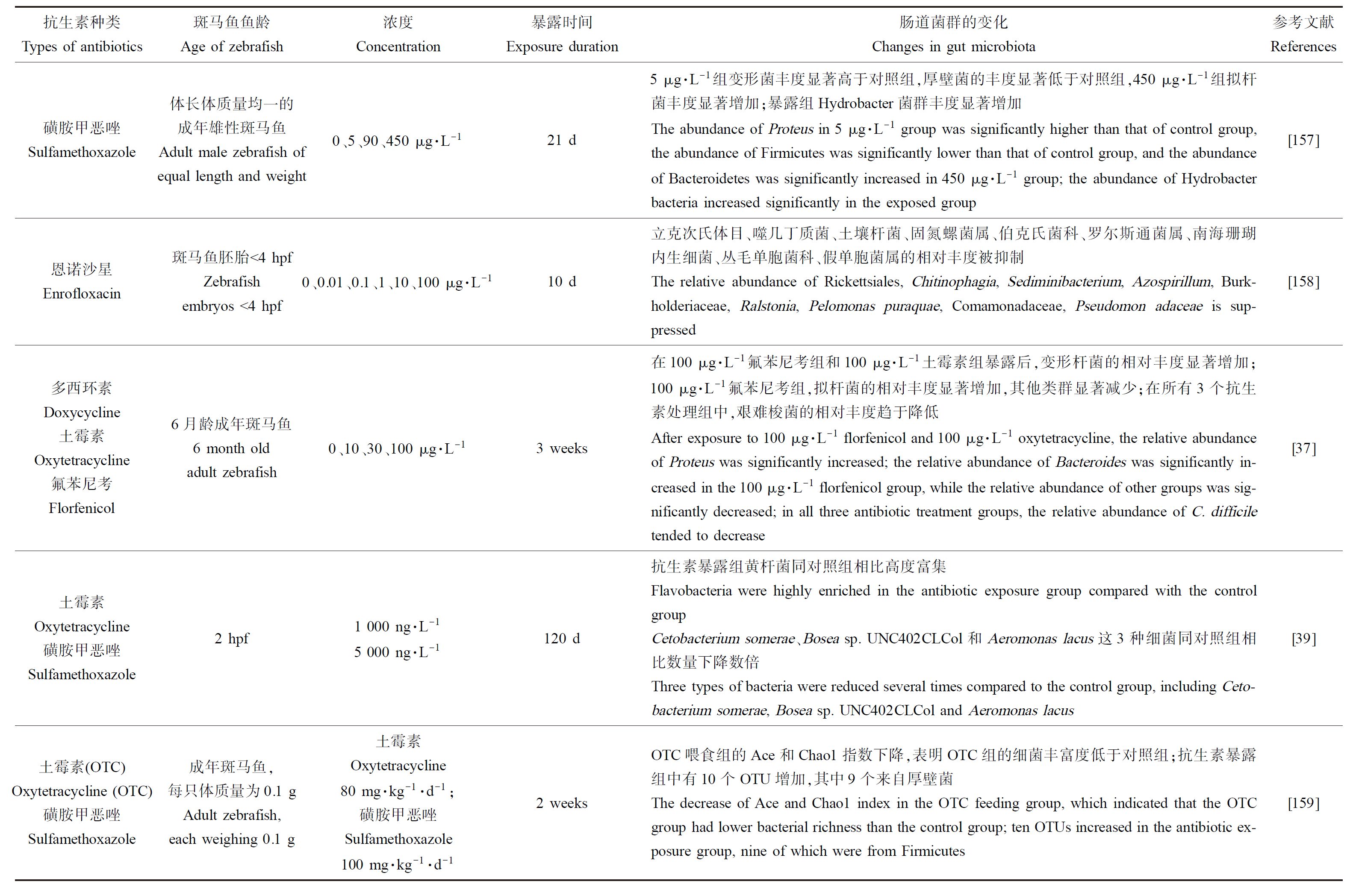
研究表明,环境浓度抗生素导致斑马鱼的肠道菌群失调(表3)。肠道菌群在调节肠上皮细胞分化、增殖、代谢功能和免疫反应、血管生成和宿主生长方面起到重要的作用[31-32]。此外,肠道菌群可以合成并释放γ-氨基丁酸、组胺、胆碱和乙酰胆碱等多种神经递质和神经肽,同时分泌细菌素和支链氨基酸或短链脂肪酸等物质,介导生理效应[33-35]。当肠道菌群紊乱时,通过肠-脑有关途径引起机体行为改变。抗生素能引起肠道菌群物种多样性的降低和改变菌体代谢物的水平和种类[36]。Qian等[37]将6月龄斑马鱼暴露在多西环素(doxycycline, DOX)、OTC和氟苯尼考(florfenicol, FF)(浓度为0、10、30、100 μg·L-1)中21 d,研究表明,同对照组相比,抗生素暴露组肠道菌群的数量和α-多样性指数均有所下降。在门水平上,100 μg·L-1 FF组和OTC组中变形杆菌(Proteus)和拟杆菌(Bacteroidaceae)的相对丰度显著增加,其他的类群显著减少。抗生素暴露组的梭杆菌门(Fusobacteria)的相对丰度均低于对照组。Zhou等[38]将体质量为0.1 g的斑马鱼分别暴露在260 ng·L-1 SMX和420 ng·L-1 OTC中6周,结果表明,同对照组相比,抗生素暴露组的Ace和Chao1指数显著降低,菌群丰富度降低。此外,较低的环境抗生素浓度也会改变斑马鱼的肠道菌群组成,Kayani等[39]用1 000 ng·L-1和5 000 ng·L-1来代表环境中残留的SMZ和OTC的抗生素浓度,将2 hpf的鱼卵暴露至120 dpf,其中,抗生素暴露组中斑马鱼肠道黄杆菌(Flavobacterium)高度富集,影响了肠道内黄杆菌的水平。
表3 抗生素对斑马鱼肠道菌群的影响
Table 3 Effects of antibiotics on intestinal microflora of zebrafish
2.2 肠道菌群的改变引起斑马鱼焦虑/抑郁样行为可能作用机制(Mechanisms for changes in intestinal flora that cause anxiety/depression-like behavior in zebrafish)
抗生素通过肠道菌群改变引起斑马鱼的焦虑/抑郁样行为(机制见图1)。文献表明抗生素所引起肠道菌群的改变可能通过5种途径影响大脑,第1种是神经系统途径[40]。第2种是神经内分泌系统途径。第3种是免疫系统途径。第4种为肠道菌群的代谢途径。第5种是氧化应激途径。
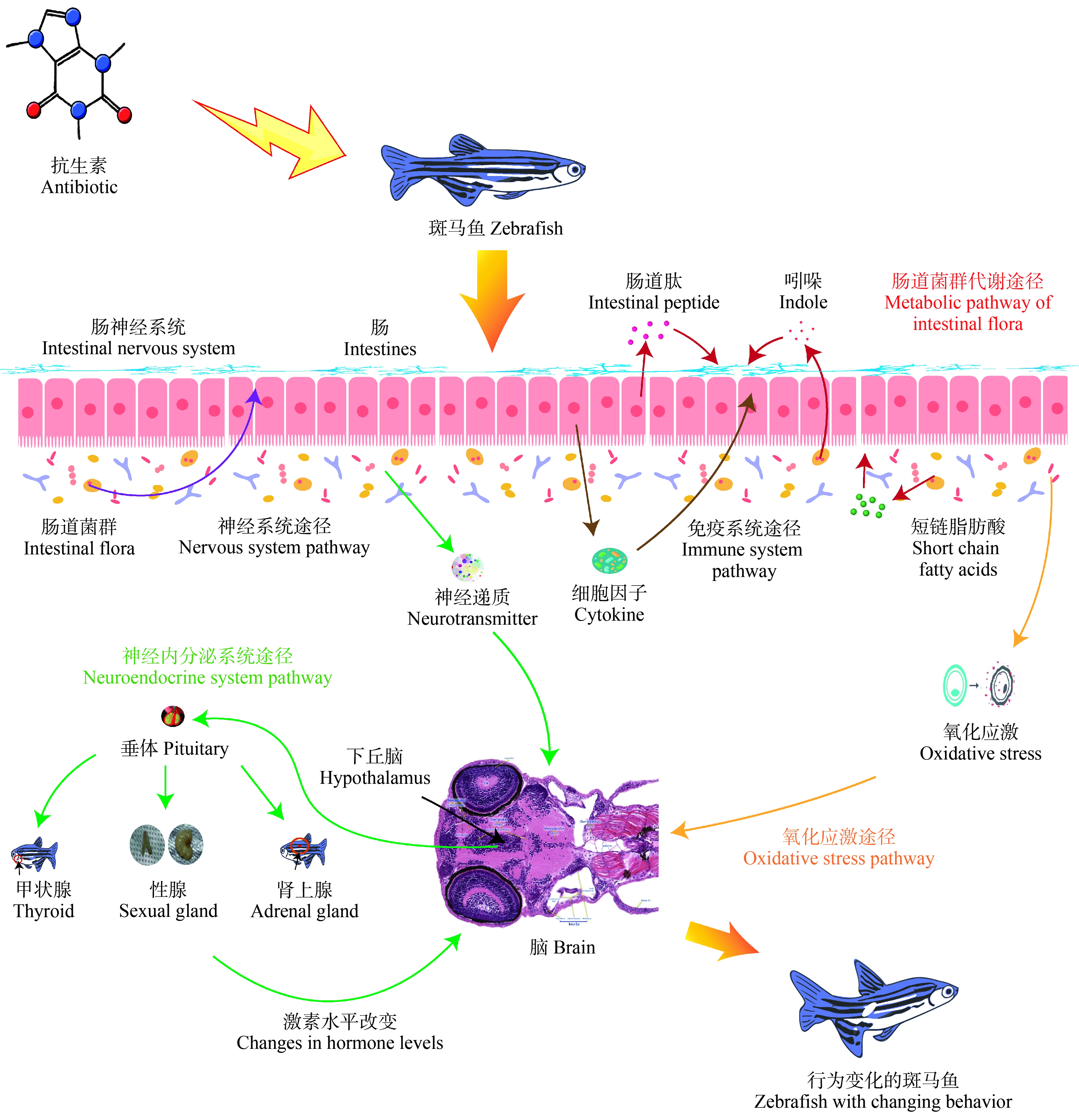
图1 抗生素引起斑马鱼行为变化可能的机制
注:图中包括神经系统途径、神经内分泌系统途径、免疫系统途径、肠道菌群代谢途径和氧化应激途径。
Fig. 1 Possible mechanism of antibiotic induced behavior change in zebrafish
Note: The figure includes nervous system pathway, neuroendocrine system pathway, immune system pathway, intestinal flora metabolic pathway and oxidative stress pathway.
2.2.1 神经系统途径
肠道菌群调控肠神经系统和中枢神经系统[41],还可通过迷走神经联系大脑传递信息[16]。抗生素能改变肠道菌群组成和丰度,对中枢神经系统和肠神经系统产生影响[42]。研究表明,肠道微生物群与焦虑/抑郁关系密切[43]。因此,抗生素引起肠道菌群的变化能够诱发机体焦虑/抑郁样行为。通常,肠腔内包含常驻菌群以及非致病菌和其他微生物。健康状态下,肠道管壁存在黏膜,菌体无法进入宿主体内[44]。当抗生素破坏肠道菌群稳定时,改变了肠道通透性,易引起肠易激综合征(irritable bowel syndrome, IBS)和炎症性肠病(inflammatory bowel disease, IBD)[45-46]。在IBS患者中,其中枢神经系统对肠道传入信号的处理及对肠神经系统的调节发生异常,IBS和IBD又同焦虑抑郁症状密不可分[47-50]。经研究发现,同时患有IBS和焦虑抑郁的人同对照组相比,其肠道菌群α-多样性偏低,变形杆菌(Proteus)、普氏菌(Prevotella copri)、拟杆菌(Bacteroidaceae)的丰度偏低[51]。抗生素影响斑马鱼肠道菌群的平衡,进而通过神经系统传递信号影响大脑,从而产生焦虑抑郁样行为。
2.2.2 神经内分泌系统途径
2.2.2.1 神经递质
肠道菌群能够产生神经递质,并且肠道菌群的代谢物可调节机体内神经递质的水平[52]。肠道菌群的失衡会影响神经递质的合成。单胺神经递质学说是抑郁症中研究最广泛,接受度最高的学说[40]。单胺类神经递质主要包含肾上腺素(epinephrine, E)、去甲肾上腺素(noradrenaline, NE)、5-羟色胺(5-hydroxytryptamine, 5-HT)、多巴胺(dopamine, DA)。研究者将健康小鼠和灌胃抑郁症患者粪菌群的小鼠海马各递质含量进行了对比,使用ELISA法测定了海马5-HT、NE和DA的水平,发现同空白对照组相比,抑郁菌群组5-HT、DA和NE水平显著降低,结果表明抑郁患者肠道的微生物群能够降低海马内神经递质的水平,引发抑郁焦虑行为。抑郁症相关的另一种学说是神经营养因子学说,脑源性神经营养因子(brain derived neurotrophic factor, BDNF)是一种具有神经营养作用的蛋白质。有研究显示,抑郁症患者体内BDNF的含量同正常水平相比,下降较多[53]。另外,乳酸菌、双歧杆菌等肠道益生菌种可以产生γ-氨基丁酸和BDNF调节机体平衡[54]。研究发现,一些细菌可能通过其代谢产物来影响肠内分泌细胞合成和释放γ-氨基丁酸、5-羟色胺、儿茶酚胺和乙酰胆碱等神经递质,还可通过血脑屏障(blood brain barrier, BBB),参与大脑中神经递质的合成循环。当肠道菌群发生改变时,其代谢物所调节的神经递质及其前体也可能发生改变,诱发神经系统疾病,引发焦虑抑郁样行为[55-56]。综上,抗生素会破坏肠道菌群的平衡及其所代谢物质种类和水平,从而影响机体平衡。当肠道菌群发生紊乱时,影响下丘脑神经递质的合成和异常表达,从而提高抑郁风险,影响情绪和运动[40]。
2.2.2.2 下丘脑-垂体-肾上腺轴(hypothalamic pituitary adrenal axis, HPA)
抑郁症患者和HPA轴过度活动存在密切联系[57-58]。HPA在动物体内是一个在面对焦虑或不适宜刺激时重要的适应性系统[59],当面对不适宜刺激时,下丘脑室旁核中的神经元会合成并且分泌促肾上腺皮质激素释放因子(corticotropin-releasing factor, CRF),随后CRF作用于垂体前叶促进促肾上腺皮质激素(adrenocorticotrophic hormone, ACTH)的合成和释放,之后,ACTH作用于肾上腺皮质产生糖皮质激素[60]。研究表明,肠道微生物群能够影响HPA轴[61],当肠道微生物的失调时会损害HPA轴,影响炎性因子和皮质醇的含量[62-65]。
糖皮质激素是HPA轴的最主要末端产物,皮质醇是糖皮质激素生物活性的代表。通过对焦虑、抑郁等患有精神障碍的患者的唾液、血浆和尿液进行分析,发现皮质醇的含量增加[66]。研究发现,抗生素可以影响斑马鱼皮质醇的含量。Gusso等[25]将8月龄斑马鱼暴露于土霉素10、20和100 mg·L-1中96 h,抗生素暴露组同对照组相比,皮质醇水平显著降低,并引起了斑马鱼的焦虑/抑郁样行为。机体在面对刺激时,皮质醇能够维持机体生理的平衡状态,对蛋白质、脂肪和糖的代谢起到重要作用。皮质醇和血浆皮质类固醇结合球蛋白(corticosteroid binding globulin, CBG)结合后,可以发挥类似组织缓冲液的作用,来抵抗皮质醇的升高所引起的非稳态状态,例如焦虑抑郁等。血浆CBG能够在血浆中储存皮质醇,当需要时释放,过多时储存[67]。抗生素影响肠道菌群的平衡,肠道菌群的紊乱会影响糖皮质激素及其活性代表皮质醇的含量,导致HPA轴的过度激活,引起负反馈调节发生障碍,丧失稳态,诱发焦虑抑郁等症状,从而引起机体行为的变化[68-70]。
2.2.2.3 下丘脑-垂体-甲状腺轴(hypothalamic pituitary thyroid axis, HPT)
肠道菌群及其代谢物能通过HPT轴影响甲状腺素含量,甲状腺素水平与焦虑/抑郁症状关系密切[71]。研究表明,焦虑/抑郁症患者的游离的甲状腺素T4(tetriodothyronine, T4)浓度约有30%的患者高于正常值,脑脊液中游离的甲状腺素T4浓度在发病期间增加,焦虑/抑郁严重患者其甲状腺素T3(triiodothyronine, T3)水平低于正常水平[72-74]。除此之外,焦虑/抑郁时促甲状腺激素(thyroid stimulating hormone, TSH)水平和对促甲状腺素释放激素(thyroid releasing hormone, TRH)的反应性均降低[75-76]。抗生素引起肠道菌群的紊乱易引起细胞壁易位,增加宿主肠道黏膜损伤几率,脂多糖等肠道菌群内毒素物质得以进入肠道内部,引起内毒素性毒血症的同时,抑制肝脏Ⅰ型碘甲状腺原氨酸脱碘酶(liver type Ⅰ iodothyronine deiodinase, D1)的活性,并刺激下丘脑和垂体前叶的Ⅱ型碘甲状腺氨酸脱碘化酶(type Ⅱ iodothyronine deiodinase, D2)将甲状腺素转化为T3。故当抗生素引起肠道菌群及其代谢物紊乱时,会影响HPT轴内相关激素水平,最终引发机体的焦虑抑郁样行为。
2.2.2.4 下丘脑-垂体-性腺轴(hypothalamic pituitary gonadal axis, HPG)
下丘脑、垂体和性腺三者之间通过促性腺激素释放激素、促性腺激素、性腺激素以及反馈和负反馈机制调控机体生殖和性行为。有研究表明,肠道微生物可直接或间接影响性类固醇激素的水平[77]。同时,Shin等[78]的实验证明性类固醇激素水平同肠道微生物组成密切相关。去除性腺的雌和雄小鼠其肠道菌群组成也会受到影响[79-80]。肠道菌群的变化会影响下丘脑,进而通过HPG轴影响性激素水平[81]。在另一项对去卵巢的女性服用黄体酮类药物实验中,黄体酮增加海马BDNF基因表达水平,减少了焦虑和抑郁样行为,肠道内乳酸杆菌水平增加[82]。此外,肠道菌群和性激素间可能存在双向作用[83],Sovijit等[82]和Audet[84]的研究提示性激素水平或性别不同所引发的焦虑/抑郁样行为或其他行为变化时,肠道菌群水平受到影响。综上所述,抗生素对肠道菌群的影响会通过HPG轴影响性激素的水平,性激素水平的改变又会作用于肠道菌群,肠道菌群变化信号传递至大脑,引起机体行为变化,产生焦虑抑郁样行为。
2.2.3 免疫系统途径
肠道菌群的失衡诱发免疫途径中细胞因子水平改变,促进炎性因子的分泌,引起肠道的炎症并导致IBD[65]。而研究发现同时患有IBD和抑郁焦虑的患者,会更容易激活炎症途径[85]。炎性因子所引发的炎症反应信号通过中枢神经系统作用于脑部,改变动物体行为。正常情况下,炎症反应是控制损伤或防止感染时对机体具有积极的作用,但抗生素的长期暴露所导致炎性因子过度表达,加剧炎症反应,破坏了肠道菌群内有益菌群的平衡,引发机体不良反应[64]。除此之外,Su等[81]的研究表明,增加益生菌和糖皮质激素联合治疗克罗恩病的患者同常规治疗的患者炎性因子显著降低的程度更高,提高乳酸杆菌等肠道益生菌的水平更显著。Desbonnet等[86]研究发现经过益生菌-双歧杆菌长期饲养的小鼠同对照组相比,双歧杆菌组能够抑制免疫细胞的产生和细胞因子的释放。双歧杆菌和瑞士乳杆菌等益生菌能够在细胞因子丝裂原的刺激下,减少白介素-6(interleukin-6, IL-6)等炎症因子的产生,减轻炎症反应[87]。IL-6和核因子κB (nuclear factor κB, NF-κB)的浓度变化也与失眠关系密切[88]。失眠能够引起免疫异常,致使IL-6炎性因子升高[89],会增加焦虑/抑郁风险。细胞因子还可以通过激活尿氨酸途径来影响色氨酸的含量,色氨酸是5-HT的前体,5-HT与大脑情绪方面联系密切。进而诱发焦虑抑郁,引起行为的变化[63]。通过上述可知,抗生素的暴露损害肠道菌群的平衡,加剧机体的炎症反应,炎症反应的信号主要通过神经传递至大脑,引起焦虑抑郁样行为变化。
2.2.4 肠道菌群代谢途径
肠道菌群的代谢物调节动物机体稳态,影响大脑及各器官功能[90]。
2.2.4.1 短链脂肪酸(short chain fatty acid, SCFA)
SCFA是碳原子数小于6的羧酸,是肠道微生物的重要代谢产物[91]。SCFA能够促进有益肠道菌群的定植,加速肠组织生长和成熟[92]、保持肠道屏障的完整,抑制肠道炎症[93]、增强肠道功能[94]、改善认知能力和脑源性疾病[95]。SCFA还能调节巨噬细胞、中性粒细胞,影响T、B细胞的分化,发挥广泛抗炎作用[91, 96]。研究表明,焦虑症患者的肠道同正常对照相比产生SCFA的细菌减少[97]。抗生素会引发肠道菌群的改变,从而导致SCFA的产量下降,在维持肠道屏障、免疫调节和脑部调节均会失衡,引起焦虑抑郁,改变行为。
2.2.4.2 吲哚
吲哚是吡咯与苯并联的化合物,通常由肠道菌群代谢色氨酸所转变而来[98],是肠道菌群代谢色氨酸的主要产物[99]。吲哚可以通过肠神经或激素将信号传递至大脑[100]。研究表明,抗生素引起肠道菌群及其代谢物水平失衡后,可能会产生过量的吲哚[99]。正常情况下,吲哚可对动物体机内的氧化应激、肠道炎症和激素分泌进行调节[101]。过量的吲哚会被肠道上皮和肝脏异种代谢酶进一步转化,形成氧化吲哚和靛红[101]。不适宜剂量的氧化吲哚会造成动物体丧失扶正反应,甚至昏迷,高剂量的靛红则可令机体产生显著抑郁行为,并损害认知功能[102-106]。Jaglin等[99]研究发现,肠道菌群极易产生吲哚的人,其患有抑郁的几率也会更大。
2.2.4.3 肠道肽
肠道是重要的内分泌器官,可分泌胰高血糖素样肽、肽YY、胆囊收缩素、CRF等多种信号分子[107]。肠道中所释放的肽类可向大脑发出信号或其受体在脑中大量表达,而且,这些肽类同焦虑抑郁等不良症状联系密切[108-109]。肠道肽的种类和浓度受肠道菌群的密切调控[110]。其中,较为典型的是血管活性肠肽(vasoactive intestinal peptide, VIP),VIP是一种在肠道中发现由28个氨基酸所组成的短蛋白,可作为神经递质和神经调节剂[111],具有神经保护活性[112]。研究表明,将VIP注入小鼠海马CA1区后,小鼠产生了焦虑样行为。将VIP 100 ng注入切除嗅球的大鼠,结果抑郁大鼠产生了抗焦虑行为。表明海马区的VIP有关神经元同情绪调节和焦虑抑郁等方面存在密切联系[111, 113]。抗生素影响肠道菌群及其代谢物的平衡,肠道肽的种类和浓度又受到肠道菌群的精确调控,影响肠道肽的表达,从而影响和脑部的信号交流,导致焦虑/抑郁样行为。
2.2.5 氧化应激途径
氧化应激是指体内氧化与抗氧化作用失衡的一种状态,倾向于氧化。研究表明,患有氧化应激型帕金森病的患者,其患有肠道炎症、便秘和肠通透性的几率增加。另外,一些肠道菌群的代谢产物同活性氧的水平直接相关。研究表明肠道菌群同氧化应激的关系十分紧密[114-116]。Ostojic[117]研究发现肠道菌群代谢产生H2的减少是引起氧化应激型帕金森病的重要因素,OHTA,S向患有氧化应激型帕金森病的大鼠给予含有50% H2的饱和水,发现可减少黑质内的氧化应激标记物[117-118],由此证明肠道菌群的某些代谢物与氧化应激有关。并且,经相关研究表明,氧化应激同焦虑/抑郁样行为存在关联[119-124],Salim等[125-126]将无毒剂量的氧化应激标记物1-丁硫宁-(S,R)-磺酰亚胺注入大鼠腹腔内,大鼠产生了焦虑样行为。给予大鼠含黄嘌呤的饮水和黄嘌呤氧化酶(此方法可增加大鼠氧化应激标记物,诱导氧化应激),发现血清皮质酮水平升高,大鼠同样产生了焦虑样行为。此外,肠道微生物还能够调节线粒体内过氧化物酶体增殖物,激活受体γ共激活因子-1α,沉默神经调节蛋白1等基因的转录,对线粒体的能量代谢和炎症反应产生影响[127]。当肠道微生物变化时影响氧化应激,造成线粒体功能障碍,从而影响中枢神经系统,引起机体行为改变[128-129]。抗生素对肠道菌群及其代谢物的影响,会引起机体产生氧化应激,氧化应激与焦虑抑郁样行为关系紧密,进而诱导机体产生行为变化。
3 展望(Expectation)
抗生素的残留和不合理使用所导致的污染是一个全球的生态环境问题,对抗生素的污染的研究要扩大广度和深度,并开展系统性的研究。由于抗生素的种类和浓度不同,对不同动物的损害部位和严重程度不一,作用机制模糊,所以无法具体判断是哪一种或多种抗生素所引起的损害。值得注意的是,斑马鱼是一种较好的神经系统模型动物,具有产卵多,价格低,对药物敏感的优点,对于评价抗生素的潜在危害性具有重要意义。未来应加强以下几方面研究。
(1)加强真实环境残留抗生素暴露情况及生物蓄积的研究。抗生素单一或联合作用于机体,机制复杂,会受到抗生素的种类、浓度和动物的种类、环境有关影响(如土壤成分、水质、植物分泌物质等)多种因素的共同影响。目前研究集中于单一或多种抗生素暴露于人造水环境和特定动物,不能阐明环境中抗生素对动物的复杂影响。未来应提出更能符合真实环境的实验设计,充分考虑抗生素同环境的相互影响和抗生素的降解,以及进入动物机体后对其的处理等方面进行系统深入的研究。而且,抗生素对动物的研究集中在受试动物,对食物链/食物网和遗传到子一代的研究较少,应增加抗生素的残留是否随食物链/网和遗传方向进行深入研究。
(2)加强多物种的对比研究。斑马鱼是一种较好的神经行为观测模型,但作为水生模式生物,水中环境因子如pH、溶解氧、硬度、盐度等均可能引起斑马鱼行为的变化。另外,行为分析上,缺少对斑马鱼行为方式的精密描述和复杂行为的定量分析。鱼作为低等脊椎动物与哺乳动物和人类相比,在生理生化等各个方面,特别是疾病发生机制等方面存在一定差异性,因此试验结果还需要在临床上进行验证。
(3)进一步加强”肠-脑”途径的研究。此外,斑马鱼虽然同人类有87%的基因相似度,然而水生动物和哺乳动物的疾病发生机制还是有区别。抗生素作用于斑马鱼通过”肠-脑”有关途径引发焦虑/抑郁行为,该机制是依据其他物种的研究和结合大量文献所做出的推测。未来应从无菌斑马鱼和微生物移植实验入手,加强”肠-脑”途径机制的深入探究。
[1] Danner M C, Robertson A, Behrends V, et al. Antibiotic pollution in surface fresh waters: Occurrence and effects [J]. The Science of the Total Environment, 2019, 664: 793-804
[2] Yeom J R, Yoon S U, Kim C G. Quantification of residual antibiotics in cow manure being spread over agricultural land and assessment of their behavioral effects on antibiotic resistant bacteria [J]. Chemosphere, 2017, 182: 771-780
[3] Xu W H, Yan W, Li X D, et al. Antibiotics in riverine runoff of the Pearl River Delta and Pearl River Estuary, China: Concentrations, mass loading and ecological risks [J]. Environmental Pollution, 2013, 182: 402-407
[4] Wei R C, Ge F, Huang S Y, et al. Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China [J]. Chemosphere, 2011, 82(10): 1408-1414
[5] Sim W J, Lee J W, Lee E S, et al. Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures [J]. Chemosphere, 2011, 82(2): 179-186
[6] Kovalakova P, Cizmas L, McDonald T J, et al. Occurrence and toxicity of antibiotics in the aquatic environment: A review [J]. Chemosphere, 2020, 251: 126351
[7] Cachat J, Canavello P, Elegante M, et al. Modeling withdrawal syndrome in zebrafish [J]. Behavioural Brain Research, 2010, 208(2): 371-376
[8] Egan R J, Bergner C L, Hart P C, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish [J]. Behavioural Brain Research, 2009, 205(1): 38-44
[9] Kalueff A V, Echevarria D J, Stewart A M. Gaining translational momentum: More zebrafish models for neuroscience research [J]. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 2014, 55: 1-6
[10] Wong K, Elegante M, Bartels B, et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio) [J]. Behavioural Brain Research, 2010, 208(2): 450-457
[11] Blaser R, Gerlai R. Behavioral phenotyping in zebrafish: Comparison of three behavioral quantification methods [J]. Behavior Research Methods, 2006, 38(3): 456-469
[12] Howe K, Clark M D, Torroja C F, et al. The zebrafish reference genome sequence and its relationship to the human genome [J]. Nature, 2013, 496(7446): 498-503
[13] Howe K, Clark M D, Torroja C F, et al. Correction: Corrigendum: The zebrafish reference genome sequence and its relationship to the human genome [J]. Nature, 2014, 505(7482): 248
[14] Kettleborough R N W, Busch-Nentwich E M, Harvey S A, et al. A systematic genome-wide analysis of zebrafish protein-coding gene function [J]. Nature, 2013, 496(7446): 494-497
[15] Mueller T, Vernier P, Wullimann M F. The adult central nervous cholinergic system of a neurogenetic model animal, the zebrafish Danio rerio [J]. Brain Research, 2004, 1011(2): 156-169
[16] Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis [J]. Frontiers in Neuroscience, 2018, 12: 49
[17] 黄倩, 曾霖, 王高祥, 等. 基于微生物-肠-脑轴理论中医调节肠道菌群治疗失眠研究进展[J]. 天津中医药, 2022, 39(4): 538-544
Huang Q, Zeng L, Wang G X, et al. Research progress in the traditional Chinese medicine treatment of insomnia by adjusting intestinal flora based on microbiota-gut-brain axis theory [J]. Tianjin Journal of Traditional Chinese Medicine, 2022, 39(4): 538-544 (in Chinese)
[18] Gonçalves C L, Vasconcelos F F P, Wessler L B, et al. Exposure to a high dose of amoxicillin causes behavioral changes and oxidative stress in young zebrafish [J]. Metabolic Brain Disease, 2020, 35(8): 1407-1416
[19] Cheng B, Jiang F, Su M L, et al. Effects of lincomycin hydrochloride on the neurotoxicity of zebrafish [J]. Ecotoxicology and Environmental Safety, 2020, 201: 110725
[20] Han E, Oh K H, Park S, et al. Analysis of behavioral changes in zebrafish (Danio rerio) larvae caused by aminoglycoside-induced damage to the lateral line and muscles [J]. Neurotoxicology, 2020, 78: 134-142
[21] Zhang Y N, Wang X D, Yin X H, et al. Toxicity assessment of combined fluoroquinolone and tetracycline exposure in zebrafish (Danio rerio) [J]. Environmental Toxicology, 2016, 31(6): 736-750
[22] Minski V T, Garbinato C, Thiel N, et al. Erythromycin in the aquatic environment: Deleterious effects on the initial development of zebrafish [J]. Journal of Toxicology and Environmental Health Part A, 2021, 84(2): 56-66
[23] Wang X D, Zheng Y S, Zhang Y N, et al. Effects of β-diketone antibiotic mixtures on behavior of zebrafish (Danio rerio) [J]. Chemosphere, 2016, 144: 2195-2205
[24] Qiu Y S, Yu K, Yu X G, et al. Long-term low-dose oxytetracycline potentially leads to neurobehavioural changes [J]. Ecotoxicology and Environmental Safety, 2021, 223: 112546
[25] Gusso D, Altenhofen S, Fritsch P M, et al. Oxytetracycline induces anxiety-like behavior in adult zebrafish [J]. Toxicology and Applied Pharmacology, 2021, 426: 115616
[26] Horzmann K A, Lin L F, Taslakjian B, et al. Anxiety-related behavior and associated brain transcriptome and epigenome alterations in adult female zebrafish exposed to atrazine during embryogenesis [J]. Chemosphere, 2022, 308(Pt 3): 136431
[27] Martinez J L. Environmental pollution by antibiotics and by antibiotic resistance determinants [J]. Environmental Pollution, 2009, 157(11): 2893-2902
[28] Almeida A R, Tacão M, Machado A L, et al. Long-term effects of oxytetracycline exposure in zebrafish: A multi-level perspective [J]. Chemosphere, 2019, 222: 333-344
[29] Petersen B D, Pereira T C B, Altenhofen S, et al. Antibiotic drugs alter zebrafish behavior [J]. Comparative Biochemistry and Physiology Toxicology &Pharmacology, 2021, 242: 108936
[30] Suryanto M E, Yang C C, Audira G, et al. Evaluation of locomotion complexity in zebrafish after exposure to twenty antibiotics by fractal dimension and entropy analysis [J]. Antibiotics, 2022, 11(8): 1059
[31] Li P P, Zhang J H, Liu X Y, et al. The function and the affecting factors of the zebrafish gut microbiota [J]. Frontiers in Microbiology, 2022, 13: 903471
[32] Xiao F S, Zhu W G, Yu Y H, et al. Interactions and stability of gut microbiota in zebrafish increase with host development [J]. Microbiology Spectrum, 2022, 10(2): e0169621
[33] Li J H, Jia H J, Cai X H, et al. An integrated catalog of reference genes in the human gut microbiome [J]. Nature Biotechnology, 2014, 32(8): 834-841
[34] Mittal R, Debs L H, Patel A P, et al. Neurotransmitters: The critical modulators regulating gut-brain axis [J]. Journal of Cellular Physiology, 2017, 232(9): 2359-2372
[35] Baxter N T, Schmidt A W, Venkataraman A, et al. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers [J]. mBio, 2019, 10(1): e02566-e02518
[36] Ramirez J, Guarner F, Bustos Fernandez L, et al. Antibiotics as major disruptors of gut microbiota [J]. Frontiers in Cellular and Infection Microbiology, 2020, 10: 572912
[37] Qian M R, Wang J M, Ji X F, et al. Sub-chronic exposure to antibiotics doxycycline, oxytetracycline or florfenicol impacts gut barrier and induces gut microbiota dysbiosis in adult zebrafish (Daino rerio) [J]. Ecotoxicology and Environmental Safety, 2021, 221: 112464
[38] Zhou L, Limbu S M, Shen M L, et al. Environmental concentrations of antibiotics impair zebrafish gut health [J]. Environmental Pollution, 2018, 235: 245-254
[39] Kayani M U R, Yu K, Qiu Y S, et al. Environmental concentrations of antibiotics alter the zebrafish gut microbiome structure and potential functions [J]. Environmental Pollution, 2021, 278: 116760
[40] 刘灿, 王宇红, 赵洪庆, 等. 微生物-肠-脑轴与抑郁症相关研究进展[J]. 医学综述, 2022, 28(2): 224-228
Liu C, Wang Y H, Zhao H Q, et al. Research advances in microbiota-gut-brain axis and depression [J]. Medical Recapitulate, 2022, 28(2): 224-228 (in Chinese)
[41] Furness J B. The enteric nervous system and neurogastroenterology [J]. Nature Reviews Gastroenterology &Hepatology, 2012, 9(5): 286-294
[42] Kuwahara A, Matsuda K, Kuwahara Y, et al. Microbiota-gut-brain axis: Enteroendocrine cells and the enteric nervous system form an interface between the microbiota and the central nervous system [J]. Biomedical Research, 2020, 41(5): 199-216
[43] Foster J A, McVey Neufeld K A. Gut-brain axis: How the microbiome influences anxiety and depression [J]. Trends in Neurosciences, 2013, 36(5): 305-312
[44] Gareau M, Silva M, Perdue M. Pathophysiological mechanisms of stress-induced intestinal damage [J]. Current Molecular Medicine, 2008, 8(4): 274-281
[45] Faure C, Thapar N, Lorenzo C D. Pediatric Neurogastroenterology: Gastrointestinal Motility and Functional Disorders in Children [M]. Cham: Springer, 2017: 385-398
[46] Mawdsley J E, Rampton D S. Psychological stress in IBD: New insights into pathogenic and therapeutic implications [J]. Gut, 2005, 54(10): 1481-1491
[47] Hu Z C, Li M X, Yao L, et al. The level and prevalence of depression and anxiety among patients with different subtypes of irritable bowel syndrome: A network meta-analysis [J]. BMC Gastroenterology, 2021, 21(1): 23
[48] Kibune Nagasako C, Garcia Montes C, Silva Lorena S L, et al. Irritable bowel syndrome subtypes: Clinical and psychological features, body mass index and comorbidities [J]. Revista Espanola De Enfermedades Digestivas, 2016, 108(2): 59-64
[49] Reigada L C, Moore M T, Martin C F, et al. Psychometric evaluation of the IBD-specific anxiety scale: A novel measure of disease-related anxiety for adolescents with IBD [J]. Journal of Pediatric Psychology, 2018, 43(4): 413-422
[50] Ahmed R M, Ali M, Akram F, et al. Anxiety, depression and IBS: Results from Lahore, Pakistan [J]. Rawal Medical Journal, 2020, 45(2): 303-306
[51] Simpson C A, Mu A, Haslam N, et al. Feeling down? A systematic review of the gut microbiota in anxiety/depression and irritable bowel syndrome [J]. Journal of Affective Disorders, 2020, 266: 429-446
[52] Liu T, Huang Z S. Evidence-based analysis of neurotransmitter modulation by gut microbiota [C]. Proceedings of the 8th International Conference on Health Information Science (HIS). Xi’an: Shaanxi Normal University, 2019
[53] Brunoni A R, Machado-Vieira R, Zarate C A Jr, et al. Assessment of non-BDNF neurotrophins and GDNF levels after depression treatment with sertraline and transcranial direct current stimulation in a factorial, randomized, sham-controlled trial (SELECT-TDCS): An exploratory analysis [J]. Progress in Neuro-Psychopharmacology &Biological Psychiatry, 2015, 56: 91-96
[54] Hao W Z, Li X J, Zhang P W, et al. A review of antibiotics, depression, and the gut microbiome [J]. Psychiatry Research, 2020, 284: 112691
[55] Chen Y J, Xu J Y, Chen Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders [J]. Nutrients, 2021, 13(6): 2099
[56] Patterson E, Cryan J F, Fitzgerald G F, et al. Gut microbiota, the pharmabiotics they produce and host health [J]. The Proceedings of the Nutrition Society, 2014, 73(4): 477-489
[57] Pariante C M, Kim R B, Makoff A, et al. Antidepressant fluoxetine enhances glucocorticoid receptor function in vitro by modulating membrane steroid transporters [J]. British Journal of Pharmacology, 2003, 139(6): 1111-1118
[58] Pariante C M, Miller A H. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment [J]. Biological Psychiatry, 2001, 49(5): 391-404
[59] Jankord R, Herman J P. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress [J]. Annals of the New York Academy of Sciences, 2008, 1148(1): 64-73
[60] Zhu L J, Liu M Y, Li H, et al. The different roles of glucocorticoids in the hippocampus and hypothalamus in chronic stress-induced HPA axis hyperactivity [J]. PLoS One, 2014, 9(5): e97689
[61] Sudo N, Chida Y, Aiba Y J, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice [J]. The Journal of Physiology, 2004, 558(Pt 1): 263-275
[62] Madeeh Hashmi A, Awais Aftab M, Mazhar N, et al. The fiery landscape of depression: A review of the inflammatory hypothesis [J]. Pakistan Journal of Medical Sciences, 2013, 29(3): 877-884
[63] Miller A H, Haroon E, Raison C L, et al. Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits [J]. Depression and Anxiety, 2013, 30(4): 297-306
[64] Salim S, Chugh G, Asghar M. Inflammation in anxiety [J]. Advances in Protein Chemistry and Structural Biology, 2012, 88: 1-25
[65] Lobionda S, Sittipo P, Kwon H Y, et al. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors [J]. Microorganisms, 2019, 7(8): 271
[66] Nemeroff C B, Vale W W. The neurobiology of depression: Inroads to treatment and new drug discovery [J]. The Journal of Clinical Psychiatry, 2005, 66(Suppl 7): 5-13
[67] Breuner C W, Orchinik M. Plasma binding proteins as mediators of corticosteroid action in vertebrates [J]. Journal of Endocrinology, 2002, 175(1): 99-112
[68] Kathol R G, Jaeckle R S, Lopez J F, et al. Pathophysiology of HPA axis abnormalities in patients with major depression: An update [J]. The American Journal of Psychiatry, 1989, 146(3): 311-317
[69] Pariante C M, Lightman S L. The HPA axis in major depression: Classical theories and new developments [J]. Trends in Neurosciences, 2008, 31(9): 464-468
[70] Rivier C, Vale W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin [J]. Nature, 1983, 305(5932): 325-327
[71] Davidsen N, Ramhøj L, Lykkebo C A, et al. PFOS-induced thyroid hormone system disrupted rats display organ-specific changes in their transcriptomes [J]. Environmental Pollution, 2022, 305: 119340
[72] Kirkegaard C. The thyrotropin response to thyrotropin-releasing hormone in endogenous depression [J]. Psychoneuroendocrinology, 1981, 6(3): 189-212
[73] Baumgartner A, Gräf K J, Kürten I, et al. The hypothalamic-pituitary-thyroid axis in psychiatric patients and healthy subjects: Parts 1-4 [J]. Psychiatry Research, 1988, 24(3): 271-332
[74] Kirkegaard C, Faber J. Free thyroxine and 3,3’,5’-triiodothyronine levels in cerebrospinal fluid in patients with endogenous depression [J]. Acta Endocrinologica, 1991, 124(2): 166-172
[75] Kirkegaard C, Faber J. The role of thyroid hormones in depression [J]. European Journal of Endocrinology, 1998, 138(1): 1-9
[76] Maes M, Vandewoude M, Maes L, et al. A revised interpretation of the TRH test results in female depressed patients. Part Ⅰ: TSH responses. Effects of severity of illness, thyroid hormones, monoamines, age, sex hormonal, corticosteroid and nutritional state [J]. Journal of Affective Disorders, 1989, 16(2-3): 203-213
[77] Jaggar M, Rea K, Spichak S, et al. You’ve got male: Sex and the microbiota-gut-brain axis across the lifespan [J]. Frontiers in Neuroendocrinology, 2020, 56: 100815
[78] Shin J H, Park Y H, Sim M, et al. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome [J]. Research in Microbiology, 2019, 170(4-5): 192-201
[79] Yurkovetskiy L, Burrows M, Khan A A, et al. Gender bias in autoimmunity is influenced by microbiota [J]. Immunity, 2013, 39(2): 400-412
[80] Kaliannan K, Robertson R C, Murphy K, et al. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice [J]. Microbiome, 2018, 6(1): 205
[81] Su H, Kang Q, Wang H H, et al. Effects of glucocorticoids combined with probiotics in treating Crohn’s disease on inflammatory factors and intestinal microflora [J]. Experimental and Therapeutic Medicine, 2018, 16(4): 2999-3003
[82] Sovijit W N, Sovijit W E, Pu S X, et al. Ovarian progesterone suppresses depression and anxiety-like behaviors by increasing the Lactobacillus population of gut microbiota in ovariectomized mice [J]. Neuroscience Research, 2021, 168: 76-82
[83] Rosser E C, de Gruijter N M, Matei D E. Mini-review: Gut-microbiota and the sex-bias in autoimmunity—Lessons learnt from animal models [J]. Frontiers in Medicine, 2022, 9: 910561
[84] Audet M C. Stress-induced disturbances along the gut microbiota-immune-brain axis and implications for mental health: Does sex matter? [J]. Frontiers in Neuroendocrinology, 2019, 54: 100772
[85] Raison C L, Capuron L, Miller A H. Cytokines sing the blues: Inflammation and the pathogenesis of depression [J]. Trends in Immunology, 2006, 27(1): 24-31
[86] Desbonnet L, Garrett L, Clarke G, et al. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat [J]. Journal of Psychiatric Research, 2008, 43(2): 164-174
[87] Tejada-Simon M V, Ustunol Z, Pestka J J. Ex vivo effects of lactobacilli, streptococci, and bifidobacteria ingestion on cytokine and nitric oxide production in a murine model [J]. Journal of Food Protection, 1999, 62(2): 162-169
[88] Miller A H, Maletic V, Raison C L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression [J]. Biological Psychiatry, 2009, 65(9): 732-741
[89] Nie L, Pan X L, Zhang X B, et al. Research on the correlation of immunity in patients with chronic insomnia [J]. Frontiers in Psychiatry, 2022, 13: 1034405
[90] Nowiński A, Ufnal M. Gut bacteria-derived molecules as mediators and markers in cardiovascular diseases. The role of the gut-blood barrier [J]. Kardiologia Polska, 2018, 76(2): 320-327
[91] Yao Y, Cai X Y, Fei W D, et al. The role of short-chain fatty acids in immunity, inflammation and metabolism [J]. Critical Reviews in Food Science and Nutrition, 2022, 62(1): 1-12
[92] Liu Z M, Roy N C, Guo Y H, et al. Human breast milk and infant formulas differentially modify the intestinal microbiota in human infants and host physiology in rats [J]. The Journal of Nutrition, 2016, 146(2): 191-199
[93] Lewis K, Lutgendorff F, Phan V, et al. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate [J]. Inflammatory Bowel Diseases, 2010, 16(7): 1138-1148
[94] Kelly C J, Zheng L, Campbell E L, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function [J]. Cell Host &Microbe, 2015, 17(5): 662-671
[95] Shi H L, Ge X, Ma X, et al. A fiber-deprived diet causes cognitive impairment and hippocampal microglia-mediated synaptic loss through the gut microbiota and metabolites [J]. Microbiome, 2021, 9(1): 223
[96] Margolis K G, Cryan J F, Mayer E A. The microbiota-gut-brain axis: From motility to mood [J]. Gastroenterology, 2021, 160(5): 1486-1501
[97] Jiang H Y, Zhang X, Yu Z H, et al. Altered gut microbiota profile in patients with generalized anxiety disorder [J]. Journal of Psychiatric Research, 2018, 104: 130-136
[98] Konopelski P, Ufnal M. Indoles - gut bacteria metabolites of tryptophan with pharmacotherapeutic potential [J]. Current Drug Metabolism, 2018, 19(10): 883-890
[99] Jaglin M, Rhimi M, Philippe C, et al. Indole, a signaling molecule produced by the gut microbiota, negatively impacts emotional behaviors in rats [J]. Frontiers in Neuroscience, 2018, 12: 216
[100] Schroeder B O, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease [J]. Nature Medicine, 2016, 22(10): 1079-1089
[101] Lee J H, Wood T K, Lee J. Roles of indole as an interspecies and interkingdom signaling molecule [J]. Trends in Microbiology, 2015, 23(11): 707-718
[102] Glover V, Bhattacharya S K, Sandler M. Isatin—A new biological factor [J]. Indian Journal of Experimental Biology, 1991, 29(1): 1-5
[103] Bhattacharya S K, Mitra S K, Acharya S B. Anxiogenic activity of isatin, a putative biological factor, in rodents [J]. Journal of Psychopharmacology, 1991, 5(3): 202-206
[104] Abel E L. Behavioral effects of isatin on open field activity and immobility in the forced swim test in rats [J]. Physiology &Behavior, 1995, 57(3): 611-613
[105] Satyan K S, Rai A, Jaiswal A K, et al. Isatin, a putative anxiogenic endocoid, induces memory dysfunction in rats [J]. Indian Journal of Experimental Biology, 1995, 33(8): 576-579
[106] Carpenedo R, Mannaioni G, Moroni F. Oxindole, a sedative tryptophan metabolite, accumulates in blood and brain of rats with acute hepatic failure [J]. Journal of Neurochemistry, 1998, 70(5): 1998-2003
[107] Dockray G J. Gastrointestinal hormones and the dialogue between gut and brain [J]. The Journal of Physiology, 2014, 592(14): 2927-2941
[108] McGonigle P. Peptide therapeutics for CNS indications [J]. Biochemical Pharmacology, 2012, 83(5): 559-566
[109] Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis [J]. Neuropeptides, 2012, 46(6): 261-274
[110] Lach G, Schellekens H, Dinan T G, et al. Anxiety, depression, and the microbiome: A role for gut peptides [J]. Neurotherapeutics, 2018, 15(1): 36-59
[111] Ivanova M, Belcheva S, Belcheva I, et al. Modulatory effect of VIP injected into hippocampal CA1 area on anxiety in olfactory bulbectomized rats [J]. Acta Neurobiologiae Experimentalis, 2014, 74(3): 317-327
[112] Gozes I, Brenneman D E. VIP: Molecular biology and neurobiological function [J]. Molecular Neurobiology, 1989, 3(4): 201-236
[113] Ivanova M, Ternianov A, Belcheva I, et al. Anxiogenic effect of vasoactive intestinal polypeptide (VIP) microinjected into the CA1 hippocampal area [J]. Comptes Rendus De L Academie Bulgare Des Sciences, 2010, 63(12): 1829-1836
[114] Shandilya S, Kumar S, Kumar Jha N, et al. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection [J]. Journal of Advanced Research, 2022, 38: 223-244
[115] Nishiwaki H, Ito M, Ishida T, et al. Meta-analysis of gut dysbiosis in Parkinson’s disease [J]. Movement Disorders, 2020, 35(9): 1626-1635
[116] Chai X Y, Diwakarla S, Pustovit R V, et al. Investigation of nerve pathways mediating colorectal dysfunction in Parkinson’s disease model produced by lesion of nigrostriatal dopaminergic neurons [J]. Neurogastroenterology and Motility, 2020, 32(9): e13893
[117] Ostojic S M. Inadequate production of H2 by gut microbiota and Parkinson disease [J]. Trends in Endocrinology &Metabolism, 2018, 29(5): 286-288
[118] Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine [J]. Pharmacology &Therapeutics, 2014, 144(1): 1-11
[119] Hovatta I, Tennant R S, Helton R, et al. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice [J]. Nature, 2005, 438(7068): 662-666
[120] Gingrich J A. Oxidative stress is the new stress [J]. Nature Medicine, 2005, 11(12): 1281-1282
[121] Masood A, Nadeem A, Mustafa S J, et al. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice [J]. Journal of Pharmacology and Experimental Therapeutics, 2008, 326(2): 369-379
[122] Souza C G, Moreira J D, Siqueira I R, et al. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior [J]. Life Sciences, 2007, 81(3): 198-203
[123] Bouayed J, Rammal H, Younos C, et al. Positive correlation between peripheral blood granulocyte oxidative status and level of anxiety in mice [J]. European Journal of Pharmacology, 2007, 564(1-3): 146-149
[124] de Oliveira M R, Silvestrin R B, Mello E Souza T, et al. Oxidative stress in the hippocampus, anxiety-like behavior and decreased locomotory and exploratory activity of adult rats: Effects of sub acute vitamin A supplementation at therapeutic doses [J]. Neurotoxicology, 2007, 28(6): 1191-1199
[125] Salim S, Sarraj N, Taneja M, et al. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats [J]. Behavioural Brain Research, 2010, 208(2): 545-552
[126] Salim S, Asghar M, Chugh G, et al. Oxidative stress: A potential recipe for anxiety, hypertension and insulin resistance [J]. Brain Research, 2010, 1359: 178-185
[127] Lin X Y, Chen Y R, Zhang P, et al. The potential mechanism of postoperative cognitive dysfunction in older people [J]. Experimental Gerontology, 2020, 130: 110791
[128] Abautret-Daly  , Dempsey E, Parra-Blanco A, et al. Gut-brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease [J]. Acta Neuropsychiatrica, 2018, 30(5): 275-296
, Dempsey E, Parra-Blanco A, et al. Gut-brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease [J]. Acta Neuropsychiatrica, 2018, 30(5): 275-296
[129] Marungruang N, Arévalo Sureda E, Lefrançoise A, et al. Impact of dietary induced precocious gut maturation on cecal microbiota and its relation to the blood-brain barrier during the postnatal period in rats [J]. Neurogastroenterology and Motility, 2018, 30(6): e13285
[130] Chen J L, Huang L L, Wang Q, et al. Antibiotics in aquaculture ponds from Guilin, South of China: Occurrence, distribution, and health risk assessment [J]. Environmental Research, 2022, 204(Pt B): 112084
[131] Fan R Q, Zhang W J, Jia L, et al. Antagonistic effects of enrofloxacin on carbendazim-induced developmental toxicity in zebrafish embryos [J]. Toxics, 2021, 9(12): 349
[132] Liu J Y, Wei T Z, Wu X, et al. Early exposure to environmental levels of sulfamethoxazole triggers immune and inflammatory response of healthy zebrafish larvae [J]. The Science of the Total Environment, 2020, 703: 134724
[133] Xue C, Zheng C, Zhao Q Q, et al. Occurrence of antibiotics and antibiotic resistance genes in cultured prawns from rice-prawn co-culture and prawn monoculture systems in China [J]. The Science of the Total Environment, 2022, 806(Pt 1): 150307
[134] Li W H, Shi Y L, Gao L H, et al. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China [J]. Chemosphere, 2012, 89(11): 1307-1315
[135] Xue J J, Lei D D, Zhao X M, et al. Antibiotic residue and toxicity assessment of wastewater during the pharmaceutical production processes [J]. Chemosphere, 2022, 291(Pt 2): 132837
[136] Geng J G, Liu X Y, Wang J, et al. Accumulation and risk assessment of antibiotics in edible plants grown in contaminated farmlands: A review [J]. Science of the Total Environment, 2022, 853: 158616
[137] Du D L, Zhou J, Zhang K Q, et al. Seasonal pollution characteristics of antibiotics on pig farms of different scales [J]. International Journal of Environmental Research and Public Health, 2022, 19(14): 8264
[138] Wei R C, He T, Zhang S X, et al. Occurrence of seventeen veterinary antibiotics and resistant bacterias in manure-fertilized vegetable farm soil in four provinces of China [J]. Chemosphere, 2019, 215: 234-240
[139] Dong B C, Li W, Xu W Y. Effects of partial organic substitution for chemical fertilizer on antibiotic residues in peri-urban agricultural soil in China [J]. Antibiotics, 2021, 10(10): 1173
[140] Wang H, Chu Y X, Fang C R. Occurrence of veterinary antibiotics in swine manure from large-scale feedlots in Zhejiang Province, China [J]. Bulletin of Environmental Contamination and Toxicology, 2017, 98(4): 472-477
[141] Pan M, Yau P C. Fate of macrolide antibiotics with different wastewater treatment technologies [J]. Water, Air, and Soil Pollution, 2021, 232(3): 103
[142] Li J, Cui M, Zhang H. Spatial and temporal variations of antibiotics in a tidal river [J]. Environmental Monitoring and Assessment, 2020, 192(6): 336
[143] Leng Y F, Xiao H L, Li Z, et al. Tetracyclines, sulfonamides and quinolones and their corresponding resistance genes in coastal areas of Beibu Gulf, China [J]. The Science of the Total Environment, 2020, 714: 136899
[144] Plhalova L, Zivna D, Bartoskova M, et al. The effects of subchronic exposure to ciprofloxacin on zebrafish (Danio rerio) [J]. Neuro Endocrinology Letters, 2014, 35(Suppl 2): 64-70
[145] Li X W, Xie Y F, Li C L, et al. Investigation of residual fluoroquinolones in a soil-vegetable system in an intensive vegetable cultivation area in Northern China [J]. The Science of the Total Environment, 2014, 468-469: 258-264
[146] Hu Y R, Jin L, Zhao Y, et al. Annual trends and health risks of antibiotics and antibiotic resistance genes in a drinking water source in East China [J]. The Science of the Total Environment, 2021, 791: 148152
[147] Wang R, Feng F, Chai Y F, et al. Screening and quantitation of residual antibiotics in two different swine wastewater treatment systems during warm and cold seasons [J]. The Science of the Total Environment, 2019, 660: 1542-1554
[148] Zhang Q, Cheng J P, Xin Q. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos [J]. Ecotoxicology, 2015, 24(4): 707-719
[149] Wang W H, Zhang W F, Liang H, et al. Seasonal distribution characteristics and health risk assessment of typical antibiotics in the Harbin section of the Songhua River Basin [J]. Environmental Technology, 2019, 40(20): 2726-2737
[150] Chen Y S, Xi X P, Xu J H, et al. Distribution patterns of antibiotic residues in an urban river catchment [J]. Water and Environment Journal, 2019, 33(1): 31-39
[151] Zhi S L, Zhou J, Yang F X, et al. Systematic analysis of occurrence and variation tendency about 58 typical veterinary antibiotics during animal wastewater disposal processes in Tianjin, China [J]. Ecotoxicology and Environmental Safety, 2018, 165: 376-385
[152] Tong L, Qin L T, Xie C, et al. Distribution of antibiotics in alluvial sediment near animal breeding areas at the Jianghan Plain, Central China [J]. Chemosphere, 2017, 186: 100-107
[153] Dong H Y, Yuan X J, Wang W D, et al. Occurrence and removal of antibiotics in ecological and conventional wastewater treatment processes: A field study [J]. Journal of Environmental Management, 2016, 178: 11-19
[154] Xi J L, Liu J, He S J, et al. Effects of norfloxacin exposure on neurodevelopment of zebrafish (Danio rerio) embryos [J]. Neurotoxicology, 2019, 72: 85-94
[155] Tong C L, Zhuo X J, Guo Y. Occurrence and risk assessment of four typical fluoroquinolone antibiotics in raw and treated sewage and in receiving waters in Hangzhou, China [J]. Journal of Agricultural and Food Chemistry, 2011, 59(13): 7303-7309
[156] Carlsson G, Patring J, Kreuger J, et al. Toxicity of 15 veterinary pharmaceuticals in zebrafish (Danio rerio) embryos [J]. Aquatic Toxicology, 2013, 126: 30-41
[157] Zheng Y, Wang Y F, Zheng M T, et al. Exposed to sulfamethoxazole induced hepatic lipid metabolism disorder and intestinal microbiota changes on zebrafish (Danio rerio) [J]. Comparative Biochemistry and Physiology Toxicology &Pharmacology, 2022, 253: 109245
[158] Qiu W H, Liu T, Liu X J, et al. Enrofloxacin induces intestinal microbiota-mediated immunosuppression in zebrafish [J]. Environmental Science &Technology, 2022, 56(12): 8428-8437
[159] Zhou L, Limbu S M, Qiao F, et al. Influence of long-term feeding antibiotics on the gut health of zebrafish [J]. Zebrafish, 2018, 15(4): 340-348