-
在中国,农村污水约占全国污水总量的30%以上[1]。与城市污水相比,农村污水具有来源分散,总量较小,缺少对污水设施的专业维护和定期监测等特点,因此,农村污水需要节能、简单、可分散化的处理方法[2]。农村污水有许多处理方法,如化粪池、人工湿地、稳定塘等,然而这些技术因占地面积大,污染物去除效率低,限制了其广泛应用。活性污泥法普遍应用于农村污水处理,但其主要存在以下问题:一是污泥膨胀,这是活性污泥工艺中普遍的问题;二是能源消耗,活性污泥工艺处理城市污水过程中曝气能耗大[3]。相较于传统活性污泥法,序批式活性污泥法(sequencing batch reactor,SBR)具有抗冲击负荷、占地面积小、污泥易于沉淀不易发生污泥膨胀等优点,但该工艺的推广与应用仍面临着曝气能耗大、氮磷去除率低等技术瓶颈。
近年来一些研究表明,使用微藻-活性污泥共生体系作为活性污泥工艺的替代方案,可以降低曝气成本[4]、同时去除氮磷等污染物且可产生有价值的生物质[5]。在SBR中引入光源可形成光序批式反应器(photosequencing batch reactors,PSBR),活性污泥和藻类协同处理污水,可以提高PSBR中氮磷等污染物的去除效率[6]。SU等[7]利用泥藻共生体系处理生活污水,当蛋白核小球藻与污泥质量比为5:1时,氮和磷去除率最高分别为(91.0±7.0)%和(93.5±2.5)%。LIANG等[8-9]利用一种含有小球藻和地衣芽孢杆菌的藻类-细菌共培养系统处理合成废水,研究结果表明,NH4+-N和TP的去除率分别可达86%和91%~93%。
然而,共培养的PSBR体系受溶解氧(dissolved oxygen,DO)、C/N、水力停留时间(hydraulic retention time,HRT)等环境条件的影响,例如溶解氧含量过低易引起污泥膨胀,若过高则能耗升高[10]。本研究在低溶解氧(DO为(0.4±0.1) mg·L−1)和短停留时间(HRT为6 h)的条件下,设置3组体系:污泥体系、藻体系、泥藻共培养体系,考察了3组体系的污染物去除效果和微生物群落特性差异,进一步揭示了体系的脱氮机理,以期为泥藻PSBR节能减排提供参考。
-
实验中污泥取自巴南区高速公路服务区污水处理设备,污泥质量浓度1.9~2.4 g·L−1,驯化污泥约10 d后接种到PSBR形成污泥体系R1,污泥质量浓度为2.24 g·L−1。本研究所采用的藻种为蛋白核小球藻(Chlorella pyrenoidosa, FACHB-9),购买自中科院水生生物所。接种蛋白核小球藻的质量浓度为14.07 g·L−1,OD680(optical density,OD)为1.79,将550 mL藻液加入PSBR形成藻体系R2,生物质量浓度为2.28 g.L−1。按照蛋白核小球藻与污泥质量比为5:1,将藻液接种到污泥形成泥藻体系R3,泥藻质量浓度为2.31 g·L−1。配制模拟废水,其组成成分为化学需氧量(chemical oxygen demand,COD)为200 mg·L−1 、24 mg·L−1 NH4+-N 、6 mg·L−1总磷(TP)、30 mg·L−1总氮 (TN)、25 mg·L−1 MgSO4·7H2O、30 mg·L−1 FeSO4.7H2O、30 mg·L−1 CaCl2。
-
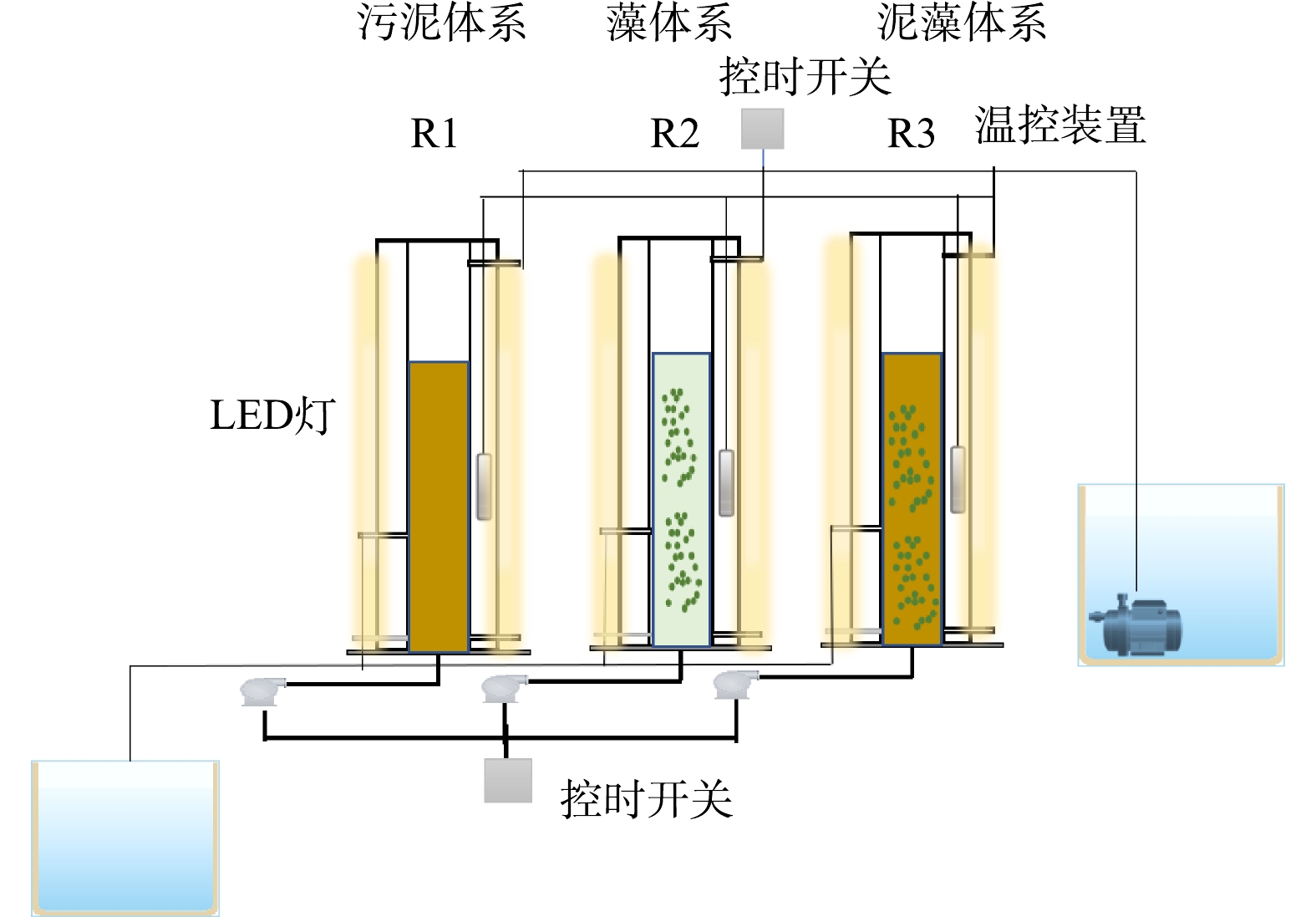
实验装置如图1所示,PSBR由双层有机玻璃圆柱组成,外层是水浴层,内置加热棒,控制温度为(25±1) ℃,反应器高45 cm,直径10 cm,高径比为4.5:1,有效体积3.53 L,每个周期排水2.7 L。反应器底部有曝气盘,连接气泵和转子流量计,控制DO为(0.4±0.1) mg·L−1,进水C/N为6~7。进、出水口分别在反应器两侧的上方和下方,进水口连接潜水泵,出水口连接抽水泵。PSBR的各个阶段都由时控开关控制。反应器的运行周期为6 h,包括:进水4 min、搅拌曝气5.5 h、沉淀5~15 min、排水5 min,每天连续运行4个周期。3个反应器在相同的条件下运行,并设置12 h:12 h的光/暗的光照周期。
-
COD采用快速消解分光光度法测定;NH4+-N采用纳氏试剂光度法测定;TN采用碱性过硫酸钾消解分光光度法测定;混合液悬浮固体浓度(MLSS)采用重量法测定;DO采用便携式溶氧仪(哈希HQ-30d)测定;OD值采用紫外分光光度法测定。
-
DNA提取和高通量测序采用MobioPowerSoil® DNA Isolation Kit提取污泥、藻、泥藻3个样品的基因组DNA。完成基因组DNA抽提后,利用1%琼脂糖凝胶电泳检测抽提的基因组DNA。依托上海美吉生物医药科技有限公司进行Illumina MiSeq高通量测序。
-
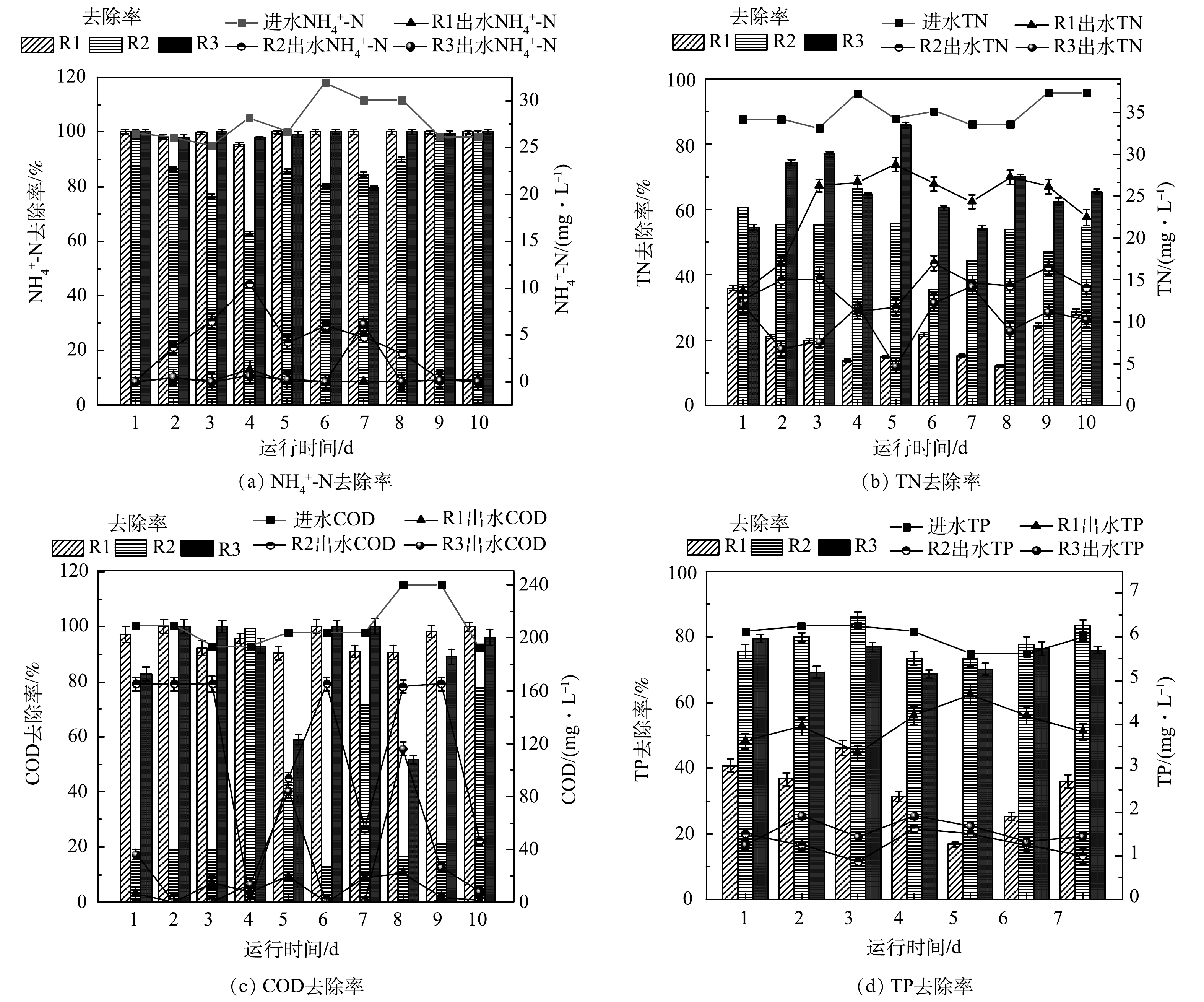
在HRT为6 h,DO为(0.4±0.1) mg·L−1的条件下,污泥体系、藻体系、泥藻体系对模拟农村废水中TN、NH4+-N、COD、TP的去除效果如图2所示。如图2(a)所示,3个体系的TN去除率依次为17.26%、49.18%和65.98%,其中泥藻体系中的TN去除率最高,较污泥体系明显提高了48.72%。这是由于较低的DO可为反硝化细菌提供缺氧的环境[11],加入蛋白核小球藻可能会增加泥藻体系脱氮功能菌的相对丰度,藻与菌协同处理污水可提升泥藻共生体系的脱氮效率。藻体系TN去除率为49.18%,远高于污泥体系。这是因为微藻可通过同化作用吸收氮,微藻吸收水体中的含氮营养物质转化为生长所必须的有机质[12]。如图2(b)所示,污泥体系、藻体系、泥藻体系的NH4+-N去除率依次为97.37%、96.80%和97.43%。有研究表明,低DO[13]和短SRT[11]有利于硝化作用,推测这可能是污泥体系NH4+-N去除率较高的原因。而藻体系中NH4+-N去除效果好是由于微藻可利用NH4+-N作为自养和异养生长的主要氮源。
如图2(c)所示,污泥体系、藻体系、泥藻体系的COD去除率依次为99.52%、18.93%和96.28%。污泥体系中COD去除率远高于藻体系,这是因为活性污泥具有良好的COD代谢能力[14]。本研究中光照的光/暗周期为12 h:12 h,停留时间为6 h,推测由于停留时间较短,藻体系未能在完整的光/暗周期中处理废水。并且由于本研究中进水所用碳源为无水乙酸钠,属于小分子有机物,容易被细菌吸收和利用[15],在污泥体系和泥藻体系中相对优势门均有变形菌门(Proteobacteria)和拟杆菌门(Bacteroidota),其属于异养细菌,主要通过消耗有机物来维持自身代谢和生长,因此,污泥体系与泥藻体系均具有较高的COD去除率。XU等[13]的研究表明,在初始COD为200 mg·L−1、C/N为10:1和5:1的条件下,泥藻体系中COD去除率分别为78.2%和80.1%。而SU等[16]的研究表明,在不同比例的泥和藻体系中,COD去除率超过90%。本研究中泥藻体系的COD去除率可达96.28%,明显高于XU等和SU等的研究结果,说明本研究的泥藻体系具有良好的COD去除效果。
如图2(d)所示,污泥体系、藻体系、泥藻体系的TP的去除率分别为37.31%、83.67%和76.49%。藻体系中TP去除率较泥藻体系提高了7.18%,这是由于TP在微藻中被同化形成藻类生物量[7]。JI等[17]研究表明,非曝气菌藻AGS体系中TP去除率达84%,其中超过70%的TP是在微藻中以聚磷酸盐的形式去除。与污泥体系相比,泥藻共生体系中TP去除率提高了39.18%,表明泥藻共培养体系对磷的去除性能优于单一污泥培养体系,这与LIANG等[8]的研究结论一致。反应体系中的DO以机械曝气为主,在菌藻共生体系中,微藻通过光合作用产生O2,在低DO条件下,微藻产生的额外O2补充了细菌对O2需求,从而提高了污染物的去除效率。此外,JAVED等[18]研究表明,活性污泥中存在的需氧细菌可以吸收微藻光合作用产生的O2,从而可间接提高微藻中所产生的氢化酶的活性,从而促进微藻产生H2。
-
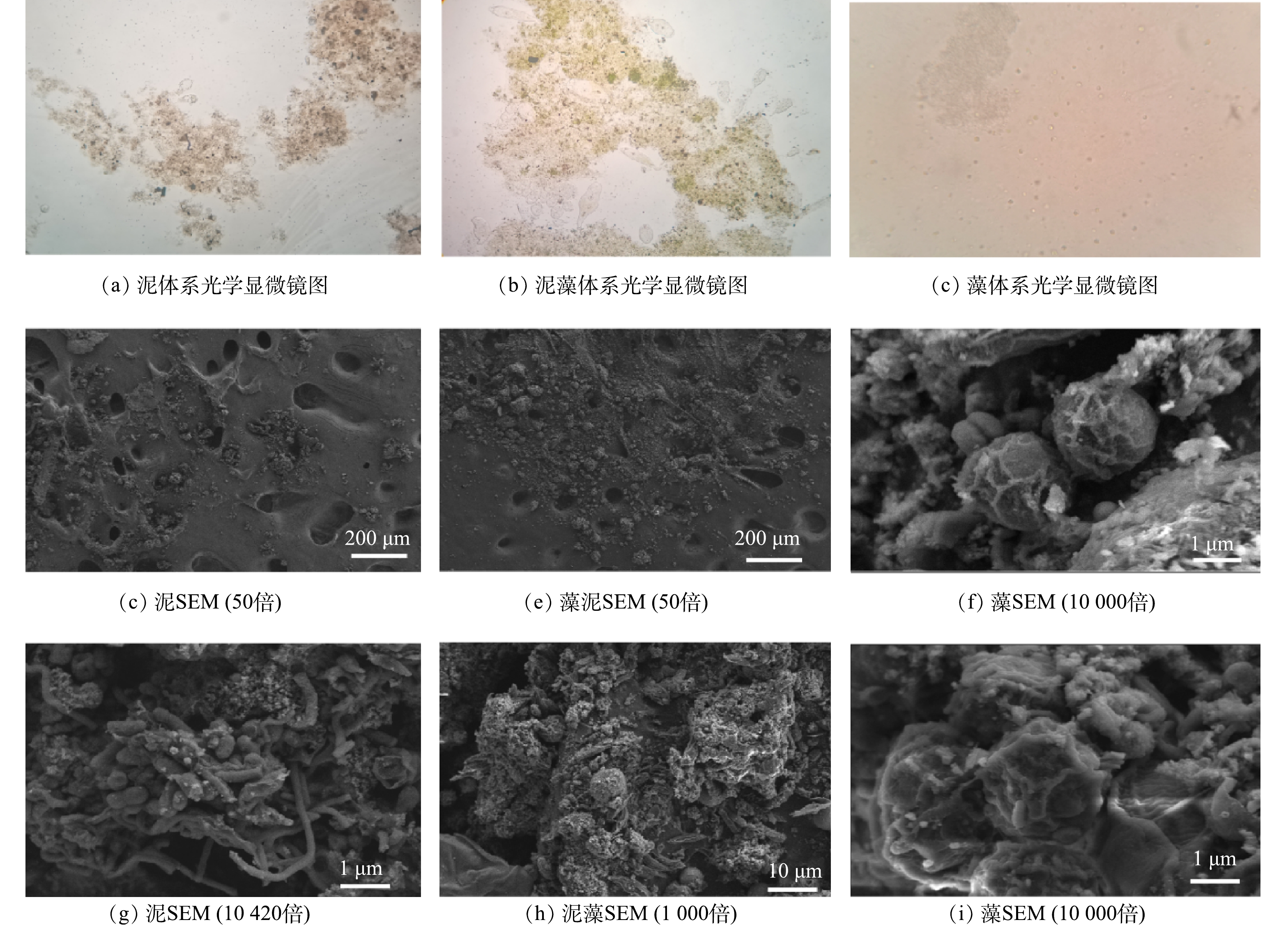
采用光学显微镜观察3个体系的微生物形态,结果如图3(a)~图3(c)所示。由图3(a)可知,污泥体系中累枝虫数量较少,污泥活性较低。由图3(b)和图3(c)可见,泥藻体系中污泥絮凝体捕获微藻,在藻体系中蛋白核小球藻呈圆形或椭圆形分散分布,表明泥藻共生体系可以提高微藻的沉降性能。与污泥体系相比,泥藻体系中累枝虫的数量增加,当活性污泥净化性能良好时会出现累枝虫、盖纤虫等,表明将蛋白核小球藻加入污泥可提高活性污泥净化性能。
利用扫描电镜观察不同体系的生物学特性,结果如图3(d)~图3(i)所示。对比图3(d)和图3(e)可知,活性污泥的表面具有空腔,可为污水气体溢出提供通道[19],将蛋白核小球藻加入污泥后,污泥的表面附着生物量增加。由图3(g)中可观察到丝状菌、杆状菌和球状菌,其中丝状细菌从絮状污泥结构中伸出,并连接在不同的絮体之间。由图3(f)和图3(i)可见,蛋白核小球藻呈分散分布。由图3(h)可见,污泥包裹蛋白核小球藻,表明污泥可以捕获微藻,提高微藻的沉降性能。
-
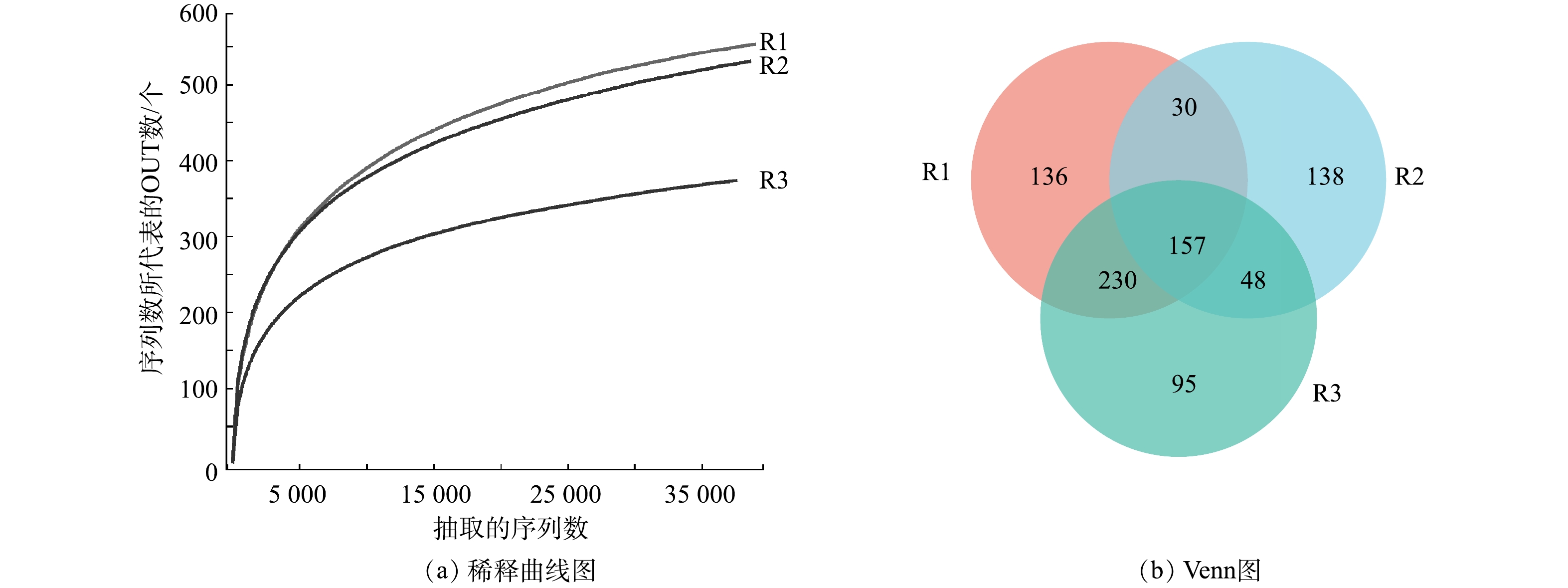
1)α多样性分析。通过微生物群落分析研究不同样品中物种丰富度及多样性的差异,结果如表1所示。3个样品的覆盖率均在99.7%以上,表明所获得的序列可以覆盖大部分微生物。Shannon指数和Simpson指数表明,污泥体系中微生物种类相对较少,泥藻体系的Shannon指数增加,表明小球藻可以丰富污泥体系微生物多样性。由所得的Ace和Chao指数可见,污泥体系和泥藻体系微生物的丰富度和多样性较高,与污染物的去除规律相一致。
图4为各体系的多样性结果。如图4(a)所示,随着测序深度增加,稀释曲线趋于平坦,可以准确地描述各体系的物种多样性。由图4(b)可见,污泥体系、藻体系、泥藻体系中的OTUs总数分别为553、373、530个,其中共享157个OTUs。这表明泥藻体系与污泥体系的OTUs差别不大,污泥可以丰富藻体系的OTUs。
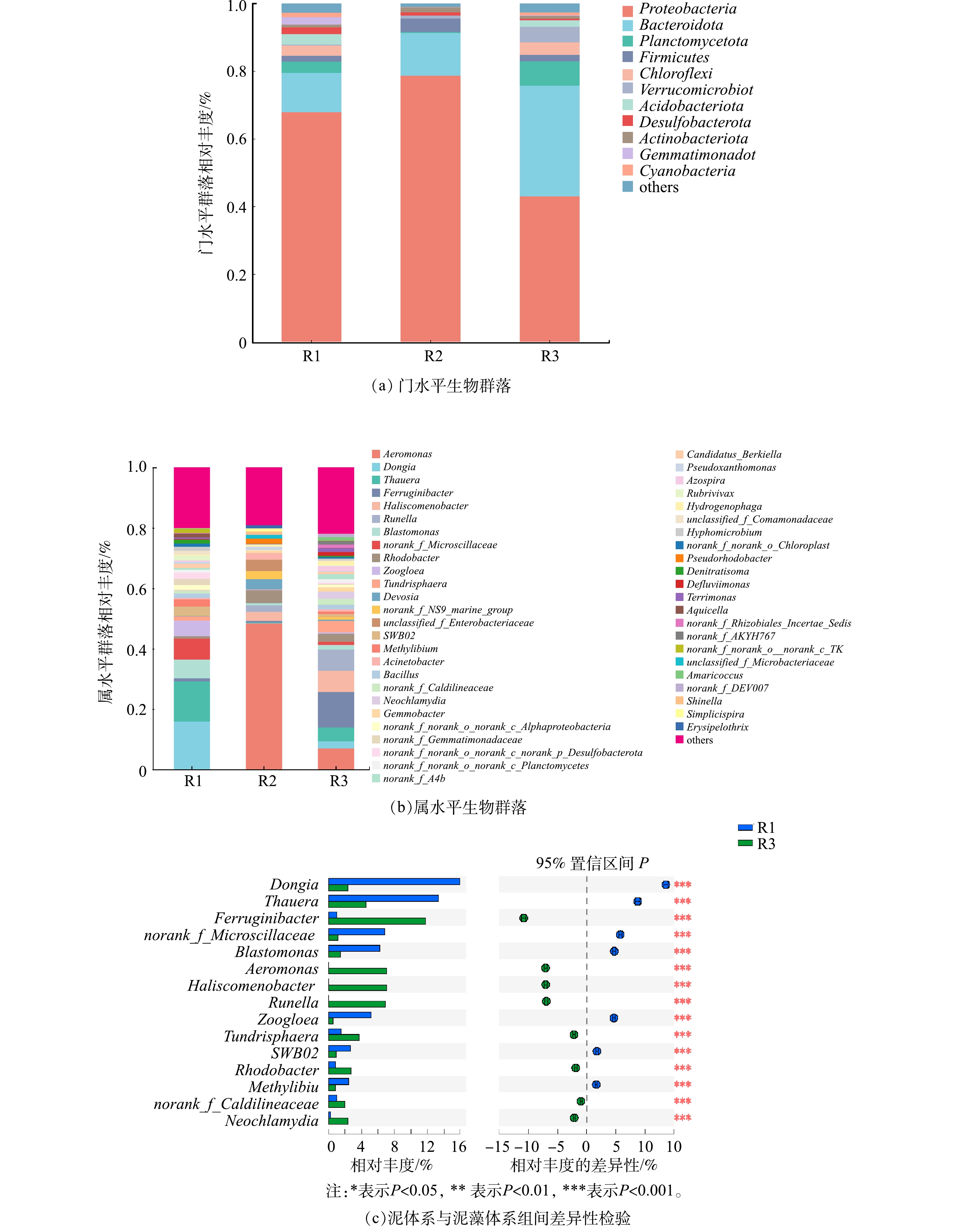
2)不同体系微生物群落分析。不同体系中群落组成和相对丰度都有差异,图5(a)和图5(b)分别表示门水平生物群落和属水平生物群落。如图5(a)所示,在污泥体系中,相对优势菌门及占比分别为变形菌门(Proteobacteria) 67.85%、拟杆菌门(Bacteroidota) 11.72%、浮霉菌门(Proteobacteria) 3.19%、酸杆菌门(Acidobacteria) 3.01%。变形菌门可参与反硝化作用[20],拟杆菌门被认为是最丰富的异养细菌,已被证明可有效降解有机物[21]。浮霉菌门可诱导厌氧脱氮[22],属于好氧异养细菌,可在含有机物和有机或无机氮的条件下进行生长繁殖,进一步去除有机物和氮[23]。在藻体系中,相对优势菌门及占比分别为变形菌门(Proteobacteria) 78.70%、拟杆菌门(Bacteroidota) 12.56%、厚壁菌门(Firmicutes) 4.02%。在泥藻体系中,相对优势菌门及占比分别为变形菌门(Proteobacteria) 43.01%、拟杆菌门(Bacteroidota) 32.72%、浮霉菌门(planctomycetota) 7.14%、疣微菌门(Verrucomicrobiota) 4.59%。与污泥体系相比,虽然泥藻体系中变形菌门占比下降了24.84%,但拟杆菌门占比增加了21%,这是因为变形菌门和拟杆菌门均属于异养菌群,而泥藻共生体系中变形菌门相对丰度低于污泥体系和藻体系,推测由于泥藻共生体系中不同菌群对碳源的严重竞争导致变形菌门相对丰度降低[24]。DING等[25]的研究表明,海洋中微藻不断地将细胞外产物包括碳水化合物、氨基酸、酶、脂质、维生素和其他元素释放到环境中供细菌利用,推测由于泥藻体系中微藻释放有机物增加了水体中有机物含量,而拟杆菌门(Bacteroidota)与蛋白质和脂类等有机物质的转换关系紧密[26],可以降解多种复杂有机物[21],从而拟杆菌门相对丰度增加。在泥藻体系中,Planctomycetota的相对丰度较污泥体系提高了4.29%,其属于好氧异养细菌,有助于微生物聚集[27],从而促进微藻沉淀。
如图5(b)所示,污泥体系中相对丰度最高的4个菌属分别是运动东秀珠氏菌(Dongia)15.94%、陶厄氏菌属(Thauera)13.34%、norank_f_Microscillaceae 6.86%、芽单胞菌属(Blastomonas)6.21%。陶厄氏菌属[28](Thauera),属于热球菌门,对环境危害具有较高的抗性[29]。Thauera是污泥体系中优势菌属,其可阻碍亚硝酸盐降解[30],导致系统的硝化和反硝化性能降低,这与污泥体系中污染物去除规律相一致。藻体系中相对丰度最高的4个属分别是气单胞菌属(Aeromonas)48.44%、红杆菌属(Rhodobacter)4.29% 、unclassified-f-Enterobacteriaceae 3.79%、德沃斯氏菌(Devosia)3.46%。藻体系中Aeromonas(48.44%)高于污泥体系,Aeromonas属于聚磷菌,具有反硝化除磷的效果[31],因此藻体系TP去除率略高于泥藻体系。红杆菌属(Rhodobacter)4.29%属于反硝化菌,可以将NH4+-N氧化为硝酸盐或亚硝酸盐[32]。藻体系不但富集了Aeromonas(48.44%)、Rhodobacter(4.29%)、Acinetobacter (2.11%)等异养硝化-好氧反硝化菌,而且微藻对氮有良好的同化作用,因此保证了藻体系对NH4+-N、TN有良好的去除效果。在泥藻体系中,相对丰度最高的4个属分别是铁锈菌属(Ferruginibacter) 11.76%、气单胞菌属(Aeromonas)7.07%、 丝状菌(Haliscomenobacter)7.05%、古字状菌属(Runella) 6.94%。
进一步对污泥体系和泥藻体系相对丰度做了students’T检验,获得组间显著性差异,结果如图5(c)所示。与污泥体系Ferruginibacter相对丰度0.97%相比,泥藻体系Ferruginibacter相对丰度11.76%显著增加(P<0.05),表明微藻可以增加反应体系中Ferruginibacter相对丰度,由于Ferruginibacter可能反映或响应无机N的可利用性[33],参与铁自养反硝化,并且YANG等[34]研究表明,Ferruginibacter在污泥体系中对Ag+具有较高的耐受性。这表明小球藻加入污泥后,不仅可以富集脱氮功能菌属,提高系统脱氮效果,还增强了活性污泥对不良环境的耐受性,使反应体系更加稳定。污泥体系未检出(相对丰度<0.01)气单胞菌属(Aeromonas)和古字状菌属(Runella),而在泥藻体系中,其相对丰度分别增长至7.07%和6.94%。值得注意的是,Aeromonas属于聚磷菌,在低温条件下具有高效反硝化脱氮性能[35],并且对Cr6+的降解有一定的作用[36],泥藻体系出现Aeromonas(7.07%),表明蛋白核小球藻可以增加Aeromonas相对丰度,提高系统的脱氮除磷性能和适应水质变化的能力。Aeromonas在藻体系和泥藻体系中相对丰度分别为48.44%和7.07%,微藻与Aeromonas形成良好的共生体系,2个体系均有优势菌Aeromonas,这不仅证明微藻可富集脱氮除磷菌Aeromonas,还为菌藻共生的研究提供了备选菌。而藻体系中Aeromonas的相对丰度高于污泥体系,同时藻又可以同化磷合成自身细胞物质,这均为藻体系TP去除率略高于泥藻体系的主要原因。Runella属于弯曲杆菌科,有研究[31]表明,其从增强生物除磷的活性污泥中分离出来,泥藻体系出现了Runella,表明小球藻加入污泥后,TP去除性能增强,推测其增强了活性污泥的除磷性能。泥藻共生体系和藻体系都出现Haliscomenobacter,相对丰度分别为7.05%和3.00%,ZHANG等[37]的研究表明,共培养藻类和Haliscomenobacter是一种高效收获微藻的方法,说明Haliscomenobacter可以固定微藻,提高微藻沉降性能,促进微藻与Haliscomenobacter形成良好的共生体系。综上所述,泥藻共生体系中富集了异养硝化-好氧反硝化菌和除磷功能菌,相对丰度分别为14.35%和14.01%,微藻与脱氮除磷功能菌可形成良好的共生关系。
-
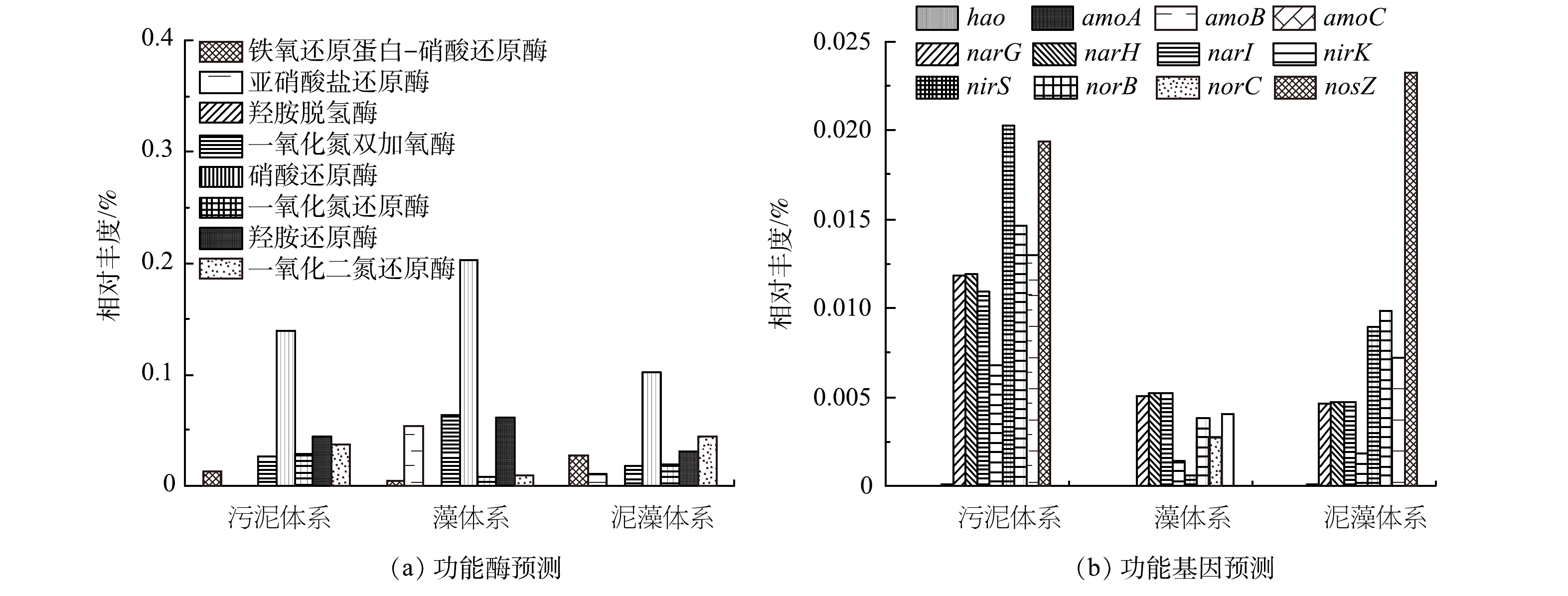
1)不同体系功能酶预测分析。根据京都基因和基因组百科全书(KEGG)数据库(http://www.genome.jp/kegg/),采用PICRUST 2对3组体系中脱氮功能酶进行了预测和注释,结果如图6(a)所示。发现了8种与脱氮相关的酶,其中包括2种硝化酶和6种反硝化酶。铁氧还原蛋白-硝酸还原酶(ferredoxin-nitrate reductase)可以将硝酸盐还原为亚硝酸盐,污泥体系、藻体系、泥藻体系中此酶含量分别为0.013%、0.0043%和0.028%。亚硝酸还原酶(nitrite reductase)可将亚硝酸盐还原为NO或者NH3,从而减轻亚硝酸盐对生物体的毒害作用,其在污泥体系、藻体系、泥藻体系中的含量分别为0.0002%、0.054%和0.011%,其中藻体系中酶含量最高。泥藻体系中亚硝酸还原酶含量比污泥体系提高了0.0108%,表明蛋白核小球藻加入污泥后可减少亚硝酸盐对生物的毒害作用,反应体系脱氮性能增强。N2O还原酶(nitrous-oxide reductase)可以将N2O还原为N2,N2O作为最重要的温室气体之一,增温潜能远高于CO2[38]。与污泥体系相比,泥藻体系中N2O还原酶含量增加了0.007%,这是因为N2O的主要代谢机制是异养反硝化[39],该研究中泥藻体系富集的反硝化菌相对丰度高于污泥体系,促进反应体系脱氮性能增强。
2)不同体系功能基因预测分析。为了获得更详细的PSBR的脱氮信息,采用PICRUST2对3组体系脱氮相关基因进行了预测和注释,结果如图6(b)所示。从预测基因中筛选出8种与反硝化相关的功能基因,包括硝酸还原基因(narG、narH、narI)、一氧化氮还原基因(norB、norC)、亚硝酸盐还原基因(nirK、nirS)和一氧化二氮还原酶基因(nosZ)。污泥体系中硝酸盐还原基因narG、narH、narI的相对丰度均高于泥藻体系,而藻体系与污泥体系的硝酸盐还原基因narG、narH、narI的相对丰度基本相同,硝酸盐还原酶可将硝酸盐还原为亚硝酸盐[40],但泥藻体系TN去除率高于污泥体系。这是由于微藻在脱氮过程中生物同化发挥主导作用[8]。本研究中泥藻体系中N2O还原基因nosZ相对丰度0.023%,高于污泥体系0.019%和藻体系0.004%。闫旭等[41]研究发现N2O的产生量与污泥中nosZ的含量成负相关,碳源和DO对含有nosZ基因的反硝化细菌有明显的影响,低DO环境和充足的碳源能够极大的促进其含量的提高,推测由于微藻释放出碳水化合物、氨基酸等有机物补充了碳源,而泥藻体系中脱氮功能菌相对丰度提高,因此,泥藻体系的nosZ基因的相对丰度升高。
-
1)当 DO为(0.4±0.1) mg·L−1,HRT为6 h时,按泥藻质量比1:5接种到PSBR中,泥藻体系中NH4+-N、TN、COD、TP去除率分别为99.91%、65.98%、96.28%、76.49%,出水达到《城镇污水处理厂排放标准》一级A标,处理效果优于污泥体系和藻体系,表明泥藻体系在低DO条件下对农村废水有良好的处理效果。
2)污泥体系、藻体系、泥藻体系优势菌属具有显著性差异,泥藻体系物种丰富度远高于藻体系,更利于污染物的去除。泥藻共生体系中富集的脱氮功能菌属和除磷功能菌属相对丰度分别为26.11%和14.01%,较污泥体系分别提高了7.60%和14.01%。
3)泥藻体系中铁氧还蛋白-硝酸还原酶的含量较污泥体系和藻体系均有明显上升,泥藻体系亚硝酸还原酶含量比污泥体系提高了0.010 8%,泥藻体系更耐亚硝酸盐毒害作用且脱氮性能有所提高。
Performance and microbial characteristics of co-culture of activated sludge and algae in SBR for rural sewage treatment under low dissolved oxygen conditions
- Received Date: 19/10/2022
- Available Online: 26/02/2023
-
Key words:
- rural domestic sewage /
- sludge-algae system /
- microbial properties /
- photo-sequencing batch bioreactor
Abstract: Aiming at the problems of high aeration and energy consumption of sequencing batch reactor (SBR), photoSequencing Batch Reactors (PSBR) were used to treat the simulated rural domestic sewage, and the efficiency of pollutant removal and microbial characteristics of sludge system, algae system and sludge-algae system were compared. The results showed that compared with the sludge system, the removal rates of TN and TP in the sludge-algae systems increased by 48.72% and 39.18%, respectively. Compared with the algae system, the removal rates of TN and COD in the sludge-algae system increased by 16.80% and 77.35%, respectively. Under the conditions of low dissolved oxygen (DO=(0.4±0.1) mg·L−1) and hydraulic retention time of 6 hours, the effluent of the sludge-algae system could reach the Class A standard of Discharge Standard of Urban Sewage Treatment Plant. The high-throughput sequencing results showed that the dominant bacterial in the sludge-algae system and sludge system were significantly different, in sludge-algae system, the phosphorus removal dominant bacteria Aeromonas of 7.07%, Runella of 6.94% and the heterotrophic nitrification- aerobic denitrification bacteria Aeromonas of 7.07%, Rhodobacter of 2.70%, and Thauera of 4.58% appeared. Compared with sludge system and algal system, the prediction results of functional enzymes and functional genes showed that the content of ferroxyroxin-nitrate reductase in the sludge-algae system increased by 0.015% and 0.0237%, respectively. The content of nitrite reductase in the sludge-algae system increased by 0.0148% compared with that in the sludge system, indicating that the addition of Chlorella pyrenoidosa to the sludge could reduce the toxic effect of nitrite on organisms, and the denitrification performance of the reaction system was enhanced. The research results can provide a theoretical reference for rural sewage treatment by sludge-algae system.





 DownLoad:
DownLoad:
