-
目前,污水中蕴含的丰富的水资源[1]、碳基能源[2]及磷元素[3]的回收已经取得了显著成效,但针对氮素营养物质回收的技术亟待进一步突破。高浓度氨氮废水因毒性强、对生态环境危害大而引起了学术界和业界的广泛关注[4],并已有成熟的处理工艺(如化学沉淀法[5]、吹脱法等[6])。而低浓度氨氮的处理问题往往容易被忽略[7],若将大量低浓度氨氮污水不经处理而直接排入环境水体,也会使自然系统无法消纳,导致水体富营养化,进而降低水体观赏价值,危害鱼类及人体健康[8]。从污水中回收低浓度氨氮的工艺受诸多条件限制[9],如折点氯化法受pH控制、易产生副产物氯胺、氯代有机物等,造成水体的二次污染[10];电化学氧化法能耗高,通常需要和其他工艺联用[11];电容去离子技术对共存的高价态离子优先吸附,降低了对氨氮的吸附效率[12];而传统生物硝化反硝化法忽略了氨氮资源属性[5-7]。离子交换技术具有交换容量高、能耗低、可再生效率高、操作过程简单、环境友好,并且能同时实现水质净化和资源原位回收等优点,因此,将离子交换技术用于低浓度氨氮回收具有明显优势。在使用传统离子交换剂的基础上,我们可以通过设计多功能化的离子交换剂,促进离子之间定向转移并实现高效调控,从而提高
${\rm{NH}}_4^{\rm{ + }}$ 的迁移速率、交换效率及分离性。因此,将离子交换技术从目前已经广泛应用的污水治理领域应用到低浓度氨氮原位富集回收领域,对于污水的全面资源化利用具有重要意义。本研究重点对3种典型的离子交换剂即沸石、粉末树脂、磁性离子交换树脂的技术原理及其在污水处理中的研究现状进行了分析总结;在此基础上,分析了3种离子交换剂在低浓度氨氮回收过程中存在的问题,并提出了相应的研究策略;同时,对3种离子交换剂的经济性进行了评价,指出了磁性离子交换树脂在工程化应用方面的优势,以期为离子交换技术从污水治理领域向低浓度氨氮资源化回收领域转换提供参考。
-
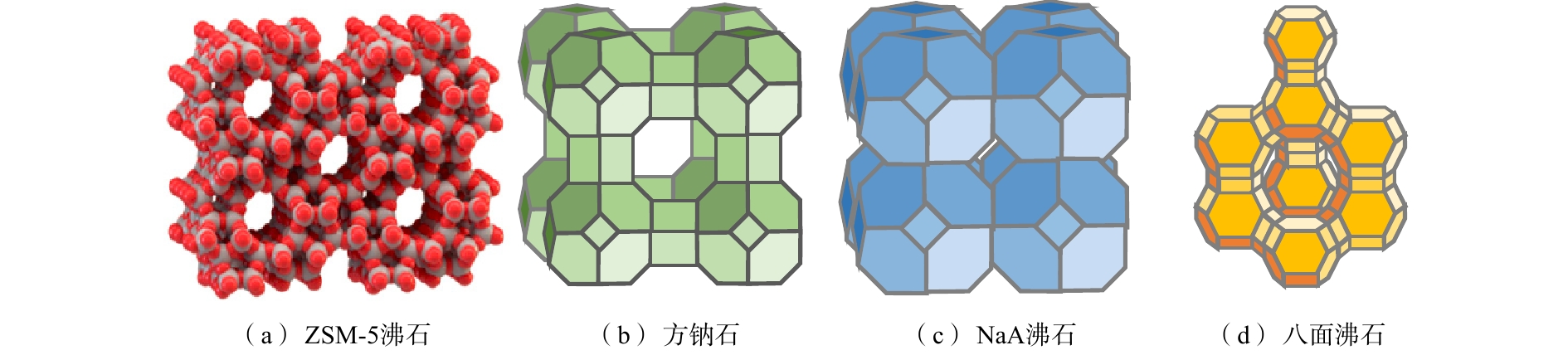
天然沸石作为一种储藏量大、分布广、成本低的矿产资源,其骨架中的阳离子与水溶液中的阳离子在交换过程中总电荷、离子数、体积保持一致,但交换的离子数目不同,从而改变了晶体内的电场,促进沸石吸附和催化性能的提升[13]。沸石骨架结构中丰富的孔穴和孔道[14](图1)能够为离子传输提供通道,促进离子转移。此外,沸石还具有环境友好兼容性,从而被广泛用作水处理过程中的吸附剂、离子交换剂、分子筛和催化剂等。
-
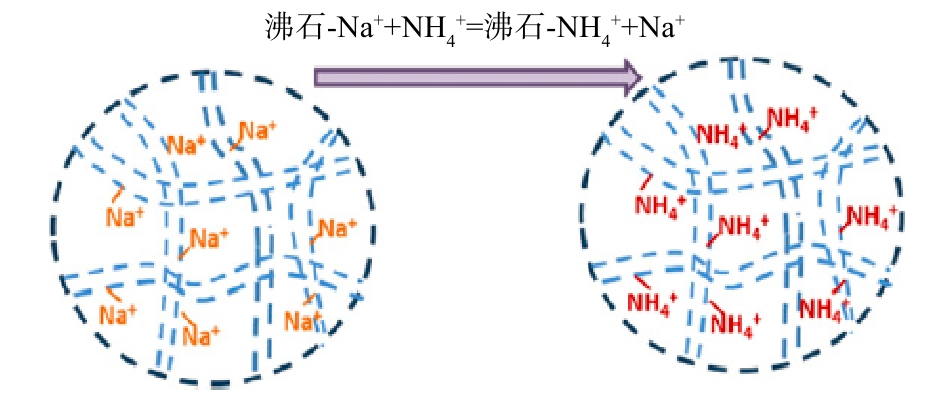
1858年,研究人员[15]首次揭示了沸石的离子交换特性,其骨架结构中的Si4+被Al3+取代,产生一个负电荷(此负电荷数目随Al3+数目增加而增加),该负电荷被沸石表面带正电荷的阳离子(Na+、K+、Ca2+、Mg2+)平衡,这些阳离子与沸石硅酸盐结构以较弱的静电作用结合,进而与溶液中的阳离子进行交换,沸石吸附
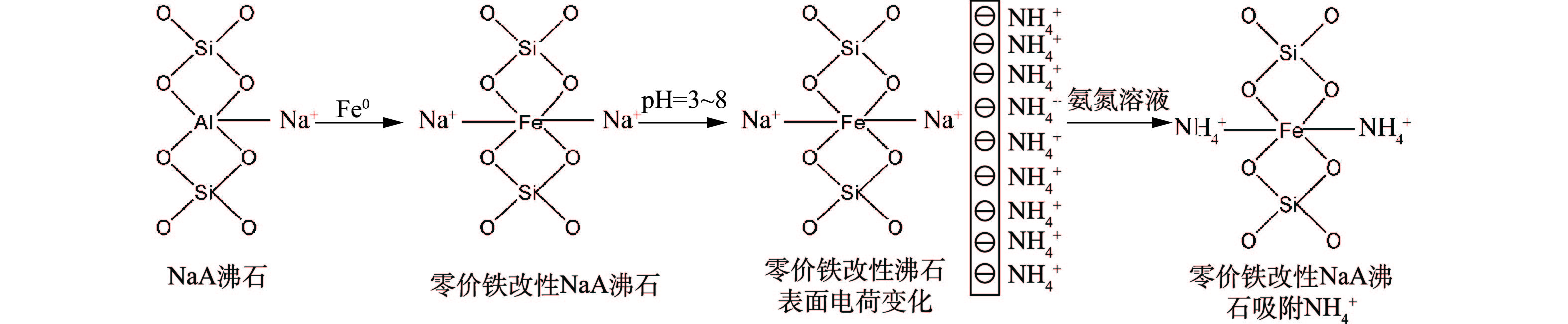
${\rm{NH}}_4^{\rm{ + }}$ 模型[16]见图2。这种离子交换作用不会因为沸石改性而改变。如图3所示,在磁性沸石制备过程中,Fe0进入沸石骨架,当氨氮溶液的pH高于等电点时,由于静电吸附作用,${\rm{NH}}_4^{\rm{ + }}$ 仍然可以自发吸附在磁性沸石表面,与Na+离子发生交换[17]。沸石进行离子交换的选择性与沸石结构、阳离子电荷数、离子水合半径及交换环境密切相关,阳离子的电荷密度越小,越容易进入沸石孔道进而发生离子交换[18]。 -
沸石来源丰富,价格便宜,对
${\rm{NH}}_4^{\rm{ + }}$ 具有较高的选择性,是一种较有潜力的针对低浓度氨氮污水的处理方法[19]。左思敏等[20]通过研究发现,天然沸石、改性沸石及人工合成沸石对脱氮、除磷、除氟、除有机污染物[21]和除重金属污染物[22-23]均具有显著效果。目前,沸石用于氨氮废水处理的研究较多。本节主要总结了具有发展潜力的天然沸石、纳米沸石及磁性沸石在氨氮回收方面的研究,并分析了3种沸石在氨氮回收过程中的优势及存在的问题,提出了磁性纳米沸石在低浓度氨氮回收中的优势。斜发沸石作为世界上存储量最丰富的天然沸石,具有明显的大孔结构,对
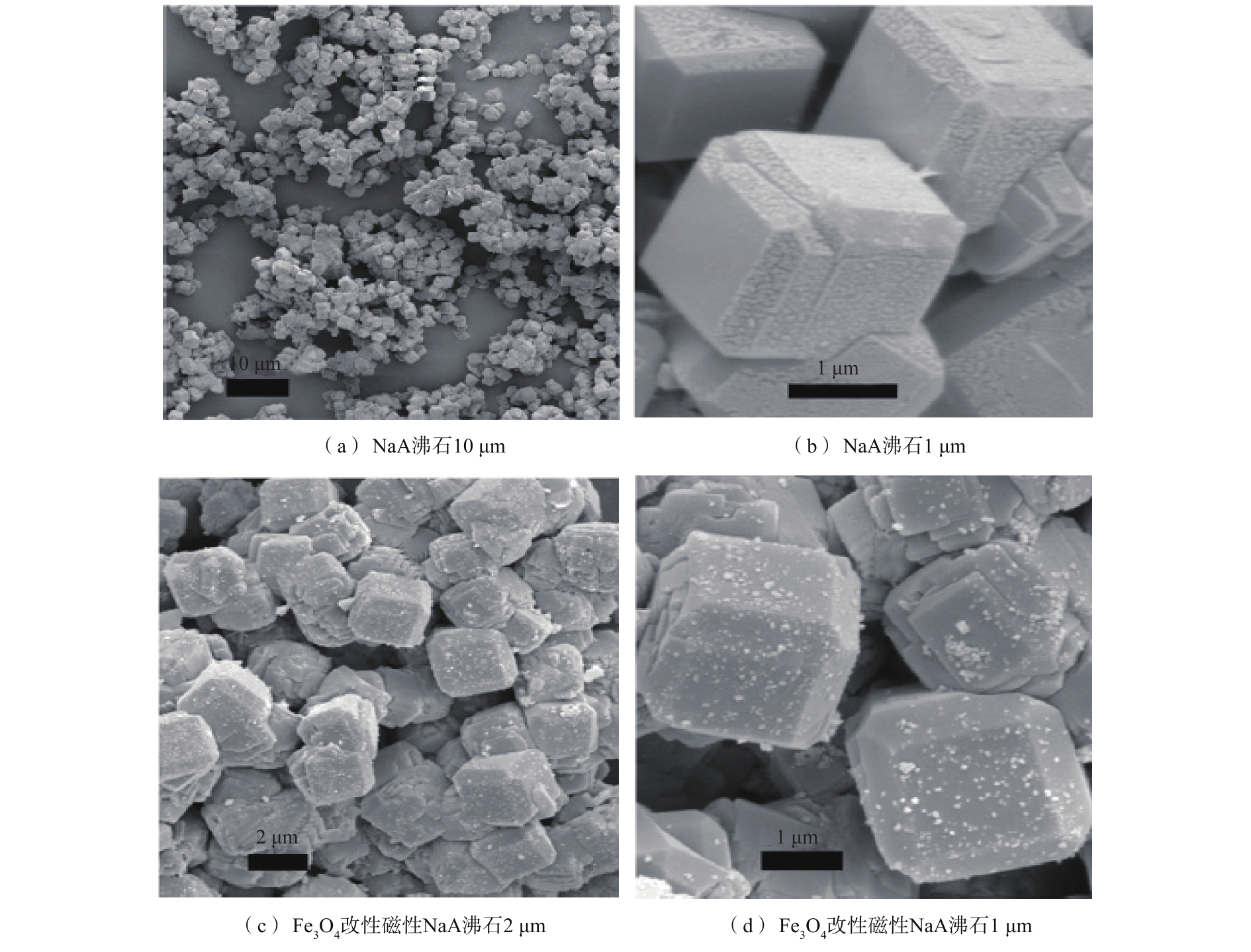
${\rm{NH}}_4^{\rm{ + }}$ 具有较高的亲和力[24-26],因此,得到了研究者广泛关注。但文献中报道的斜发沸石对氨氮的离子交换容量仅为0.15~1.70 mmol·g−1[27],因此,为了提高天然沸石的吸附效率,很多研究者尝试利用酸、盐、碱、超声、微波等单独及耦合物理化学法对天然沸石进行改性[28-31],通过改变沸石表面形貌、提高比表面积、降低平均孔径来提高膜传质效率,从而提升氨氮吸附容量。相对于天然沸石,人工合成沸石的显著优势在于可以根据使用目的,有针对性地设计及调控沸石结构,提高沸石有效成分含量,进而有助于离子交换反应的发生。人工沸石的合成通常以廉价易得的稻壳、天然高岭石、硅藻土、粉煤灰等作为原料,在含碱性硅酸铝前驱体的高温水热反应釜中完成[32-34]。其中人工合成的纳米沸石具有晶粒小、孔道短、比表面积大等特征,从而表现出优异的体积效应、表面效应[35]和离子交换性能。NAWOG等[36]利用谷壳灰合成NaA型纳米沸石,其粒径主要分布在100~400 nm之间,与微米级颗粒的NaA沸石相比,氨氮的去除率提高了14%。此外,通过在沸石制备过程中加入磁核-Fe3O4的前驱体或利用零价铁对沸石进行改性,能够解决复杂多相体系中沸石分离难的问题[37],有效提高沸石再生和循环利用效率,从而达到安全处理被吸附的有毒废物或回收有价值资源的目的。在此过程中,适量Fe3O4(低于3.4%)的加入不会改变沸石原有结构(图4)及其对氨氮的去除性能[38];而零价铁对沸石改性过程也不会对其离子交换性能产生影响[17]。有研究表明,沸石对氨氮吸附过程多数属于吸热、自发进行且符合假二级吸附动力学模型[24, 34, 39],此过程受沸石结构及操作条件的影响。天然沸石、改性沸石及人工合成沸石对氨氮吸附性能及沸石再生工艺对比结果如表1所示。基于上述分析,天然沸石存在交换容量低,沸石改性过程需要消耗酸、碱化学药品等问题,因此,纳米沸石及磁性沸石对低浓度氨氮回收更具发展潜力。但纳米沸石很难从溶液中快速分离,而磁性沸石交换容量随着Fe3O4负载量的增加而降低,这些问题仍然限制了沸石材料在氮素资源化回收方面的应用。此外,在通常情况下,
${\rm{NH}}_4^{\rm{ + }}$ 离子半径小于斜发沸石和一些人工合成沸石的孔径(如4A沸石及八面沸石等),导致这些沸石对${\rm{NH}}_4^{\rm{ + }}$ 具有很强的选择性吸附能力,但这种选择性会随着共存阳离子价态的升高、同价态阳离子半径的减小而降低[18]。因此,如何通过进一步调控沸石孔径结构,针对性地设计高氨氮选择性磁性纳米沸石,是提高沸石材料利用率和氨氮集中处理效率的有效措施之一。 -
离子交换树脂诞生于20世纪30年代,由不溶性的三维空间网状高分子骨架、连接在骨架上的离子功能基团和带相反电荷的可交换离子组成。与天然沸石和人工合成沸石相比,离子交换树脂具有较强的吸附能力和良好的化学、机械稳定性,在氨氮回收方面具有明显优势。但传统离子交换树脂存在吸附容量低、分离过程复杂繁琐等问题[40],因此,研究者通过采取减小树脂粒珠尺寸、加入无机磁性材料等措施,开发了具有高交换容量的粉末树脂和同时具有高吸附容量和沉降速率的磁性离子交换树脂,二者分别在火电厂冷凝水精处理系统[41]及天然有机物的去除等方面具有显著优势[42]。但目前将粉末树脂及磁性离子交换树脂用于氨氮回收方面的研究相对较少。因此,本节在对这2种树脂的应用现状进行总结的基础上,重点讨论了两者对低浓度氨氮回收的可行性及相应的解决方案。
-
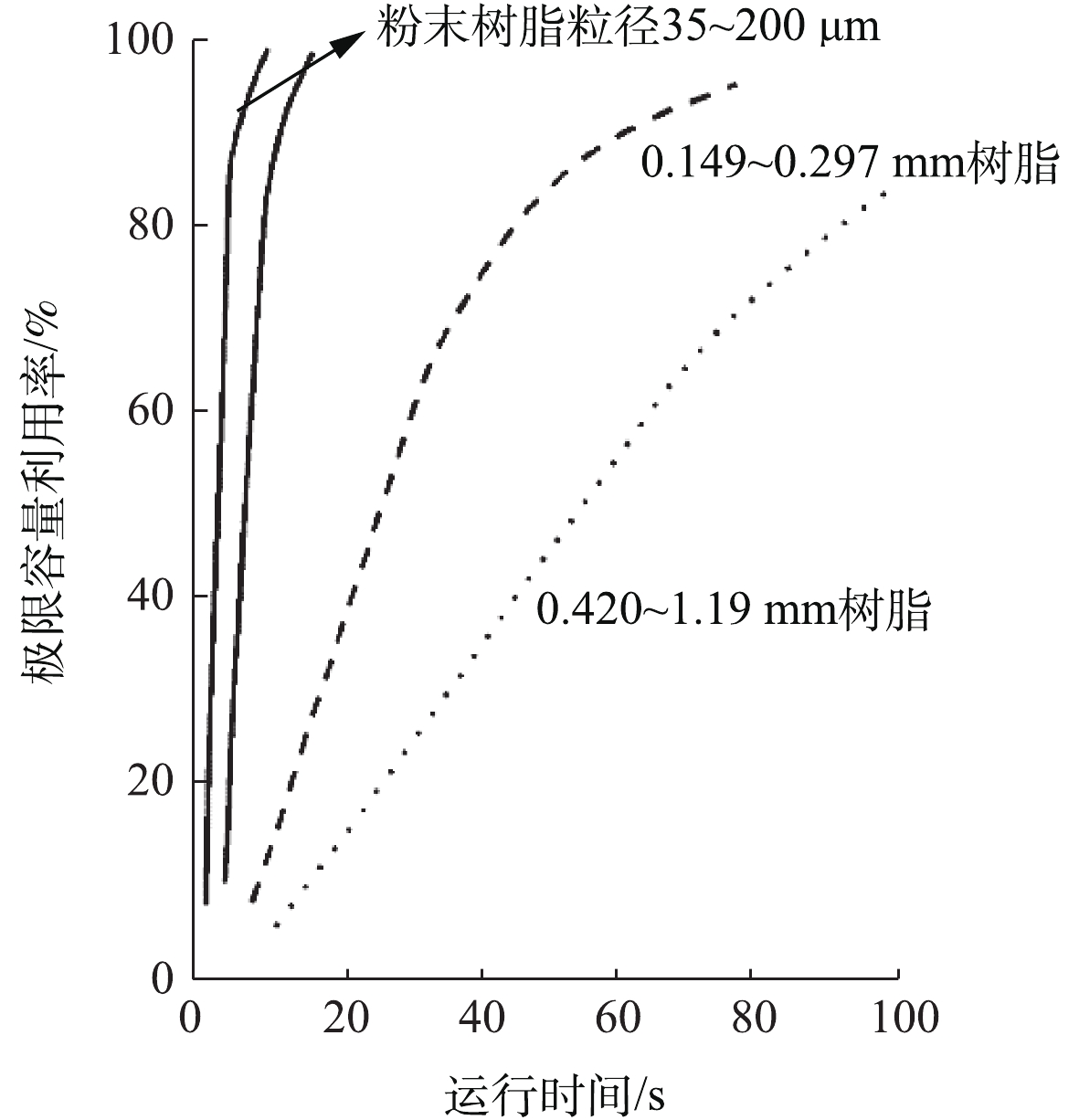
20世纪60年代初,Graver水处理公司率先将离子交换树脂颗粒磨成粉末,制成粉末离子交换树脂。常见粉末树脂的粒径(35~200 μm)[43]比传统离子交换树脂粒径(400~600 μm)小,故具有更大的比表面积。如图5所示,与标准尺寸微球、小尺寸微球的树脂相比,粉末状强碱性阴离子树脂具有更快的离子交换速率[44]。LEVENDUSKY[45]研究证实了粉末树脂的反应速率大约是标准珠状树脂的100倍,故少量粉末树脂就可以实现对目标离子的高效去除。粉末树脂的实际交换容量可达理论交换容量的60%~95%,有效减少了树脂再生次数[41]。
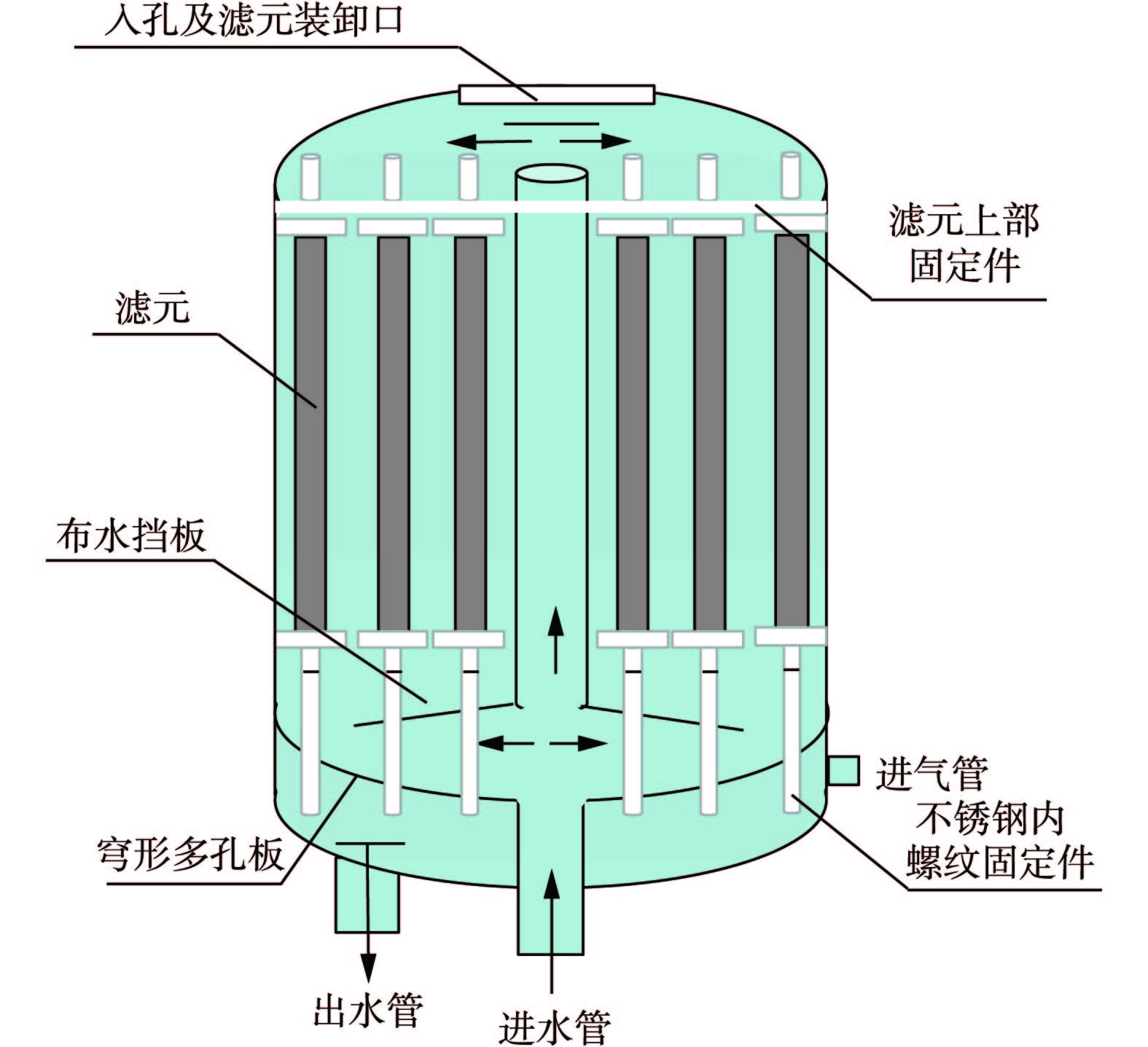
1)粉末树脂过滤器技术原理。1966年,Graver水处理公司的研究人员设计了粉末树脂过滤器系统[45](如图6所示),并进行了实际应用。粉末树脂过滤器是集过滤、除盐及除铁功能于一体的水处理设备,主要工作原理包括过滤、吸附和离子交换[46]。由于粉末树脂过滤器由覆盖过滤器发展而来,因此,其具有覆盖过滤器的过滤和吸附功能。在实际涂覆粉末树脂过滤器时,将具有离子交换功能的粉末树脂和过滤作用纤维粉以一定配比混合,旨在提高粉末树脂使用效率的同时,达到快速净化水质的目的[41, 47]。此外,粉末树脂的吸附能力很强,对水中悬浮物和胶体硅的去除更为有利。采用粉末树脂替代纸浆后,除铁率从60%~70%提高至80%~90%,胶体硅的去除率可达99%[46]。
2)粉末树脂在凝结水处理中的应用及处理低浓度氨氮可行性。目前,粉末树脂主要应用于火电厂凝结水精处理系统,技术发展较为成熟[41, 46, 48-49]。首先,火电厂凝结水水质较好,含盐量低、温度高(60~70 ℃)、
${\rm{HCO}}_3^ - $ 浓度高、铁含量较高(<1 000 μg·L−1),因此,很薄的粉末树脂涂层就能够使其全交换容量得到充分发挥[41],适用于凝结水精处理系统的频繁启动;而粉末树脂耐热性好、比表面积大、吸附容量高,因此,进水与树脂之间的有效接触面积大、反应速度快,故能保证较高品质出水,有利于促进凝结水回用。其次,粉末树脂用量少,因此,从经济性角度考虑,吸附饱和后直接爆膜抛弃,减少了传统树脂的再生过程,也不会出现由于凝结水温度升高而造成树脂老化问题[43]。粉末树脂过滤器技术的显著优势在于其系统简单、无树脂分离再生等设备、占地少、适应性强、一次性投资小、运行控制灵活方便且无废酸碱水排放[48]。此外,相对于普通颗粒状离子交换树脂,粉末树脂过滤器的压力损耗对系统运行产生的影响很小。这是因为火电厂凝结水中电导率很低,基本没有悬浮物,所以其滤元和树脂层的总压力损耗低,不超过0.1 MPa。因此,这部分压损可以忽略不计。而当粉末树脂覆盖过滤器铁含量或进出口压差达到设定值时,视作粉末树脂失效,须对其进行爆膜清洗,重新铺膜[47],因此,压力损耗不会再增大。在操作过程中,可以根据实际水质条件,将粉末树脂过滤器(可以仅铺纤维粉起过滤作用)和高速混床精处理系统联用,兼具过滤和除盐的双重功效[49]。目前,火电厂已经利用国产粉末树脂代替了美国陶氏公司或Graver Technologies公司的进口粉末树脂(国产和进口粉末树脂的性能参数[50]如表2所示),从实际火电厂的运行结果对比分析,采用国产粉末树脂的精处理系统运行周期长,周期产水量大,从而可降低运行成本[50]。此外,粉末树脂还可用于自来水、工业用水及泉水中的放射性Sr的原位预浓缩[51],能够简化测定大容量(>100 L)淡水样品中放射性锶的方法。基于上述分析,将粉末树脂应用于污水中低浓度氨氮回收领域,须考虑以下几个问题。首先,粉末树脂质量轻、粒径小,传统的流化床实验装置易造成树脂堆积或流失,不能使之与进水有较好接触,建议根据粉末树脂覆盖过滤器原理及实际操作条件,设计新型反应器系统。这是因为粉末树脂过滤器中粉末树脂的流失问题并不常见。如果出现此类问题,一般是由于滤元的断裂或者绕线松动引起的,但是滤元有明确的使用年限,通常需要在此期限前更换,因此能有效避免树脂流失。其次,粉末树脂过滤器在铺膜后,须启动保持泵护膜至少8 h后再投入运行,能有效防止树脂脱落[52];而且粉末树脂覆盖过滤器在备用情况下,护膜泵也一直保持运行状态(流量为31 m3·h−1),护膜泵进出口与粉末树脂覆盖过滤器的出水管和进水管相连接,利用循环水压将粉末树脂压在滤元表面,从而避免其脱落[47]。以上措施能有效避免粉末树脂流失,能够为设计新型反应器提供参考。第三,由于凝结水水质较好,每次铺膜树脂用量很少,因此,失效后直接爆膜,并不会造成严重经济损失。但在低浓度氨氮回收过程中,若对吸附饱和的粉末树脂直接抛弃,运行费用较高。以城市污水中的氨氮为例,其浓度为20 mg·L−1左右,但同时存在其他阳离子(如Na+ 72 mg·L−1、
${\rm{NH}}_4^{\rm{ + }}$ -N 20 mg·L−1、K+ 10 mg·L−1、Mg2+ 34 mg·L−1、Ca2+ 102 mg·L−1[53]),故总离子浓度是凝结水中铁离子浓度[41]的500~4 000倍左右。因此,粉末树脂在回收低浓度氨氮过程中用量较大,故在技术推广及现场实际操作等方面,需要进一步研究与探索。最后,由于凝结水含盐及含铁量低,较薄涂层的粉末树脂覆盖过滤器能满足生产运行要求,但将其用于污水中低浓度氨氮吸附时,需要根据进水离子浓度及出水指标,在合适的滤膜厚度阻力下,优化粉末树脂装填量,或者增加混床和分床系统,以提高氨氮回收效率,同时使出水水质达标。此外,可以根据进水水质及目标污染物特性,在涂覆层掺杂沸石等高氨氮选择性离子交换介质,提高氨氮的吸附效率,针对性地设计涂覆层是未来探索和研究的方向。 -
与沸石、粉末树脂及传统离子交换树脂相比,磁性离子交换树脂的突出优势在于通过引入磁核(γ-Fe2O3或者Fe3O4),强化珠粒间团聚成更大的颗粒,依靠树脂珠粒间的相互吸引力、重力及外部磁场作用,显著提高树脂沉降速率(10~20 m·h−1)[54],实现树脂与溶液快速分离,提高树脂回收率(99%[55])。此外,磁性离子交换树脂的粒径小(直径150~180 μm,比传统的小2~5倍)、比表面积大、反应速率快、吸附容量及再生效率高、性能稳定[56]。在操作工艺上,磁性树脂采用全混式操作工艺代替传统固定床工艺,使处理水量不受固定床交换容量限制,并可以通过改变树脂投加量实现对出水水质可控化调节[57],从而有效解决传统离子交换树脂吸附速率慢、操作设备及工艺繁冗的问题[42, 58-59]。磁性离子交换树脂包括磁性阴离子交换树脂和磁性阳离子交换树脂。本节首先阐述了污水处理领域应用更为广泛的磁性阴离子交换树脂的原理及应用现状;并对磁性阳离子交换树脂的应用现状进行了总结,分析了其在低浓度氨氮废水处理的可行性和突出优势,提出了针对低浓度氨氮回收的磁性阳离子交换树脂未来可以突破的研究方向。
1)磁性阴离子交换树脂及技术原理。澳大利亚Orica公司率先生产的MIEX®磁性阴离子交换树脂,是一种具有大孔结构的以聚丙烯为基体的强碱性阴离子交换树脂,其孔隙率为77%,平均孔直径为2.0 nm。聚合结构包含季胺基团和65%的含水量,交换容量为2.55 mmol·g−1,80%树脂的过筛粒径为200 μm,BET表面积为43.5 m2·g−1[60-61]。它的大孔结构由中度交联的丙烯酸骨架构成,能够有效抵抗物理磨损和有机物的污染[62]。此外,SHUANG等[63]通过悬浮聚合、氨解和烷基化反应,制备了具有较高交换容量的新型磁性阴离子交换树脂NDMP。
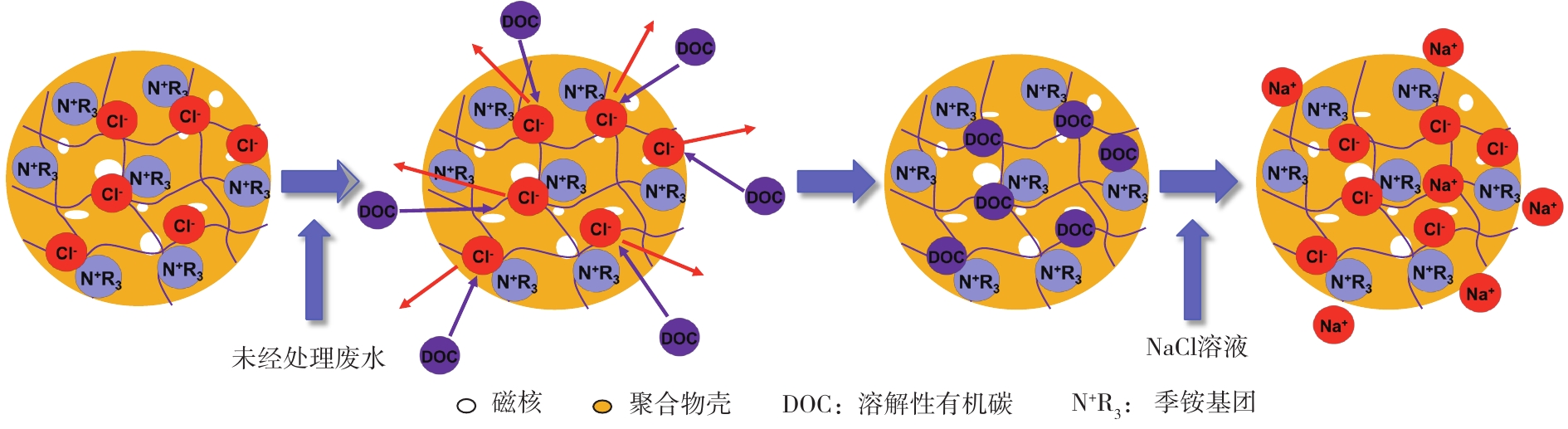
磁性阴离子交换树脂的离子交换原理[64]如图7所示。吸附过程是利用树脂聚合物壳表面活性位点上的Cl−或者
${\rm{HCO}}_3^ - $ ,与溶液中带负电荷基团(—COOH、—OH)的有机物或者阴离子(如Br−、${\rm{NO}}_3^ - $ 、${\rm{SO}}_4^{2 - }$ 等)发生离子交换;同时,一些抗生素和树脂之间可形成氢键,其有助于树脂吸附容量的提高[65]。解吸过程可利用2 mol·L−1 NaCl[65]或50 g·L−1 NaHCO3[66]溶液实现树脂再生,根据水质的不同,也可以加入质量分数为1% 的NaOH溶液,进而促进溶解性有机碳脱附[62, 67]。2)磁性阴离子树脂在水处理中的应用。磁性阴离子交换树脂目前已在饮用水[68]、市政污水、有机工业废水(印染、纸浆)处理中实现了工程化应用[57]。大量研究已经证实,磁性阴离子交换树脂对天然有机质[42]、消毒副产物前驱体[56]、无机阴离子(如Br−[42],
${\rm{ClO}}_4^ - $ [69])以及低含量污染物(包括抗生素[65]、农药[70-71]、微量毒性金属元素[61, 72])和染料[63, 73]等都有良好的去除效果。但磁性阴离子交换树脂对浊度、病原体和其他特殊污染物去除效果较差,因此,可将其作为预处理工艺与其他处理技术(如膜、混凝、臭氧、活性炭)联用,以降低处理成本、提高出水水质。磁性阴离子交换树脂与混凝工艺联用,能够加快初始絮状物的聚合,减少铁铝混凝剂用量[74-75]。MIEX®和膜工艺联用能使有机物去除率提高40%~50%[76],但MIEX®并不能减少短期膜污染[77]。这是因为溶解性有机物的性质,包括分子质量、有机物的组成、亲疏水性等均会对膜污染产生影响[78]。JUTAPORN等[79]也证实了MIEX®不能去除造成短期低压膜污染的微生物的溶解性有机物。因此,要减少膜污染,需要提高树脂对微生物溶解性有机碳的选择性,或者将MIEX®与生物活性炭、混凝剂联用,去除胶质类溶解性有机物及生物高聚物,延长膜使用寿命[80-82]。但在此过程中,应考虑成本问题。MIEX®与臭氧工艺联用,能有效降低致癌物(${\rm{BrO}}_3^ - $ )生成的可能性并提高O3的使用效率[83],降低微生物的失活率[84]。此外,磁性阴离子交换树脂还可以和颗粒活性炭联用,以减少高分子质量有机质对活性炭孔的阻塞,提高杀虫剂的去除率[70]。因此,面对日益严格的污水排放标准,将磁性离子交换树脂与其他工艺组合更有利于其在污水处理领域未来的应用。3)磁性阳离子交换树脂及技术原理。相对于磁性阴离子交换树脂,磁性阳离子交换树脂在污水处理中的研究较为有限。目前报道的磁性阳离子交换树脂主要是指含有羧酸基的大孔结构聚丙烯酸型树脂,如Orica公司研发的MIEX-Na型树脂[85]、南京大学研发的MCER型[86]和MWA06型树脂[87]等。
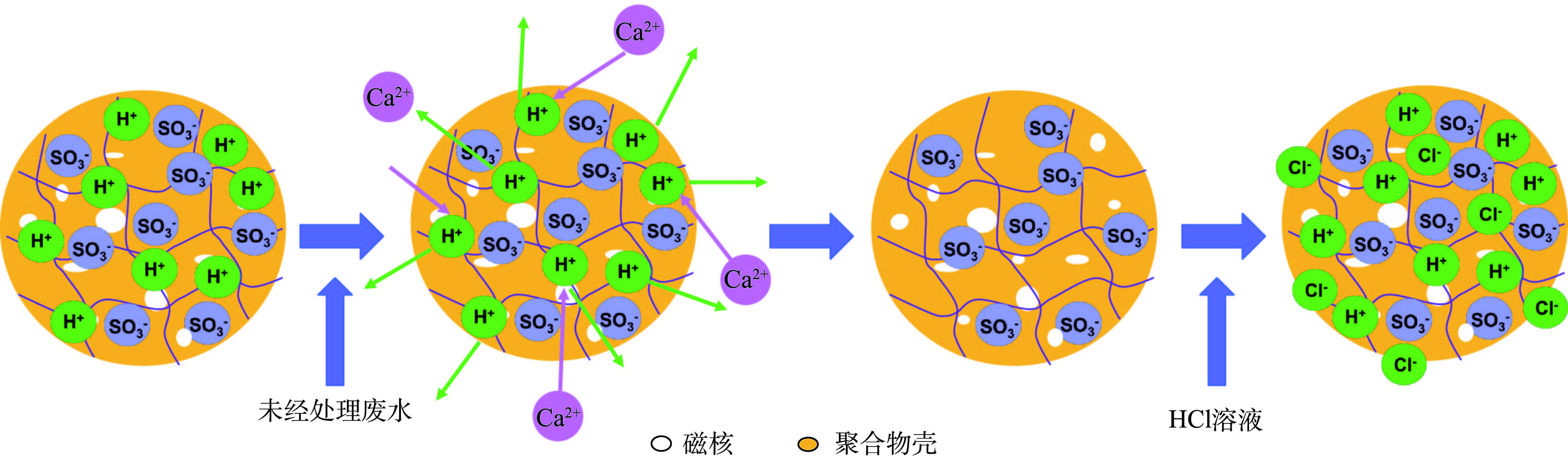
磁性阳离子交换树脂的技术原理和磁性阴离子交换树脂的技术原理类似,区别是利用聚合物壳表面上的阳离子,如H+或者Na+与溶液中的Ca2+、Mg2+、
${\rm{NH}}_4^{\rm{ + }}$ 、Cu2+、Ni2+等阳离子之间发生离子交换反应(图8),然后在磁场作用力下发生凝聚沉降,与原水溶液快速分离[88]。APELL等[89]研究发现,MIEX-Na磁性阳离子交换树脂在盐溶液中再生后,吸附效率明显降低。FU等[40]也证实磁性阳离子交换树脂在HCl(pH=2)溶液中的脱附效率是其在NaCl(20%)溶液中的3.5倍。因此,为了保证磁性阳离子交换树脂的重复使用率,可利用酸性介质进行再生,但在此过程中需要考虑铁离子的浸出问题。4)磁性阳离子交换树脂在重金属废水处理中的应用及对低浓度氨氮回收可行性分析。磁性阳离子交换树脂对饮用水中Ca2+、Mg2+[89-90]及工业废水中重金属离子去除具有显著效果。FU等[40]制备的NDMC型树脂对Cu2+的平衡吸附量可以达到4.18 mmol·g−1,是商用树脂C106和IRC-748吸附量的2倍,初始吸附速率是二者的4~8倍(初始浓度为500 mg·L−1)。吸附饱和的NDMC树脂在HCl溶液(pH=2)中再生后,其脱附率可以达到100%,且可以连续使用10次。为了提高树脂的耐酸性和亲油性,LI等[88]利用二甲基二乙氧基硅烷对γ-Fe2O3表面改性,并通过2次聚合反应获得更大的树脂比表面积和更多的交换位点,利用此MCER树脂对实际电镀废水中的Cu2+和Ni2+进行了处理,结果表明其吸附容量明显增加(pH为4~6,>2.34 mmol·g−1),且出水不含毒性(TUa:急性毒性单位<0.3)。此外,ZHANG等[87]利用钛酸酯偶联剂(乙烯基三甲氧基硅烷)对γ-Fe2O3表面进行了改性,得到的MWA06树脂的交换容量为8.44 mmol·g−1,该树脂在pH为2~6性能稳定,可重复使用10次,对Ni2+吸附效率基本不变。为了同时去除有毒污染物及重金属离子,FU等[91]将磁性阴离子交换树脂和磁性阳离子交换树脂混合,利用Cu2+和Ni2+与磁性阳离子交换树脂之间的离子交换反应、Cu2+和单宁酸之间的络合-桥联反应以及单宁酸和磁性阴离子交换树脂之间的离子交换和氢键作用,同时实现了对3种污染物的高效去除。将磁性阴离子交换树脂(200床体积MIEX-Cl)和磁性阳离子交换树脂(83床体积A200C-Na)联用,对膜浓缩水中的溶解性有机物、硬度的去除率可以达到85%左右[92]。这种联用工艺能使2种树脂在同一个反应器中进行离子交换,有效降低了操作的复杂性,减少了设备占地面积,能够节约成本。
磁性阳离子交换树脂的缺点在于吸附容量较低(如MIEX-Na的吸附容量仅约为2.55 mmol·g−1[89-90]),再生后吸附容量下降明显,机械强度差,容易造成二次污染[88, 93-94]。南京大学研发的MCEX型树脂,其吸附容量可以达到8.80 mmol·g−1,且其交换容量、机械性能和分离效率较MIEX-Na树脂有所提高[40, 87]。目前,磁性阳离子交换树脂主要用于重金属离子回收领域,在氨氮回收领域的研究较少。这可能是因为,虽然磁性阳离子交换树脂对氨氮的吸附容量较沸石有所提高,而且性能稳定,便于重复使用,但其对
${\rm{NH}}_4^{\rm{ + }}$ 的吸附选择性较差。BOYER等[85]发现,MIEX-Na树脂对垃圾填埋渗滤液中的总氮去除效果并不显著,这是因为垃圾渗滤液中的总氮并不主要以${\rm{NH}}_4^{\rm{ + }}$ 离子形式存在,而且溶液中含有的高浓度背景阳离子(如Na+、Mg2+、Ca2+)会与${\rm{NH}}_4^{\rm{ + }}$ 形成竞争吸附。因此,如何提高树脂对氨氮的选择性,不仅是磁性阳离子交换树脂的问题,也是离子交换树脂的共性问题。彭佳乐[95]制备的铜基螯合型阳离子交换树脂(R-Cu)对氨氮选择性强、吸附容量大。利用该树脂处理高浓度氨氮废水,在Na+和Ca2+等竞争阳离子共同存在的情况下,通过控制吸附过程中的pH(9.5~10.0),仍然能达到较高的氨氮吸附容量(1.08~1.60 mmol·g−1),出水氨氮浓度低于1 mg·L−1。周康根等[96]对R-Cu树脂的氨氮解析过程进行分析,发现HCl对氨氮的解吸效率高达93%,使吸附材料可重复使用5次,且对氨氮吸附性能稳定。这些研究为制备高氨氮吸附选择性的磁性鳌合阳离子交换树脂,奠定了坚实基础。因此,基于磁性阳离子交换树脂已有优点,通过对树脂孔径结构进行调控,可以达到筛分${\rm{NH}}_4^{\rm{ + }}$ 的目的;或者通过制备含过渡金属基的磁性树脂[97],利用过渡金属与氨分子之间的特殊配位能力,同时实现氨氮的高选择性吸附及树脂快速分离回收[96]。 -
滤料的再生工艺及再生液的妥善处理是影响滤料重复使用的重要因素。目前,沸石再生的方法主要有化学法(包括电化学法)和生物法。常见的化学法一般是利用NaCl、HCl或NaOH等单独或两两组合溶液(加入酸或碱的目的是提高再生效率,减小所需盐溶液的体积),在烧杯实验中对沸石进行再生,通过搅拌使其充分发生离子交换反应,然后再用纯水冲洗,最后干燥。王志杰等[53]利用快速小柱实验装置对沸石进行再生,将0.17 mol·L−1 NaCl再生液从吸附柱底部进入,使其与沸石之间充分发生离子交换反应。但即使使用高于强酸树脂3倍的再生液体积,也不能使天然沸石完全再生,其出水氨氮浓度仍然高达57.2 mg·L−1。SARIOGLU等[98]利用1 mol·L−1 NaCl溶液对沸石吸附柱再生,通过控制溶液pH和再生液流速,提高沸石再生效率,但再生后沸石的吸附容量仍然降低了31.52%。这说明虽然化学法对沸石的再生过程简单,但即使在不同的反应器中,沸石也很难完全解吸。而该过程中产生的高盐高氨氮再生废液会造成二次污染,需要进一步处理。因此,为了实现沸石再生及高浓度氨氮同步去除,HUANG等[16]在同一个反应器中,首先利用沸石对
${\rm{NH}}_4^{\rm{ + }}$ 进行完全吸附,然后排出部分处理液后再加入NaClO溶液,结果表明,99%的${\rm{NH}}_4^{\rm{ + }}$ 能够被快速氧化(10 min)为N2,不会有${\rm{NH}}_4^{\rm{ + }}$ 残留在再生液中。再生后的沸石与用NaCl改性的新鲜沸石的吸附氨氮的能力相当。ZHANG等[99]通过长期沸石吸附${\rm{NH}}_4^{\rm{ + }}$ 及再生实验,证实了NaClO和NaCl的混合溶液对沸石的再生能力高于单独使用NaCl或者NaClO溶液对沸石的再生能力。这主要是因为混合溶液能提高沸石的微孔性,有利于${\rm{NH}}_4^{\rm{ + }}$ 的脱附并被氧化为N2和${\rm{NO}}_3^ - $ 。此方法的优势是沸石再生过程简单,不受溶液温度及被交换的${\rm{NH}}_4^{\rm{ + }}$ 量的影响,且能实现沸石再生及高浓度氨氮同步去除,无二次污染。但该方法的缺点是将${\rm{NH}}_4^{\rm{ + }}$ 转换为N2,未能对其回收利用。为了节约化学药剂及重复使用再生液,在有Cl−存在的条件下,利用电化学反应使其生成HClO,再将${\rm{NH}}_4^{\rm{ + }}$ 氧化为N2,也能够同步实现沸石再生及${\rm{NH}}_4^{\rm{ + }}$ 去除[100]。但是沸石的电化学再生过程需要复杂的设备和较高的能耗,这可能会增加项目运行和废水处理的成本。沸石的生物再生实质上是化学和生物再生的结合,首先利用NaNO3溶液使沸石再生,然后利用硝化菌的硝化作用将${\rm{NH}}_4^{\rm{ + }}$ 氧化为${\rm{NO}}_3^ - $ 。徐丽花等[101]通过比较硝化污泥对沸石直接再生、硝化污泥分别在同一个反应器及2个反应器对再生液再生3个工艺流程,发现先对沸石再生,然后对再生液进行硝化反应,能够避免硝化菌与沸石的接触,缩短再生时间,减少硝化池体积。与化学再生法相比,沸石的生物再生工艺是一种环保、节能的方法,但是该工艺复杂,再生过程需要严格控制各种参数(如pH、溶解氧、温度、添加碳源等),而且污泥可能会使沸石表面快速污染,降低${\rm{NH}}_4^{\rm{ + }}$ 的交换效率[100]。而粉末树脂在火电厂冷凝水精处理系统中是一次性使用,无粉末树脂分离及再生工艺[48],失效的粉末树脂直接爆膜,爆膜后的失效树脂一般是深埋或者焚烧[43]。目前还没有粉末树脂、磁性阳离子交换树脂针对低浓度氨氮吸附及再生的报道,但二者的再生过程也属于离子交换反应,因此,可以根据实际运行情况,从上述化学法、电化学法以及生物法中优选合适的工艺对滤料进行再生。此外,沸石、粉末树脂及磁性阳离子交换树脂再生后,产生的高盐或强酸及氨氮浓缩液处理是未来离子交换工艺中亟需解决的重要问题。目前,较常用的方法包括排入大海、钻孔注入岩石层、垃圾填埋及利用太阳光蒸发。但这些方法并没有对浓缩后的无机及有机资源分别进行回收,未达到资源最优化利用的目的。有研究已经证实,利用含NaClO的溶液对沸石再生后,产生的废液只有盐酸,因此,可以纯化后进一步利用[16]。而在电化学再生过程中,通过改变NaCl的浓度,能使
${\rm{NH}}_4^{\rm{ + }}$ 主要氧化为N2而不生成其他物质,因此,再生液可以重复使用[100]。生物再生工艺中产生的富含${\rm{NO}}_3^ - $ 的溶液可以排入农田或反硝化池,进一步利用或循环使用[101]。因此,通过开发新工艺,同时实现滤料再生和废液处理,设计合理工艺对高含盐或者含酸氨氮浓缩液进行分离、纯化,生成高纯度产品,或者合成具有高附加值的液体肥料是未来研究的重要内容。表3主要比较了目前这3种滤料在低浓度氨氮吸附方面的研究现状、市场价格及优缺点,未来可有针对性地解决每种材料存在的缺点,有助于提高其应用价值。 -
相对于粉末树脂和磁性离子交换树脂,天然沸石资源储量丰富,价格低廉。表3主要比较了目前这3种滤料在低浓度氨氮吸附方面的主要研究结果及市场价格,未来针对性的解决每种材料存在的缺点,有助于提高其应用价值。全球每年约开采沸石300×104~400×104 t,其中中国开采量为200×104 t[14]。普通沸石价格为210~480 元·t−1,研磨较细的沸石价格为350~800 元·t−1。若以廉价的谷壳灰作为人工沸石的制备原料,能够有效降低成本。磁性沸石制备过程中的主要费用支出在于原料铁盐和亚铁盐,因此,确定适宜的Fe3O4的浸入量是节省磁性沸石制备费用及提高沸石性能的关键。国产粉末阴、阳离子树脂的价格分别约为45 000元·t−1和80 000元·t−1,相对沸石价格较贵,因为目前没有实际工程中的运行数据,故很难对运行费用进行精确计算。中国水务投资有限公司及焦茹媛等[102]通过对磁性离子交换技术的运行费用进行计算,发现主要运行费用包括盐耗(用于树脂再生)、树脂流失和电耗。其中盐耗费用为0.02元·t−1,树脂费用为0.08元·t−1,电耗为0.02元·t−1,设备折旧费用为0.03元,合计运行费用为0.15元·t−1。此结果与卢宁等[103]根据烧杯实验和中试实验,计算出利用MIEX®树脂去除黄浦江原水中有机物运行成本为0.16元·t−1,基本一致。潘若平等[104]对澳大利亚Wanneroo水厂中MIEX®工艺、活性炭及臭氧+活性炭工艺的经济性进行了评价,以DOC去除率达到40%~50%为标准,发现MIEX®总成本是后两者成本的1/3。
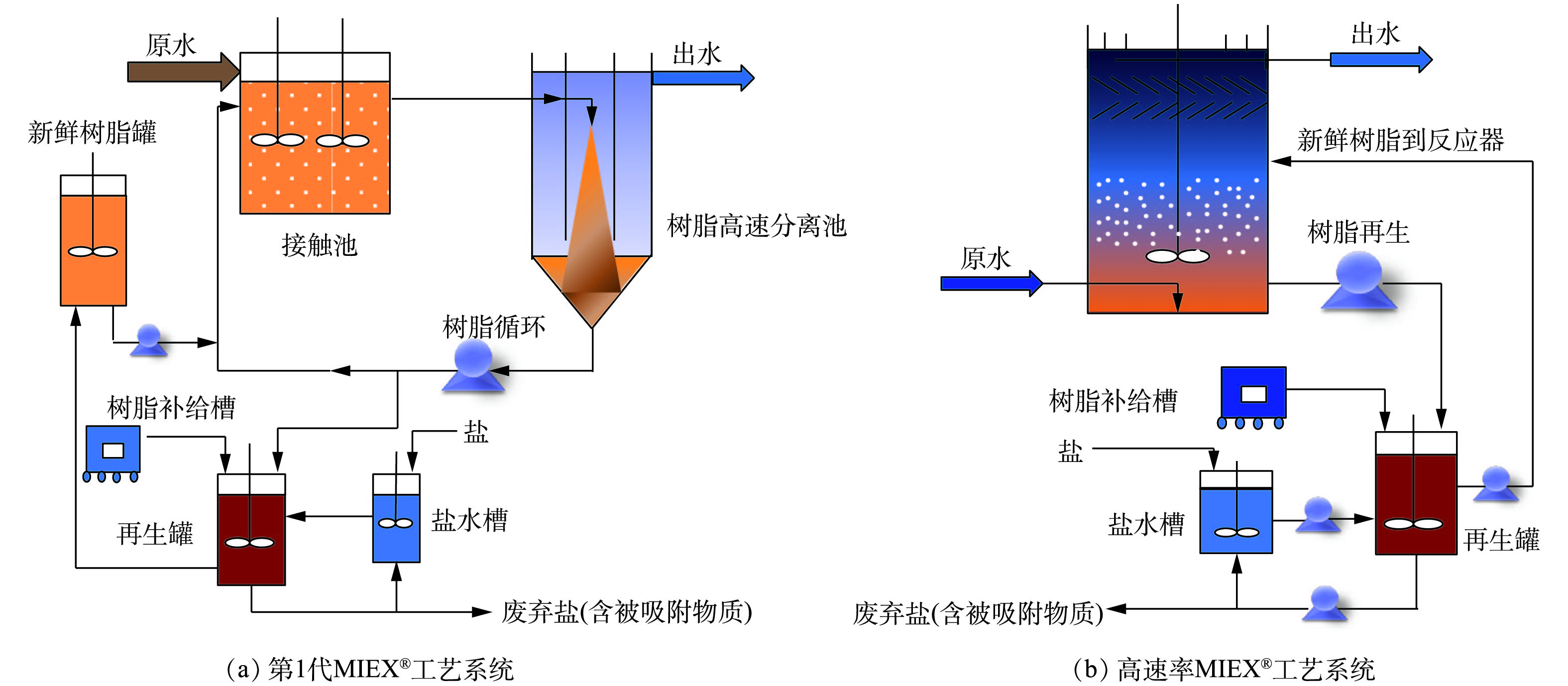
MIEX®技术工程化运行工艺成熟,其优势是进水指标范围宽、操作压力低、工艺设计灵活、占地面积小、出水水质高,有利于对低浓度氨氮进行回收。2种不同MIEX®工艺系统见图9。第1代MIEX®工艺主要包括3部分[105]:接触池、高速沉降池和再生池(图9(a))。该工艺的缺点是接触池中树脂浓度低,树脂再生过程流失损失大,整体工艺占地面积大。目前广泛应用的高速率MIEX®工艺流程将接触池和分离池合并(图9(b)),克服了第1代工艺占地大的缺点。该工艺布水采用升流式设计,流动树脂层浓度为100~150 mL·L−1,接触时间为5 min,搅拌器缓慢旋转保持树脂分散,并且使其不会从反应器顶部流出,上部的斜板用来减少树脂流失,出水从反应器顶部流出。与第1代工艺相比,高速MIEX®工艺流程可以提高树脂的反应浓度,减少接触时间和树脂的损失量,有利于低浓度氨氮回收方案的实施。
-
1)关于天然沸石、人工合成沸石对氨氮的去除方面已有大量研究,但如何在进一步提高离子交换容量和稳定性的同时降低成本,仍然是未来需要解决的问题。
2)粉末离子交换树脂吸附容量高,但对
${\rm{NH}}_4^{\rm{ + }}$ 选择性差,价格较贵。因此,需要设计合理的反应器及高选择性涂覆材料,以提高${\rm{NH}}_4^{\rm{ + }}$ 离子交换效率、降低运行成本。3)磁性阴离子交换树脂工艺发展成熟,磁性阳离子交换树脂对氨氮的吸附效率较低。利用配体交换理论,制备含过渡金属基的磁性鳌合阳离子树脂,是实现氨氮的高选择性回收的有效途径之一。
4)离子交换剂再生后产生的浓缩液的处理问题,也是未来具有发展前景的研究方向之一。
5)磁性离子树脂的工程化应用条件成熟,有利于污水中低浓度氨氮回收方案的实施。
典型离子交换水处理技术在低浓度氨氮回收中的应用分析
Analysis of the application of typical ion exchange water treatment technology in low concentration ammonia nitrogen recovery
-
摘要: 离子交换技术具有交换容量高、能耗低、可再生效率高、操作过程简单、环境友好,并且能同时实现水质净化和资源原位回收等优势,在水处理领域应用十分广泛。在文献及工程调研的基础上,综述了3种典型离子交换剂:沸石、粉末树脂、磁性离子交换树脂的技术原理及其在污水处理中的应用现状;分析了三者在低浓度氨氮回收过程中存在的问题,并提出了相应的研究策略;评价了3种离子交换剂在工程化应用中的经济性,主要介绍了成熟的磁性离子交换树脂工艺;通过分析可知,将离子交换技术从污水治理领域应用到污水中低浓度氨氮原位富集回收领域,对污水全面资源化利用具有重要意义。Abstract: Ion exchange technology has a broad application prospects in the field of water treatment due to its advantages of high exchange capacity, low energy consumption, high regenerative efficiency, simple operation, environmental friendliness and simultaneous water purification and in situ resources recovery. Based on the analysis of literatures and engineering cases, the technical principles of three typical ion exchangers, such as zeolite, powder resin and magnetic ion exchange resin and their application in wastewater treatment were reviewed. The problems of the three ion exchangers for low concentration ammonia nitrogen recovery were analyzed and their corresponding solutions were proposed. Then their economics in engineering application were evaluated and the mature magnetic ion exchange resin process was mainly introduced. The author believes that the application of ion exchange technology from the field of sewage treatment to the in situ enrichment and recovery of low concentration ammonia nitrogen is of great significance to the comprehensive utilization of sewage resources.
-
Key words:
- ion exchange /
- zeolite /
- powder resin /
- magnetic resin /
- low concentration ammonia nitrogen
-

-
表 1 天然沸石、改性沸石及人工合成沸石对氨氮吸附性能及再生工艺对比
Table 1. Comparison of ammonium adsorption performance and regeneration process of natural zeolite, modified zeolite and synthetic zeolite
沸石类型 初始氨氮浓度/
(mg·L−1)温度/
℃接触时间/
min氨氮去
除率/%吸附容量/
(mmol·g−1)沸石再生工艺 再生率/% 来源 斜发沸石 2~94 — 240 — 1.03 0.5 mol·L−1 NaCl (pH=11~12) 99.00 [25] 斜发沸石 573~2 290 25 180 — 0.60 生物反硝化 95.50 [26] 天然沸石 5~80 25 100 97.00 0.25 1% NaCl溶液 95.10 [39] NaCl改性沸石 6~20 25 1 440 — 0.46 5 g·L−1 NaCl+NaOH 95.20 [19] NaCl改性沸石 320~1 120 — 120 93.80 — HClO 99.00 [16] 纳米零价铁改性沸石 200~3 000 30~50 — — 2.78 — — [17] 纳米NaA沸石 2~50 — 120 90.00 3.32 — — [36] Fe3O4改性NaA沸石 63.75 16~70 120 82.53 0.58 — — [38] 注:—为无文献报道。 表 2 进口粉末树脂与国产粉末树脂性能对比
Table 2. Comparison of properties between imported and domestic powder resins
样品名称 基准型 含水量/% 交换容量/(mmol·g−1) 国产阳树脂(HC2009111) 铵型 43.56 4.85 国产阴树脂(HC2009112) 氢氧型 62.94 4.71 进口混样树脂 (HC2009113) 铵型与氢氧型 48.32 — 进口混样树脂 (HC2009113) 铵型 — 4.25 进口混样树脂 (HC2009113) 氢氧型 — 3.50 注:—为无文献报道。 表 3 沸石、粉末树脂及磁性阳离子交换树脂针对低浓度氨氮吸附性能对比
Table 3. Comparison of adsorption performance of zeolite, powder resin and magnetic cation exchange resin for low concentration ammonia nitrogen
材料 初始氨
氮浓度/
(mg·L−1)残余氨
氮浓度/
(mg·L−1)吸附容量/
(mmol·g−1)静态实验
主要结论动态实验
主要结论假二级动力学
速率常数/
(g·(mg·min)−1)来源 土耳其斜发沸石 25 13 0.45 自发、放热 — 0.03 [24] 天然沸石 5~50 0.25~4 0.26 去除率90% 床体积128 BV — [53] 改性沸石 5~50 0~2.5 0.32 去除率90 床体积368 BV — [53] 天然沸石 20 <5 0.38 — 床体积15 L 0.002 5 [19] 改性沸石 20 <5 0.53 — 床体积40 L 0.03 [19] 天然沸石 5~12 0~5 1.08 去除率70%~90% — — [98] 改性沸石 5~12 0~5 1.32 去除率70%~90% — — [98] 合成沸石 12.3 3.16 — 自发、放热 — 2.21×10−3 [34] 粉末树脂 — — — — — — — 磁性阳离子交换树脂 — — — — — — — 注:天然沸石市场价格为210~480 元·t−1;研磨较细沸石市场价格为350~800 元·t−1;粉末树脂市场价格为8×104 元·t−1;磁性阳离子交换树脂市场价格为5×104~6×104 元·t−1[59];—为无文献报道。 -
[1] QIN H J, KEKRE K A, TAO G H, et al. New option of MBR-RO process for production of NEWater from domestic sewage[J]. Journal of Membrane Science, 2006, 272(1/2): 70-77. [2] JIN Z, GONG H, TEMMINK H, et al. Efficient sewage pre-concentration with combined coagulation microfiltration for organic matter recovery[J]. Chemical Engineering Journal, 2016, 292: 130-138. doi: 10.1016/j.cej.2016.02.024 [3] CUSICK R D, LOGAN B E. Phosphate recovery as struvite within a single chamber microbial electrolysis cell[J]. Bioresource Technology, 2012, 107: 110-115. doi: 10.1016/j.biortech.2011.12.038 [4] 张冬娜, 张冬娜, 宋永会, 等. 利用丝光沸石吸附高浓度氨氮的研究[J]. 安全与环境学报, 2006, 6(5): 17-20. doi: 10.3969/j.issn.1009-6094.2006.05.006 [5] CHEN Y Q, TANG J J, LI W L, et al. Thermal decomposition of magnesium ammonium phosphate and adsorption properties of its pyrolysis products toward ammonia nitrogen[J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 497-503. doi: 10.1016/S1003-6326(15)63630-5 [6] 赵贤广, 李武, 王金龙, 等. 高浓度氨氮废水处理与氨资源化新技术[J]. 工业水处理, 2011, 31(12): 31-34. doi: 10.11894/1005-829x.2011.31(12).31 [7] 李丹, 沈存花, 刘佛财, 等. 低浓度氨氮废水处理技术研究进展[J]. 应用化工, 2018, 47(6): 1274-1280. doi: 10.3969/j.issn.1671-3206.2018.06.048 [8] 曾青云, 薛丽燕, 曾繁钢, 等. 氨氮废水处理技术的研究现状[J]. 有色金属科学与工程, 2018, 9(4): 83-87. [9] 胡雪飞, 黄万抚. 氨氮废水处理技术研究进展[J]. 金属矿山, 2017(7): 199-203. [10] 李婵君, 贺剑明. 折点加氯法处理深度处理低氨氮废水[J]. 广东化工, 2013, 40(20): 43-44. doi: 10.3969/j.issn.1007-1865.2013.20.023 [11] 王鹏, 刘伟藻, 方汉平. 电化学氧化与厌氧技术联用处理垃圾渗沥水[J]. 环境科学, 2001, 22(5): 70-72. doi: 10.3321/j.issn:0250-3301.2001.05.016 [12] FANG K, GONG H, HE W Y, et al. Recovering ammonia from municipal wastewater by flow-electrode capacitive deionization[J]. Chemical Engineering Journal, 2018, 348: 301-309. doi: 10.1016/j.cej.2018.04.128 [13] 孔庆瑞, 左建. 天然沸石在环境保护上的应用[J]. 铁道劳动安全卫生与环保, 1987(2): 21. [14] HUAN J, KANKANAMGE N R, CHOW C, et al. Removing ammonium from water and wastewater using cost-effective adsorbents: A review[J]. Journal of Environmental Sciences (China), 2018, 63: 174-197. doi: 10.1016/j.jes.2017.09.009 [15] MUMPTON F A. Natural zeolites: A new industrial mineral commodity[M]//SAND L B, MUMPTON F A. Natural Zeolites, Occurrence, Properties, Use. New York: Pergamon Press, 1976: 285-302. [16] HUANG H, YANG L, XUE Q, et al. Removal of ammonium from swine wastewater by zeolite combined with chlorination for regeneration[J]. Journal of Environmental Management, 2015, 160: 333-341. [17] LIU M M, XI B D, HOU L A, et al. Magnetic multi-functional nano-fly ash-derived zeolite composites for environmental applications[J]. Journal of Materials Chemistry A, 2013, 41(1): 12617-12626. [18] 安莹, 王志伟, 张一帆, 等. 天然沸石吸附氨氮的影响因素[J]. 环境工程学报, 2013, 7(10): 3927-3932. [19] 唐登勇, 郑正, 郭照冰. 改性沸石吸附低浓度氨氮废水及其脱附的研究[J]. 环境工程学报, 2011, 5(2): 293-296. [20] 左思敏, 荆肇乾, 陶梦妮, 等. 天然沸石和改性沸石在废水处理中的应用研究[J]. 应用化工, 2019, 48(5): 1136-1145. [21] LIU M M, AN D, HOU L A, et al. Zero valent iron particles impregnated zeolite X composites for adsorption of tetracycline in aquatic environment[J]. RSC Advances, 2015, 125(5): 103480-103487. [22] OLIVEIRA L C, PETKOWICZ D I, SMANIOTTO A, et al. Magnetic zeolites: A new adsorbent for removal of metallic contaminants from water[J]. Water Research, 2004, 38: 3699-3704. doi: 10.1016/j.watres.2004.06.008 [23] YUAN M, XIE T, YAN G, et al. Effective removal of Pb2+ from aqueous solutions by magnetically modified zeolite[J]. Powder Technology, 2018, 332: 234-241. doi: 10.1016/j.powtec.2018.03.043 [24] KARADAG D, KOC Y, TURAN M, et al. Removal of ammonium ion from aqueous solution using natural Turkish clinoptilolite[J]. Journal of Hazardous Materials, 2006, 136: 604-609. doi: 10.1016/j.jhazmat.2005.12.042 [25] DU Q, LIU S, CAO Z, et al. Ammonia removal from aqueous solution using natural Chinese clinoptilolite[J]. Separation and Purification Technology, 2005, 44: 229-234. doi: 10.1016/j.seppur.2004.04.011 [26] MARTINS T H, SOUZA T S O, FORESTI E. Ammonium removal from landfill leachate by clinoptilolite adsorption followed by bioregeneration[J]. Journal of Environmental Chemical Engineering, 2017, 5(1): 63-68. doi: 10.1016/j.jece.2016.11.024 [27] WANG S B, PENG Y L. Natural zeolites as effective adsorbents in water and wastewater treatment[J]. Chemical Engineering Journal, 2010, 156: 11-24. doi: 10.1016/j.cej.2009.10.029 [28] 王萌, 房春生, 颜昌宙, 等. 沸石的改性及其对氨氮吸附特征[J]. 环境科学研究, 2012, 25(9): 1025-1029. [29] 曲珍杰, 徐宁. 超声与改性沸石强化处理高浓度氨氮废水[J]. 净水技术, 2017, 36(7): 20-26. [30] 薛永强, 石太宏, 陈栩迪, 等. 沸石改性及其对水中氨氮去除实验研究[J]. 广东化工, 2015, 23(42): 146-148. [31] ZIELINSKI M, ZIELINSKA M, DEBOWSKI M. Ammonium removal on zeolite modified by ultrasound[J]. Desalination and Water Treatment, 2015, 57: 8748-8753. [32] 王曦, 张雪峰, 阙耀华, 等. 粉煤灰水热法合成沸石及其对氨氮吸附性能的研究[J]. 环境工程, 2012, 30(5): 13-16. doi: 10.3969/j.issn.1671-1556.2012.05.004 [33] 宋卫军, 谢妤. 稻壳沸石的合成及其对氨氮的吸附/脱附动力学[J]. 环境工程学报, 2016, 10(9): 4745-4752. doi: 10.12030/j.cjee.201601043 [34] SHABAN M, ABUKHADRA M R, NASIEF F M, et al. Removal of ammonia from aqueous solutions, ground water, and wastewater using mechanically activated clinoptilolite and synthetic zeolite-A: Kinetic and equilibrium studies[J]. Water, Air & Soil Pollution, 2017, 228: 450-466. [35] 王水利, 葛岭梅, 杨建利. 纳米沸石的合成及影响因素[J]. 纳米加工工艺, 2005(2): 182-188. [36] NAWOG M A, MUHID M N M, MALEK N A N N, et al. Eco-friendly synthesis of nanozeolite NaA from rice husk ash and its efficiency in removing ammonium ions[J]. Key Engineering Materials, 2013, 594-595: 168-172. doi: 10.4028/www.scientific.net/KEM.594-595 [37] 袁明亮, 谭美易, 闫冠杰. 磁性X沸石的合成及其性能[J]. 过程工程学报, 2009, 9(6): 1210-1215. doi: 10.3321/j.issn:1009-606X.2009.06.031 [38] LIU H, PENG S, SHU L, et al. Effect of Fe3O4 addition on removal of ammonium by zeolite NaA[J]. Journal of Colloid and Interface Science, 2013, 390: 204-210. doi: 10.1016/j.jcis.2012.09.010 [39] WIDIASTUTI N, WU H, ANG H M, et al. Removal of ammonium from greywater using natural zeolite[J]. Desalination, 2011, 277: 15-23. doi: 10.1016/j.desal.2011.03.030 [40] FU L, SHUAN C, LIU F, et al. Rapid removal of copper with magnetic poly-acrylic weak acid resin: Quantitative role of bead radius on ion exchange[J]. Journal of Hazardous Materials, 2014, 272: 102-111. doi: 10.1016/j.jhazmat.2014.02.047 [41] 查方林, 刘凯, 吴俊杰, 等. 粉末树脂覆盖过滤器在超临界直接空冷机组中的应用[J]. 湖南电力, 2015, 35(6): 48-52. doi: 10.3969/j.issn.1008-0198.2015.06.013 [42] BOYER T H, SINGER P C. A pilot-scale evaluation of magnetic ion exchange treatment for removal of natural organic material and inorganic anions[J]. Water Research, 2006, 40: 2865-2876. doi: 10.1016/j.watres.2006.05.022 [43] 周志丹. 粉末树脂过滤技术在直接空冷机组凝结水精处理中的应用[J]. 吉林电力, 2014, 42(5): 8-9. doi: 10.3969/j.issn.1009-5306.2014.05.003 [44] YARNELL P A. Powdered resins: Continuous ion exchange[M]//YARNELL P A. Encyclopedia of Separation Science. Elsevier, 2015: 3973-398. [45] LEVENDUSKY J A. Process for purifying liquid and particulate ion exchange material used therefor: 3250702A[P]. 1966-03-08. [46] 陈磊. 粉末树脂过滤器在凝结水精处理系统中的应用[J]. 设备管理与维修, 2009(6): 22-24. doi: 10.3969/j.issn.1001-0599.2009.06.010 [47] 李长海, 党小建, 裴胜, 等. 粉末树脂过滤器在凝结水精处理系统中的应用[J]. 给水排水, 2015, 41(5): 51-54. doi: 10.3969/j.issn.1002-8471.2015.05.012 [48] 史勉. 粉末树脂过滤器在直接空冷机组凝结水精处理系统中的应用[J]. 电力建设, 2009, 30(6): 69-71. doi: 10.3969/j.issn.1000-7229.2009.06.018 [49] 涂孝飞, 张建斌, 杨彦科. 粉末树脂过滤器+高速混床精处理系统在超临界空冷机组的应用[J]. 水处理技术, 2014, 40(6): 117-122. [50] 王应高, 刘金明, 靳丽霞, 等. 国产粉末离子交换树脂的工业应用[J]. 热力发电, 2009, 38(10): 64-67. doi: 10.3969/j.issn.1002-3364.2009.10.064 [51] TOMITA J, YAMAOTO M, NOZAKI T, et al. Determination of low-level radiostrontium, with emphasis on in situ pre-concentration of Sr from large volume of freshwater sample using Powdex resin[J]. Journal of Environmental Radioactivity, 2015, 146: 88-93. doi: 10.1016/j.jenvrad.2015.04.011 [52] 张红. 凝结水精处理覆盖过滤器漏粉末树脂的原因及处理措施[J]. 清洗世界, 2015, 31(5): 42-45. doi: 10.3969/j.issn.1671-8909.2015.05.011 [53] 王志杰, 宫徽, 王凯军. 离子交换富集回收生活污水超滤膜滤后出水的氨氮[J]. 环境工程学报, 2017, 11(5): 2633-2639. doi: 10.12030/j.cjee.201608036 [54] KITIS M, İLKER HARMAN B, YIGIT N O, et al. The removal of natural organic matter from selected Turkish source waters using magnetic ion exchange resin (MIEX®)[J]. Reactive and Functional Polymers, 2007, 67: 1495-1504. doi: 10.1016/j.reactfunctpolym.2007.07.037 [55] FEARING D A, BANKS J, GUYETAND S, et al. Combination of ferric and MIEXs for the treatment of a humic rich water[J]. Water Research, 2004, 38: 2551-2558. doi: 10.1016/j.watres.2004.02.020 [56] BOYER T H, SINGER P C. Bench-scale testing of a magnetic ion exchange resin for removal of disinfection by-product precursors[J]. Water Research, 2005, 39: 1265-1276. doi: 10.1016/j.watres.2005.01.002 [57] 张全兴, 张政朴, 李爱民, 等. 我国离子交换与吸附树脂的发展历程回顾与展望[J]. 高分子学报, 2018(7): 814-828. doi: 10.11777/j.issn1000-3304.2018.17317 [58] JOHNSON C J, SINGER P C. Impact of a magnetic ion exchange resin on ozone demand and bromate formation during drinking water treatment[J]. Water Research, 2004, 38: 3738-3750. doi: 10.1016/j.watres.2004.06.021 [59] 王琼杰, 王津南, 李爱民, 等. MIEX®在饮用水净化中的应用研究进展[J]. 离子交换与吸附, 2012, 28(3): 282-288. [60] BOYER T H, SINGER P C, AIKEN G R. Removal of dissolved organic matter by anion exchange effect of dissolved organic matter properties[J]. Environmental Science & Technology, 2008, 42: 7431-7437. [61] HANS R, SENANAYAKE G, DHARMASIRI L C S, et al. A preliminary batch study of sorption kinetics of Cr(VI) ions from aqueous solutions by a magnetic ion exchange (MIEX®) resin and determination of film/pore diffusivity[J]. Hydrometallurgy, 2016, 164: 208-218. doi: 10.1016/j.hydromet.2016.06.007 [62] KITIS M, HARMAN B I, YIGIT N O, et al. The removal of natural organic matter from selected Turkish source waters using magnetic ion exchange resin (MIEX®)[J]. Reactive & Functional Polymers, 2007, 67: 1495-1504. [63] SHUANG C, LI P, LI A, et al. Quaternized magnetic microspheres for the efficient removal of reactive dyes[J]. Water Research, 2012, 46: 4417-4426. doi: 10.1016/j.watres.2012.05.052 [64] NGUVEN T V, ZHANG R, VIGNESWARAN S, et al. Removal of organic matter from effluents by magnetic ion exchange (MIEX®)[J]. Desalination, 2011, 276: 96-102. doi: 10.1016/j.desal.2011.03.028 [65] WANG T Y, PAN X, BEN W W, et al. Adsorptive removal of antibiotics from water using magnetic ion exchange resin[J]. Journal of Environmental Sciences, 2017, 52: 111-117. doi: 10.1016/j.jes.2016.03.017 [66] SLUNJSKI M, CADEE K, TATTERSALL J. MIEX® resin water treatment process[J]. Proceedings of Aquatech, 2000, 26: 29. [67] HU X W, CHEN K, LAI X K, et al. Treatment of pretreated coal gasification wastewater (CGW) by magnetic polyacrylic anion exchange resin[J]. Journal of Environmental Chemical Engineering, 2016, 4: 2040-2044. doi: 10.1016/j.jece.2016.02.018 [68] SHI P, MA R, ZHOU Q. Chemical and bioanalytical assessments on drinking water treatments by quaternized magnetic microspheres[J]. Journal of Hazardous Materials, 2015, 285: 53-60. doi: 10.1016/j.jhazmat.2014.09.047 [69] TANG Y L, LIANG S, GUO H C, et al. Adsorptive characteristics of perchlorate from aqueous solutions by MIEX resin[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2013, 417: 26-31. [70] HUMBERT H, GALLARD H, SUTY H, et al. Natural organic matter (NOM) and pesticides removal using a combination of ion exchange resin and powdered activated carbon (PAC)[J]. Water Research, 2008, 42: 1635-1643. doi: 10.1016/j.watres.2007.10.012 [71] DING L, LU X, DENG H P, et al. Adsorptive removal of 2, 4-dichlorophenoxyacetic acid (2, 4-D) from aqueous solutions using MIEX resin[J]. Industrial & Engineering Chemistry Research, 2012, 51: 11226-11235. [72] JHA A K, BOSE A, DOWNEY J P. Removal of As (V) and Cr (VI) ions from aqueous solution using a continuous, hybrid field-gradient magnetic separation device[J]. Separation Science and Technology, 2006, 41: 3297-3312. doi: 10.1080/01496390600915007 [73] SONG W, GAO B, XU X, et al. Adsorption-desorption behavior of magnetic amine/Fe3O4 functionalized biopolymer resin towards anionic dyes from wastewater[J]. Bioresource Technology, 2016, 210: 123-130. doi: 10.1016/j.biortech.2016.01.078 [74] BRAUN K, CRUAUX L, FABRIS R, et al. Comparison of coagulation and MIEX pre-treatment processes for bacterial and turbidity removal, utilizing real-time optical monitoring techniques[J]. Environmental Technology, 2014, 35: 1038-1045. doi: 10.1080/09593330.2013.859737 [75] ALLPIKE B P, HEITZ A, JOLL C A, et al. Size exclusion chromatography to characerise DOC removal in drinking water treatment[J]. Environmental Science & Technology, 2005, 39: 2334-2342. [76] ZHANG R, VIGNESWARAN S, NGO H, et al. Fluidized bed magnetic ion exchange (MIEX®) as pre-treatment process for a submerged membrane reactor in wastewater treatment and reuse[J]. Desalination, 2008, 227: 85-93. doi: 10.1016/j.desal.2007.05.037 [77] HUMBERT H, GALLARD H, JACQUEMET V, et al. Combination of coagulation and ion exchange for the reduction of UF fouling properties of a high DOC content surface water[J]. Water Research, 2007, 41: 3803-3811. doi: 10.1016/j.watres.2007.06.009 [78] CHO J, AMY G, PELLEGRINO J. Membrane filtration of natural organic matter factors and mechanisms affecting rejection and flux decline with charged ultrafiltration (UF) membrane[J]. Journal of Membrane Science, 2000, 164: 89-110. doi: 10.1016/S0376-7388(99)00176-3 [79] JUTAPORN P, SINGER P C, CORY R M, et al. Minimization of short-term low-pressure membrane fouling using a magnetic ion exchange (MIEX®) resin[J]. Water Research, 2016, 98: 225-234. doi: 10.1016/j.watres.2016.04.007 [80] FABRIS R, LEE E K, CHOW CW K, et al. Pre-treatments to reduce fouling of low pressure micro-filtration (MF) membranes[J]. Journal of Membrane Science, 2007, 289: 231-240. doi: 10.1016/j.memsci.2006.12.003 [81] HUANG H, CHO H H, JACANGELO J G, et al. Mechanisms of membrane fouling control by integrated magnetic ion exchange and coagulation[J]. Environmental Science & Technology, 2012, 46: 10711-10717. [82] PRAMANIK B K, RODDICK F A, FAN L. Combining coagulation/MIEX with biological activated carbon treatment to control organic fouling in the microfiltration of secondary effluent[J]. Membranes, 2016, 6: 39-53. doi: 10.3390/membranes6030039 [83] PLEWA M J, SIMMONS J E, RICHARDSON S D, et al. Mammalian cell cytotoxicity and genotoxicity of the haloacetic acids, a major class of drinking water disinfection by-products[J]. Environmental and Molecular Mutagenesis, 2010, 51: 871-878. doi: 10.1002/em.v51:8/9 [84] WERT E C, EDWARDS-BRANDT J C, SINGER P C, et al. Evaluating magnetic ion exchange resin MIEX pretreatment to increase ozone disinfection and reduce bromate formation[J]. Ozone: Science & Engineering, 2005, 27: 371-379. [85] BOYER T H, GRAF K C, COMSTOCK S E H, et al. Magnetic ion exchange treatment of stabilized landfill leachate[J]. Chemosphere, 2011, 83: 1220-1227. doi: 10.1016/j.chemosphere.2011.03.040 [86] LI Q M, WANG Z, LI Q, et al. Competition and enhancement effect in coremoval of atenolol and copper by an easily regenerative magnetic cation exchange resin[J]. Chemosphere, 2017, 179: 1-9. doi: 10.1016/j.chemosphere.2017.03.005 [87] ZHANG M C, LI A M, ZHOU Q, et al. Preparation and high reusability of a novel acid-resistant magnetic weak acid resin for Ni2+ removal[J]. Industrial & Engineering Chemistry Research, 2014, 53: 340-345. [88] LI Q, FU L, WANG Z, et al. Synthesis and characterization of a novel magnetic cation exchange resin and its application for efficient removal of Cu2+ and Ni2+ from aqueous solutions[J]. Journal of Cleaner Production, 2017, 165: 801-810. doi: 10.1016/j.jclepro.2017.06.150 [89] APELL J N, BOYER T H. Combined ion exchange treatment for removal of dissolved organic matter and hardness[J]. Water Research, 2010, 44: 2419-2430. doi: 10.1016/j.watres.2010.01.004 [90] INDARAWIS K, BOYER T H. Alkaline earth metal cation exchange: Effect of mobile counterion and dissolved organic matter[J]. Environmental Science & Technology, 2012, 46: 4591-4598. [91] FU L C, LIU F Q, MA Y, et al. High-efficient technique to simultaneous removal of Cu(II), Ni(II) and tannic acid with magnetic resins: Complex mechanism behind integrative application[J]. Chemical Engineering Journal, 2015, 263: 83-91. doi: 10.1016/j.cej.2014.11.041 [92] COMSTOCK S E H, BOYER T H. Combined magnetic ion exchange and cation exchange for removal of DOC and hardness[J]. Chemical Engineering Journal, 2014, 241: 366-375. doi: 10.1016/j.cej.2013.10.073 [93] ARIAS-PAIC M, CAWLEY K M, BYG S, et al. Enhanced DOC removal using anion and cation ion exchange resins[J]. Water Research, 2016, 88: 981-989. doi: 10.1016/j.watres.2015.11.019 [94] NEALE P A, MASTRUP M, BORGMANN T, et al. Sorption of micropollutant estrone to a water treatment ion exchange resin[J]. Journal of Environmental Monitoring, 2010, 12: 311-317. doi: 10.1039/B913338K [95] 彭佳乐. 新型铜基离子交换树脂处理氨氮废水的研究[D]. 长沙: 中南大学, 2009. [96] 周康根, 陈泉洲, 姜科, 等. 铜负载螯合树脂对氨氮的解吸及循环吸附性能[J]. 中南大学学报, 2015, 46(11): 3999-4003. doi: 10.11817/j.issn.1672-7207.2015.11.004 [97] 李曦, 靳艳巧, 张超灿. 磁性螯合树脂的悬浮缩聚合成及吸附性能研究[J]. 武汉理工大学学报, 2004, 26(11): 1-4. doi: 10.3321/j.issn:1671-4431.2004.11.001 [98] SARIOGLU M. Removal of ammonium from municipal wastewater using natural Turkish (Dogantepe) zeolite[J]. Separation and Purification Technology, 2005, 41: 1-11. doi: 10.1016/j.seppur.2004.03.008 [99] ZHANG W, ZHOU Z, AN Y, et al. Optimization for zeolite regeneration and nitrogen removal performance of a hypochlorite-chloride regenerant[J]. Chemosphere, 2017, 178: 565-572. doi: 10.1016/j.chemosphere.2017.03.091 [100] LEI X, LI M, ZHANG Z, et al. Electrochemical regeneration of zeolites and the removal of ammonia[J]. Journal of Hazardous Materials, 2009, 169: 746-750. doi: 10.1016/j.jhazmat.2009.04.012 [101] 徐丽花, 周琪. 沸石去除废水中氨氮及其再生[J]. 中国给水排水, 2003, 19(3): 24-26. doi: 10.3321/j.issn:1000-4602.2003.03.008 [102] 焦茹媛, 许志珍, 王东升. 磁性离子交换(MIEX)树脂的研究与应用现状[J]. 中国给水排水, 2015, 31(6): 1-6. [103] 卢宁, 张东, 潘为平, 等. 磁性离子交换树指MIEX去除黄浦江原水中有机物[J]. 净水技术, 2011, 30(1): 25-48. doi: 10.3969/j.issn.1009-0177.2011.01.007 [104] 潘若平, 邓慧萍. 磁性离子交换树脂在饮用水预处理中的应用[J]. 工业用水与废水, 2009, 40(2): 63-67. doi: 10.3969/j.issn.1009-2455.2009.02.018 [105] SHORROCK K, DRAGE B. A pilot plant evaluation of the magnetic ion exchanges process for the removal of dissolved organic carbon at Draycote water treatment works[J]. Water and Environment Journal, 2006, 20: 65-70. doi: 10.1111/wej.2006.20.issue-2 -



 下载:
下载: