-
化学镀镍是通过还原剂提供电子,使得金属离子还原为金属单质镀在镀件表面的工艺[1],化学镀镍电镀液中主要以次磷酸盐为还原剂,硫酸镍提供镍离子,由有机酸或者盐类作为络合剂。化学镀镍废水主要来源是化学镍镀件的漂洗水,其主要成分为高浓度镍离子、次磷酸盐和亚磷酸盐、难降解有机物[2]。现有的处理技术主要有离子交换法[3]、膜分离反渗透法[4]、化学沉淀法[5]和高级氧化技术。离子交换法和膜分离法由于运行要求高,膜易受污染以及离子交换剂饱和再生等限制,难以大规模运用。对于络合镍,由于络合剂能与镍离子稳定结合,很难通过传统的化学破络及沉淀方法彻底去除。高级氧化技术操作方便,处理效果好,在处理含镍废水中广泛应用。刘洋[6]采用类芬顿氧化处理化学镀镍废水,XU等[7]采用电催化处理化学镀镍工艺废水,均取得一定的效果。但上述工艺均存在一定弊端,芬顿氧化污泥产生量大,电催化技术处理规模小,对设备要求高。因此,急须寻找一种稳定、高效的方法降解水中络合镍和有机物。
臭氧具有氧化性强、操作简单、无二次污染等优点,被广泛应用于水处理中[8-9]。臭氧氧化降解有机物的途径分为直接反应和间接反应[10]。直接反应是指臭氧直接和有机物进行反应,具有较强的选择性,不能很好地降解有机物[11];间接反应是指通过催化作用引发臭氧分解生成具有强氧化性的 · OH, · OH再与有机污染物发生反应。 · OH (E0=2.8 V)比O3(E0=2.07 V)具有更高的氧化还原电位,可以和水中大部分的有机物反应,因此,臭氧催化氧化技术在水处理领域具有独特的优势[12-13]。
本研究采用臭氧催化氧化工艺处理化学镀镍废水,考察了臭氧催化氧化对化学镀镍废水的处理效果,研究了臭氧投加量、反应pH和反应时间对出水COD和UV254的影响;对最优反应条件下臭氧催化氧化出水进行化学沉淀,通过检测进出水总镍的变化,考察臭氧催化氧化对络合态镍的破络效果。本研究对处理前后废水的生物毒性进行了探讨,为化学镀镍废水的处理提供了参考。
-
实验所用废水为江苏常州某电镀厂化学镍生产车间一级漂洗水,该车间电镀液有硫酸镍、次磷酸钠、柠檬酸钠、乙酸、氯化铵等物质组成。废水中有机物主要来源于镀液中添加的稳定剂、络合剂和光亮剂,主要物质包含烯炳基磺酸钠、聚乙二醇、柠檬酸、十二烷基磺酸钠、硫脲衍生物和未知名称的含氮杂环类物质等,水中镍离子与络合剂结合形成复杂的络合离子。实验用废水COD为532 mg·L−1、总镍为78.2 mg·L−1、TP为64.2 mg·L−1、正磷酸根为1.2 mg·L−1、pH为5.0,废水呈绿色。
-
为去除陶粒表面油脂,将粒径为3~5 mm的陶粒用质量分数为8%的NaOH溶液浸泡,之后再用质量分数为13%的稀硝酸溶液浸渍1~2 h,最后用去离子水洗净至出水中性后烘干,备用。以钛酸四丁酯为前驱体,将其与无水乙醇和抑制剂冰醋酸混合,再加入适量水,得溶液溶胶A。将一定量的硝酸锰、硝酸铁加入到无水乙醇中,配成溶液B,用酸调节pH至3.0。边搅拌边将溶液B滴加到溶液溶胶A中,室温下搅拌,使钛酸四丁酯充分水解,得到溶液溶胶C。将预处理后的陶粒浸渍在制备好的溶液溶胶C中12 h,然后将溶液蒸发、干燥后,置于马弗炉中,在500 ℃条件下,焙烧4 h,即得负载型Fe2O3-TiO2-MnO2/Al2O3催化剂[14]。
-
1)臭氧催化氧化实验。实验工艺流程如图1所示。实验在室温(25±2) ℃下进行。臭氧反应器高度为2 500 mm,直径为200 mm,为有机玻璃材质,其中臭氧催化剂的填充率为50%,催化剂在填充前用废水浸泡直至吸附饱和。臭氧催化氧化采用静态实验的方式,底部采用刚玉微孔曝气盘,孔径为50 μm,臭氧发生器(CF-G-3-010g,青岛国林实业股份有限公司)以99.9%的纯氧为气源,进气气体流速通过阀门调节,尾气采用KI溶液吸收。通过内循环泵将反应器内废水形成回流,从而使废水均匀地与催化剂接触,内循环泵流速控制为5 L·min−1。通过调节臭氧进气浓度控制臭氧投加量,取不同反应时间的水样分析污染物的去除率。
2)化学沉淀实验。取适量臭氧催化氧化出水于烧杯中,用Ca(OH)2调节pH至10,向烧杯中分别加入聚合氯化铝(PAC)和聚丙烯酰胺(PAM),PAC加药量为300 mg·L−1,PAM加药量为5 mg·L−1。将烧杯置于磁力搅拌器中,快速磁力搅拌15 min后,再慢速搅拌40 min,静置沉淀后,取上清液,通过定性滤纸过滤得到水样。考察不同臭氧投加量、不同初始pH条件下水样中镍浓度的变化。
-
COD、BOD、TP和正磷酸根等指标的测定采用标准方法[15];pH采用酸度计(pHB-2,上海雷磁仪器厂)测定;UV254和全波段扫描采用紫外分光光度计(DR6000,HACH)测定;镍浓度采用火焰原子吸收分光光度法测定(GB/T 5750.6-2006),所用仪器型号为(TAS-990MFG,北京普析通用仪器厂)。活性污泥比耗氧速率(SOUR)的检测见文献中的方法[16]。
-
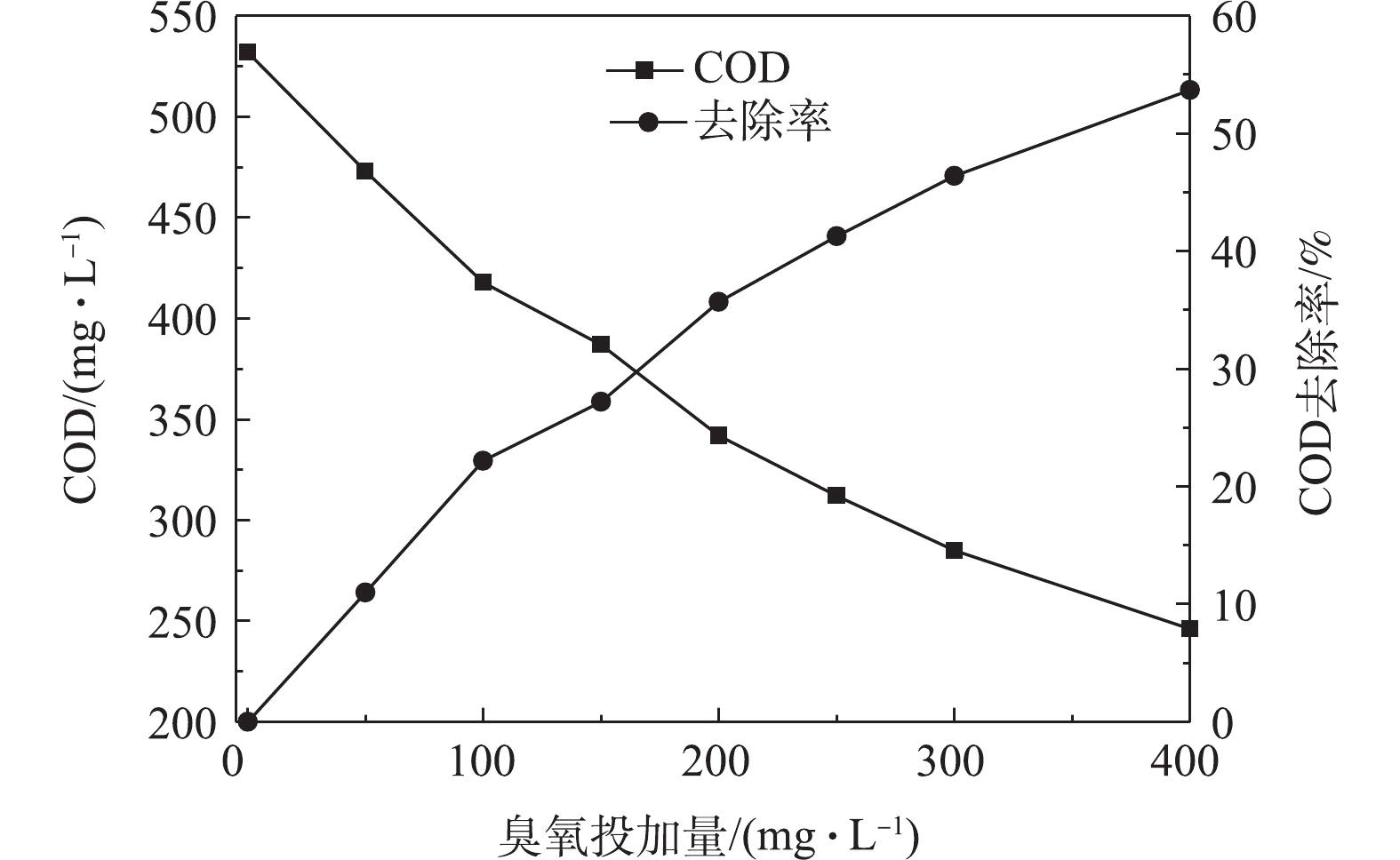
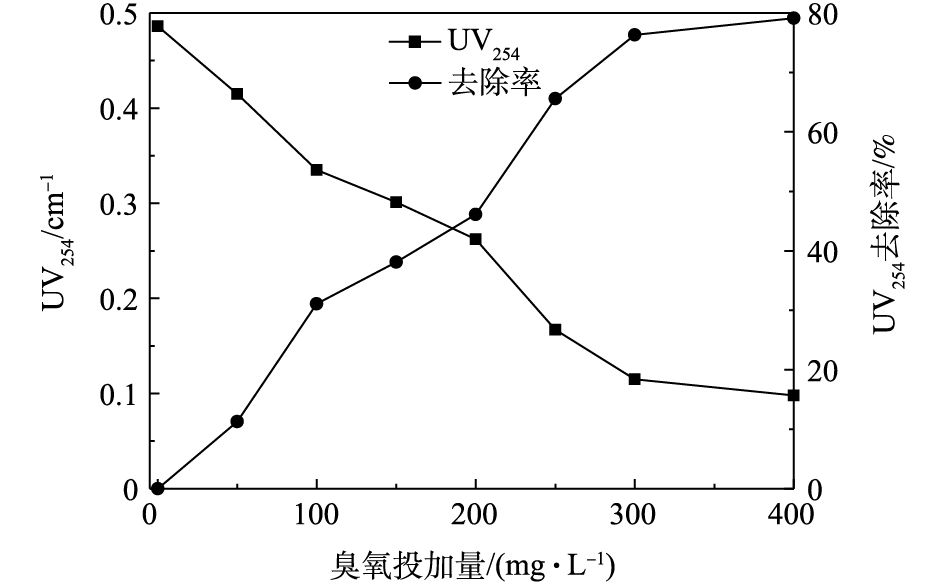
1)臭氧投加量的影响。臭氧催化氧化实验在室温下进行,废水初始pH为5,反应时间为60 min,通过调节臭氧进气浓度控制臭氧的投加量,图2和图3为不同臭氧投加量条件下水中有机物的降解情况。由图2可知,随着臭氧投加量的增加,水中COD的去除率不断升高,当臭氧投加量增至400 mg·L−1时,COD由532 mg·L−1降至246 mg·L−1,UV254由0.468降至0.098,COD和UV254的去除率分别升至53.7%和79.1%。COD和UV254的去除率随着臭氧投加量的增加而逐步升高,当臭氧投加量增到300 mg·L−1时,COD和UV254的去除率开始趋于平缓,臭氧的利用率开始下降。臭氧催化氧化主要通过臭氧催化产生 · OH,通过 · OH氧化水中有机物。随着臭氧投加量的增加,有机物不断被降解,随着水中可被氧化的有机物的减少, · OH与有机物发生碰撞的概率降低[17],从而导致臭氧的利用率下降。
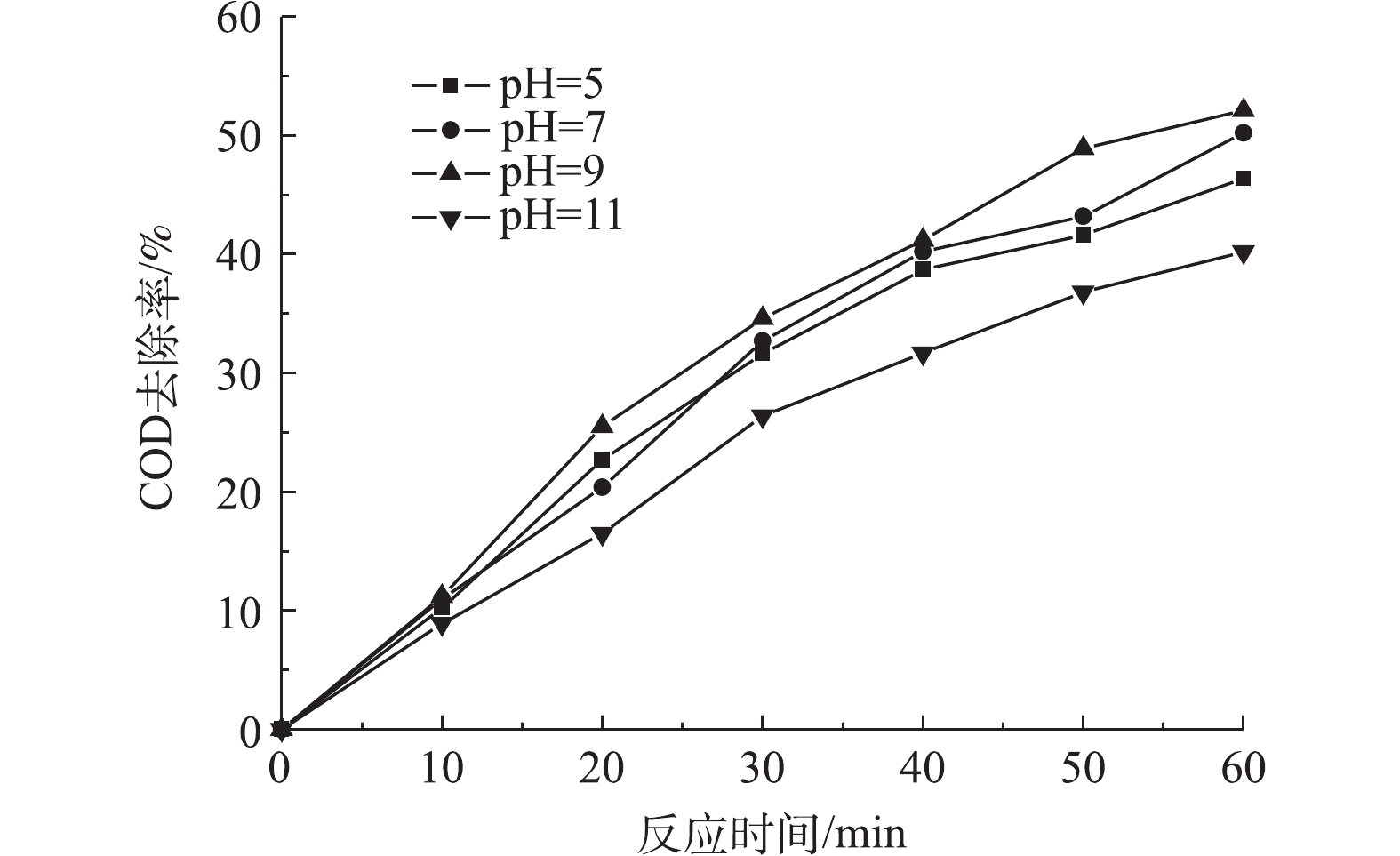
2)溶液初始pH的影响。实验选取了4个不同的pH,用NaOH调节废水pH,臭氧催化氧化时间为60 min,臭氧的投加量为300 mg·L−1。由图4可知,初始pH对臭氧催化氧化降解COD的影响较大,随着pH的升高,COD的去除率呈上升趋势,当初始pH从5升到9时,COD的去除率从46.4%升至52.1%。臭氧间接氧化较多发生在中、碱性条件下,臭氧在碱性条件下很不稳定,易促进其自身分解[18],可通过自发的一系列链式反应生成强氧化性的 · OH[19],更有利于COD的去除。此外随着废水pH的升高,水中OH−浓度增加,加剧了臭氧分解产生 · OH。当废水pH进一步升高至11时,COD的去除率下降至40.2%,这可能是因为臭氧催化剂的零电荷点在pH=9附近,酸性或过碱性条件都影响了催化剂表面羟基的电荷形态[20]。此外,由于pH的升高,导致羟基自由基的淬灭速率提高,降低了与有机物的接触概率,导致COD去除率的降低[21]。
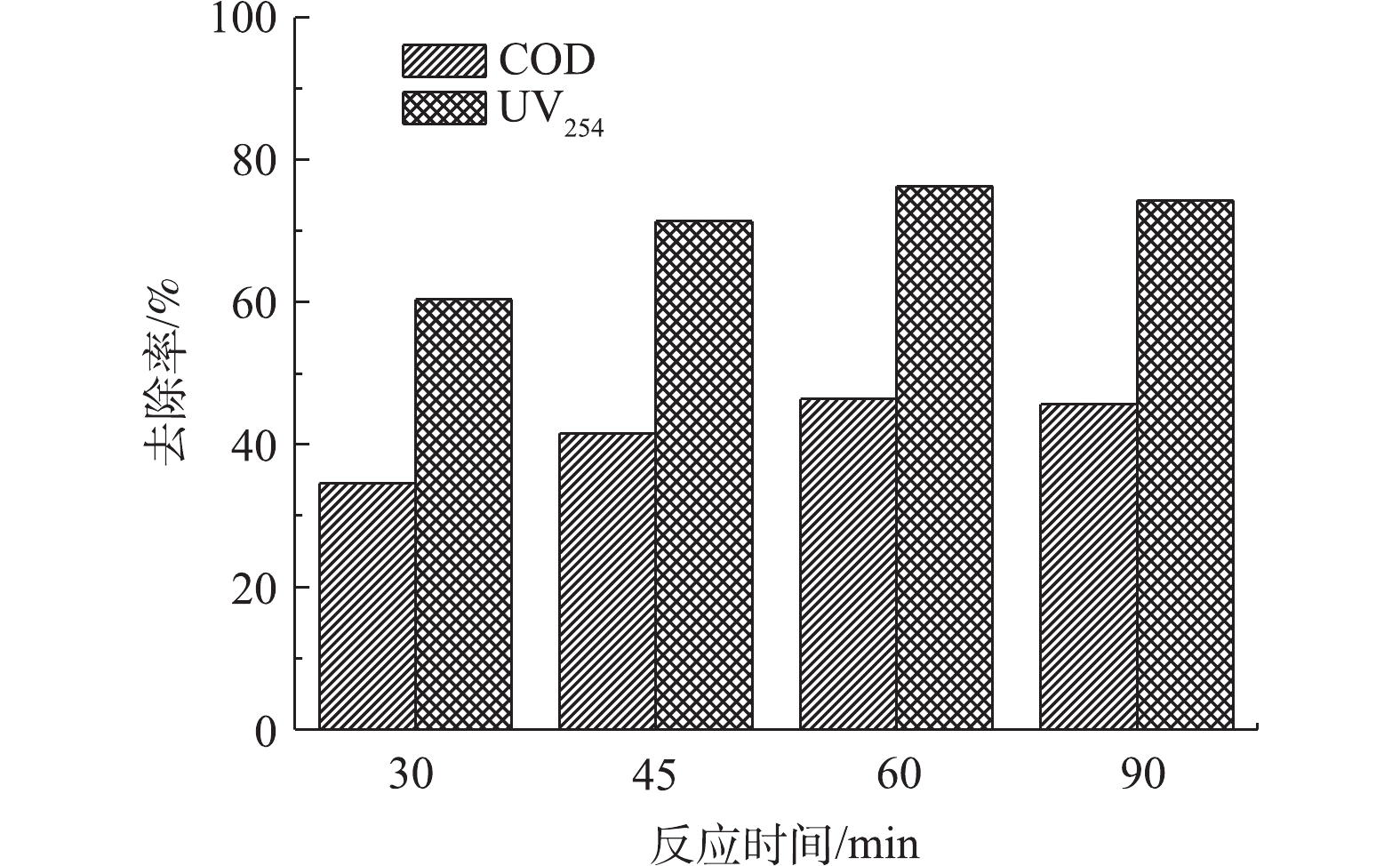
3)反应时间的影响。臭氧催化氧化实验在室温下进行,在废水初始pH为 5,通过调节臭氧进气浓度控制臭氧投加量为300 mg·L−1的条件下,考察了臭氧催化氧化时间对有机物去除效果的影响,结果如图5所示。当反应时间从30 min增至60 min时,COD和UV254的去除率分别从34.6%、60.4%升高到46.4%、76.3%,有机物的去除率随反应时间的延迟而增加。这是由于 · OH在水中的寿命很短[22],大约为10−9 s,臭氧催化氧化中 · OH氧化反应实际发生在催化剂周围,在传质不充分的反应条件下,部分 · OH在未知有机物发生反应之前就已经被淬灭。当臭氧催化氧化时间缩短时,单位时间内臭氧投加量加大,产生的 · OH增多,导致大量 · OH未参与反应就淬灭,从而导致有机物去除率下降。当反应时间到达一定的临界点时,再延长反应时间,其去除率增加不再明显,臭氧催化氧化反应从60 min延长至90 min时,COD和UV254的去除率基本不变。
-
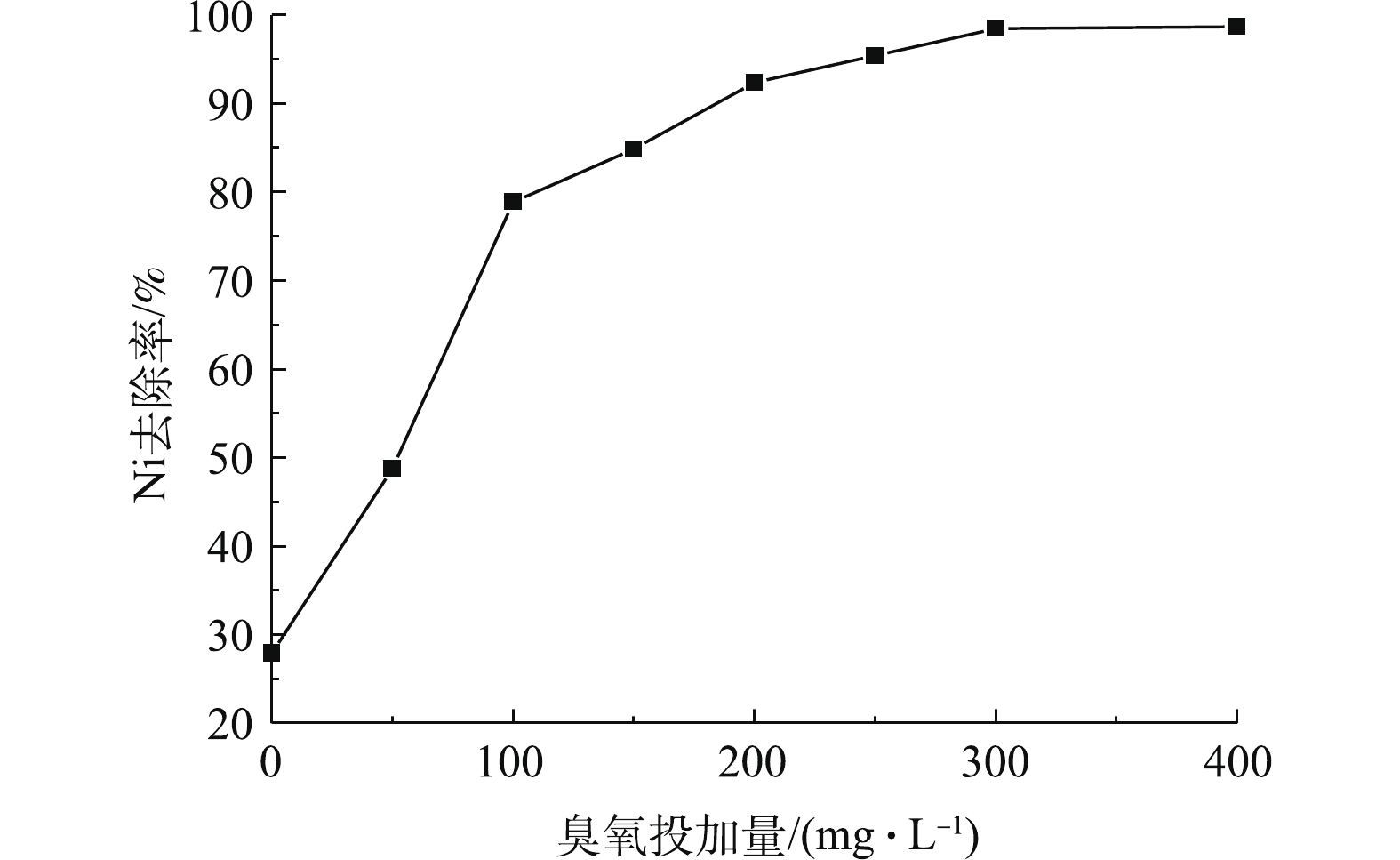
1)臭氧投加量对镍去除的影响。在室温条件下,对废水进行臭氧催化氧化实验,在反应降解60 min后,对氧化出水进行混凝、过滤。混凝反应条件:将氧化出水pH调节至10,投加PAC、PAM进行混凝、过滤,PAC和PAM投加量分别为300 mg·L−1和5 mg·L−1。检测水中镍的去除率,考察了不同反应条件下的破络效果。由图6可见,在未投加臭氧的情况下,对废水进行混凝过滤,镍的去除率达到27.9%,表明水中部分镍以游离态的形式存在,含量达到21.8 mg·L−1。随着臭氧投加量的增加,镍的去除率逐渐提高,在臭氧投加量为0~100 mg·L−1时,镍的去除率显著升高,当投加量为100 mg·L−1时,镍的去除率可达到78.9%。随着臭氧投加量继续增加,臭氧的破络合效率开始减慢,随着臭氧投加量增到300 mg·L−1时,镍的去除率提高至98.5%。当臭氧投加量超过300 mg·L−1时,镍的去除率基本保持不变,臭氧投加量增到400 mg·L−1时,镍的去除率仅升至98.7%。水中络合基团易被臭氧催化氧化生成的 · OH氧化分解,络合态镍转换为游离态的镍离子,随着臭氧投加量的增加,络合基团浓度逐渐变小,与羟基自由基发生接触的概率越来越低。因此,随着臭氧投加量的不断加大,镍的去除率呈现急剧升高、平稳升高和缓慢升高3个阶段,这和关智杰等[23]的研究结果一致。
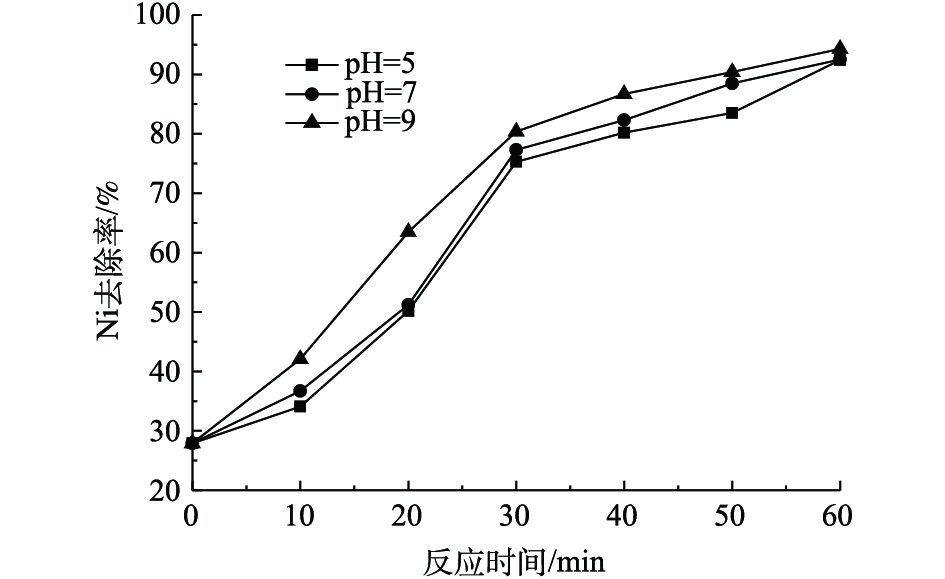
2)初始pH对镍去除的影响。反应在室温条件下进行,臭氧投加量为200 mg·L−1,反应降解时间为60 min,取不同时间段出水进行混凝、过滤。混凝反应条件:将氧化出水pH调节至10,PAC和PAM投加量分别为300 mg·L−1和5 mg·L−1。调节水样初始pH进行反应,考察了不同初始pH对出水中镍处理效果,结果如图7所示。随着初始pH的升高,镍离子的去除逐渐增加,当初始pH为5~9时,镍离子的去除率保持在92.4%以上,这可能因为在碱性环境下,破络和产生的镍离子和OH−反应,产生细小的沉淀物,阻止了镍离子和水中有机物进行二次络合。臭氧催化反应前30 min,随着初始pH升高,镍离子的去除率增加显著,反应后30 min镍离子的去除率增加缓慢,这也和上文中COD的降解结果相吻合。
-
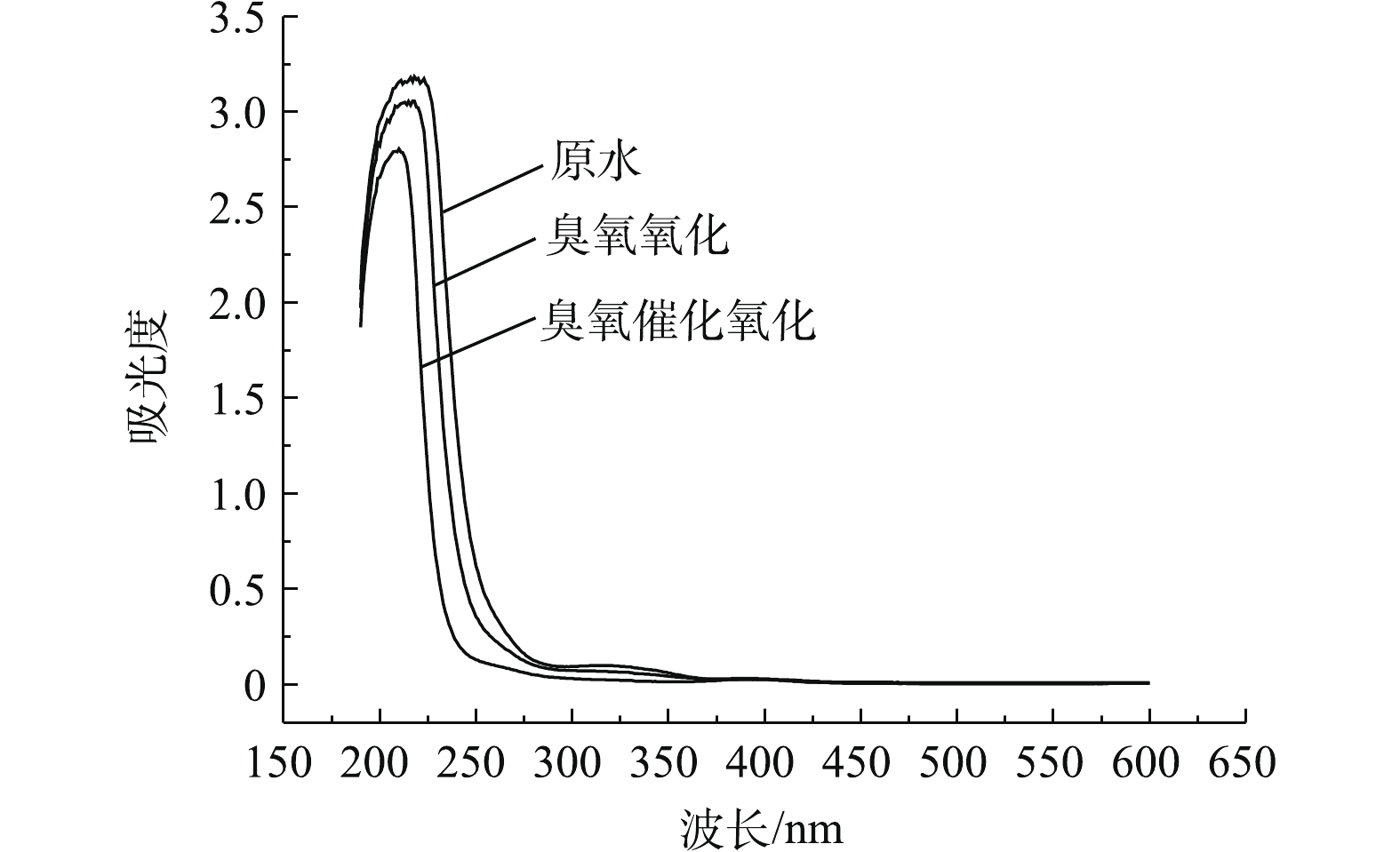
对臭氧催化氧化进出水进行了紫外全波段扫描(190~600 nm),实验结果如图8所示。臭氧氧化实验条件为:废水初始pH为 5,臭氧投加量均为300 mg·L−1,反应时间为60 min。由图8可知,废水在200~250 nm处有较强的吸收峰,这说明水中含有共轭双键或饱和脂肪烃[24];在250~300 nm处出现一定强度的紫外吸收,说明水中含有苯环[25];在320 nm波段又出现一个微弱的吸收峰,说明水中存在较大的共轭体系。经60 min氧化降解后,各吸收峰强度出现不同程度的下降,表明臭氧氧化和臭氧催化氧化对化学镀镍废水均有一定的降解效果,而臭氧催化氧化的降解效果更为明显。可以看出,臭氧催化氧化出水各波段的吸收峰强度均明显下降,并有一定程度的蓝移,位于250~300 nm处的紫外吸收和320 nm处的吸收峰基本消失,这种变化表明水中的含苯环类物质和共轭结构被破坏,其可能分解成更小的分子碎片。
-
根据微生物酶系统受到抑制的机理,SOUR是污泥活性的重要指标[26-27],可以间接反映废水的生物毒性。取最优反应条件下臭氧催化氧化进出水,用活性污泥法处理,测定污泥的比耗氧速率,同时用葡萄糖溶液配制同等COD浓度的对照样本,结果见表1和表2。
活性污泥的SOUR值随着进水有机物的减少会下降。由表1可知,化学镀镍原水对污泥有明显的抑制作用,原水和葡萄糖溶液的SOUR比值仅有0.31;由表2可知,化学镀镍废水经臭氧催化氧化后,出水和葡萄糖溶液的SOUR比值升高到0.63,废水对污泥虽然有抑制作用,但没有原水明显。通过检测臭氧氧化进出水的BOD可以发现,原水的B/C仅为0.12,臭氧催化氧化出水B/C比0.36,经臭氧催化氧化后出水B/C提高了2倍,间接证实了水中大分子物质经臭氧催化氧化后发生了一系列的开环断链反应,转化为易降解的小分子[28],为后续进一步生化处理提供了条件。
-
1)臭氧催化氧化处理化学镀镍废水,对水中COD的去除和对化学镍的破络合均具有明显效果。在最佳的反应条件下,COD从532 mg·L−1下降至285 mg·L−1,去除率可达到46.4%,镍的去除率最高可达到98.7%。
2)化学镀镍废水中存在共轭双键或饱和脂肪烃、苯环类物质和共轭体系,经臭氧催化氧化后各波段的吸收峰强度均大幅度下降,位于250~300 nm处的紫外吸收和320 nm处的吸收峰基本消失,水中的苯环类物质和共轭结构被破坏,并分解成更小的分子碎片。
3)经臭氧催化氧化后,废水和葡萄糖溶液的SOUR比值从0.31升高到0.63,废水的生物毒性大幅降低,废水的可生化性提高,出水B/C由原来的0.12提高到0.36,为后续进一步生化处理提供了条件。
臭氧催化氧化处理化学镀镍废水
Treatment of electroless nickel-plating wastewater by ozone catalytic oxidation
-
摘要: 采用臭氧催化氧化工艺处理化学镀镍废水,以Fe2O3-TiO2-MnO2/A12O3作为臭氧催化剂,考察了不同反应条件下臭氧催化氧化对化学镀镍废水的影响。结果表明,在初始pH为9,臭氧投加量为300 mg·L−1,反应时间为60 min的最佳反应条件下,水中COD可从532 mg·L−1下降至285 mg·L−1,去除率达到46.4%。臭氧催化氧化对化学镍具有较好的破络效果,在初始pH为9,臭氧投加量为200 mg·L−1,反应为60 min后进行混凝过滤,水中镍的去除率可达到86.7%。紫外全波段扫描分析发现,经臭氧催化氧化后,各波段的吸收峰均有大幅度下降,位于254 nm和320 nm处的吸收峰基本消失,说明水中的苯环类物质和共轭结构被破坏。经臭氧催化氧化后,废水的生物毒性大幅降低,废水的可生化性提高,出水B/C由原来的0.12提高到0.36,为后续进一步生化处理提供了条件。Abstract: Electroless nickel plating wastewater was treated by ozone catalytic oxidation with a type of Fe2O3-TiO2-MnO2/A12O3 catalyst. The effects of different reaction conditions on the treatment performance were studied. The results showed that COD in wastewater decreased from 532 mg·L−1 to 285 mg·L−1, and the corresponding removal efficiency reached 46.4% under the optimize conditions: initial pH=9, O3 dosage of 300 mg·L−1 and oxidation time of 60 min. Ozone catalytic oxidation also presented a good effect on the breakage of the complexes for electroless nickel-plating wastewater. The Ni removal rate reached 86.7% after coagulation and filtration of ozone oxidized wastewater at initial pH=9, O3 dosage of 200 mg·L−1 and oxidation time of 60 min. Ultraviolet spectrum analysis showed that the absorption peaks of each band decreased significantly after ozone catalytic oxidation, and the absorption peaks at 254 nm and 320 nm almost disappeared, which indicates that the benzene-ring species and conjugated structure in the wastewater were destroyed. After ozone-catalyzed oxidation, the biological toxicity of wastewater was greatly reduced, and its biodegradability was improved. The B/C ratio of effluent increased from 0.12 to 0.36, which was beneficial for further biochemical treatment.
-

-
表 1 原水的活性污泥的好氧速率、MLSS及比耗氧速率
Table 1. Oxygen uptake rate, MLSS and specific oxygen consumption rate of activated sludge in raw wastewater
样品 OUR/(mg·(L·min)−1) MLSS/(mg·L−1) SOUR/(mg·(g·h)−1) 原水 0.142 2 2.20 3.87 对照样本 0.460 8 2.22 12.45 表 2 氧化出水的活性污泥的好氧速率、MLSS及比耗氧速率
Table 2. Oxygen uptake rate, MLSS and specific oxygen consumption rate of activated sludge in oxidized wastewater effluent
样品 OUR/(mg·(L·min)−1) MLSS/(mg·L−1) SOUR/(mg·(g·h)−1) 原水 0.200 5 2.10 5.73 对照样本 0.321 4 2.12 9.10 -
[1] BULASARA V K, THAKURIA H, UPPALURI R, et al. Combinatorial performance characteristics of agitated nickel hypophosphite electroless plating baths[J]. Journal of Materials Processing Technology, 2011, 211(9): 1488-1499. doi: 10.1016/j.jmatprotec.2011.03.022 [2] SHAO Z, CAI Z, HU R, et al. The study of electroless nickel plating directly on magnesium alloy[J]. Surface & Coatings Technology, 2014, 249: 42-47. [3] 符丽纯, 戴建军, 陈利芳, 等. 基于树脂吸附的电镀废水深度处理工程实例[J]. 水处理技术, 2018, 44(1): 128-131. [4] 程仁振, 邱立平, 刘贵彩, 等. 陶瓷膜-反渗透工艺用于电镀废水深度处理[J]. 中国给水排水, 2018, 34(14): 41-45. [5] SHI Y J, LIN C P, HUANG Y H, et al. Application of Fered-Fenton and chemical precipitation process for the treatment of electroless nickel plating wastewater[J]. Separation and Purification Technology, 2013, 104: 100-105. doi: 10.1016/j.seppur.2012.11.025 [6] 刘洋. 化学镀镍废水中污染物去除工艺的研究[D]. 广州: 华南理工大学, 2015. [7] XU G R, SHEN T, MA Y Y, et al. Treatment of wastewater discharged from electroless nickel plating process by high-voltage pulsed electrocoagulation[J]. Electroplating & Finishing, 2017, 23(36): 1284-1287. [8] HUANG Y X, LUO M Y, XU Z H. Catalytic ozonation of organic contaminants in petrochemical wastewater with iron-nickel foam as catalyst[J]. Separation and Purification Technology, 2019, 221: 269-278. doi: 10.1016/j.seppur.2019.03.073 [9] LI X F, CHEN W Y, MA L M, et al. Industrial wastewater advanced treatment via catalytic ozonation with an Fe-based catalyst[J]. Chemosphere, 2018, 195: 336-343. doi: 10.1016/j.chemosphere.2017.12.080 [10] ZHUANG H, HAN H, JIA S, et al. Advanced treatment of biologically pretreated coal gasification wastewater using a novel anoxic moving bed biofilm reactor (ANMBBR)-biological aerated filter (BAF) system[J]. Bioresource Technology, 2014, 157: 223-230. doi: 10.1016/j.biortech.2014.01.105 [11] 涂勇, 张耀辉, 徐军, 等. 臭氧对化工园区废水厂二级出水的选择性氧化[J]. 环境工程学报, 2015, 9(11): 2595-2300. [12] ZHUANG H, HAN H, HOU B, et al. Heterogeneous catalytic ozonation of biologically pretreated Lurgi coal gasification wastewater using sewage sludge based activated carbon supported manganese and ferric oxides as catalysts[J]. Bioresource Technology, 2014, 166: 178-186. doi: 10.1016/j.biortech.2014.05.056 [13] HUANG G, PAN F, FAN G, et al. Application of heterogeneous catalytic ozonation as a tertiary treatment of effluent of biologically treated tannery wastewater[J]. Journal of Environmental Science and Health, 2016, 51(8): 626-633. [14] 张耀辉, 涂勇, 唐敏, 等. Fe2O3-TiO2-MnO2/A12O3催化臭氧化催化剂的制备及表征[J]. 中国环境科学, 2016, 36(10): 3003-3009. doi: 10.3969/j.issn.1000-6923.2016.10.023 [15] 国家环境保护总局. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社, 2002. [16] 荣宏伟, 李健中, 张可方. 铜对活性污泥微生物活性影响研究[J]. 环境工程学报, 2010, 4(8): 1709-1713. [17] 徐军, 涂勇, 武倩, 等. 臭氧、臭氧/双氧水催化氧化深度处理化工废水[J]. 工业水处理, 2017, 37(4): 62-65. doi: 10.11894/1005-829x.2017.37(4).015 [18] TAMURA H, TANAKA A, KY M, et al. Surface hydroxyl site densities on metal oxides as a measure for the ion-exchange capacity[J]. Journal of Colloid & Interface Science, 1999, 209(1): 225-231. [19] NADEZHDIN A D. Mechanism of ozone decomposition in water: Therole of termination[J]. Industrial & Engineering Chemistry Research, 1988, 27(4): 548-550. [20] 陈炜彧, 李旭芳, 马鲁铭. 铁基催化剂催化臭氧深度处理煤化工废水[J]. 环境工程学报, 2018, 12(1): 86-92. doi: 10.12030/j.cjee.201706031 [21] 王兵, 周望, 任宏洋, 等. MgO催化臭氧氧化降解苯酚机理研究[J]. 环境科学学报, 2016, 36(11): 4009-4016. [22] 王海燕, 蒋展鹏, 杨宏伟. 电助光催化氧化过程中羟基自由基(OH·)的定量分析[J]. 环境工程学报, 2008, 2(2): 225-228. [23] 关智杰, 郭艳平, 区雪连, 等. 臭氧预破络-重金属捕集耦合体系高效去除废水中络合态镍的机理研究[J]. 环境科学学报, 2019, 39(6): 1754-1762. [24] 张雪, 丁鑫, 杨浈, 等. 腐殖质氧化还原官能团测定新方法[J]. 环境化学, 2016, 35(10): 2106-2116. [25] 蒋绍阶, 刘宗源. UV254作为水处理中有机物控制指标的意义[J]. 重庆建筑大学学报, 2002, 24(2): 61-65. [26] 荣宏伟, 张耀坤, 张朝升, 等. INT·ETS活性及AUR和SOUR表征污泥活性的比较[J]. 环境科学研究, 2016, 29(5): 767-773. [27] 杨茜, 于茵, 周岳溪, 等. 石化工业园区有毒废水来源识别研究[J]. 环境科学, 2014, 35(12): 4582-4588. [28] 周洪政, 刘平, 张静, 等. 微气泡臭氧催化氧化-生化耦合处理难降解含氮杂环芳烃[J]. 中国环境科学, 2017, 37(8): 2978-2985. doi: 10.3969/j.issn.1000-6923.2017.08.021 -




 下载:
下载: