-
地下水是我国饮用水的重要来源,我国《生活饮用水卫生标准》(GB 5749-2006)中规定氟离子浓度不超过1.0 mg·L−1;当饮用水中氟离子浓度超标时,会引起骨质疏松、斑釉症,甚至出现氟中毒现象[1-3]。我国高含氟地下水分别广泛,影响人口众多,随着我国工业的发展,地下水遭到不同程度的污染,地下水本底氟浓度超标区与区域污染物浓度叠加进一步推高地下水氟污染物范围和浓度限值,如内蒙古河套地区和山东高密地区,地下水含氟量为2~9 mg·L−1,部分地区的含氟量超过10 mg·L−1[4-5],而高含氟地下水势必对人群健康造成严重影响。因此,开展高含氟地下水除氟技术研究显得尤为重要。
电絮凝除氟作为一种深度除氟工艺,因其除氟效率高、占地面积小、产泥量少、成本低而被国内外学者关注。目前,关于电絮凝除氟的研究包括极板材质、影响因素、组合工艺处理及作用机理等[6-14]。邵坚等[8]通过在双铝电絮凝中引入锌电极以构建锌铝电絮凝体系并用于饮用水处理,通过控制锌铝比为1∶3,出水氟离子浓度为0.82 mg·L−1,除氟效率高达85.2%,且系统对CODMn和浊度去除效果要优于双铝电极。ESSADKI等[9]发现除氟过程符合一级吸附动力学模型,并发现吸附动力学常数与电流密度、初始氟浓度、pH及反应器水力条件有关,当电流密度由2.86 mA·cm−2增至17.1 mA·cm−2时,动力学常数由0.052 min−1增长至0.117 min−1。电絮凝体系中离子种类同样影响除氟效果,有研究[10-11]表明,在电絮凝体系中适当引入Ca2+、Mg2+有助于除氟效率的提高,但两者作用机理有所不同,通过回归分析发现Mg2+可以与铝、氟形成化合物而改变铝氟比,而Ca2+不参与化合物形成。为实现电絮凝体系产生的含氟沉淀絮体与水的快速分离,有研究[12-13]尝试通过电絮凝与微滤、超滤系统结合,结果表明,电絮凝与膜分离技术的结合不仅提高了电絮凝除氟效率,而且显著减小了装置占地,但由此也会引发连续运行中的膜污染问题,通过控制装置运行方式,调节运行参数如pH,可以减缓膜污染,实现系统长周期稳定运行[14]。目前,关于电絮凝除氟研究对象多集中于饮用水和地下水,但初始氟浓度一般低于5 mg·L−1,并且关于电絮凝除氟动力学研究相对较少。
针对高含氟地下水,探讨电絮凝除氟影响因素及除氟动力学,不仅可以优化除氟效率,而且为该类废水工程化提供技术参考。本研究采用双铝电极电絮凝装置处理人工模拟高含氟地下水,对电絮凝除氟效率影响因素,包括电流密度、pH、极板间距及进水氟浓度进行了考察,对电絮凝除氟过程和除氟动力学进行了研究,并对电絮凝除氟动力学影响因素进行了探讨,为电絮凝深度处理高含氟地下水应用和除氟反应器开发提供技术依据。
-
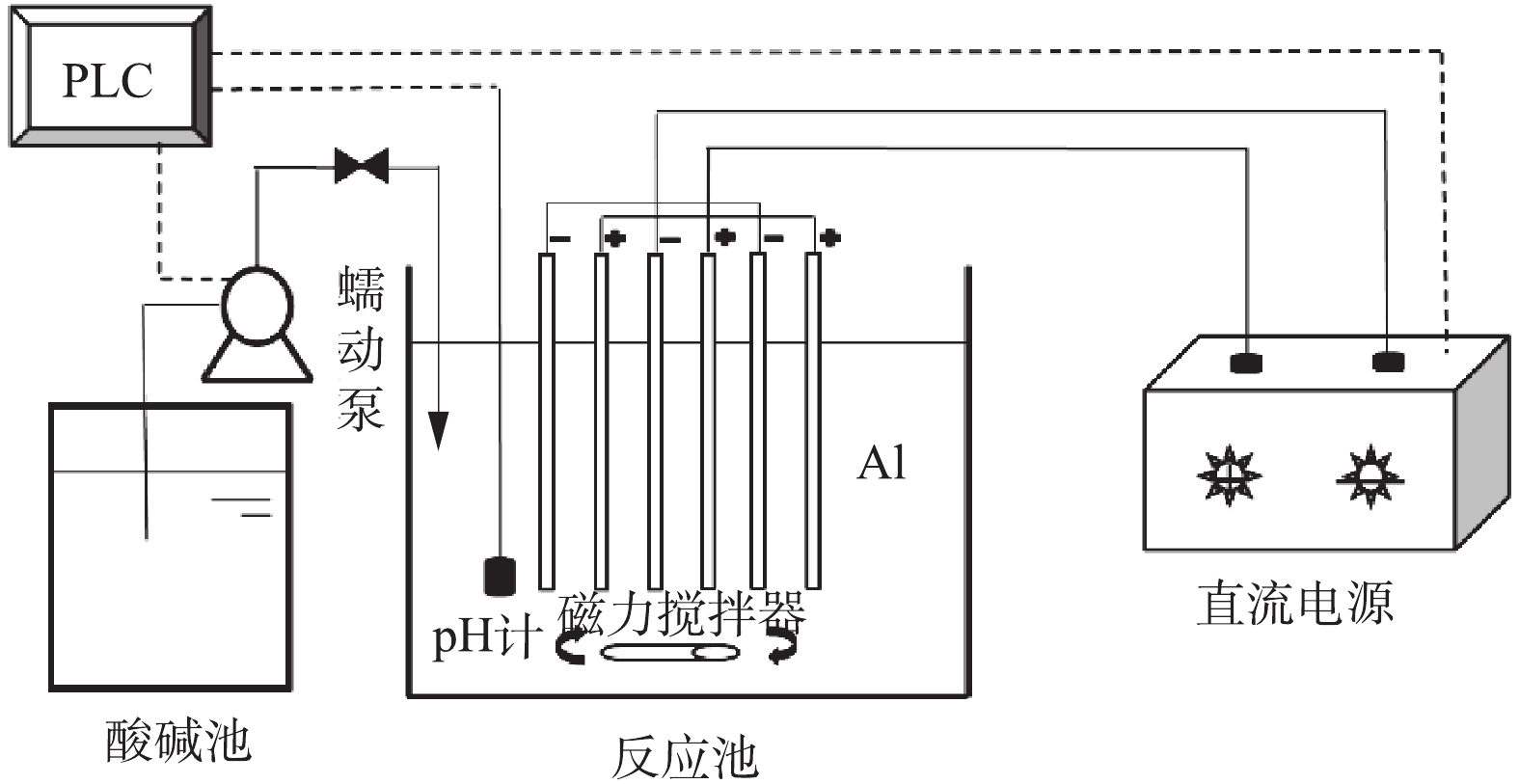
本实验装置如图1所示。采用双铝电极电絮凝装置,电极板采用单极式排列,初始极板间距为10 mm,具体可根据实验需求调整,阴、阳极板有效面积均为0.01 m2;电絮凝反应池采用有机玻璃制成,反应池有效容积为1 L。反应池内装有pH和温控探头,通过PLC可设定反应池pH,并控制蠕动泵酸碱加入量,保证反应池维持设定pH,酸碱池分2格,分别配置2 mol·L−1的HCl和NaOH溶液,反应池底部设置有磁力搅拌器和恒温控制器。直流电源采用恒流源稳压控制模式,规格为(0~3 000) mA/(0~30) V,可根据实验条件调节输出电流。
-
电絮凝除氟主要利用铝板作为阳极,通过阴、阳极加载直流电使阳极逐渐释放铝离子,一部分与水中氟离子结合成铝氟络合物,另一部分在阴极碱性环境产生氢氧化铝絮体;在阴极由于还原作用产生氢气,通过吸附、络合沉淀、气浮作用实现氟离子去除。反应原理[15]如式(1)~式(5)所示。阳极反应见式(1),阴极反应见式(2)。
由式(4)和式(5)可知,氟离子的去除与溶液中Al3+浓度和形态有直接关系,而铝离子与氟离子络合形态和铝离子在溶液中形态与pH有直接关系,因此,pH对电絮凝除氟效果同样有较大影响[16]。
-
本研究用水来自山东省某石化企业厂区地下水,经分析检测地下水氟化物浓度约为8.1~14.2 mg·L−1,Na+为189~206 mg·L−1,K+为15~30 mg·L−1,Ca2+为50~55 mg·L−1,Mg2+为36~39 mg·L−1,
${\rm{SO}}_4^{2 - }$ 为145~158 mg·L−1,pH为6.72~7.35,电导率为1.51~1.62 mS·cm−1。实验用水采用KF、MgSO4、CaCl2、NaCl及去离子水进行人工模拟高含氟地下水。电絮凝出水定期检测取样,分析化验前经0.22 μm抽滤膜过滤。采用哈希分光光度法测定氟离子浓度;采用便携式电导仪测定电导率;采用自动pH计测定pH;絮凝物XRD采用X射线衍射仪;元素分析采用X射线荧光光谱分析仪。
-
一般情况下,铝盐混凝除氟过程中铝离子浓度直接影响除氟效果。在电絮凝体系下,根据法拉第电解定律,阳极释放溶解性铝离子浓度与加载电流、反应器体积及反应时间存在联系[6],换算关系见式(6)。
式中:ρ(Al3+)为溶解性Al3+浓度,mg·L−1;M为铝的摩尔质量,27 g·mol−1;I为加载电流,A;t为反应时间,s;n为溶解态铝化合价,n=3;F为法拉第常数,F=96 485 C·mol−1;V为反应池有效容积,m3;K=M/nF为常数。本研究中的电流密度为单位体积液体所加载电流。因此,溶液中溶解性Al3+浓度取决于所加载电流密度和反应时间。
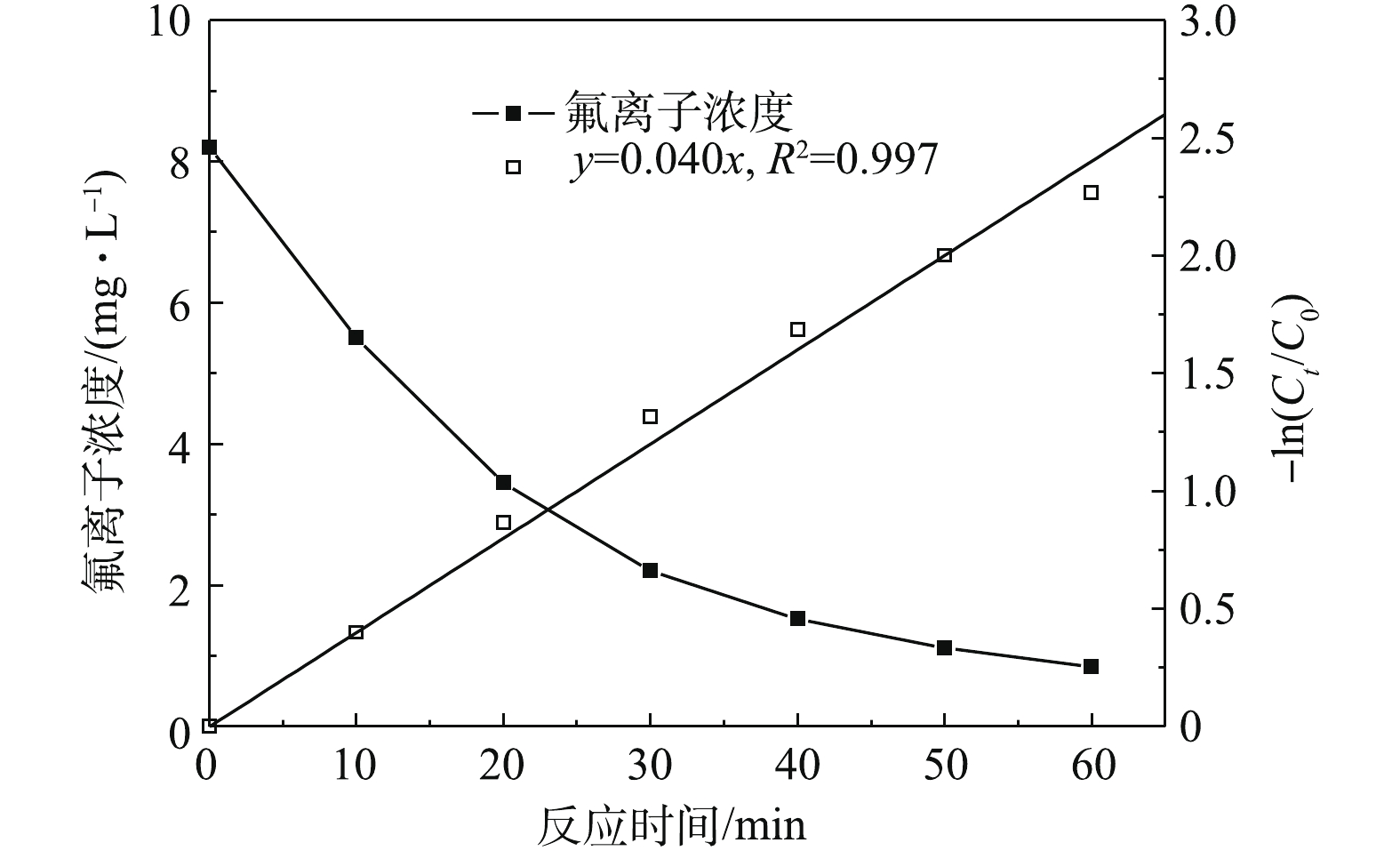
图2为pH=6.0~7.0,电流密度为300 A·m−3条件下,电絮凝除氟过程中氟离子浓度随时间变化状况。由图2可知,氟离子初始浓度约为8.2 mg·L−1,随着反应时间的延长,氟离子浓度逐渐降低,这主要因为在同一电流密度下,随着反应时间的增加,体系中释放铝离子增加,铝离子可以为氟离子提供更多的络合位点,同时铝离子增加有助于提高混凝效果。当反应时间延长至60 min时,氟离子浓度约为0.85 mg·L−1,去除率为89.63%,出水满足《生活饮用水卫生标准》(GB 5749-2006)一级标准。此外,由图2可知,随着氟离子浓度降低,氟离子去除速率减慢,通过Origin 8.0拟合发现,氟离子去除过程符合一级反应动力学模型,如式(7)所示。
式中:Ct为氟离子浓度,mg·L−1;C0为初始氟离子浓度,mg·L−1;一级动力学反应常数k=0.040 min−1,R2=0.997。因此,当出水氟离子浓度Ct=1.0 mg·L−1时,根据式(7)可得式(8)。
在其他条件不变情况下,电絮凝反应时间仅取决于初始氟离子浓度C0和动力学反应常数k。
-
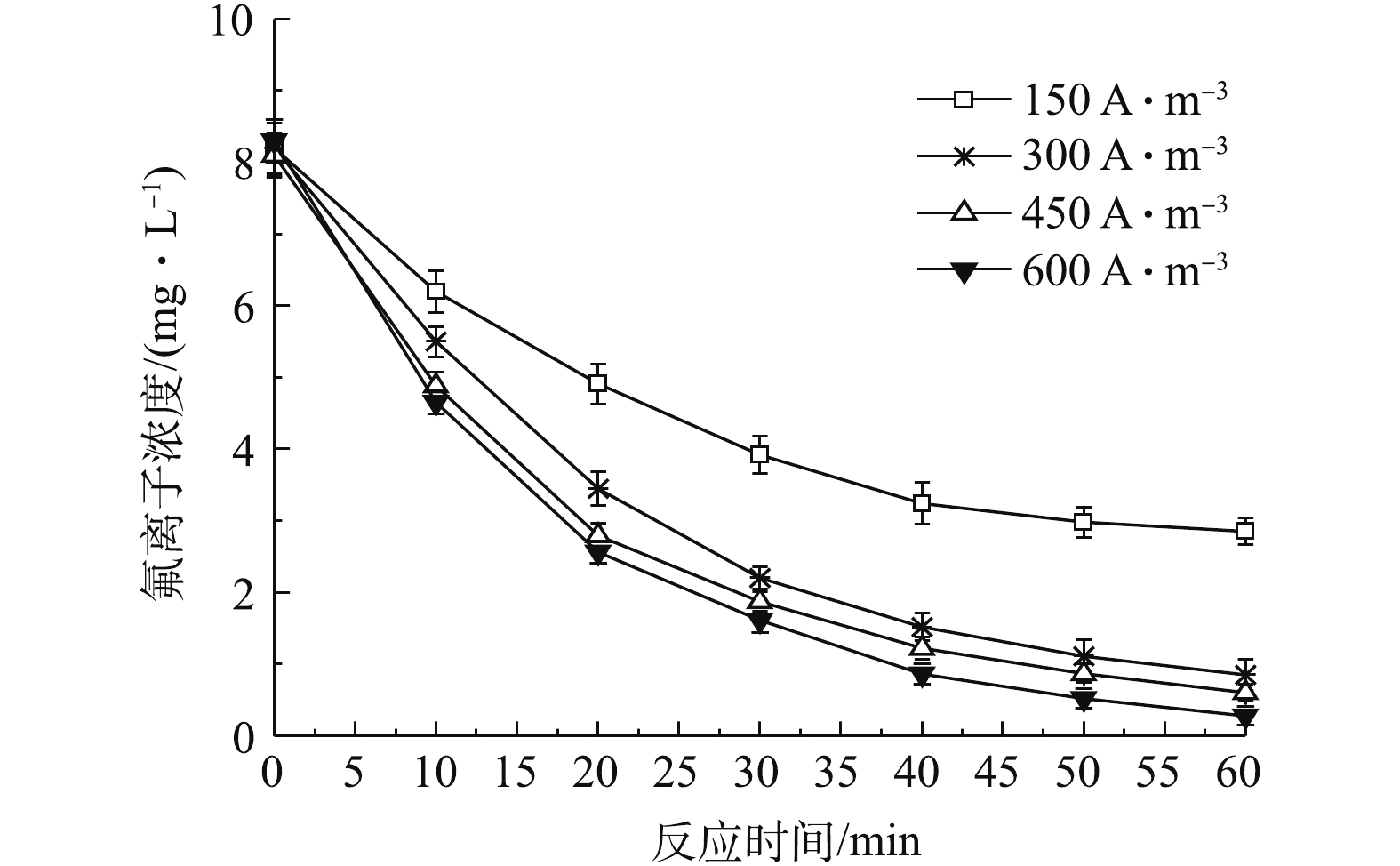
根据式(6)可知,单位时间溶液中溶解性铝离子浓度与电流密度存在线性关系,因此,不同电流密度下氟离子去除效率有所不同。图3为pH=6.0~7.0,初始氟离子浓度为8.1~8.3 mg·L−1,电流密度分别为150、300、450和600 A·m−3条件下,氟离子浓度随时间的变化状况。由图3可知,相同电流密度下,随着反应时间的延长,氟离子浓度逐渐降低;而在相同反应时间下,随着电流密度的提高,氟离子浓度逐渐降低,这与式(6)相符,因为阳极溶解性铝离子浓度取决于电解时间和电流密度大小,而较高的铝离子浓度显然有利于氟离子去除。不仅如此,由图3可知,在前30 min内,氟离子浓度由初始8 mg·L−1降至2 mg·L−1左右;而后30 min内,氟离子浓度仅从2 mg·L−1降到1 mg·L−1左右,这不利于提高电絮凝除氟效率。由式(8)可知,当电流密度分别为150、300、450和600 A·m−3,氟离子平均浓度为8.2 mg·L−1时,为使出水氟离子浓度小于1.0 mg·L−1,所需理论反应时间分别为105.2、52.6、46.8和38.3 min,也即在相同氟离子去除率下,提高电流密度可显著缩短除氟反应时间。
此外,由图4可知,随着电流密度的提高,氟离子去除一级反应动力学常数变大。这说明随着电流密度的提高,氟离子去除速率增大。但值得注意的是,当电流密度为150 A·m−3时,一级反应动力学常数k仅为0.020 min−1,远小于电流密度为300、450和600 A·m−3时的动力学常数。这主要由于在此电流密度下,铝离子释放较少,一方面不能为此浓度下氟离子提供足够的络合位点,另一方面较少氢氧化铝絮体也不利于沉淀发生,导致此条件下去除效果较差[9]。当电流密度增至300 A·m−3以上时,此时体系内能够产生足够的铝离子,使之与氟离子发生络合、沉淀;当电流密度进一步增加时,铝离子数量进一步增加,但由于氟离子浓度不变,此时大量铝离子只起到絮凝沉淀作用。去除单位质量氟所需消耗的能耗可采用式(9)进行计算。
式中:W为去除1 g氟所需能耗,kWh·g−1;J为电流密度,A·m−3;U为槽电压,V;t为反应时间,h,C0为初始氟浓度,mg·L−1;C1为出水氟浓度,取1.0 mg·L−1。
当电流密度分别为150、300、450和600 A·m−3时,极间电压分别为3.87、4.32、4.95和5.57 V。经式(9)核算可知,在上述不同电流密度下去除单位质量氟能耗分别为0.141 4、0.157 8、0.241 3和0.296 3 kWh·g−1,即去除单位质量氟化物所需能耗随电流密度的提高而增加。由于在电流密度为150 A·m−3时,所需反应停留时间过长,这不利于降低除氟装置尺寸和投资成本,因此,确定最佳操作电流密度应为300 A·m−3。
-
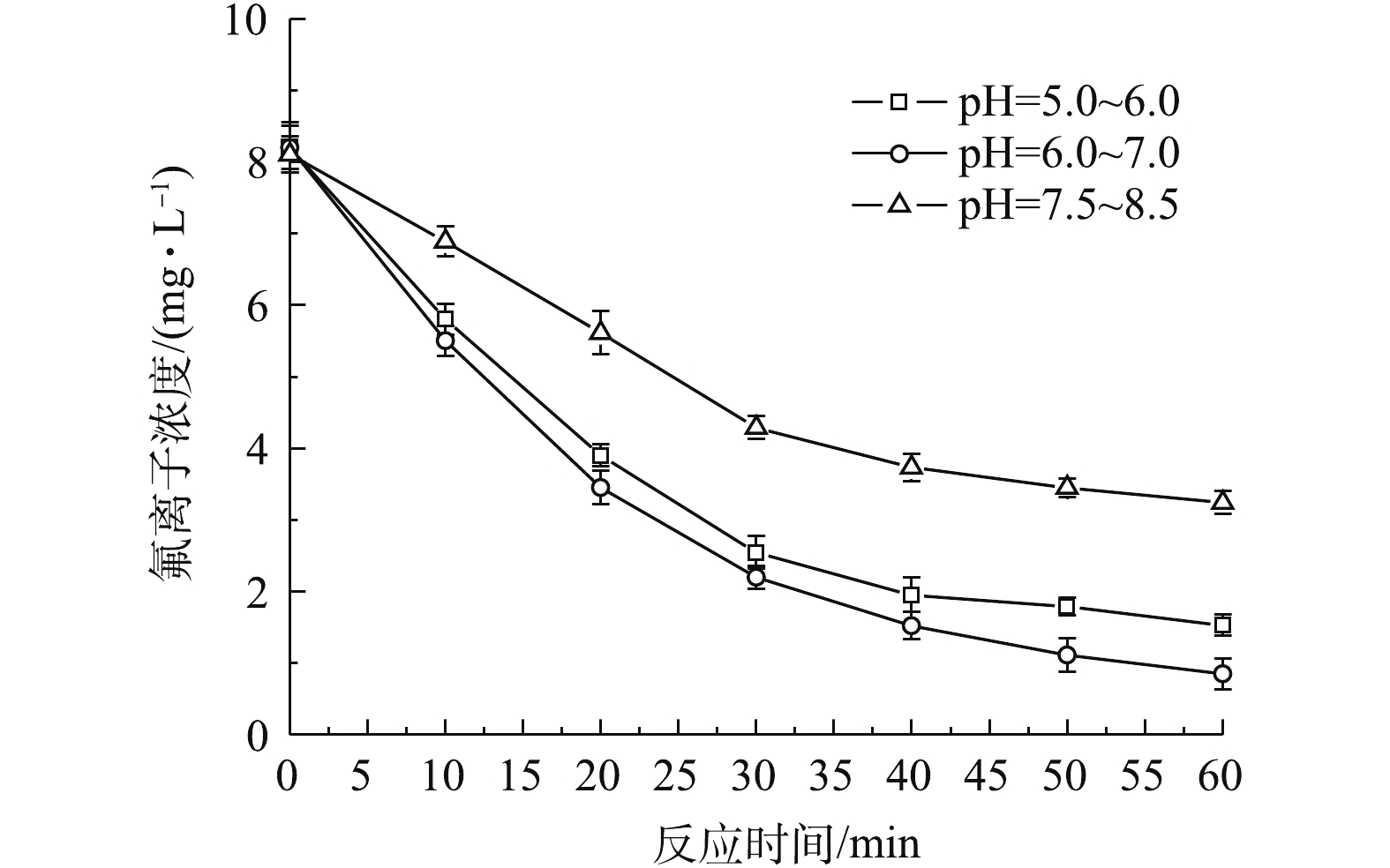
pH不仅影响铝离子与氟离子络合状态,而且影响铝离子在溶液中的形态,进而影响除氟的过程。图5反映了电流密度为300 A·m−3,pH=5.0~6.0、6.0~7.0和7.5~8.5条件下氟离子浓度随时间的变化。由图5可知,不同pH下氟离子浓度均随反应时间的增加而降低,但氟离子去除过程存在明显差异。在相同的反应时间下,在pH=6.0~7.0时,氟离子去除效果最好,在pH=5.0~6.0时次之;在pH=7.5~8.5时最差,并且在pH=7.5~8.5时氟离子出水浓度明显高于前两者。这主要因为:当pH为5.0~6.0时,体系中存在大量H+,大多铝离子以水解态存在,包括Al3+、
${\rm{Al}}\left( {{\rm{OH}}} \right)_2^ + $ 、Al(OH)2+等,该种形态铝离子形成絮体比表面较小,与氟离子结合能力较弱,造成氟离子去除效果较差;当pH为7.5~8.5时,氢氧化铝将发生解离生成溶解态铝,此时与氟离子结合能力最弱,氟离子去除效果最差[17];而在pH为6.0~7.0时,此时体系中存在大量无定形凝胶型氢氧化铝,具有较大比表面积,能够提供较多结合位点与氟离子形成铝氟络合物,且此时絮体吸附容量最大,最终通过吸附、网捕及共沉淀等方式实现氟离子去除[16]。因此,一般在中性偏酸条件下,电絮凝除氟效率最高,在后续电絮凝实验中pH均控制在6.0~7.0。 -
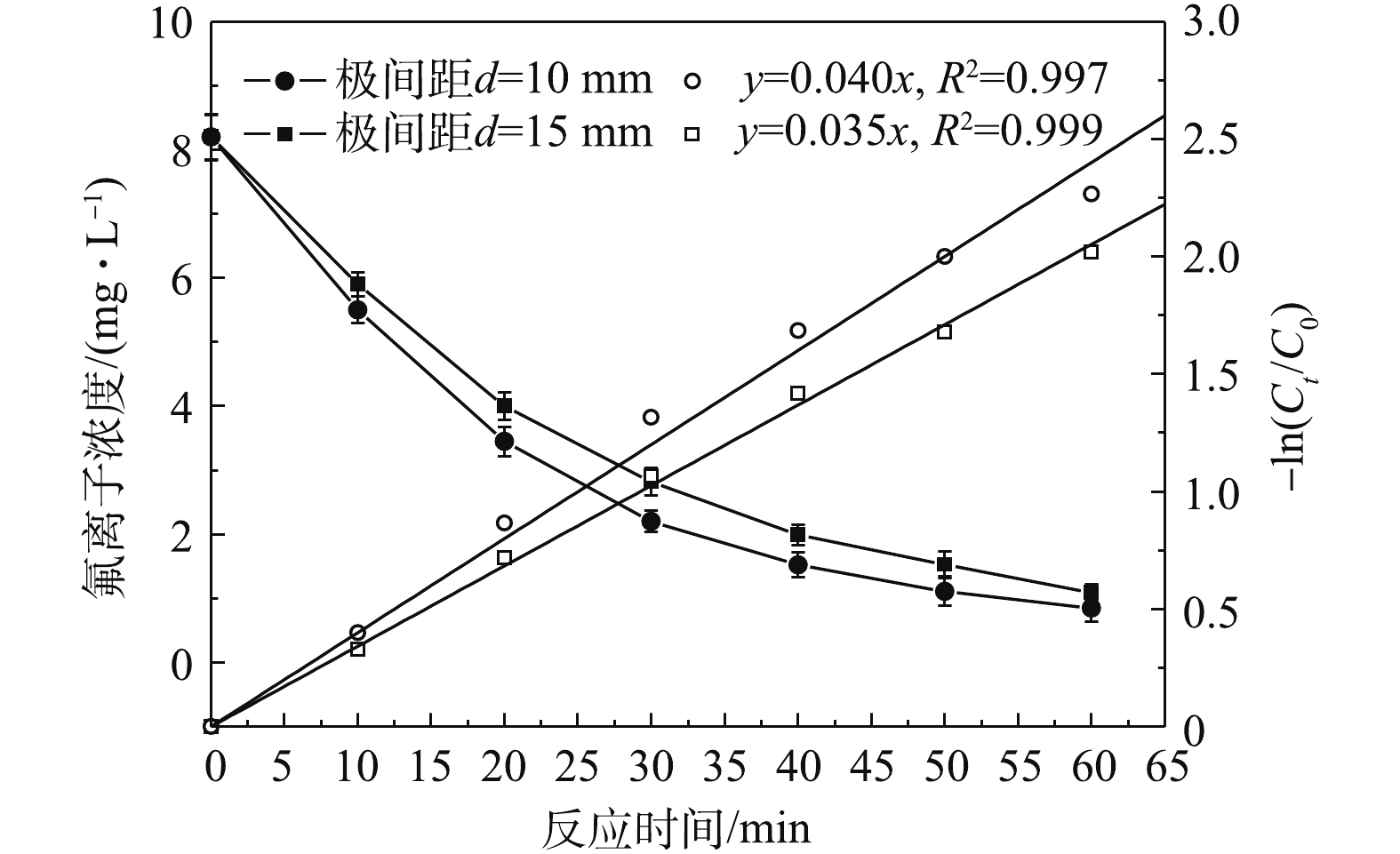
在双铝电絮凝体系下,铝板间距不仅影响极间水力条件,而且影响槽电压,进而影响阴阳极反应和体系运行能耗。图6反映了pH=6.0~7.0,初始氟离子浓度为8.2 mg·L−1,电流密度为300 A·m−3,极间距分别为10 mm和15 mm下氟离子浓度随时间的变化。由图6可知,随着极板间距由10 mm增至15 mm,相同时间内剩余氟离子浓度小幅升高,氟离子一级反应动力学常数随极板间距增大由0.040 min−1降低至0.035 min−1,这与EMAMJOMEH等[6]的研究结果相符。这可能由于随着极板间距的增大,阳极区域铝离子浓度相对降低,铝基聚合物与氟离子相互作用减弱所致。此外,图7为不同极间距下反应器含氟絮凝物XRD谱图。由图7可知,在不同极间距下的絮体结构出现部分衍射峰,且出峰位置形态相差不大,这说明不同极间距下絮体物质结构相差不大,经与标准谱图对比,该絮体为铝基氧化物;但由于出峰较宽,该絮凝物结晶程度不好,基本属于无定型形态,这与在此条件下产生的主要为无定形凝胶型氢氧化铝相吻合。不仅如此,随着极板间距由10 mm增至15 mm,电絮凝单元加载电压由4.32 V增至5.45 V,并且为达到相同的处理效果,根据式(8)可知,在极间距增大情况下,反应时间由52.6 min延长至60.1 min,由式(9)可知,在相同电流密度下,当极间距为10 mm和15 mm时,去除单位质量氟的电耗分别为0.157 8 kWh·g−1和0.227 5 kWh·g−1,能耗增加了44%,即极板间距的增大不利于节约运行成本。因此,在进行其他因素实验时,将极板间距设定为10 mm。
-
在电流密度为300 A·m−3,极板间距为10 mm,pH=6.0~7.0的条件下,考察了进水初始氟浓度对除氟效果影响,结果如图8所示。此外,对不同初始氟浓度下反应器所产生絮体进行了元素分析,结果如表1所示。由图8可知,当氟离子初始浓度分别为8.3、10.2和14.2 mg·L−1时,在反应60 min后,出水氟离子浓度分别为0.85、1.22和1.80 mg·L−1。由表1可知,在不同氟离子初始浓度下所形成的含氟絮体,除了氟元素相差较大外,其他元素相差不大,均可看作为铝氟络合物AlnFm(OH)3n-m和氢氧化铝Aln(OH)3n的混合物。经核算,3种初始氟浓度下絮体中铝氟比(摩尔比)分别为24.3∶1、12.7∶1和8.7∶1。因此,在相同电流密度下,溶液中释放铝离子浓度保持一定,当初始氟离子浓度增加时,铝氟比下降,造成铝离子不能够提供足够络合位点,致使除氟效果下降。另外,当初始氟离子浓度较高时,氟离子去除一级反应常数减小,这进一步地说明当铝离子浓度在限制浓度以下时,将影响除氟进程。根据式(8)可知,为保证此种条件下出水氟离子浓度达标,应适当延长反应时间。但由图8可知,单位时间内较高的进水初始氟浓度去除量明显高于低浓度初始氟浓度,这说明在铝离子浓度一定时,所形成的凝胶絮体与氟离子络合、吸附更加饱和、彻底,即在此条件下铝离子利用效率更高[18]。
-
1)双铝电絮凝除氟过程遵循一级反应动力学模型,并且在一定条件下,电絮凝除氟理论反应时间取决于初始氟离子浓度和动力学反应常数,而除氟动力学常数随着电流密度增大而增大,随极板间距和初始氟离子浓度增大而减小。
2)当电流密度为300 A·m−3,pH为6.0~7.0,极间距为10 mm时,电絮凝除氟能效最高,出水氟浓度为0.85 mg·L−1,去除率约为89.56%,单位质量氟去除能耗为0.157 8 kWh·g−1。
3)在相同条件下,较高的初始氟浓度使铝氟比下降,导致除氟效果下降,但此时所产生凝胶絮体与氟离子络合、吸附更加彻底,铝离子利用效率更高。
双铝电极电絮凝处理高含氟地下水的影响因素及动力学分析
Influencing factors and kinetics analysis of electrocoagulation with bipolar aluminum electrodes treating high fluorine groundwater
-
摘要: 为探求电絮凝处理高含氟地下水工艺技术参数及其除氟动力学,采用双铝电极电絮凝装置处理人工模拟高含氟地下水,研究了双铝电絮凝除氟过程及其除氟动力学模型,分别考察了电流密度、pH、极板间距及初始氟浓度对电絮凝除氟过程影响。结果表明,电絮凝除氟过程符合一级反应动力学模型,理论所需除氟时间取决于初始氟浓度和除氟动力学常数,而除氟动力学常数受电流密度、极板间距和初始氟浓度影响;当电流密度为300 A·m−3,pH为6.0~7.0,极间距为10 mm时,双铝电絮凝除氟能效最高,氟离子去除率为89.56%,能耗为0.157 8 kWh·g−1;但较高的初始氟浓度容易使铝氟比下降,导致除氟效果下降,不利于除氟过程。以上结果可为电絮凝处理高含氟地下水工程化应用和除氟反应器开发提供参考。Abstract: Aiming to determine the process parameters for electrocoagulation treating high fluorine groundwater and its de-fluorine kinetics, the electrocoagulation with bipolar aluminum electrodes was used to treat high fluorine groundwater and study its de-fluorine process and kinetic model. The effects of current density, pH, electrode distance and initial fluorine concentration on the de-fluorine process by electrocoagulation were also investigated, respectively. The results showed that the electrocoagulation de-fluorine process followed the first-order kinetics model. The theoretical time of de-fluorine depended on the initial fluorine concentration and the kinetic constant, while the kinetic constant of de-fluorine was affected by current density, electrode distance and initial fluorine concentration. The highest fluorine removal efficiency by electrocoagulation with bipolar aluminum electrodes reached 89.56% with energy consumption of 0.157 8 kWh·g−1 when the operational parameters of current density, pH and electrode distance maintained at 300 A·m−3, 6.0~7.0 and 10 mm, respectively. However, the aluminum-fluorine ratio decreased with the increase of the initial fluorine concentration, resulting in a decrease in the fluorine removal efficiency, which was not conducive to de-fluorine by electrocoagulation. This research provides a theoretical basis for the practical application of electrocoagulation treating high fluorine groundwater and the development of de-fluorine reactor.
-

-
表 1 不同初始氟浓度下絮体元素分析
Table 1. Elementary composition of flocs under different initial fluoride concentration
初始氟浓度/
(mg·L−1)质量分数/% 含氟絮体对应的化学结构 F Na Mg Al K Ca S Cl O 合计 8.2 0.75 5.64 1.08 25.6 0.635 1.97 4.18 12.0 47.9 99.76 FAl24.3O76.7H58.4Na6.3K0.4Ca1.3Mg1.2Cl8.7S3.4 10.2 1.38 5.15 0.98 25.0 0.690 2.06 4.32 11.8 48.0 99.38 FAl12.7O41.1H32.7Na3.1K0.2Ca0.7Mg0.6Cl4.5S1.8 14.3 2.04 4.54 0.91 25.0 0.647 2.14 4.65 12.9 46.5 99.32 FAl8.7O27.2H21.0Na1.8K0.2Ca0.5Mg0.4Cl3.4S1.4 -
[1] PRENTICE A. Diet, nutrition and the prevention of osteoporosis[J]. Public Health Nutrition, 2004, 7(1A): 227-243. doi: 10.1079/PHN2003590 [2] JAGTAP S, YENKIE M K, LABHSETWAR N, et al. Fluoride in drinking water and defluoridation of water[J]. Chemical Reviews, 2012, 112(4): 2454-2466. doi: 10.1021/cr2002855 [3] MOHAPATRA M, ANAND S, MISHRA B K, et al. Review of fluoride removal from drinking water[J]. Journal of Environmental Management, 2009, 91(1): 67-77. doi: 10.1016/j.jenvman.2009.08.015 [4] 毛若愚, 郭华明, 贾永峰, 等. 内蒙古河套盆地含氟地下水分布特点及成因[J]. 地学前缘, 2016, 23(2): 260-268. [5] 李彩霞. 山东省高密地区高氟地下水的成因浅析[J]. 山东国土资源, 2007, 23(8): 8-11. doi: 10.3969/j.issn.1672-6979.2007.08.003 [6] EMAMJOMEH M M, SIVAKUMAR M. An empirical model for defluoridation by batch monopolar electrocagulation/flotation (ECF) process[J]. Journal of Hazardous Materials, 2006, 131: 118-125. doi: 10.1016/j.jhazmat.2005.09.030 [7] 张道勇, 潘响亮, 穆桂金, 等. 双极铝电极电絮凝法去除地下水中氟的实验研究[J]. 水处理技术, 2008, 34(5): 46-49. [8] 邵坚, 陆建红, 邹仲勋, 等. 锌铝电极电絮凝法处理高氟饮用水的研究[J]. 中国给水排水, 2009, 25(15): 100-102. doi: 10.3321/j.issn:1000-4602.2009.15.029 [9] ESSADKI A H, GOURICH B, AZZI M, et al. Kinetic study of defluoridation of drinking water by electrocoagulation/electroflotation in a stirred tank reactor and in an external-loop airlift reactor[J]. Chemical Engineering Journal, 2010, 164(1): 106-114. doi: 10.1016/j.cej.2010.08.037 [10] GOVINDAN K, RAJA M, UMA MAHESHWARI S, et al. Comparison and understanding of fluoride removal mechanism in Ca2+, Mg2+ and Al3+ ion assisted electrocoagulation process using Fe and Al electrodes[J]. Journal of Environmental Chemical Engineering, 2015, 3(3): 1784-1793. doi: 10.1016/j.jece.2015.06.014 [11] ZHAO H, ZHAO B, YANG W, et al. Effects of Ca2+ and Mg2+ on defluoridation in the electrocoagulation process[J]. Environmental Science & Technology, 2010, 44(23): 9112-9116. [12] 李静波, 赵璇, 李福志. 电絮凝-微滤去除饮用水中的氟[J]. 清华大学学报(自然科学版), 2007, 47(9): 1495-1497. doi: 10.3321/j.issn:1000-0054.2007.09.025 [13] 王树青, 胡承志, 杨春风, 等. 电絮凝-超滤除氟工艺参数优化研究[J]. 环境工程学报, 2015, 9(7): 3093-3098. doi: 10.12030/j.cjee.20150704 [14] 梁言, 杨小明, 孙境求, 等. 电絮凝-超滤除氟控铝工艺参数优化[J]. 环境工程学报, 2018, 12(11): 3020-3027. doi: 10.12030/j.cjee.201805096 [15] HU C Y, LO S L, KUAN W H. Effects of co-existing anions on fluoride removal in electrocoagulation (EC) process using aluminum electrodes[J]. Water Research, 2003, 37(18): 4513-4523. doi: 10.1016/S0043-1354(03)00378-6 [16] 卢建杭, 刘维屏, 郑巍, 等. 铝盐混凝去除氟离子的作用机理探讨[J]. 环境科学学报, 2000, 20(6): 709-713. doi: 10.3321/j.issn:0253-2468.2000.06.009 [17] PITTER P. Forms of occurrence of fluorine in drinking water[J]. Water Research, 1985, 19(3): 281-284. doi: 10.1016/0043-1354(85)90086-7 [18] THAKUR L S, MONDAL P. Simultaneous arsenic and fluoride removal from synthetic and real groundwater by electrocoagulation process: Parametric and cost evaluation[J]. Journal of Environmental Management, 2017, 190: 102-112. -



 下载:
下载: