-
污水的脱氮过程主要由微生物完成,温度与有机碳浓度是影响微生物处理效率的主要因素。微生物生长的最佳水温是20~35 ℃,当水温在此范围之外时,反硝化细菌的增殖代谢速率将降低,致使反硝化速率也降低[1]。此外,菌体生长过程中需要有机物作为碳源提供其生长和反硝化过程所必需的能源,当碳源不足时,不能为反硝化提供充足的能量,反应进行不彻底且造成中间产物亚硝酸盐的积累[2]。近年来,我国污水厂进水COD/N为3~5,有机物浓度偏低,导致传统的反硝化工艺脱氮不彻底[3-5]。铁碳微电解技术由于其对碳源的需求小、处理效果好、成本低廉以及操作维护方便等优点被国内外学者广泛关注[6]。自养反硝化菌可利用铁碳微电解颗粒通过原电池反应生成的Fe2+和[H]为电子供体进行自养反硝化反应[7-8],但是对于COD/N<3~5的污水,若单独使用铁碳微电解技术会在处理中出现亚硝态氮积累的现象[9]。为提高生化工艺的脱氮效果,大多数污水厂采用外加碳源的方式。投加固体碳源一方面可作为微生物的载体,另一方面可补充异养反硝化所需的碳源,从而获得较高的脱氮除磷效率[10]。近年来,自养反硝化与异养反硝化的结合成为一种新的发展趋势。有研究[11]发现,自养反硝化和异养反硝化在同一体系内可以协同完成完全反硝化过程。为提高对低COD/N污水的脱氮除磷效果,本研究采用铁碳微电解耦合固相反硝化强化生物脱氮除磷系统,通过在系统内投加铁碳微电解填料以及固体碳源颗粒,实现了自养/异养反硝化耦合脱氮,使得氮、磷被有效地去除。对此,分别考察了HRT、DO、pH对耦合系统脱氮除磷性能的影响,对门、纲、属3个水平上的微生物群落结构进行了多样性分析,从微生物生态学角度解析铁碳微电解-自养/异养反硝化除磷协同机制。
全文HTML
-
实验用固体碳源选取玉米芯,采自阜蒙县生态园。将玉米芯蒸煮后洗净,切割成体积约为1 cm3的方块,放入烘箱以80 ℃进行干燥。然后将玉米芯置于1.5%的NaOH溶液中浸没24 h,最后洗净、中和、烘干、密封保存。
在制备铁碳微电解填料时,将纳米级铁粉与活性炭按1∶2混合,同时加入一定量石膏粉、铜粉和催化剂,然后加适量水制成粒径约为10 mm的球形颗粒,置于真空环境中干燥24 h后,再放入真空管式炉,以900 ℃的高温进行烧结,待自然冷却之后密封保存。
在配制实验用水时,以葡萄糖为碳源、硝酸钾和氯化铵为氮源、磷酸二氢钾为磷源,人工配制低C/N污水。控制进水C/N为1.5,
${\rm{NH}}_4^ + $ -N质量浓度为50 mg·L−1,${\rm{NO}}_3^ - $ -N质量浓度为25 mg·L−1,TP质量浓度为(3.5±0.5) mg·L−1;配水时加入1 mg·L−1酵母膏和1 ml·L−1微量元素溶液;使用NaOH和H2SO4调节pH。实验中所用SBR为圆柱体形, 由透明有机玻璃制成, 高为300 mm,内径为200 mm,实际工作体积为6.8 L。实验所用的固体碳源填料投加量为25 g·L−1,铁碳微电解填料的投加量为28 g·L−1,将2种填料装入反应器中,进水由反应器底部依次流经铁碳微电解填料层、固体碳源填料层后出水。反应周期包括进水5 min,微曝气反应6 h,沉淀110 min,出水5 min,1个完整周期为8 h,每天运行3个周期,换水比为1∶5。反应温度控制在20~25 ℃。
-
${\rm{NH}}_4^ + $ -N、${\rm{NO}}_3^ - $ -N、${\rm{NO}}_2^ - $ -N、TN、TP等指标采用标准方法测定。${\rm{NH}}_4^ + $ -N采用纳氏试剂分光光度法测定,${\rm{NO}}_3^ - $ -N采用紫外分光光度法测定,${\rm{NO}}_2^ - $ -N采用N -(1-萘基)-乙二胺光度法测定,TN采用碱性过硫酸钾-紫外分光光度法测定,TP采用过硫酸钾消解-钼锑抗分光光度法测定,COD、DO、pH分别采用重铬酸钾法、便携式溶解氧仪、pH计测定。 -
在耦合系统运行稳定时,取出适量的铁碳微电解填料以及玉米芯,装入50 mL的离心管中,再加入一定量蒸馏水振荡,采集铁碳填料上和玉米芯上脱落的微生物样品(分别记为FC和CC),同时采集系统内悬浮的微生物样品(记为SS),6 000 r·min−1高速离心后,密封并置于−20 ℃低温冰箱中保存待测。
取不同微生物离心样品,使用E.Z.N.ATM Mag-Bind Soil DNA Kit试剂盒(OMEGA)进行DNA提取。利用1%琼脂糖凝胶电泳检测抽提的基因组DNA。而后进行PCR扩增,扩增过程与WANG等[12]的研究过程一致,扩增后切胶回收PCR产物并进行电泳检测。使用Qubit3.0 DNA检测试剂盒精确定量回收DNA,每个样品DNA量取10 ng,基因测序和序列处理与朱文优[13]研究方法一致,最后将OUT代表序列与 Silva 数据库比对,并在门、纲、属3个水平统计每个样品的群落组成,最后对各样本数据的质量进行质控过滤,得到各样本有效数据,然后对数据进行优化处理。在数据处理及统计完成后,进行 Alpha多样性分析、物种分类分析和菌群差异分析。
1.1. 实验材料
1.2. 分析项目
1.3. 微生物多样性分析
-
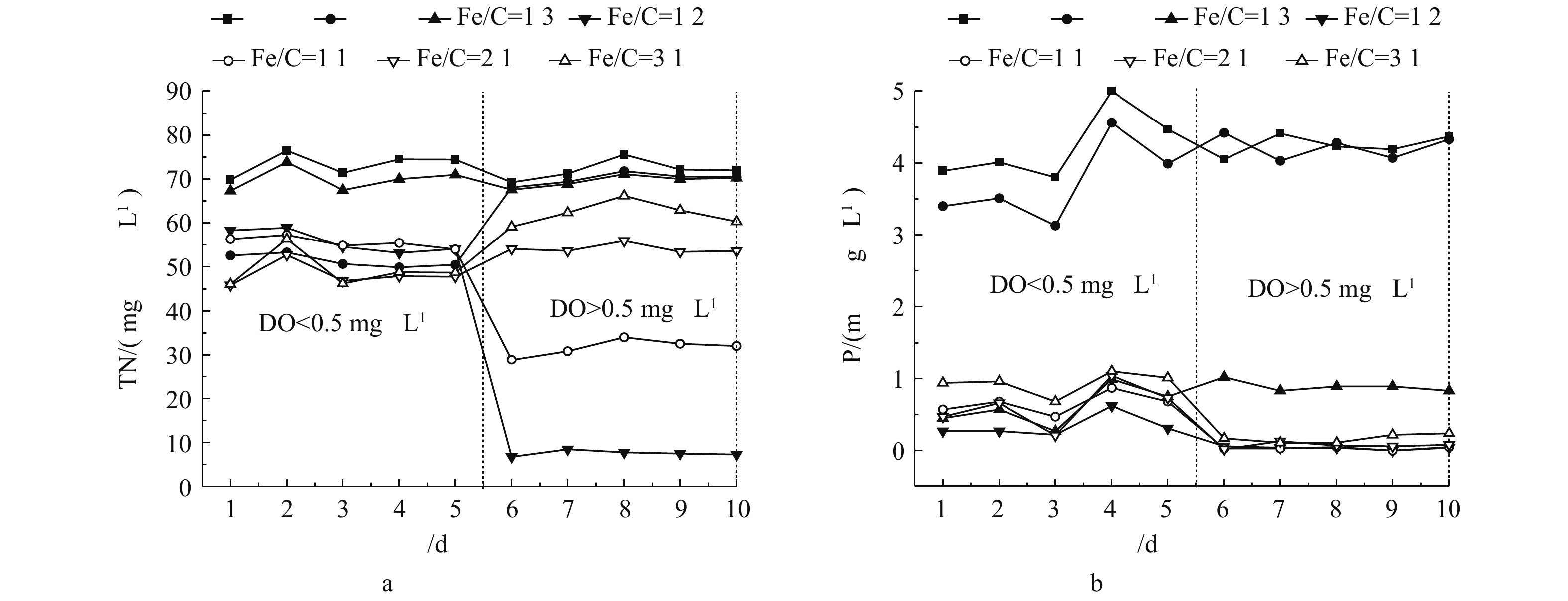
图1反映了溶解氧(DO)浓度小于0.5 mg·L−1、大于0.5 mg·L−1时不同铁碳比例的铁碳微电解填料处理后的出水TN、TP浓度变化结果。由图1可知,当系统内DO<0.5 mg·L−1时,各组分TN去除效果并不理想,主要是因为铁碳微电解作用将硝态氮氧化还原为氨氮;而氨氮由于缺少溶解氧,无法进行硝化作用;当系统内DO>0.5 mg·L−1时,经Fe/C比为1∶2的铁碳微电解填料处理的出水TN浓度最低,稳定于7.13 mg·L−1,去除率为89.81%,此时磷的去除率为99.1%。由于铁碳微电解填料对氮的去除效果不佳,故须建立铁碳微电解-固体碳源耦合系统,加强对氮、磷的去除。
-
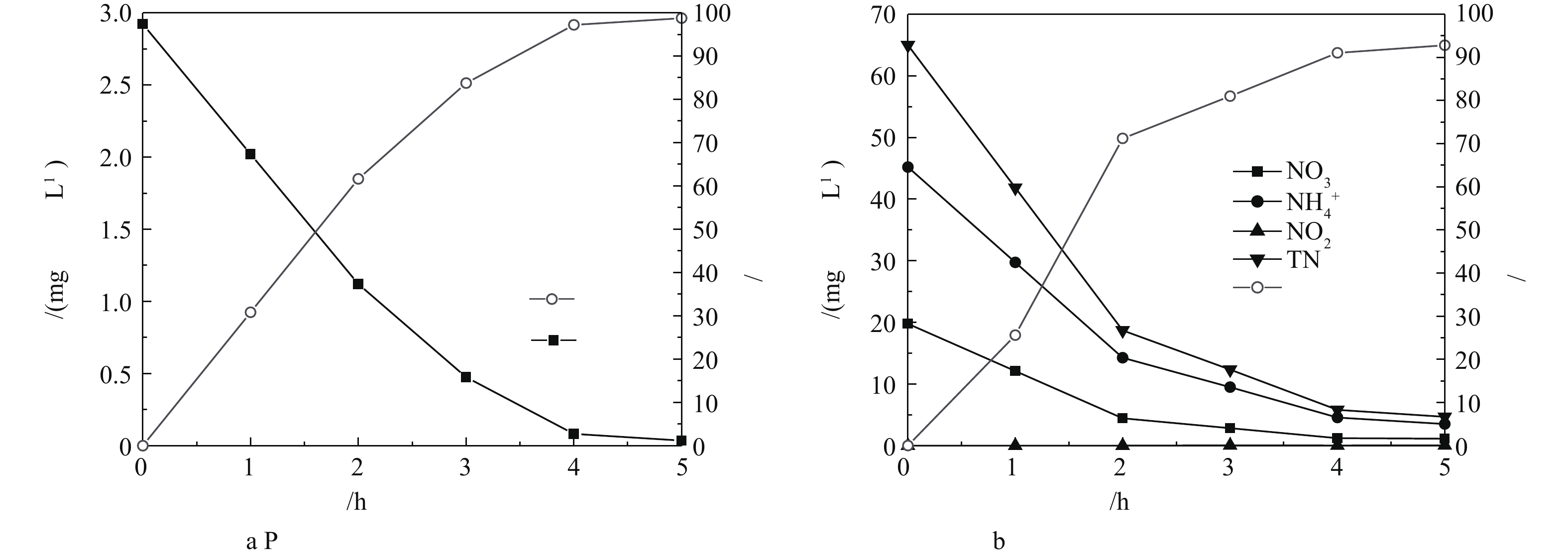
控制耦合系统进水溶解氧(DO)浓度为(2.0±0.1) mg·L−1、pH为7.0±0.1,在HRT为2、3、4、5 h时分别测定出水中的
${\rm{NH}}_4^ + $ -N、${\rm{NO}}_3^ - $ -N、TN、TP的浓度,结果如图2所示。由图2可知,${\rm{NO}}_3^ - $ -N的浓度从0 h到2 h大幅降低,0~2 h时去除速率为7.5 mg·(L·h)−1,2~4 h时去除速率降为2.0 mg·(L·h)−1,4 h后去除速率接近为0 mg·(L·h)−1,此时水中${\rm{NO}}_3^ - $ -N的浓度为1.5 mg·(L·h)−1。${\rm{NH}}_4^ + $ -N浓度也随着反应时间的延长而减小,4 h后水中${\rm{NH}}_4^ + $ -N的浓度为1.9 mg·(L·h)−1。${\rm{NO}}_2^ - $ -N在反应过程中并未出现积累的现象。TN浓度的变化趋势与${\rm{NO}}_3^ - $ -N和${\rm{NH}}_4^ + $ -N大致相同,反应4 h后,去除速率由22 mg·(L·h)−1降低到6.5 mg·(L·h)−1。水中TP的去除速率由0.9 mg·(L·h)−1下降到0.4 mg·(L·h)−1,直至趋于稳定。反应前2 h,氮、磷污染物浓度较高,在与玉米芯、铁碳微电解填料和活性污泥充分接触后,获得较高的去除速率。反应初期,${\rm{NH}}_4^ + $ -N去除速率高于${\rm{NO}}_3^ - $ -N去除速率,主要因为进水C/N比低,有机碳源不足,难以进行异养反硝化。此时铁碳微电解产生的氢、Fe2+为自养反硝化过程提供了电子供体,随着固体碳源玉米芯的缓慢降解释放碳源,为异养反硝化提供较为充足的电子供体,异养反硝化产生的CO2被自养反硝化菌作为无机碳源加以利用[11]。在自养反硝化菌与异养反硝化菌的共同作用下将氮从系统中去除,磷与铁碳微电解反应溶出的铁离子接触反应生成沉淀而去除[14-15];当HRT继续增加时,系统内N、P污染物浓度较低,去除速率逐渐降低[16]。因此,铁碳微电解耦合固相反硝化强化生物脱氮除磷系统的最佳HRT为4.0 h。 -
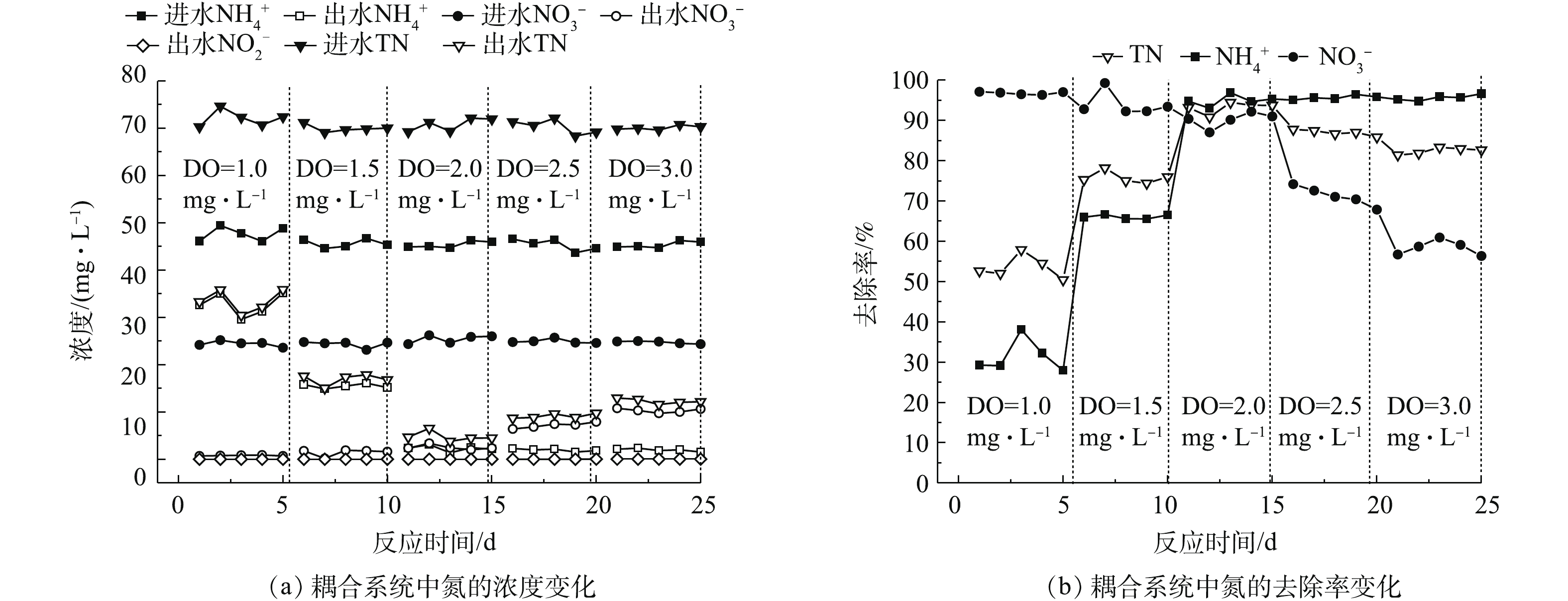
由于DO对于系统除磷效果的影响很小[17],因此,本节只考察进水DO对于耦合系统脱氮效果的影响,控制进水DO的浓度分别为(1.0±0.1)、(1.5±0.1)、(2.0±0.1)、(2.5±0.1)和(3.0±0.1) mg·L−1,HRT为4.0 h、进水pH为7.0±0.1,结果如图3所示。由图3(a)可知,当DO为(1.0±0.1)、(1.5±0.1)、(2.0±0.1)、(2.5±0.1)和(3.0±0.1) mg·L−1时,出水
${\rm{NH}}_4^ + $ -N浓度分别为32.74、15.48、2.28、1.96和1.97 mg·L−1,出水${\rm{NO}}_3^ - $ -N浓度分别为0.78、1.45、2.50、7.17和10.30 mg·L−1。分析认为,${\rm{NH}}_4^ + $ -N在硝化细菌的作用下可转化为${\rm{NO}}_2^ - $ -N以及${\rm{NO}}_3^ - $ -N,一部分${\rm{NH}}_4^ + $ -N水解电离,NH3在曝气作用下吹脱;${\rm{NO}}_3^ - $ -N的减少与反硝化脱氮以及化学还原有关。由图3(b)可知,${\rm{NH}}_4^ + $ -N的去除率随DO浓度的增加由29.58%升高到95.63%,直至趋于稳定;而在DO浓度超过(2.0±0.1) mg·L−1时,${\rm{NO}}_3^ - $ -N的去除率由93.48%降低到56.72%,这是由于DO对于${\rm{NH}}_4^ + $ -N在硝化细菌的作用下转化有积极作用,而当底物浓度一定,DO浓度增加至一定值时,${\rm{NH}}_4^ + $ -N的去除率将不再随DO浓度的增大而升高;在中性条件下,DO浓度较大时,电子的大量消耗抑制了自养反硝化过程,同时生成了大量的碱,降低反硝化细菌的活性。此外,DO浓度不宜过大,否则会对填料表面生物膜产生冲刷,使微生物不易于附着,从而影响系统的脱氮效果。因此,铁碳微电解耦合固相反硝化强化生物脱氮除磷系统适宜的DO浓度为(2.0±0.1) mg·L−1。 -
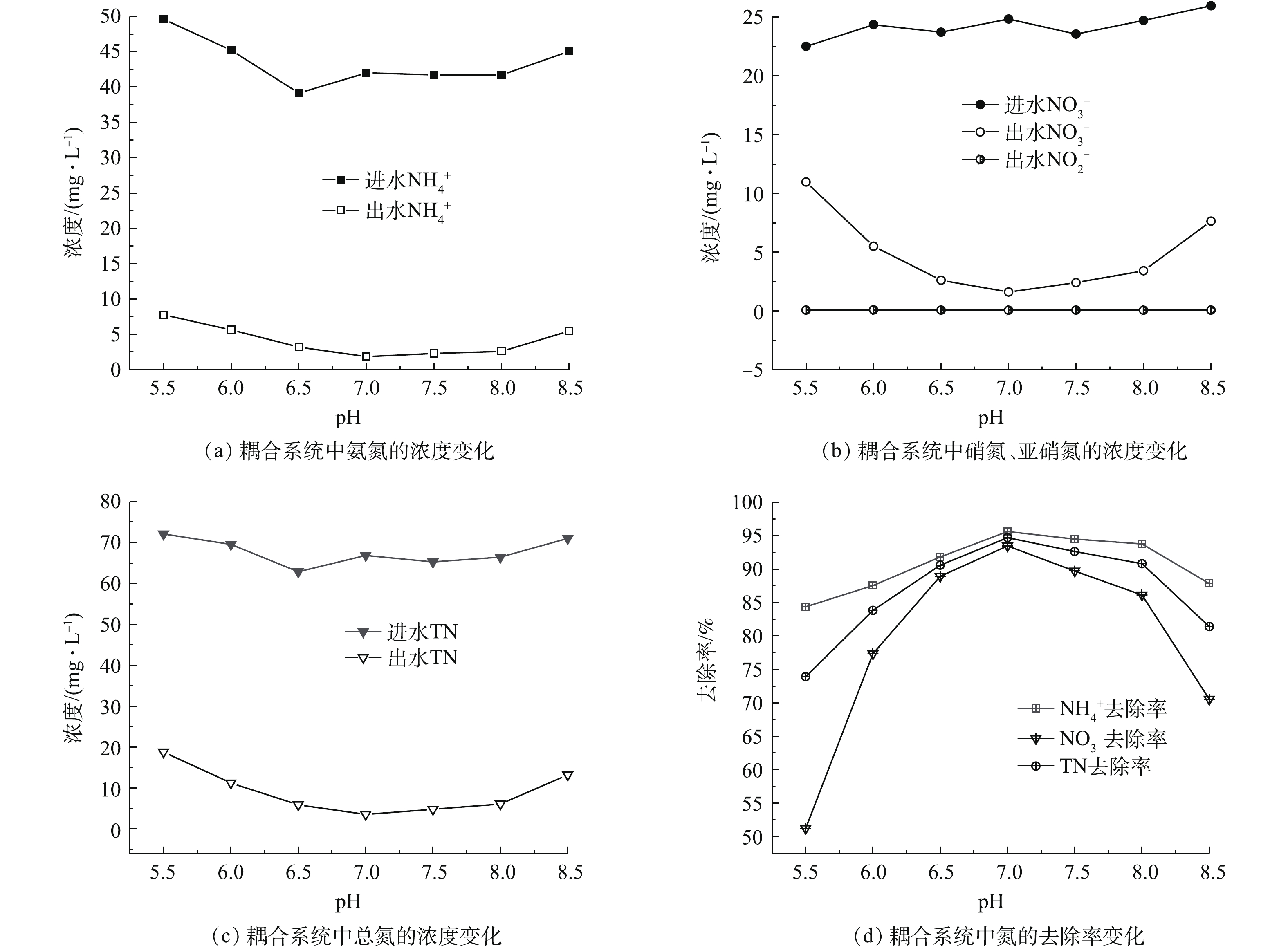
为考察进水pH对耦合系统脱氮除磷效果的影响,控制HRT、进水DO浓度分别为4.0 h、(2.0±0.1) mg·L−1,以NaOH和H2SO4调节进水pH分别为5.5±0.1、6.0±0.1、6.5±0.1、7.0±0.1、7.5±0.1、8.0±0.1、8.5±0.1,结果如图4所示。由图4可知,
${\rm{NH}}_4^ + $ -N的去除率随pH的升高由83.13%升高至95.63%,当pH超过7.0±0.1时,${\rm{NH}}_4^ + $ -N去除率由95.63%下降至89.05%;同时${\rm{NO}}_3^ - $ -N的去除率也随pH的升高而升高,在pH超过7.0±0.1时,${\rm{NO}}_3^ - $ -N去除率由93.48%下降至70.35%;${\rm{NO}}_2^ - $ -N在反应过程中一直处于较低水平,未出现积累;TN的去除率在pH为7.0±0.1时达到最高,为94.72%。分析认为,硝化细菌和氨化细菌适宜pH为7.5~8.5,反硝化细菌适宜pH为7.0~8.0。当进水pH不在所适范围内,硝化细菌、氨化细菌以及反硝化细菌的活性将会受到抑制,使得耦合系统脱氮效果下降。因此,耦合系统的适宜进水pH为7.0±0.1,此时,${\rm{NH}}_4^ + $ -N、${\rm{NO}}_3^ - $ -N、TN以及TP的去除率分别为95.63%、93.48%、94.72%以及99.10%。 -
高通量测序技术作为新型微生物种群鉴定技术,具有分析结果准确、高速、高自动化和高灵敏度等特点,能够准确分析生物的多样性,故广泛应用于环境微生物鉴定领域[18]。因此,本研究采用Miseq高通量测序技术对铁碳微电解-固相反硝化耦合系统中的微生物结构进行分析。
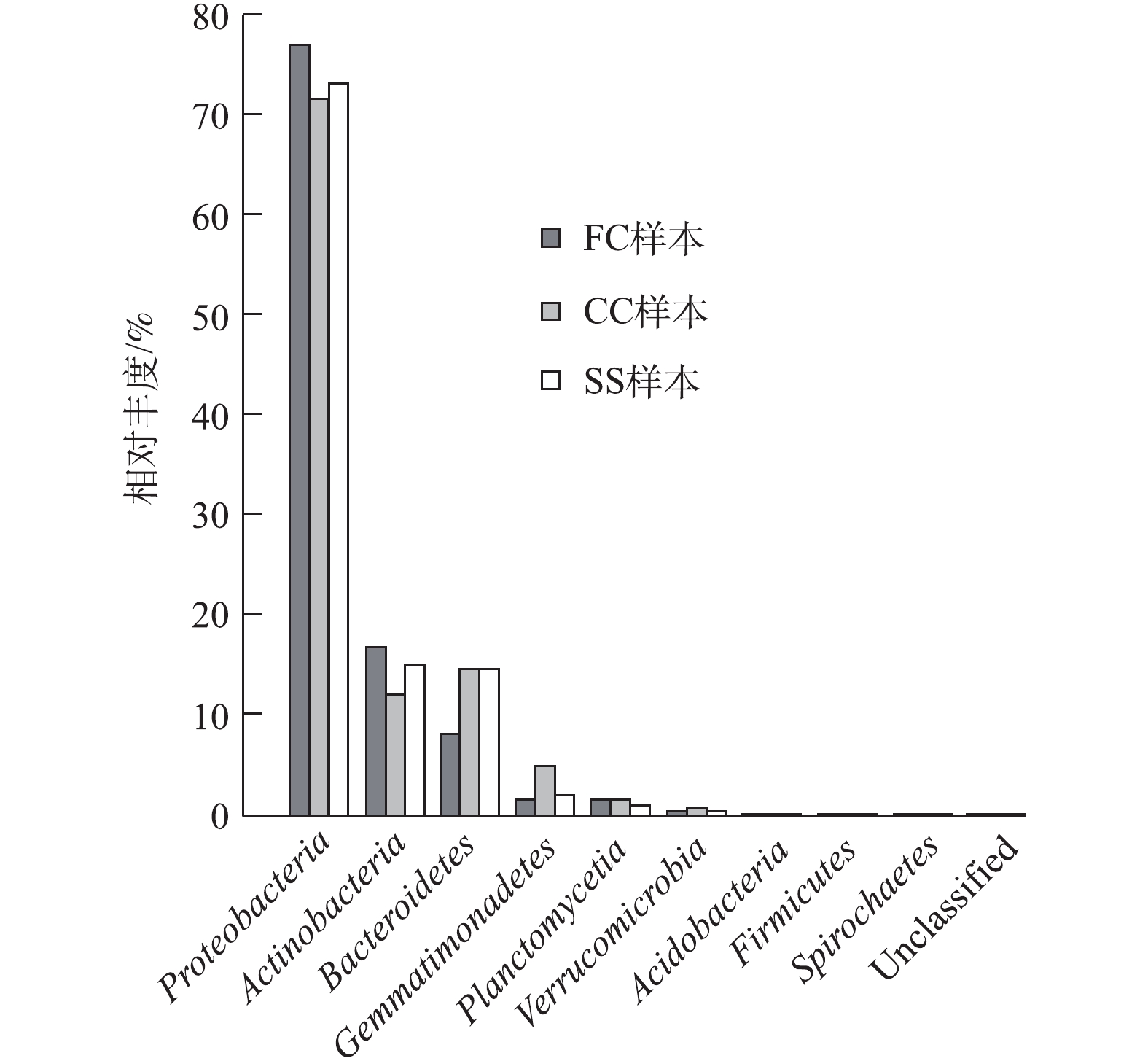
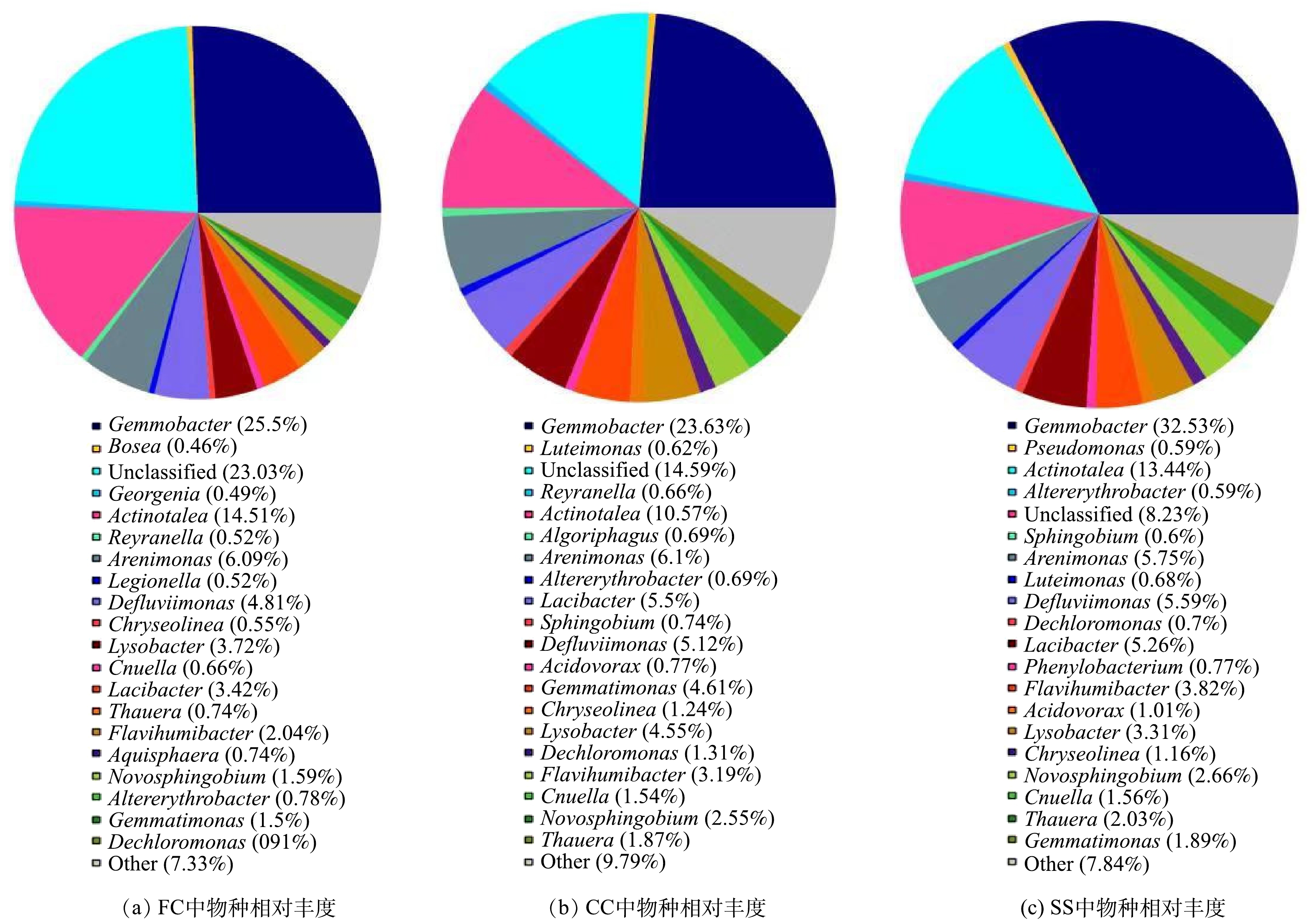
1)微生物群落结构比较分析。图5为耦合系统在门水平上的物种相对丰度,表1为在门水平上,各样本中主要种群的分布情况。由图5和表1可知,3个样本(FC、CC、SS)中的优势菌门为具有反硝化脱氮功能的变形菌门Proteobacteria,在3个样本(FC、CC、SS)中分别占样本总数的72.66%、67.43%、68.66%。变形菌是污水处理中最为重要的微生物之一,对污染物生物降解有着重要作用,大多数反硝化菌属于变形菌门Proteobacteria[19-20],说明系统的脱氮能力稳定。放线菌门Actinobacteria在FC、SS中的相对丰度显著高于CC。拟杆菌门Bacteroidetes在CC、SS中的相对丰度显著高于FC。芽单胞菌门Gemmatimonadetes在CC中的相对丰度为4.61%,显著高于FC、SS。分析认为,Gemmatimonadetes可分解纤维素类物质,作用于固体碳源的释碳过程[21],该过程主要发生在CC中。
图6为耦合系统在纲水平上的物种相对丰度情况,表2为在纲水平上各样本中主要种群的分布情况。由图6和表2可知,α-变形纲Alphaproteobacteria在3个样本(FC、CC、SS)中所占样本总数最高,分别为36.73%、37.21%、46.18%,在SS中的相对丰度显著高于FC、CC。FC中的γ-变形菌纲Gammaproteobacteria的相对丰度显著高于CC和SS,对于有机物的降解以及碳、氮、硫等元素的循环起关键性作用,同时对于脱氮过程也发挥着重要作用,对于污水处理较具优势[22-24]。鞘脂杆菌纲Sphingobacteriia在3个样本(FC、CC、SS)中所占样本总数分别为6.47%、11.12%、11.39%,在CC和SS中的相对丰度显著高于FC。CC中的Gemmatimonadetes相对丰度明显高于FC和SS,所占样本比例为生物除磷主要发生在CC上。
为进一步阐明耦合系统运行过程中细菌群落的变化情况,在属的水平上,选取系统中占有比例较多的几种具有脱氮除磷功能的菌属进行分析,结果如图7和表3所示。由图7和表3可知,芽植杆菌属Gemmobacter在FC、CC、SS中的相对丰度分别为25.50%、23.64%、32.53%,对于异养反硝化过程起到重要作用[25]。铁矿砂单胞杆菌属Arenimonas在FC、CC、SS中的相对丰度分别为6.09%、6.10%、5.75%,是最重要的自养反硝化菌属[26]。Thauera菌属在CC、SS中的相对丰度显著高于FC,这是由于Thauera属于异养反硝化菌[27],而异养反硝化过程主要发生在CC中,SS中由于固体碳源颗粒的脱落也会发生异养反硝化反应,它们的存在为耦合系统脱氮除磷效果提供了保障。Flavihumibacter菌属可以参与碳循环,同时代谢碳水化合物[28],出芽单孢菌属Gemmatimonas与除磷密切相关,新硝氨醇菌属Novosphingobium能够充分降解木质素,在CC、SS中的相对丰度高于FC。由图7和表3的分析可知,耦合系统中微生物群落在不同位置,其特征也有所不同,故处理工艺与微生物群落结构之间存在着显著的映射关系。
2)物种组成多样性分析。Alpha多样性可以定量地反映微生物群落的多样性,包括物种丰富度指数和物种多样性指数,结果如表4所示。经过质量控制后,得到优化序列分别为39 586、47 036、46 114条,平均长度分别为416.34、416.36、414.09 bp。在97%的相似度下,FC、CC、SS共得到了4 557个OTUs,其中FC中有1 544个,CC中有1 947个,SS中有1 700个。Shannon、ACE、Chao1、Simpson 指数表明系统中脱氮除磷功能区细菌群落的丰富度,其中丰富度指数ACE和Chao1可通过估算群落中所含OTU数目的指数来反映耦合系统中种群的丰富度,数值越大,表明其所含物种总数越多。由表4可知,3个样品的Chao1、ACE指数均为CC>SS、FC,表明固体碳源上的细菌群落丰富度最高,这可能是由于系统脱氮的效果主要来自于固相反硝化过程。覆盖率越高,样本中序列没有被测出的概率就越低,实际反映了测序结果是否可以代表样本的真实情况;Shannon指数可反映群落种类多样性,指数越大,耦合系统内群落的复杂程度越高。
2.1. 铁碳微电解填料的脱氮除磷效果
2.2. 水力停留时间(HRT)对耦合系统脱氮除磷效果的影响
2.3. DO对耦合系统脱氮效果的影响
2.4. pH对耦合系统脱氮除磷效果的影响
2.5. 耦合系统微生物群落分析
-
1)耦合系统在最佳运行参数的条件下,氨氮、硝态氮、总氮、总磷的去除率分别为95.63%、93.48%、94.72%、99.10%。
2)高通量测序分析表明,耦合系统中异养反硝化细菌(Gemmobacter、Defluviimonas、Thauera、Dechloromonas)的相对丰度处于较高水平,同时存在着自养反硝化细菌Arenimonas以及能够分解纤维素、半纤维素、木质素相关的细菌(Actinotalea、Chryseolinea、Novosphingobium),这说明系统内脱氮除磷的过程是自养反硝化与异养反硝化相结合,且为多种微生物的协同作用。







 下载:
下载: