-
随着我国工业化的快速发展,工业废水的排放量在显著增长[1]。尤其是煤化工、石化、制药等废水处理不当,将会对环境造成严重的危害。在煤化工、石化和制药废水中,酚类化合物是一类常见的有机污染物,其中的间甲酚因其具有较大的毒性和强烈的腐蚀性,对生物体具有直接或潜在的危害,其直接进入人体会引起蛋白质的团聚和变性,同时抑制环氧化酶的活性和血小板的凝结,进而影响中枢神经系统[2],间甲酚已被多国环保机构列入优先控制污染物的名单中。因此,对含有间甲酚废水的处理、转化和降解的研究已引起广泛的关注[3-6]。常用的间甲酚废水处理方法有生化法[7]、吸附法[8]和高级氧化法(催化臭氧氧化[9]、电催化氧化[10]、催化过氧化氢氧化[11])。由于传统方法对间甲酚的去除效果并不理想,高级氧化方法(advanced oxidation processes)被认为是处理间甲酚废水最常用的方法。
众所周知,臭氧对有机污染物具有较强的降解和矿化能力[12]。催化臭氧氧化技术的核心为催化剂,目前,在催化臭氧氧化中常用的非均相催化剂有活性炭类、活性金属铁、金属氧化物、分子筛和天然矿物等[13-14]。在这些非均相催化剂的作用下,臭氧可以更有效地和有机物发生反应,以实现污染物的降解和矿化。然而上述非均相催化剂在应用于催化臭氧氧化反应时,仍存在一定问题,如催化剂稳定性不够、活性组分易流失、催化效率不高等[15]。
钙钛矿类混合金属氧化物具有明确的晶体结构,通用单元分子式为ABO3,式中的A表示稀有或碱土金属,B表示过渡金属,因其结构的复杂性和多样性,可作为催化剂应用于各类催化反应中,如光催化、燃料电池、三效催化剂、VOCs的治理和催化湿式过氧化物的氧化等[16]。这种催化剂在高温和腐蚀性介质中是稳定的,同时B位元素位于晶体结构的中心,可以防止活性组分的流失,这对于钙钛矿催化剂的活性和结构稳定性至关重要。笔者前期的研究发现,钙钛矿材料也可被用于催化臭氧氧化技术中,许多学者也已经证明了钙钛矿在催化臭氧氧化反应中有着较好的活性[15, 17-20]。RIVAS等[21]将LaTi0.15Cu0.85O3用于催化臭氧氧化丙酮酸的实验中,在重复使用3次后,其活性甚至有所提高。因此,有必要进一步研究钙钛矿型催化剂在降解污染物中的应用情况。锆酸钙(CaZrO3)复合材料是一种重要的钙钛矿材料,常应用于发光材料[22-23]、湿度传感器[24]、陶瓷电容器[25]和超高温下的保护材料[26]等。与其他钙钛矿氧化物不同,有关其在催化臭氧氧化反应中的研究较少。因此,有必要对CaZrO3复合材料在废水处理中的应用及催化机制进行详细研究,以便更深入地了解其催化性能。
本研究采用共沉淀法制备了一系列Ca-Zr复合材料,在不同焙烧温度下,对复合材料进行了焙烧。鉴于制备方法对钙钛矿的结构性质和相纯度的决定性影响,考察了合成条件(主要是焙烧温度)对降解间甲酚催化性能的影响,且将使用XRD、SEM、TEM等表征手段对不同焙烧温度下制备的Ca-Zr复合材料催化剂的结构、形貌和组分进行了分析,对其在催化臭氧氧化中的催化活性、机理和稳定性进行阐释。
全文HTML
-
所用实验药品为ZrOCl·8H2O、CaCl2、(NH4)2C2O4·H2O、NH3·H2O(25%~28%)。钙锆比例设置为1.1∶1,称取3.631 g ZrOCl·8H2O和1.418 5 g CaCl2溶于100 mL超纯水中,将 (NH4)2C2O4·H2O溶于5 mL氨水中,同时,添加0.1 g聚乙二醇(PEG)。将上述2种溶液混合后,可产生白色乳胶状沉淀,充分反应后,在磁力搅拌器上搅拌1 h,将反应后的溶液在100 ℃下进行24 h老化,再进行真空抽滤,并用去离子水洗涤3次。将过滤后的沉淀于120 ℃下干燥12 h,之后在700~1 200 ℃下焙烧4 h,制备得到Ca-Zr复合材料。
-
X-射线衍射测试采用德国布鲁克X射线衍射仪(Bruker D8 Focus型),CuKa辐射源,宽角度扫描范围2θ=10°~90°;使用Quante400F(FEI)场发射扫描电镜仪表征催化剂的样品形貌,工作电压为20 kV;使用WRT-1D微机热天平进行样品热重分析,温度为25~1 200 ℃;采用美国麦克仪器公司生产的Chemisorb 2720型脉冲化学吸附仪对催化剂的还原特性进行表征,称取0.10 g的催化剂样品于石英管中,在Ar气流中预处理15 min,由室温升至300 ℃后,保温30 min,然后降至室温,切换为10% H2/Ar混合气体,混合气流速为20 m2·min−1,待基线平稳后,以10 ℃·min−1升温至1 000 ℃,由热导池检测器检测出耗氢信号;采用高分辨透射电镜(Jeol 2100F HRTEM)进行样品的形貌表征;采用VG Scientific ESCA-3000光谱仪上进行X射线光电子能谱(XPS)表征。
-
在250 mL的间歇反应器中进行催化臭氧氧化反应。反应过程中每隔2 min取样1.5 mL,连续取样10次,使用0.45 µm滤膜过滤后,测定出水的间甲酚浓度。间甲酚浓度采用大连依利特分析仪器有限公司生产的HPLC-P1201型高效液相色谱进行分析,所用色谱柱为C18色谱柱(4.6 mm×250 mm,5 µm),流动相为甲醇∶水=80∶20(体积比),流速为1.0 mL·min−1,检测波长为272 nm。
1.1. 实验原料
1.2. 催化剂表征
1.3. 催化剂评价
-
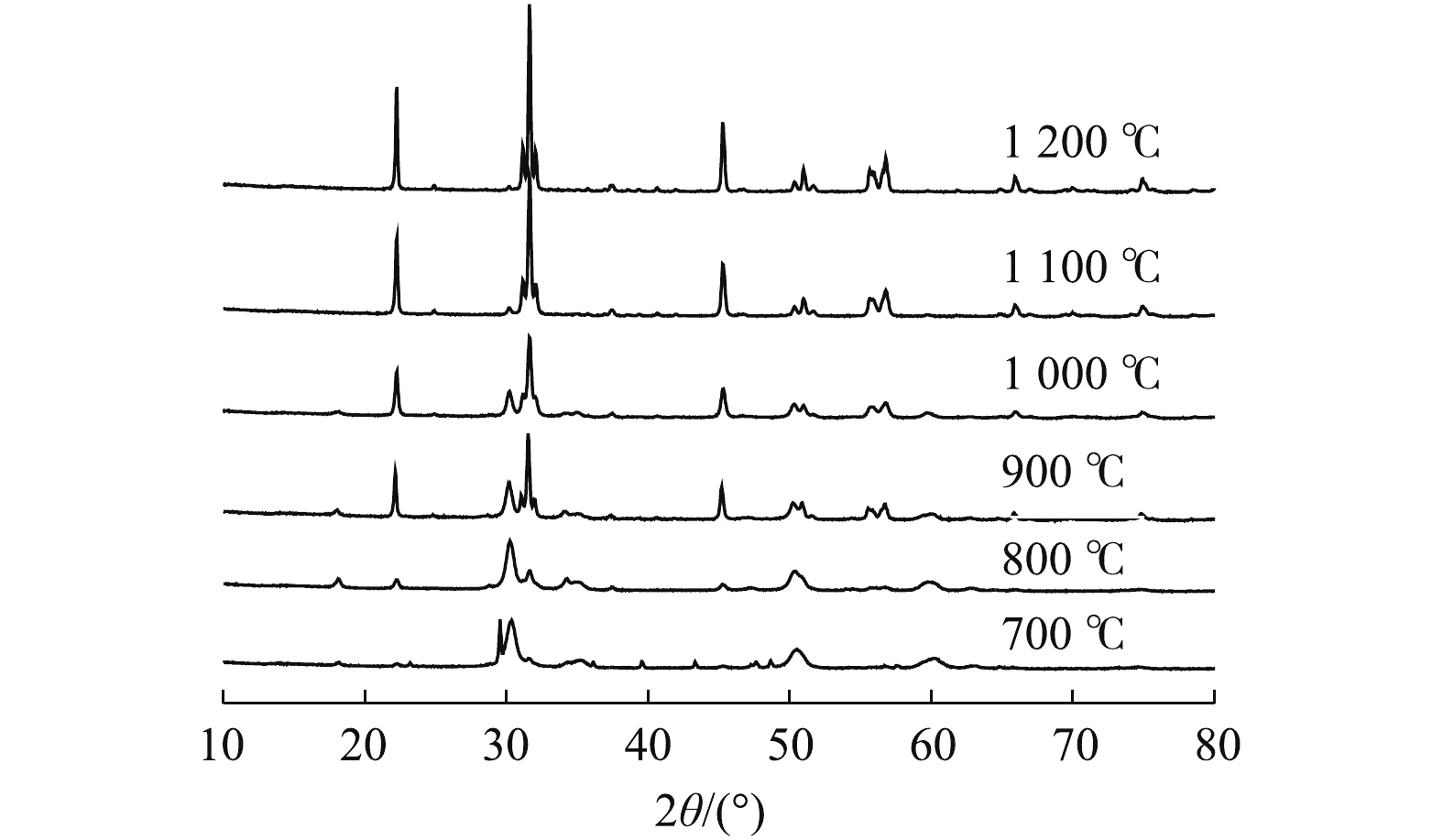
图1为在不同温度下制备出的样品的XRD表征结果。由图1可知,所合成的催化剂为典型的斜方晶系锆酸钙材料,且锆酸钙衍射峰强度随着焙烧温度的升高不断增强。此外,材料中还残存少量立方晶系的ZrO2晶体和六方晶系的Ca(OH)2晶体。结果表明,在不同焙烧温度下所得到的样品晶型有显著的变化,当焙烧温度升高到1 000 ℃以上时,所制备出的样品晶型较纯,为斜方晶系CaZrO3(PDF#35-0790)。
-
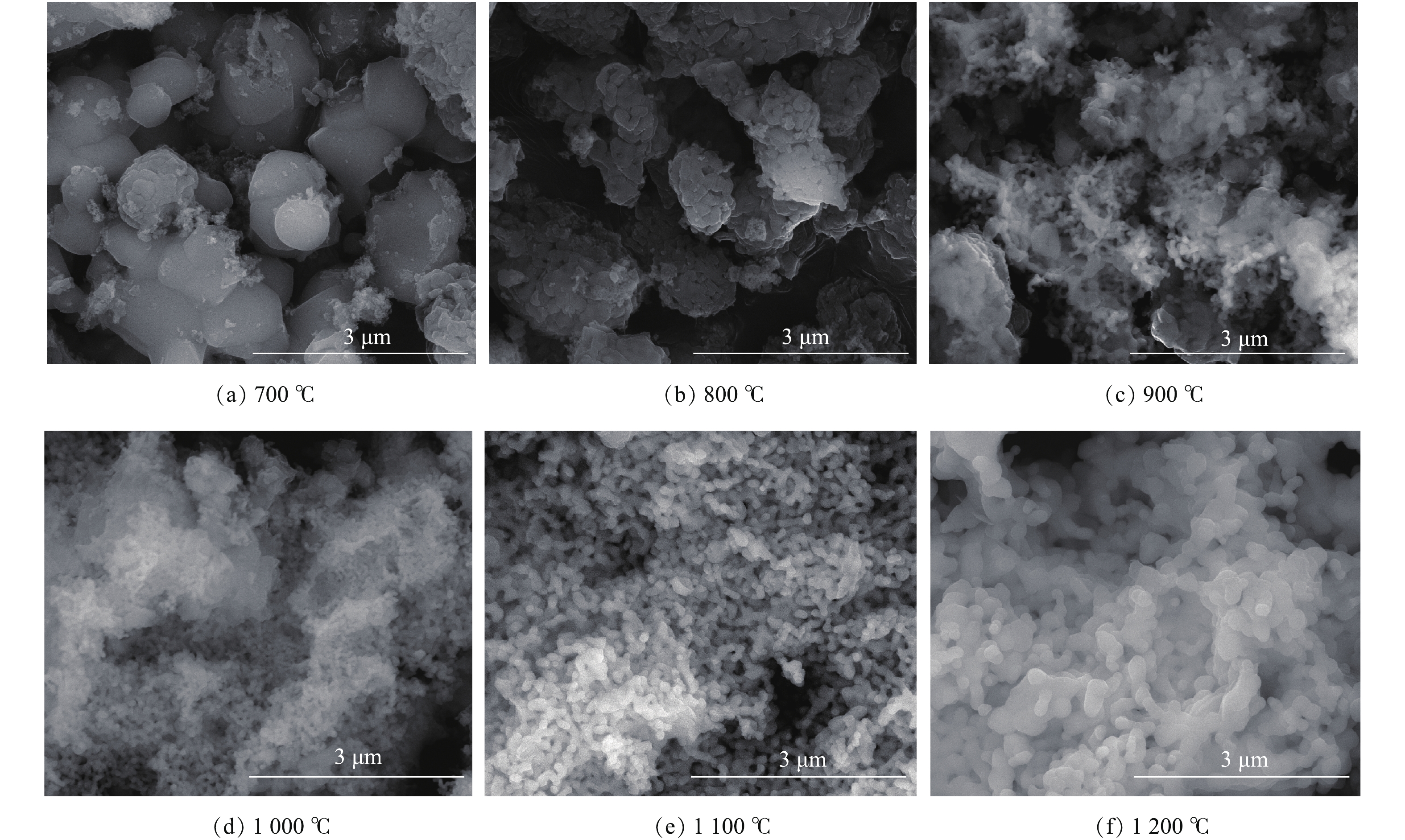
图2为在不同焙烧温度下所制得样品的形貌表征结果。由图2(a)可知,当焙烧温度为700 ℃时,样品颗粒近球形,大小不均匀,粒径尺寸较大,结合图1可知,这些大的颗粒为ZrO2;当焙烧温度达到800 ℃时,样品颗粒有变小的趋势(图2(b));当升高焙烧温度达到1 100 ℃以上时(图2(e)),颗粒形状大小变得更加均匀。结合物相分析结果,当焙烧温度在1 000 ℃以上时,样品组分主要为CaZrO3,由此可知,所制备的催化剂大小均一,且分散均匀。
-
图3为干燥后未焙烧的样品的热重-差热分析结果。由图3可知,TG曲线上有3次较为明显的失重,对应的DTA曲线在100 ℃左右出现1个小的吸热峰,这主要是由于样品中水的脱附引起的[27- 28]。在200 ℃左右出现的峰可以归因为样品失掉结晶水和CaC2O4转化成CaCO3所引起的;在490 ℃左右出现的峰可能是由无定形的ZrO2转化成介稳态的ZrO2导致的;在850 ℃左右出现的峰是稳定态的ZrO2与CaCO3在高温下反应生成CaZrO3。综上所述,在焙烧过程中温度的控制尤为重要,样品焙烧温度应不能低于800 ℃,才能合成结晶良好的CaZrO3,这与XRD谱图中所显示的物相变化趋势相一致。
-
为了探究在催化臭氧氧化过程中臭氧对样品的影响,使用O3对样品进行了预处理(吹扫3 h),并对其进行H2-TPR分析(图4)。由图4所知,在700 ℃和800 ℃制备的样品所对应的还原峰在700~800 ℃,这可能是由于样品中的CaCO3还原导致的;当焙烧温度大于900 ℃时,样品还原峰位置均在600~700 ℃,这可能由于样品中的CaO还原导致的。经臭氧预处理后的样品与未经预处理的样品在被还原时,虽然在耗氢量上有一定的差别,但其还原峰位置并未发生改变。由此可以推测,所制备样品结构具有较强的稳定性。由BRIK等[29]所做的第一性原理计算结果也证实了此结论。
-
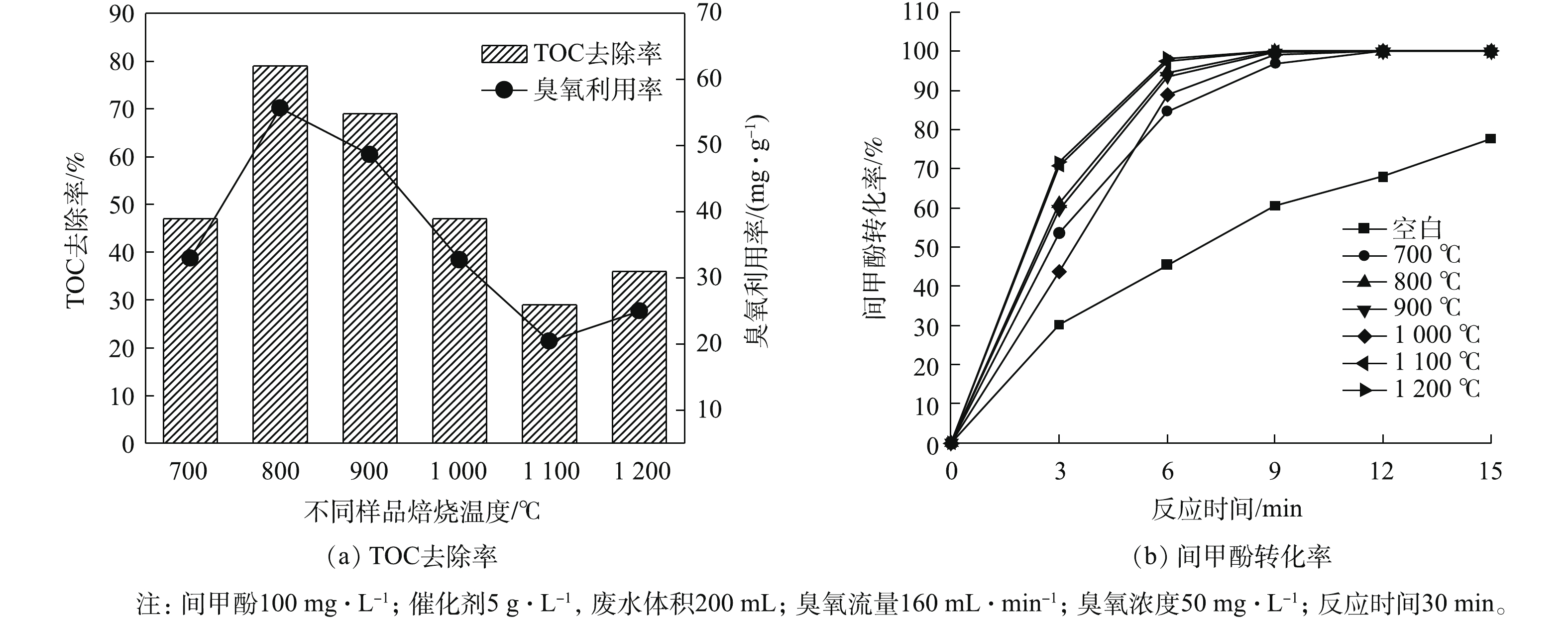
通过对样品的一系列表征发现,焙烧温度对催化剂的表面结构和性质具有显著的影响。为探究焙烧温度对催化剂催化臭氧氧化活性的影响,以间甲酚为底物,使用催化臭氧氧化方法对催化效果进行了评价,结果如图5所示。由图5可知,所合成的样品具有明显的催化活性,且随焙烧温度的升高,催化剂活性呈现出先升高后降低的趋势。其中单独臭氧反应TOC去除率为15%,臭氧利用率在10%左右。在焙烧温度为800 ℃时,TOC的去除率达到了79%,同时臭氧的利用率与TOC的去除率变化趋势相一致。即使对TOC去除率最低的样品也优于单独臭氧反应体系所对应的去除率。结合图1可知,当焙烧温度为800 ℃时,认为样品为ZrO2、Ca(OH)2和CaZrO3的复合材料,可能是三者的共同作用导致材料具有较高的催化活性。当焙烧温度升高到1 100 ℃时,CaZrO3晶型占比升高,Ca(OH)2晶型消失。通过催化臭氧氧化间甲酚结果可知其TOC的去除率有所下降,说明ZrO2和CaZrO3的复合材料不利于催化臭氧氧化反应。在1 200 ℃下焙烧的样品相较于1 100 ℃焙烧样品TOC去除率有所上升,可能样品中ZrO2含量的降低有利于臭氧反应。在催化臭氧氧化反应过程中,间甲酚转化率结果如图5(b)所示。由图5(b)可知,相较于单独的臭氧反应,加入样品均有利于间甲酚的催化氧化,效果最佳的为在1 100 ℃ 和1 200 ℃下焙烧的样品,这说明CaZrO3在间甲酚转化方面表现优异。在800 ℃和900 ℃下焙烧出的复合材料对间甲酚的转化率仅次于以上2种条件下所制备的样品,说明Ca-Zr复合材料对间甲酚的转化表现出较好的性能。
-
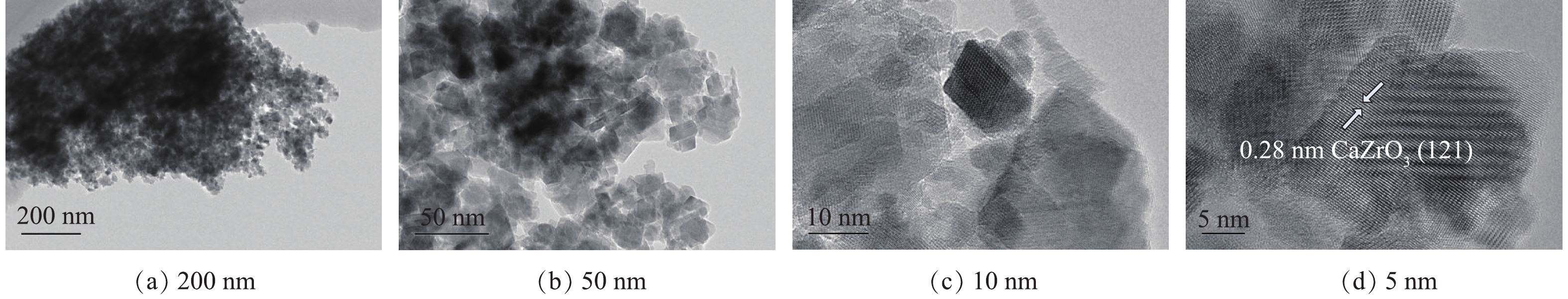
由于在800 ℃焙烧出的样品催化臭氧氧化活性最佳,为进一步确定该材料的表面形貌,使用高分辨透射电镜(HRTEM)对该条件下的样品进行表征,结果见图6。由图6(a)和6(b)可知,所制备复合材料样品由许多不规则的纳米颗粒组成。通过对选定的区域进行观察,发现了纳米颗粒的晶格图像(图6(c)和图6(d)),经过分析计算,样品具有0.29 nm的特征晶格间距,这说明样品的高暴露晶面为CaZrO3的(121)晶面。
-
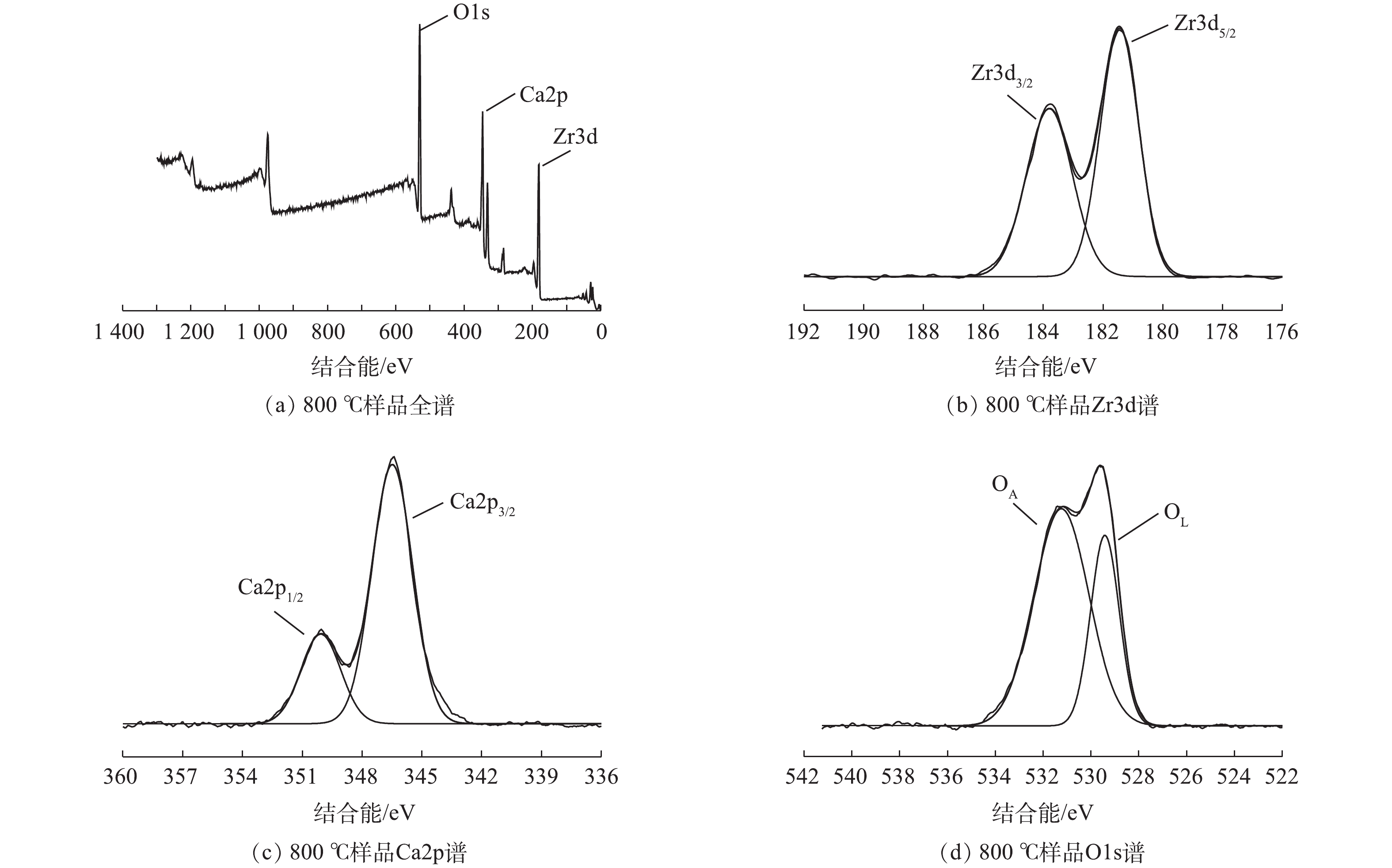
为了进一步探究所制备样品表面的元素状态,对其进行XPS表征,结果见图7。将Zr3d图谱解卷积成2个峰,其结合能分别为183.7 eV和181.4 eV,其分别对应Zr3d3/2和Zr3d5/2,这是由CaZrO3中的阳离子Zr4+引起的。将Ca2p图谱解卷积成2个峰,其结合能分别为350.0 eV和346.5 eV,其分别对应Ca2p1/2和Ca2p3/2。这些峰是由于Ca2+氧化态所导致的[30]。样品O1s图谱分别在结合能为529.4 eV和531.1 eV处出现峰值,分别归因于表面晶格氧(OL)、表面吸附氧(OA)或表面羟基(OH)物种[15, 31]。晶格氧和表面羟基在氧化反应中起重要作用。晶格氧失去电子,被氧化成O2并形成氧空穴,在富氧状态下,氧空穴导电被还原成晶格氧,两者之间的循环转变确保了催化活性[32]。另一方面,可能是由于表面羟基基团在起重要作用。
2.1. 物相分析(XRD)
2.2. 形貌(SEM)结果分析
2.3. 热重分析(TG-DTA)
2.4. 程序升温还原(H2-TPR)分析
2.5. 焙烧温度对催化臭氧氧化间甲酚的影响
2.6. 高分辨透射电镜(TEM)
2.7. X射线光电子能谱分析(XPS)
-
1)合成复合材料的XRD分析结果表明:当焙烧温度≤1 000 ℃时,为Ca(OH)2、CaZrO3和ZrO2的复合材料;当焙烧温度≥1 100 ℃时,为ZrO2和CaZrO3的复合材料。通过形貌(SEM)分析,制备出的样品随着焙烧温度的上升,颗粒粒径随之改变,呈现出先增大后变小的趋势,并且逐渐出现均匀的现象。与物相分析结果相吻合,焙烧温度对样品的制备有着较大影响。热重分析证实了这一点,焙烧温度应在800 ℃以上,CaZrO3晶型占比逐渐升高。H2-TPR结果表明O3对催化剂结构产生影响较小。
2)以间甲酚的催化臭氧氧化降解反应作为探针反应,对合成出的催化剂性能进行评价。结果表明,所制备催化剂显示出较好的催化活性,焙烧温度在800 ℃时,所得样品TOC的去效果最优,去除率达到79%以上。
3)对800 ℃焙烧的样品进行TEM分析,结果显示,样品由纳米颗粒组成,其晶格间距为0.29 nm,即高暴露晶面为CaZrO3(121)晶面。对样品进行XPS表征,分析其表面的化学组成,结果显示,在催化臭氧氧化过程中,晶格氧与表面羟基物种起到重要的作用。



 下载:
下载: