-
氮氧化物(NOx)会造成地面臭氧、酸雨、富营养化等一系列环境问题,也可对人体造成直接危害。传统的NH3选择性催化还原NO(NH3-SCR)技术广泛应用于固定源脱硝,但其存在氨腐蚀、泄漏等诸多问题,易对环境造成二次污染。烃类选择性催化还原NO(HC-SCR)是一种新型烟气脱硝技术,其优点在于烃类化合物HC既是尾气中的燃烧产物,又可作为消除NO的还原剂,可实现2种污染物的同时消除,故具有较好的研究价值和应用潜力。近年来,以黏土材料为原料,通过金属氧化物在黏土层间形成“支柱”从而制备的柱撑黏土(pillared clay, PILC)类催化剂受到了研究人员的广泛关注。柱撑黏土材料是一种由金属阳离子通过离子交换取代黏土原料的层间阳离子从而得到的一种微孔材料。经高温焙烧后,层间金属阳离子转化为具有体相氧化物结构的金属氧化物纳米颗粒,这些颗粒固定在黏土的层间(也就是所谓“柱”),使黏土层间形成微观孔道。柱撑黏土具有孔隙结构,其比表面积大且水热稳定性强,是一种优良的催化剂载体[1-2]。国内外研究者针对金属修饰柱撑黏土类催化剂进行了大量的研究,发现该类催化剂具有较好的HC-SCR催化性能,研究结果如表1所示。通过比较可以发现,课题组前期制备并研究的铁修饰铝柱撑黏土(Fe/Al-PILC)催化剂表面的NO转化率高且反应温度低[11],说明Fe/Al-PILC具有很好的C3H6-SCR催化活性,值得进一步深入研究。
蒙脱土[(MxnH2O)(Al2-xMgx)(Si4O10)(OH)2]为一种具有2∶1型片层状硅铝酸盐结构的层状黏土,其单元层由2层Si—O四面体中间夹着一层Al-O八面体构成。蒙脱土结构比较松散,结构单元层之间以分子力连接,可制备不同性质的柱撑黏土催化剂。在蒙脱土的四面体片中,部分Si4+可被A13+置换。当黏土片层所带的电荷密度一定时,黏土层间距将会随着金属柱撑阳离子体积的增大而扩大,从而获得不同孔径的孔隙结构。Al-PILC柱撑黏土是通过AlCl3水解产生的[Al13O4(OH)24(H2O)12]7+,与蒙脱土层间吸附的阳离子之间进行离子交换制得的[12]。在锻烧过程中,这些[Al13O4(OH)24(H2O)12]7+无机阳离子在高温下经过脱水反应,在蒙脱土层间形成稳定的Al2O3金属氧化物簇,这些Al2O3簇像柱子一样矗立于单元结构层之间,从而增加了蒙脱土的层间距使得载体材料(Al-PILC)的比表面积大幅提高。在制备过程中,Al3+与黏土原料的比例或黏土层间负载的Al3+的量将影响Al-PILC柱撑黏土的物理化学性质,从而影响其脱硝的催化活性。此外,前期的研究[13]发现,不同的焙烧温度对催化剂的孔隙结构和表面活性组分的晶体形态造成了一定的影响。因此,本研究在前期研究工作的基础上,研究了柱撑Al3+与黏土比例和焙烧温度这2个因素对Fe/Al-PILC催化剂表面C3H6-SCR性能的影响,研究结果对于进一步优化催化剂的合成工艺具有一定的参考意义。
全文HTML
-
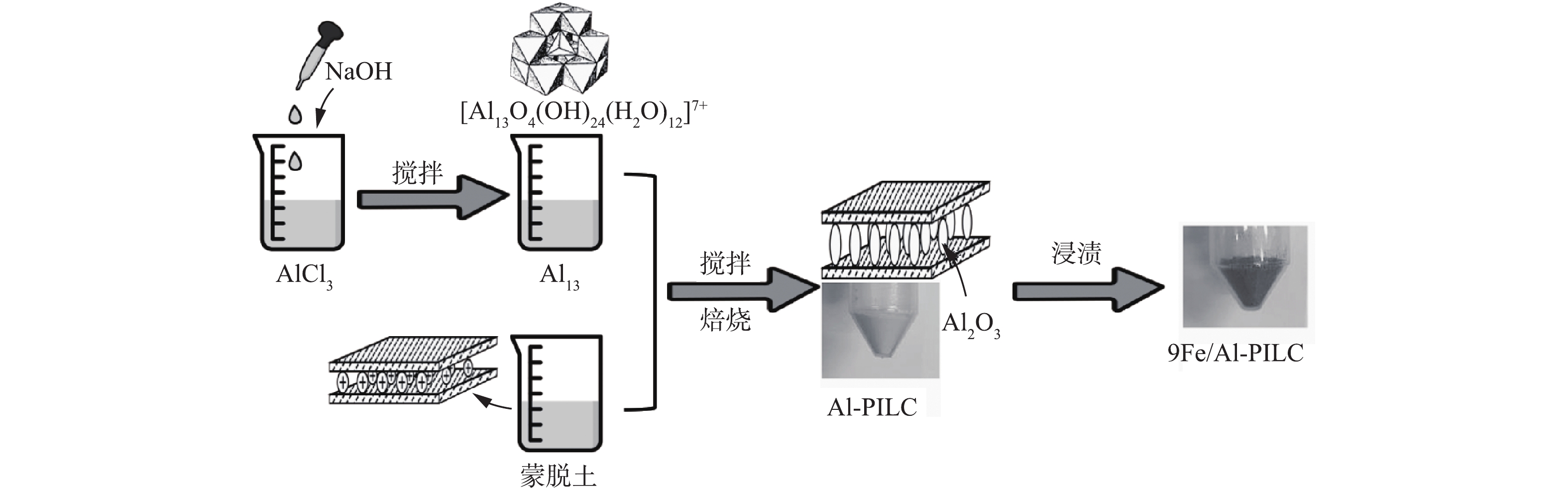
Fe/Al-PILC催化剂的制备过程如图1所示。铝柱撑剂的制备方法参考之前的研究[11]。配置浓度为1.6 mol·L−1的NaOH溶液,将其滴加到浓度为0.8 mol·L−1的AlCl3溶液中,使得OH−/Al3+=2.0,在滴加过程中,须持续搅拌混合溶液,最终获得铝溶胶,作为金属离子柱撑剂。
在制备Al-PILC时,将金属离子柱撑剂缓慢加入到一定浓度的蒙脱土悬浮溶液中,将Al3+与蒙脱土原料的比例(下文简称Al3+/蒙脱土)控制在5~40 mmol·g−1,接着在室温下剧烈搅拌24 h,使Al3+通过离子交换作用进入到蒙脱土层间。然后将混合物离心分离,去离子水洗涤至无Cl−,干燥后,在400~700 ℃焙烧5 h得到Al-PILC-X-T,其中:X为Al3+/蒙脱土的摩尔比值(X=5,10,20,40),T为焙烧温度(T=300、500、700 ℃)。
在制备催化剂Fe/Al-PILC时,本研究使用了浸渍法负载金属活性组分。室温下,将Al-PILC载体浸入不同浓度的Fe(NO3)3溶液中,剧烈搅拌24 h后离心分离,最后置于马弗炉中,在500 ℃下焙烧5 h,得到mFe/Al-PILC-X催化剂样品(m为催化剂中铁的质量百分比,根据前期研究结果[11],本研究的催化剂中铁的质量分数大约为9%)。
-
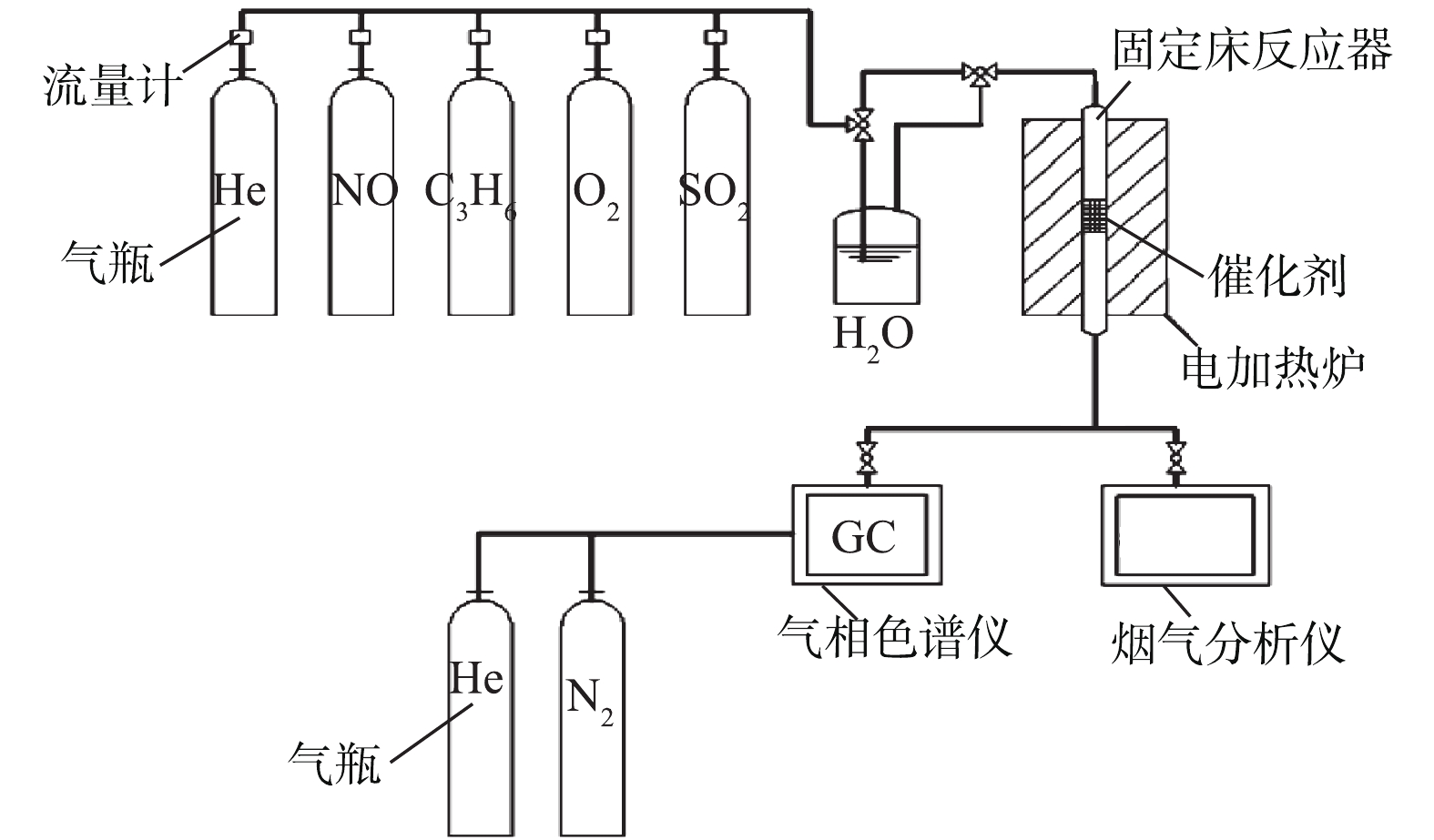
催化剂的C3H6-SCR实验系统如图2所示。反应系统包括气体控制系统、微反应器与气体在线检测系统。不同反应气体由质量流量计单独控制流量。固定床微反应器为竖直放置石英管,内径8 mm,催化剂样品(0.4 g)放置于反应器中部,气体流向向上。反应温度为200~550 ℃,反应气体组分包括0.3% C3H6、0.05% NO、1% O2、He配平,总流量为200 mL·min−1,空速为18 000 h−1。烟气分析仪(ECOM-J2KN, Germany)装备电化学传感器,用以检测出口气体中的NO、NO2与NOx浓度。NO测量范围为0~0.5%,精度为±0.000 5%,分辨率为0.000 1%;NO2测量范围为0~0.1%,精度为±0.000 5%,分辨率为0.000 1%。C3H6与N2浓度分别由气相色谱仪(GC-4000A)配备的KB-Al2O3/Na2SO4毛细管柱火焰电离检测器(FID)和5A分子筛柱热导检测器(TCD)进行在线监测。
-
催化剂样品的比表面积大小与孔分布范围通过氮气吸附-脱附 (BET) 实验进行分析,采用了Micromeritics 公司的ASAP2460型吸附仪进行测试,比表面积大小通过BET方程计算, 孔容与孔径分布则采用BJH模型计算,测试前,催化剂在N2气氛中300 ℃下处理3 h。X射线衍射(XRD)表征由Rigaku公司的D/max-2550PC分析,目标靶为Cu Kα辐射源(λ=0.154 168 nm)。ICP表征运用电感耦合等离子体发射光谱测量,仪器型号为美国Leeman公司生产的Prodigy型电感耦合等离子体原子发射仪,分辨率≤0.005 nm,精密度(RSD)(n=10)<1.0%。H2-TPR表征由气相色谱仪GC-4000A测试,热导检测器TCD。每次实验样品用量200 mg。实验前,样品在300 ℃的N2气氛中预处理30 min。待样品冷却至室温后通入N2/H2(20 mL·min−1,5% H2),升温速率3 ℃·min−1,升温至805 ℃,每升温15 ℃进行采样。紫外吸收光谱(UV-vis)由UV3600仪器测试,测量波长为200~800 nm,测试过程运用漫反射积分球的方法进行测定。吡啶吸附红外光谱(Py-IR)由美国PE公司生产的FT-IR Frontier型仪器检测,在吡啶吸附前,样品在500 ℃真空中预处理1 h,降温后采集样品背景。接着降温至40 ℃,吡啶吸附催化剂15 min。然后进行20 min脱附过程,吸附较弱的吡啶分子在真空环境中逐渐脱离样品表面。脱附温度分别为150 ℃和300 ℃。
-
NO转化率、C3H6转化率、N2选择性计算方法见式(1)~式(3)。
式中:RNO为NO转化率;
$R_{{\rm{C}}_3{\rm{H}}_6} $ 为C3H6转化率;$S_{\rm{N}_2} $ 为N2选择性;$C_{{\rm{NO}},{\rm{in}}} $ 为进口NO浓度;CNO, out为出口NO浓度;$R_{{\rm{C}}_3{\rm{H}}_6,{\rm{in}}} $ 为进口C3H6浓度;$R_{{\rm{C}}_3{\rm{H}}_6,{\rm{out}}} $ 为出口C3H6浓度;$C_{\rm{N}_2,{\rm{out}}} $ 为出口N2浓度。
1.1. 催化剂的制备
1.2. 催化剂的活性测试
1.3. 催化剂的表征分析
1.4. 计算方法
-
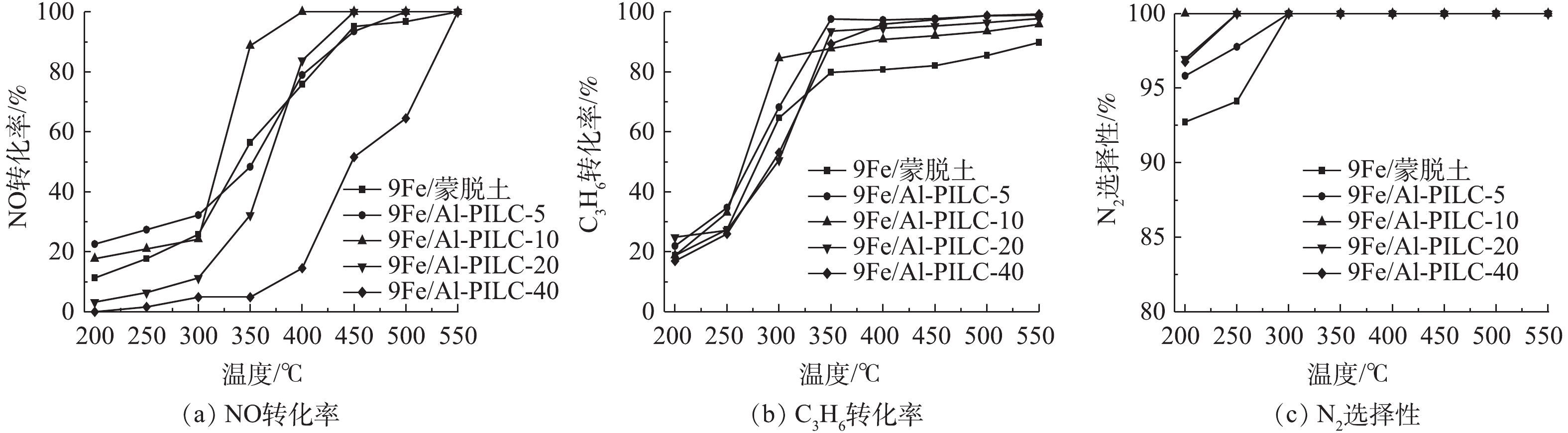
图3反映了不同Al含量的9Fe/Al-PILC系列催化剂的NO转化率、C3H6转化率和 N2选择性随反应温度的变化结果。当温度<300 ℃时,催化剂SCR活性较低;而在300~400 ℃时,脱硝效率迅速上升;在400 ℃时,脱硝效率可达100%,并可持续保持至550 ℃。当催化剂样品的Al3+/蒙脱土由5 mmol·g−1增加到20 mmol·g−1时,400 ℃时的最大NO转化率有所提高;而当Al3+/蒙脱土增加到40 mmol·g−1时,NO的转化率明显下降。由此可见,当Al3+/蒙脱土为10 mmol·g−1时,NO的转化率最高。Fe/Al-PILC催化剂对C3H6-SCR的转化率曲线具有相似的变化规律,当反应温度为250~350 ℃时,催化活性相较高于Fe/蒙脱土催化剂(Fe直接负载于未经柱撑的蒙脱土原样)。在200~300 ℃时,有最高的N2选择性的催化剂是9Fe/Al-PILC-10。具有不同Al加入量的催化剂(焙烧温度为500 ℃)选择性催化还原NO的活性高低排序为9Fe/Al-PILC-10>9Fe/Al-PILC-20>9Fe/Al-PILC-5>9Fe/蒙脱土>9Fe/Al-PILC-40。
-
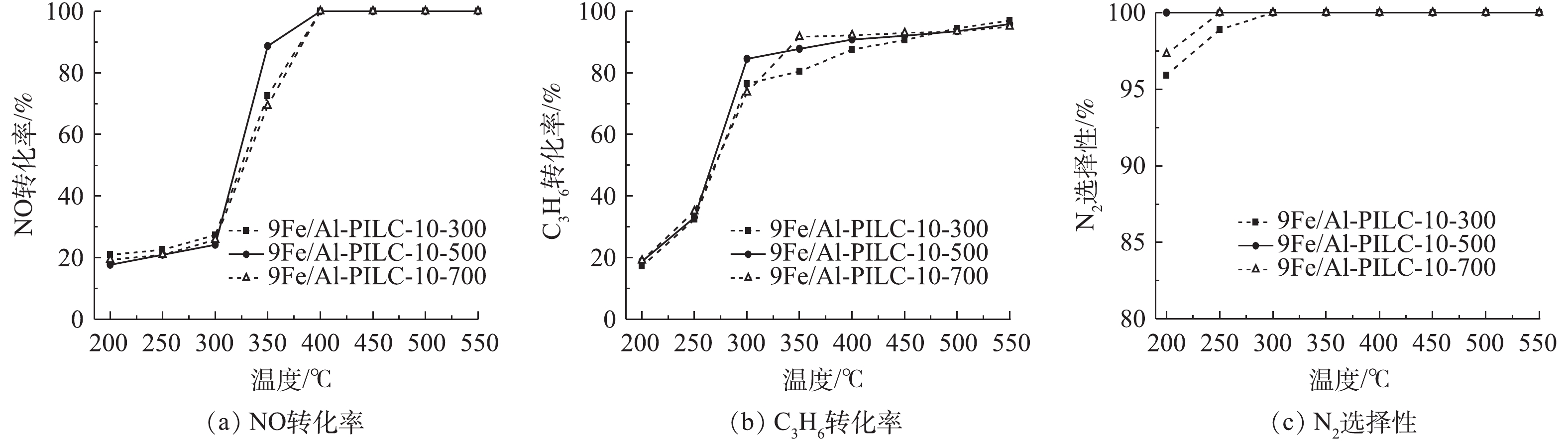
柱撑黏土载体在高温焙烧过程中会形成孔隙结构,这有助于催化活性组分的在载体表面的均匀分布。焙烧温度不仅会影响柱撑黏土层间金属氧化物簇的形态和结构,也会影响层间金属氧化物活性组分的存在形式。有的样品层间结构在较高的温度下可能会发生坍塌。因此,研究不同的焙烧温度对Fe/Al-PILC催化剂的SCR催化性能影响是有必要的。选用活性最佳的Al3+/蒙脱土 (Al3+/蒙脱土=10),并在不同温度下(300、500、700 ℃)焙烧制备了催化剂载体,负载活性组分Fe后进行催化剂的活性测试,结果如图4所示。由图4(a)可知,在300~400 ℃催化剂样品的脱硝效率上升较为明显,当温度达到400 ℃时,最大NO转化率可达100%,并可持续保持至550 ℃。此外,结果表明,在500 ℃焙烧下的9Fe/Al-PILC-10-500催化剂在350 ℃取得85%的NO转化率,相比其他2组催化剂样品,脱硝率略高。在200~300 ℃时,500 ℃焙烧的9Fe/Al-PILC-10-500催化剂样品具有最佳的N2选择性(图4(c))。
-
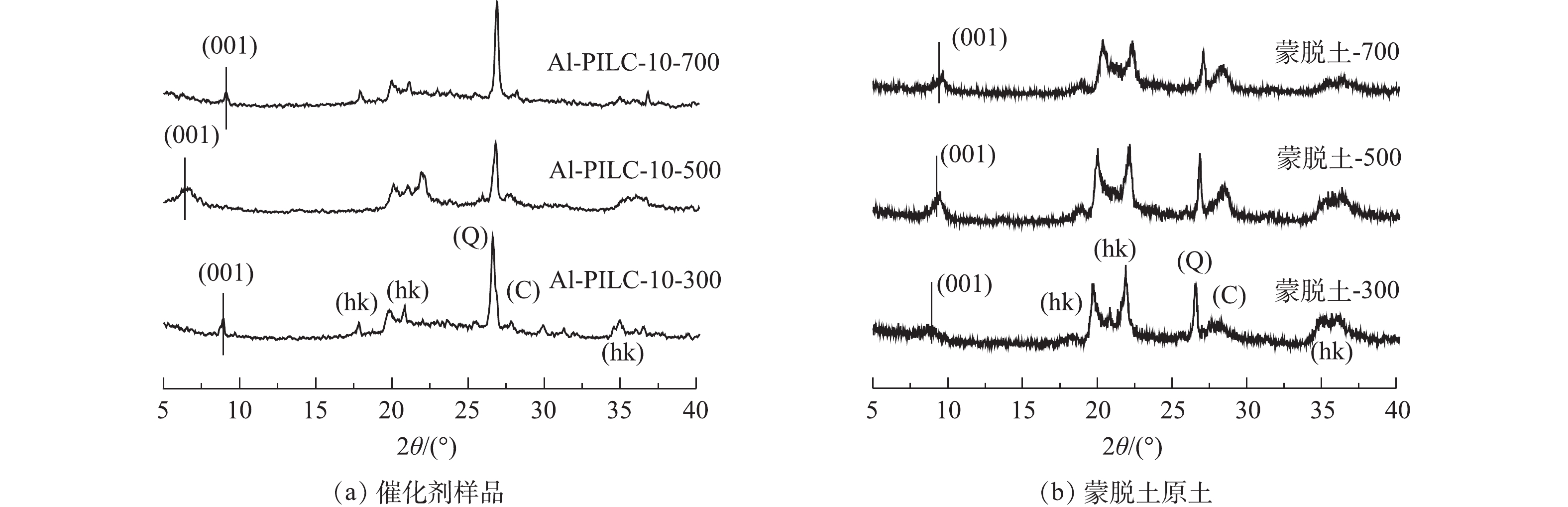
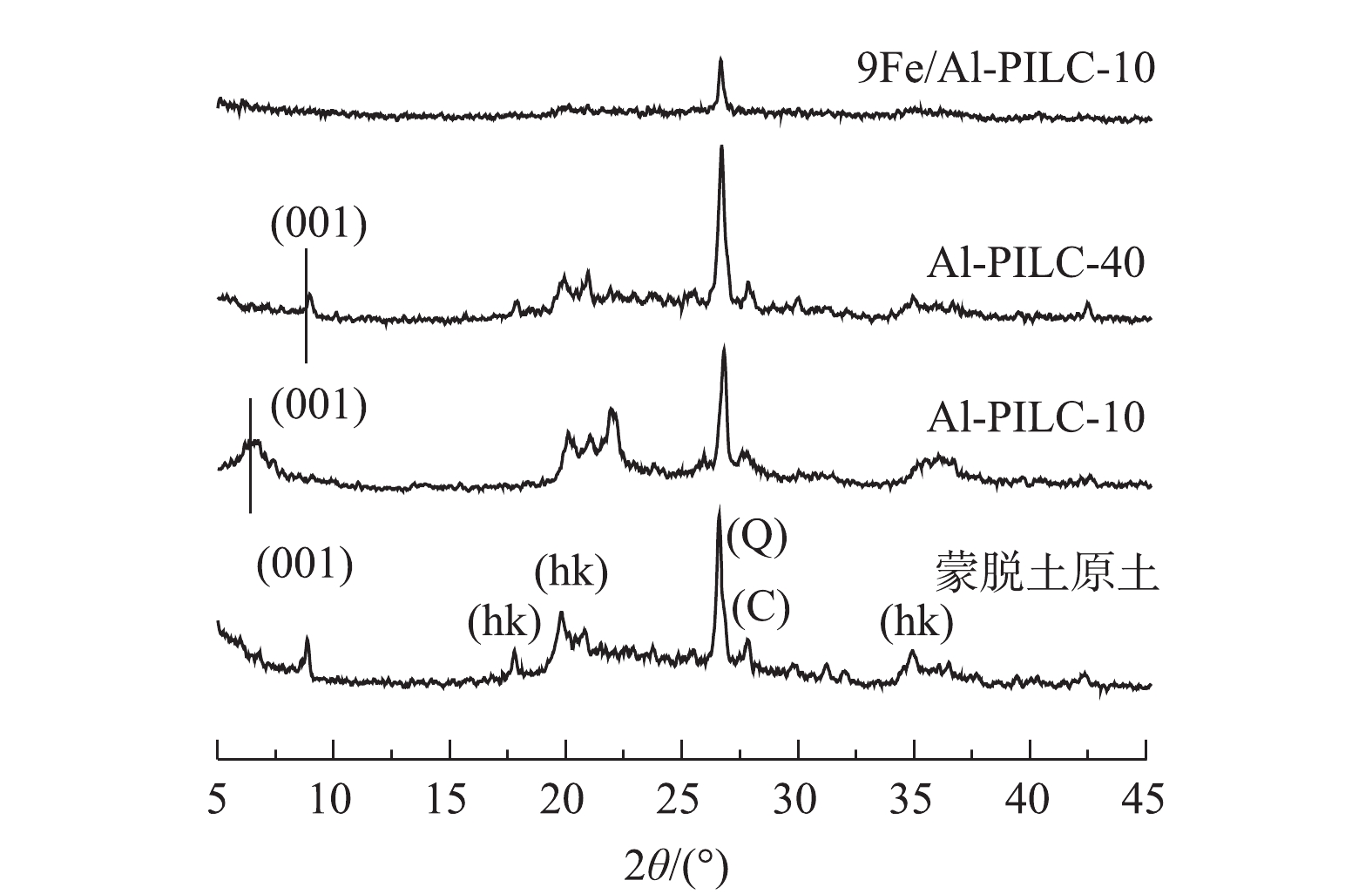
图5(a)反映了在300、500、700 ℃下焙烧的Al-PILC-10的XRD测试结果。可以看出,在不同的焙烧温度下所制备的Al-PILC-10载体均保持蒙脱土原有的晶形结构。在XRD谱图中,存在蒙脱土原样的d(001)晶面衍射峰,衍射角2θ为18°与35°附近的特征峰对应于蒙脱土原样的(hk)晶面的衍射峰。此外,衍射角2θ位于26.6°和27.9°的特征峰分别对应于石英(101)晶面(Q)和白硅石(C)的衍射峰[14]。Al-PILC-10-500载体的d(001)晶面特征衍射峰向低衍射角方向偏移至2θ为6.7°,对应层间距为1.31 nm。然而,Al-PILC-10-700样品与Al-PILC-10-300样品的d(001)晶面特征衍射峰分别位于9.1°与8.9°,对应的层间距分别为0.97 nm与0.99 nm。考虑到蒙脱土的晶层厚度约为0.96 nm,所以在300 ℃和700 ℃下焙烧的Al-PILC载体层间距离较小,仅有500 ℃下焙烧的Al-PILC-10载体的层间结构被Al2O3柱有效地撑开,形成柱撑黏土结构。作为对比,蒙脱土原土在300、500、700 ℃下焙烧后的XRD谱图如图5(b)所示。可以发现,经700 ℃焙烧后,蒙脱土仍能保持原有晶体结构,这说明焙烧温度主要对铝柱撑黏土(Al-PILC)层间的氧化物柱产生影响。有研究[10]表明,当焙烧温度较高时,蒙脱土层间的氧化物柱结构容易遭到破坏,从而导致层间柱撑塌陷。此外,300 ℃下焙烧的Al-PILC载体也未形成有效柱撑,这可能是由于在该温度下层间[Al13O4(OH)24(H2O)12]7+离子未能完全脱羟基形成高强度的Al2O3柱,导致了层间柱撑的失败。在图4催化剂活性测试结果中,9Fe/Al-PILC-10-500的催化活性最高, 由于Al-PILC-10-300与Al-PILC-10-700层间柱撑塌陷导致制备的催化剂C3H6-SCR活性下降。
图6反映了500 ℃焙烧的铝柱撑黏土及负载铁后的催化剂的XRD结果。由图6可以发现,Al-PILC-10样品的d(001)峰位置相较Al-PILC-40样品明显向左偏移,这说明Al-PILC-10样品具有较大的层间距,柱撑效果更佳。此外,柱撑后的Al-PILC载体在20°附近依旧出现了蒙脱土的二维(hk)晶面的衍射特征峰,这说明蒙脱土层状结构未被破坏。在9Fe/Al-PILC-10催化剂的XRD谱图中未能找到明显的铁相应氧化物衍射特征峰,这表明催化剂表面的活性组分铁物种分散均匀或以无定形态氧化铁物种存在。
-
通过ICP表征,检测了不同铝柱撑剂加入量的Al-PILC载体(500 ℃焙烧)中Al元素的含量,结果如下:蒙脱土原土(29.05 mg·g−1)、Al-PILC-5 (57.68 mg·g−1)、Al-PILC-10 (119.84 mg·g−1)、Al-PILC-20 (109.35 mg·g−1)、Al-PILC-40 (107.56 mg·g−1)。当Al3+/蒙脱土比例由5 mmol·g−1增大到10 mmol·g−1时,蒙脱土中Al3+的含量增大;当继续增大到40 mmol·g−1时,蒙脱土中Al3+的含量并未继续增大。结合Al-PILC-10与Al-PILC-40的XRD结果(图6),可以看出,这是由于柱撑液中的Al3+/蒙脱土比增大至40 mmol·g−1时,溶液中形成的[Al13O4(OH)24(H2O)12]7+物种容易发生团聚,阻碍其通过离子交换进入蒙脱土层间。这也就解释了Al-PILC-40层间距小,柱撑效果不佳的原因。
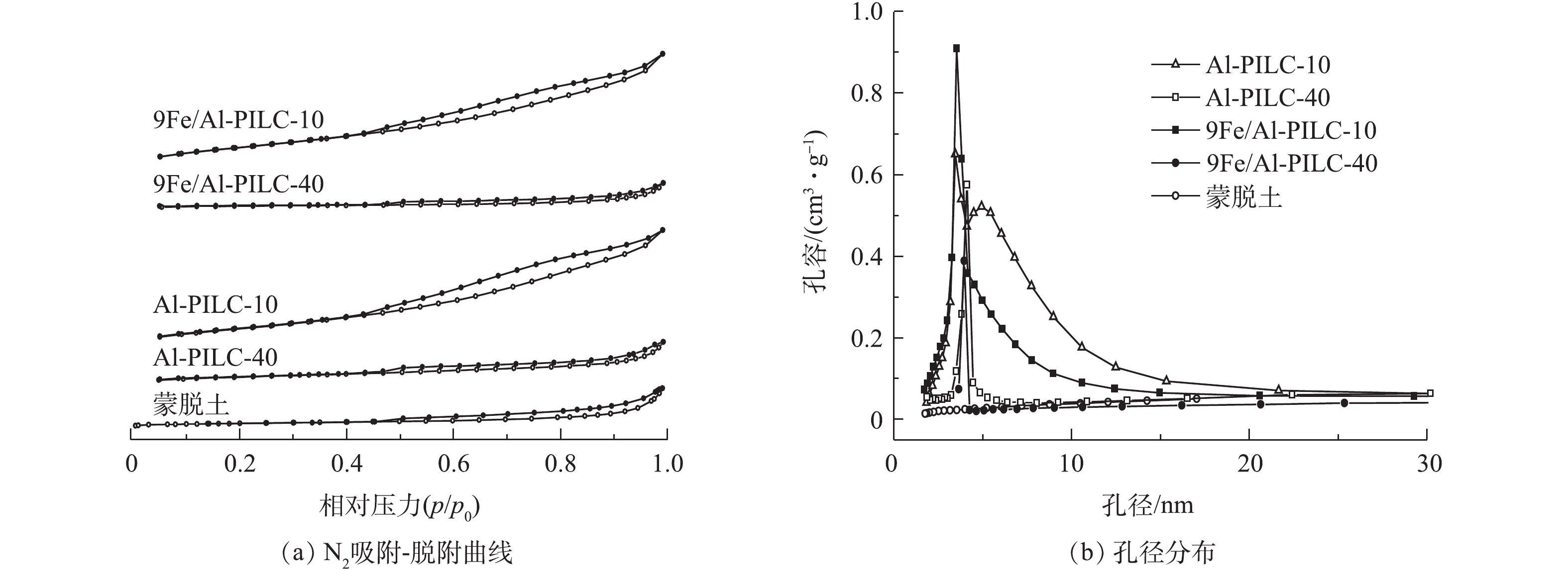
图7反映了吸附-脱附曲线与孔径大小分布测试结果。图7(a)为在500 ℃焙烧的催化剂以及载体的N2吸附脱附等温曲线。样品的吸附等温曲线属于IV型吸附等温线,这说明Fe/Al-PILC催化剂中存在微孔与介孔结构[15]。在蒙脱土原样的吸附等温线中,当相对压力p/p0<0.8时,吸附量几乎不随相对压力的增加而增加,呈现出吸附饱和,表明材料的孔道较小或为无孔结构[16];当p/p0>0.8时,蒙脱土原料的N2吸附量明显增加,直至接近饱和蒸汽压也未出现吸附饱和现象,这说明较高相对压力下出现了毛细孔凝聚,然而由于蒙脱土的孔容较小,仅为0.099 cm3·g−1(表2),所以毛细孔凝聚导致的吸附量增加并非十分明显。蒙脱土经过Al3+柱撑后的吸附等温线变化较为明显,由图7(a)可知:当相对压力p/p0<0.4时,柱撑黏土载体(Al-PILC-10和Al-PILC-40)的吸附等温线较为平缓,这表明N2在载体的微孔道中发生多层吸附和毛细管凝聚;而当p/p0>0.4时,微小孔道中N2充满后吸附量上升较为明显。
脱附过程在高压部分与吸附相比出现一定的滞后,脱附等温线中的滞后环属于H3型,孔结构为双锥形管状毛细孔[16]。当p/p0为0.40~0.45时,出现了吸附与脱附曲线的闭合现象, 说明材料内部孔存在部分堵塞的毛细管状孔道结构[17]。此外,我们着重比较了不同Al3+/蒙脱土比例制备的催化剂载体Al-PILC-10与Al-PILC-40样品后发现,前者具有较高的Al含量和比表面积,故Al-PILC-10的柱撑效果较好。从结果中还可以发现,负载活性组分铁后,9Fe/Al-PILC-10比表面积基本不变,孔容和平均孔径下降,这可能是由于铁物种进入到Al-PILC-10的孔道内部造成的。样品的孔径分布如图7(b)所示。可以看出,催化剂及其载体的孔径分布均集中在3~5 nm,且孔隙结构可能以介孔为主。
通过比较可以发现,Al-PILC-10样品具有最大的孔容与比表面积:一方面,这有利于反应过程中气体分子的吸附;另一方面,有利于活性组分较为分散地负载于载体表面,暴露更多的反应活性位点,从而提高催化剂的催化性能。结合催化剂活性的测试结果,以Al-PILC-10为载体制备的9Fe/Al-PILC-10催化剂表现出最佳的C3H6-SCR活性是合理的。
-
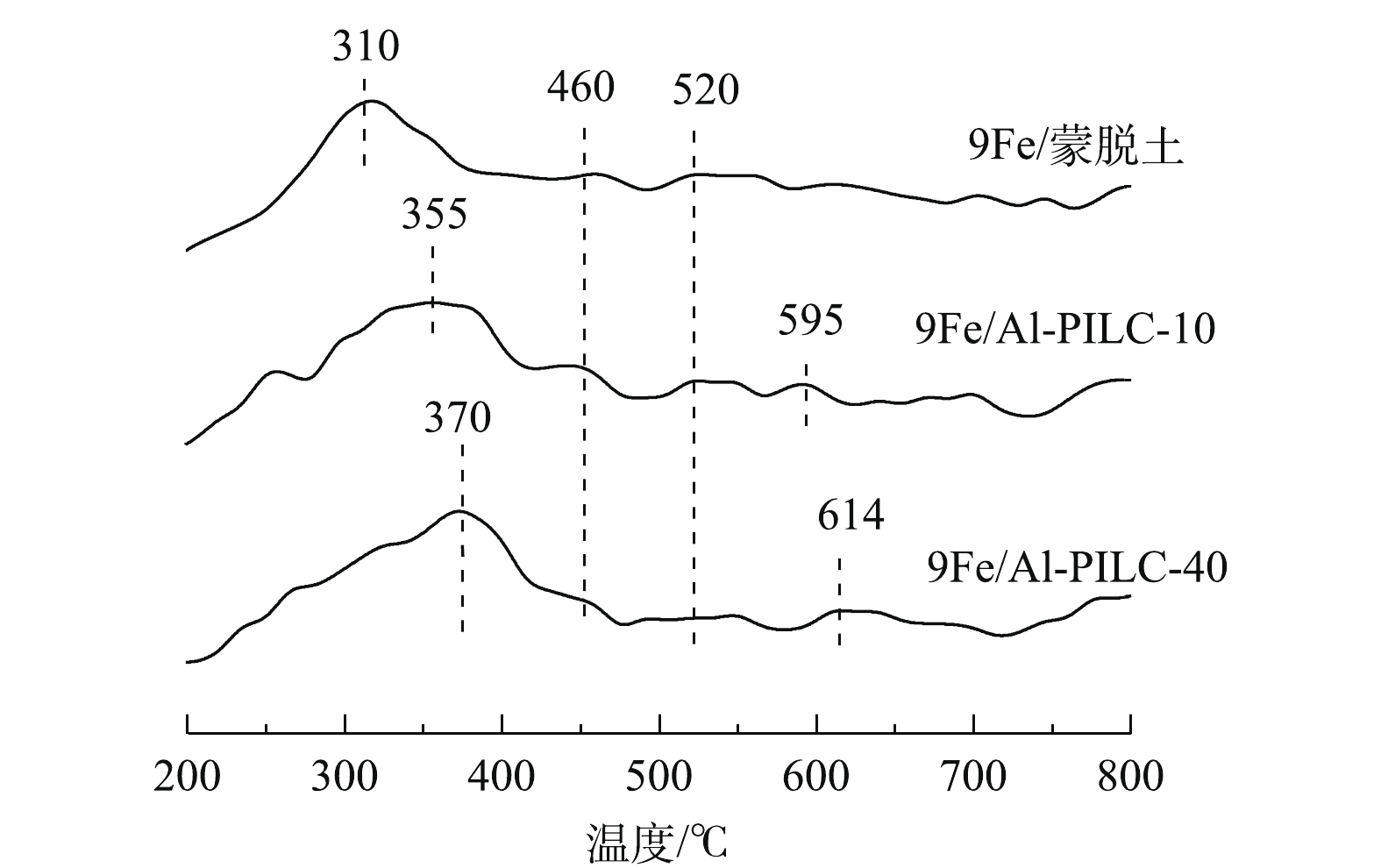
图8反映了500 ℃焙烧的催化剂的H2-TPR表征结果。以不同Al3+/蒙脱土比制备的Al-PILC为载体,负载活性组分铁的9Fe/Al-PILC催化剂有较为相似的TPR谱图。可以看出,各个催化剂样品的H2还原主峰均位于350 ℃附近,对应于Fe2O3→Fe3O4的还原反应。此外,520 ℃和595 ℃附近的还原峰分别对应于Fe3O4→FeO→Fe的还原反应,而位于460 ℃左右的还原峰可能是由催化剂载体骨架中部分难以被还原的Fe3+离子(Fe—O—Si键)造成的。OLIVEIRA等[18]发现,对于α-Fe2O3的H2-TPR图谱,460 ℃附近的还原峰对应的Fe3+→Fe2+还原过程。由图8可知,350 ℃附近的H2还原峰温度高低顺序为9Fe/Al-PILC-40>9Fe/Al-PILC-10>9Fe/蒙脱土。其中,9Fe/蒙脱土样品具有较低的还原峰温度,这是由于Fe直接负载于未经柱撑的蒙脱土原样,会造成大量Fe2O3物种团聚。VALVERDE等[3]研究发现,表面发生CuO团聚现象的Cu修饰Ti柱撑黏土催化剂会具有较低的H2-TPR还原峰温度,这是由于团聚的金属氧化物颗粒体积大,易于被H2还原。
-
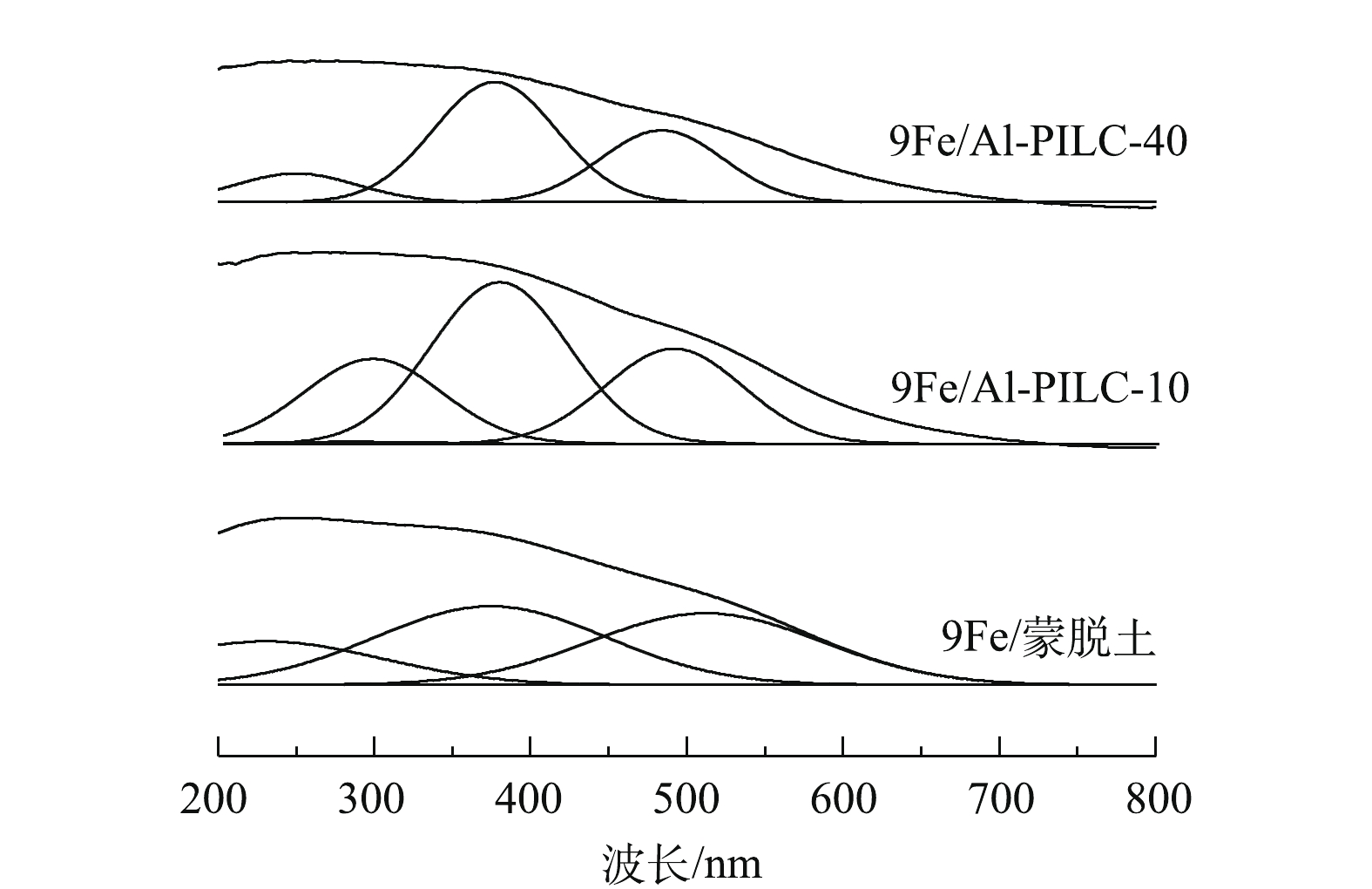
图9为500 ℃焙烧催化剂样品的UV-vis谱图。由图9可知,催化剂中存在3类铁物种。其中,在波长位于200~300 nm,中心位置约255 nm宽带的特征峰对应Fe3+离子与四面体氧配体间的dП-pП低能荷移跃迁;300~400 nm宽带的特征峰代表八面体配位低聚态FexOy物种;而高于400 nm宽带区域的特征峰则对应高聚态Fe2O3簇物种。通过分峰拟合发现,不同催化剂样品表面低聚态FexOy物种对应吸收峰强度顺序为9Fe/Al-PILC-10>9Fe/Al-PILC-40>9Fe/蒙脱土。相关研究[19]发现,催化剂表面的低聚态FexOy物种主要影响催化剂的中低温SCR活性,这可解释H2-TPR表征结果中9Fe/Al-PILC-10相较9Fe/Al-PILC-40催化剂样品还原峰温度较低的原因。结合图3中催化剂活性测试结果可以推测,9Fe/Al-PILC-10催化剂样品中形成的大量低聚态FexOy物种有助于提升催化剂的C3H6-SCR活性。
-
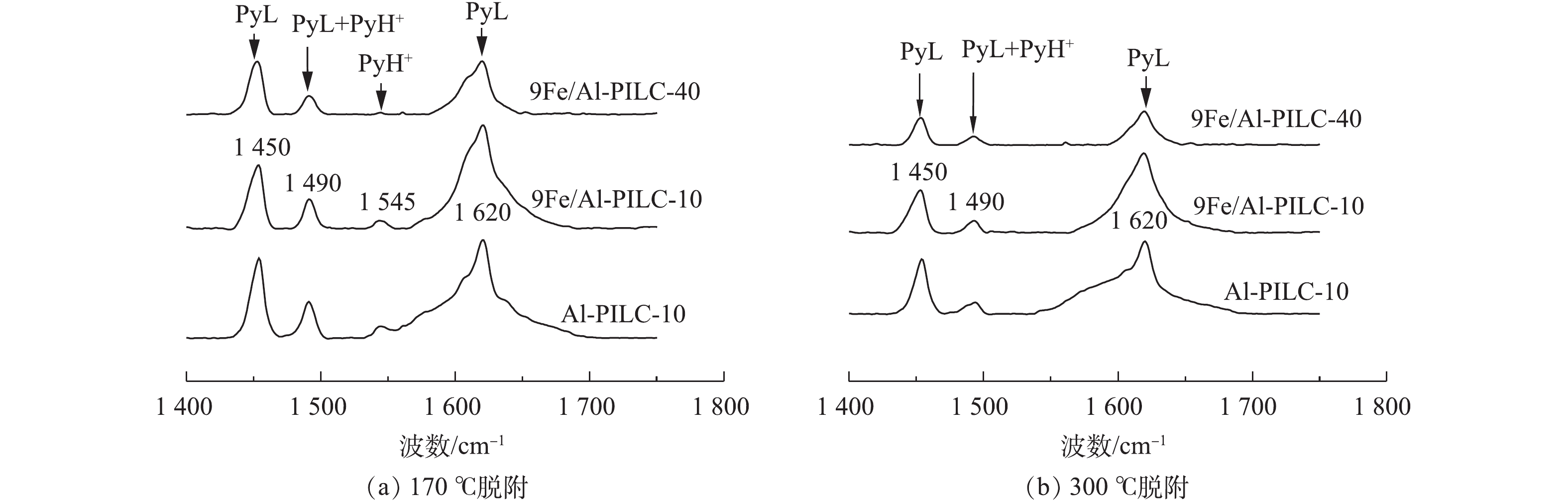
催化剂表面的酸性位特性可通过吡啶吸附红外光谱技术分析。500 ℃焙烧的催化剂样品的Py-IR谱图如图10所示,图10(a)与图10(b)分别对应脱附温度为150 ℃与300 ℃时的测试结果。位于1 450 cm−1和1 620 cm−1宽带处的特征峰对应于催化剂表面Lewis酸性位点与吡啶分子形成的PyL吸收峰,并且Lewis酸性位点数量与1 450 cm−1宽带处特征峰强度呈正比关系。位于1 545 cm−1宽带处的特征峰对应于催化剂表面Brønsted酸性位点与吡啶分子所形成的PyH+吸收峰,同理,Brønsted酸性位点数量与1 545 cm−1处的吸收峰强度呈正比关系。此外,位于1 490 cm−1处的特征峰为Lewis与Brønsted酸性位点的共同峰。
YANG等[20]首先报道了在HC-SCR反应中,柱撑黏土催化剂表面的Brønsted酸可促进烃类化合物的活化。MOSQUEDA等[21]提出Brønsted酸性位是分子筛催化剂表面烃类脱氢活化的反应位点。根据已有研究[22],高效的HC-SCR催化剂应同时含有活性金属(金属氧化物)以及高强度的Brønsted酸性位点。LI等[23-24]的研究结果表明, Lewis酸和Brønsted酸都有利于选择性催化还原NO。由表3可知,蒙脱土原料表面几乎不存在Brønsted酸性位点[25],然而经过Al3+柱撑后的Al-PILC-10材料表面Brønsted酸量明显有所增加,这是由于在柱撑黏土层间形成了大量≡Si—O—Al≡结构[10]。进一步在载体表面负载活性组分Fe后,可观察到9Fe/Al-PILC-10催化剂表面的Brønsted酸量再次增加,这可能与≡Fe—O—Al≡键结构的形成有关[10]。通过比较可以发现,在150 ℃脱附温度下,9Fe/Al-PILC-10表面的Brønsted酸含量显著高于9Fe/Al-PILC-40,这与Al-PILC-10载体中Al含量较高相关,故Al3+/蒙脱土比例的改变可对催化剂表面的酸性位性质产生影响。结合催化性能实验结果(图3(a))可以发现,9Fe/Al-PILC-10催化剂表面形成的大量Brønsted酸性位点应该有助于提升催化剂的C3H6-SCR催化活性,使其表现出最佳的NO脱除效率。
2.1. Al含量对9Fe/Al-PILC的C3H6-SCR特性的影响
2.2. 焙烧温度对9Fe/Al-PILC-10脱硝活性的影响
2.3. 催化剂物相的XRD表征
2.4. BET和ICP表征
2.5. H2-TPR表征
2.6. UV-vis表征
2.7. Py-IR表征
-
1)研究制备了不同Al3+/蒙脱土比例的铝柱撑蒙脱土负载铁离子催化剂9Fe/Al-PILC,考察了其催化C3H6选择性还原NO的特性。结果表明,催化剂活性高低排序为9Fe/Al-PILC-10>9Fe/Al-PILC-20>9Fe/Al-PILC-5>9Fe/蒙脱土>9Fe/Al-PILC-40。Al3+/蒙脱土比例为10 mmol·g−1时,制备的9Fe/Al-PILC-10催化剂活性最高,在400 ℃时的脱硝效率可达100%。
2)催化剂的表征结果表明,当Al3+/蒙脱土比例为10 mmol·g−1时, Al-PILC-10载体的比表面积、孔容和平均孔径均较大,表现出最佳的柱撑效果。以此为载体制备的9Fe/Al-PILC-10催化剂具有较大的比表面积和较好的中低温氧化还原性能,催化剂表面形成了较多的低聚态FexOy物种以及Brønsted酸性位点,有利于促进催化剂表面C3H6-SCR反应的进行,从而提高催化的反应活性。
3)当焙烧温度为500 ℃时,所制备的Al-PILC-10柱撑效果最佳,以此为载体制备的9Fe/Al-PILC-10-500催化剂具有最佳的C3H6-SCR活性。XRD表征结果表明,300 ℃和700 ℃下焙烧的Al-PILC-10-300与Al-PILC-10-700载体发生层间柱撑塌陷,从而导致制备的催化剂活性下降。



 下载:
下载: